User login
CPR for EHRs
The truth about EHR systems is that their implementation is never easy. It's a lot of work. It takes time and money, and despite the best laid plans there will be trauma and frustration. So expecting problems to arise is key to keeping perspective.
When our office implemented an electronic health records system in 2000, our system crashed 25–75 times a day for 5 months, and we lost patient data each time. I repeat: We lost patient data each time. Extensive troubleshooting ensued. Ceiling tiles were ripped out to see if the fluorescent lights were interfering with the network cables, a consultant was brought in, and our server and network were reinstalled. Finally, the cause of the crashes was determined to be a bug in our Microsoft program. As nightmarish as this situation was, I would say that such technology challenges were nothing compared to challenges in managing processes and people.
From a process perspective, a common mistake involves attempting to make the EHR system conform to what is done with paper. The whole point is to imagine a process that can help your office save time and money instead of mirroring what you did for years with a paper-based system.
Staff challenges are by far the toughest ones to manage because they require changing the minds and habits of individuals who don't feel comfortable giving up paper-based processes. Persistent naysayers can sabotage EHR implementation by convincing others that the changes cannot be made. Over the years, four of five staff members have left. When new staff members were hired, we emphasized the fact that our office was computerized and those individuals have successfully adapted to a paperless system. Among the lessons we've learned over the years are these:
▸ Don't skimp on training. When you're spending thousands of dollars on an EHR system it's tempting to shave costs and training may appear to be part of the discretionary spending budget. But giving training short shrift can cost you a lot more than you saved in the long run.
Even if you're the most technologically savvy physician, avoid the “I can do it all” mentality. Your time is best spent seeing patients and making money. Make sure that others are well trained so you feel comfortable delegating EHR responsibilities.
▸ Train the Luddites last. Once you've worked out all the kinks in the training process with those who are most comfortable using computers, it'll go a lot more smoothly for those who are less tech-savvy. Don't let anyone opt out of training. That can cost tens of thousands of dollars in the long run.
▸ Include everyone in brainstorming sessions. While no one likes meetings, get everyone involved in implementation meetings, not just the doctors and the office manager, because you will get good ideas from everyone. In addition, if they are involved in the brainstorming sessions, they are far more likely to adopt new behaviors.
▸ It doesn't have to be perfect. During the transition phase to an EHR system, there's a temptation to try to make everything perfect. Soon after we went live with our system, I spent a lot of time checking electronic charts to make sure the staff had included consultation notes. It was really a wasted step, because 99% of the time they had done it. In the rare event that the notes don't get into the chart, it doesn't affect patient care. The key is knowing when to accept a process as good enough and move on.
▸ Get a leader. You need a leader with a vision to organize the troubleshooting, both to build support and to keep everyone on track. The most common cause of EHR failure is lack of a leader.
The truth about EHR systems is that their implementation is never easy. It's a lot of work. It takes time and money, and despite the best laid plans there will be trauma and frustration. So expecting problems to arise is key to keeping perspective.
When our office implemented an electronic health records system in 2000, our system crashed 25–75 times a day for 5 months, and we lost patient data each time. I repeat: We lost patient data each time. Extensive troubleshooting ensued. Ceiling tiles were ripped out to see if the fluorescent lights were interfering with the network cables, a consultant was brought in, and our server and network were reinstalled. Finally, the cause of the crashes was determined to be a bug in our Microsoft program. As nightmarish as this situation was, I would say that such technology challenges were nothing compared to challenges in managing processes and people.
From a process perspective, a common mistake involves attempting to make the EHR system conform to what is done with paper. The whole point is to imagine a process that can help your office save time and money instead of mirroring what you did for years with a paper-based system.
Staff challenges are by far the toughest ones to manage because they require changing the minds and habits of individuals who don't feel comfortable giving up paper-based processes. Persistent naysayers can sabotage EHR implementation by convincing others that the changes cannot be made. Over the years, four of five staff members have left. When new staff members were hired, we emphasized the fact that our office was computerized and those individuals have successfully adapted to a paperless system. Among the lessons we've learned over the years are these:
▸ Don't skimp on training. When you're spending thousands of dollars on an EHR system it's tempting to shave costs and training may appear to be part of the discretionary spending budget. But giving training short shrift can cost you a lot more than you saved in the long run.
Even if you're the most technologically savvy physician, avoid the “I can do it all” mentality. Your time is best spent seeing patients and making money. Make sure that others are well trained so you feel comfortable delegating EHR responsibilities.
▸ Train the Luddites last. Once you've worked out all the kinks in the training process with those who are most comfortable using computers, it'll go a lot more smoothly for those who are less tech-savvy. Don't let anyone opt out of training. That can cost tens of thousands of dollars in the long run.
▸ Include everyone in brainstorming sessions. While no one likes meetings, get everyone involved in implementation meetings, not just the doctors and the office manager, because you will get good ideas from everyone. In addition, if they are involved in the brainstorming sessions, they are far more likely to adopt new behaviors.
▸ It doesn't have to be perfect. During the transition phase to an EHR system, there's a temptation to try to make everything perfect. Soon after we went live with our system, I spent a lot of time checking electronic charts to make sure the staff had included consultation notes. It was really a wasted step, because 99% of the time they had done it. In the rare event that the notes don't get into the chart, it doesn't affect patient care. The key is knowing when to accept a process as good enough and move on.
▸ Get a leader. You need a leader with a vision to organize the troubleshooting, both to build support and to keep everyone on track. The most common cause of EHR failure is lack of a leader.
The truth about EHR systems is that their implementation is never easy. It's a lot of work. It takes time and money, and despite the best laid plans there will be trauma and frustration. So expecting problems to arise is key to keeping perspective.
When our office implemented an electronic health records system in 2000, our system crashed 25–75 times a day for 5 months, and we lost patient data each time. I repeat: We lost patient data each time. Extensive troubleshooting ensued. Ceiling tiles were ripped out to see if the fluorescent lights were interfering with the network cables, a consultant was brought in, and our server and network were reinstalled. Finally, the cause of the crashes was determined to be a bug in our Microsoft program. As nightmarish as this situation was, I would say that such technology challenges were nothing compared to challenges in managing processes and people.
From a process perspective, a common mistake involves attempting to make the EHR system conform to what is done with paper. The whole point is to imagine a process that can help your office save time and money instead of mirroring what you did for years with a paper-based system.
Staff challenges are by far the toughest ones to manage because they require changing the minds and habits of individuals who don't feel comfortable giving up paper-based processes. Persistent naysayers can sabotage EHR implementation by convincing others that the changes cannot be made. Over the years, four of five staff members have left. When new staff members were hired, we emphasized the fact that our office was computerized and those individuals have successfully adapted to a paperless system. Among the lessons we've learned over the years are these:
▸ Don't skimp on training. When you're spending thousands of dollars on an EHR system it's tempting to shave costs and training may appear to be part of the discretionary spending budget. But giving training short shrift can cost you a lot more than you saved in the long run.
Even if you're the most technologically savvy physician, avoid the “I can do it all” mentality. Your time is best spent seeing patients and making money. Make sure that others are well trained so you feel comfortable delegating EHR responsibilities.
▸ Train the Luddites last. Once you've worked out all the kinks in the training process with those who are most comfortable using computers, it'll go a lot more smoothly for those who are less tech-savvy. Don't let anyone opt out of training. That can cost tens of thousands of dollars in the long run.
▸ Include everyone in brainstorming sessions. While no one likes meetings, get everyone involved in implementation meetings, not just the doctors and the office manager, because you will get good ideas from everyone. In addition, if they are involved in the brainstorming sessions, they are far more likely to adopt new behaviors.
▸ It doesn't have to be perfect. During the transition phase to an EHR system, there's a temptation to try to make everything perfect. Soon after we went live with our system, I spent a lot of time checking electronic charts to make sure the staff had included consultation notes. It was really a wasted step, because 99% of the time they had done it. In the rare event that the notes don't get into the chart, it doesn't affect patient care. The key is knowing when to accept a process as good enough and move on.
▸ Get a leader. You need a leader with a vision to organize the troubleshooting, both to build support and to keep everyone on track. The most common cause of EHR failure is lack of a leader.
A New Revenue Source?
A recently expanded palliative care program at University Hospital in Salt Lake City is the latest window into ancillary revenue streams hospitalists can tap during the continuing economic crisis.
Stephen Bekanich, MD, a hospitalist and medical director of the Utah center's palliative-care team, says his program saves money for the hospital and increases the value of its hospitalists. In October, Dr. Bekanich's team expanded into outpatient clinic treatment one half-day a week. While he plans to study the revenue generated through that month before expanding further, Dr. Bekanich thinks palliative-care teams are a strong revenue source for hospitalists.
"It’s a natural tie-in," he says. "The people that probably win the most with having palliative care established within the hospital is the hospital itself. We see that in our patient satisfaction."
Palliative care can lower hospital expenditures by cutting down on costly procedures that may not improve a patient's quality of life, as well as by trimming lengths of stay, Dr. Bekanich says. In the first half of 2008, Dr. Bekanich's team saved University Hospital about $600,000. The team’s 2008 budget was roughly $330,000 for about 500 encounters.
There are obstacles, though. Because palliative care is a recognized specialty, starting a program requires a hospitalist who is willing—and able—to become certified and hospital administration willing to front expenditures.
"The idea of starting this from scratch is going to become more difficult," Dr. Bekanich says, "but it’s worthwhile."
A recently expanded palliative care program at University Hospital in Salt Lake City is the latest window into ancillary revenue streams hospitalists can tap during the continuing economic crisis.
Stephen Bekanich, MD, a hospitalist and medical director of the Utah center's palliative-care team, says his program saves money for the hospital and increases the value of its hospitalists. In October, Dr. Bekanich's team expanded into outpatient clinic treatment one half-day a week. While he plans to study the revenue generated through that month before expanding further, Dr. Bekanich thinks palliative-care teams are a strong revenue source for hospitalists.
"It’s a natural tie-in," he says. "The people that probably win the most with having palliative care established within the hospital is the hospital itself. We see that in our patient satisfaction."
Palliative care can lower hospital expenditures by cutting down on costly procedures that may not improve a patient's quality of life, as well as by trimming lengths of stay, Dr. Bekanich says. In the first half of 2008, Dr. Bekanich's team saved University Hospital about $600,000. The team’s 2008 budget was roughly $330,000 for about 500 encounters.
There are obstacles, though. Because palliative care is a recognized specialty, starting a program requires a hospitalist who is willing—and able—to become certified and hospital administration willing to front expenditures.
"The idea of starting this from scratch is going to become more difficult," Dr. Bekanich says, "but it’s worthwhile."
A recently expanded palliative care program at University Hospital in Salt Lake City is the latest window into ancillary revenue streams hospitalists can tap during the continuing economic crisis.
Stephen Bekanich, MD, a hospitalist and medical director of the Utah center's palliative-care team, says his program saves money for the hospital and increases the value of its hospitalists. In October, Dr. Bekanich's team expanded into outpatient clinic treatment one half-day a week. While he plans to study the revenue generated through that month before expanding further, Dr. Bekanich thinks palliative-care teams are a strong revenue source for hospitalists.
"It’s a natural tie-in," he says. "The people that probably win the most with having palliative care established within the hospital is the hospital itself. We see that in our patient satisfaction."
Palliative care can lower hospital expenditures by cutting down on costly procedures that may not improve a patient's quality of life, as well as by trimming lengths of stay, Dr. Bekanich says. In the first half of 2008, Dr. Bekanich's team saved University Hospital about $600,000. The team’s 2008 budget was roughly $330,000 for about 500 encounters.
There are obstacles, though. Because palliative care is a recognized specialty, starting a program requires a hospitalist who is willing—and able—to become certified and hospital administration willing to front expenditures.
"The idea of starting this from scratch is going to become more difficult," Dr. Bekanich says, "but it’s worthwhile."
Ringing in the New Year
Garth King, MD, a hospitalist and medical director at Southwest General Medical Center in Lafayette, La., wasn’t surprised he was treating an inebriated 17-year-old who came to the hospital with his mother this past New Year's Eve. The intoxicated 14-year-old who came in shortly after, however, did throw him slightly off guard.
"We usually just send them to the emergency room, where they are monitored," Dr. King says. "It's a waste of resources to admit them."
Kenneth Patrick, MD, a hospitalist and ICU director at Chestnut Hill Hospital in Philadelphia, says alcohol-related conditions, including gastritis and pancreatitis, are the most common cases he sees on New Year's Eve and New Year's Day. The second-most common, he says, are fractures caused by slipping on ice or snow.
"New Year's Day is the busiest day of the year for inpatients," Dr. Patrick says.
National data on daily hospital visits don't exist, but Dr. King agrees with Dr. Patrick's assessment. He says the number of patients his group normally sees doubles between Christmas Eve and New Year’s Day. "In residency, I remember this would happen," he says. "It would seem like family members would bring in their family members, just because."
If you are one of the unfortunate members of your HM group scheduled to work next holiday season, Dr. Patrick offers a little advice: "Stay well-hydrated and get lots of rest, because you will be busy."
Garth King, MD, a hospitalist and medical director at Southwest General Medical Center in Lafayette, La., wasn’t surprised he was treating an inebriated 17-year-old who came to the hospital with his mother this past New Year's Eve. The intoxicated 14-year-old who came in shortly after, however, did throw him slightly off guard.
"We usually just send them to the emergency room, where they are monitored," Dr. King says. "It's a waste of resources to admit them."
Kenneth Patrick, MD, a hospitalist and ICU director at Chestnut Hill Hospital in Philadelphia, says alcohol-related conditions, including gastritis and pancreatitis, are the most common cases he sees on New Year's Eve and New Year's Day. The second-most common, he says, are fractures caused by slipping on ice or snow.
"New Year's Day is the busiest day of the year for inpatients," Dr. Patrick says.
National data on daily hospital visits don't exist, but Dr. King agrees with Dr. Patrick's assessment. He says the number of patients his group normally sees doubles between Christmas Eve and New Year’s Day. "In residency, I remember this would happen," he says. "It would seem like family members would bring in their family members, just because."
If you are one of the unfortunate members of your HM group scheduled to work next holiday season, Dr. Patrick offers a little advice: "Stay well-hydrated and get lots of rest, because you will be busy."
Garth King, MD, a hospitalist and medical director at Southwest General Medical Center in Lafayette, La., wasn’t surprised he was treating an inebriated 17-year-old who came to the hospital with his mother this past New Year's Eve. The intoxicated 14-year-old who came in shortly after, however, did throw him slightly off guard.
"We usually just send them to the emergency room, where they are monitored," Dr. King says. "It's a waste of resources to admit them."
Kenneth Patrick, MD, a hospitalist and ICU director at Chestnut Hill Hospital in Philadelphia, says alcohol-related conditions, including gastritis and pancreatitis, are the most common cases he sees on New Year's Eve and New Year's Day. The second-most common, he says, are fractures caused by slipping on ice or snow.
"New Year's Day is the busiest day of the year for inpatients," Dr. Patrick says.
National data on daily hospital visits don't exist, but Dr. King agrees with Dr. Patrick's assessment. He says the number of patients his group normally sees doubles between Christmas Eve and New Year’s Day. "In residency, I remember this would happen," he says. "It would seem like family members would bring in their family members, just because."
If you are one of the unfortunate members of your HM group scheduled to work next holiday season, Dr. Patrick offers a little advice: "Stay well-hydrated and get lots of rest, because you will be busy."
Methicillin‐resistant Staphylococcus aureus bacteremia due to prostatic abscess
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
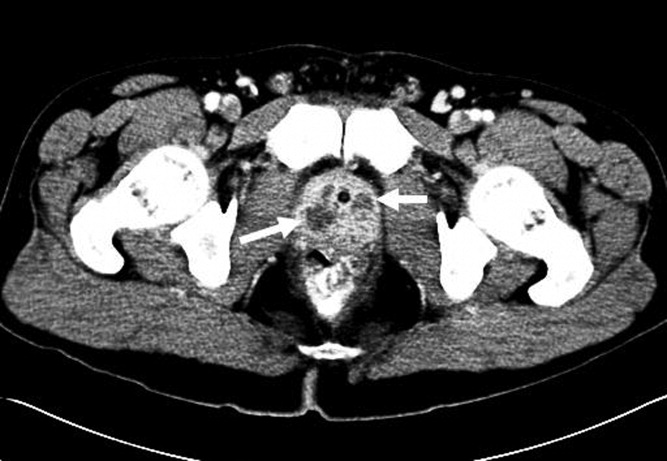
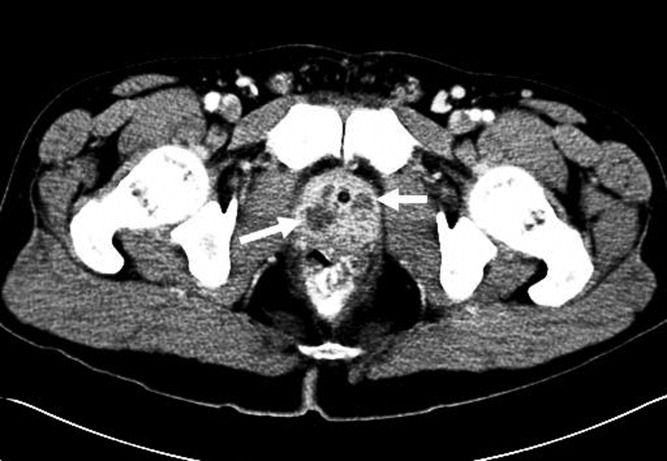
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.
Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.

Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
Community‐associated methicillin‐resistant Staphylococcus aureus (MRSA) infection is an evolving disease that is changing medical practice. MRSA has become the most frequent cause of skin and soft‐tissue infections presenting to most emergency departments in the United States.1 In comparison with methicillin‐sensitive S. aureus, community‐associated MRSA is more likely to present as soft‐tissue abscesses or necrotizing pneumonia.2 In 2005 alone, 94,360 invasive MRSA infections were estimated to have occurred in the United States, most of which were associated with MRSA bacteremia.3 In the hospital, MRSA infections are associated with greater lengths of stay, higher mortality, and increased costs.3
We report a patient with persistent MRSA bacteremia due to a prostatic abscess. Prostatitis or prostatic abscess with MRSA has rarely been reported. Resolution of the bacteremia was achieved only after drainage of the abscess. This case highlights the importance of recognizing this clinical condition and draining any MRSA‐associated abscesses. In addition, the abscess‐forming characteristics of MRSA may suggest that the incidence of prostatic abscess due to this microbe is on the rise.
CASE REPORT
A 40‐year‐old human immunodeficiency viruspositive man presented with a 10‐day history of intermittent fever, urinary hesitancy, weak urinary stream, and intermittent abdominal pain relieved following urination. He denied dysuria, hematuria, chest pain, dyspnea, nausea, weight loss, diarrhea, or decreased functional status. His last CD4 count was 528/L on highly active antiretroviral therapy 1 year prior to presentation; however, he had run out of medications several months prior to presentation. His medical history was also significant for incision and drainage of skin abscesses with unknown microbiology several months prior to presentation. He currently used tobacco but denied illicit drug use. He was sexually abstinent for over a year prior to presentation but had a history of having unprotected sex with men.
On physical examination, his vital signs were as follows: temperature, 37.8C; blood pressure, 133/84 mm Hg; heart rate, 107 beats/minute; respiration rate, 16 breaths/minute; and oxygen saturation, 100% on room air. He was under no distress. His heart sound was tachycardic and regular with no murmur noted. Lung sounds were clear. Abdominal examination revealed no tenderness or organomegaly. His prostate was boggy, minimally tender, and slightly enlarged. The rest of his physical examination was normal. The white blood cell count was 7.5 109/L with 79% neutrophils. The serum chemistry was normal. The prostate‐specific antigen level was 2.9 ng/mL. A repeat CD4 count was 140/L. Urinalysis revealed large leukocyte esterase, no nitrites, and 60 white blood cells per high‐power field. He was diagnosed with prostatitis and discharged on levofloxacin.
The subsequent day, he returned to the emergency department with an inability to void. A Foley catheter was placed with resolution of his symptoms. Later that day, blood cultures from his initial admission grew MRSA in 2 out of 4 bottles, and he was admitted to the hospital. A review of the prior urine culture showed no growth. Computed tomography (CT) of the abdomen and pelvis showed an enlarged prostate with multiple noncommunicating peripherally enhancing hypodensities consistent with prostatic abscesses (arrows in Figure 1). These abscesses were associated with periprostatic fat stranding, edematous seminal vesicles, diffuse urinary bladder wall thickening, and mild inguinal adenopathy. Therapy with intravenous vancomycin was initiated.

Over the next 6 days, the patient continued to have intermittent fevers. Blood cultures continued to grow MRSA. Gentamicin was added for possible synergy on hospital day 3 without improvement. A transesophageal echocardiogram was normal. On hospital day 6, repeat CT showed no change in the size of the prostatic abscesses but an increased capsular bulge along the right mid gland. The urology service was consulted. They performed bedside transperineal drainage of the largest abscess with transrectal ultrasound guidance. No indwelling drain was placed. A culture of the purulent drainage grew MRSA. Three days after the surgical drainage, the patient was afebrile, urinary symptoms had resolved, and serial blood cultures remained negative. He was discharged on hospital day 13 to complete a 4‐week course of intravenous vancomycin. The patient missed an appointment for follow‐up imaging.
DISCUSSION
We report a case of community‐associated MRSA bacteremia secondary to a prostatic abscess, as confirmed by cultures from serum and of the abscess. S. aureus bacteremia often stems from infections of the respiratory tract, skin, and soft tissues, endocarditis, or infections of indwelling devices.4S. aureus can then create embolic foci, including in the prostate, which can serve as sources of persistent bacteremia. However, new evidence suggests community‐associated MRSA might be sexually transmitted among men who have sex with men.5 An MRSA prostatic abscess could then potentially be related to an ascending urinary tract infection or translocation from the gastrointestinal tract.
The vast majority of prostatitis cases prostatic abscesses are caused by Escherichia coli and other gram‐negative bacilli.6 Staphylococcus species are less common, and MRSA isolates have been described as a rare etiologic agent. Only four other case reports describing MRSA bacteremia associated with prostatitis or prostatic abscesses have been published.710 Both our patient and the three other case reports in the literature with prostatic abscesses and MRSA bacteremia required drainage of the abscess as well as antibiotics for resolution. The other reported cases involved drainage by transurethral resection of the prostate,79 whereas our case used transperineal drainage of the prostatic abscess via transrectal ultrasound guidance. In addition to intravenous antibiotics, drainage appears to be a key therapeutic measure for resolution of MRSA prostatic abscesses.
With the increasing incidence of community‐associated and healthcare‐associated MRSA infections, the incidence of prostatitis or prostatic abscesses secondary to MRSA may be increasing. MRSA seems to have a specific predilection for abscess formation in skin and soft‐tissue infections and pneumonia.2 Clinicians must be alert to a potentially higher frequency of MRSA as a cause of prostatic abscesses and prostatitis.
In the evaluation of a patient with persistent MRSA bacteremia, the potential for the prostate as a source of infection should be considered. Urinary symptoms may be subtle. Clinicians should have a low threshold for imaging studies such as CT to evaluate for a possible source of MRSA bacteremia. If a prostatic abscess is found, prompt surgical drainage or debridement is necessary for cure.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.
- ,,, et al.Methicillin‐resistant S. aureus infections among patients in the emergency department.N Engl J Med.2006;355:666–674.
- ,,, et al.Comparison of both clinical features and mortality risk associated with bacteremia due to community‐acquired methicillin‐resistant Staphylococcus aureus and methicillin‐susceptible S. aureus.Clin Infect Dis.2008;46:799–806.
- ,,, et al.Invasive methicillin‐resistant Staphylococcus aureus infections in the United States.JAMA.2007;298:1763–1771.
- ,,,,,.The current spectrum of Staphylococcus aureus infection in a tertiary care hospital.Medicine (Baltimore).1994;73:186–208.
- ,,, et al.Emergence of multidrug‐resistant, community‐associated, methicillin‐resistant Staphylococcus aureus clone USA300 in men who have sex with men.Ann Intern Med.2008;148:249–257.
- ,,, et al.Prostatic abscess in the antibiotic era.Rev Infect Dis.1988;10:239–249.
- ,,.Community‐acquired methicillin‐resistant Staphylococcus aureus prostatic abscess.Urology.2004;64:808–810.
- ,,.Persistent methicillin‐resistant Staphylococcus aureus bacteremia due to a prostatic abscess.Scand J Infect Dis.2003;35:273–274.
- ,.Dual perinephric and prostatic abscesses from methicillin resistant Staphylococcus aureus.South Med J.2007;100:515–516.
- ,.Methicillin‐resistant Staphylococcus aureus prostatitis.Urology.2007;69:779.e1–779.e3.
Calcinosis universalis
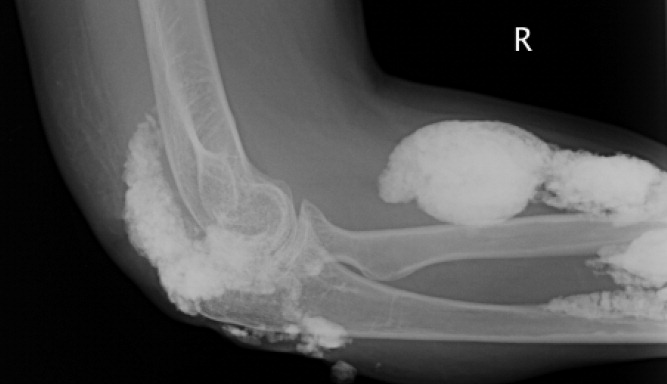
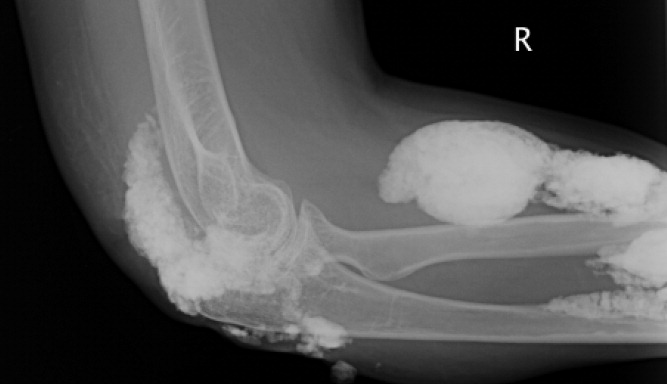
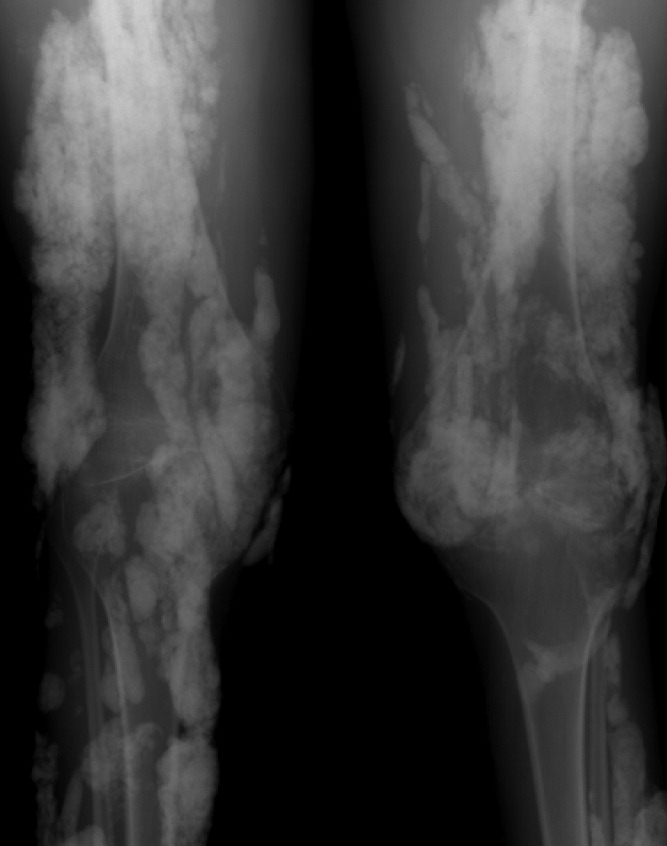
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.
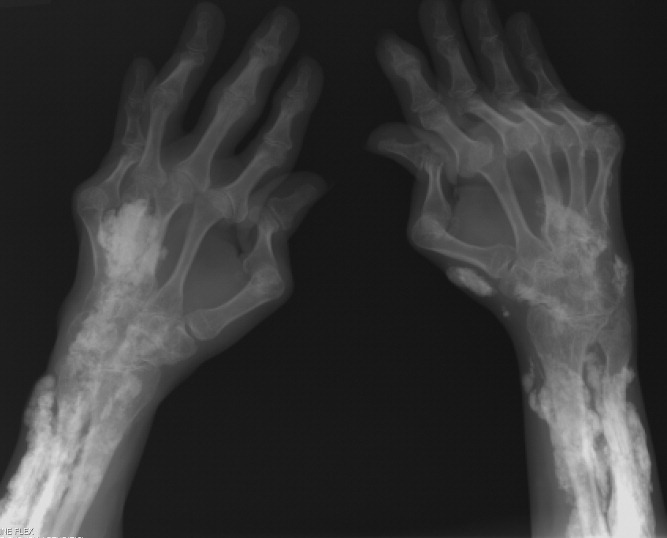
Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2
Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
Evaluation of glycemic control following discontinuation of an intensive insulin protocol
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
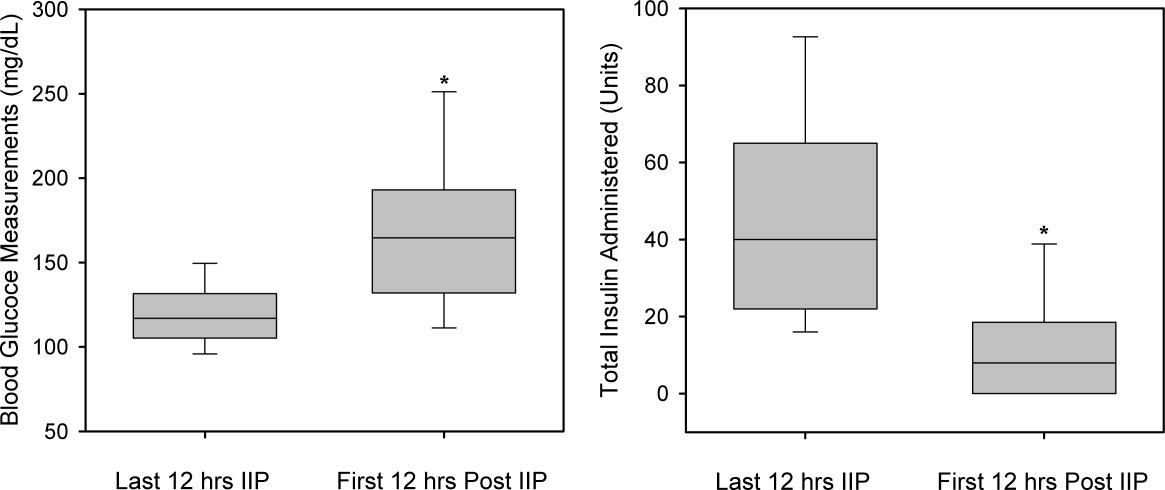
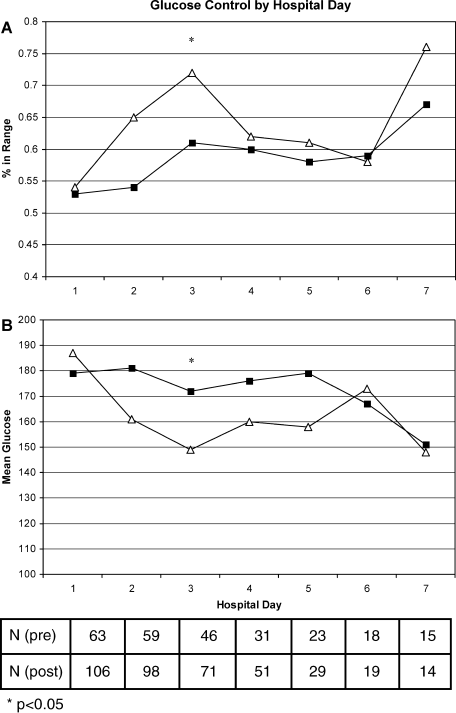
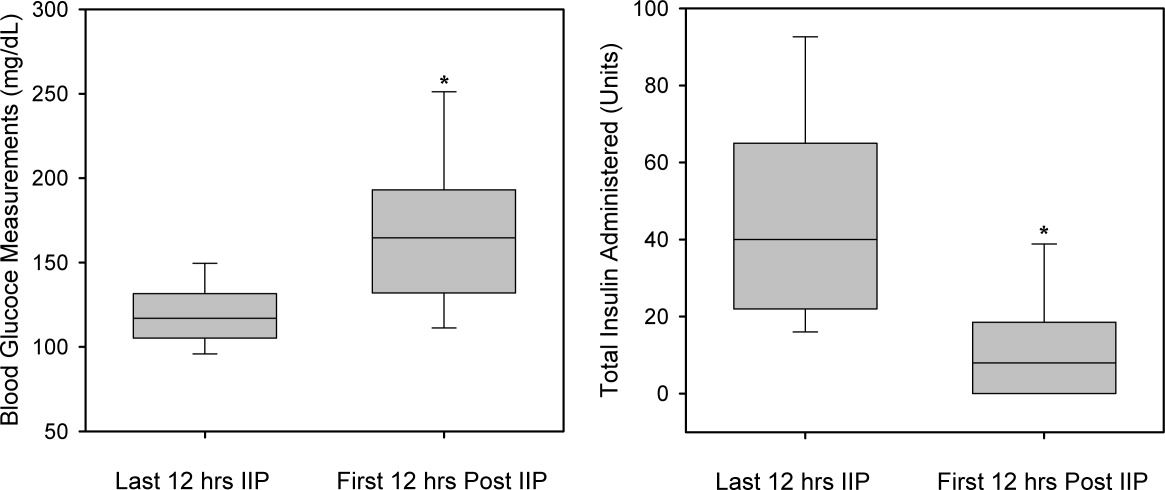
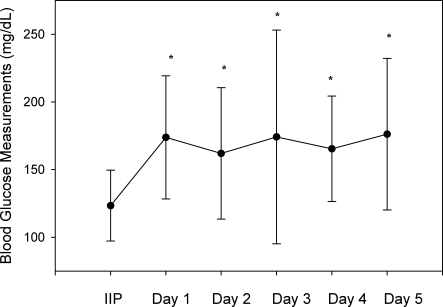
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.
| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.


| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.


| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
Copyright © 2009 Society of Hospital Medicine
Pericardial Effusion
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
Improving Inpatient Glycemic Control
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.
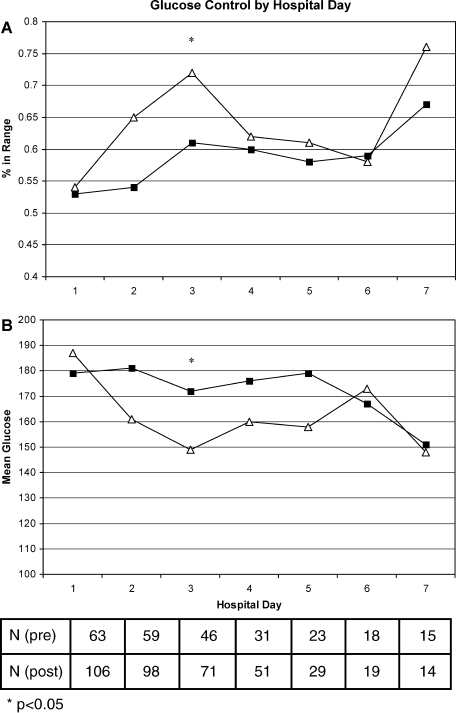
DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Copyright © 2009 Society of Hospital Medicine
Welcome to My World
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Improved Glycemic Control and Hypoglycemia
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.
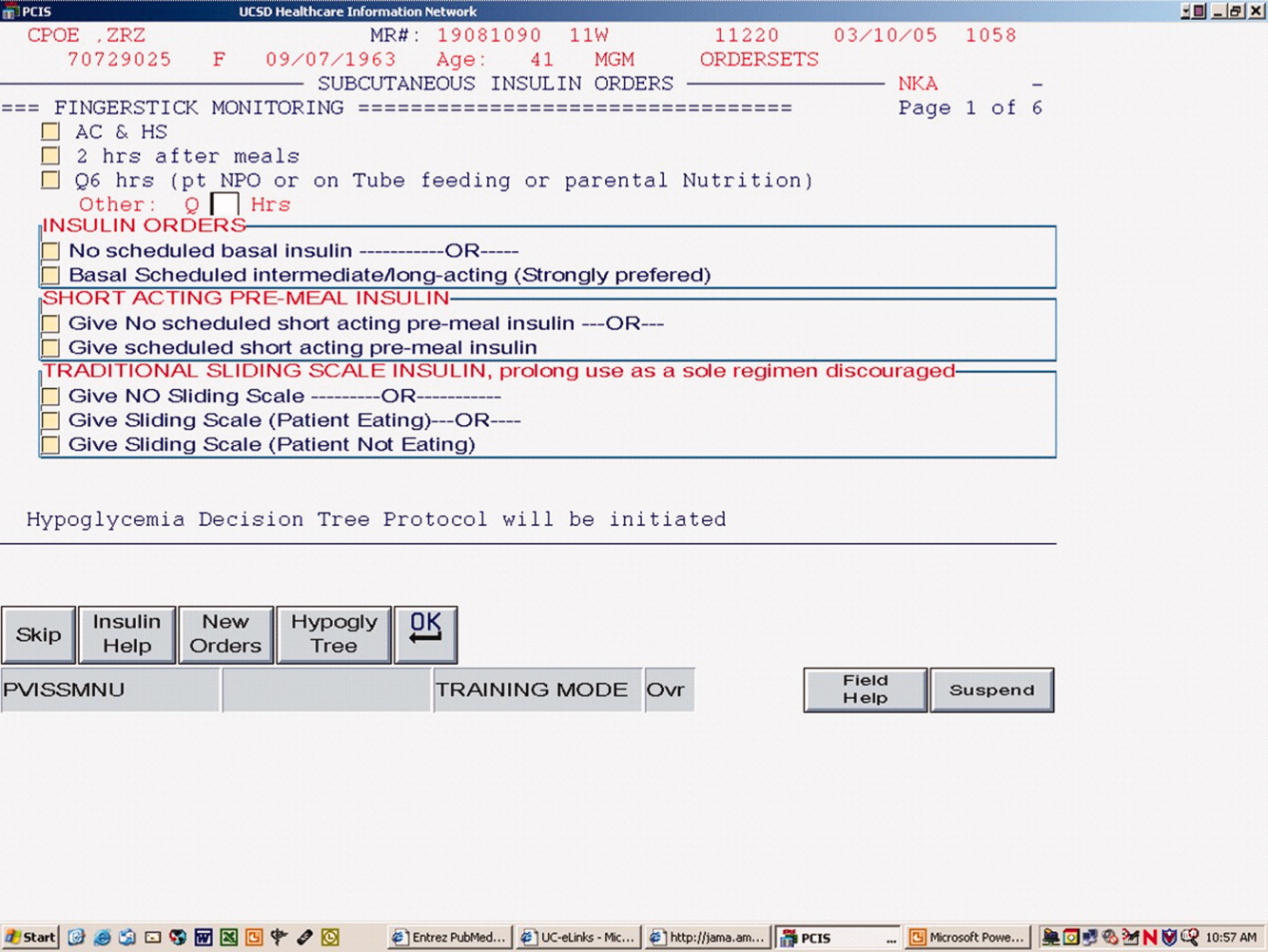
Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.
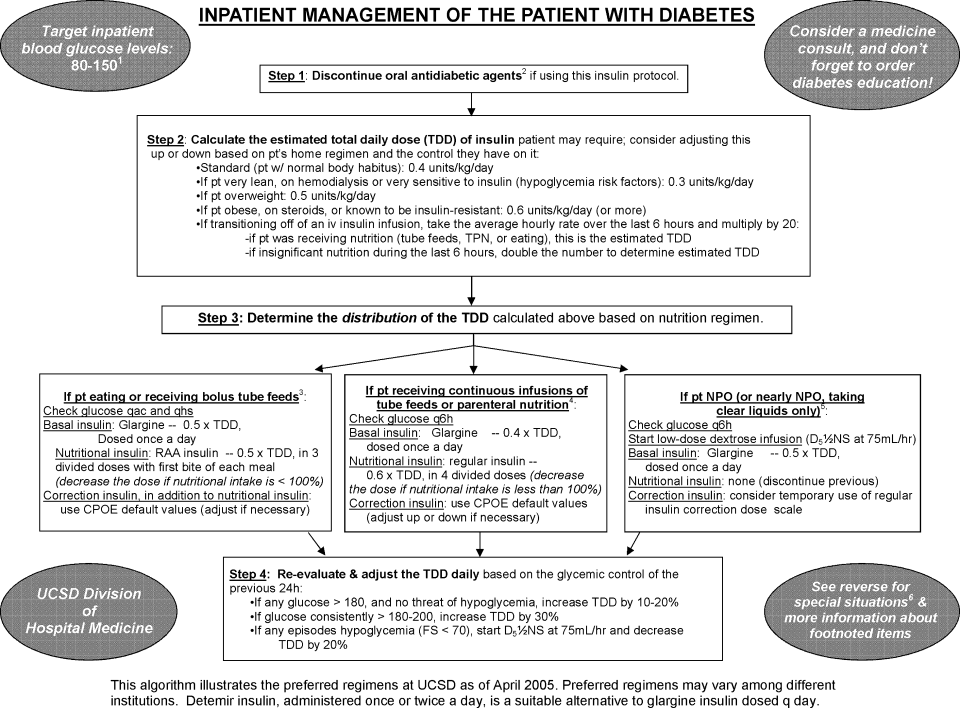

Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.
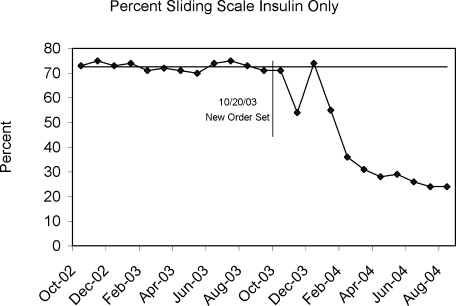
Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.
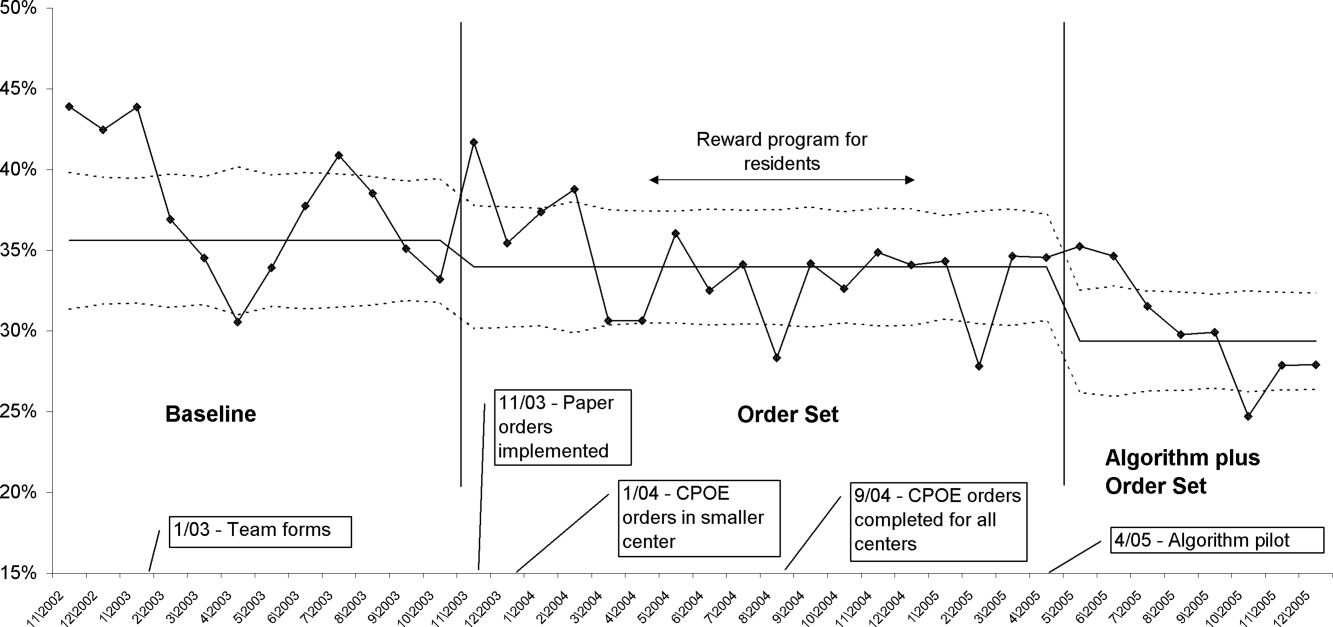
Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Copyright © 2009 Society of Hospital Medicine