User login
Medicare policy tweak on LVADs may reduce access to transplant
A recent change in Medicare policy designed to increase access to left ventricular assist devices (LVADs) may have had the unintended consequence of increasing inequalities in access to heart transplant for patients with advanced heart failure.
In December 2020, the Centers for Medicare & Medicaid Services relaxed restrictions on centers that implant LVADs but don’t perform heart transplants. Specifically, they dropped the requirement that LVAD-only centers obtain permission from a Medicare-approved heart transplant center authorizing LVAD implantation with “bridge-to-transplant” (BTT) intent, meaning the patient is a transplant candidate.
While the relaxed requirement has the potential to increase access to LVADs for appropriate patients, a look back at 22,221 LVAD recipients found that patients who received LVADs at transplant-capable centers had a 79% higher likelihood of receiving a BTT LVAD designation.
The 2-year heart transplant rate following LVAD implant was 25.6% for patients who received an LVAD at a heart transplant center, compared with 11.9% at LVAD-only centers.
Thomas Cascino, MD, with University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, and colleagues reported their findings in JAMA Network Open.
Differential assessment?
Nontransplant LVAD centers are increasing in number in the United States now that the CMS has made establishing an LVAD-only center easier.
“Although there should be enthusiasm for the potential of LVAD-only centers to increase access to LVAD, it appears that receiving an LVAD at a center that does not perform transplants results in differential assessment of transplant eligibility at the time of LVAD implant and inequities in receipt of transplant,” Dr. Cascino and colleagues said.
“Being cared for at a center that does not perform heart transplant should not result in a lesser chance to receive a heart transplant,” Dr. Cascino added in a university news release. “Our study shows that this disparity existed before the policy change, and we think it will likely grow larger now that there is less collaboration.”
The CMS policy will likely “further challenge equity in access to transplant for patients seeking care at nontransplant centers and may have the unintended consequence of contributing to increasing inequities in access to transplants, as has been feared,” the researchers wrote.
They also note that recent changes in the adult heart allocation system under the United Network for Organ Sharing have significantly reduced the likelihood of transplant after durable LVAD implant unless candidates are listed as being at higher urgency status owing to an LVAD complication or clinical deterioration.
“The reality is that durable LVADs are much less likely to be a bridge to the best therapy (that is, transplant) in the current allocation system. As a result, there is a critical need to select appropriate durable LVAD and transplant candidates at the initial evaluation,” the authors said.
“This puts the onus on the transplant community to select appropriate LVAD and transplant candidates during the initial evaluation. We need a system in which any patient can walk into the same hospital and get the right therapy for them,” Dr. Cascino added in the news release.
The research was supported in part through funding from the University of Michigan Health department of cardiac surgery and the National Institutes of Health, National Heart, Lung, and Blood Institute. Dr. Cascino has received grants from Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A recent change in Medicare policy designed to increase access to left ventricular assist devices (LVADs) may have had the unintended consequence of increasing inequalities in access to heart transplant for patients with advanced heart failure.
In December 2020, the Centers for Medicare & Medicaid Services relaxed restrictions on centers that implant LVADs but don’t perform heart transplants. Specifically, they dropped the requirement that LVAD-only centers obtain permission from a Medicare-approved heart transplant center authorizing LVAD implantation with “bridge-to-transplant” (BTT) intent, meaning the patient is a transplant candidate.
While the relaxed requirement has the potential to increase access to LVADs for appropriate patients, a look back at 22,221 LVAD recipients found that patients who received LVADs at transplant-capable centers had a 79% higher likelihood of receiving a BTT LVAD designation.
The 2-year heart transplant rate following LVAD implant was 25.6% for patients who received an LVAD at a heart transplant center, compared with 11.9% at LVAD-only centers.
Thomas Cascino, MD, with University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, and colleagues reported their findings in JAMA Network Open.
Differential assessment?
Nontransplant LVAD centers are increasing in number in the United States now that the CMS has made establishing an LVAD-only center easier.
“Although there should be enthusiasm for the potential of LVAD-only centers to increase access to LVAD, it appears that receiving an LVAD at a center that does not perform transplants results in differential assessment of transplant eligibility at the time of LVAD implant and inequities in receipt of transplant,” Dr. Cascino and colleagues said.
“Being cared for at a center that does not perform heart transplant should not result in a lesser chance to receive a heart transplant,” Dr. Cascino added in a university news release. “Our study shows that this disparity existed before the policy change, and we think it will likely grow larger now that there is less collaboration.”
The CMS policy will likely “further challenge equity in access to transplant for patients seeking care at nontransplant centers and may have the unintended consequence of contributing to increasing inequities in access to transplants, as has been feared,” the researchers wrote.
They also note that recent changes in the adult heart allocation system under the United Network for Organ Sharing have significantly reduced the likelihood of transplant after durable LVAD implant unless candidates are listed as being at higher urgency status owing to an LVAD complication or clinical deterioration.
“The reality is that durable LVADs are much less likely to be a bridge to the best therapy (that is, transplant) in the current allocation system. As a result, there is a critical need to select appropriate durable LVAD and transplant candidates at the initial evaluation,” the authors said.
“This puts the onus on the transplant community to select appropriate LVAD and transplant candidates during the initial evaluation. We need a system in which any patient can walk into the same hospital and get the right therapy for them,” Dr. Cascino added in the news release.
The research was supported in part through funding from the University of Michigan Health department of cardiac surgery and the National Institutes of Health, National Heart, Lung, and Blood Institute. Dr. Cascino has received grants from Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A recent change in Medicare policy designed to increase access to left ventricular assist devices (LVADs) may have had the unintended consequence of increasing inequalities in access to heart transplant for patients with advanced heart failure.
In December 2020, the Centers for Medicare & Medicaid Services relaxed restrictions on centers that implant LVADs but don’t perform heart transplants. Specifically, they dropped the requirement that LVAD-only centers obtain permission from a Medicare-approved heart transplant center authorizing LVAD implantation with “bridge-to-transplant” (BTT) intent, meaning the patient is a transplant candidate.
While the relaxed requirement has the potential to increase access to LVADs for appropriate patients, a look back at 22,221 LVAD recipients found that patients who received LVADs at transplant-capable centers had a 79% higher likelihood of receiving a BTT LVAD designation.
The 2-year heart transplant rate following LVAD implant was 25.6% for patients who received an LVAD at a heart transplant center, compared with 11.9% at LVAD-only centers.
Thomas Cascino, MD, with University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, and colleagues reported their findings in JAMA Network Open.
Differential assessment?
Nontransplant LVAD centers are increasing in number in the United States now that the CMS has made establishing an LVAD-only center easier.
“Although there should be enthusiasm for the potential of LVAD-only centers to increase access to LVAD, it appears that receiving an LVAD at a center that does not perform transplants results in differential assessment of transplant eligibility at the time of LVAD implant and inequities in receipt of transplant,” Dr. Cascino and colleagues said.
“Being cared for at a center that does not perform heart transplant should not result in a lesser chance to receive a heart transplant,” Dr. Cascino added in a university news release. “Our study shows that this disparity existed before the policy change, and we think it will likely grow larger now that there is less collaboration.”
The CMS policy will likely “further challenge equity in access to transplant for patients seeking care at nontransplant centers and may have the unintended consequence of contributing to increasing inequities in access to transplants, as has been feared,” the researchers wrote.
They also note that recent changes in the adult heart allocation system under the United Network for Organ Sharing have significantly reduced the likelihood of transplant after durable LVAD implant unless candidates are listed as being at higher urgency status owing to an LVAD complication or clinical deterioration.
“The reality is that durable LVADs are much less likely to be a bridge to the best therapy (that is, transplant) in the current allocation system. As a result, there is a critical need to select appropriate durable LVAD and transplant candidates at the initial evaluation,” the authors said.
“This puts the onus on the transplant community to select appropriate LVAD and transplant candidates during the initial evaluation. We need a system in which any patient can walk into the same hospital and get the right therapy for them,” Dr. Cascino added in the news release.
The research was supported in part through funding from the University of Michigan Health department of cardiac surgery and the National Institutes of Health, National Heart, Lung, and Blood Institute. Dr. Cascino has received grants from Johnson & Johnson.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
If we care about cancer patients, we must care about climate change
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
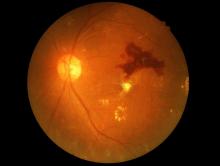
Eye check important before starting semaglutide for diabetes
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
FDA okays Tidepool Loop app to help guide insulin delivery
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
Clarity on torsemide vs. furosemide in HF: TRANSFORM-HF published
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Children with autism but no intellectual disability may be falling through the cracks
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
FROM PEDIATRICS
Pre-existing radiographic damage influence response to secukinumab in PsA
Key clinical point: Pre-existing radiographic damage was substantially prevalent in patients with psoriatic arthritis (PsA), with secukinumab therapy being associated with the inhibition of joint tenderness and swelling; however, high baseline radiographic damage reduced the likelihood of achieving minimal disease activity (MDA).
Major finding: Overall, 86% and 60% of patients had erosion and joint space narrowing (JSN) scores of >0, respectively. At week 16 and 52, 150 and 300 mg secukinumab reduced tender and swollen joint counts across all values of baseline erosion and JSN scores; however, patients with higher baseline erosion and JSN scores were less likely to achieve MDA.
Study details: This was a post hoc analysis of two phase 3 trials, FUTURE 1 and FUTURE 5, including 1554 patients with PsA who received 300 or 150 mg secukinumab with or without a loading dose.
Disclosures: This study received funding from Novartis Pharma AG. Four authors declared being employees of or owning stocks in Novartis. Seven authors declared ties with various sources, including Novartis.
Source: Mease P et al. Quantification of pre-existing radiographic damage and its relationship with joint activity and long-term clinical outcomes with secukinumab therapy in patients with psoriatic arthritis. Arthritis Res Ther. 2022;24(1):283 (Dec 28). Doi: 10.1186/s13075-022-02944-1
Key clinical point: Pre-existing radiographic damage was substantially prevalent in patients with psoriatic arthritis (PsA), with secukinumab therapy being associated with the inhibition of joint tenderness and swelling; however, high baseline radiographic damage reduced the likelihood of achieving minimal disease activity (MDA).
Major finding: Overall, 86% and 60% of patients had erosion and joint space narrowing (JSN) scores of >0, respectively. At week 16 and 52, 150 and 300 mg secukinumab reduced tender and swollen joint counts across all values of baseline erosion and JSN scores; however, patients with higher baseline erosion and JSN scores were less likely to achieve MDA.
Study details: This was a post hoc analysis of two phase 3 trials, FUTURE 1 and FUTURE 5, including 1554 patients with PsA who received 300 or 150 mg secukinumab with or without a loading dose.
Disclosures: This study received funding from Novartis Pharma AG. Four authors declared being employees of or owning stocks in Novartis. Seven authors declared ties with various sources, including Novartis.
Source: Mease P et al. Quantification of pre-existing radiographic damage and its relationship with joint activity and long-term clinical outcomes with secukinumab therapy in patients with psoriatic arthritis. Arthritis Res Ther. 2022;24(1):283 (Dec 28). Doi: 10.1186/s13075-022-02944-1
Key clinical point: Pre-existing radiographic damage was substantially prevalent in patients with psoriatic arthritis (PsA), with secukinumab therapy being associated with the inhibition of joint tenderness and swelling; however, high baseline radiographic damage reduced the likelihood of achieving minimal disease activity (MDA).
Major finding: Overall, 86% and 60% of patients had erosion and joint space narrowing (JSN) scores of >0, respectively. At week 16 and 52, 150 and 300 mg secukinumab reduced tender and swollen joint counts across all values of baseline erosion and JSN scores; however, patients with higher baseline erosion and JSN scores were less likely to achieve MDA.
Study details: This was a post hoc analysis of two phase 3 trials, FUTURE 1 and FUTURE 5, including 1554 patients with PsA who received 300 or 150 mg secukinumab with or without a loading dose.
Disclosures: This study received funding from Novartis Pharma AG. Four authors declared being employees of or owning stocks in Novartis. Seven authors declared ties with various sources, including Novartis.
Source: Mease P et al. Quantification of pre-existing radiographic damage and its relationship with joint activity and long-term clinical outcomes with secukinumab therapy in patients with psoriatic arthritis. Arthritis Res Ther. 2022;24(1):283 (Dec 28). Doi: 10.1186/s13075-022-02944-1
PsA-related uveitis: Real-world data on epidemiology and clinical features
Key clinical point: Uveitis related to psoriatic arthritis (PsA) had a prevalence of approximately 5%, presented as an acute and recurrent disease with anterior and unilateral involvement, and was associated with impaired quality of life and increased functional disability.
Major finding: Uveitis onset was acute in all cases, with 50% being recurrent and 80% being anterior and unilateral. Patients with vs without uveitis had a higher PsA impact of Disease Score (P = .001) and Bath Ankylosing Spondylitis Functional Index (
Study details: This retrospective longitudinal study included 406 patients with PsA, of which 4.9% of patients experienced ≥1 episode of uveitis.
Disclosures: This study did not receive any specific funding. Several authors declared receiving grants, research support, consulting fees, or honoraria for participation in company-sponsored speaker's bureaus from various sources.
Source: De Vicente Delmás A et al. Uveitis in psoriatic arthritis: Study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781 (Jan 12). Doi: 10.1136/rmdopen-2022-002781
Key clinical point: Uveitis related to psoriatic arthritis (PsA) had a prevalence of approximately 5%, presented as an acute and recurrent disease with anterior and unilateral involvement, and was associated with impaired quality of life and increased functional disability.
Major finding: Uveitis onset was acute in all cases, with 50% being recurrent and 80% being anterior and unilateral. Patients with vs without uveitis had a higher PsA impact of Disease Score (P = .001) and Bath Ankylosing Spondylitis Functional Index (
Study details: This retrospective longitudinal study included 406 patients with PsA, of which 4.9% of patients experienced ≥1 episode of uveitis.
Disclosures: This study did not receive any specific funding. Several authors declared receiving grants, research support, consulting fees, or honoraria for participation in company-sponsored speaker's bureaus from various sources.
Source: De Vicente Delmás A et al. Uveitis in psoriatic arthritis: Study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781 (Jan 12). Doi: 10.1136/rmdopen-2022-002781
Key clinical point: Uveitis related to psoriatic arthritis (PsA) had a prevalence of approximately 5%, presented as an acute and recurrent disease with anterior and unilateral involvement, and was associated with impaired quality of life and increased functional disability.
Major finding: Uveitis onset was acute in all cases, with 50% being recurrent and 80% being anterior and unilateral. Patients with vs without uveitis had a higher PsA impact of Disease Score (P = .001) and Bath Ankylosing Spondylitis Functional Index (
Study details: This retrospective longitudinal study included 406 patients with PsA, of which 4.9% of patients experienced ≥1 episode of uveitis.
Disclosures: This study did not receive any specific funding. Several authors declared receiving grants, research support, consulting fees, or honoraria for participation in company-sponsored speaker's bureaus from various sources.
Source: De Vicente Delmás A et al. Uveitis in psoriatic arthritis: Study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781 (Jan 12). Doi: 10.1136/rmdopen-2022-002781
Musculoskeletal ultrasound improves accuracy of early PsA diagnosis
Key clinical point: Targeted musculoskeletal ultrasound performed by trained dermatologists improved the accuracy of early psoriatic arthritis (PsA) diagnosis and may eventually decrease referral to rheumatologists.
Major finding: Use of musculoskeletal ultrasound changed the sensitivity and specificity of early PsA screening strategy from 88.2% (95% CI 58.1%-94.6%) and 54.4% (95% CI 44.8%-64.1%) to 70.6% (95% CI 38.4%-81.9%) and 90.4% (95% CI 83.9%-95.6%), respectively. Overall, 45 of the 46 patients were cleared of preliminary diagnosis-based PsA suspicion after musculoskeletal ultrasound.
Study details: This was a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia, of which 19 patients were diagnosed with PsA by a rheumatologist.
Disclosures: This study was supported by Novartis Pharma. The authors declared no conflicts of interest.
Source: Grobelski J et al. Prospective double-blind study on the value of musculoskeletal ultrasound by dermatologists as a screening instrument for psoriatic arthritis. Rheumatology (Oxford). 2022 (Dec 22). Doi: 10.1093/rheumatology/keac702
Key clinical point: Targeted musculoskeletal ultrasound performed by trained dermatologists improved the accuracy of early psoriatic arthritis (PsA) diagnosis and may eventually decrease referral to rheumatologists.
Major finding: Use of musculoskeletal ultrasound changed the sensitivity and specificity of early PsA screening strategy from 88.2% (95% CI 58.1%-94.6%) and 54.4% (95% CI 44.8%-64.1%) to 70.6% (95% CI 38.4%-81.9%) and 90.4% (95% CI 83.9%-95.6%), respectively. Overall, 45 of the 46 patients were cleared of preliminary diagnosis-based PsA suspicion after musculoskeletal ultrasound.
Study details: This was a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia, of which 19 patients were diagnosed with PsA by a rheumatologist.
Disclosures: This study was supported by Novartis Pharma. The authors declared no conflicts of interest.
Source: Grobelski J et al. Prospective double-blind study on the value of musculoskeletal ultrasound by dermatologists as a screening instrument for psoriatic arthritis. Rheumatology (Oxford). 2022 (Dec 22). Doi: 10.1093/rheumatology/keac702
Key clinical point: Targeted musculoskeletal ultrasound performed by trained dermatologists improved the accuracy of early psoriatic arthritis (PsA) diagnosis and may eventually decrease referral to rheumatologists.
Major finding: Use of musculoskeletal ultrasound changed the sensitivity and specificity of early PsA screening strategy from 88.2% (95% CI 58.1%-94.6%) and 54.4% (95% CI 44.8%-64.1%) to 70.6% (95% CI 38.4%-81.9%) and 90.4% (95% CI 83.9%-95.6%), respectively. Overall, 45 of the 46 patients were cleared of preliminary diagnosis-based PsA suspicion after musculoskeletal ultrasound.
Study details: This was a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia, of which 19 patients were diagnosed with PsA by a rheumatologist.
Disclosures: This study was supported by Novartis Pharma. The authors declared no conflicts of interest.
Source: Grobelski J et al. Prospective double-blind study on the value of musculoskeletal ultrasound by dermatologists as a screening instrument for psoriatic arthritis. Rheumatology (Oxford). 2022 (Dec 22). Doi: 10.1093/rheumatology/keac702
Minorities with epilepsy blocked from receiving ‘highest quality of care’
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY CLINICAL PRACTICE