User login
Adding veliparib to cisplatin improves PFS in BRCA-like metastatic TNBC
Key clinical point: In patients with germline BRCA1/2-wildtype metastatic triple-negative breast cancer (TNBC) with a BRCA-like phenotype, cisplatin plus veliparib significantly improved progression-free survival (PFS) without causing any unprecedented adverse events.
Major finding: PFS was significantly improved with cisplatin+veliparib vs cisplatin+placebo (hazard ratio 0.57; log-rank P = .01) in patients with BRCA-like TNBC, but not in germline BRCA1/2-mutated (P = .54) and non-BRCA-like (P = .57) groups. No new toxicity signals were observed.
Study details: Findings are from the phase 2 S1416 study including 320 patients with metastatic TNBC (n = 305) or estrogen receptor (ER)-positive/progesterone receptor (PR)-positive/both ER and PR positive, human epidermal growth factor receptor 2-negative BC (n = 15) who were randomly assigned to receive cisplatin with either veliparib or placebo.
Disclosures: This study was funded by the US National Cancer Institute and other sources. Some authors declared receiving grants, payments, or honoraria from; serving on advisory boards for; or having other financial or non-financial ties with several sources.
Source: Rodler E et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2023 (Jan 6). Doi: 10.1016/S1470-2045(22)00739-2
Key clinical point: In patients with germline BRCA1/2-wildtype metastatic triple-negative breast cancer (TNBC) with a BRCA-like phenotype, cisplatin plus veliparib significantly improved progression-free survival (PFS) without causing any unprecedented adverse events.
Major finding: PFS was significantly improved with cisplatin+veliparib vs cisplatin+placebo (hazard ratio 0.57; log-rank P = .01) in patients with BRCA-like TNBC, but not in germline BRCA1/2-mutated (P = .54) and non-BRCA-like (P = .57) groups. No new toxicity signals were observed.
Study details: Findings are from the phase 2 S1416 study including 320 patients with metastatic TNBC (n = 305) or estrogen receptor (ER)-positive/progesterone receptor (PR)-positive/both ER and PR positive, human epidermal growth factor receptor 2-negative BC (n = 15) who were randomly assigned to receive cisplatin with either veliparib or placebo.
Disclosures: This study was funded by the US National Cancer Institute and other sources. Some authors declared receiving grants, payments, or honoraria from; serving on advisory boards for; or having other financial or non-financial ties with several sources.
Source: Rodler E et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2023 (Jan 6). Doi: 10.1016/S1470-2045(22)00739-2
Key clinical point: In patients with germline BRCA1/2-wildtype metastatic triple-negative breast cancer (TNBC) with a BRCA-like phenotype, cisplatin plus veliparib significantly improved progression-free survival (PFS) without causing any unprecedented adverse events.
Major finding: PFS was significantly improved with cisplatin+veliparib vs cisplatin+placebo (hazard ratio 0.57; log-rank P = .01) in patients with BRCA-like TNBC, but not in germline BRCA1/2-mutated (P = .54) and non-BRCA-like (P = .57) groups. No new toxicity signals were observed.
Study details: Findings are from the phase 2 S1416 study including 320 patients with metastatic TNBC (n = 305) or estrogen receptor (ER)-positive/progesterone receptor (PR)-positive/both ER and PR positive, human epidermal growth factor receptor 2-negative BC (n = 15) who were randomly assigned to receive cisplatin with either veliparib or placebo.
Disclosures: This study was funded by the US National Cancer Institute and other sources. Some authors declared receiving grants, payments, or honoraria from; serving on advisory boards for; or having other financial or non-financial ties with several sources.
Source: Rodler E et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2023 (Jan 6). Doi: 10.1016/S1470-2045(22)00739-2
Contralateral BC risk elevated in women with germline pathogenic variants
Key clinical point: Women with invasive breast cancer (BC) who have germline pathogenic variants (PV) in BRCA1, BRCA2, CHEK2, or PALB2 have 2-3 times higher risk for contralateral BC than those without these PVs.
Major finding: The overall risk for contralateral BC was significantly elevated in all women with germline PV in BRCA1 (hazard ratio [HR] 2.7; P < .001), BRCA2 (HR 3.0; P < .001), and CHEK2 (HR 1.9; P = .03), and in the subset of women with estrogen receptor-negative BC and germline PV in PALB2 (HR 2.9; P = .006).
Study details: Findings are from an analysis of the CARRIERS study including 15,104 women with invasive BC who underwent ipsilateral surgery.
Disclosures: This study was supported by US National Institutes of Health grants and other sources. The authors declared serving as consultants or advisors and on speakers’ bureaus; receiving research funding, travel, accommodation expenses, or honoraria; and having other ties with several sources.
Source: Yadav S, Boddicker NJ, et al. Contralateral breast cancer risk among carriers of germline pathogenic variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J Clin Oncol. 2023 (Jan 9). Doi: 10.1200/JCO.22.01239
Key clinical point: Women with invasive breast cancer (BC) who have germline pathogenic variants (PV) in BRCA1, BRCA2, CHEK2, or PALB2 have 2-3 times higher risk for contralateral BC than those without these PVs.
Major finding: The overall risk for contralateral BC was significantly elevated in all women with germline PV in BRCA1 (hazard ratio [HR] 2.7; P < .001), BRCA2 (HR 3.0; P < .001), and CHEK2 (HR 1.9; P = .03), and in the subset of women with estrogen receptor-negative BC and germline PV in PALB2 (HR 2.9; P = .006).
Study details: Findings are from an analysis of the CARRIERS study including 15,104 women with invasive BC who underwent ipsilateral surgery.
Disclosures: This study was supported by US National Institutes of Health grants and other sources. The authors declared serving as consultants or advisors and on speakers’ bureaus; receiving research funding, travel, accommodation expenses, or honoraria; and having other ties with several sources.
Source: Yadav S, Boddicker NJ, et al. Contralateral breast cancer risk among carriers of germline pathogenic variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J Clin Oncol. 2023 (Jan 9). Doi: 10.1200/JCO.22.01239
Key clinical point: Women with invasive breast cancer (BC) who have germline pathogenic variants (PV) in BRCA1, BRCA2, CHEK2, or PALB2 have 2-3 times higher risk for contralateral BC than those without these PVs.
Major finding: The overall risk for contralateral BC was significantly elevated in all women with germline PV in BRCA1 (hazard ratio [HR] 2.7; P < .001), BRCA2 (HR 3.0; P < .001), and CHEK2 (HR 1.9; P = .03), and in the subset of women with estrogen receptor-negative BC and germline PV in PALB2 (HR 2.9; P = .006).
Study details: Findings are from an analysis of the CARRIERS study including 15,104 women with invasive BC who underwent ipsilateral surgery.
Disclosures: This study was supported by US National Institutes of Health grants and other sources. The authors declared serving as consultants or advisors and on speakers’ bureaus; receiving research funding, travel, accommodation expenses, or honoraria; and having other ties with several sources.
Source: Yadav S, Boddicker NJ, et al. Contralateral breast cancer risk among carriers of germline pathogenic variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J Clin Oncol. 2023 (Jan 9). Doi: 10.1200/JCO.22.01239
Regorafenib: New option for advanced gastroesophageal cancer?
SAN FRANCISCO – New data showing improved survival suggest that regorafenib (Stivarga) may offer a new treatment option in these patients.
“Regorafenib significantly improves survival compared with placebo in patients with refractory AGOC, delaying deterioration in global quality of life,” said lead author Nick Pavlakis, PhD, MBBS, Royal North Shore Hospital, St. Leonards, Australia. “There were also no new toxicity signals.”
He emphasized that benefit was consistent in all preplanned subgroups. “This offers a new treatment option in this setting,” Dr. Pavlakis said.
He presented the findings at the ASCO Gastrointestinal Cancers Symposium 2023.
Regorafenib is an oral multikinase inhibitor targeting kinases involved in angiogenesis (VEGFR1-3, TIE-2), tumor microenvironment (PDGFR-beta, FGFR), and oncogenesis (RAF, RET, and KIT). It is already approved for several indications including metastatic colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma.
AGOC could be a new indication for the drug. Use in this patient population was explored in an earlier trial, Dr. Pavlakis commented.
“We previously demonstrated the regorafenib prolonged progression-free survival in the INTEGRATE phase 2 trial,” he said. “Based on those results, we undertook the phase 3 INTEGRATE II study. The goal was to examine if regorafenib improves overall survival after failure of at least two lines of treatment.”
However, Dr. Pavlakis noted that during the conduct of this study, there was a change of practice in gastric cancer. “There was an evolution of new evidence in support of additional chemotherapy and immune checkpoint inhibitors such as nivolumab, and very interesting data on the combination of nivolumab and regorafenib from a study being conducted in Japan. So we amended the protocol and evolved the INTEGRATE II to INTEGRATE IIa, to continue evaluating regorafenib versus placebo, and then to evolve to the INTEGRATE IIb study to evaluate regorafenib plus nivolumab versus chemotherapy,” he explained.
The results he presented at the meeting came from the phase 3 INTEGRATE II part of this trial. The other part of the trial, INTEGRATE IIb, is still accruing.
A total of 251 patients were enrolled from five countries and stratified by tumor location, geographic location (Asia vs. “rest of the world”), and prior treatment with VEGF inhibitors.
The cohort was randomly assigned to receive 160 mg regorafenib and best supportive care or placebo plus BSC.
After 238 events, the overall survival hazard ratio was 0.68. “The survival benefit of regorafenib was best observed in the 12-month survival of 19% versus 6%,” said Pavlakis.
The median overall survival for regorafenib versus placebo was 4.5 versus 4.0 months (HR, 0.70; P = .011).
After preplanned adjustment for multiplicity, there were no statistically significant differences across regions (Asia vs. non-Asia) or other prespecified subgroups.
In the pooled analysis, which included study populations from both the INTEGRATE and INTEGRATE IIa populations, the median overall survival was 5.0 versus 4.1 (HR, 0.70; P = .001).
Regorafenib also improved progression-free survival (median PFS, 1.8 vs. 1.6 months; HR, 0.52; P < .0001) and it delayed deterioration in global quality of life as compared with placebo (P = .0043).
Toxicity was similar to that previously reported in other studies, and adverse events were mostly grade 1 and 2.
A building block?
In a comment, Pamela Kunz, MD, director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and Yale Cancer Center, New Haven, Conn., said that she would consider the results with regorafenib in this setting as statistically significant, but with questionable clinical significance.
“The median overall survival difference 0.5 months or about 2 weeks is very modest, especially when taking into consideration the side effect profile,” she said. “I think that regorafenib as a building block for the additional phase 3 study is more interesting.”
“There is data that suggests synergy between VEGF inhibitors and immune checkpoint inhibitors, so I am eager to see the results of INEGRATE IIb [exploring regorafenib use with nivolumab],” Dr. Kunz added.
The study is sponsored by the Australasian Gastro-Intestinal Trials Group. Dr. Pavlakis reported relationships with Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Merck, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Kunz reported relationships with Ipsen, Novartis (Advanced Accelerator Applications), Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – New data showing improved survival suggest that regorafenib (Stivarga) may offer a new treatment option in these patients.
“Regorafenib significantly improves survival compared with placebo in patients with refractory AGOC, delaying deterioration in global quality of life,” said lead author Nick Pavlakis, PhD, MBBS, Royal North Shore Hospital, St. Leonards, Australia. “There were also no new toxicity signals.”
He emphasized that benefit was consistent in all preplanned subgroups. “This offers a new treatment option in this setting,” Dr. Pavlakis said.
He presented the findings at the ASCO Gastrointestinal Cancers Symposium 2023.
Regorafenib is an oral multikinase inhibitor targeting kinases involved in angiogenesis (VEGFR1-3, TIE-2), tumor microenvironment (PDGFR-beta, FGFR), and oncogenesis (RAF, RET, and KIT). It is already approved for several indications including metastatic colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma.
AGOC could be a new indication for the drug. Use in this patient population was explored in an earlier trial, Dr. Pavlakis commented.
“We previously demonstrated the regorafenib prolonged progression-free survival in the INTEGRATE phase 2 trial,” he said. “Based on those results, we undertook the phase 3 INTEGRATE II study. The goal was to examine if regorafenib improves overall survival after failure of at least two lines of treatment.”
However, Dr. Pavlakis noted that during the conduct of this study, there was a change of practice in gastric cancer. “There was an evolution of new evidence in support of additional chemotherapy and immune checkpoint inhibitors such as nivolumab, and very interesting data on the combination of nivolumab and regorafenib from a study being conducted in Japan. So we amended the protocol and evolved the INTEGRATE II to INTEGRATE IIa, to continue evaluating regorafenib versus placebo, and then to evolve to the INTEGRATE IIb study to evaluate regorafenib plus nivolumab versus chemotherapy,” he explained.
The results he presented at the meeting came from the phase 3 INTEGRATE II part of this trial. The other part of the trial, INTEGRATE IIb, is still accruing.
A total of 251 patients were enrolled from five countries and stratified by tumor location, geographic location (Asia vs. “rest of the world”), and prior treatment with VEGF inhibitors.
The cohort was randomly assigned to receive 160 mg regorafenib and best supportive care or placebo plus BSC.
After 238 events, the overall survival hazard ratio was 0.68. “The survival benefit of regorafenib was best observed in the 12-month survival of 19% versus 6%,” said Pavlakis.
The median overall survival for regorafenib versus placebo was 4.5 versus 4.0 months (HR, 0.70; P = .011).
After preplanned adjustment for multiplicity, there were no statistically significant differences across regions (Asia vs. non-Asia) or other prespecified subgroups.
In the pooled analysis, which included study populations from both the INTEGRATE and INTEGRATE IIa populations, the median overall survival was 5.0 versus 4.1 (HR, 0.70; P = .001).
Regorafenib also improved progression-free survival (median PFS, 1.8 vs. 1.6 months; HR, 0.52; P < .0001) and it delayed deterioration in global quality of life as compared with placebo (P = .0043).
Toxicity was similar to that previously reported in other studies, and adverse events were mostly grade 1 and 2.
A building block?
In a comment, Pamela Kunz, MD, director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and Yale Cancer Center, New Haven, Conn., said that she would consider the results with regorafenib in this setting as statistically significant, but with questionable clinical significance.
“The median overall survival difference 0.5 months or about 2 weeks is very modest, especially when taking into consideration the side effect profile,” she said. “I think that regorafenib as a building block for the additional phase 3 study is more interesting.”
“There is data that suggests synergy between VEGF inhibitors and immune checkpoint inhibitors, so I am eager to see the results of INEGRATE IIb [exploring regorafenib use with nivolumab],” Dr. Kunz added.
The study is sponsored by the Australasian Gastro-Intestinal Trials Group. Dr. Pavlakis reported relationships with Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Merck, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Kunz reported relationships with Ipsen, Novartis (Advanced Accelerator Applications), Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – New data showing improved survival suggest that regorafenib (Stivarga) may offer a new treatment option in these patients.
“Regorafenib significantly improves survival compared with placebo in patients with refractory AGOC, delaying deterioration in global quality of life,” said lead author Nick Pavlakis, PhD, MBBS, Royal North Shore Hospital, St. Leonards, Australia. “There were also no new toxicity signals.”
He emphasized that benefit was consistent in all preplanned subgroups. “This offers a new treatment option in this setting,” Dr. Pavlakis said.
He presented the findings at the ASCO Gastrointestinal Cancers Symposium 2023.
Regorafenib is an oral multikinase inhibitor targeting kinases involved in angiogenesis (VEGFR1-3, TIE-2), tumor microenvironment (PDGFR-beta, FGFR), and oncogenesis (RAF, RET, and KIT). It is already approved for several indications including metastatic colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma.
AGOC could be a new indication for the drug. Use in this patient population was explored in an earlier trial, Dr. Pavlakis commented.
“We previously demonstrated the regorafenib prolonged progression-free survival in the INTEGRATE phase 2 trial,” he said. “Based on those results, we undertook the phase 3 INTEGRATE II study. The goal was to examine if regorafenib improves overall survival after failure of at least two lines of treatment.”
However, Dr. Pavlakis noted that during the conduct of this study, there was a change of practice in gastric cancer. “There was an evolution of new evidence in support of additional chemotherapy and immune checkpoint inhibitors such as nivolumab, and very interesting data on the combination of nivolumab and regorafenib from a study being conducted in Japan. So we amended the protocol and evolved the INTEGRATE II to INTEGRATE IIa, to continue evaluating regorafenib versus placebo, and then to evolve to the INTEGRATE IIb study to evaluate regorafenib plus nivolumab versus chemotherapy,” he explained.
The results he presented at the meeting came from the phase 3 INTEGRATE II part of this trial. The other part of the trial, INTEGRATE IIb, is still accruing.
A total of 251 patients were enrolled from five countries and stratified by tumor location, geographic location (Asia vs. “rest of the world”), and prior treatment with VEGF inhibitors.
The cohort was randomly assigned to receive 160 mg regorafenib and best supportive care or placebo plus BSC.
After 238 events, the overall survival hazard ratio was 0.68. “The survival benefit of regorafenib was best observed in the 12-month survival of 19% versus 6%,” said Pavlakis.
The median overall survival for regorafenib versus placebo was 4.5 versus 4.0 months (HR, 0.70; P = .011).
After preplanned adjustment for multiplicity, there were no statistically significant differences across regions (Asia vs. non-Asia) or other prespecified subgroups.
In the pooled analysis, which included study populations from both the INTEGRATE and INTEGRATE IIa populations, the median overall survival was 5.0 versus 4.1 (HR, 0.70; P = .001).
Regorafenib also improved progression-free survival (median PFS, 1.8 vs. 1.6 months; HR, 0.52; P < .0001) and it delayed deterioration in global quality of life as compared with placebo (P = .0043).
Toxicity was similar to that previously reported in other studies, and adverse events were mostly grade 1 and 2.
A building block?
In a comment, Pamela Kunz, MD, director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and Yale Cancer Center, New Haven, Conn., said that she would consider the results with regorafenib in this setting as statistically significant, but with questionable clinical significance.
“The median overall survival difference 0.5 months or about 2 weeks is very modest, especially when taking into consideration the side effect profile,” she said. “I think that regorafenib as a building block for the additional phase 3 study is more interesting.”
“There is data that suggests synergy between VEGF inhibitors and immune checkpoint inhibitors, so I am eager to see the results of INEGRATE IIb [exploring regorafenib use with nivolumab],” Dr. Kunz added.
The study is sponsored by the Australasian Gastro-Intestinal Trials Group. Dr. Pavlakis reported relationships with Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Merck, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Kunz reported relationships with Ipsen, Novartis (Advanced Accelerator Applications), Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
AT ASCO GI 2023
Novel resuscitation for patients with nonshockable rhythms in cardiac arrest
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
How should PRAME be used to evaluate melanocytic lesions?
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
AT MELANOMA 2023
More type 2 diabetes deaths from cancer than heart disease
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.
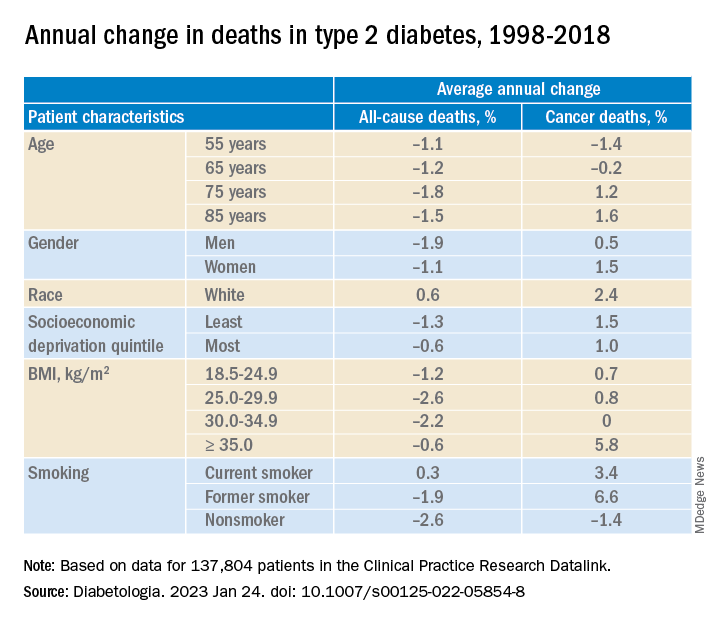
In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
FDA panel backs shift toward one-dose COVID shot
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
STS, new president apologize for predecessor’s speech amid Twitter backlash
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
Is preeclampsia a cardiovascular time bomb for mothers?
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY

