User login
Management of Volume Overload
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
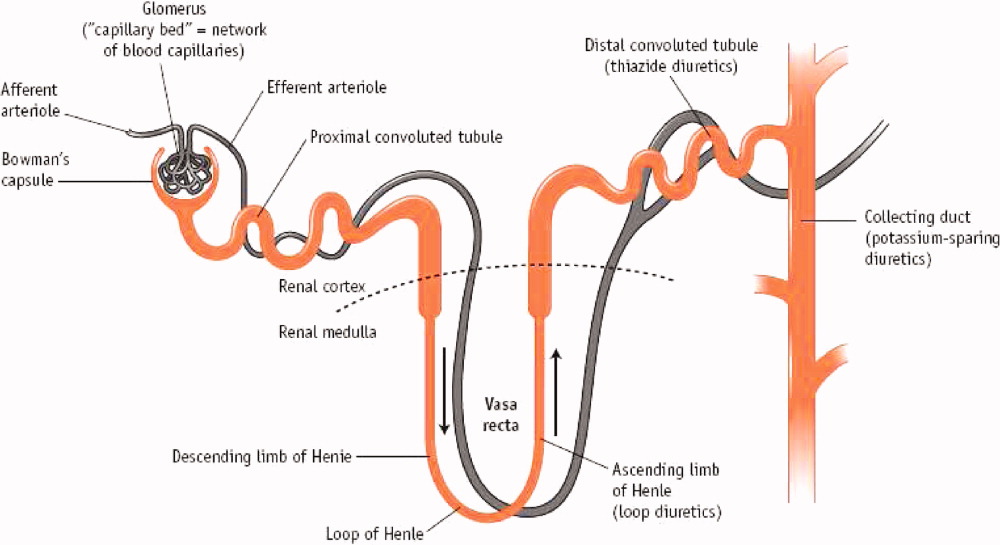
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14
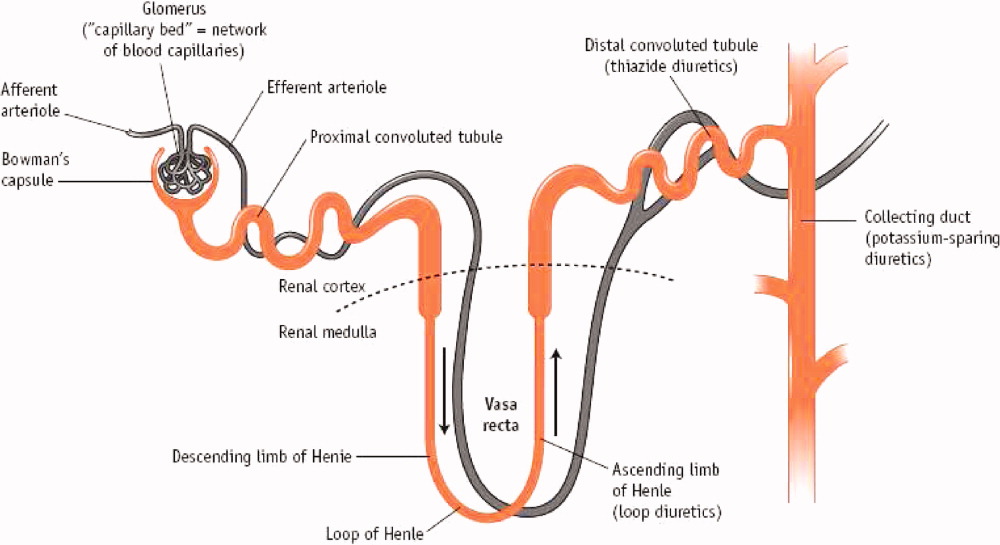
Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P < 0.001), despite increased urine output (P < 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
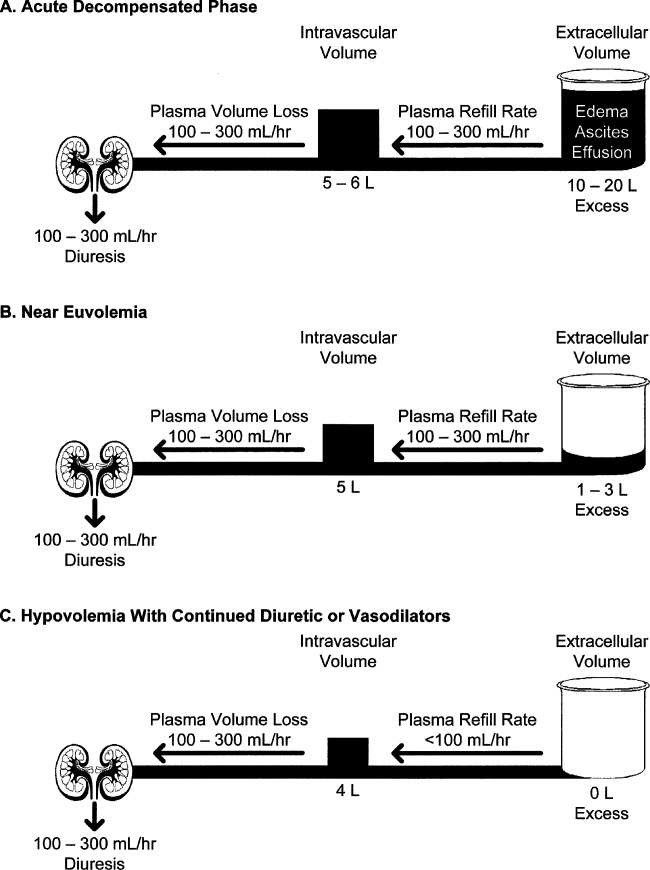
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.
INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14

Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P < 0.001), despite increased urine output (P < 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.

INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14

Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P < 0.001), despite increased urine output (P < 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.

INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
Multidisciplinary Management of ADHF
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.
A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.
An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.

A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.

An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.

A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.

An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
Bridging the Evidence/Practice Gap
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.
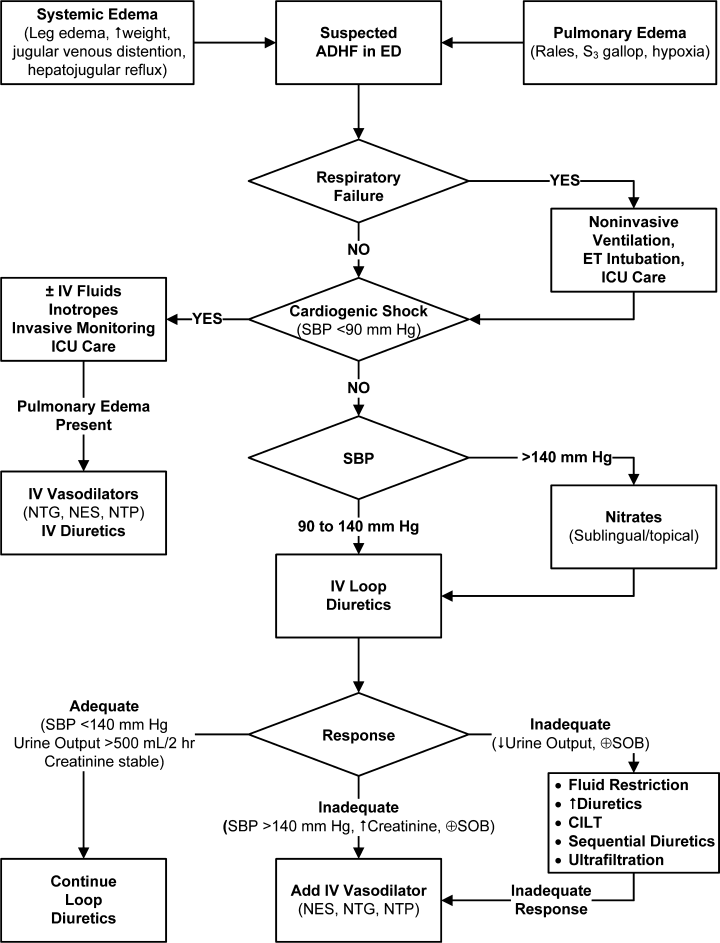
Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2
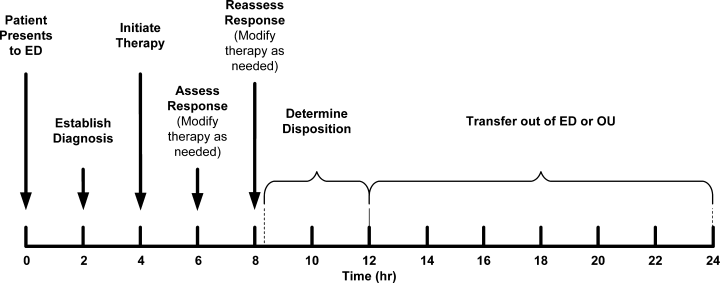
If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
Economic Outlook Not Good for Nonprofit Providers
Despite record hospital profits a year ago, Moody's Investors Service lowered the outlook for nonprofit hospitals from "stable" to "negative" in a report issued Nov. 11 in lieu of the recent downturn in the U.S. economy.
Steven Liu, MD, founder and CEO of Atlanta-based hospitalist group Ingenous Med, says the downgrade has "tremendous repercussions" for hospitalists. As hospitals start to lose revenue and look at ways to cut costs, one of the big-ticket items administrators will look at are the subsidies paid to hospitalists, which account for 40% to 50% of hospitalists' revenue, he says.
To combat arguments for decreases in subsidies, hospital medicine group (HMG) leaders need to have data supporting the value of their practice in order to show the various benefits they provide the hospital in terms of growth and quality of care.
Dr. Liu also says HMGs must maximize or protect revenue, and examine expenses carefully. Groups building or expanding must ensure that there are enough patients to care for in order to justify expenses, he says.
"Recession is a time where you focus on the quality of your organization, and it is less a time for taking on contracts and hiring physicians," he says.
Moody's provides research data and analytic tools for assessing credit risk, and publishes market-leading credit opinions. For more information, download the report at www.moodys.com.
Despite record hospital profits a year ago, Moody's Investors Service lowered the outlook for nonprofit hospitals from "stable" to "negative" in a report issued Nov. 11 in lieu of the recent downturn in the U.S. economy.
Steven Liu, MD, founder and CEO of Atlanta-based hospitalist group Ingenous Med, says the downgrade has "tremendous repercussions" for hospitalists. As hospitals start to lose revenue and look at ways to cut costs, one of the big-ticket items administrators will look at are the subsidies paid to hospitalists, which account for 40% to 50% of hospitalists' revenue, he says.
To combat arguments for decreases in subsidies, hospital medicine group (HMG) leaders need to have data supporting the value of their practice in order to show the various benefits they provide the hospital in terms of growth and quality of care.
Dr. Liu also says HMGs must maximize or protect revenue, and examine expenses carefully. Groups building or expanding must ensure that there are enough patients to care for in order to justify expenses, he says.
"Recession is a time where you focus on the quality of your organization, and it is less a time for taking on contracts and hiring physicians," he says.
Moody's provides research data and analytic tools for assessing credit risk, and publishes market-leading credit opinions. For more information, download the report at www.moodys.com.
Despite record hospital profits a year ago, Moody's Investors Service lowered the outlook for nonprofit hospitals from "stable" to "negative" in a report issued Nov. 11 in lieu of the recent downturn in the U.S. economy.
Steven Liu, MD, founder and CEO of Atlanta-based hospitalist group Ingenous Med, says the downgrade has "tremendous repercussions" for hospitalists. As hospitals start to lose revenue and look at ways to cut costs, one of the big-ticket items administrators will look at are the subsidies paid to hospitalists, which account for 40% to 50% of hospitalists' revenue, he says.
To combat arguments for decreases in subsidies, hospital medicine group (HMG) leaders need to have data supporting the value of their practice in order to show the various benefits they provide the hospital in terms of growth and quality of care.
Dr. Liu also says HMGs must maximize or protect revenue, and examine expenses carefully. Groups building or expanding must ensure that there are enough patients to care for in order to justify expenses, he says.
"Recession is a time where you focus on the quality of your organization, and it is less a time for taking on contracts and hiring physicians," he says.
Moody's provides research data and analytic tools for assessing credit risk, and publishes market-leading credit opinions. For more information, download the report at www.moodys.com.
Please, Wash Your Hands
Approximately 13 out of every 1,000 hospitalized patients are infected with Clostridium difficile (C. diff), a new study reports.
The study, by the Association for Professionals in Infection Control and Epidemiology (APIC), surveyed 12,000 patients in 648 medical facilities. Nearly 1,500 patients (12.5%) were identified with C. diff, a bacterium that causes diarrhea and more serious intestinal conditions. The occurrence rate was between 6.5 and 20 times higher than previous estimates.
Infection control is a part of hospitalist training, however, preventive efforts have been stagnant, says William Ford, MD, section chief of hospital medicine for Temple University Hospital in Philadelphia. Temple already has begun answering APIC's call for intensified infection control with a hand washing outreach protocol. Over a two-month period, hospitalists volunteered to administer five-minute PowerPoint presentations to the nursing staff during all shifts, reminding staff of the importance of washing their hands for at least 30 seconds with warm water and soap, and using friction when doing so.
The hospital has implemented a poster campaign with photos and step-by-step instructions regarding the proper hand-washing technique.
Although some disagree, hand washing has been shown to decrease the transmission of nosocomial infections, Dr. Ford says. "Hand washing," he says, "is the first line of defense for C. diff infections."
The study will be published in the January issue of American Journal of Infection Control.
Approximately 13 out of every 1,000 hospitalized patients are infected with Clostridium difficile (C. diff), a new study reports.
The study, by the Association for Professionals in Infection Control and Epidemiology (APIC), surveyed 12,000 patients in 648 medical facilities. Nearly 1,500 patients (12.5%) were identified with C. diff, a bacterium that causes diarrhea and more serious intestinal conditions. The occurrence rate was between 6.5 and 20 times higher than previous estimates.
Infection control is a part of hospitalist training, however, preventive efforts have been stagnant, says William Ford, MD, section chief of hospital medicine for Temple University Hospital in Philadelphia. Temple already has begun answering APIC's call for intensified infection control with a hand washing outreach protocol. Over a two-month period, hospitalists volunteered to administer five-minute PowerPoint presentations to the nursing staff during all shifts, reminding staff of the importance of washing their hands for at least 30 seconds with warm water and soap, and using friction when doing so.
The hospital has implemented a poster campaign with photos and step-by-step instructions regarding the proper hand-washing technique.
Although some disagree, hand washing has been shown to decrease the transmission of nosocomial infections, Dr. Ford says. "Hand washing," he says, "is the first line of defense for C. diff infections."
The study will be published in the January issue of American Journal of Infection Control.
Approximately 13 out of every 1,000 hospitalized patients are infected with Clostridium difficile (C. diff), a new study reports.
The study, by the Association for Professionals in Infection Control and Epidemiology (APIC), surveyed 12,000 patients in 648 medical facilities. Nearly 1,500 patients (12.5%) were identified with C. diff, a bacterium that causes diarrhea and more serious intestinal conditions. The occurrence rate was between 6.5 and 20 times higher than previous estimates.
Infection control is a part of hospitalist training, however, preventive efforts have been stagnant, says William Ford, MD, section chief of hospital medicine for Temple University Hospital in Philadelphia. Temple already has begun answering APIC's call for intensified infection control with a hand washing outreach protocol. Over a two-month period, hospitalists volunteered to administer five-minute PowerPoint presentations to the nursing staff during all shifts, reminding staff of the importance of washing their hands for at least 30 seconds with warm water and soap, and using friction when doing so.
The hospital has implemented a poster campaign with photos and step-by-step instructions regarding the proper hand-washing technique.
Although some disagree, hand washing has been shown to decrease the transmission of nosocomial infections, Dr. Ford says. "Hand washing," he says, "is the first line of defense for C. diff infections."
The study will be published in the January issue of American Journal of Infection Control.
Hospitalists Improve Care, Efficiency
Better patient outcomes have been seen in hospitals that employ the hospitalist model of care, reports a new study in Human Resource Management (2008;47(4):729—755).
"This study will resonate among hospitalists as something to reinforce what their intuitions have told them," says Joe Miller, study co-author and SHM's executive advisor to the CEO. Although a lot of other studies have shown hospitalists to be more efficient, this is the first to try to understand why, he says.
The study attributes the success of the hospitalist model to a concept known as relational coordination, in which members of the healthcare team are assessed based on their coordination with other team members. In the study, performance outcomes were analyzed in more than 6,000 cases at Newton-Wellesley Hospital in Newton, Mass. between July 2001 and July 2003. On the days when the attending physician was a hospitalist, as opposed to a primary care physician, the relational coordination between the care team—meaning the strength of the communication and relationships between physicians and the other care providers—was statistically significantly higher. This translated into decreased length of hospital stay, reduced hospital costs by $655 per patient, a 41.8% reduction in the risk of patient readmittance, and a 13.2% improvement in coordination.
"Hospitals are being asked to share their performance results and it's being acknowledged that delivering their service requires coordination and cooperation among the various players." Miller says. "I think this study may stimulate more research, and it may stimulate hospital executives to examine the hospitalist program within their organization to achieve these types of results."
Better patient outcomes have been seen in hospitals that employ the hospitalist model of care, reports a new study in Human Resource Management (2008;47(4):729—755).
"This study will resonate among hospitalists as something to reinforce what their intuitions have told them," says Joe Miller, study co-author and SHM's executive advisor to the CEO. Although a lot of other studies have shown hospitalists to be more efficient, this is the first to try to understand why, he says.
The study attributes the success of the hospitalist model to a concept known as relational coordination, in which members of the healthcare team are assessed based on their coordination with other team members. In the study, performance outcomes were analyzed in more than 6,000 cases at Newton-Wellesley Hospital in Newton, Mass. between July 2001 and July 2003. On the days when the attending physician was a hospitalist, as opposed to a primary care physician, the relational coordination between the care team—meaning the strength of the communication and relationships between physicians and the other care providers—was statistically significantly higher. This translated into decreased length of hospital stay, reduced hospital costs by $655 per patient, a 41.8% reduction in the risk of patient readmittance, and a 13.2% improvement in coordination.
"Hospitals are being asked to share their performance results and it's being acknowledged that delivering their service requires coordination and cooperation among the various players." Miller says. "I think this study may stimulate more research, and it may stimulate hospital executives to examine the hospitalist program within their organization to achieve these types of results."
Better patient outcomes have been seen in hospitals that employ the hospitalist model of care, reports a new study in Human Resource Management (2008;47(4):729—755).
"This study will resonate among hospitalists as something to reinforce what their intuitions have told them," says Joe Miller, study co-author and SHM's executive advisor to the CEO. Although a lot of other studies have shown hospitalists to be more efficient, this is the first to try to understand why, he says.
The study attributes the success of the hospitalist model to a concept known as relational coordination, in which members of the healthcare team are assessed based on their coordination with other team members. In the study, performance outcomes were analyzed in more than 6,000 cases at Newton-Wellesley Hospital in Newton, Mass. between July 2001 and July 2003. On the days when the attending physician was a hospitalist, as opposed to a primary care physician, the relational coordination between the care team—meaning the strength of the communication and relationships between physicians and the other care providers—was statistically significantly higher. This translated into decreased length of hospital stay, reduced hospital costs by $655 per patient, a 41.8% reduction in the risk of patient readmittance, and a 13.2% improvement in coordination.
"Hospitals are being asked to share their performance results and it's being acknowledged that delivering their service requires coordination and cooperation among the various players." Miller says. "I think this study may stimulate more research, and it may stimulate hospital executives to examine the hospitalist program within their organization to achieve these types of results."
Physician Shortage Continues
With a new survey reporting more than 150,000 primary care doctors are expected to reduce the number of patients they see or stop practicing altogether within the next three years, hospitalist programs need to focus on their retention rates, one hospital medicine group leader says.
"In this industry, we need to do more to make sure people realize that this is a career platform, and not just a placeholder for them to move on to another job," says Adam Singer, MD, CEO and CMO of IPC: The Hospitalist Company. "We're seeing a lot of this in hospitals. A lot of people use this as a one-year job in order to get a fellowship, and so we are seeing that a large percentage of the doctors that actually come, leave in order to go on to another career."
To increase retention, HM programs need to make sure young doctors in residency are better educated about the benefits of hospital medicine, such as higher incomes and the exciting short-term, high-impact relationships that appeal to young physicians, Dr. Singer says. Additionally, HM needs to advocate more medical school slots to assist in the creation of more physicians, he says.
The survey, "The Physicians' Perspective: Medical Practice in 2008," was released Nov. 18 by The Physician's Foundation. Additional findings include:
• 76% of physicians said they are either at "full capacity" or "overextended and overworked;"
• 45% said they would retire today if they had the financial means; and
• 60% would not recommend medicine as a career to young people.
For more information on the survey, visit http://www.physiciansfoundations.org.
With a new survey reporting more than 150,000 primary care doctors are expected to reduce the number of patients they see or stop practicing altogether within the next three years, hospitalist programs need to focus on their retention rates, one hospital medicine group leader says.
"In this industry, we need to do more to make sure people realize that this is a career platform, and not just a placeholder for them to move on to another job," says Adam Singer, MD, CEO and CMO of IPC: The Hospitalist Company. "We're seeing a lot of this in hospitals. A lot of people use this as a one-year job in order to get a fellowship, and so we are seeing that a large percentage of the doctors that actually come, leave in order to go on to another career."
To increase retention, HM programs need to make sure young doctors in residency are better educated about the benefits of hospital medicine, such as higher incomes and the exciting short-term, high-impact relationships that appeal to young physicians, Dr. Singer says. Additionally, HM needs to advocate more medical school slots to assist in the creation of more physicians, he says.
The survey, "The Physicians' Perspective: Medical Practice in 2008," was released Nov. 18 by The Physician's Foundation. Additional findings include:
• 76% of physicians said they are either at "full capacity" or "overextended and overworked;"
• 45% said they would retire today if they had the financial means; and
• 60% would not recommend medicine as a career to young people.
For more information on the survey, visit http://www.physiciansfoundations.org.
With a new survey reporting more than 150,000 primary care doctors are expected to reduce the number of patients they see or stop practicing altogether within the next three years, hospitalist programs need to focus on their retention rates, one hospital medicine group leader says.
"In this industry, we need to do more to make sure people realize that this is a career platform, and not just a placeholder for them to move on to another job," says Adam Singer, MD, CEO and CMO of IPC: The Hospitalist Company. "We're seeing a lot of this in hospitals. A lot of people use this as a one-year job in order to get a fellowship, and so we are seeing that a large percentage of the doctors that actually come, leave in order to go on to another career."
To increase retention, HM programs need to make sure young doctors in residency are better educated about the benefits of hospital medicine, such as higher incomes and the exciting short-term, high-impact relationships that appeal to young physicians, Dr. Singer says. Additionally, HM needs to advocate more medical school slots to assist in the creation of more physicians, he says.
The survey, "The Physicians' Perspective: Medical Practice in 2008," was released Nov. 18 by The Physician's Foundation. Additional findings include:
• 76% of physicians said they are either at "full capacity" or "overextended and overworked;"
• 45% said they would retire today if they had the financial means; and
• 60% would not recommend medicine as a career to young people.
For more information on the survey, visit http://www.physiciansfoundations.org.
The latest research you need to know
Literature at a Glance
- Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
- Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality.
- Targeting normal glycated hemoglobin levels with a gliclazide-based regimen does not have an effect on preventing major macrovascular events.
- Non-invasive ventilation has no effect on short-term mortality or rates of tracheal intubation and admission to ICU
- Routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes.
- No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
- Initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing.
- High BNP or NT-pro-BNP levels can differentiate patients who are at a higher risk of complicated hospital course and short-term mortality.
- Use of a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Tight Glucose Control in the Intensive Care Unit (ICU) Setting Does Not Reduce Short-Term Mortality
Clinical question: Does tight glucose control for critically ill patients affect mortality?
Background: Intensive glucose control for adult ICU patients has been advocated by numerous professional societies and adopted worldwide as a means to reduce mortality of critically ill patients. Evidence from multiple randomized controlled trials of tight glucose control in the ICU setting, however, shows mixed results.
Study Design: Meta-analysis of randomized controlled trials.
Setting: 29 studies involving 8,432 critically ill patients.
Synopsis: This study evaluated 29 trials involving critically ill adult patients randomized to tight glucose control versus usual care. Comparing these patients, there was no significant difference in short-term mortality (<30 days). Stratification of trials by level of glucose control (very tight <110 mg/dL versus moderately tight <150 mg/dL) and by ICU setting (surgical, medical, or mixed medical-surgical) did not affect mortality.
Tight glucose control was associated with a reduced risk of septicemia, but only in surgical patients. There was no association between tight control and a new need for dialysis, consistent across all ICU settings, as well as with both levels of glucose control. Finally, there was an increased risk of hypoglycemia (<40 mg/dL) with tight control, higher in patients who received very tight control versus those who received moderately tight control.
Limitations of the studies evaluated in this meta-analysis include difficulties with consistently maintaining tight glucose control. Twenty one percent of the trials did not achieve a mean glucose level within 5 mg/dL of the goal. This, along with a lack of standardization in reporting glucose control, makes study comparison problematic.
Bottom Line: Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
Citation: Wiener, RS, Wiener DC, Larson, RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933-944.
Intensive Glucose-Lowering Therapy Increases Mortality in High-Risk Diabetic Patients
Clinical Question: Does intensive glucose-lowering therapy reduce cardiac events in high-risk diabetic patients?
Background: Epidemiologic studies have suggested the risk of cardiovascular disease increases with higher levels of glycated hemoglobin in patients with type-2 diabetes. No definitive data from randomized trials exist to test the effect of intensive glucose-lowering therapy on the rate of cardiovascular events in high-risk diabetic patients.
Study Design: Multicenter randomized controlled trial led by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group.
Setting: 77 clinical centers in the U.S. and Canada.
Synopsis: 10,251 diabetic patients with established cardiovascular disease or additional cardiovascular risk factors, and median glycated hemoglobin level of 8.1%, received either intensive therapy (targeting glycated hemoglobin level <6.0%) or standard therapy (targeting level from 7.0% to 7.9%). The primary outcome was a composite of non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.
Data indicated intensive therapy did decrease the rate of non-fatal myocardial infarctions, however, it did not significantly reduce the primary composite of major cardiovascular events. Moreover, intensive therapy resulted in a significant increase in death from cardiovascular causes, as well as a relative increase of 22% of death from any cause, during follow up of three and a half years. Due to this finding, the intensive therapy regimen was discontinued 17 months before the scheduled end of the study.
Analysis of the data has not identified a cause for the unexpected increased mortality in the intensive therapy group, and has not shown any medication or combination of medications to be responsible.
Bottom Line: Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality and did not significantly reduce major cardiovascular events.
Citation: Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type-2 diabetes. N Engl J Med. 2008;358:2545-2559.
Intensive Glucose Control Reduces Nephropathy but Has No Effect on Major Cardiovascular Events
Clinical Question: Does intensive glucose-lowering therapy decrease major macrovascular and microvascular events in high-risk diabetic patients?
Background: Prospective studies show a direct association between elevated glycated hemoglobin levels in diabetics and increased risk of vascular events. However, definitive evidence from randomized trials about the role of intensive glucose control in preventing vascular disease in diabetics is lacking.
Study Design: Multicenter randomized controlled trial led by the ADVANCE Collaborative Group.
Setting: 215 clinical centers in 20 countries from Asia, Australia, Europe, and North America.
Synopsis: 11,140 diabetic patients received either standard glucose therapy or intensive glucose therapy using gliclazide, as well as other drugs, to reach a targeted glycated hemoglobin of 6.5% or less. The primary outcome was a composite of major macrovascular and microvascular events, including nonfatal myocardial infarction (MI), nonfatal stroke, death from cardiovascular causes, nephropathy, and retinopathy.
Intensive glucose-lowering therapy, as compared to standard therapy, resulted in a 21% relative reduction of new or worsening nephropathy. There was no significant effect on the rate of MI, strokes, death from cardiovascular causes, or retinopathy. Furthermore, intensive glucose control was associated with an increased risk of severe hypoglycemia and increased rate of hospitalization. In contrast to the ACCORD study, intensive therapy did not result in an increase in mortality.
Bottom Line: While targeting normal glycated hemoglobin levels with a gliclazide-based regimen reduced the rate of nephropathy, this strategy did not have an effect on preventing major macrovascular events.
Citation: ADVANCE collaborative group. Intensive blood glucose control and vascular outcomes in patients with type-2 diabetes. N Eng J Med. 2008;358:2560-2572.
Non-invasive Ventilation Does Not Improve Short-Term Mortality in Patients with Acute Cardiogenic Pulmonary Edema
Clinical Question: Does non-invasive ventilation improve survival for patients with acute cardiogenic pulmonary edema?
Background: Acute cardiogenic pulmonary edema is a common medical emergency, but only small trials address outcomes of non-invasive methods of ventilation.
Study Design: A prospective, randomized control study.
Setting: 26 emergency rooms and hospitals in the United Kingdom.
Synopsis: 1,069 patients with a clinical diagnosis of acute cardiogenic pulmonary edema in the emergency room were randomized to one of three treatment strategies: standard oxygen therapy, continuous positive airway pressure (CPAP), or non-invasive intermittent positive pressure ventilation (NIPPV).
There was no significant difference in the seven-day mortality between patients receiving standard oxygen therapy (9.8%) and those treated with non-invasive ventilation (9.5%). Additionally, there was no significant difference in the combined end point of death or intubation within seven days between patients receiving CPAP and NIPPV—the primary end points of the study.
While non-invasive ventilation was associated with greater reductions in dyspnea, heart rate, acidosis, and hypercapnia than was standard oxygen therapy, rates of other secondary outcomes—including tracheal intubation, admission to the critical care unit, myocardial infarction, and 30-day mortality—were similar.
Bottom Line: Although non-invasive ventilation rapidly improves respiratory distress and metabolic disturbances for patients with acute cardiogenic pulmonary edema, it has no effect on short-term mortality or rates of tracheal intubation and admission to ICU.
Citation: Gray A, Goodacre S, Newby D, et al. Non-invasive ventilation in acute cardiogenic pulmonary edema. N Eng J Med. 2008;359:142-151.
Rhythm Control in Patients with Atrial Fibrillation and CHF Does Not Improve Mortality
Clinical Question: Does the restoration of sinus rhythm in patients with atrial fibrillation and heart failure reduce mortality from cardiovascular causes?
Background: Recent data show rhythm control provides no benefit over rate control among patients with atrial fibrillation, but limited information is available regarding its applicability to patients with heart failure.
Study Design: Multicenter, prospective, double-blind, randomized trial.
Settings: 123 medical centers worldwide.
Synopsis:1,376 patients with a left ventricular ejection fraction of 35% or less, symptoms of heart failure, and a history of atrial fibrillation were randomized (allocation not concealed) either to a rhythm-control (e.g., conversion to sinus rhythm) or rate-control strategy. The primary outcome measured was the time to death from cardiovascular causes. Secondary outcomes included death from any cause, stroke, worsening heart failure, hospitalization, quality of life, cost of therapy, and a composite of death from cardiovascular causes, stroke or worsening heart failure.
Study follow up succeeded with 94% of enrolled patients completing follow up (median=47 months for survivors) or dying. Amiodarone was the drug used most often in the rhythm-control group. Ninety percent of patients received angiotensin converting enzyme inhibitors or angiotensin II receptor blocker, and 90% received anticoagulation.
Among patients with atrial fibrillation and congestive heart failure, the number of deaths from cardiovascular causes was similar in the rate (25%) and rhythm-control group (27%). Furthermore, there were no significant differences in important secondary outcomes, including death from any cause, worsening heart failure, or stroke.
Bottom Line: For patients with heart failure from systolic dysfunction and atrial fibrillation, the routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes as compared with a rate-control strategy.
Citation: Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
No Benefit from Intensive Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (AKI)
Clinical Question: Does intensive renal replacement therapy, as compared with a conventional treatment strategy, affect outcomes in critically ill patients?
Background: The optimal timing for the initiation, method, and dosing of renal replacement therapy among patients with AKI remains uncertain. Previous single-center studies limited to single methods of renal replacement therapy have suggested more intensive therapy is associated with improved survival. These results, however, have been inconsistent.
Study Design: Randomized controlled trial.
Setting: Veterans Affairs hospitals and university affiliated medical centers.
Synopsis: This study randomized 1,124 of 4,340 eligible critically ill adults with AKI to receive either intensive renal replacement therapy or a less intensive, more conventional approach. The primary end point of death at day 60 from any cause was 53.6% with intensive therapy and 51.5 % with less intensive therapy (odds ratio, 1.09; 95% CI 0.86 to 1.40; p=0.47). No significant differences were found in secondary end points, including rate of recovery of kidney function, duration of renal-replacement therapy, or evolution of non-renal organ failure.
This study demonstrates providing hemodialysis more frequently than three times per week to hemodynamically stable patients, or providing continuous renal replacement therapy at an effluent flow rate of more than 20 ml/kg/hour to hemodynamically unstable patients, does not improve outcomes. It should be noted, however, the less-intensive treatment strategy in this study provides a dose of renal replacement therapy exceeding the normal dose given in usual care.
Bottom Line: No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
Citation: Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7-20.
Elderly Patients and Females with Acute Coronary Syndrome (ACS) Often Receive Excess Doses of Heparin
Clinical Question: What initial dosing of unfractionated heparin (UFH) for patients with non-ST-segment elevation (NSTE) acute coronary syndrome is most frequently used and how does it affect risk of bleeding?
Background: UFH is commonly used in clinical practice for NSTE ACS, but a wide variability continues to exist in dosing protocols. Clinical studies have shown UFH dosing based on weight provides more effective early anticoagulation. However, the relationship between increasing weight-adjusted doses of UFH and the risk of bleeding has not been well described.
Study Design: Retrospective cohort study.
Setting: 420 U.S. hospitals involved in the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation) initiative.
Synopsis: This study used data from patients enrolled in the CRUSADE initiative to investigate UFH dosing amongst 24,021 patients presenting with NSTE ACS. The study showed despite a recommendation to use weight-adjusted UFH dosing for NSTE ACS, there is a continued preference for a fixed dosing regimen of 5,000 U bolus and 1,000 U/hr initial infusion.
This fixed dose means women and the elderly are more likely to receive an excess UFH dose due to their lower body weight. The study found 35% of the group received excess weight-adjusted doses of UFH.
A clear relationship is present between excess weight-adjusted UFH doses and major bleeding, with the risk of major bleeding increasing particularly when UFH dose exceeds the recommended dosing of 70 U/kg bolus and 15 U/kg per hour infusion.
These observations support the need to follow guidelines on weight-adjusted UFH dosing in order to minimize the risk of bleeding in patients with NSTE ACS.
Bottom line: The initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing, leading to higher rates of bleeding, particularly among women and the elderly.
Citation: Melloni C, Alexander KP, Chen AY et al. Unfractionated heparin dosing and risk of major bleeding in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;156(2):209-215.
Elevated BNP Level Is a Marker for Higher Risk of Adverse Outcomes in Patients with Pulmonary Embolism
Clinical Question: Can elevated BNP levels predict adverse outcomes in patients with acute pulmonary embolism (PE)?
Background: Plasma brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) often are elevated in patients with PE and right ventricular (RV) dysfunction. The finding of RV dysfunction on echocardiography is an indicator of poor outcome in these patients. The role of BNP levels to differentiate patients with PE who are at higher risk of adverse events and poor clinical outcomes has not been determined.
Study Design: Meta analysis of prospective studies.
Settings: 13 studies involving 1,132 patients.
Synopsis: Elevated levels of BNP or NT-pro-BNP were noted in 51% of patients with acute PE. These patients had a higher rate of complicated inpatient course, as well as a higher risk of 30-day mortality (odds ratio 6.8; 95% CI 4.4-10; odds-ratio 7.6; 95% CI 3.4-17). Additionally, increased BNP levels were significantly associated with RV dysfunction (p<0.0001).
While elevated BNP levels may serve as a marker for increased risk of adverse outcomes, the investigators stress these levels alone should not be used to pursue more aggressive treatment strategies. Elevation of these markers is nonspecific and can be secondary to pre-existing heart, lung, or kidney disease, or older age. Further studies are needed to determine the role of BNP in risk stratifying patients with acute PE to different forms of therapy.
Bottom Line: High BNP or NT-pro-BNP levels can differentiate patients with PE who are at a higher risk of complicated hospital course and short-term mortality.
Citation: Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425-430.
Silver-Coated Endotracheal Tubes Reduce Incidence of Ventilator-Associated Pneumonia (VAP)
Clinical Question: Can silver-coated endotracheal tubes reduce the incidence of VAP?
Background: Given the high morbidity linked to VAP, prevention strategies have been sought. Silver has broad-spectrum antimicrobial activity in vitro. Thus, a silver-coated endotracheal tube (ET) was designed to help prevent biofilm formation and bacterial colonization.
Study Design: A prospective, randomized, single-blind, controlled study.
Settings: 54 centers in North America.
Synopsis: Out of 9,417 potentially eligible patients, 2,003 patients expected to require mechanical ventilation for 24 hours or longer were randomized to undergo intubation with endotracheal tubes with and without silver coating. The primary outcome was incidence of VAP based on quantitative culture of bronchoalveolar lavage fluid.
The rate of microbiologically confirmed VAP in patients intubated for 24 hours or longer with the silver-coated ET tube was 4.8%, as compared to 7.5% in the control group. Using silver-coated ET tubes resulted in a 35.9% relative risk reduction of VAP incidence. Furthermore, the silver-coated ET tube was associated with a delayed time to VAP occurrence. Other outcomes, including length of intubation, duration of hospital stay, mortality, and frequency of adverse events, however, did not show statistically significant differences between the two groups.
Bottom Line: Using a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Citation: Kollef MH, Afessa B, Anzueta A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805-813.
Literature at a Glance
- Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
- Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality.
- Targeting normal glycated hemoglobin levels with a gliclazide-based regimen does not have an effect on preventing major macrovascular events.
- Non-invasive ventilation has no effect on short-term mortality or rates of tracheal intubation and admission to ICU
- Routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes.
- No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
- Initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing.
- High BNP or NT-pro-BNP levels can differentiate patients who are at a higher risk of complicated hospital course and short-term mortality.
- Use of a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Tight Glucose Control in the Intensive Care Unit (ICU) Setting Does Not Reduce Short-Term Mortality
Clinical question: Does tight glucose control for critically ill patients affect mortality?
Background: Intensive glucose control for adult ICU patients has been advocated by numerous professional societies and adopted worldwide as a means to reduce mortality of critically ill patients. Evidence from multiple randomized controlled trials of tight glucose control in the ICU setting, however, shows mixed results.
Study Design: Meta-analysis of randomized controlled trials.
Setting: 29 studies involving 8,432 critically ill patients.
Synopsis: This study evaluated 29 trials involving critically ill adult patients randomized to tight glucose control versus usual care. Comparing these patients, there was no significant difference in short-term mortality (<30 days). Stratification of trials by level of glucose control (very tight <110 mg/dL versus moderately tight <150 mg/dL) and by ICU setting (surgical, medical, or mixed medical-surgical) did not affect mortality.
Tight glucose control was associated with a reduced risk of septicemia, but only in surgical patients. There was no association between tight control and a new need for dialysis, consistent across all ICU settings, as well as with both levels of glucose control. Finally, there was an increased risk of hypoglycemia (<40 mg/dL) with tight control, higher in patients who received very tight control versus those who received moderately tight control.
Limitations of the studies evaluated in this meta-analysis include difficulties with consistently maintaining tight glucose control. Twenty one percent of the trials did not achieve a mean glucose level within 5 mg/dL of the goal. This, along with a lack of standardization in reporting glucose control, makes study comparison problematic.
Bottom Line: Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
Citation: Wiener, RS, Wiener DC, Larson, RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933-944.
Intensive Glucose-Lowering Therapy Increases Mortality in High-Risk Diabetic Patients
Clinical Question: Does intensive glucose-lowering therapy reduce cardiac events in high-risk diabetic patients?
Background: Epidemiologic studies have suggested the risk of cardiovascular disease increases with higher levels of glycated hemoglobin in patients with type-2 diabetes. No definitive data from randomized trials exist to test the effect of intensive glucose-lowering therapy on the rate of cardiovascular events in high-risk diabetic patients.
Study Design: Multicenter randomized controlled trial led by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group.
Setting: 77 clinical centers in the U.S. and Canada.
Synopsis: 10,251 diabetic patients with established cardiovascular disease or additional cardiovascular risk factors, and median glycated hemoglobin level of 8.1%, received either intensive therapy (targeting glycated hemoglobin level <6.0%) or standard therapy (targeting level from 7.0% to 7.9%). The primary outcome was a composite of non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.
Data indicated intensive therapy did decrease the rate of non-fatal myocardial infarctions, however, it did not significantly reduce the primary composite of major cardiovascular events. Moreover, intensive therapy resulted in a significant increase in death from cardiovascular causes, as well as a relative increase of 22% of death from any cause, during follow up of three and a half years. Due to this finding, the intensive therapy regimen was discontinued 17 months before the scheduled end of the study.
Analysis of the data has not identified a cause for the unexpected increased mortality in the intensive therapy group, and has not shown any medication or combination of medications to be responsible.
Bottom Line: Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality and did not significantly reduce major cardiovascular events.
Citation: Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type-2 diabetes. N Engl J Med. 2008;358:2545-2559.
Intensive Glucose Control Reduces Nephropathy but Has No Effect on Major Cardiovascular Events
Clinical Question: Does intensive glucose-lowering therapy decrease major macrovascular and microvascular events in high-risk diabetic patients?
Background: Prospective studies show a direct association between elevated glycated hemoglobin levels in diabetics and increased risk of vascular events. However, definitive evidence from randomized trials about the role of intensive glucose control in preventing vascular disease in diabetics is lacking.
Study Design: Multicenter randomized controlled trial led by the ADVANCE Collaborative Group.
Setting: 215 clinical centers in 20 countries from Asia, Australia, Europe, and North America.
Synopsis: 11,140 diabetic patients received either standard glucose therapy or intensive glucose therapy using gliclazide, as well as other drugs, to reach a targeted glycated hemoglobin of 6.5% or less. The primary outcome was a composite of major macrovascular and microvascular events, including nonfatal myocardial infarction (MI), nonfatal stroke, death from cardiovascular causes, nephropathy, and retinopathy.
Intensive glucose-lowering therapy, as compared to standard therapy, resulted in a 21% relative reduction of new or worsening nephropathy. There was no significant effect on the rate of MI, strokes, death from cardiovascular causes, or retinopathy. Furthermore, intensive glucose control was associated with an increased risk of severe hypoglycemia and increased rate of hospitalization. In contrast to the ACCORD study, intensive therapy did not result in an increase in mortality.
Bottom Line: While targeting normal glycated hemoglobin levels with a gliclazide-based regimen reduced the rate of nephropathy, this strategy did not have an effect on preventing major macrovascular events.
Citation: ADVANCE collaborative group. Intensive blood glucose control and vascular outcomes in patients with type-2 diabetes. N Eng J Med. 2008;358:2560-2572.
Non-invasive Ventilation Does Not Improve Short-Term Mortality in Patients with Acute Cardiogenic Pulmonary Edema
Clinical Question: Does non-invasive ventilation improve survival for patients with acute cardiogenic pulmonary edema?
Background: Acute cardiogenic pulmonary edema is a common medical emergency, but only small trials address outcomes of non-invasive methods of ventilation.
Study Design: A prospective, randomized control study.
Setting: 26 emergency rooms and hospitals in the United Kingdom.
Synopsis: 1,069 patients with a clinical diagnosis of acute cardiogenic pulmonary edema in the emergency room were randomized to one of three treatment strategies: standard oxygen therapy, continuous positive airway pressure (CPAP), or non-invasive intermittent positive pressure ventilation (NIPPV).
There was no significant difference in the seven-day mortality between patients receiving standard oxygen therapy (9.8%) and those treated with non-invasive ventilation (9.5%). Additionally, there was no significant difference in the combined end point of death or intubation within seven days between patients receiving CPAP and NIPPV—the primary end points of the study.
While non-invasive ventilation was associated with greater reductions in dyspnea, heart rate, acidosis, and hypercapnia than was standard oxygen therapy, rates of other secondary outcomes—including tracheal intubation, admission to the critical care unit, myocardial infarction, and 30-day mortality—were similar.
Bottom Line: Although non-invasive ventilation rapidly improves respiratory distress and metabolic disturbances for patients with acute cardiogenic pulmonary edema, it has no effect on short-term mortality or rates of tracheal intubation and admission to ICU.
Citation: Gray A, Goodacre S, Newby D, et al. Non-invasive ventilation in acute cardiogenic pulmonary edema. N Eng J Med. 2008;359:142-151.
Rhythm Control in Patients with Atrial Fibrillation and CHF Does Not Improve Mortality
Clinical Question: Does the restoration of sinus rhythm in patients with atrial fibrillation and heart failure reduce mortality from cardiovascular causes?
Background: Recent data show rhythm control provides no benefit over rate control among patients with atrial fibrillation, but limited information is available regarding its applicability to patients with heart failure.
Study Design: Multicenter, prospective, double-blind, randomized trial.
Settings: 123 medical centers worldwide.
Synopsis:1,376 patients with a left ventricular ejection fraction of 35% or less, symptoms of heart failure, and a history of atrial fibrillation were randomized (allocation not concealed) either to a rhythm-control (e.g., conversion to sinus rhythm) or rate-control strategy. The primary outcome measured was the time to death from cardiovascular causes. Secondary outcomes included death from any cause, stroke, worsening heart failure, hospitalization, quality of life, cost of therapy, and a composite of death from cardiovascular causes, stroke or worsening heart failure.
Study follow up succeeded with 94% of enrolled patients completing follow up (median=47 months for survivors) or dying. Amiodarone was the drug used most often in the rhythm-control group. Ninety percent of patients received angiotensin converting enzyme inhibitors or angiotensin II receptor blocker, and 90% received anticoagulation.
Among patients with atrial fibrillation and congestive heart failure, the number of deaths from cardiovascular causes was similar in the rate (25%) and rhythm-control group (27%). Furthermore, there were no significant differences in important secondary outcomes, including death from any cause, worsening heart failure, or stroke.
Bottom Line: For patients with heart failure from systolic dysfunction and atrial fibrillation, the routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes as compared with a rate-control strategy.
Citation: Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
No Benefit from Intensive Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (AKI)
Clinical Question: Does intensive renal replacement therapy, as compared with a conventional treatment strategy, affect outcomes in critically ill patients?
Background: The optimal timing for the initiation, method, and dosing of renal replacement therapy among patients with AKI remains uncertain. Previous single-center studies limited to single methods of renal replacement therapy have suggested more intensive therapy is associated with improved survival. These results, however, have been inconsistent.
Study Design: Randomized controlled trial.
Setting: Veterans Affairs hospitals and university affiliated medical centers.
Synopsis: This study randomized 1,124 of 4,340 eligible critically ill adults with AKI to receive either intensive renal replacement therapy or a less intensive, more conventional approach. The primary end point of death at day 60 from any cause was 53.6% with intensive therapy and 51.5 % with less intensive therapy (odds ratio, 1.09; 95% CI 0.86 to 1.40; p=0.47). No significant differences were found in secondary end points, including rate of recovery of kidney function, duration of renal-replacement therapy, or evolution of non-renal organ failure.
This study demonstrates providing hemodialysis more frequently than three times per week to hemodynamically stable patients, or providing continuous renal replacement therapy at an effluent flow rate of more than 20 ml/kg/hour to hemodynamically unstable patients, does not improve outcomes. It should be noted, however, the less-intensive treatment strategy in this study provides a dose of renal replacement therapy exceeding the normal dose given in usual care.
Bottom Line: No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
Citation: Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7-20.
Elderly Patients and Females with Acute Coronary Syndrome (ACS) Often Receive Excess Doses of Heparin
Clinical Question: What initial dosing of unfractionated heparin (UFH) for patients with non-ST-segment elevation (NSTE) acute coronary syndrome is most frequently used and how does it affect risk of bleeding?
Background: UFH is commonly used in clinical practice for NSTE ACS, but a wide variability continues to exist in dosing protocols. Clinical studies have shown UFH dosing based on weight provides more effective early anticoagulation. However, the relationship between increasing weight-adjusted doses of UFH and the risk of bleeding has not been well described.
Study Design: Retrospective cohort study.
Setting: 420 U.S. hospitals involved in the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation) initiative.
Synopsis: This study used data from patients enrolled in the CRUSADE initiative to investigate UFH dosing amongst 24,021 patients presenting with NSTE ACS. The study showed despite a recommendation to use weight-adjusted UFH dosing for NSTE ACS, there is a continued preference for a fixed dosing regimen of 5,000 U bolus and 1,000 U/hr initial infusion.
This fixed dose means women and the elderly are more likely to receive an excess UFH dose due to their lower body weight. The study found 35% of the group received excess weight-adjusted doses of UFH.
A clear relationship is present between excess weight-adjusted UFH doses and major bleeding, with the risk of major bleeding increasing particularly when UFH dose exceeds the recommended dosing of 70 U/kg bolus and 15 U/kg per hour infusion.
These observations support the need to follow guidelines on weight-adjusted UFH dosing in order to minimize the risk of bleeding in patients with NSTE ACS.
Bottom line: The initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing, leading to higher rates of bleeding, particularly among women and the elderly.
Citation: Melloni C, Alexander KP, Chen AY et al. Unfractionated heparin dosing and risk of major bleeding in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;156(2):209-215.
Elevated BNP Level Is a Marker for Higher Risk of Adverse Outcomes in Patients with Pulmonary Embolism
Clinical Question: Can elevated BNP levels predict adverse outcomes in patients with acute pulmonary embolism (PE)?
Background: Plasma brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) often are elevated in patients with PE and right ventricular (RV) dysfunction. The finding of RV dysfunction on echocardiography is an indicator of poor outcome in these patients. The role of BNP levels to differentiate patients with PE who are at higher risk of adverse events and poor clinical outcomes has not been determined.
Study Design: Meta analysis of prospective studies.
Settings: 13 studies involving 1,132 patients.
Synopsis: Elevated levels of BNP or NT-pro-BNP were noted in 51% of patients with acute PE. These patients had a higher rate of complicated inpatient course, as well as a higher risk of 30-day mortality (odds ratio 6.8; 95% CI 4.4-10; odds-ratio 7.6; 95% CI 3.4-17). Additionally, increased BNP levels were significantly associated with RV dysfunction (p<0.0001).
While elevated BNP levels may serve as a marker for increased risk of adverse outcomes, the investigators stress these levels alone should not be used to pursue more aggressive treatment strategies. Elevation of these markers is nonspecific and can be secondary to pre-existing heart, lung, or kidney disease, or older age. Further studies are needed to determine the role of BNP in risk stratifying patients with acute PE to different forms of therapy.
Bottom Line: High BNP or NT-pro-BNP levels can differentiate patients with PE who are at a higher risk of complicated hospital course and short-term mortality.
Citation: Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425-430.
Silver-Coated Endotracheal Tubes Reduce Incidence of Ventilator-Associated Pneumonia (VAP)
Clinical Question: Can silver-coated endotracheal tubes reduce the incidence of VAP?
Background: Given the high morbidity linked to VAP, prevention strategies have been sought. Silver has broad-spectrum antimicrobial activity in vitro. Thus, a silver-coated endotracheal tube (ET) was designed to help prevent biofilm formation and bacterial colonization.
Study Design: A prospective, randomized, single-blind, controlled study.
Settings: 54 centers in North America.
Synopsis: Out of 9,417 potentially eligible patients, 2,003 patients expected to require mechanical ventilation for 24 hours or longer were randomized to undergo intubation with endotracheal tubes with and without silver coating. The primary outcome was incidence of VAP based on quantitative culture of bronchoalveolar lavage fluid.
The rate of microbiologically confirmed VAP in patients intubated for 24 hours or longer with the silver-coated ET tube was 4.8%, as compared to 7.5% in the control group. Using silver-coated ET tubes resulted in a 35.9% relative risk reduction of VAP incidence. Furthermore, the silver-coated ET tube was associated with a delayed time to VAP occurrence. Other outcomes, including length of intubation, duration of hospital stay, mortality, and frequency of adverse events, however, did not show statistically significant differences between the two groups.
Bottom Line: Using a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Citation: Kollef MH, Afessa B, Anzueta A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805-813.
Literature at a Glance
- Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
- Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality.
- Targeting normal glycated hemoglobin levels with a gliclazide-based regimen does not have an effect on preventing major macrovascular events.
- Non-invasive ventilation has no effect on short-term mortality or rates of tracheal intubation and admission to ICU
- Routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes.
- No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
- Initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing.
- High BNP or NT-pro-BNP levels can differentiate patients who are at a higher risk of complicated hospital course and short-term mortality.
- Use of a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Tight Glucose Control in the Intensive Care Unit (ICU) Setting Does Not Reduce Short-Term Mortality
Clinical question: Does tight glucose control for critically ill patients affect mortality?
Background: Intensive glucose control for adult ICU patients has been advocated by numerous professional societies and adopted worldwide as a means to reduce mortality of critically ill patients. Evidence from multiple randomized controlled trials of tight glucose control in the ICU setting, however, shows mixed results.
Study Design: Meta-analysis of randomized controlled trials.
Setting: 29 studies involving 8,432 critically ill patients.
Synopsis: This study evaluated 29 trials involving critically ill adult patients randomized to tight glucose control versus usual care. Comparing these patients, there was no significant difference in short-term mortality (<30 days). Stratification of trials by level of glucose control (very tight <110 mg/dL versus moderately tight <150 mg/dL) and by ICU setting (surgical, medical, or mixed medical-surgical) did not affect mortality.
Tight glucose control was associated with a reduced risk of septicemia, but only in surgical patients. There was no association between tight control and a new need for dialysis, consistent across all ICU settings, as well as with both levels of glucose control. Finally, there was an increased risk of hypoglycemia (<40 mg/dL) with tight control, higher in patients who received very tight control versus those who received moderately tight control.
Limitations of the studies evaluated in this meta-analysis include difficulties with consistently maintaining tight glucose control. Twenty one percent of the trials did not achieve a mean glucose level within 5 mg/dL of the goal. This, along with a lack of standardization in reporting glucose control, makes study comparison problematic.
Bottom Line: Tight glucose control in critically ill patients is not associated with reduction in short-term mortality, but it is associated with an increased risk of hypoglycemia.
Citation: Wiener, RS, Wiener DC, Larson, RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933-944.
Intensive Glucose-Lowering Therapy Increases Mortality in High-Risk Diabetic Patients
Clinical Question: Does intensive glucose-lowering therapy reduce cardiac events in high-risk diabetic patients?
Background: Epidemiologic studies have suggested the risk of cardiovascular disease increases with higher levels of glycated hemoglobin in patients with type-2 diabetes. No definitive data from randomized trials exist to test the effect of intensive glucose-lowering therapy on the rate of cardiovascular events in high-risk diabetic patients.
Study Design: Multicenter randomized controlled trial led by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group.
Setting: 77 clinical centers in the U.S. and Canada.
Synopsis: 10,251 diabetic patients with established cardiovascular disease or additional cardiovascular risk factors, and median glycated hemoglobin level of 8.1%, received either intensive therapy (targeting glycated hemoglobin level <6.0%) or standard therapy (targeting level from 7.0% to 7.9%). The primary outcome was a composite of non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.
Data indicated intensive therapy did decrease the rate of non-fatal myocardial infarctions, however, it did not significantly reduce the primary composite of major cardiovascular events. Moreover, intensive therapy resulted in a significant increase in death from cardiovascular causes, as well as a relative increase of 22% of death from any cause, during follow up of three and a half years. Due to this finding, the intensive therapy regimen was discontinued 17 months before the scheduled end of the study.
Analysis of the data has not identified a cause for the unexpected increased mortality in the intensive therapy group, and has not shown any medication or combination of medications to be responsible.
Bottom Line: Intensive glucose-lowering therapy in diabetic patients at high risk for cardiovascular events increased mortality and did not significantly reduce major cardiovascular events.
Citation: Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type-2 diabetes. N Engl J Med. 2008;358:2545-2559.
Intensive Glucose Control Reduces Nephropathy but Has No Effect on Major Cardiovascular Events
Clinical Question: Does intensive glucose-lowering therapy decrease major macrovascular and microvascular events in high-risk diabetic patients?
Background: Prospective studies show a direct association between elevated glycated hemoglobin levels in diabetics and increased risk of vascular events. However, definitive evidence from randomized trials about the role of intensive glucose control in preventing vascular disease in diabetics is lacking.
Study Design: Multicenter randomized controlled trial led by the ADVANCE Collaborative Group.
Setting: 215 clinical centers in 20 countries from Asia, Australia, Europe, and North America.
Synopsis: 11,140 diabetic patients received either standard glucose therapy or intensive glucose therapy using gliclazide, as well as other drugs, to reach a targeted glycated hemoglobin of 6.5% or less. The primary outcome was a composite of major macrovascular and microvascular events, including nonfatal myocardial infarction (MI), nonfatal stroke, death from cardiovascular causes, nephropathy, and retinopathy.
Intensive glucose-lowering therapy, as compared to standard therapy, resulted in a 21% relative reduction of new or worsening nephropathy. There was no significant effect on the rate of MI, strokes, death from cardiovascular causes, or retinopathy. Furthermore, intensive glucose control was associated with an increased risk of severe hypoglycemia and increased rate of hospitalization. In contrast to the ACCORD study, intensive therapy did not result in an increase in mortality.
Bottom Line: While targeting normal glycated hemoglobin levels with a gliclazide-based regimen reduced the rate of nephropathy, this strategy did not have an effect on preventing major macrovascular events.
Citation: ADVANCE collaborative group. Intensive blood glucose control and vascular outcomes in patients with type-2 diabetes. N Eng J Med. 2008;358:2560-2572.
Non-invasive Ventilation Does Not Improve Short-Term Mortality in Patients with Acute Cardiogenic Pulmonary Edema
Clinical Question: Does non-invasive ventilation improve survival for patients with acute cardiogenic pulmonary edema?
Background: Acute cardiogenic pulmonary edema is a common medical emergency, but only small trials address outcomes of non-invasive methods of ventilation.
Study Design: A prospective, randomized control study.
Setting: 26 emergency rooms and hospitals in the United Kingdom.
Synopsis: 1,069 patients with a clinical diagnosis of acute cardiogenic pulmonary edema in the emergency room were randomized to one of three treatment strategies: standard oxygen therapy, continuous positive airway pressure (CPAP), or non-invasive intermittent positive pressure ventilation (NIPPV).
There was no significant difference in the seven-day mortality between patients receiving standard oxygen therapy (9.8%) and those treated with non-invasive ventilation (9.5%). Additionally, there was no significant difference in the combined end point of death or intubation within seven days between patients receiving CPAP and NIPPV—the primary end points of the study.
While non-invasive ventilation was associated with greater reductions in dyspnea, heart rate, acidosis, and hypercapnia than was standard oxygen therapy, rates of other secondary outcomes—including tracheal intubation, admission to the critical care unit, myocardial infarction, and 30-day mortality—were similar.
Bottom Line: Although non-invasive ventilation rapidly improves respiratory distress and metabolic disturbances for patients with acute cardiogenic pulmonary edema, it has no effect on short-term mortality or rates of tracheal intubation and admission to ICU.
Citation: Gray A, Goodacre S, Newby D, et al. Non-invasive ventilation in acute cardiogenic pulmonary edema. N Eng J Med. 2008;359:142-151.
Rhythm Control in Patients with Atrial Fibrillation and CHF Does Not Improve Mortality
Clinical Question: Does the restoration of sinus rhythm in patients with atrial fibrillation and heart failure reduce mortality from cardiovascular causes?
Background: Recent data show rhythm control provides no benefit over rate control among patients with atrial fibrillation, but limited information is available regarding its applicability to patients with heart failure.
Study Design: Multicenter, prospective, double-blind, randomized trial.
Settings: 123 medical centers worldwide.
Synopsis:1,376 patients with a left ventricular ejection fraction of 35% or less, symptoms of heart failure, and a history of atrial fibrillation were randomized (allocation not concealed) either to a rhythm-control (e.g., conversion to sinus rhythm) or rate-control strategy. The primary outcome measured was the time to death from cardiovascular causes. Secondary outcomes included death from any cause, stroke, worsening heart failure, hospitalization, quality of life, cost of therapy, and a composite of death from cardiovascular causes, stroke or worsening heart failure.
Study follow up succeeded with 94% of enrolled patients completing follow up (median=47 months for survivors) or dying. Amiodarone was the drug used most often in the rhythm-control group. Ninety percent of patients received angiotensin converting enzyme inhibitors or angiotensin II receptor blocker, and 90% received anticoagulation.
Among patients with atrial fibrillation and congestive heart failure, the number of deaths from cardiovascular causes was similar in the rate (25%) and rhythm-control group (27%). Furthermore, there were no significant differences in important secondary outcomes, including death from any cause, worsening heart failure, or stroke.
Bottom Line: For patients with heart failure from systolic dysfunction and atrial fibrillation, the routine use of a rhythm-control strategy does not reduce the rate of death from cardiovascular causes as compared with a rate-control strategy.
Citation: Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
No Benefit from Intensive Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (AKI)
Clinical Question: Does intensive renal replacement therapy, as compared with a conventional treatment strategy, affect outcomes in critically ill patients?
Background: The optimal timing for the initiation, method, and dosing of renal replacement therapy among patients with AKI remains uncertain. Previous single-center studies limited to single methods of renal replacement therapy have suggested more intensive therapy is associated with improved survival. These results, however, have been inconsistent.
Study Design: Randomized controlled trial.
Setting: Veterans Affairs hospitals and university affiliated medical centers.
Synopsis: This study randomized 1,124 of 4,340 eligible critically ill adults with AKI to receive either intensive renal replacement therapy or a less intensive, more conventional approach. The primary end point of death at day 60 from any cause was 53.6% with intensive therapy and 51.5 % with less intensive therapy (odds ratio, 1.09; 95% CI 0.86 to 1.40; p=0.47). No significant differences were found in secondary end points, including rate of recovery of kidney function, duration of renal-replacement therapy, or evolution of non-renal organ failure.
This study demonstrates providing hemodialysis more frequently than three times per week to hemodynamically stable patients, or providing continuous renal replacement therapy at an effluent flow rate of more than 20 ml/kg/hour to hemodynamically unstable patients, does not improve outcomes. It should be noted, however, the less-intensive treatment strategy in this study provides a dose of renal replacement therapy exceeding the normal dose given in usual care.
Bottom Line: No clinical benefit exists in employing intensive renal replacement over a conventional approach in critically ill patients.
Citation: Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7-20.
Elderly Patients and Females with Acute Coronary Syndrome (ACS) Often Receive Excess Doses of Heparin
Clinical Question: What initial dosing of unfractionated heparin (UFH) for patients with non-ST-segment elevation (NSTE) acute coronary syndrome is most frequently used and how does it affect risk of bleeding?
Background: UFH is commonly used in clinical practice for NSTE ACS, but a wide variability continues to exist in dosing protocols. Clinical studies have shown UFH dosing based on weight provides more effective early anticoagulation. However, the relationship between increasing weight-adjusted doses of UFH and the risk of bleeding has not been well described.
Study Design: Retrospective cohort study.
Setting: 420 U.S. hospitals involved in the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation) initiative.
Synopsis: This study used data from patients enrolled in the CRUSADE initiative to investigate UFH dosing amongst 24,021 patients presenting with NSTE ACS. The study showed despite a recommendation to use weight-adjusted UFH dosing for NSTE ACS, there is a continued preference for a fixed dosing regimen of 5,000 U bolus and 1,000 U/hr initial infusion.
This fixed dose means women and the elderly are more likely to receive an excess UFH dose due to their lower body weight. The study found 35% of the group received excess weight-adjusted doses of UFH.
A clear relationship is present between excess weight-adjusted UFH doses and major bleeding, with the risk of major bleeding increasing particularly when UFH dose exceeds the recommended dosing of 70 U/kg bolus and 15 U/kg per hour infusion.
These observations support the need to follow guidelines on weight-adjusted UFH dosing in order to minimize the risk of bleeding in patients with NSTE ACS.
Bottom line: The initial UFH bolus and infusion dosing used for NSTE ACS often exceeds recommended weight-adjusted dosing, leading to higher rates of bleeding, particularly among women and the elderly.
Citation: Melloni C, Alexander KP, Chen AY et al. Unfractionated heparin dosing and risk of major bleeding in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;156(2):209-215.
Elevated BNP Level Is a Marker for Higher Risk of Adverse Outcomes in Patients with Pulmonary Embolism
Clinical Question: Can elevated BNP levels predict adverse outcomes in patients with acute pulmonary embolism (PE)?
Background: Plasma brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) often are elevated in patients with PE and right ventricular (RV) dysfunction. The finding of RV dysfunction on echocardiography is an indicator of poor outcome in these patients. The role of BNP levels to differentiate patients with PE who are at higher risk of adverse events and poor clinical outcomes has not been determined.
Study Design: Meta analysis of prospective studies.
Settings: 13 studies involving 1,132 patients.
Synopsis: Elevated levels of BNP or NT-pro-BNP were noted in 51% of patients with acute PE. These patients had a higher rate of complicated inpatient course, as well as a higher risk of 30-day mortality (odds ratio 6.8; 95% CI 4.4-10; odds-ratio 7.6; 95% CI 3.4-17). Additionally, increased BNP levels were significantly associated with RV dysfunction (p<0.0001).
While elevated BNP levels may serve as a marker for increased risk of adverse outcomes, the investigators stress these levels alone should not be used to pursue more aggressive treatment strategies. Elevation of these markers is nonspecific and can be secondary to pre-existing heart, lung, or kidney disease, or older age. Further studies are needed to determine the role of BNP in risk stratifying patients with acute PE to different forms of therapy.
Bottom Line: High BNP or NT-pro-BNP levels can differentiate patients with PE who are at a higher risk of complicated hospital course and short-term mortality.
Citation: Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425-430.
Silver-Coated Endotracheal Tubes Reduce Incidence of Ventilator-Associated Pneumonia (VAP)
Clinical Question: Can silver-coated endotracheal tubes reduce the incidence of VAP?
Background: Given the high morbidity linked to VAP, prevention strategies have been sought. Silver has broad-spectrum antimicrobial activity in vitro. Thus, a silver-coated endotracheal tube (ET) was designed to help prevent biofilm formation and bacterial colonization.
Study Design: A prospective, randomized, single-blind, controlled study.
Settings: 54 centers in North America.
Synopsis: Out of 9,417 potentially eligible patients, 2,003 patients expected to require mechanical ventilation for 24 hours or longer were randomized to undergo intubation with endotracheal tubes with and without silver coating. The primary outcome was incidence of VAP based on quantitative culture of bronchoalveolar lavage fluid.
The rate of microbiologically confirmed VAP in patients intubated for 24 hours or longer with the silver-coated ET tube was 4.8%, as compared to 7.5% in the control group. Using silver-coated ET tubes resulted in a 35.9% relative risk reduction of VAP incidence. Furthermore, the silver-coated ET tube was associated with a delayed time to VAP occurrence. Other outcomes, including length of intubation, duration of hospital stay, mortality, and frequency of adverse events, however, did not show statistically significant differences between the two groups.
Bottom Line: Using a silver-coated ET tube reduces the incidence of VAP, as well as delays time to VAP occurrence.
Citation: Kollef MH, Afessa B, Anzueta A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805-813.
New Faces in Key Places
Healthcare quality and patient safety is a hot topic in hospitals across the country, as well as here at The Society of Hospital Medicine (SHM). It seems like every day we hear of new regulatory requirements from the Centers for Medicare and Medicaid Services (CMS), The Joint Commission, and state health departments, or requirements from other health care organizations, including insurance companies. It’s hard to keep up with it all.
To help hospitals with their quality initiatives, SHM recently beefed up its Quality Initiatives Department by adding three new staff members, including myself. I’d like to introduce you to our newest members. My name is Jane Kelly-Cummings, and I joined SHM in July as the senior director of Quality Initiatives. I’m responsible for the strategic planning, development and implementation of quality initiatives for the society and the staff liaison for the Hospital Quality and Patient Safety Committee. I’ve been a registered nurse (RN) for more than 20 years and have been working in the quality world for more than a decade.
Linda Boclair joined SHM in June as director of Quality Initiatives. She is responsible for proposal/grant writing, managing select quality initiative projects, and department operations. She has a background in medical technology and industrial relations/organizational behavior. Linda currently is working on a medication reconciliation project, an advisory panel on pharmacoeconomics, and she is working on the new co-management taskforce.
Lauren Valentino also joined SHM in June as the departments project coordinator. She is responsible for supporting the Quality Initiatives department through project planning and implementation. Lauren also is a support resource for the society, providing documentation and coordination to the committees, task forces, and various special interest groups.
The new staffers join Joy M. Wittnebert-Barnosky, senior project manager; Tina Budnitz, senior advisor for Quality Initiatives; and Kathleen Kerr, project manager of QI Mentored Implementation in rounding out SHM’s new Quality Initiatives Department.
In order to best meet the quality initiatives needs of our members, we have been developing SHM’s quality initiatives strategic plan. We had the chance to share the plan with SHM board members at the bi-annual board meeting in October. The board received the strategic plan well, and the group is looking forward to an update at the January board meeting. SHM’s strategic quality initiative planning began in October 2007, when a group of SHM leaders met to begin the process of developing a 10-year strategic plan for quality initiatives. The output of this meeting led to six areas of focus, which we refer to as core strategies:
- Develop education programs and technical support tools in quality improvement or patient safety;
- Advance a national quality agenda for hospitals and hospitalists;
- Facilitate cultural change and develop initiatives to promote hospital medicine’s role in quality initiatives within the C-suite at our nation’s hospitals;
- Evaluate effectiveness of current offerings in quality improvement or patient safety;
- Promote adoption of health information technologies to advance patient safety and quality improvement; and
- Promote and support the new science of QI (i.e., develop a research agenda).
Linking these focus areas with SHM’s Core Competencies in Hospital Medicine and current national industry quality initiatives, such as The Joint Commission’s National Patient Safety Goals and Core Measures, is the basic foundation for SHM’s strategic quality plan. This plan will allow SHM to become more proactive in our approach to quality initiatives for the next one, three, five, and 10 years. It will help us focus on the areas identified as needs for hospitalists and hospital medicine.
Plan Breakdown
I would like to tell you about some of the newest quality initiatives currently underway. First is an advisory board on pharmacoeconomics. We are pulling together 11 industry leaders, including CEOs, chief financial officers, chief medical officers, pharmacists, and thought leaders in quality. This advisory board will be evaluating the standard operating procedures in hospitals across the country regarding the use of pharmacoeconomics in decisions to utilize medications, especially newer and more expensive agents, using venous thromboembolism (VTE) prevention as a case example.
We have assembled another advisory board, which is just underway, to look at complicated skin and skin structure infections (cSSSI). This board will include hospitalists, infection control physicians, emergency room physicians, pharmacists, wound care nurses, and quality improvement experts.
Lastly, we have a second VTE project charged with the development of an automated, electronic query for a major commercial clinical information system. The project team is tasked to develop and demonstrate how a system can dramatically increase the prevalence of VTE prophylaxis in hospitals where it will be piloted.
Collaborative Efforts
I also would like to share a few of the other exciting activities the quality department has been up to recently. SHM is in the process of collaborating on a book with the Joint Commission Resources on Hospitalists and Patient Safety. The book is scheduled for release this spring, so be on the lookout. We also are writing an article for the The Physician Executive, which is the journal of the American College of Physician Executives. The article focuses on how quality, patient safety, and patient satisfaction are becoming priorities for physician executives, and how hospitalists are a critical element of a strategy to address this priority. We also are talking with other organizations, such as the American Hospital Association and United Health Group, about potential collaborative work in the future.
So, you can see things at SHM headquarters are anything but dull. The entire QI department—both the veterans and new staff—are looking forward to helping you make your quality and patient safety initiatives more successful and improve the care of patients throughout the country. TH
Healthcare quality and patient safety is a hot topic in hospitals across the country, as well as here at The Society of Hospital Medicine (SHM). It seems like every day we hear of new regulatory requirements from the Centers for Medicare and Medicaid Services (CMS), The Joint Commission, and state health departments, or requirements from other health care organizations, including insurance companies. It’s hard to keep up with it all.
To help hospitals with their quality initiatives, SHM recently beefed up its Quality Initiatives Department by adding three new staff members, including myself. I’d like to introduce you to our newest members. My name is Jane Kelly-Cummings, and I joined SHM in July as the senior director of Quality Initiatives. I’m responsible for the strategic planning, development and implementation of quality initiatives for the society and the staff liaison for the Hospital Quality and Patient Safety Committee. I’ve been a registered nurse (RN) for more than 20 years and have been working in the quality world for more than a decade.
Linda Boclair joined SHM in June as director of Quality Initiatives. She is responsible for proposal/grant writing, managing select quality initiative projects, and department operations. She has a background in medical technology and industrial relations/organizational behavior. Linda currently is working on a medication reconciliation project, an advisory panel on pharmacoeconomics, and she is working on the new co-management taskforce.
Lauren Valentino also joined SHM in June as the departments project coordinator. She is responsible for supporting the Quality Initiatives department through project planning and implementation. Lauren also is a support resource for the society, providing documentation and coordination to the committees, task forces, and various special interest groups.
The new staffers join Joy M. Wittnebert-Barnosky, senior project manager; Tina Budnitz, senior advisor for Quality Initiatives; and Kathleen Kerr, project manager of QI Mentored Implementation in rounding out SHM’s new Quality Initiatives Department.
In order to best meet the quality initiatives needs of our members, we have been developing SHM’s quality initiatives strategic plan. We had the chance to share the plan with SHM board members at the bi-annual board meeting in October. The board received the strategic plan well, and the group is looking forward to an update at the January board meeting. SHM’s strategic quality initiative planning began in October 2007, when a group of SHM leaders met to begin the process of developing a 10-year strategic plan for quality initiatives. The output of this meeting led to six areas of focus, which we refer to as core strategies:
- Develop education programs and technical support tools in quality improvement or patient safety;
- Advance a national quality agenda for hospitals and hospitalists;
- Facilitate cultural change and develop initiatives to promote hospital medicine’s role in quality initiatives within the C-suite at our nation’s hospitals;
- Evaluate effectiveness of current offerings in quality improvement or patient safety;
- Promote adoption of health information technologies to advance patient safety and quality improvement; and
- Promote and support the new science of QI (i.e., develop a research agenda).
Linking these focus areas with SHM’s Core Competencies in Hospital Medicine and current national industry quality initiatives, such as The Joint Commission’s National Patient Safety Goals and Core Measures, is the basic foundation for SHM’s strategic quality plan. This plan will allow SHM to become more proactive in our approach to quality initiatives for the next one, three, five, and 10 years. It will help us focus on the areas identified as needs for hospitalists and hospital medicine.
Plan Breakdown
I would like to tell you about some of the newest quality initiatives currently underway. First is an advisory board on pharmacoeconomics. We are pulling together 11 industry leaders, including CEOs, chief financial officers, chief medical officers, pharmacists, and thought leaders in quality. This advisory board will be evaluating the standard operating procedures in hospitals across the country regarding the use of pharmacoeconomics in decisions to utilize medications, especially newer and more expensive agents, using venous thromboembolism (VTE) prevention as a case example.
We have assembled another advisory board, which is just underway, to look at complicated skin and skin structure infections (cSSSI). This board will include hospitalists, infection control physicians, emergency room physicians, pharmacists, wound care nurses, and quality improvement experts.
Lastly, we have a second VTE project charged with the development of an automated, electronic query for a major commercial clinical information system. The project team is tasked to develop and demonstrate how a system can dramatically increase the prevalence of VTE prophylaxis in hospitals where it will be piloted.
Collaborative Efforts
I also would like to share a few of the other exciting activities the quality department has been up to recently. SHM is in the process of collaborating on a book with the Joint Commission Resources on Hospitalists and Patient Safety. The book is scheduled for release this spring, so be on the lookout. We also are writing an article for the The Physician Executive, which is the journal of the American College of Physician Executives. The article focuses on how quality, patient safety, and patient satisfaction are becoming priorities for physician executives, and how hospitalists are a critical element of a strategy to address this priority. We also are talking with other organizations, such as the American Hospital Association and United Health Group, about potential collaborative work in the future.
So, you can see things at SHM headquarters are anything but dull. The entire QI department—both the veterans and new staff—are looking forward to helping you make your quality and patient safety initiatives more successful and improve the care of patients throughout the country. TH
Healthcare quality and patient safety is a hot topic in hospitals across the country, as well as here at The Society of Hospital Medicine (SHM). It seems like every day we hear of new regulatory requirements from the Centers for Medicare and Medicaid Services (CMS), The Joint Commission, and state health departments, or requirements from other health care organizations, including insurance companies. It’s hard to keep up with it all.
To help hospitals with their quality initiatives, SHM recently beefed up its Quality Initiatives Department by adding three new staff members, including myself. I’d like to introduce you to our newest members. My name is Jane Kelly-Cummings, and I joined SHM in July as the senior director of Quality Initiatives. I’m responsible for the strategic planning, development and implementation of quality initiatives for the society and the staff liaison for the Hospital Quality and Patient Safety Committee. I’ve been a registered nurse (RN) for more than 20 years and have been working in the quality world for more than a decade.
Linda Boclair joined SHM in June as director of Quality Initiatives. She is responsible for proposal/grant writing, managing select quality initiative projects, and department operations. She has a background in medical technology and industrial relations/organizational behavior. Linda currently is working on a medication reconciliation project, an advisory panel on pharmacoeconomics, and she is working on the new co-management taskforce.
Lauren Valentino also joined SHM in June as the departments project coordinator. She is responsible for supporting the Quality Initiatives department through project planning and implementation. Lauren also is a support resource for the society, providing documentation and coordination to the committees, task forces, and various special interest groups.
The new staffers join Joy M. Wittnebert-Barnosky, senior project manager; Tina Budnitz, senior advisor for Quality Initiatives; and Kathleen Kerr, project manager of QI Mentored Implementation in rounding out SHM’s new Quality Initiatives Department.
In order to best meet the quality initiatives needs of our members, we have been developing SHM’s quality initiatives strategic plan. We had the chance to share the plan with SHM board members at the bi-annual board meeting in October. The board received the strategic plan well, and the group is looking forward to an update at the January board meeting. SHM’s strategic quality initiative planning began in October 2007, when a group of SHM leaders met to begin the process of developing a 10-year strategic plan for quality initiatives. The output of this meeting led to six areas of focus, which we refer to as core strategies:
- Develop education programs and technical support tools in quality improvement or patient safety;
- Advance a national quality agenda for hospitals and hospitalists;
- Facilitate cultural change and develop initiatives to promote hospital medicine’s role in quality initiatives within the C-suite at our nation’s hospitals;
- Evaluate effectiveness of current offerings in quality improvement or patient safety;
- Promote adoption of health information technologies to advance patient safety and quality improvement; and
- Promote and support the new science of QI (i.e., develop a research agenda).
Linking these focus areas with SHM’s Core Competencies in Hospital Medicine and current national industry quality initiatives, such as The Joint Commission’s National Patient Safety Goals and Core Measures, is the basic foundation for SHM’s strategic quality plan. This plan will allow SHM to become more proactive in our approach to quality initiatives for the next one, three, five, and 10 years. It will help us focus on the areas identified as needs for hospitalists and hospital medicine.
Plan Breakdown
I would like to tell you about some of the newest quality initiatives currently underway. First is an advisory board on pharmacoeconomics. We are pulling together 11 industry leaders, including CEOs, chief financial officers, chief medical officers, pharmacists, and thought leaders in quality. This advisory board will be evaluating the standard operating procedures in hospitals across the country regarding the use of pharmacoeconomics in decisions to utilize medications, especially newer and more expensive agents, using venous thromboembolism (VTE) prevention as a case example.
We have assembled another advisory board, which is just underway, to look at complicated skin and skin structure infections (cSSSI). This board will include hospitalists, infection control physicians, emergency room physicians, pharmacists, wound care nurses, and quality improvement experts.
Lastly, we have a second VTE project charged with the development of an automated, electronic query for a major commercial clinical information system. The project team is tasked to develop and demonstrate how a system can dramatically increase the prevalence of VTE prophylaxis in hospitals where it will be piloted.
Collaborative Efforts
I also would like to share a few of the other exciting activities the quality department has been up to recently. SHM is in the process of collaborating on a book with the Joint Commission Resources on Hospitalists and Patient Safety. The book is scheduled for release this spring, so be on the lookout. We also are writing an article for the The Physician Executive, which is the journal of the American College of Physician Executives. The article focuses on how quality, patient safety, and patient satisfaction are becoming priorities for physician executives, and how hospitalists are a critical element of a strategy to address this priority. We also are talking with other organizations, such as the American Hospital Association and United Health Group, about potential collaborative work in the future.
So, you can see things at SHM headquarters are anything but dull. The entire QI department—both the veterans and new staff—are looking forward to helping you make your quality and patient safety initiatives more successful and improve the care of patients throughout the country. TH
Medicare Modifications
Most hospitalists vividly recall Congress overriding President Bush’s July veto to avert a hefty, 10.6% cut in Medicare Part B payments to physicians. That memorable, last-minute save (instead of a pay cut, Congress increased Part B payments by 1.1%) was just a tiny part of some important legislation. The Medicare Improvements for Patients and Providers Act (MIPPA) includes myriad provisions addressing Medicare benefits, protections for low-income beneficiaries, changes for providers, data collection requirements for correcting healthcare disparities, and much more.
Hospitalists will be particularly interested in a handful of the provisions outlined in MIPPA, some of which impact them directly and others that will affect hospitals and clinical care, and still more whose outcomes remain to be seen.
For example, MIPPA is the legislation that extends the Physician Quality Reporting Initiative (PQRI) for two years, offering a bonus payment in 2009 and 2010 of 2% (up from 1.5%) of total Medicare allowed charges. It also directs the Centers for Medicare and Medicaid Services (CMS) to publicly post the list of providers who participate in the PQRI. (See “A Permanent PQRI” in the October 2008 issue of The Hospitalist.)
MIPPA also requires CMS to establish a program to promote widespread adoption of electronic prescribing, as outlined in “e-Prescription for Success?” in the September 2008 issue of The Hospitalist. Reporting on e-prescribing is not likely to apply to hospitalists, says Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City and a member of SHM’s Public Policy Committee. “Of course, it depends on whether the hospital uses it, but no one can say whether a hospitalist will get a benefit for reporting on e-prescribing,” he says.
Lucrative Changes to E&M Codes
One provision directly impacting hospitalists is MIPPA’s changes to payments for inpatient evaluation and management codes (E&M codes). According to Laura Allendorf, SHM’s senior advisor for advocacy and government affairs, this change will result in an estimated 3% average gain in total Medicare payments to hospitalists, or $5,000 to $6,000 annually—on top of the 1.1% payment update. (It’s important to note the final 2009 physician fee schedule, published in November, could change the overall impact for individual members.) E&M payments from some private payers also could increase, since many base their fees on Medicare’s fee schedule.
Quality Research Initiatives
MIPPA requires the establishment or continuation of several quality research initiatives, designed to help CMS determine new models of efficiency of care and cost efficiency.
One of these initiatives is Patient-Centered Medical Home (PCMH), a care model that facilitates partnerships between individual patients and their personal physicians, and when appropriate, the patient’s family. Care is facilitated by registries, information technology, health information exchange, and other means to assure patients get the indicated care when and where they need and want it in a culturally and linguistically appropriate manner. MIPPA grants new funding and expanded authority for CMS’ Medical Home Demonstration Project—if certain quality and/or savings targets are achieved.
“We’ve talked a bit about Patient-Centered Medical Home,” says Dr. Flansbaum of SHM’s Public Policy Committee. “From a political standpoint, it’s a feel-good agenda item with a lot of bipartisan support. The notion of this is here, but operationalizing it—getting it to work—is an entirely different story.” By definition, PCMH will revolve around primary care physicians, and the role and responsibilities of any hospitalists involved is yet unknown—as is the reimbursement model. “This is so far away right now, it’s a notion that needs to be turned into a theory that needs to be turned into a paradigm, to paraphrase Woody Allen,” Dr. Flansbaum says.
Another initiative greenlighted by MIPPA is comparative effectiveness research, or CER. It examines the effectiveness of different therapies for a specific medical condition, or for a specific set of patients, to determine the best option. It may involve comparing competing medications, or may analyze different treatment approaches such as surgery, devices, and drug therapies. MIPPA requires the Institute of Medicine report on best practices for the review of comparative effectiveness research and the development of clinical protocols.
“Obviously, the medical device companies and the pharmaceutical companies are against this,” Dr. Flansbaum says. “But it would be helpful for physicians, because it would give some guidance in certain gray-area treatments, such as: Is this drug appropriate in treating an end-stage cancer patient?” And as far as the nation’s health care system goes, he explains, “I think we need comparative effectiveness. We can’t continue as we are—on the net, we’re going broke—our current healthcare system can’t afford to keep going.”
Not the Only Game in Town
One interesting provision of MIPPA revokes “the unique authority of the Joint Commission to deem hospitals in compliance with the Medicare Conditions of Participation,” meaning hospital compliance is an open market—subject to approval from CMS, of course.
“The Joint Commission has been the gold standard for hospitals for a long, long time,” Dr. Flansbaum points out. “Now that they’ve opened that up, DNV (Det Norske Veritas Healthcare, Inc.) [for example], can compete with the Joint Commission to certify hospitals.”
What will this mean for hospitals? Probably not much in the short term. “I believe only 15 hospitals have DNV certifications, and that all of those also have a Joint Commission certification,” Dr. Flansbaum says, adding “[DNV and the Joint Commission] have a different approach; it’s like the ACT and the SAT. Both are used for college entrance exams, but the SAT is still mostly the gold standard, like the Joint Commission. But who knows? That could change … and if it does, well, competition is good.”
Some of the MIPPA provisions, such as the quality research initiatives, could end up shaping the future of healthcare. Others, such as the continuation of PQRI, may lead to new payment models for physicians.
Only time will tell which provisions will truly improve efficiency and costs—and which will impact hospital medicine in particular. TH
Jane Jerrard is a medical writer based in Chicago.
Most hospitalists vividly recall Congress overriding President Bush’s July veto to avert a hefty, 10.6% cut in Medicare Part B payments to physicians. That memorable, last-minute save (instead of a pay cut, Congress increased Part B payments by 1.1%) was just a tiny part of some important legislation. The Medicare Improvements for Patients and Providers Act (MIPPA) includes myriad provisions addressing Medicare benefits, protections for low-income beneficiaries, changes for providers, data collection requirements for correcting healthcare disparities, and much more.
Hospitalists will be particularly interested in a handful of the provisions outlined in MIPPA, some of which impact them directly and others that will affect hospitals and clinical care, and still more whose outcomes remain to be seen.
For example, MIPPA is the legislation that extends the Physician Quality Reporting Initiative (PQRI) for two years, offering a bonus payment in 2009 and 2010 of 2% (up from 1.5%) of total Medicare allowed charges. It also directs the Centers for Medicare and Medicaid Services (CMS) to publicly post the list of providers who participate in the PQRI. (See “A Permanent PQRI” in the October 2008 issue of The Hospitalist.)
MIPPA also requires CMS to establish a program to promote widespread adoption of electronic prescribing, as outlined in “e-Prescription for Success?” in the September 2008 issue of The Hospitalist. Reporting on e-prescribing is not likely to apply to hospitalists, says Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City and a member of SHM’s Public Policy Committee. “Of course, it depends on whether the hospital uses it, but no one can say whether a hospitalist will get a benefit for reporting on e-prescribing,” he says.
Lucrative Changes to E&M Codes
One provision directly impacting hospitalists is MIPPA’s changes to payments for inpatient evaluation and management codes (E&M codes). According to Laura Allendorf, SHM’s senior advisor for advocacy and government affairs, this change will result in an estimated 3% average gain in total Medicare payments to hospitalists, or $5,000 to $6,000 annually—on top of the 1.1% payment update. (It’s important to note the final 2009 physician fee schedule, published in November, could change the overall impact for individual members.) E&M payments from some private payers also could increase, since many base their fees on Medicare’s fee schedule.
Quality Research Initiatives
MIPPA requires the establishment or continuation of several quality research initiatives, designed to help CMS determine new models of efficiency of care and cost efficiency.
One of these initiatives is Patient-Centered Medical Home (PCMH), a care model that facilitates partnerships between individual patients and their personal physicians, and when appropriate, the patient’s family. Care is facilitated by registries, information technology, health information exchange, and other means to assure patients get the indicated care when and where they need and want it in a culturally and linguistically appropriate manner. MIPPA grants new funding and expanded authority for CMS’ Medical Home Demonstration Project—if certain quality and/or savings targets are achieved.
“We’ve talked a bit about Patient-Centered Medical Home,” says Dr. Flansbaum of SHM’s Public Policy Committee. “From a political standpoint, it’s a feel-good agenda item with a lot of bipartisan support. The notion of this is here, but operationalizing it—getting it to work—is an entirely different story.” By definition, PCMH will revolve around primary care physicians, and the role and responsibilities of any hospitalists involved is yet unknown—as is the reimbursement model. “This is so far away right now, it’s a notion that needs to be turned into a theory that needs to be turned into a paradigm, to paraphrase Woody Allen,” Dr. Flansbaum says.
Another initiative greenlighted by MIPPA is comparative effectiveness research, or CER. It examines the effectiveness of different therapies for a specific medical condition, or for a specific set of patients, to determine the best option. It may involve comparing competing medications, or may analyze different treatment approaches such as surgery, devices, and drug therapies. MIPPA requires the Institute of Medicine report on best practices for the review of comparative effectiveness research and the development of clinical protocols.
“Obviously, the medical device companies and the pharmaceutical companies are against this,” Dr. Flansbaum says. “But it would be helpful for physicians, because it would give some guidance in certain gray-area treatments, such as: Is this drug appropriate in treating an end-stage cancer patient?” And as far as the nation’s health care system goes, he explains, “I think we need comparative effectiveness. We can’t continue as we are—on the net, we’re going broke—our current healthcare system can’t afford to keep going.”
Not the Only Game in Town
One interesting provision of MIPPA revokes “the unique authority of the Joint Commission to deem hospitals in compliance with the Medicare Conditions of Participation,” meaning hospital compliance is an open market—subject to approval from CMS, of course.
“The Joint Commission has been the gold standard for hospitals for a long, long time,” Dr. Flansbaum points out. “Now that they’ve opened that up, DNV (Det Norske Veritas Healthcare, Inc.) [for example], can compete with the Joint Commission to certify hospitals.”
What will this mean for hospitals? Probably not much in the short term. “I believe only 15 hospitals have DNV certifications, and that all of those also have a Joint Commission certification,” Dr. Flansbaum says, adding “[DNV and the Joint Commission] have a different approach; it’s like the ACT and the SAT. Both are used for college entrance exams, but the SAT is still mostly the gold standard, like the Joint Commission. But who knows? That could change … and if it does, well, competition is good.”
Some of the MIPPA provisions, such as the quality research initiatives, could end up shaping the future of healthcare. Others, such as the continuation of PQRI, may lead to new payment models for physicians.
Only time will tell which provisions will truly improve efficiency and costs—and which will impact hospital medicine in particular. TH
Jane Jerrard is a medical writer based in Chicago.
Most hospitalists vividly recall Congress overriding President Bush’s July veto to avert a hefty, 10.6% cut in Medicare Part B payments to physicians. That memorable, last-minute save (instead of a pay cut, Congress increased Part B payments by 1.1%) was just a tiny part of some important legislation. The Medicare Improvements for Patients and Providers Act (MIPPA) includes myriad provisions addressing Medicare benefits, protections for low-income beneficiaries, changes for providers, data collection requirements for correcting healthcare disparities, and much more.
Hospitalists will be particularly interested in a handful of the provisions outlined in MIPPA, some of which impact them directly and others that will affect hospitals and clinical care, and still more whose outcomes remain to be seen.
For example, MIPPA is the legislation that extends the Physician Quality Reporting Initiative (PQRI) for two years, offering a bonus payment in 2009 and 2010 of 2% (up from 1.5%) of total Medicare allowed charges. It also directs the Centers for Medicare and Medicaid Services (CMS) to publicly post the list of providers who participate in the PQRI. (See “A Permanent PQRI” in the October 2008 issue of The Hospitalist.)
MIPPA also requires CMS to establish a program to promote widespread adoption of electronic prescribing, as outlined in “e-Prescription for Success?” in the September 2008 issue of The Hospitalist. Reporting on e-prescribing is not likely to apply to hospitalists, says Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City and a member of SHM’s Public Policy Committee. “Of course, it depends on whether the hospital uses it, but no one can say whether a hospitalist will get a benefit for reporting on e-prescribing,” he says.
Lucrative Changes to E&M Codes
One provision directly impacting hospitalists is MIPPA’s changes to payments for inpatient evaluation and management codes (E&M codes). According to Laura Allendorf, SHM’s senior advisor for advocacy and government affairs, this change will result in an estimated 3% average gain in total Medicare payments to hospitalists, or $5,000 to $6,000 annually—on top of the 1.1% payment update. (It’s important to note the final 2009 physician fee schedule, published in November, could change the overall impact for individual members.) E&M payments from some private payers also could increase, since many base their fees on Medicare’s fee schedule.
Quality Research Initiatives
MIPPA requires the establishment or continuation of several quality research initiatives, designed to help CMS determine new models of efficiency of care and cost efficiency.
One of these initiatives is Patient-Centered Medical Home (PCMH), a care model that facilitates partnerships between individual patients and their personal physicians, and when appropriate, the patient’s family. Care is facilitated by registries, information technology, health information exchange, and other means to assure patients get the indicated care when and where they need and want it in a culturally and linguistically appropriate manner. MIPPA grants new funding and expanded authority for CMS’ Medical Home Demonstration Project—if certain quality and/or savings targets are achieved.
“We’ve talked a bit about Patient-Centered Medical Home,” says Dr. Flansbaum of SHM’s Public Policy Committee. “From a political standpoint, it’s a feel-good agenda item with a lot of bipartisan support. The notion of this is here, but operationalizing it—getting it to work—is an entirely different story.” By definition, PCMH will revolve around primary care physicians, and the role and responsibilities of any hospitalists involved is yet unknown—as is the reimbursement model. “This is so far away right now, it’s a notion that needs to be turned into a theory that needs to be turned into a paradigm, to paraphrase Woody Allen,” Dr. Flansbaum says.
Another initiative greenlighted by MIPPA is comparative effectiveness research, or CER. It examines the effectiveness of different therapies for a specific medical condition, or for a specific set of patients, to determine the best option. It may involve comparing competing medications, or may analyze different treatment approaches such as surgery, devices, and drug therapies. MIPPA requires the Institute of Medicine report on best practices for the review of comparative effectiveness research and the development of clinical protocols.
“Obviously, the medical device companies and the pharmaceutical companies are against this,” Dr. Flansbaum says. “But it would be helpful for physicians, because it would give some guidance in certain gray-area treatments, such as: Is this drug appropriate in treating an end-stage cancer patient?” And as far as the nation’s health care system goes, he explains, “I think we need comparative effectiveness. We can’t continue as we are—on the net, we’re going broke—our current healthcare system can’t afford to keep going.”
Not the Only Game in Town
One interesting provision of MIPPA revokes “the unique authority of the Joint Commission to deem hospitals in compliance with the Medicare Conditions of Participation,” meaning hospital compliance is an open market—subject to approval from CMS, of course.
“The Joint Commission has been the gold standard for hospitals for a long, long time,” Dr. Flansbaum points out. “Now that they’ve opened that up, DNV (Det Norske Veritas Healthcare, Inc.) [for example], can compete with the Joint Commission to certify hospitals.”
What will this mean for hospitals? Probably not much in the short term. “I believe only 15 hospitals have DNV certifications, and that all of those also have a Joint Commission certification,” Dr. Flansbaum says, adding “[DNV and the Joint Commission] have a different approach; it’s like the ACT and the SAT. Both are used for college entrance exams, but the SAT is still mostly the gold standard, like the Joint Commission. But who knows? That could change … and if it does, well, competition is good.”
Some of the MIPPA provisions, such as the quality research initiatives, could end up shaping the future of healthcare. Others, such as the continuation of PQRI, may lead to new payment models for physicians.
Only time will tell which provisions will truly improve efficiency and costs—and which will impact hospital medicine in particular. TH
Jane Jerrard is a medical writer based in Chicago.