User login
Partnering for pulmonary fibrosis
The CHEST Foundation raises awareness for the most common interstitial lung disease.
On August 27, the CHEST Foundation and the Feldman Family Foundation will be hosting the 9th annual Irv Feldman Texas Hold ‘Em Tournament & Casino Night fundraiser supporting patient access and the provision of better quality of life for patients battling the interstitial lung disease – pulmonary fibrosis.
“My dad, Irv, had pulmonary fibrosis and deeply loved to play poker. It was always a family activity, and it continued through when he got sick. We played at his kitchen table when he couldn’t leave the house, and we even brought cards and chips to his hospital and rehab rooms,” said Mitch Feldman, President of the Feldman Family Foundation and member of the CHEST Foundation Board of Trustees. “During these few hours of poker play, he all but forgot about his illness and showed virtually no symptoms of the disease. In his honor, we created an event where people would come together to have fun playing poker while raising money for the disease [that] so deeply impacted our family.”
Through years of hosting the event, the Feldman family and the CHEST Foundation secured funding to develop a pulmonary fibrosis patient education resource hub that serves as a resource for those newly diagnosed and living with this disease. The Feldman Family and the CHEST Foundation continue to raise funds to support both early diagnosis and closing the gap between diagnosis and beginning treatment.
Partnering to address gaps
Affecting around 400,000 people in the United States, ILDs are frequently misdiagnosed as more common lung diseases. Some studies show that reaching an appropriate diagnosis for rarer lung diseases can take upwards of several years.
To begin addressing the issue of delays in diagnosis, the American College of Chest Physicians (CHEST) and Three Lakes Foundation are collaborating on a multiphase educational initiative aiming to reduce the time it takes to identify interstitial lung diseases like pulmonary fibrosis.
The initiative is called “Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients” to highlight the collaboration of pulmonary and primary care medicine. A steering committee of medical experts – including pulmonologists, primary care physicians, and a nursing professional – will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“By having experts from both pulmonary and primary care medicine as members of the steering committee, we are bringing together the pieces of the puzzle that is a complex diagnosis,” said Bridging Specialties steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
To learn more about the Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients initiative and to sign up for updates, visit https://tinyurl.com/2p92ha6r.
For ticket and donation information to the Irv Feldman Texas Hold ‘Em Tournament & Casino Night, visit the CHEST Foundation website at foundation.chestnet.org.
The CHEST Foundation raises awareness for the most common interstitial lung disease.
On August 27, the CHEST Foundation and the Feldman Family Foundation will be hosting the 9th annual Irv Feldman Texas Hold ‘Em Tournament & Casino Night fundraiser supporting patient access and the provision of better quality of life for patients battling the interstitial lung disease – pulmonary fibrosis.
“My dad, Irv, had pulmonary fibrosis and deeply loved to play poker. It was always a family activity, and it continued through when he got sick. We played at his kitchen table when he couldn’t leave the house, and we even brought cards and chips to his hospital and rehab rooms,” said Mitch Feldman, President of the Feldman Family Foundation and member of the CHEST Foundation Board of Trustees. “During these few hours of poker play, he all but forgot about his illness and showed virtually no symptoms of the disease. In his honor, we created an event where people would come together to have fun playing poker while raising money for the disease [that] so deeply impacted our family.”
Through years of hosting the event, the Feldman family and the CHEST Foundation secured funding to develop a pulmonary fibrosis patient education resource hub that serves as a resource for those newly diagnosed and living with this disease. The Feldman Family and the CHEST Foundation continue to raise funds to support both early diagnosis and closing the gap between diagnosis and beginning treatment.
Partnering to address gaps
Affecting around 400,000 people in the United States, ILDs are frequently misdiagnosed as more common lung diseases. Some studies show that reaching an appropriate diagnosis for rarer lung diseases can take upwards of several years.
To begin addressing the issue of delays in diagnosis, the American College of Chest Physicians (CHEST) and Three Lakes Foundation are collaborating on a multiphase educational initiative aiming to reduce the time it takes to identify interstitial lung diseases like pulmonary fibrosis.
The initiative is called “Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients” to highlight the collaboration of pulmonary and primary care medicine. A steering committee of medical experts – including pulmonologists, primary care physicians, and a nursing professional – will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“By having experts from both pulmonary and primary care medicine as members of the steering committee, we are bringing together the pieces of the puzzle that is a complex diagnosis,” said Bridging Specialties steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
To learn more about the Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients initiative and to sign up for updates, visit https://tinyurl.com/2p92ha6r.
For ticket and donation information to the Irv Feldman Texas Hold ‘Em Tournament & Casino Night, visit the CHEST Foundation website at foundation.chestnet.org.
The CHEST Foundation raises awareness for the most common interstitial lung disease.
On August 27, the CHEST Foundation and the Feldman Family Foundation will be hosting the 9th annual Irv Feldman Texas Hold ‘Em Tournament & Casino Night fundraiser supporting patient access and the provision of better quality of life for patients battling the interstitial lung disease – pulmonary fibrosis.
“My dad, Irv, had pulmonary fibrosis and deeply loved to play poker. It was always a family activity, and it continued through when he got sick. We played at his kitchen table when he couldn’t leave the house, and we even brought cards and chips to his hospital and rehab rooms,” said Mitch Feldman, President of the Feldman Family Foundation and member of the CHEST Foundation Board of Trustees. “During these few hours of poker play, he all but forgot about his illness and showed virtually no symptoms of the disease. In his honor, we created an event where people would come together to have fun playing poker while raising money for the disease [that] so deeply impacted our family.”
Through years of hosting the event, the Feldman family and the CHEST Foundation secured funding to develop a pulmonary fibrosis patient education resource hub that serves as a resource for those newly diagnosed and living with this disease. The Feldman Family and the CHEST Foundation continue to raise funds to support both early diagnosis and closing the gap between diagnosis and beginning treatment.
Partnering to address gaps
Affecting around 400,000 people in the United States, ILDs are frequently misdiagnosed as more common lung diseases. Some studies show that reaching an appropriate diagnosis for rarer lung diseases can take upwards of several years.
To begin addressing the issue of delays in diagnosis, the American College of Chest Physicians (CHEST) and Three Lakes Foundation are collaborating on a multiphase educational initiative aiming to reduce the time it takes to identify interstitial lung diseases like pulmonary fibrosis.
The initiative is called “Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients” to highlight the collaboration of pulmonary and primary care medicine. A steering committee of medical experts – including pulmonologists, primary care physicians, and a nursing professional – will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“By having experts from both pulmonary and primary care medicine as members of the steering committee, we are bringing together the pieces of the puzzle that is a complex diagnosis,” said Bridging Specialties steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
To learn more about the Bridging SpecialtiesTM: Timely Diagnosis for ILD Patients initiative and to sign up for updates, visit https://tinyurl.com/2p92ha6r.
For ticket and donation information to the Irv Feldman Texas Hold ‘Em Tournament & Casino Night, visit the CHEST Foundation website at foundation.chestnet.org.
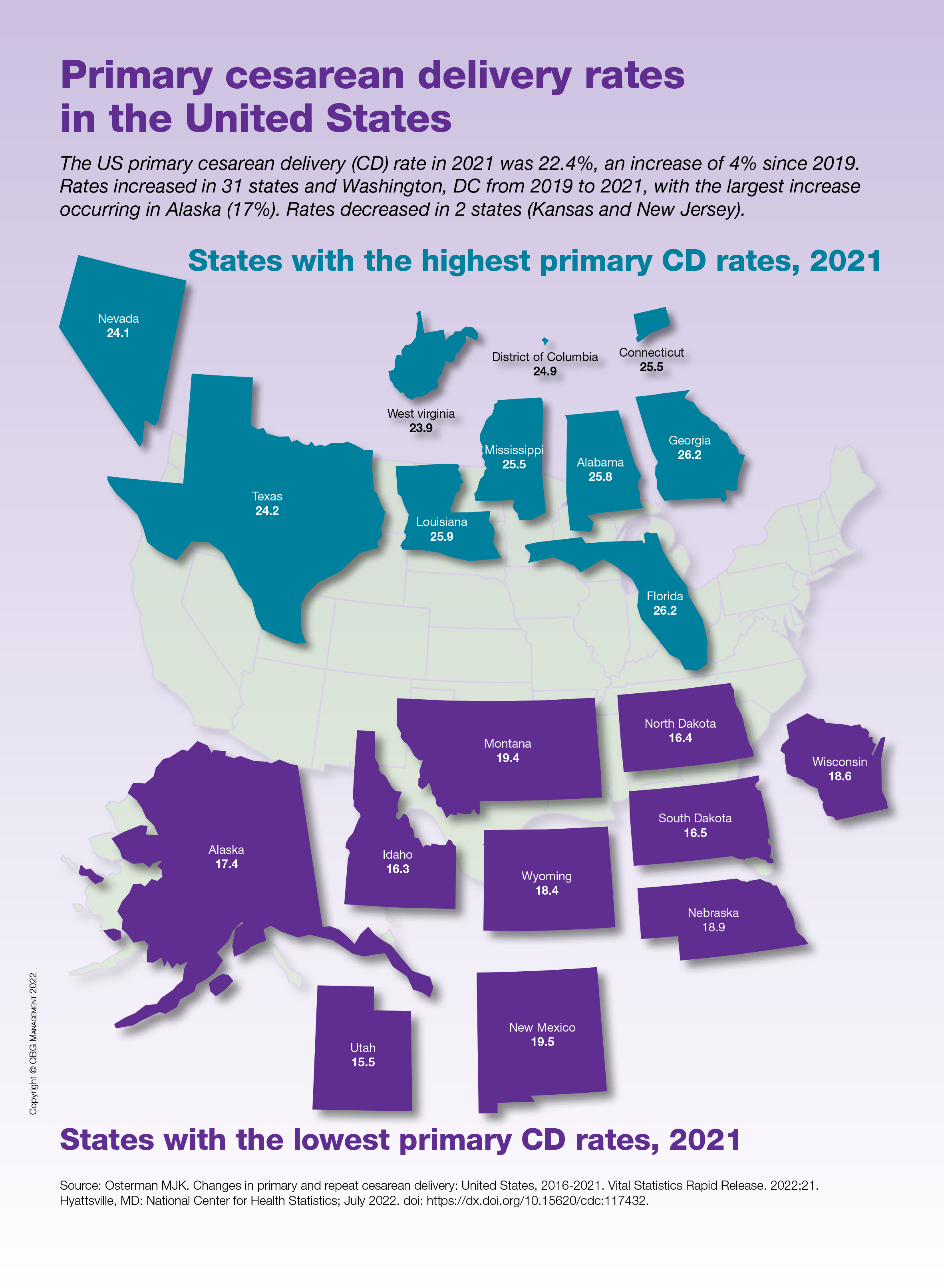
Primary cesarean delivery rates in the United States


Positive phase 3 results for novel schizophrenia drug
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
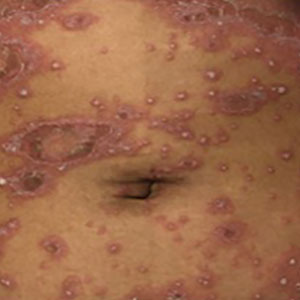
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
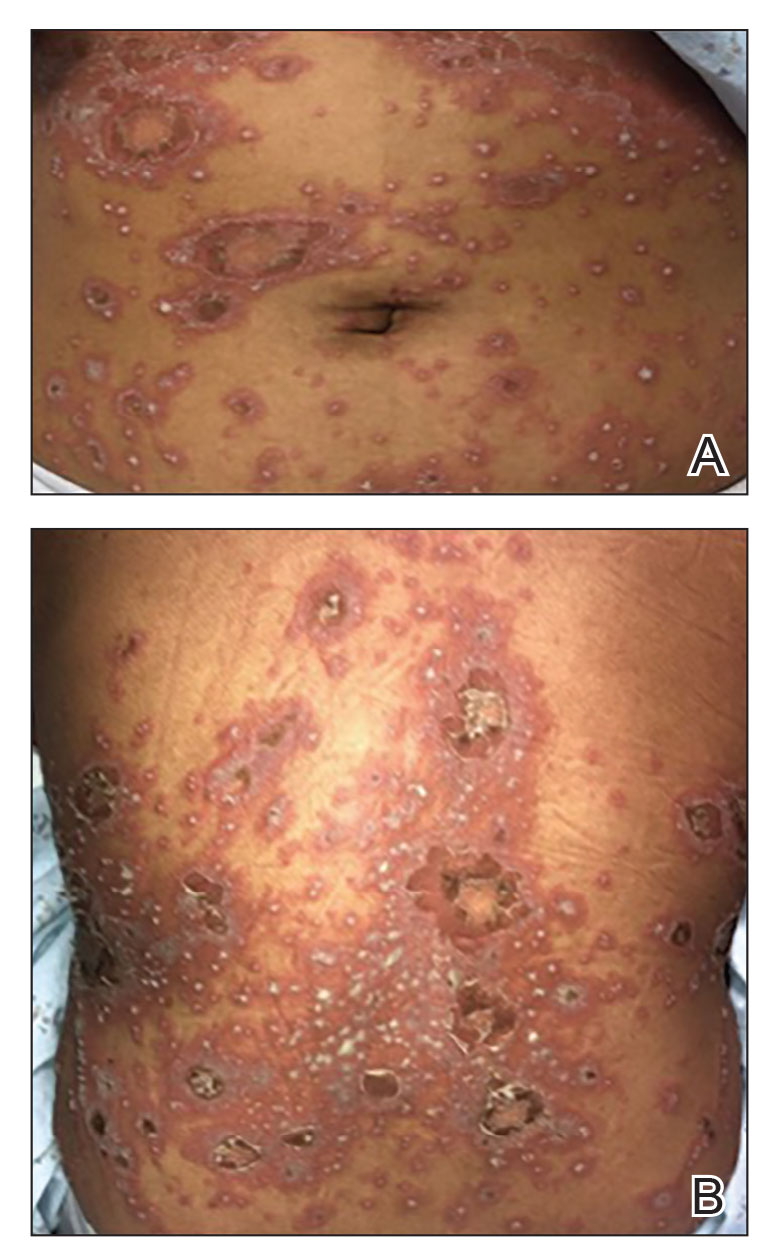
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
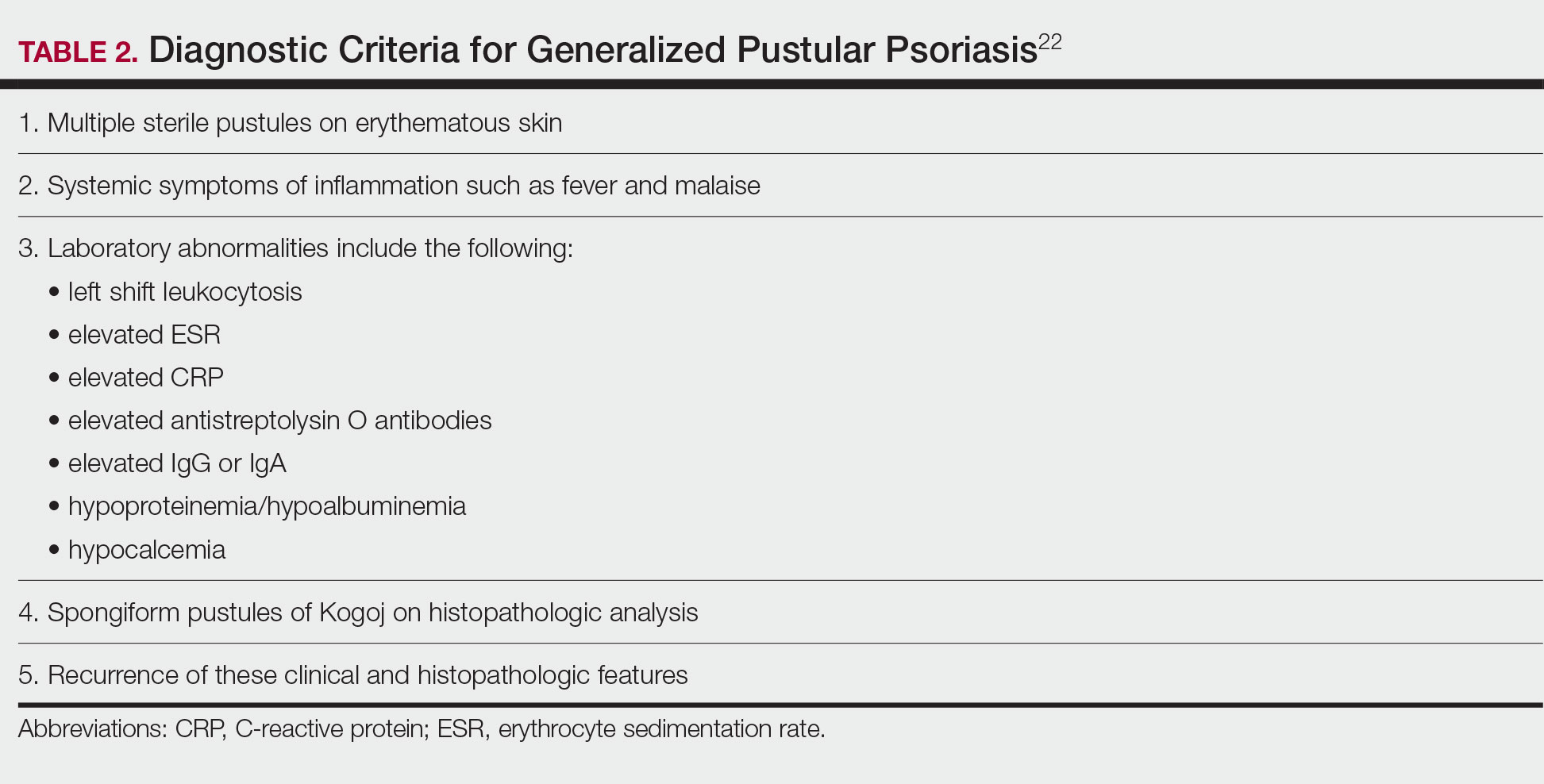
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
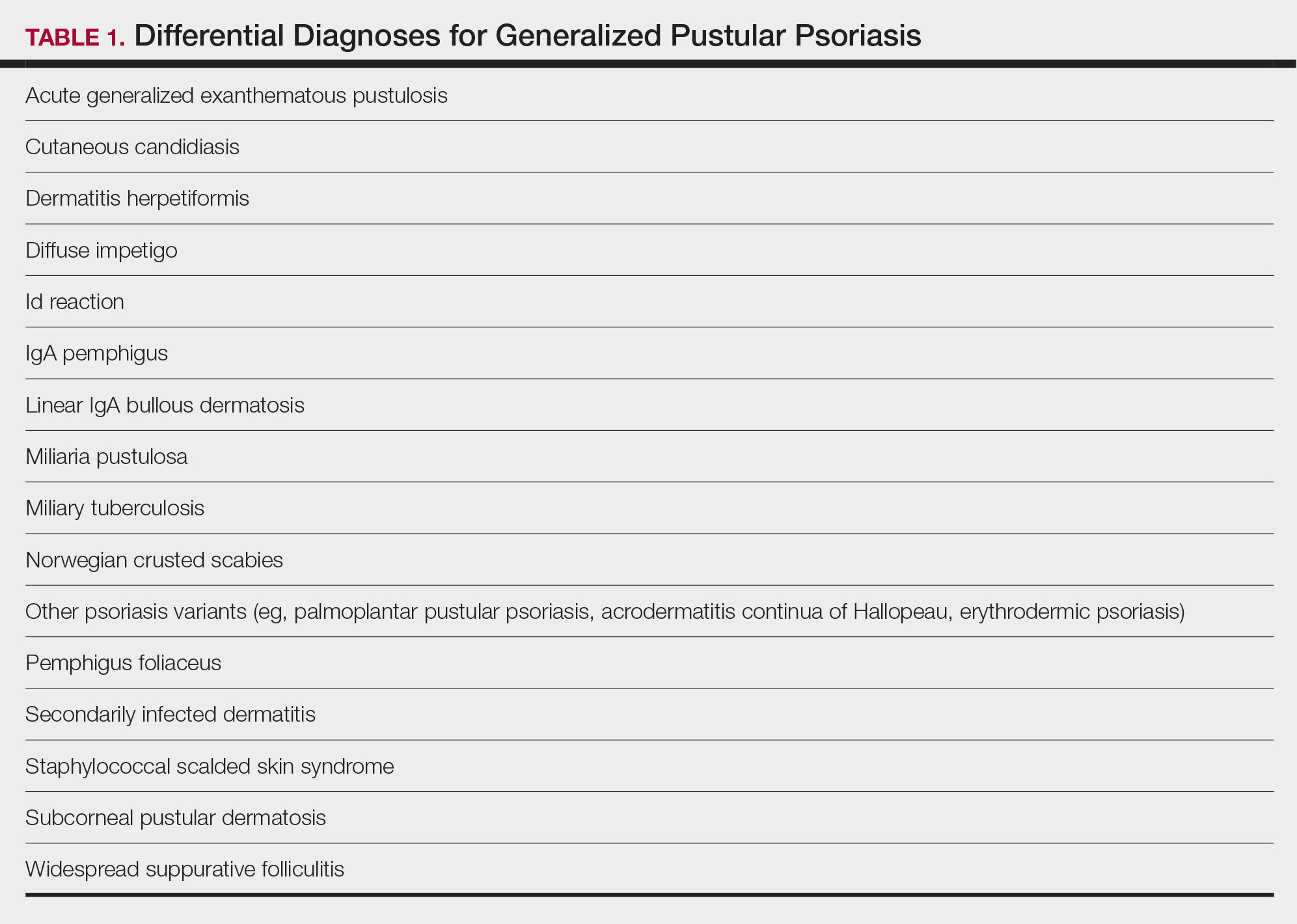
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Racial Disparities in the Diagnosis of Psoriasis
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.
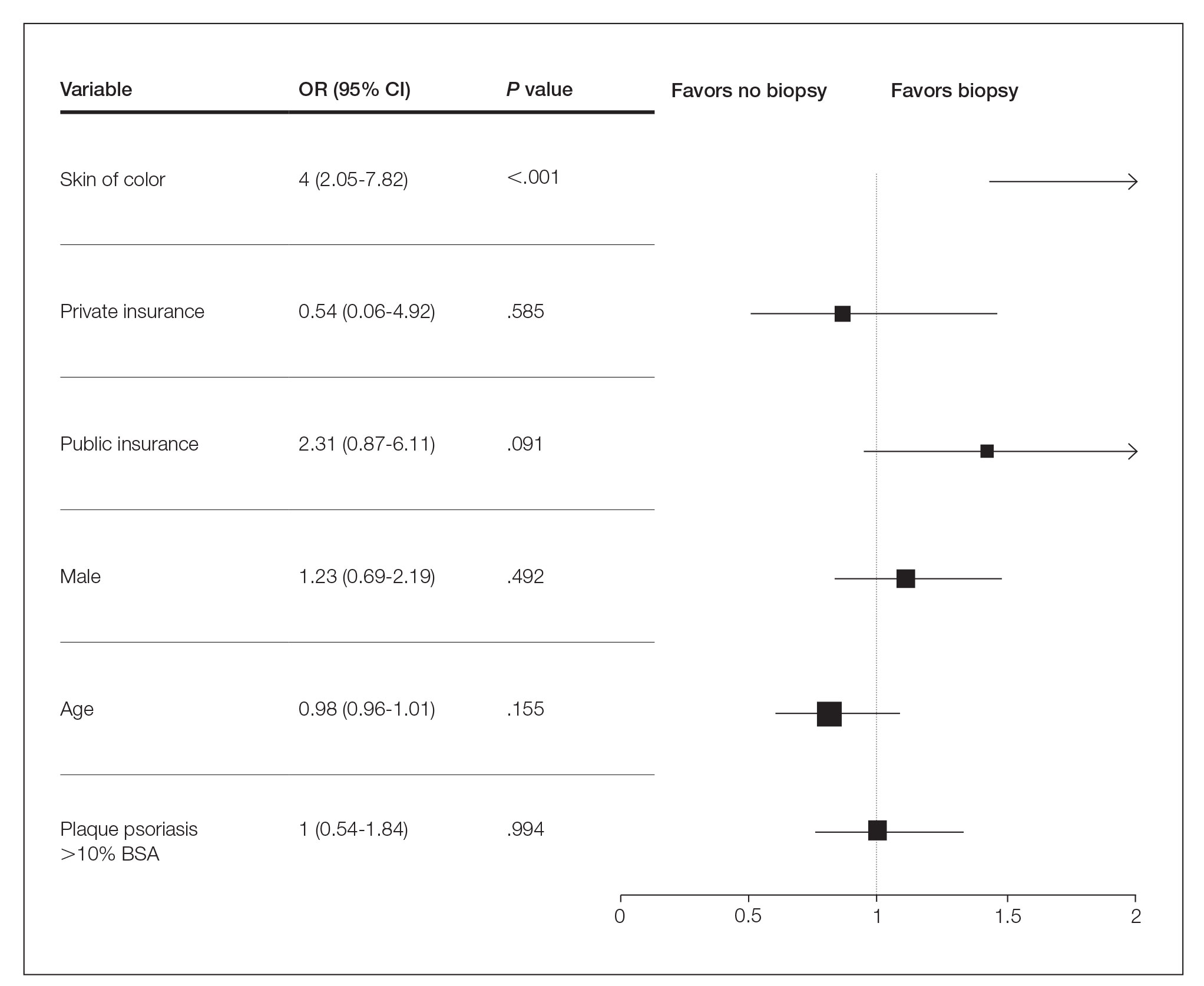
Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).
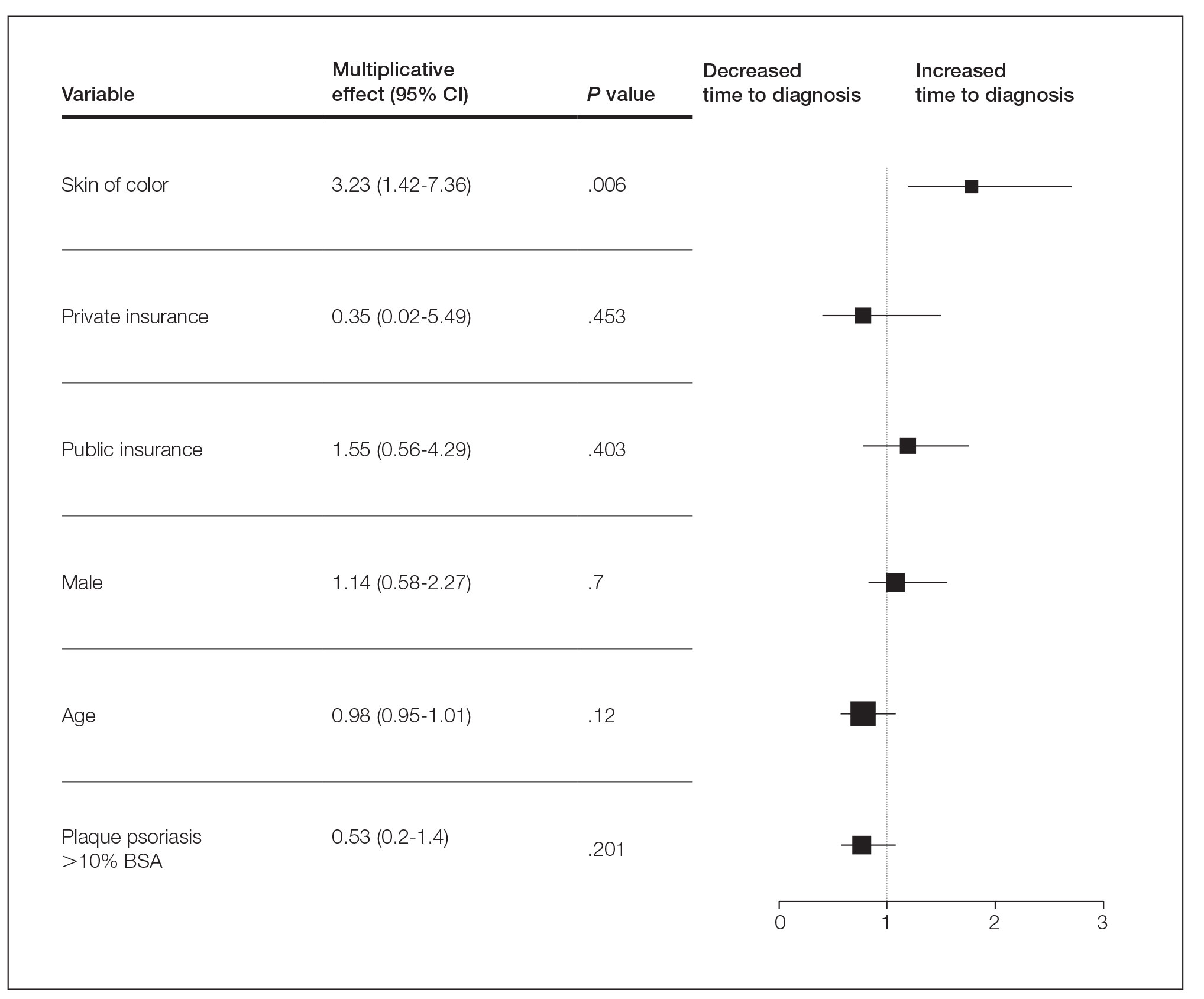
Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
Practice Points
- Skin of color (SOC) patients can wait 3 times longer to receive a diagnosis of psoriasis than non-SOC patients.
- Patients with SOC more often present with severe forms of psoriasis and are more likely to have palmoplantar psoriasis.
- Skin of color patients can be 4 times as likely to require a biopsy to confirm psoriasis diagnosis compared to non-SOC patients.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Guidance From the National Psoriasis Foundation COVID-19 Task Force
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
FDA authorizes intradermal use of Jynneos vaccine for monkeypox
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
Tobramycin tames infection in bronchiectasis
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST
In one state, pandemic tamped down lice and scabies cases
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
FROM PEDIATRIC DERMATOLOGY