User login
Pure Mucinous Breast Cancer Shows Better Survival Rates Than Other Subtypes
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Xanthelasma Not Linked to Heart Diseases, Study Finds
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Association Between Pruritus and Fibromyalgia: Results of a Population-Based, Cross-Sectional Study
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.
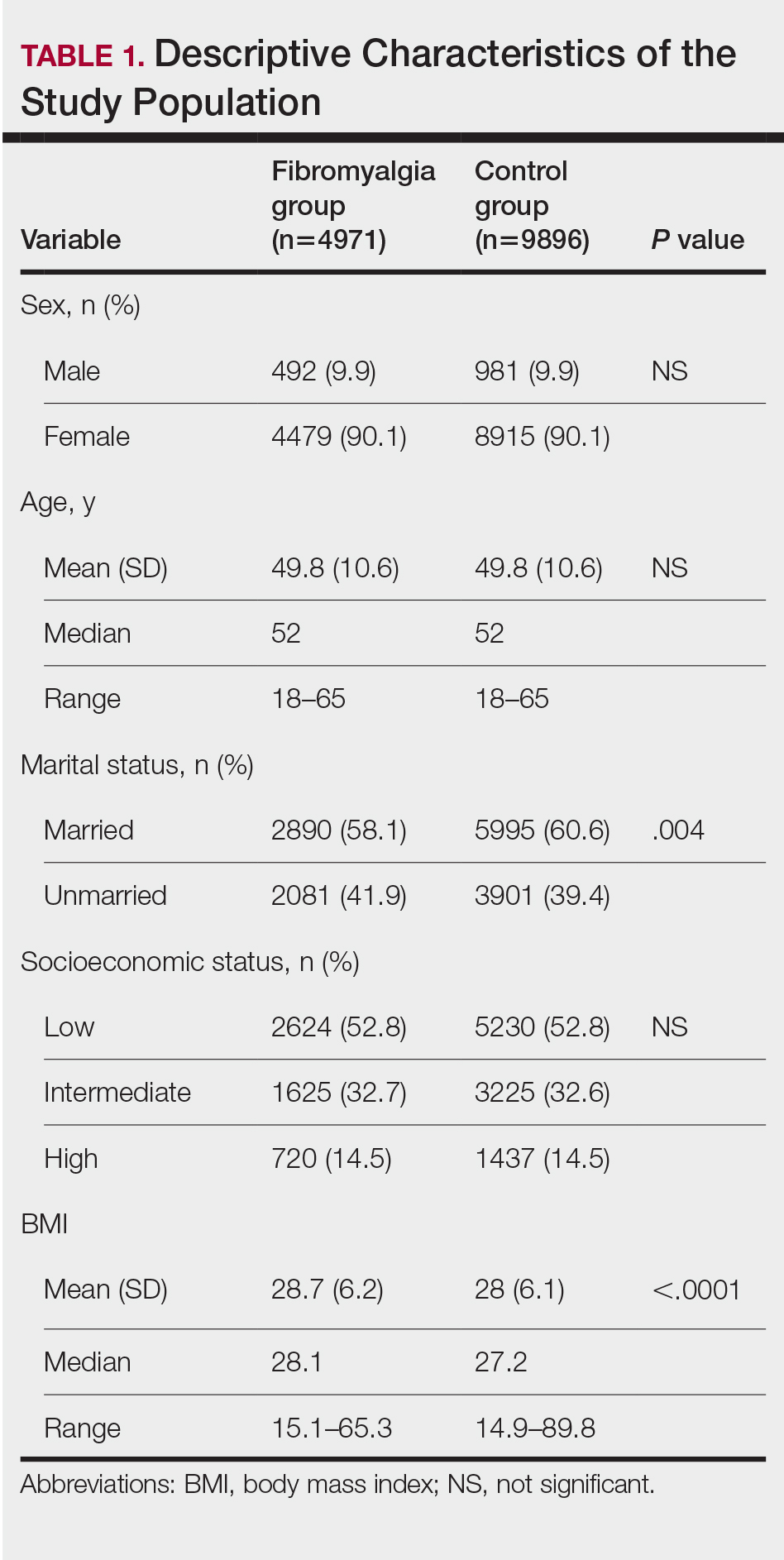
We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).
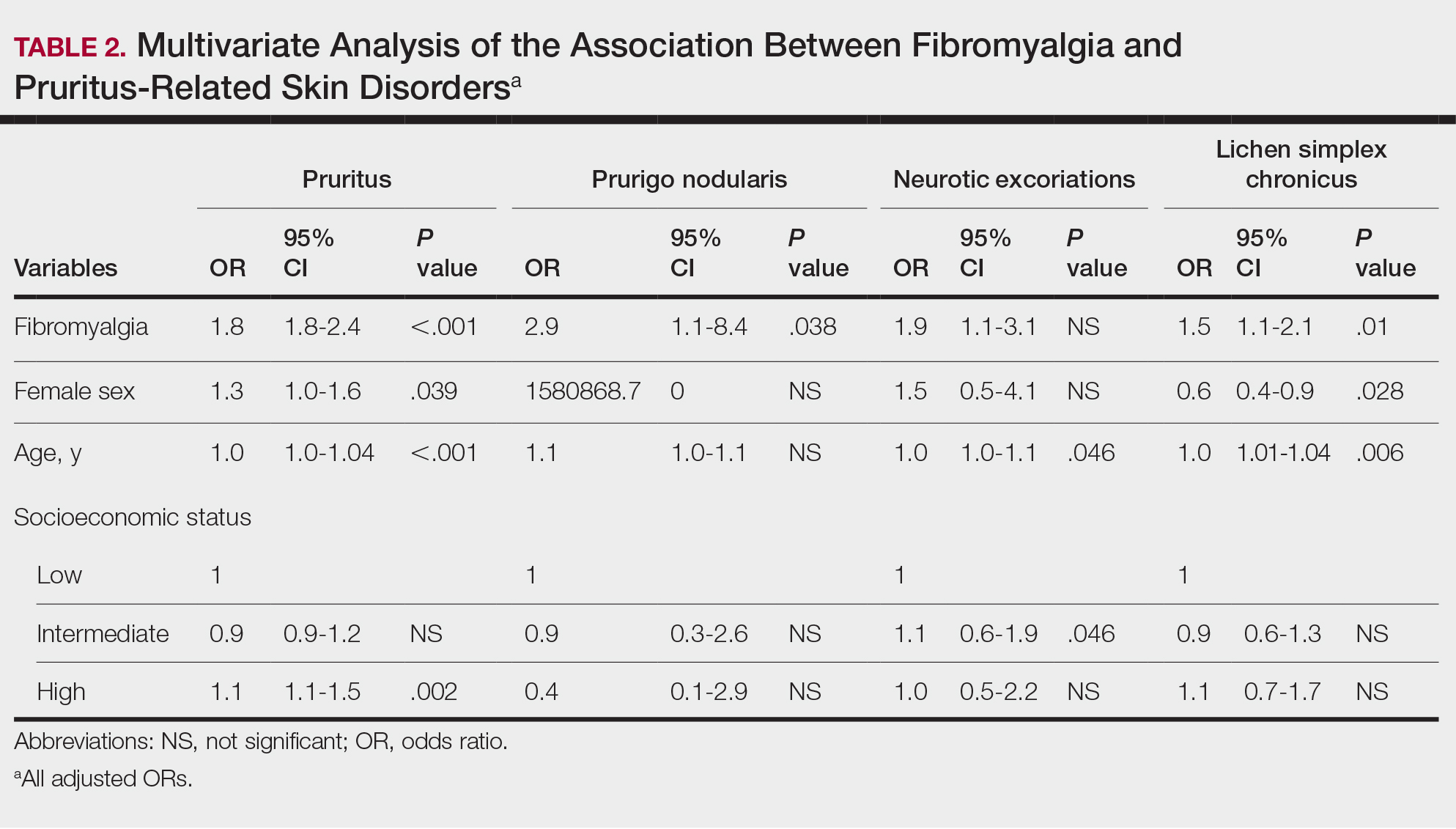
Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).
Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Practice Points
- Dermatologists should be aware of the connection between fibromyalgia, pruritus, and related conditions to improve patient care.
- The association between fibromyalgia and pruritus underscores the importance of employing multidisciplinary treatment strategies for managing these conditions.
Are Your Patients Using Any of These Six Potentially Hepatotoxic Botanicals?
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
New Study Links Sweetener to Heart Risk: What to Know
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Painful Plaque on the Forearm
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
A 30-year-old woman presented to the dermatology clinic with lesions on the right forearm of 2 years’ duration. Her medical history was unremarkable. She reported working as a chef and caring for multiple pets in her home, including 3 cats, 6 fish tanks, 3 dogs, and 3 lizards. Physical examination revealed a painful, indurated, red-violaceous plaque on the right forearm with satellite pink nodules that had been slowly migrating proximally up the forearm. An outside excisional biopsy performed 1 year prior had shown suppurative granulomatous dermatitis with negative stains for infectious organisms and negative tissue cultures. At that time, the patient was diagnosed with ruptured folliculitis; however, a subsequent lack of clinical improvement prompted her to seek a second opinion at our clinic.
Cannabis Overuse Linked to Increased Risk for Head and Neck Cancer
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Snare Tip Soft Coagulation Leaves Clean Margins After Resection
according to a recent study.
Since STSC was faster to apply than APC and results in lower cost and plastic waste (because of APC requiring an additional catheter), STSC was the preferred option.
“The reduction in recurrence rate with thermal margin treatment is arguably the most important development in endoscopic mucosal resection in the past 2 decades,” said lead author Douglas Rex, MD, AGAF, a distinguished professor emeritus at the Indiana University School of Medicine and director of endoscopy at Indiana University Hospitals, both in Indianapolis.
“Margin thermal therapy with STSC should now be standard treatment after piecemeal EMR in the colorectum,” he said. “Before applying STSC, the endoscopist must ensure that the entire lesion is resected down to the submucosa. Then STSC should be aggressively applied to 100% of the margin.”
The study was published in Clinical Gastroenterology and Hepatology .
Comparing Treatments
Dr. Rex and colleagues performed a randomized three-arm trial in nine U.S. centers, comparing STSC with APC and no margin treatment in patients undergoing colorectal EMR of nonpedunculated lesions of 15 mm or greater.
All lesions underwent conventional injection and snare resection EMR using electrocautery, but the endoscopist chose the injection fluid and snare type and size. Areas with residual polyp that weren’t removable by snare resection because of flat shape or fibrosis were removed by hot or cold avulsion. After that, patients were randomized to one of the three arms.
Patients were scheduled for a follow-up appointment six months after the initial EMR. Any visible recurrence was resected using methods at the discretion of the endoscopist, and if no visible recurrence was present, EMR site biopsies were recommended.
Among 384 patients with 414 lesions, 308 patients with 328 lesions completed at least one follow-up appointment. The median interval to the first follow-up was 6.4 months, ranging from 2 to 37 months. The primary endpoint was the presence of recurrent or residual polyp at first follow-up.
The median polyp size was 25 mm, and 65 of the 414 polyps (15.7%) were 15-19 mm in size. Overall, 14.8% of lesions were resected en bloc, with no difference between the study arms.
The proportion of lesions with residual polyp at first follow-up was 4.6% with STSC, 9.3% with APC, and 21.4% among control subjects with no margin treatment.
The odds of having a residual polyp at first follow-up were lower for STSC and APC when compared with control subjects (odds ratio [OR] of 0.182 and 0.341, or P = .001 and P = .01, respectively). There wasn’t a significant difference in the odds of recurrence between STSC and APC (OR, 1.874).
In 259 lesions in 248 patients that were 20 mm or greater, the recurrence rates at first follow-up were 5.9% for STSC, 10.1% for APC, and 25.9% for the control group. In these lesions, STSC and APC remained associated with a lower risk of recurrence versus the control (OR, 0.18 and 0.323, respectively). The difference in recurrence rates between STSC and APC wasn’t significant.
Even still, STSC took less time to apply than APC, with a median time of 3.35 minutes vs 4.08 minutes.
The rates of adverse events were low, with no difference between the three arms. There were no immediate or delayed perforations in any arm, and the overall occurrence of delayed bleeding was low at 3.6%.
“I think STSC won the trial because it was numerically (though not statistically) superior to APC, was faster to apply, and using STSC results in lower cost and less plastic compared to APC,” Dr. Rex said.
Additional Considerations
Based on charges at the nine U.S. centers and a survey of two manufacturers, APC catheters typically cost $175-$275 each, the study authors wrote, noting that APC results in increased cost, plastic waste because of the catheter, and carbon emissions associated with its manufacture.
“What we’re seeing — now over several trials — is STSC appears to be the most effective method of treating the edges, and it’s inexpensive because it uses the same device used for snare resection, so there’s no incremental cost for the device,” said Michael Wallace, MD, professor of medicine and director of the digestive diseases research program at Mayo Clinic, Jacksonville, Florida.
Dr. Wallace, who wasn’t involved with this study, has researched thermal ablation after EMR, including both the margins and the base.
“The single most important message now is that patients shouldn’t be getting surgical resections for endoscopically treatable polyps,” he said. “We see many patients who are told they need to get surgery, but overwhelmingly, the data shows we can remove polyps without surgery.”
Dr. Rex and several authors declared fees and grants from numerous companies outside of this study. Dr. Wallace reported no relevant disclosures.
according to a recent study.
Since STSC was faster to apply than APC and results in lower cost and plastic waste (because of APC requiring an additional catheter), STSC was the preferred option.
“The reduction in recurrence rate with thermal margin treatment is arguably the most important development in endoscopic mucosal resection in the past 2 decades,” said lead author Douglas Rex, MD, AGAF, a distinguished professor emeritus at the Indiana University School of Medicine and director of endoscopy at Indiana University Hospitals, both in Indianapolis.
“Margin thermal therapy with STSC should now be standard treatment after piecemeal EMR in the colorectum,” he said. “Before applying STSC, the endoscopist must ensure that the entire lesion is resected down to the submucosa. Then STSC should be aggressively applied to 100% of the margin.”
The study was published in Clinical Gastroenterology and Hepatology .
Comparing Treatments
Dr. Rex and colleagues performed a randomized three-arm trial in nine U.S. centers, comparing STSC with APC and no margin treatment in patients undergoing colorectal EMR of nonpedunculated lesions of 15 mm or greater.
All lesions underwent conventional injection and snare resection EMR using electrocautery, but the endoscopist chose the injection fluid and snare type and size. Areas with residual polyp that weren’t removable by snare resection because of flat shape or fibrosis were removed by hot or cold avulsion. After that, patients were randomized to one of the three arms.
Patients were scheduled for a follow-up appointment six months after the initial EMR. Any visible recurrence was resected using methods at the discretion of the endoscopist, and if no visible recurrence was present, EMR site biopsies were recommended.
Among 384 patients with 414 lesions, 308 patients with 328 lesions completed at least one follow-up appointment. The median interval to the first follow-up was 6.4 months, ranging from 2 to 37 months. The primary endpoint was the presence of recurrent or residual polyp at first follow-up.
The median polyp size was 25 mm, and 65 of the 414 polyps (15.7%) were 15-19 mm in size. Overall, 14.8% of lesions were resected en bloc, with no difference between the study arms.
The proportion of lesions with residual polyp at first follow-up was 4.6% with STSC, 9.3% with APC, and 21.4% among control subjects with no margin treatment.
The odds of having a residual polyp at first follow-up were lower for STSC and APC when compared with control subjects (odds ratio [OR] of 0.182 and 0.341, or P = .001 and P = .01, respectively). There wasn’t a significant difference in the odds of recurrence between STSC and APC (OR, 1.874).
In 259 lesions in 248 patients that were 20 mm or greater, the recurrence rates at first follow-up were 5.9% for STSC, 10.1% for APC, and 25.9% for the control group. In these lesions, STSC and APC remained associated with a lower risk of recurrence versus the control (OR, 0.18 and 0.323, respectively). The difference in recurrence rates between STSC and APC wasn’t significant.
Even still, STSC took less time to apply than APC, with a median time of 3.35 minutes vs 4.08 minutes.
The rates of adverse events were low, with no difference between the three arms. There were no immediate or delayed perforations in any arm, and the overall occurrence of delayed bleeding was low at 3.6%.
“I think STSC won the trial because it was numerically (though not statistically) superior to APC, was faster to apply, and using STSC results in lower cost and less plastic compared to APC,” Dr. Rex said.
Additional Considerations
Based on charges at the nine U.S. centers and a survey of two manufacturers, APC catheters typically cost $175-$275 each, the study authors wrote, noting that APC results in increased cost, plastic waste because of the catheter, and carbon emissions associated with its manufacture.
“What we’re seeing — now over several trials — is STSC appears to be the most effective method of treating the edges, and it’s inexpensive because it uses the same device used for snare resection, so there’s no incremental cost for the device,” said Michael Wallace, MD, professor of medicine and director of the digestive diseases research program at Mayo Clinic, Jacksonville, Florida.
Dr. Wallace, who wasn’t involved with this study, has researched thermal ablation after EMR, including both the margins and the base.
“The single most important message now is that patients shouldn’t be getting surgical resections for endoscopically treatable polyps,” he said. “We see many patients who are told they need to get surgery, but overwhelmingly, the data shows we can remove polyps without surgery.”
Dr. Rex and several authors declared fees and grants from numerous companies outside of this study. Dr. Wallace reported no relevant disclosures.
according to a recent study.
Since STSC was faster to apply than APC and results in lower cost and plastic waste (because of APC requiring an additional catheter), STSC was the preferred option.
“The reduction in recurrence rate with thermal margin treatment is arguably the most important development in endoscopic mucosal resection in the past 2 decades,” said lead author Douglas Rex, MD, AGAF, a distinguished professor emeritus at the Indiana University School of Medicine and director of endoscopy at Indiana University Hospitals, both in Indianapolis.
“Margin thermal therapy with STSC should now be standard treatment after piecemeal EMR in the colorectum,” he said. “Before applying STSC, the endoscopist must ensure that the entire lesion is resected down to the submucosa. Then STSC should be aggressively applied to 100% of the margin.”
The study was published in Clinical Gastroenterology and Hepatology .
Comparing Treatments
Dr. Rex and colleagues performed a randomized three-arm trial in nine U.S. centers, comparing STSC with APC and no margin treatment in patients undergoing colorectal EMR of nonpedunculated lesions of 15 mm or greater.
All lesions underwent conventional injection and snare resection EMR using electrocautery, but the endoscopist chose the injection fluid and snare type and size. Areas with residual polyp that weren’t removable by snare resection because of flat shape or fibrosis were removed by hot or cold avulsion. After that, patients were randomized to one of the three arms.
Patients were scheduled for a follow-up appointment six months after the initial EMR. Any visible recurrence was resected using methods at the discretion of the endoscopist, and if no visible recurrence was present, EMR site biopsies were recommended.
Among 384 patients with 414 lesions, 308 patients with 328 lesions completed at least one follow-up appointment. The median interval to the first follow-up was 6.4 months, ranging from 2 to 37 months. The primary endpoint was the presence of recurrent or residual polyp at first follow-up.
The median polyp size was 25 mm, and 65 of the 414 polyps (15.7%) were 15-19 mm in size. Overall, 14.8% of lesions were resected en bloc, with no difference between the study arms.
The proportion of lesions with residual polyp at first follow-up was 4.6% with STSC, 9.3% with APC, and 21.4% among control subjects with no margin treatment.
The odds of having a residual polyp at first follow-up were lower for STSC and APC when compared with control subjects (odds ratio [OR] of 0.182 and 0.341, or P = .001 and P = .01, respectively). There wasn’t a significant difference in the odds of recurrence between STSC and APC (OR, 1.874).
In 259 lesions in 248 patients that were 20 mm or greater, the recurrence rates at first follow-up were 5.9% for STSC, 10.1% for APC, and 25.9% for the control group. In these lesions, STSC and APC remained associated with a lower risk of recurrence versus the control (OR, 0.18 and 0.323, respectively). The difference in recurrence rates between STSC and APC wasn’t significant.
Even still, STSC took less time to apply than APC, with a median time of 3.35 minutes vs 4.08 minutes.
The rates of adverse events were low, with no difference between the three arms. There were no immediate or delayed perforations in any arm, and the overall occurrence of delayed bleeding was low at 3.6%.
“I think STSC won the trial because it was numerically (though not statistically) superior to APC, was faster to apply, and using STSC results in lower cost and less plastic compared to APC,” Dr. Rex said.
Additional Considerations
Based on charges at the nine U.S. centers and a survey of two manufacturers, APC catheters typically cost $175-$275 each, the study authors wrote, noting that APC results in increased cost, plastic waste because of the catheter, and carbon emissions associated with its manufacture.
“What we’re seeing — now over several trials — is STSC appears to be the most effective method of treating the edges, and it’s inexpensive because it uses the same device used for snare resection, so there’s no incremental cost for the device,” said Michael Wallace, MD, professor of medicine and director of the digestive diseases research program at Mayo Clinic, Jacksonville, Florida.
Dr. Wallace, who wasn’t involved with this study, has researched thermal ablation after EMR, including both the margins and the base.
“The single most important message now is that patients shouldn’t be getting surgical resections for endoscopically treatable polyps,” he said. “We see many patients who are told they need to get surgery, but overwhelmingly, the data shows we can remove polyps without surgery.”
Dr. Rex and several authors declared fees and grants from numerous companies outside of this study. Dr. Wallace reported no relevant disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Gastroenterologists Can Play a Critical Role in Obesity Management
according to a series of presentations during the American Gastroenterological Association (AGA) Postgraduate Course held at Digestive Disease Week® (DDW) in May.
Gastroenterologists can step up as part of a multidisciplinary response to provide treatment — with a range of lifestyle interventions, pharmacological options, and bariatric endoscopic possibilities — based on a patient’s needs and preferences.
“Obesity is in our clinics. We’re usually the first line of obesity, and that’s why we need to know it, learn how to manage it, and understand the complications,” said Andres Acosta, MD, an associate professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota, and principal investigator of Mayo’s Precision Medicine for Obesity Laboratory.
Obesity tops the charts as the most significant chronic disease in the world, affecting 130 million patients in the United States and 1 billion globally, he said, and those numbers will only climb higher in coming years. By 2030, the United States is projected to have an obesity prevalence of 50% and overweight prevalence of 80%, with every state having a prevalence greater than 35%.
The alarming prevalence rates matter not because of aesthetics or personal preference, he noted, but because of the major associations with premature death, cardiovascular disease, stroke, type 2 diabetes, numerous cancers, and 280 other diseases.
“Choose the organ you like, and obesity is a major contributor to its most important disease,” Dr. Acosta said. “Obesity affects every single disease and every single organ in the gastrointestinal system, so it’s essential that we actually manage this.”
Based on current recommendations focused on body mass index (BMI), diet, exercise, and behavioral therapy are suggested for a BMI of 25 or higher, followed by pharmacotherapy for a BMI greater than 27 with comorbidities, endoscopic procedures for a BMI greater than 30, and surgical options for a BMI greater than 40 or BMI greater than 30 with comorbidities. At each step, clinicians can start shared decision-making conversations with patients about the best options for them.
“We’re moving from a pyramid approach where we tell patients to choose one intervention toward multidisciplinary programs where we offer interventions in combination,” Dr. Acosta said, recommending AGA’s POWER - Practice Guide on Obesity and Weight Management Education and Resources . Other AGA resources for physicians treating patients with obesity include the AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity , and the Obesity Resource Center on the AGA website .
Progress in Pharmacotherapy
In recent years, developments focused on glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide and tirzepatide, have “changed the conversation about obesity,” Dr. Acosta said. For the first time, medications not only reduce weight but also cardiovascular disease risks, which were previously only observed with bariatric surgery.
Additional GLP-1 options are in research pipelines. During the next 3 years, for instance, more medications will focus on how the gut signals to the brain through intestinal hormones, targeting GLP-1, glucose-dependent insulinotropic polypeptide, and other receptors. Leading the pipeline, Eli Lilly’s retatrutide shows promise, with weight loss and comorbidity improvement reported similar to or better than tirzepatide. Additional data from phase 3 trials are forthcoming.
In clinical practice, major conversations remain about gastrointestinal side effects, particularly gastroparesis, that may pose a risk for aspiration in upper endoscopy. Gastroenterologists should feel comfortable about managing these types of side effects when starting patients on these medications, Dr. Acosta said, but also continue to ask questions about side effects and the latest research developments.
Of course, major obstacles remain regarding patient access, insurance coverage, cost-effective options, and heterogeneous patient responses. At the Mayo Clinic, Dr. Acosta and colleagues are researching and targeting obesity phenotypes — such as the “hungry gut” or “hungry brain” — to improve weight loss outcomes and patient adherence.
Ultimately, he said, the most important obstacle is our healthcare system. “We cannot afford to manage obesity with expensive procedures or expensive medications.”
Efficacy of Endobariatrics
For patients with a BMI of 30 or higher, minimally invasive bariatric endoscopic procedures can lead to weight loss, improvement in metabolic outcomes, and fewer adverse events compared to bariatric surgery, said Violeta Popov, MD, director of bariatric endoscopy at the New York Veterans Affairs Harbor Healthcare System in New York City.
For example, intragastric balloons — marketed under the names Orbera and Spatz — work by altering the rate of gastric emptying. They’re placed temporarily and removed after several months, and Spatz can be adjusted while in place, either by removing or adding volume if needed. Data show that associated weight loss can lead to improvements in insulin resistance, visceral obesity, dyslipidemia, high blood pressure, liver enzymes, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH).
Although the majority of patients undergoing minimally invasive procedures do experience adverse events such as nausea and vomiting, symptoms tend to subside in the first few weeks, Dr. Popov said. At the same time, gastroesophageal reflux disease (GERD) can worsen in patients who have experienced it, so proton pump inhibitors are recommended for as long as the balloon is inserted.
Endoscopic sleeve gastroplasty has become the most prevalent endobariatric method in Dr. Popov’s practice during the past few years. The procedure uses full thickness sutures placed with an endoscopic suturing device called OverStitch, to decrease the size of the opening into the stomach. In previous trials, patients lost up to 40 pounds, and more than 80% maintained the lost weight up to 5 years. The procedure, which showed no worsening of GERD, works by preserving gastric contractility while delaying gastric emptying.
Dr. Popov noted one of the main challenges is training and credentialing, with many patients not having access to those who can perform these procedures. As a diplomate of the American Board of Obesity Medicine, Dr. Popov highlighted the need for bariatric endoscopy fellowships or training during GI fellowships, post-fellowship hands-on courses, and competency training with simulators.
“It’s not just technical competency in performing a procedure — it’s also the administrative work of setting up a multidisciplinary program,” she said. “It’s very important to understand obesity as a disease and learn how to manage it.”
Monitoring MASLD
Linked strongly to insulin resistance, MASLD prevalence is increasing worldwide as obesity increases, reaching 30% in the United States and even higher among certain patient populations, said Sonali Paul, MD, an assistant professor of medicine and hepatologist at the Center for Liver Diseases at the University of Chicago Medicine in Illinois.
The good news is that the associations between MASLD and obesity also move the other way — if patients lose weight and improve cardiovascular risk factors, MASLD can improve as well. Notably, steatosis can disappear at 3% weight loss, inflammation decreases at 5% weight loss, MASH resolution occurs at 7% weight loss, and fibrosis improves at 10% weight loss.
Primarily, Dr. Paul and colleagues have focused on lifestyle interventions, especially diet, by working carefully with dietitians. A modified Mediterranean diet with olive oil and monounsaturated fats can decrease steatosis on MRI, as compared with a high-fat/low-carb diet, and it also appears to decrease mortality, cardiovascular disease, and obesity. As part of the modified diet, carbohydrates are limited to 30 grams per meal per day.
“We really want to tailor the diet to cultural and personal preferences,” she said. “I’m South Asian, and when I tell my South Asian patients not to eat rice, they don’t love that, so we work with them to meet them where they are.”
Dr. Paul recommends physical activity interventions, proper sleep hygiene, treatment of obstructive sleep apnea, pharmacological options, and bariatric solutions to reduce weight, improve insulin resistance, and target MASLD risk factors. For instance, recent phase 2b studies indicate semaglutide can lead to MASH resolution, with phase 3 trial data expected by the end of 2024.
In addition, resmetirom, a liver-directed thyroid hormone receptor beta selective agonist — the first Food and Drug Administration–approved drug for MASH — achieved both primary endpoints of MASH resolution and fibrosis improvement. American Association for the Study of Liver Diseases guidelines are forthcoming about who should use the drug, Dr. Paul said.
“In terms of the paradigm that I think about with MASLD, we want to target other causes and diagnose advanced fibrosis, treat risk factors, and target MASH through treatment,” she said.
Considering the Community Perspective
Community-based clinicians face a unique set of challenges when addressing obesity through a multidisciplinary approach and longitudinal care, but it remains vital as more practices see increased patient loads with obesity-related GI comorbidities, said Pooja Singhal, MD, assistant professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and founder/president of Oklahoma Gastro Health and Wellness.
Dr. Singhal noted obesity-related associations with earlier presentations of GERD, elevated liver enzymes, MASLD, MASH, IBS, IBD, gallbladder disease, colon polyps, and GI cancers.
“Gastroenterologists, as most of us are board-certified internists, are in a unique position to offer both pharmacotherapy and endoscopic treatment,” she said. “The GI comorbidities provide an opportunity for early intervention, and we’re seeing a lot of side effects of antiobesity medications, so whether we like it or not, we are involved.”
The best practices at the community level start with a patient-centric approach, Dr. Singhal said. Although clinicians are already time constrained and focused on addressing GI-related comorbidities, using the 5A’s framework can help:
- Asking if the patient is ready to talk
- Assessing for factors contributing to obesity
- Advising them of treatment options
- Agreeing on goals based on shared decision-making
- Assisting or Arranging the agreed-on plan.
During the assessment phase, Dr. Singhal suggested not only looking at medical and physical values but also secondary causes of weight gain, including the patient’s relationship with food, micronutrient deficiencies, psychosocial concerns, body image disorders, and triggers for eating.
During the advising phase, clinicians should consider multiple targets — such as diet, physical activity, and behavior — with a supervised and structured approach. Dr. Singhal and colleagues include a meal plan, aerobic activity, resistance training, behavior modification of eating habits, sleep hygiene, and patient self-monitoring through smartphone apps and wearables. Pharmacotherapy may be relevant and effective for some patients but less accessible for many, she noted.
Above all, Dr. Singhal recommended training through the American Board of Obesity Medicine, major GI society guidelines and conferences, American Society for Gastrointestinal Endoscopy STAR courses, and connecting with a multidisciplinary team of dietitians, coaches, physical therapists, and other GI specialists when possible.
“Most importantly, we’re dealing with decades of stigma and bias around this disease, where ‘you are what you eat,’ ” she said. “This mentality of ‘I can lose weight without help’ is a real challenge.”
according to a series of presentations during the American Gastroenterological Association (AGA) Postgraduate Course held at Digestive Disease Week® (DDW) in May.
Gastroenterologists can step up as part of a multidisciplinary response to provide treatment — with a range of lifestyle interventions, pharmacological options, and bariatric endoscopic possibilities — based on a patient’s needs and preferences.
“Obesity is in our clinics. We’re usually the first line of obesity, and that’s why we need to know it, learn how to manage it, and understand the complications,” said Andres Acosta, MD, an associate professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota, and principal investigator of Mayo’s Precision Medicine for Obesity Laboratory.
Obesity tops the charts as the most significant chronic disease in the world, affecting 130 million patients in the United States and 1 billion globally, he said, and those numbers will only climb higher in coming years. By 2030, the United States is projected to have an obesity prevalence of 50% and overweight prevalence of 80%, with every state having a prevalence greater than 35%.
The alarming prevalence rates matter not because of aesthetics or personal preference, he noted, but because of the major associations with premature death, cardiovascular disease, stroke, type 2 diabetes, numerous cancers, and 280 other diseases.
“Choose the organ you like, and obesity is a major contributor to its most important disease,” Dr. Acosta said. “Obesity affects every single disease and every single organ in the gastrointestinal system, so it’s essential that we actually manage this.”
Based on current recommendations focused on body mass index (BMI), diet, exercise, and behavioral therapy are suggested for a BMI of 25 or higher, followed by pharmacotherapy for a BMI greater than 27 with comorbidities, endoscopic procedures for a BMI greater than 30, and surgical options for a BMI greater than 40 or BMI greater than 30 with comorbidities. At each step, clinicians can start shared decision-making conversations with patients about the best options for them.
“We’re moving from a pyramid approach where we tell patients to choose one intervention toward multidisciplinary programs where we offer interventions in combination,” Dr. Acosta said, recommending AGA’s POWER - Practice Guide on Obesity and Weight Management Education and Resources . Other AGA resources for physicians treating patients with obesity include the AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity , and the Obesity Resource Center on the AGA website .
Progress in Pharmacotherapy
In recent years, developments focused on glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide and tirzepatide, have “changed the conversation about obesity,” Dr. Acosta said. For the first time, medications not only reduce weight but also cardiovascular disease risks, which were previously only observed with bariatric surgery.
Additional GLP-1 options are in research pipelines. During the next 3 years, for instance, more medications will focus on how the gut signals to the brain through intestinal hormones, targeting GLP-1, glucose-dependent insulinotropic polypeptide, and other receptors. Leading the pipeline, Eli Lilly’s retatrutide shows promise, with weight loss and comorbidity improvement reported similar to or better than tirzepatide. Additional data from phase 3 trials are forthcoming.
In clinical practice, major conversations remain about gastrointestinal side effects, particularly gastroparesis, that may pose a risk for aspiration in upper endoscopy. Gastroenterologists should feel comfortable about managing these types of side effects when starting patients on these medications, Dr. Acosta said, but also continue to ask questions about side effects and the latest research developments.
Of course, major obstacles remain regarding patient access, insurance coverage, cost-effective options, and heterogeneous patient responses. At the Mayo Clinic, Dr. Acosta and colleagues are researching and targeting obesity phenotypes — such as the “hungry gut” or “hungry brain” — to improve weight loss outcomes and patient adherence.
Ultimately, he said, the most important obstacle is our healthcare system. “We cannot afford to manage obesity with expensive procedures or expensive medications.”
Efficacy of Endobariatrics
For patients with a BMI of 30 or higher, minimally invasive bariatric endoscopic procedures can lead to weight loss, improvement in metabolic outcomes, and fewer adverse events compared to bariatric surgery, said Violeta Popov, MD, director of bariatric endoscopy at the New York Veterans Affairs Harbor Healthcare System in New York City.
For example, intragastric balloons — marketed under the names Orbera and Spatz — work by altering the rate of gastric emptying. They’re placed temporarily and removed after several months, and Spatz can be adjusted while in place, either by removing or adding volume if needed. Data show that associated weight loss can lead to improvements in insulin resistance, visceral obesity, dyslipidemia, high blood pressure, liver enzymes, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH).
Although the majority of patients undergoing minimally invasive procedures do experience adverse events such as nausea and vomiting, symptoms tend to subside in the first few weeks, Dr. Popov said. At the same time, gastroesophageal reflux disease (GERD) can worsen in patients who have experienced it, so proton pump inhibitors are recommended for as long as the balloon is inserted.
Endoscopic sleeve gastroplasty has become the most prevalent endobariatric method in Dr. Popov’s practice during the past few years. The procedure uses full thickness sutures placed with an endoscopic suturing device called OverStitch, to decrease the size of the opening into the stomach. In previous trials, patients lost up to 40 pounds, and more than 80% maintained the lost weight up to 5 years. The procedure, which showed no worsening of GERD, works by preserving gastric contractility while delaying gastric emptying.
Dr. Popov noted one of the main challenges is training and credentialing, with many patients not having access to those who can perform these procedures. As a diplomate of the American Board of Obesity Medicine, Dr. Popov highlighted the need for bariatric endoscopy fellowships or training during GI fellowships, post-fellowship hands-on courses, and competency training with simulators.
“It’s not just technical competency in performing a procedure — it’s also the administrative work of setting up a multidisciplinary program,” she said. “It’s very important to understand obesity as a disease and learn how to manage it.”
Monitoring MASLD
Linked strongly to insulin resistance, MASLD prevalence is increasing worldwide as obesity increases, reaching 30% in the United States and even higher among certain patient populations, said Sonali Paul, MD, an assistant professor of medicine and hepatologist at the Center for Liver Diseases at the University of Chicago Medicine in Illinois.
The good news is that the associations between MASLD and obesity also move the other way — if patients lose weight and improve cardiovascular risk factors, MASLD can improve as well. Notably, steatosis can disappear at 3% weight loss, inflammation decreases at 5% weight loss, MASH resolution occurs at 7% weight loss, and fibrosis improves at 10% weight loss.
Primarily, Dr. Paul and colleagues have focused on lifestyle interventions, especially diet, by working carefully with dietitians. A modified Mediterranean diet with olive oil and monounsaturated fats can decrease steatosis on MRI, as compared with a high-fat/low-carb diet, and it also appears to decrease mortality, cardiovascular disease, and obesity. As part of the modified diet, carbohydrates are limited to 30 grams per meal per day.
“We really want to tailor the diet to cultural and personal preferences,” she said. “I’m South Asian, and when I tell my South Asian patients not to eat rice, they don’t love that, so we work with them to meet them where they are.”
Dr. Paul recommends physical activity interventions, proper sleep hygiene, treatment of obstructive sleep apnea, pharmacological options, and bariatric solutions to reduce weight, improve insulin resistance, and target MASLD risk factors. For instance, recent phase 2b studies indicate semaglutide can lead to MASH resolution, with phase 3 trial data expected by the end of 2024.
In addition, resmetirom, a liver-directed thyroid hormone receptor beta selective agonist — the first Food and Drug Administration–approved drug for MASH — achieved both primary endpoints of MASH resolution and fibrosis improvement. American Association for the Study of Liver Diseases guidelines are forthcoming about who should use the drug, Dr. Paul said.
“In terms of the paradigm that I think about with MASLD, we want to target other causes and diagnose advanced fibrosis, treat risk factors, and target MASH through treatment,” she said.
Considering the Community Perspective
Community-based clinicians face a unique set of challenges when addressing obesity through a multidisciplinary approach and longitudinal care, but it remains vital as more practices see increased patient loads with obesity-related GI comorbidities, said Pooja Singhal, MD, assistant professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and founder/president of Oklahoma Gastro Health and Wellness.
Dr. Singhal noted obesity-related associations with earlier presentations of GERD, elevated liver enzymes, MASLD, MASH, IBS, IBD, gallbladder disease, colon polyps, and GI cancers.
“Gastroenterologists, as most of us are board-certified internists, are in a unique position to offer both pharmacotherapy and endoscopic treatment,” she said. “The GI comorbidities provide an opportunity for early intervention, and we’re seeing a lot of side effects of antiobesity medications, so whether we like it or not, we are involved.”
The best practices at the community level start with a patient-centric approach, Dr. Singhal said. Although clinicians are already time constrained and focused on addressing GI-related comorbidities, using the 5A’s framework can help:
- Asking if the patient is ready to talk
- Assessing for factors contributing to obesity
- Advising them of treatment options
- Agreeing on goals based on shared decision-making
- Assisting or Arranging the agreed-on plan.
During the assessment phase, Dr. Singhal suggested not only looking at medical and physical values but also secondary causes of weight gain, including the patient’s relationship with food, micronutrient deficiencies, psychosocial concerns, body image disorders, and triggers for eating.
During the advising phase, clinicians should consider multiple targets — such as diet, physical activity, and behavior — with a supervised and structured approach. Dr. Singhal and colleagues include a meal plan, aerobic activity, resistance training, behavior modification of eating habits, sleep hygiene, and patient self-monitoring through smartphone apps and wearables. Pharmacotherapy may be relevant and effective for some patients but less accessible for many, she noted.
Above all, Dr. Singhal recommended training through the American Board of Obesity Medicine, major GI society guidelines and conferences, American Society for Gastrointestinal Endoscopy STAR courses, and connecting with a multidisciplinary team of dietitians, coaches, physical therapists, and other GI specialists when possible.
“Most importantly, we’re dealing with decades of stigma and bias around this disease, where ‘you are what you eat,’ ” she said. “This mentality of ‘I can lose weight without help’ is a real challenge.”
according to a series of presentations during the American Gastroenterological Association (AGA) Postgraduate Course held at Digestive Disease Week® (DDW) in May.
Gastroenterologists can step up as part of a multidisciplinary response to provide treatment — with a range of lifestyle interventions, pharmacological options, and bariatric endoscopic possibilities — based on a patient’s needs and preferences.
“Obesity is in our clinics. We’re usually the first line of obesity, and that’s why we need to know it, learn how to manage it, and understand the complications,” said Andres Acosta, MD, an associate professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota, and principal investigator of Mayo’s Precision Medicine for Obesity Laboratory.
Obesity tops the charts as the most significant chronic disease in the world, affecting 130 million patients in the United States and 1 billion globally, he said, and those numbers will only climb higher in coming years. By 2030, the United States is projected to have an obesity prevalence of 50% and overweight prevalence of 80%, with every state having a prevalence greater than 35%.
The alarming prevalence rates matter not because of aesthetics or personal preference, he noted, but because of the major associations with premature death, cardiovascular disease, stroke, type 2 diabetes, numerous cancers, and 280 other diseases.
“Choose the organ you like, and obesity is a major contributor to its most important disease,” Dr. Acosta said. “Obesity affects every single disease and every single organ in the gastrointestinal system, so it’s essential that we actually manage this.”
Based on current recommendations focused on body mass index (BMI), diet, exercise, and behavioral therapy are suggested for a BMI of 25 or higher, followed by pharmacotherapy for a BMI greater than 27 with comorbidities, endoscopic procedures for a BMI greater than 30, and surgical options for a BMI greater than 40 or BMI greater than 30 with comorbidities. At each step, clinicians can start shared decision-making conversations with patients about the best options for them.
“We’re moving from a pyramid approach where we tell patients to choose one intervention toward multidisciplinary programs where we offer interventions in combination,” Dr. Acosta said, recommending AGA’s POWER - Practice Guide on Obesity and Weight Management Education and Resources . Other AGA resources for physicians treating patients with obesity include the AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity , and the Obesity Resource Center on the AGA website .
Progress in Pharmacotherapy
In recent years, developments focused on glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide and tirzepatide, have “changed the conversation about obesity,” Dr. Acosta said. For the first time, medications not only reduce weight but also cardiovascular disease risks, which were previously only observed with bariatric surgery.
Additional GLP-1 options are in research pipelines. During the next 3 years, for instance, more medications will focus on how the gut signals to the brain through intestinal hormones, targeting GLP-1, glucose-dependent insulinotropic polypeptide, and other receptors. Leading the pipeline, Eli Lilly’s retatrutide shows promise, with weight loss and comorbidity improvement reported similar to or better than tirzepatide. Additional data from phase 3 trials are forthcoming.
In clinical practice, major conversations remain about gastrointestinal side effects, particularly gastroparesis, that may pose a risk for aspiration in upper endoscopy. Gastroenterologists should feel comfortable about managing these types of side effects when starting patients on these medications, Dr. Acosta said, but also continue to ask questions about side effects and the latest research developments.
Of course, major obstacles remain regarding patient access, insurance coverage, cost-effective options, and heterogeneous patient responses. At the Mayo Clinic, Dr. Acosta and colleagues are researching and targeting obesity phenotypes — such as the “hungry gut” or “hungry brain” — to improve weight loss outcomes and patient adherence.
Ultimately, he said, the most important obstacle is our healthcare system. “We cannot afford to manage obesity with expensive procedures or expensive medications.”
Efficacy of Endobariatrics
For patients with a BMI of 30 or higher, minimally invasive bariatric endoscopic procedures can lead to weight loss, improvement in metabolic outcomes, and fewer adverse events compared to bariatric surgery, said Violeta Popov, MD, director of bariatric endoscopy at the New York Veterans Affairs Harbor Healthcare System in New York City.
For example, intragastric balloons — marketed under the names Orbera and Spatz — work by altering the rate of gastric emptying. They’re placed temporarily and removed after several months, and Spatz can be adjusted while in place, either by removing or adding volume if needed. Data show that associated weight loss can lead to improvements in insulin resistance, visceral obesity, dyslipidemia, high blood pressure, liver enzymes, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH).
Although the majority of patients undergoing minimally invasive procedures do experience adverse events such as nausea and vomiting, symptoms tend to subside in the first few weeks, Dr. Popov said. At the same time, gastroesophageal reflux disease (GERD) can worsen in patients who have experienced it, so proton pump inhibitors are recommended for as long as the balloon is inserted.
Endoscopic sleeve gastroplasty has become the most prevalent endobariatric method in Dr. Popov’s practice during the past few years. The procedure uses full thickness sutures placed with an endoscopic suturing device called OverStitch, to decrease the size of the opening into the stomach. In previous trials, patients lost up to 40 pounds, and more than 80% maintained the lost weight up to 5 years. The procedure, which showed no worsening of GERD, works by preserving gastric contractility while delaying gastric emptying.
Dr. Popov noted one of the main challenges is training and credentialing, with many patients not having access to those who can perform these procedures. As a diplomate of the American Board of Obesity Medicine, Dr. Popov highlighted the need for bariatric endoscopy fellowships or training during GI fellowships, post-fellowship hands-on courses, and competency training with simulators.
“It’s not just technical competency in performing a procedure — it’s also the administrative work of setting up a multidisciplinary program,” she said. “It’s very important to understand obesity as a disease and learn how to manage it.”
Monitoring MASLD
Linked strongly to insulin resistance, MASLD prevalence is increasing worldwide as obesity increases, reaching 30% in the United States and even higher among certain patient populations, said Sonali Paul, MD, an assistant professor of medicine and hepatologist at the Center for Liver Diseases at the University of Chicago Medicine in Illinois.
The good news is that the associations between MASLD and obesity also move the other way — if patients lose weight and improve cardiovascular risk factors, MASLD can improve as well. Notably, steatosis can disappear at 3% weight loss, inflammation decreases at 5% weight loss, MASH resolution occurs at 7% weight loss, and fibrosis improves at 10% weight loss.
Primarily, Dr. Paul and colleagues have focused on lifestyle interventions, especially diet, by working carefully with dietitians. A modified Mediterranean diet with olive oil and monounsaturated fats can decrease steatosis on MRI, as compared with a high-fat/low-carb diet, and it also appears to decrease mortality, cardiovascular disease, and obesity. As part of the modified diet, carbohydrates are limited to 30 grams per meal per day.
“We really want to tailor the diet to cultural and personal preferences,” she said. “I’m South Asian, and when I tell my South Asian patients not to eat rice, they don’t love that, so we work with them to meet them where they are.”
Dr. Paul recommends physical activity interventions, proper sleep hygiene, treatment of obstructive sleep apnea, pharmacological options, and bariatric solutions to reduce weight, improve insulin resistance, and target MASLD risk factors. For instance, recent phase 2b studies indicate semaglutide can lead to MASH resolution, with phase 3 trial data expected by the end of 2024.
In addition, resmetirom, a liver-directed thyroid hormone receptor beta selective agonist — the first Food and Drug Administration–approved drug for MASH — achieved both primary endpoints of MASH resolution and fibrosis improvement. American Association for the Study of Liver Diseases guidelines are forthcoming about who should use the drug, Dr. Paul said.
“In terms of the paradigm that I think about with MASLD, we want to target other causes and diagnose advanced fibrosis, treat risk factors, and target MASH through treatment,” she said.
Considering the Community Perspective
Community-based clinicians face a unique set of challenges when addressing obesity through a multidisciplinary approach and longitudinal care, but it remains vital as more practices see increased patient loads with obesity-related GI comorbidities, said Pooja Singhal, MD, assistant professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and founder/president of Oklahoma Gastro Health and Wellness.
Dr. Singhal noted obesity-related associations with earlier presentations of GERD, elevated liver enzymes, MASLD, MASH, IBS, IBD, gallbladder disease, colon polyps, and GI cancers.
“Gastroenterologists, as most of us are board-certified internists, are in a unique position to offer both pharmacotherapy and endoscopic treatment,” she said. “The GI comorbidities provide an opportunity for early intervention, and we’re seeing a lot of side effects of antiobesity medications, so whether we like it or not, we are involved.”
The best practices at the community level start with a patient-centric approach, Dr. Singhal said. Although clinicians are already time constrained and focused on addressing GI-related comorbidities, using the 5A’s framework can help:
- Asking if the patient is ready to talk
- Assessing for factors contributing to obesity
- Advising them of treatment options
- Agreeing on goals based on shared decision-making
- Assisting or Arranging the agreed-on plan.
During the assessment phase, Dr. Singhal suggested not only looking at medical and physical values but also secondary causes of weight gain, including the patient’s relationship with food, micronutrient deficiencies, psychosocial concerns, body image disorders, and triggers for eating.
During the advising phase, clinicians should consider multiple targets — such as diet, physical activity, and behavior — with a supervised and structured approach. Dr. Singhal and colleagues include a meal plan, aerobic activity, resistance training, behavior modification of eating habits, sleep hygiene, and patient self-monitoring through smartphone apps and wearables. Pharmacotherapy may be relevant and effective for some patients but less accessible for many, she noted.
Above all, Dr. Singhal recommended training through the American Board of Obesity Medicine, major GI society guidelines and conferences, American Society for Gastrointestinal Endoscopy STAR courses, and connecting with a multidisciplinary team of dietitians, coaches, physical therapists, and other GI specialists when possible.
“Most importantly, we’re dealing with decades of stigma and bias around this disease, where ‘you are what you eat,’ ” she said. “This mentality of ‘I can lose weight without help’ is a real challenge.”