User login
Atopic dermatitis is positively linked with the risk for juvenile idiopathic arthritis
Key clinical point: Children and adolescents with atopic dermatitis (AD) are at an increased risk of developing juvenile idiopathic arthritis (JIA).
Major finding: Patients with AD vs control individuals had an increased risk for JIA (adjusted odds ratio [aOR] 1.58; 95% CI 1.41-1.77), especially for psoriatic JIA (aOR 2.75; 95% CI 1.64-4.60).
Study details: This population-based register study included 70,584 patients with AD aged <18 years at the time of the first AD diagnosis and 270,783 age- and sex-matched control individuals without AD.
Disclosures: This study did not receive any funding. Some authors declared serving as investigators for or receiving educational grants, speaker honoraria, or consultation honoraria from various organizations.
Source: Keskitalo PL et al. Juvenile idiopathic arthritis in children and adolescents with atopic dermatitis: A Finnish nationwide registry study. J Am Acad Dermatol. 2023 (Jan 3). Doi: 10.1016/j.jaad.2022.12.025
Key clinical point: Children and adolescents with atopic dermatitis (AD) are at an increased risk of developing juvenile idiopathic arthritis (JIA).
Major finding: Patients with AD vs control individuals had an increased risk for JIA (adjusted odds ratio [aOR] 1.58; 95% CI 1.41-1.77), especially for psoriatic JIA (aOR 2.75; 95% CI 1.64-4.60).
Study details: This population-based register study included 70,584 patients with AD aged <18 years at the time of the first AD diagnosis and 270,783 age- and sex-matched control individuals without AD.
Disclosures: This study did not receive any funding. Some authors declared serving as investigators for or receiving educational grants, speaker honoraria, or consultation honoraria from various organizations.
Source: Keskitalo PL et al. Juvenile idiopathic arthritis in children and adolescents with atopic dermatitis: A Finnish nationwide registry study. J Am Acad Dermatol. 2023 (Jan 3). Doi: 10.1016/j.jaad.2022.12.025
Key clinical point: Children and adolescents with atopic dermatitis (AD) are at an increased risk of developing juvenile idiopathic arthritis (JIA).
Major finding: Patients with AD vs control individuals had an increased risk for JIA (adjusted odds ratio [aOR] 1.58; 95% CI 1.41-1.77), especially for psoriatic JIA (aOR 2.75; 95% CI 1.64-4.60).
Study details: This population-based register study included 70,584 patients with AD aged <18 years at the time of the first AD diagnosis and 270,783 age- and sex-matched control individuals without AD.
Disclosures: This study did not receive any funding. Some authors declared serving as investigators for or receiving educational grants, speaker honoraria, or consultation honoraria from various organizations.
Source: Keskitalo PL et al. Juvenile idiopathic arthritis in children and adolescents with atopic dermatitis: A Finnish nationwide registry study. J Am Acad Dermatol. 2023 (Jan 3). Doi: 10.1016/j.jaad.2022.12.025
Atopic dermatitis: No association between dupilumab use and malignancy
Key clinical point: Treatment of patients with atopic dermatitis (AD) with dupilumab is not associated with the development of primary or recurrent malignancy.
Major finding: Dupilumab-exposed and unexposed patients had comparable incidence rates of primary malignancies (adjusted hazard ratio [aHR], 1.010; P = .946), keratinocyte cancers (aHR, 0.994; P = .973), and recurrent cancers (aHR, 0.828; P = .758) per 1,000 person-years.
Study details: This single-center 5-year retrospective study included 9,707 patients with AD, of which 1,627 patients received dupilumab treatment.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants for and/or receiving research funds from various organizations.
Source: Owji S et al. No association between dupilumab use and short-term cancer development in atopic dermatitis patients. J Allergy Clin Immunol Pract. 2022 (Dec 26). Doi: 10.1016/j.jaip.2022.12.018.
Key clinical point: Treatment of patients with atopic dermatitis (AD) with dupilumab is not associated with the development of primary or recurrent malignancy.
Major finding: Dupilumab-exposed and unexposed patients had comparable incidence rates of primary malignancies (adjusted hazard ratio [aHR], 1.010; P = .946), keratinocyte cancers (aHR, 0.994; P = .973), and recurrent cancers (aHR, 0.828; P = .758) per 1,000 person-years.
Study details: This single-center 5-year retrospective study included 9,707 patients with AD, of which 1,627 patients received dupilumab treatment.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants for and/or receiving research funds from various organizations.
Source: Owji S et al. No association between dupilumab use and short-term cancer development in atopic dermatitis patients. J Allergy Clin Immunol Pract. 2022 (Dec 26). Doi: 10.1016/j.jaip.2022.12.018.
Key clinical point: Treatment of patients with atopic dermatitis (AD) with dupilumab is not associated with the development of primary or recurrent malignancy.
Major finding: Dupilumab-exposed and unexposed patients had comparable incidence rates of primary malignancies (adjusted hazard ratio [aHR], 1.010; P = .946), keratinocyte cancers (aHR, 0.994; P = .973), and recurrent cancers (aHR, 0.828; P = .758) per 1,000 person-years.
Study details: This single-center 5-year retrospective study included 9,707 patients with AD, of which 1,627 patients received dupilumab treatment.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants for and/or receiving research funds from various organizations.
Source: Owji S et al. No association between dupilumab use and short-term cancer development in atopic dermatitis patients. J Allergy Clin Immunol Pract. 2022 (Dec 26). Doi: 10.1016/j.jaip.2022.12.018.
Childhood atopic dermatitis is associated with increased fatigue
Key clinical point: Fatigue is a common symptom in children with atopic dermatitis (AD), particularly those with moderate-to-severe AD, and thus should be considered in clinical practice and trials.
Major finding: Most children had no (38.6%) or mild (32.1%) parent-proxy fatigue, but 27.2% had moderate fatigue and 2.0% had severe fatigue. Higher proportions of children with moderate-to-severe parent-proxy Patient-Reported Outcome Measurement Information System Pediatric fatigue scores were those with moderate (25.7%/1.4%) and severe (39.3%/5.4%) AD vs mild AD (18.0%/0.0%), as determined by Investigator’s Global Assessment, especially those with 5-6 (44.4%/0.0%) or 7 (44.2%/5.2%) nights of sleep disturbance from eczema.
Study details: This cross-sectional observational study included 248 children aged 0-17 years with AD.
Disclosures: This study was funded by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
Source: Rangel SM et al. Prevalence and associations of fatigue in childhood atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2022 (Dec 21). Doi: 10.1111/jdv.18819
Key clinical point: Fatigue is a common symptom in children with atopic dermatitis (AD), particularly those with moderate-to-severe AD, and thus should be considered in clinical practice and trials.
Major finding: Most children had no (38.6%) or mild (32.1%) parent-proxy fatigue, but 27.2% had moderate fatigue and 2.0% had severe fatigue. Higher proportions of children with moderate-to-severe parent-proxy Patient-Reported Outcome Measurement Information System Pediatric fatigue scores were those with moderate (25.7%/1.4%) and severe (39.3%/5.4%) AD vs mild AD (18.0%/0.0%), as determined by Investigator’s Global Assessment, especially those with 5-6 (44.4%/0.0%) or 7 (44.2%/5.2%) nights of sleep disturbance from eczema.
Study details: This cross-sectional observational study included 248 children aged 0-17 years with AD.
Disclosures: This study was funded by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
Source: Rangel SM et al. Prevalence and associations of fatigue in childhood atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2022 (Dec 21). Doi: 10.1111/jdv.18819
Key clinical point: Fatigue is a common symptom in children with atopic dermatitis (AD), particularly those with moderate-to-severe AD, and thus should be considered in clinical practice and trials.
Major finding: Most children had no (38.6%) or mild (32.1%) parent-proxy fatigue, but 27.2% had moderate fatigue and 2.0% had severe fatigue. Higher proportions of children with moderate-to-severe parent-proxy Patient-Reported Outcome Measurement Information System Pediatric fatigue scores were those with moderate (25.7%/1.4%) and severe (39.3%/5.4%) AD vs mild AD (18.0%/0.0%), as determined by Investigator’s Global Assessment, especially those with 5-6 (44.4%/0.0%) or 7 (44.2%/5.2%) nights of sleep disturbance from eczema.
Study details: This cross-sectional observational study included 248 children aged 0-17 years with AD.
Disclosures: This study was funded by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
Source: Rangel SM et al. Prevalence and associations of fatigue in childhood atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2022 (Dec 21). Doi: 10.1111/jdv.18819
Prolonged dupilumab therapy is safe and effective in moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab maintained efficacy against moderate-to-severe atopic dermatitis (AD) with no new safety signals over 104 weeks; however, it was ineffective in treating head-and-neck AD.
Major finding: The median Eczema Area and Severity Index score decreased significantly from 18.0 at baseline to 2.0 at week 52 and 1.7 at week 104 (both P < .0001); 35% of patients reported an adverse event, with conjunctivitis being the most common (25%). Although the 104-week treatment persistence rate was 86%, the majority of patients still had AD in the head-and-neck area.
Study details: This real-world study included 347 adult patients with moderate-to-severe AD who received dupilumab and were registered in the prospective Severe and ChRonic Atopic dermatitis Treatment CoHort (SCRATCH) registry during 2017-2022.
Disclosures: The SCRATCH registry was supported by research grants from Sanofi-Genzyme and Pfizer. Some authors reported ties with various organizations, including Sanofi-Genzyme and Pfizer.
Source: Vittrup I et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: Ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jan 6). Doi: 10.1111/jdv.18849
Key clinical point: Dupilumab maintained efficacy against moderate-to-severe atopic dermatitis (AD) with no new safety signals over 104 weeks; however, it was ineffective in treating head-and-neck AD.
Major finding: The median Eczema Area and Severity Index score decreased significantly from 18.0 at baseline to 2.0 at week 52 and 1.7 at week 104 (both P < .0001); 35% of patients reported an adverse event, with conjunctivitis being the most common (25%). Although the 104-week treatment persistence rate was 86%, the majority of patients still had AD in the head-and-neck area.
Study details: This real-world study included 347 adult patients with moderate-to-severe AD who received dupilumab and were registered in the prospective Severe and ChRonic Atopic dermatitis Treatment CoHort (SCRATCH) registry during 2017-2022.
Disclosures: The SCRATCH registry was supported by research grants from Sanofi-Genzyme and Pfizer. Some authors reported ties with various organizations, including Sanofi-Genzyme and Pfizer.
Source: Vittrup I et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: Ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jan 6). Doi: 10.1111/jdv.18849
Key clinical point: Dupilumab maintained efficacy against moderate-to-severe atopic dermatitis (AD) with no new safety signals over 104 weeks; however, it was ineffective in treating head-and-neck AD.
Major finding: The median Eczema Area and Severity Index score decreased significantly from 18.0 at baseline to 2.0 at week 52 and 1.7 at week 104 (both P < .0001); 35% of patients reported an adverse event, with conjunctivitis being the most common (25%). Although the 104-week treatment persistence rate was 86%, the majority of patients still had AD in the head-and-neck area.
Study details: This real-world study included 347 adult patients with moderate-to-severe AD who received dupilumab and were registered in the prospective Severe and ChRonic Atopic dermatitis Treatment CoHort (SCRATCH) registry during 2017-2022.
Disclosures: The SCRATCH registry was supported by research grants from Sanofi-Genzyme and Pfizer. Some authors reported ties with various organizations, including Sanofi-Genzyme and Pfizer.
Source: Vittrup I et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: Ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jan 6). Doi: 10.1111/jdv.18849
Upadacitinib is effective in treating difficult-to-treat moderate-to-severe atopic dermatitis
Key clinical point: Upadacitinib is effective and safe for the treatment of patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the percentages of patients achieving the Eczema Area and Severity Index (EASI) 50, EASI 75, EASI 90, and EASI 100 were 94.29%, 91.43%, 74.29%, and 60.0%, respectively; 91.43% of patients achieved a ≥4-point decrease in itch-numerical rating scale score. No severe adverse events were reported.
Study details: This single-center retrospective real-life study included 38 patients aged ≥12 years with moderate-to-severe AD and inadequate response, intolerance, or contraindications to cyclosporine or dupilumab who had received upadacitinib (15/30 mg daily) for ≥8 weeks; 35 patients received the treatment for 16 weeks.
Disclosures: This study was supported by grants from Fondazione Roma, Italian Ministry of Health (Rome, Italy), “Ricerca Finalizzata” project. Some authors reported ties with various organizations.
Source: Gargiulo L et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: A single-center 16-week study. Dermatol Ther (Heidelb). 2023 (Jan 9). Doi: 10.1007/s13555-022-00882-z
Key clinical point: Upadacitinib is effective and safe for the treatment of patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the percentages of patients achieving the Eczema Area and Severity Index (EASI) 50, EASI 75, EASI 90, and EASI 100 were 94.29%, 91.43%, 74.29%, and 60.0%, respectively; 91.43% of patients achieved a ≥4-point decrease in itch-numerical rating scale score. No severe adverse events were reported.
Study details: This single-center retrospective real-life study included 38 patients aged ≥12 years with moderate-to-severe AD and inadequate response, intolerance, or contraindications to cyclosporine or dupilumab who had received upadacitinib (15/30 mg daily) for ≥8 weeks; 35 patients received the treatment for 16 weeks.
Disclosures: This study was supported by grants from Fondazione Roma, Italian Ministry of Health (Rome, Italy), “Ricerca Finalizzata” project. Some authors reported ties with various organizations.
Source: Gargiulo L et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: A single-center 16-week study. Dermatol Ther (Heidelb). 2023 (Jan 9). Doi: 10.1007/s13555-022-00882-z
Key clinical point: Upadacitinib is effective and safe for the treatment of patients with difficult-to-treat moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the percentages of patients achieving the Eczema Area and Severity Index (EASI) 50, EASI 75, EASI 90, and EASI 100 were 94.29%, 91.43%, 74.29%, and 60.0%, respectively; 91.43% of patients achieved a ≥4-point decrease in itch-numerical rating scale score. No severe adverse events were reported.
Study details: This single-center retrospective real-life study included 38 patients aged ≥12 years with moderate-to-severe AD and inadequate response, intolerance, or contraindications to cyclosporine or dupilumab who had received upadacitinib (15/30 mg daily) for ≥8 weeks; 35 patients received the treatment for 16 weeks.
Disclosures: This study was supported by grants from Fondazione Roma, Italian Ministry of Health (Rome, Italy), “Ricerca Finalizzata” project. Some authors reported ties with various organizations.
Source: Gargiulo L et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: A single-center 16-week study. Dermatol Ther (Heidelb). 2023 (Jan 9). Doi: 10.1007/s13555-022-00882-z
Long-term integrated safety of baricitinib in moderate-to-severe atopic dermatitis
Key clinical point: The safety profile of baricitinib in patients with moderate-to-severe atopic dermatitis (AD) was similar to that reported in earlier analyses; major adverse cardiovascular event (MACE), pulmonary embolism (PE), and malignancy were within the background range.
Major finding: The adjusted incidence rate/100 patient-years at risk for any infection was 67.2 and that for herpes simplex, herpes zoster, opportunistic infections, serious adverse events, MACE, PE, and 14 malignancies excluding nonmelanoma skin cancer was 6.7, 2.8, 0.3, 5.2, 0.15, 0.06, and 0.3, respectively.
Study details: This updated integrated analysis of eight clinical trials included 2636 patients with moderate-to-severe AD treated with ≥1 baricitinib dose (1/2/4 mg) through 3.9 years (4628.4 patient-years of exposure).
Disclosures: Baricitinib is developed by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Five authors declared being employees and stockholders of Eli Lilly.
Source: Bieber T et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: An updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2022 (Dec 22). Doi: 10.1080/09546634.2022.2161812
Key clinical point: The safety profile of baricitinib in patients with moderate-to-severe atopic dermatitis (AD) was similar to that reported in earlier analyses; major adverse cardiovascular event (MACE), pulmonary embolism (PE), and malignancy were within the background range.
Major finding: The adjusted incidence rate/100 patient-years at risk for any infection was 67.2 and that for herpes simplex, herpes zoster, opportunistic infections, serious adverse events, MACE, PE, and 14 malignancies excluding nonmelanoma skin cancer was 6.7, 2.8, 0.3, 5.2, 0.15, 0.06, and 0.3, respectively.
Study details: This updated integrated analysis of eight clinical trials included 2636 patients with moderate-to-severe AD treated with ≥1 baricitinib dose (1/2/4 mg) through 3.9 years (4628.4 patient-years of exposure).
Disclosures: Baricitinib is developed by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Five authors declared being employees and stockholders of Eli Lilly.
Source: Bieber T et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: An updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2022 (Dec 22). Doi: 10.1080/09546634.2022.2161812
Key clinical point: The safety profile of baricitinib in patients with moderate-to-severe atopic dermatitis (AD) was similar to that reported in earlier analyses; major adverse cardiovascular event (MACE), pulmonary embolism (PE), and malignancy were within the background range.
Major finding: The adjusted incidence rate/100 patient-years at risk for any infection was 67.2 and that for herpes simplex, herpes zoster, opportunistic infections, serious adverse events, MACE, PE, and 14 malignancies excluding nonmelanoma skin cancer was 6.7, 2.8, 0.3, 5.2, 0.15, 0.06, and 0.3, respectively.
Study details: This updated integrated analysis of eight clinical trials included 2636 patients with moderate-to-severe AD treated with ≥1 baricitinib dose (1/2/4 mg) through 3.9 years (4628.4 patient-years of exposure).
Disclosures: Baricitinib is developed by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Five authors declared being employees and stockholders of Eli Lilly.
Source: Bieber T et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: An updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2022 (Dec 22). Doi: 10.1080/09546634.2022.2161812
Insomnia diagnosis and treatment across the lifespan
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings, and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59
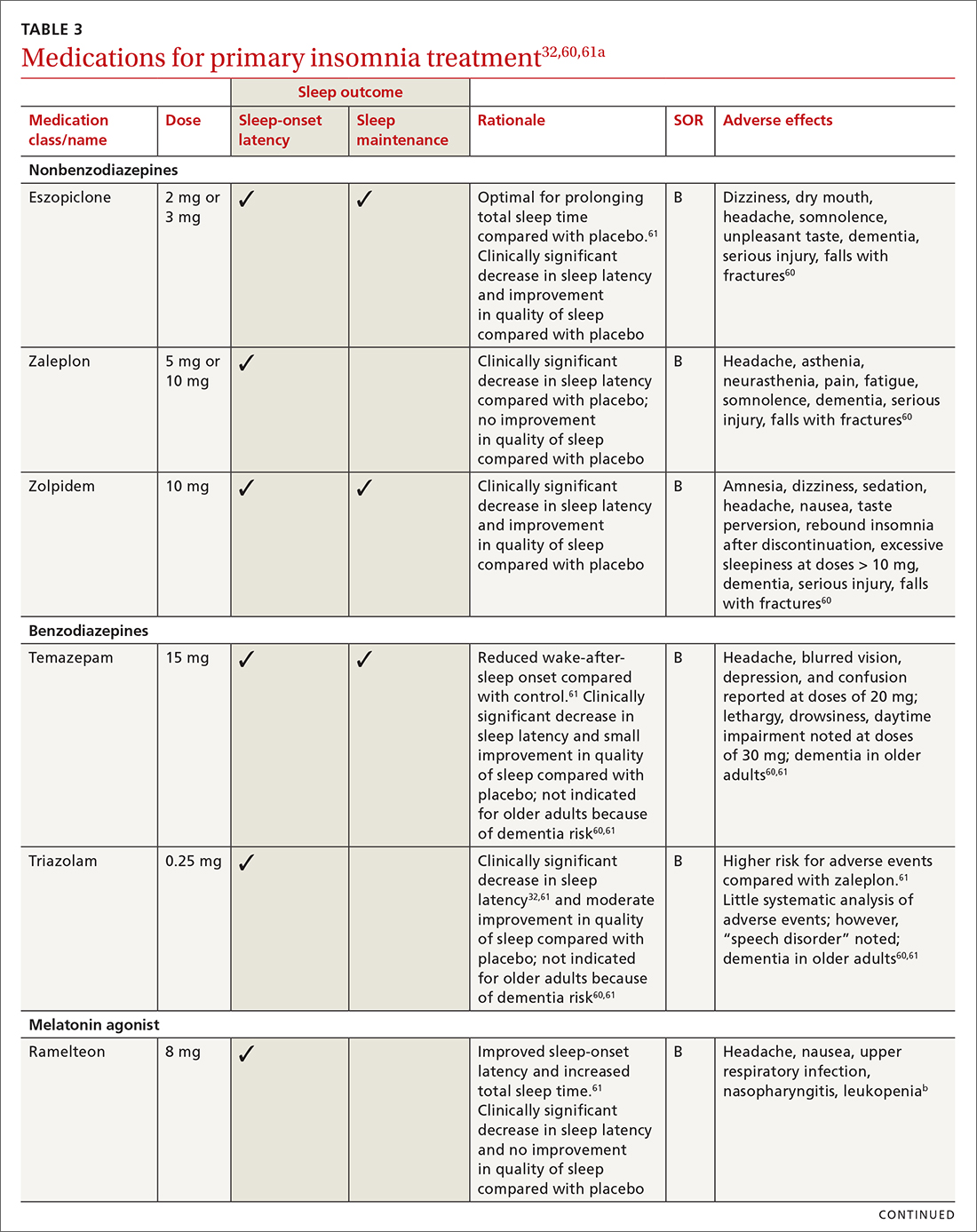
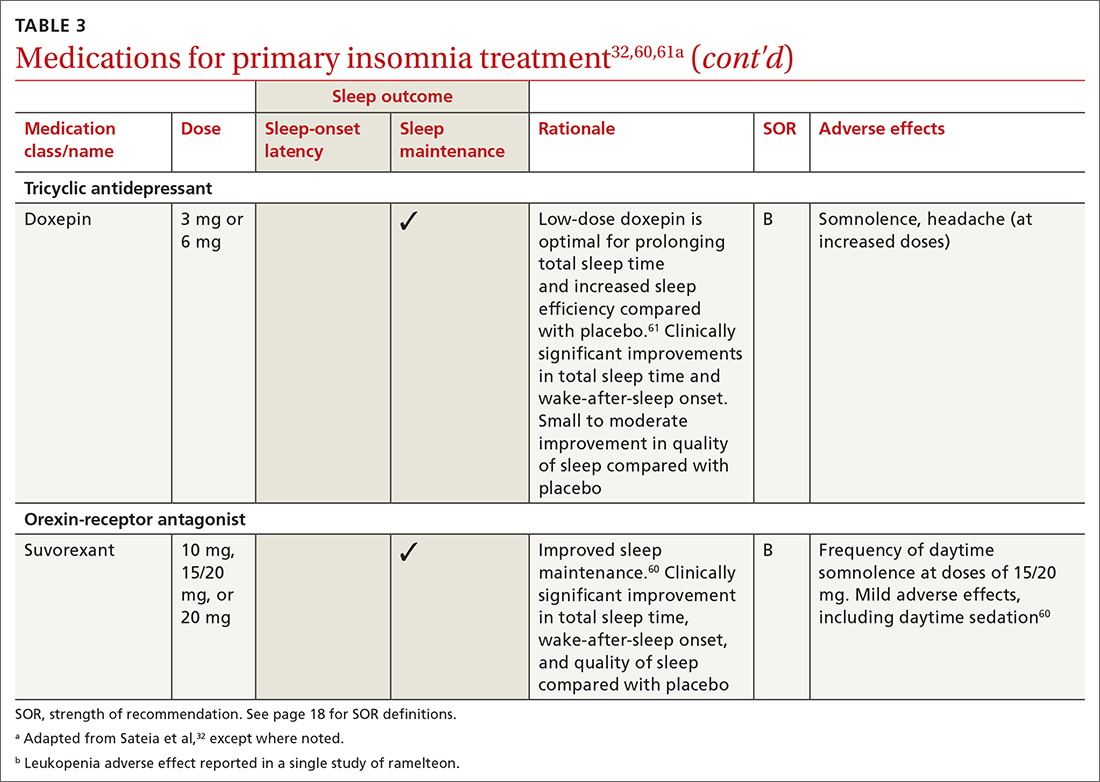
Pharmacologic interventions
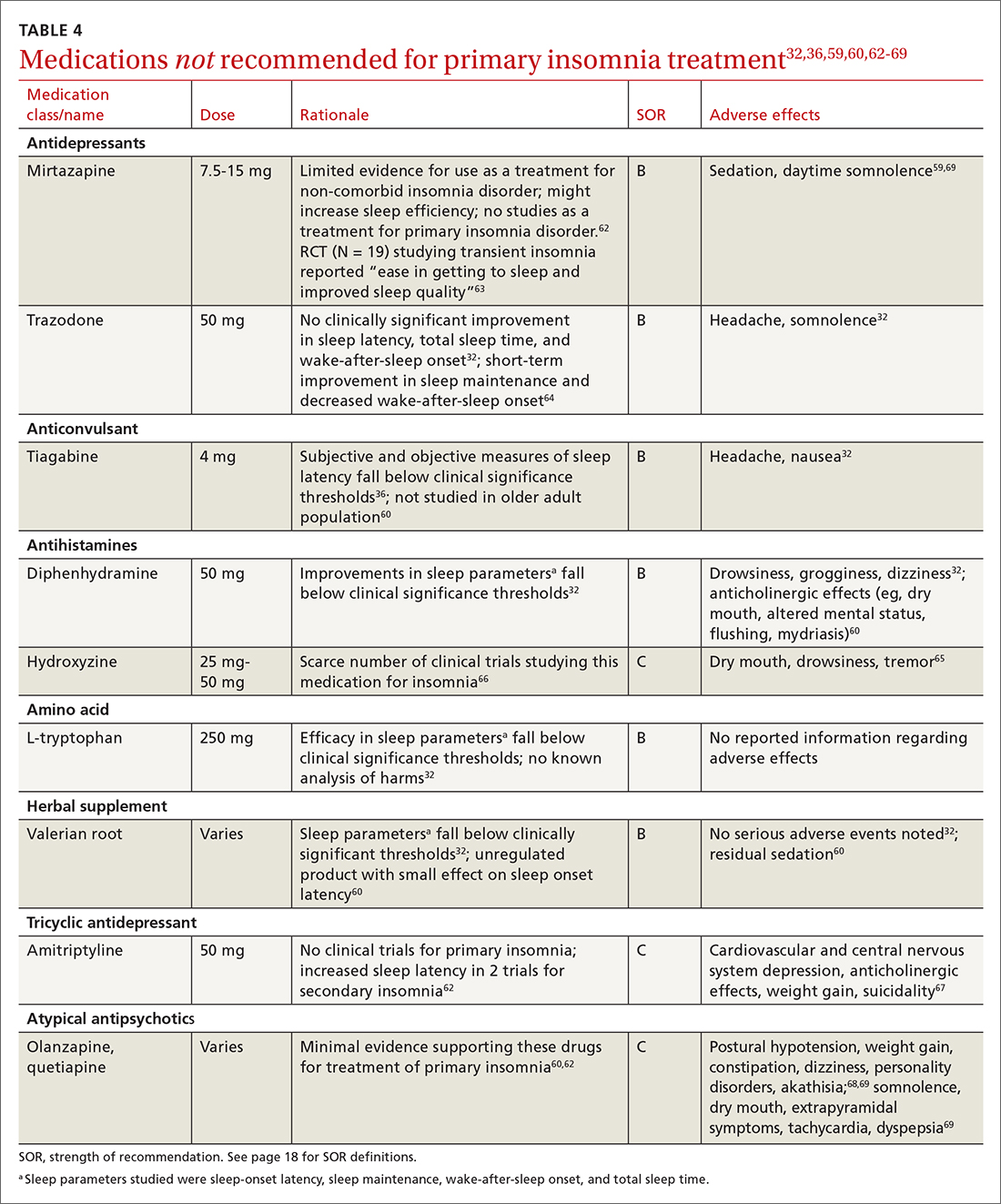
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61) are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32
Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings, and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61) are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings, and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61) are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
PRACTICE RECOMMENDATIONS
› Use a standard validated screening tool for the diagnosis of insomnia in all age groups. A
› Employ nonpharmacologic interventions as first-line treatment for insomnia in all populations. A
› Utilize sleep hygiene or cognitive behavioral therapy for insomnia in adolescents and all adults. A
› Initiate independent cognitive or behavioral therapies with younger children. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baricitinib offers a long-term treatment option for moderate-to-severe atopic dermatitis
Key clinical point: Over 68-week continuous treatment, 4 mg and 2 mg baricitinib plus topical corticosteroids (TCS) showed clinically meaningful efficacy in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The proportions of patients with a validated Investigator’s Global Assessment for AD score of 0/1 at weeks 32/68 in the 4 mg baricitinib intent-to-treat, 4 mg baricitinib responder or partial responder (RPR), and 2 mg baricitinib RPR cohorts were 21.6%/23.5%, 31.7%/34.9%, and 45.3%/30.2%, respectively; Eczema Area and Severity Index 75 response rates were 46.1%/43.1%, 57.1%/49.2%, and 69.8%/58.5%, respectively.
Study details: This ongoing extension study of BREEZE-AD7 (BREEZE-AD3) included 292 patients with moderate-to-severe AD, of which RPR receiving 2 mg baricitinib +TCS/4 mg baricitinib + TCS continued their original treatment, nonresponders receiving 2 mg baricitinib were reassigned to receive 2 mg or 4 mg baricitinib, and nonresponders receiving 4 mg baricitinib continued their treatment.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Four authors declared being employees and stockholders of Eli Lilly.
Source: Silverberg JI et al. Long-term efficacy (up to 68 weeks) of baricitinib in combination with topical corticosteroids in adult patients with moderate-to-severe atopic dermatitis: Analysis of treatment responders, partial responders and nonresponders originating from study BREEZE-AD7. J Eur Acad Dermatol Venereol. 2023 (Dec 14, 2022). Doi: 10.1111/jdv.18816
Key clinical point: Over 68-week continuous treatment, 4 mg and 2 mg baricitinib plus topical corticosteroids (TCS) showed clinically meaningful efficacy in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The proportions of patients with a validated Investigator’s Global Assessment for AD score of 0/1 at weeks 32/68 in the 4 mg baricitinib intent-to-treat, 4 mg baricitinib responder or partial responder (RPR), and 2 mg baricitinib RPR cohorts were 21.6%/23.5%, 31.7%/34.9%, and 45.3%/30.2%, respectively; Eczema Area and Severity Index 75 response rates were 46.1%/43.1%, 57.1%/49.2%, and 69.8%/58.5%, respectively.
Study details: This ongoing extension study of BREEZE-AD7 (BREEZE-AD3) included 292 patients with moderate-to-severe AD, of which RPR receiving 2 mg baricitinib +TCS/4 mg baricitinib + TCS continued their original treatment, nonresponders receiving 2 mg baricitinib were reassigned to receive 2 mg or 4 mg baricitinib, and nonresponders receiving 4 mg baricitinib continued their treatment.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Four authors declared being employees and stockholders of Eli Lilly.
Source: Silverberg JI et al. Long-term efficacy (up to 68 weeks) of baricitinib in combination with topical corticosteroids in adult patients with moderate-to-severe atopic dermatitis: Analysis of treatment responders, partial responders and nonresponders originating from study BREEZE-AD7. J Eur Acad Dermatol Venereol. 2023 (Dec 14, 2022). Doi: 10.1111/jdv.18816
Key clinical point: Over 68-week continuous treatment, 4 mg and 2 mg baricitinib plus topical corticosteroids (TCS) showed clinically meaningful efficacy in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: The proportions of patients with a validated Investigator’s Global Assessment for AD score of 0/1 at weeks 32/68 in the 4 mg baricitinib intent-to-treat, 4 mg baricitinib responder or partial responder (RPR), and 2 mg baricitinib RPR cohorts were 21.6%/23.5%, 31.7%/34.9%, and 45.3%/30.2%, respectively; Eczema Area and Severity Index 75 response rates were 46.1%/43.1%, 57.1%/49.2%, and 69.8%/58.5%, respectively.
Study details: This ongoing extension study of BREEZE-AD7 (BREEZE-AD3) included 292 patients with moderate-to-severe AD, of which RPR receiving 2 mg baricitinib +TCS/4 mg baricitinib + TCS continued their original treatment, nonresponders receiving 2 mg baricitinib were reassigned to receive 2 mg or 4 mg baricitinib, and nonresponders receiving 4 mg baricitinib continued their treatment.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Some authors reported ties with various organizations, including Eli Lilly. Four authors declared being employees and stockholders of Eli Lilly.
Source: Silverberg JI et al. Long-term efficacy (up to 68 weeks) of baricitinib in combination with topical corticosteroids in adult patients with moderate-to-severe atopic dermatitis: Analysis of treatment responders, partial responders and nonresponders originating from study BREEZE-AD7. J Eur Acad Dermatol Venereol. 2023 (Dec 14, 2022). Doi: 10.1111/jdv.18816
Abrocitinib rapidly relieves itch in moderate-to-severe atopic dermatitis
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) experienced a significantly greater reduction in itch as early as 4 days after treatment with 200 mg abrocitinib compared with dupilumab and placebo.
Major finding: At day 4 after treatment, a significantly higher proportion of patients achieved a ≥4-point improvement in Peak Pruritus Numerical Rating Scale score in the 200 mg abrocitinib group (18.6%) than in the placebo (6.0%; P < .003) and dupilumab (5.6%; P < .001) groups.
Study details: This post hoc analysis of JADE COMPARE included 837 adult patients with moderate-to-severe AD who were randomly assigned to receive oral abrocitinib (200 or 100 mg), subcutaneous dupilumab (300 mg), or placebo with medicated topical therapy for 16 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors reported ties with various organizations, including Pfizer. Six authors declared being current or former employees and shareholders of Pfizer.
Source: Ständer S et al. Early itch response with abrocitinib is associated with later efficacy outcomes in patients with moderate-to-severe atopic dermatitis: Subgroup analysis of the randomized phase III JADE COMPARE trial. Am J Clin Dermatol. 2022 (Dec 13). Doi: 10.1007/s40257-022-00738-4
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) experienced a significantly greater reduction in itch as early as 4 days after treatment with 200 mg abrocitinib compared with dupilumab and placebo.
Major finding: At day 4 after treatment, a significantly higher proportion of patients achieved a ≥4-point improvement in Peak Pruritus Numerical Rating Scale score in the 200 mg abrocitinib group (18.6%) than in the placebo (6.0%; P < .003) and dupilumab (5.6%; P < .001) groups.
Study details: This post hoc analysis of JADE COMPARE included 837 adult patients with moderate-to-severe AD who were randomly assigned to receive oral abrocitinib (200 or 100 mg), subcutaneous dupilumab (300 mg), or placebo with medicated topical therapy for 16 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors reported ties with various organizations, including Pfizer. Six authors declared being current or former employees and shareholders of Pfizer.
Source: Ständer S et al. Early itch response with abrocitinib is associated with later efficacy outcomes in patients with moderate-to-severe atopic dermatitis: Subgroup analysis of the randomized phase III JADE COMPARE trial. Am J Clin Dermatol. 2022 (Dec 13). Doi: 10.1007/s40257-022-00738-4
Key clinical point: Patients with moderate-to-severe atopic dermatitis (AD) experienced a significantly greater reduction in itch as early as 4 days after treatment with 200 mg abrocitinib compared with dupilumab and placebo.
Major finding: At day 4 after treatment, a significantly higher proportion of patients achieved a ≥4-point improvement in Peak Pruritus Numerical Rating Scale score in the 200 mg abrocitinib group (18.6%) than in the placebo (6.0%; P < .003) and dupilumab (5.6%; P < .001) groups.
Study details: This post hoc analysis of JADE COMPARE included 837 adult patients with moderate-to-severe AD who were randomly assigned to receive oral abrocitinib (200 or 100 mg), subcutaneous dupilumab (300 mg), or placebo with medicated topical therapy for 16 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors reported ties with various organizations, including Pfizer. Six authors declared being current or former employees and shareholders of Pfizer.
Source: Ständer S et al. Early itch response with abrocitinib is associated with later efficacy outcomes in patients with moderate-to-severe atopic dermatitis: Subgroup analysis of the randomized phase III JADE COMPARE trial. Am J Clin Dermatol. 2022 (Dec 13). Doi: 10.1007/s40257-022-00738-4
Shared decision-making (when you’re wearing the paper gown)
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
