User login
Ibrutinib associated with decreased circulating malignant cells and restored T-cell function in CLL patients
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
FROM LEUKEMIA RESEARCH
Beat AML: Precision medicine strategy feasible, superior to SOC for AML
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
FROM NATURE MEDICINE
Rising IBD rates in minorities heighten need for awareness, strategies to close treatment gaps
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
FROM GASTROENTEROLOGY
Template Design and Analysis: Integrating Informatics Solutions to Improve Clinical Documentation
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
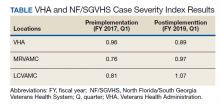
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
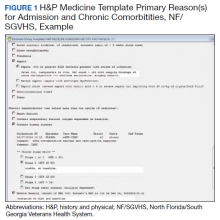
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
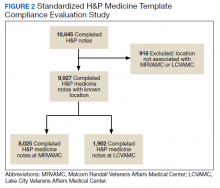
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
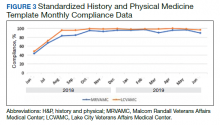
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Triple combination therapy for cystic fibrosis linked to plunging hospitalizations
.
The triple combination therapy elexacaftor/tezacaftor/ivacaftor was associated with a near elimination of hospital stays in one hospital in Oregon, according to a new report. The hospital savings still weren’t nearly enough to pay for the cost of therapy, but the study underscores what many institutions have observed and adds a new layer to the view of quality of life improvements that the new therapy brings.
“After we started prescribing it, we noticed pretty quickly that hospitalizations appeared to be declining after patients started triple combination therapy, and we were hearing [similar reports] from other centers as well. We wanted to quantify this,” Eric C. Walter, MD, a pulmonologist at the Kaiser Permanente Cystic Fibrosis Clinic in Portland, Ore., said during a presentation of the results at the virtual North American Cystic Fibrosis Conference.
“We’re seeing that across the board in real practice, the number of cystic fibrosis patients that have to be hospitalized since starting this triple combination has gone down,” Robert Giusti, MD, said in an interview. “When they’ve had pulmonary exacerbations in the past, it was frequently because they failed outpatient antibiotics, but I think with triple combination therapy, if they do get sick, the likelihood is they will respond to oral antibiotics, so they may not need that prolonged IV course in the hospital.” Dr. Giusti is clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center. He was not involved in the study.
The therapy gained Food and Drug Administration approval in 2019 for the treatment of individuals with CF who are aged 12 years and older, and who have at least one copy of the F508del mutation. Its cost is about $317,000 per year within the Kaiser Permanente system, according to Dr. Walter. His group compared hospitalization days for CF-related diagnoses from Jan. 1 through Aug. 31, 2020, before and after initiation of triple combination therapy.
Of 47 eligible patients, 32 initiated therapy during the study period; 38% had severe lung disease, defined by forced expiratory volume in 1 second (FEV1) value less than 40%. In 2020, before initiation of therapy, there were an average of 27 hospital days per month, all among patients with severe lung disease.
Among the therapy group, there were no hospitalizations after initiation of therapy through Aug. 31. Dr. Walter noted that the first hospitalization of a patient on triple combination therapy didn’t occur until early October.
At an average daily cost of $6,700, the researchers calculated that triple combination therapy saved about $189,000 per month in this group of patients. Comparing numbers to previous years, in which some patients with FEV1 greater than 40% were hospitalized, the researchers calculated that the therapy saved about $151,000 per month among individuals with severe lung disease: Patients with severe lung disease contributed about 80% to total hospital costs.
The drug itself for the whole group cost $845,000, dwarfing the $189,000 savings overall. But among patients with severe disease, hospitalization savings were about $151,000 per month, while the drug cost in this group was $316,800 per month.
Cost savings are important, but the improvement in quality of life for a patient – avoiding hospitalization, fewer impacts on work and education – should not be overlooked, according to Ryan Perkins, MD, a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, who moderated the session. “Some of these aren’t things people typically quantify and assign a price tag to,” Dr. Perkins said in an interview.
A big limitation of the work is that it was conducted during the COVID-19 pandemic, which may have reduced hospitalizations. “We did have patients that called in, told us they were sick, that they needed to be treated for an exacerbation but didn’t want to go to the hospital,” said Dr. Walter. To help adjust for this, Dr. Walter’s team plans to compare intravenous antibiotic exposure before and after triple combination therapy, reasoning that it could help clarify the pandemic’s impact on hospitalizations.
Dr. Walter, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
SOURCE: Walter E et al. NACFC 2020. Abstract 795.
.
The triple combination therapy elexacaftor/tezacaftor/ivacaftor was associated with a near elimination of hospital stays in one hospital in Oregon, according to a new report. The hospital savings still weren’t nearly enough to pay for the cost of therapy, but the study underscores what many institutions have observed and adds a new layer to the view of quality of life improvements that the new therapy brings.
“After we started prescribing it, we noticed pretty quickly that hospitalizations appeared to be declining after patients started triple combination therapy, and we were hearing [similar reports] from other centers as well. We wanted to quantify this,” Eric C. Walter, MD, a pulmonologist at the Kaiser Permanente Cystic Fibrosis Clinic in Portland, Ore., said during a presentation of the results at the virtual North American Cystic Fibrosis Conference.
“We’re seeing that across the board in real practice, the number of cystic fibrosis patients that have to be hospitalized since starting this triple combination has gone down,” Robert Giusti, MD, said in an interview. “When they’ve had pulmonary exacerbations in the past, it was frequently because they failed outpatient antibiotics, but I think with triple combination therapy, if they do get sick, the likelihood is they will respond to oral antibiotics, so they may not need that prolonged IV course in the hospital.” Dr. Giusti is clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center. He was not involved in the study.
The therapy gained Food and Drug Administration approval in 2019 for the treatment of individuals with CF who are aged 12 years and older, and who have at least one copy of the F508del mutation. Its cost is about $317,000 per year within the Kaiser Permanente system, according to Dr. Walter. His group compared hospitalization days for CF-related diagnoses from Jan. 1 through Aug. 31, 2020, before and after initiation of triple combination therapy.
Of 47 eligible patients, 32 initiated therapy during the study period; 38% had severe lung disease, defined by forced expiratory volume in 1 second (FEV1) value less than 40%. In 2020, before initiation of therapy, there were an average of 27 hospital days per month, all among patients with severe lung disease.
Among the therapy group, there were no hospitalizations after initiation of therapy through Aug. 31. Dr. Walter noted that the first hospitalization of a patient on triple combination therapy didn’t occur until early October.
At an average daily cost of $6,700, the researchers calculated that triple combination therapy saved about $189,000 per month in this group of patients. Comparing numbers to previous years, in which some patients with FEV1 greater than 40% were hospitalized, the researchers calculated that the therapy saved about $151,000 per month among individuals with severe lung disease: Patients with severe lung disease contributed about 80% to total hospital costs.
The drug itself for the whole group cost $845,000, dwarfing the $189,000 savings overall. But among patients with severe disease, hospitalization savings were about $151,000 per month, while the drug cost in this group was $316,800 per month.
Cost savings are important, but the improvement in quality of life for a patient – avoiding hospitalization, fewer impacts on work and education – should not be overlooked, according to Ryan Perkins, MD, a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, who moderated the session. “Some of these aren’t things people typically quantify and assign a price tag to,” Dr. Perkins said in an interview.
A big limitation of the work is that it was conducted during the COVID-19 pandemic, which may have reduced hospitalizations. “We did have patients that called in, told us they were sick, that they needed to be treated for an exacerbation but didn’t want to go to the hospital,” said Dr. Walter. To help adjust for this, Dr. Walter’s team plans to compare intravenous antibiotic exposure before and after triple combination therapy, reasoning that it could help clarify the pandemic’s impact on hospitalizations.
Dr. Walter, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
SOURCE: Walter E et al. NACFC 2020. Abstract 795.
.
The triple combination therapy elexacaftor/tezacaftor/ivacaftor was associated with a near elimination of hospital stays in one hospital in Oregon, according to a new report. The hospital savings still weren’t nearly enough to pay for the cost of therapy, but the study underscores what many institutions have observed and adds a new layer to the view of quality of life improvements that the new therapy brings.
“After we started prescribing it, we noticed pretty quickly that hospitalizations appeared to be declining after patients started triple combination therapy, and we were hearing [similar reports] from other centers as well. We wanted to quantify this,” Eric C. Walter, MD, a pulmonologist at the Kaiser Permanente Cystic Fibrosis Clinic in Portland, Ore., said during a presentation of the results at the virtual North American Cystic Fibrosis Conference.
“We’re seeing that across the board in real practice, the number of cystic fibrosis patients that have to be hospitalized since starting this triple combination has gone down,” Robert Giusti, MD, said in an interview. “When they’ve had pulmonary exacerbations in the past, it was frequently because they failed outpatient antibiotics, but I think with triple combination therapy, if they do get sick, the likelihood is they will respond to oral antibiotics, so they may not need that prolonged IV course in the hospital.” Dr. Giusti is clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center. He was not involved in the study.
The therapy gained Food and Drug Administration approval in 2019 for the treatment of individuals with CF who are aged 12 years and older, and who have at least one copy of the F508del mutation. Its cost is about $317,000 per year within the Kaiser Permanente system, according to Dr. Walter. His group compared hospitalization days for CF-related diagnoses from Jan. 1 through Aug. 31, 2020, before and after initiation of triple combination therapy.
Of 47 eligible patients, 32 initiated therapy during the study period; 38% had severe lung disease, defined by forced expiratory volume in 1 second (FEV1) value less than 40%. In 2020, before initiation of therapy, there were an average of 27 hospital days per month, all among patients with severe lung disease.
Among the therapy group, there were no hospitalizations after initiation of therapy through Aug. 31. Dr. Walter noted that the first hospitalization of a patient on triple combination therapy didn’t occur until early October.
At an average daily cost of $6,700, the researchers calculated that triple combination therapy saved about $189,000 per month in this group of patients. Comparing numbers to previous years, in which some patients with FEV1 greater than 40% were hospitalized, the researchers calculated that the therapy saved about $151,000 per month among individuals with severe lung disease: Patients with severe lung disease contributed about 80% to total hospital costs.
The drug itself for the whole group cost $845,000, dwarfing the $189,000 savings overall. But among patients with severe disease, hospitalization savings were about $151,000 per month, while the drug cost in this group was $316,800 per month.
Cost savings are important, but the improvement in quality of life for a patient – avoiding hospitalization, fewer impacts on work and education – should not be overlooked, according to Ryan Perkins, MD, a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, who moderated the session. “Some of these aren’t things people typically quantify and assign a price tag to,” Dr. Perkins said in an interview.
A big limitation of the work is that it was conducted during the COVID-19 pandemic, which may have reduced hospitalizations. “We did have patients that called in, told us they were sick, that they needed to be treated for an exacerbation but didn’t want to go to the hospital,” said Dr. Walter. To help adjust for this, Dr. Walter’s team plans to compare intravenous antibiotic exposure before and after triple combination therapy, reasoning that it could help clarify the pandemic’s impact on hospitalizations.
Dr. Walter, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
SOURCE: Walter E et al. NACFC 2020. Abstract 795.
FROM NACFC 2020
Study supports genetic testing in older women with breast cancer
according to researchers.
The prevalence of pathogenic variants in genes predisposing women to breast cancer was 3.18% among women with breast cancer and 1.48% among women without breast cancer in a case-control study of 26,707 women older than 65 years.
Variants in BRCA1/2, CHEK2, and PALB2 were significantly associated with increased breast cancer risk. The residual risk of breast cancer for women aged 66-85 years was 18.3% for BRCA1, 18.6% for BRCA2, 14.9% for CHEK2, and 15.8% for PALB2. In comparison, the residual risk of breast cancer for the general population was 6.8%, according to Surveillance, Epidemiology, and End Results data.
The investigators noted that women who develop breast cancer beyond 65 years of age – a large percentage of the breast cancer population – do not often qualify for genetic testing, but the frequency of pathogenic variants “is not negligible in this population” and significantly elevates remaining lifetime risk.
The data from this study “can be used to reevaluate cancer screening and additional risk management strategies for women over the age of 65,” investigator Nicholas Boddicker, PhD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in a poster presentation.
The researchers presented their findings at the American Society of Human Genetics Virtual Meeting 2020.
Results may inform guidelines
National guidelines generally recommend screening for genetic variants when women develop breast cancer early in life or if they have a family history of breast cancer, but there has been controversy about whether those screening recommendations should be expanded, Dr. Boddicker and colleagues noted. The team thinks data from their study should help inform the discussion.
“We had an idea that the prevalence of these mutations in this population was not going to be zero, but I am not sure we were thinking that it was going to be over 3%. We believe these data will assist with reassessing genetic testing guidelines,” Dr. Boddicker said in an interview.
He said expanding genetic screening to include older women would have clinical implications. Women found to have pathogenic variants could perhaps undergo MRI surveillance in addition to mammography. If they are especially high risk, prophylactic mastectomy could be considered. Also, newer treatments hinge on the presence of pathogenic variants, such as PARP inhibitors for HER2-negative metastatic breast cancer with BRCA mutations.
Current testing limits ‘ridiculous’
“This is an excellent study and shows that even women over 65 have significant risk of breast cancer if they have a pathogenic variant. The variant could absolutely affect their treatment,” said Peter Beitsch, MD, a breast cancer surgical oncologist at the Dallas Surgical Group.
Dr. Beitsch was the lead author of a study, published in the Journal of Clinical Oncology in 2019, that showed that nearly half of breast cancer patients with a clinically actionable pathogenic variant were missed by current testing guidelines.
“All patients with a diagnosis of breast cancer [should] undergo expanded panel testing,” Dr. Beitsch and colleagues concluded in the paper.
Dr. Beitsch said current limitations on genetic screening make “no common sense. It’s OK to genetically test a woman who is 64 years and 11 months, but not 1 month later? Obviously ridiculous,” he said when asked for comment on the new report.
“The bigger impact is on their relatives,” Dr. Beitsch added. “Identifying people (men and women) with the same pathogenic variant can potentially save lives from more intensive screening or even prevent a cancer by doing prophylactic mastectomies. Male relatives have increased incidence of cancers with pathogenic variants in many of these genes.”
Screening for those variants could “lead to earlier detection or prevention,” Dr. Beitsch said.
As Dr. Boddicker noted, however, there is the question of who would pay for expanded screening and how to counsel patients who, despite increased risk, may never develop cancer.
Study details and next steps
The study included 13,762 women with breast cancer who were older than 65 years and 12,945 age-matched controls without breast cancer. A multigene amplicon-based panel was used to identify 12 known pathogenic variants in breast cancer–predisposing genes.
The women were part of the CARRIERS consortium, which pools breast cancer patients from case-control studies. Overall, 82.6% of subjects were non-Hispanic White, 25.6% of breast cancer patients and 17.9% of control subjects had a positive family history, and the mean age was 72.8 years (range, 66-94.3 years).
Across the entire study population, 0.48% of subjects had variants in ATM, 0.18% in BRCA1, 0.49% in BRCA2, 0.67% in CHEK2, and 0.23% in PALB2.
After adjustment for age, race, and family history, pathogenic variants in BRCA1 increased the risk of cancer more than threefold (odds ratio, 3.37), with similar findings for BRCA2 (OR, 2.64), PALB2 (OR, 3.09), and CHEK2 (OR, 2.13). ATM variants were not associated with a significantly increased risk of breast cancer (OR, 1.38).
Dr. Boddicker said the researchers’ next steps are to incorporate polygenic risk scores into the analyses and further investigate the impact of race.
The study is funded by the National Institutes of Health. Dr. Boddicker and Dr. Beitsch didn’t have any disclosures.
SOURCE: Boddicker NJ et al. ASHG 2020, Abstract 2412.
according to researchers.
The prevalence of pathogenic variants in genes predisposing women to breast cancer was 3.18% among women with breast cancer and 1.48% among women without breast cancer in a case-control study of 26,707 women older than 65 years.
Variants in BRCA1/2, CHEK2, and PALB2 were significantly associated with increased breast cancer risk. The residual risk of breast cancer for women aged 66-85 years was 18.3% for BRCA1, 18.6% for BRCA2, 14.9% for CHEK2, and 15.8% for PALB2. In comparison, the residual risk of breast cancer for the general population was 6.8%, according to Surveillance, Epidemiology, and End Results data.
The investigators noted that women who develop breast cancer beyond 65 years of age – a large percentage of the breast cancer population – do not often qualify for genetic testing, but the frequency of pathogenic variants “is not negligible in this population” and significantly elevates remaining lifetime risk.
The data from this study “can be used to reevaluate cancer screening and additional risk management strategies for women over the age of 65,” investigator Nicholas Boddicker, PhD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in a poster presentation.
The researchers presented their findings at the American Society of Human Genetics Virtual Meeting 2020.
Results may inform guidelines
National guidelines generally recommend screening for genetic variants when women develop breast cancer early in life or if they have a family history of breast cancer, but there has been controversy about whether those screening recommendations should be expanded, Dr. Boddicker and colleagues noted. The team thinks data from their study should help inform the discussion.
“We had an idea that the prevalence of these mutations in this population was not going to be zero, but I am not sure we were thinking that it was going to be over 3%. We believe these data will assist with reassessing genetic testing guidelines,” Dr. Boddicker said in an interview.
He said expanding genetic screening to include older women would have clinical implications. Women found to have pathogenic variants could perhaps undergo MRI surveillance in addition to mammography. If they are especially high risk, prophylactic mastectomy could be considered. Also, newer treatments hinge on the presence of pathogenic variants, such as PARP inhibitors for HER2-negative metastatic breast cancer with BRCA mutations.
Current testing limits ‘ridiculous’
“This is an excellent study and shows that even women over 65 have significant risk of breast cancer if they have a pathogenic variant. The variant could absolutely affect their treatment,” said Peter Beitsch, MD, a breast cancer surgical oncologist at the Dallas Surgical Group.
Dr. Beitsch was the lead author of a study, published in the Journal of Clinical Oncology in 2019, that showed that nearly half of breast cancer patients with a clinically actionable pathogenic variant were missed by current testing guidelines.
“All patients with a diagnosis of breast cancer [should] undergo expanded panel testing,” Dr. Beitsch and colleagues concluded in the paper.
Dr. Beitsch said current limitations on genetic screening make “no common sense. It’s OK to genetically test a woman who is 64 years and 11 months, but not 1 month later? Obviously ridiculous,” he said when asked for comment on the new report.
“The bigger impact is on their relatives,” Dr. Beitsch added. “Identifying people (men and women) with the same pathogenic variant can potentially save lives from more intensive screening or even prevent a cancer by doing prophylactic mastectomies. Male relatives have increased incidence of cancers with pathogenic variants in many of these genes.”
Screening for those variants could “lead to earlier detection or prevention,” Dr. Beitsch said.
As Dr. Boddicker noted, however, there is the question of who would pay for expanded screening and how to counsel patients who, despite increased risk, may never develop cancer.
Study details and next steps
The study included 13,762 women with breast cancer who were older than 65 years and 12,945 age-matched controls without breast cancer. A multigene amplicon-based panel was used to identify 12 known pathogenic variants in breast cancer–predisposing genes.
The women were part of the CARRIERS consortium, which pools breast cancer patients from case-control studies. Overall, 82.6% of subjects were non-Hispanic White, 25.6% of breast cancer patients and 17.9% of control subjects had a positive family history, and the mean age was 72.8 years (range, 66-94.3 years).
Across the entire study population, 0.48% of subjects had variants in ATM, 0.18% in BRCA1, 0.49% in BRCA2, 0.67% in CHEK2, and 0.23% in PALB2.
After adjustment for age, race, and family history, pathogenic variants in BRCA1 increased the risk of cancer more than threefold (odds ratio, 3.37), with similar findings for BRCA2 (OR, 2.64), PALB2 (OR, 3.09), and CHEK2 (OR, 2.13). ATM variants were not associated with a significantly increased risk of breast cancer (OR, 1.38).
Dr. Boddicker said the researchers’ next steps are to incorporate polygenic risk scores into the analyses and further investigate the impact of race.
The study is funded by the National Institutes of Health. Dr. Boddicker and Dr. Beitsch didn’t have any disclosures.
SOURCE: Boddicker NJ et al. ASHG 2020, Abstract 2412.
according to researchers.
The prevalence of pathogenic variants in genes predisposing women to breast cancer was 3.18% among women with breast cancer and 1.48% among women without breast cancer in a case-control study of 26,707 women older than 65 years.
Variants in BRCA1/2, CHEK2, and PALB2 were significantly associated with increased breast cancer risk. The residual risk of breast cancer for women aged 66-85 years was 18.3% for BRCA1, 18.6% for BRCA2, 14.9% for CHEK2, and 15.8% for PALB2. In comparison, the residual risk of breast cancer for the general population was 6.8%, according to Surveillance, Epidemiology, and End Results data.
The investigators noted that women who develop breast cancer beyond 65 years of age – a large percentage of the breast cancer population – do not often qualify for genetic testing, but the frequency of pathogenic variants “is not negligible in this population” and significantly elevates remaining lifetime risk.
The data from this study “can be used to reevaluate cancer screening and additional risk management strategies for women over the age of 65,” investigator Nicholas Boddicker, PhD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in a poster presentation.
The researchers presented their findings at the American Society of Human Genetics Virtual Meeting 2020.
Results may inform guidelines
National guidelines generally recommend screening for genetic variants when women develop breast cancer early in life or if they have a family history of breast cancer, but there has been controversy about whether those screening recommendations should be expanded, Dr. Boddicker and colleagues noted. The team thinks data from their study should help inform the discussion.
“We had an idea that the prevalence of these mutations in this population was not going to be zero, but I am not sure we were thinking that it was going to be over 3%. We believe these data will assist with reassessing genetic testing guidelines,” Dr. Boddicker said in an interview.
He said expanding genetic screening to include older women would have clinical implications. Women found to have pathogenic variants could perhaps undergo MRI surveillance in addition to mammography. If they are especially high risk, prophylactic mastectomy could be considered. Also, newer treatments hinge on the presence of pathogenic variants, such as PARP inhibitors for HER2-negative metastatic breast cancer with BRCA mutations.
Current testing limits ‘ridiculous’
“This is an excellent study and shows that even women over 65 have significant risk of breast cancer if they have a pathogenic variant. The variant could absolutely affect their treatment,” said Peter Beitsch, MD, a breast cancer surgical oncologist at the Dallas Surgical Group.
Dr. Beitsch was the lead author of a study, published in the Journal of Clinical Oncology in 2019, that showed that nearly half of breast cancer patients with a clinically actionable pathogenic variant were missed by current testing guidelines.
“All patients with a diagnosis of breast cancer [should] undergo expanded panel testing,” Dr. Beitsch and colleagues concluded in the paper.
Dr. Beitsch said current limitations on genetic screening make “no common sense. It’s OK to genetically test a woman who is 64 years and 11 months, but not 1 month later? Obviously ridiculous,” he said when asked for comment on the new report.
“The bigger impact is on their relatives,” Dr. Beitsch added. “Identifying people (men and women) with the same pathogenic variant can potentially save lives from more intensive screening or even prevent a cancer by doing prophylactic mastectomies. Male relatives have increased incidence of cancers with pathogenic variants in many of these genes.”
Screening for those variants could “lead to earlier detection or prevention,” Dr. Beitsch said.
As Dr. Boddicker noted, however, there is the question of who would pay for expanded screening and how to counsel patients who, despite increased risk, may never develop cancer.
Study details and next steps
The study included 13,762 women with breast cancer who were older than 65 years and 12,945 age-matched controls without breast cancer. A multigene amplicon-based panel was used to identify 12 known pathogenic variants in breast cancer–predisposing genes.
The women were part of the CARRIERS consortium, which pools breast cancer patients from case-control studies. Overall, 82.6% of subjects were non-Hispanic White, 25.6% of breast cancer patients and 17.9% of control subjects had a positive family history, and the mean age was 72.8 years (range, 66-94.3 years).
Across the entire study population, 0.48% of subjects had variants in ATM, 0.18% in BRCA1, 0.49% in BRCA2, 0.67% in CHEK2, and 0.23% in PALB2.
After adjustment for age, race, and family history, pathogenic variants in BRCA1 increased the risk of cancer more than threefold (odds ratio, 3.37), with similar findings for BRCA2 (OR, 2.64), PALB2 (OR, 3.09), and CHEK2 (OR, 2.13). ATM variants were not associated with a significantly increased risk of breast cancer (OR, 1.38).
Dr. Boddicker said the researchers’ next steps are to incorporate polygenic risk scores into the analyses and further investigate the impact of race.
The study is funded by the National Institutes of Health. Dr. Boddicker and Dr. Beitsch didn’t have any disclosures.
SOURCE: Boddicker NJ et al. ASHG 2020, Abstract 2412.
FROM ASHG 2020
The evidence for noncosmetic uses of botulinum toxin
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.
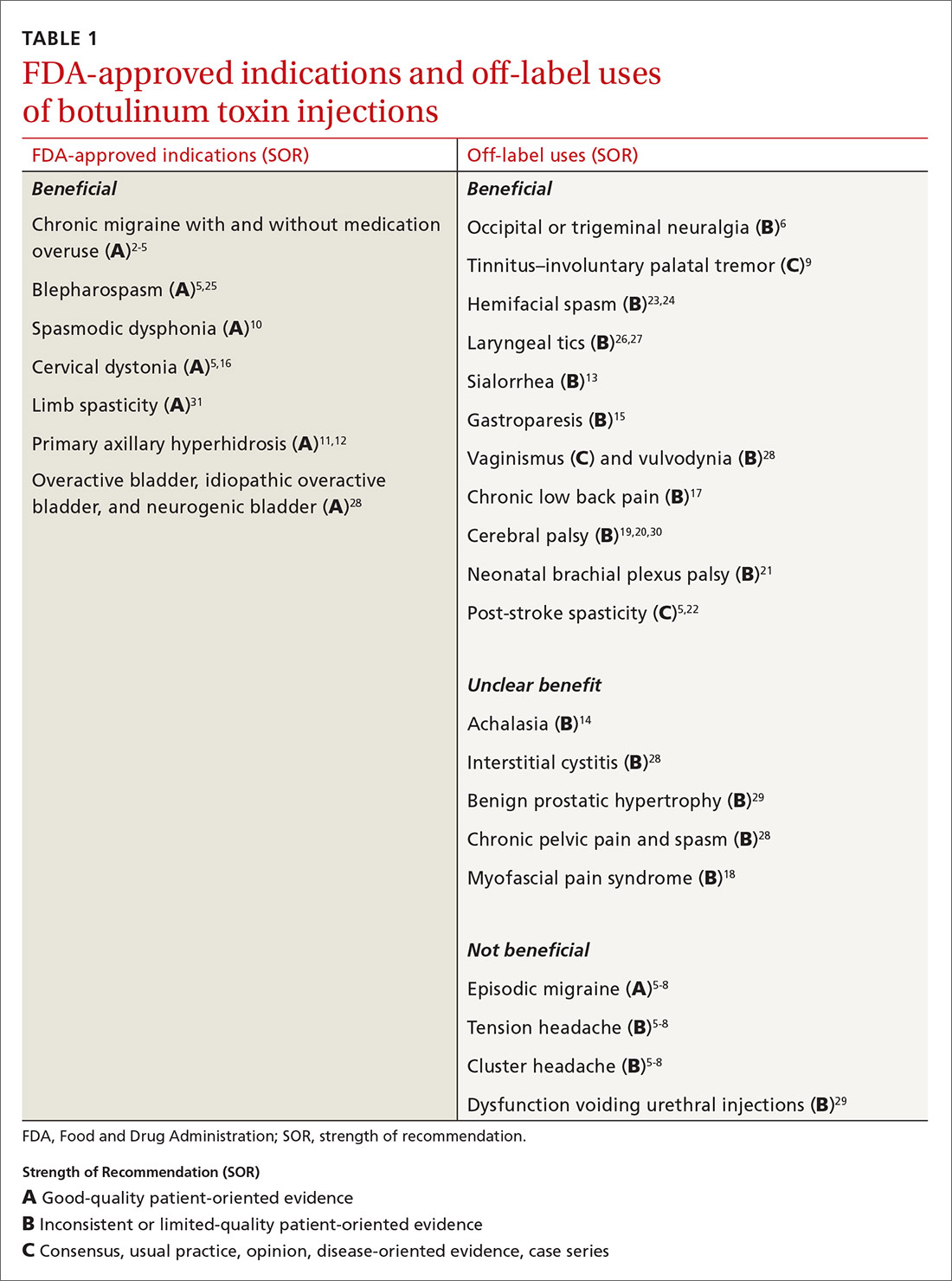
But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).
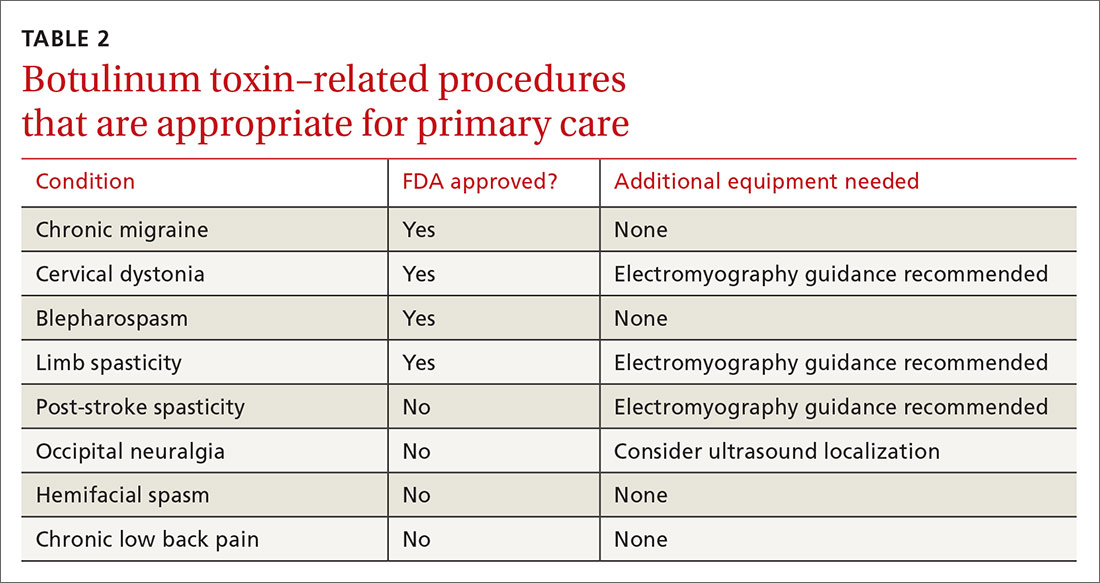
Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.

But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).

Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
Mention the word “botulinum toxin” and one’s mind is likely to go to the big business of cosmetic procedures. Among the 15.7 minimally invasive cosmetic procedures performed in 2017, botulinum toxin type A (BoNT-A) made up the largest share, with 7.23 million procedures.1 However, botulinum toxin—which was first recognized for the ability to paralyze muscles through decreased release of acetylcholine—also has many pain-related and noncosmetic uses; some are approved by the US Food and Drug Administration (FDA) and others are off-label (see TABLE 12-31). This review provides an evidence-based look at these uses, from those that have good evidence to support them—including chronic migraine and overactive bladder—to those that have limited (or no) evidence to support them—such as chronic pelvic pain and cluster headache.

But before we get into the evidence behind specific uses for botulinum toxin, let’s review the available options and the potential risks they pose.
Many options
Although botulinum toxin is produced by Clostridium botulinum, the synthetic process to produce pharmaceuticals is patented and branded. BoNT-A is 1 of 7 recognized serotypes derived from C botulinum; some examples of BoNT-A include onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA. Clinically, the differences are minor, but they do allow for use of other brands if a patient becomes intolerant to the selected therapy. Treatment doses and costs for each brand vary.
Training. Primary care providers can obtain didactic training from pharmaceutical companies as well as skills training through workshops on botulinum toxin. Credentialed providers can perform some procedures in the primary care setting (TABLE 2).

Adverse effects also vary depending on the formulation and the sites injected. Patients generally tolerate the procedure well, with discomfort from injections and localized bleeding as the major complaints. However, systemic events such as anaphylaxis and antibody development can occur. Depending on the formulation injected, the molecule can migrate and cause weakness in adjacent muscles, leading to undesired effects. Compensatory muscles can become strained, resulting in pain. Serious complications such as pneumonia and death have occurred with injection of botulinum toxin in or around the neck.
A note about pain management. In addition to muscle relaxation, analgesic properties are among the identified benefits of BoNT-A injections.32,33 BoNT-A suppresses the release of norepinephrine, substance P, and glutamate, which reduces pain sensitization.32 However, the extent of ongoing research involving BoNT-A uses in pain management exceeds the scope of this article. Some pain-related indications will be discussed, but the focus will be on other noncosmetic uses.
Headache disorders
Chronic migraine affects 1.3% to 2.2% of the population and is defined as headaches occurring ≥ 15 days (≥ 8 migrainous days) per month.2 To qualify for BoNT-A treatment, patients must have tried 2 prophylactic medications that failed to provide relief, and their headaches must last at least 4 hours. Injections every 12 weeks with 5 U in each of 31 prescribed sites is effective, as shown in the PREEMPT 2 study2 with external verification.3 The 24-week, double-blind, placebo-controlled study showed that BoNT-A treatment reduced headache days by 9 days (P < .001) and migraine days by 8.7 days (P < .001)2 and, at 108 weeks, injections reduced headache days by 10.7 days (P < .0001).4,5
Continue to: Episodic migraine, tension headache, and cluster headaches
Episodic migraine, tension headache, and cluster headaches. There is no significant BoNT-A-related pain reduction in episodic migraine (n = 1838; 0.05 headaches/mo; 95% CI, –0.26 to –0.36) or tension headaches (n = 675; –1.43 headaches/mo; 95% CI, –3.13 to –0.27).5,6 For cluster headaches, a single prospective study with low enrollment showed no consistent benefit,7 while a pilot study showed some improvement, with reduction of attacks by 50% in half of subjects.8
Occipital neuralgia and trigeminal neuralgia entail paroxysmal, brief, shock-like pain without associated deficits affecting the respective nerve distributions. Multiple prospective and double-blind placebo-controlled studies with relatively low enrollment show consistent improvement in pain intensity, number of pain-free days, analgesic consumption, and headache frequency with BoNT-A added to nerve blocks.6
ENT disorders
Tinnitus by involuntary palatal tremor causes a discontinuous clicking noise. Palatal tremor can be treated with BoNT-A 15 U to tensor veli palatini and levator veli muscles to provide temporary relief for 2 to 6 months.9
Spasmodic dysphonia and voice tremor are the result of laryngeal hyperkinesis, and BoNT-A has been deemed the gold standard of treatment. BoNT-A is administered via bilateral injection of the thyroarytenoid muscles for patients with adductor-type spasmodic dysphonia and of the posterior cricoarytenoid muscles for those with the abductor type. A series of 1300 patients (predominantly with the adductor type) treated with BoNT-A showed a 100% improvement in symptoms for 6 to 15 weeks. Patients with abductor-type spasmodic dysphonia were found to have 89% improvement in Voice Related Quality of Life Index score.10
Secretory disorders
Primary axillary hyperhidrosis (PAH) is an idiopathic excessive production of sweat occurring for at least 6 months, typically with onset before age 25 years. PAH can cause significant psychosocial and physical impairment. Current treatments include topical aluminum chloride, systemic anticholinergics, and thoracic sympathectomy, which can provide temporary relief but are not well tolerated.
Continue to: BoNT-A treatment is efficacious...
BoNT-A treatment is efficacious, safe, and improves quality of life for PAH patients. A 52-week, multicenter, double-blind, randomized, placebo-controlled study showed significant reductions in symptom severity, decreased sweating at rest by gravimetric testing, and improvements in self-reported quality of life.11 A 10-year retrospective study in patients ages 12 years and older showed a 75% to 100% improvement in hyperhidrosis, with a median treatment effect duration of 7 months.12
Sialorrhea, or hypersalivation, is typically associated with neurological conditions such as cerebral palsy, amyotrophic lateral sclerosis, Parkinson disease, and posttraumatic brain injuries. It typically is treated with anticholinergic drugs, surgery, and irradiation of salivary glands, which can have significant adverse effects and complications. In a randomized blinded study, BoNT-A injections in the parotid and submandibular glands resulted in a dramatic reduction of sialorrhea and were safe and well tolerated.13
Gastric disorders
Achalasia is a syndrome of aperistalsis and incomplete lower esophageal sphincter (LES) relaxation with a “bird beak” appearance on barium swallow. Patients who meet diagnostic criteria are treated with pneumatic dilation or myotomy; however, some patients demonstrate symptoms of achalasia but don’t meet the diagnostic criteria. In these patients, BoNT-A injection in the LES provides symptomatic relief. In a case series, LES BoNT-A injections 20 U were used as a decision tool in whether to proceed with definitive treatment.14
Gastroparesis is a disorder of impaired gastric motility without mechanical obstruction. Pyloric sphincter BoNT-A injections are useful in refractory patients. Multiple prospective, noncontrolled (4), retrospective (3), and randomized placebo-controlled (2), studies with limited enrollment showed benefit for 37.5% to 100% of patients receiving BoNT-A injections of 80 to 200 U.15
Musculoskeletal disorders
Cervical dystonia (CD) entails involuntary contractions of the neck and upper shoulder musculature, causing abnormal neck, shoulder, and head posturing. BoNT-A is first-line treatment for CD.5 BoNT-A is more efficacious than trihexyphenidyl based on multiple large, high-quality studies.16
Continue to: Chronic low back pain
Chronic low back pain (CLBP) is defined as back pain persisting ≥ 12 weeks. More than 80% of adults have had at least 1 episode of back pain in their lifetime.
Myofascial pain syndrome (MPS) consists of myofascial trigger points (palpable, tender nodules that produce pain) with multiple pathophysiological etiologies that include dysfunctional acetylcholine activity, which releases nociceptive neurotransmitters. Studies have yielded inconsistent effects of BoNT-A on MPS.18
Spastic disorders
Cerebral palsy (CP) involves altered muscle tone, posture, and movement secondary to central motor dysfunction with spasticity. Evaluation of BoNT-A as an adjunctive therapy in CP has been extensive and conflicting. A prospective cohort study evaluating gastrocsoleus BoNT-A injections along with gait analysis in 37 children with CP showed no significant improvements.30 In 60 children with CP who received BoNT-A injections, there was improvement in muscle tone and range of motion, while gait improved in patients up to (but not after) age 7 years.19 A multicenter Dutch study of 65 children compared BoNT-A injections in addition to a comprehensive rehabilitation program vs rehabilitation alone, with no difference identified.20
Neonatal brachial plexus palsy (NBPP) is damage to the brachial plexus as a result of trauma during the perinatal period. It is typically self-resolving but can cause residual functional impairment. Surgery is recommended for serious injuries or if functional recovery is not achieved within 9 months. Off-label use of BoNT-A has been shown to be effective in relieving muscle contractures and imbalance, but data are limited and there have only been small studies performed.21 A retrospective cohort study of 59 patients with NBPP who received BoNT-A injections showed improved range of motion and function of the affected extremity. Moreover, surgical intervention was deferred, modified, or averted in patients who were under consideration for more invasive treatment.21
Post-stroke spasticity can be temporarily relieved with the use of BoNT-A injections. Several studies have examined the effect of BoNT-A coupled with rehabilitation programs vs injections alone in the treatment of post-stroke spasticity. Devier et al found that improvements in spasticity scores did not differ between groups; however, implementing rehabilitation after BoNT-A injections was associated with improved function compared to injection alone.31 A 2018 randomized, double-blind, placebo-controlled trial demonstrated improvements in both treatment groups: those who received BoNT-A plus targeted rehab regimen and those who received saline injection plus rehab.22 In this case, it appears BoNT-A acts as more of an adjunct to physical therapy in the treatment of post-stroke spasticity.5
Continue to: Hemifacial spasm
Hemifacial spasm is an involuntary, brief, irregular unilateral (sometimes bilateral) spasm of the face in the distribution of the facial nerve. Injections with BoNT-A have been deemed effective by the American Academy of Neurology.23 A 16-year retrospective study examined the efficacy and adverse effects of BoNT-A in the treatment of hemifacial spasm in 113 patients with a mean age of 63.1 years; it demonstrated high efficacy and mild temporary adverse effects.24 The duration of improvement averaged 16 weeks; pretarsal injections had better results than preseptal injections; and there were no differences between the commercial brands.
Blepharospasm is a focal dystonia marked by excessive blinking and involuntary eye closures due to overexcitability of orbicularis oculi and periocular muscles, and BoNT-A is the treatment of choice.5,25 A retrospective review of 19 patients with blepharospasm who were treated with BoNT-A for more than 5 years found that BoNT-A is a stable and effective treatment with an adverse event rate of 4%. Additionally, there were no differences found in clinical efficacy between the 4 BoNT-A brands on the market.25
Laryngeal tics can cause significant psychosocial distress for patients. This condition is characterized by involuntary, recurrent rhythmic sounds that are often preceded by premonitory urges that are relieved by the behavior. An open-label, uncontrolled, confirmatory study with 30 subjects showed that bilateral vocal cord BoNT-A injections resulted in 93% improvement in vocal tics.26 A subsequent study highlighted case histories of 2 patients with laryngeal tics who received thyroarytenoid muscle BoNT-A injections and had marked reduction in symptoms and premonitory sensations.27 Although these small studies have suggested possible effectiveness of BoNT-A for laryngeal tics, there is no high-quality evidence.
Urologic disorders
Overactive, idiopathic overactive, or neurogenic bladder causes increased urinary frequency, urgency, and nocturia without infectious etiology; they can be a result of neurologic dysregulation, detrusor overactivity, or idiopathic causes. Intravesical BoNT-A injection of 100 to 300 U has been found effective for symptoms refractory to anticholinergic and lifestyle therapy, with increased cystometric capacity (229.1 to 427 mL, P < .00001), decreased maximum detrusor pressure (60.7 to 26.1 cm H2O, P < .00001), and resolution of urgency in 87% of patients (P < .001).28
Interstitial cystitis, also known as painful bladder syndrome, is characterized by reduced bladder emptying, urethral pressure, and residual urine pressure, with symptoms of increased urinary frequency without infection. Intravesicular BoNT-A injections have not consistently been effective in treatment of this condition.28
Continue to: Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome
Dysfunctional voiding, urethral sphincter overactivity, and Fowler syndrome involve urethral sphincter spasticity with difficulty passing urine and possibly retention. Urethral sphincter injections of 100 U BoNT-A improved flow rates and decreased residual volume. A randomized, double-blinded, placebo-controlled study showed a significantly improved International Prostate Symptom Score (IPSS), quality of life index, maximum flow rate, voided volume, and decreased detrusor voiding pressure at 1 month.29
Benign prostatic hypertrophy (BPH) is a very common condition leading to outlet obstruction. The mainstays of treatment are 5-α-reductase inhibitors, α-adrenergic blockers, and surgical removal.
Gynecologic disorders
Vaginismus is the involuntary, recurrent, or persistent contraction of the perineal muscles surrounding the outer third of the vagina; it is classified by 4 progressively more severe degrees of intensity. Levator ani, bulbospongiosus, bulbocavernosus, pubococcygeus, and/or puborectalis muscle BoNT-A injections have shown benefits in decreasing resistance to vaginal exams (95.8%) and the ability to achieve satisfactory sexual intercourse after first injection (75%-100%). Effects were transient for up to 15.4% of patients requiring repeat injections.28
Vulvodynia is vulvar pain and orgasmic difficulties and has been treated with bulbospongiosus muscle BoNT-A injections in retrospective studies. A single randomized, double-blinded, placebo-controlled study showed significantly improved pain scores after 1 to 2 injection series.28
Chronic pelvic pain is a syndrome of somatic functional or regional pain, which can be caused by the spasm of the pelvic musculature with or without trigger points. Patients with pain refractory to treatment have been treated with levator ani injections. A retrospective cohort study found 79.3% of patients experienced pain relief and 20.7% reported improved symptoms. In a double-blind, randomized, placebo-controlled trial, pelvic floor muscles were injected with 80 U BoNT or saline, and symptoms were evaluated along with vaginal manometry. BoNT was associated with a reduction in some pain but not as much as placebo, while vaginal pressures decreased more with BoNT than with placebo.28
Blake Busey, DO, FAAFP, Texas Tech University of Health Sciences El Paso–Transmountain, 2000B Transmountain Road, Suite B400, El Paso, TX 79911; [email protected]
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
1. American Society of Plastic Surgeons. New statistics reveal the shape of plastic surgery [news release]. March 1, 2018. www.plasticsurgery.org/news/press-releases/new-statistics-reveal-the-shape-of-plastic-surgery. Accessed October 23, 2020.
2. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalgia. 2010;30:804-814.
3. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616.
4. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826.
6. Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7:3818-3844.
7. Sostak P, Krause P, Förderreuther S, et al. Botulinum toxin type-A therapy in cluster headache: an open study. J Headache Pain. 2007;8:236-241.
8. Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503-509.
9. Mandavia R, Dessouky O, Dhar V, et al. The use of botulinum toxin in otorhinolaryngology: an updated review. Clin Otolaryngol. 2014;39:203-209.
10. Klein AM, Stong BC, Wise J, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:421-423.
11. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596-599.
12. Rosen R, Stewart T. Results of a 10-year follow-up study of botulinum toxin A therapy for primary axillary hyperhidrosis in Australia. Intern Med J. 2018;48:343-347.
13. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins (Basel). 2018;10:55.
14. Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
15. Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc. 2015;7:790-798.
16. Zakin E, Simpson D. Evidence on botulinum toxin in selected disorders. Toxicon. 2018;147:134-140.
17. Jabbari B, Ney J, Sichani A, et al. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264.
18. Climent JM, Kuan TS, Fenollosa P, et al. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459.
19. Mirska A, Cybula K, Okurowska-Zawada B, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517-522.
20. Schasfoort F, Pangalila R, Sneekes EM, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732-742.
21. Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6:1107-1119.
22. Prazeres A, Lira M, Aguiar P, et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol Int. 2018;10:7385.
23. Simpson DM, Blitzer A, Brashear A, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
24. Sorgun MH, Yilmaz R, Akin YA, et al. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22:1319-1325.
25. Lee S, Park S, Lew H. Long-term efficacy of botulinum neurotoxin-A treatment for essential blepharospasm. Korean J Ophthalmol. 2018;32:1-7.
26. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
27. Vincent DA Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22:251-256.
28. Moga MA, Dimienescu OG, Balan A, et al. Therapeutic approaches of botulinum toxin in gynecology. Toxins (Basel) 2018;10:169.
29. Jhang J-F, Kuo H-C. Novel applications of onabotulinumtoxinA in lower urinary tract dysfunction. Toxins (Basel). 2018;10:260.
30. Hastings-Ison T, Sangeux M, Thomason P, et al. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: a prospective kinematic study. J Child Orthop. 2018;12:390-397.
31. Devier D, Harnar J, Lopez L, et al. Rehabilitation plus onabotulinumtoxina improves motor function over onabotulinumtoxina alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins. 2017;9:216.
32. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24:1-6.
33. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes—an evidence based review. Toxicon. 2018;147:120-128.
PRACTICE RECOMMENDATIONS
› Do not use botulinum toxin for episodic migraine, tension headache, or cluster headaches. B
› Consider off-label use of botulinum toxin for select patients with occipital and trigeminal neuralgia, gastroparesis, vaginismus, benign prostatic hypertrophy, neonatal brachial plexus palsy, post-stroke spasticity, and hemifacial spasm. B
› Consider the use of botulinum toxin as an adjunct in chronic low back pain management. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
New eGFR equation ‘less biased’ by age, kidney function; some disagree
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Case of the inappropriate endoscopy referral
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
FDA clears smartphone app to interrupt PTSD-related nightmares
The Food and Drug Administration has cleared for marketing a smartphone app that can detect and interrupt nightmares in adults with posttraumatic stress disorder (PTSD).
The NightWare app, from Minneapolis-based NightWare Inc., runs on the Apple Watch and Apple iPhone.
During sleep, Apple Watch sensors monitor heart rate and body movement. These data are used to create a unique sleep profile using a proprietary algorithm.
When the NightWare app detects that a patient is experiencing a nightmare based on changes in heart rate and movement, it provides slight vibrations through the Apple Watch to arouse the patient and interrupt the nightmare, without fully awakening the patient, the company notes.
NightWare is available by prescription only and is intended for use in adults aged 22 years and older with PTSD.
“Sleep is an essential part of a person’s daily routine. However, certain adults who have a nightmare disorder or who experience nightmares from PTSD are not able to get the rest they need,” Carlos Peña, PhD, director, Office of Neurological and Physical Medicine Devices, Center for Devices and Radiological Health at the FDA, said in a news release.
This authorization “offers a new, low-risk treatment option that uses digital technology in an effort to provide temporary relief from sleep disturbance related to nightmares,” said Dr. Peña.
NightWare was tested in a 30-day randomized, sham-controlled trial of 70 patients. Patients in the sham group wore the device, but no vibrations were provided.
Both the sham and active groups showed improvement in sleep on standard sleep scales, with the active group showing greater improvement than sham. “The evidence demonstrated the probable benefits outweighed the probable risks,” the FDA said in a statement.
and other recommended therapies for PTSD-associated nightmares and nightmare disorder, the agency said.
NightWare was granted breakthrough device designation for the treatment of nightmares in patients with PTSD. The device reviewed through the de novo premarket pathway, a regulatory pathway for some low- to moderate-risk devices of a new type.
Along with this marketing authorization, the FDA is establishing “special controls” designed to provide a “reasonable assurance of safety and effectiveness for tests of this type,” the agency said.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing a smartphone app that can detect and interrupt nightmares in adults with posttraumatic stress disorder (PTSD).
The NightWare app, from Minneapolis-based NightWare Inc., runs on the Apple Watch and Apple iPhone.
During sleep, Apple Watch sensors monitor heart rate and body movement. These data are used to create a unique sleep profile using a proprietary algorithm.
When the NightWare app detects that a patient is experiencing a nightmare based on changes in heart rate and movement, it provides slight vibrations through the Apple Watch to arouse the patient and interrupt the nightmare, without fully awakening the patient, the company notes.
NightWare is available by prescription only and is intended for use in adults aged 22 years and older with PTSD.
“Sleep is an essential part of a person’s daily routine. However, certain adults who have a nightmare disorder or who experience nightmares from PTSD are not able to get the rest they need,” Carlos Peña, PhD, director, Office of Neurological and Physical Medicine Devices, Center for Devices and Radiological Health at the FDA, said in a news release.
This authorization “offers a new, low-risk treatment option that uses digital technology in an effort to provide temporary relief from sleep disturbance related to nightmares,” said Dr. Peña.
NightWare was tested in a 30-day randomized, sham-controlled trial of 70 patients. Patients in the sham group wore the device, but no vibrations were provided.
Both the sham and active groups showed improvement in sleep on standard sleep scales, with the active group showing greater improvement than sham. “The evidence demonstrated the probable benefits outweighed the probable risks,” the FDA said in a statement.
and other recommended therapies for PTSD-associated nightmares and nightmare disorder, the agency said.
NightWare was granted breakthrough device designation for the treatment of nightmares in patients with PTSD. The device reviewed through the de novo premarket pathway, a regulatory pathway for some low- to moderate-risk devices of a new type.
Along with this marketing authorization, the FDA is establishing “special controls” designed to provide a “reasonable assurance of safety and effectiveness for tests of this type,” the agency said.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing a smartphone app that can detect and interrupt nightmares in adults with posttraumatic stress disorder (PTSD).
The NightWare app, from Minneapolis-based NightWare Inc., runs on the Apple Watch and Apple iPhone.
During sleep, Apple Watch sensors monitor heart rate and body movement. These data are used to create a unique sleep profile using a proprietary algorithm.
When the NightWare app detects that a patient is experiencing a nightmare based on changes in heart rate and movement, it provides slight vibrations through the Apple Watch to arouse the patient and interrupt the nightmare, without fully awakening the patient, the company notes.
NightWare is available by prescription only and is intended for use in adults aged 22 years and older with PTSD.
“Sleep is an essential part of a person’s daily routine. However, certain adults who have a nightmare disorder or who experience nightmares from PTSD are not able to get the rest they need,” Carlos Peña, PhD, director, Office of Neurological and Physical Medicine Devices, Center for Devices and Radiological Health at the FDA, said in a news release.
This authorization “offers a new, low-risk treatment option that uses digital technology in an effort to provide temporary relief from sleep disturbance related to nightmares,” said Dr. Peña.
NightWare was tested in a 30-day randomized, sham-controlled trial of 70 patients. Patients in the sham group wore the device, but no vibrations were provided.
Both the sham and active groups showed improvement in sleep on standard sleep scales, with the active group showing greater improvement than sham. “The evidence demonstrated the probable benefits outweighed the probable risks,” the FDA said in a statement.
and other recommended therapies for PTSD-associated nightmares and nightmare disorder, the agency said.
NightWare was granted breakthrough device designation for the treatment of nightmares in patients with PTSD. The device reviewed through the de novo premarket pathway, a regulatory pathway for some low- to moderate-risk devices of a new type.
Along with this marketing authorization, the FDA is establishing “special controls” designed to provide a “reasonable assurance of safety and effectiveness for tests of this type,” the agency said.
A version of this article originally appeared on Medscape.com.