User login
Dermatologist arrested for allegedly poisoning radiologist husband
It is a story that has quickly gone viral around the world:
Yue Yu, MD, aged 45, was booked into the Orange County Jail on Aug. 4, after Irvine Police had been called to her residence that day by her husband, Jack Chen, MD, 53, a radiologist. Dr. Chen provided the police with video evidence that he said showed Dr. Yu pouring a drain-opening chemical into his hot lemonade drink.
“The victim sustained significant internal injuries but is expected to recover,” the Irvine police department said in a statement.
Dr. Yu was released after paying a $30,000 bond and has not been formally charged, according to the New York Post.
In a statement to the court on Aug. 5, Dr. Chen said he and the couple’s two children had long suffered verbal abuse from his wife and her mother, according to the Post. Multiple news organizations reported that Dr. Chen filed for divorce and also for a restraining order against Dr. Yu on that day.
After feeling ill for months – and being diagnosed with ulcers and esophageal inflammation – Dr. Chen reportedly set up video cameras in the couple’s house. He said he caught Dr. Yu on camera pouring something into his drink on several occasions in July.
According to NBC News, Dr. Yu’s attorney, David E. Wohl, said that Dr. Yu “vehemently and unequivocally denies ever attempting to poison her husband or anyone else.”
Dr. Yu received her medical degree from Washington University in St. Louis in 2006 and has no disciplinary actions against her, according to the Medical Board of California. She was head of dermatology at Mission Heritage Medical Group, but her name and information have been scrubbed from that group’s website. Mission Heritage is affiliated with Providence Mission Hospital. A spokesperson for the hospital told NBC News that it is cooperating with the police investigation and that no patients are in danger.
The dermatologist is due to report back to court in November, NBC News said.
A version of this article first appeared on Medscape.com.
It is a story that has quickly gone viral around the world:
Yue Yu, MD, aged 45, was booked into the Orange County Jail on Aug. 4, after Irvine Police had been called to her residence that day by her husband, Jack Chen, MD, 53, a radiologist. Dr. Chen provided the police with video evidence that he said showed Dr. Yu pouring a drain-opening chemical into his hot lemonade drink.
“The victim sustained significant internal injuries but is expected to recover,” the Irvine police department said in a statement.
Dr. Yu was released after paying a $30,000 bond and has not been formally charged, according to the New York Post.
In a statement to the court on Aug. 5, Dr. Chen said he and the couple’s two children had long suffered verbal abuse from his wife and her mother, according to the Post. Multiple news organizations reported that Dr. Chen filed for divorce and also for a restraining order against Dr. Yu on that day.
After feeling ill for months – and being diagnosed with ulcers and esophageal inflammation – Dr. Chen reportedly set up video cameras in the couple’s house. He said he caught Dr. Yu on camera pouring something into his drink on several occasions in July.
According to NBC News, Dr. Yu’s attorney, David E. Wohl, said that Dr. Yu “vehemently and unequivocally denies ever attempting to poison her husband or anyone else.”
Dr. Yu received her medical degree from Washington University in St. Louis in 2006 and has no disciplinary actions against her, according to the Medical Board of California. She was head of dermatology at Mission Heritage Medical Group, but her name and information have been scrubbed from that group’s website. Mission Heritage is affiliated with Providence Mission Hospital. A spokesperson for the hospital told NBC News that it is cooperating with the police investigation and that no patients are in danger.
The dermatologist is due to report back to court in November, NBC News said.
A version of this article first appeared on Medscape.com.
It is a story that has quickly gone viral around the world:
Yue Yu, MD, aged 45, was booked into the Orange County Jail on Aug. 4, after Irvine Police had been called to her residence that day by her husband, Jack Chen, MD, 53, a radiologist. Dr. Chen provided the police with video evidence that he said showed Dr. Yu pouring a drain-opening chemical into his hot lemonade drink.
“The victim sustained significant internal injuries but is expected to recover,” the Irvine police department said in a statement.
Dr. Yu was released after paying a $30,000 bond and has not been formally charged, according to the New York Post.
In a statement to the court on Aug. 5, Dr. Chen said he and the couple’s two children had long suffered verbal abuse from his wife and her mother, according to the Post. Multiple news organizations reported that Dr. Chen filed for divorce and also for a restraining order against Dr. Yu on that day.
After feeling ill for months – and being diagnosed with ulcers and esophageal inflammation – Dr. Chen reportedly set up video cameras in the couple’s house. He said he caught Dr. Yu on camera pouring something into his drink on several occasions in July.
According to NBC News, Dr. Yu’s attorney, David E. Wohl, said that Dr. Yu “vehemently and unequivocally denies ever attempting to poison her husband or anyone else.”
Dr. Yu received her medical degree from Washington University in St. Louis in 2006 and has no disciplinary actions against her, according to the Medical Board of California. She was head of dermatology at Mission Heritage Medical Group, but her name and information have been scrubbed from that group’s website. Mission Heritage is affiliated with Providence Mission Hospital. A spokesperson for the hospital told NBC News that it is cooperating with the police investigation and that no patients are in danger.
The dermatologist is due to report back to court in November, NBC News said.
A version of this article first appeared on Medscape.com.
Long COVID’s grip will likely tighten as infections continue
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
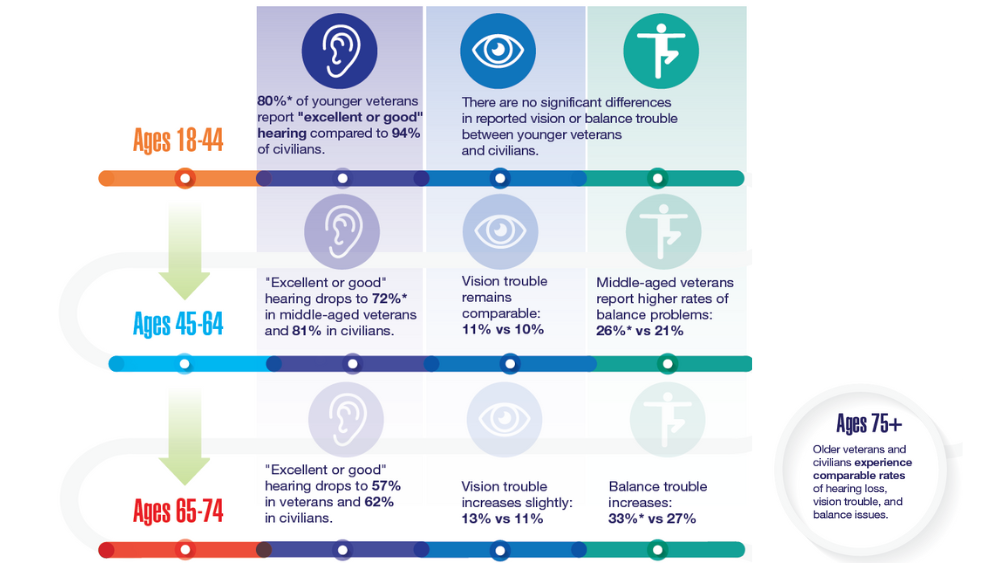
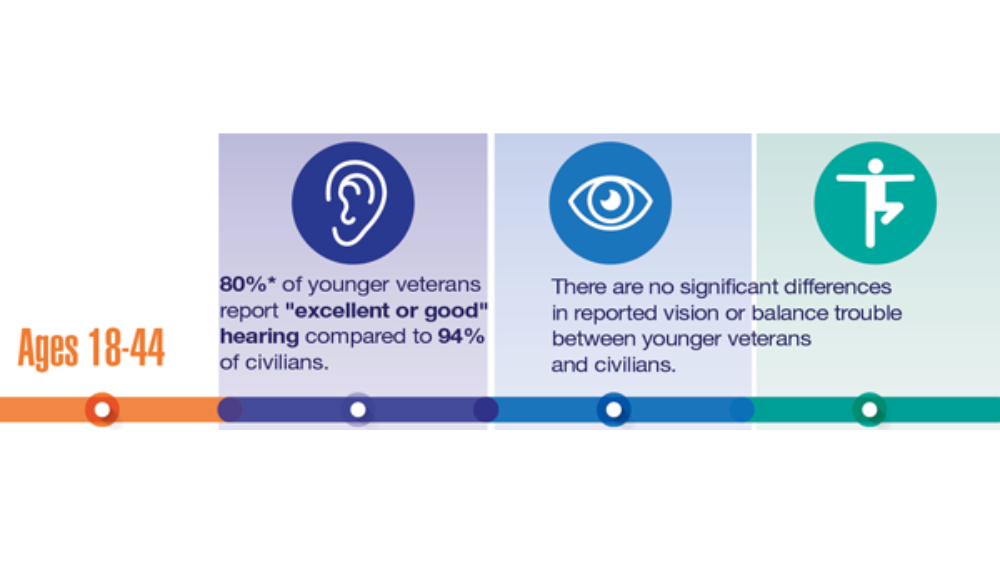
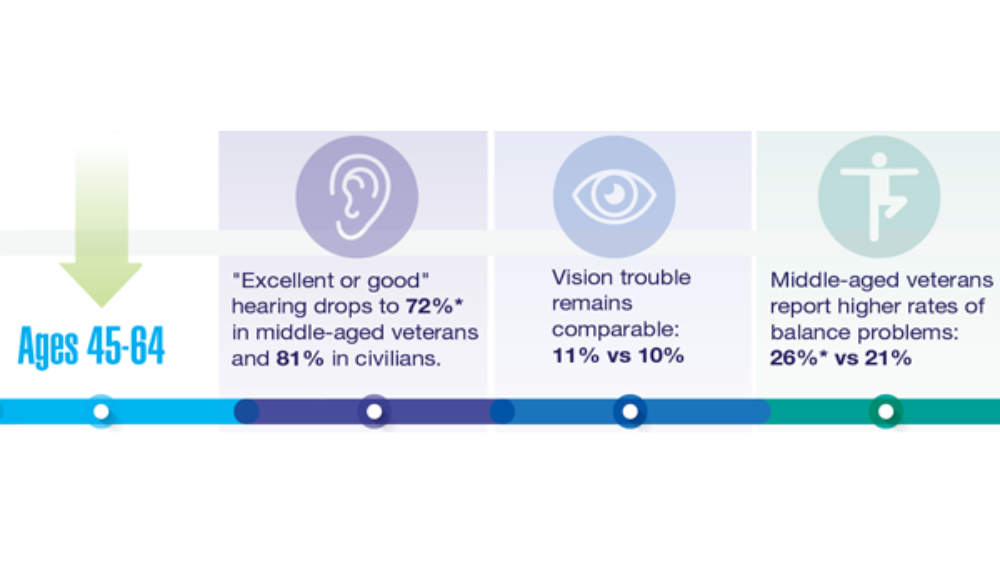
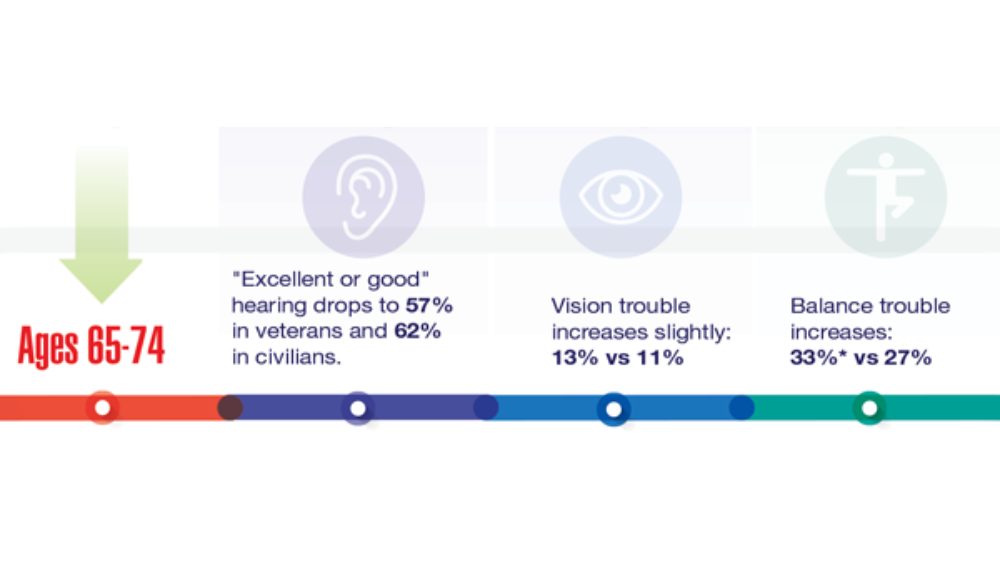
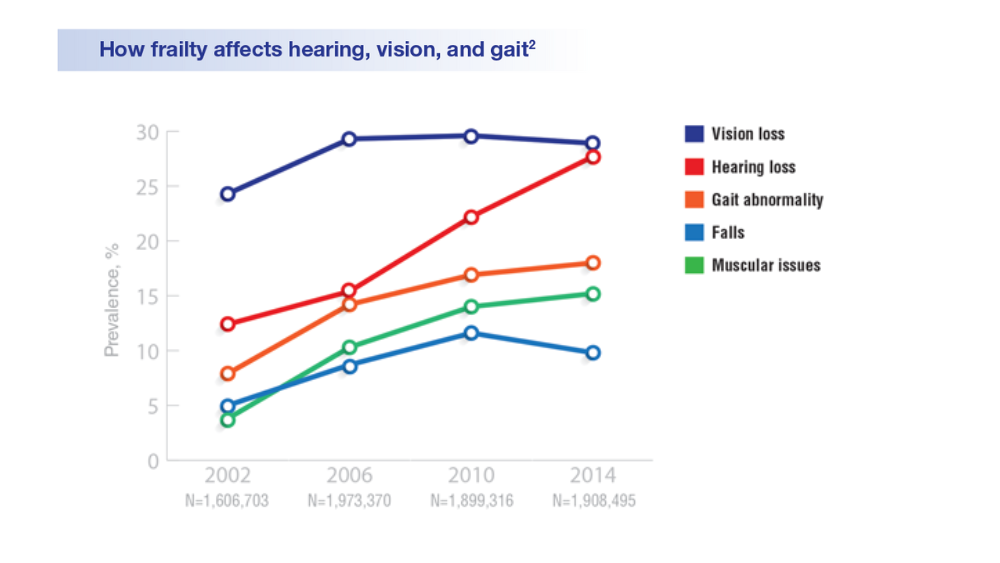
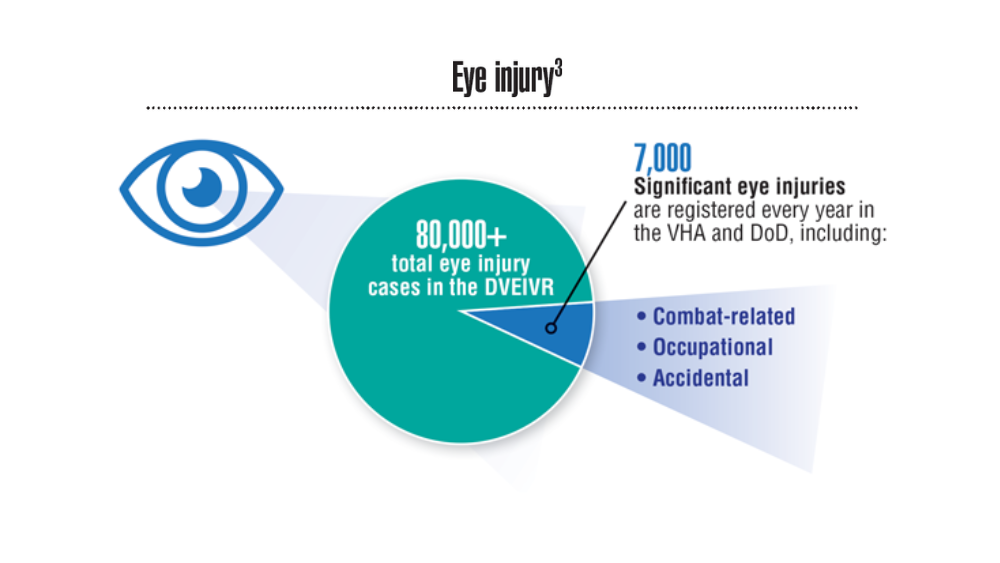
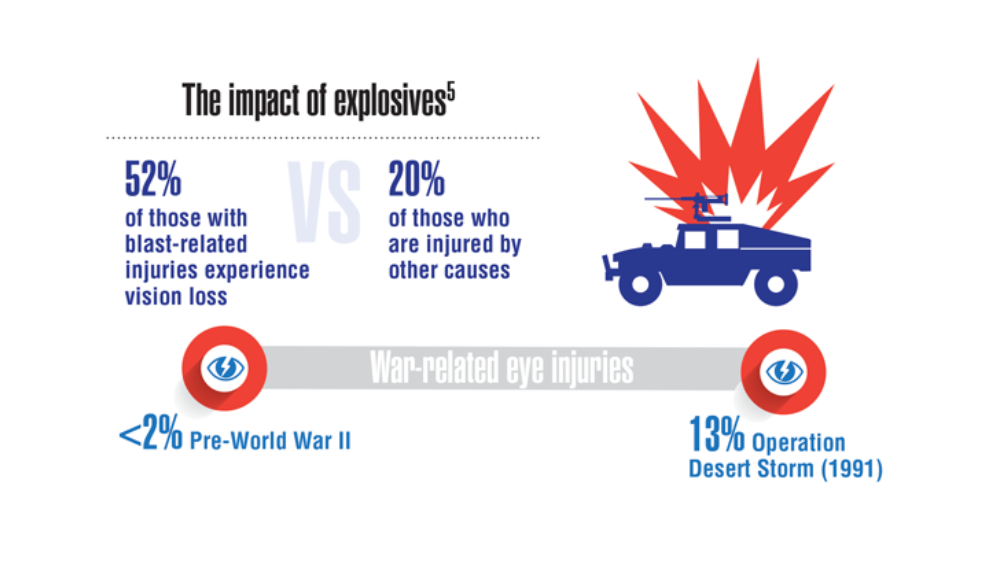
Federal Health Care Data Trends 2022: Hearing, Vision, and Balance
- Lucas JW, Zelaya CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Natl Health Stat Report. 2020;(142):1-8. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
- Shrauner W, Lord EM, Nguyen XMT, et al. Frailty and cardiovascular mortality in more than 3 million US veterans. Eur Heart J. 2022;43(8):818-826. https://doi.org/10.1093/eurheartj/ehab850
- Defense Health Agency, Vision Center of Excellence (email, March 23, 2022).
- Frick KD, Singman EL. Cost of military eye injury and vision impairment related to traumatic brain injury: 2001–2017. Mil Med. 2019;184(5-6):e338. http://doi.org/10.1093/milmed/usy420
- Hussain SF, Raza Z, Cash ATG, et al. Traumatic brain injury and sight loss in military and veteran populations – a review. Mil Med Res. 2021;8(1):42. http://doi.org/10.1186/s40779-021-00334-3
- Lucas JW, Zelaya CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Natl Health Stat Report. 2020;(142):1-8. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
- Shrauner W, Lord EM, Nguyen XMT, et al. Frailty and cardiovascular mortality in more than 3 million US veterans. Eur Heart J. 2022;43(8):818-826. https://doi.org/10.1093/eurheartj/ehab850
- Defense Health Agency, Vision Center of Excellence (email, March 23, 2022).
- Frick KD, Singman EL. Cost of military eye injury and vision impairment related to traumatic brain injury: 2001–2017. Mil Med. 2019;184(5-6):e338. http://doi.org/10.1093/milmed/usy420
- Hussain SF, Raza Z, Cash ATG, et al. Traumatic brain injury and sight loss in military and veteran populations – a review. Mil Med Res. 2021;8(1):42. http://doi.org/10.1186/s40779-021-00334-3
- Lucas JW, Zelaya CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Natl Health Stat Report. 2020;(142):1-8. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
- Shrauner W, Lord EM, Nguyen XMT, et al. Frailty and cardiovascular mortality in more than 3 million US veterans. Eur Heart J. 2022;43(8):818-826. https://doi.org/10.1093/eurheartj/ehab850
- Defense Health Agency, Vision Center of Excellence (email, March 23, 2022).
- Frick KD, Singman EL. Cost of military eye injury and vision impairment related to traumatic brain injury: 2001–2017. Mil Med. 2019;184(5-6):e338. http://doi.org/10.1093/milmed/usy420
- Hussain SF, Raza Z, Cash ATG, et al. Traumatic brain injury and sight loss in military and veteran populations – a review. Mil Med Res. 2021;8(1):42. http://doi.org/10.1186/s40779-021-00334-3
‘Shocking’ and persistent gap in treatment for opioid addiction
The vast majority of Americans with opioid use disorder (OUD) do not receive potentially lifesaving medications.
Drugs such as methadone, buprenorphine, and extended-release naltrexone have been shown to reduce opioid overdoses by more than 50%. Yet a new analysis shows that only about 1 in 10 people living with OUD receive these medications.
“Even though it’s not especially surprising, it’s still disturbing and shocking in a way that we have just made such little progress on this huge issue,” study investigator Noa Krawczyk, PhD, with the Center for Opioid Epidemiology and Policy, department of population health, NYU Langone, told this news organization.
The study was published online in the International Journal of Drug Policy.
Increased urgency
Despite efforts to increase capacity for OUD treatment in the United States, how receipt of treatment compares to need for treatment remains unclear.
Dr. Krawczyk and colleagues examined the gap between new estimates of OUD prevalence and treatment at the national and state levels from 2010 through 2019.
“,” the investigators write.
Adjusted estimates suggest that past-year OUD affected roughly 7.63 million individuals in the United States (2,773 per 100,000), yet only about 1.02 million received medication (365 per 100,000), they note.
Overall, there was a 106% increase in receipt of medications for OUD across the United States from 2010 to 2019 and a 5% increase from 2018 to 2019.
Yet, as of 2019, 87% of people with OUD were not receiving medication.
“While the number of people getting treatment doubled over the last decade, it’s nowhere near the amount of people who are still struggling with an opioid use disorder, and the urgency of the problem has become much worse because of the worsening fentanyl crisis and the lethality of the drug supply,” said Dr. Krawczyk.
The study also showed wide variation in past-year OUD prevalence and treatment across the United States.
Past-year OUD rates were highest in Washington, D.C., and lowest in Minnesota. Receipt of treatment was lowest in South Dakota and highest in Vermont.
However, in all 50 states and Washington, D.C., past-year OUD prevalence was greater than rates of medication use. As of 2019, the largest treatment gaps were in Iowa, North Dakota, and Washington, D.C. The smallest treatment gaps were in Connecticut, Maryland, and Rhode Island.
Long road ahead
“Even in states with the smallest treatment gaps, at least 50% of people who could benefit from medications for opioid use disorder are still not receiving them,” senior author Magdalena Cerdá, DrPH, director of the Center for Opioid Epidemiology and Policy in the department of population health at NYU Langone Health, said in a statement.
“We have a long way to go in reducing stigma surrounding treatment and in devising the types of policies and programs we need to ensure these medications reach the people who need them the most,” Dr. Cerdá added.
Access to OUD treatment is an ongoing problem in the United States.
“A lot of areas don’t have specialty treatment programs that provide methadone, or they might not have addiction-trained providers who are willing to prescribe buprenorphine or have a waiver to prescribe buprenorphine, so a lot places are really struggling with where people can get treatment,” said Dr. Krawczyk.
Recent data show that 46% of counties lack an OUD medication provider, and 32% have no specialty programs to treat substance use disorders.
Dr. Krawczyk and colleagues note that COVID-19–related policy changes and recently proposed legislation to allow more flexible and convenient access to OUD treatment may be a first step toward expanding access to this lifesaving treatment.
But improving initial access to medication for OUD is “only the first step – our research and health systems have a long way to go in addressing the needs of people with OUD to support retention in treatment and services to effectively reduce overdose and improve long-term health and well-being,” the researchers write.
The study was supported by the NYU Center for Epidemiology and Policy. Dr. Krawczyk has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vast majority of Americans with opioid use disorder (OUD) do not receive potentially lifesaving medications.
Drugs such as methadone, buprenorphine, and extended-release naltrexone have been shown to reduce opioid overdoses by more than 50%. Yet a new analysis shows that only about 1 in 10 people living with OUD receive these medications.
“Even though it’s not especially surprising, it’s still disturbing and shocking in a way that we have just made such little progress on this huge issue,” study investigator Noa Krawczyk, PhD, with the Center for Opioid Epidemiology and Policy, department of population health, NYU Langone, told this news organization.
The study was published online in the International Journal of Drug Policy.
Increased urgency
Despite efforts to increase capacity for OUD treatment in the United States, how receipt of treatment compares to need for treatment remains unclear.
Dr. Krawczyk and colleagues examined the gap between new estimates of OUD prevalence and treatment at the national and state levels from 2010 through 2019.
“,” the investigators write.
Adjusted estimates suggest that past-year OUD affected roughly 7.63 million individuals in the United States (2,773 per 100,000), yet only about 1.02 million received medication (365 per 100,000), they note.
Overall, there was a 106% increase in receipt of medications for OUD across the United States from 2010 to 2019 and a 5% increase from 2018 to 2019.
Yet, as of 2019, 87% of people with OUD were not receiving medication.
“While the number of people getting treatment doubled over the last decade, it’s nowhere near the amount of people who are still struggling with an opioid use disorder, and the urgency of the problem has become much worse because of the worsening fentanyl crisis and the lethality of the drug supply,” said Dr. Krawczyk.
The study also showed wide variation in past-year OUD prevalence and treatment across the United States.
Past-year OUD rates were highest in Washington, D.C., and lowest in Minnesota. Receipt of treatment was lowest in South Dakota and highest in Vermont.
However, in all 50 states and Washington, D.C., past-year OUD prevalence was greater than rates of medication use. As of 2019, the largest treatment gaps were in Iowa, North Dakota, and Washington, D.C. The smallest treatment gaps were in Connecticut, Maryland, and Rhode Island.
Long road ahead
“Even in states with the smallest treatment gaps, at least 50% of people who could benefit from medications for opioid use disorder are still not receiving them,” senior author Magdalena Cerdá, DrPH, director of the Center for Opioid Epidemiology and Policy in the department of population health at NYU Langone Health, said in a statement.
“We have a long way to go in reducing stigma surrounding treatment and in devising the types of policies and programs we need to ensure these medications reach the people who need them the most,” Dr. Cerdá added.
Access to OUD treatment is an ongoing problem in the United States.
“A lot of areas don’t have specialty treatment programs that provide methadone, or they might not have addiction-trained providers who are willing to prescribe buprenorphine or have a waiver to prescribe buprenorphine, so a lot places are really struggling with where people can get treatment,” said Dr. Krawczyk.
Recent data show that 46% of counties lack an OUD medication provider, and 32% have no specialty programs to treat substance use disorders.
Dr. Krawczyk and colleagues note that COVID-19–related policy changes and recently proposed legislation to allow more flexible and convenient access to OUD treatment may be a first step toward expanding access to this lifesaving treatment.
But improving initial access to medication for OUD is “only the first step – our research and health systems have a long way to go in addressing the needs of people with OUD to support retention in treatment and services to effectively reduce overdose and improve long-term health and well-being,” the researchers write.
The study was supported by the NYU Center for Epidemiology and Policy. Dr. Krawczyk has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vast majority of Americans with opioid use disorder (OUD) do not receive potentially lifesaving medications.
Drugs such as methadone, buprenorphine, and extended-release naltrexone have been shown to reduce opioid overdoses by more than 50%. Yet a new analysis shows that only about 1 in 10 people living with OUD receive these medications.
“Even though it’s not especially surprising, it’s still disturbing and shocking in a way that we have just made such little progress on this huge issue,” study investigator Noa Krawczyk, PhD, with the Center for Opioid Epidemiology and Policy, department of population health, NYU Langone, told this news organization.
The study was published online in the International Journal of Drug Policy.
Increased urgency
Despite efforts to increase capacity for OUD treatment in the United States, how receipt of treatment compares to need for treatment remains unclear.
Dr. Krawczyk and colleagues examined the gap between new estimates of OUD prevalence and treatment at the national and state levels from 2010 through 2019.
“,” the investigators write.
Adjusted estimates suggest that past-year OUD affected roughly 7.63 million individuals in the United States (2,773 per 100,000), yet only about 1.02 million received medication (365 per 100,000), they note.
Overall, there was a 106% increase in receipt of medications for OUD across the United States from 2010 to 2019 and a 5% increase from 2018 to 2019.
Yet, as of 2019, 87% of people with OUD were not receiving medication.
“While the number of people getting treatment doubled over the last decade, it’s nowhere near the amount of people who are still struggling with an opioid use disorder, and the urgency of the problem has become much worse because of the worsening fentanyl crisis and the lethality of the drug supply,” said Dr. Krawczyk.
The study also showed wide variation in past-year OUD prevalence and treatment across the United States.
Past-year OUD rates were highest in Washington, D.C., and lowest in Minnesota. Receipt of treatment was lowest in South Dakota and highest in Vermont.
However, in all 50 states and Washington, D.C., past-year OUD prevalence was greater than rates of medication use. As of 2019, the largest treatment gaps were in Iowa, North Dakota, and Washington, D.C. The smallest treatment gaps were in Connecticut, Maryland, and Rhode Island.
Long road ahead
“Even in states with the smallest treatment gaps, at least 50% of people who could benefit from medications for opioid use disorder are still not receiving them,” senior author Magdalena Cerdá, DrPH, director of the Center for Opioid Epidemiology and Policy in the department of population health at NYU Langone Health, said in a statement.
“We have a long way to go in reducing stigma surrounding treatment and in devising the types of policies and programs we need to ensure these medications reach the people who need them the most,” Dr. Cerdá added.
Access to OUD treatment is an ongoing problem in the United States.
“A lot of areas don’t have specialty treatment programs that provide methadone, or they might not have addiction-trained providers who are willing to prescribe buprenorphine or have a waiver to prescribe buprenorphine, so a lot places are really struggling with where people can get treatment,” said Dr. Krawczyk.
Recent data show that 46% of counties lack an OUD medication provider, and 32% have no specialty programs to treat substance use disorders.
Dr. Krawczyk and colleagues note that COVID-19–related policy changes and recently proposed legislation to allow more flexible and convenient access to OUD treatment may be a first step toward expanding access to this lifesaving treatment.
But improving initial access to medication for OUD is “only the first step – our research and health systems have a long way to go in addressing the needs of people with OUD to support retention in treatment and services to effectively reduce overdose and improve long-term health and well-being,” the researchers write.
The study was supported by the NYU Center for Epidemiology and Policy. Dr. Krawczyk has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF DRUG POLICY
Pro-life ob.gyns. say Dobbs not end of abortion struggle
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
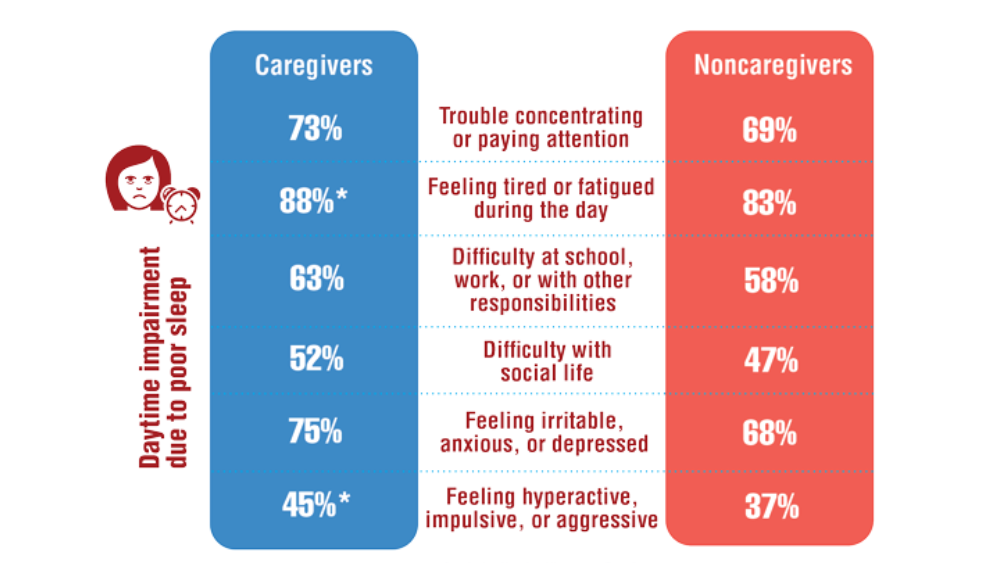
Federal Health Care Data Trends 2022: Sleep Disorders
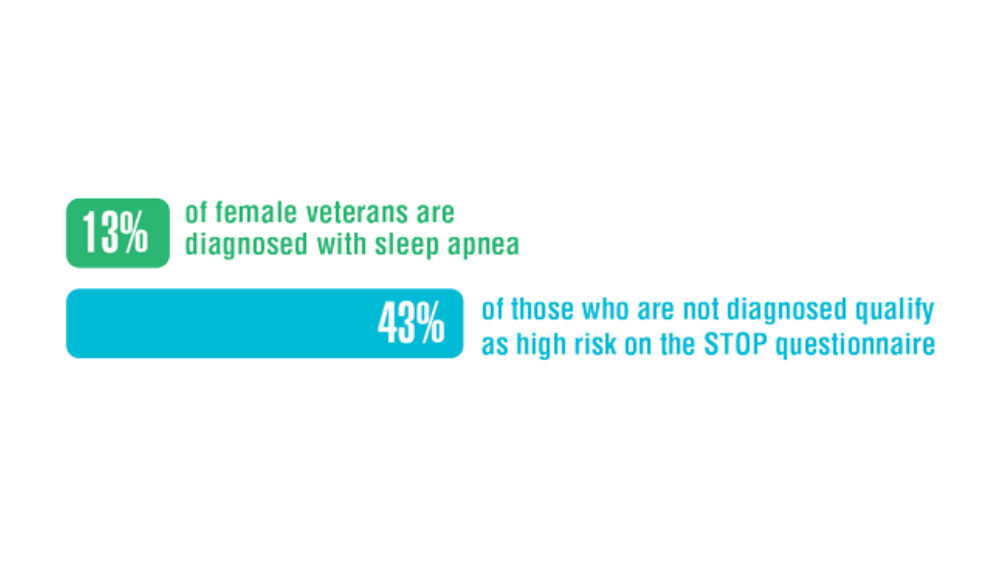
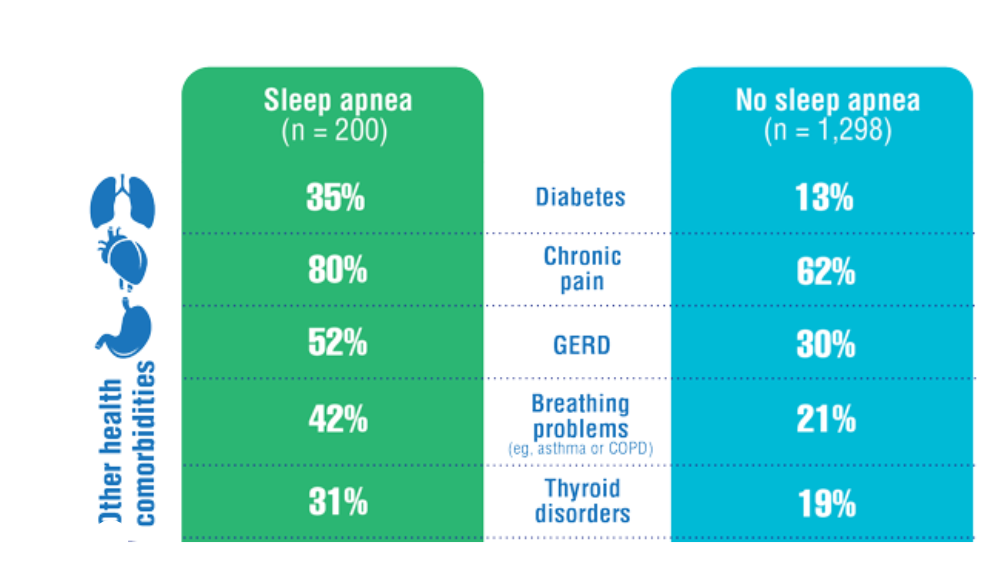
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
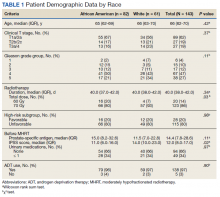
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
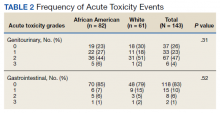
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
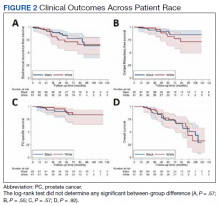
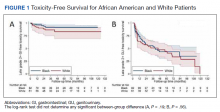
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
MERIT: Endoscopic sleeve gastroplasty shows ‘very impressive’ outcomes in randomized clinical trial
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
FROM THE LANCET
Federal Health Care Data Trends 2022: Vaccinations
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022