User login
Transitions in Inpatient Hyperglycemia
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
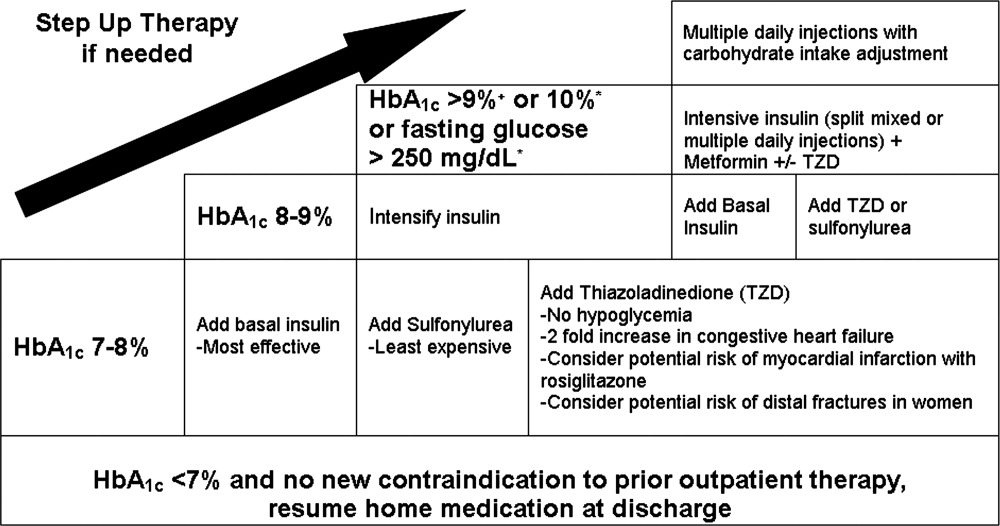
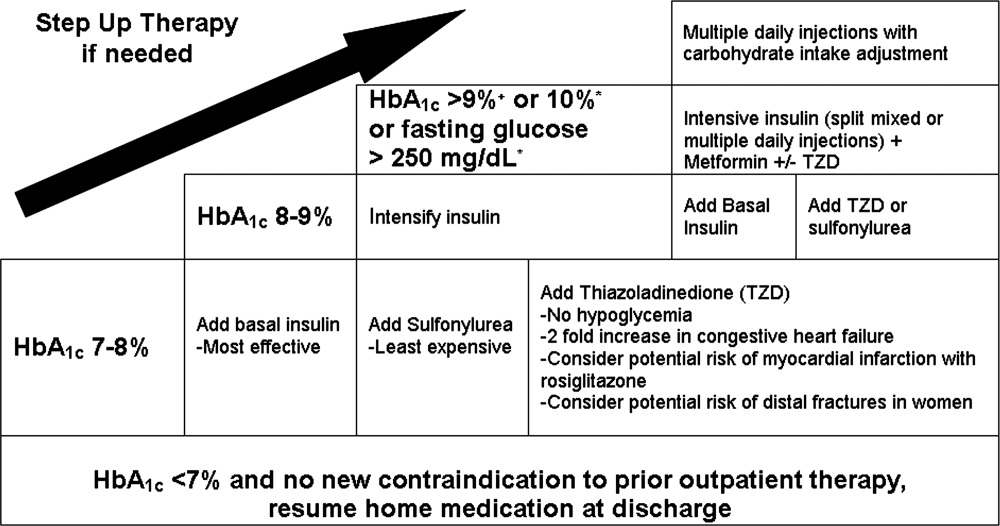
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448
Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
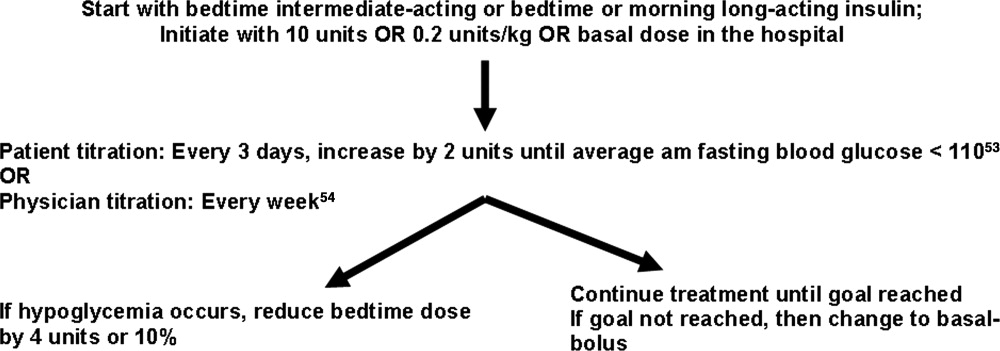
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55
With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.