User login
Safe Transitions and Congregate Living in the Age of COVID-19: A Retrospective Cohort Study
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
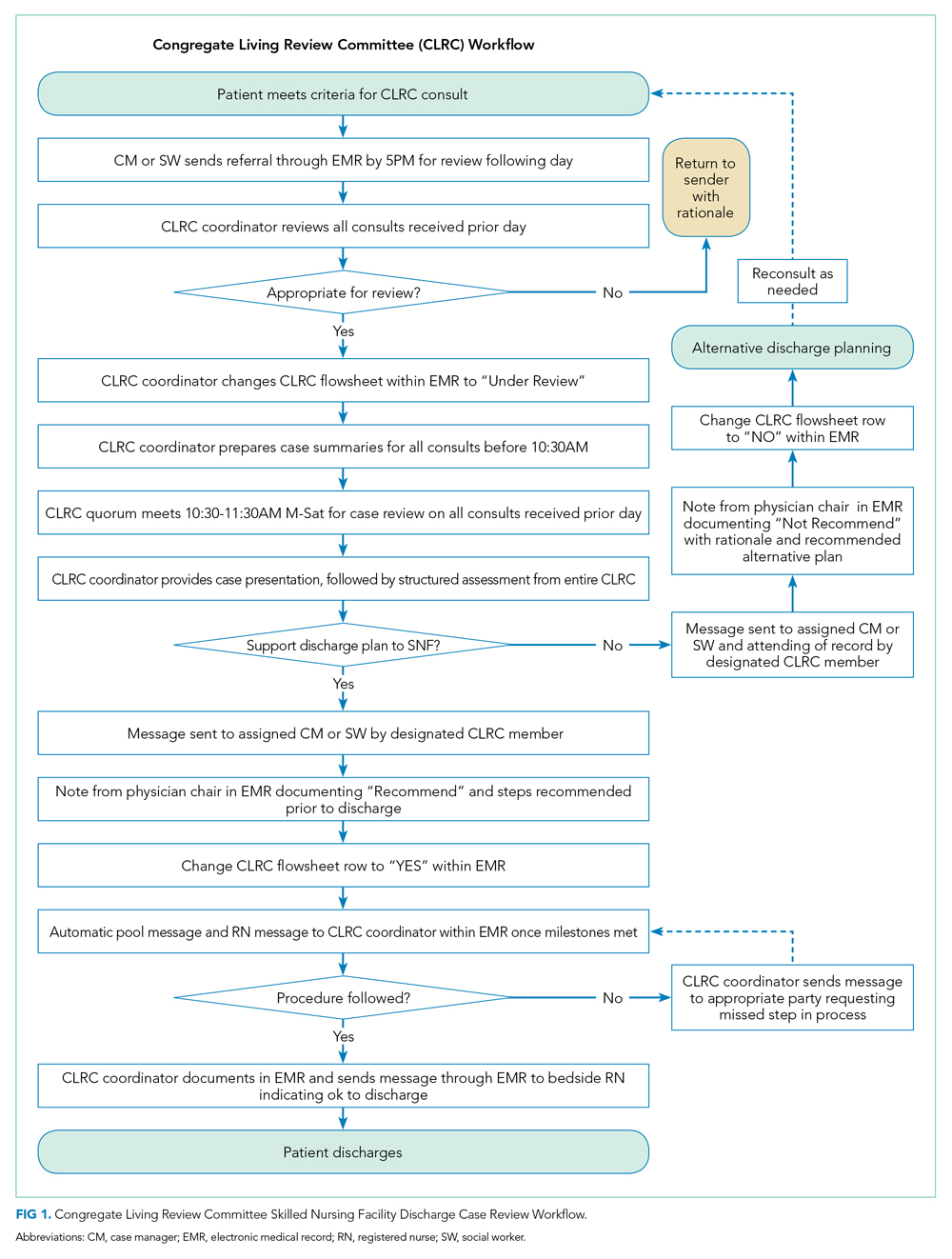
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
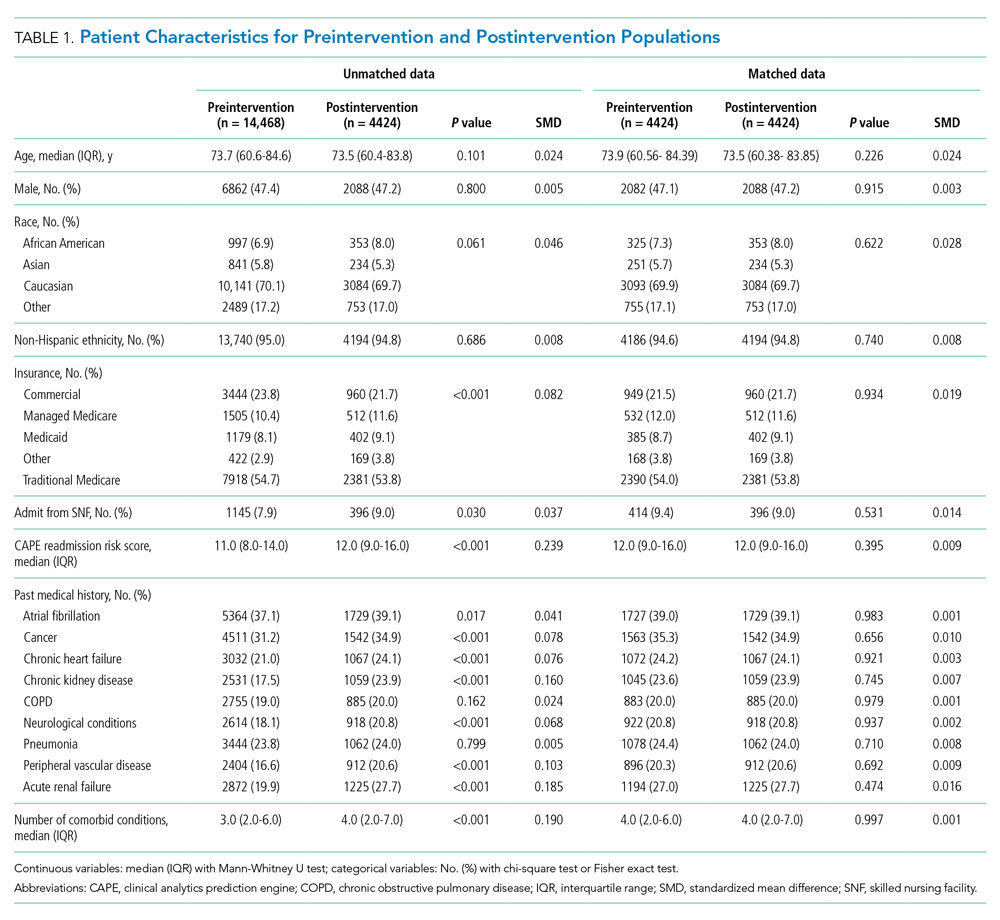
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2. 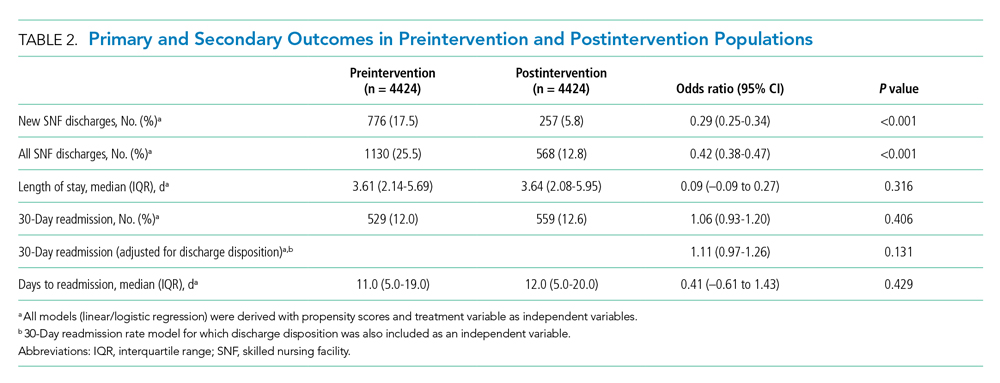
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.

Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.

During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2. 

DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
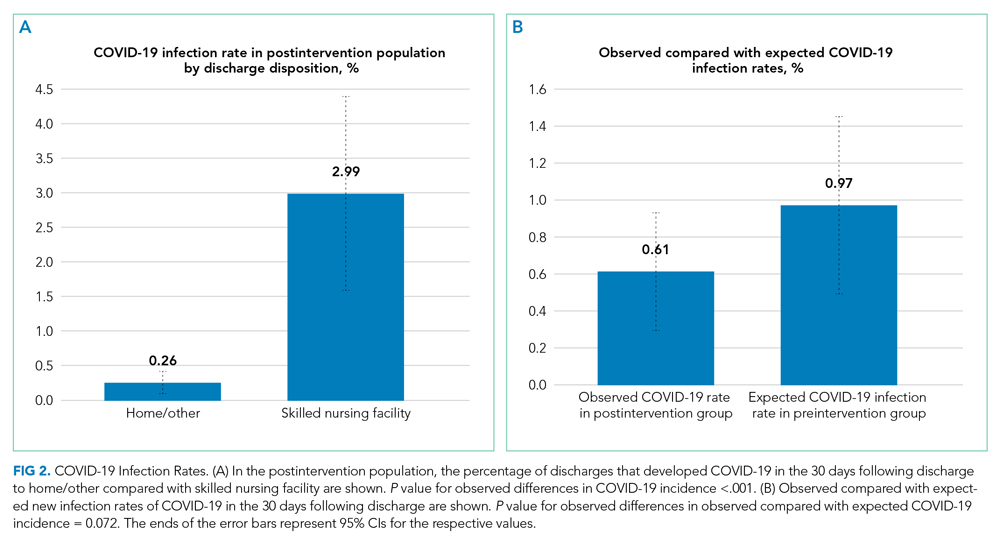
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.

Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.

During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2. 

DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
© 2021 Society of Hospital Medicine
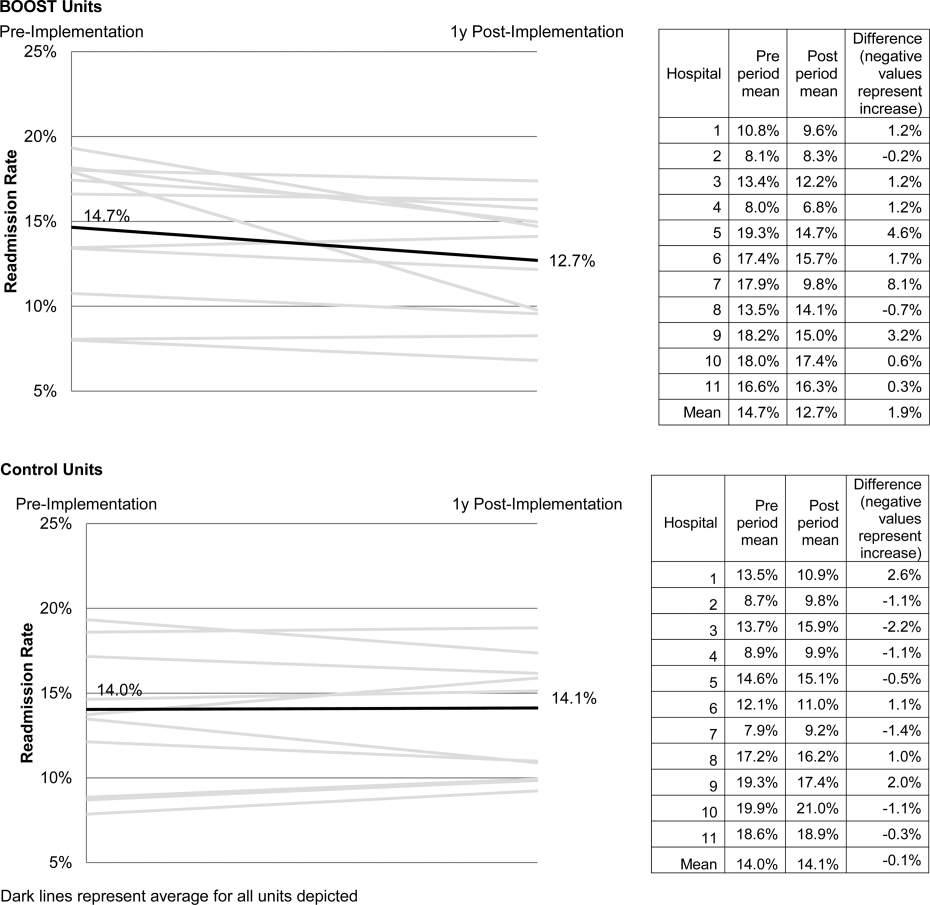
Project BOOST
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).
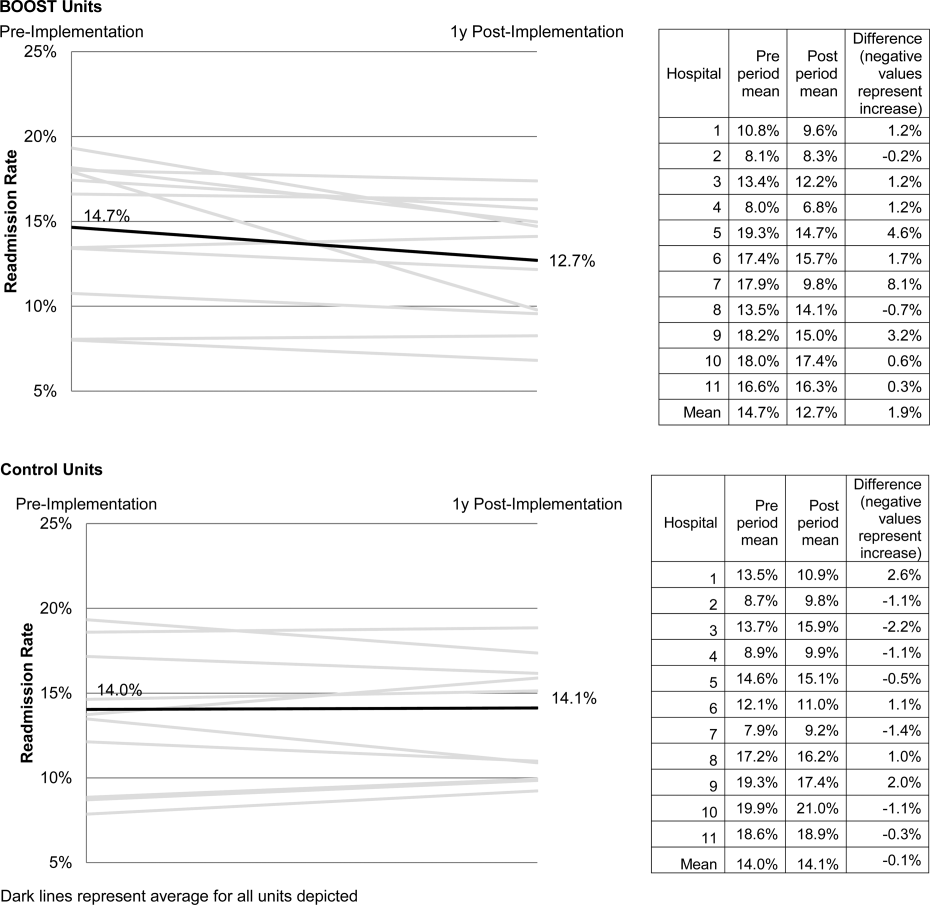
DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Copyright © 2013 Society of Hospital Medicine
Medication Reconciliation: A Consensus Statement From Stakeholders
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Recommendations for Hospitalist Handoffs
Handoffs during hospitalization from one provider to another represent critical transition points in patient care.1 In‐hospital handoffs are a frequent occurrence, with 1 teaching hospital reporting 4000 handoffs daily for a total of 1.6 million per year.2
Incomplete or poor‐quality handoffs have been implicated as a source of adverse events and near misses in hospitalized patients.35 Standardizing the handoff process may improve patient safety during care transitions.6 In 2006, the Joint Commission issued a National Patient Safety Goal that requires care providers to adopt a standardized approach for handoff communications, including an opportunity to ask and respond to questions about a patient's care.7 The reductions in resident work hours by the Accreditation Council for Graduate Medical Education (ACGME) has also resulted in a greater number and greater scrutiny of handoffs in teaching hospitals.8, 9
In response to these issues, and because handoffs are a core competency for hospitalists, the Society of Hospital Medicine (SHM)convened a task force.10 Our goal was to develop a set of recommendations for handoffs that would be applicable in both community and academic settings; among physicians (hospitalists, internists, subspecialists, residents), nurse practitioners, and physicians assistants; and across roles including serving as the primary provider of hospital care, comanager, or consultant. This work focuses on handoffs that occur at shift change and service change.11 Shift changes are transitions of care between an outgoing provider and an incoming provider that occur at the end of the outgoing provider's continuous on‐duty period. Service changesa special type of shift changeare transitions of care between an outgoing provider and an incoming provider that occur when an outgoing provider is leaving a rotation or period of consecutive daily care for patients on the same service.
For this initiative, transfers of care in which the patient is moving from one patient area to another (eg, Emergency Department to inpatient floor, or floor to intensive care unit [ICU]) were excluded since they likely require unique consideration given their cross‐disciplinary and multispecialty nature. Likewise, transitions of care at hospital admission and discharge were also excluded because recommendations for discharge are already summarized in 2 complementary reports.12, 13
To develop recommendations for handoffs at routine shift change and service changes, the Handoff Task Force performed a systematic review of the literature to develop initial recommendations, obtained feedback from hospital‐based clinicians in addition to a panel of handoff experts, and finalized handoff recommendations, as well as a proposed research agenda, for the SHM.
Methods
The SHM Healthcare Quality and Patient Safety (HQPS) Committee convened the Handoff Task Force, which was comprised of 6 geographically diverse, predominantly academic hospitalists with backgrounds in education, patient safety, health communication, evidence‐based medicine, and handoffs. The Task Force then engaged a panel of 4 content experts selected for their work on handoffs in the fields of nursing, information technology, human factors engineering, and hospital medicine. Similar to clinical guideline development by professional societies, the Task Force used a combination of evidence‐based review and expert opinions to propose recommendations.
Literature Review
A PubMed search was performed for English language articles published from January 1975 to January 2007, using the following keywords: handover or handoff or hand‐off or shift change or signout or sign‐out. Articles were eligible if they presented results from a controlled intervention to improve handoffs at shift change or service change, by any health profession. Articles that appeared potentially relevant based on their title were retrieved for full‐text review and included if deemed eligible by at least 2 reviewers. Additional studies were obtained through the Agency for Healthcare Research and Quality (AHRQ) Patient Safety Network,14 using the category Safety target and subcategory Discontinuities, gaps, and hand‐off problems. Finally, the expert panel reviewed the results of the literature review and suggested additional articles.
Eligible studies were abstracted by individual members of the Handoff Task Force using a structured form (Appendix Figure 1), and abstractions were verified by a second member. Handoff‐related outcome measures were categorized as referring to (1) patient outcomes, (2) staff outcomes, or (3) system outcomes. Because studies included those from nursing and other industries, interventions were evaluated by abstractors for their applicability to routine hospitalist handoffs. The literature review was supplemented by review of expert consensus or policy white papers that described recommendations for handoffs. The list of white papers was generated utilizing a common internet search engine (Google;
Peer and Expert Panel Review
The Task Force generated draft recommendations, which were revised through interactive discussions until consensus was achieved. These recommendations were then presented at a workshop to an audience of approximately 300 hospitalists, case managers, nurses, and pharmacists at the 2007 SHM Annual Meeting.
During the workshop, participants were asked to cast up to 3 votes for recommendations that should be removed. Those recommendations that received more than 20 votes for removal were then discussed. Participants also had the opportunity to anonymously suggest new recommendations or revisions using index cards, which were reviewed by 2 workshop faculty, assembled into themes, and immediately presented to the group. Through group discussion of prevalent themes, additional recommendations were developed.
Four content experts were then asked to review a draft paper that summarized the literature review, discussion at the SHM meeting, and handoff recommendations. Their input regarding the process, potential gaps in the literature, and additional items of relevance, was incorporated into this final manuscript.
Final Review by SHM Board and Rating each Recommendation
A working paper was reviewed and approved by the Board of the SHM in early January 2008. With Board input, the Task Force adopted the American College of Cardiology/American Heart Association (ACC/AHA) framework to rate each recommendation because of its appropriateness, ease of use, and familiarity to hospital‐based physicians.15 Recommendations are rated as Class I (effective), IIa (conflicting findings but weight of evidence supports use), IIb (conflicting findings but weight of evidence does not support use), or III (not effective). The Level of Evidence behind each recommendation is graded as A (from multiple large randomized controlled trials), B (from smaller or limited randomized trials, or nonrandomized studies), or C (based primarily on expert consensus). A recommendation with Level of Evidence B or C should not imply that the recommendation is not supported.15
Results
Literature Review
Of the 374 articles identified by the electronic search of PubMed and the AHRQ Patient Safety Network, 109 were retrieved for detailed review, and 10 of these met the criteria for inclusion (Figure 1). Of these studies, 3 were derived from nursing literature and the remaining were tests of technology solutions or structured templates (Table 1).1618, 20, 22, 3842 No studies examined hospitalist handoffs. All eligible studies concerned shift change. There were no studies of service change. Only 1 study was a randomized controlled trial; the rest were pre‐post studies with historical controls or a controlled simulation. All reports were single‐site studies. Most outcomes were staff‐related or system‐related, with only 2 studies using patient outcomes.
| Author (Year) | Study Design | Intervention | Setting and Study Population | Target | Outcomes |
|---|---|---|---|---|---|
| |||||
| Nursing | |||||
| Kelly22 (2005) | Pre‐post | Change to walk‐round handover (at bedside) from baseline (control) | 12‐bed rehab unit with 18 nurses and 10 patients | Staff, patient | 11/18 nurses felt more or much more informed and involved; 8/10 patients felt more involved |
| Pothier et al.20 (2005) | Controlled simulation | Compared pure verbal to verbal with note‐taking to verbal plus typed content | Handover of 12 simulated patients over 5 cycles | System (data loss) | Minimal data loss with typed content, compared to 31% data retained with note‐taking, and no data retained with verbal only |
| Wallum38 (1995) | Pre‐post | Change from oral handover (baseline) to written template read with exchange | 20 nurses in a geriatric dementia ward | Staff | 83% of nurses felt care plans followed better; 88% knew care plans better |
| Technology or structured template | |||||
| Cheah et al.39 (2005) | Pre‐post | Electronic template with free‐text entry compared to baseline | 14 UK Surgery residents | Staff | 100% (14) of residents rated electronic system as desirable, but 7 (50%) reported that information was not updated |
| Lee et al.40 (1996) | Pre‐post | Standardized signout card for interns to transmit information during handoffs compared to handwritten (baseline) | Inpatient cardiology service at IM residency program in Minnesota with 19 new interns over a 3‐month period | Staff | Intervention interns (n = 10) reported poor sign‐out less often than controls (n = 9) [intervention 8 nights (5.8%) vs. control 17 nights (14.9%); P = 0.016] |
| Kannry and Moore18 (1999) | Pre‐post | Compared web‐based signout program to usual system (baseline) | An academic teaching hospital in New York (34 patients admitted in 1997; 40 patients admitted in 1998) | System | Improved provider identification (86% web signout vs. 57% hospital census) |
| Petersen et al.17 (1998) | Pre‐post | 4 months of computerized signouts compared to baseline period (control) | 3747 patients admitted to the medical service at an academic teaching hospital | Patient | Preventable adverse events (ADE) decreased (1.7% to 1.2%, P 0.10); risk of cross‐cover physician for ADE eliminated |
| Ram and Block41 (1993) | Pre‐post | Compared handwritten (baseline) to computer‐generated | Family medicine residents at 2 academic teaching hospitals [Buffalo (n = 16) and Pittsburgh (n = 16)] | Staff | Higher satisfaction after electronic signout, but complaints with burden of data entry and need to keep information updated |
| Van Eaton et al.42 (2004) | Pre‐post | Use of UW Cores links sign‐out to list for rounds and IS data | 28 surgical and medical residents at 2 teaching hospitals | System | At 6 months, 66% of patients entered in system (adoption) |
| Van Eaton et al.16 (2005) | Prospective, randomized, crossover study. | Compared UW Cores* integrated system compared to usual system | 14 inpatient resident teams (6 surgery, 8 IM) at 2 teaching hospitals for 5 months | Staff, system | 50% reduction in the perceived time spent copying data [from 24% to 12% (P 0.0001)] and number of patients missed on rounds (2.5 vs. 5 patients/team/month, P = 0.0001); improved signout quality (69.6% agree or strongly agree); and improved continuity of care (66.1% agree or strongly agree) |

Overall, the literature presented supports the use of a verbal handoff supplemented with written documentation in a structured format or technology solution. The 2 most rigorous studies were led by Van Eaton et al.16 and Petersen et al.17 and focused on evaluating technology solutions. Van Eaton et al.16 performed a randomized controlled trial of a locally created rounding template with 161 surgical residents. This template downloads certain information (lab values and recent vital signs) from the hospital system into a sign‐out sheet and allows residents to enter notes about diagnoses, allergies, medications and to‐do items. When implemented, the investigators found the number of patients missed on rounds decreased by 50%. Residents reported an increase of 40% in the amount of time available to pre‐round, due largely to not having to copy data such as vital signs. They reported a decrease in rounding time by 3 hours per week, and this was perceived as helping them meet the ACGME 80 hours work rules. Lastly, the residents reported a higher quality of sign‐outs from their peers and perceived an overall improvement in continuity of care. Petersen and colleagues implemented a computerized sign‐out (auto‐imported medications, name, room number) in an internal medicine residency to improve continuity of care during cross‐coverage and decrease adverse events.17 Prior to the intervention, the frequency of preventable adverse events was 1.7% and it was significantly associated with cross‐coverage. Preventable adverse events were identified using a confidential self‐report system that was also validated by clinician review. After the intervention, the frequency of preventable adverse events dropped to 1.2% (P 0.1), and cross‐coverage was no longer associated with preventable adverse events. In other studies, technological solutions also improved provider identification and staff communication.18, 19 Together, these technology‐based intervention studies suggest that a computerized sign‐out with auto‐imported fields has the ability to improve physician efficiency and also improve inpatient care (reduction in number of patients missed on rounds, decrease in preventable adverse events).
Studies from nursing demonstrated that supplementing a verbal exchange with written information improved transfer of information, compared to verbal exchange alone.20 One of these studies rated the transfer of information using videotaped simulated handoff cases.21 Last, 1 nursing study that more directly involved patients in the handoff process resulted in improved nursing knowledge and greater patient empowerment (Table 1).22
White papers or consensus statements originated from international and national consortia in patient safety including the Australian Council for Safety and Quality in Healthcare,23 the Junior Doctors Committee of the British Medical Association,24 University Health Consortium,25 the Department of Defense Patient Safety Program,26 and The Joint Commission.27 Several common themes were prevalent in all white papers. First, there exists a need to train new personnel on how to perform an effective handoff. Second, efforts should be undertaken to ensure adequate time for handoffs and reduce interruptions during handoffs. Third, several of the papers supported verbal exchange that facilitates interactive questioning, focuses on ill patients, and delineates actions to be taken. Lastly, content should be updated to ensure transfer of the latest clinical information.
Peer Review at SHM Meeting of Preliminary Handoff Recommendations
In the presentation of preliminary handoff recommendations to over 300 attendees at the SHM Annual Meeting in 2007, 2 recommendations were supported unanimously: (1) a formal recognized handoff plan should be instituted at end of shift or change in service; and (2) ill patients should be given priority during verbal exchange.
During the workshop, discussion focused on three recommendations of concern, or those that received greater than 20 negative votes by participants. The proposed recommendation that raised the most objections (48 negative votes) was that interruptions be limited. Audience members expressed that it was hard to expect that interruptions would be limited given the busy workplace in the absence of endorsing a separate room and time. This recommendation was ultimately deleted.
The 2 other debated recommendations, which were retained after discussion, were ensuring adequate time for handoffs and using an interactive process during verbal communication. Several attendees stated that ensuring adequate time for handoffs may be difficult without setting a specific time. Others questioned the need for interactive verbal communication, and endorsed leaving a handoff by voicemail with a phone number or pager to answer questions. However, this type of asynchronous communication (senders and receivers not present at the same time) was not desirable or consistent with the Joint Commission's National Patient Safety Goal.
Two new recommendations were proposed from anonymous input and incorporated in the final recommendations, including (a) all patients should be on the sign‐out, and (b) sign‐outs should be accessible from a centralized location. Another recommendation proposed at the Annual Meeting was to institute feedback for poor sign‐outs, but this was not added to the final recommendations after discussion at the meeting and with content experts about the difficulty of maintaining anonymity in small hospitalist groups. Nevertheless, this should not preclude informal feedback among practitioners.
Anonymous commentary also yielded several major themes regarding handoff improvements and areas of uncertainty that merit future work. Several hospitalists described the need to delineate specific content domains for handoffs including, for example, code status, allergies, discharge plan, and parental contact information in the case of pediatric care. However, due to the variability in hospitalist programs and health systems and the general lack of evidence in this area, the Task Force opted to avoid recommending specific content domains which may have limited applicability in certain settings and little support from the literature. Several questions were raised regarding the legal status of written sign‐outs, and whether sign‐outs, especially those that are web‐based, are compliant with the Healthcare Information Portability and Accountability Act (HIPAA). Hospitalists also questioned the appropriate number of patients to be handed off safely. Promoting efficient technology solutions that reduce documentation burden, such as linking the most current progress note to the sign‐out, was also proposed. Concerns were also raised about promoting safe handoffs when using moonlighting or rotating physicians, who may be less invested in the continuity of the patients' overall care.
Expert Panel Review
The final version of the Task Force recommendations incorporates feedback provided by the expert panel. In particular, the expert panel favored the use of the term, recommendations, rather than standards, minimum acceptable practices, or best practices. While the distinction may appear semantic, the Task Force and expert panel acknowledge that the current state of scientific knowledge regarding hospital handoffs is limited. Although an evidence‐based process informed the development of these recommendations, they are not a legal standard for practice. Additional research may allow for refinement of recommendations and development of more formal handoff standards.
The expert panel also highlighted the need to provide tools to hospitalist programs to facilitate the adoption of these recommendations. For example, recommendations for content exchange are difficult to adopt if groups do not already use a written template. The panel also commented on the need to consider the possible consequences if efforts are undertaken to include handoff documents (whether paper or electronic) as part of the medical record. While formalizing handoff documents may raise their quality, it is also possible that handoff documents become less helpful by either excluding the most candid impression regarding a patient's status or by encouraging hospitalists to provide too much detail. Privacy and confidentiality of paper‐based systems, in particular, were also questioned.
Additional Recommendations for Service Change
Patient handoffs during a change of service are a routine part of hospitalist care. Since service change is a type of shift change, the handoff recommendations for shift change do apply. Unlike shift change, service changes involve a more significant transfer of responsibility. Therefore, the Task Force recommends also that the incoming hospitalist be readily identified in the medical record or chart as the new provider, so that relevant clinical information can be communicated to the correct physician. This program‐level recommendation can be met by an electronic or paper‐based system that correctly identifies the current primary inpatient physician.
Final Handoff Recommendations
The final handoff recommendations are shown in Figure 2. The recommendations were designed to be consistent with the overall finding of the literature review, which supports the use of a verbal handoff supplemented with written documentation or a technological solution in a structured format. With the exception of 1 recommendation that is specific to service changes, all recommendations are designed to refer to shift changes and service changes. One overarching recommendation refers to the need for a formally recognized handoff plan at a shift change or change of service. The remaining 12 recommendations are divided into 4 that refer to hospitalist groups or programs, 3 that refer to verbal exchange, and 5 that refer to content exchange. The distinction is an important one because program‐level recommendations require organizational support and buy‐in to promote clinician participation and adherence. The 4 program recommendations also form the necessary framework for the remaining recommendations. For example, the second program recommendation describes the need for a standardized template or technology solution for accessing and recording patient information during the handoff. After a program adopts such a mechanism for exchanging patient information, the specific details for use and maintenance are outlined in greater detail in content exchange recommendations.

Because of the limited trials of handoff strategies, none of the recommendations are supported with level of evidence A (multiple numerous randomized controlled trials). In fact, with the exception of using a template or technology solution which was supported with level of evidence B, all handoff recommendations were supported with C level of evidence. The recommendations, however, were rated as Class I (effective) because there were no conflicting expert opinions or studies (Figure 2).
Discussion
In summary, our review of the literature supports the use of face‐to‐face verbal handoffs that are aided by the use of structured template to guide exchange of information. Furthermore, the development of these recommendations is the first effort of its kind for hospitalist handoffs and a movement towards standardizing the handoff process. While these recommendations are meant to provide structure to the hospitalist handoff process, the use and implementation by individual hospitalist programs may require more specific detail than these recommendations provide. Local modifications can allow for improved acceptance and adoption by practicing hospitalists. These recommendations can also help guide teaching efforts for academic hospitalists who are responsible for supervising residents.
The limitations of these recommendations related to lack of evidence in this field. Studies suffered from small size, poor description of methods, and a paucity of controlled interventions. The described technology solutions are not standardized or commercially available. Only 1 study included patient outcomes.28 There are no multicenter studies, studies of hospitalist handoffs, or studies to guide inclusion of specific content. Randomized controlled trials, interrupted time series analyses, and other rigorous study designs are needed in both teaching and non‐teaching settings to evaluate these recommendations and other approaches to improving handoffs. Ideally, these studies would occur through multicenter collaboratives and with human factors researchers familiar with mixed methods approaches to evaluate how and why interventions work.29 Efforts should focus on developing surrogate measures that are sensitive to handoff quality and related to important patient outcomes. The results of future studies should be used to refine the present recommendations. Locating new literature could be facilitated through the introduction of Medical Subject Heading for the term handoff by the National Library of Medicine. After completing this systematic review and developing the handoff recommendations described here, a few other noteworthy articles have been published on this topic, to which we refer interested readers. Several of these studies demonstrate that standardizing content and process during medical or surgical intern sign‐out improves resident confidence with handoffs,30 resident perceptions of accuracy and completeness of signout,31 and perceptions of patient safety.32 Another prospective audiotape study of 12 days of resident signout of clinical information demonstrated that poor quality oral sign‐outs was associated with an increased risk of post‐call resident reported signout‐related problems.5 Lastly, 1 nursing study demonstrated improved staff reports of safety, efficiency, and teamwork after a change from verbal reporting in an isolated room to bedside handover.33 Overall, these additional studies continue to support the current recommendations presented in this paper and do not significantly impact the conclusions of our literature review.
While lacking specific content domain recommendations, this report can be used as a starting point to guide development of self and peer assessment of hospitalist handoff quality. Development and validation of such assessments is especially important and can be incorporated into efforts to certify hospitalists through the recently approved certificate of focused practice in hospital medicine by the American Board of Internal Medicine (ABIM). Initiatives by several related organizations may help guide these effortsThe Joint Commission, the ABIM's Stepping Up to the Plate (SUTTP) Alliance, the Institute for Healthcare Improvement, the Information Transfer and Communication Practices (ITCP) Project for surgical care transitions, and the Hospital at Night (H@N) Program sponsored by the United Kingdom's National Health Service.3437 Professional medical organizations can also serve as powerful mediators of change in this area, not only by raising the visibility of handoffs, but also by mobilizing research funding. Patients and their caregivers may also play an important role in increasing awareness and education in this area. Future efforts should target handoffs not addressed in this initiative, such as transfers from emergency departments to inpatient care units, or between ICUs and the medical floor.
Conclusion
With the growth of hospital medicine and the increased acuity of inpatients, improving handoffs becomes an important part of ensuring patient safety. The goal of the SHM Handoffs Task Force was to begin to standardize handoffs at change of shift and change of servicea fundamental activity of hospitalists. These recommendations build on the limited literature in surgery, nursing, and medical informatics and provide a starting point for promoting safe and seamless in‐hospital handoffs for practitioners of Hospital Medicine.
Acknowledgements
The authors also acknowledge Tina Budnitz and the Healthcare Quality and Safety Committee of the Society of Hospital Medicine. Last, they are indebted to the staff support provided by Shannon Roach from the Society of Hospital Medicine.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80(12):1094–1099.
- ... AHRQ WebM167(19):2030–2036.
- ,,,,.Communication failures in patient signout and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,,,.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- ,,, et al.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16:125–132.
- Joint Commission. 2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June2009.
- ,,,.Transfers of patient care between house staff on internal medicine wards: a national survey.Arch Intern Med.2006;166(11):1173–1177.
- ,.Re‐framing continuity of care for this century.Qual Saf Health Care.2005;14(6):394–396.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(suppl 1):48–56.
- ,,, et al.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary‐care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Transition of care for hospitalized elderly patients: development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- Discontinuities, Gaps, and Hand‐Off Problems. AHRQ PSNet Patient Safety Network. Available at: http://www.psnet.ahrq.gov/content.aspx?taxonomyID=412. Accessed June2009.
- Manual for ACC/AHA Guideline Writing Committees. Methodologies and Policies from the ACC/AHA Task Force on Practice Guidelines. Available at: http://circ.ahajournals.org/manual/manual_IIstep6.shtml. Accessed June2009.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200(4):538–545.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.MediSign: using a web‐based SignOut System to improve provider identification.Proc AMIA Symp.1999:550–554.
- ,.Using a computerized sign‐out system to improve physician‐nurse communication.Jt Comm J Qual Patient Saf.2006;32(1):32–36.
- ,,,.Pilot study to show the loss of important data in nursing handover.Br J Nurs.2005;14(20):1090–1093.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- .Change from an office‐based to a walk‐around handover system.Nurs Times.2005;101(10):34–35.
- Clinical Handover and Patient Safety. Literature review report. Australian Council for Safety and Quality in Health Care. Available at: http://www.health.gov.au/internet/safety/publishing.nsf/Content/AA1369AD4AC5FC2ACA2571BF0081CD95/$File/clinhovrlitrev.pdf. Accessed June2009.
- Safe Handover: Safe Patients. Guidance on clinical handover for clinicians and managers. Junior Doctors Committee, British Medical Association. Available at: http://www.bma.org.uk/ap.nsf/AttachmentsByTitle/PDFsafehandover/$FILE/safehandover.pdf. Accessed June2009.
- University HealthSystem Consortium (UHC).UHC Best Practice Recommendation: Patient Hand Off Communication White Paper, May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- Healthcare Communications Toolkit to Improve Transitions in Care. Department of Defense Patient Safety Program. Available at: http://dodpatientsafety.usuhs.mil/files/Handoff_Toolkit.pdf. Accessed June2009.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission announces 2006 national patient safety goals for ambulatory care and office‐based surgery organizations. Available at: http://www.jcaho.org/news+room/news+release+archives/06_npsg_amb_obs.htm. Accessed June2009.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- .Communication strategies from high‐reliability organizations: translation is hard work.Ann Surg.2007;245(2):170–172.
- ,,, et al.A structured handoff program for interns.Acad Med.2009;84(3):347–352.
- ,,, et al.Simple standardized patient handoff system that increases accuracy and completeness.J Surg Educ.2008;65(6):476–485.
- ,,.Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward.Teach Learn Med.2009;21(2):121–126.
- ,,,,,.Bedside handover: quality improvement strategy to “transform care at the bedside”.J Nurs Care Qual.2009;24(2):136–142.
- Pillow M, ed.Improving Handoff Communications.Chicago:Joint Commission Resources;2007.
- American Board of Internal Medicine Foundation. Step Up To The Plate. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed June2009.
- ,,, et al.Surgeon information transfer and communication: factors affecting quality and efficiency of inpatient care.Ann Surg.2007;245(2):159–169.
- Hospital at Night. Available at: http://www.healthcareworkforce.nhs.uk/hospitalatnight.html. Accessed June2009.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- ,,,.Electronic medical handover: towards safer medical care.Med J Aust.2005;183(7):369–372.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11(12):753–755.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
Handoffs during hospitalization from one provider to another represent critical transition points in patient care.1 In‐hospital handoffs are a frequent occurrence, with 1 teaching hospital reporting 4000 handoffs daily for a total of 1.6 million per year.2
Incomplete or poor‐quality handoffs have been implicated as a source of adverse events and near misses in hospitalized patients.35 Standardizing the handoff process may improve patient safety during care transitions.6 In 2006, the Joint Commission issued a National Patient Safety Goal that requires care providers to adopt a standardized approach for handoff communications, including an opportunity to ask and respond to questions about a patient's care.7 The reductions in resident work hours by the Accreditation Council for Graduate Medical Education (ACGME) has also resulted in a greater number and greater scrutiny of handoffs in teaching hospitals.8, 9
In response to these issues, and because handoffs are a core competency for hospitalists, the Society of Hospital Medicine (SHM)convened a task force.10 Our goal was to develop a set of recommendations for handoffs that would be applicable in both community and academic settings; among physicians (hospitalists, internists, subspecialists, residents), nurse practitioners, and physicians assistants; and across roles including serving as the primary provider of hospital care, comanager, or consultant. This work focuses on handoffs that occur at shift change and service change.11 Shift changes are transitions of care between an outgoing provider and an incoming provider that occur at the end of the outgoing provider's continuous on‐duty period. Service changesa special type of shift changeare transitions of care between an outgoing provider and an incoming provider that occur when an outgoing provider is leaving a rotation or period of consecutive daily care for patients on the same service.
For this initiative, transfers of care in which the patient is moving from one patient area to another (eg, Emergency Department to inpatient floor, or floor to intensive care unit [ICU]) were excluded since they likely require unique consideration given their cross‐disciplinary and multispecialty nature. Likewise, transitions of care at hospital admission and discharge were also excluded because recommendations for discharge are already summarized in 2 complementary reports.12, 13
To develop recommendations for handoffs at routine shift change and service changes, the Handoff Task Force performed a systematic review of the literature to develop initial recommendations, obtained feedback from hospital‐based clinicians in addition to a panel of handoff experts, and finalized handoff recommendations, as well as a proposed research agenda, for the SHM.
Methods
The SHM Healthcare Quality and Patient Safety (HQPS) Committee convened the Handoff Task Force, which was comprised of 6 geographically diverse, predominantly academic hospitalists with backgrounds in education, patient safety, health communication, evidence‐based medicine, and handoffs. The Task Force then engaged a panel of 4 content experts selected for their work on handoffs in the fields of nursing, information technology, human factors engineering, and hospital medicine. Similar to clinical guideline development by professional societies, the Task Force used a combination of evidence‐based review and expert opinions to propose recommendations.
Literature Review
A PubMed search was performed for English language articles published from January 1975 to January 2007, using the following keywords: handover or handoff or hand‐off or shift change or signout or sign‐out. Articles were eligible if they presented results from a controlled intervention to improve handoffs at shift change or service change, by any health profession. Articles that appeared potentially relevant based on their title were retrieved for full‐text review and included if deemed eligible by at least 2 reviewers. Additional studies were obtained through the Agency for Healthcare Research and Quality (AHRQ) Patient Safety Network,14 using the category Safety target and subcategory Discontinuities, gaps, and hand‐off problems. Finally, the expert panel reviewed the results of the literature review and suggested additional articles.
Eligible studies were abstracted by individual members of the Handoff Task Force using a structured form (Appendix Figure 1), and abstractions were verified by a second member. Handoff‐related outcome measures were categorized as referring to (1) patient outcomes, (2) staff outcomes, or (3) system outcomes. Because studies included those from nursing and other industries, interventions were evaluated by abstractors for their applicability to routine hospitalist handoffs. The literature review was supplemented by review of expert consensus or policy white papers that described recommendations for handoffs. The list of white papers was generated utilizing a common internet search engine (Google;
Peer and Expert Panel Review
The Task Force generated draft recommendations, which were revised through interactive discussions until consensus was achieved. These recommendations were then presented at a workshop to an audience of approximately 300 hospitalists, case managers, nurses, and pharmacists at the 2007 SHM Annual Meeting.
During the workshop, participants were asked to cast up to 3 votes for recommendations that should be removed. Those recommendations that received more than 20 votes for removal were then discussed. Participants also had the opportunity to anonymously suggest new recommendations or revisions using index cards, which were reviewed by 2 workshop faculty, assembled into themes, and immediately presented to the group. Through group discussion of prevalent themes, additional recommendations were developed.
Four content experts were then asked to review a draft paper that summarized the literature review, discussion at the SHM meeting, and handoff recommendations. Their input regarding the process, potential gaps in the literature, and additional items of relevance, was incorporated into this final manuscript.
Final Review by SHM Board and Rating each Recommendation
A working paper was reviewed and approved by the Board of the SHM in early January 2008. With Board input, the Task Force adopted the American College of Cardiology/American Heart Association (ACC/AHA) framework to rate each recommendation because of its appropriateness, ease of use, and familiarity to hospital‐based physicians.15 Recommendations are rated as Class I (effective), IIa (conflicting findings but weight of evidence supports use), IIb (conflicting findings but weight of evidence does not support use), or III (not effective). The Level of Evidence behind each recommendation is graded as A (from multiple large randomized controlled trials), B (from smaller or limited randomized trials, or nonrandomized studies), or C (based primarily on expert consensus). A recommendation with Level of Evidence B or C should not imply that the recommendation is not supported.15
Results
Literature Review
Of the 374 articles identified by the electronic search of PubMed and the AHRQ Patient Safety Network, 109 were retrieved for detailed review, and 10 of these met the criteria for inclusion (Figure 1). Of these studies, 3 were derived from nursing literature and the remaining were tests of technology solutions or structured templates (Table 1).1618, 20, 22, 3842 No studies examined hospitalist handoffs. All eligible studies concerned shift change. There were no studies of service change. Only 1 study was a randomized controlled trial; the rest were pre‐post studies with historical controls or a controlled simulation. All reports were single‐site studies. Most outcomes were staff‐related or system‐related, with only 2 studies using patient outcomes.
| Author (Year) | Study Design | Intervention | Setting and Study Population | Target | Outcomes |
|---|---|---|---|---|---|
| |||||
| Nursing | |||||
| Kelly22 (2005) | Pre‐post | Change to walk‐round handover (at bedside) from baseline (control) | 12‐bed rehab unit with 18 nurses and 10 patients | Staff, patient | 11/18 nurses felt more or much more informed and involved; 8/10 patients felt more involved |
| Pothier et al.20 (2005) | Controlled simulation | Compared pure verbal to verbal with note‐taking to verbal plus typed content | Handover of 12 simulated patients over 5 cycles | System (data loss) | Minimal data loss with typed content, compared to 31% data retained with note‐taking, and no data retained with verbal only |
| Wallum38 (1995) | Pre‐post | Change from oral handover (baseline) to written template read with exchange | 20 nurses in a geriatric dementia ward | Staff | 83% of nurses felt care plans followed better; 88% knew care plans better |
| Technology or structured template | |||||
| Cheah et al.39 (2005) | Pre‐post | Electronic template with free‐text entry compared to baseline | 14 UK Surgery residents | Staff | 100% (14) of residents rated electronic system as desirable, but 7 (50%) reported that information was not updated |
| Lee et al.40 (1996) | Pre‐post | Standardized signout card for interns to transmit information during handoffs compared to handwritten (baseline) | Inpatient cardiology service at IM residency program in Minnesota with 19 new interns over a 3‐month period | Staff | Intervention interns (n = 10) reported poor sign‐out less often than controls (n = 9) [intervention 8 nights (5.8%) vs. control 17 nights (14.9%); P = 0.016] |
| Kannry and Moore18 (1999) | Pre‐post | Compared web‐based signout program to usual system (baseline) | An academic teaching hospital in New York (34 patients admitted in 1997; 40 patients admitted in 1998) | System | Improved provider identification (86% web signout vs. 57% hospital census) |
| Petersen et al.17 (1998) | Pre‐post | 4 months of computerized signouts compared to baseline period (control) | 3747 patients admitted to the medical service at an academic teaching hospital | Patient | Preventable adverse events (ADE) decreased (1.7% to 1.2%, P 0.10); risk of cross‐cover physician for ADE eliminated |
| Ram and Block41 (1993) | Pre‐post | Compared handwritten (baseline) to computer‐generated | Family medicine residents at 2 academic teaching hospitals [Buffalo (n = 16) and Pittsburgh (n = 16)] | Staff | Higher satisfaction after electronic signout, but complaints with burden of data entry and need to keep information updated |
| Van Eaton et al.42 (2004) | Pre‐post | Use of UW Cores links sign‐out to list for rounds and IS data | 28 surgical and medical residents at 2 teaching hospitals | System | At 6 months, 66% of patients entered in system (adoption) |
| Van Eaton et al.16 (2005) | Prospective, randomized, crossover study. | Compared UW Cores* integrated system compared to usual system | 14 inpatient resident teams (6 surgery, 8 IM) at 2 teaching hospitals for 5 months | Staff, system | 50% reduction in the perceived time spent copying data [from 24% to 12% (P 0.0001)] and number of patients missed on rounds (2.5 vs. 5 patients/team/month, P = 0.0001); improved signout quality (69.6% agree or strongly agree); and improved continuity of care (66.1% agree or strongly agree) |

Overall, the literature presented supports the use of a verbal handoff supplemented with written documentation in a structured format or technology solution. The 2 most rigorous studies were led by Van Eaton et al.16 and Petersen et al.17 and focused on evaluating technology solutions. Van Eaton et al.16 performed a randomized controlled trial of a locally created rounding template with 161 surgical residents. This template downloads certain information (lab values and recent vital signs) from the hospital system into a sign‐out sheet and allows residents to enter notes about diagnoses, allergies, medications and to‐do items. When implemented, the investigators found the number of patients missed on rounds decreased by 50%. Residents reported an increase of 40% in the amount of time available to pre‐round, due largely to not having to copy data such as vital signs. They reported a decrease in rounding time by 3 hours per week, and this was perceived as helping them meet the ACGME 80 hours work rules. Lastly, the residents reported a higher quality of sign‐outs from their peers and perceived an overall improvement in continuity of care. Petersen and colleagues implemented a computerized sign‐out (auto‐imported medications, name, room number) in an internal medicine residency to improve continuity of care during cross‐coverage and decrease adverse events.17 Prior to the intervention, the frequency of preventable adverse events was 1.7% and it was significantly associated with cross‐coverage. Preventable adverse events were identified using a confidential self‐report system that was also validated by clinician review. After the intervention, the frequency of preventable adverse events dropped to 1.2% (P 0.1), and cross‐coverage was no longer associated with preventable adverse events. In other studies, technological solutions also improved provider identification and staff communication.18, 19 Together, these technology‐based intervention studies suggest that a computerized sign‐out with auto‐imported fields has the ability to improve physician efficiency and also improve inpatient care (reduction in number of patients missed on rounds, decrease in preventable adverse events).
Studies from nursing demonstrated that supplementing a verbal exchange with written information improved transfer of information, compared to verbal exchange alone.20 One of these studies rated the transfer of information using videotaped simulated handoff cases.21 Last, 1 nursing study that more directly involved patients in the handoff process resulted in improved nursing knowledge and greater patient empowerment (Table 1).22
White papers or consensus statements originated from international and national consortia in patient safety including the Australian Council for Safety and Quality in Healthcare,23 the Junior Doctors Committee of the British Medical Association,24 University Health Consortium,25 the Department of Defense Patient Safety Program,26 and The Joint Commission.27 Several common themes were prevalent in all white papers. First, there exists a need to train new personnel on how to perform an effective handoff. Second, efforts should be undertaken to ensure adequate time for handoffs and reduce interruptions during handoffs. Third, several of the papers supported verbal exchange that facilitates interactive questioning, focuses on ill patients, and delineates actions to be taken. Lastly, content should be updated to ensure transfer of the latest clinical information.
Peer Review at SHM Meeting of Preliminary Handoff Recommendations
In the presentation of preliminary handoff recommendations to over 300 attendees at the SHM Annual Meeting in 2007, 2 recommendations were supported unanimously: (1) a formal recognized handoff plan should be instituted at end of shift or change in service; and (2) ill patients should be given priority during verbal exchange.
During the workshop, discussion focused on three recommendations of concern, or those that received greater than 20 negative votes by participants. The proposed recommendation that raised the most objections (48 negative votes) was that interruptions be limited. Audience members expressed that it was hard to expect that interruptions would be limited given the busy workplace in the absence of endorsing a separate room and time. This recommendation was ultimately deleted.
The 2 other debated recommendations, which were retained after discussion, were ensuring adequate time for handoffs and using an interactive process during verbal communication. Several attendees stated that ensuring adequate time for handoffs may be difficult without setting a specific time. Others questioned the need for interactive verbal communication, and endorsed leaving a handoff by voicemail with a phone number or pager to answer questions. However, this type of asynchronous communication (senders and receivers not present at the same time) was not desirable or consistent with the Joint Commission's National Patient Safety Goal.
Two new recommendations were proposed from anonymous input and incorporated in the final recommendations, including (a) all patients should be on the sign‐out, and (b) sign‐outs should be accessible from a centralized location. Another recommendation proposed at the Annual Meeting was to institute feedback for poor sign‐outs, but this was not added to the final recommendations after discussion at the meeting and with content experts about the difficulty of maintaining anonymity in small hospitalist groups. Nevertheless, this should not preclude informal feedback among practitioners.
Anonymous commentary also yielded several major themes regarding handoff improvements and areas of uncertainty that merit future work. Several hospitalists described the need to delineate specific content domains for handoffs including, for example, code status, allergies, discharge plan, and parental contact information in the case of pediatric care. However, due to the variability in hospitalist programs and health systems and the general lack of evidence in this area, the Task Force opted to avoid recommending specific content domains which may have limited applicability in certain settings and little support from the literature. Several questions were raised regarding the legal status of written sign‐outs, and whether sign‐outs, especially those that are web‐based, are compliant with the Healthcare Information Portability and Accountability Act (HIPAA). Hospitalists also questioned the appropriate number of patients to be handed off safely. Promoting efficient technology solutions that reduce documentation burden, such as linking the most current progress note to the sign‐out, was also proposed. Concerns were also raised about promoting safe handoffs when using moonlighting or rotating physicians, who may be less invested in the continuity of the patients' overall care.
Expert Panel Review
The final version of the Task Force recommendations incorporates feedback provided by the expert panel. In particular, the expert panel favored the use of the term, recommendations, rather than standards, minimum acceptable practices, or best practices. While the distinction may appear semantic, the Task Force and expert panel acknowledge that the current state of scientific knowledge regarding hospital handoffs is limited. Although an evidence‐based process informed the development of these recommendations, they are not a legal standard for practice. Additional research may allow for refinement of recommendations and development of more formal handoff standards.
The expert panel also highlighted the need to provide tools to hospitalist programs to facilitate the adoption of these recommendations. For example, recommendations for content exchange are difficult to adopt if groups do not already use a written template. The panel also commented on the need to consider the possible consequences if efforts are undertaken to include handoff documents (whether paper or electronic) as part of the medical record. While formalizing handoff documents may raise their quality, it is also possible that handoff documents become less helpful by either excluding the most candid impression regarding a patient's status or by encouraging hospitalists to provide too much detail. Privacy and confidentiality of paper‐based systems, in particular, were also questioned.
Additional Recommendations for Service Change
Patient handoffs during a change of service are a routine part of hospitalist care. Since service change is a type of shift change, the handoff recommendations for shift change do apply. Unlike shift change, service changes involve a more significant transfer of responsibility. Therefore, the Task Force recommends also that the incoming hospitalist be readily identified in the medical record or chart as the new provider, so that relevant clinical information can be communicated to the correct physician. This program‐level recommendation can be met by an electronic or paper‐based system that correctly identifies the current primary inpatient physician.
Final Handoff Recommendations
The final handoff recommendations are shown in Figure 2. The recommendations were designed to be consistent with the overall finding of the literature review, which supports the use of a verbal handoff supplemented with written documentation or a technological solution in a structured format. With the exception of 1 recommendation that is specific to service changes, all recommendations are designed to refer to shift changes and service changes. One overarching recommendation refers to the need for a formally recognized handoff plan at a shift change or change of service. The remaining 12 recommendations are divided into 4 that refer to hospitalist groups or programs, 3 that refer to verbal exchange, and 5 that refer to content exchange. The distinction is an important one because program‐level recommendations require organizational support and buy‐in to promote clinician participation and adherence. The 4 program recommendations also form the necessary framework for the remaining recommendations. For example, the second program recommendation describes the need for a standardized template or technology solution for accessing and recording patient information during the handoff. After a program adopts such a mechanism for exchanging patient information, the specific details for use and maintenance are outlined in greater detail in content exchange recommendations.

Because of the limited trials of handoff strategies, none of the recommendations are supported with level of evidence A (multiple numerous randomized controlled trials). In fact, with the exception of using a template or technology solution which was supported with level of evidence B, all handoff recommendations were supported with C level of evidence. The recommendations, however, were rated as Class I (effective) because there were no conflicting expert opinions or studies (Figure 2).
Discussion
In summary, our review of the literature supports the use of face‐to‐face verbal handoffs that are aided by the use of structured template to guide exchange of information. Furthermore, the development of these recommendations is the first effort of its kind for hospitalist handoffs and a movement towards standardizing the handoff process. While these recommendations are meant to provide structure to the hospitalist handoff process, the use and implementation by individual hospitalist programs may require more specific detail than these recommendations provide. Local modifications can allow for improved acceptance and adoption by practicing hospitalists. These recommendations can also help guide teaching efforts for academic hospitalists who are responsible for supervising residents.
The limitations of these recommendations related to lack of evidence in this field. Studies suffered from small size, poor description of methods, and a paucity of controlled interventions. The described technology solutions are not standardized or commercially available. Only 1 study included patient outcomes.28 There are no multicenter studies, studies of hospitalist handoffs, or studies to guide inclusion of specific content. Randomized controlled trials, interrupted time series analyses, and other rigorous study designs are needed in both teaching and non‐teaching settings to evaluate these recommendations and other approaches to improving handoffs. Ideally, these studies would occur through multicenter collaboratives and with human factors researchers familiar with mixed methods approaches to evaluate how and why interventions work.29 Efforts should focus on developing surrogate measures that are sensitive to handoff quality and related to important patient outcomes. The results of future studies should be used to refine the present recommendations. Locating new literature could be facilitated through the introduction of Medical Subject Heading for the term handoff by the National Library of Medicine. After completing this systematic review and developing the handoff recommendations described here, a few other noteworthy articles have been published on this topic, to which we refer interested readers. Several of these studies demonstrate that standardizing content and process during medical or surgical intern sign‐out improves resident confidence with handoffs,30 resident perceptions of accuracy and completeness of signout,31 and perceptions of patient safety.32 Another prospective audiotape study of 12 days of resident signout of clinical information demonstrated that poor quality oral sign‐outs was associated with an increased risk of post‐call resident reported signout‐related problems.5 Lastly, 1 nursing study demonstrated improved staff reports of safety, efficiency, and teamwork after a change from verbal reporting in an isolated room to bedside handover.33 Overall, these additional studies continue to support the current recommendations presented in this paper and do not significantly impact the conclusions of our literature review.
While lacking specific content domain recommendations, this report can be used as a starting point to guide development of self and peer assessment of hospitalist handoff quality. Development and validation of such assessments is especially important and can be incorporated into efforts to certify hospitalists through the recently approved certificate of focused practice in hospital medicine by the American Board of Internal Medicine (ABIM). Initiatives by several related organizations may help guide these effortsThe Joint Commission, the ABIM's Stepping Up to the Plate (SUTTP) Alliance, the Institute for Healthcare Improvement, the Information Transfer and Communication Practices (ITCP) Project for surgical care transitions, and the Hospital at Night (H@N) Program sponsored by the United Kingdom's National Health Service.3437 Professional medical organizations can also serve as powerful mediators of change in this area, not only by raising the visibility of handoffs, but also by mobilizing research funding. Patients and their caregivers may also play an important role in increasing awareness and education in this area. Future efforts should target handoffs not addressed in this initiative, such as transfers from emergency departments to inpatient care units, or between ICUs and the medical floor.
Conclusion
With the growth of hospital medicine and the increased acuity of inpatients, improving handoffs becomes an important part of ensuring patient safety. The goal of the SHM Handoffs Task Force was to begin to standardize handoffs at change of shift and change of servicea fundamental activity of hospitalists. These recommendations build on the limited literature in surgery, nursing, and medical informatics and provide a starting point for promoting safe and seamless in‐hospital handoffs for practitioners of Hospital Medicine.
Acknowledgements
The authors also acknowledge Tina Budnitz and the Healthcare Quality and Safety Committee of the Society of Hospital Medicine. Last, they are indebted to the staff support provided by Shannon Roach from the Society of Hospital Medicine.
Handoffs during hospitalization from one provider to another represent critical transition points in patient care.1 In‐hospital handoffs are a frequent occurrence, with 1 teaching hospital reporting 4000 handoffs daily for a total of 1.6 million per year.2
Incomplete or poor‐quality handoffs have been implicated as a source of adverse events and near misses in hospitalized patients.35 Standardizing the handoff process may improve patient safety during care transitions.6 In 2006, the Joint Commission issued a National Patient Safety Goal that requires care providers to adopt a standardized approach for handoff communications, including an opportunity to ask and respond to questions about a patient's care.7 The reductions in resident work hours by the Accreditation Council for Graduate Medical Education (ACGME) has also resulted in a greater number and greater scrutiny of handoffs in teaching hospitals.8, 9
In response to these issues, and because handoffs are a core competency for hospitalists, the Society of Hospital Medicine (SHM)convened a task force.10 Our goal was to develop a set of recommendations for handoffs that would be applicable in both community and academic settings; among physicians (hospitalists, internists, subspecialists, residents), nurse practitioners, and physicians assistants; and across roles including serving as the primary provider of hospital care, comanager, or consultant. This work focuses on handoffs that occur at shift change and service change.11 Shift changes are transitions of care between an outgoing provider and an incoming provider that occur at the end of the outgoing provider's continuous on‐duty period. Service changesa special type of shift changeare transitions of care between an outgoing provider and an incoming provider that occur when an outgoing provider is leaving a rotation or period of consecutive daily care for patients on the same service.
For this initiative, transfers of care in which the patient is moving from one patient area to another (eg, Emergency Department to inpatient floor, or floor to intensive care unit [ICU]) were excluded since they likely require unique consideration given their cross‐disciplinary and multispecialty nature. Likewise, transitions of care at hospital admission and discharge were also excluded because recommendations for discharge are already summarized in 2 complementary reports.12, 13
To develop recommendations for handoffs at routine shift change and service changes, the Handoff Task Force performed a systematic review of the literature to develop initial recommendations, obtained feedback from hospital‐based clinicians in addition to a panel of handoff experts, and finalized handoff recommendations, as well as a proposed research agenda, for the SHM.
Methods
The SHM Healthcare Quality and Patient Safety (HQPS) Committee convened the Handoff Task Force, which was comprised of 6 geographically diverse, predominantly academic hospitalists with backgrounds in education, patient safety, health communication, evidence‐based medicine, and handoffs. The Task Force then engaged a panel of 4 content experts selected for their work on handoffs in the fields of nursing, information technology, human factors engineering, and hospital medicine. Similar to clinical guideline development by professional societies, the Task Force used a combination of evidence‐based review and expert opinions to propose recommendations.
Literature Review
A PubMed search was performed for English language articles published from January 1975 to January 2007, using the following keywords: handover or handoff or hand‐off or shift change or signout or sign‐out. Articles were eligible if they presented results from a controlled intervention to improve handoffs at shift change or service change, by any health profession. Articles that appeared potentially relevant based on their title were retrieved for full‐text review and included if deemed eligible by at least 2 reviewers. Additional studies were obtained through the Agency for Healthcare Research and Quality (AHRQ) Patient Safety Network,14 using the category Safety target and subcategory Discontinuities, gaps, and hand‐off problems. Finally, the expert panel reviewed the results of the literature review and suggested additional articles.
Eligible studies were abstracted by individual members of the Handoff Task Force using a structured form (Appendix Figure 1), and abstractions were verified by a second member. Handoff‐related outcome measures were categorized as referring to (1) patient outcomes, (2) staff outcomes, or (3) system outcomes. Because studies included those from nursing and other industries, interventions were evaluated by abstractors for their applicability to routine hospitalist handoffs. The literature review was supplemented by review of expert consensus or policy white papers that described recommendations for handoffs. The list of white papers was generated utilizing a common internet search engine (Google;
Peer and Expert Panel Review
The Task Force generated draft recommendations, which were revised through interactive discussions until consensus was achieved. These recommendations were then presented at a workshop to an audience of approximately 300 hospitalists, case managers, nurses, and pharmacists at the 2007 SHM Annual Meeting.
During the workshop, participants were asked to cast up to 3 votes for recommendations that should be removed. Those recommendations that received more than 20 votes for removal were then discussed. Participants also had the opportunity to anonymously suggest new recommendations or revisions using index cards, which were reviewed by 2 workshop faculty, assembled into themes, and immediately presented to the group. Through group discussion of prevalent themes, additional recommendations were developed.
Four content experts were then asked to review a draft paper that summarized the literature review, discussion at the SHM meeting, and handoff recommendations. Their input regarding the process, potential gaps in the literature, and additional items of relevance, was incorporated into this final manuscript.
Final Review by SHM Board and Rating each Recommendation
A working paper was reviewed and approved by the Board of the SHM in early January 2008. With Board input, the Task Force adopted the American College of Cardiology/American Heart Association (ACC/AHA) framework to rate each recommendation because of its appropriateness, ease of use, and familiarity to hospital‐based physicians.15 Recommendations are rated as Class I (effective), IIa (conflicting findings but weight of evidence supports use), IIb (conflicting findings but weight of evidence does not support use), or III (not effective). The Level of Evidence behind each recommendation is graded as A (from multiple large randomized controlled trials), B (from smaller or limited randomized trials, or nonrandomized studies), or C (based primarily on expert consensus). A recommendation with Level of Evidence B or C should not imply that the recommendation is not supported.15
Results
Literature Review
Of the 374 articles identified by the electronic search of PubMed and the AHRQ Patient Safety Network, 109 were retrieved for detailed review, and 10 of these met the criteria for inclusion (Figure 1). Of these studies, 3 were derived from nursing literature and the remaining were tests of technology solutions or structured templates (Table 1).1618, 20, 22, 3842 No studies examined hospitalist handoffs. All eligible studies concerned shift change. There were no studies of service change. Only 1 study was a randomized controlled trial; the rest were pre‐post studies with historical controls or a controlled simulation. All reports were single‐site studies. Most outcomes were staff‐related or system‐related, with only 2 studies using patient outcomes.
| Author (Year) | Study Design | Intervention | Setting and Study Population | Target | Outcomes |
|---|---|---|---|---|---|
| |||||
| Nursing | |||||
| Kelly22 (2005) | Pre‐post | Change to walk‐round handover (at bedside) from baseline (control) | 12‐bed rehab unit with 18 nurses and 10 patients | Staff, patient | 11/18 nurses felt more or much more informed and involved; 8/10 patients felt more involved |
| Pothier et al.20 (2005) | Controlled simulation | Compared pure verbal to verbal with note‐taking to verbal plus typed content | Handover of 12 simulated patients over 5 cycles | System (data loss) | Minimal data loss with typed content, compared to 31% data retained with note‐taking, and no data retained with verbal only |
| Wallum38 (1995) | Pre‐post | Change from oral handover (baseline) to written template read with exchange | 20 nurses in a geriatric dementia ward | Staff | 83% of nurses felt care plans followed better; 88% knew care plans better |
| Technology or structured template | |||||
| Cheah et al.39 (2005) | Pre‐post | Electronic template with free‐text entry compared to baseline | 14 UK Surgery residents | Staff | 100% (14) of residents rated electronic system as desirable, but 7 (50%) reported that information was not updated |
| Lee et al.40 (1996) | Pre‐post | Standardized signout card for interns to transmit information during handoffs compared to handwritten (baseline) | Inpatient cardiology service at IM residency program in Minnesota with 19 new interns over a 3‐month period | Staff | Intervention interns (n = 10) reported poor sign‐out less often than controls (n = 9) [intervention 8 nights (5.8%) vs. control 17 nights (14.9%); P = 0.016] |
| Kannry and Moore18 (1999) | Pre‐post | Compared web‐based signout program to usual system (baseline) | An academic teaching hospital in New York (34 patients admitted in 1997; 40 patients admitted in 1998) | System | Improved provider identification (86% web signout vs. 57% hospital census) |
| Petersen et al.17 (1998) | Pre‐post | 4 months of computerized signouts compared to baseline period (control) | 3747 patients admitted to the medical service at an academic teaching hospital | Patient | Preventable adverse events (ADE) decreased (1.7% to 1.2%, P 0.10); risk of cross‐cover physician for ADE eliminated |
| Ram and Block41 (1993) | Pre‐post | Compared handwritten (baseline) to computer‐generated | Family medicine residents at 2 academic teaching hospitals [Buffalo (n = 16) and Pittsburgh (n = 16)] | Staff | Higher satisfaction after electronic signout, but complaints with burden of data entry and need to keep information updated |
| Van Eaton et al.42 (2004) | Pre‐post | Use of UW Cores links sign‐out to list for rounds and IS data | 28 surgical and medical residents at 2 teaching hospitals | System | At 6 months, 66% of patients entered in system (adoption) |
| Van Eaton et al.16 (2005) | Prospective, randomized, crossover study. | Compared UW Cores* integrated system compared to usual system | 14 inpatient resident teams (6 surgery, 8 IM) at 2 teaching hospitals for 5 months | Staff, system | 50% reduction in the perceived time spent copying data [from 24% to 12% (P 0.0001)] and number of patients missed on rounds (2.5 vs. 5 patients/team/month, P = 0.0001); improved signout quality (69.6% agree or strongly agree); and improved continuity of care (66.1% agree or strongly agree) |

Overall, the literature presented supports the use of a verbal handoff supplemented with written documentation in a structured format or technology solution. The 2 most rigorous studies were led by Van Eaton et al.16 and Petersen et al.17 and focused on evaluating technology solutions. Van Eaton et al.16 performed a randomized controlled trial of a locally created rounding template with 161 surgical residents. This template downloads certain information (lab values and recent vital signs) from the hospital system into a sign‐out sheet and allows residents to enter notes about diagnoses, allergies, medications and to‐do items. When implemented, the investigators found the number of patients missed on rounds decreased by 50%. Residents reported an increase of 40% in the amount of time available to pre‐round, due largely to not having to copy data such as vital signs. They reported a decrease in rounding time by 3 hours per week, and this was perceived as helping them meet the ACGME 80 hours work rules. Lastly, the residents reported a higher quality of sign‐outs from their peers and perceived an overall improvement in continuity of care. Petersen and colleagues implemented a computerized sign‐out (auto‐imported medications, name, room number) in an internal medicine residency to improve continuity of care during cross‐coverage and decrease adverse events.17 Prior to the intervention, the frequency of preventable adverse events was 1.7% and it was significantly associated with cross‐coverage. Preventable adverse events were identified using a confidential self‐report system that was also validated by clinician review. After the intervention, the frequency of preventable adverse events dropped to 1.2% (P 0.1), and cross‐coverage was no longer associated with preventable adverse events. In other studies, technological solutions also improved provider identification and staff communication.18, 19 Together, these technology‐based intervention studies suggest that a computerized sign‐out with auto‐imported fields has the ability to improve physician efficiency and also improve inpatient care (reduction in number of patients missed on rounds, decrease in preventable adverse events).
Studies from nursing demonstrated that supplementing a verbal exchange with written information improved transfer of information, compared to verbal exchange alone.20 One of these studies rated the transfer of information using videotaped simulated handoff cases.21 Last, 1 nursing study that more directly involved patients in the handoff process resulted in improved nursing knowledge and greater patient empowerment (Table 1).22
White papers or consensus statements originated from international and national consortia in patient safety including the Australian Council for Safety and Quality in Healthcare,23 the Junior Doctors Committee of the British Medical Association,24 University Health Consortium,25 the Department of Defense Patient Safety Program,26 and The Joint Commission.27 Several common themes were prevalent in all white papers. First, there exists a need to train new personnel on how to perform an effective handoff. Second, efforts should be undertaken to ensure adequate time for handoffs and reduce interruptions during handoffs. Third, several of the papers supported verbal exchange that facilitates interactive questioning, focuses on ill patients, and delineates actions to be taken. Lastly, content should be updated to ensure transfer of the latest clinical information.
Peer Review at SHM Meeting of Preliminary Handoff Recommendations
In the presentation of preliminary handoff recommendations to over 300 attendees at the SHM Annual Meeting in 2007, 2 recommendations were supported unanimously: (1) a formal recognized handoff plan should be instituted at end of shift or change in service; and (2) ill patients should be given priority during verbal exchange.
During the workshop, discussion focused on three recommendations of concern, or those that received greater than 20 negative votes by participants. The proposed recommendation that raised the most objections (48 negative votes) was that interruptions be limited. Audience members expressed that it was hard to expect that interruptions would be limited given the busy workplace in the absence of endorsing a separate room and time. This recommendation was ultimately deleted.
The 2 other debated recommendations, which were retained after discussion, were ensuring adequate time for handoffs and using an interactive process during verbal communication. Several attendees stated that ensuring adequate time for handoffs may be difficult without setting a specific time. Others questioned the need for interactive verbal communication, and endorsed leaving a handoff by voicemail with a phone number or pager to answer questions. However, this type of asynchronous communication (senders and receivers not present at the same time) was not desirable or consistent with the Joint Commission's National Patient Safety Goal.
Two new recommendations were proposed from anonymous input and incorporated in the final recommendations, including (a) all patients should be on the sign‐out, and (b) sign‐outs should be accessible from a centralized location. Another recommendation proposed at the Annual Meeting was to institute feedback for poor sign‐outs, but this was not added to the final recommendations after discussion at the meeting and with content experts about the difficulty of maintaining anonymity in small hospitalist groups. Nevertheless, this should not preclude informal feedback among practitioners.
Anonymous commentary also yielded several major themes regarding handoff improvements and areas of uncertainty that merit future work. Several hospitalists described the need to delineate specific content domains for handoffs including, for example, code status, allergies, discharge plan, and parental contact information in the case of pediatric care. However, due to the variability in hospitalist programs and health systems and the general lack of evidence in this area, the Task Force opted to avoid recommending specific content domains which may have limited applicability in certain settings and little support from the literature. Several questions were raised regarding the legal status of written sign‐outs, and whether sign‐outs, especially those that are web‐based, are compliant with the Healthcare Information Portability and Accountability Act (HIPAA). Hospitalists also questioned the appropriate number of patients to be handed off safely. Promoting efficient technology solutions that reduce documentation burden, such as linking the most current progress note to the sign‐out, was also proposed. Concerns were also raised about promoting safe handoffs when using moonlighting or rotating physicians, who may be less invested in the continuity of the patients' overall care.
Expert Panel Review
The final version of the Task Force recommendations incorporates feedback provided by the expert panel. In particular, the expert panel favored the use of the term, recommendations, rather than standards, minimum acceptable practices, or best practices. While the distinction may appear semantic, the Task Force and expert panel acknowledge that the current state of scientific knowledge regarding hospital handoffs is limited. Although an evidence‐based process informed the development of these recommendations, they are not a legal standard for practice. Additional research may allow for refinement of recommendations and development of more formal handoff standards.
The expert panel also highlighted the need to provide tools to hospitalist programs to facilitate the adoption of these recommendations. For example, recommendations for content exchange are difficult to adopt if groups do not already use a written template. The panel also commented on the need to consider the possible consequences if efforts are undertaken to include handoff documents (whether paper or electronic) as part of the medical record. While formalizing handoff documents may raise their quality, it is also possible that handoff documents become less helpful by either excluding the most candid impression regarding a patient's status or by encouraging hospitalists to provide too much detail. Privacy and confidentiality of paper‐based systems, in particular, were also questioned.
Additional Recommendations for Service Change
Patient handoffs during a change of service are a routine part of hospitalist care. Since service change is a type of shift change, the handoff recommendations for shift change do apply. Unlike shift change, service changes involve a more significant transfer of responsibility. Therefore, the Task Force recommends also that the incoming hospitalist be readily identified in the medical record or chart as the new provider, so that relevant clinical information can be communicated to the correct physician. This program‐level recommendation can be met by an electronic or paper‐based system that correctly identifies the current primary inpatient physician.
Final Handoff Recommendations
The final handoff recommendations are shown in Figure 2. The recommendations were designed to be consistent with the overall finding of the literature review, which supports the use of a verbal handoff supplemented with written documentation or a technological solution in a structured format. With the exception of 1 recommendation that is specific to service changes, all recommendations are designed to refer to shift changes and service changes. One overarching recommendation refers to the need for a formally recognized handoff plan at a shift change or change of service. The remaining 12 recommendations are divided into 4 that refer to hospitalist groups or programs, 3 that refer to verbal exchange, and 5 that refer to content exchange. The distinction is an important one because program‐level recommendations require organizational support and buy‐in to promote clinician participation and adherence. The 4 program recommendations also form the necessary framework for the remaining recommendations. For example, the second program recommendation describes the need for a standardized template or technology solution for accessing and recording patient information during the handoff. After a program adopts such a mechanism for exchanging patient information, the specific details for use and maintenance are outlined in greater detail in content exchange recommendations.

Because of the limited trials of handoff strategies, none of the recommendations are supported with level of evidence A (multiple numerous randomized controlled trials). In fact, with the exception of using a template or technology solution which was supported with level of evidence B, all handoff recommendations were supported with C level of evidence. The recommendations, however, were rated as Class I (effective) because there were no conflicting expert opinions or studies (Figure 2).
Discussion
In summary, our review of the literature supports the use of face‐to‐face verbal handoffs that are aided by the use of structured template to guide exchange of information. Furthermore, the development of these recommendations is the first effort of its kind for hospitalist handoffs and a movement towards standardizing the handoff process. While these recommendations are meant to provide structure to the hospitalist handoff process, the use and implementation by individual hospitalist programs may require more specific detail than these recommendations provide. Local modifications can allow for improved acceptance and adoption by practicing hospitalists. These recommendations can also help guide teaching efforts for academic hospitalists who are responsible for supervising residents.
The limitations of these recommendations related to lack of evidence in this field. Studies suffered from small size, poor description of methods, and a paucity of controlled interventions. The described technology solutions are not standardized or commercially available. Only 1 study included patient outcomes.28 There are no multicenter studies, studies of hospitalist handoffs, or studies to guide inclusion of specific content. Randomized controlled trials, interrupted time series analyses, and other rigorous study designs are needed in both teaching and non‐teaching settings to evaluate these recommendations and other approaches to improving handoffs. Ideally, these studies would occur through multicenter collaboratives and with human factors researchers familiar with mixed methods approaches to evaluate how and why interventions work.29 Efforts should focus on developing surrogate measures that are sensitive to handoff quality and related to important patient outcomes. The results of future studies should be used to refine the present recommendations. Locating new literature could be facilitated through the introduction of Medical Subject Heading for the term handoff by the National Library of Medicine. After completing this systematic review and developing the handoff recommendations described here, a few other noteworthy articles have been published on this topic, to which we refer interested readers. Several of these studies demonstrate that standardizing content and process during medical or surgical intern sign‐out improves resident confidence with handoffs,30 resident perceptions of accuracy and completeness of signout,31 and perceptions of patient safety.32 Another prospective audiotape study of 12 days of resident signout of clinical information demonstrated that poor quality oral sign‐outs was associated with an increased risk of post‐call resident reported signout‐related problems.5 Lastly, 1 nursing study demonstrated improved staff reports of safety, efficiency, and teamwork after a change from verbal reporting in an isolated room to bedside handover.33 Overall, these additional studies continue to support the current recommendations presented in this paper and do not significantly impact the conclusions of our literature review.
While lacking specific content domain recommendations, this report can be used as a starting point to guide development of self and peer assessment of hospitalist handoff quality. Development and validation of such assessments is especially important and can be incorporated into efforts to certify hospitalists through the recently approved certificate of focused practice in hospital medicine by the American Board of Internal Medicine (ABIM). Initiatives by several related organizations may help guide these effortsThe Joint Commission, the ABIM's Stepping Up to the Plate (SUTTP) Alliance, the Institute for Healthcare Improvement, the Information Transfer and Communication Practices (ITCP) Project for surgical care transitions, and the Hospital at Night (H@N) Program sponsored by the United Kingdom's National Health Service.3437 Professional medical organizations can also serve as powerful mediators of change in this area, not only by raising the visibility of handoffs, but also by mobilizing research funding. Patients and their caregivers may also play an important role in increasing awareness and education in this area. Future efforts should target handoffs not addressed in this initiative, such as transfers from emergency departments to inpatient care units, or between ICUs and the medical floor.
Conclusion
With the growth of hospital medicine and the increased acuity of inpatients, improving handoffs becomes an important part of ensuring patient safety. The goal of the SHM Handoffs Task Force was to begin to standardize handoffs at change of shift and change of servicea fundamental activity of hospitalists. These recommendations build on the limited literature in surgery, nursing, and medical informatics and provide a starting point for promoting safe and seamless in‐hospital handoffs for practitioners of Hospital Medicine.
Acknowledgements
The authors also acknowledge Tina Budnitz and the Healthcare Quality and Safety Committee of the Society of Hospital Medicine. Last, they are indebted to the staff support provided by Shannon Roach from the Society of Hospital Medicine.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80(12):1094–1099.
- ... AHRQ WebM167(19):2030–2036.
- ,,,,.Communication failures in patient signout and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,,,.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- ,,, et al.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16:125–132.
- Joint Commission. 2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June2009.
- ,,,.Transfers of patient care between house staff on internal medicine wards: a national survey.Arch Intern Med.2006;166(11):1173–1177.
- ,.Re‐framing continuity of care for this century.Qual Saf Health Care.2005;14(6):394–396.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(suppl 1):48–56.
- ,,, et al.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary‐care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Transition of care for hospitalized elderly patients: development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- Discontinuities, Gaps, and Hand‐Off Problems. AHRQ PSNet Patient Safety Network. Available at: http://www.psnet.ahrq.gov/content.aspx?taxonomyID=412. Accessed June2009.
- Manual for ACC/AHA Guideline Writing Committees. Methodologies and Policies from the ACC/AHA Task Force on Practice Guidelines. Available at: http://circ.ahajournals.org/manual/manual_IIstep6.shtml. Accessed June2009.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200(4):538–545.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.MediSign: using a web‐based SignOut System to improve provider identification.Proc AMIA Symp.1999:550–554.
- ,.Using a computerized sign‐out system to improve physician‐nurse communication.Jt Comm J Qual Patient Saf.2006;32(1):32–36.
- ,,,.Pilot study to show the loss of important data in nursing handover.Br J Nurs.2005;14(20):1090–1093.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- .Change from an office‐based to a walk‐around handover system.Nurs Times.2005;101(10):34–35.
- Clinical Handover and Patient Safety. Literature review report. Australian Council for Safety and Quality in Health Care. Available at: http://www.health.gov.au/internet/safety/publishing.nsf/Content/AA1369AD4AC5FC2ACA2571BF0081CD95/$File/clinhovrlitrev.pdf. Accessed June2009.
- Safe Handover: Safe Patients. Guidance on clinical handover for clinicians and managers. Junior Doctors Committee, British Medical Association. Available at: http://www.bma.org.uk/ap.nsf/AttachmentsByTitle/PDFsafehandover/$FILE/safehandover.pdf. Accessed June2009.
- University HealthSystem Consortium (UHC).UHC Best Practice Recommendation: Patient Hand Off Communication White Paper, May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- Healthcare Communications Toolkit to Improve Transitions in Care. Department of Defense Patient Safety Program. Available at: http://dodpatientsafety.usuhs.mil/files/Handoff_Toolkit.pdf. Accessed June2009.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission announces 2006 national patient safety goals for ambulatory care and office‐based surgery organizations. Available at: http://www.jcaho.org/news+room/news+release+archives/06_npsg_amb_obs.htm. Accessed June2009.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- .Communication strategies from high‐reliability organizations: translation is hard work.Ann Surg.2007;245(2):170–172.
- ,,, et al.A structured handoff program for interns.Acad Med.2009;84(3):347–352.
- ,,, et al.Simple standardized patient handoff system that increases accuracy and completeness.J Surg Educ.2008;65(6):476–485.
- ,,.Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward.Teach Learn Med.2009;21(2):121–126.
- ,,,,,.Bedside handover: quality improvement strategy to “transform care at the bedside”.J Nurs Care Qual.2009;24(2):136–142.
- Pillow M, ed.Improving Handoff Communications.Chicago:Joint Commission Resources;2007.
- American Board of Internal Medicine Foundation. Step Up To The Plate. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed June2009.
- ,,, et al.Surgeon information transfer and communication: factors affecting quality and efficiency of inpatient care.Ann Surg.2007;245(2):159–169.
- Hospital at Night. Available at: http://www.healthcareworkforce.nhs.uk/hospitalatnight.html. Accessed June2009.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- ,,,.Electronic medical handover: towards safer medical care.Med J Aust.2005;183(7):369–372.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11(12):753–755.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80(12):1094–1099.
- ... AHRQ WebM167(19):2030–2036.
- ,,,,.Communication failures in patient signout and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,,,.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- ,,, et al.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16:125–132.
- Joint Commission. 2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June2009.
- ,,,.Transfers of patient care between house staff on internal medicine wards: a national survey.Arch Intern Med.2006;166(11):1173–1177.
- ,.Re‐framing continuity of care for this century.Qual Saf Health Care.2005;14(6):394–396.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(suppl 1):48–56.
- ,,, et al.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary‐care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Transition of care for hospitalized elderly patients: development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- Discontinuities, Gaps, and Hand‐Off Problems. AHRQ PSNet Patient Safety Network. Available at: http://www.psnet.ahrq.gov/content.aspx?taxonomyID=412. Accessed June2009.
- Manual for ACC/AHA Guideline Writing Committees. Methodologies and Policies from the ACC/AHA Task Force on Practice Guidelines. Available at: http://circ.ahajournals.org/manual/manual_IIstep6.shtml. Accessed June2009.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200(4):538–545.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.MediSign: using a web‐based SignOut System to improve provider identification.Proc AMIA Symp.1999:550–554.
- ,.Using a computerized sign‐out system to improve physician‐nurse communication.Jt Comm J Qual Patient Saf.2006;32(1):32–36.
- ,,,.Pilot study to show the loss of important data in nursing handover.Br J Nurs.2005;14(20):1090–1093.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- .Change from an office‐based to a walk‐around handover system.Nurs Times.2005;101(10):34–35.
- Clinical Handover and Patient Safety. Literature review report. Australian Council for Safety and Quality in Health Care. Available at: http://www.health.gov.au/internet/safety/publishing.nsf/Content/AA1369AD4AC5FC2ACA2571BF0081CD95/$File/clinhovrlitrev.pdf. Accessed June2009.
- Safe Handover: Safe Patients. Guidance on clinical handover for clinicians and managers. Junior Doctors Committee, British Medical Association. Available at: http://www.bma.org.uk/ap.nsf/AttachmentsByTitle/PDFsafehandover/$FILE/safehandover.pdf. Accessed June2009.
- University HealthSystem Consortium (UHC).UHC Best Practice Recommendation: Patient Hand Off Communication White Paper, May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- Healthcare Communications Toolkit to Improve Transitions in Care. Department of Defense Patient Safety Program. Available at: http://dodpatientsafety.usuhs.mil/files/Handoff_Toolkit.pdf. Accessed June2009.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission announces 2006 national patient safety goals for ambulatory care and office‐based surgery organizations. Available at: http://www.jcaho.org/news+room/news+release+archives/06_npsg_amb_obs.htm. Accessed June2009.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- .Communication strategies from high‐reliability organizations: translation is hard work.Ann Surg.2007;245(2):170–172.
- ,,, et al.A structured handoff program for interns.Acad Med.2009;84(3):347–352.
- ,,, et al.Simple standardized patient handoff system that increases accuracy and completeness.J Surg Educ.2008;65(6):476–485.
- ,,.Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward.Teach Learn Med.2009;21(2):121–126.
- ,,,,,.Bedside handover: quality improvement strategy to “transform care at the bedside”.J Nurs Care Qual.2009;24(2):136–142.
- Pillow M, ed.Improving Handoff Communications.Chicago:Joint Commission Resources;2007.
- American Board of Internal Medicine Foundation. Step Up To The Plate. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed June2009.
- ,,, et al.Surgeon information transfer and communication: factors affecting quality and efficiency of inpatient care.Ann Surg.2007;245(2):159–169.
- Hospital at Night. Available at: http://www.healthcareworkforce.nhs.uk/hospitalatnight.html. Accessed June2009.
- .Using care plans to replace the handover.Nurs Stand.1995;9(32):24–26.
- ,,,.Electronic medical handover: towards safer medical care.Med J Aust.2005;183(7):369–372.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11(12):753–755.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
SHM Medication Reconciliation Survey Results
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
Transitions in Inpatient Hyperglycemia
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448
Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55
With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Contributors to Patient Care Mistakes
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).
The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Copyright © 2008 Society of Hospital Medicine