User login
CT scan changes indicate increased mortality risk in ever-smokers
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
FROM CHEST
Climate change can worsen more than half of infectious diseases
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Severe illness rising as vaccination effort stalls
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
ORATOR2 mute on best de-escalation therapy for low-risk HPV+ oropharynx cancers
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
FROM JAMA ONCOLOGY
Drug-resistant epilepsy needs earlier surgical referral
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
FROM EPILEPSIA
Funding of cosmetic clinical trials linked to racial/ethnic disparity
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.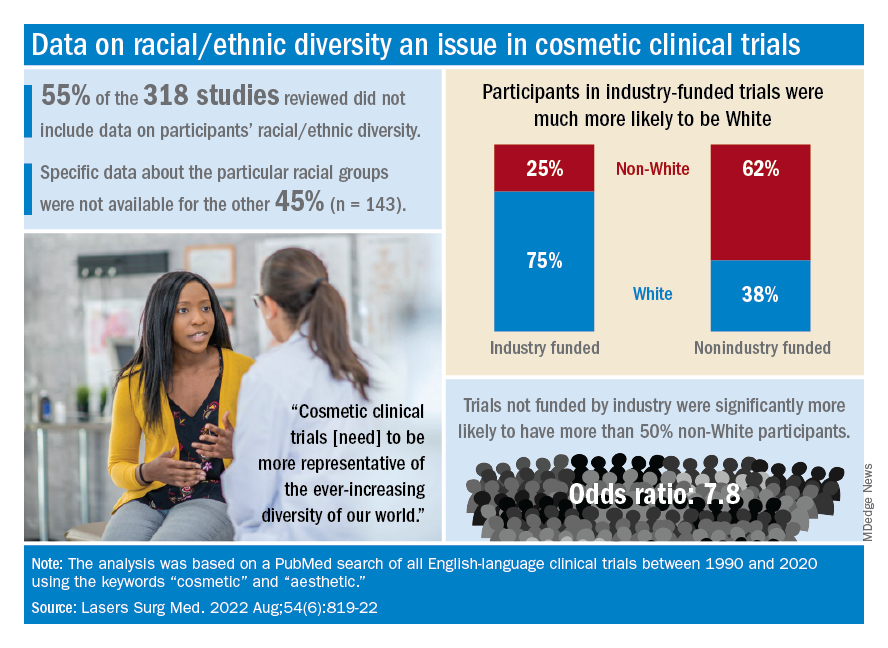
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
FROM LASERS IN SURGERY AND MEDICINE
Vaginal birth possible in 50% of women with low-lying placenta
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Vonoprazan-based therapy for resistant H. pylori superior to standard care
A look at the data behind the FDA approval
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
A look at the data behind the FDA approval
A look at the data behind the FDA approval
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
FROM GASTROENTEROLOGY
Jury out on synbiotics for kids with GI disorders
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION
Genetic counseling for cancer often costs patients nothing
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM

