User login
Just Like Rock and Roll, Topical Medications for Psoriasis Are Here to Stay
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
Adjunctive Use of Halobetasol Propionate–Tazarotene in Biologic-Experienced Patients With Psoriasis
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).

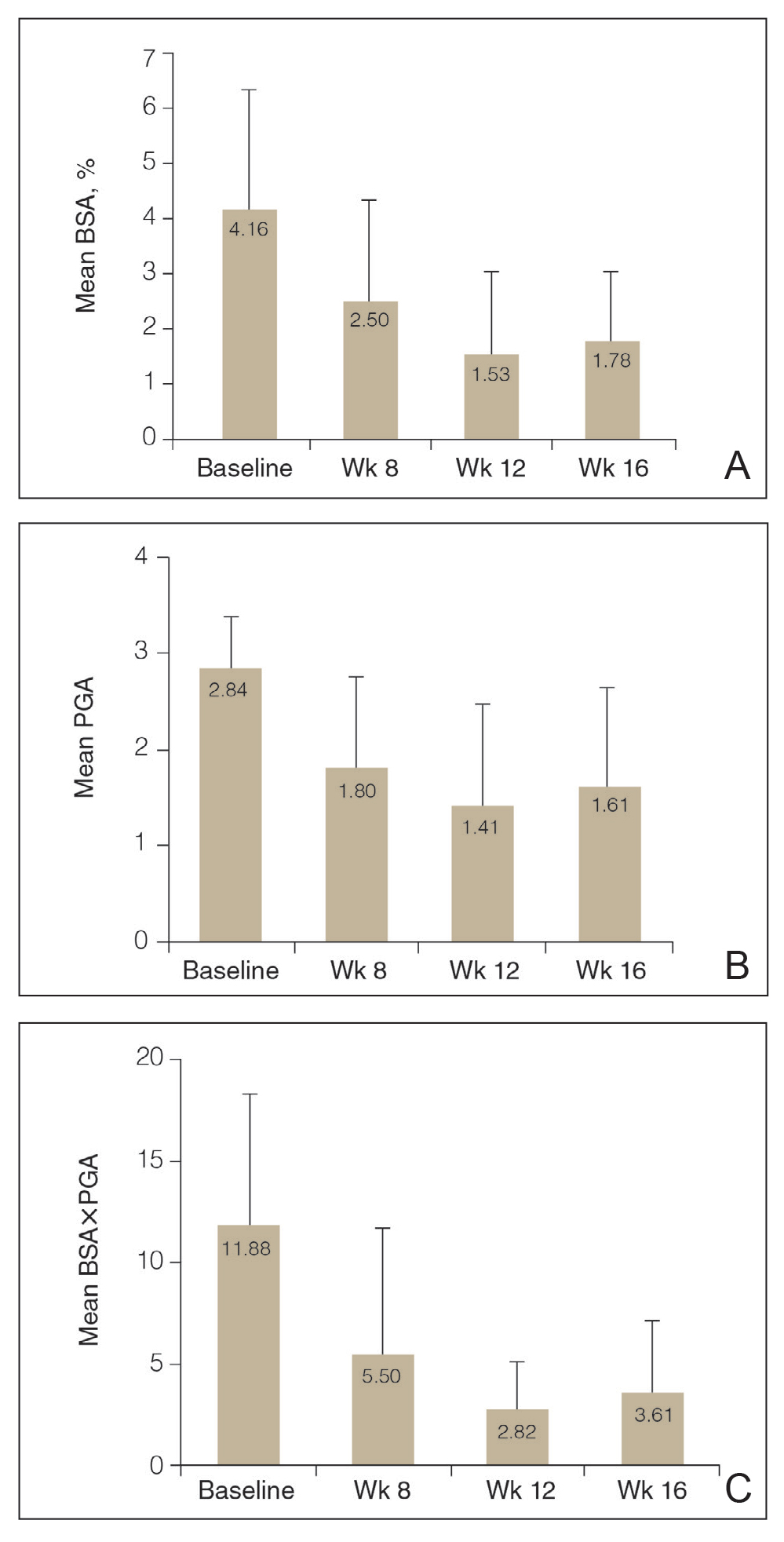
The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).
The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
![Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16 Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16](https://cdn.mdedge.com/files/s3fs-public/Bagel_0222_2.JPG)
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
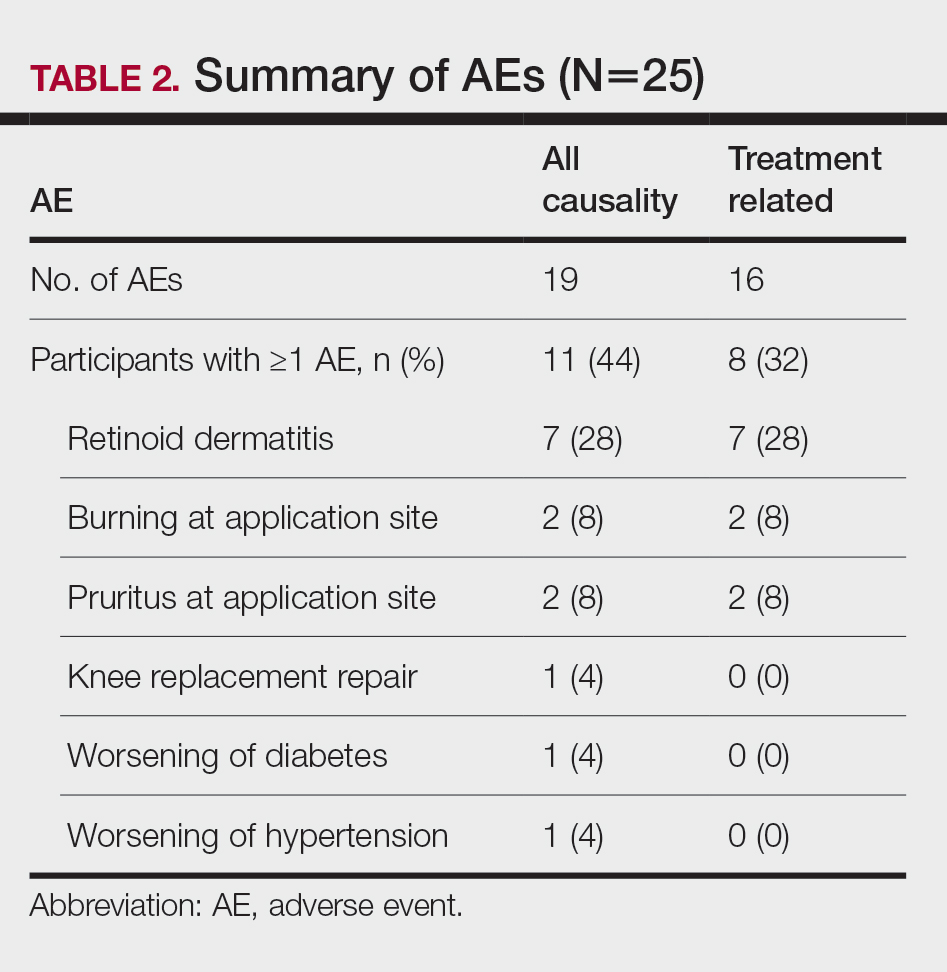
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.
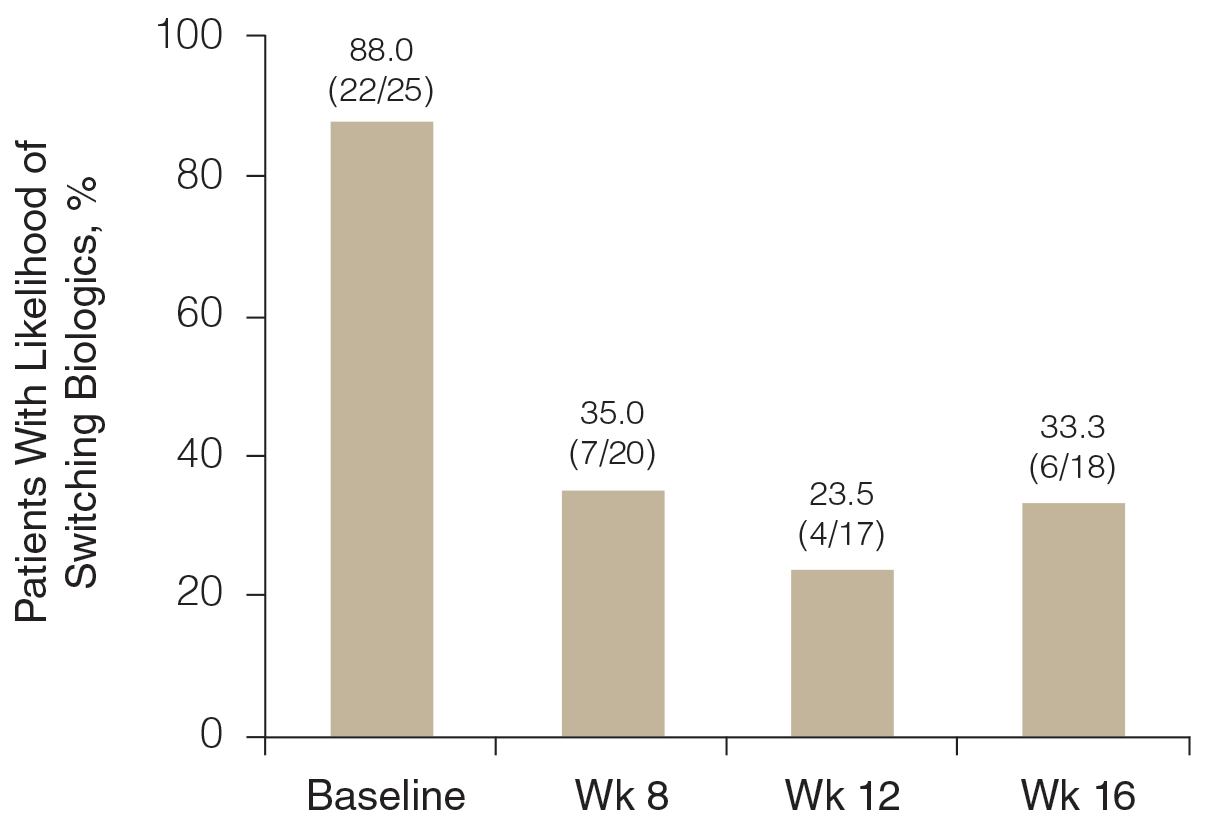
Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).
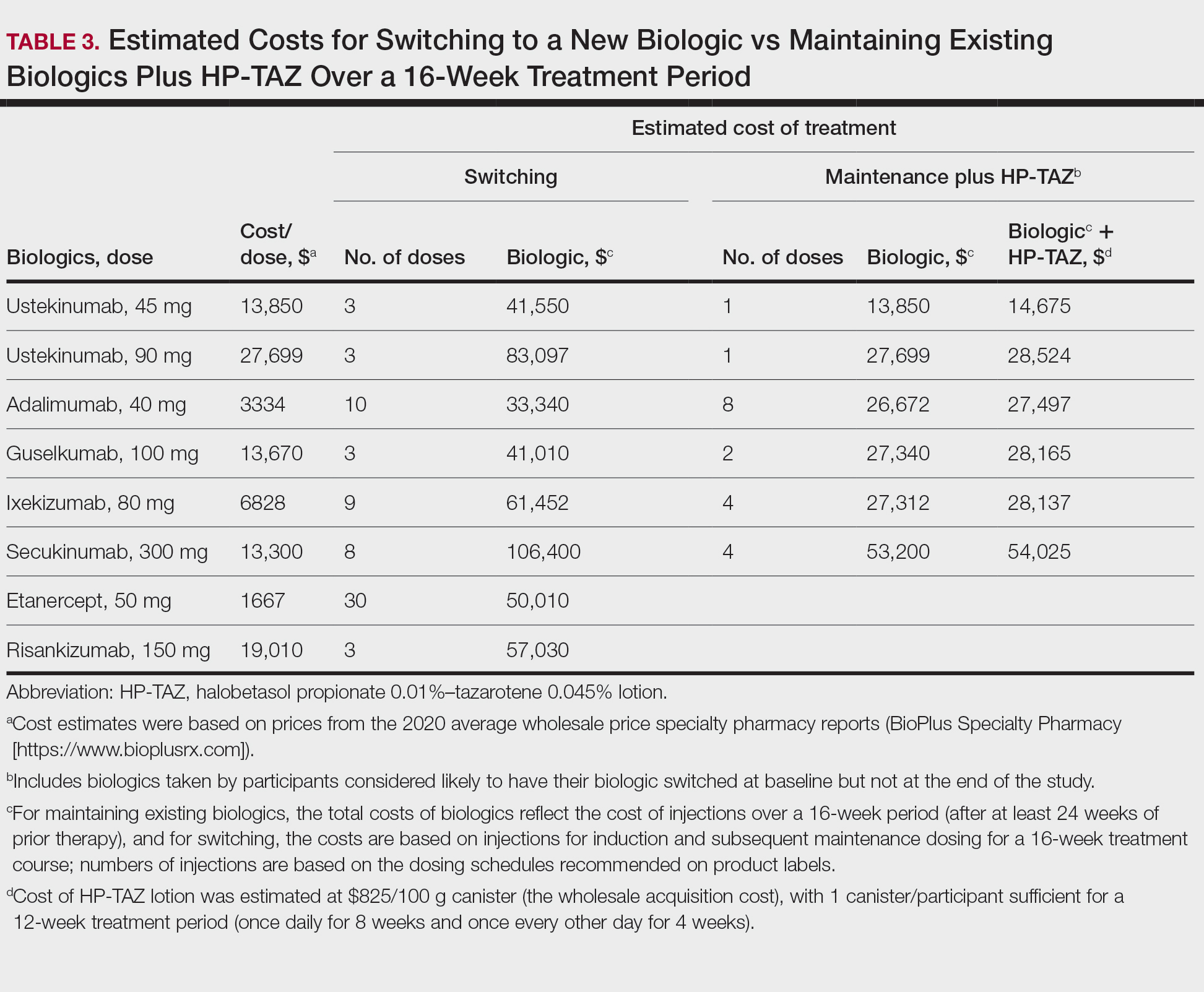
Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.
Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).

The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).

The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
![Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16 Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16](https://cdn.mdedge.com/files/s3fs-public/Bagel_0222_2.JPG)
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.

Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).

Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.

Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).

The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).

The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
![Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16 Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16](https://cdn.mdedge.com/files/s3fs-public/Bagel_0222_2.JPG)
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.

Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).

Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.

Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
Practice Points
- Although monotherapy with biologic agents is effective to treat psoriasis, some patients do not achieve a satisfactory response.
- Adjunctive therapy with halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion can improve responses to biologic treatment in patients whose psoriasis symptoms could not be adequately controlled by biologic monotherapy.
- Adjunctive use of HP-TAZ lotion in addition to biologics was well tolerated.
- Compared with switching to a new biologic regimen, adding a topical regimen of HP-TAZ lotion to ongoing biologics may be a more cost-effective approach to enhance treatment effects.
Guttate Psoriasis Following COVID-19 Infection
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.
The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.

The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.

The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Practice Points
- Guttate psoriasis is the only type of psoriasis that originates from viral infection.
- Dysregulation of proinflammatory cytokines during COVID-19 infection in our patient led to development of guttate psoriasis 3 weeks later.
Hairstyling Practices to Prevent Hair Damage and Alopecia in Women of African Descent
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)

Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)

Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Chewing xylitol gum may modestly reduce preterm birth
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
FROM THE PREGNANCY MEETING
The differences between IBS-C and CIC
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
Lifestyle likely responsible for obesity in children, not mother’s BMI
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
FROM BMC MEDICINE
A test for cannabis-caused impairment
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
EMA gives green light to new CAR T-cell therapy
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.
Screen time in first year may raise autism risk at age 3
Boys exposed to at least 2 hours a day of screen time by 1 year of age were significantly more likely to have an autism spectrum disorder (ASD) diagnosis at 3 years, based on data from more than 80,000 children.
The World Health Organization and the American Academy of Pediatrics recommend against any screen time for infants up to 1 year of age and 18 months of age, respectively, wrote Megumi Kushima, MA, of the University of Yamanashi (Japan), and colleagues on behalf of the Japan Environment and Children’s Study Group.
The extent to which screen time duration in infancy is associated with subsequent ASD diagnosis remains unclear, the researchers said. The ongoing COVID-19 pandemic has increased screen time among children worldwide, which makes an examination of the impact of screen time on children’s health an important public health issue.
In a study published in JAMA Pediatrics, the researchers recruited pregnant women between 2011 and 2014; data were analyzed in December 2020. The final study population included 84,030 mother-child pairs. The primary exposure of screen time at 1 year of age was assessed by questionnaire, in which mothers were asked to report their number of hours they let their child watch TV or DVDs daily. Responses were none (no screen time), less than 1 hour, 1 hour or more but less than 2 hours, 2 hours or more but less than 4 hours, and 4 hours or more.
The primary outcome was ASD diagnosis at 3 years of age, and mothers were asked via questionnaire whether their 3-year-old had been diagnosed with ASD from age 2.
The study was conducted by the Japan Environment and Children’s Study Group at 15 regional centers across Japan.
Overall, 330 children had received an ASD diagnosis at age 3 years, a prevalence of 0.4%. Of these, 251 (76%) were boys, and 79 (24%) were girls. Independent of ASD, the most common response for screen time was less than 1 hour, which was reported by 27,707 mothers. The proportion of children with ASD at age 3 increased as screen time at age 1 increased, the percentages were 5.8%, 22.3%, 30.2%, and 31.7% for children with no screen time, less than 1 hour, 1 to less than 2 hours, and 2 to less than 4 hours, respectively. The percentage of children with ASD diagnoses who had 4 hours or more of daily screen time was 10%.
Logistic regression analysis showed that longer screen time at age 1 year was significantly associated with higher odds of ASD at 3 years in boys, but not in girls. The researchers controlled for variables including maternal maltreatment and children’s predisposition to ASD. Among boys, the adjusted odds ratios for screen times of less than 1 hour, 1 hour to less than 2 hours, 2 hours to less than 4 hours, and more than 4 hours were 1.38, 2.16, 3.48, and 3.02, respectively.
Screen time at age 3 years was not associated with ASD diagnosis at age 3 years, potentially “because the association with environmental factors on brain development varies with age,” the researchers noted.
The study findings were limited by several factors including the reliance on parental reports of screen time and potential for reporting bias, the researchers noted. Other limitations included the possible missed diagnoses of mild ASD cases at 3 years, and the inability to consider variables such as childcare environment, living conditions, diseases, genetics, and disabilities.
However, the results were strengthened by the large study population and examination of screen time in early childhood, they said. More research is needed to examine other factors that contribute to the association between ASD and screen time, but given the rapid increase in device use in children, “it is necessary to review its health effects on infants and control excessive screen time.”
Strong study, but some gaps appear
The study is strong in many respects, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
However, “what I am not sure they addressed is that children on the spectrum are often not entertained by basic toys and may be hard to manage behaviorally,” Dr. Kinsella said. Consequently, parents may be more inclined to offer screen time as a way to pacify children with behavioral difficulties. “Parents also may see that their children are happier interacting with devices, so they may be more apt to let them continue with screen time.We know screen time is not good for the developing brain, however; I worry that the message from this study is that screen time causes autism in boys.
“What I would have liked to know from the parents who allowed more screen time was why they were offering it,” Dr. Kinsella said. “Was it because their child was difficult behaviorally or because that is the one place that they seemed to have satisfaction? To me, that would indicate the reverse hypothesis.” That said, the study findings “remind us to counsel families about screen time, especially in the age of COVID-19. Kids are home much longer than usual, which ultimately leads to more screen time.”
The study was funded by the Japanese Ministry of Environment. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
Boys exposed to at least 2 hours a day of screen time by 1 year of age were significantly more likely to have an autism spectrum disorder (ASD) diagnosis at 3 years, based on data from more than 80,000 children.
The World Health Organization and the American Academy of Pediatrics recommend against any screen time for infants up to 1 year of age and 18 months of age, respectively, wrote Megumi Kushima, MA, of the University of Yamanashi (Japan), and colleagues on behalf of the Japan Environment and Children’s Study Group.
The extent to which screen time duration in infancy is associated with subsequent ASD diagnosis remains unclear, the researchers said. The ongoing COVID-19 pandemic has increased screen time among children worldwide, which makes an examination of the impact of screen time on children’s health an important public health issue.
In a study published in JAMA Pediatrics, the researchers recruited pregnant women between 2011 and 2014; data were analyzed in December 2020. The final study population included 84,030 mother-child pairs. The primary exposure of screen time at 1 year of age was assessed by questionnaire, in which mothers were asked to report their number of hours they let their child watch TV or DVDs daily. Responses were none (no screen time), less than 1 hour, 1 hour or more but less than 2 hours, 2 hours or more but less than 4 hours, and 4 hours or more.
The primary outcome was ASD diagnosis at 3 years of age, and mothers were asked via questionnaire whether their 3-year-old had been diagnosed with ASD from age 2.
The study was conducted by the Japan Environment and Children’s Study Group at 15 regional centers across Japan.
Overall, 330 children had received an ASD diagnosis at age 3 years, a prevalence of 0.4%. Of these, 251 (76%) were boys, and 79 (24%) were girls. Independent of ASD, the most common response for screen time was less than 1 hour, which was reported by 27,707 mothers. The proportion of children with ASD at age 3 increased as screen time at age 1 increased, the percentages were 5.8%, 22.3%, 30.2%, and 31.7% for children with no screen time, less than 1 hour, 1 to less than 2 hours, and 2 to less than 4 hours, respectively. The percentage of children with ASD diagnoses who had 4 hours or more of daily screen time was 10%.
Logistic regression analysis showed that longer screen time at age 1 year was significantly associated with higher odds of ASD at 3 years in boys, but not in girls. The researchers controlled for variables including maternal maltreatment and children’s predisposition to ASD. Among boys, the adjusted odds ratios for screen times of less than 1 hour, 1 hour to less than 2 hours, 2 hours to less than 4 hours, and more than 4 hours were 1.38, 2.16, 3.48, and 3.02, respectively.
Screen time at age 3 years was not associated with ASD diagnosis at age 3 years, potentially “because the association with environmental factors on brain development varies with age,” the researchers noted.
The study findings were limited by several factors including the reliance on parental reports of screen time and potential for reporting bias, the researchers noted. Other limitations included the possible missed diagnoses of mild ASD cases at 3 years, and the inability to consider variables such as childcare environment, living conditions, diseases, genetics, and disabilities.
However, the results were strengthened by the large study population and examination of screen time in early childhood, they said. More research is needed to examine other factors that contribute to the association between ASD and screen time, but given the rapid increase in device use in children, “it is necessary to review its health effects on infants and control excessive screen time.”
Strong study, but some gaps appear
The study is strong in many respects, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
However, “what I am not sure they addressed is that children on the spectrum are often not entertained by basic toys and may be hard to manage behaviorally,” Dr. Kinsella said. Consequently, parents may be more inclined to offer screen time as a way to pacify children with behavioral difficulties. “Parents also may see that their children are happier interacting with devices, so they may be more apt to let them continue with screen time.We know screen time is not good for the developing brain, however; I worry that the message from this study is that screen time causes autism in boys.
“What I would have liked to know from the parents who allowed more screen time was why they were offering it,” Dr. Kinsella said. “Was it because their child was difficult behaviorally or because that is the one place that they seemed to have satisfaction? To me, that would indicate the reverse hypothesis.” That said, the study findings “remind us to counsel families about screen time, especially in the age of COVID-19. Kids are home much longer than usual, which ultimately leads to more screen time.”
The study was funded by the Japanese Ministry of Environment. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
Boys exposed to at least 2 hours a day of screen time by 1 year of age were significantly more likely to have an autism spectrum disorder (ASD) diagnosis at 3 years, based on data from more than 80,000 children.
The World Health Organization and the American Academy of Pediatrics recommend against any screen time for infants up to 1 year of age and 18 months of age, respectively, wrote Megumi Kushima, MA, of the University of Yamanashi (Japan), and colleagues on behalf of the Japan Environment and Children’s Study Group.
The extent to which screen time duration in infancy is associated with subsequent ASD diagnosis remains unclear, the researchers said. The ongoing COVID-19 pandemic has increased screen time among children worldwide, which makes an examination of the impact of screen time on children’s health an important public health issue.
In a study published in JAMA Pediatrics, the researchers recruited pregnant women between 2011 and 2014; data were analyzed in December 2020. The final study population included 84,030 mother-child pairs. The primary exposure of screen time at 1 year of age was assessed by questionnaire, in which mothers were asked to report their number of hours they let their child watch TV or DVDs daily. Responses were none (no screen time), less than 1 hour, 1 hour or more but less than 2 hours, 2 hours or more but less than 4 hours, and 4 hours or more.
The primary outcome was ASD diagnosis at 3 years of age, and mothers were asked via questionnaire whether their 3-year-old had been diagnosed with ASD from age 2.
The study was conducted by the Japan Environment and Children’s Study Group at 15 regional centers across Japan.
Overall, 330 children had received an ASD diagnosis at age 3 years, a prevalence of 0.4%. Of these, 251 (76%) were boys, and 79 (24%) were girls. Independent of ASD, the most common response for screen time was less than 1 hour, which was reported by 27,707 mothers. The proportion of children with ASD at age 3 increased as screen time at age 1 increased, the percentages were 5.8%, 22.3%, 30.2%, and 31.7% for children with no screen time, less than 1 hour, 1 to less than 2 hours, and 2 to less than 4 hours, respectively. The percentage of children with ASD diagnoses who had 4 hours or more of daily screen time was 10%.
Logistic regression analysis showed that longer screen time at age 1 year was significantly associated with higher odds of ASD at 3 years in boys, but not in girls. The researchers controlled for variables including maternal maltreatment and children’s predisposition to ASD. Among boys, the adjusted odds ratios for screen times of less than 1 hour, 1 hour to less than 2 hours, 2 hours to less than 4 hours, and more than 4 hours were 1.38, 2.16, 3.48, and 3.02, respectively.
Screen time at age 3 years was not associated with ASD diagnosis at age 3 years, potentially “because the association with environmental factors on brain development varies with age,” the researchers noted.
The study findings were limited by several factors including the reliance on parental reports of screen time and potential for reporting bias, the researchers noted. Other limitations included the possible missed diagnoses of mild ASD cases at 3 years, and the inability to consider variables such as childcare environment, living conditions, diseases, genetics, and disabilities.
However, the results were strengthened by the large study population and examination of screen time in early childhood, they said. More research is needed to examine other factors that contribute to the association between ASD and screen time, but given the rapid increase in device use in children, “it is necessary to review its health effects on infants and control excessive screen time.”
Strong study, but some gaps appear
The study is strong in many respects, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
However, “what I am not sure they addressed is that children on the spectrum are often not entertained by basic toys and may be hard to manage behaviorally,” Dr. Kinsella said. Consequently, parents may be more inclined to offer screen time as a way to pacify children with behavioral difficulties. “Parents also may see that their children are happier interacting with devices, so they may be more apt to let them continue with screen time.We know screen time is not good for the developing brain, however; I worry that the message from this study is that screen time causes autism in boys.
“What I would have liked to know from the parents who allowed more screen time was why they were offering it,” Dr. Kinsella said. “Was it because their child was difficult behaviorally or because that is the one place that they seemed to have satisfaction? To me, that would indicate the reverse hypothesis.” That said, the study findings “remind us to counsel families about screen time, especially in the age of COVID-19. Kids are home much longer than usual, which ultimately leads to more screen time.”
The study was funded by the Japanese Ministry of Environment. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
FROM JAMA PEDIATRICS