User login
Erythema Nodosum Triggered by a Bite From a Copperhead Snake
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
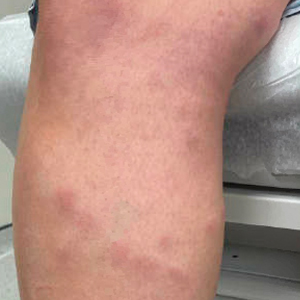
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).
Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
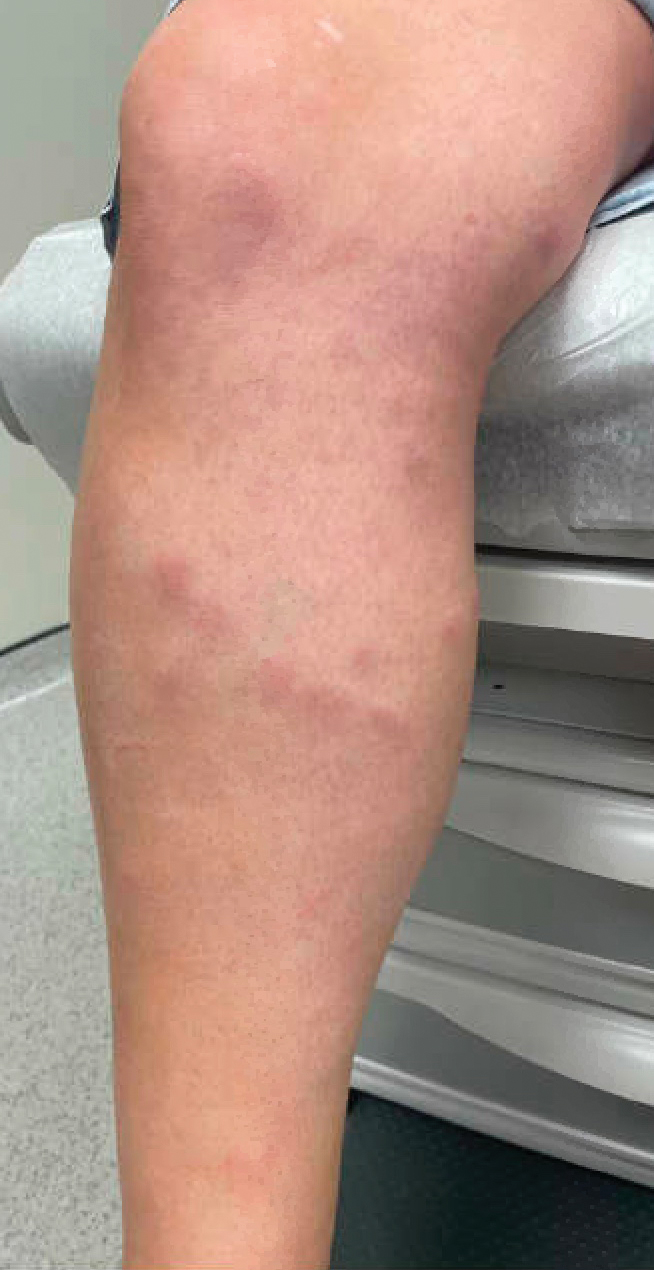
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.
Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
Practice Points
- Erythema nodosum (EN) can occur following snakebites from pit vipers such as the eastern copperhead.
- The acute phase of EN is neutrophilic and responds to colchicine. The chronic phase of EN is granulomatous and responds best to rest and elevation as well as nonsteroidal anti-inflammatory drugs and iodides.
Distinguishing Generalized Bullous Fixed Drug Eruption From SJS/TEN: A Retrospective Study on Clinical and Demographic Features
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
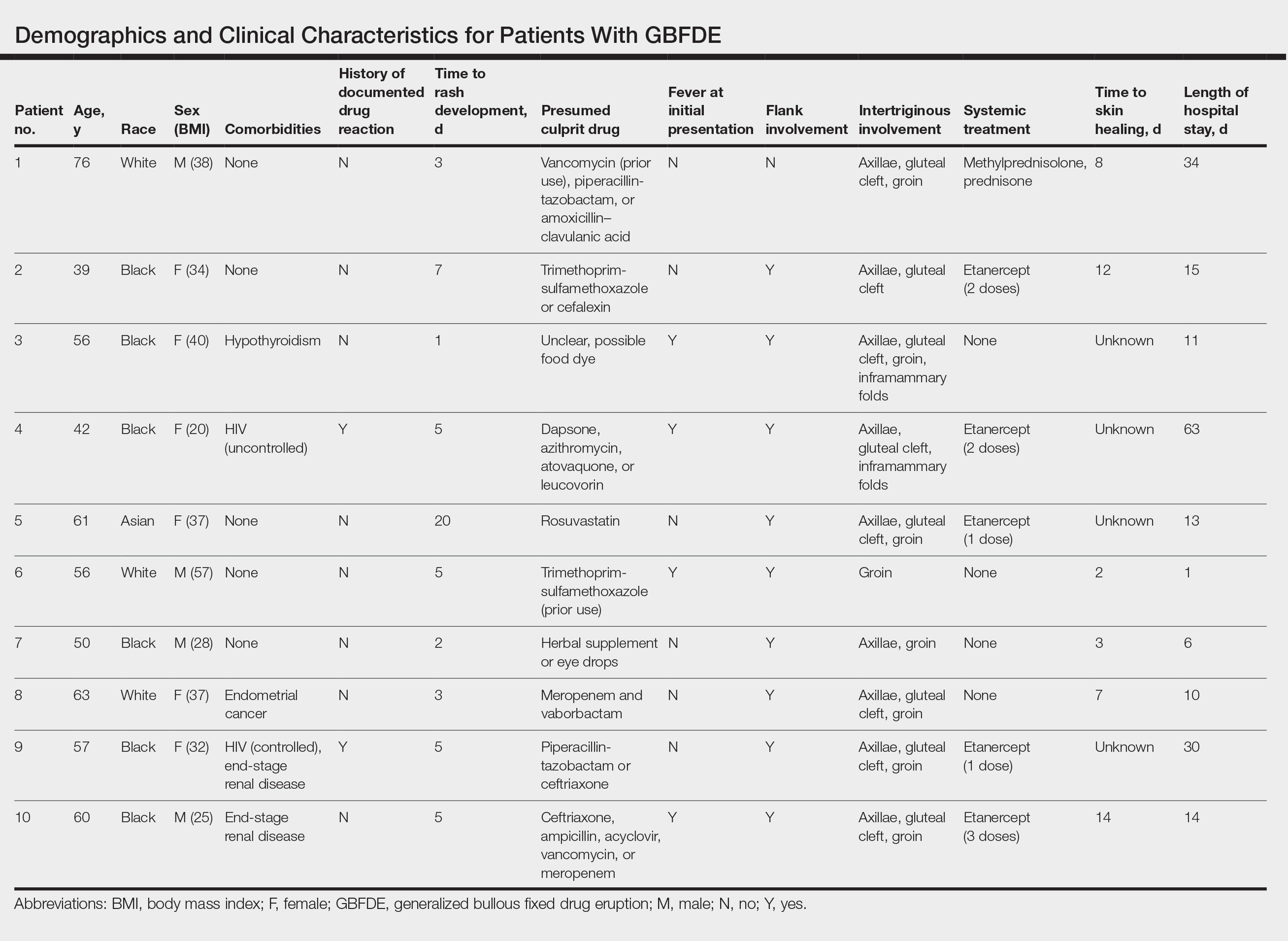
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).
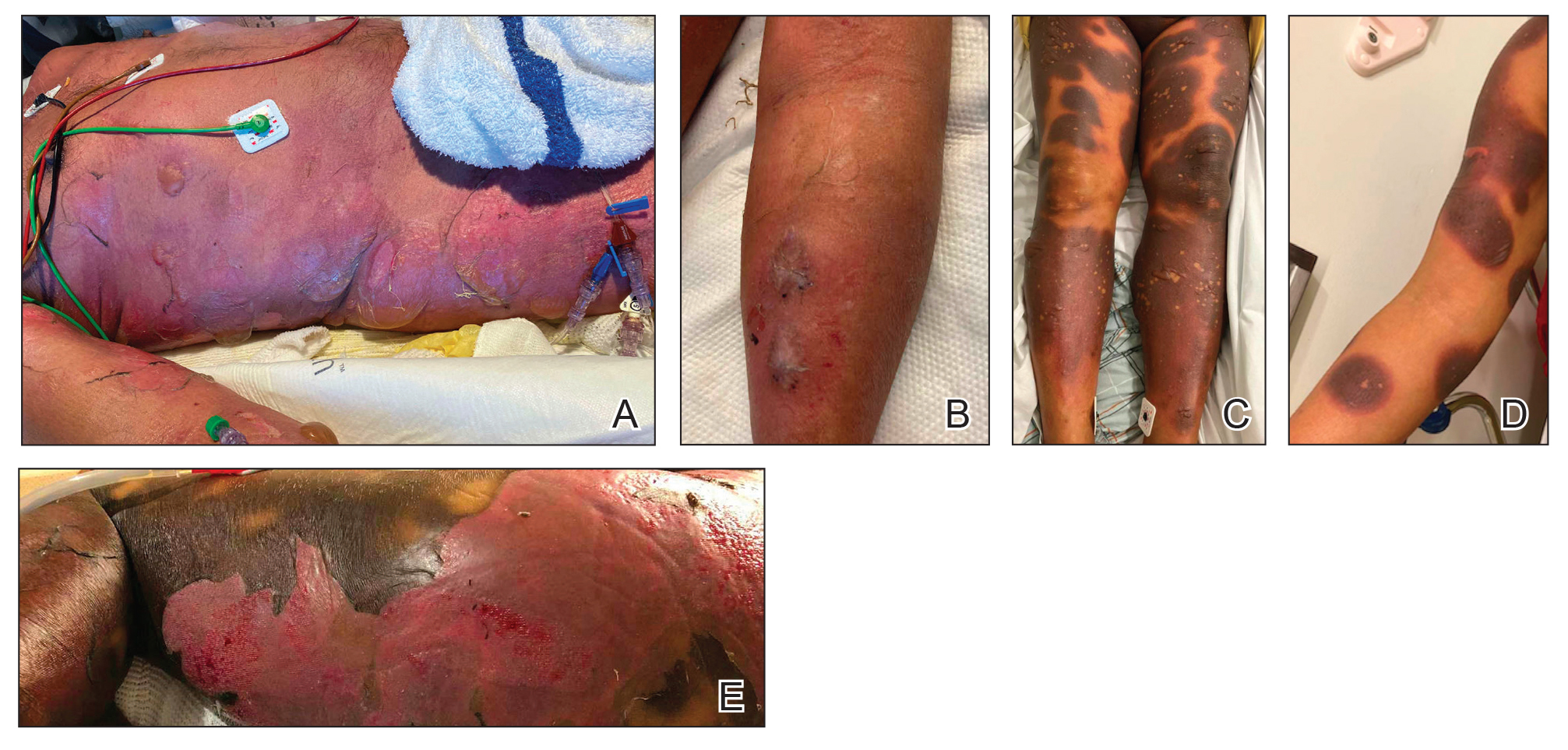
This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
PRACTICE POINTS
- Distinguishing features of generalized bullous fixed
drug eruption (GBFDE) may include truncal and proximal predilection with early intertriginous blistering. - Etanercept is a viable treatment option for GBFDE.
Comment on “Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination”
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
A Whiff of Trouble: Navigating Allergic Contact Dermatitis to Fragrance
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Practice Points
- Fragrance allergy is common due to daily exposure from many sources, ranging from personal care products and cosmetics to cleaning products, foods/spices, and workplace materials.
- More than 100 different fragrances can cause contact allergy, but patch testing in routine practice usually is limited to a few key screening allergens with important limitations.
- Fragrance avoidance is challenging, and comprehensive patient education is critical, including the provision of a list of safe products that are truly fragrance free.
Turning Late-Night Advice into Big Business: Two Nurses’ Story
Fevers? Vomiting? Fussiness? How to manage the first night home from the hospital? These are just a few of the hundreds of questions from parents that Atlanta, Georgia–based pediatric nurses Jennifer Walker and Laura Hunter answered well into the night.
It was the mid-1990s, and theirs was the only practice in town that offered on-call nurse responses around the clock. Ms. Hunter and Ms. Walker alternated work-from-home shifts, chatting with many of the practice’s families.
The pair answered the same questions from panicked parents over and over. And they found themselves bridging the gap between medical advice and parenting advice when supporting families.
“Parents were calling us at 2:00 in the morning with all kinds of things they were worried about, and that’s where Moms on Call was born,” Ms. Walker said.
A few decades later, Ms. Walker and Ms. Hunter turned that experience, empathy, and expertise into a thriving business. Moms on Call is often referred to as the “instruction manual for babies,” and the two nurses have consulted with more than 10,000 families. Along the way, they’ve sold more than a million copies of multiple books, created a deep well of online resources, and trained others in their techniques.
So how did they do it?
A Folder, a Swaddle, and a Mission
Ms. Walker and Ms. Hunter literally wrote the book on helping people in the trenches of new parenthood. But it wasn’t quite a book at first. “It was a folder we printed off the computer with those questions coming in,” Ms. Hunter recalled. The nurses developed a way to approach each call with a specific outline of protocols they had designed.
“What if we just go to the [patient’s] house and help them figure that out?” Ms. Walker remembered one of the pediatricians she worked with suggesting in 2002. For example, Ms. Hunter’s swaddle technique that calmed even the fussiest babies worked much better if it was demonstrated in person.
The two embarked on home visits with new parents. But their advice would be practical, not medical. Because they were not classified as traveling nurses, they drew a “definitive line” that they wouldn’t be discussing “major medical issues.”
“Going into the homes here in Atlanta, taking that folder, clipping nails, doing baths, discussing feeding — whether you were doing bottles or breastfeeding — we were going to help parents where they were,” Ms. Hunter said.
The physicians they worked with began recommending their services. Ms. Walker jokes that they didn’t know what they were doing at first; they considered giving their first client their money back. But parents needed what they were delivering, which was advice, validation, and confidence in their parenting.
Just 6-8 weeks into their initiative, other practices started to inquire about whether the nurses could do the same thing for them.
It was a solution to the problem of the 15-minute office visit. “We were helping with those questions so that when [babies] came in for their well visits, those questions were already answered. Not only did we go into their homes, but we supported them in the months after we left,” Ms. Hunter said.
The Ripple Effect
The outcomes were astonishing. “Babies were sleeping through the night. Parents were more confident. We didn’t expect the results, and we were shocked at how consistent it was,” Ms. Walker said. “Laura and I used to call each other in disbelief after we would put these basic principles in place and partner with parents.”
Local pediatricians were grateful for the help. But for the nurses, it was about walking alongside families. The two have countless stories of desperate parents, marriages “on the brink of disaster,” moments when they realized their work was having a ripple effect.
One military family stands out in Ms. Walker’s memory. “The father was fighting for our country overseas, and his wife was struggling alone at home.”
But support from Moms on Call had a powerful impact. “When [the father] came home, he presented Laura with a flag and a beautiful personal note expressing his gratitude,” Ms. Walker said. “Once his wife had a partner to help and felt confident and well rested, his heart could rest as well. We did what he couldn’t, and it made all the difference. After all, that’s what he was fighting for in the first place.”
The Gambler Calls
After just 1 or 2 years as Moms on Call, Ms. Walker and Ms. Hunter got an unexpected call from none other than celebrity singer Kenny Rogers, who needed help with his twins.
“I was flipping through the folder, and he said: ‘It’s not copyrighted. It’ll be copyrighted tomorrow morning,’ ” Ms. Hunter recalled.
Mr. Rogers’ attorneys called the next day to provide all the information. “He said: ‘Y’all have got something here. Send this folder to a self-publishing company. Throw up a website. It’ll cost you a few thousand bucks,’” said Ms. Hunter. The business was officially born in 2004.
More of Mr. Rogers’ advice: “You can’t hit a bull’s-eye if you don’t throw a few darts. This is worth throwing a few darts at.”
‘They Don’t Teach You That in Nursing School’
The two nurses reimagined their all-knowing folder as a book with a DVD in the back. Because how do you teach parents how to suction noses without showing it? They also wanted to use an outline format — simpler for exhausted parents who just needed to get the information quickly. A few publishers pushed back on these ideas. But the nurses persisted and self-published the first edition.
The original website was basic. Ms. Walker’s Aunt Janet put it together. But grateful clients were Ms. Walker and Ms. Hunter’s best marketing tool, spreading the word to friends and family. The message: Parents know their own children best and can be empowered to help their own kids, rather than leaning on professionals alone.
A community of families also helped them navigate starting a business. A client who was a mergers and acquisitions lawyer helped them form their LLC. “They don’t teach you that in nursing school,” Ms. Walker said.
Ms. Walker added that they made mistakes. “Not everyone that we encountered viewed or felt the same way about growing a business that is primarily focused on helping families. Sometimes that meant offering services at no charge. Or saying no to certain partnerships that didn’t align with our business model.”
Ms. Walker and Ms. Hunter had an eye on equity in creating multiple ways to access their advice at various price points. They started by charging around $75 for an in-home visit. (Now, if one of the CEOs comes out, it’s around $1000.) But the books, app, and online resources support those who can’t access that, as do an additional 10 in-home consultants around the country.
Along the way, moments told them they were going in the right direction and helped them define their purpose. “It is having a client ‘buy’ us as their go-to [baby] shower gift. It is being able to provide and support a clinic in Kenya or military families around the world. It is helping families realize that they can sleep — that they aren’t alone,” Ms. Walker said.
On Call 24/7 in the Car, in the Checkout Line ...
The early days of Moms on Call were also a juggling act. As Ms. Walker and Ms. Hunter balanced work and home with 10 of their own kids between the two of them, they took calls wherever they were. A friend and caller once joked that she could tell Ms. Hunter was checking out at the grocery store while she advised her on her very sick son’s vomiting.
“We were still trying to take care of the kids, run the house, and neither one of us had nannies or night nurses or housekeepers,” Ms. Hunter said. “But being on call allowed us to still be at home.”
Ms. Walker remembers taking calls on the way to ball games with her own kids, who by 8 years old could recite the advice for a baby’s fever from the back of the car. “It was like a family affair, and our kids got to see how that works and see their moms in action,” she said.
Through it all, Ms. Walker and Ms. Hunter’s motivation came from knowing that thousands of parents were begging for help — and they had an answer.
“Our shoulders have absorbed so many tears of parents who were exhausted and hurting, some who had been lied to or told their child would never sleep or had to be raised a certain way. When someone steals that confidence, especially from a brand-new parent overwhelmed by information, it makes us want to shout the truth from every rooftop and digital channel available,” Ms. Walker added.
Do You Have a Business Idea?
“Boots on the ground” healthcare professionals often see new opportunities to serve patients who might be falling through the cracks of the healthcare system. While not all will become a full-blown business, Ms. Hunter encourages them to break down their idea into “bite-sized pieces.” Just have the next conversation.
“Ask the people around you and the people who are brought to you,” Ms. Hunter said. When the two nurses look back, they see how those pieces of the puzzle were meant to come together. “Ask everyone you know,” Ms. Hunter advised. “And talk to the people you are taking care of. It’s possible they have a gift that will help you get to the next bite-sized piece.”
In short — develop a network of people who believe in your idea. Prioritize those relationships and see where they can take you.
The close relationship between Ms. Walker and Ms. Hunter, as business partners and friends, has also been crucial. They joke that they finish each other’s sentences and sandwiches. “You have to fight for that — we prioritize [that relationship]” too, Ms. Walker said.
Finally, remember why you are doing what you do, Ms. Walker said. “These are the people we help: Wonderful people with jobs that serve us all — the airplane pilot, the anesthesiologist, the pediatrician, the single dad. They are all parents who have felt alone and exhausted. In those lonely moments of a parent’s heart where they fear they are doing the wrong thing, we want to be the voice of hope,” she added. “We let them know that if they ever wondered if they were doing it right, well, only good parents wonder that.”
A version of this article appeared on Medscape.com.
Fevers? Vomiting? Fussiness? How to manage the first night home from the hospital? These are just a few of the hundreds of questions from parents that Atlanta, Georgia–based pediatric nurses Jennifer Walker and Laura Hunter answered well into the night.
It was the mid-1990s, and theirs was the only practice in town that offered on-call nurse responses around the clock. Ms. Hunter and Ms. Walker alternated work-from-home shifts, chatting with many of the practice’s families.
The pair answered the same questions from panicked parents over and over. And they found themselves bridging the gap between medical advice and parenting advice when supporting families.
“Parents were calling us at 2:00 in the morning with all kinds of things they were worried about, and that’s where Moms on Call was born,” Ms. Walker said.
A few decades later, Ms. Walker and Ms. Hunter turned that experience, empathy, and expertise into a thriving business. Moms on Call is often referred to as the “instruction manual for babies,” and the two nurses have consulted with more than 10,000 families. Along the way, they’ve sold more than a million copies of multiple books, created a deep well of online resources, and trained others in their techniques.
So how did they do it?
A Folder, a Swaddle, and a Mission
Ms. Walker and Ms. Hunter literally wrote the book on helping people in the trenches of new parenthood. But it wasn’t quite a book at first. “It was a folder we printed off the computer with those questions coming in,” Ms. Hunter recalled. The nurses developed a way to approach each call with a specific outline of protocols they had designed.
“What if we just go to the [patient’s] house and help them figure that out?” Ms. Walker remembered one of the pediatricians she worked with suggesting in 2002. For example, Ms. Hunter’s swaddle technique that calmed even the fussiest babies worked much better if it was demonstrated in person.
The two embarked on home visits with new parents. But their advice would be practical, not medical. Because they were not classified as traveling nurses, they drew a “definitive line” that they wouldn’t be discussing “major medical issues.”
“Going into the homes here in Atlanta, taking that folder, clipping nails, doing baths, discussing feeding — whether you were doing bottles or breastfeeding — we were going to help parents where they were,” Ms. Hunter said.
The physicians they worked with began recommending their services. Ms. Walker jokes that they didn’t know what they were doing at first; they considered giving their first client their money back. But parents needed what they were delivering, which was advice, validation, and confidence in their parenting.
Just 6-8 weeks into their initiative, other practices started to inquire about whether the nurses could do the same thing for them.
It was a solution to the problem of the 15-minute office visit. “We were helping with those questions so that when [babies] came in for their well visits, those questions were already answered. Not only did we go into their homes, but we supported them in the months after we left,” Ms. Hunter said.
The Ripple Effect
The outcomes were astonishing. “Babies were sleeping through the night. Parents were more confident. We didn’t expect the results, and we were shocked at how consistent it was,” Ms. Walker said. “Laura and I used to call each other in disbelief after we would put these basic principles in place and partner with parents.”
Local pediatricians were grateful for the help. But for the nurses, it was about walking alongside families. The two have countless stories of desperate parents, marriages “on the brink of disaster,” moments when they realized their work was having a ripple effect.
One military family stands out in Ms. Walker’s memory. “The father was fighting for our country overseas, and his wife was struggling alone at home.”
But support from Moms on Call had a powerful impact. “When [the father] came home, he presented Laura with a flag and a beautiful personal note expressing his gratitude,” Ms. Walker said. “Once his wife had a partner to help and felt confident and well rested, his heart could rest as well. We did what he couldn’t, and it made all the difference. After all, that’s what he was fighting for in the first place.”
The Gambler Calls
After just 1 or 2 years as Moms on Call, Ms. Walker and Ms. Hunter got an unexpected call from none other than celebrity singer Kenny Rogers, who needed help with his twins.
“I was flipping through the folder, and he said: ‘It’s not copyrighted. It’ll be copyrighted tomorrow morning,’ ” Ms. Hunter recalled.
Mr. Rogers’ attorneys called the next day to provide all the information. “He said: ‘Y’all have got something here. Send this folder to a self-publishing company. Throw up a website. It’ll cost you a few thousand bucks,’” said Ms. Hunter. The business was officially born in 2004.
More of Mr. Rogers’ advice: “You can’t hit a bull’s-eye if you don’t throw a few darts. This is worth throwing a few darts at.”
‘They Don’t Teach You That in Nursing School’
The two nurses reimagined their all-knowing folder as a book with a DVD in the back. Because how do you teach parents how to suction noses without showing it? They also wanted to use an outline format — simpler for exhausted parents who just needed to get the information quickly. A few publishers pushed back on these ideas. But the nurses persisted and self-published the first edition.
The original website was basic. Ms. Walker’s Aunt Janet put it together. But grateful clients were Ms. Walker and Ms. Hunter’s best marketing tool, spreading the word to friends and family. The message: Parents know their own children best and can be empowered to help their own kids, rather than leaning on professionals alone.
A community of families also helped them navigate starting a business. A client who was a mergers and acquisitions lawyer helped them form their LLC. “They don’t teach you that in nursing school,” Ms. Walker said.
Ms. Walker added that they made mistakes. “Not everyone that we encountered viewed or felt the same way about growing a business that is primarily focused on helping families. Sometimes that meant offering services at no charge. Or saying no to certain partnerships that didn’t align with our business model.”
Ms. Walker and Ms. Hunter had an eye on equity in creating multiple ways to access their advice at various price points. They started by charging around $75 for an in-home visit. (Now, if one of the CEOs comes out, it’s around $1000.) But the books, app, and online resources support those who can’t access that, as do an additional 10 in-home consultants around the country.
Along the way, moments told them they were going in the right direction and helped them define their purpose. “It is having a client ‘buy’ us as their go-to [baby] shower gift. It is being able to provide and support a clinic in Kenya or military families around the world. It is helping families realize that they can sleep — that they aren’t alone,” Ms. Walker said.
On Call 24/7 in the Car, in the Checkout Line ...
The early days of Moms on Call were also a juggling act. As Ms. Walker and Ms. Hunter balanced work and home with 10 of their own kids between the two of them, they took calls wherever they were. A friend and caller once joked that she could tell Ms. Hunter was checking out at the grocery store while she advised her on her very sick son’s vomiting.
“We were still trying to take care of the kids, run the house, and neither one of us had nannies or night nurses or housekeepers,” Ms. Hunter said. “But being on call allowed us to still be at home.”
Ms. Walker remembers taking calls on the way to ball games with her own kids, who by 8 years old could recite the advice for a baby’s fever from the back of the car. “It was like a family affair, and our kids got to see how that works and see their moms in action,” she said.
Through it all, Ms. Walker and Ms. Hunter’s motivation came from knowing that thousands of parents were begging for help — and they had an answer.
“Our shoulders have absorbed so many tears of parents who were exhausted and hurting, some who had been lied to or told their child would never sleep or had to be raised a certain way. When someone steals that confidence, especially from a brand-new parent overwhelmed by information, it makes us want to shout the truth from every rooftop and digital channel available,” Ms. Walker added.
Do You Have a Business Idea?
“Boots on the ground” healthcare professionals often see new opportunities to serve patients who might be falling through the cracks of the healthcare system. While not all will become a full-blown business, Ms. Hunter encourages them to break down their idea into “bite-sized pieces.” Just have the next conversation.
“Ask the people around you and the people who are brought to you,” Ms. Hunter said. When the two nurses look back, they see how those pieces of the puzzle were meant to come together. “Ask everyone you know,” Ms. Hunter advised. “And talk to the people you are taking care of. It’s possible they have a gift that will help you get to the next bite-sized piece.”
In short — develop a network of people who believe in your idea. Prioritize those relationships and see where they can take you.
The close relationship between Ms. Walker and Ms. Hunter, as business partners and friends, has also been crucial. They joke that they finish each other’s sentences and sandwiches. “You have to fight for that — we prioritize [that relationship]” too, Ms. Walker said.
Finally, remember why you are doing what you do, Ms. Walker said. “These are the people we help: Wonderful people with jobs that serve us all — the airplane pilot, the anesthesiologist, the pediatrician, the single dad. They are all parents who have felt alone and exhausted. In those lonely moments of a parent’s heart where they fear they are doing the wrong thing, we want to be the voice of hope,” she added. “We let them know that if they ever wondered if they were doing it right, well, only good parents wonder that.”
A version of this article appeared on Medscape.com.
Fevers? Vomiting? Fussiness? How to manage the first night home from the hospital? These are just a few of the hundreds of questions from parents that Atlanta, Georgia–based pediatric nurses Jennifer Walker and Laura Hunter answered well into the night.
It was the mid-1990s, and theirs was the only practice in town that offered on-call nurse responses around the clock. Ms. Hunter and Ms. Walker alternated work-from-home shifts, chatting with many of the practice’s families.
The pair answered the same questions from panicked parents over and over. And they found themselves bridging the gap between medical advice and parenting advice when supporting families.
“Parents were calling us at 2:00 in the morning with all kinds of things they were worried about, and that’s where Moms on Call was born,” Ms. Walker said.
A few decades later, Ms. Walker and Ms. Hunter turned that experience, empathy, and expertise into a thriving business. Moms on Call is often referred to as the “instruction manual for babies,” and the two nurses have consulted with more than 10,000 families. Along the way, they’ve sold more than a million copies of multiple books, created a deep well of online resources, and trained others in their techniques.
So how did they do it?
A Folder, a Swaddle, and a Mission
Ms. Walker and Ms. Hunter literally wrote the book on helping people in the trenches of new parenthood. But it wasn’t quite a book at first. “It was a folder we printed off the computer with those questions coming in,” Ms. Hunter recalled. The nurses developed a way to approach each call with a specific outline of protocols they had designed.
“What if we just go to the [patient’s] house and help them figure that out?” Ms. Walker remembered one of the pediatricians she worked with suggesting in 2002. For example, Ms. Hunter’s swaddle technique that calmed even the fussiest babies worked much better if it was demonstrated in person.
The two embarked on home visits with new parents. But their advice would be practical, not medical. Because they were not classified as traveling nurses, they drew a “definitive line” that they wouldn’t be discussing “major medical issues.”
“Going into the homes here in Atlanta, taking that folder, clipping nails, doing baths, discussing feeding — whether you were doing bottles or breastfeeding — we were going to help parents where they were,” Ms. Hunter said.
The physicians they worked with began recommending their services. Ms. Walker jokes that they didn’t know what they were doing at first; they considered giving their first client their money back. But parents needed what they were delivering, which was advice, validation, and confidence in their parenting.
Just 6-8 weeks into their initiative, other practices started to inquire about whether the nurses could do the same thing for them.
It was a solution to the problem of the 15-minute office visit. “We were helping with those questions so that when [babies] came in for their well visits, those questions were already answered. Not only did we go into their homes, but we supported them in the months after we left,” Ms. Hunter said.
The Ripple Effect
The outcomes were astonishing. “Babies were sleeping through the night. Parents were more confident. We didn’t expect the results, and we were shocked at how consistent it was,” Ms. Walker said. “Laura and I used to call each other in disbelief after we would put these basic principles in place and partner with parents.”
Local pediatricians were grateful for the help. But for the nurses, it was about walking alongside families. The two have countless stories of desperate parents, marriages “on the brink of disaster,” moments when they realized their work was having a ripple effect.
One military family stands out in Ms. Walker’s memory. “The father was fighting for our country overseas, and his wife was struggling alone at home.”
But support from Moms on Call had a powerful impact. “When [the father] came home, he presented Laura with a flag and a beautiful personal note expressing his gratitude,” Ms. Walker said. “Once his wife had a partner to help and felt confident and well rested, his heart could rest as well. We did what he couldn’t, and it made all the difference. After all, that’s what he was fighting for in the first place.”
The Gambler Calls
After just 1 or 2 years as Moms on Call, Ms. Walker and Ms. Hunter got an unexpected call from none other than celebrity singer Kenny Rogers, who needed help with his twins.
“I was flipping through the folder, and he said: ‘It’s not copyrighted. It’ll be copyrighted tomorrow morning,’ ” Ms. Hunter recalled.
Mr. Rogers’ attorneys called the next day to provide all the information. “He said: ‘Y’all have got something here. Send this folder to a self-publishing company. Throw up a website. It’ll cost you a few thousand bucks,’” said Ms. Hunter. The business was officially born in 2004.
More of Mr. Rogers’ advice: “You can’t hit a bull’s-eye if you don’t throw a few darts. This is worth throwing a few darts at.”
‘They Don’t Teach You That in Nursing School’
The two nurses reimagined their all-knowing folder as a book with a DVD in the back. Because how do you teach parents how to suction noses without showing it? They also wanted to use an outline format — simpler for exhausted parents who just needed to get the information quickly. A few publishers pushed back on these ideas. But the nurses persisted and self-published the first edition.
The original website was basic. Ms. Walker’s Aunt Janet put it together. But grateful clients were Ms. Walker and Ms. Hunter’s best marketing tool, spreading the word to friends and family. The message: Parents know their own children best and can be empowered to help their own kids, rather than leaning on professionals alone.
A community of families also helped them navigate starting a business. A client who was a mergers and acquisitions lawyer helped them form their LLC. “They don’t teach you that in nursing school,” Ms. Walker said.
Ms. Walker added that they made mistakes. “Not everyone that we encountered viewed or felt the same way about growing a business that is primarily focused on helping families. Sometimes that meant offering services at no charge. Or saying no to certain partnerships that didn’t align with our business model.”
Ms. Walker and Ms. Hunter had an eye on equity in creating multiple ways to access their advice at various price points. They started by charging around $75 for an in-home visit. (Now, if one of the CEOs comes out, it’s around $1000.) But the books, app, and online resources support those who can’t access that, as do an additional 10 in-home consultants around the country.
Along the way, moments told them they were going in the right direction and helped them define their purpose. “It is having a client ‘buy’ us as their go-to [baby] shower gift. It is being able to provide and support a clinic in Kenya or military families around the world. It is helping families realize that they can sleep — that they aren’t alone,” Ms. Walker said.
On Call 24/7 in the Car, in the Checkout Line ...
The early days of Moms on Call were also a juggling act. As Ms. Walker and Ms. Hunter balanced work and home with 10 of their own kids between the two of them, they took calls wherever they were. A friend and caller once joked that she could tell Ms. Hunter was checking out at the grocery store while she advised her on her very sick son’s vomiting.
“We were still trying to take care of the kids, run the house, and neither one of us had nannies or night nurses or housekeepers,” Ms. Hunter said. “But being on call allowed us to still be at home.”
Ms. Walker remembers taking calls on the way to ball games with her own kids, who by 8 years old could recite the advice for a baby’s fever from the back of the car. “It was like a family affair, and our kids got to see how that works and see their moms in action,” she said.
Through it all, Ms. Walker and Ms. Hunter’s motivation came from knowing that thousands of parents were begging for help — and they had an answer.
“Our shoulders have absorbed so many tears of parents who were exhausted and hurting, some who had been lied to or told their child would never sleep or had to be raised a certain way. When someone steals that confidence, especially from a brand-new parent overwhelmed by information, it makes us want to shout the truth from every rooftop and digital channel available,” Ms. Walker added.
Do You Have a Business Idea?
“Boots on the ground” healthcare professionals often see new opportunities to serve patients who might be falling through the cracks of the healthcare system. While not all will become a full-blown business, Ms. Hunter encourages them to break down their idea into “bite-sized pieces.” Just have the next conversation.
“Ask the people around you and the people who are brought to you,” Ms. Hunter said. When the two nurses look back, they see how those pieces of the puzzle were meant to come together. “Ask everyone you know,” Ms. Hunter advised. “And talk to the people you are taking care of. It’s possible they have a gift that will help you get to the next bite-sized piece.”
In short — develop a network of people who believe in your idea. Prioritize those relationships and see where they can take you.
The close relationship between Ms. Walker and Ms. Hunter, as business partners and friends, has also been crucial. They joke that they finish each other’s sentences and sandwiches. “You have to fight for that — we prioritize [that relationship]” too, Ms. Walker said.
Finally, remember why you are doing what you do, Ms. Walker said. “These are the people we help: Wonderful people with jobs that serve us all — the airplane pilot, the anesthesiologist, the pediatrician, the single dad. They are all parents who have felt alone and exhausted. In those lonely moments of a parent’s heart where they fear they are doing the wrong thing, we want to be the voice of hope,” she added. “We let them know that if they ever wondered if they were doing it right, well, only good parents wonder that.”
A version of this article appeared on Medscape.com.
Low Vitamin D Levels May Worsen Gastroparesis Symptoms
TOPLINE:
Over half of patients with symptoms of gastroparesis have low vitamin D levels, which is linked to heightened nausea, vomiting, and gastric neuromuscular dysfunction.
METHODOLOGY:
- In this observational study, researchers evaluated 513 patients, aged 18 years or older, with symptoms of gastroparesis who are included in the registry of the Gastroparesis Clinical Research Consortium. Patients were enrolled across seven tertiary clinical centers in the United States.
- Patients’ vitamin D levels were measured at enrollment in the registry. Gastroparesis Cardinal Symptom Index, gastric emptying scintigraphy, and electrogastrography before and after a water load satiety test (WLST) were measured.
- Low vitamin D levels were defined as 25-hydroxy vitamin D levels < 30 ng/mL, with 20 to < 30 ng/mL considered insufficient and < 20 ng/mL considered deficient.
- The aims of the study were to determine the prevalence of low vitamin D levels in patients with gastroparesis and to examine the relationships among vitamin D levels, symptoms, gastric emptying rate, and gastric myoelectric activity in response to WLST in patients with gastroparesis and those with symptoms but normal gastric emptying.
TAKEAWAY:
- Of the 513 patients with gastroparesis symptoms, 288 patients (56.1%) had low vitamin D levels, with levels being insufficient in 156 patients and deficient in 132 patients. The prevalence of low vitamin D levels was similar in patients with gastroparesis (54.8%) and with normal gastric emptying (59.9%).
- Low vitamin D levels were associated with significantly higher nausea subscores and individual retching scores in patients with gastroparesis and with normal gastric emptying and with higher individual vomiting scores in patients with gastroparesis. It was not associated with increased fullness or bloating in either group.
- Patients with gastroparesis and low vitamin D levels showed higher gastric retention of a solid, low-fat meal at 4 hours than those with normal vitamin D levels (36% retention vs 31% retention; P = .05) but not at 1 or 2 hours.
- Patients with normal gastric emptying and insufficient and deficient vitamin D levels had increased bradygastria and decreased three-cycles-per-minute gastric myoelectrical activity before ingesting a water load compared with those with normal vitamin D levels (P = .004 for both). After ingesting a water load, they had increased tachygastria (P = .01).
IN PRACTICE:
“This study lays the framework for the next level of investigation, replenishment of vitamin D in patients with symptoms of gastroparesis who have low vitamin D levels and see if this improves their gastric neuromuscular dysfunction and symptoms of gastroparesis. Until this study is performed, we advocate assessing vitamin D levels in patients with symptoms of gastroparesis and treatment with exogenous vitamin D if the patient is deficient in vitamin D,” the authors wrote.
SOURCE:
The study, led by Kenneth L. Koch, MD, from the Section of Gastroenterology, Wake Forest University, Winston-Salem, North Carolina, was published online (2024 Jun 14. doi: 10.1007/s10620-024-08520-8) in Digestive Diseases and Sciences.
LIMITATIONS:
The performance of gastric emptying tests and vitamin D level measurements only at registry enrollment could influence the associations observed. Researchers did not compare vitamin D levels with actual dietary intake or explore the correlation between vitamin D levels and symptoms over time. Limitations also included the study’s exploratory nature in which multiple comparisons were made.
DISCLOSURES:
The Gastroparesis Clinical Research Consortium is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences. Dr. Koch declared being a shareholder of 3CPM, a diagnostic gastroenterology device company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Over half of patients with symptoms of gastroparesis have low vitamin D levels, which is linked to heightened nausea, vomiting, and gastric neuromuscular dysfunction.
METHODOLOGY:
- In this observational study, researchers evaluated 513 patients, aged 18 years or older, with symptoms of gastroparesis who are included in the registry of the Gastroparesis Clinical Research Consortium. Patients were enrolled across seven tertiary clinical centers in the United States.
- Patients’ vitamin D levels were measured at enrollment in the registry. Gastroparesis Cardinal Symptom Index, gastric emptying scintigraphy, and electrogastrography before and after a water load satiety test (WLST) were measured.
- Low vitamin D levels were defined as 25-hydroxy vitamin D levels < 30 ng/mL, with 20 to < 30 ng/mL considered insufficient and < 20 ng/mL considered deficient.
- The aims of the study were to determine the prevalence of low vitamin D levels in patients with gastroparesis and to examine the relationships among vitamin D levels, symptoms, gastric emptying rate, and gastric myoelectric activity in response to WLST in patients with gastroparesis and those with symptoms but normal gastric emptying.
TAKEAWAY:
- Of the 513 patients with gastroparesis symptoms, 288 patients (56.1%) had low vitamin D levels, with levels being insufficient in 156 patients and deficient in 132 patients. The prevalence of low vitamin D levels was similar in patients with gastroparesis (54.8%) and with normal gastric emptying (59.9%).
- Low vitamin D levels were associated with significantly higher nausea subscores and individual retching scores in patients with gastroparesis and with normal gastric emptying and with higher individual vomiting scores in patients with gastroparesis. It was not associated with increased fullness or bloating in either group.
- Patients with gastroparesis and low vitamin D levels showed higher gastric retention of a solid, low-fat meal at 4 hours than those with normal vitamin D levels (36% retention vs 31% retention; P = .05) but not at 1 or 2 hours.
- Patients with normal gastric emptying and insufficient and deficient vitamin D levels had increased bradygastria and decreased three-cycles-per-minute gastric myoelectrical activity before ingesting a water load compared with those with normal vitamin D levels (P = .004 for both). After ingesting a water load, they had increased tachygastria (P = .01).
IN PRACTICE:
“This study lays the framework for the next level of investigation, replenishment of vitamin D in patients with symptoms of gastroparesis who have low vitamin D levels and see if this improves their gastric neuromuscular dysfunction and symptoms of gastroparesis. Until this study is performed, we advocate assessing vitamin D levels in patients with symptoms of gastroparesis and treatment with exogenous vitamin D if the patient is deficient in vitamin D,” the authors wrote.
SOURCE:
The study, led by Kenneth L. Koch, MD, from the Section of Gastroenterology, Wake Forest University, Winston-Salem, North Carolina, was published online (2024 Jun 14. doi: 10.1007/s10620-024-08520-8) in Digestive Diseases and Sciences.
LIMITATIONS:
The performance of gastric emptying tests and vitamin D level measurements only at registry enrollment could influence the associations observed. Researchers did not compare vitamin D levels with actual dietary intake or explore the correlation between vitamin D levels and symptoms over time. Limitations also included the study’s exploratory nature in which multiple comparisons were made.
DISCLOSURES:
The Gastroparesis Clinical Research Consortium is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences. Dr. Koch declared being a shareholder of 3CPM, a diagnostic gastroenterology device company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Over half of patients with symptoms of gastroparesis have low vitamin D levels, which is linked to heightened nausea, vomiting, and gastric neuromuscular dysfunction.
METHODOLOGY:
- In this observational study, researchers evaluated 513 patients, aged 18 years or older, with symptoms of gastroparesis who are included in the registry of the Gastroparesis Clinical Research Consortium. Patients were enrolled across seven tertiary clinical centers in the United States.
- Patients’ vitamin D levels were measured at enrollment in the registry. Gastroparesis Cardinal Symptom Index, gastric emptying scintigraphy, and electrogastrography before and after a water load satiety test (WLST) were measured.
- Low vitamin D levels were defined as 25-hydroxy vitamin D levels < 30 ng/mL, with 20 to < 30 ng/mL considered insufficient and < 20 ng/mL considered deficient.
- The aims of the study were to determine the prevalence of low vitamin D levels in patients with gastroparesis and to examine the relationships among vitamin D levels, symptoms, gastric emptying rate, and gastric myoelectric activity in response to WLST in patients with gastroparesis and those with symptoms but normal gastric emptying.
TAKEAWAY:
- Of the 513 patients with gastroparesis symptoms, 288 patients (56.1%) had low vitamin D levels, with levels being insufficient in 156 patients and deficient in 132 patients. The prevalence of low vitamin D levels was similar in patients with gastroparesis (54.8%) and with normal gastric emptying (59.9%).
- Low vitamin D levels were associated with significantly higher nausea subscores and individual retching scores in patients with gastroparesis and with normal gastric emptying and with higher individual vomiting scores in patients with gastroparesis. It was not associated with increased fullness or bloating in either group.
- Patients with gastroparesis and low vitamin D levels showed higher gastric retention of a solid, low-fat meal at 4 hours than those with normal vitamin D levels (36% retention vs 31% retention; P = .05) but not at 1 or 2 hours.
- Patients with normal gastric emptying and insufficient and deficient vitamin D levels had increased bradygastria and decreased three-cycles-per-minute gastric myoelectrical activity before ingesting a water load compared with those with normal vitamin D levels (P = .004 for both). After ingesting a water load, they had increased tachygastria (P = .01).
IN PRACTICE:
“This study lays the framework for the next level of investigation, replenishment of vitamin D in patients with symptoms of gastroparesis who have low vitamin D levels and see if this improves their gastric neuromuscular dysfunction and symptoms of gastroparesis. Until this study is performed, we advocate assessing vitamin D levels in patients with symptoms of gastroparesis and treatment with exogenous vitamin D if the patient is deficient in vitamin D,” the authors wrote.
SOURCE:
The study, led by Kenneth L. Koch, MD, from the Section of Gastroenterology, Wake Forest University, Winston-Salem, North Carolina, was published online (2024 Jun 14. doi: 10.1007/s10620-024-08520-8) in Digestive Diseases and Sciences.
LIMITATIONS:
The performance of gastric emptying tests and vitamin D level measurements only at registry enrollment could influence the associations observed. Researchers did not compare vitamin D levels with actual dietary intake or explore the correlation between vitamin D levels and symptoms over time. Limitations also included the study’s exploratory nature in which multiple comparisons were made.
DISCLOSURES:
The Gastroparesis Clinical Research Consortium is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences. Dr. Koch declared being a shareholder of 3CPM, a diagnostic gastroenterology device company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Vulvar Inflammatory Dermatoses: New Approaches for Diagnosis and Treatment
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
Light Therapy, Phototherapy, Photobiomodulation: New Ways to Heal With Light
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
Did Statin Decision-Making Just Get Harder?
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Optimizing Patient Care With Teledermatology: Improving Access, Efficiency, and Satisfaction
Telemedicine interest, which was relatively quiescent prior to the COVID-19 pandemic, has surged in popularity in the past few years.1 It can now be utilized seamlessly in dermatology practices to deliver exceptional patient care while reducing costs and travel time and offering dermatologists flexibility and improved work-life balance. Teledermatology applications include synchronous, asynchronous, and hybrid platforms.2 For synchronous teledermatology, patient visits are carried out in real time with audio and video technology.3 For asynchronous teledermatology—also known as the store-and-forward model—the dermatologist receives the patient’s history and photographs and then renders an assessment and treatment plan.2 Hybrid teledermatology uses real-time audio and video conferencing for history taking, assessment and treatment plan, and patient education, with photographs sent asynchronously.3 Telemedicine may not be initially intuitive or easy to integrate into clinical practice, but with time and effort, it will complement your dermatology practice, making it run more efficiently.
Patient Satisfaction With Teledermatology
Studies generally have shown very high patient satisfaction rates and shorter wait times with teledermatology vs in-person visits; for example, in a systematic review of 15 teledermatology studies including 7781 patients, more than 80% of participants reported high satisfaction with their telemedicine visit, with up to 92% reporting that they would choose to do a televisit again.4 In a retrospective analysis of 615 Zocdoc physicians, 65% of whom were dermatologists, mean wait times were 2.4 days for virtual appointments compared with 11.7 days for in-person appointments.5 Similarly, in a retrospective single-institution study, mean wait times for televisits were 14.3 days compared with 34.7 days for in-person referrals.6
Follow-Up Visits for Nail Disorders Via Teledermatology
Teledermatology may be particularly well suited for treating patients with nail disorders. In a prospective observational study, Onyeka et al7 accessed 813 images from 63 dermatology patients via teledermatology over a 6-month period to assess distance, focus, brightness, background, and image quality; of them, 83% were rated as high quality. Notably, images of nail disorders, skin growths, or pigmentation disorders were rated as having better image quality than images of inflammatory skin conditions (odds ratio [OR], 4.2-12.9 [P<.005]).7 In a retrospective study of 107 telemedicine visits for nail disorders during the COVID-19 pandemic, patients with longitudinal melanonychia were recommended for in-person visits for physical examination and dermoscopy, as were patients with suspected onychomycosis, who required nail plate sampling for diagnostic confirmation; however, approximately half of visits did not require in-person follow-up, including those patients with confirmed onychomycosis.8 Onychomycosis patients could be examined for clinical improvement and counseled on medication compliance via telemedicine. Other patients who did not require in-person follow-ups were those with traumatic nail disorders such as subungual hematoma and retronychia as well as those with body‐focused repetitive behaviors, including habit-tic nail deformity, onychophagia, and onychotillomania.8
Patients undergoing nail biopsies to rule out malignancies or to diagnose inflammatory nail disorders also may be managed via telemedicine. Patients for whom nail biopsies are recommended often are anxious about the procedure, which may be due to portrayal of nail trauma in the media9 or lack of accurate information on nail biopsies online.10 Therefore, counseling via telemedicine about the details of the procedure in a patient-friendly way (eg, showing an animated video and narrating it11) can allay anxiety without the inconvenience, cost, and time missed from work associated with traveling to an in-person visit. In addition, postoperative counseling ideally is performed via telemedicine because complications following nail procedures are uncommon. In a retrospective study of 502 patients who underwent a nail biopsy at a single academic center, only 14 developed surgical site infections within 8 days on average (range, 5–13 days), with a higher infection risk in patients with type 2 diabetes mellitus (P<.0003).12
Advantages and Limitations
There are many benefits to incorporating telemedicine into dermatology practices, including reduced overhead costs, convenience and time saved for patients, and flexibility and improved work-life balance for dermatologists. In addition, because the number of in-person visits seen generally is fixed due to space constraints and work-hour restrictions, delegating follow-up visits to telemedicine can free up in-person slots for new patients and those needing procedures. However, there also are some inherent limitations to telemedicine: technology access, vision or hearing difficulties or low digital health literacy, or language barriers. In the prospective observational study by Onyeka et al7 analyzing 813 teledermatology images, patients aged 65 to 74 years sent in more clinically useful images (OR, 7.9) and images that were more often in focus (OR, 2.6) compared with patients older than 85 years.
Final Thoughts
Incorporation of telemedicine into dermatologic practice is a valuable tool for triaging patients with acute issues, improving patient care and health care access, making practices more efficient, and improving dermatologist flexibility and work-life balance. Further development of teledermatology to provide access to underserved populations prioritizing dermatologist reimbursement and progress on technologic innovations will make teledermatology even more useful in the coming years.
- He A, Ti Kim T, Nguyen KD. Utilization of teledermatology services for dermatological diagnoses during the COVID-19 pandemic. Arch Dermatol Res. 2023;315:1059-1062.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Wang RH, Barbieri JS, Kovarik CL, et al. Synchronous and asynchronous teledermatology: a narrative review of strengths and limitations. J Telemed Telecare. 2022;28:533-538.
- Miller J, Jones E. Shaping the future of teledermatology: a literature review of patient and provider satisfaction with synchronous teledermatology during the COVID-19 pandemic. Clin Exp Dermatol. 2022;47:1903-1909.
- Gu L, Xiang L, Lipner SR. Analysis of availability of online dermatology appointments during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:517-520.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an eConsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2019;83:1633-1638.
- Onyeka S, Kim J, Eid E, et al. Quality of images submitted by older patients to a teledermatology platform. Abstract presented at the Society of Investigative Dermatology Annual Meeting; May 15-18, 2024; Dallas, TX.
- Chang MJ, Stewart CR, Lipner SR. Retrospective study of nail telemedicine visits during the COVID-19 pandemic. Dermatol Ther. 2021;34:E14630.
- Albucker SJ, Falotico JM, Lipner SR. A real nail biter: a cross-sectional study of 75 nail trauma scenes in international films and television series. J Cutan Med Surg. 2023;27:288-291.
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Hill RC, Ho B, Lipner SR. Assuaging patient anxiety about nail biopsies with an animated educational video. J Am Acad Dermatol. Published online March 29, 2024. doi:10.1016/j.jaad.2024.03.031.
- Axler E, Lu A, Darrell M, et al. Surgical site infections are uncommon following nail biopsies in a single-center case-control study of 502 patients. J Am Acad Dermatol. Published online May 15, 2024. doi:10.1016/j.jaad.2024.05.017
Telemedicine interest, which was relatively quiescent prior to the COVID-19 pandemic, has surged in popularity in the past few years.1 It can now be utilized seamlessly in dermatology practices to deliver exceptional patient care while reducing costs and travel time and offering dermatologists flexibility and improved work-life balance. Teledermatology applications include synchronous, asynchronous, and hybrid platforms.2 For synchronous teledermatology, patient visits are carried out in real time with audio and video technology.3 For asynchronous teledermatology—also known as the store-and-forward model—the dermatologist receives the patient’s history and photographs and then renders an assessment and treatment plan.2 Hybrid teledermatology uses real-time audio and video conferencing for history taking, assessment and treatment plan, and patient education, with photographs sent asynchronously.3 Telemedicine may not be initially intuitive or easy to integrate into clinical practice, but with time and effort, it will complement your dermatology practice, making it run more efficiently.
Patient Satisfaction With Teledermatology
Studies generally have shown very high patient satisfaction rates and shorter wait times with teledermatology vs in-person visits; for example, in a systematic review of 15 teledermatology studies including 7781 patients, more than 80% of participants reported high satisfaction with their telemedicine visit, with up to 92% reporting that they would choose to do a televisit again.4 In a retrospective analysis of 615 Zocdoc physicians, 65% of whom were dermatologists, mean wait times were 2.4 days for virtual appointments compared with 11.7 days for in-person appointments.5 Similarly, in a retrospective single-institution study, mean wait times for televisits were 14.3 days compared with 34.7 days for in-person referrals.6
Follow-Up Visits for Nail Disorders Via Teledermatology
Teledermatology may be particularly well suited for treating patients with nail disorders. In a prospective observational study, Onyeka et al7 accessed 813 images from 63 dermatology patients via teledermatology over a 6-month period to assess distance, focus, brightness, background, and image quality; of them, 83% were rated as high quality. Notably, images of nail disorders, skin growths, or pigmentation disorders were rated as having better image quality than images of inflammatory skin conditions (odds ratio [OR], 4.2-12.9 [P<.005]).7 In a retrospective study of 107 telemedicine visits for nail disorders during the COVID-19 pandemic, patients with longitudinal melanonychia were recommended for in-person visits for physical examination and dermoscopy, as were patients with suspected onychomycosis, who required nail plate sampling for diagnostic confirmation; however, approximately half of visits did not require in-person follow-up, including those patients with confirmed onychomycosis.8 Onychomycosis patients could be examined for clinical improvement and counseled on medication compliance via telemedicine. Other patients who did not require in-person follow-ups were those with traumatic nail disorders such as subungual hematoma and retronychia as well as those with body‐focused repetitive behaviors, including habit-tic nail deformity, onychophagia, and onychotillomania.8
Patients undergoing nail biopsies to rule out malignancies or to diagnose inflammatory nail disorders also may be managed via telemedicine. Patients for whom nail biopsies are recommended often are anxious about the procedure, which may be due to portrayal of nail trauma in the media9 or lack of accurate information on nail biopsies online.10 Therefore, counseling via telemedicine about the details of the procedure in a patient-friendly way (eg, showing an animated video and narrating it11) can allay anxiety without the inconvenience, cost, and time missed from work associated with traveling to an in-person visit. In addition, postoperative counseling ideally is performed via telemedicine because complications following nail procedures are uncommon. In a retrospective study of 502 patients who underwent a nail biopsy at a single academic center, only 14 developed surgical site infections within 8 days on average (range, 5–13 days), with a higher infection risk in patients with type 2 diabetes mellitus (P<.0003).12
Advantages and Limitations
There are many benefits to incorporating telemedicine into dermatology practices, including reduced overhead costs, convenience and time saved for patients, and flexibility and improved work-life balance for dermatologists. In addition, because the number of in-person visits seen generally is fixed due to space constraints and work-hour restrictions, delegating follow-up visits to telemedicine can free up in-person slots for new patients and those needing procedures. However, there also are some inherent limitations to telemedicine: technology access, vision or hearing difficulties or low digital health literacy, or language barriers. In the prospective observational study by Onyeka et al7 analyzing 813 teledermatology images, patients aged 65 to 74 years sent in more clinically useful images (OR, 7.9) and images that were more often in focus (OR, 2.6) compared with patients older than 85 years.
Final Thoughts
Incorporation of telemedicine into dermatologic practice is a valuable tool for triaging patients with acute issues, improving patient care and health care access, making practices more efficient, and improving dermatologist flexibility and work-life balance. Further development of teledermatology to provide access to underserved populations prioritizing dermatologist reimbursement and progress on technologic innovations will make teledermatology even more useful in the coming years.
Telemedicine interest, which was relatively quiescent prior to the COVID-19 pandemic, has surged in popularity in the past few years.1 It can now be utilized seamlessly in dermatology practices to deliver exceptional patient care while reducing costs and travel time and offering dermatologists flexibility and improved work-life balance. Teledermatology applications include synchronous, asynchronous, and hybrid platforms.2 For synchronous teledermatology, patient visits are carried out in real time with audio and video technology.3 For asynchronous teledermatology—also known as the store-and-forward model—the dermatologist receives the patient’s history and photographs and then renders an assessment and treatment plan.2 Hybrid teledermatology uses real-time audio and video conferencing for history taking, assessment and treatment plan, and patient education, with photographs sent asynchronously.3 Telemedicine may not be initially intuitive or easy to integrate into clinical practice, but with time and effort, it will complement your dermatology practice, making it run more efficiently.
Patient Satisfaction With Teledermatology
Studies generally have shown very high patient satisfaction rates and shorter wait times with teledermatology vs in-person visits; for example, in a systematic review of 15 teledermatology studies including 7781 patients, more than 80% of participants reported high satisfaction with their telemedicine visit, with up to 92% reporting that they would choose to do a televisit again.4 In a retrospective analysis of 615 Zocdoc physicians, 65% of whom were dermatologists, mean wait times were 2.4 days for virtual appointments compared with 11.7 days for in-person appointments.5 Similarly, in a retrospective single-institution study, mean wait times for televisits were 14.3 days compared with 34.7 days for in-person referrals.6
Follow-Up Visits for Nail Disorders Via Teledermatology
Teledermatology may be particularly well suited for treating patients with nail disorders. In a prospective observational study, Onyeka et al7 accessed 813 images from 63 dermatology patients via teledermatology over a 6-month period to assess distance, focus, brightness, background, and image quality; of them, 83% were rated as high quality. Notably, images of nail disorders, skin growths, or pigmentation disorders were rated as having better image quality than images of inflammatory skin conditions (odds ratio [OR], 4.2-12.9 [P<.005]).7 In a retrospective study of 107 telemedicine visits for nail disorders during the COVID-19 pandemic, patients with longitudinal melanonychia were recommended for in-person visits for physical examination and dermoscopy, as were patients with suspected onychomycosis, who required nail plate sampling for diagnostic confirmation; however, approximately half of visits did not require in-person follow-up, including those patients with confirmed onychomycosis.8 Onychomycosis patients could be examined for clinical improvement and counseled on medication compliance via telemedicine. Other patients who did not require in-person follow-ups were those with traumatic nail disorders such as subungual hematoma and retronychia as well as those with body‐focused repetitive behaviors, including habit-tic nail deformity, onychophagia, and onychotillomania.8
Patients undergoing nail biopsies to rule out malignancies or to diagnose inflammatory nail disorders also may be managed via telemedicine. Patients for whom nail biopsies are recommended often are anxious about the procedure, which may be due to portrayal of nail trauma in the media9 or lack of accurate information on nail biopsies online.10 Therefore, counseling via telemedicine about the details of the procedure in a patient-friendly way (eg, showing an animated video and narrating it11) can allay anxiety without the inconvenience, cost, and time missed from work associated with traveling to an in-person visit. In addition, postoperative counseling ideally is performed via telemedicine because complications following nail procedures are uncommon. In a retrospective study of 502 patients who underwent a nail biopsy at a single academic center, only 14 developed surgical site infections within 8 days on average (range, 5–13 days), with a higher infection risk in patients with type 2 diabetes mellitus (P<.0003).12
Advantages and Limitations
There are many benefits to incorporating telemedicine into dermatology practices, including reduced overhead costs, convenience and time saved for patients, and flexibility and improved work-life balance for dermatologists. In addition, because the number of in-person visits seen generally is fixed due to space constraints and work-hour restrictions, delegating follow-up visits to telemedicine can free up in-person slots for new patients and those needing procedures. However, there also are some inherent limitations to telemedicine: technology access, vision or hearing difficulties or low digital health literacy, or language barriers. In the prospective observational study by Onyeka et al7 analyzing 813 teledermatology images, patients aged 65 to 74 years sent in more clinically useful images (OR, 7.9) and images that were more often in focus (OR, 2.6) compared with patients older than 85 years.
Final Thoughts
Incorporation of telemedicine into dermatologic practice is a valuable tool for triaging patients with acute issues, improving patient care and health care access, making practices more efficient, and improving dermatologist flexibility and work-life balance. Further development of teledermatology to provide access to underserved populations prioritizing dermatologist reimbursement and progress on technologic innovations will make teledermatology even more useful in the coming years.
- He A, Ti Kim T, Nguyen KD. Utilization of teledermatology services for dermatological diagnoses during the COVID-19 pandemic. Arch Dermatol Res. 2023;315:1059-1062.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Wang RH, Barbieri JS, Kovarik CL, et al. Synchronous and asynchronous teledermatology: a narrative review of strengths and limitations. J Telemed Telecare. 2022;28:533-538.
- Miller J, Jones E. Shaping the future of teledermatology: a literature review of patient and provider satisfaction with synchronous teledermatology during the COVID-19 pandemic. Clin Exp Dermatol. 2022;47:1903-1909.
- Gu L, Xiang L, Lipner SR. Analysis of availability of online dermatology appointments during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:517-520.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an eConsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2019;83:1633-1638.
- Onyeka S, Kim J, Eid E, et al. Quality of images submitted by older patients to a teledermatology platform. Abstract presented at the Society of Investigative Dermatology Annual Meeting; May 15-18, 2024; Dallas, TX.
- Chang MJ, Stewart CR, Lipner SR. Retrospective study of nail telemedicine visits during the COVID-19 pandemic. Dermatol Ther. 2021;34:E14630.
- Albucker SJ, Falotico JM, Lipner SR. A real nail biter: a cross-sectional study of 75 nail trauma scenes in international films and television series. J Cutan Med Surg. 2023;27:288-291.
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Hill RC, Ho B, Lipner SR. Assuaging patient anxiety about nail biopsies with an animated educational video. J Am Acad Dermatol. Published online March 29, 2024. doi:10.1016/j.jaad.2024.03.031.
- Axler E, Lu A, Darrell M, et al. Surgical site infections are uncommon following nail biopsies in a single-center case-control study of 502 patients. J Am Acad Dermatol. Published online May 15, 2024. doi:10.1016/j.jaad.2024.05.017
- He A, Ti Kim T, Nguyen KD. Utilization of teledermatology services for dermatological diagnoses during the COVID-19 pandemic. Arch Dermatol Res. 2023;315:1059-1062.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Wang RH, Barbieri JS, Kovarik CL, et al. Synchronous and asynchronous teledermatology: a narrative review of strengths and limitations. J Telemed Telecare. 2022;28:533-538.
- Miller J, Jones E. Shaping the future of teledermatology: a literature review of patient and provider satisfaction with synchronous teledermatology during the COVID-19 pandemic. Clin Exp Dermatol. 2022;47:1903-1909.
- Gu L, Xiang L, Lipner SR. Analysis of availability of online dermatology appointments during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:517-520.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an eConsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2019;83:1633-1638.
- Onyeka S, Kim J, Eid E, et al. Quality of images submitted by older patients to a teledermatology platform. Abstract presented at the Society of Investigative Dermatology Annual Meeting; May 15-18, 2024; Dallas, TX.
- Chang MJ, Stewart CR, Lipner SR. Retrospective study of nail telemedicine visits during the COVID-19 pandemic. Dermatol Ther. 2021;34:E14630.
- Albucker SJ, Falotico JM, Lipner SR. A real nail biter: a cross-sectional study of 75 nail trauma scenes in international films and television series. J Cutan Med Surg. 2023;27:288-291.
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Hill RC, Ho B, Lipner SR. Assuaging patient anxiety about nail biopsies with an animated educational video. J Am Acad Dermatol. Published online March 29, 2024. doi:10.1016/j.jaad.2024.03.031.
- Axler E, Lu A, Darrell M, et al. Surgical site infections are uncommon following nail biopsies in a single-center case-control study of 502 patients. J Am Acad Dermatol. Published online May 15, 2024. doi:10.1016/j.jaad.2024.05.017
Practice Points
- Incorporation of telemedicine into dermatologic practice can improve patient access, reduce costs, and offer dermatologists flexibility and improved work-life balance.
- Patient satisfaction with telemedicine is exceedingly high, and teledermatology may be particularly well suited for caring for patients with nail disorders.