User login
HIV stigma persists globally, according to Harris poll
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Yescarta label updated: Prophylactic steroids to prevent CRS
This is a chimeric antigen receptor T-cell product indicated for use in adult patients with relapsed or refractory large B-cell lymphoma (LBCL) after two or more lines of systemic therapy.
The product label carries a black box warning that CRS is a potentially fatal complication. With the update, the new labeling advises clinicians to “consider the use of prophylactic corticosteroid in patients after weighing the potential benefits and risks ... to delay the onset and decrease the duration of CRS.”
However, labeling also notes that “prophylactic corticosteroids ... may result in [a] higher grade of neurologic toxicities or prolongation of neurologic toxicities,” another potentially fatal complication noted in the black box warning.
The addition of prophylactic corticosteroids to labeling was based on data from 39 patients with relapsed or refractory LBCL who received dexamethasone 10 mg orally once daily for 3 days starting prior to Yescarta infusion. In this cohort, 31 of the 39 patients (79%) developed CRS, at which point they were managed with tocilizumab and/or corticosteroids. No one developed grade 3 or higher CRS. The median time to CRS onset was 5 days and the median duration was 4 days, according to labeling.
In contrast, in another cohort of 41 patients who were started on tocilizumab and/or corticosteroids only after becoming symptomatic, the overall incidence of CRS was 93% (38/41), with a median onset at 2 days, median duration of 7 days, and two patients who developed grade 3 CRS.
Prophylactic steroids do not compromise the activity of the cell therapy, Kite said in a press release.
“These new data will enable doctors to more easily and confidently manage treatment for patients,” said Frank Neumann, MD, PhD, the company’s global head of clinical development.
Yescarta is currently under review in the United States and Europe for second-line use in relapsed or refractory LBCL, Kite noted.
A version of this article first appeared on Medscape.com.
This is a chimeric antigen receptor T-cell product indicated for use in adult patients with relapsed or refractory large B-cell lymphoma (LBCL) after two or more lines of systemic therapy.
The product label carries a black box warning that CRS is a potentially fatal complication. With the update, the new labeling advises clinicians to “consider the use of prophylactic corticosteroid in patients after weighing the potential benefits and risks ... to delay the onset and decrease the duration of CRS.”
However, labeling also notes that “prophylactic corticosteroids ... may result in [a] higher grade of neurologic toxicities or prolongation of neurologic toxicities,” another potentially fatal complication noted in the black box warning.
The addition of prophylactic corticosteroids to labeling was based on data from 39 patients with relapsed or refractory LBCL who received dexamethasone 10 mg orally once daily for 3 days starting prior to Yescarta infusion. In this cohort, 31 of the 39 patients (79%) developed CRS, at which point they were managed with tocilizumab and/or corticosteroids. No one developed grade 3 or higher CRS. The median time to CRS onset was 5 days and the median duration was 4 days, according to labeling.
In contrast, in another cohort of 41 patients who were started on tocilizumab and/or corticosteroids only after becoming symptomatic, the overall incidence of CRS was 93% (38/41), with a median onset at 2 days, median duration of 7 days, and two patients who developed grade 3 CRS.
Prophylactic steroids do not compromise the activity of the cell therapy, Kite said in a press release.
“These new data will enable doctors to more easily and confidently manage treatment for patients,” said Frank Neumann, MD, PhD, the company’s global head of clinical development.
Yescarta is currently under review in the United States and Europe for second-line use in relapsed or refractory LBCL, Kite noted.
A version of this article first appeared on Medscape.com.
This is a chimeric antigen receptor T-cell product indicated for use in adult patients with relapsed or refractory large B-cell lymphoma (LBCL) after two or more lines of systemic therapy.
The product label carries a black box warning that CRS is a potentially fatal complication. With the update, the new labeling advises clinicians to “consider the use of prophylactic corticosteroid in patients after weighing the potential benefits and risks ... to delay the onset and decrease the duration of CRS.”
However, labeling also notes that “prophylactic corticosteroids ... may result in [a] higher grade of neurologic toxicities or prolongation of neurologic toxicities,” another potentially fatal complication noted in the black box warning.
The addition of prophylactic corticosteroids to labeling was based on data from 39 patients with relapsed or refractory LBCL who received dexamethasone 10 mg orally once daily for 3 days starting prior to Yescarta infusion. In this cohort, 31 of the 39 patients (79%) developed CRS, at which point they were managed with tocilizumab and/or corticosteroids. No one developed grade 3 or higher CRS. The median time to CRS onset was 5 days and the median duration was 4 days, according to labeling.
In contrast, in another cohort of 41 patients who were started on tocilizumab and/or corticosteroids only after becoming symptomatic, the overall incidence of CRS was 93% (38/41), with a median onset at 2 days, median duration of 7 days, and two patients who developed grade 3 CRS.
Prophylactic steroids do not compromise the activity of the cell therapy, Kite said in a press release.
“These new data will enable doctors to more easily and confidently manage treatment for patients,” said Frank Neumann, MD, PhD, the company’s global head of clinical development.
Yescarta is currently under review in the United States and Europe for second-line use in relapsed or refractory LBCL, Kite noted.
A version of this article first appeared on Medscape.com.
Open-label placebo improves symptoms in pediatric IBS and functional abdominal pain
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
“I didn’t want to meet you.” Dispelling myths about palliative care
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
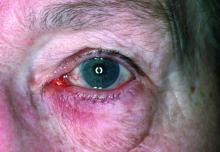
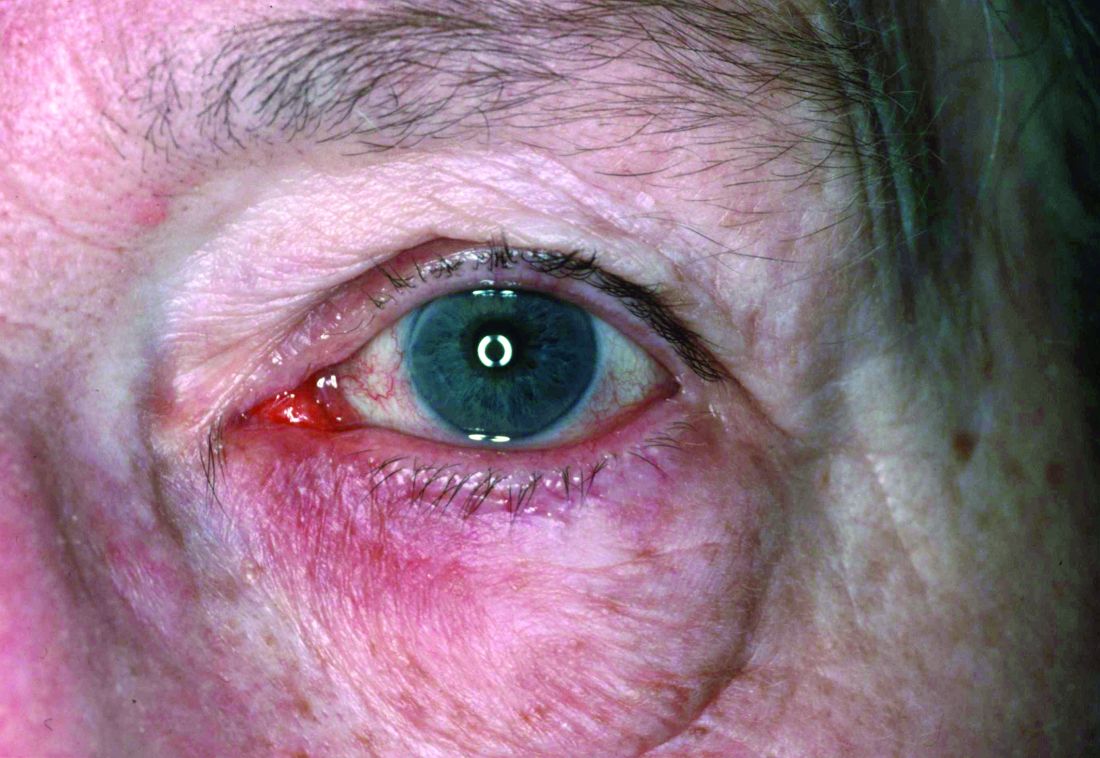
Dryness, conjunctival telangiectasia among ocular symptoms common in rosacea
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL OPTHALMOLOGY
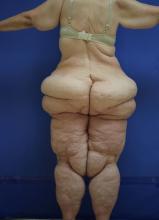
Lipedema: A potentially devastating, often unrecognized disease
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
FROM ODAC 2022
Severe Acute Systemic Reaction After the First Injections of Ixekizumab
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.

At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).

Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.

At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).

Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Practice Points
- Psoriasis is an autoimmune disorder with a predominance of CD4+ and CD8+ T cells that release cytokines, such as tumor necrosis factor 11α and interleukins, which promote inflammation in the skin and joints and is associated with systemic inflammation predisposing patients to cardiovascular disease.
- Common adverse effects of most biologic medications for psoriasis include injection-site pain and rash, fever, malaise, back pain, urticaria and flushing, edema, dyspnea, and nausea.
- Ixekizumab is a humanized IL-17A antagonist intended for adults with moderate to severe psoriasis. Certain rare side effects specific to ixekizumab include inflammatory bowel disease, thrombocytopenia, severe injection-site reactions, and candidiasis.
- Acute onycholysis and acute exacerbation of arthritis/dactylitis are rare side effects of ixekizumab therapy.
Scleral Plaques in Nephrogenic Systemic Fibrosis
To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2
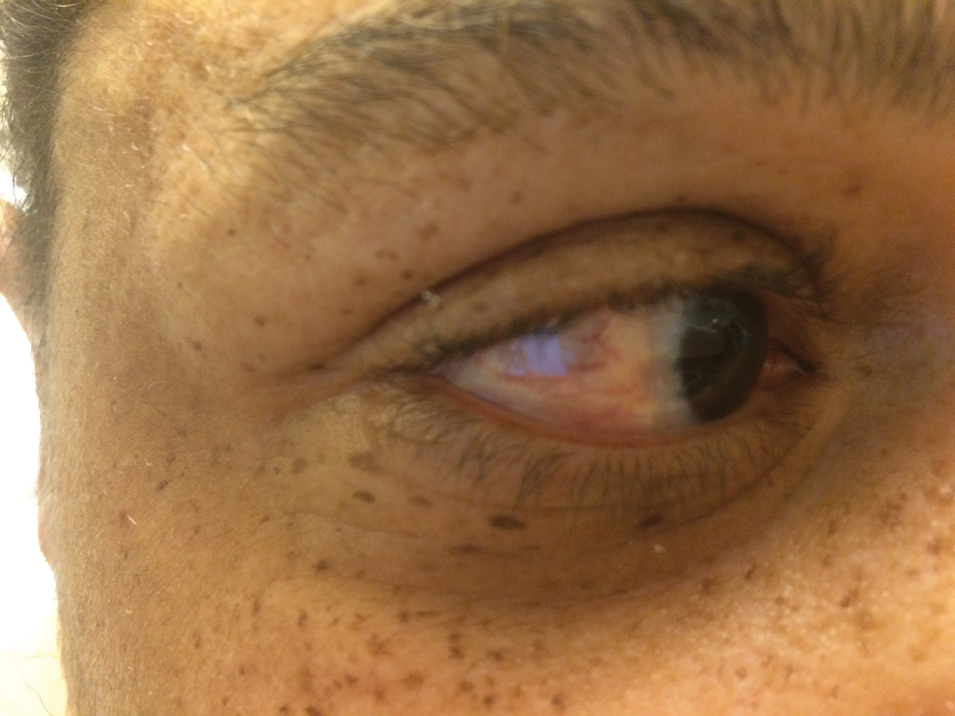
- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.

Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2

To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.

Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2

- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
Practice Points
- It is important to examine the eyes in a patient with sclerotic skin changes on physical examination.
- The presence of yellow scleral plaques strongly is associated with a diagnosis of nephrogenic systemic fibrosis.
Clinical Edge Journal Scan Commentary: Migraine February 2022
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).
Clinical Edge Journal Scan Commentary: Migraine February 2022
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).
Most practitioners recommend a host of non-medical therapeutic options to their patients with migraine. The best studied and safest, most effective supplements remain magnesium, riboflavin/B2, and CoQ10. Alpha-lipoic acid (ALA) is a supplement with both antioxidant and anti-inflammatory effects that has showed positive protective effects in a number of medical conditions, including diabetes and episodes of oxidative stress. One migraine study1 evaluated serum ALA levels and found over 90% of people with migraine to deficient. This study sought to observe the potential benefit of supplementation with ALA in patients with episodic migraine.
This was a randomized, double-blind placebo-controlled trial over the course of 3 months. In this study, 92 female subjects with episodic migraine (defined as experiencing >2 but <15 days of headache per month) were recruited and randomized to receiving 300 mg ALA twice daily or placebo. Patients with chronic migraine, in menopause, pregnant, or lactating were excluded, as were patients with the presence of other chronic medical issues, or patients who had taken antioxidant supplements in the previous 4 months.
The primary outcomes of migraine severity, frequency, and Headache Impact Test (HIT-6) score were found to be significantly improved in the intervention group; duration of headache was not significantly different. Biochemical analysis of the two groups did show a difference in the lactate level of the intervention group, and this was considered a secondary outcome. Relevant side effects were primarily gastrointestinal, including stomach pain (higher in the placebo group), increased appetite, and constipation.
There is a great interest in finding effective non-medical treatments for migraine. These are frequently used as an adjunct to other preventive medications, or potentially as a stand-alone treatment for low frequency migraine. Many patients prefer non-medical options as well, and unfortunately many of the treatments they read about online or in less scientific spaces are unproven or unsafe. Supplementation remains an important part of migraine treatment for many practitioners and patients.
This study argues that ALA can be considered a safe and effective treatment for episodic migraine. When patients ask about non-medical options, ALA can be an additional treatment worth considering. Many patients are already taking multiple supplements before seeing their specialist, and this article informs us that there may be some treatment benefit for this supplement as well. We may not be recommending this supplement alone as a preventive treatment for migraine, but we can add a new non-medical option to consider to our mix.
Using preventive medication in pediatrics is now more controversial than it had been previously. The well known The Childhood and Adolescent Migraine Prevention (CHAMP) trial2 surprised many in the field by revealing that were no significant differences in headache frequency or disability when comparing children with migraine who received preventive medications or placebo. The CHAMP trial spotlighted the effect of non-medical therapies (cognitive behavioral therapy, biofeedback) and education. Many pediatric specialists have altered their practice paradigm in response to these results and have been more reticent to prescribe preventive medications for children with migraine. This is due to concern for potential side effects in light of the absence of direct benefit.
In an observational study of pediatric migraine,3 the investigators followed 186 children with migraine over a 3-year period to determine if the use of a number of preventive medications addresses disability (measured by Pediatric Migraine Disability Assessment [PedMIDAS]) as well as frequency, severity and duration of migraine. Other bothersome features of migraine were followed including the presence of nausea, vomiting, photophobia, analgesic use, and the side effects of the preventive medication.
The preventive medications used were cyproheptadine, flunarazine, propranolol, and topiramate—all at weight based doses. It is important to note that amitriptyline was not used in the study and there was no placebo group. This was a Turkish population, the median age was 14, and 63% were female, all of which are appropriate for a pediatric migraine study. Treatment efficacy was defined as a 50% reduction of symptoms. This was achieved in 90% of subjects in the topiramate group, 75% in the propranolol group, and 52-53% in the flunarazine and cyproheptadine groups.
Medication side effects were divided into minor or significant side effects. The only significant side effect noted was 3% of patient with palpitations; minor side effects were changes in appetite and drowsiness. More than half (57%) of patients taking topiramate experienced some side effect, 51% of the cyproheptadine group did as well, and the propranolol and flunarazine groups were noted to have side effects in 22% and 13%, respectively. Overall, 31.7% of patients had some side effect.
PedMIDAS scores improved significantly with the use of preventive medications; migraine frequency improved significantly as well, especially in the topiramate group. This study argues for the use of preventive medications in pediatric migraine. One of the most commonly used medications for migraine prevention was not investigated unfortunately. Amitriptyline is widely considered a safe and effective migraine prophylactic medication, especially at low doses. One important takeaway is the frequency of side effects at all, and especially with topiramate. It is unclear how many patients stopped their preventive medications due to a side effect. In light of this study, propranolol, which is often overlooked, might be considered a better choice for children with migraine.
Most of the patients with migraine we see are in their most productive years. Migraine disability can be a major difficulty for our patients, especially as it relates to work. The American Migraine Foundation and American Headache Society have both recently taken on initiatives that relate to migraine in the workplace. Migraine epidemiologic studies have shown that people with migraine are more likely to experience a negative impact on their careers, and migraine disability scores weigh time absent from work as well as lower function at work. Many people with migraine are concerned that having migraine may hold them back from being hired or achieving promotion.
Autio et al performed a retrospective analysis of occupationally active patients treated at a single provider (the Finnish health clinic Terveystalo).4 The authors first looked for erenumab responders, who they defined as patients who received two prescriptions for erenumab and no other calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) medication. These patients were followed for 12 months, and their data was compared to the 12-month period prior to initiating erenumab. The authors evaluated headache-related sick days, all-cause sick days, healthcare visits, and prescriptions for all medications based on a registry. This registry also provided an age- and sex-matched control group of patients with migraine not taking any CGRP mAb medication.
A total of 162 patients were included, 82 in the erenumab responder group. Headache-related sick days decreased by 74%, and headache-related healthcare visits decreased by 44%. Triptan prescription use decreased by 31.5%; all-cause sick days and healthcare visits differences were not statistically significant.
Prevention remains key in improving our patients’ quality of life and a large factor in this is their work life. This study shows that intervention with erenumab significantly decreases migraine-related absenteeism. It could be argued that the other CGRP mAb medications may have the same effect, as can many other preventive therapies. It can also be argued that even with this data we can only assume that patients function better at work with preventive therapies. Further studies will also look at the degree that “presenteeism” plays in the workplace—people who show up to work but are functioning at a lesser extent due to migraine. That said, this is an important step towards recognizing the burden migraine disability has on our patients’ work life, and the extent that prevention can improve their quality of life.
References
- Kelishadi MR et al. The beneficial effect of Alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci Rep. 2022;12:271 (Jan 7).
- Powers SW et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115-124. Doi: 10.1056/NEJMoa1610384.
- Tekin H, Edem P. Effects and side effects of migraine prophylaxis in children. Pediatr Int. 2021 (Dec 14).
- Autio H et al. Erenumab decreases headache-related sick leave days and health care visits: a retrospective real-world study in working patients with migraine. Neurol Ther. 2021 (Dec 10).