User login
PPI Prophylaxis Prevents GI Bleed in Ventilated Patients
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Investing in Future Discovery
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 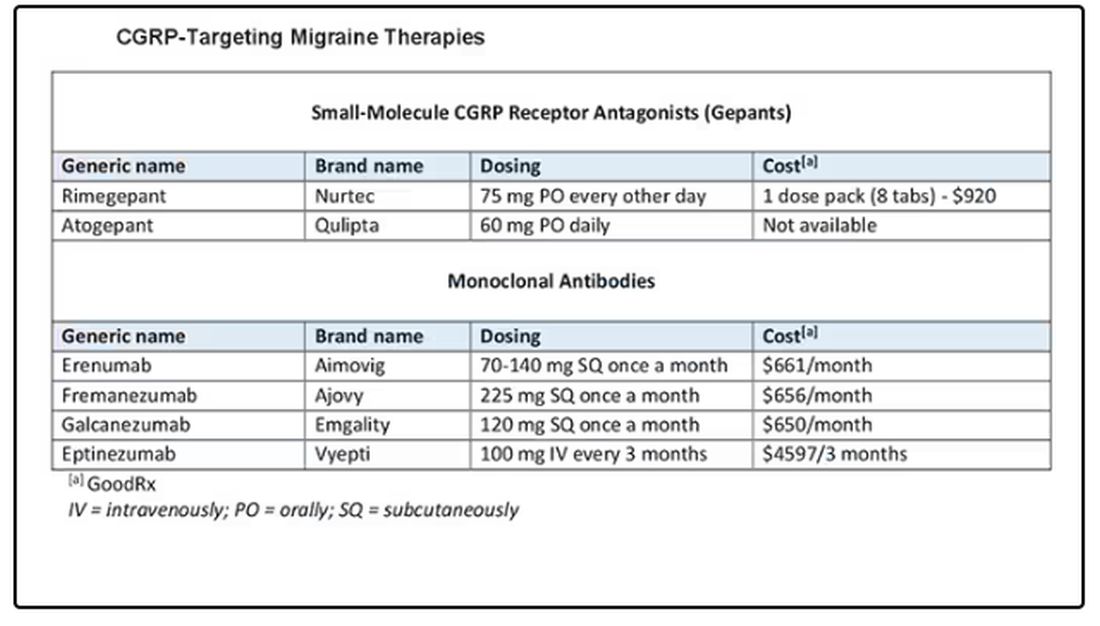
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
Late ERCP After Cholecystectomy Linked with Worse Outcomes
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Environment More Than Genes Affects Age of IBD Diagnosis
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Immunotherapy May Be Overused in Dying Patients With Cancer
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Acid Blockers Appear Superior to PPIs in Erosive Esophagitis
TOPLINE:
, a meta-analysis suggests.
METHODOLOGY:
- Researchers conducted a database search up to May 31, 2023, for randomized controlled trials of potassium-competitive acid blockers and PPIs for the treatment of erosive esophagitis. They included 34 trials in a systematic review and a network meta-analysis comparing the efficacy of the two medication classes in this patient population.
- The trials included 25,054 patients with erosive esophagitis, and the treatments involved were standard or double doses of potassium-competitive acid blockers (tegoprazan, vonoprazan, keverprazan, and fexuprazan), PPIs (esomeprazole, ilaprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole), or placebo.
- The researchers compared the healing rates at 4 and 8 weeks.
TAKEAWAY:
- The main analysis found that both potassium-competitive acid blockers and PPIs showed better healing rates at 4 and 8 weeks than placebo. This finding held up in a subgroup analysis of patients with and without severe erosive esophagitis at both time points.
- For most treatments, the pooled healing rates at 8 weeks were significantly higher than those at 4 weeks.
- In the main analysis, ilaprazole 10 mg once daily had the best healing rate (surface under the cumulative ranking curve [SUCRA], 89.3) at 4 weeks, followed by vonoprazan 40 mg once daily (SUCRA, 86.7). At 8 weeks, keverprazan 20 mg once daily ranked best (SUCRA, 84.7), followed by ilaprazole 10 mg once daily (SUCRA, 82.0).
- The subgroup analysis found that healing rates were higher with most potassium-competitive acid blockers than with PPIs, particularly for patients with severe erosive esophagitis. Keverprazan 20 mg daily was found to have the highest healing rate at 8 weeks for both severe and non-severe erosive esophagitis, and vonoprazan 40 mg daily had a relatively higher healing rate at 4 weeks.
IN PRACTICE:
The finding that most potassium-competitive acid blockers showed a higher healing rate than PPIs, particularly for patients with severe erosive esophagitis, “may help inform future directions of treatment,” the authors wrote. But high-quality randomized controlled trials are required to confirm potassium-competitive acid blockers’ healing effect in patients with erosive esophagitis, they added.
SOURCE:
The study, led by Yin Liu of the Henan Cancer Hospital (Affiliated Cancer Hospital of Zhengzhou University), Zhengzhou, and Zhifeng Gao of the Department of Gastroenterology, The First People’s Hospital of Xuzhou, Xuzhou, China, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
The limitations of the study included heterogeneity and bias across included studies, a lack of head-to-head trials for all included treatments, and insufficient reporting on outcomes based on the severity of erosive esophagitis.
DISCLOSURES:
The authors received no financial support for the study. There were no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a meta-analysis suggests.
METHODOLOGY:
- Researchers conducted a database search up to May 31, 2023, for randomized controlled trials of potassium-competitive acid blockers and PPIs for the treatment of erosive esophagitis. They included 34 trials in a systematic review and a network meta-analysis comparing the efficacy of the two medication classes in this patient population.
- The trials included 25,054 patients with erosive esophagitis, and the treatments involved were standard or double doses of potassium-competitive acid blockers (tegoprazan, vonoprazan, keverprazan, and fexuprazan), PPIs (esomeprazole, ilaprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole), or placebo.
- The researchers compared the healing rates at 4 and 8 weeks.
TAKEAWAY:
- The main analysis found that both potassium-competitive acid blockers and PPIs showed better healing rates at 4 and 8 weeks than placebo. This finding held up in a subgroup analysis of patients with and without severe erosive esophagitis at both time points.
- For most treatments, the pooled healing rates at 8 weeks were significantly higher than those at 4 weeks.
- In the main analysis, ilaprazole 10 mg once daily had the best healing rate (surface under the cumulative ranking curve [SUCRA], 89.3) at 4 weeks, followed by vonoprazan 40 mg once daily (SUCRA, 86.7). At 8 weeks, keverprazan 20 mg once daily ranked best (SUCRA, 84.7), followed by ilaprazole 10 mg once daily (SUCRA, 82.0).
- The subgroup analysis found that healing rates were higher with most potassium-competitive acid blockers than with PPIs, particularly for patients with severe erosive esophagitis. Keverprazan 20 mg daily was found to have the highest healing rate at 8 weeks for both severe and non-severe erosive esophagitis, and vonoprazan 40 mg daily had a relatively higher healing rate at 4 weeks.
IN PRACTICE:
The finding that most potassium-competitive acid blockers showed a higher healing rate than PPIs, particularly for patients with severe erosive esophagitis, “may help inform future directions of treatment,” the authors wrote. But high-quality randomized controlled trials are required to confirm potassium-competitive acid blockers’ healing effect in patients with erosive esophagitis, they added.
SOURCE:
The study, led by Yin Liu of the Henan Cancer Hospital (Affiliated Cancer Hospital of Zhengzhou University), Zhengzhou, and Zhifeng Gao of the Department of Gastroenterology, The First People’s Hospital of Xuzhou, Xuzhou, China, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
The limitations of the study included heterogeneity and bias across included studies, a lack of head-to-head trials for all included treatments, and insufficient reporting on outcomes based on the severity of erosive esophagitis.
DISCLOSURES:
The authors received no financial support for the study. There were no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a meta-analysis suggests.
METHODOLOGY:
- Researchers conducted a database search up to May 31, 2023, for randomized controlled trials of potassium-competitive acid blockers and PPIs for the treatment of erosive esophagitis. They included 34 trials in a systematic review and a network meta-analysis comparing the efficacy of the two medication classes in this patient population.
- The trials included 25,054 patients with erosive esophagitis, and the treatments involved were standard or double doses of potassium-competitive acid blockers (tegoprazan, vonoprazan, keverprazan, and fexuprazan), PPIs (esomeprazole, ilaprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole), or placebo.
- The researchers compared the healing rates at 4 and 8 weeks.
TAKEAWAY:
- The main analysis found that both potassium-competitive acid blockers and PPIs showed better healing rates at 4 and 8 weeks than placebo. This finding held up in a subgroup analysis of patients with and without severe erosive esophagitis at both time points.
- For most treatments, the pooled healing rates at 8 weeks were significantly higher than those at 4 weeks.
- In the main analysis, ilaprazole 10 mg once daily had the best healing rate (surface under the cumulative ranking curve [SUCRA], 89.3) at 4 weeks, followed by vonoprazan 40 mg once daily (SUCRA, 86.7). At 8 weeks, keverprazan 20 mg once daily ranked best (SUCRA, 84.7), followed by ilaprazole 10 mg once daily (SUCRA, 82.0).
- The subgroup analysis found that healing rates were higher with most potassium-competitive acid blockers than with PPIs, particularly for patients with severe erosive esophagitis. Keverprazan 20 mg daily was found to have the highest healing rate at 8 weeks for both severe and non-severe erosive esophagitis, and vonoprazan 40 mg daily had a relatively higher healing rate at 4 weeks.
IN PRACTICE:
The finding that most potassium-competitive acid blockers showed a higher healing rate than PPIs, particularly for patients with severe erosive esophagitis, “may help inform future directions of treatment,” the authors wrote. But high-quality randomized controlled trials are required to confirm potassium-competitive acid blockers’ healing effect in patients with erosive esophagitis, they added.
SOURCE:
The study, led by Yin Liu of the Henan Cancer Hospital (Affiliated Cancer Hospital of Zhengzhou University), Zhengzhou, and Zhifeng Gao of the Department of Gastroenterology, The First People’s Hospital of Xuzhou, Xuzhou, China, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
The limitations of the study included heterogeneity and bias across included studies, a lack of head-to-head trials for all included treatments, and insufficient reporting on outcomes based on the severity of erosive esophagitis.
DISCLOSURES:
The authors received no financial support for the study. There were no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How Clinicians Can Help Patients Navigate Psychedelics/Microdosing
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Anxiety Linked to a Threefold Increased Risk for Dementia
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Modest Gains Shown in Breast Cancer Immunotherapy Trials
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.










