User login
Prolonged Drug-Induced Hypersensitivity Syndrome/DRESS With Alopecia Areata and Autoimmune Thyroiditis
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
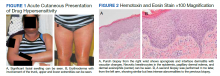
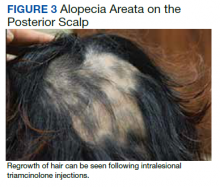
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
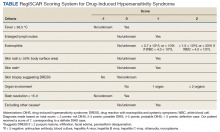
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
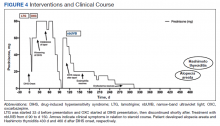
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Federal Health Care Data Trends 2022: HIV Care in the VA
- Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480. http://doi.org/10.1097/QAI.0000000000000615
- VA HIV Testing Information for Health Care Providers. US Department of Veterans Affairs. January 2021. Accessed March 4, 2022. https://www.hiv.va.gov/pdf/GetChecked-FactSheet-Providers-2021-508.pdf
- Associated Press. Judge rules US Military can’t discharge HIV-positive troops. ABC News. Published April 10, 2022. Accessed May 4, 2022. https://abcnews.go.com/Health/wireStory/judge-rules-us-military-discharge-hiv-positive-troops-84000771
- Bokhour BG, Bolton RE, Asch SM, et al. How should we organize care for patients with human immunodeficiency virus and comorbidities? A multisite qualitative study of human immunodeficiency virus care in the United States Department of Veterans Affairs. Med Care. 2021;59(8):727-735. http://doi.org/10.1097/MLR.0000000000001563
- Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593-1601. http://doi.org/10.1086/523577
- Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480. http://doi.org/10.1097/QAI.0000000000000615
- VA HIV Testing Information for Health Care Providers. US Department of Veterans Affairs. January 2021. Accessed March 4, 2022. https://www.hiv.va.gov/pdf/GetChecked-FactSheet-Providers-2021-508.pdf
- Associated Press. Judge rules US Military can’t discharge HIV-positive troops. ABC News. Published April 10, 2022. Accessed May 4, 2022. https://abcnews.go.com/Health/wireStory/judge-rules-us-military-discharge-hiv-positive-troops-84000771
- Bokhour BG, Bolton RE, Asch SM, et al. How should we organize care for patients with human immunodeficiency virus and comorbidities? A multisite qualitative study of human immunodeficiency virus care in the United States Department of Veterans Affairs. Med Care. 2021;59(8):727-735. http://doi.org/10.1097/MLR.0000000000001563
- Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593-1601. http://doi.org/10.1086/523577
- Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480. http://doi.org/10.1097/QAI.0000000000000615
- VA HIV Testing Information for Health Care Providers. US Department of Veterans Affairs. January 2021. Accessed March 4, 2022. https://www.hiv.va.gov/pdf/GetChecked-FactSheet-Providers-2021-508.pdf
- Associated Press. Judge rules US Military can’t discharge HIV-positive troops. ABC News. Published April 10, 2022. Accessed May 4, 2022. https://abcnews.go.com/Health/wireStory/judge-rules-us-military-discharge-hiv-positive-troops-84000771
- Bokhour BG, Bolton RE, Asch SM, et al. How should we organize care for patients with human immunodeficiency virus and comorbidities? A multisite qualitative study of human immunodeficiency virus care in the United States Department of Veterans Affairs. Med Care. 2021;59(8):727-735. http://doi.org/10.1097/MLR.0000000000001563
- Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593-1601. http://doi.org/10.1086/523577
Saddled with med school debt, yet left out of loan forgiveness plans
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.
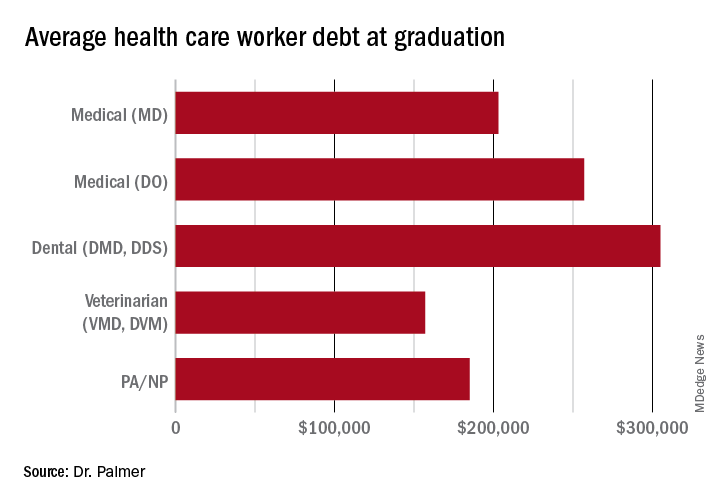
The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
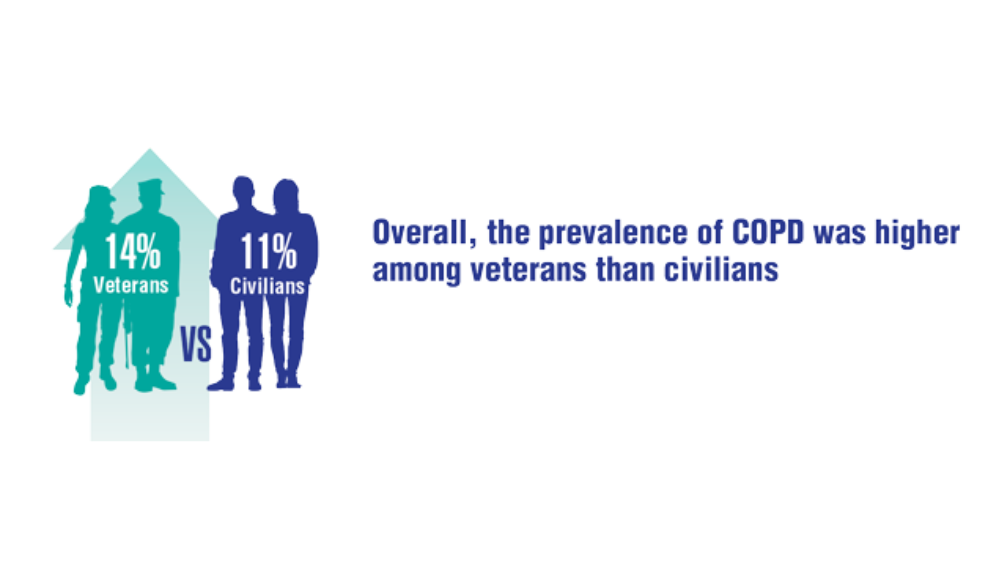
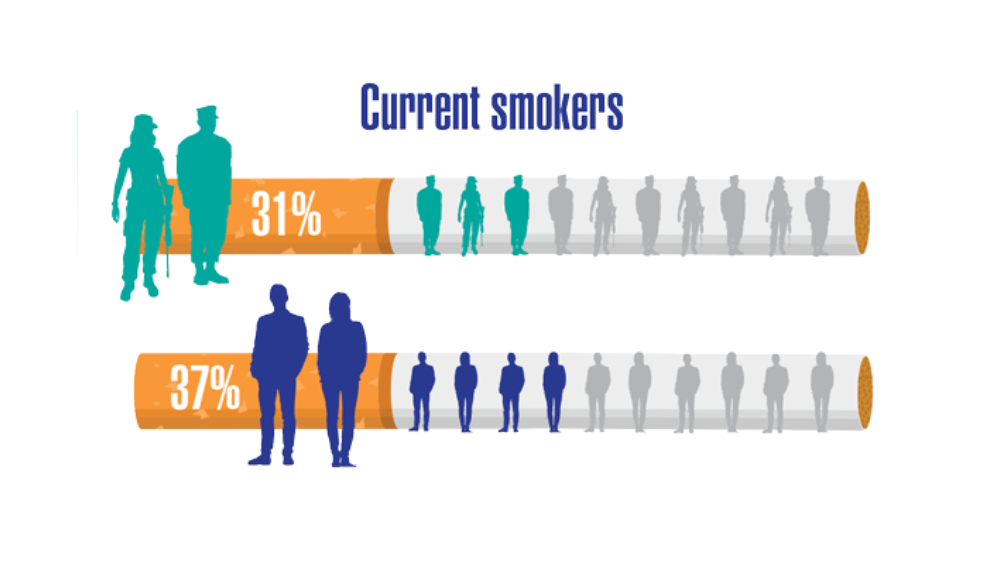
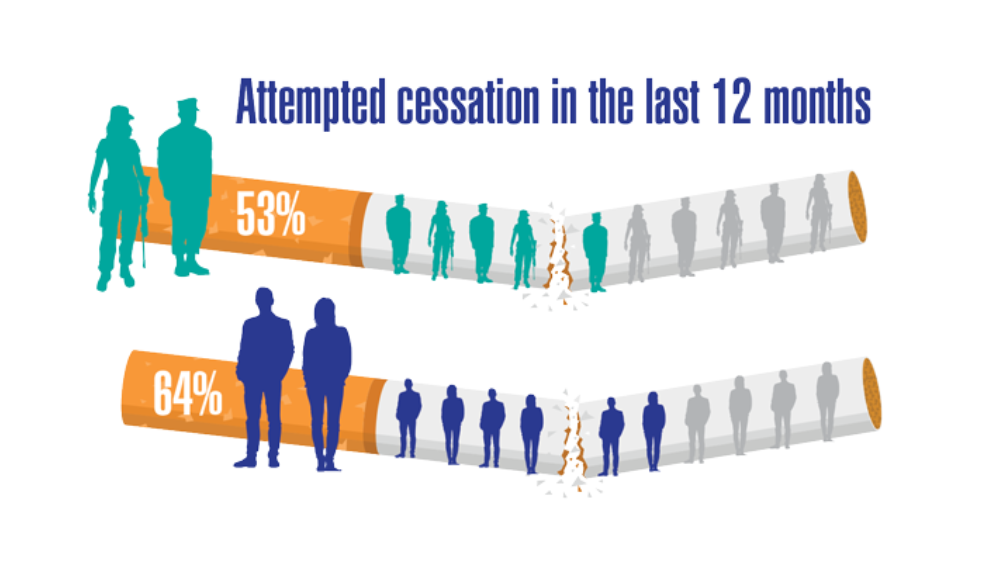
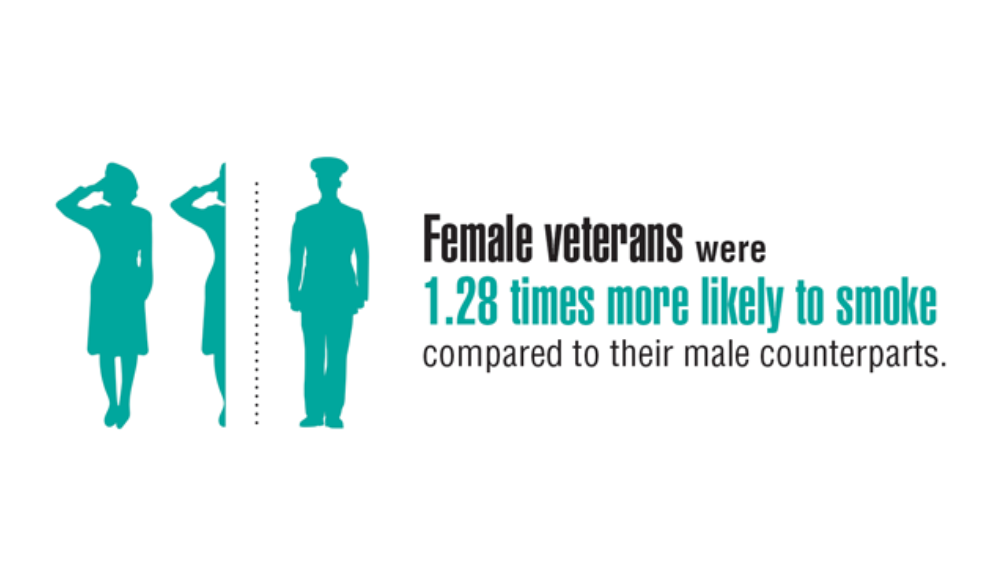
Federal Health Care Data Trends 2022: Respiratory Illnesses
- Federal Register. Presumptive service connection for respiratory conditions due to exposure to particulate matter. Published August 5, 2021. Accessed April 6, 2022. https://www.govinfo.gov/content/pkg/FR-2021-08-05/pdf/2021-16693.pdf
- Rivera AC, Powell TM, Boyko EJ, et al. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144.
- Rinne ST, Elwy AR, Liu CF et al. Implementation of guideline-based therapy for chronic obstructive pulmonary disease: differences between men and women veterans. Chron Respir Dis. 2017;14(4):385-391. http://doi.org/10.1177/1479972317702141
- Greiner B, Ottwell R, Corcoran A, Hartwell M. Smoking and physical activity patterns of US Military veterans with chronic obstructive pulmonary disease: an analysis of 2017 behavioral risk factor surveillance system. Mil Med. 2021;186:e1-5. http://doi.org/10.1093/milmed/usaa330
- Federal Register. Presumptive service connection for respiratory conditions due to exposure to particulate matter. Published August 5, 2021. Accessed April 6, 2022. https://www.govinfo.gov/content/pkg/FR-2021-08-05/pdf/2021-16693.pdf
- Rivera AC, Powell TM, Boyko EJ, et al. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144.
- Rinne ST, Elwy AR, Liu CF et al. Implementation of guideline-based therapy for chronic obstructive pulmonary disease: differences between men and women veterans. Chron Respir Dis. 2017;14(4):385-391. http://doi.org/10.1177/1479972317702141
- Greiner B, Ottwell R, Corcoran A, Hartwell M. Smoking and physical activity patterns of US Military veterans with chronic obstructive pulmonary disease: an analysis of 2017 behavioral risk factor surveillance system. Mil Med. 2021;186:e1-5. http://doi.org/10.1093/milmed/usaa330
- Federal Register. Presumptive service connection for respiratory conditions due to exposure to particulate matter. Published August 5, 2021. Accessed April 6, 2022. https://www.govinfo.gov/content/pkg/FR-2021-08-05/pdf/2021-16693.pdf
- Rivera AC, Powell TM, Boyko EJ, et al. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144.
- Rinne ST, Elwy AR, Liu CF et al. Implementation of guideline-based therapy for chronic obstructive pulmonary disease: differences between men and women veterans. Chron Respir Dis. 2017;14(4):385-391. http://doi.org/10.1177/1479972317702141
- Greiner B, Ottwell R, Corcoran A, Hartwell M. Smoking and physical activity patterns of US Military veterans with chronic obstructive pulmonary disease: an analysis of 2017 behavioral risk factor surveillance system. Mil Med. 2021;186:e1-5. http://doi.org/10.1093/milmed/usaa330
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.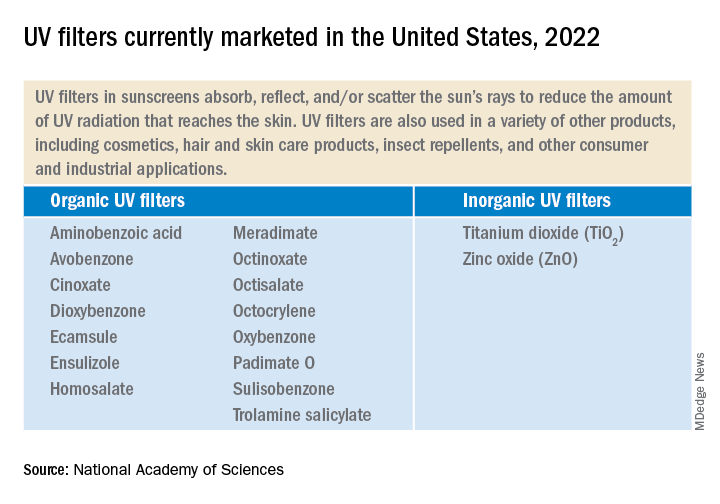
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In RA, tofacitinib shows higher infection rate than TNF inhibitors
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) are more likely to develop infections than are those who take a tumor necrosis factor inhibitor (TNFi), results of an industry-sponsored randomized controlled trial suggest.
The Janus kinase (JAK) inhibitor tofacitinib and TNFi biologics are common RA treatments that, along with factors including age, disease activity, and comorbidities, can put patients with RA at increased risk for infections.
“In this secondary analysis of the ORAL Surveillance trial, infections were increased with tofacitinib, compared with TNFi,” study coauthor Deepak L. Bhatt, MD, MPH, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston, explained in an interview.
As reported in Annals of the Rheumatic Diseases, Dr. Bhatt and colleagues performed a subanalysis of the final dataset from the phase 3b/4 open-label safety trial of tofacitinib in RA conducted between March 2014 and July 2020, in 345 study locations worldwide.
Study participants were 50 years of age or older with moderate to severe RA who were taking methotrexate but having inadequate symptom control. They had at least one cardiovascular risk factor such as being a current smoker or having hypertension, past heart attack, family history of coronary heart disease, high cholesterol, diabetes mellitus, or extra-articular RA. Patients with current or recent infection, clinically significant laboratory abnormalities, or pregnancy, were excluded from the study.
In the study, 1,455 participants received oral tofacitinib 5 mg twice per day; 1,456 received oral tofacitinib 10 mg twice per day; and 1,451 were treated with subcutaneous TNFi (40 mg subcutaneous adalimumab [Humira] injection every 2 weeks in the United States, Puerto Rico, and Canada; and 50 mg subcutaneous etanercept [Enbrel] injection every week in all other countries. Participants continued their prestudy stable dose of methotrexate if clinically indicated.
The researchers calculated incidence rates and hazard ratios for infections, overall and by age (50-64 years, compared with 65 years and older). They calculated probabilities of infection using Kaplan-Meier estimates and identified infection risk factors through Cox modeling.
They found higher infection rates, serious infection events (SIEs), and nonserious infections (NSIs) with tofacitinib than with TNFi, including:
- Patients taking tofacitinib 5 mg (HR, 1.17; 95% confidence interval, 0.92-1.50) and 10 mg (HR, 1.48; 95% CI, 1.17-1.87) were at greater risk for SIEs.
- Patients older than 65 who were taking tofacitinib 10 mg had increased IRs and HRs for all infections and for SIEs, compared with those aged 50-64.
- The probability of a SIE rose from month 18 onward in participants taking tofacitinib 5 mg, as well as before month 6 in those taking tofacitinib 10 mg.
- In both tofacitinib groups, the probability of NSI increased before month 6.
The most common risk factors for SIEs were higher age, baseline opioid use, history of chronic lung disease, and time-dependent oral corticosteroid use. Risk factors for NSIs were female sex, history of chronic lung disease or infection, history of smoking, as well as time-dependent higher Disease Activity Score in 28 joints and C-reactive protein score.
‘Best information to date’
Michael George, MD, MSCE, assistant professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, welcomed the study’s results.
“This study provides the best information to date on the risk of infection with the JAK inhibitor tofacitinib, compared to a TNF inhibitor,” Dr. George, who was not involved in the study, said in an interview. “It is rare to have such a large randomized trial with an active comparator focused on safety. This is a major strength.
“Being able to quantify the amount of increased risk will help with shared decision-making when counseling patients,” he added.
Dr. George said that, while the small overall risk may not be clinically meaningful for younger, healthier patients, trying biologics such as TNFi before tofacitinib may be optimal for high-risk patients who are older or have comorbidities.
Dr. Bhatt agreed.
“In deciding on appropriate therapies for RA (or other conditions where tofacitinib is used), it is important for the prescribing physician to explain the risks to the patient and weigh them against the potential benefits,” he advised.
Dr. Bhatt noted that increased infection is not the first risk that’s been linked with tofacitinib.
“ORAL Surveillance was designed primarily to assess cardiovascular safety and showed higher rates of cardiovascular events such as myocardial infarction and pulmonary embolism, as well as cancer, with tofacitinib,” he explained.
He recommended further related research.
“Randomized trials are needed to determine the best ways to treat conditions such as RA while trying to minimize cardiovascular, cancer, and infectious risks,” he said.
The study was sponsored by Pfizer. All authors reported financial involvements with Pfizer; most have financial involvements with other pharmaceutical companies as well; four authors are employees of Pfizer and three are also stockholders in the company. Dr. George reported involvements with the pharmaceutical industry.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) are more likely to develop infections than are those who take a tumor necrosis factor inhibitor (TNFi), results of an industry-sponsored randomized controlled trial suggest.
The Janus kinase (JAK) inhibitor tofacitinib and TNFi biologics are common RA treatments that, along with factors including age, disease activity, and comorbidities, can put patients with RA at increased risk for infections.
“In this secondary analysis of the ORAL Surveillance trial, infections were increased with tofacitinib, compared with TNFi,” study coauthor Deepak L. Bhatt, MD, MPH, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston, explained in an interview.
As reported in Annals of the Rheumatic Diseases, Dr. Bhatt and colleagues performed a subanalysis of the final dataset from the phase 3b/4 open-label safety trial of tofacitinib in RA conducted between March 2014 and July 2020, in 345 study locations worldwide.
Study participants were 50 years of age or older with moderate to severe RA who were taking methotrexate but having inadequate symptom control. They had at least one cardiovascular risk factor such as being a current smoker or having hypertension, past heart attack, family history of coronary heart disease, high cholesterol, diabetes mellitus, or extra-articular RA. Patients with current or recent infection, clinically significant laboratory abnormalities, or pregnancy, were excluded from the study.
In the study, 1,455 participants received oral tofacitinib 5 mg twice per day; 1,456 received oral tofacitinib 10 mg twice per day; and 1,451 were treated with subcutaneous TNFi (40 mg subcutaneous adalimumab [Humira] injection every 2 weeks in the United States, Puerto Rico, and Canada; and 50 mg subcutaneous etanercept [Enbrel] injection every week in all other countries. Participants continued their prestudy stable dose of methotrexate if clinically indicated.
The researchers calculated incidence rates and hazard ratios for infections, overall and by age (50-64 years, compared with 65 years and older). They calculated probabilities of infection using Kaplan-Meier estimates and identified infection risk factors through Cox modeling.
They found higher infection rates, serious infection events (SIEs), and nonserious infections (NSIs) with tofacitinib than with TNFi, including:
- Patients taking tofacitinib 5 mg (HR, 1.17; 95% confidence interval, 0.92-1.50) and 10 mg (HR, 1.48; 95% CI, 1.17-1.87) were at greater risk for SIEs.
- Patients older than 65 who were taking tofacitinib 10 mg had increased IRs and HRs for all infections and for SIEs, compared with those aged 50-64.
- The probability of a SIE rose from month 18 onward in participants taking tofacitinib 5 mg, as well as before month 6 in those taking tofacitinib 10 mg.
- In both tofacitinib groups, the probability of NSI increased before month 6.
The most common risk factors for SIEs were higher age, baseline opioid use, history of chronic lung disease, and time-dependent oral corticosteroid use. Risk factors for NSIs were female sex, history of chronic lung disease or infection, history of smoking, as well as time-dependent higher Disease Activity Score in 28 joints and C-reactive protein score.
‘Best information to date’
Michael George, MD, MSCE, assistant professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, welcomed the study’s results.
“This study provides the best information to date on the risk of infection with the JAK inhibitor tofacitinib, compared to a TNF inhibitor,” Dr. George, who was not involved in the study, said in an interview. “It is rare to have such a large randomized trial with an active comparator focused on safety. This is a major strength.
“Being able to quantify the amount of increased risk will help with shared decision-making when counseling patients,” he added.
Dr. George said that, while the small overall risk may not be clinically meaningful for younger, healthier patients, trying biologics such as TNFi before tofacitinib may be optimal for high-risk patients who are older or have comorbidities.
Dr. Bhatt agreed.
“In deciding on appropriate therapies for RA (or other conditions where tofacitinib is used), it is important for the prescribing physician to explain the risks to the patient and weigh them against the potential benefits,” he advised.
Dr. Bhatt noted that increased infection is not the first risk that’s been linked with tofacitinib.
“ORAL Surveillance was designed primarily to assess cardiovascular safety and showed higher rates of cardiovascular events such as myocardial infarction and pulmonary embolism, as well as cancer, with tofacitinib,” he explained.
He recommended further related research.
“Randomized trials are needed to determine the best ways to treat conditions such as RA while trying to minimize cardiovascular, cancer, and infectious risks,” he said.
The study was sponsored by Pfizer. All authors reported financial involvements with Pfizer; most have financial involvements with other pharmaceutical companies as well; four authors are employees of Pfizer and three are also stockholders in the company. Dr. George reported involvements with the pharmaceutical industry.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) are more likely to develop infections than are those who take a tumor necrosis factor inhibitor (TNFi), results of an industry-sponsored randomized controlled trial suggest.
The Janus kinase (JAK) inhibitor tofacitinib and TNFi biologics are common RA treatments that, along with factors including age, disease activity, and comorbidities, can put patients with RA at increased risk for infections.
“In this secondary analysis of the ORAL Surveillance trial, infections were increased with tofacitinib, compared with TNFi,” study coauthor Deepak L. Bhatt, MD, MPH, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston, explained in an interview.
As reported in Annals of the Rheumatic Diseases, Dr. Bhatt and colleagues performed a subanalysis of the final dataset from the phase 3b/4 open-label safety trial of tofacitinib in RA conducted between March 2014 and July 2020, in 345 study locations worldwide.
Study participants were 50 years of age or older with moderate to severe RA who were taking methotrexate but having inadequate symptom control. They had at least one cardiovascular risk factor such as being a current smoker or having hypertension, past heart attack, family history of coronary heart disease, high cholesterol, diabetes mellitus, or extra-articular RA. Patients with current or recent infection, clinically significant laboratory abnormalities, or pregnancy, were excluded from the study.
In the study, 1,455 participants received oral tofacitinib 5 mg twice per day; 1,456 received oral tofacitinib 10 mg twice per day; and 1,451 were treated with subcutaneous TNFi (40 mg subcutaneous adalimumab [Humira] injection every 2 weeks in the United States, Puerto Rico, and Canada; and 50 mg subcutaneous etanercept [Enbrel] injection every week in all other countries. Participants continued their prestudy stable dose of methotrexate if clinically indicated.
The researchers calculated incidence rates and hazard ratios for infections, overall and by age (50-64 years, compared with 65 years and older). They calculated probabilities of infection using Kaplan-Meier estimates and identified infection risk factors through Cox modeling.
They found higher infection rates, serious infection events (SIEs), and nonserious infections (NSIs) with tofacitinib than with TNFi, including:
- Patients taking tofacitinib 5 mg (HR, 1.17; 95% confidence interval, 0.92-1.50) and 10 mg (HR, 1.48; 95% CI, 1.17-1.87) were at greater risk for SIEs.
- Patients older than 65 who were taking tofacitinib 10 mg had increased IRs and HRs for all infections and for SIEs, compared with those aged 50-64.
- The probability of a SIE rose from month 18 onward in participants taking tofacitinib 5 mg, as well as before month 6 in those taking tofacitinib 10 mg.
- In both tofacitinib groups, the probability of NSI increased before month 6.
The most common risk factors for SIEs were higher age, baseline opioid use, history of chronic lung disease, and time-dependent oral corticosteroid use. Risk factors for NSIs were female sex, history of chronic lung disease or infection, history of smoking, as well as time-dependent higher Disease Activity Score in 28 joints and C-reactive protein score.
‘Best information to date’
Michael George, MD, MSCE, assistant professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, welcomed the study’s results.
“This study provides the best information to date on the risk of infection with the JAK inhibitor tofacitinib, compared to a TNF inhibitor,” Dr. George, who was not involved in the study, said in an interview. “It is rare to have such a large randomized trial with an active comparator focused on safety. This is a major strength.
“Being able to quantify the amount of increased risk will help with shared decision-making when counseling patients,” he added.
Dr. George said that, while the small overall risk may not be clinically meaningful for younger, healthier patients, trying biologics such as TNFi before tofacitinib may be optimal for high-risk patients who are older or have comorbidities.
Dr. Bhatt agreed.
“In deciding on appropriate therapies for RA (or other conditions where tofacitinib is used), it is important for the prescribing physician to explain the risks to the patient and weigh them against the potential benefits,” he advised.
Dr. Bhatt noted that increased infection is not the first risk that’s been linked with tofacitinib.
“ORAL Surveillance was designed primarily to assess cardiovascular safety and showed higher rates of cardiovascular events such as myocardial infarction and pulmonary embolism, as well as cancer, with tofacitinib,” he explained.
He recommended further related research.
“Randomized trials are needed to determine the best ways to treat conditions such as RA while trying to minimize cardiovascular, cancer, and infectious risks,” he said.
The study was sponsored by Pfizer. All authors reported financial involvements with Pfizer; most have financial involvements with other pharmaceutical companies as well; four authors are employees of Pfizer and three are also stockholders in the company. Dr. George reported involvements with the pharmaceutical industry.
FROM ANNALS OF THE RHEUMATIC DISEASES
Weight-loss surgery has a big effect on marriage
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
FROM ANNALS OF SURGERY
Young adults who learn how to cook eat more veggies
Obesity remains a significant risk factor for numerous diseases, and is often a problem in young adults, who often fall back on fast food and other less-healthy meals associated with a lower quality diet, lead author Carol S. O’Neal, PhD, of the University of Louisville (Ky.), said in an interview.
Previous research involving Social Cognitive Theory and goal-setting to promote self-efficacy and behavior changes has shown success in improving eating habits in young adults, but adding video technology for an additional education element has not been well studied, Dr. O’Neal and colleagues wrote in the Journal of Nutrition Education and Behavior.
Methods and results
In the study, 138 college students aged 18-40 years participated in a 15-week pilot intervention course at a large, metropolitan university. The course included lectures on a topic, such as carbohydrates, and included skill-based activities, such as how to read an ingredient list, and discussion of how these skills could improve healthier eating and meet nutrition goals, such as eating more whole grains.
A total of 77 completed the study in person, and 61 participated online. The majority (59%) were college sophomores, 74% were White, and 82% were female.
The course engaged the students in weekly food challenges to apply their knowledge and develop better eating habits and behaviors. The challenges were accompanied by cooking videos related to each week’s topic, such as how to make overnight oats for the healthy carbohydrates/whole grains week.
Students also selected two goals each week, such as choosing whole grain foods to increase fiber consumption, from a list of 10-15 goals, and were required to write weekly reflections to track their progress toward these goals. Goal-setting was based on the strategy of creating goals that are specific, measurable, attainable, realistic, and time-bound (the SMART method).
The main outcomes were increased consumption of fruits and vegetables, improved skills in cooking and healthy eating, and improved attitudes about healthy cooking and eating. The researchers surveyed the students to determine whether these outcomes were met.
Students participating in the study indicated that they met the goal of eating at least five servings of fruits and vegetables per day more often after the course than before, the researchers wrote.
By the course’s end, the students showed significant increases in consumption of fruits and vegetables (P < .001 for both), and in the self-efficacy related to consumption of produce (P = .004); cooking (P = .002;, and using more fruits, vegetables, and seasonings rather than salt in cooking (P = .001).
A review of the students’ written reflections illustrated positive behavior changes such as planning meals before shopping, preparing meals in advance on weekends, taking lunch to school, and using herbs and spices, the researchers noted.
“Self-directed SMART goals set you up for success by making goals specific, measurable, achievable, realistic, and timely,” Dr. O’Neal said in an interview. “The SMART method helps push you further, gives you a sense of direction, and helps you organize and reach your goals,” but self-monitoring and social support are also needed for success. The takeaway message for clinicians is that use of a self-directed goal-setting strategy may be more effective at changing dietary behaviors and promoting self-efficacy than a traditional dietary prescription.
In addition, “this model could be used to address a variety of health outcomes in dietetics, health education and community health programs,” said Dr. O’Neal. “I think the key components of this intervention are teaching SMART goal setting, self-monitoring, and social support of successes. I see time as a main barrier, but this barrier could be reduced for populations who are able to use online learning. Our intervention was successful for in-person and online learning.”
Other areas for future research include evaluation of progress that combines quantitative data and qualitative reflections, she said.
Real-world applications
“Clinicians have limited time to address behavioral counseling, and this study offers an opportunity to reach patients not only in class sessions, but virtually,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
Although the findings from the study are not new, the knowledge can be used by clinicians to help promote behavior change. The study also showcased the use of additional tools, such as weekly food challenges, to impact college students who often consume high-fat diets in nonmedical settings, Dr. Jay said.
For consumers, the real-world implications are exciting, Dr. Jay said.
“People are increasingly attempting to “eat healthy” and despite clinicians wanting to impact healthy eating, limited office visits may not be conducive to behavioral change,” she said.
The current study was important as a way to identify tactics to improve the diet and nutrition of young adults, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the University of Wisconsin–Madison, said in an interview.
The study findings of increased fruit and vegetable consumption were not surprising, as the study population may have been more highly motivated to improve their diets, Dr. Thew said. However, she was surprised to see the significant improvement in cooking attitudes and cooking self-efficiency after the intervention. “This tells me that we need to offer more opportunities to educate young adults on how to cook to improve diet outcomes.”
The message for clinicians is to encourage and support young adults to learn cooking skills to promote healthier eating, said Dr. Thew.
“When patients have confidence in their ability to cook, they will explore more food options and consequently improve their diets,” she emphasized. “As clinicians, we need to advocate for nutrition education and promote cooking classes that are accessible to all if we hope to reduce obesity and improve our patients’ diets.”
Limitations
The study findings were limited by several factors including the use of a convenience sample that might not represent all college students, the reliance on self-reports, the inability to account for the impact of demographic factors, and the lack of a control group, the researchers wrote.
“Larger prospective studies are needed,” given the limitations of the pilot design and short study period, Dr. Jay noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Jay and Dr. Thew had no financial conflicts to disclose.
Obesity remains a significant risk factor for numerous diseases, and is often a problem in young adults, who often fall back on fast food and other less-healthy meals associated with a lower quality diet, lead author Carol S. O’Neal, PhD, of the University of Louisville (Ky.), said in an interview.
Previous research involving Social Cognitive Theory and goal-setting to promote self-efficacy and behavior changes has shown success in improving eating habits in young adults, but adding video technology for an additional education element has not been well studied, Dr. O’Neal and colleagues wrote in the Journal of Nutrition Education and Behavior.
Methods and results
In the study, 138 college students aged 18-40 years participated in a 15-week pilot intervention course at a large, metropolitan university. The course included lectures on a topic, such as carbohydrates, and included skill-based activities, such as how to read an ingredient list, and discussion of how these skills could improve healthier eating and meet nutrition goals, such as eating more whole grains.
A total of 77 completed the study in person, and 61 participated online. The majority (59%) were college sophomores, 74% were White, and 82% were female.
The course engaged the students in weekly food challenges to apply their knowledge and develop better eating habits and behaviors. The challenges were accompanied by cooking videos related to each week’s topic, such as how to make overnight oats for the healthy carbohydrates/whole grains week.
Students also selected two goals each week, such as choosing whole grain foods to increase fiber consumption, from a list of 10-15 goals, and were required to write weekly reflections to track their progress toward these goals. Goal-setting was based on the strategy of creating goals that are specific, measurable, attainable, realistic, and time-bound (the SMART method).
The main outcomes were increased consumption of fruits and vegetables, improved skills in cooking and healthy eating, and improved attitudes about healthy cooking and eating. The researchers surveyed the students to determine whether these outcomes were met.
Students participating in the study indicated that they met the goal of eating at least five servings of fruits and vegetables per day more often after the course than before, the researchers wrote.
By the course’s end, the students showed significant increases in consumption of fruits and vegetables (P < .001 for both), and in the self-efficacy related to consumption of produce (P = .004); cooking (P = .002;, and using more fruits, vegetables, and seasonings rather than salt in cooking (P = .001).
A review of the students’ written reflections illustrated positive behavior changes such as planning meals before shopping, preparing meals in advance on weekends, taking lunch to school, and using herbs and spices, the researchers noted.
“Self-directed SMART goals set you up for success by making goals specific, measurable, achievable, realistic, and timely,” Dr. O’Neal said in an interview. “The SMART method helps push you further, gives you a sense of direction, and helps you organize and reach your goals,” but self-monitoring and social support are also needed for success. The takeaway message for clinicians is that use of a self-directed goal-setting strategy may be more effective at changing dietary behaviors and promoting self-efficacy than a traditional dietary prescription.
In addition, “this model could be used to address a variety of health outcomes in dietetics, health education and community health programs,” said Dr. O’Neal. “I think the key components of this intervention are teaching SMART goal setting, self-monitoring, and social support of successes. I see time as a main barrier, but this barrier could be reduced for populations who are able to use online learning. Our intervention was successful for in-person and online learning.”
Other areas for future research include evaluation of progress that combines quantitative data and qualitative reflections, she said.
Real-world applications
“Clinicians have limited time to address behavioral counseling, and this study offers an opportunity to reach patients not only in class sessions, but virtually,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
Although the findings from the study are not new, the knowledge can be used by clinicians to help promote behavior change. The study also showcased the use of additional tools, such as weekly food challenges, to impact college students who often consume high-fat diets in nonmedical settings, Dr. Jay said.
For consumers, the real-world implications are exciting, Dr. Jay said.
“People are increasingly attempting to “eat healthy” and despite clinicians wanting to impact healthy eating, limited office visits may not be conducive to behavioral change,” she said.
The current study was important as a way to identify tactics to improve the diet and nutrition of young adults, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the University of Wisconsin–Madison, said in an interview.
The study findings of increased fruit and vegetable consumption were not surprising, as the study population may have been more highly motivated to improve their diets, Dr. Thew said. However, she was surprised to see the significant improvement in cooking attitudes and cooking self-efficiency after the intervention. “This tells me that we need to offer more opportunities to educate young adults on how to cook to improve diet outcomes.”
The message for clinicians is to encourage and support young adults to learn cooking skills to promote healthier eating, said Dr. Thew.
“When patients have confidence in their ability to cook, they will explore more food options and consequently improve their diets,” she emphasized. “As clinicians, we need to advocate for nutrition education and promote cooking classes that are accessible to all if we hope to reduce obesity and improve our patients’ diets.”
Limitations
The study findings were limited by several factors including the use of a convenience sample that might not represent all college students, the reliance on self-reports, the inability to account for the impact of demographic factors, and the lack of a control group, the researchers wrote.
“Larger prospective studies are needed,” given the limitations of the pilot design and short study period, Dr. Jay noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Jay and Dr. Thew had no financial conflicts to disclose.
Obesity remains a significant risk factor for numerous diseases, and is often a problem in young adults, who often fall back on fast food and other less-healthy meals associated with a lower quality diet, lead author Carol S. O’Neal, PhD, of the University of Louisville (Ky.), said in an interview.
Previous research involving Social Cognitive Theory and goal-setting to promote self-efficacy and behavior changes has shown success in improving eating habits in young adults, but adding video technology for an additional education element has not been well studied, Dr. O’Neal and colleagues wrote in the Journal of Nutrition Education and Behavior.
Methods and results
In the study, 138 college students aged 18-40 years participated in a 15-week pilot intervention course at a large, metropolitan university. The course included lectures on a topic, such as carbohydrates, and included skill-based activities, such as how to read an ingredient list, and discussion of how these skills could improve healthier eating and meet nutrition goals, such as eating more whole grains.
A total of 77 completed the study in person, and 61 participated online. The majority (59%) were college sophomores, 74% were White, and 82% were female.
The course engaged the students in weekly food challenges to apply their knowledge and develop better eating habits and behaviors. The challenges were accompanied by cooking videos related to each week’s topic, such as how to make overnight oats for the healthy carbohydrates/whole grains week.
Students also selected two goals each week, such as choosing whole grain foods to increase fiber consumption, from a list of 10-15 goals, and were required to write weekly reflections to track their progress toward these goals. Goal-setting was based on the strategy of creating goals that are specific, measurable, attainable, realistic, and time-bound (the SMART method).
The main outcomes were increased consumption of fruits and vegetables, improved skills in cooking and healthy eating, and improved attitudes about healthy cooking and eating. The researchers surveyed the students to determine whether these outcomes were met.
Students participating in the study indicated that they met the goal of eating at least five servings of fruits and vegetables per day more often after the course than before, the researchers wrote.
By the course’s end, the students showed significant increases in consumption of fruits and vegetables (P < .001 for both), and in the self-efficacy related to consumption of produce (P = .004); cooking (P = .002;, and using more fruits, vegetables, and seasonings rather than salt in cooking (P = .001).
A review of the students’ written reflections illustrated positive behavior changes such as planning meals before shopping, preparing meals in advance on weekends, taking lunch to school, and using herbs and spices, the researchers noted.
“Self-directed SMART goals set you up for success by making goals specific, measurable, achievable, realistic, and timely,” Dr. O’Neal said in an interview. “The SMART method helps push you further, gives you a sense of direction, and helps you organize and reach your goals,” but self-monitoring and social support are also needed for success. The takeaway message for clinicians is that use of a self-directed goal-setting strategy may be more effective at changing dietary behaviors and promoting self-efficacy than a traditional dietary prescription.
In addition, “this model could be used to address a variety of health outcomes in dietetics, health education and community health programs,” said Dr. O’Neal. “I think the key components of this intervention are teaching SMART goal setting, self-monitoring, and social support of successes. I see time as a main barrier, but this barrier could be reduced for populations who are able to use online learning. Our intervention was successful for in-person and online learning.”
Other areas for future research include evaluation of progress that combines quantitative data and qualitative reflections, she said.
Real-world applications
“Clinicians have limited time to address behavioral counseling, and this study offers an opportunity to reach patients not only in class sessions, but virtually,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
Although the findings from the study are not new, the knowledge can be used by clinicians to help promote behavior change. The study also showcased the use of additional tools, such as weekly food challenges, to impact college students who often consume high-fat diets in nonmedical settings, Dr. Jay said.
For consumers, the real-world implications are exciting, Dr. Jay said.
“People are increasingly attempting to “eat healthy” and despite clinicians wanting to impact healthy eating, limited office visits may not be conducive to behavioral change,” she said.
The current study was important as a way to identify tactics to improve the diet and nutrition of young adults, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the University of Wisconsin–Madison, said in an interview.
The study findings of increased fruit and vegetable consumption were not surprising, as the study population may have been more highly motivated to improve their diets, Dr. Thew said. However, she was surprised to see the significant improvement in cooking attitudes and cooking self-efficiency after the intervention. “This tells me that we need to offer more opportunities to educate young adults on how to cook to improve diet outcomes.”
The message for clinicians is to encourage and support young adults to learn cooking skills to promote healthier eating, said Dr. Thew.
“When patients have confidence in their ability to cook, they will explore more food options and consequently improve their diets,” she emphasized. “As clinicians, we need to advocate for nutrition education and promote cooking classes that are accessible to all if we hope to reduce obesity and improve our patients’ diets.”
Limitations
The study findings were limited by several factors including the use of a convenience sample that might not represent all college students, the reliance on self-reports, the inability to account for the impact of demographic factors, and the lack of a control group, the researchers wrote.
“Larger prospective studies are needed,” given the limitations of the pilot design and short study period, Dr. Jay noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Jay and Dr. Thew had no financial conflicts to disclose.
FROM THE JOURNAL OF NUTRITION EDUCATION AND BEHAVIOR
Federal Health Care Data Trends 2022
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Regular fasting linked to less severe COVID: Study
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
FROM BMJ NUTRITION, PREVENTION & HEALTH