User login
Why do some people escape infection that sickens others?
During the COVID-19 pandemic, we’ve seen this play out time and time again when whole families get sick except for one or two fortunate family members. And at so-called superspreader events that infect many, a lucky few typically walk away with their health intact. Did the virus never enter their bodies? Or do some people have natural resistance to pathogens they’ve never been exposed to before encoded in their genes?
Resistance to infectious disease is much more than a scientific curiosity and studying how it works can be a path to curb future outbreaks.
“In the event that we could identify what makes some people resistant, that immediately opens avenues for therapeutics that we could apply in all those other people who do suffer from the disease,” says András Spaan, MD, a microbiologist at Rockefeller University in New York.
Dr. Spaan is part of an international effort to identify genetic variations that spare people from becoming infected with SARS-CoV-2, the virus that causes COVID-19.
There’s far more research on what drives the tendency to get infectious diseases than on resistance to them. But a few researchers are investigating resistance to some of the world’s most common and deadly infectious diseases, and in a few cases, they’ve already translated these insights into treatments.
Perhaps the strongest example of how odd genes of just a few people can inspire treatments to help many comes from research on the human immunodeficiency virus (HIV), the virus that causes acquired immune deficiency syndrome (AIDS).
A genetic quirk
In the mid-1990s, several groups of researchers independently identified a mutation in a gene called CCR5 linked to resistance to HIV infection.
The gene encodes a protein on the surface of some white blood cells that helps set up the movement of other immune cells to fight infections. HIV, meanwhile, uses the CCR5 protein to help it enter the white blood cells that it infects.
The mutation, known as delta 32, results in a shorter than usual protein that doesn’t reach the surface of the cell. People who carry two copies of the delta 32 form of CCR5 do not have any CCR5 protein on the outside of their white blood cells.
Researchers, led by molecular immunologist Philip Murphy, MD, at the National Institute of Allergy and Infectious Diseases in Bethesda, Md, showed in 1997 that people with two copies of the mutation were unusually common among a group of men who were at especially high risk of HIV exposure, but had never contracted the virus. And out of more than 700 HIV-positive people, none carried two copies of CCR5 delta 32.
Pharmaceutical companies used these insights to develop drugs to block CCR5 and delay the development of AIDS. For instance, the drug maraviroc, marketed by Pfizer, was approved for use in HIV-positive people in 2007.
Only a few examples of this kind of inborn, genetically determined complete resistance to infection have ever been heard of. All of them involve cell-surface molecules that are believed to help a virus or other pathogen gain entry to the cell.
Locking out illness
“The first step for any intracellular pathogen is getting inside the cell. And if you’re missing the doorway, then the virus can’t accomplish the first step in its life cycle,” Dr. Murphy says. “Getting inside is fundamental.”
Changes in cell-surface molecules can also make someone more likely to have an infection or severe disease. One such group of cell-surface molecules that have been linked to both increasing and decreasing the risk of various infections are histo-blood group antigens. The most familiar members of this group are the molecules that define blood types A, B, and O.
Scientists have also identified one example of total resistance to infection involving these molecules. In 2003, researchers showed that people who lack a functional copy of a gene known as FUT2 cannot be infected with Norwalk virus, one of more than 30 viruses in the norovirus family that cause illness in the digestive tract.
The gene FUT2 encodes an enzyme that determines whether or not blood group antigens are found in a person’s saliva and other body fluids as well as on their red blood cells.
“It didn’t matter how many virus particles we challenged an individual with, if they did not have that first enzyme, they did not get infected,” says researcher Lisa Lindesmith, a virologist at the University of North Carolina in Chapel Hill.
No norovirus
Norwalk is a relatively rare type of norovirus. But FUT2 deficiency also provides some protection against the most common strains of norovirus, known as GII.4, which have periodically swept across the world over the past quarter-century. These illnesses take an especially heavy toll on children in the developing world, causing malnutrition and contributing to infant and child deaths.
But progress in translating these insights about genetic resistance into drugs or other things that could reduce the burden of noroviruses has been slow.
“The biggest barrier here is lack of ability to study the virus outside of humans,” Lindesmith says.
Noroviruses are very difficult to grow in the lab, “and there’s no small animal model of gastrointestinal illness caused by the viruses.”
We are clearly making giant strides in improving those skills,” says Lindesmith. “But we are just not quite there yet.”
In the years before COVID-19 emerged, tuberculosis was responsible for the largest number of annual worldwide deaths from an infectious disease. It’s a lung disease caused by the bacterium Mycobacterium tuberculosis, and it has been a pandemic for thousands of years.
Some 85%-95% of people with intact immune systems who are infected with TB control the infection and never get active lung disease. And some people who have intense, continuing exposure to the bacterium, which is spread through droplets and aerosols from people with active lung disease, apparently never become infected at all.
Thwarting uberculosis
Understanding the ways of these different forms of resistance could help in the search for vaccines, treatments, and other ways to fight tuberculosis, says Elouise Kroon, MD, a graduate student at Stellenbosch University in Cape Town, South Africa.
“What makes it particularly hard to study is the fact that there is no gold standard to measure infection,” she says. “So, what we do is infer infection from two different types of tests” -- a skin test and a blood test that measure different kinds of immune response to molecules from the bacterium.
Dr. Kroon and other researchers have studied resistance to infection by following people living in the same household as those with active lung disease or people who live and work in crowded conditions in high-risk communities. But not all such studies have used the same definition of so-called resisters, documented exposure in the same way, or followed up to ensure that people continue to test negative over the long term.
The best clue that has emerged from studies so far links resistance to infection to certain variations in immune molecules known as HLA class II antigens, says Marlo Möller, PhD, a professor in the TB Host Genetics Research Group at Stellenbosch University.
“That always seems to pop up everywhere. But the rest is not so obvious,” she says. “A lot of the studies don’t find the same thing. It’s different in different populations,” which may be a result of the long evolutionary history between tuberculosis and humans, as well as the fact that different strains of the bacterium are prevalent in different parts of the world.
COVID-19 is a much newer infectious disease, but teasing out how it contributes to both severe illness and resistance to infection is still a major task.
Overcoming COVID
Early in the pandemic, research by the COVID Human Genetic Effort, the international consortium that Dr. Spaan is part of, linked severe COVID-19 pneumonia to the lack of immune molecules known as type I interferons and to antibodies produced by the body that destroy these molecules. Together, these mechanisms explain about one-fifth of severe COVID-19 cases, the researchers reported in 2021.
A few studies by other groups have explored resistance to COVID-19 infection, suggesting that reduced risk of contracting the virus is tied to certain blood group factors. People with Type O blood appear to be at slightly reduced risk of infection, for example.
But the studies done so far are designed to find common genetic variations, which generally have a small effect on resistance. Now, genetic researchers are launching an effort to identify genetic resistance factors with a big effect, even if they are vanishingly rare.
The group is recruiting people who did not become infected with COVID-19 despite heavy exposure, such as those living in households where all the other members got sick or people who were exposed to a superspreader event but did not become ill. As with tuberculosis, being certain that someone has not been infected with the virus can be tricky, but the team is using several blood tests to home in on the people most likely to have escaped infection.
They plan to sequence the genomes of these people to identify things that strongly affect infection risk, then do more laboratory studies to try to tease out the means of resistance.
Their work is inspired by earlier efforts to uncover inborn resistance to infections, Dr. Spaan says. Despite the lack of known examples of such resistance, he is optimistic about the possibilities. Those earlier efforts took place in “a different epoch,” before there were rapid sequencing technologies, Dr. Spaan says.
“Now we have modern technologies to do this more systematically.”
The emergence of viral variants such as the Delta and Omicron COVID strains raises the stakes of the work, he continues.
“The need to unravel these inborn mechanisms of resistance to COVID has become even more important because of these new variants and the anticipation that we will have COVID with us for years.”
A version of this article first appeared on WebMD.com.
During the COVID-19 pandemic, we’ve seen this play out time and time again when whole families get sick except for one or two fortunate family members. And at so-called superspreader events that infect many, a lucky few typically walk away with their health intact. Did the virus never enter their bodies? Or do some people have natural resistance to pathogens they’ve never been exposed to before encoded in their genes?
Resistance to infectious disease is much more than a scientific curiosity and studying how it works can be a path to curb future outbreaks.
“In the event that we could identify what makes some people resistant, that immediately opens avenues for therapeutics that we could apply in all those other people who do suffer from the disease,” says András Spaan, MD, a microbiologist at Rockefeller University in New York.
Dr. Spaan is part of an international effort to identify genetic variations that spare people from becoming infected with SARS-CoV-2, the virus that causes COVID-19.
There’s far more research on what drives the tendency to get infectious diseases than on resistance to them. But a few researchers are investigating resistance to some of the world’s most common and deadly infectious diseases, and in a few cases, they’ve already translated these insights into treatments.
Perhaps the strongest example of how odd genes of just a few people can inspire treatments to help many comes from research on the human immunodeficiency virus (HIV), the virus that causes acquired immune deficiency syndrome (AIDS).
A genetic quirk
In the mid-1990s, several groups of researchers independently identified a mutation in a gene called CCR5 linked to resistance to HIV infection.
The gene encodes a protein on the surface of some white blood cells that helps set up the movement of other immune cells to fight infections. HIV, meanwhile, uses the CCR5 protein to help it enter the white blood cells that it infects.
The mutation, known as delta 32, results in a shorter than usual protein that doesn’t reach the surface of the cell. People who carry two copies of the delta 32 form of CCR5 do not have any CCR5 protein on the outside of their white blood cells.
Researchers, led by molecular immunologist Philip Murphy, MD, at the National Institute of Allergy and Infectious Diseases in Bethesda, Md, showed in 1997 that people with two copies of the mutation were unusually common among a group of men who were at especially high risk of HIV exposure, but had never contracted the virus. And out of more than 700 HIV-positive people, none carried two copies of CCR5 delta 32.
Pharmaceutical companies used these insights to develop drugs to block CCR5 and delay the development of AIDS. For instance, the drug maraviroc, marketed by Pfizer, was approved for use in HIV-positive people in 2007.
Only a few examples of this kind of inborn, genetically determined complete resistance to infection have ever been heard of. All of them involve cell-surface molecules that are believed to help a virus or other pathogen gain entry to the cell.
Locking out illness
“The first step for any intracellular pathogen is getting inside the cell. And if you’re missing the doorway, then the virus can’t accomplish the first step in its life cycle,” Dr. Murphy says. “Getting inside is fundamental.”
Changes in cell-surface molecules can also make someone more likely to have an infection or severe disease. One such group of cell-surface molecules that have been linked to both increasing and decreasing the risk of various infections are histo-blood group antigens. The most familiar members of this group are the molecules that define blood types A, B, and O.
Scientists have also identified one example of total resistance to infection involving these molecules. In 2003, researchers showed that people who lack a functional copy of a gene known as FUT2 cannot be infected with Norwalk virus, one of more than 30 viruses in the norovirus family that cause illness in the digestive tract.
The gene FUT2 encodes an enzyme that determines whether or not blood group antigens are found in a person’s saliva and other body fluids as well as on their red blood cells.
“It didn’t matter how many virus particles we challenged an individual with, if they did not have that first enzyme, they did not get infected,” says researcher Lisa Lindesmith, a virologist at the University of North Carolina in Chapel Hill.
No norovirus
Norwalk is a relatively rare type of norovirus. But FUT2 deficiency also provides some protection against the most common strains of norovirus, known as GII.4, which have periodically swept across the world over the past quarter-century. These illnesses take an especially heavy toll on children in the developing world, causing malnutrition and contributing to infant and child deaths.
But progress in translating these insights about genetic resistance into drugs or other things that could reduce the burden of noroviruses has been slow.
“The biggest barrier here is lack of ability to study the virus outside of humans,” Lindesmith says.
Noroviruses are very difficult to grow in the lab, “and there’s no small animal model of gastrointestinal illness caused by the viruses.”
We are clearly making giant strides in improving those skills,” says Lindesmith. “But we are just not quite there yet.”
In the years before COVID-19 emerged, tuberculosis was responsible for the largest number of annual worldwide deaths from an infectious disease. It’s a lung disease caused by the bacterium Mycobacterium tuberculosis, and it has been a pandemic for thousands of years.
Some 85%-95% of people with intact immune systems who are infected with TB control the infection and never get active lung disease. And some people who have intense, continuing exposure to the bacterium, which is spread through droplets and aerosols from people with active lung disease, apparently never become infected at all.
Thwarting uberculosis
Understanding the ways of these different forms of resistance could help in the search for vaccines, treatments, and other ways to fight tuberculosis, says Elouise Kroon, MD, a graduate student at Stellenbosch University in Cape Town, South Africa.
“What makes it particularly hard to study is the fact that there is no gold standard to measure infection,” she says. “So, what we do is infer infection from two different types of tests” -- a skin test and a blood test that measure different kinds of immune response to molecules from the bacterium.
Dr. Kroon and other researchers have studied resistance to infection by following people living in the same household as those with active lung disease or people who live and work in crowded conditions in high-risk communities. But not all such studies have used the same definition of so-called resisters, documented exposure in the same way, or followed up to ensure that people continue to test negative over the long term.
The best clue that has emerged from studies so far links resistance to infection to certain variations in immune molecules known as HLA class II antigens, says Marlo Möller, PhD, a professor in the TB Host Genetics Research Group at Stellenbosch University.
“That always seems to pop up everywhere. But the rest is not so obvious,” she says. “A lot of the studies don’t find the same thing. It’s different in different populations,” which may be a result of the long evolutionary history between tuberculosis and humans, as well as the fact that different strains of the bacterium are prevalent in different parts of the world.
COVID-19 is a much newer infectious disease, but teasing out how it contributes to both severe illness and resistance to infection is still a major task.
Overcoming COVID
Early in the pandemic, research by the COVID Human Genetic Effort, the international consortium that Dr. Spaan is part of, linked severe COVID-19 pneumonia to the lack of immune molecules known as type I interferons and to antibodies produced by the body that destroy these molecules. Together, these mechanisms explain about one-fifth of severe COVID-19 cases, the researchers reported in 2021.
A few studies by other groups have explored resistance to COVID-19 infection, suggesting that reduced risk of contracting the virus is tied to certain blood group factors. People with Type O blood appear to be at slightly reduced risk of infection, for example.
But the studies done so far are designed to find common genetic variations, which generally have a small effect on resistance. Now, genetic researchers are launching an effort to identify genetic resistance factors with a big effect, even if they are vanishingly rare.
The group is recruiting people who did not become infected with COVID-19 despite heavy exposure, such as those living in households where all the other members got sick or people who were exposed to a superspreader event but did not become ill. As with tuberculosis, being certain that someone has not been infected with the virus can be tricky, but the team is using several blood tests to home in on the people most likely to have escaped infection.
They plan to sequence the genomes of these people to identify things that strongly affect infection risk, then do more laboratory studies to try to tease out the means of resistance.
Their work is inspired by earlier efforts to uncover inborn resistance to infections, Dr. Spaan says. Despite the lack of known examples of such resistance, he is optimistic about the possibilities. Those earlier efforts took place in “a different epoch,” before there were rapid sequencing technologies, Dr. Spaan says.
“Now we have modern technologies to do this more systematically.”
The emergence of viral variants such as the Delta and Omicron COVID strains raises the stakes of the work, he continues.
“The need to unravel these inborn mechanisms of resistance to COVID has become even more important because of these new variants and the anticipation that we will have COVID with us for years.”
A version of this article first appeared on WebMD.com.
During the COVID-19 pandemic, we’ve seen this play out time and time again when whole families get sick except for one or two fortunate family members. And at so-called superspreader events that infect many, a lucky few typically walk away with their health intact. Did the virus never enter their bodies? Or do some people have natural resistance to pathogens they’ve never been exposed to before encoded in their genes?
Resistance to infectious disease is much more than a scientific curiosity and studying how it works can be a path to curb future outbreaks.
“In the event that we could identify what makes some people resistant, that immediately opens avenues for therapeutics that we could apply in all those other people who do suffer from the disease,” says András Spaan, MD, a microbiologist at Rockefeller University in New York.
Dr. Spaan is part of an international effort to identify genetic variations that spare people from becoming infected with SARS-CoV-2, the virus that causes COVID-19.
There’s far more research on what drives the tendency to get infectious diseases than on resistance to them. But a few researchers are investigating resistance to some of the world’s most common and deadly infectious diseases, and in a few cases, they’ve already translated these insights into treatments.
Perhaps the strongest example of how odd genes of just a few people can inspire treatments to help many comes from research on the human immunodeficiency virus (HIV), the virus that causes acquired immune deficiency syndrome (AIDS).
A genetic quirk
In the mid-1990s, several groups of researchers independently identified a mutation in a gene called CCR5 linked to resistance to HIV infection.
The gene encodes a protein on the surface of some white blood cells that helps set up the movement of other immune cells to fight infections. HIV, meanwhile, uses the CCR5 protein to help it enter the white blood cells that it infects.
The mutation, known as delta 32, results in a shorter than usual protein that doesn’t reach the surface of the cell. People who carry two copies of the delta 32 form of CCR5 do not have any CCR5 protein on the outside of their white blood cells.
Researchers, led by molecular immunologist Philip Murphy, MD, at the National Institute of Allergy and Infectious Diseases in Bethesda, Md, showed in 1997 that people with two copies of the mutation were unusually common among a group of men who were at especially high risk of HIV exposure, but had never contracted the virus. And out of more than 700 HIV-positive people, none carried two copies of CCR5 delta 32.
Pharmaceutical companies used these insights to develop drugs to block CCR5 and delay the development of AIDS. For instance, the drug maraviroc, marketed by Pfizer, was approved for use in HIV-positive people in 2007.
Only a few examples of this kind of inborn, genetically determined complete resistance to infection have ever been heard of. All of them involve cell-surface molecules that are believed to help a virus or other pathogen gain entry to the cell.
Locking out illness
“The first step for any intracellular pathogen is getting inside the cell. And if you’re missing the doorway, then the virus can’t accomplish the first step in its life cycle,” Dr. Murphy says. “Getting inside is fundamental.”
Changes in cell-surface molecules can also make someone more likely to have an infection or severe disease. One such group of cell-surface molecules that have been linked to both increasing and decreasing the risk of various infections are histo-blood group antigens. The most familiar members of this group are the molecules that define blood types A, B, and O.
Scientists have also identified one example of total resistance to infection involving these molecules. In 2003, researchers showed that people who lack a functional copy of a gene known as FUT2 cannot be infected with Norwalk virus, one of more than 30 viruses in the norovirus family that cause illness in the digestive tract.
The gene FUT2 encodes an enzyme that determines whether or not blood group antigens are found in a person’s saliva and other body fluids as well as on their red blood cells.
“It didn’t matter how many virus particles we challenged an individual with, if they did not have that first enzyme, they did not get infected,” says researcher Lisa Lindesmith, a virologist at the University of North Carolina in Chapel Hill.
No norovirus
Norwalk is a relatively rare type of norovirus. But FUT2 deficiency also provides some protection against the most common strains of norovirus, known as GII.4, which have periodically swept across the world over the past quarter-century. These illnesses take an especially heavy toll on children in the developing world, causing malnutrition and contributing to infant and child deaths.
But progress in translating these insights about genetic resistance into drugs or other things that could reduce the burden of noroviruses has been slow.
“The biggest barrier here is lack of ability to study the virus outside of humans,” Lindesmith says.
Noroviruses are very difficult to grow in the lab, “and there’s no small animal model of gastrointestinal illness caused by the viruses.”
We are clearly making giant strides in improving those skills,” says Lindesmith. “But we are just not quite there yet.”
In the years before COVID-19 emerged, tuberculosis was responsible for the largest number of annual worldwide deaths from an infectious disease. It’s a lung disease caused by the bacterium Mycobacterium tuberculosis, and it has been a pandemic for thousands of years.
Some 85%-95% of people with intact immune systems who are infected with TB control the infection and never get active lung disease. And some people who have intense, continuing exposure to the bacterium, which is spread through droplets and aerosols from people with active lung disease, apparently never become infected at all.
Thwarting uberculosis
Understanding the ways of these different forms of resistance could help in the search for vaccines, treatments, and other ways to fight tuberculosis, says Elouise Kroon, MD, a graduate student at Stellenbosch University in Cape Town, South Africa.
“What makes it particularly hard to study is the fact that there is no gold standard to measure infection,” she says. “So, what we do is infer infection from two different types of tests” -- a skin test and a blood test that measure different kinds of immune response to molecules from the bacterium.
Dr. Kroon and other researchers have studied resistance to infection by following people living in the same household as those with active lung disease or people who live and work in crowded conditions in high-risk communities. But not all such studies have used the same definition of so-called resisters, documented exposure in the same way, or followed up to ensure that people continue to test negative over the long term.
The best clue that has emerged from studies so far links resistance to infection to certain variations in immune molecules known as HLA class II antigens, says Marlo Möller, PhD, a professor in the TB Host Genetics Research Group at Stellenbosch University.
“That always seems to pop up everywhere. But the rest is not so obvious,” she says. “A lot of the studies don’t find the same thing. It’s different in different populations,” which may be a result of the long evolutionary history between tuberculosis and humans, as well as the fact that different strains of the bacterium are prevalent in different parts of the world.
COVID-19 is a much newer infectious disease, but teasing out how it contributes to both severe illness and resistance to infection is still a major task.
Overcoming COVID
Early in the pandemic, research by the COVID Human Genetic Effort, the international consortium that Dr. Spaan is part of, linked severe COVID-19 pneumonia to the lack of immune molecules known as type I interferons and to antibodies produced by the body that destroy these molecules. Together, these mechanisms explain about one-fifth of severe COVID-19 cases, the researchers reported in 2021.
A few studies by other groups have explored resistance to COVID-19 infection, suggesting that reduced risk of contracting the virus is tied to certain blood group factors. People with Type O blood appear to be at slightly reduced risk of infection, for example.
But the studies done so far are designed to find common genetic variations, which generally have a small effect on resistance. Now, genetic researchers are launching an effort to identify genetic resistance factors with a big effect, even if they are vanishingly rare.
The group is recruiting people who did not become infected with COVID-19 despite heavy exposure, such as those living in households where all the other members got sick or people who were exposed to a superspreader event but did not become ill. As with tuberculosis, being certain that someone has not been infected with the virus can be tricky, but the team is using several blood tests to home in on the people most likely to have escaped infection.
They plan to sequence the genomes of these people to identify things that strongly affect infection risk, then do more laboratory studies to try to tease out the means of resistance.
Their work is inspired by earlier efforts to uncover inborn resistance to infections, Dr. Spaan says. Despite the lack of known examples of such resistance, he is optimistic about the possibilities. Those earlier efforts took place in “a different epoch,” before there were rapid sequencing technologies, Dr. Spaan says.
“Now we have modern technologies to do this more systematically.”
The emergence of viral variants such as the Delta and Omicron COVID strains raises the stakes of the work, he continues.
“The need to unravel these inborn mechanisms of resistance to COVID has become even more important because of these new variants and the anticipation that we will have COVID with us for years.”
A version of this article first appeared on WebMD.com.
Antibody mix may prevent COVID symptoms in some asymptomatic people
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA
Future respiratory infection risk raised by early life virus exposure
Many factors influence a child’s subsequent susceptibility to respiratory tract infection (RTI), including breastfeeding, crowded conditions, and exposure to environmental tobacco. Now researchers have found that asymptomatic viral infection in the first days of a baby’s life are linked to a greater risk of respiratory infections in later life.
The new research, published in Nature Microbiology, was conducted as part of the Microbiome Utrecht Infant Study (MUIS), a healthy infant birth cohort study that’s been running for 6 years.
In their study, the authors explained how the respiratory tract is “populated by a specialized microbial ecosystem, which is seeded during and directly following birth,” adding that, “despite recognition of many host and environmental factors known to modulate RTI susceptibility, the mechanism by which a child develops recurrent or severe RTIs, while others remain healthy, remains largely unknown”.
Researchers from the University of Edinburgh and University Medical Centre Utrecht (the Netherlands) examined nasal mucosa samples of 114 babies at various times from birth until 12 months of age. They then analyzed the gene activity of the babies’ nasal mucosa, the microbes present in the lining of the nose, and any viruses that infected the children.
Interferon-related mucosal gene activity
The researchers described how the microbiome – the community of microbes in the body – of a newborn baby can be influenced by many things, including delivery method, breastfeeding, antibiotics and the hospital environment. They highlighted how viruses were found to interact with a newborn’s immune system and microbiome in a way that affected both a child’s risk, and number, of subsequent infections.
They explained how when a viral infection was detected in the first days after birth, which they said largely occurred asymptomatically, specific mucosal genes were activated – genes involved with interferons – coinciding with a change in the composition of the microbiome, promoting the growth of potentially harmful microbes.
“The interferon-related gene activity caused by an early first viral infection is thought to create a proinflammatory environment that makes babies susceptible to future infections,” they said, adding that in their study they have demonstrated that “first asymptomatic viral encounters were associated with increased interferon signaling, and preceded the development of disadvantageous respiratory microbiota profiles and clinical RTIs”.
Proinflammatory and microbiologically perturbed environment
Debby Bogaert, PhD, chair of paediatric medicine at the University of Edinburgh, said: “We were surprised to see viral infections occur so early in life, and go mostly unnoticed, probably because the infant’s immune system is in what is known as a state of tolerance after birth. Despite this, these infections seem to affect a normal immune development, which is important to know.”
The authors wrote that their data supports the hypothesis that first viral encounters trigger an interferon-associated proinflammatory environment, which then further drives airway inflammation and symptomatology in a “self-enforcing positive feedback loop”. They said that this “proinflammatory and microbiologically perturbed environment in turn renders an individual more vulnerable to recurrent viral-induced RTIs”.
Wouter de Steenhuijsen, PhD, postdoctoral investigator at University Medical Centre Utrecht, said: “Although further work will be needed to confirm the causality of our findings, the data from this study indicate that early-life encounters with respiratory viruses – especially during the first days of life – may set the tone for subsequent non-beneficial host-microbe interactions, which are related to an infection risk and possibly long term respiratory health.”
Dr. Bogaert added: “Only from birth onwards will an infant start to develop its microbiome. Limiting the number of viral encounters in those first days to weeks of life might be essential for a healthy immune and microbiome development, and consequently long term respiratory health.”
A version of this article first appeared on Medscape UK.
Many factors influence a child’s subsequent susceptibility to respiratory tract infection (RTI), including breastfeeding, crowded conditions, and exposure to environmental tobacco. Now researchers have found that asymptomatic viral infection in the first days of a baby’s life are linked to a greater risk of respiratory infections in later life.
The new research, published in Nature Microbiology, was conducted as part of the Microbiome Utrecht Infant Study (MUIS), a healthy infant birth cohort study that’s been running for 6 years.
In their study, the authors explained how the respiratory tract is “populated by a specialized microbial ecosystem, which is seeded during and directly following birth,” adding that, “despite recognition of many host and environmental factors known to modulate RTI susceptibility, the mechanism by which a child develops recurrent or severe RTIs, while others remain healthy, remains largely unknown”.
Researchers from the University of Edinburgh and University Medical Centre Utrecht (the Netherlands) examined nasal mucosa samples of 114 babies at various times from birth until 12 months of age. They then analyzed the gene activity of the babies’ nasal mucosa, the microbes present in the lining of the nose, and any viruses that infected the children.
Interferon-related mucosal gene activity
The researchers described how the microbiome – the community of microbes in the body – of a newborn baby can be influenced by many things, including delivery method, breastfeeding, antibiotics and the hospital environment. They highlighted how viruses were found to interact with a newborn’s immune system and microbiome in a way that affected both a child’s risk, and number, of subsequent infections.
They explained how when a viral infection was detected in the first days after birth, which they said largely occurred asymptomatically, specific mucosal genes were activated – genes involved with interferons – coinciding with a change in the composition of the microbiome, promoting the growth of potentially harmful microbes.
“The interferon-related gene activity caused by an early first viral infection is thought to create a proinflammatory environment that makes babies susceptible to future infections,” they said, adding that in their study they have demonstrated that “first asymptomatic viral encounters were associated with increased interferon signaling, and preceded the development of disadvantageous respiratory microbiota profiles and clinical RTIs”.
Proinflammatory and microbiologically perturbed environment
Debby Bogaert, PhD, chair of paediatric medicine at the University of Edinburgh, said: “We were surprised to see viral infections occur so early in life, and go mostly unnoticed, probably because the infant’s immune system is in what is known as a state of tolerance after birth. Despite this, these infections seem to affect a normal immune development, which is important to know.”
The authors wrote that their data supports the hypothesis that first viral encounters trigger an interferon-associated proinflammatory environment, which then further drives airway inflammation and symptomatology in a “self-enforcing positive feedback loop”. They said that this “proinflammatory and microbiologically perturbed environment in turn renders an individual more vulnerable to recurrent viral-induced RTIs”.
Wouter de Steenhuijsen, PhD, postdoctoral investigator at University Medical Centre Utrecht, said: “Although further work will be needed to confirm the causality of our findings, the data from this study indicate that early-life encounters with respiratory viruses – especially during the first days of life – may set the tone for subsequent non-beneficial host-microbe interactions, which are related to an infection risk and possibly long term respiratory health.”
Dr. Bogaert added: “Only from birth onwards will an infant start to develop its microbiome. Limiting the number of viral encounters in those first days to weeks of life might be essential for a healthy immune and microbiome development, and consequently long term respiratory health.”
A version of this article first appeared on Medscape UK.
Many factors influence a child’s subsequent susceptibility to respiratory tract infection (RTI), including breastfeeding, crowded conditions, and exposure to environmental tobacco. Now researchers have found that asymptomatic viral infection in the first days of a baby’s life are linked to a greater risk of respiratory infections in later life.
The new research, published in Nature Microbiology, was conducted as part of the Microbiome Utrecht Infant Study (MUIS), a healthy infant birth cohort study that’s been running for 6 years.
In their study, the authors explained how the respiratory tract is “populated by a specialized microbial ecosystem, which is seeded during and directly following birth,” adding that, “despite recognition of many host and environmental factors known to modulate RTI susceptibility, the mechanism by which a child develops recurrent or severe RTIs, while others remain healthy, remains largely unknown”.
Researchers from the University of Edinburgh and University Medical Centre Utrecht (the Netherlands) examined nasal mucosa samples of 114 babies at various times from birth until 12 months of age. They then analyzed the gene activity of the babies’ nasal mucosa, the microbes present in the lining of the nose, and any viruses that infected the children.
Interferon-related mucosal gene activity
The researchers described how the microbiome – the community of microbes in the body – of a newborn baby can be influenced by many things, including delivery method, breastfeeding, antibiotics and the hospital environment. They highlighted how viruses were found to interact with a newborn’s immune system and microbiome in a way that affected both a child’s risk, and number, of subsequent infections.
They explained how when a viral infection was detected in the first days after birth, which they said largely occurred asymptomatically, specific mucosal genes were activated – genes involved with interferons – coinciding with a change in the composition of the microbiome, promoting the growth of potentially harmful microbes.
“The interferon-related gene activity caused by an early first viral infection is thought to create a proinflammatory environment that makes babies susceptible to future infections,” they said, adding that in their study they have demonstrated that “first asymptomatic viral encounters were associated with increased interferon signaling, and preceded the development of disadvantageous respiratory microbiota profiles and clinical RTIs”.
Proinflammatory and microbiologically perturbed environment
Debby Bogaert, PhD, chair of paediatric medicine at the University of Edinburgh, said: “We were surprised to see viral infections occur so early in life, and go mostly unnoticed, probably because the infant’s immune system is in what is known as a state of tolerance after birth. Despite this, these infections seem to affect a normal immune development, which is important to know.”
The authors wrote that their data supports the hypothesis that first viral encounters trigger an interferon-associated proinflammatory environment, which then further drives airway inflammation and symptomatology in a “self-enforcing positive feedback loop”. They said that this “proinflammatory and microbiologically perturbed environment in turn renders an individual more vulnerable to recurrent viral-induced RTIs”.
Wouter de Steenhuijsen, PhD, postdoctoral investigator at University Medical Centre Utrecht, said: “Although further work will be needed to confirm the causality of our findings, the data from this study indicate that early-life encounters with respiratory viruses – especially during the first days of life – may set the tone for subsequent non-beneficial host-microbe interactions, which are related to an infection risk and possibly long term respiratory health.”
Dr. Bogaert added: “Only from birth onwards will an infant start to develop its microbiome. Limiting the number of viral encounters in those first days to weeks of life might be essential for a healthy immune and microbiome development, and consequently long term respiratory health.”
A version of this article first appeared on Medscape UK.
FROM NATURE MICROBIOLOGY
Chronic respiratory conditions occur more often in RSV vs. flu
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
FROM CHEST
HIV stigma persists globally, according to Harris poll
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four decades into the AIDS epidemic and for some, it’s as if gains in awareness, advances in prevention and treatment, and the concept of undetected equals untransmissable (U=U) never happened. In its place,
Accordingly, findings from a Harris poll conducted Oct. 13-18, 2021, among 5,047 adults (18 and older) residing in Australia, Portugal, the United Kingdom, and the United States, reveal that 88% of those surveyed believe that negative perceptions toward people living with HIV persist even though HIV infection can be effectively managed with antiretroviral therapy (ART). Conversely, three-quarters (76%) are unaware of U=U, and the fact that someone with HIV who is taking effective treatment cannot pass it on to their partner. Two-thirds incorrectly believe that a person living with HIV can pass it onto their baby, even when they are ART adherent.
“The survey made me think of people who work in HIV clinics, and how much of a bubble I think that we in the HIV field live in,” Nneka Nwokolo, MBBS, senior global medical director at ViiV Healthcare, London, and practicing consultant in sexual health and HIV medicine, told this news organization. “I think that we generally feel that everyone knows as much as we do or feels the way that we do.”
Misconceptions abound across the globe
The online survey, which was commissioned by ViiV Healthcare, also highlights that one in five adults do not know that anyone can acquire HIV regardless of lifestyle, thereby perpetuating the stereotype that HIV is a disease that only affects certain populations, such as men who have sex with men (MSM) or transgender women (TGW).
Pervasive stereotypes and stigmatization only serve to magnify preexisting social inequities that affect access to appropriate care. A recent editorial published in the journal AIDS and Behavior underscores that stigma experienced by marginalized populations in particular (for example, Black MSM, TGW) is directly linked to decreased access to and use of effective HIV prevention and treatment services. Additionally, once stigma becomes internalized, it might further affect overall well-being, mental health, and social support.
“One of the most significant consequences of the ongoing stigma is that people are scared to test and then they end up coming to services late [when] they’re really ill,” explained Dr. Nwokolo. “It goes back to the early days when HIV was a death sentence ... it’s still there. I have one patient who to this day hates the fact that he has HIV, that he has to come to the clinic – it’s a reminder of why he hates himself.”
Great strides in testing and advances in treatment might be helping to reframe HIV as a chronic but treatable and preventable disease. Nevertheless, survey findings also revealed that nearly three out of five adults incorrectly believe that a person living with HIV will have a shorter lifespan than someone who is HIV negative, even if they are on effective treatment.
These beliefs are especially true among Dr. Nwokolo’s patient base, most of whom are Africans who’ve immigrated to the United Kingdom from countries that have been devastated by the HIV epidemic. “Those who’ve never tested are reluctant to do so because they are afraid that they will have the same outcome as the people that they know that they’ve left behind,” she said.
HIV stigma in the era of 90-90-90
While there has been progress toward achieving UN AID’s 90-90-90 targets (that is, 90% living with HIV know their status, 90% who know their status are on ART, and 90% of people on ART are virally suppressed), exclusion and isolation – the key hallmarks of stigma – may ultimately be the most important barriers preventing a lofty goal to end the AIDS epidemic by the year 2030.
“Here we are, 40 years in and we are still facing such ignorance, some stigma,” Carl Schmid, MBA, former cochair of the Presidential Advisory Council on HIV/AIDS, and executive director of HIV+Policy Institute, told this news organization. “It’s gotten better, but it is really putting a damper on people being tested, getting treated, getting access to PrEP.” Mr. Schmid was not involved in the Harris Poll.
Mr. Schmid also said that, in addition to broader outreach and education as well as dissemination of information about HIV and AIDS from the White House and other government leaders, physician involvement is essential.
“They’re the ones that need to step up. They have to talk about sex with their patients, [but] they don’t do that, especially in the South among certain populations,” he noted.
Data support the unique challenges faced by at-risk individuals living in the southern United States. Not only do Southern states account for roughly half of all new HIV cases annually, but Black MSM and Black women account for the majority of new diagnoses, according to the Centers for Disease Control and Prevention. Data have also demonstrated discrimination and prejudice toward people with HIV persist among many medical professionals in the South (especially those working in rural areas).
But this is not only a Southern problem; a 2018 review of studies in clinicians across the United States published in AIDS Patient Care and STDs linked provider fear of acquiring HIV through occupational exposure to reduced quality of care, refusal of care, and anxiety, especially among providers with limited awareness of PrEP. Discordant attitudes around making a priority to address HIV-related stigma versus other health care needs also reduced overall care delivery and patient experience.
“I think that the first thing that we as HIV clinicians can and should do – and is definitely within our power to do – is to educate our peers about HIV,” Dr. Nwokolo said, “HIV has gone off the radar, but it’s still out there.”
The study was commissioned by Viiv Healthcare. Dr. Nwokolo is an employee of ViiV Healthcare. Mr. Schmid disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in COVID-19 Outcomes Among Patients With Type 1 Diabetes: First vs Later Surges
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.
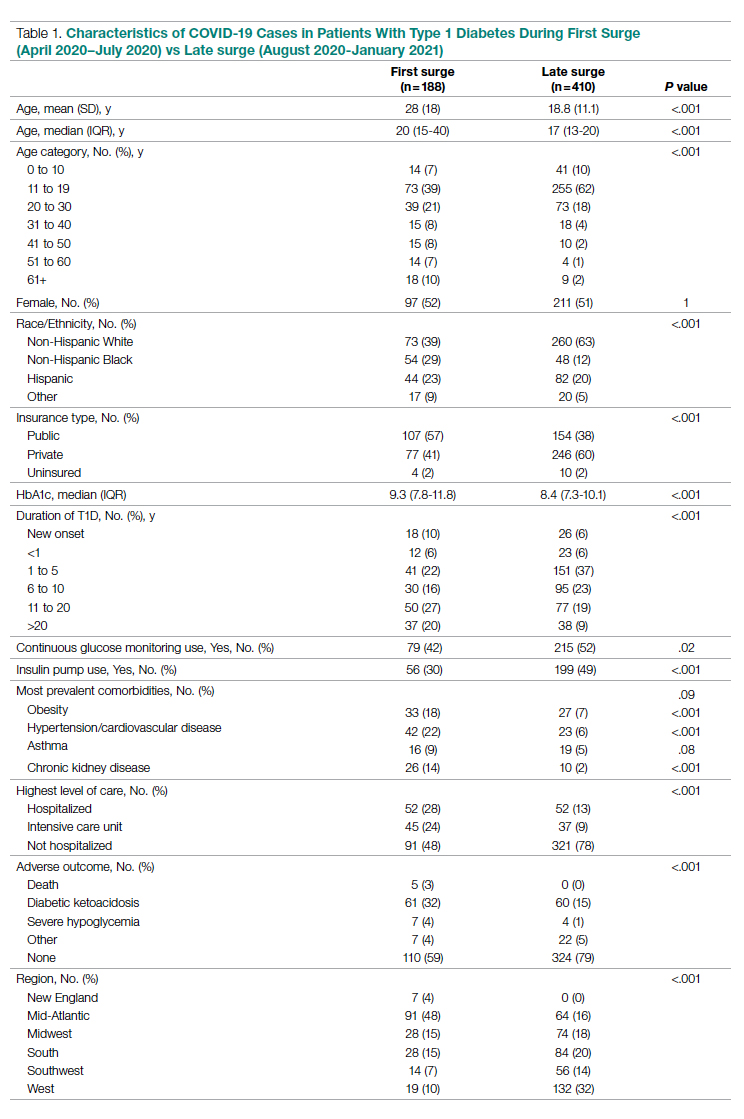
Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.
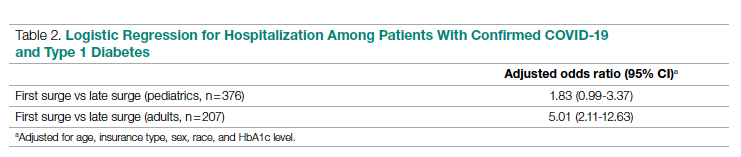
Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
Role and Experience of a Subintensive Care Unit in Caring for Patients With COVID-19 in Italy: The CO-RESP Study
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.
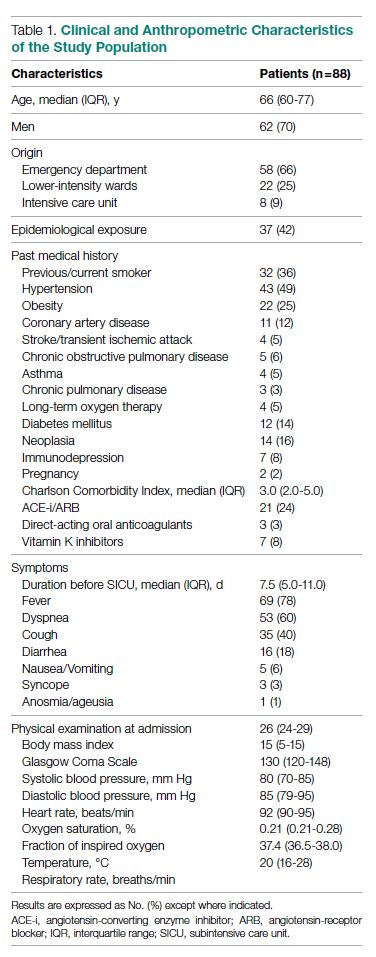
Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).
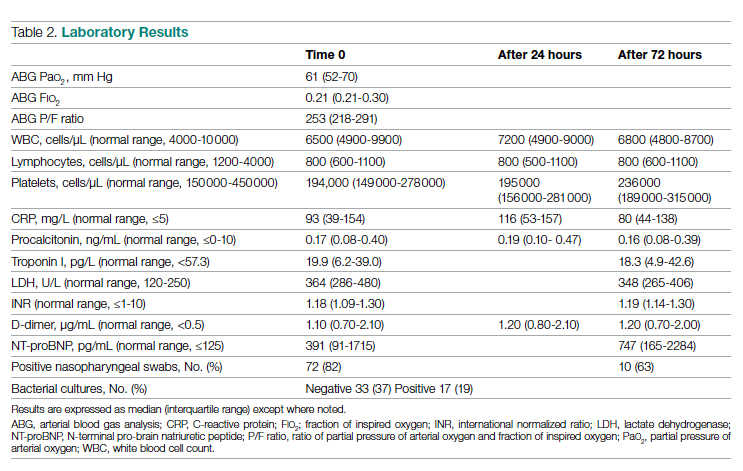
Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.
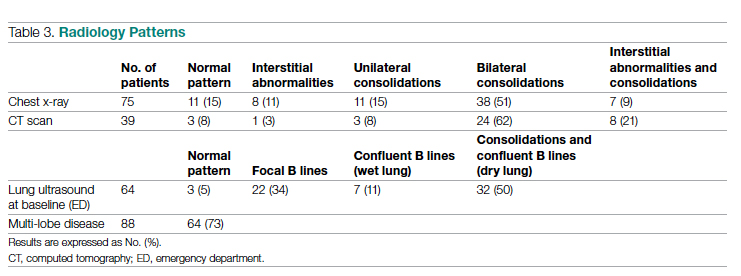
Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.
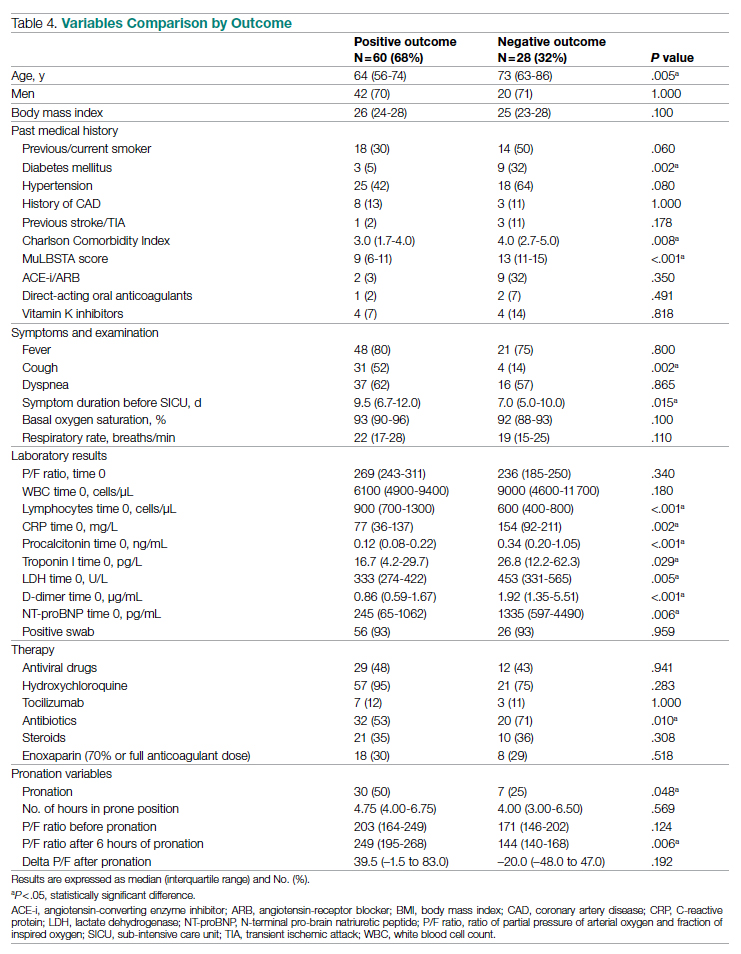
Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
Structural Ableism: Defining Standards of Care Amid Crisis and Inequity
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”
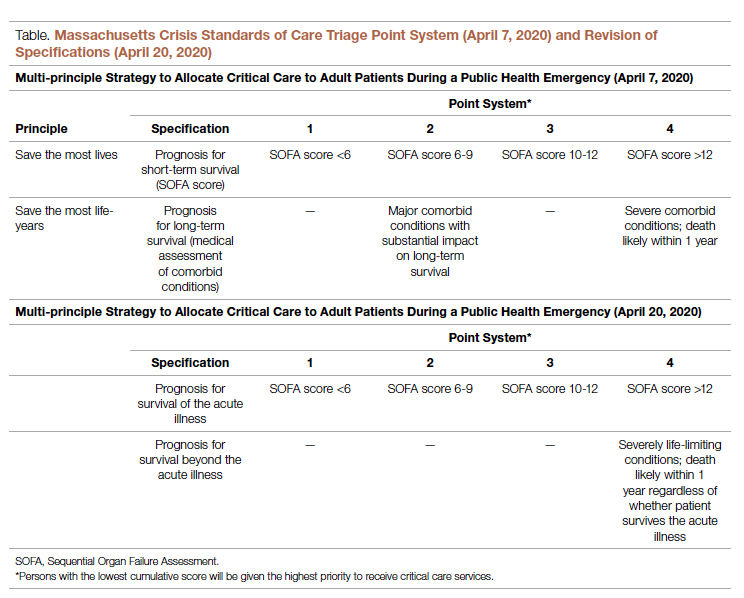
Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Children and COVID-19: The Omicron tide may have turned
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
CDC issues new pneumococcal vaccine recommendations for adults
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MMWR