User login
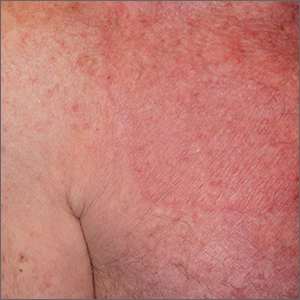
Lebrikizumab+topical corticosteroid shows promise in moderate-to-severe atopic dermatitis
Key clinical point: Compared with topical corticosteroids (TCS) alone, lebrikizumab+TCS significantly improved outcomes in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, a significantly higher proportion of patients in the lebrikizumab+TCS vs placebo+TCS group achieved an Investigator’s Global Assessment score of 0 or 1 (41.2% vs 22.1%; P = .01) and a 75% improvement in the Eczema Area and Severity Index (69.5% vs 42.2%; P < .001). The frequencies of patient-reported serious adverse events (AE) were similar in both groups (<2%); most treatment-emergent AE were of mild or moderate severity.
Study details: Findings are from a multicenter phase 3 study, ADhere, including 211 patients aged ≥ 12 years with moderate-to-severe AD who were randomly assigned to receive lebrikizumab+TCS (n = 145) or placebo+TCS (n = 66).
Disclosures: This study was sponsored by Dermira, Inc; a wholly-owned subsidiary of Eli Lilly and Company. Some authors reported ties with various organizations, including Eli Lilly. Six authors declared being employees or stockholders of Eli Lilly.
Source: Simpson EL et al for the ADhere Investigators. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: A randomized clinical trial (ADhere). JAMA Dermatol. 2023 (Jan 11). Doi: 10.1001/jamadermatol.2022.5534
Key clinical point: Compared with topical corticosteroids (TCS) alone, lebrikizumab+TCS significantly improved outcomes in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, a significantly higher proportion of patients in the lebrikizumab+TCS vs placebo+TCS group achieved an Investigator’s Global Assessment score of 0 or 1 (41.2% vs 22.1%; P = .01) and a 75% improvement in the Eczema Area and Severity Index (69.5% vs 42.2%; P < .001). The frequencies of patient-reported serious adverse events (AE) were similar in both groups (<2%); most treatment-emergent AE were of mild or moderate severity.
Study details: Findings are from a multicenter phase 3 study, ADhere, including 211 patients aged ≥ 12 years with moderate-to-severe AD who were randomly assigned to receive lebrikizumab+TCS (n = 145) or placebo+TCS (n = 66).
Disclosures: This study was sponsored by Dermira, Inc; a wholly-owned subsidiary of Eli Lilly and Company. Some authors reported ties with various organizations, including Eli Lilly. Six authors declared being employees or stockholders of Eli Lilly.
Source: Simpson EL et al for the ADhere Investigators. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: A randomized clinical trial (ADhere). JAMA Dermatol. 2023 (Jan 11). Doi: 10.1001/jamadermatol.2022.5534
Key clinical point: Compared with topical corticosteroids (TCS) alone, lebrikizumab+TCS significantly improved outcomes in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, a significantly higher proportion of patients in the lebrikizumab+TCS vs placebo+TCS group achieved an Investigator’s Global Assessment score of 0 or 1 (41.2% vs 22.1%; P = .01) and a 75% improvement in the Eczema Area and Severity Index (69.5% vs 42.2%; P < .001). The frequencies of patient-reported serious adverse events (AE) were similar in both groups (<2%); most treatment-emergent AE were of mild or moderate severity.
Study details: Findings are from a multicenter phase 3 study, ADhere, including 211 patients aged ≥ 12 years with moderate-to-severe AD who were randomly assigned to receive lebrikizumab+TCS (n = 145) or placebo+TCS (n = 66).
Disclosures: This study was sponsored by Dermira, Inc; a wholly-owned subsidiary of Eli Lilly and Company. Some authors reported ties with various organizations, including Eli Lilly. Six authors declared being employees or stockholders of Eli Lilly.
Source: Simpson EL et al for the ADhere Investigators. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: A randomized clinical trial (ADhere). JAMA Dermatol. 2023 (Jan 11). Doi: 10.1001/jamadermatol.2022.5534
How to integrate shared decision-making into your practice
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
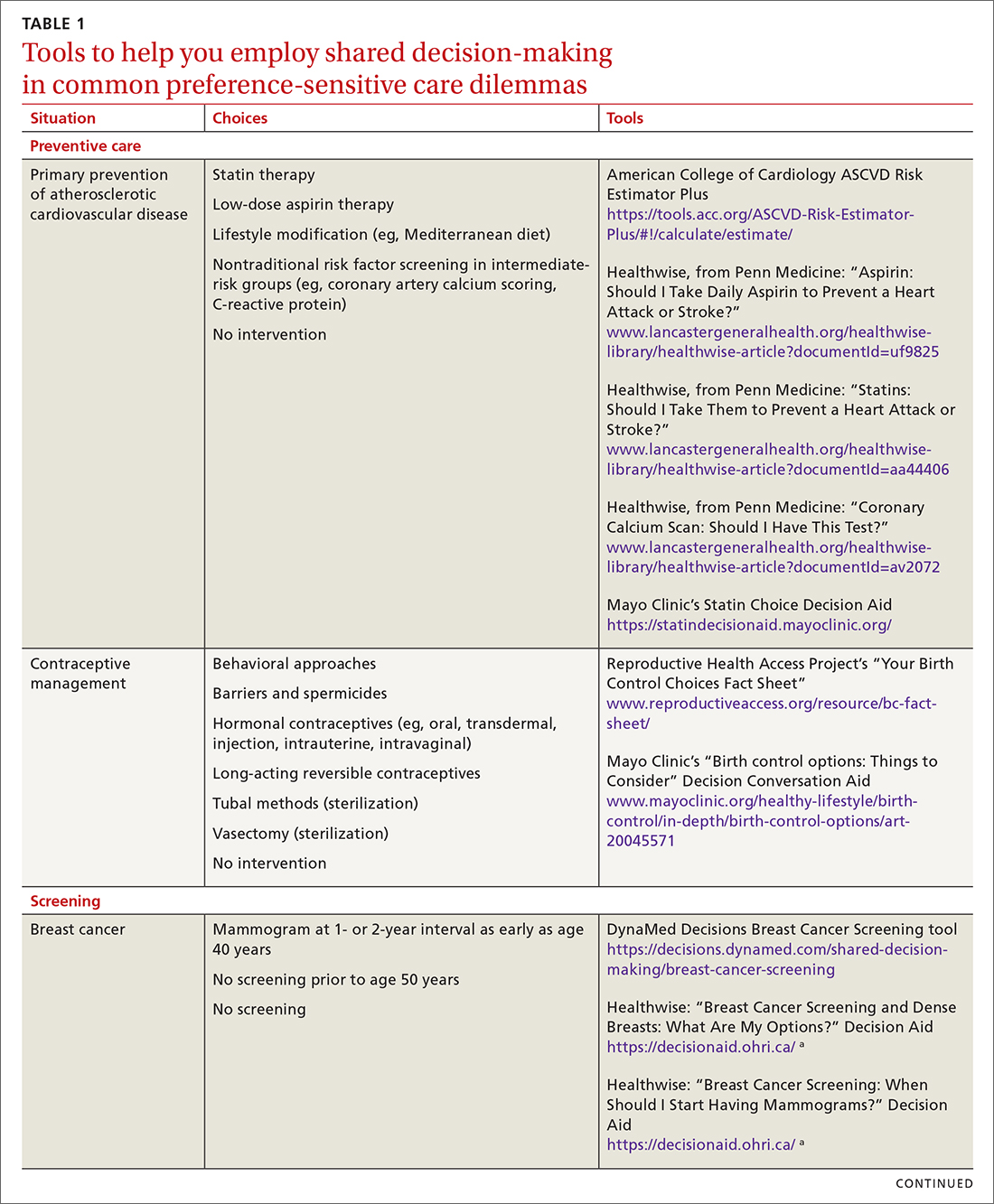
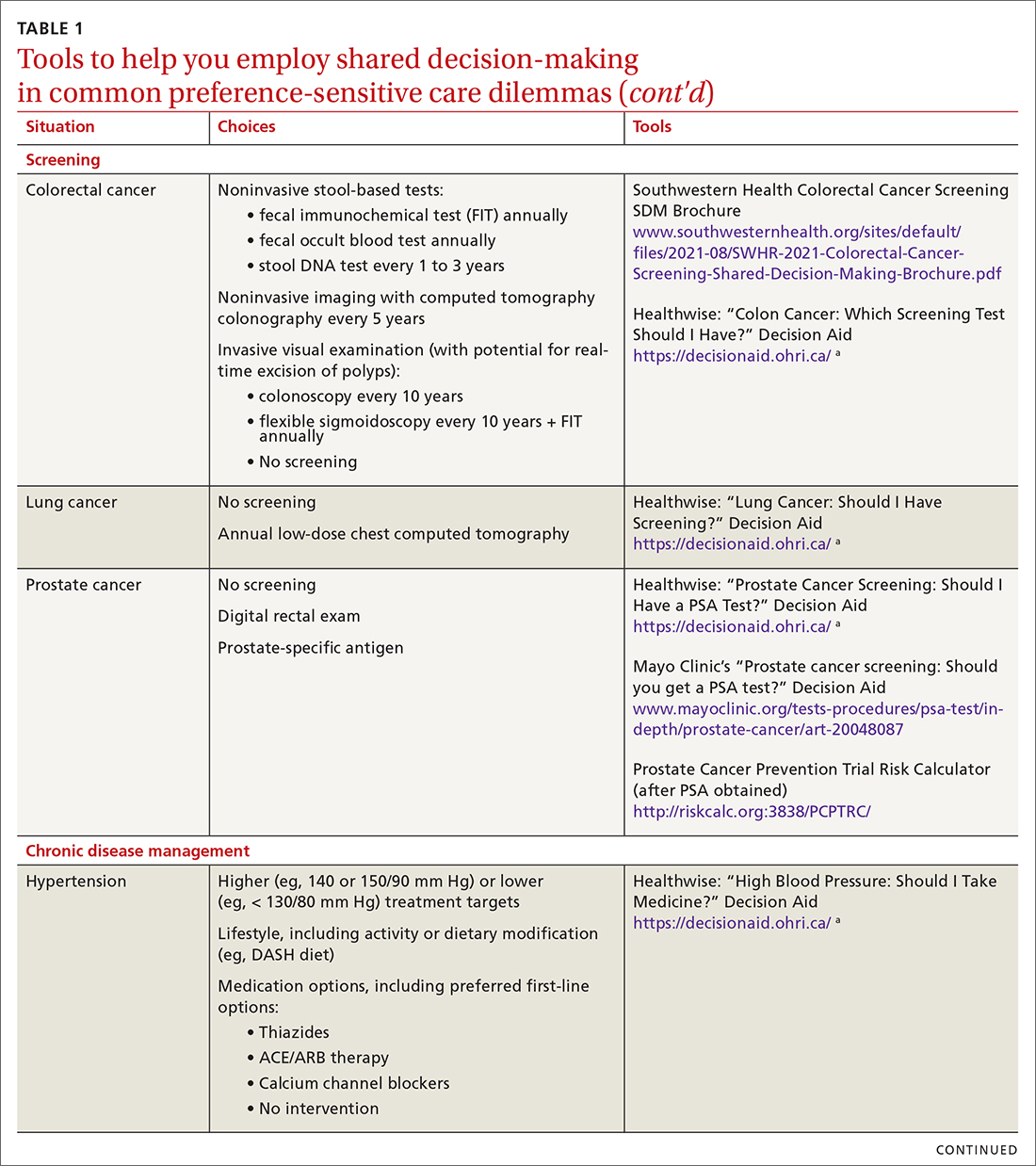
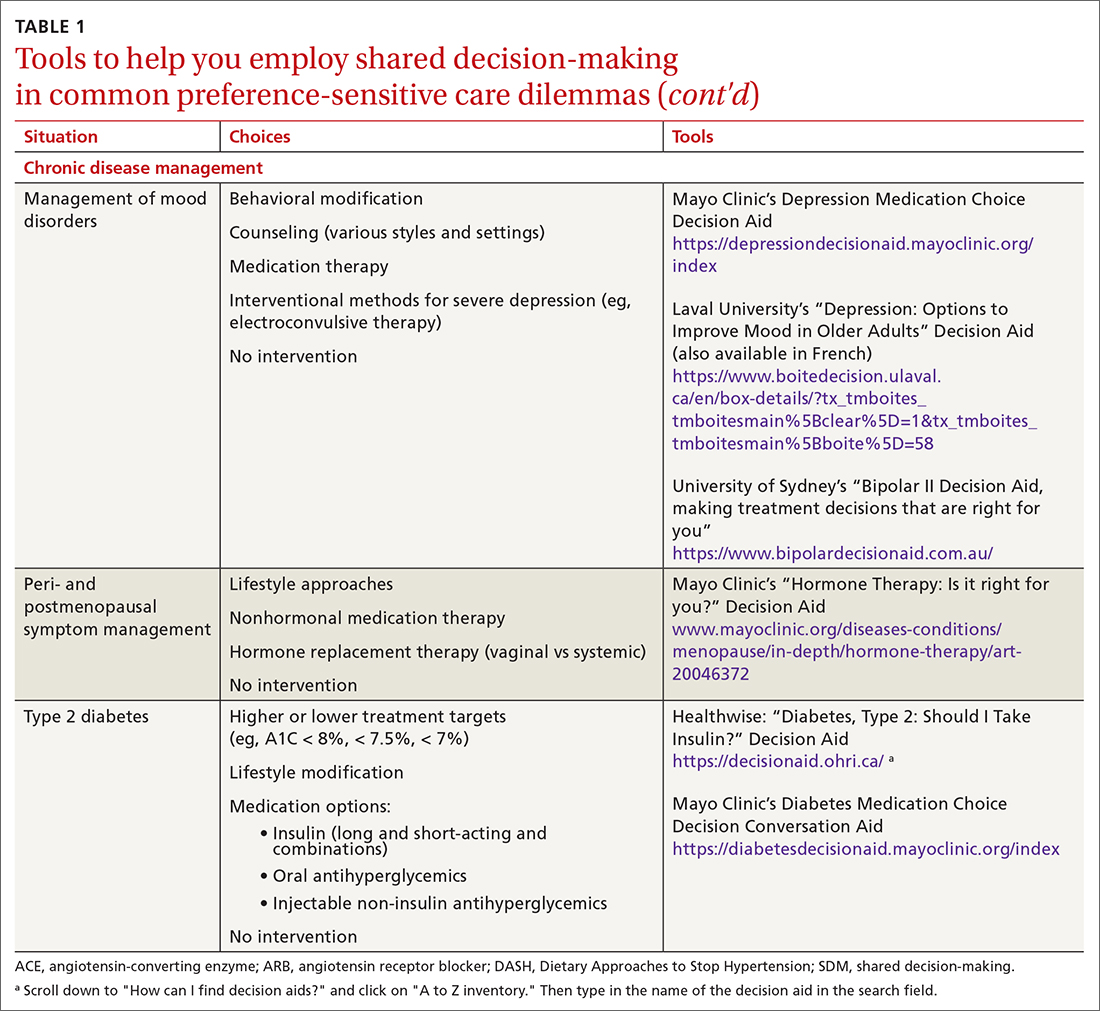
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.
How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.
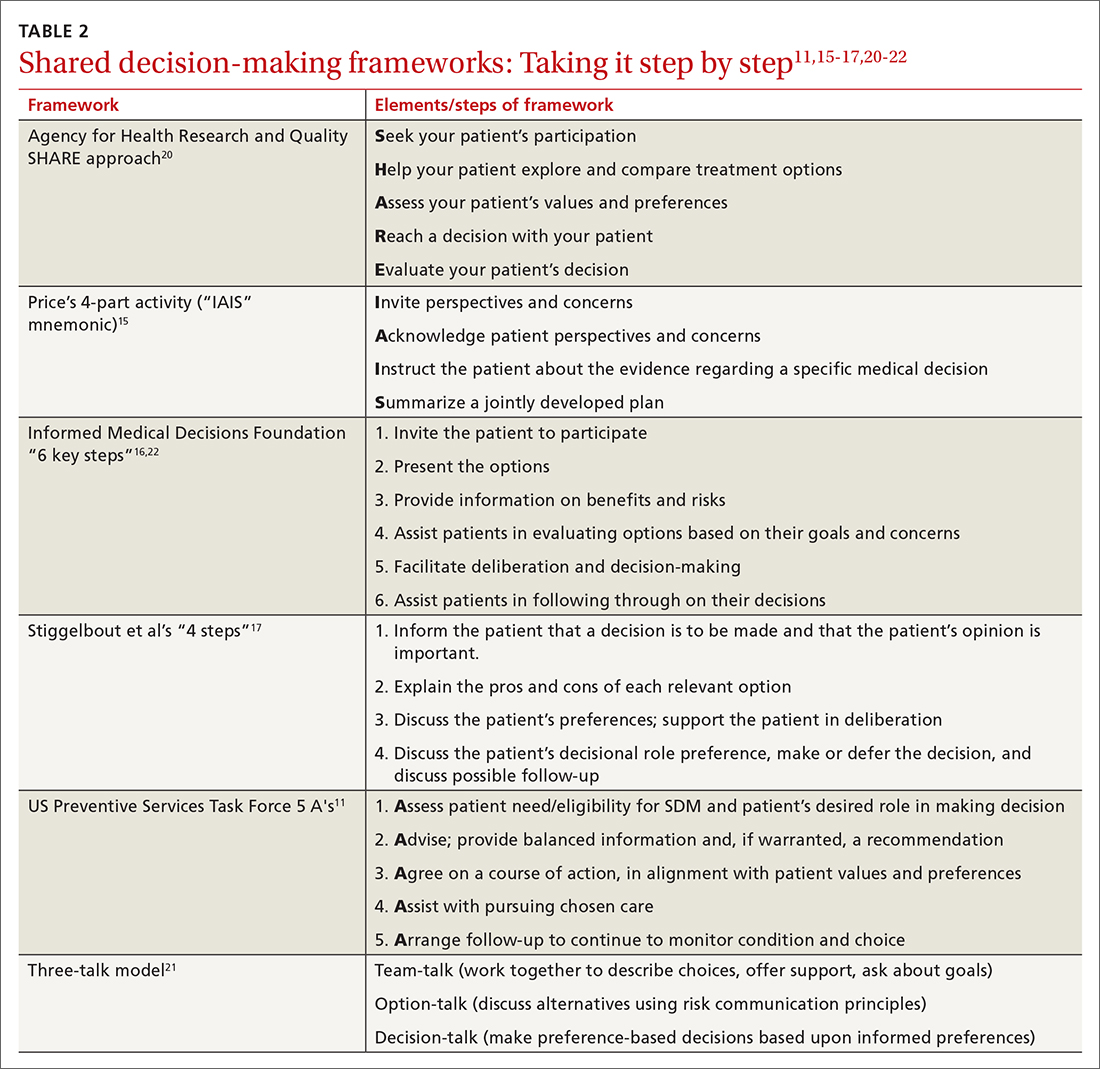
These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
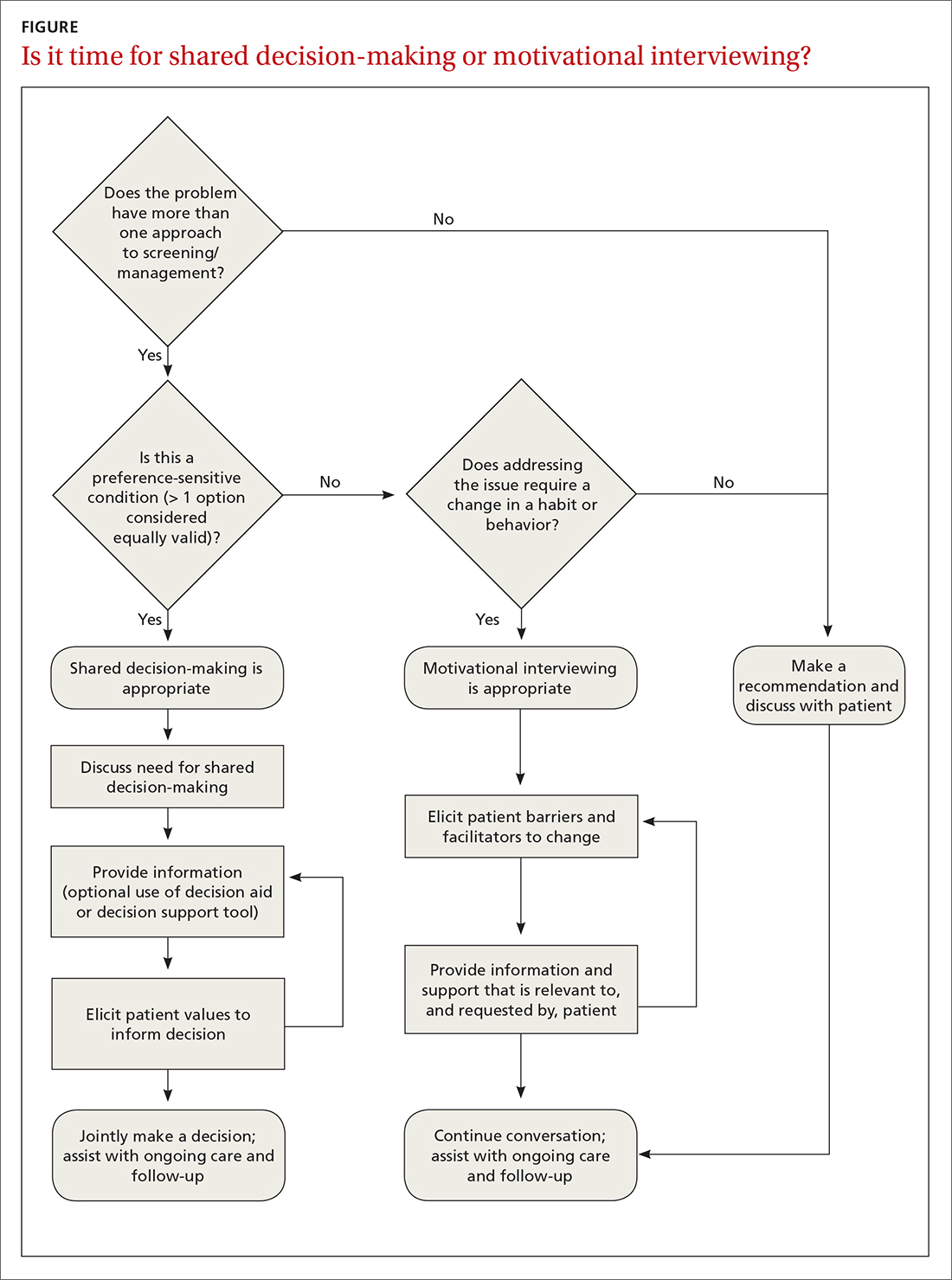
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.
Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.



How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.

These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.

Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.



How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.

These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.

Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
42-year-old man • altered mental status • vomiting • agitation • Dx?
THE CASE
A 42-year-old man with a history of bipolar disorder with psychotic features, asthma, and chronic pain was brought to the emergency department (ED) by his father due to altered mental status, coughing, and vomiting. The patient was unable to recall events earlier in the day in detail but stated that he remembered using his inhaler for his cough, which seemed to precipitate his vomiting. The patient’s home medications were listed as albuterol 90 mcg, methadone 90 mg/d, and quetiapine 100 mg.
While in the ED, the patient was tachycardic (heart rate, 102 bpm), but all other vital signs were normal. He was agitated and at one point required restraints. On exam, he had epigastric tenderness to palpation, and his lungs were clear to auscultation bilaterally.
Blood work was notable for an elevated lipase level of 729 U/L (normal range, 0-160 U/L). Complete blood count, comprehensive metabolic panel, urinalysis, chest x-ray, and alcohol levels were unremarkable. Computed tomography of the abdomen/pelvis and ultrasound of the abdomen showed excess stool and gallbladder sludge without cholecystitis.
The patient was treated symptomatically with intravenous fluids, ondansetron, and lorazepam. He was admitted with a working diagnosis of acute pancreatitis and possible acute psychosis in the setting of schizophrenia.
A few hours after presentation, the patient returned to his baseline mental status. Over the next 24 hours, his lipase level trended down to normal.
THE DIAGNOSIS
After the patient’s discharge, the pharmacist from his primary care provider’s office called as part of the routine post-hospital follow-up and a medication reconciliation was performed. During this call, the patient stated he had used 2 different nasal sprays prior to his ED presentation.
The pharmacist asked him to read the names of each medication. He related the first was naloxone and the second, fluticasone (neither of which was included on his medication list). Upon further questioning, the pharmacist elicited clarification from the patient that he had, in fact, taken 2 doses of naloxone, shortly after which his vomiting began.
Continue to: This additional history...
This additional history suggested the patient’s true diagnosis was acute opioid withdrawal precipitated by his accidental self-administration of naloxone.
DISCUSSION
Naloxone is a pure mu-opioid receptor antagonist that is used for opioid overdose.1 In the past decade, in response to the opioid epidemic, naloxone has become increasingly available in the community as a way of decreasing opioid-related deaths.1,2 The US Food and Drug Administration recommends that all patients who are prescribed opioids for pain or opioid use disorder, as well as those who are at increased risk for opioid overdose, should be prescribed naloxone and educated on its use. Patients who received a naloxone prescription from their primary care provider have been found to have 47% fewer opioid-related ED visits.3
Quick effects, potential for complications. Use of naloxone can rapidly induce opioid withdrawal symptoms, including gastrointestinal effects, tachycardia, and agitation, as well as diaphoresis, shivering, lacrimation, tremor, anxiety, mydriasis, and hypertension. Naloxone use can also lead to severe complications, such as violent behaviors, ventricular tachycardia or fibrillation, asystole, or pulmonary edema, in the period immediately following administration.4 These effects most often subside within 20 to 60 minutes after administration of naloxone, as the antagonist effect wears off.
The treatment of naloxone toxicity is supportive, with particular attention paid to the patient’s mental and respiratory status.
Our patient was advised by his primary care physician on the proper use of all of his medications, including nasal sprays. The clinic pharmacist also met with him for an additional educational session on the proper use of naloxone.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Given the widespread use of naloxone, proper education and counselling regarding this medication is crucial. Patients should be advised of what to expect after its use. In addition, physicians should always maintain updated patient medication lists, ensuring that they include naloxone if it has been prescribed for use as needed for opioid reversal, to assist in the emergency treatment of affected patients.5
CORRESPONDENCE
Erik Weitz, DO, Troy Beaumont Family Medicine Residency, 44250 Dequindre Road, Sterling Heights, MI 48314; [email protected]
1. Parkin S, Neale J, Brown C, et al. Opioid overdose reversals using naloxone in New York City by people who use opioids: implications for public health and overdose harm reduction approaches from a qualitative study. Int J Drug Policy. 2020;79:102751. doi: 10.1016/j.drugpo.2020.102751
2. Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63-88. doi: 10.1177/2042098617744161
3. Coffin PO, Behar E, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-52. doi: 10.7326/M15-2771
4. Osterwalder JJ. Naloxone—for intoxications with intravenous heroin and heroin mixtures—harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409-416. doi: 10.3109/15563659609013811
5. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397-403. doi: 10.7326/0003-4819-158-5-201303051-00006
THE CASE
A 42-year-old man with a history of bipolar disorder with psychotic features, asthma, and chronic pain was brought to the emergency department (ED) by his father due to altered mental status, coughing, and vomiting. The patient was unable to recall events earlier in the day in detail but stated that he remembered using his inhaler for his cough, which seemed to precipitate his vomiting. The patient’s home medications were listed as albuterol 90 mcg, methadone 90 mg/d, and quetiapine 100 mg.
While in the ED, the patient was tachycardic (heart rate, 102 bpm), but all other vital signs were normal. He was agitated and at one point required restraints. On exam, he had epigastric tenderness to palpation, and his lungs were clear to auscultation bilaterally.
Blood work was notable for an elevated lipase level of 729 U/L (normal range, 0-160 U/L). Complete blood count, comprehensive metabolic panel, urinalysis, chest x-ray, and alcohol levels were unremarkable. Computed tomography of the abdomen/pelvis and ultrasound of the abdomen showed excess stool and gallbladder sludge without cholecystitis.
The patient was treated symptomatically with intravenous fluids, ondansetron, and lorazepam. He was admitted with a working diagnosis of acute pancreatitis and possible acute psychosis in the setting of schizophrenia.
A few hours after presentation, the patient returned to his baseline mental status. Over the next 24 hours, his lipase level trended down to normal.
THE DIAGNOSIS
After the patient’s discharge, the pharmacist from his primary care provider’s office called as part of the routine post-hospital follow-up and a medication reconciliation was performed. During this call, the patient stated he had used 2 different nasal sprays prior to his ED presentation.
The pharmacist asked him to read the names of each medication. He related the first was naloxone and the second, fluticasone (neither of which was included on his medication list). Upon further questioning, the pharmacist elicited clarification from the patient that he had, in fact, taken 2 doses of naloxone, shortly after which his vomiting began.
Continue to: This additional history...
This additional history suggested the patient’s true diagnosis was acute opioid withdrawal precipitated by his accidental self-administration of naloxone.
DISCUSSION
Naloxone is a pure mu-opioid receptor antagonist that is used for opioid overdose.1 In the past decade, in response to the opioid epidemic, naloxone has become increasingly available in the community as a way of decreasing opioid-related deaths.1,2 The US Food and Drug Administration recommends that all patients who are prescribed opioids for pain or opioid use disorder, as well as those who are at increased risk for opioid overdose, should be prescribed naloxone and educated on its use. Patients who received a naloxone prescription from their primary care provider have been found to have 47% fewer opioid-related ED visits.3
Quick effects, potential for complications. Use of naloxone can rapidly induce opioid withdrawal symptoms, including gastrointestinal effects, tachycardia, and agitation, as well as diaphoresis, shivering, lacrimation, tremor, anxiety, mydriasis, and hypertension. Naloxone use can also lead to severe complications, such as violent behaviors, ventricular tachycardia or fibrillation, asystole, or pulmonary edema, in the period immediately following administration.4 These effects most often subside within 20 to 60 minutes after administration of naloxone, as the antagonist effect wears off.
The treatment of naloxone toxicity is supportive, with particular attention paid to the patient’s mental and respiratory status.
Our patient was advised by his primary care physician on the proper use of all of his medications, including nasal sprays. The clinic pharmacist also met with him for an additional educational session on the proper use of naloxone.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Given the widespread use of naloxone, proper education and counselling regarding this medication is crucial. Patients should be advised of what to expect after its use. In addition, physicians should always maintain updated patient medication lists, ensuring that they include naloxone if it has been prescribed for use as needed for opioid reversal, to assist in the emergency treatment of affected patients.5
CORRESPONDENCE
Erik Weitz, DO, Troy Beaumont Family Medicine Residency, 44250 Dequindre Road, Sterling Heights, MI 48314; [email protected]
THE CASE
A 42-year-old man with a history of bipolar disorder with psychotic features, asthma, and chronic pain was brought to the emergency department (ED) by his father due to altered mental status, coughing, and vomiting. The patient was unable to recall events earlier in the day in detail but stated that he remembered using his inhaler for his cough, which seemed to precipitate his vomiting. The patient’s home medications were listed as albuterol 90 mcg, methadone 90 mg/d, and quetiapine 100 mg.
While in the ED, the patient was tachycardic (heart rate, 102 bpm), but all other vital signs were normal. He was agitated and at one point required restraints. On exam, he had epigastric tenderness to palpation, and his lungs were clear to auscultation bilaterally.
Blood work was notable for an elevated lipase level of 729 U/L (normal range, 0-160 U/L). Complete blood count, comprehensive metabolic panel, urinalysis, chest x-ray, and alcohol levels were unremarkable. Computed tomography of the abdomen/pelvis and ultrasound of the abdomen showed excess stool and gallbladder sludge without cholecystitis.
The patient was treated symptomatically with intravenous fluids, ondansetron, and lorazepam. He was admitted with a working diagnosis of acute pancreatitis and possible acute psychosis in the setting of schizophrenia.
A few hours after presentation, the patient returned to his baseline mental status. Over the next 24 hours, his lipase level trended down to normal.
THE DIAGNOSIS
After the patient’s discharge, the pharmacist from his primary care provider’s office called as part of the routine post-hospital follow-up and a medication reconciliation was performed. During this call, the patient stated he had used 2 different nasal sprays prior to his ED presentation.
The pharmacist asked him to read the names of each medication. He related the first was naloxone and the second, fluticasone (neither of which was included on his medication list). Upon further questioning, the pharmacist elicited clarification from the patient that he had, in fact, taken 2 doses of naloxone, shortly after which his vomiting began.
Continue to: This additional history...
This additional history suggested the patient’s true diagnosis was acute opioid withdrawal precipitated by his accidental self-administration of naloxone.
DISCUSSION
Naloxone is a pure mu-opioid receptor antagonist that is used for opioid overdose.1 In the past decade, in response to the opioid epidemic, naloxone has become increasingly available in the community as a way of decreasing opioid-related deaths.1,2 The US Food and Drug Administration recommends that all patients who are prescribed opioids for pain or opioid use disorder, as well as those who are at increased risk for opioid overdose, should be prescribed naloxone and educated on its use. Patients who received a naloxone prescription from their primary care provider have been found to have 47% fewer opioid-related ED visits.3
Quick effects, potential for complications. Use of naloxone can rapidly induce opioid withdrawal symptoms, including gastrointestinal effects, tachycardia, and agitation, as well as diaphoresis, shivering, lacrimation, tremor, anxiety, mydriasis, and hypertension. Naloxone use can also lead to severe complications, such as violent behaviors, ventricular tachycardia or fibrillation, asystole, or pulmonary edema, in the period immediately following administration.4 These effects most often subside within 20 to 60 minutes after administration of naloxone, as the antagonist effect wears off.
The treatment of naloxone toxicity is supportive, with particular attention paid to the patient’s mental and respiratory status.
Our patient was advised by his primary care physician on the proper use of all of his medications, including nasal sprays. The clinic pharmacist also met with him for an additional educational session on the proper use of naloxone.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Given the widespread use of naloxone, proper education and counselling regarding this medication is crucial. Patients should be advised of what to expect after its use. In addition, physicians should always maintain updated patient medication lists, ensuring that they include naloxone if it has been prescribed for use as needed for opioid reversal, to assist in the emergency treatment of affected patients.5
CORRESPONDENCE
Erik Weitz, DO, Troy Beaumont Family Medicine Residency, 44250 Dequindre Road, Sterling Heights, MI 48314; [email protected]
1. Parkin S, Neale J, Brown C, et al. Opioid overdose reversals using naloxone in New York City by people who use opioids: implications for public health and overdose harm reduction approaches from a qualitative study. Int J Drug Policy. 2020;79:102751. doi: 10.1016/j.drugpo.2020.102751
2. Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63-88. doi: 10.1177/2042098617744161
3. Coffin PO, Behar E, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-52. doi: 10.7326/M15-2771
4. Osterwalder JJ. Naloxone—for intoxications with intravenous heroin and heroin mixtures—harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409-416. doi: 10.3109/15563659609013811
5. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397-403. doi: 10.7326/0003-4819-158-5-201303051-00006
1. Parkin S, Neale J, Brown C, et al. Opioid overdose reversals using naloxone in New York City by people who use opioids: implications for public health and overdose harm reduction approaches from a qualitative study. Int J Drug Policy. 2020;79:102751. doi: 10.1016/j.drugpo.2020.102751
2. Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63-88. doi: 10.1177/2042098617744161
3. Coffin PO, Behar E, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-52. doi: 10.7326/M15-2771
4. Osterwalder JJ. Naloxone—for intoxications with intravenous heroin and heroin mixtures—harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409-416. doi: 10.3109/15563659609013811
5. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397-403. doi: 10.7326/0003-4819-158-5-201303051-00006
Severe health diagnoses drive suicide risk
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH–EUROPE
The longevity gene: Healthy mutant reverses heart aging
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Put down the electronics after a concussion?
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
PRACTICE CHANGER
Advise your teenaged and young adult patients with concussion to avoid electronic screens in the first 48 hours after a concussion to minimize time to symptom resolution.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized clinical trial.1
Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediatrics.2021.2782
Circular patch on chest
A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6

A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6
Despite ongoing challenges, experts are optimistic about the future of MS therapy
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
Preoperative preparation for gender-affirming vaginoplasty surgery
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
Patients with COPD at higher risk of death 1 year after surgery
Patients with chronic obstructive pulmonary disease (COPD) are more likely to die within a year of undergoing elective surgery and to incur higher health care costs than are similar patients without COPD, data suggest.
An analysis of close to a million patient records found that, after adjustment for sociodemographic factors, procedure type, and comorbidities, patients with COPD were 26% more likely to die in the year after surgery than were those without COPD. Moreover, COPD was associated with a 4.6% increase in health care costs.
Previous studies have evaluated outcomes for the first 30 days after surgery. Those data “may not adequately capture the overall burden of surgery and how long it may take patients to recover,” study author Ashwin Sankar, MD, a clinician-investigator at St. Michael’s Hospital and assistant professor of anesthesia at the University of Toronto, told this news organization.
“We found that COPD often coexists with other conditions, like diabetes, coronary artery disease, and frailty,” Dr. Sankar added.
The study was published online in the Canadian Medical Association Journal.
Additional recovery support
The authors analyzed data from 932,616 patients who underwent intermediate-risk to high-risk elective noncardiac surgeries from 2005 to 2019 in Ontario. Procedures included carotid endarterectomy, open or endovascular abdominal aortic aneurysm repair, peripheral arterial bypass, total hip replacement, total knee replacement, shoulder surgery, large-bowel surgery, partial liver resection, pancreaticoduodenectomy, gastrectomy, esophagectomy, nephrectomy, cystectomy, prostatectomy, and hysterectomy.
The researchers quantified the associations of COPD with survival and costs. Their analyses included partial adjustment for sociodemographic factors and procedure type and full adjustment, which included comorbidities.
The primary outcome was all-cause death in the year after surgery; the secondary outcome was total health care costs in that year.
The mean age of the population was 65 years, and 60% of patients were women. A total of 170,482 (18%) patients had COPD. Compared with those without COPD, the patients with COPD were older and were more likely to be male, to be in a lower income quintile, to be residents of long-term care facilities, and to have been admitted to the hospital before surgery. They were also more likely to have comorbidities, including coronary artery disease, heart failure, and lung cancer.
A larger proportion of patients with COPD had frailty and medium to high comorbidity. They also more frequently underwent orthopedic, open upper abdominal, and vascular surgery.
During the year after surgery, 52,021 (5.6%) patients died, including 18,007 (10.6%) with COPD and 34,014 (4.5%) without. Those with COPD were more likely to die within 30 days of surgery (3.4% vs 1.2%).
For patients with COPD, the partially adjusted hazard ratio (HR) was 1.61 for risk of death; the fully adjusted HR was 1.26. COPD also was associated with a partially adjusted relative increase of 13.1% in health care costs and an increase of 4.6% with full adjustment.
Frailty, cancer, and procedure type were factors that modified the association between COPD and outcomes. “Procedures such as open aortic and upper abdominal surgery are associated with higher postoperative risks irrespective of COPD status, whereas others, such as orthopedic and lower abdominal surgery, may be of significantly greater risk for patients with COPD,” the authors wrote. “Our results suggest that perioperative management of patients with COPD requires careful consideration of the multiple domains that contribute to their elevated perioperative risk.
“Our finding that patients with COPD are at risk beyond 30 days after surgery suggests that it may be worthwhile to additionally support these patients’ recovery well beyond the first month after the procedure,” said Dr. Sankar.
Shared decision-making
Commenting on the study, William Whalen, MD, a pulmonary critical care specialist at Weill Cornell Medicine in New York, said, “I echo the authors’ sentiments that these findings highlight how chronically ill COPD patients are, which may be playing a role in the elevated mortality seen in this study.”
One caveat is in regard to the interpretation of the interaction effects of the study, he said. “Clinicians are unlikely to send patients who are frail or have multiple comorbidities to overly complex surgeries. Therefore, these effects may be misestimated due to selection bias.”
Two questions remain after reading the study, he added. “The first is how the degree of obstruction (i.e., the severity of COPD) impacts long-term mortality. Previous observational studies in nonsurgical COPD patients have shown increased mortality as the severity of obstruction increases. The second is how much of the long-term mortality observed in this study is related to respiratory disease from COPD. Patients with COPD are complex, and many die from nonrespiratory-related causes.”
Dr. Whalen suggests that discussion be held with the surgical team about the long-term morbidity and mortality with and without surgical intervention. Such a discussion could inform a shared decision-making process with the patient.
“Some procedures may be necessary to reduce immediate mortality, such as aortic aneurysmal repair, so [the risk of] longer-term mortality may be more acceptable in this setting,” he said. “Less straightforward are procedures that may improve quality of life. Would a patient accept an increased long-term mortality [risk] if that meant living without orthopedic-related pain?”
The study was funded by the Government of Ontario. Dr. Sankar and Dr. Whalen have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with chronic obstructive pulmonary disease (COPD) are more likely to die within a year of undergoing elective surgery and to incur higher health care costs than are similar patients without COPD, data suggest.
An analysis of close to a million patient records found that, after adjustment for sociodemographic factors, procedure type, and comorbidities, patients with COPD were 26% more likely to die in the year after surgery than were those without COPD. Moreover, COPD was associated with a 4.6% increase in health care costs.
Previous studies have evaluated outcomes for the first 30 days after surgery. Those data “may not adequately capture the overall burden of surgery and how long it may take patients to recover,” study author Ashwin Sankar, MD, a clinician-investigator at St. Michael’s Hospital and assistant professor of anesthesia at the University of Toronto, told this news organization.
“We found that COPD often coexists with other conditions, like diabetes, coronary artery disease, and frailty,” Dr. Sankar added.
The study was published online in the Canadian Medical Association Journal.
Additional recovery support
The authors analyzed data from 932,616 patients who underwent intermediate-risk to high-risk elective noncardiac surgeries from 2005 to 2019 in Ontario. Procedures included carotid endarterectomy, open or endovascular abdominal aortic aneurysm repair, peripheral arterial bypass, total hip replacement, total knee replacement, shoulder surgery, large-bowel surgery, partial liver resection, pancreaticoduodenectomy, gastrectomy, esophagectomy, nephrectomy, cystectomy, prostatectomy, and hysterectomy.
The researchers quantified the associations of COPD with survival and costs. Their analyses included partial adjustment for sociodemographic factors and procedure type and full adjustment, which included comorbidities.
The primary outcome was all-cause death in the year after surgery; the secondary outcome was total health care costs in that year.
The mean age of the population was 65 years, and 60% of patients were women. A total of 170,482 (18%) patients had COPD. Compared with those without COPD, the patients with COPD were older and were more likely to be male, to be in a lower income quintile, to be residents of long-term care facilities, and to have been admitted to the hospital before surgery. They were also more likely to have comorbidities, including coronary artery disease, heart failure, and lung cancer.
A larger proportion of patients with COPD had frailty and medium to high comorbidity. They also more frequently underwent orthopedic, open upper abdominal, and vascular surgery.
During the year after surgery, 52,021 (5.6%) patients died, including 18,007 (10.6%) with COPD and 34,014 (4.5%) without. Those with COPD were more likely to die within 30 days of surgery (3.4% vs 1.2%).
For patients with COPD, the partially adjusted hazard ratio (HR) was 1.61 for risk of death; the fully adjusted HR was 1.26. COPD also was associated with a partially adjusted relative increase of 13.1% in health care costs and an increase of 4.6% with full adjustment.
Frailty, cancer, and procedure type were factors that modified the association between COPD and outcomes. “Procedures such as open aortic and upper abdominal surgery are associated with higher postoperative risks irrespective of COPD status, whereas others, such as orthopedic and lower abdominal surgery, may be of significantly greater risk for patients with COPD,” the authors wrote. “Our results suggest that perioperative management of patients with COPD requires careful consideration of the multiple domains that contribute to their elevated perioperative risk.
“Our finding that patients with COPD are at risk beyond 30 days after surgery suggests that it may be worthwhile to additionally support these patients’ recovery well beyond the first month after the procedure,” said Dr. Sankar.
Shared decision-making
Commenting on the study, William Whalen, MD, a pulmonary critical care specialist at Weill Cornell Medicine in New York, said, “I echo the authors’ sentiments that these findings highlight how chronically ill COPD patients are, which may be playing a role in the elevated mortality seen in this study.”
One caveat is in regard to the interpretation of the interaction effects of the study, he said. “Clinicians are unlikely to send patients who are frail or have multiple comorbidities to overly complex surgeries. Therefore, these effects may be misestimated due to selection bias.”
Two questions remain after reading the study, he added. “The first is how the degree of obstruction (i.e., the severity of COPD) impacts long-term mortality. Previous observational studies in nonsurgical COPD patients have shown increased mortality as the severity of obstruction increases. The second is how much of the long-term mortality observed in this study is related to respiratory disease from COPD. Patients with COPD are complex, and many die from nonrespiratory-related causes.”
Dr. Whalen suggests that discussion be held with the surgical team about the long-term morbidity and mortality with and without surgical intervention. Such a discussion could inform a shared decision-making process with the patient.
“Some procedures may be necessary to reduce immediate mortality, such as aortic aneurysmal repair, so [the risk of] longer-term mortality may be more acceptable in this setting,” he said. “Less straightforward are procedures that may improve quality of life. Would a patient accept an increased long-term mortality [risk] if that meant living without orthopedic-related pain?”
The study was funded by the Government of Ontario. Dr. Sankar and Dr. Whalen have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with chronic obstructive pulmonary disease (COPD) are more likely to die within a year of undergoing elective surgery and to incur higher health care costs than are similar patients without COPD, data suggest.
An analysis of close to a million patient records found that, after adjustment for sociodemographic factors, procedure type, and comorbidities, patients with COPD were 26% more likely to die in the year after surgery than were those without COPD. Moreover, COPD was associated with a 4.6% increase in health care costs.
Previous studies have evaluated outcomes for the first 30 days after surgery. Those data “may not adequately capture the overall burden of surgery and how long it may take patients to recover,” study author Ashwin Sankar, MD, a clinician-investigator at St. Michael’s Hospital and assistant professor of anesthesia at the University of Toronto, told this news organization.
“We found that COPD often coexists with other conditions, like diabetes, coronary artery disease, and frailty,” Dr. Sankar added.
The study was published online in the Canadian Medical Association Journal.
Additional recovery support
The authors analyzed data from 932,616 patients who underwent intermediate-risk to high-risk elective noncardiac surgeries from 2005 to 2019 in Ontario. Procedures included carotid endarterectomy, open or endovascular abdominal aortic aneurysm repair, peripheral arterial bypass, total hip replacement, total knee replacement, shoulder surgery, large-bowel surgery, partial liver resection, pancreaticoduodenectomy, gastrectomy, esophagectomy, nephrectomy, cystectomy, prostatectomy, and hysterectomy.
The researchers quantified the associations of COPD with survival and costs. Their analyses included partial adjustment for sociodemographic factors and procedure type and full adjustment, which included comorbidities.
The primary outcome was all-cause death in the year after surgery; the secondary outcome was total health care costs in that year.
The mean age of the population was 65 years, and 60% of patients were women. A total of 170,482 (18%) patients had COPD. Compared with those without COPD, the patients with COPD were older and were more likely to be male, to be in a lower income quintile, to be residents of long-term care facilities, and to have been admitted to the hospital before surgery. They were also more likely to have comorbidities, including coronary artery disease, heart failure, and lung cancer.
A larger proportion of patients with COPD had frailty and medium to high comorbidity. They also more frequently underwent orthopedic, open upper abdominal, and vascular surgery.
During the year after surgery, 52,021 (5.6%) patients died, including 18,007 (10.6%) with COPD and 34,014 (4.5%) without. Those with COPD were more likely to die within 30 days of surgery (3.4% vs 1.2%).
For patients with COPD, the partially adjusted hazard ratio (HR) was 1.61 for risk of death; the fully adjusted HR was 1.26. COPD also was associated with a partially adjusted relative increase of 13.1% in health care costs and an increase of 4.6% with full adjustment.
Frailty, cancer, and procedure type were factors that modified the association between COPD and outcomes. “Procedures such as open aortic and upper abdominal surgery are associated with higher postoperative risks irrespective of COPD status, whereas others, such as orthopedic and lower abdominal surgery, may be of significantly greater risk for patients with COPD,” the authors wrote. “Our results suggest that perioperative management of patients with COPD requires careful consideration of the multiple domains that contribute to their elevated perioperative risk.
“Our finding that patients with COPD are at risk beyond 30 days after surgery suggests that it may be worthwhile to additionally support these patients’ recovery well beyond the first month after the procedure,” said Dr. Sankar.
Shared decision-making
Commenting on the study, William Whalen, MD, a pulmonary critical care specialist at Weill Cornell Medicine in New York, said, “I echo the authors’ sentiments that these findings highlight how chronically ill COPD patients are, which may be playing a role in the elevated mortality seen in this study.”
One caveat is in regard to the interpretation of the interaction effects of the study, he said. “Clinicians are unlikely to send patients who are frail or have multiple comorbidities to overly complex surgeries. Therefore, these effects may be misestimated due to selection bias.”
Two questions remain after reading the study, he added. “The first is how the degree of obstruction (i.e., the severity of COPD) impacts long-term mortality. Previous observational studies in nonsurgical COPD patients have shown increased mortality as the severity of obstruction increases. The second is how much of the long-term mortality observed in this study is related to respiratory disease from COPD. Patients with COPD are complex, and many die from nonrespiratory-related causes.”
Dr. Whalen suggests that discussion be held with the surgical team about the long-term morbidity and mortality with and without surgical intervention. Such a discussion could inform a shared decision-making process with the patient.
“Some procedures may be necessary to reduce immediate mortality, such as aortic aneurysmal repair, so [the risk of] longer-term mortality may be more acceptable in this setting,” he said. “Less straightforward are procedures that may improve quality of life. Would a patient accept an increased long-term mortality [risk] if that meant living without orthopedic-related pain?”
The study was funded by the Government of Ontario. Dr. Sankar and Dr. Whalen have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.