User login
Supporting Faculty Development in Hospital Medicine: Design and Implementation of a Personalized Structured Mentoring Program
The lack of mentorship in hospital medicine has been previously documented,1-3 but there is scant literature about solutions to the problem.4 In other disciplines, data suggest that the guidance of a mentor has a positive influence on academic productivity and professional satisfaction. Mentored faculty at all levels in their careers are more successful at producing peer-reviewed publications, procuring grant support, and maintaining confidence in their career trajectory.
METHODS
The mentorship program was implemented from October 2015 to June 2016 in the Hospital Medicine Unit (HMU) of the Massachusetts General Hospital (MGH), a teaching affiliate of Harvard Medical School.
Program Goals, Design, and Development
Participants
Mentees had to be hired at >0.5 full-time equivalent and have 3 years or fewer of hospitalist experience.
Mentor–Mentee Matching
Mentors were paired with 1 or 2 mentees. Participant information such as history of mentorship and areas of interest for mentorship was collected. Two authors matched mentors and mentees to maximize similarities in these areas. Four mentors were paired with 2 mentees each, and 12 mentors were paired with 1 mentee each.
Mentorship Training Sessions
The program provided 3 mentorship-training lunch sessions for both mentees and mentors during the 9-month program. To enrich attendance, mentees were provided coverage for their clinical duties. The initial training session provided an opportunity to meet, articulate expectations and challenges, and develop action plans with individualized goals for the mentoring relationship. The second training session occurred at the midpoint. Pairs considered their mentorship status, evaluated their progress, and discussed strategies for optimizing their experience. At the final training session, participants reflected on their mentoring relationships, identified their extended network of mentoring support, and set expectations regarding whether the mentoring relationship would continue.
Mentorship Meetings
In addition to the training sessions, mentee–mentor pairs were expected to meet a minimum of 2 times during the formal mentorship program. CFD experts performed participant outreach via e-mail to assess progress. Mentees were given dining facility gift cards to support meetings with their mentors.
Program Evaluation
Statistical Analysis
RESULTS
Program Participation and Response Rate
Of the 25 eligible mentees, 16 (64%) participated in the mentorship program. Of the 20 eligible mentors, 12 (60%) participated. One participating mentee and 1 mentor left the institution during the intervention period. Fourteen mentees (response rate: 88%) and 9 mentors (response rate: 75%) completed the preintervention survey. Ten mentees (response rate: 63%) and 8 mentors (response rate: 67%) completed the postintervention survey.
Mentor Characteristics
Mentorship Meetings and the Mentorship Network
All participants attended at least 2 of the 3 trainings. For the mentees who completed the postintervention survey, 9 (90%) met with their mentors 3 or more additional times, and 8 (80%) were connected by their mentor to at least 1 additional faculty mentor.
Perceptions and Overall Satisfaction with Mentorship
Prior to starting the mentoring relationship, 86% of mentees and 78% of mentors anticipated that differing career goals would be a challenge to a successful mentor–mentee relationship. At the end of the program, only 30% of mentees and 38% of mentors felt that such differences were a challenge. Ninety percent of mentees and 88% of mentors were satisfied or very satisfied with their mentorship match. Forty-three percent of mentees felt supported by the HMU prior to the mentorship program, while 90% felt supported after the program. All the mentees agreed that future HMU faculty should participate in a similar program.
Impact of Mentorship on Critical Domains
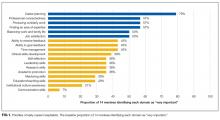
At baseline, the following domains were most commonly rated as very important by mentees: career planning, professional connectedness, producing scholarly work, finding an area of expertise, balancing work and family life, and job satisfaction (Figure 1). There was a significant improvement in composite satisfaction scores after completion of the mentorship program (54.5 ± 6.2 vs 65 ± 14.9, P = 0.02). The influence of the mentorship program on all domains is shown in Figure 2.
DISCUSSION
Our pilot structured mentorship program for junior hospitalists was feasible and led to improved satisfaction in select key career domains. Other mentoring or faculty coaching programs have been studied in several fields of medicine10-12; however, to our knowledge, there have not been published data studying a structured mentorship program for junior faculty in hospital medicine. Our intervention prioritized not only optimizing mentorship matches but also formalizing training sessions led by content experts.
After experiencing a structured mentoring relationship, most mentees felt a greater sense of support, were satisfied with their mentoring experiences, were connected to additional faculty, and had significant improvement in satisfaction in key career domains. Satisfaction with other self-identified “very important” domains, including scholarly activity, finding an area of expertise, job satisfaction, and work and family-life balance, did not significantly improve by the end of the program.
Perceived challenges to mentoring did not persist to the same degree with the implementation of a structured program. This highlights the importance of building mentorship skill sets (such as mentoring across differences and goal setting) through expert-led training sessions and perhaps also the importance of matching based on career goals.
CONCLUSION
Effective and sustainable career development requires mentorship.
Acknowledgments
The authors would like to thank each of the participants in the HMU Mentorship Program and the MGH CFD and Division of General Internal Medicine for supporting this effort.
Disclosure
Funding was provided by the MGH DGIM and CFD. Dr. Regina O’Neill reports the following relevant financial relationship: Massachusetts General Hospital Center for Faculty Development (consultant). All other authors report no other financial or other conflicts of interest to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5-9. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23-27. PubMed
3. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1-2. PubMed
4. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314-318. PubMed
5. Berk RA, Berg J, Mortimer R, Walton-Moss B, Yeo TP. Measuring the effectiveness of faculty mentoring relationships. Acad Med. 2005;80:66-71. PubMed
6. Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78:328-334. PubMed
7. Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112:336-341. PubMed
8. Steven A, Oxley J, Fleming WG. Mentoring for NHS doctors: perceived benefits across the personal-professional interface. J R Soc Med. 2008;101:552-557. PubMed
9. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359:1921-1931. PubMed
10. Pololi LH, Knight SM, Dennis K, Frankel RM. Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program. Acad Med. 2002;77:377-384. PubMed
11. Sambunjak D, Straus SE, Marusic A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115. PubMed
12. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161-166. PubMed
The lack of mentorship in hospital medicine has been previously documented,1-3 but there is scant literature about solutions to the problem.4 In other disciplines, data suggest that the guidance of a mentor has a positive influence on academic productivity and professional satisfaction. Mentored faculty at all levels in their careers are more successful at producing peer-reviewed publications, procuring grant support, and maintaining confidence in their career trajectory.
METHODS
The mentorship program was implemented from October 2015 to June 2016 in the Hospital Medicine Unit (HMU) of the Massachusetts General Hospital (MGH), a teaching affiliate of Harvard Medical School.
Program Goals, Design, and Development
Participants
Mentees had to be hired at >0.5 full-time equivalent and have 3 years or fewer of hospitalist experience.
Mentor–Mentee Matching
Mentors were paired with 1 or 2 mentees. Participant information such as history of mentorship and areas of interest for mentorship was collected. Two authors matched mentors and mentees to maximize similarities in these areas. Four mentors were paired with 2 mentees each, and 12 mentors were paired with 1 mentee each.
Mentorship Training Sessions
The program provided 3 mentorship-training lunch sessions for both mentees and mentors during the 9-month program. To enrich attendance, mentees were provided coverage for their clinical duties. The initial training session provided an opportunity to meet, articulate expectations and challenges, and develop action plans with individualized goals for the mentoring relationship. The second training session occurred at the midpoint. Pairs considered their mentorship status, evaluated their progress, and discussed strategies for optimizing their experience. At the final training session, participants reflected on their mentoring relationships, identified their extended network of mentoring support, and set expectations regarding whether the mentoring relationship would continue.
Mentorship Meetings
In addition to the training sessions, mentee–mentor pairs were expected to meet a minimum of 2 times during the formal mentorship program. CFD experts performed participant outreach via e-mail to assess progress. Mentees were given dining facility gift cards to support meetings with their mentors.
Program Evaluation
Statistical Analysis
RESULTS
Program Participation and Response Rate
Of the 25 eligible mentees, 16 (64%) participated in the mentorship program. Of the 20 eligible mentors, 12 (60%) participated. One participating mentee and 1 mentor left the institution during the intervention period. Fourteen mentees (response rate: 88%) and 9 mentors (response rate: 75%) completed the preintervention survey. Ten mentees (response rate: 63%) and 8 mentors (response rate: 67%) completed the postintervention survey.
Mentor Characteristics
Mentorship Meetings and the Mentorship Network
All participants attended at least 2 of the 3 trainings. For the mentees who completed the postintervention survey, 9 (90%) met with their mentors 3 or more additional times, and 8 (80%) were connected by their mentor to at least 1 additional faculty mentor.
Perceptions and Overall Satisfaction with Mentorship
Prior to starting the mentoring relationship, 86% of mentees and 78% of mentors anticipated that differing career goals would be a challenge to a successful mentor–mentee relationship. At the end of the program, only 30% of mentees and 38% of mentors felt that such differences were a challenge. Ninety percent of mentees and 88% of mentors were satisfied or very satisfied with their mentorship match. Forty-three percent of mentees felt supported by the HMU prior to the mentorship program, while 90% felt supported after the program. All the mentees agreed that future HMU faculty should participate in a similar program.
Impact of Mentorship on Critical Domains
At baseline, the following domains were most commonly rated as very important by mentees: career planning, professional connectedness, producing scholarly work, finding an area of expertise, balancing work and family life, and job satisfaction (Figure 1). There was a significant improvement in composite satisfaction scores after completion of the mentorship program (54.5 ± 6.2 vs 65 ± 14.9, P = 0.02). The influence of the mentorship program on all domains is shown in Figure 2.
DISCUSSION
Our pilot structured mentorship program for junior hospitalists was feasible and led to improved satisfaction in select key career domains. Other mentoring or faculty coaching programs have been studied in several fields of medicine10-12; however, to our knowledge, there have not been published data studying a structured mentorship program for junior faculty in hospital medicine. Our intervention prioritized not only optimizing mentorship matches but also formalizing training sessions led by content experts.
After experiencing a structured mentoring relationship, most mentees felt a greater sense of support, were satisfied with their mentoring experiences, were connected to additional faculty, and had significant improvement in satisfaction in key career domains. Satisfaction with other self-identified “very important” domains, including scholarly activity, finding an area of expertise, job satisfaction, and work and family-life balance, did not significantly improve by the end of the program.
Perceived challenges to mentoring did not persist to the same degree with the implementation of a structured program. This highlights the importance of building mentorship skill sets (such as mentoring across differences and goal setting) through expert-led training sessions and perhaps also the importance of matching based on career goals.
CONCLUSION
Effective and sustainable career development requires mentorship.
Acknowledgments
The authors would like to thank each of the participants in the HMU Mentorship Program and the MGH CFD and Division of General Internal Medicine for supporting this effort.
Disclosure
Funding was provided by the MGH DGIM and CFD. Dr. Regina O’Neill reports the following relevant financial relationship: Massachusetts General Hospital Center for Faculty Development (consultant). All other authors report no other financial or other conflicts of interest to disclose.
The lack of mentorship in hospital medicine has been previously documented,1-3 but there is scant literature about solutions to the problem.4 In other disciplines, data suggest that the guidance of a mentor has a positive influence on academic productivity and professional satisfaction. Mentored faculty at all levels in their careers are more successful at producing peer-reviewed publications, procuring grant support, and maintaining confidence in their career trajectory.
METHODS
The mentorship program was implemented from October 2015 to June 2016 in the Hospital Medicine Unit (HMU) of the Massachusetts General Hospital (MGH), a teaching affiliate of Harvard Medical School.
Program Goals, Design, and Development
Participants
Mentees had to be hired at >0.5 full-time equivalent and have 3 years or fewer of hospitalist experience.
Mentor–Mentee Matching
Mentors were paired with 1 or 2 mentees. Participant information such as history of mentorship and areas of interest for mentorship was collected. Two authors matched mentors and mentees to maximize similarities in these areas. Four mentors were paired with 2 mentees each, and 12 mentors were paired with 1 mentee each.
Mentorship Training Sessions
The program provided 3 mentorship-training lunch sessions for both mentees and mentors during the 9-month program. To enrich attendance, mentees were provided coverage for their clinical duties. The initial training session provided an opportunity to meet, articulate expectations and challenges, and develop action plans with individualized goals for the mentoring relationship. The second training session occurred at the midpoint. Pairs considered their mentorship status, evaluated their progress, and discussed strategies for optimizing their experience. At the final training session, participants reflected on their mentoring relationships, identified their extended network of mentoring support, and set expectations regarding whether the mentoring relationship would continue.
Mentorship Meetings
In addition to the training sessions, mentee–mentor pairs were expected to meet a minimum of 2 times during the formal mentorship program. CFD experts performed participant outreach via e-mail to assess progress. Mentees were given dining facility gift cards to support meetings with their mentors.
Program Evaluation
Statistical Analysis
RESULTS
Program Participation and Response Rate
Of the 25 eligible mentees, 16 (64%) participated in the mentorship program. Of the 20 eligible mentors, 12 (60%) participated. One participating mentee and 1 mentor left the institution during the intervention period. Fourteen mentees (response rate: 88%) and 9 mentors (response rate: 75%) completed the preintervention survey. Ten mentees (response rate: 63%) and 8 mentors (response rate: 67%) completed the postintervention survey.
Mentor Characteristics
Mentorship Meetings and the Mentorship Network
All participants attended at least 2 of the 3 trainings. For the mentees who completed the postintervention survey, 9 (90%) met with their mentors 3 or more additional times, and 8 (80%) were connected by their mentor to at least 1 additional faculty mentor.
Perceptions and Overall Satisfaction with Mentorship
Prior to starting the mentoring relationship, 86% of mentees and 78% of mentors anticipated that differing career goals would be a challenge to a successful mentor–mentee relationship. At the end of the program, only 30% of mentees and 38% of mentors felt that such differences were a challenge. Ninety percent of mentees and 88% of mentors were satisfied or very satisfied with their mentorship match. Forty-three percent of mentees felt supported by the HMU prior to the mentorship program, while 90% felt supported after the program. All the mentees agreed that future HMU faculty should participate in a similar program.
Impact of Mentorship on Critical Domains
At baseline, the following domains were most commonly rated as very important by mentees: career planning, professional connectedness, producing scholarly work, finding an area of expertise, balancing work and family life, and job satisfaction (Figure 1). There was a significant improvement in composite satisfaction scores after completion of the mentorship program (54.5 ± 6.2 vs 65 ± 14.9, P = 0.02). The influence of the mentorship program on all domains is shown in Figure 2.
DISCUSSION
Our pilot structured mentorship program for junior hospitalists was feasible and led to improved satisfaction in select key career domains. Other mentoring or faculty coaching programs have been studied in several fields of medicine10-12; however, to our knowledge, there have not been published data studying a structured mentorship program for junior faculty in hospital medicine. Our intervention prioritized not only optimizing mentorship matches but also formalizing training sessions led by content experts.
After experiencing a structured mentoring relationship, most mentees felt a greater sense of support, were satisfied with their mentoring experiences, were connected to additional faculty, and had significant improvement in satisfaction in key career domains. Satisfaction with other self-identified “very important” domains, including scholarly activity, finding an area of expertise, job satisfaction, and work and family-life balance, did not significantly improve by the end of the program.
Perceived challenges to mentoring did not persist to the same degree with the implementation of a structured program. This highlights the importance of building mentorship skill sets (such as mentoring across differences and goal setting) through expert-led training sessions and perhaps also the importance of matching based on career goals.
CONCLUSION
Effective and sustainable career development requires mentorship.
Acknowledgments
The authors would like to thank each of the participants in the HMU Mentorship Program and the MGH CFD and Division of General Internal Medicine for supporting this effort.
Disclosure
Funding was provided by the MGH DGIM and CFD. Dr. Regina O’Neill reports the following relevant financial relationship: Massachusetts General Hospital Center for Faculty Development (consultant). All other authors report no other financial or other conflicts of interest to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5-9. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23-27. PubMed
3. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1-2. PubMed
4. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314-318. PubMed
5. Berk RA, Berg J, Mortimer R, Walton-Moss B, Yeo TP. Measuring the effectiveness of faculty mentoring relationships. Acad Med. 2005;80:66-71. PubMed
6. Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78:328-334. PubMed
7. Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112:336-341. PubMed
8. Steven A, Oxley J, Fleming WG. Mentoring for NHS doctors: perceived benefits across the personal-professional interface. J R Soc Med. 2008;101:552-557. PubMed
9. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359:1921-1931. PubMed
10. Pololi LH, Knight SM, Dennis K, Frankel RM. Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program. Acad Med. 2002;77:377-384. PubMed
11. Sambunjak D, Straus SE, Marusic A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115. PubMed
12. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161-166. PubMed
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5-9. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23-27. PubMed
3. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1-2. PubMed
4. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314-318. PubMed
5. Berk RA, Berg J, Mortimer R, Walton-Moss B, Yeo TP. Measuring the effectiveness of faculty mentoring relationships. Acad Med. 2005;80:66-71. PubMed
6. Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78:328-334. PubMed
7. Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112:336-341. PubMed
8. Steven A, Oxley J, Fleming WG. Mentoring for NHS doctors: perceived benefits across the personal-professional interface. J R Soc Med. 2008;101:552-557. PubMed
9. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359:1921-1931. PubMed
10. Pololi LH, Knight SM, Dennis K, Frankel RM. Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program. Acad Med. 2002;77:377-384. PubMed
11. Sambunjak D, Straus SE, Marusic A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115. PubMed
12. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161-166. PubMed
© 2018 Society of Hospital Medicine
Critical Literature 2014
Keeping up with the medical literature in a field as broad as hospital medicine is a daunting task. In 2014 alone, there were over 9200 articles published in top‐tier internal medicine journals.[1] The authors have selected articles from among these top journals using a nonsystematic process that involved reviewing articles brought to their attention via colleagues, literature searches, and online services. The focus was to identify articles that would be of importance to the field of hospital medicine for their potential to be practice changing, provocative, or iconoclastic. After culling through hundreds of titles and abstracts, 46 articles were reviewed by both authors in full text, and ultimately 14 were selected for presentation here. Table 1 summarizes the key points.
|
| 1. Now that neprolysin inhibitors are approved by the FDA, hospitalists will see them prescribed as an alternative to ACE‐inhibitors given their impressive benefits in cardiovascular mortality and heart failure hospitalizations. |
| 2. Current evidence suggests that intravenous contrast given with CT scans may not significantly alter the incidence of acute kidney injury, its associated mortality, or the need for hemodialysis. |
| 3. The CAM‐S score is an important tool for prognostication in delirious patients. Those patients with high CAM‐S scores should be considered for goals of care conversations. |
| 4. The melatonin agonist, ramelteon, shows promise for lowering incident delirium among elderly medical patients, though larger trials are still needed. |
| 5. Polyethylene glycol may be an excellent alternative to lactulose for patients with acute hepatic encephalopathy once larger studies are done, as it is well tolerated and shows faster resolution of symptoms. |
| 6. Nonselective ‐blockers should no longer be offered to cirrhotic patients after they develop spontaneous bacterial peritonitis, as they are associated with increased mortality and acute kidney injury. |
| 7. Current guidelines regarding prophylaxis against VTE in medical inpatients likely result in nonbeneficial use of medications for this purpose. It remains unclear which high‐risk populations do benefit from pharmacologic prophylaxis. |
| 8. DOACs are as effective and are safer than conventional therapy for treatment of VTE, though they are not recommended in patients with GFR <30 mL/min. |
| 9. DOACs are more effective and are safer (though they may increase risk of gastrointestinal bleeding) than conventional therapy in patients with AF. |
| 10. DOACs are as safe and more effective than conventional therapy in elderly patients with VTE or AF, being mindful of dosing recommendations in this population. |
| 11. Two new once‐weekly antibiotics, dalbavancin and oritavancin, approved for skin and soft tissue infections, appear noninferior to vancomycin and have the potential to shorten hospitalizations and, in doing so, may decrease cost. |
| 12. Offering family members of a patient undergoing CPR the opportunity to observe has durable impact on meaningful short‐ and long‐term psychological outcomes. Clinicians should strongly consider making this offer. |
AN APPROACHING PARADIGM SHIFT IN THE TREATMENT FOR HEART FAILURE
McMurray J, Packer M, Desai A, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:9931004.
Background
The last drug approved by the Food and Drug Administration (FDA) for heart failure (HF) was 10 years ago.[2] The new PARADIGM (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) heart failure study comparing a novel combination drug of a neprilysin inhibitor and angiotensin receptor blocker (ARB) to an angiotensin‐converting enzyme (ACE) inhibitor has cardiologists considering a possible change in the HF treatment algorithm. Neprilysin is a naturally occurring enzyme that breaks down the protective vasoactive peptides (brain natriuretic peptide, atrial natriuretic peptide, and bradykinin) made by the heart and the body in HF. These vasoactive peptides function to increase vasodilation and block sodium and water reabsorption. This novel neprilysin inhibitor extends the life of these vasoactive peptides, thus enhancing their effect. By inhibiting both neprilysin and the renin‐angiotensin system, there should be additional improvement in HF management. The neprilysin inhibitor was combined with an ARB instead of an ACE inhibitor because of significant angioedema seen in earlier phase trials when combined with an ACE inhibitor. This is believed related to increases in bradykinin due to both agents.
Findings
In this multicenter, blinded, randomized trial, over 10,000 patients with known HF (ejection fraction<35%, New York Heart Association class II or higher) went through 2 run‐in periods to ensure tolerance of both enalapril and the study drug, a combination of a neprilysin inhibitor and valsartan (neprilysin‐I/ARB). Eventually 8442 patients underwent randomization to either enalapril (10 mg twice a day) or neprilysin‐I/ARB (200 mg twice a day). The primary outcome was a combination of cardiovascular mortality and heart failure hospitalizations. The trial was stopped early at 27 months because of overwhelming benefit with neprilysin‐I/ARB (21.8% vs 26.5%; P<0.001). There was a 20% reduction specifically in cardiovascular mortality (13.3% vs 16.5%; hazard ratio [HR]: 0.80; P<0.001). The number needed to treat (NNT) was 32. There was also a 21% reduction in the risk of hospitalization (P<0.001). More patients with neprilysin‐I/ARB had symptomatic hypotension (14% vs 9.2%; P<0.001) but patients on the ACE inhibitor experienced more cough, hyperkalemia, and increases in their serum creatinine.
Cautions
There are 2 reasons clinicians may not see the same results in practice. First, the trial was stopped early, which can sometimes exaggerate benefits.[3] Second, the 2 run‐in periods eliminated patients who could not tolerate the medications at the trial doses. Additionally, although the study's authors were independent, the trial was funded by a pharmaceutical company.
Implications
This new combination drug of a neprilysin inhibitor and valsartan shows great promise at reducing cardiovascular mortality and hospitalizations for heart failure compared to enalapril alone. Given the high morbidity and mortality of heart failure, having a new agent in the treatment algorithm will be useful to patients and physicians. The drug was just approved by the FDA in July 2015 and will likely be offered as an alternative to ACE inhibitors.
VENOUS CONTRAST‐INDUCED NEPHROTOXICITY: IS THERE REALLY A RISK?
McDonald J, McDonald R, Carter R, et al. Risk of intravenous contrast material‐mediated acute kidney injury: a propensity score‐matched study stratified by baseline‐estimated glomerular filtration rate. Radiology. 2014;271(1):6573.
McDonald R, McDonald J, Carter R, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014;273(3):714725.
Background
It is a common practice to withhold intravenous contrast material from computed tomography (CT) scans in patients with even moderately poor renal function out of concern for causing contrast‐induced nephropathy (CIN). Our understanding of CIN is based largely on observational studies and outcomes of cardiac catheterizations, where larger amounts of contrast are given intra‐arterially into an atherosclerotic aorta.[4] The exact mechanism of injury is not clear, possibly from direct tubule toxicity or renal vasoconstriction.[5] CIN is defined as a rise in creatinine >0.5 mg/dL or >25% rise in serum creatinine 24 to 48 hours after receiving intravenous contrast. Although it is usually self‐limited, there is concern that patients who develop CIN have an increase risk of dialysis and death.[6] In the last few years, radiologists have started to question whether the risk of CIN is overstated. A recent meta‐analysis of 13 studies demonstrated a similar likelihood of acute kidney injury in patients regardless of receiving intravenous contrast.[7] If the true incidence of CIN after venous contrast is actually lower, this raises the question of whether we are unnecessarily withholding contrast from CTs and thereby reducing their diagnostic accuracy. Two 2014 observational studies provide additional evidence that the concern for CIN may be overstated.
Findings
The 2 Mayo Clinic studies used the same database. They looked at all patients who underwent a contrast‐enhanced or unenhanced thoracic, abdominal, or pelvic CT between January 2000 and December 2010 at the Mayo Clinic. After limiting the data to patients with pre‐ and post‐CT creatinine measurements and excluding anyone on dialysis, with preexisting acute kidney injury, or who had received additional contrast within 14 days, they ended up with 41,229 patients, mostly inpatients. All of the patients were assigned propensity scores based on risk factors for the development of CIN and whether they would likely receive contrast. The patients were then subdivided into 4 renal function subgroups based on estimated glomerular filtration rate (eGFR). The patients who received contrast were matched based on their propensity scores to those who did not received contrast within their eGFR subgroups. Unmatched patients were eliminated, leaving a cohort of 12,508 matched patients. The outcome of the first article was acute kidney injury (AKI) defined as a rise in creatinine >0.5 mg/dL at 24 to 48 hours. Though AKI rose with worsening eGFR subgroups (eGFR > 90 [1.2%] vs eGFR < 30 [14%]), the rates of AKI were the same regardless of contrast exposure. There was no statistical difference in any of the eGFR subgroups. The second study looked at important clinical outcomesdeath and the need for dialysis. There was no statistical difference for emergent dialysis (odds ratio [OR]: 0.96, P=0.89) or 30‐day mortality (HR: 0.97; P=0.45) regardless of whether the patients received contrast or not.
Cautions
In propensity matching, unmeasured confounders can bias the results. However, the issue of whether venous contrast causes CIN will unlikely be settled in a randomized controlled trial. For patients with severe renal failure (eGFR < 30), there were far fewer patients in this subgroup, making it harder to draw conclusions. The amount of venous contrast given was not provided. Finally, this study evaluated intravenous contrast for CTs, not intra‐arterial contrast.
Implications
These 2 studies raise doubt as to whether the incidence of AKI after contrast‐enhanced CT can be attributed to the contrast itself. What exactly causes the rise in creatinine is probably multifactorial including lab variation, hydration, blood pressure changes, nephrotoxic drugs, and comorbid disease. In trying to decide whether to obtain a contrast‐enhanced CT for patients with chronic kidney dysfunction, these studies provide more evidence to consider in the decision‐making process. A conversation with the radiologist about the benefits gained from using contrast in an individual patient may be of value.
PREVENTION AND PROGNOSIS OF INPATIENT DELIRIUM
Hatta K, Yasuhiro K, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo controlled trial. JAMA Psych. 2014;71(4):397403.
A new melatonin agonist dramatically improves delirium incidence.
Background
Numerous medications and therapeutic approaches have been studied to prevent incident delirium in hospitalized medical and surgical patients with varying success. Many of the tested medications also have the potential for significant undesirable side effects. An earlier small trial of melatonin appeared to have impressive efficacy for this purpose and be well tolerated, but the substance is not regulated by the FDA.[8] Ramelteon, a melatonin receptor agonist, is approved by the FDA for insomnia, and authors hypothesized that it, too, may be effective in delirium prevention.
Findings
This study was a multicenter, single‐blinded, randomized controlled trial of the melatonin‐agonist ramelteon versus placebo in elderly patients admitted to the hospital ward or ICU with serious medical conditions. Researchers excluded intubated patients or those with Lewy body dementia, psychiatric disorders, and severe liver disease. Patients received either ramelteon or placebo nightly for up to a week, and the primary end point was incident delirium as determined by a blinded observer using a validated assessment tool. Sixty‐seven patients were enrolled. The baseline characteristics in the arms of the trial were similar. In the placebo arm, 11 of 34 patients (32%) developed delirium during the 7‐day observation period. In the ramelteon arm, 1 of 33 (3%) developed delirium (P=0.003). The rate of drug discontinuation was the same in each arm.
Cautions
This study is small, and the single‐blinded design (the physicians and patients knew which group they were in but the observers did not) limits the validity of these results, mandating a larger double‐blinded trial.
Implications
Ramelteon showed a dramatic impact on preventing incident delirium on elderly hospitalized patients with serious medical conditions admitted to the ward or intensive care unit (ICU) (nonintubated) in this small study. If larger trials concur with the impact of this well‐tolerated and inexpensive medication, the potential for delirium incidence reduction could have a dramatic impact on how care for delirium‐vulnerable patients is conducted as well as the systems‐level costs associated with delirium care. Further studies of this class of medications are needed to more definitively establish its value in delirium prevention.
THE CONFUSION ASSESSMENT METHOD SEVERITY SCORE CAN QUANTIFY PROGNOSIS FOR DELIRIOUS MEDICAL INPATIENTS
Innoye SK, Kosar CM, Tommet D, et al. The CAM‐S: development and validation of a new scoring system for delirium in 2 cohorts. Ann Intern Med. 2014;160:526533.
Background
Delirium is common in hospitalized elderly patients, and numerous studies show that there are both short‐ and long‐term implications of developing delirium. Using well studied and validated tools has made identifying delirium fairly straightforward, yet its treatment remains difficult. Additionally, differentiating which patients will have a simpler clinical course from those at risk for a more morbid one has proved challenging. Using the Confusion Assessment Method (CAM), both in its short (4‐item) and long (10‐item) forms, as the basis for a prognostication tool, would allow for future research on treatment to have a scale against which to measure impact, and would allow clinicians to anticipate which patients were more likely to have difficult clinical courses.
Findings
The CAM Severity (CAM‐S) score was derived in 1219 subjects participating in 2 ongoing studies: 1 included high‐risk medical inpatients 70 years old or older, and the other included similarly aged patients undergoing major orthopedic, general, or vascular surgeries. Outcomes data were not available for the surgical patients. The CAM items were rated as either present/absent or absent/mild/severe, depending on the item, with an associated score attached to each item such that the 4‐item CAM had a score of 0 to 7 and the 10‐item CAM 0 to 19 (Table 2). Clinical outcomes from the medical patients cohort showed a dose response with increasing CAM‐S scores with respect to length of stay, adjusted cost, combined 90‐day end points of skilled nursing facility placement or death, and 90‐day mortality. Specifically, for patients with a CAM‐S (short form) score of 5 to 7, the 90‐day rate of death or nursing home residence was 62%, whereas the 90‐day postdischarge mortality rate was 36%.
| The CAM | The CAM‐S | |
|---|---|---|
| ||
| Acute onset with fluctuating course | Absent | 0 |
| Present | 1 | |
| Inattention or distractability | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Disorganized thinking, illogical or unclear ideas | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Alteration of consciousness | Absent | 0 |
| Mild | 0 | |
| Severe | 2 | |
| Total | 07 | |
Cautions
The CAM‐S, like the CAM, may work less well in patients with hypoactive delirium. This scale has been applied in a surgical cohort, but study outcomes were not presented in this article. This absence limits our ability to apply these results to a surgical population presently.
Implications
This study demonstrates that in medical inpatients, the CAM‐S is effective for prognostication. Moreover, the study points out that high‐scoring patients on the CAM‐S have quite poor prognoses, with more than one‐third dying by 3 months. This finding suggests that an important use of the CAM‐S is to identify patients about whom goals of care discussions should be held and end‐of‐life planning initiated if not previously done.
GET EXCITED ABOUT HEPATIC ENCEPHALOPATHY AGAINA NEW POSSIBLE TREATMENT
Rahimi R, Singal A, Cuthbert J, et al. Lactulose vs polyethylene glycol 3350‐electrolyte solution for treatment of overt hepatic encephalopathy. The HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):17271733.
Background
Lactulose has been the principle treatment for acute hepatic encephalopathy (HE) since 1966.[9] It theoretically works by lowering the pH of the colon and trapping ammonia as ammonium, which is then expelled. Alternatively, it may simply decrease transit time through the colon. In fact, earlier treatments for HE were cathartics such as magnesium salts. Unfortunately 20% tp 30% of patients are poor responders to lactulose, and patients do not like it. This new study tests whether a modern‐day cathartic, polyethylene glycol, works as well as lactulose.
Findings
In this unblinded, randomized controlled trial, patients presenting to the emergency department with acute HE were assigned to either lactulose 20 to 30 g for a minimum of 3 doses over 24 hours or 4 L of polyethylene glycol (PEG) over 4 hours. The2 groups were similar in severity and etiology of liver disease. Patients were allowed to have received 1 dose of lactulose given in the emergency department prior to study enrollment. They were excluded if taking rifaximin. The primary outcome was improvement in the hepatic encephalopathy scoring algorithm (HESA) by 1 grade at 24 hours.[10] The algorithm scores HE from 0 (no clinical findings of HE) to 5 (comatose). Initial mean HESA scores in the 2 groups were identical (2.3).
In the lactulose group, 13/25 (52%) improved by at least 1 HESA score at 24 hours. Two patients (8%) completely cleared with a HESA score of 0. In comparison, 21/23 (91%) in the PEG group improved at 24 hours, and 10/23 (43%) had cleared with a HESA score of 0 (P<0.01). The median time to HE resolution was 2 days in the lactulose group compared with 1 day in the PEG group (P=0.01). There were no differences in serious adverse events. The majority (76%) of the PEG group received the full 4 L of PEG.
Cautions
The main limitations of the trial were the small sample size, that it was a single‐center study, and the fact it was unblinded. Additionally, 80% of the PEG group received 1 dose of lactulose prior to enrollment. Statistically, more patients in the PEG group developed hypokalemia, which can worsen HE. Therefore, if PEG is used for acute HE, potassium will need to be monitored.
Implications
The results are intriguing and may represent a new possible treatment for acute HE once larger studies are done. Interestingly, the ammonia level dropped further in the lactulose group than the PEG group, yet there was more cognitive improvement in the PEG group. This raises questions about the role of ammonia and catharsis in HE. Although lactulose and rifaximin continue to be the standard of care, cathartics may be returning as a viable alternative.
SHOULD ‐BLOCKERS BE STOPPED IN PATIENTS WITH CIRRHOSIS WHEN SPONTANEOUS BACTERIAL PERITONITIS OCCURS?
Mandorfer M, Bota S, Schwabi P, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:16801690.
Background
Nonselective ‐blockers (NSBBs) are considered the aspirin of hepatologists, as they are used for primary and secondary prevention of variceal bleeds in patients with cirrhosis.[11] Since the 1980s, their benefit in reducing bleeding risk has been known, and more recently there has been evidence that they may reduce the risk of developing ascites in patients with compensated cirrhosis. Yet, there has been some contradictory evidence suggesting reduced survival in patients with decompensated cirrhosis and infections on NSBBs. This has led to the window hypothesis of NSBBs in cirrhosis, where NSBBs are beneficial only during a certain window period during the progression of cirrhosis.[12] Early on in cirrhosis, before the development of varices or ascites, NSBBs have no benefit. As cirrhosis progresses and portal hypertension develops, NSBBs play a major role in reducing bleeding from varices. However, in advanced cirrhosis, NSBBs may become harmful. In theory, they block the body's attempt to increase cardiac output during situations of increased physiologic stress, resulting in decreased mean arterial pressure and perfusion. This, in turn, causes end‐organ damage and increased risk of death. When exactly this NSBB window closes is unclear. A 2014 study suggests the window should close when patients develop spontaneous bacterial peritonitis (SBP).
Findings
This retrospective study followed 607 consecutive patients seen at a liver transplant center in Vienna, Austria, from 2006 to 2011. All of the patients were followed from the time of their first paracentesis. They were excluded if SBP was diagnosed during the first paracentesis. Patients were grouped based on whether they took an NSBB. As expected, more patients on an NSBB had varices (90% vs 62%; P<0.001) and a lower mean heart rate (77.5 vs 83.9 beats/minute; P<0.001). However, the 2 groups were similar in mean arterial pressure, systolic blood pressure, Model for End‐Stage Liver Disease score (17.5), Childs Pugh Score (CPS) (50% were C), and in the etiology of cirrhosis (55% were from alcoholic liver disease). They followed the patients for development of SBP. The primary outcome was transplant‐free survival. For the patients who never developed SBP, there was a 25% reduction in the risk of death for those on an NSBB adjusted for varices and CPS stage (HR=0.75, P=0.027). However, for the 182 patients who developed SBP, those on an NSBB had a 58% increase risk of death, again adjusted for varices and CPS stage (HR=1.58, P=0.014). Among the patients who developed SBP, there was a higher risk of hepatorenal syndrome (HRS) within 90 days for those on an NSBB (24% vs 11%, P=0.027). Although the mean arterial pressures (MAP) had been similar in the 2 groups before SBP, after the development of SBP, those on an NSBB had a significantly lower MAP (77.2 vs 82.6 mm Hg, P=0.005).
Cautions
This is a retrospective study, and although the authors controlled for varices and CPS, it is still possible the 2 groups were not similar. Whether patients were actually taking the NSBB is unknown, and doses of the NSBB were variable.
Implications
This study provides more evidence for the NSBB window hypothesis in the treatment of patients with cirrhosis. It suggests that the window on NSBB closes when patients develop SBP, as NSBBs appear to increase mortality and the risk of HRS. Thus, NSBB therapy should probably be discontinued in cirrhotic patients developing SBP. The question is for how long? The editorial accompanying the article says permanently.[13]
VTE PROPHYLAXIS FOR MEDICAL INPATIENTS: IS IT A THING OF THE PAST?
Flanders SA, Greene T, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism. A cohort study. JAMA Intern Med. 2014;174(10):15771584.
Background
Based on early research studies, many quality and regulatory organizations have stressed the importance of assessing hospitalized patients' venous thromboembolism (VTE) risk and prophylaxing those patients at increased risk either pharmacologically or mechanically. In 2011, a meta‐analysis of 40 studies of medical and stroke patients including approximately 52,000 patients failed to demonstrate a mortality benefit, showing that for every 3 pulmonary embolisms (PEs) prevented, it caused 4 major bleeding episodes per 1000 patients.[14] A second study in 2011, a multicenter, randomized controlled trial with medically complex patients deemed high risk for VTE, also failed to demonstrate a mortality benefit.[15] Despite these and other trials showing questionable benefit, guidelines continue to recommend that high‐risk medical patients should get pharmacologic prophylaxis against VTE.
Findings
This retrospective cohort trial retrospectively evaluated a cohort of 20,794 medical patients (non‐ICU) across 35 hospitals, excluding those with a Caprini score of <2 (ie, low risk for VTE). The authors divided the hospitals into tertiles based on adherence to VTE prophylaxis guidelines. Patients were followed to 90 days after hospitalization with telephone calls (reaching 56%) and chart reviews (100% reviewed) to identify clinically evident VTE events, excluding those that occurred within the first 3 days of index hospitalization. The study identified no statistically significant differences among the tertiles in terms of VTE rates, either in the hospital or at 90 days, though the overall VTE event rate was low. Interestingly, 85% of events took place postdischarge. Subgroup analyses also failed to identify a population of medical patients who benefited from prophylaxis.
Cautions
Debate about whether the Caprini risk score is the best available VTE risk scoring system exists. This study also excluded surgical and ICU patients.
Implications
This trial adds to the mounting literature suggesting that current guidelines‐based pharmacologic VTE prophylaxis for medical patients may offer no clear benefit in terms of incident VTE events or mortality. Although it is not yet time to abandon VTE prophylaxis completely, this study does raise the important question of whether it is time to revisit the quality guidelines and regulatory standards around VTE prophylaxis in medical inpatients. It also highlights the difficulty in assessing medical patients for their VTE risk. Though this study is provocative and important for its real‐world setting, further studies are required.
OUT WITH THE OLD AND IN WITH THE NEW? SHOULD DIRECT ORAL ANTICOAGULANTS BE OUR FIRST CHOICE FOR CARING FOR PATIENTS WITH VTE AND ATRIAL FIBRILLATION?
van Es N, Coppens M, Schulman S. et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):19681975.
For patients with acute VTE, direct oral anticoagulants work as well and are safer.
Background
There have been 6 large published randomized controlled trials of direct oral anticoagulants (DOACs) versus vitamin K antagonists (VKAs) in patients with acute VTE. Study sizes range from approximately 2500 to over 8000 subjects. All showed no significant difference between the arms with respect to efficacy (VTE or VTE‐related death) but had variable results with respect to major bleeding risk, a major concern given the nonreversibility of this group of medications. Additionally, subgroup analysis within these studies was challenging given sample size issues.
Findings
These 6 studies were combined in a meta‐analysis to address the DOACs' overall efficacy and safety profile, as well as looking in prespecified subgroups. The meta‐analysis included data from over 27,000 patients, evenly divided between DOACs (edoxaban, apixaban, rivaroxaban, and dabigatran) and VKAs, with the time in the therapeutic range (TTR) in the VKA arm being 64%. Overall, the primary efficacy endpoint (VTE and VTE‐related death) was similar (DOACs relative tisk [RR]=0.90; 95% confidence interval [CI]: 0.77‐1.06) but major bleeding (DOACs RR=0.61; 95% CI: 0.45‐0.83; NNT=150) and combined fatal and intracranial bleeding (DOACs RR=0.37; 95% CI: 0.27‐0.68; NNT=314) favored the DOACs. In subgroup analysis, there was no efficacy difference between the therapeutic groups in the subset specifically with DVT or with PE, or with patients weighing >100 kg, though safety data in these subsets were not evaluable. Patients with creatinine clearances of 30 to 49 mL/min demonstrated similar efficacy in both treatment arms, and the safety analysis in this subset with moderate renal impairment was better in the DOAC arm. Cancer patients achieved better efficacy with similar safety with the DOACs, whereas elderly patients achieved both better safety and efficacy with DOACs.
Cautions
As yet, there are inadequate data on patients with more advanced renal failure (creatinine clearance <30 mL/min) to advise using DOACs in that subset. Also, as there were no data comparing cancer patients with VTE that investigated DOACs versus low molecular weight heparins (the standard of care rather than warfarin since the CLOT [Comparison of Low‐molecular‐weight heparin versus Oral anticoagulant Therapy] trial[16]), the current meta‐analysis does not yet answer whether DOACs should be used in this population despite the efficacy benefit noted in the subgroup analysis.
Implications
This large meta‐analysis strongly suggests we can achieve comparable treatment efficacy from the DOACs as with VKAs, with better safety profiles in patients with acute VTE. In the subset of patients with moderate renal impairment (creatinine clearance 3049 mL/min), it appears safe and effective to choose DOACs.
IN PATIENTS WITH ATRIAL FIBRILLATION, DOACs APPEAR MORE EFFECTIVE THAN VKAs WITH COMPARABLE OR BETTER SAFETY PROFILES
Ruff CT, Guigliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet. 2014;383(9921):955962.
Background
Adding to the previously published meta‐analyses of the original phase 3 randomized trials regarding the DOACs' impact on the atrial fibrillation (AF) treatment safety and efficacy literature relative to VKAs, a 2013 trial, ENGAGE AF‐TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial FibrillationThrombolysis in Myocardial Infarction 48), with edoxaban was published and warrants inclusion to have a better opportunity to glean important subgroup information.[17]
Findings
This meta‐analysis included data on 71,683 patients, 42,411 in the DOAC arm and 29,272 in the warfarin arm, as 2 of the trials were3‐arm studies, comparing warfarin to a high dose and a low dose of the DOAC. Meta‐analyses of the 4 trials were broken down into a high‐dose subsetthe 2 high‐dose arms and the standard doses used in the other 2 trialsand a low‐dose subsetthe 2 low‐dose arms and the standard doses used in the other 2 trials. With respect to the efficacy endpoint (incident stroke or systemic embolization), the high‐dose subset analyses of the DOACs yielded a 19% reduction (P<0.0001; NNT=142) relative to the VKAs. The safety endpoint of major bleeding in this analysis identified a 14% reduction in the DOAC group that was nonsignificant (P=0.06). Within the high‐dose subset, analyses favored DOACs with respect to hemorrhagic stroke (51% reduction; P<0.0001; NNT=220), intracranial hemorrhage (52% reduction; P<0.0001; NNT=132), and overall mortality (10% reduction; P=0.0003; NNT=129), whereas they increased the risk of gastrointestinal bleeding (25% increase; P=0.043; NNH=185). There was no significant difference between DOACs and warfarin with respect to ischemic stroke. The low‐dose subset had similar overall results with even fewer hemorrhage strokes balancing a higher incidence of ischemic strokes in the DOAC arm than in warfarin. Other important subgroup analyses suggest the safety and efficacy impact of DOACs is significant for VKA‐naive and experienced patients, though only statistically so for VKA‐naive patients. Additionally, the anticoagulation centers included in the study that had a TTR <66% seemed to gain a safety advantage from the DOACs, whereas both TTR groups (<66% and 66%) appeared to achieve an efficacy benefit from DOACs.
Cautions
There are not sufficient data to suggest routinely switching patients tolerating and well managed on VKAs to DOACs for AF.
Implications
DOACs reduce stroke and systemic emboli in patients with AF without increasing intracranial bleeding or hemorrhagic stroke, though at the cost of increased gastrointestinal bleeding in patients on the high‐dose regimens. Those patients on the low‐dose regimens have even a lower hemorrhagic stroke risk, the benefit of which is negated by a higher than VKA risk of ischemic strokes. Centers with lower TTRs (and perhaps by extrapolation, those patients with more difficulty staying in the therapeutic range) may gain more benefit by switching. New patients on treatment for AF should strongly be considered for DOAC therapy as the first line.
IN ELDERLY PATIENTS, THE DOACs APPEAR TO OFFER IMPROVED EFFICACY WITHOUT SACRIFICING SAFETY
Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from meta‐analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857864.
Background
The prevalence of AF rises with age, as does the prevalence of malignancy, limited mobility, and other comorbidities that increase the risk for VTEs. These factors may also increase the risk of bleeding with conventional therapy with heparins and VKAs. As such, understanding the implications of using DOACs in the elderly population is important.
Findings
This meta‐analysis included the elderly (age 75 years) subset of patients from existing AF treatment and VTE treatment and prophylaxis randomized trials comparing DOACs with VKAs, low‐molecular‐weight heparin (LMWH), aspirin, or placebo. The primary safety outcome was major bleeding. For AF trials, the efficacy endpoint was stroke or systemic embolization, whereas in VTE trials it was VTE or VTE‐related death. Authors were able to extract data on 25,031 patients across 10 trials that evaluated rivaroxaban, apixaban, and dabigatran (not edoxaban), with follow‐up data ranging from 35 days to 2 years. For safety outcomes, the 2 arms showed no statistical difference (DOAC: 6.4%; conventional therapy: 6.3%; OR: 1.02; 95% CI: 0.73‐1.43). For efficacy endpoints in VTE studies, DOACs were more effective (3.7% vs 7.0%; OR: 0.45; 95% CI: 0.27‐77; NNT=30). For AF, the efficacy analysis favored DOACs also (3.3% vs 4.7%; OR: 0.65; 95% CI: 0.48‐0.87; NNT=71). When analyzed by the efficacy of the individual DOAC, rivaroxaban and apixaban both appeared to outperform the VKA/LMWH arm for both VTE and AF treatment, whereas data on dabigatran were only available for AF, also showing an efficacy benefit. Individual DOAC analyses for safety endpoints showed all the 3 to be similar to VKA/LMWH.
Cautions
Authors note, however, that coexisting low body weight and renal insufficiency may influence dosing choices in this population. There are specific dosage recommendations in the elderly for some DOACs.
Implications
The use of DOACs in patients aged 75 years and older appears to confer a substantial efficacy advantage when used for treatment of VTE and AF patients. The safety data presented in this meta‐analysis suggest that this class is comparable to VKA/LMWH medications.
CHANGING INPATIENT MANAGEMENT OF SKIN INFECTIONS
Boucher, H, Wilcox M, Talbot G, et al. Once‐weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:21692179.
Corey G, Kabler, H, Mahra P, et al. Single‐dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:21802190.
Background
There are over 870,000 hospital admissions yearly for skin infection, making it one of most common reasons for hospitalization in the United States.[18] Management often requires lengthy treatments with intravenous antibiotics, especially with the emergence of methicillin‐resistant Staphylococcus aureus. Results from 2 large randomized, double‐blinded, multicenter clinical trials were published looking at new once‐weekly intravenous antibiotics. Dalbavancin and oritavancin are both lipoglycopeptides in the same family as vancomycin. What is unique is that their serum drug concentrations exceed the minimum inhibitor concentrations for over a week. Both drugs were compared in noninferiority trials to vancomycin. The studies had similar outcomes. The dalbavancin results are presented below.
Findings
Researchers randomized 1312 patients with significant cellulitis, large abscess, or wound infection. Patients also had fever, leukocytosis, or bandemia, and the infection had to be deemed severe enough to require a minimum of 3 days of intravenous antibiotics. The patients could not have received any prior antibiotics. Over 80% of the patients had fevers, and more than half met the criteria for systemic inflammatory response syndrome. Patients were randomized to either dalbavancin (on day 1 and day 8) or vancomycin every 12 hours (1 gm or 15 mg/kg), with both groups receiving placebo dosing of the other drug. The blinded physicians could decide to switch to oral agent (placebo or linezolid in the vancomycin group) anytime after day 3, and the physicians could stop antibiotics anytime after day 10. Otherwise, all patients received 14 days of antibiotics.
The FDA‐approved outcome was cessation of spread of erythema at 48 to 72 hours and no fever at 3 independent readings. Results were similar in the dalbavancin group compared to the vancomycinlinezolid group (79.7% vs 79.8%). Dalbavancin was deemed noninferior to vancomycin. Blinded investigator's assessment of treatment success at 2 weeks was also similar (96% vs 96.7%, respectively). More treatment‐related adverse events occurred in the vancomycinlinezolid group (183 vs 139; P=0.02) and more deaths occurred in the vancomycin group (7 vs 1; P=0.03).
Cautions
These antibiotics have only been shown effective for complicated, acute bacterial skin infections. Their performance for other gram‐positive infections is unknown. In the future, it is possible that patients with severe skin infections will receive a dose of these antibiotics on hospital day 1 and be sent home with close follow‐up. However, that study has not been done yet to confirm efficacy and safety. Though the drugs appear safe, there needs to be more clinical use before they become standard of care, especially because of the long half‐life. Finally, these drugs are very expensive and provide broad spectrum gram‐positive coverage. They are not meant for a simple cellulitis.
Implications
These 2 new once‐weekly antibioticsdalbavancin and oritavancinare noninferior to vancomycin for acute bacterial skin infections. They provide alternative treatment choices for managing patients with significant infections requiring hospitalization. In the future, they may change the need for hospitalization of these patients or significantly reduce their length of stay. Though expensive, a significant reduction in hospitalization will offset costs.
SHOULD THEY STAY OR SHOULD THEY GO? FAMILY PRESENCE DURING CPR MAY IMPROVE THE GRIEF PROCESS DURABLY
Jabre P, Tazarourte K, Azoulay E, et al. Offering the opportunity for family to be present during cardiopulmonary resuscitation: 1 year assessment. Intensive Care Med. 2014;40:981987.
Background
In 2013, a French study randomized adult family members of a patient undergoing cardiopulmonary resuscitation (CPR) occurring at home to either be invited to stay and watch the resuscitation or to have no specific invitation offered.[19] At 90 days, this study revealed that those who were invited to watch (and 79% did) had fewer symptoms of post‐traumatic stress disorder (PTSD) (27% vs 37%) and anxiety (15% vs 23%), though not depression, than did the group not offered the opportunity to watch (though 43% watched anyway). There were 570 subjects (family members) in the trial, of whom a greater number in the control arm declined to participate in a 90‐day follow‐up due to emotional distress. Notably, only 4% of the patients in this study undergoing CPR survived to day 28. Whether the apparent positive psychological impact of the offer to watch CPR for families was durable remained in question.
Findings
The study group followed the families up to 1 year. At that time, dropout rates were similar (with the assumption, as in the prior study, that those who dropped out of either arm had PTSD symptoms). At follow‐up, subjects were again assessed for PTSD, anxiety, and depression symptoms as well as for meeting criteria for having had a major depressive episode or complicated grief. Four hundred eight of the original 570 subjects were able to undergo reevaluation. The 1‐year results showed the group offered the chance to watch CPR had fewer PTSD symptoms (20% vs 32%) and depression symptoms (10% vs 16%), as well as fewer major depressive episodes (23% vs 31%) and less complicated grief (21% vs 36%) but without a durable impact on anxiety symptoms.
Cautions
The resuscitation efforts in question here occurred out of hospital (in the home). Part of the protocol for those family members observing CPR was that a clinician was assigned to stay with them and explain the resuscitation process as it occurred.
Implications
It is postulated that having the chance to observe CPR, if desired, may help the grieving process. This study clearly raises a question about the wisdom of routinely escorting patient's families out of the room during resuscitative efforts. It seems likely that the durable and important psychological effects observed in this study for family members would similarly persist in emergency department and inpatient settings, where staff can be with patients' families to talk them through the events they are witnessing. It is time to ask families if they prefer to stay and watch CPR and not automatically move them to a waiting room.
Disclosure: Nothing to report.
- Journals in the 2014 release of the JCR. Available at: http://scientific.thomsonreuters.com/imgblast/JCRFullCovlist-2014.pdf. Accessed August 28, 2015.
- Neprilysin inhibition—a novel therapy for heart failure. N Engl J Med. 2014;371(11):1062–1064.
- , , , et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA. 2010;303(12):1180–1187.
- , Intravenous contrast medium‐induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology 2010;256(1):21–28.
- , , Pathophysiology of contrast medium‐induced nephropathy. Kidney Int. 2005;68(1):14–22.
- , Contrast‐induced acute kidney injury: short‐ and long‐term implications. Semin Nephrol. 2011;31(3):300–309.
- , , , et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta‐analysis. Radiology. 2013;267(1):119–128.
- , , , , , Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694.
- , , Lactulose in the treatment of chronic portal‐systemic encephalopathy. A double‐blind clinical trial. N Engl J Med. 1969;281(8):408–412.
- , , , et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104(6):1392–1400.
- , The changing role of beta‐blocker therapy in patients with cirrhosis. J Hepatol. 2014;60(3):643–653.
- , , , The window hypothesis: haemodynamic and non‐haemodynamic effects of beta‐blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61(7):967–969.
- , When should the beta‐blocker window in cirrhosis close? Gastroenterology. 2014;146(7):1597–1599.
- , , , Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , , , ; LIFENOX Investigators. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365(26):2463–2472.
- , , , et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153.
- , , , et al.; ENGAGE AF‐TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Pharmacology and the treatment of complicated skin and skin‐structure infections. N Engl J Med. 2014;370(23):2238–2239.
- , , , et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368(11):1008–1018.
Keeping up with the medical literature in a field as broad as hospital medicine is a daunting task. In 2014 alone, there were over 9200 articles published in top‐tier internal medicine journals.[1] The authors have selected articles from among these top journals using a nonsystematic process that involved reviewing articles brought to their attention via colleagues, literature searches, and online services. The focus was to identify articles that would be of importance to the field of hospital medicine for their potential to be practice changing, provocative, or iconoclastic. After culling through hundreds of titles and abstracts, 46 articles were reviewed by both authors in full text, and ultimately 14 were selected for presentation here. Table 1 summarizes the key points.
|
| 1. Now that neprolysin inhibitors are approved by the FDA, hospitalists will see them prescribed as an alternative to ACE‐inhibitors given their impressive benefits in cardiovascular mortality and heart failure hospitalizations. |
| 2. Current evidence suggests that intravenous contrast given with CT scans may not significantly alter the incidence of acute kidney injury, its associated mortality, or the need for hemodialysis. |
| 3. The CAM‐S score is an important tool for prognostication in delirious patients. Those patients with high CAM‐S scores should be considered for goals of care conversations. |
| 4. The melatonin agonist, ramelteon, shows promise for lowering incident delirium among elderly medical patients, though larger trials are still needed. |
| 5. Polyethylene glycol may be an excellent alternative to lactulose for patients with acute hepatic encephalopathy once larger studies are done, as it is well tolerated and shows faster resolution of symptoms. |
| 6. Nonselective ‐blockers should no longer be offered to cirrhotic patients after they develop spontaneous bacterial peritonitis, as they are associated with increased mortality and acute kidney injury. |
| 7. Current guidelines regarding prophylaxis against VTE in medical inpatients likely result in nonbeneficial use of medications for this purpose. It remains unclear which high‐risk populations do benefit from pharmacologic prophylaxis. |
| 8. DOACs are as effective and are safer than conventional therapy for treatment of VTE, though they are not recommended in patients with GFR <30 mL/min. |
| 9. DOACs are more effective and are safer (though they may increase risk of gastrointestinal bleeding) than conventional therapy in patients with AF. |
| 10. DOACs are as safe and more effective than conventional therapy in elderly patients with VTE or AF, being mindful of dosing recommendations in this population. |
| 11. Two new once‐weekly antibiotics, dalbavancin and oritavancin, approved for skin and soft tissue infections, appear noninferior to vancomycin and have the potential to shorten hospitalizations and, in doing so, may decrease cost. |
| 12. Offering family members of a patient undergoing CPR the opportunity to observe has durable impact on meaningful short‐ and long‐term psychological outcomes. Clinicians should strongly consider making this offer. |
AN APPROACHING PARADIGM SHIFT IN THE TREATMENT FOR HEART FAILURE
McMurray J, Packer M, Desai A, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:9931004.
Background
The last drug approved by the Food and Drug Administration (FDA) for heart failure (HF) was 10 years ago.[2] The new PARADIGM (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) heart failure study comparing a novel combination drug of a neprilysin inhibitor and angiotensin receptor blocker (ARB) to an angiotensin‐converting enzyme (ACE) inhibitor has cardiologists considering a possible change in the HF treatment algorithm. Neprilysin is a naturally occurring enzyme that breaks down the protective vasoactive peptides (brain natriuretic peptide, atrial natriuretic peptide, and bradykinin) made by the heart and the body in HF. These vasoactive peptides function to increase vasodilation and block sodium and water reabsorption. This novel neprilysin inhibitor extends the life of these vasoactive peptides, thus enhancing their effect. By inhibiting both neprilysin and the renin‐angiotensin system, there should be additional improvement in HF management. The neprilysin inhibitor was combined with an ARB instead of an ACE inhibitor because of significant angioedema seen in earlier phase trials when combined with an ACE inhibitor. This is believed related to increases in bradykinin due to both agents.
Findings
In this multicenter, blinded, randomized trial, over 10,000 patients with known HF (ejection fraction<35%, New York Heart Association class II or higher) went through 2 run‐in periods to ensure tolerance of both enalapril and the study drug, a combination of a neprilysin inhibitor and valsartan (neprilysin‐I/ARB). Eventually 8442 patients underwent randomization to either enalapril (10 mg twice a day) or neprilysin‐I/ARB (200 mg twice a day). The primary outcome was a combination of cardiovascular mortality and heart failure hospitalizations. The trial was stopped early at 27 months because of overwhelming benefit with neprilysin‐I/ARB (21.8% vs 26.5%; P<0.001). There was a 20% reduction specifically in cardiovascular mortality (13.3% vs 16.5%; hazard ratio [HR]: 0.80; P<0.001). The number needed to treat (NNT) was 32. There was also a 21% reduction in the risk of hospitalization (P<0.001). More patients with neprilysin‐I/ARB had symptomatic hypotension (14% vs 9.2%; P<0.001) but patients on the ACE inhibitor experienced more cough, hyperkalemia, and increases in their serum creatinine.
Cautions
There are 2 reasons clinicians may not see the same results in practice. First, the trial was stopped early, which can sometimes exaggerate benefits.[3] Second, the 2 run‐in periods eliminated patients who could not tolerate the medications at the trial doses. Additionally, although the study's authors were independent, the trial was funded by a pharmaceutical company.
Implications
This new combination drug of a neprilysin inhibitor and valsartan shows great promise at reducing cardiovascular mortality and hospitalizations for heart failure compared to enalapril alone. Given the high morbidity and mortality of heart failure, having a new agent in the treatment algorithm will be useful to patients and physicians. The drug was just approved by the FDA in July 2015 and will likely be offered as an alternative to ACE inhibitors.
VENOUS CONTRAST‐INDUCED NEPHROTOXICITY: IS THERE REALLY A RISK?
McDonald J, McDonald R, Carter R, et al. Risk of intravenous contrast material‐mediated acute kidney injury: a propensity score‐matched study stratified by baseline‐estimated glomerular filtration rate. Radiology. 2014;271(1):6573.
McDonald R, McDonald J, Carter R, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014;273(3):714725.
Background
It is a common practice to withhold intravenous contrast material from computed tomography (CT) scans in patients with even moderately poor renal function out of concern for causing contrast‐induced nephropathy (CIN). Our understanding of CIN is based largely on observational studies and outcomes of cardiac catheterizations, where larger amounts of contrast are given intra‐arterially into an atherosclerotic aorta.[4] The exact mechanism of injury is not clear, possibly from direct tubule toxicity or renal vasoconstriction.[5] CIN is defined as a rise in creatinine >0.5 mg/dL or >25% rise in serum creatinine 24 to 48 hours after receiving intravenous contrast. Although it is usually self‐limited, there is concern that patients who develop CIN have an increase risk of dialysis and death.[6] In the last few years, radiologists have started to question whether the risk of CIN is overstated. A recent meta‐analysis of 13 studies demonstrated a similar likelihood of acute kidney injury in patients regardless of receiving intravenous contrast.[7] If the true incidence of CIN after venous contrast is actually lower, this raises the question of whether we are unnecessarily withholding contrast from CTs and thereby reducing their diagnostic accuracy. Two 2014 observational studies provide additional evidence that the concern for CIN may be overstated.
Findings
The 2 Mayo Clinic studies used the same database. They looked at all patients who underwent a contrast‐enhanced or unenhanced thoracic, abdominal, or pelvic CT between January 2000 and December 2010 at the Mayo Clinic. After limiting the data to patients with pre‐ and post‐CT creatinine measurements and excluding anyone on dialysis, with preexisting acute kidney injury, or who had received additional contrast within 14 days, they ended up with 41,229 patients, mostly inpatients. All of the patients were assigned propensity scores based on risk factors for the development of CIN and whether they would likely receive contrast. The patients were then subdivided into 4 renal function subgroups based on estimated glomerular filtration rate (eGFR). The patients who received contrast were matched based on their propensity scores to those who did not received contrast within their eGFR subgroups. Unmatched patients were eliminated, leaving a cohort of 12,508 matched patients. The outcome of the first article was acute kidney injury (AKI) defined as a rise in creatinine >0.5 mg/dL at 24 to 48 hours. Though AKI rose with worsening eGFR subgroups (eGFR > 90 [1.2%] vs eGFR < 30 [14%]), the rates of AKI were the same regardless of contrast exposure. There was no statistical difference in any of the eGFR subgroups. The second study looked at important clinical outcomesdeath and the need for dialysis. There was no statistical difference for emergent dialysis (odds ratio [OR]: 0.96, P=0.89) or 30‐day mortality (HR: 0.97; P=0.45) regardless of whether the patients received contrast or not.
Cautions
In propensity matching, unmeasured confounders can bias the results. However, the issue of whether venous contrast causes CIN will unlikely be settled in a randomized controlled trial. For patients with severe renal failure (eGFR < 30), there were far fewer patients in this subgroup, making it harder to draw conclusions. The amount of venous contrast given was not provided. Finally, this study evaluated intravenous contrast for CTs, not intra‐arterial contrast.
Implications
These 2 studies raise doubt as to whether the incidence of AKI after contrast‐enhanced CT can be attributed to the contrast itself. What exactly causes the rise in creatinine is probably multifactorial including lab variation, hydration, blood pressure changes, nephrotoxic drugs, and comorbid disease. In trying to decide whether to obtain a contrast‐enhanced CT for patients with chronic kidney dysfunction, these studies provide more evidence to consider in the decision‐making process. A conversation with the radiologist about the benefits gained from using contrast in an individual patient may be of value.
PREVENTION AND PROGNOSIS OF INPATIENT DELIRIUM
Hatta K, Yasuhiro K, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo controlled trial. JAMA Psych. 2014;71(4):397403.
A new melatonin agonist dramatically improves delirium incidence.
Background
Numerous medications and therapeutic approaches have been studied to prevent incident delirium in hospitalized medical and surgical patients with varying success. Many of the tested medications also have the potential for significant undesirable side effects. An earlier small trial of melatonin appeared to have impressive efficacy for this purpose and be well tolerated, but the substance is not regulated by the FDA.[8] Ramelteon, a melatonin receptor agonist, is approved by the FDA for insomnia, and authors hypothesized that it, too, may be effective in delirium prevention.
Findings
This study was a multicenter, single‐blinded, randomized controlled trial of the melatonin‐agonist ramelteon versus placebo in elderly patients admitted to the hospital ward or ICU with serious medical conditions. Researchers excluded intubated patients or those with Lewy body dementia, psychiatric disorders, and severe liver disease. Patients received either ramelteon or placebo nightly for up to a week, and the primary end point was incident delirium as determined by a blinded observer using a validated assessment tool. Sixty‐seven patients were enrolled. The baseline characteristics in the arms of the trial were similar. In the placebo arm, 11 of 34 patients (32%) developed delirium during the 7‐day observation period. In the ramelteon arm, 1 of 33 (3%) developed delirium (P=0.003). The rate of drug discontinuation was the same in each arm.
Cautions
This study is small, and the single‐blinded design (the physicians and patients knew which group they were in but the observers did not) limits the validity of these results, mandating a larger double‐blinded trial.
Implications
Ramelteon showed a dramatic impact on preventing incident delirium on elderly hospitalized patients with serious medical conditions admitted to the ward or intensive care unit (ICU) (nonintubated) in this small study. If larger trials concur with the impact of this well‐tolerated and inexpensive medication, the potential for delirium incidence reduction could have a dramatic impact on how care for delirium‐vulnerable patients is conducted as well as the systems‐level costs associated with delirium care. Further studies of this class of medications are needed to more definitively establish its value in delirium prevention.
THE CONFUSION ASSESSMENT METHOD SEVERITY SCORE CAN QUANTIFY PROGNOSIS FOR DELIRIOUS MEDICAL INPATIENTS
Innoye SK, Kosar CM, Tommet D, et al. The CAM‐S: development and validation of a new scoring system for delirium in 2 cohorts. Ann Intern Med. 2014;160:526533.
Background
Delirium is common in hospitalized elderly patients, and numerous studies show that there are both short‐ and long‐term implications of developing delirium. Using well studied and validated tools has made identifying delirium fairly straightforward, yet its treatment remains difficult. Additionally, differentiating which patients will have a simpler clinical course from those at risk for a more morbid one has proved challenging. Using the Confusion Assessment Method (CAM), both in its short (4‐item) and long (10‐item) forms, as the basis for a prognostication tool, would allow for future research on treatment to have a scale against which to measure impact, and would allow clinicians to anticipate which patients were more likely to have difficult clinical courses.
Findings
The CAM Severity (CAM‐S) score was derived in 1219 subjects participating in 2 ongoing studies: 1 included high‐risk medical inpatients 70 years old or older, and the other included similarly aged patients undergoing major orthopedic, general, or vascular surgeries. Outcomes data were not available for the surgical patients. The CAM items were rated as either present/absent or absent/mild/severe, depending on the item, with an associated score attached to each item such that the 4‐item CAM had a score of 0 to 7 and the 10‐item CAM 0 to 19 (Table 2). Clinical outcomes from the medical patients cohort showed a dose response with increasing CAM‐S scores with respect to length of stay, adjusted cost, combined 90‐day end points of skilled nursing facility placement or death, and 90‐day mortality. Specifically, for patients with a CAM‐S (short form) score of 5 to 7, the 90‐day rate of death or nursing home residence was 62%, whereas the 90‐day postdischarge mortality rate was 36%.
| The CAM | The CAM‐S | |
|---|---|---|
| ||
| Acute onset with fluctuating course | Absent | 0 |
| Present | 1 | |
| Inattention or distractability | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Disorganized thinking, illogical or unclear ideas | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Alteration of consciousness | Absent | 0 |
| Mild | 0 | |
| Severe | 2 | |
| Total | 07 | |
Cautions
The CAM‐S, like the CAM, may work less well in patients with hypoactive delirium. This scale has been applied in a surgical cohort, but study outcomes were not presented in this article. This absence limits our ability to apply these results to a surgical population presently.
Implications
This study demonstrates that in medical inpatients, the CAM‐S is effective for prognostication. Moreover, the study points out that high‐scoring patients on the CAM‐S have quite poor prognoses, with more than one‐third dying by 3 months. This finding suggests that an important use of the CAM‐S is to identify patients about whom goals of care discussions should be held and end‐of‐life planning initiated if not previously done.
GET EXCITED ABOUT HEPATIC ENCEPHALOPATHY AGAINA NEW POSSIBLE TREATMENT
Rahimi R, Singal A, Cuthbert J, et al. Lactulose vs polyethylene glycol 3350‐electrolyte solution for treatment of overt hepatic encephalopathy. The HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):17271733.
Background
Lactulose has been the principle treatment for acute hepatic encephalopathy (HE) since 1966.[9] It theoretically works by lowering the pH of the colon and trapping ammonia as ammonium, which is then expelled. Alternatively, it may simply decrease transit time through the colon. In fact, earlier treatments for HE were cathartics such as magnesium salts. Unfortunately 20% tp 30% of patients are poor responders to lactulose, and patients do not like it. This new study tests whether a modern‐day cathartic, polyethylene glycol, works as well as lactulose.
Findings
In this unblinded, randomized controlled trial, patients presenting to the emergency department with acute HE were assigned to either lactulose 20 to 30 g for a minimum of 3 doses over 24 hours or 4 L of polyethylene glycol (PEG) over 4 hours. The2 groups were similar in severity and etiology of liver disease. Patients were allowed to have received 1 dose of lactulose given in the emergency department prior to study enrollment. They were excluded if taking rifaximin. The primary outcome was improvement in the hepatic encephalopathy scoring algorithm (HESA) by 1 grade at 24 hours.[10] The algorithm scores HE from 0 (no clinical findings of HE) to 5 (comatose). Initial mean HESA scores in the 2 groups were identical (2.3).
In the lactulose group, 13/25 (52%) improved by at least 1 HESA score at 24 hours. Two patients (8%) completely cleared with a HESA score of 0. In comparison, 21/23 (91%) in the PEG group improved at 24 hours, and 10/23 (43%) had cleared with a HESA score of 0 (P<0.01). The median time to HE resolution was 2 days in the lactulose group compared with 1 day in the PEG group (P=0.01). There were no differences in serious adverse events. The majority (76%) of the PEG group received the full 4 L of PEG.
Cautions
The main limitations of the trial were the small sample size, that it was a single‐center study, and the fact it was unblinded. Additionally, 80% of the PEG group received 1 dose of lactulose prior to enrollment. Statistically, more patients in the PEG group developed hypokalemia, which can worsen HE. Therefore, if PEG is used for acute HE, potassium will need to be monitored.
Implications
The results are intriguing and may represent a new possible treatment for acute HE once larger studies are done. Interestingly, the ammonia level dropped further in the lactulose group than the PEG group, yet there was more cognitive improvement in the PEG group. This raises questions about the role of ammonia and catharsis in HE. Although lactulose and rifaximin continue to be the standard of care, cathartics may be returning as a viable alternative.
SHOULD ‐BLOCKERS BE STOPPED IN PATIENTS WITH CIRRHOSIS WHEN SPONTANEOUS BACTERIAL PERITONITIS OCCURS?
Mandorfer M, Bota S, Schwabi P, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:16801690.
Background
Nonselective ‐blockers (NSBBs) are considered the aspirin of hepatologists, as they are used for primary and secondary prevention of variceal bleeds in patients with cirrhosis.[11] Since the 1980s, their benefit in reducing bleeding risk has been known, and more recently there has been evidence that they may reduce the risk of developing ascites in patients with compensated cirrhosis. Yet, there has been some contradictory evidence suggesting reduced survival in patients with decompensated cirrhosis and infections on NSBBs. This has led to the window hypothesis of NSBBs in cirrhosis, where NSBBs are beneficial only during a certain window period during the progression of cirrhosis.[12] Early on in cirrhosis, before the development of varices or ascites, NSBBs have no benefit. As cirrhosis progresses and portal hypertension develops, NSBBs play a major role in reducing bleeding from varices. However, in advanced cirrhosis, NSBBs may become harmful. In theory, they block the body's attempt to increase cardiac output during situations of increased physiologic stress, resulting in decreased mean arterial pressure and perfusion. This, in turn, causes end‐organ damage and increased risk of death. When exactly this NSBB window closes is unclear. A 2014 study suggests the window should close when patients develop spontaneous bacterial peritonitis (SBP).
Findings
This retrospective study followed 607 consecutive patients seen at a liver transplant center in Vienna, Austria, from 2006 to 2011. All of the patients were followed from the time of their first paracentesis. They were excluded if SBP was diagnosed during the first paracentesis. Patients were grouped based on whether they took an NSBB. As expected, more patients on an NSBB had varices (90% vs 62%; P<0.001) and a lower mean heart rate (77.5 vs 83.9 beats/minute; P<0.001). However, the 2 groups were similar in mean arterial pressure, systolic blood pressure, Model for End‐Stage Liver Disease score (17.5), Childs Pugh Score (CPS) (50% were C), and in the etiology of cirrhosis (55% were from alcoholic liver disease). They followed the patients for development of SBP. The primary outcome was transplant‐free survival. For the patients who never developed SBP, there was a 25% reduction in the risk of death for those on an NSBB adjusted for varices and CPS stage (HR=0.75, P=0.027). However, for the 182 patients who developed SBP, those on an NSBB had a 58% increase risk of death, again adjusted for varices and CPS stage (HR=1.58, P=0.014). Among the patients who developed SBP, there was a higher risk of hepatorenal syndrome (HRS) within 90 days for those on an NSBB (24% vs 11%, P=0.027). Although the mean arterial pressures (MAP) had been similar in the 2 groups before SBP, after the development of SBP, those on an NSBB had a significantly lower MAP (77.2 vs 82.6 mm Hg, P=0.005).
Cautions
This is a retrospective study, and although the authors controlled for varices and CPS, it is still possible the 2 groups were not similar. Whether patients were actually taking the NSBB is unknown, and doses of the NSBB were variable.
Implications
This study provides more evidence for the NSBB window hypothesis in the treatment of patients with cirrhosis. It suggests that the window on NSBB closes when patients develop SBP, as NSBBs appear to increase mortality and the risk of HRS. Thus, NSBB therapy should probably be discontinued in cirrhotic patients developing SBP. The question is for how long? The editorial accompanying the article says permanently.[13]
VTE PROPHYLAXIS FOR MEDICAL INPATIENTS: IS IT A THING OF THE PAST?
Flanders SA, Greene T, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism. A cohort study. JAMA Intern Med. 2014;174(10):15771584.
Background
Based on early research studies, many quality and regulatory organizations have stressed the importance of assessing hospitalized patients' venous thromboembolism (VTE) risk and prophylaxing those patients at increased risk either pharmacologically or mechanically. In 2011, a meta‐analysis of 40 studies of medical and stroke patients including approximately 52,000 patients failed to demonstrate a mortality benefit, showing that for every 3 pulmonary embolisms (PEs) prevented, it caused 4 major bleeding episodes per 1000 patients.[14] A second study in 2011, a multicenter, randomized controlled trial with medically complex patients deemed high risk for VTE, also failed to demonstrate a mortality benefit.[15] Despite these and other trials showing questionable benefit, guidelines continue to recommend that high‐risk medical patients should get pharmacologic prophylaxis against VTE.
Findings
This retrospective cohort trial retrospectively evaluated a cohort of 20,794 medical patients (non‐ICU) across 35 hospitals, excluding those with a Caprini score of <2 (ie, low risk for VTE). The authors divided the hospitals into tertiles based on adherence to VTE prophylaxis guidelines. Patients were followed to 90 days after hospitalization with telephone calls (reaching 56%) and chart reviews (100% reviewed) to identify clinically evident VTE events, excluding those that occurred within the first 3 days of index hospitalization. The study identified no statistically significant differences among the tertiles in terms of VTE rates, either in the hospital or at 90 days, though the overall VTE event rate was low. Interestingly, 85% of events took place postdischarge. Subgroup analyses also failed to identify a population of medical patients who benefited from prophylaxis.
Cautions
Debate about whether the Caprini risk score is the best available VTE risk scoring system exists. This study also excluded surgical and ICU patients.
Implications
This trial adds to the mounting literature suggesting that current guidelines‐based pharmacologic VTE prophylaxis for medical patients may offer no clear benefit in terms of incident VTE events or mortality. Although it is not yet time to abandon VTE prophylaxis completely, this study does raise the important question of whether it is time to revisit the quality guidelines and regulatory standards around VTE prophylaxis in medical inpatients. It also highlights the difficulty in assessing medical patients for their VTE risk. Though this study is provocative and important for its real‐world setting, further studies are required.
OUT WITH THE OLD AND IN WITH THE NEW? SHOULD DIRECT ORAL ANTICOAGULANTS BE OUR FIRST CHOICE FOR CARING FOR PATIENTS WITH VTE AND ATRIAL FIBRILLATION?
van Es N, Coppens M, Schulman S. et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):19681975.
For patients with acute VTE, direct oral anticoagulants work as well and are safer.
Background
There have been 6 large published randomized controlled trials of direct oral anticoagulants (DOACs) versus vitamin K antagonists (VKAs) in patients with acute VTE. Study sizes range from approximately 2500 to over 8000 subjects. All showed no significant difference between the arms with respect to efficacy (VTE or VTE‐related death) but had variable results with respect to major bleeding risk, a major concern given the nonreversibility of this group of medications. Additionally, subgroup analysis within these studies was challenging given sample size issues.
Findings
These 6 studies were combined in a meta‐analysis to address the DOACs' overall efficacy and safety profile, as well as looking in prespecified subgroups. The meta‐analysis included data from over 27,000 patients, evenly divided between DOACs (edoxaban, apixaban, rivaroxaban, and dabigatran) and VKAs, with the time in the therapeutic range (TTR) in the VKA arm being 64%. Overall, the primary efficacy endpoint (VTE and VTE‐related death) was similar (DOACs relative tisk [RR]=0.90; 95% confidence interval [CI]: 0.77‐1.06) but major bleeding (DOACs RR=0.61; 95% CI: 0.45‐0.83; NNT=150) and combined fatal and intracranial bleeding (DOACs RR=0.37; 95% CI: 0.27‐0.68; NNT=314) favored the DOACs. In subgroup analysis, there was no efficacy difference between the therapeutic groups in the subset specifically with DVT or with PE, or with patients weighing >100 kg, though safety data in these subsets were not evaluable. Patients with creatinine clearances of 30 to 49 mL/min demonstrated similar efficacy in both treatment arms, and the safety analysis in this subset with moderate renal impairment was better in the DOAC arm. Cancer patients achieved better efficacy with similar safety with the DOACs, whereas elderly patients achieved both better safety and efficacy with DOACs.
Cautions
As yet, there are inadequate data on patients with more advanced renal failure (creatinine clearance <30 mL/min) to advise using DOACs in that subset. Also, as there were no data comparing cancer patients with VTE that investigated DOACs versus low molecular weight heparins (the standard of care rather than warfarin since the CLOT [Comparison of Low‐molecular‐weight heparin versus Oral anticoagulant Therapy] trial[16]), the current meta‐analysis does not yet answer whether DOACs should be used in this population despite the efficacy benefit noted in the subgroup analysis.
Implications
This large meta‐analysis strongly suggests we can achieve comparable treatment efficacy from the DOACs as with VKAs, with better safety profiles in patients with acute VTE. In the subset of patients with moderate renal impairment (creatinine clearance 3049 mL/min), it appears safe and effective to choose DOACs.
IN PATIENTS WITH ATRIAL FIBRILLATION, DOACs APPEAR MORE EFFECTIVE THAN VKAs WITH COMPARABLE OR BETTER SAFETY PROFILES
Ruff CT, Guigliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet. 2014;383(9921):955962.
Background
Adding to the previously published meta‐analyses of the original phase 3 randomized trials regarding the DOACs' impact on the atrial fibrillation (AF) treatment safety and efficacy literature relative to VKAs, a 2013 trial, ENGAGE AF‐TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial FibrillationThrombolysis in Myocardial Infarction 48), with edoxaban was published and warrants inclusion to have a better opportunity to glean important subgroup information.[17]
Findings
This meta‐analysis included data on 71,683 patients, 42,411 in the DOAC arm and 29,272 in the warfarin arm, as 2 of the trials were3‐arm studies, comparing warfarin to a high dose and a low dose of the DOAC. Meta‐analyses of the 4 trials were broken down into a high‐dose subsetthe 2 high‐dose arms and the standard doses used in the other 2 trialsand a low‐dose subsetthe 2 low‐dose arms and the standard doses used in the other 2 trials. With respect to the efficacy endpoint (incident stroke or systemic embolization), the high‐dose subset analyses of the DOACs yielded a 19% reduction (P<0.0001; NNT=142) relative to the VKAs. The safety endpoint of major bleeding in this analysis identified a 14% reduction in the DOAC group that was nonsignificant (P=0.06). Within the high‐dose subset, analyses favored DOACs with respect to hemorrhagic stroke (51% reduction; P<0.0001; NNT=220), intracranial hemorrhage (52% reduction; P<0.0001; NNT=132), and overall mortality (10% reduction; P=0.0003; NNT=129), whereas they increased the risk of gastrointestinal bleeding (25% increase; P=0.043; NNH=185). There was no significant difference between DOACs and warfarin with respect to ischemic stroke. The low‐dose subset had similar overall results with even fewer hemorrhage strokes balancing a higher incidence of ischemic strokes in the DOAC arm than in warfarin. Other important subgroup analyses suggest the safety and efficacy impact of DOACs is significant for VKA‐naive and experienced patients, though only statistically so for VKA‐naive patients. Additionally, the anticoagulation centers included in the study that had a TTR <66% seemed to gain a safety advantage from the DOACs, whereas both TTR groups (<66% and 66%) appeared to achieve an efficacy benefit from DOACs.
Cautions
There are not sufficient data to suggest routinely switching patients tolerating and well managed on VKAs to DOACs for AF.
Implications
DOACs reduce stroke and systemic emboli in patients with AF without increasing intracranial bleeding or hemorrhagic stroke, though at the cost of increased gastrointestinal bleeding in patients on the high‐dose regimens. Those patients on the low‐dose regimens have even a lower hemorrhagic stroke risk, the benefit of which is negated by a higher than VKA risk of ischemic strokes. Centers with lower TTRs (and perhaps by extrapolation, those patients with more difficulty staying in the therapeutic range) may gain more benefit by switching. New patients on treatment for AF should strongly be considered for DOAC therapy as the first line.
IN ELDERLY PATIENTS, THE DOACs APPEAR TO OFFER IMPROVED EFFICACY WITHOUT SACRIFICING SAFETY
Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from meta‐analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857864.
Background
The prevalence of AF rises with age, as does the prevalence of malignancy, limited mobility, and other comorbidities that increase the risk for VTEs. These factors may also increase the risk of bleeding with conventional therapy with heparins and VKAs. As such, understanding the implications of using DOACs in the elderly population is important.
Findings
This meta‐analysis included the elderly (age 75 years) subset of patients from existing AF treatment and VTE treatment and prophylaxis randomized trials comparing DOACs with VKAs, low‐molecular‐weight heparin (LMWH), aspirin, or placebo. The primary safety outcome was major bleeding. For AF trials, the efficacy endpoint was stroke or systemic embolization, whereas in VTE trials it was VTE or VTE‐related death. Authors were able to extract data on 25,031 patients across 10 trials that evaluated rivaroxaban, apixaban, and dabigatran (not edoxaban), with follow‐up data ranging from 35 days to 2 years. For safety outcomes, the 2 arms showed no statistical difference (DOAC: 6.4%; conventional therapy: 6.3%; OR: 1.02; 95% CI: 0.73‐1.43). For efficacy endpoints in VTE studies, DOACs were more effective (3.7% vs 7.0%; OR: 0.45; 95% CI: 0.27‐77; NNT=30). For AF, the efficacy analysis favored DOACs also (3.3% vs 4.7%; OR: 0.65; 95% CI: 0.48‐0.87; NNT=71). When analyzed by the efficacy of the individual DOAC, rivaroxaban and apixaban both appeared to outperform the VKA/LMWH arm for both VTE and AF treatment, whereas data on dabigatran were only available for AF, also showing an efficacy benefit. Individual DOAC analyses for safety endpoints showed all the 3 to be similar to VKA/LMWH.
Cautions
Authors note, however, that coexisting low body weight and renal insufficiency may influence dosing choices in this population. There are specific dosage recommendations in the elderly for some DOACs.
Implications
The use of DOACs in patients aged 75 years and older appears to confer a substantial efficacy advantage when used for treatment of VTE and AF patients. The safety data presented in this meta‐analysis suggest that this class is comparable to VKA/LMWH medications.
CHANGING INPATIENT MANAGEMENT OF SKIN INFECTIONS
Boucher, H, Wilcox M, Talbot G, et al. Once‐weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:21692179.
Corey G, Kabler, H, Mahra P, et al. Single‐dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:21802190.
Background
There are over 870,000 hospital admissions yearly for skin infection, making it one of most common reasons for hospitalization in the United States.[18] Management often requires lengthy treatments with intravenous antibiotics, especially with the emergence of methicillin‐resistant Staphylococcus aureus. Results from 2 large randomized, double‐blinded, multicenter clinical trials were published looking at new once‐weekly intravenous antibiotics. Dalbavancin and oritavancin are both lipoglycopeptides in the same family as vancomycin. What is unique is that their serum drug concentrations exceed the minimum inhibitor concentrations for over a week. Both drugs were compared in noninferiority trials to vancomycin. The studies had similar outcomes. The dalbavancin results are presented below.
Findings
Researchers randomized 1312 patients with significant cellulitis, large abscess, or wound infection. Patients also had fever, leukocytosis, or bandemia, and the infection had to be deemed severe enough to require a minimum of 3 days of intravenous antibiotics. The patients could not have received any prior antibiotics. Over 80% of the patients had fevers, and more than half met the criteria for systemic inflammatory response syndrome. Patients were randomized to either dalbavancin (on day 1 and day 8) or vancomycin every 12 hours (1 gm or 15 mg/kg), with both groups receiving placebo dosing of the other drug. The blinded physicians could decide to switch to oral agent (placebo or linezolid in the vancomycin group) anytime after day 3, and the physicians could stop antibiotics anytime after day 10. Otherwise, all patients received 14 days of antibiotics.
The FDA‐approved outcome was cessation of spread of erythema at 48 to 72 hours and no fever at 3 independent readings. Results were similar in the dalbavancin group compared to the vancomycinlinezolid group (79.7% vs 79.8%). Dalbavancin was deemed noninferior to vancomycin. Blinded investigator's assessment of treatment success at 2 weeks was also similar (96% vs 96.7%, respectively). More treatment‐related adverse events occurred in the vancomycinlinezolid group (183 vs 139; P=0.02) and more deaths occurred in the vancomycin group (7 vs 1; P=0.03).
Cautions
These antibiotics have only been shown effective for complicated, acute bacterial skin infections. Their performance for other gram‐positive infections is unknown. In the future, it is possible that patients with severe skin infections will receive a dose of these antibiotics on hospital day 1 and be sent home with close follow‐up. However, that study has not been done yet to confirm efficacy and safety. Though the drugs appear safe, there needs to be more clinical use before they become standard of care, especially because of the long half‐life. Finally, these drugs are very expensive and provide broad spectrum gram‐positive coverage. They are not meant for a simple cellulitis.
Implications
These 2 new once‐weekly antibioticsdalbavancin and oritavancinare noninferior to vancomycin for acute bacterial skin infections. They provide alternative treatment choices for managing patients with significant infections requiring hospitalization. In the future, they may change the need for hospitalization of these patients or significantly reduce their length of stay. Though expensive, a significant reduction in hospitalization will offset costs.
SHOULD THEY STAY OR SHOULD THEY GO? FAMILY PRESENCE DURING CPR MAY IMPROVE THE GRIEF PROCESS DURABLY
Jabre P, Tazarourte K, Azoulay E, et al. Offering the opportunity for family to be present during cardiopulmonary resuscitation: 1 year assessment. Intensive Care Med. 2014;40:981987.
Background
In 2013, a French study randomized adult family members of a patient undergoing cardiopulmonary resuscitation (CPR) occurring at home to either be invited to stay and watch the resuscitation or to have no specific invitation offered.[19] At 90 days, this study revealed that those who were invited to watch (and 79% did) had fewer symptoms of post‐traumatic stress disorder (PTSD) (27% vs 37%) and anxiety (15% vs 23%), though not depression, than did the group not offered the opportunity to watch (though 43% watched anyway). There were 570 subjects (family members) in the trial, of whom a greater number in the control arm declined to participate in a 90‐day follow‐up due to emotional distress. Notably, only 4% of the patients in this study undergoing CPR survived to day 28. Whether the apparent positive psychological impact of the offer to watch CPR for families was durable remained in question.
Findings
The study group followed the families up to 1 year. At that time, dropout rates were similar (with the assumption, as in the prior study, that those who dropped out of either arm had PTSD symptoms). At follow‐up, subjects were again assessed for PTSD, anxiety, and depression symptoms as well as for meeting criteria for having had a major depressive episode or complicated grief. Four hundred eight of the original 570 subjects were able to undergo reevaluation. The 1‐year results showed the group offered the chance to watch CPR had fewer PTSD symptoms (20% vs 32%) and depression symptoms (10% vs 16%), as well as fewer major depressive episodes (23% vs 31%) and less complicated grief (21% vs 36%) but without a durable impact on anxiety symptoms.
Cautions
The resuscitation efforts in question here occurred out of hospital (in the home). Part of the protocol for those family members observing CPR was that a clinician was assigned to stay with them and explain the resuscitation process as it occurred.
Implications
It is postulated that having the chance to observe CPR, if desired, may help the grieving process. This study clearly raises a question about the wisdom of routinely escorting patient's families out of the room during resuscitative efforts. It seems likely that the durable and important psychological effects observed in this study for family members would similarly persist in emergency department and inpatient settings, where staff can be with patients' families to talk them through the events they are witnessing. It is time to ask families if they prefer to stay and watch CPR and not automatically move them to a waiting room.
Disclosure: Nothing to report.
Keeping up with the medical literature in a field as broad as hospital medicine is a daunting task. In 2014 alone, there were over 9200 articles published in top‐tier internal medicine journals.[1] The authors have selected articles from among these top journals using a nonsystematic process that involved reviewing articles brought to their attention via colleagues, literature searches, and online services. The focus was to identify articles that would be of importance to the field of hospital medicine for their potential to be practice changing, provocative, or iconoclastic. After culling through hundreds of titles and abstracts, 46 articles were reviewed by both authors in full text, and ultimately 14 were selected for presentation here. Table 1 summarizes the key points.
|
| 1. Now that neprolysin inhibitors are approved by the FDA, hospitalists will see them prescribed as an alternative to ACE‐inhibitors given their impressive benefits in cardiovascular mortality and heart failure hospitalizations. |
| 2. Current evidence suggests that intravenous contrast given with CT scans may not significantly alter the incidence of acute kidney injury, its associated mortality, or the need for hemodialysis. |
| 3. The CAM‐S score is an important tool for prognostication in delirious patients. Those patients with high CAM‐S scores should be considered for goals of care conversations. |
| 4. The melatonin agonist, ramelteon, shows promise for lowering incident delirium among elderly medical patients, though larger trials are still needed. |
| 5. Polyethylene glycol may be an excellent alternative to lactulose for patients with acute hepatic encephalopathy once larger studies are done, as it is well tolerated and shows faster resolution of symptoms. |
| 6. Nonselective ‐blockers should no longer be offered to cirrhotic patients after they develop spontaneous bacterial peritonitis, as they are associated with increased mortality and acute kidney injury. |
| 7. Current guidelines regarding prophylaxis against VTE in medical inpatients likely result in nonbeneficial use of medications for this purpose. It remains unclear which high‐risk populations do benefit from pharmacologic prophylaxis. |
| 8. DOACs are as effective and are safer than conventional therapy for treatment of VTE, though they are not recommended in patients with GFR <30 mL/min. |
| 9. DOACs are more effective and are safer (though they may increase risk of gastrointestinal bleeding) than conventional therapy in patients with AF. |
| 10. DOACs are as safe and more effective than conventional therapy in elderly patients with VTE or AF, being mindful of dosing recommendations in this population. |
| 11. Two new once‐weekly antibiotics, dalbavancin and oritavancin, approved for skin and soft tissue infections, appear noninferior to vancomycin and have the potential to shorten hospitalizations and, in doing so, may decrease cost. |
| 12. Offering family members of a patient undergoing CPR the opportunity to observe has durable impact on meaningful short‐ and long‐term psychological outcomes. Clinicians should strongly consider making this offer. |
AN APPROACHING PARADIGM SHIFT IN THE TREATMENT FOR HEART FAILURE
McMurray J, Packer M, Desai A, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:9931004.
Background
The last drug approved by the Food and Drug Administration (FDA) for heart failure (HF) was 10 years ago.[2] The new PARADIGM (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) heart failure study comparing a novel combination drug of a neprilysin inhibitor and angiotensin receptor blocker (ARB) to an angiotensin‐converting enzyme (ACE) inhibitor has cardiologists considering a possible change in the HF treatment algorithm. Neprilysin is a naturally occurring enzyme that breaks down the protective vasoactive peptides (brain natriuretic peptide, atrial natriuretic peptide, and bradykinin) made by the heart and the body in HF. These vasoactive peptides function to increase vasodilation and block sodium and water reabsorption. This novel neprilysin inhibitor extends the life of these vasoactive peptides, thus enhancing their effect. By inhibiting both neprilysin and the renin‐angiotensin system, there should be additional improvement in HF management. The neprilysin inhibitor was combined with an ARB instead of an ACE inhibitor because of significant angioedema seen in earlier phase trials when combined with an ACE inhibitor. This is believed related to increases in bradykinin due to both agents.
Findings
In this multicenter, blinded, randomized trial, over 10,000 patients with known HF (ejection fraction<35%, New York Heart Association class II or higher) went through 2 run‐in periods to ensure tolerance of both enalapril and the study drug, a combination of a neprilysin inhibitor and valsartan (neprilysin‐I/ARB). Eventually 8442 patients underwent randomization to either enalapril (10 mg twice a day) or neprilysin‐I/ARB (200 mg twice a day). The primary outcome was a combination of cardiovascular mortality and heart failure hospitalizations. The trial was stopped early at 27 months because of overwhelming benefit with neprilysin‐I/ARB (21.8% vs 26.5%; P<0.001). There was a 20% reduction specifically in cardiovascular mortality (13.3% vs 16.5%; hazard ratio [HR]: 0.80; P<0.001). The number needed to treat (NNT) was 32. There was also a 21% reduction in the risk of hospitalization (P<0.001). More patients with neprilysin‐I/ARB had symptomatic hypotension (14% vs 9.2%; P<0.001) but patients on the ACE inhibitor experienced more cough, hyperkalemia, and increases in their serum creatinine.
Cautions
There are 2 reasons clinicians may not see the same results in practice. First, the trial was stopped early, which can sometimes exaggerate benefits.[3] Second, the 2 run‐in periods eliminated patients who could not tolerate the medications at the trial doses. Additionally, although the study's authors were independent, the trial was funded by a pharmaceutical company.
Implications
This new combination drug of a neprilysin inhibitor and valsartan shows great promise at reducing cardiovascular mortality and hospitalizations for heart failure compared to enalapril alone. Given the high morbidity and mortality of heart failure, having a new agent in the treatment algorithm will be useful to patients and physicians. The drug was just approved by the FDA in July 2015 and will likely be offered as an alternative to ACE inhibitors.
VENOUS CONTRAST‐INDUCED NEPHROTOXICITY: IS THERE REALLY A RISK?
McDonald J, McDonald R, Carter R, et al. Risk of intravenous contrast material‐mediated acute kidney injury: a propensity score‐matched study stratified by baseline‐estimated glomerular filtration rate. Radiology. 2014;271(1):6573.
McDonald R, McDonald J, Carter R, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014;273(3):714725.
Background
It is a common practice to withhold intravenous contrast material from computed tomography (CT) scans in patients with even moderately poor renal function out of concern for causing contrast‐induced nephropathy (CIN). Our understanding of CIN is based largely on observational studies and outcomes of cardiac catheterizations, where larger amounts of contrast are given intra‐arterially into an atherosclerotic aorta.[4] The exact mechanism of injury is not clear, possibly from direct tubule toxicity or renal vasoconstriction.[5] CIN is defined as a rise in creatinine >0.5 mg/dL or >25% rise in serum creatinine 24 to 48 hours after receiving intravenous contrast. Although it is usually self‐limited, there is concern that patients who develop CIN have an increase risk of dialysis and death.[6] In the last few years, radiologists have started to question whether the risk of CIN is overstated. A recent meta‐analysis of 13 studies demonstrated a similar likelihood of acute kidney injury in patients regardless of receiving intravenous contrast.[7] If the true incidence of CIN after venous contrast is actually lower, this raises the question of whether we are unnecessarily withholding contrast from CTs and thereby reducing their diagnostic accuracy. Two 2014 observational studies provide additional evidence that the concern for CIN may be overstated.
Findings
The 2 Mayo Clinic studies used the same database. They looked at all patients who underwent a contrast‐enhanced or unenhanced thoracic, abdominal, or pelvic CT between January 2000 and December 2010 at the Mayo Clinic. After limiting the data to patients with pre‐ and post‐CT creatinine measurements and excluding anyone on dialysis, with preexisting acute kidney injury, or who had received additional contrast within 14 days, they ended up with 41,229 patients, mostly inpatients. All of the patients were assigned propensity scores based on risk factors for the development of CIN and whether they would likely receive contrast. The patients were then subdivided into 4 renal function subgroups based on estimated glomerular filtration rate (eGFR). The patients who received contrast were matched based on their propensity scores to those who did not received contrast within their eGFR subgroups. Unmatched patients were eliminated, leaving a cohort of 12,508 matched patients. The outcome of the first article was acute kidney injury (AKI) defined as a rise in creatinine >0.5 mg/dL at 24 to 48 hours. Though AKI rose with worsening eGFR subgroups (eGFR > 90 [1.2%] vs eGFR < 30 [14%]), the rates of AKI were the same regardless of contrast exposure. There was no statistical difference in any of the eGFR subgroups. The second study looked at important clinical outcomesdeath and the need for dialysis. There was no statistical difference for emergent dialysis (odds ratio [OR]: 0.96, P=0.89) or 30‐day mortality (HR: 0.97; P=0.45) regardless of whether the patients received contrast or not.
Cautions
In propensity matching, unmeasured confounders can bias the results. However, the issue of whether venous contrast causes CIN will unlikely be settled in a randomized controlled trial. For patients with severe renal failure (eGFR < 30), there were far fewer patients in this subgroup, making it harder to draw conclusions. The amount of venous contrast given was not provided. Finally, this study evaluated intravenous contrast for CTs, not intra‐arterial contrast.
Implications
These 2 studies raise doubt as to whether the incidence of AKI after contrast‐enhanced CT can be attributed to the contrast itself. What exactly causes the rise in creatinine is probably multifactorial including lab variation, hydration, blood pressure changes, nephrotoxic drugs, and comorbid disease. In trying to decide whether to obtain a contrast‐enhanced CT for patients with chronic kidney dysfunction, these studies provide more evidence to consider in the decision‐making process. A conversation with the radiologist about the benefits gained from using contrast in an individual patient may be of value.
PREVENTION AND PROGNOSIS OF INPATIENT DELIRIUM
Hatta K, Yasuhiro K, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo controlled trial. JAMA Psych. 2014;71(4):397403.
A new melatonin agonist dramatically improves delirium incidence.
Background
Numerous medications and therapeutic approaches have been studied to prevent incident delirium in hospitalized medical and surgical patients with varying success. Many of the tested medications also have the potential for significant undesirable side effects. An earlier small trial of melatonin appeared to have impressive efficacy for this purpose and be well tolerated, but the substance is not regulated by the FDA.[8] Ramelteon, a melatonin receptor agonist, is approved by the FDA for insomnia, and authors hypothesized that it, too, may be effective in delirium prevention.
Findings
This study was a multicenter, single‐blinded, randomized controlled trial of the melatonin‐agonist ramelteon versus placebo in elderly patients admitted to the hospital ward or ICU with serious medical conditions. Researchers excluded intubated patients or those with Lewy body dementia, psychiatric disorders, and severe liver disease. Patients received either ramelteon or placebo nightly for up to a week, and the primary end point was incident delirium as determined by a blinded observer using a validated assessment tool. Sixty‐seven patients were enrolled. The baseline characteristics in the arms of the trial were similar. In the placebo arm, 11 of 34 patients (32%) developed delirium during the 7‐day observation period. In the ramelteon arm, 1 of 33 (3%) developed delirium (P=0.003). The rate of drug discontinuation was the same in each arm.
Cautions
This study is small, and the single‐blinded design (the physicians and patients knew which group they were in but the observers did not) limits the validity of these results, mandating a larger double‐blinded trial.
Implications
Ramelteon showed a dramatic impact on preventing incident delirium on elderly hospitalized patients with serious medical conditions admitted to the ward or intensive care unit (ICU) (nonintubated) in this small study. If larger trials concur with the impact of this well‐tolerated and inexpensive medication, the potential for delirium incidence reduction could have a dramatic impact on how care for delirium‐vulnerable patients is conducted as well as the systems‐level costs associated with delirium care. Further studies of this class of medications are needed to more definitively establish its value in delirium prevention.
THE CONFUSION ASSESSMENT METHOD SEVERITY SCORE CAN QUANTIFY PROGNOSIS FOR DELIRIOUS MEDICAL INPATIENTS
Innoye SK, Kosar CM, Tommet D, et al. The CAM‐S: development and validation of a new scoring system for delirium in 2 cohorts. Ann Intern Med. 2014;160:526533.
Background
Delirium is common in hospitalized elderly patients, and numerous studies show that there are both short‐ and long‐term implications of developing delirium. Using well studied and validated tools has made identifying delirium fairly straightforward, yet its treatment remains difficult. Additionally, differentiating which patients will have a simpler clinical course from those at risk for a more morbid one has proved challenging. Using the Confusion Assessment Method (CAM), both in its short (4‐item) and long (10‐item) forms, as the basis for a prognostication tool, would allow for future research on treatment to have a scale against which to measure impact, and would allow clinicians to anticipate which patients were more likely to have difficult clinical courses.
Findings
The CAM Severity (CAM‐S) score was derived in 1219 subjects participating in 2 ongoing studies: 1 included high‐risk medical inpatients 70 years old or older, and the other included similarly aged patients undergoing major orthopedic, general, or vascular surgeries. Outcomes data were not available for the surgical patients. The CAM items were rated as either present/absent or absent/mild/severe, depending on the item, with an associated score attached to each item such that the 4‐item CAM had a score of 0 to 7 and the 10‐item CAM 0 to 19 (Table 2). Clinical outcomes from the medical patients cohort showed a dose response with increasing CAM‐S scores with respect to length of stay, adjusted cost, combined 90‐day end points of skilled nursing facility placement or death, and 90‐day mortality. Specifically, for patients with a CAM‐S (short form) score of 5 to 7, the 90‐day rate of death or nursing home residence was 62%, whereas the 90‐day postdischarge mortality rate was 36%.
| The CAM | The CAM‐S | |
|---|---|---|
| ||
| Acute onset with fluctuating course | Absent | 0 |
| Present | 1 | |
| Inattention or distractability | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Disorganized thinking, illogical or unclear ideas | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Alteration of consciousness | Absent | 0 |
| Mild | 0 | |
| Severe | 2 | |
| Total | 07 | |
Cautions
The CAM‐S, like the CAM, may work less well in patients with hypoactive delirium. This scale has been applied in a surgical cohort, but study outcomes were not presented in this article. This absence limits our ability to apply these results to a surgical population presently.
Implications
This study demonstrates that in medical inpatients, the CAM‐S is effective for prognostication. Moreover, the study points out that high‐scoring patients on the CAM‐S have quite poor prognoses, with more than one‐third dying by 3 months. This finding suggests that an important use of the CAM‐S is to identify patients about whom goals of care discussions should be held and end‐of‐life planning initiated if not previously done.
GET EXCITED ABOUT HEPATIC ENCEPHALOPATHY AGAINA NEW POSSIBLE TREATMENT
Rahimi R, Singal A, Cuthbert J, et al. Lactulose vs polyethylene glycol 3350‐electrolyte solution for treatment of overt hepatic encephalopathy. The HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):17271733.
Background
Lactulose has been the principle treatment for acute hepatic encephalopathy (HE) since 1966.[9] It theoretically works by lowering the pH of the colon and trapping ammonia as ammonium, which is then expelled. Alternatively, it may simply decrease transit time through the colon. In fact, earlier treatments for HE were cathartics such as magnesium salts. Unfortunately 20% tp 30% of patients are poor responders to lactulose, and patients do not like it. This new study tests whether a modern‐day cathartic, polyethylene glycol, works as well as lactulose.
Findings
In this unblinded, randomized controlled trial, patients presenting to the emergency department with acute HE were assigned to either lactulose 20 to 30 g for a minimum of 3 doses over 24 hours or 4 L of polyethylene glycol (PEG) over 4 hours. The2 groups were similar in severity and etiology of liver disease. Patients were allowed to have received 1 dose of lactulose given in the emergency department prior to study enrollment. They were excluded if taking rifaximin. The primary outcome was improvement in the hepatic encephalopathy scoring algorithm (HESA) by 1 grade at 24 hours.[10] The algorithm scores HE from 0 (no clinical findings of HE) to 5 (comatose). Initial mean HESA scores in the 2 groups were identical (2.3).
In the lactulose group, 13/25 (52%) improved by at least 1 HESA score at 24 hours. Two patients (8%) completely cleared with a HESA score of 0. In comparison, 21/23 (91%) in the PEG group improved at 24 hours, and 10/23 (43%) had cleared with a HESA score of 0 (P<0.01). The median time to HE resolution was 2 days in the lactulose group compared with 1 day in the PEG group (P=0.01). There were no differences in serious adverse events. The majority (76%) of the PEG group received the full 4 L of PEG.
Cautions
The main limitations of the trial were the small sample size, that it was a single‐center study, and the fact it was unblinded. Additionally, 80% of the PEG group received 1 dose of lactulose prior to enrollment. Statistically, more patients in the PEG group developed hypokalemia, which can worsen HE. Therefore, if PEG is used for acute HE, potassium will need to be monitored.
Implications
The results are intriguing and may represent a new possible treatment for acute HE once larger studies are done. Interestingly, the ammonia level dropped further in the lactulose group than the PEG group, yet there was more cognitive improvement in the PEG group. This raises questions about the role of ammonia and catharsis in HE. Although lactulose and rifaximin continue to be the standard of care, cathartics may be returning as a viable alternative.
SHOULD ‐BLOCKERS BE STOPPED IN PATIENTS WITH CIRRHOSIS WHEN SPONTANEOUS BACTERIAL PERITONITIS OCCURS?
Mandorfer M, Bota S, Schwabi P, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:16801690.
Background
Nonselective ‐blockers (NSBBs) are considered the aspirin of hepatologists, as they are used for primary and secondary prevention of variceal bleeds in patients with cirrhosis.[11] Since the 1980s, their benefit in reducing bleeding risk has been known, and more recently there has been evidence that they may reduce the risk of developing ascites in patients with compensated cirrhosis. Yet, there has been some contradictory evidence suggesting reduced survival in patients with decompensated cirrhosis and infections on NSBBs. This has led to the window hypothesis of NSBBs in cirrhosis, where NSBBs are beneficial only during a certain window period during the progression of cirrhosis.[12] Early on in cirrhosis, before the development of varices or ascites, NSBBs have no benefit. As cirrhosis progresses and portal hypertension develops, NSBBs play a major role in reducing bleeding from varices. However, in advanced cirrhosis, NSBBs may become harmful. In theory, they block the body's attempt to increase cardiac output during situations of increased physiologic stress, resulting in decreased mean arterial pressure and perfusion. This, in turn, causes end‐organ damage and increased risk of death. When exactly this NSBB window closes is unclear. A 2014 study suggests the window should close when patients develop spontaneous bacterial peritonitis (SBP).
Findings
This retrospective study followed 607 consecutive patients seen at a liver transplant center in Vienna, Austria, from 2006 to 2011. All of the patients were followed from the time of their first paracentesis. They were excluded if SBP was diagnosed during the first paracentesis. Patients were grouped based on whether they took an NSBB. As expected, more patients on an NSBB had varices (90% vs 62%; P<0.001) and a lower mean heart rate (77.5 vs 83.9 beats/minute; P<0.001). However, the 2 groups were similar in mean arterial pressure, systolic blood pressure, Model for End‐Stage Liver Disease score (17.5), Childs Pugh Score (CPS) (50% were C), and in the etiology of cirrhosis (55% were from alcoholic liver disease). They followed the patients for development of SBP. The primary outcome was transplant‐free survival. For the patients who never developed SBP, there was a 25% reduction in the risk of death for those on an NSBB adjusted for varices and CPS stage (HR=0.75, P=0.027). However, for the 182 patients who developed SBP, those on an NSBB had a 58% increase risk of death, again adjusted for varices and CPS stage (HR=1.58, P=0.014). Among the patients who developed SBP, there was a higher risk of hepatorenal syndrome (HRS) within 90 days for those on an NSBB (24% vs 11%, P=0.027). Although the mean arterial pressures (MAP) had been similar in the 2 groups before SBP, after the development of SBP, those on an NSBB had a significantly lower MAP (77.2 vs 82.6 mm Hg, P=0.005).
Cautions
This is a retrospective study, and although the authors controlled for varices and CPS, it is still possible the 2 groups were not similar. Whether patients were actually taking the NSBB is unknown, and doses of the NSBB were variable.
Implications
This study provides more evidence for the NSBB window hypothesis in the treatment of patients with cirrhosis. It suggests that the window on NSBB closes when patients develop SBP, as NSBBs appear to increase mortality and the risk of HRS. Thus, NSBB therapy should probably be discontinued in cirrhotic patients developing SBP. The question is for how long? The editorial accompanying the article says permanently.[13]
VTE PROPHYLAXIS FOR MEDICAL INPATIENTS: IS IT A THING OF THE PAST?
Flanders SA, Greene T, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism. A cohort study. JAMA Intern Med. 2014;174(10):15771584.
Background
Based on early research studies, many quality and regulatory organizations have stressed the importance of assessing hospitalized patients' venous thromboembolism (VTE) risk and prophylaxing those patients at increased risk either pharmacologically or mechanically. In 2011, a meta‐analysis of 40 studies of medical and stroke patients including approximately 52,000 patients failed to demonstrate a mortality benefit, showing that for every 3 pulmonary embolisms (PEs) prevented, it caused 4 major bleeding episodes per 1000 patients.[14] A second study in 2011, a multicenter, randomized controlled trial with medically complex patients deemed high risk for VTE, also failed to demonstrate a mortality benefit.[15] Despite these and other trials showing questionable benefit, guidelines continue to recommend that high‐risk medical patients should get pharmacologic prophylaxis against VTE.
Findings
This retrospective cohort trial retrospectively evaluated a cohort of 20,794 medical patients (non‐ICU) across 35 hospitals, excluding those with a Caprini score of <2 (ie, low risk for VTE). The authors divided the hospitals into tertiles based on adherence to VTE prophylaxis guidelines. Patients were followed to 90 days after hospitalization with telephone calls (reaching 56%) and chart reviews (100% reviewed) to identify clinically evident VTE events, excluding those that occurred within the first 3 days of index hospitalization. The study identified no statistically significant differences among the tertiles in terms of VTE rates, either in the hospital or at 90 days, though the overall VTE event rate was low. Interestingly, 85% of events took place postdischarge. Subgroup analyses also failed to identify a population of medical patients who benefited from prophylaxis.
Cautions
Debate about whether the Caprini risk score is the best available VTE risk scoring system exists. This study also excluded surgical and ICU patients.
Implications
This trial adds to the mounting literature suggesting that current guidelines‐based pharmacologic VTE prophylaxis for medical patients may offer no clear benefit in terms of incident VTE events or mortality. Although it is not yet time to abandon VTE prophylaxis completely, this study does raise the important question of whether it is time to revisit the quality guidelines and regulatory standards around VTE prophylaxis in medical inpatients. It also highlights the difficulty in assessing medical patients for their VTE risk. Though this study is provocative and important for its real‐world setting, further studies are required.
OUT WITH THE OLD AND IN WITH THE NEW? SHOULD DIRECT ORAL ANTICOAGULANTS BE OUR FIRST CHOICE FOR CARING FOR PATIENTS WITH VTE AND ATRIAL FIBRILLATION?
van Es N, Coppens M, Schulman S. et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):19681975.
For patients with acute VTE, direct oral anticoagulants work as well and are safer.
Background
There have been 6 large published randomized controlled trials of direct oral anticoagulants (DOACs) versus vitamin K antagonists (VKAs) in patients with acute VTE. Study sizes range from approximately 2500 to over 8000 subjects. All showed no significant difference between the arms with respect to efficacy (VTE or VTE‐related death) but had variable results with respect to major bleeding risk, a major concern given the nonreversibility of this group of medications. Additionally, subgroup analysis within these studies was challenging given sample size issues.
Findings
These 6 studies were combined in a meta‐analysis to address the DOACs' overall efficacy and safety profile, as well as looking in prespecified subgroups. The meta‐analysis included data from over 27,000 patients, evenly divided between DOACs (edoxaban, apixaban, rivaroxaban, and dabigatran) and VKAs, with the time in the therapeutic range (TTR) in the VKA arm being 64%. Overall, the primary efficacy endpoint (VTE and VTE‐related death) was similar (DOACs relative tisk [RR]=0.90; 95% confidence interval [CI]: 0.77‐1.06) but major bleeding (DOACs RR=0.61; 95% CI: 0.45‐0.83; NNT=150) and combined fatal and intracranial bleeding (DOACs RR=0.37; 95% CI: 0.27‐0.68; NNT=314) favored the DOACs. In subgroup analysis, there was no efficacy difference between the therapeutic groups in the subset specifically with DVT or with PE, or with patients weighing >100 kg, though safety data in these subsets were not evaluable. Patients with creatinine clearances of 30 to 49 mL/min demonstrated similar efficacy in both treatment arms, and the safety analysis in this subset with moderate renal impairment was better in the DOAC arm. Cancer patients achieved better efficacy with similar safety with the DOACs, whereas elderly patients achieved both better safety and efficacy with DOACs.
Cautions
As yet, there are inadequate data on patients with more advanced renal failure (creatinine clearance <30 mL/min) to advise using DOACs in that subset. Also, as there were no data comparing cancer patients with VTE that investigated DOACs versus low molecular weight heparins (the standard of care rather than warfarin since the CLOT [Comparison of Low‐molecular‐weight heparin versus Oral anticoagulant Therapy] trial[16]), the current meta‐analysis does not yet answer whether DOACs should be used in this population despite the efficacy benefit noted in the subgroup analysis.
Implications
This large meta‐analysis strongly suggests we can achieve comparable treatment efficacy from the DOACs as with VKAs, with better safety profiles in patients with acute VTE. In the subset of patients with moderate renal impairment (creatinine clearance 3049 mL/min), it appears safe and effective to choose DOACs.
IN PATIENTS WITH ATRIAL FIBRILLATION, DOACs APPEAR MORE EFFECTIVE THAN VKAs WITH COMPARABLE OR BETTER SAFETY PROFILES
Ruff CT, Guigliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet. 2014;383(9921):955962.
Background
Adding to the previously published meta‐analyses of the original phase 3 randomized trials regarding the DOACs' impact on the atrial fibrillation (AF) treatment safety and efficacy literature relative to VKAs, a 2013 trial, ENGAGE AF‐TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial FibrillationThrombolysis in Myocardial Infarction 48), with edoxaban was published and warrants inclusion to have a better opportunity to glean important subgroup information.[17]
Findings
This meta‐analysis included data on 71,683 patients, 42,411 in the DOAC arm and 29,272 in the warfarin arm, as 2 of the trials were3‐arm studies, comparing warfarin to a high dose and a low dose of the DOAC. Meta‐analyses of the 4 trials were broken down into a high‐dose subsetthe 2 high‐dose arms and the standard doses used in the other 2 trialsand a low‐dose subsetthe 2 low‐dose arms and the standard doses used in the other 2 trials. With respect to the efficacy endpoint (incident stroke or systemic embolization), the high‐dose subset analyses of the DOACs yielded a 19% reduction (P<0.0001; NNT=142) relative to the VKAs. The safety endpoint of major bleeding in this analysis identified a 14% reduction in the DOAC group that was nonsignificant (P=0.06). Within the high‐dose subset, analyses favored DOACs with respect to hemorrhagic stroke (51% reduction; P<0.0001; NNT=220), intracranial hemorrhage (52% reduction; P<0.0001; NNT=132), and overall mortality (10% reduction; P=0.0003; NNT=129), whereas they increased the risk of gastrointestinal bleeding (25% increase; P=0.043; NNH=185). There was no significant difference between DOACs and warfarin with respect to ischemic stroke. The low‐dose subset had similar overall results with even fewer hemorrhage strokes balancing a higher incidence of ischemic strokes in the DOAC arm than in warfarin. Other important subgroup analyses suggest the safety and efficacy impact of DOACs is significant for VKA‐naive and experienced patients, though only statistically so for VKA‐naive patients. Additionally, the anticoagulation centers included in the study that had a TTR <66% seemed to gain a safety advantage from the DOACs, whereas both TTR groups (<66% and 66%) appeared to achieve an efficacy benefit from DOACs.
Cautions
There are not sufficient data to suggest routinely switching patients tolerating and well managed on VKAs to DOACs for AF.
Implications
DOACs reduce stroke and systemic emboli in patients with AF without increasing intracranial bleeding or hemorrhagic stroke, though at the cost of increased gastrointestinal bleeding in patients on the high‐dose regimens. Those patients on the low‐dose regimens have even a lower hemorrhagic stroke risk, the benefit of which is negated by a higher than VKA risk of ischemic strokes. Centers with lower TTRs (and perhaps by extrapolation, those patients with more difficulty staying in the therapeutic range) may gain more benefit by switching. New patients on treatment for AF should strongly be considered for DOAC therapy as the first line.
IN ELDERLY PATIENTS, THE DOACs APPEAR TO OFFER IMPROVED EFFICACY WITHOUT SACRIFICING SAFETY
Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from meta‐analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857864.
Background
The prevalence of AF rises with age, as does the prevalence of malignancy, limited mobility, and other comorbidities that increase the risk for VTEs. These factors may also increase the risk of bleeding with conventional therapy with heparins and VKAs. As such, understanding the implications of using DOACs in the elderly population is important.
Findings
This meta‐analysis included the elderly (age 75 years) subset of patients from existing AF treatment and VTE treatment and prophylaxis randomized trials comparing DOACs with VKAs, low‐molecular‐weight heparin (LMWH), aspirin, or placebo. The primary safety outcome was major bleeding. For AF trials, the efficacy endpoint was stroke or systemic embolization, whereas in VTE trials it was VTE or VTE‐related death. Authors were able to extract data on 25,031 patients across 10 trials that evaluated rivaroxaban, apixaban, and dabigatran (not edoxaban), with follow‐up data ranging from 35 days to 2 years. For safety outcomes, the 2 arms showed no statistical difference (DOAC: 6.4%; conventional therapy: 6.3%; OR: 1.02; 95% CI: 0.73‐1.43). For efficacy endpoints in VTE studies, DOACs were more effective (3.7% vs 7.0%; OR: 0.45; 95% CI: 0.27‐77; NNT=30). For AF, the efficacy analysis favored DOACs also (3.3% vs 4.7%; OR: 0.65; 95% CI: 0.48‐0.87; NNT=71). When analyzed by the efficacy of the individual DOAC, rivaroxaban and apixaban both appeared to outperform the VKA/LMWH arm for both VTE and AF treatment, whereas data on dabigatran were only available for AF, also showing an efficacy benefit. Individual DOAC analyses for safety endpoints showed all the 3 to be similar to VKA/LMWH.
Cautions
Authors note, however, that coexisting low body weight and renal insufficiency may influence dosing choices in this population. There are specific dosage recommendations in the elderly for some DOACs.
Implications
The use of DOACs in patients aged 75 years and older appears to confer a substantial efficacy advantage when used for treatment of VTE and AF patients. The safety data presented in this meta‐analysis suggest that this class is comparable to VKA/LMWH medications.
CHANGING INPATIENT MANAGEMENT OF SKIN INFECTIONS
Boucher, H, Wilcox M, Talbot G, et al. Once‐weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:21692179.
Corey G, Kabler, H, Mahra P, et al. Single‐dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:21802190.
Background
There are over 870,000 hospital admissions yearly for skin infection, making it one of most common reasons for hospitalization in the United States.[18] Management often requires lengthy treatments with intravenous antibiotics, especially with the emergence of methicillin‐resistant Staphylococcus aureus. Results from 2 large randomized, double‐blinded, multicenter clinical trials were published looking at new once‐weekly intravenous antibiotics. Dalbavancin and oritavancin are both lipoglycopeptides in the same family as vancomycin. What is unique is that their serum drug concentrations exceed the minimum inhibitor concentrations for over a week. Both drugs were compared in noninferiority trials to vancomycin. The studies had similar outcomes. The dalbavancin results are presented below.
Findings
Researchers randomized 1312 patients with significant cellulitis, large abscess, or wound infection. Patients also had fever, leukocytosis, or bandemia, and the infection had to be deemed severe enough to require a minimum of 3 days of intravenous antibiotics. The patients could not have received any prior antibiotics. Over 80% of the patients had fevers, and more than half met the criteria for systemic inflammatory response syndrome. Patients were randomized to either dalbavancin (on day 1 and day 8) or vancomycin every 12 hours (1 gm or 15 mg/kg), with both groups receiving placebo dosing of the other drug. The blinded physicians could decide to switch to oral agent (placebo or linezolid in the vancomycin group) anytime after day 3, and the physicians could stop antibiotics anytime after day 10. Otherwise, all patients received 14 days of antibiotics.
The FDA‐approved outcome was cessation of spread of erythema at 48 to 72 hours and no fever at 3 independent readings. Results were similar in the dalbavancin group compared to the vancomycinlinezolid group (79.7% vs 79.8%). Dalbavancin was deemed noninferior to vancomycin. Blinded investigator's assessment of treatment success at 2 weeks was also similar (96% vs 96.7%, respectively). More treatment‐related adverse events occurred in the vancomycinlinezolid group (183 vs 139; P=0.02) and more deaths occurred in the vancomycin group (7 vs 1; P=0.03).
Cautions
These antibiotics have only been shown effective for complicated, acute bacterial skin infections. Their performance for other gram‐positive infections is unknown. In the future, it is possible that patients with severe skin infections will receive a dose of these antibiotics on hospital day 1 and be sent home with close follow‐up. However, that study has not been done yet to confirm efficacy and safety. Though the drugs appear safe, there needs to be more clinical use before they become standard of care, especially because of the long half‐life. Finally, these drugs are very expensive and provide broad spectrum gram‐positive coverage. They are not meant for a simple cellulitis.
Implications
These 2 new once‐weekly antibioticsdalbavancin and oritavancinare noninferior to vancomycin for acute bacterial skin infections. They provide alternative treatment choices for managing patients with significant infections requiring hospitalization. In the future, they may change the need for hospitalization of these patients or significantly reduce their length of stay. Though expensive, a significant reduction in hospitalization will offset costs.
SHOULD THEY STAY OR SHOULD THEY GO? FAMILY PRESENCE DURING CPR MAY IMPROVE THE GRIEF PROCESS DURABLY
Jabre P, Tazarourte K, Azoulay E, et al. Offering the opportunity for family to be present during cardiopulmonary resuscitation: 1 year assessment. Intensive Care Med. 2014;40:981987.
Background
In 2013, a French study randomized adult family members of a patient undergoing cardiopulmonary resuscitation (CPR) occurring at home to either be invited to stay and watch the resuscitation or to have no specific invitation offered.[19] At 90 days, this study revealed that those who were invited to watch (and 79% did) had fewer symptoms of post‐traumatic stress disorder (PTSD) (27% vs 37%) and anxiety (15% vs 23%), though not depression, than did the group not offered the opportunity to watch (though 43% watched anyway). There were 570 subjects (family members) in the trial, of whom a greater number in the control arm declined to participate in a 90‐day follow‐up due to emotional distress. Notably, only 4% of the patients in this study undergoing CPR survived to day 28. Whether the apparent positive psychological impact of the offer to watch CPR for families was durable remained in question.
Findings
The study group followed the families up to 1 year. At that time, dropout rates were similar (with the assumption, as in the prior study, that those who dropped out of either arm had PTSD symptoms). At follow‐up, subjects were again assessed for PTSD, anxiety, and depression symptoms as well as for meeting criteria for having had a major depressive episode or complicated grief. Four hundred eight of the original 570 subjects were able to undergo reevaluation. The 1‐year results showed the group offered the chance to watch CPR had fewer PTSD symptoms (20% vs 32%) and depression symptoms (10% vs 16%), as well as fewer major depressive episodes (23% vs 31%) and less complicated grief (21% vs 36%) but without a durable impact on anxiety symptoms.
Cautions
The resuscitation efforts in question here occurred out of hospital (in the home). Part of the protocol for those family members observing CPR was that a clinician was assigned to stay with them and explain the resuscitation process as it occurred.
Implications
It is postulated that having the chance to observe CPR, if desired, may help the grieving process. This study clearly raises a question about the wisdom of routinely escorting patient's families out of the room during resuscitative efforts. It seems likely that the durable and important psychological effects observed in this study for family members would similarly persist in emergency department and inpatient settings, where staff can be with patients' families to talk them through the events they are witnessing. It is time to ask families if they prefer to stay and watch CPR and not automatically move them to a waiting room.
Disclosure: Nothing to report.
- Journals in the 2014 release of the JCR. Available at: http://scientific.thomsonreuters.com/imgblast/JCRFullCovlist-2014.pdf. Accessed August 28, 2015.
- Neprilysin inhibition—a novel therapy for heart failure. N Engl J Med. 2014;371(11):1062–1064.
- , , , et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA. 2010;303(12):1180–1187.
- , Intravenous contrast medium‐induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology 2010;256(1):21–28.
- , , Pathophysiology of contrast medium‐induced nephropathy. Kidney Int. 2005;68(1):14–22.
- , Contrast‐induced acute kidney injury: short‐ and long‐term implications. Semin Nephrol. 2011;31(3):300–309.
- , , , et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta‐analysis. Radiology. 2013;267(1):119–128.
- , , , , , Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694.
- , , Lactulose in the treatment of chronic portal‐systemic encephalopathy. A double‐blind clinical trial. N Engl J Med. 1969;281(8):408–412.
- , , , et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104(6):1392–1400.
- , The changing role of beta‐blocker therapy in patients with cirrhosis. J Hepatol. 2014;60(3):643–653.
- , , , The window hypothesis: haemodynamic and non‐haemodynamic effects of beta‐blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61(7):967–969.
- , When should the beta‐blocker window in cirrhosis close? Gastroenterology. 2014;146(7):1597–1599.
- , , , Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , , , ; LIFENOX Investigators. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365(26):2463–2472.
- , , , et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153.
- , , , et al.; ENGAGE AF‐TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Pharmacology and the treatment of complicated skin and skin‐structure infections. N Engl J Med. 2014;370(23):2238–2239.
- , , , et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368(11):1008–1018.
- Journals in the 2014 release of the JCR. Available at: http://scientific.thomsonreuters.com/imgblast/JCRFullCovlist-2014.pdf. Accessed August 28, 2015.
- Neprilysin inhibition—a novel therapy for heart failure. N Engl J Med. 2014;371(11):1062–1064.
- , , , et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA. 2010;303(12):1180–1187.
- , Intravenous contrast medium‐induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology 2010;256(1):21–28.
- , , Pathophysiology of contrast medium‐induced nephropathy. Kidney Int. 2005;68(1):14–22.
- , Contrast‐induced acute kidney injury: short‐ and long‐term implications. Semin Nephrol. 2011;31(3):300–309.
- , , , et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta‐analysis. Radiology. 2013;267(1):119–128.
- , , , , , Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694.
- , , Lactulose in the treatment of chronic portal‐systemic encephalopathy. A double‐blind clinical trial. N Engl J Med. 1969;281(8):408–412.
- , , , et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104(6):1392–1400.
- , The changing role of beta‐blocker therapy in patients with cirrhosis. J Hepatol. 2014;60(3):643–653.
- , , , The window hypothesis: haemodynamic and non‐haemodynamic effects of beta‐blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61(7):967–969.
- , When should the beta‐blocker window in cirrhosis close? Gastroenterology. 2014;146(7):1597–1599.
- , , , Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , , , ; LIFENOX Investigators. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365(26):2463–2472.
- , , , et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153.
- , , , et al.; ENGAGE AF‐TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Pharmacology and the treatment of complicated skin and skin‐structure infections. N Engl J Med. 2014;370(23):2238–2239.
- , , , et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368(11):1008–1018.
Project BOOST
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).
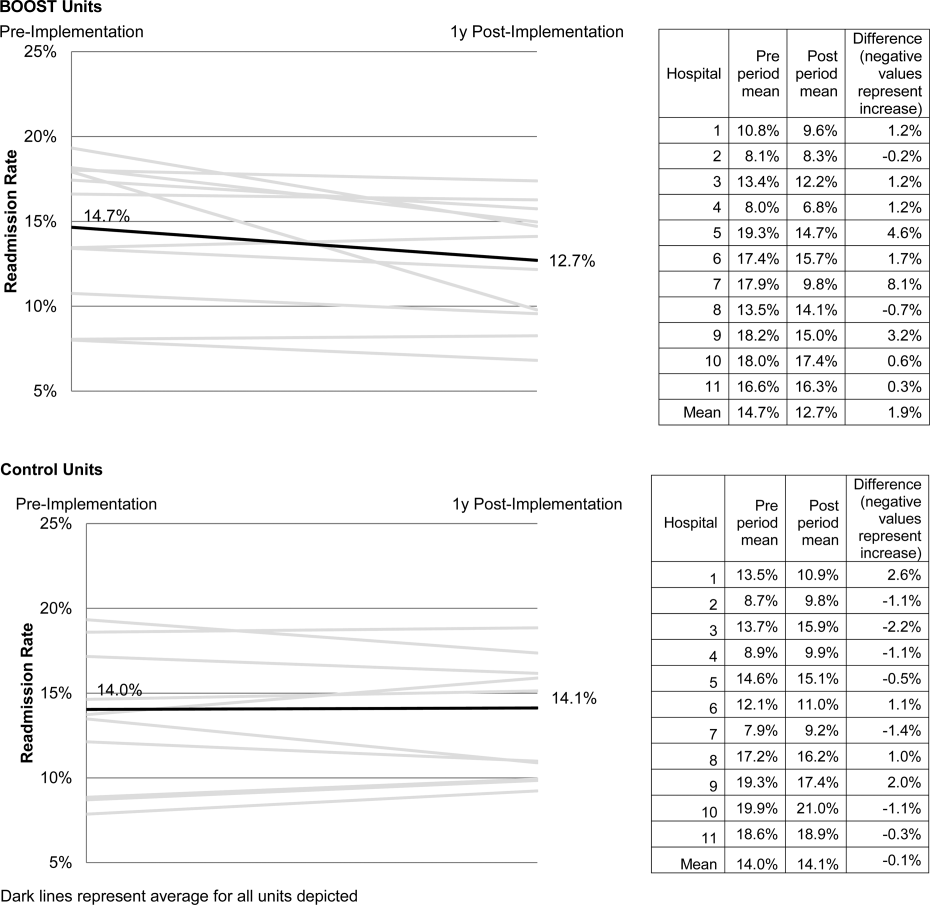
DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Copyright © 2013 Society of Hospital Medicine
Medication Reconciliation: A Consensus Statement From Stakeholders
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Post‐Discharge Inpatients With Depressive Symptoms
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).
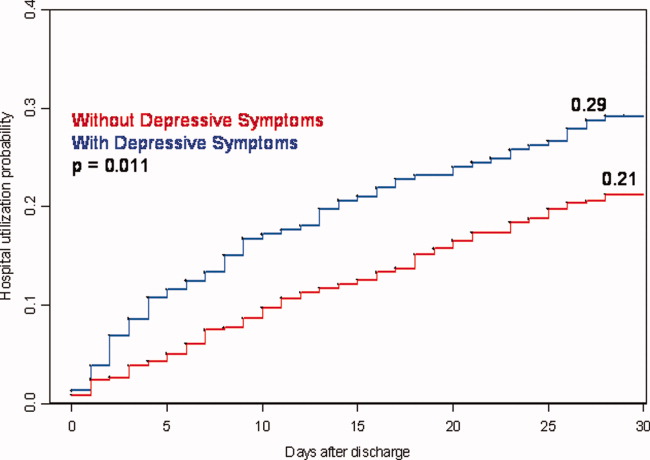
Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).

Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).

Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
Copyright © 2010 Society of Hospital Medicine
SHM Medication Reconciliation Survey Results
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | <0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | <0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | <0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | <0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | <0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | <0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | <0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | <0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | <0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
Routine Rapid HIV Testing / Greenwald
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.
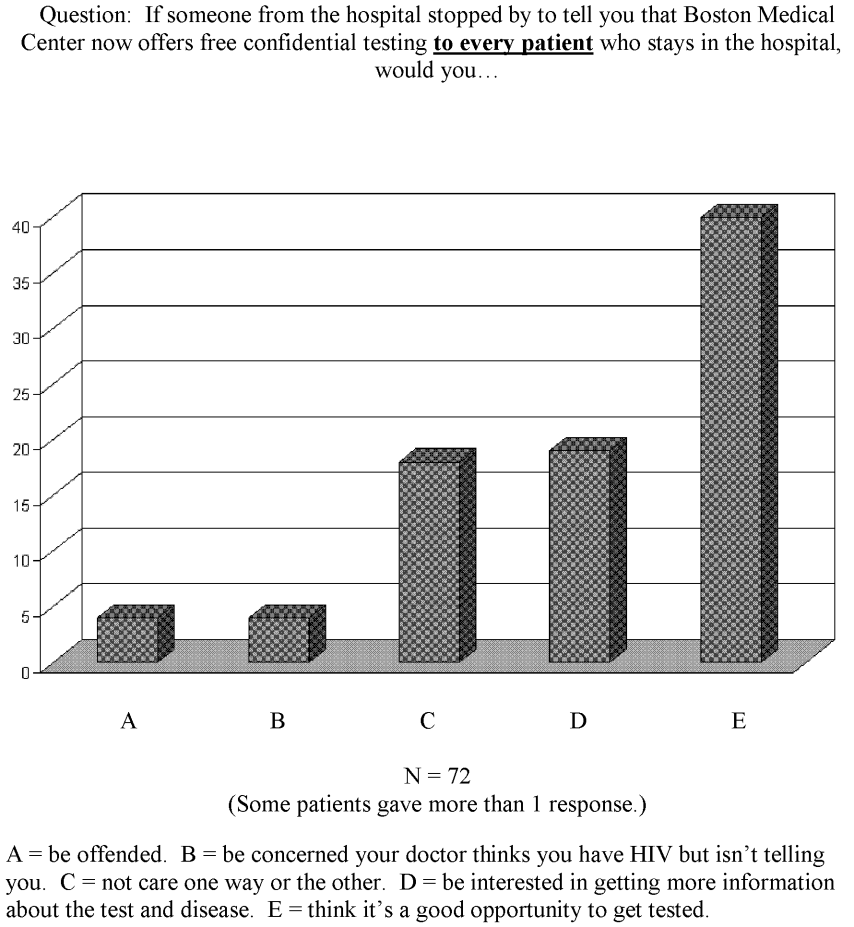
From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.