User login
Confirmed: Pembro plus chemo as first-line standard of care for esophageal cancer
An interim analysis of the KEYNOTE-590 study, published in 2020, found that the combination of pembrolizumab and chemotherapy in the first-line setting proved superior to chemotherapy alone in all outcome measures.
The updated analysis, which adds 12 months of follow-up data, shows “first-line pembrolizumab plus chemotherapy continued to provide clinically meaningful benefits in all patients with locally advanced and metastatic esophageal cancer, including [gastroesophageal junction] adenocarcinoma,” said lead author Jean-Philippe Metges, MD, of the CHU Brest-Institut de Cancerologie et d’Hematologie ARPEGO Network, Brest, France.
Similar quality of life and safety data were also observed with pembrolizumab plus chemotherapy versus chemotherapy alone, Dr. Metges added.
“These longer-term data further support first-line pembrolizumab plus chemotherapy as a new standard of care in patients with locally advanced and metastatic esophageal cancer,” he said.
The updated analysis was presented at the 2022 Gastrointestinal Cancers Symposium.
Pembro for esophageal cancer
Pembrolizumab first received regulatory approval in 2019 as monotherapy in the second-line setting to treat recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus in tumors with programmed death–ligand 1 (PD-L1) expression.
In response to the interim KEYNOTE-590 data, the FDA expanded the indication in 2021, granting accelerated approval for pembrolizumab in combination with platinum and fluoropyrimidine-based chemotherapy in the first-line setting for patients who were not candidates for surgical resection or definitive chemoradiotherapy.
The updated KEYNOTE-590 data lend greater weight for the use of pembrolizumab plus chemotherapy as first-line standard of care in advanced esophageal cancer.
In the analysis, a total of 749 eligible patients with previously untreated locally advanced, unresectable, or metastatic esophageal squamous cell carcinoma (ESCC), adenocarcinoma, or Siewert type 1 esophagogastric junction adenocarcinoma, regardless of PD-L1 status, were randomly assigned (1:1) to pembrolizumab 200 mg or placebo plus 5-fluorouracil and cisplatin once every 3 weeks for up to 35 cycles.
The authors evaluated overall survival in all patients as well as subgroups including those with ESCC, ESCC PD-L1 combined positive score ≥10 tumors, and PD-L1 CPS ≥10 tumors. The research team also looked at progression-free survival in most groups and overall response rate, duration of response, safety, and health-related quality of life.
Treatment continued until progression, unacceptable toxicity, withdrawal, or until 2 years, with no crossover permitted.
At the median follow-up of 34.8 months, median overall survival was longer for all patients receiving the combination therapy (hazard ratio, 0.73) as well as patients with ESCC (HR, 0.73), ESCC CPS ≥10 (HR, 0.59), CPS ≥10 (HR, 0.64), and adenocarcinoma (HR, 0.73).
For progression-free survival, pembrolizumab plus chemotherapy was superior in all patients (HR, 0.64), the ESCC group (HR, 0.65), as well as the PD-L1 CPS ≥10 tumor group (HR, 0.51).
The 24-month overall survival in all patients was also notably higher for those receiving the combination therapy – 26.3% versus 16.1% – as was 24-month progression-free survival – 11.6% versus 3.3%.
The overall response rate was 45.0% in the combination group, with 25 complete responses (6.7%), versus 29.3% in the control group, with 9 complete responses (2.4%). The median duration of response was 8.3 months in the combination group versus 6.0 months in the chemotherapy group. About 20% of patients in the combination group had a response rate lasting 24 months or longer, compared with 6% who received chemotherapy alone.
As for safety, grade 3-5 drug-related adverse events were similar in both arms – 72% for the combination versus 68% for chemotherapy alone. However, more patients in the combination group discontinued treatment because of drug-related adverse events – 21% versus 12%.
No additional or surprise adverse events occurred with the longer follow-up, plus quality of life was comparable between groups, Dr. Metges noted.
Stefano Cascinu, MD, Università Vita-Salute, San Raffaele Hospital, Milan, who was not involved in the analysis, reiterated that this update confirms the findings from earlier analyses and shows a benefit across all subgroups.
“One of the most relevant findings was that 20% of patients were responding for more than 24 months,” he said. “It is also important that a similar quality of life was maintained.”
Although Dr. Cascinu emphasized that this is a landmark trial in advanced esophageal and gastric cancers, he indicated to several points that remain to be investigated. These include the reproducibility of the findings in common clinical situations – such as a patient with impaired performance status, malnutrition, or peritoneal involvement – as well as the role of PD-L1.
The efficacy of the combination therapy across all subgroups led to a wide FDA approval, though the European Medicines Agency limited its approval to patients with PD-L1 CPS ≥10 tumors.
“Even though all subgroups did well, patients with [PD-L1] CPS ≥10 did better,” said Dr. Cascinu. “[And] in reality, the benefit may only be driven by a specific subpopulation.”
Dr. Cascinu added: “PD-L1 may be a negative biomarker and may be informative about the magnitude of benefit. This may be useful to discuss with patients regarding the expected benefit [of this therapeutic option].”
The study was supported by Merck & Co. Dr. Metges reported receiving payment for travel, accommodations, expenses from Amgen, LEO Pharma, and MSD Oncology, and receiving honoraria from Bristol-Myers Squibb, Lilly, Novartis, Sanofi, and Syncore. Dr. Cascinu has disclosed honoraria from BMS, Lilly, MSD Oncology, and others, as well as a consulting or advisory role for many of these same manufacturers and serving on the speakers’ bureau of Lilly and SERVIER. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
An interim analysis of the KEYNOTE-590 study, published in 2020, found that the combination of pembrolizumab and chemotherapy in the first-line setting proved superior to chemotherapy alone in all outcome measures.
The updated analysis, which adds 12 months of follow-up data, shows “first-line pembrolizumab plus chemotherapy continued to provide clinically meaningful benefits in all patients with locally advanced and metastatic esophageal cancer, including [gastroesophageal junction] adenocarcinoma,” said lead author Jean-Philippe Metges, MD, of the CHU Brest-Institut de Cancerologie et d’Hematologie ARPEGO Network, Brest, France.
Similar quality of life and safety data were also observed with pembrolizumab plus chemotherapy versus chemotherapy alone, Dr. Metges added.
“These longer-term data further support first-line pembrolizumab plus chemotherapy as a new standard of care in patients with locally advanced and metastatic esophageal cancer,” he said.
The updated analysis was presented at the 2022 Gastrointestinal Cancers Symposium.
Pembro for esophageal cancer
Pembrolizumab first received regulatory approval in 2019 as monotherapy in the second-line setting to treat recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus in tumors with programmed death–ligand 1 (PD-L1) expression.
In response to the interim KEYNOTE-590 data, the FDA expanded the indication in 2021, granting accelerated approval for pembrolizumab in combination with platinum and fluoropyrimidine-based chemotherapy in the first-line setting for patients who were not candidates for surgical resection or definitive chemoradiotherapy.
The updated KEYNOTE-590 data lend greater weight for the use of pembrolizumab plus chemotherapy as first-line standard of care in advanced esophageal cancer.
In the analysis, a total of 749 eligible patients with previously untreated locally advanced, unresectable, or metastatic esophageal squamous cell carcinoma (ESCC), adenocarcinoma, or Siewert type 1 esophagogastric junction adenocarcinoma, regardless of PD-L1 status, were randomly assigned (1:1) to pembrolizumab 200 mg or placebo plus 5-fluorouracil and cisplatin once every 3 weeks for up to 35 cycles.
The authors evaluated overall survival in all patients as well as subgroups including those with ESCC, ESCC PD-L1 combined positive score ≥10 tumors, and PD-L1 CPS ≥10 tumors. The research team also looked at progression-free survival in most groups and overall response rate, duration of response, safety, and health-related quality of life.
Treatment continued until progression, unacceptable toxicity, withdrawal, or until 2 years, with no crossover permitted.
At the median follow-up of 34.8 months, median overall survival was longer for all patients receiving the combination therapy (hazard ratio, 0.73) as well as patients with ESCC (HR, 0.73), ESCC CPS ≥10 (HR, 0.59), CPS ≥10 (HR, 0.64), and adenocarcinoma (HR, 0.73).
For progression-free survival, pembrolizumab plus chemotherapy was superior in all patients (HR, 0.64), the ESCC group (HR, 0.65), as well as the PD-L1 CPS ≥10 tumor group (HR, 0.51).
The 24-month overall survival in all patients was also notably higher for those receiving the combination therapy – 26.3% versus 16.1% – as was 24-month progression-free survival – 11.6% versus 3.3%.
The overall response rate was 45.0% in the combination group, with 25 complete responses (6.7%), versus 29.3% in the control group, with 9 complete responses (2.4%). The median duration of response was 8.3 months in the combination group versus 6.0 months in the chemotherapy group. About 20% of patients in the combination group had a response rate lasting 24 months or longer, compared with 6% who received chemotherapy alone.
As for safety, grade 3-5 drug-related adverse events were similar in both arms – 72% for the combination versus 68% for chemotherapy alone. However, more patients in the combination group discontinued treatment because of drug-related adverse events – 21% versus 12%.
No additional or surprise adverse events occurred with the longer follow-up, plus quality of life was comparable between groups, Dr. Metges noted.
Stefano Cascinu, MD, Università Vita-Salute, San Raffaele Hospital, Milan, who was not involved in the analysis, reiterated that this update confirms the findings from earlier analyses and shows a benefit across all subgroups.
“One of the most relevant findings was that 20% of patients were responding for more than 24 months,” he said. “It is also important that a similar quality of life was maintained.”
Although Dr. Cascinu emphasized that this is a landmark trial in advanced esophageal and gastric cancers, he indicated to several points that remain to be investigated. These include the reproducibility of the findings in common clinical situations – such as a patient with impaired performance status, malnutrition, or peritoneal involvement – as well as the role of PD-L1.
The efficacy of the combination therapy across all subgroups led to a wide FDA approval, though the European Medicines Agency limited its approval to patients with PD-L1 CPS ≥10 tumors.
“Even though all subgroups did well, patients with [PD-L1] CPS ≥10 did better,” said Dr. Cascinu. “[And] in reality, the benefit may only be driven by a specific subpopulation.”
Dr. Cascinu added: “PD-L1 may be a negative biomarker and may be informative about the magnitude of benefit. This may be useful to discuss with patients regarding the expected benefit [of this therapeutic option].”
The study was supported by Merck & Co. Dr. Metges reported receiving payment for travel, accommodations, expenses from Amgen, LEO Pharma, and MSD Oncology, and receiving honoraria from Bristol-Myers Squibb, Lilly, Novartis, Sanofi, and Syncore. Dr. Cascinu has disclosed honoraria from BMS, Lilly, MSD Oncology, and others, as well as a consulting or advisory role for many of these same manufacturers and serving on the speakers’ bureau of Lilly and SERVIER. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
An interim analysis of the KEYNOTE-590 study, published in 2020, found that the combination of pembrolizumab and chemotherapy in the first-line setting proved superior to chemotherapy alone in all outcome measures.
The updated analysis, which adds 12 months of follow-up data, shows “first-line pembrolizumab plus chemotherapy continued to provide clinically meaningful benefits in all patients with locally advanced and metastatic esophageal cancer, including [gastroesophageal junction] adenocarcinoma,” said lead author Jean-Philippe Metges, MD, of the CHU Brest-Institut de Cancerologie et d’Hematologie ARPEGO Network, Brest, France.
Similar quality of life and safety data were also observed with pembrolizumab plus chemotherapy versus chemotherapy alone, Dr. Metges added.
“These longer-term data further support first-line pembrolizumab plus chemotherapy as a new standard of care in patients with locally advanced and metastatic esophageal cancer,” he said.
The updated analysis was presented at the 2022 Gastrointestinal Cancers Symposium.
Pembro for esophageal cancer
Pembrolizumab first received regulatory approval in 2019 as monotherapy in the second-line setting to treat recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus in tumors with programmed death–ligand 1 (PD-L1) expression.
In response to the interim KEYNOTE-590 data, the FDA expanded the indication in 2021, granting accelerated approval for pembrolizumab in combination with platinum and fluoropyrimidine-based chemotherapy in the first-line setting for patients who were not candidates for surgical resection or definitive chemoradiotherapy.
The updated KEYNOTE-590 data lend greater weight for the use of pembrolizumab plus chemotherapy as first-line standard of care in advanced esophageal cancer.
In the analysis, a total of 749 eligible patients with previously untreated locally advanced, unresectable, or metastatic esophageal squamous cell carcinoma (ESCC), adenocarcinoma, or Siewert type 1 esophagogastric junction adenocarcinoma, regardless of PD-L1 status, were randomly assigned (1:1) to pembrolizumab 200 mg or placebo plus 5-fluorouracil and cisplatin once every 3 weeks for up to 35 cycles.
The authors evaluated overall survival in all patients as well as subgroups including those with ESCC, ESCC PD-L1 combined positive score ≥10 tumors, and PD-L1 CPS ≥10 tumors. The research team also looked at progression-free survival in most groups and overall response rate, duration of response, safety, and health-related quality of life.
Treatment continued until progression, unacceptable toxicity, withdrawal, or until 2 years, with no crossover permitted.
At the median follow-up of 34.8 months, median overall survival was longer for all patients receiving the combination therapy (hazard ratio, 0.73) as well as patients with ESCC (HR, 0.73), ESCC CPS ≥10 (HR, 0.59), CPS ≥10 (HR, 0.64), and adenocarcinoma (HR, 0.73).
For progression-free survival, pembrolizumab plus chemotherapy was superior in all patients (HR, 0.64), the ESCC group (HR, 0.65), as well as the PD-L1 CPS ≥10 tumor group (HR, 0.51).
The 24-month overall survival in all patients was also notably higher for those receiving the combination therapy – 26.3% versus 16.1% – as was 24-month progression-free survival – 11.6% versus 3.3%.
The overall response rate was 45.0% in the combination group, with 25 complete responses (6.7%), versus 29.3% in the control group, with 9 complete responses (2.4%). The median duration of response was 8.3 months in the combination group versus 6.0 months in the chemotherapy group. About 20% of patients in the combination group had a response rate lasting 24 months or longer, compared with 6% who received chemotherapy alone.
As for safety, grade 3-5 drug-related adverse events were similar in both arms – 72% for the combination versus 68% for chemotherapy alone. However, more patients in the combination group discontinued treatment because of drug-related adverse events – 21% versus 12%.
No additional or surprise adverse events occurred with the longer follow-up, plus quality of life was comparable between groups, Dr. Metges noted.
Stefano Cascinu, MD, Università Vita-Salute, San Raffaele Hospital, Milan, who was not involved in the analysis, reiterated that this update confirms the findings from earlier analyses and shows a benefit across all subgroups.
“One of the most relevant findings was that 20% of patients were responding for more than 24 months,” he said. “It is also important that a similar quality of life was maintained.”
Although Dr. Cascinu emphasized that this is a landmark trial in advanced esophageal and gastric cancers, he indicated to several points that remain to be investigated. These include the reproducibility of the findings in common clinical situations – such as a patient with impaired performance status, malnutrition, or peritoneal involvement – as well as the role of PD-L1.
The efficacy of the combination therapy across all subgroups led to a wide FDA approval, though the European Medicines Agency limited its approval to patients with PD-L1 CPS ≥10 tumors.
“Even though all subgroups did well, patients with [PD-L1] CPS ≥10 did better,” said Dr. Cascinu. “[And] in reality, the benefit may only be driven by a specific subpopulation.”
Dr. Cascinu added: “PD-L1 may be a negative biomarker and may be informative about the magnitude of benefit. This may be useful to discuss with patients regarding the expected benefit [of this therapeutic option].”
The study was supported by Merck & Co. Dr. Metges reported receiving payment for travel, accommodations, expenses from Amgen, LEO Pharma, and MSD Oncology, and receiving honoraria from Bristol-Myers Squibb, Lilly, Novartis, Sanofi, and Syncore. Dr. Cascinu has disclosed honoraria from BMS, Lilly, MSD Oncology, and others, as well as a consulting or advisory role for many of these same manufacturers and serving on the speakers’ bureau of Lilly and SERVIER. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
FROM GI CANCERS SYMPOSIUM 2022
Cancer, infection risk higher in transplant patients than rejection
“It’s important to have immunosuppression to protect people from rejection, but we wanted to be able to say, ‘What are the other causes of kidney failure that we might be able to identify that help improve longer-term outcomes’,” coauthor Andrew Bentall, MBChB, MD, a Mayo Clinic nephrologist, told this news organization.
“And I think the main thing we found is that we need to differentiate people into two groups,” he said, including younger, nondiabetic patients who develop graft failure due to alloimmunity and older often diabetic patients “who are less likely to have a rejection episode but who are still at high risk for death from a malignancy or infection so maybe we can modify their immunosuppression, for example, and reduce their mortality risk which could be very helpful.”
The study was published online Jan. 17 in Transplantation Direct.
Cohort study
The cohort was made up of 5,752 consecutive kidney transplant recipients treated at one of three Mayo Clinic sites. The mean age of recipients was 53.8 years and one-quarter were 65 years of age or older. “At the time of transplantation, 69.8% were on dialysis, and 10.3% had received a prior kidney,” of which half were from a deceased donor, the authors note.
Almost all patients received tacrolimus as part of their maintenance immunosuppressive regimen. At a median follow-up of 3.5 years, overall graft failure occurred in 21.6% of patients, including death with a functioning graft (DWFG) in 12% and graft failure in 9.6% of patients. The most common causes of DWFG included malignancy at 20.0%, followed closely by infection at 19.7%, investigators note.
Cardiac disease was the cause of DWFG in 12.6% of patients, and the cause was unknown in 37%. Of those patients who died with a functioning graft, 12.3% died within the first year of transplantation. Roughly 45% died between 1 and 5 years later, and 42% died more than 5 years after transplantation.
On multivariable analysis, independent predictors of DWFG included:
- Older age at transplantation (hazard ratio, 1.75; P < .001)
- Male sex (HR, 1.34; P < .001)
- Dialysis prior to transplant (HR, 1.49; P < .001)
- Diabetes as a cause of end-stage renal disease (ESRD) (HR, 1.88; P < .001)
- Prednisone use as maintenance therapy (HR, 1.34; P = .008)
Graft failure
Of patients who had graft failure, almost one-quarter occurred within the first year of transplantation, about 42% occurred 1 to 5 years later, and a third occurred more than 5 years later.
Most patients (39%) who went on to graft failure did so as a result of “alloimmunity”, a term investigators used to cover all types of rejection, with a smaller number of graft failures being caused by glomerular diseases, at 18.6%, and renal tubular injury, at 13.9%.
“In the first year after transplantation, surgical complications and primary nonfunction of the allograft caused 60.3% ... of graft losses,” the authors point out. Beyond the first year, alloimmunity accounted for approximately half of the cases of graft failure, investigators note.
In the multivariable analysis for overall graft failure, risk factors included:
- Young recipient age (HR, 0.80; P < .001)
- History of a previous kidney transplant (HR, 1.33; P = .042)
- Dialysis at time of transplantation (HR, 1.54; P < .001)
- Black recipient race (HR, 1.40; P = .006)
- Black donor race (HR, 1.35; P = .038)
- Diabetes as a cause of ESRD (HR, 1.40; P = .002)
- HLA mismatch (HR, 1.27; P < .001)
- Delayed graft function (HR, 2.20; P < .001)
“Over time, DWFG was more common than graft failure,” the authors note.
Modifiable risk factors
As Dr. Bentall acknowledged, not all risk factors contributing to DWFG or graft failure are modifiable. However, diabetes – which stood out as a risk factor for both DWFG and graft failure – is potentially modifiable before patients reach ESRD, as he suggested. Diabetes is currently a cause for up to 40% of all ESRD cases in the United States.
“We can’t necessarily always reverse the diabetes, but there are significant new medications that can be used along with weight loss strategies to improve diabetes control,” he noted.
Similarly, it’s well established that patients who come into transplantation with a body mass index in excess of 30 kg/m2 have more scarring and damage to the kidney 5- and 10-years post-transplantation than healthy weight patients, as Dr. Bentall observed. “Again, this is a key modifiable component, and it fits into diabetes intervention strategies as well,” he emphasized. The use of prednisone as maintenance immunosuppressive therapy similarly emerged as a risk factor for DWFG.
Transplant recipients who receive prednisone may well be a higher risk population to begin with, “but we are also using prednisone in our older patients because we try to use less induction immunosuppression at the time of transplantation. So if we can try and get people off prednisone, that may lessen their risk of infection and subsequent mortality,” Dr. Bentall noted.
A version of this article first appeared on Medscape.com.
“It’s important to have immunosuppression to protect people from rejection, but we wanted to be able to say, ‘What are the other causes of kidney failure that we might be able to identify that help improve longer-term outcomes’,” coauthor Andrew Bentall, MBChB, MD, a Mayo Clinic nephrologist, told this news organization.
“And I think the main thing we found is that we need to differentiate people into two groups,” he said, including younger, nondiabetic patients who develop graft failure due to alloimmunity and older often diabetic patients “who are less likely to have a rejection episode but who are still at high risk for death from a malignancy or infection so maybe we can modify their immunosuppression, for example, and reduce their mortality risk which could be very helpful.”
The study was published online Jan. 17 in Transplantation Direct.
Cohort study
The cohort was made up of 5,752 consecutive kidney transplant recipients treated at one of three Mayo Clinic sites. The mean age of recipients was 53.8 years and one-quarter were 65 years of age or older. “At the time of transplantation, 69.8% were on dialysis, and 10.3% had received a prior kidney,” of which half were from a deceased donor, the authors note.
Almost all patients received tacrolimus as part of their maintenance immunosuppressive regimen. At a median follow-up of 3.5 years, overall graft failure occurred in 21.6% of patients, including death with a functioning graft (DWFG) in 12% and graft failure in 9.6% of patients. The most common causes of DWFG included malignancy at 20.0%, followed closely by infection at 19.7%, investigators note.
Cardiac disease was the cause of DWFG in 12.6% of patients, and the cause was unknown in 37%. Of those patients who died with a functioning graft, 12.3% died within the first year of transplantation. Roughly 45% died between 1 and 5 years later, and 42% died more than 5 years after transplantation.
On multivariable analysis, independent predictors of DWFG included:
- Older age at transplantation (hazard ratio, 1.75; P < .001)
- Male sex (HR, 1.34; P < .001)
- Dialysis prior to transplant (HR, 1.49; P < .001)
- Diabetes as a cause of end-stage renal disease (ESRD) (HR, 1.88; P < .001)
- Prednisone use as maintenance therapy (HR, 1.34; P = .008)
Graft failure
Of patients who had graft failure, almost one-quarter occurred within the first year of transplantation, about 42% occurred 1 to 5 years later, and a third occurred more than 5 years later.
Most patients (39%) who went on to graft failure did so as a result of “alloimmunity”, a term investigators used to cover all types of rejection, with a smaller number of graft failures being caused by glomerular diseases, at 18.6%, and renal tubular injury, at 13.9%.
“In the first year after transplantation, surgical complications and primary nonfunction of the allograft caused 60.3% ... of graft losses,” the authors point out. Beyond the first year, alloimmunity accounted for approximately half of the cases of graft failure, investigators note.
In the multivariable analysis for overall graft failure, risk factors included:
- Young recipient age (HR, 0.80; P < .001)
- History of a previous kidney transplant (HR, 1.33; P = .042)
- Dialysis at time of transplantation (HR, 1.54; P < .001)
- Black recipient race (HR, 1.40; P = .006)
- Black donor race (HR, 1.35; P = .038)
- Diabetes as a cause of ESRD (HR, 1.40; P = .002)
- HLA mismatch (HR, 1.27; P < .001)
- Delayed graft function (HR, 2.20; P < .001)
“Over time, DWFG was more common than graft failure,” the authors note.
Modifiable risk factors
As Dr. Bentall acknowledged, not all risk factors contributing to DWFG or graft failure are modifiable. However, diabetes – which stood out as a risk factor for both DWFG and graft failure – is potentially modifiable before patients reach ESRD, as he suggested. Diabetes is currently a cause for up to 40% of all ESRD cases in the United States.
“We can’t necessarily always reverse the diabetes, but there are significant new medications that can be used along with weight loss strategies to improve diabetes control,” he noted.
Similarly, it’s well established that patients who come into transplantation with a body mass index in excess of 30 kg/m2 have more scarring and damage to the kidney 5- and 10-years post-transplantation than healthy weight patients, as Dr. Bentall observed. “Again, this is a key modifiable component, and it fits into diabetes intervention strategies as well,” he emphasized. The use of prednisone as maintenance immunosuppressive therapy similarly emerged as a risk factor for DWFG.
Transplant recipients who receive prednisone may well be a higher risk population to begin with, “but we are also using prednisone in our older patients because we try to use less induction immunosuppression at the time of transplantation. So if we can try and get people off prednisone, that may lessen their risk of infection and subsequent mortality,” Dr. Bentall noted.
A version of this article first appeared on Medscape.com.
“It’s important to have immunosuppression to protect people from rejection, but we wanted to be able to say, ‘What are the other causes of kidney failure that we might be able to identify that help improve longer-term outcomes’,” coauthor Andrew Bentall, MBChB, MD, a Mayo Clinic nephrologist, told this news organization.
“And I think the main thing we found is that we need to differentiate people into two groups,” he said, including younger, nondiabetic patients who develop graft failure due to alloimmunity and older often diabetic patients “who are less likely to have a rejection episode but who are still at high risk for death from a malignancy or infection so maybe we can modify their immunosuppression, for example, and reduce their mortality risk which could be very helpful.”
The study was published online Jan. 17 in Transplantation Direct.
Cohort study
The cohort was made up of 5,752 consecutive kidney transplant recipients treated at one of three Mayo Clinic sites. The mean age of recipients was 53.8 years and one-quarter were 65 years of age or older. “At the time of transplantation, 69.8% were on dialysis, and 10.3% had received a prior kidney,” of which half were from a deceased donor, the authors note.
Almost all patients received tacrolimus as part of their maintenance immunosuppressive regimen. At a median follow-up of 3.5 years, overall graft failure occurred in 21.6% of patients, including death with a functioning graft (DWFG) in 12% and graft failure in 9.6% of patients. The most common causes of DWFG included malignancy at 20.0%, followed closely by infection at 19.7%, investigators note.
Cardiac disease was the cause of DWFG in 12.6% of patients, and the cause was unknown in 37%. Of those patients who died with a functioning graft, 12.3% died within the first year of transplantation. Roughly 45% died between 1 and 5 years later, and 42% died more than 5 years after transplantation.
On multivariable analysis, independent predictors of DWFG included:
- Older age at transplantation (hazard ratio, 1.75; P < .001)
- Male sex (HR, 1.34; P < .001)
- Dialysis prior to transplant (HR, 1.49; P < .001)
- Diabetes as a cause of end-stage renal disease (ESRD) (HR, 1.88; P < .001)
- Prednisone use as maintenance therapy (HR, 1.34; P = .008)
Graft failure
Of patients who had graft failure, almost one-quarter occurred within the first year of transplantation, about 42% occurred 1 to 5 years later, and a third occurred more than 5 years later.
Most patients (39%) who went on to graft failure did so as a result of “alloimmunity”, a term investigators used to cover all types of rejection, with a smaller number of graft failures being caused by glomerular diseases, at 18.6%, and renal tubular injury, at 13.9%.
“In the first year after transplantation, surgical complications and primary nonfunction of the allograft caused 60.3% ... of graft losses,” the authors point out. Beyond the first year, alloimmunity accounted for approximately half of the cases of graft failure, investigators note.
In the multivariable analysis for overall graft failure, risk factors included:
- Young recipient age (HR, 0.80; P < .001)
- History of a previous kidney transplant (HR, 1.33; P = .042)
- Dialysis at time of transplantation (HR, 1.54; P < .001)
- Black recipient race (HR, 1.40; P = .006)
- Black donor race (HR, 1.35; P = .038)
- Diabetes as a cause of ESRD (HR, 1.40; P = .002)
- HLA mismatch (HR, 1.27; P < .001)
- Delayed graft function (HR, 2.20; P < .001)
“Over time, DWFG was more common than graft failure,” the authors note.
Modifiable risk factors
As Dr. Bentall acknowledged, not all risk factors contributing to DWFG or graft failure are modifiable. However, diabetes – which stood out as a risk factor for both DWFG and graft failure – is potentially modifiable before patients reach ESRD, as he suggested. Diabetes is currently a cause for up to 40% of all ESRD cases in the United States.
“We can’t necessarily always reverse the diabetes, but there are significant new medications that can be used along with weight loss strategies to improve diabetes control,” he noted.
Similarly, it’s well established that patients who come into transplantation with a body mass index in excess of 30 kg/m2 have more scarring and damage to the kidney 5- and 10-years post-transplantation than healthy weight patients, as Dr. Bentall observed. “Again, this is a key modifiable component, and it fits into diabetes intervention strategies as well,” he emphasized. The use of prednisone as maintenance immunosuppressive therapy similarly emerged as a risk factor for DWFG.
Transplant recipients who receive prednisone may well be a higher risk population to begin with, “but we are also using prednisone in our older patients because we try to use less induction immunosuppression at the time of transplantation. So if we can try and get people off prednisone, that may lessen their risk of infection and subsequent mortality,” Dr. Bentall noted.
A version of this article first appeared on Medscape.com.
FROM TRANSPLANTATION DIRECT
Some U.S. women not getting ET for curable breast cancer
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Researchers eye cannabis for gynecologic pain
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Vitamin D shows no survival benefit in nondeficient elderly
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Clinical Edge Journal Scan Commentary: Multiple Sclerosis February 2022
In another study (Maniscalco GT et al) exploring vaccine efficacy in 149 PwMS, treatment with interferon (IFN)-beta 1A resulted in improved anti-spike IgG specific humoral response levels than was seen in healthy controls response (median, 1,916 vs 1,089; P = .029) whereas reduced anti-spike IgG levels were significantly lower in patients treated with Cladribine (P = .002), Fingolimod (P < .0001), or Ocrelizumab (P < .0001). Clinical decisions regarding DMT treatment choice and DMT change focused solely on relapse rate and MRI are now insufficient without considering and incorporating vaccine response into the decision-making process. Further information across all DMT’s is needed to allow improved decision making regarding DMT choice. Confounding this problem is the frequency of unrecognized cognitive impairment (CI) in PwMS and the impact CI has on the shared decision-making process beyond EDSS.
In another study (Cavaco S et al) regarding CI in 408 PwMS, the presence of cognitive dysfunction was not only predictive of a higher risk for conversion from relapsing-remitting disease to progressive disease (adjusted odds ratio, 2.29; P = .043) and shorter survival (e.g. higher risk for death), (adjusted hazard ratio, 3.07; P = .006). The impact of such CI and progressive CI on vaccine hesitancy is unknown. Monitoring disease impact and change in cognitive function in PwMS remains another great unmet need in routine care of PwMS and evaluating the impact of CI on vaccine hesitancy and the shared decision-making process also requires further exploration and incorporation into routine care. Care of PwMS and the choice of DMT should hinge not only considerations about efficacy and safety but now must also incorporate patient vaccine hesitancy and response to vaccination.
In another study (Maniscalco GT et al) exploring vaccine efficacy in 149 PwMS, treatment with interferon (IFN)-beta 1A resulted in improved anti-spike IgG specific humoral response levels than was seen in healthy controls response (median, 1,916 vs 1,089; P = .029) whereas reduced anti-spike IgG levels were significantly lower in patients treated with Cladribine (P = .002), Fingolimod (P < .0001), or Ocrelizumab (P < .0001). Clinical decisions regarding DMT treatment choice and DMT change focused solely on relapse rate and MRI are now insufficient without considering and incorporating vaccine response into the decision-making process. Further information across all DMT’s is needed to allow improved decision making regarding DMT choice. Confounding this problem is the frequency of unrecognized cognitive impairment (CI) in PwMS and the impact CI has on the shared decision-making process beyond EDSS.
In another study (Cavaco S et al) regarding CI in 408 PwMS, the presence of cognitive dysfunction was not only predictive of a higher risk for conversion from relapsing-remitting disease to progressive disease (adjusted odds ratio, 2.29; P = .043) and shorter survival (e.g. higher risk for death), (adjusted hazard ratio, 3.07; P = .006). The impact of such CI and progressive CI on vaccine hesitancy is unknown. Monitoring disease impact and change in cognitive function in PwMS remains another great unmet need in routine care of PwMS and evaluating the impact of CI on vaccine hesitancy and the shared decision-making process also requires further exploration and incorporation into routine care. Care of PwMS and the choice of DMT should hinge not only considerations about efficacy and safety but now must also incorporate patient vaccine hesitancy and response to vaccination.
In another study (Maniscalco GT et al) exploring vaccine efficacy in 149 PwMS, treatment with interferon (IFN)-beta 1A resulted in improved anti-spike IgG specific humoral response levels than was seen in healthy controls response (median, 1,916 vs 1,089; P = .029) whereas reduced anti-spike IgG levels were significantly lower in patients treated with Cladribine (P = .002), Fingolimod (P < .0001), or Ocrelizumab (P < .0001). Clinical decisions regarding DMT treatment choice and DMT change focused solely on relapse rate and MRI are now insufficient without considering and incorporating vaccine response into the decision-making process. Further information across all DMT’s is needed to allow improved decision making regarding DMT choice. Confounding this problem is the frequency of unrecognized cognitive impairment (CI) in PwMS and the impact CI has on the shared decision-making process beyond EDSS.
In another study (Cavaco S et al) regarding CI in 408 PwMS, the presence of cognitive dysfunction was not only predictive of a higher risk for conversion from relapsing-remitting disease to progressive disease (adjusted odds ratio, 2.29; P = .043) and shorter survival (e.g. higher risk for death), (adjusted hazard ratio, 3.07; P = .006). The impact of such CI and progressive CI on vaccine hesitancy is unknown. Monitoring disease impact and change in cognitive function in PwMS remains another great unmet need in routine care of PwMS and evaluating the impact of CI on vaccine hesitancy and the shared decision-making process also requires further exploration and incorporation into routine care. Care of PwMS and the choice of DMT should hinge not only considerations about efficacy and safety but now must also incorporate patient vaccine hesitancy and response to vaccination.
Omicron subvariant 1.5 times more contagious than Omicron
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
Surrogate endpoints acceptable in AML trials, says FDA
The Food and Drug Administration has been harshly criticized for using surrogate endpoints in clinical trials in its approval of new drugs, and especially so in oncology, where critics have argued that the only truly meaningful endpoint is overall survival (OS).
But the FDA is now fighting back and is arguing that in certain cases surrogate endpoints do translate to overall survival benefits. A case in point is in clinical trials of new treatments being investigated in patients newly diagnosed with acute myeloid leukemia (AML).
The results show that these particular surrogate endpoints do have real value in predicting the efficacy of drugs for the treatment of newly diagnosed AML, they concluded.
“To our knowledge, our results represent the first direct examination of the relationship of response rate and EFS to OS using trial-level and patient-level data in patients with newly diagnosed AML treated with intensive induction chemotherapy,” the FDA investigators commented.
“The results support the FDA’s acceptance of EFS as a clinical benefit endpoint supportive of traditional approval for treatments with curative intent,” they added.
The analysis was published online on Dec. 10, 2021, in the Journal of Clinical Oncology.
“The central finding of the article, that both EFS and the CR rate are reliably associated with improved OS, is of immediate relevance and indicates that these parameters represent acceptable and appropriate surrogate endpoints for clinical trials of AML,” Courtney DiNardo, MD, University of Texas MD Anderson Cancer Center, Houston, and Daniel Pollyea, MD, University of Colorado at Denver, Aurora, wrote in an accompanying editorial.
“The establishment of CR and EFS as appropriate surrogate endpoints for patients with newly diagnosed AML receiving intensive chemotherapy will allow earlier evaluation of novel therapies and speed the delivery of safe and effective therapies to our patients,” they added.
Analysis of clinical trials submitted for approval
The FDA investigators conducted an analysis of eight trials that had been submitted to the agency for licensing approval between 2007 and 2011. Together, the trials included a total of 4,482 patients with newly diagnosed AML.
Five trials evaluated gemtuzumab ozogamicin (Mylotarg), while two trials evaluated a daunorubicin and cytarabine liposome injection, also known as CPX-351 (Vyxeos), and one trial evaluated midostaurin (RYDAPT).
“All were approved in combination with, or for use as, intensive induction chemotherapy in patients with newly diagnosed AML,” the team wrote. Both trial-level and patient-level associations between responses, EFS, and OS were evaluated.
The association between the hazard ratio for OS and the odds ratio for CR at the trial level was “moderate.” The association between the HR for OS and the OR for lesser response rates – namely, CR with incomplete hematologic recovery (CRi) and CR with incomplete platelet recovery (CRp) – was similarly moderate, as investigators note.
On the other hand, while the associations between the HR for OS and the HR for EFS were again moderate, “on the basis of the harmonized primary definition of EFS across trials, the association became stronger,” the authors reported. The harmonized definition of EFS across the trials included time from random assignment to treatment failure, relapse from CR, or death from any cause, whichever occurred earlier.
The FDA authors cautioned that a significant number of patients who relapsed did not die during the course of the clinical trial, resulting in a considerably longer OS, compared with EFS in some patients. However, to further explore the relationship between response and survival, a patient-level analysis of response – namely, CR versus CRi or CRp versus no response – was performed.”Patients who achieved a CR had a [27%] better OS, compared with patients whose best response was CRi or CRp,” the authors noted.
Patients who achieved a CRi or a CRp as their best response still had a 54% better OS, compared with patients who achieved no response, irrespective of the treatment received, they added.
Interestingly enough, a small number of patients who had no response to treatment also experienced prolonged survival, possibly because of successful second-line therapy, the authors speculated.
Effective salvage therapies
Commenting further in their editorial, Dr. DiNardo and Dr. Pollyea wrote that, given that there are now multiple effective salvage therapies for the treatment of AML, an OS endpoint no longer solely reflects the effectiveness of an initial therapy, as survival will also be affected by subsequent lines of AML-directed treatment.
“Accordingly, OS should no longer be considered the sole determinant of the value of a new therapy,” the editorialists emphasized.
Furthermore, as the treatment of AML is increasingly based on biologically defined and differentially targeted subsets, “the required sample sizes and timelines to run a proper randomized, phase 3 study for an OS end point of a rare AML subset become logistically untenable,” they wrote.
That said, the editorialists felt the fact that FDA employees performed this meta-analysis at all was “highly laudable.” “It is, moreover, gratifying to know that the experiences of clinical trial participants can be maximized beyond the original contributions made to the studies in which they originally volunteered,” the editorialists observed.
“In the United States, this example should inspire investigators and industry partners to prioritize similar analyses with their [own] data sets,” they added.
Dr. Norsworthy, Dr. DiNardo, and Dr. Pollyea disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has been harshly criticized for using surrogate endpoints in clinical trials in its approval of new drugs, and especially so in oncology, where critics have argued that the only truly meaningful endpoint is overall survival (OS).
But the FDA is now fighting back and is arguing that in certain cases surrogate endpoints do translate to overall survival benefits. A case in point is in clinical trials of new treatments being investigated in patients newly diagnosed with acute myeloid leukemia (AML).
The results show that these particular surrogate endpoints do have real value in predicting the efficacy of drugs for the treatment of newly diagnosed AML, they concluded.
“To our knowledge, our results represent the first direct examination of the relationship of response rate and EFS to OS using trial-level and patient-level data in patients with newly diagnosed AML treated with intensive induction chemotherapy,” the FDA investigators commented.
“The results support the FDA’s acceptance of EFS as a clinical benefit endpoint supportive of traditional approval for treatments with curative intent,” they added.
The analysis was published online on Dec. 10, 2021, in the Journal of Clinical Oncology.
“The central finding of the article, that both EFS and the CR rate are reliably associated with improved OS, is of immediate relevance and indicates that these parameters represent acceptable and appropriate surrogate endpoints for clinical trials of AML,” Courtney DiNardo, MD, University of Texas MD Anderson Cancer Center, Houston, and Daniel Pollyea, MD, University of Colorado at Denver, Aurora, wrote in an accompanying editorial.
“The establishment of CR and EFS as appropriate surrogate endpoints for patients with newly diagnosed AML receiving intensive chemotherapy will allow earlier evaluation of novel therapies and speed the delivery of safe and effective therapies to our patients,” they added.
Analysis of clinical trials submitted for approval
The FDA investigators conducted an analysis of eight trials that had been submitted to the agency for licensing approval between 2007 and 2011. Together, the trials included a total of 4,482 patients with newly diagnosed AML.
Five trials evaluated gemtuzumab ozogamicin (Mylotarg), while two trials evaluated a daunorubicin and cytarabine liposome injection, also known as CPX-351 (Vyxeos), and one trial evaluated midostaurin (RYDAPT).
“All were approved in combination with, or for use as, intensive induction chemotherapy in patients with newly diagnosed AML,” the team wrote. Both trial-level and patient-level associations between responses, EFS, and OS were evaluated.
The association between the hazard ratio for OS and the odds ratio for CR at the trial level was “moderate.” The association between the HR for OS and the OR for lesser response rates – namely, CR with incomplete hematologic recovery (CRi) and CR with incomplete platelet recovery (CRp) – was similarly moderate, as investigators note.
On the other hand, while the associations between the HR for OS and the HR for EFS were again moderate, “on the basis of the harmonized primary definition of EFS across trials, the association became stronger,” the authors reported. The harmonized definition of EFS across the trials included time from random assignment to treatment failure, relapse from CR, or death from any cause, whichever occurred earlier.
The FDA authors cautioned that a significant number of patients who relapsed did not die during the course of the clinical trial, resulting in a considerably longer OS, compared with EFS in some patients. However, to further explore the relationship between response and survival, a patient-level analysis of response – namely, CR versus CRi or CRp versus no response – was performed.”Patients who achieved a CR had a [27%] better OS, compared with patients whose best response was CRi or CRp,” the authors noted.
Patients who achieved a CRi or a CRp as their best response still had a 54% better OS, compared with patients who achieved no response, irrespective of the treatment received, they added.
Interestingly enough, a small number of patients who had no response to treatment also experienced prolonged survival, possibly because of successful second-line therapy, the authors speculated.
Effective salvage therapies
Commenting further in their editorial, Dr. DiNardo and Dr. Pollyea wrote that, given that there are now multiple effective salvage therapies for the treatment of AML, an OS endpoint no longer solely reflects the effectiveness of an initial therapy, as survival will also be affected by subsequent lines of AML-directed treatment.
“Accordingly, OS should no longer be considered the sole determinant of the value of a new therapy,” the editorialists emphasized.
Furthermore, as the treatment of AML is increasingly based on biologically defined and differentially targeted subsets, “the required sample sizes and timelines to run a proper randomized, phase 3 study for an OS end point of a rare AML subset become logistically untenable,” they wrote.
That said, the editorialists felt the fact that FDA employees performed this meta-analysis at all was “highly laudable.” “It is, moreover, gratifying to know that the experiences of clinical trial participants can be maximized beyond the original contributions made to the studies in which they originally volunteered,” the editorialists observed.
“In the United States, this example should inspire investigators and industry partners to prioritize similar analyses with their [own] data sets,” they added.
Dr. Norsworthy, Dr. DiNardo, and Dr. Pollyea disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has been harshly criticized for using surrogate endpoints in clinical trials in its approval of new drugs, and especially so in oncology, where critics have argued that the only truly meaningful endpoint is overall survival (OS).
But the FDA is now fighting back and is arguing that in certain cases surrogate endpoints do translate to overall survival benefits. A case in point is in clinical trials of new treatments being investigated in patients newly diagnosed with acute myeloid leukemia (AML).
The results show that these particular surrogate endpoints do have real value in predicting the efficacy of drugs for the treatment of newly diagnosed AML, they concluded.
“To our knowledge, our results represent the first direct examination of the relationship of response rate and EFS to OS using trial-level and patient-level data in patients with newly diagnosed AML treated with intensive induction chemotherapy,” the FDA investigators commented.
“The results support the FDA’s acceptance of EFS as a clinical benefit endpoint supportive of traditional approval for treatments with curative intent,” they added.
The analysis was published online on Dec. 10, 2021, in the Journal of Clinical Oncology.
“The central finding of the article, that both EFS and the CR rate are reliably associated with improved OS, is of immediate relevance and indicates that these parameters represent acceptable and appropriate surrogate endpoints for clinical trials of AML,” Courtney DiNardo, MD, University of Texas MD Anderson Cancer Center, Houston, and Daniel Pollyea, MD, University of Colorado at Denver, Aurora, wrote in an accompanying editorial.
“The establishment of CR and EFS as appropriate surrogate endpoints for patients with newly diagnosed AML receiving intensive chemotherapy will allow earlier evaluation of novel therapies and speed the delivery of safe and effective therapies to our patients,” they added.
Analysis of clinical trials submitted for approval
The FDA investigators conducted an analysis of eight trials that had been submitted to the agency for licensing approval between 2007 and 2011. Together, the trials included a total of 4,482 patients with newly diagnosed AML.
Five trials evaluated gemtuzumab ozogamicin (Mylotarg), while two trials evaluated a daunorubicin and cytarabine liposome injection, also known as CPX-351 (Vyxeos), and one trial evaluated midostaurin (RYDAPT).
“All were approved in combination with, or for use as, intensive induction chemotherapy in patients with newly diagnosed AML,” the team wrote. Both trial-level and patient-level associations between responses, EFS, and OS were evaluated.
The association between the hazard ratio for OS and the odds ratio for CR at the trial level was “moderate.” The association between the HR for OS and the OR for lesser response rates – namely, CR with incomplete hematologic recovery (CRi) and CR with incomplete platelet recovery (CRp) – was similarly moderate, as investigators note.
On the other hand, while the associations between the HR for OS and the HR for EFS were again moderate, “on the basis of the harmonized primary definition of EFS across trials, the association became stronger,” the authors reported. The harmonized definition of EFS across the trials included time from random assignment to treatment failure, relapse from CR, or death from any cause, whichever occurred earlier.
The FDA authors cautioned that a significant number of patients who relapsed did not die during the course of the clinical trial, resulting in a considerably longer OS, compared with EFS in some patients. However, to further explore the relationship between response and survival, a patient-level analysis of response – namely, CR versus CRi or CRp versus no response – was performed.”Patients who achieved a CR had a [27%] better OS, compared with patients whose best response was CRi or CRp,” the authors noted.
Patients who achieved a CRi or a CRp as their best response still had a 54% better OS, compared with patients who achieved no response, irrespective of the treatment received, they added.
Interestingly enough, a small number of patients who had no response to treatment also experienced prolonged survival, possibly because of successful second-line therapy, the authors speculated.
Effective salvage therapies
Commenting further in their editorial, Dr. DiNardo and Dr. Pollyea wrote that, given that there are now multiple effective salvage therapies for the treatment of AML, an OS endpoint no longer solely reflects the effectiveness of an initial therapy, as survival will also be affected by subsequent lines of AML-directed treatment.
“Accordingly, OS should no longer be considered the sole determinant of the value of a new therapy,” the editorialists emphasized.
Furthermore, as the treatment of AML is increasingly based on biologically defined and differentially targeted subsets, “the required sample sizes and timelines to run a proper randomized, phase 3 study for an OS end point of a rare AML subset become logistically untenable,” they wrote.
That said, the editorialists felt the fact that FDA employees performed this meta-analysis at all was “highly laudable.” “It is, moreover, gratifying to know that the experiences of clinical trial participants can be maximized beyond the original contributions made to the studies in which they originally volunteered,” the editorialists observed.
“In the United States, this example should inspire investigators and industry partners to prioritize similar analyses with their [own] data sets,” they added.
Dr. Norsworthy, Dr. DiNardo, and Dr. Pollyea disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
A dermatologist-led model for CVD prevention in psoriasis may be feasible
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
FROM JAMA DERMATOLOGY
Assessing imminent suicide risk: What about future planning?
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.
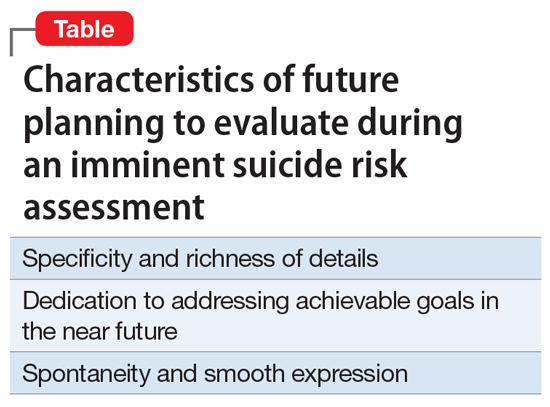
Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.

Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.

Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.









