User login
Doctor I-Don’t-Know
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Rheumatoid Arthritis May Raise Lung Cancer Risk, Particularly in Those With ILD
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
US Experience With Infliximab Biosimilars Suggests Need for More Development Incentives
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Remission or Not, Biologics May Mitigate Cardiovascular Risks of RA
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Nonmelanoma Skin Cancer: Encouraging Data on Laser Treatment
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
The Digital Side Effects
On July 19, what was supposed to be a harmless software upgrade brought down a huge chunk of the health care, banking, flight, and travel systems.
While my dinky little practice wasn’t affected, several of my patients were in other ways. Tests that had to be rescheduled, flights canceled ... inconveniences, but not life altering.
Things are allegedly fixed (at least until next time) but there may be fallout down the road. People who had delayed medical procedures could have a different prognosis depending on what the results showed when they were done. Hopefully this won’t happen.
But it’s a reminder of how vulnerable our whole world is to disruption of the internet, not to mention the power grid and software systems. Paper is time consuming, and takes up a lot of space, but as long as you have a decent pen and enough light to read it you’re fine.
I’m not saying we should go back to paper. It’s more expensive in the long run, takes up shelf and closet space, kills trees, has to be shredded after a time, and turns yellow around the edges. It also makes it a pain to copy and transfer records. With paper I wouldn’t be able to take all my charts with me to refer to when I leave town on a busman’s holiday. The benefits of digital far outstrip paper or we wouldn’t have switched in the first place.
But it’s still kind of scary to realize how much we depend on software to keep things running smoothly. The events of July 19 were unintentional. Someone looking to cause real trouble could do worse — and there are plenty out there who would love to — and we’re putting our faith in companies like CrowdStrike to protect us from them.
But, on the flip side, we’re asking others to do the same. We often use the phrase “trust me, I’m a doctor,” in jest, but the point is there. People come to us because we have knowledge and training they don’t, and they’re hoping we can help them. We spent a lot of time getting to the point where we can hang up a sign that says so. And we, like everyone else, are not infallible.
We’re individuals, not machines. Both are fallible, though in different ways. In CrowdStrike’s case the machines didn’t fail, they just did what the humans told them to do. Which didn’t work.
The bottom line is that even the most well-meaning will make mistakes.
But it’s still pretty scary because, even unintentionally, there will be a next time. And between now and then our world will become even more dependent on these systems. None of us want to go back to the preconnected era, it’s too much a part of our daily lives.
Like the long list of potential side effects on any drug we prescribe, it’s a trade-off that we’ve accepted. And at this point we aren’t going back.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
On July 19, what was supposed to be a harmless software upgrade brought down a huge chunk of the health care, banking, flight, and travel systems.
While my dinky little practice wasn’t affected, several of my patients were in other ways. Tests that had to be rescheduled, flights canceled ... inconveniences, but not life altering.
Things are allegedly fixed (at least until next time) but there may be fallout down the road. People who had delayed medical procedures could have a different prognosis depending on what the results showed when they were done. Hopefully this won’t happen.
But it’s a reminder of how vulnerable our whole world is to disruption of the internet, not to mention the power grid and software systems. Paper is time consuming, and takes up a lot of space, but as long as you have a decent pen and enough light to read it you’re fine.
I’m not saying we should go back to paper. It’s more expensive in the long run, takes up shelf and closet space, kills trees, has to be shredded after a time, and turns yellow around the edges. It also makes it a pain to copy and transfer records. With paper I wouldn’t be able to take all my charts with me to refer to when I leave town on a busman’s holiday. The benefits of digital far outstrip paper or we wouldn’t have switched in the first place.
But it’s still kind of scary to realize how much we depend on software to keep things running smoothly. The events of July 19 were unintentional. Someone looking to cause real trouble could do worse — and there are plenty out there who would love to — and we’re putting our faith in companies like CrowdStrike to protect us from them.
But, on the flip side, we’re asking others to do the same. We often use the phrase “trust me, I’m a doctor,” in jest, but the point is there. People come to us because we have knowledge and training they don’t, and they’re hoping we can help them. We spent a lot of time getting to the point where we can hang up a sign that says so. And we, like everyone else, are not infallible.
We’re individuals, not machines. Both are fallible, though in different ways. In CrowdStrike’s case the machines didn’t fail, they just did what the humans told them to do. Which didn’t work.
The bottom line is that even the most well-meaning will make mistakes.
But it’s still pretty scary because, even unintentionally, there will be a next time. And between now and then our world will become even more dependent on these systems. None of us want to go back to the preconnected era, it’s too much a part of our daily lives.
Like the long list of potential side effects on any drug we prescribe, it’s a trade-off that we’ve accepted. And at this point we aren’t going back.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
On July 19, what was supposed to be a harmless software upgrade brought down a huge chunk of the health care, banking, flight, and travel systems.
While my dinky little practice wasn’t affected, several of my patients were in other ways. Tests that had to be rescheduled, flights canceled ... inconveniences, but not life altering.
Things are allegedly fixed (at least until next time) but there may be fallout down the road. People who had delayed medical procedures could have a different prognosis depending on what the results showed when they were done. Hopefully this won’t happen.
But it’s a reminder of how vulnerable our whole world is to disruption of the internet, not to mention the power grid and software systems. Paper is time consuming, and takes up a lot of space, but as long as you have a decent pen and enough light to read it you’re fine.
I’m not saying we should go back to paper. It’s more expensive in the long run, takes up shelf and closet space, kills trees, has to be shredded after a time, and turns yellow around the edges. It also makes it a pain to copy and transfer records. With paper I wouldn’t be able to take all my charts with me to refer to when I leave town on a busman’s holiday. The benefits of digital far outstrip paper or we wouldn’t have switched in the first place.
But it’s still kind of scary to realize how much we depend on software to keep things running smoothly. The events of July 19 were unintentional. Someone looking to cause real trouble could do worse — and there are plenty out there who would love to — and we’re putting our faith in companies like CrowdStrike to protect us from them.
But, on the flip side, we’re asking others to do the same. We often use the phrase “trust me, I’m a doctor,” in jest, but the point is there. People come to us because we have knowledge and training they don’t, and they’re hoping we can help them. We spent a lot of time getting to the point where we can hang up a sign that says so. And we, like everyone else, are not infallible.
We’re individuals, not machines. Both are fallible, though in different ways. In CrowdStrike’s case the machines didn’t fail, they just did what the humans told them to do. Which didn’t work.
The bottom line is that even the most well-meaning will make mistakes.
But it’s still pretty scary because, even unintentionally, there will be a next time. And between now and then our world will become even more dependent on these systems. None of us want to go back to the preconnected era, it’s too much a part of our daily lives.
Like the long list of potential side effects on any drug we prescribe, it’s a trade-off that we’ve accepted. And at this point we aren’t going back.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Nemolizumab Benefits Seen in Adults, Teens With Atopic Dermatitis
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
These Four Factors Account for 18 Years of Life Expectancy
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
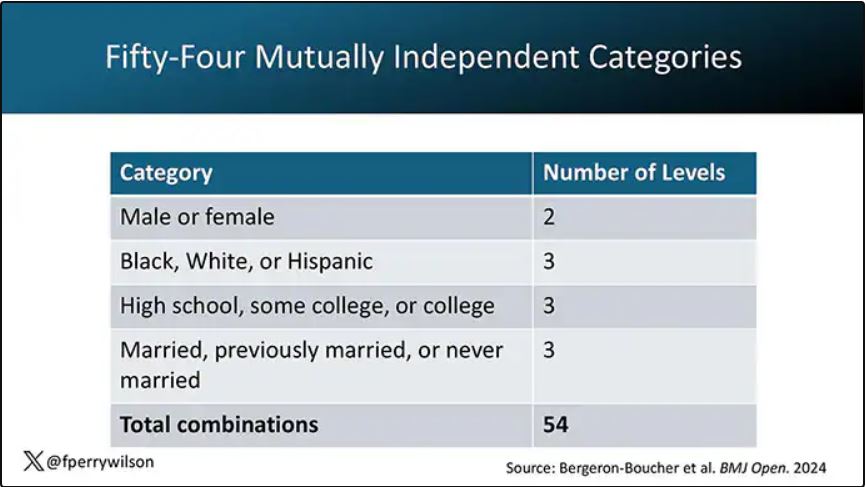
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.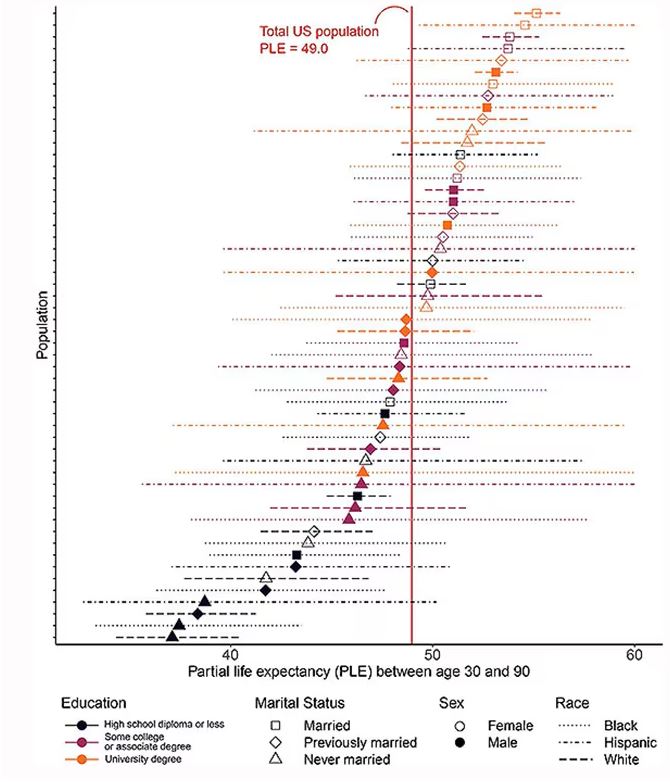
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.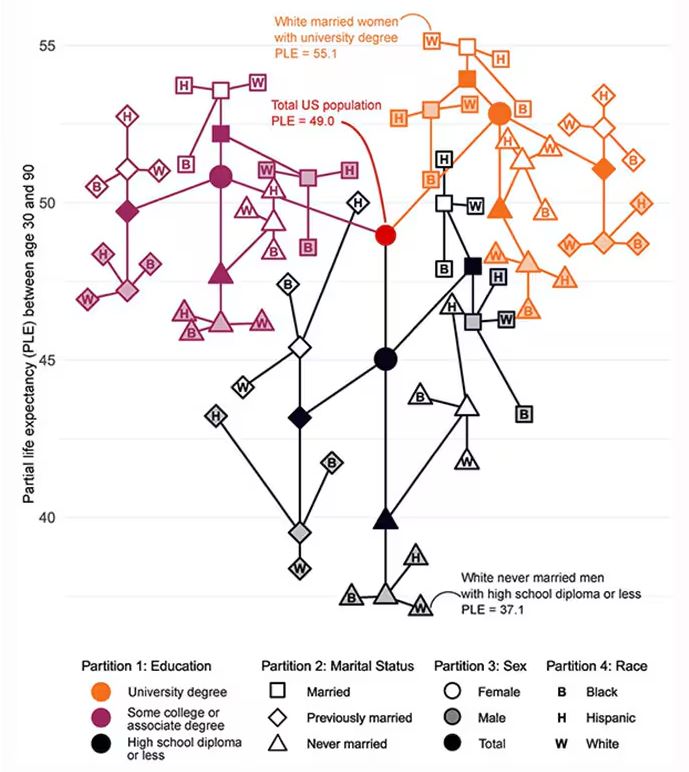
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.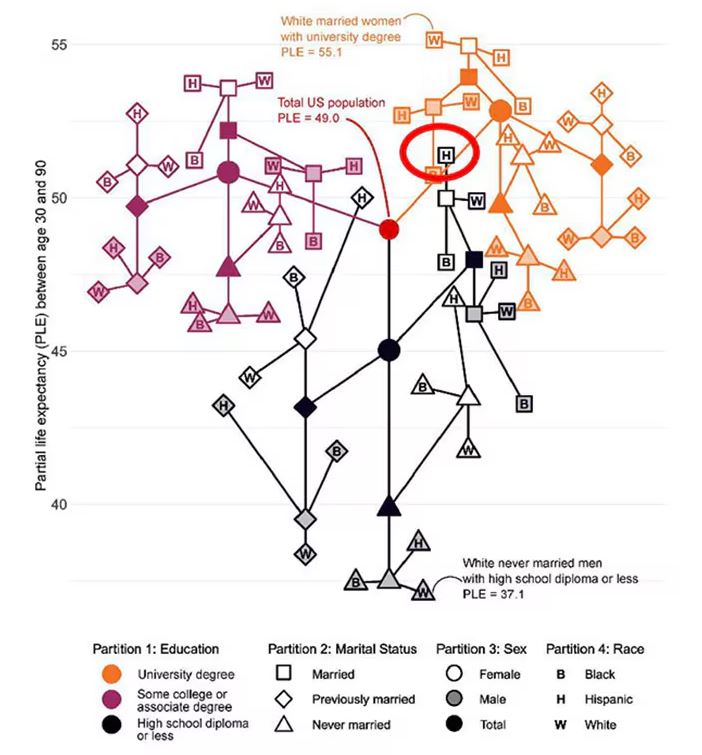
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.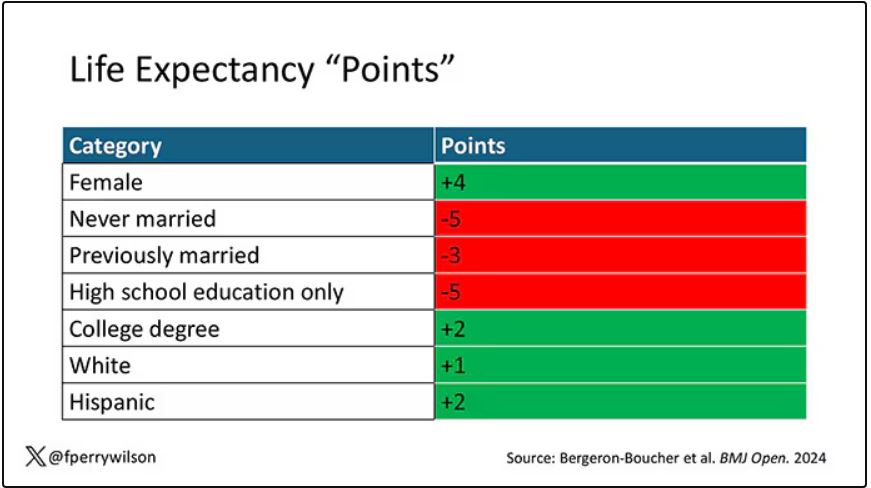
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.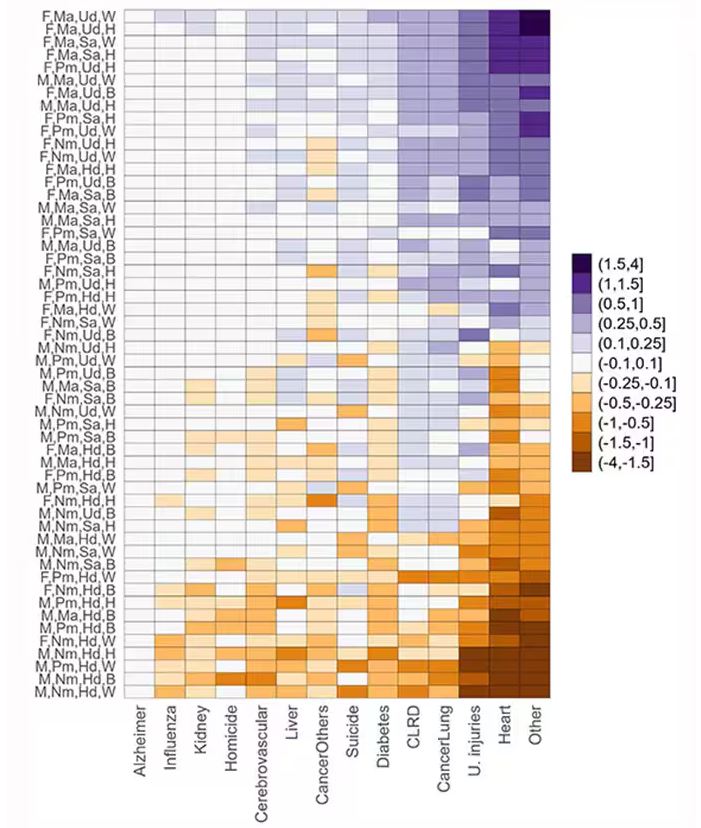
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall. 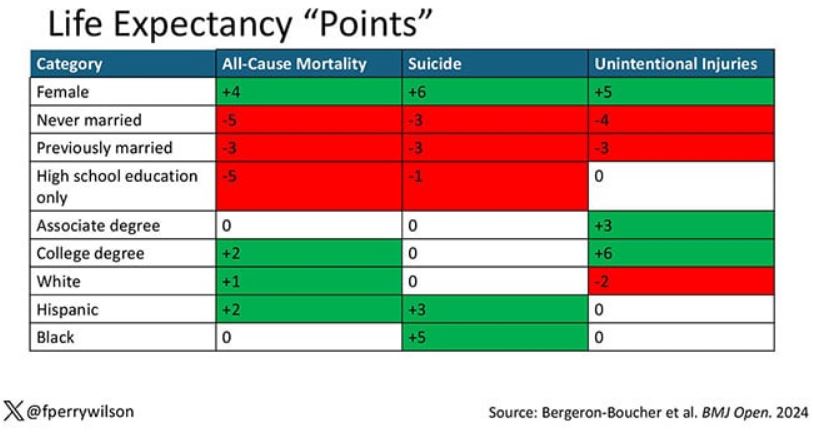
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall. 
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall. 
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recommendations From a Pediatric Dermatologist on Using AI in Daily Practice
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Study Identifies Oral Antibiotics Linked to Severe Cutaneous Reactions
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA