User login
Perceptions of Hospital Discharge Software
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
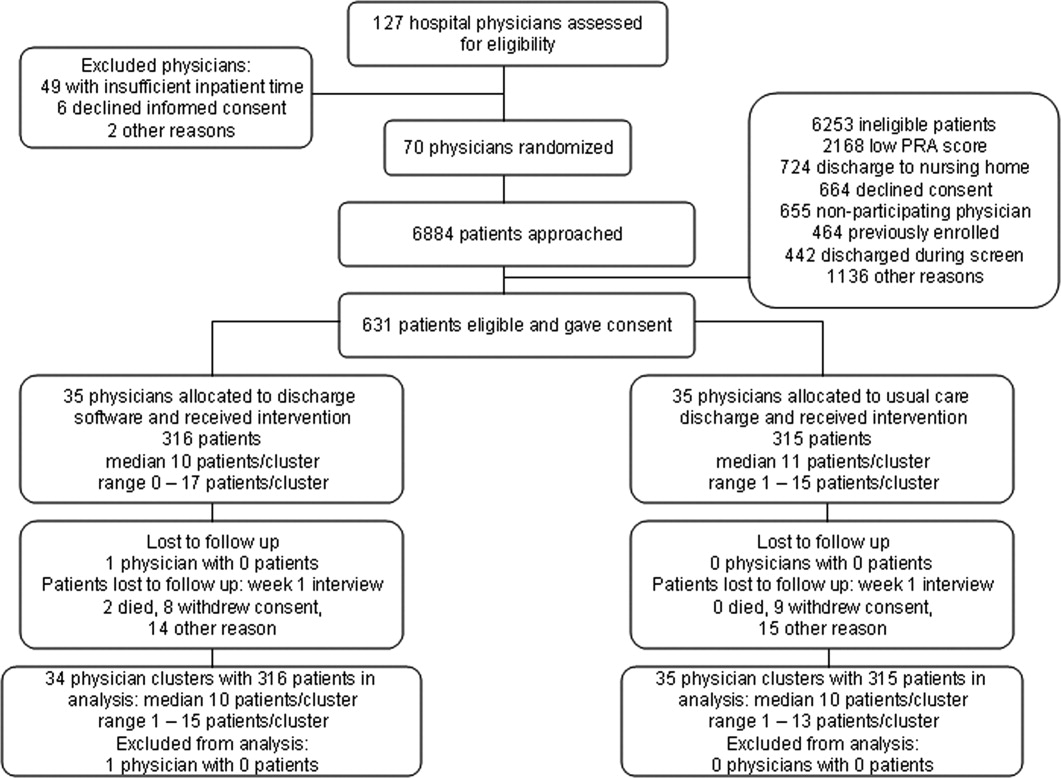
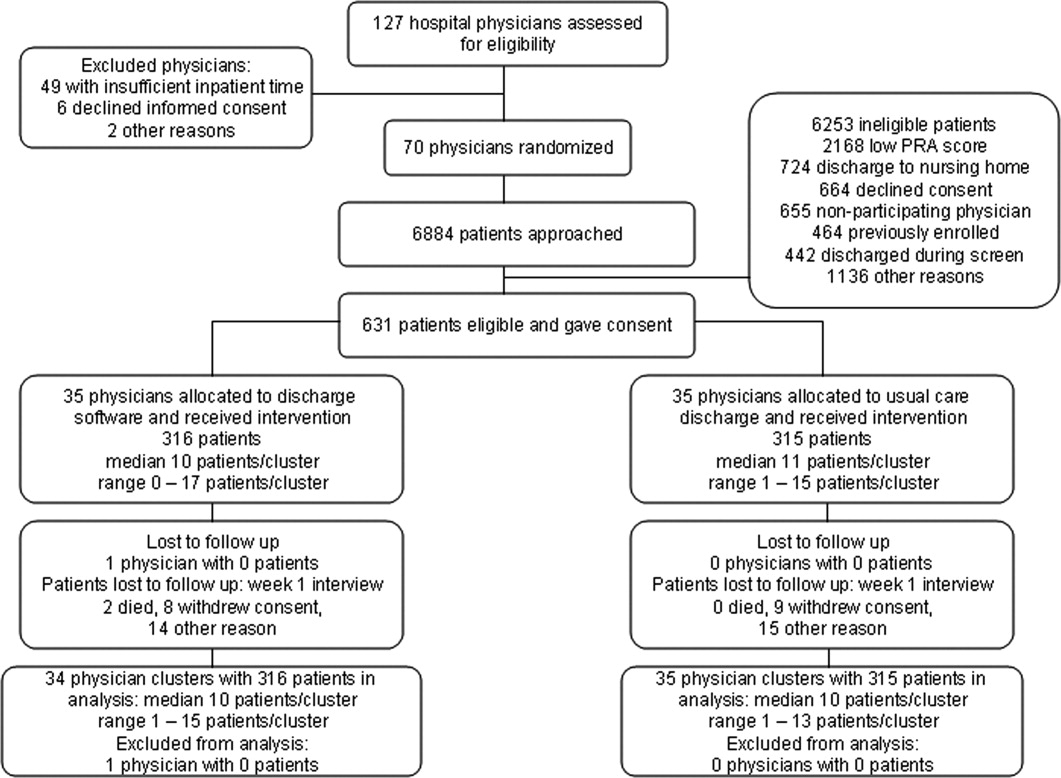
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.
| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
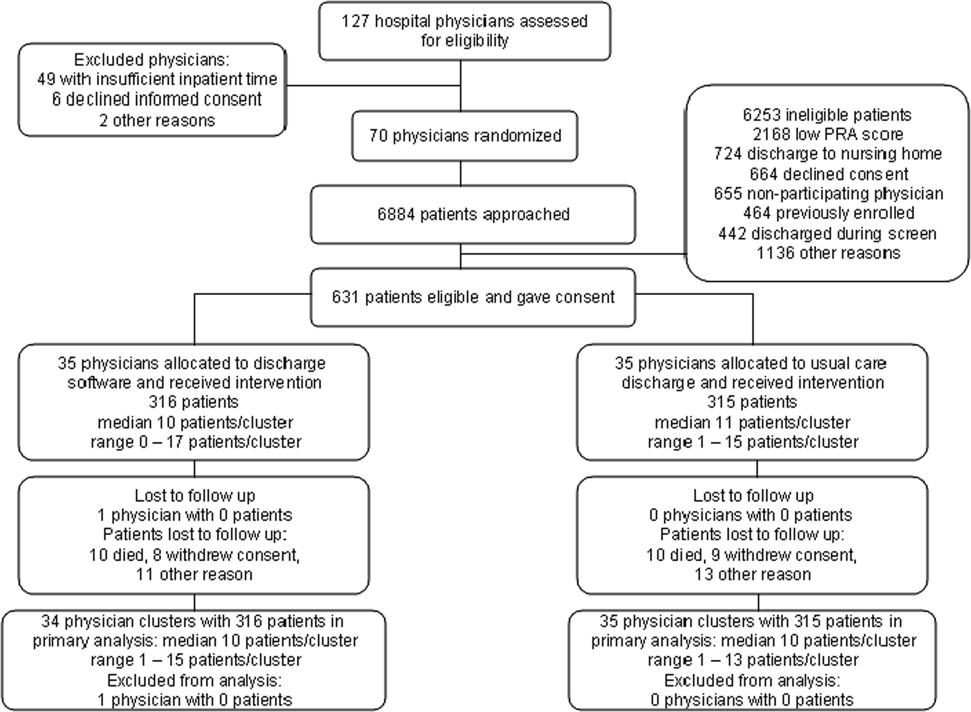
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
Copyright © 2009 Society of Hospital Medicine
Discharge Software and Readmissions
Adverse events occur to patients after their discharge from acute care hospitals.1, 2 Most of these injuries are adverse drug events, procedure‐related events, nosocomial infections, or falls.1 Postdischarge adverse events are associated with several days of symptoms, nonpermanent disability, emergency department visits, or hospital readmission.1, 3 When adverse events are preventable or ameliorable, the most common root cause is poor communication between hospital personnel and either the patient or the outpatient primary care physician.1 In addition, there may be deficits in discharge processes related to assessment and communication of unresolved problems.1 Systematic reviews have shown that discharge communication is an inefficient and error‐prone process.46
One potential solution to poor discharge communication is health information technology.7 An example of technology is discharge software with a computerized physician order entry (CPOE) system. By definition, a CPOE system is a computer‐based system that automates direct entry of orders by physicians and ensures standardized, legible, and complete orders.8 The benefits of CPOE have been tested in other inpatient settings.8, 9 It is logical to consider software applications with CPOE for discharge interventions.7
Several mechanisms explain the potential benefit of discharge software with CPOE.7 Applications with CPOE decrease medication errors.8, 10 Software with decision support could prompt physicians to enter posthospitalization appointment dates and orders for preventive services.11, 12 Discharge software could facilitate medication reconciliation and generate patient instructions and information.4, 1315 The potential benefits of discharge software with CPOE provide a rationale for clinical trials to measure benefits.
Previous studies addressed discharge applications of health information technology. Observational studies recorded outcomes such as physician satisfaction.16, 17 Prior randomized clinical trials measured quality and timeliness of discharge summaries.18 However, these previous trials did not assess clinically relevant outcomes like readmissions, emergency department visits, or adverse events. We performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The rationale for our clustered design complied with recommendations from a systematic review of discharge interventions.5 Our objective was to assess the benefit of discharge software with CPOE when used to discharge patients at high risk for repeat admission. After the intervention, we compared the rates of hospital readmission, emergency department visits, and postdischarge adverse events due to medical management.
Methods
The trial design was a cluster randomized, controlled trial with blinded outcome assessment. Follow‐up occurred until 6 months after discharge from index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
The cluster definition was the hospital physician. Patients discharged by the physician comprised the cluster. Hospital physicians and patients were enrolled between November 2004 and January 2007. Internal medicine resident or attending physicians were eligible. We excluded hospital physicians if their assignments to inpatient duties were less than 2 months during the 27‐month enrollment period. The rationale for the physician exclusion was a consequence of the patient enrollment rate of 3 to 5 patients per physician per month. Physicians with brief assignments could not achieve the goal of 9 or more patients per cluster. After physicians gave informed consent to screen their patients, trained research coordinators applied inclusion and exclusion criteria and obtained informed consent from patients. Research personnel identified all consecutive, unique, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) score 0.40.19, 20 The Pra score came from a logistic model of age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. Other details about exclusion criteria have been published.21 If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was a CPOE software application that facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. Details about the discharge software appeared in a previous publication.7 Software features included required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software prompted the discharging physician to enter pending tests and order tests after discharge. Hospital physicians used the software on the day of discharge and automatically generated 4 discharge documents. The first document was a personalized letter to the outpatient physician with discharge diagnoses, reconciled medication list, diet and activity instructions, patient education materials provided, and follow‐up appointments and studies. Second, the software printed legible prescriptions along with specific information for the dispensing pharmacist about changes and deletions in the patient's previous regimen. Third, the software created patient instructions with addresses and telephone numbers for follow‐up appointments and tests. Fourth, the software printed a legible discharge order including all of the aforementioned information.
The control intervention was the usual care discharge process as described previously.7 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. A previous publication gave details about the standard care available to all patients regardless of intervention.7
Randomization
The unit of randomization was the hospital physician who performed the discharge process. Random allocation was to discharge software or usual care discharge process. The randomization ratio was 1:1, the block size was 2, and there was no stratification or matching. There was concealed allocation and details are available from the investigators. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients. Likewise, it was not possible to conceal the outcome ascertainment, including readmission, from the hospital physicians.
All hospital physicians received training on the usual care discharge process. Physicians assigned to discharge software completed additional training via multimedia demonstration with 1‐on‐1 coaching as needed. Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. After informed consent, patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge from the index hospitalization.
The baseline assessment of patient characteristics occurred during the index hospitalization. Trained data abstractors recorded patient demographic data plus variables to calculate the Pra score for probability for repeat admission. We recorded additional variables because of their possible association with readmission.15, 2229 Data came from the patient or proxy for physical functioning and mental health (SF‐36, Version 2; Medical Outcomes Trust, Boston, MA). Other data for predictor variables came from interviews or hospital records.
Outcome Assessment
The primary study outcome was the proportion of patients readmitted at least once within 6 months after the index hospitalization. Readmission was for any reason and included observation and full admission status. Secondary outcomes were emergency department visits that did not result in hospital admission. Outcome assessment occurred at the patient level. We obtained data for readmissions and emergency department visits from 6 hospitals in central Illinois where study patients were likely to seek care. We validated readmissions and emergency department visits via patient/proxy telephone interviews that occurred 6 months after index hospital discharge. Interviewers were blind to intervention assignment. We evaluated the adequacy of the blind and asked interviewers to guess the patient's intervention assignment.
Another secondary outcome was the proportion of patients who experienced an adverse event related to medical management within 1 month after discharge. For adverse event ascertainment, we employed the process of Forster et al.1, 2 Within 20 to 40 days after discharge, an internal medicine physician performed telephone interviews with the patient or proxy. The interviewer recorded symptoms, drug information, other treatment, hospital readmissions, and emergency department visits. Another physician compiled case summaries from interview data and information abstracted from the electronic medical record, including dictated discharge summaries from the index hospitalization and postdischarge emergency department visits, diagnostic test results, and readmission reports. Two additional internal medicine physicians adjudicated each case summary separately. We counted adverse events only when adjudicators agreed that medical management probably or definitely caused the event. The initial rating by each adjudicator revealed moderate‐to‐good agreement (Kappa = 0.52).30 When initial adjudications were discordant, then adjudicators met and resolved all discrepancies. The adjudicators also scored the severity of the adverse event. The severity scale options were serious laboratory abnormality only, 1 day of symptoms, several days of symptoms, nonpermanent disability, permanent disability, or death. The adjudicators also scored the adverse event as preventable (yes/no), ameliorable (yes/no), and recorded system problems associated with preventable and ameliorable adverse events.1 For adverse drug events, the adjudicators recorded preventability categories defined by previous investigators.31 We designed the adverse event outcome ascertainment as a blinded process. We evaluated the success of the blind and asked adjudicators to guess the patient's intervention assignment.
Sample Size
The sample size analysis employed several assumptions regarding the proportion of readmitted patients. The estimated readmission rate after usual care was 37%.24, 3236 The minimum clinically relevant difference in readmission rates was 13%, an empirical boundary for quantitative significance.37 Estimates for intracluster correlation were not available when we designed the trial. We projected intracluster correlations with low, medium, and high values. The cluster number and size were selected to maintain test significance level, 1‐sided alpha, <0.05 and power >80%. The sample size assumed no interim analysis. The initial sample size estimates were 11 physician clusters per intervention with 25 patients per cluster. During the first 2 months of patient recruitment, we observed that we could not consistently achieve clusters with 25 patients. We recalculated the sample size. Using the same assumptions, we found we could achieve similar test significance and power with 35 physician clusters per intervention and 9 patients per cluster. The sample size calculator was nQuery (Statistical Solutions, Saugus, MA).
Statistical Methods
Analyses were performed with SPSS PC (Version 15.0.1; SPSS Inc, Chicago, IL). Using descriptive statistics, we reported baseline variables as means and standard deviations (SD) for interval variables, and percentages for categorical variables. For outcome variables, we utilized the principle of intention‐to‐treat and assumed patient exposure to the intervention randomly assigned to their discharging physician. We inspected scatter plots and correlations for all variables to test assumptions regarding normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. When assumptions failed, we stratified variables (median or thirds) or performed transformations to satisfy assumptions. For patient‐level outcome variables, we calculated intracluster correlation coefficients. The assessment of the blind was unaffected by the cluster assumption so we used the chi‐square procedure. For analysis of time to event, we used Kaplan‐Meier plots.
The primary hypothesis was a significant decrease in the primary readmission outcome for patients assigned to discharge software. We tested the primary hypothesis with generalized estimating equations that corrected for clustering by hospital physician and adjusted for covariates that predicted readmission. The intervention variable was discharge software versus usual care handwritten discharge. We reported parameter estimates of the intervention variable coefficient and Wald 95% confidence interval (95% CI) with and without correction for cluster. For the secondary, patient‐level outcomes, we performed similar analyses with generalized estimating equations that corrected for clustering by hospital physician.
During covariate analysis, we screened all baseline variables for their correlation with readmission. The variable with the highest correlation and P value <0.05 entered initially in the general estimating equation. After initial variable entry, we evaluated subsequent variables with partial correlations that controlled for variables entered previously. At each iterative step, we entered into the model the variable with the highest partial correlation and P value <0.05.
In exploratory analyses, we examined intervention group differences within strata defined by covariates that predicted readmission. We used generalized estimating equations and adjusted for the other covariates that predicted readmission.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. The physician characteristics appear in Table 1. Most of the hospital physicians were interns in the first year of postgraduate training (58.6%; 41/70). We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). The most common reason for hospital physician exclusion applied to resident physicians in their last months of training before graduation or emergency department residents temporarily assigned to internal medicine training. We approached 6,884 patients during their index hospitalization. After excluding 6,253 ineligible patients, we enrolled and followed 631 patients who received the discharge intervention (Figure 1). During 6 months of follow‐up, a small proportion of patients died (3%; 20/631). Hospital records were available for deceased patients and they were included in the analysis. A small proportion (6%; 41/631) of patients withdrew consent or left the trial for other reasons during 6 months. There was no differential dropout between the interventions. Protocol deviations were rare (0.5%; 3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly‐assigned hospital physicians and their patients are in Table 1.
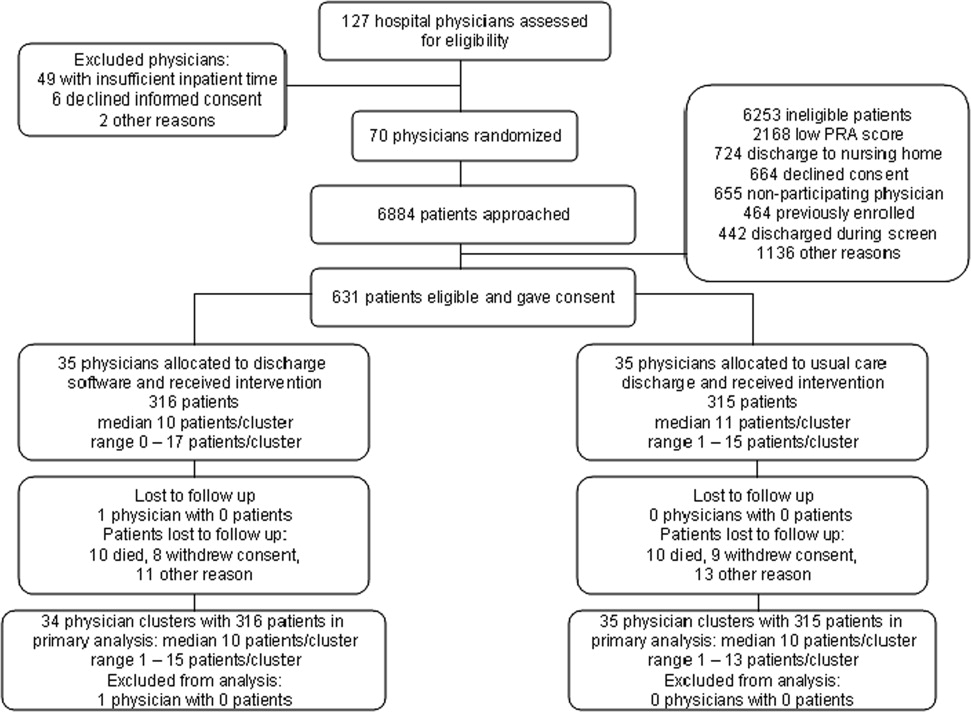
| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | (n = 35) | (n = 35) |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics | (n = 316) | (n = 315) |
| Gender, female, n (%) | 180 (57.0) | 168 (53.3) |
| Age, years, n (%) | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Race, n (%) | ||
| Caucasian | 239 (75.6) | 229 (72.7) |
| Black | 72 (22.8) | 85 (27.0) |
| Other | 5 (1.6) | 1 (0.3) |
| Self‐rated health status, n (%) | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus, n (%) | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease, n (%) | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease, n (%) | 133 (42.1) | 120 (38.1) |
| Heart failure, n (%) | 80 (25.3) | 67 (21.3) |
| Informal caregiver available, yes, n (%) | 313 (99.1) | 313 (99.4) |
| Taking loop diuretic, n (%) | 110 (34.8) | 88 (27.9) |
| Physical functioning from SF‐36, n (%) | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental health from SF‐36, n (%) | ||
| Lowest third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Hospital admissions during year prior to index admission, n (%) | ||
| 0 or 1 | 247 (78.2) | 224 (71.1) |
| 2 or more | 69 (21.8) | 91 (28.9) |
| Emergency department visits during 6 months before index admission, n (%) | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Outpatient doctor or clinic visits during year prior to index admission | ||
| 0 to 4 | 97 (30.7%) | 77 (24.4%) |
| 5 to 8 | 68 (21.5%) | 81 (25.7%) |
| 9 to 12 | 82 (25.9%) | 84 (26.7%) |
| 13 or more | 69 (21.8%) | 73 (23.2%) |
| Insurance or payor | ||
| Medicare, age less than 65 years | 18 (5.7%) | 13 (4.1%) |
| Medicare, age 65 years and older | 56 (17.7%) | 40 (12.7%) |
| Medicaid, age less than 65 years | 98 (31.0%) | 130 (41.3%) |
| Medicaid, age 65 years and older | 17 (5.4%) | 20 (6.3%) |
| Commercial or veteran | 85 (26.9%) | 61 (19.4%) |
| Self‐pay | 42 (13.3%) | 51 (16.2%) |
| Religious participation | ||
| Never | 159 (50.3%) | 164 (52.1%) |
| 1‐24 times per year | 55 (17.4%) | 51 (16.2%) |
| 1‐7 times per week | 102 (32.3%) | 100 (31.7%) |
| Volunteer activity, 1 or more hour/month | 31 (9.8%) | 39 (12.4%) |
| Employment status | ||
| Not working | 229 (72.5%) | 233 (74.4%)* |
| Part‐time (<37.5 hours/week) | 30 (9.5%) | 25 (8.0%)* |
| Full‐time (at least 37.5 hour/week) | 57 (18.0%) | 55 (17.6%)* |
| Number of discharge medications, mean (SD) | 10.5 (4.8) | 9.9 (5.1) |
| Severity of illness, mean (SD) | 1.8 (1.2) | 1.6 (1.3) |
| Charlson‐Deyo comorbidity, mean (SD) | 1.7 (1.4) | 1.6 (1.9) |
| Index hospital length of stay, days, mean (SD) | 3.9 (3.5) | 3.5 (3.5) |
| Blood urea nitrogen, mean (SD) | 17.9 (12.9) | 19.1 (12.9) |
| Probability of repeat admission, Pra, mean (SD) | 0.486 (0.072) | 0.495 (0.076) |
We asked outpatient physicians about their receipt of discharge communication from hospital physicians. The text of the question was, How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? We mailed the question 10 days after discharge to outpatient physicians designated by patients enrolled in the study. Among patients in the discharge software group, 75.0% (237/316) of their outpatient physicians responded to the question. The response rate was 80.6% (254/315) from physicians who followed patients in the usual care group. Respondents from the discharge software group said within 1‐2 days or within a week for 56.0% (177/316) of patients. Respondents from the usual care group said within 1‐2 days or within a week for 57.1% (180/315) of patients. The difference between the intervention groups, 1.1% (95% CI, 9.2% to 6.9%), was not significant.
The primary, prespecified, outcome of the study was the proportion of patients with at least 1 readmission to the hospital. After intervention with discharge software versus usual care, there was no significant difference in readmission rates (Table 2) or time to first readmission (Figure 2). We screened all baseline variables in Table 1 and sought predictors of readmission to employ in adjusted models. For example, we evaluated physician level of training because we wondered if experience or seniority affected readmission when hospital physicians used the discharge software or usual care discharge. The candidate variable, physician level of training, did not correlate with readmission (rho = 0.066; P = 0.100), so it was dropped from subsequent analyses. After screening all variables in Table 1, we found 4 independent predictors of readmission: previous hospitalizations, previous emergency department visits, heart failure, and physical function. Generalized estimating equations for readmission that adjusted for predictor variables confirmed a negligible parameter estimate for the discharge intervention variable coefficient (Table 2).
| Outcome | Discharge Software, n (%) | Usual Care, n (%) | Parameter Estimate Without Cluster Correction Intervention Coefficient (95% CI) | P Value | Parameter Estimate With Cluster Correction Intervention Coefficient (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Readmitted within 6 months | 117 (37.0%) | 119 (37.8%) | 0.005* (0.076, 0.067) | 0.897 | 0.005* (0.074, 0.065) | 0.894 |
| Emergency department visit within 6 months | 112 (35.4%) | 128 (40.6%) | 0.052 (0.128, 0.024) | 0.179 | 0.052 (0.115, 0.011) | 0.108 |
| Adverse event within 1 month | 23 (7.3%) | 23 (7.3%) | 0.003 (0.037, 0.043) | 0.886 | 0.003 (0.037, 0.043) | 0.884 |
We evaluated emergency department visits that were unrelated to readmission as secondary, prespecified, outcomes. The results were similar to readmission results. While the proportion of patients with at least 1 emergency department visit was lower for the discharge software intervention, the difference with usual care was not significant (Table 2). There was no significant difference between interventions for time to first emergency department visit (Figure 3).
Postdischarge adverse events were secondary, prespecified, outcomes. Data for adverse event adjudication were available for 98% (309/316) of discharge software patients and 97% (307/315) of usual care patients. Within 1 month after discharge, 46 patients had adverse events probably or definitely related to medical management. Two patients had 2 events and 1 patient had 3 events. For analysis, we randomly selected 1 event per patient. When comparing patients assigned to discharge software versus usual care, there were no differences in adverse events related to medical management (Table 2). Most of the events were possible adverse drug events (74%; 34/46). The adverse event severity was several days of symptoms or nonpermanent disability for 76% (35/46) of the adverse events. Adjudicators rated 26% (12/46) of the adverse events as preventable and 46% (21/46) as ameliorable. The absolute numbers of events were small. There were no differences between discharge software and usual care patients within adverse event strata defined by type, severity, preventable, or ameliorable (Table 3). For most of the patients with adverse events, the adjudicators could not identify a system problem or preventability category (Table 3). When a deficiency was evident, there was no pattern to suggest a significant difference between discharge software patients versus usual care patients.
| Discharge Software (n) | Usual Care Discharge (n) | |
|---|---|---|
| ||
| At least 1 adverse event | 23 | 23 |
| Preventable adverse event | 7 | 5 |
| Ameliorable adverse event | 9 | 12 |
| Adverse event severity | ||
| Serious laboratory abnormality only or 1 day of symptoms | 5 | 5 |
| Several days of symptoms or nonpermanent disability | 18 | 17 |
| Permanent disability or death | 0 | 1 |
| Adverse event by type | ||
| Possible adverse drug event | 17 | 17 |
| Procedure‐related injury | 2 | 1 |
| Therapeutic error | 4 | 4 |
| Diagnostic error | 0 | 1 |
| System problems associated with preventable or ameliorable adverse events | ||
| Inadequate patient education regarding the medical condition or its treatment | 0 | 1 |
| Poor communication between patient and physician | 2 | 1 |
| Poor communication between hospital and community physicians | 0 | 0 |
| Inadequate monitoring of the patient's illness after discharge | 0 | 6 |
| Inadequate monitoring of the patient's treatment after discharge | 2 | 6 |
| No emergency contact number given to patient to call about problems | 0 | 0 |
| Patient with problems getting prescribed medications immediately | 1 | 0 |
| Inadequate home services | 0 | 0 |
| Delayed follow‐up care | 0 | 3 |
| Premature hospital discharge | 1 | 2 |
| Adverse drug event (ADE) preventability categories | ||
| Drug involved in the ADE inappropriate for the clinical condition | 2 | 4 |
| Dose, route, or frequency inappropriate for age, weight, creatinine clearance, or disease | 1 | 2 |
| Failure to obtain required lab tests and/or drug levels | 1 | 2 |
| Prior history of an adverse event or allergy to the drug | 1 | 2 |
| Drug‐drug interaction involved in the ADE | 2 | 0 |
| Toxic serum drug level documented | 0 | 0 |
| Noncompliance involved in the ADE | 0 | 1 |
When we designed the trial, we assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for readmissions, emergency department visits, and adverse events. For all of these outcomes, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on confidence intervals for intervention coefficients (Table 2).
We performed an exploratory stratified analysis. We evaluated the intervention effect on readmission within subgroups defined by covariates that predicted readmission (Table 4). When the intervention groups were compared within baseline categories of previous hospitalizations, previous emergency department visits, heart failure, and physical functioning, there was a consistent pattern with no differential effect by intervention assignment. None of the intervention coefficients were statistically significant (Table 4).
| Subgroup | Discharge Software Readmitted n/n (%) | Usual Care Readmitted n/n (%) | Adjusted Parameter Estimate Intervention Coefficient (95% CI) |
|---|---|---|---|
| |||
| Hospital admissions during year prior to index admission | |||
| 0 or 1 | 77/247 (31.2) | 73/224 (32.6) | 0.025 (0.095, 0.045)* |
| 2 or more | 40/69 (58.0) | 46/91 (50.5) | 0.059 (0.090, 0.208)* |
| Emergency department visits during 6 months before index admission | |||
| 0 or 1 | 64/194 (33.0) | 45/168 (26.8) | 0.033 (0.047, 0.113) |
| 2 or more | 53/122 (43.4) | 74/147 (50.3) | 0.071 (0.188, 0.046) |
| Heart failure | |||
| Present | 40/80 (50.0) | 36/67 (53.7) | 0.024 (0.224, 0.177) |
| Absent | 77/236 (32.6) | 83/248 (33.5) | 0.000 (0.076, 0.075) |
| Physical functioning from SF‐36 | |||
| Lowest third | 55/128 (43.0) | 59/121 (48.8) | 0.032 (0.161, 0.096) |
| Upper two‐thirds | 62/188 (33.0) | 60/194 (30.9) | 0.012 (0.071, 0.095) |
Assessment of the Success of the Blind
We evaluated the adequacy of the blind for outcome assessors who interviewed patients or adjudicated adverse events. The guesses of outcomes assessors were unrelated to true intervention assignment (all P values >0.097). We interpreted the blind as adequate for outcome assessors who recorded readmissions, emergency department visits, and adverse events.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software versus usual care handwritten discharge. The discharge software with CPOE implemented elements of high‐quality discharge planning and communication endorsed by the National Quality Forum and systematic reviews.6, 38 Despite theoretical benefits, our discharge software intervention did not reduce readmissions or emergency department visits. What were potential explanations for our results? We assumed an association between postdischarge adverse events and readmissions or emergency department visits.1 Our failure to reduce adverse events might explain the failure to reduce readmissions or emergency department visits. Another potential explanation was related to adverse drug events. Other investigators showed most postdischarge adverse events were adverse drug events and our data confirmed previous studies.1, 2 Medication reconciliation at discharge was a potential mechanism for adverse drug event reduction.14 Medication reconciliation was the standard at the study hospital, so it was unethical to deny reconciliation to patients assigned to either intervention.39 Required medication reconciliation in both groups, by its known effect on preventable adverse drug events, might have reduced the event rates in both groups.14 This possibility is supported by the low rate of adverse events observed in our study compared with other studies.1 We speculate that the low background rate of adverse events at the study hospital may have minimized events in both the discharge software and usual care groups and prevented detection of software benefits, if present.39
One limitation of our study may have been the discharge software. The automated decision support in our software lacked features that might have improved outcomes. For example, the software did not generate a list of diagnostic test results that were pending at the time of discharge. Our software relied on prompts to the physician user that did not specify which tests were pending. The software did not perform error checks on the discharge orders to warn physicians about drug‐drug interactions, therapeutic duplications, or missing items (eg, immunizations, drugs, education). The absence of these software enhancements made our discharge process vulnerable to the lapses and slips of the physician user. Whether or not such enhancements affect clinically relevant outcomes remains a testable hypothesis for future studies.
Another limitation of our study was the outpatient physician response. Discharge software did not increase the proportion of outpatient physicians who said they received communication within 7 days after hospital discharge. Our intervention addressed the sending partner but not the receiving partner in the communication dyad. Our discharge software was not designed to change information flow within the outpatient physician office. We do not know if discharge communication arrived and remained unnoticed until the patient called or visited the outpatient clinic. Future studies of discharge communication should consider a closed loop design to assure receipt and comprehension.
When we designed our study, we expected at least some variance between patient clusters attributable to the physician who performed the discharge. Our analysis of intracluster correlation revealed negligible variance. We speculate the highly‐standardized discharge process implemented by discharge software and usual care at our hospital resulted in minimal variance. Future studies of discharge interventions may consider designs that avoid cluster randomization.
In conclusion, a discharge software application of CPOE did not affect readmissions, emergency department visits, or adverse events after discharge.
Acknowledgements
The authors thank Howard S. Cohen, MD, for his review of the trial protocol and the manuscript.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170(3):345–349.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2(2):58–68.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14(2):109–119.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139(1):31–39.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,,,.Compliance with post‐hospitalization follow‐up visits: rationing by inconvenience?Ethn Dis.1999;9(3):387–395.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345(13):965–970.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,.Closing the loop of patient care—a clinical trial of a computerized discharge medication program.Proc Annu Symp Comput Appl Med Care.1994:841–845.
- ,,,.A comprehensive inpatient discharge system.Proc AMIA Annu Fall Symp.1996:699–703.
- Agency for Healthcare Research and Quality. Making health care safer: a critical analysis of patient safety practices, subchapter 42.3. Discharge summaries and follow‐up. Available at: http://www.ahrq.gov/clinic/ptsafety/chap42b. htm#42.3. Accessed January 2009.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43(4):374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45(5):614–617.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3(6):446–454.
- .SF‐36 health survey update.Spine.2000;25(24):3130–3139.
- ,,,,,.Development of a method to identify seniors at high risk for high hospital utilization.Med Care.2002;40(9):782–793.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: arandomized clinical trial.JAMA.1999;281(7):613–620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,.Identification of factors associated with hospital readmission and development of a predictive model.Health Serv Res.1992;27(1):81–101.
- ,.Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool?Health Serv Res.2000;34(7):1469–1489.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,.The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit.Am J Manag Care.2000;6(8):925–933.
- ,,,.Clinical Epidemiology: A Basic Science for Clinical Medicine.2nd ed.Boston:Little, Brown;1991.
- ,,,,.Identifying clinically significant preventable adverse drug events through a hospital's database of adverse drug reaction reports.Am J Health Syst Pharm.2002;59(18):1742–1749.
- ,,,,,.A pharmacy discharge plan for hospitalized elderly patients—a randomized controlled trial.Age Ageing.2001;30(1):33–40.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16(3):289–293.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Can readmission after stroke be prevented? Results of a randomized clinical study: a postdischarge follow‐up service for stroke survivors.Stroke.2000;31(5):1038–1045.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–147.
- ,,.Indexes and boundaries for “quantitative significance” in statistical decisions.J Clin Epidemiol.1990;43(12):1273–1284.
- National Quality Forum. Safe Practices for Better Healthcare 2006 Update, A Consensus Report, Safe Practice 11: Discharge Systems. Available at: http://qualityforum.org/pdf/reports/safe_practices/txsppublic.pdf. Accessed January 2009.
- ,.OSF Healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
Adverse events occur to patients after their discharge from acute care hospitals.1, 2 Most of these injuries are adverse drug events, procedure‐related events, nosocomial infections, or falls.1 Postdischarge adverse events are associated with several days of symptoms, nonpermanent disability, emergency department visits, or hospital readmission.1, 3 When adverse events are preventable or ameliorable, the most common root cause is poor communication between hospital personnel and either the patient or the outpatient primary care physician.1 In addition, there may be deficits in discharge processes related to assessment and communication of unresolved problems.1 Systematic reviews have shown that discharge communication is an inefficient and error‐prone process.46
One potential solution to poor discharge communication is health information technology.7 An example of technology is discharge software with a computerized physician order entry (CPOE) system. By definition, a CPOE system is a computer‐based system that automates direct entry of orders by physicians and ensures standardized, legible, and complete orders.8 The benefits of CPOE have been tested in other inpatient settings.8, 9 It is logical to consider software applications with CPOE for discharge interventions.7
Several mechanisms explain the potential benefit of discharge software with CPOE.7 Applications with CPOE decrease medication errors.8, 10 Software with decision support could prompt physicians to enter posthospitalization appointment dates and orders for preventive services.11, 12 Discharge software could facilitate medication reconciliation and generate patient instructions and information.4, 1315 The potential benefits of discharge software with CPOE provide a rationale for clinical trials to measure benefits.
Previous studies addressed discharge applications of health information technology. Observational studies recorded outcomes such as physician satisfaction.16, 17 Prior randomized clinical trials measured quality and timeliness of discharge summaries.18 However, these previous trials did not assess clinically relevant outcomes like readmissions, emergency department visits, or adverse events. We performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The rationale for our clustered design complied with recommendations from a systematic review of discharge interventions.5 Our objective was to assess the benefit of discharge software with CPOE when used to discharge patients at high risk for repeat admission. After the intervention, we compared the rates of hospital readmission, emergency department visits, and postdischarge adverse events due to medical management.
Methods
The trial design was a cluster randomized, controlled trial with blinded outcome assessment. Follow‐up occurred until 6 months after discharge from index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
The cluster definition was the hospital physician. Patients discharged by the physician comprised the cluster. Hospital physicians and patients were enrolled between November 2004 and January 2007. Internal medicine resident or attending physicians were eligible. We excluded hospital physicians if their assignments to inpatient duties were less than 2 months during the 27‐month enrollment period. The rationale for the physician exclusion was a consequence of the patient enrollment rate of 3 to 5 patients per physician per month. Physicians with brief assignments could not achieve the goal of 9 or more patients per cluster. After physicians gave informed consent to screen their patients, trained research coordinators applied inclusion and exclusion criteria and obtained informed consent from patients. Research personnel identified all consecutive, unique, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) score 0.40.19, 20 The Pra score came from a logistic model of age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. Other details about exclusion criteria have been published.21 If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was a CPOE software application that facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. Details about the discharge software appeared in a previous publication.7 Software features included required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software prompted the discharging physician to enter pending tests and order tests after discharge. Hospital physicians used the software on the day of discharge and automatically generated 4 discharge documents. The first document was a personalized letter to the outpatient physician with discharge diagnoses, reconciled medication list, diet and activity instructions, patient education materials provided, and follow‐up appointments and studies. Second, the software printed legible prescriptions along with specific information for the dispensing pharmacist about changes and deletions in the patient's previous regimen. Third, the software created patient instructions with addresses and telephone numbers for follow‐up appointments and tests. Fourth, the software printed a legible discharge order including all of the aforementioned information.
The control intervention was the usual care discharge process as described previously.7 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. A previous publication gave details about the standard care available to all patients regardless of intervention.7
Randomization
The unit of randomization was the hospital physician who performed the discharge process. Random allocation was to discharge software or usual care discharge process. The randomization ratio was 1:1, the block size was 2, and there was no stratification or matching. There was concealed allocation and details are available from the investigators. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients. Likewise, it was not possible to conceal the outcome ascertainment, including readmission, from the hospital physicians.
All hospital physicians received training on the usual care discharge process. Physicians assigned to discharge software completed additional training via multimedia demonstration with 1‐on‐1 coaching as needed. Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. After informed consent, patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge from the index hospitalization.
The baseline assessment of patient characteristics occurred during the index hospitalization. Trained data abstractors recorded patient demographic data plus variables to calculate the Pra score for probability for repeat admission. We recorded additional variables because of their possible association with readmission.15, 2229 Data came from the patient or proxy for physical functioning and mental health (SF‐36, Version 2; Medical Outcomes Trust, Boston, MA). Other data for predictor variables came from interviews or hospital records.
Outcome Assessment
The primary study outcome was the proportion of patients readmitted at least once within 6 months after the index hospitalization. Readmission was for any reason and included observation and full admission status. Secondary outcomes were emergency department visits that did not result in hospital admission. Outcome assessment occurred at the patient level. We obtained data for readmissions and emergency department visits from 6 hospitals in central Illinois where study patients were likely to seek care. We validated readmissions and emergency department visits via patient/proxy telephone interviews that occurred 6 months after index hospital discharge. Interviewers were blind to intervention assignment. We evaluated the adequacy of the blind and asked interviewers to guess the patient's intervention assignment.
Another secondary outcome was the proportion of patients who experienced an adverse event related to medical management within 1 month after discharge. For adverse event ascertainment, we employed the process of Forster et al.1, 2 Within 20 to 40 days after discharge, an internal medicine physician performed telephone interviews with the patient or proxy. The interviewer recorded symptoms, drug information, other treatment, hospital readmissions, and emergency department visits. Another physician compiled case summaries from interview data and information abstracted from the electronic medical record, including dictated discharge summaries from the index hospitalization and postdischarge emergency department visits, diagnostic test results, and readmission reports. Two additional internal medicine physicians adjudicated each case summary separately. We counted adverse events only when adjudicators agreed that medical management probably or definitely caused the event. The initial rating by each adjudicator revealed moderate‐to‐good agreement (Kappa = 0.52).30 When initial adjudications were discordant, then adjudicators met and resolved all discrepancies. The adjudicators also scored the severity of the adverse event. The severity scale options were serious laboratory abnormality only, 1 day of symptoms, several days of symptoms, nonpermanent disability, permanent disability, or death. The adjudicators also scored the adverse event as preventable (yes/no), ameliorable (yes/no), and recorded system problems associated with preventable and ameliorable adverse events.1 For adverse drug events, the adjudicators recorded preventability categories defined by previous investigators.31 We designed the adverse event outcome ascertainment as a blinded process. We evaluated the success of the blind and asked adjudicators to guess the patient's intervention assignment.
Sample Size
The sample size analysis employed several assumptions regarding the proportion of readmitted patients. The estimated readmission rate after usual care was 37%.24, 3236 The minimum clinically relevant difference in readmission rates was 13%, an empirical boundary for quantitative significance.37 Estimates for intracluster correlation were not available when we designed the trial. We projected intracluster correlations with low, medium, and high values. The cluster number and size were selected to maintain test significance level, 1‐sided alpha, <0.05 and power >80%. The sample size assumed no interim analysis. The initial sample size estimates were 11 physician clusters per intervention with 25 patients per cluster. During the first 2 months of patient recruitment, we observed that we could not consistently achieve clusters with 25 patients. We recalculated the sample size. Using the same assumptions, we found we could achieve similar test significance and power with 35 physician clusters per intervention and 9 patients per cluster. The sample size calculator was nQuery (Statistical Solutions, Saugus, MA).
Statistical Methods
Analyses were performed with SPSS PC (Version 15.0.1; SPSS Inc, Chicago, IL). Using descriptive statistics, we reported baseline variables as means and standard deviations (SD) for interval variables, and percentages for categorical variables. For outcome variables, we utilized the principle of intention‐to‐treat and assumed patient exposure to the intervention randomly assigned to their discharging physician. We inspected scatter plots and correlations for all variables to test assumptions regarding normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. When assumptions failed, we stratified variables (median or thirds) or performed transformations to satisfy assumptions. For patient‐level outcome variables, we calculated intracluster correlation coefficients. The assessment of the blind was unaffected by the cluster assumption so we used the chi‐square procedure. For analysis of time to event, we used Kaplan‐Meier plots.
The primary hypothesis was a significant decrease in the primary readmission outcome for patients assigned to discharge software. We tested the primary hypothesis with generalized estimating equations that corrected for clustering by hospital physician and adjusted for covariates that predicted readmission. The intervention variable was discharge software versus usual care handwritten discharge. We reported parameter estimates of the intervention variable coefficient and Wald 95% confidence interval (95% CI) with and without correction for cluster. For the secondary, patient‐level outcomes, we performed similar analyses with generalized estimating equations that corrected for clustering by hospital physician.
During covariate analysis, we screened all baseline variables for their correlation with readmission. The variable with the highest correlation and P value <0.05 entered initially in the general estimating equation. After initial variable entry, we evaluated subsequent variables with partial correlations that controlled for variables entered previously. At each iterative step, we entered into the model the variable with the highest partial correlation and P value <0.05.
In exploratory analyses, we examined intervention group differences within strata defined by covariates that predicted readmission. We used generalized estimating equations and adjusted for the other covariates that predicted readmission.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. The physician characteristics appear in Table 1. Most of the hospital physicians were interns in the first year of postgraduate training (58.6%; 41/70). We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). The most common reason for hospital physician exclusion applied to resident physicians in their last months of training before graduation or emergency department residents temporarily assigned to internal medicine training. We approached 6,884 patients during their index hospitalization. After excluding 6,253 ineligible patients, we enrolled and followed 631 patients who received the discharge intervention (Figure 1). During 6 months of follow‐up, a small proportion of patients died (3%; 20/631). Hospital records were available for deceased patients and they were included in the analysis. A small proportion (6%; 41/631) of patients withdrew consent or left the trial for other reasons during 6 months. There was no differential dropout between the interventions. Protocol deviations were rare (0.5%; 3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly‐assigned hospital physicians and their patients are in Table 1.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | (n = 35) | (n = 35) |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics | (n = 316) | (n = 315) |
| Gender, female, n (%) | 180 (57.0) | 168 (53.3) |
| Age, years, n (%) | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Race, n (%) | ||
| Caucasian | 239 (75.6) | 229 (72.7) |
| Black | 72 (22.8) | 85 (27.0) |
| Other | 5 (1.6) | 1 (0.3) |
| Self‐rated health status, n (%) | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus, n (%) | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease, n (%) | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease, n (%) | 133 (42.1) | 120 (38.1) |
| Heart failure, n (%) | 80 (25.3) | 67 (21.3) |
| Informal caregiver available, yes, n (%) | 313 (99.1) | 313 (99.4) |
| Taking loop diuretic, n (%) | 110 (34.8) | 88 (27.9) |
| Physical functioning from SF‐36, n (%) | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental health from SF‐36, n (%) | ||
| Lowest third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Hospital admissions during year prior to index admission, n (%) | ||
| 0 or 1 | 247 (78.2) | 224 (71.1) |
| 2 or more | 69 (21.8) | 91 (28.9) |
| Emergency department visits during 6 months before index admission, n (%) | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Outpatient doctor or clinic visits during year prior to index admission | ||
| 0 to 4 | 97 (30.7%) | 77 (24.4%) |
| 5 to 8 | 68 (21.5%) | 81 (25.7%) |
| 9 to 12 | 82 (25.9%) | 84 (26.7%) |
| 13 or more | 69 (21.8%) | 73 (23.2%) |
| Insurance or payor | ||
| Medicare, age less than 65 years | 18 (5.7%) | 13 (4.1%) |
| Medicare, age 65 years and older | 56 (17.7%) | 40 (12.7%) |
| Medicaid, age less than 65 years | 98 (31.0%) | 130 (41.3%) |
| Medicaid, age 65 years and older | 17 (5.4%) | 20 (6.3%) |
| Commercial or veteran | 85 (26.9%) | 61 (19.4%) |
| Self‐pay | 42 (13.3%) | 51 (16.2%) |
| Religious participation | ||
| Never | 159 (50.3%) | 164 (52.1%) |
| 1‐24 times per year | 55 (17.4%) | 51 (16.2%) |
| 1‐7 times per week | 102 (32.3%) | 100 (31.7%) |
| Volunteer activity, 1 or more hour/month | 31 (9.8%) | 39 (12.4%) |
| Employment status | ||
| Not working | 229 (72.5%) | 233 (74.4%)* |
| Part‐time (<37.5 hours/week) | 30 (9.5%) | 25 (8.0%)* |
| Full‐time (at least 37.5 hour/week) | 57 (18.0%) | 55 (17.6%)* |
| Number of discharge medications, mean (SD) | 10.5 (4.8) | 9.9 (5.1) |
| Severity of illness, mean (SD) | 1.8 (1.2) | 1.6 (1.3) |
| Charlson‐Deyo comorbidity, mean (SD) | 1.7 (1.4) | 1.6 (1.9) |
| Index hospital length of stay, days, mean (SD) | 3.9 (3.5) | 3.5 (3.5) |
| Blood urea nitrogen, mean (SD) | 17.9 (12.9) | 19.1 (12.9) |
| Probability of repeat admission, Pra, mean (SD) | 0.486 (0.072) | 0.495 (0.076) |
We asked outpatient physicians about their receipt of discharge communication from hospital physicians. The text of the question was, How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? We mailed the question 10 days after discharge to outpatient physicians designated by patients enrolled in the study. Among patients in the discharge software group, 75.0% (237/316) of their outpatient physicians responded to the question. The response rate was 80.6% (254/315) from physicians who followed patients in the usual care group. Respondents from the discharge software group said within 1‐2 days or within a week for 56.0% (177/316) of patients. Respondents from the usual care group said within 1‐2 days or within a week for 57.1% (180/315) of patients. The difference between the intervention groups, 1.1% (95% CI, 9.2% to 6.9%), was not significant.
The primary, prespecified, outcome of the study was the proportion of patients with at least 1 readmission to the hospital. After intervention with discharge software versus usual care, there was no significant difference in readmission rates (Table 2) or time to first readmission (Figure 2). We screened all baseline variables in Table 1 and sought predictors of readmission to employ in adjusted models. For example, we evaluated physician level of training because we wondered if experience or seniority affected readmission when hospital physicians used the discharge software or usual care discharge. The candidate variable, physician level of training, did not correlate with readmission (rho = 0.066; P = 0.100), so it was dropped from subsequent analyses. After screening all variables in Table 1, we found 4 independent predictors of readmission: previous hospitalizations, previous emergency department visits, heart failure, and physical function. Generalized estimating equations for readmission that adjusted for predictor variables confirmed a negligible parameter estimate for the discharge intervention variable coefficient (Table 2).

| Outcome | Discharge Software, n (%) | Usual Care, n (%) | Parameter Estimate Without Cluster Correction Intervention Coefficient (95% CI) | P Value | Parameter Estimate With Cluster Correction Intervention Coefficient (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Readmitted within 6 months | 117 (37.0%) | 119 (37.8%) | 0.005* (0.076, 0.067) | 0.897 | 0.005* (0.074, 0.065) | 0.894 |
| Emergency department visit within 6 months | 112 (35.4%) | 128 (40.6%) | 0.052 (0.128, 0.024) | 0.179 | 0.052 (0.115, 0.011) | 0.108 |
| Adverse event within 1 month | 23 (7.3%) | 23 (7.3%) | 0.003 (0.037, 0.043) | 0.886 | 0.003 (0.037, 0.043) | 0.884 |
We evaluated emergency department visits that were unrelated to readmission as secondary, prespecified, outcomes. The results were similar to readmission results. While the proportion of patients with at least 1 emergency department visit was lower for the discharge software intervention, the difference with usual care was not significant (Table 2). There was no significant difference between interventions for time to first emergency department visit (Figure 3).

Postdischarge adverse events were secondary, prespecified, outcomes. Data for adverse event adjudication were available for 98% (309/316) of discharge software patients and 97% (307/315) of usual care patients. Within 1 month after discharge, 46 patients had adverse events probably or definitely related to medical management. Two patients had 2 events and 1 patient had 3 events. For analysis, we randomly selected 1 event per patient. When comparing patients assigned to discharge software versus usual care, there were no differences in adverse events related to medical management (Table 2). Most of the events were possible adverse drug events (74%; 34/46). The adverse event severity was several days of symptoms or nonpermanent disability for 76% (35/46) of the adverse events. Adjudicators rated 26% (12/46) of the adverse events as preventable and 46% (21/46) as ameliorable. The absolute numbers of events were small. There were no differences between discharge software and usual care patients within adverse event strata defined by type, severity, preventable, or ameliorable (Table 3). For most of the patients with adverse events, the adjudicators could not identify a system problem or preventability category (Table 3). When a deficiency was evident, there was no pattern to suggest a significant difference between discharge software patients versus usual care patients.
| Discharge Software (n) | Usual Care Discharge (n) | |
|---|---|---|
| ||
| At least 1 adverse event | 23 | 23 |
| Preventable adverse event | 7 | 5 |
| Ameliorable adverse event | 9 | 12 |
| Adverse event severity | ||
| Serious laboratory abnormality only or 1 day of symptoms | 5 | 5 |
| Several days of symptoms or nonpermanent disability | 18 | 17 |
| Permanent disability or death | 0 | 1 |
| Adverse event by type | ||
| Possible adverse drug event | 17 | 17 |
| Procedure‐related injury | 2 | 1 |
| Therapeutic error | 4 | 4 |
| Diagnostic error | 0 | 1 |
| System problems associated with preventable or ameliorable adverse events | ||
| Inadequate patient education regarding the medical condition or its treatment | 0 | 1 |
| Poor communication between patient and physician | 2 | 1 |
| Poor communication between hospital and community physicians | 0 | 0 |
| Inadequate monitoring of the patient's illness after discharge | 0 | 6 |
| Inadequate monitoring of the patient's treatment after discharge | 2 | 6 |
| No emergency contact number given to patient to call about problems | 0 | 0 |
| Patient with problems getting prescribed medications immediately | 1 | 0 |
| Inadequate home services | 0 | 0 |
| Delayed follow‐up care | 0 | 3 |
| Premature hospital discharge | 1 | 2 |
| Adverse drug event (ADE) preventability categories | ||
| Drug involved in the ADE inappropriate for the clinical condition | 2 | 4 |
| Dose, route, or frequency inappropriate for age, weight, creatinine clearance, or disease | 1 | 2 |
| Failure to obtain required lab tests and/or drug levels | 1 | 2 |
| Prior history of an adverse event or allergy to the drug | 1 | 2 |
| Drug‐drug interaction involved in the ADE | 2 | 0 |
| Toxic serum drug level documented | 0 | 0 |
| Noncompliance involved in the ADE | 0 | 1 |
When we designed the trial, we assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for readmissions, emergency department visits, and adverse events. For all of these outcomes, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on confidence intervals for intervention coefficients (Table 2).
We performed an exploratory stratified analysis. We evaluated the intervention effect on readmission within subgroups defined by covariates that predicted readmission (Table 4). When the intervention groups were compared within baseline categories of previous hospitalizations, previous emergency department visits, heart failure, and physical functioning, there was a consistent pattern with no differential effect by intervention assignment. None of the intervention coefficients were statistically significant (Table 4).
| Subgroup | Discharge Software Readmitted n/n (%) | Usual Care Readmitted n/n (%) | Adjusted Parameter Estimate Intervention Coefficient (95% CI) |
|---|---|---|---|
| |||
| Hospital admissions during year prior to index admission | |||
| 0 or 1 | 77/247 (31.2) | 73/224 (32.6) | 0.025 (0.095, 0.045)* |
| 2 or more | 40/69 (58.0) | 46/91 (50.5) | 0.059 (0.090, 0.208)* |
| Emergency department visits during 6 months before index admission | |||
| 0 or 1 | 64/194 (33.0) | 45/168 (26.8) | 0.033 (0.047, 0.113) |
| 2 or more | 53/122 (43.4) | 74/147 (50.3) | 0.071 (0.188, 0.046) |
| Heart failure | |||
| Present | 40/80 (50.0) | 36/67 (53.7) | 0.024 (0.224, 0.177) |
| Absent | 77/236 (32.6) | 83/248 (33.5) | 0.000 (0.076, 0.075) |
| Physical functioning from SF‐36 | |||
| Lowest third | 55/128 (43.0) | 59/121 (48.8) | 0.032 (0.161, 0.096) |
| Upper two‐thirds | 62/188 (33.0) | 60/194 (30.9) | 0.012 (0.071, 0.095) |
Assessment of the Success of the Blind
We evaluated the adequacy of the blind for outcome assessors who interviewed patients or adjudicated adverse events. The guesses of outcomes assessors were unrelated to true intervention assignment (all P values >0.097). We interpreted the blind as adequate for outcome assessors who recorded readmissions, emergency department visits, and adverse events.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software versus usual care handwritten discharge. The discharge software with CPOE implemented elements of high‐quality discharge planning and communication endorsed by the National Quality Forum and systematic reviews.6, 38 Despite theoretical benefits, our discharge software intervention did not reduce readmissions or emergency department visits. What were potential explanations for our results? We assumed an association between postdischarge adverse events and readmissions or emergency department visits.1 Our failure to reduce adverse events might explain the failure to reduce readmissions or emergency department visits. Another potential explanation was related to adverse drug events. Other investigators showed most postdischarge adverse events were adverse drug events and our data confirmed previous studies.1, 2 Medication reconciliation at discharge was a potential mechanism for adverse drug event reduction.14 Medication reconciliation was the standard at the study hospital, so it was unethical to deny reconciliation to patients assigned to either intervention.39 Required medication reconciliation in both groups, by its known effect on preventable adverse drug events, might have reduced the event rates in both groups.14 This possibility is supported by the low rate of adverse events observed in our study compared with other studies.1 We speculate that the low background rate of adverse events at the study hospital may have minimized events in both the discharge software and usual care groups and prevented detection of software benefits, if present.39
One limitation of our study may have been the discharge software. The automated decision support in our software lacked features that might have improved outcomes. For example, the software did not generate a list of diagnostic test results that were pending at the time of discharge. Our software relied on prompts to the physician user that did not specify which tests were pending. The software did not perform error checks on the discharge orders to warn physicians about drug‐drug interactions, therapeutic duplications, or missing items (eg, immunizations, drugs, education). The absence of these software enhancements made our discharge process vulnerable to the lapses and slips of the physician user. Whether or not such enhancements affect clinically relevant outcomes remains a testable hypothesis for future studies.
Another limitation of our study was the outpatient physician response. Discharge software did not increase the proportion of outpatient physicians who said they received communication within 7 days after hospital discharge. Our intervention addressed the sending partner but not the receiving partner in the communication dyad. Our discharge software was not designed to change information flow within the outpatient physician office. We do not know if discharge communication arrived and remained unnoticed until the patient called or visited the outpatient clinic. Future studies of discharge communication should consider a closed loop design to assure receipt and comprehension.
When we designed our study, we expected at least some variance between patient clusters attributable to the physician who performed the discharge. Our analysis of intracluster correlation revealed negligible variance. We speculate the highly‐standardized discharge process implemented by discharge software and usual care at our hospital resulted in minimal variance. Future studies of discharge interventions may consider designs that avoid cluster randomization.
In conclusion, a discharge software application of CPOE did not affect readmissions, emergency department visits, or adverse events after discharge.
Acknowledgements
The authors thank Howard S. Cohen, MD, for his review of the trial protocol and the manuscript.
Adverse events occur to patients after their discharge from acute care hospitals.1, 2 Most of these injuries are adverse drug events, procedure‐related events, nosocomial infections, or falls.1 Postdischarge adverse events are associated with several days of symptoms, nonpermanent disability, emergency department visits, or hospital readmission.1, 3 When adverse events are preventable or ameliorable, the most common root cause is poor communication between hospital personnel and either the patient or the outpatient primary care physician.1 In addition, there may be deficits in discharge processes related to assessment and communication of unresolved problems.1 Systematic reviews have shown that discharge communication is an inefficient and error‐prone process.46
One potential solution to poor discharge communication is health information technology.7 An example of technology is discharge software with a computerized physician order entry (CPOE) system. By definition, a CPOE system is a computer‐based system that automates direct entry of orders by physicians and ensures standardized, legible, and complete orders.8 The benefits of CPOE have been tested in other inpatient settings.8, 9 It is logical to consider software applications with CPOE for discharge interventions.7
Several mechanisms explain the potential benefit of discharge software with CPOE.7 Applications with CPOE decrease medication errors.8, 10 Software with decision support could prompt physicians to enter posthospitalization appointment dates and orders for preventive services.11, 12 Discharge software could facilitate medication reconciliation and generate patient instructions and information.4, 1315 The potential benefits of discharge software with CPOE provide a rationale for clinical trials to measure benefits.
Previous studies addressed discharge applications of health information technology. Observational studies recorded outcomes such as physician satisfaction.16, 17 Prior randomized clinical trials measured quality and timeliness of discharge summaries.18 However, these previous trials did not assess clinically relevant outcomes like readmissions, emergency department visits, or adverse events. We performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The rationale for our clustered design complied with recommendations from a systematic review of discharge interventions.5 Our objective was to assess the benefit of discharge software with CPOE when used to discharge patients at high risk for repeat admission. After the intervention, we compared the rates of hospital readmission, emergency department visits, and postdischarge adverse events due to medical management.
Methods
The trial design was a cluster randomized, controlled trial with blinded outcome assessment. Follow‐up occurred until 6 months after discharge from index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
The cluster definition was the hospital physician. Patients discharged by the physician comprised the cluster. Hospital physicians and patients were enrolled between November 2004 and January 2007. Internal medicine resident or attending physicians were eligible. We excluded hospital physicians if their assignments to inpatient duties were less than 2 months during the 27‐month enrollment period. The rationale for the physician exclusion was a consequence of the patient enrollment rate of 3 to 5 patients per physician per month. Physicians with brief assignments could not achieve the goal of 9 or more patients per cluster. After physicians gave informed consent to screen their patients, trained research coordinators applied inclusion and exclusion criteria and obtained informed consent from patients. Research personnel identified all consecutive, unique, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) score 0.40.19, 20 The Pra score came from a logistic model of age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. Other details about exclusion criteria have been published.21 If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was a CPOE software application that facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. Details about the discharge software appeared in a previous publication.7 Software features included required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software prompted the discharging physician to enter pending tests and order tests after discharge. Hospital physicians used the software on the day of discharge and automatically generated 4 discharge documents. The first document was a personalized letter to the outpatient physician with discharge diagnoses, reconciled medication list, diet and activity instructions, patient education materials provided, and follow‐up appointments and studies. Second, the software printed legible prescriptions along with specific information for the dispensing pharmacist about changes and deletions in the patient's previous regimen. Third, the software created patient instructions with addresses and telephone numbers for follow‐up appointments and tests. Fourth, the software printed a legible discharge order including all of the aforementioned information.
The control intervention was the usual care discharge process as described previously.7 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. A previous publication gave details about the standard care available to all patients regardless of intervention.7
Randomization
The unit of randomization was the hospital physician who performed the discharge process. Random allocation was to discharge software or usual care discharge process. The randomization ratio was 1:1, the block size was 2, and there was no stratification or matching. There was concealed allocation and details are available from the investigators. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients. Likewise, it was not possible to conceal the outcome ascertainment, including readmission, from the hospital physicians.
All hospital physicians received training on the usual care discharge process. Physicians assigned to discharge software completed additional training via multimedia demonstration with 1‐on‐1 coaching as needed. Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. After informed consent, patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge from the index hospitalization.
The baseline assessment of patient characteristics occurred during the index hospitalization. Trained data abstractors recorded patient demographic data plus variables to calculate the Pra score for probability for repeat admission. We recorded additional variables because of their possible association with readmission.15, 2229 Data came from the patient or proxy for physical functioning and mental health (SF‐36, Version 2; Medical Outcomes Trust, Boston, MA). Other data for predictor variables came from interviews or hospital records.
Outcome Assessment
The primary study outcome was the proportion of patients readmitted at least once within 6 months after the index hospitalization. Readmission was for any reason and included observation and full admission status. Secondary outcomes were emergency department visits that did not result in hospital admission. Outcome assessment occurred at the patient level. We obtained data for readmissions and emergency department visits from 6 hospitals in central Illinois where study patients were likely to seek care. We validated readmissions and emergency department visits via patient/proxy telephone interviews that occurred 6 months after index hospital discharge. Interviewers were blind to intervention assignment. We evaluated the adequacy of the blind and asked interviewers to guess the patient's intervention assignment.
Another secondary outcome was the proportion of patients who experienced an adverse event related to medical management within 1 month after discharge. For adverse event ascertainment, we employed the process of Forster et al.1, 2 Within 20 to 40 days after discharge, an internal medicine physician performed telephone interviews with the patient or proxy. The interviewer recorded symptoms, drug information, other treatment, hospital readmissions, and emergency department visits. Another physician compiled case summaries from interview data and information abstracted from the electronic medical record, including dictated discharge summaries from the index hospitalization and postdischarge emergency department visits, diagnostic test results, and readmission reports. Two additional internal medicine physicians adjudicated each case summary separately. We counted adverse events only when adjudicators agreed that medical management probably or definitely caused the event. The initial rating by each adjudicator revealed moderate‐to‐good agreement (Kappa = 0.52).30 When initial adjudications were discordant, then adjudicators met and resolved all discrepancies. The adjudicators also scored the severity of the adverse event. The severity scale options were serious laboratory abnormality only, 1 day of symptoms, several days of symptoms, nonpermanent disability, permanent disability, or death. The adjudicators also scored the adverse event as preventable (yes/no), ameliorable (yes/no), and recorded system problems associated with preventable and ameliorable adverse events.1 For adverse drug events, the adjudicators recorded preventability categories defined by previous investigators.31 We designed the adverse event outcome ascertainment as a blinded process. We evaluated the success of the blind and asked adjudicators to guess the patient's intervention assignment.
Sample Size
The sample size analysis employed several assumptions regarding the proportion of readmitted patients. The estimated readmission rate after usual care was 37%.24, 3236 The minimum clinically relevant difference in readmission rates was 13%, an empirical boundary for quantitative significance.37 Estimates for intracluster correlation were not available when we designed the trial. We projected intracluster correlations with low, medium, and high values. The cluster number and size were selected to maintain test significance level, 1‐sided alpha, <0.05 and power >80%. The sample size assumed no interim analysis. The initial sample size estimates were 11 physician clusters per intervention with 25 patients per cluster. During the first 2 months of patient recruitment, we observed that we could not consistently achieve clusters with 25 patients. We recalculated the sample size. Using the same assumptions, we found we could achieve similar test significance and power with 35 physician clusters per intervention and 9 patients per cluster. The sample size calculator was nQuery (Statistical Solutions, Saugus, MA).
Statistical Methods
Analyses were performed with SPSS PC (Version 15.0.1; SPSS Inc, Chicago, IL). Using descriptive statistics, we reported baseline variables as means and standard deviations (SD) for interval variables, and percentages for categorical variables. For outcome variables, we utilized the principle of intention‐to‐treat and assumed patient exposure to the intervention randomly assigned to their discharging physician. We inspected scatter plots and correlations for all variables to test assumptions regarding normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. When assumptions failed, we stratified variables (median or thirds) or performed transformations to satisfy assumptions. For patient‐level outcome variables, we calculated intracluster correlation coefficients. The assessment of the blind was unaffected by the cluster assumption so we used the chi‐square procedure. For analysis of time to event, we used Kaplan‐Meier plots.
The primary hypothesis was a significant decrease in the primary readmission outcome for patients assigned to discharge software. We tested the primary hypothesis with generalized estimating equations that corrected for clustering by hospital physician and adjusted for covariates that predicted readmission. The intervention variable was discharge software versus usual care handwritten discharge. We reported parameter estimates of the intervention variable coefficient and Wald 95% confidence interval (95% CI) with and without correction for cluster. For the secondary, patient‐level outcomes, we performed similar analyses with generalized estimating equations that corrected for clustering by hospital physician.
During covariate analysis, we screened all baseline variables for their correlation with readmission. The variable with the highest correlation and P value <0.05 entered initially in the general estimating equation. After initial variable entry, we evaluated subsequent variables with partial correlations that controlled for variables entered previously. At each iterative step, we entered into the model the variable with the highest partial correlation and P value <0.05.
In exploratory analyses, we examined intervention group differences within strata defined by covariates that predicted readmission. We used generalized estimating equations and adjusted for the other covariates that predicted readmission.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. The physician characteristics appear in Table 1. Most of the hospital physicians were interns in the first year of postgraduate training (58.6%; 41/70). We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). The most common reason for hospital physician exclusion applied to resident physicians in their last months of training before graduation or emergency department residents temporarily assigned to internal medicine training. We approached 6,884 patients during their index hospitalization. After excluding 6,253 ineligible patients, we enrolled and followed 631 patients who received the discharge intervention (Figure 1). During 6 months of follow‐up, a small proportion of patients died (3%; 20/631). Hospital records were available for deceased patients and they were included in the analysis. A small proportion (6%; 41/631) of patients withdrew consent or left the trial for other reasons during 6 months. There was no differential dropout between the interventions. Protocol deviations were rare (0.5%; 3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly‐assigned hospital physicians and their patients are in Table 1.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | (n = 35) | (n = 35) |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics | (n = 316) | (n = 315) |
| Gender, female, n (%) | 180 (57.0) | 168 (53.3) |
| Age, years, n (%) | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Race, n (%) | ||
| Caucasian | 239 (75.6) | 229 (72.7) |
| Black | 72 (22.8) | 85 (27.0) |
| Other | 5 (1.6) | 1 (0.3) |
| Self‐rated health status, n (%) | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus, n (%) | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease, n (%) | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease, n (%) | 133 (42.1) | 120 (38.1) |
| Heart failure, n (%) | 80 (25.3) | 67 (21.3) |
| Informal caregiver available, yes, n (%) | 313 (99.1) | 313 (99.4) |
| Taking loop diuretic, n (%) | 110 (34.8) | 88 (27.9) |
| Physical functioning from SF‐36, n (%) | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental health from SF‐36, n (%) | ||
| Lowest third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Hospital admissions during year prior to index admission, n (%) | ||
| 0 or 1 | 247 (78.2) | 224 (71.1) |
| 2 or more | 69 (21.8) | 91 (28.9) |
| Emergency department visits during 6 months before index admission, n (%) | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Outpatient doctor or clinic visits during year prior to index admission | ||
| 0 to 4 | 97 (30.7%) | 77 (24.4%) |
| 5 to 8 | 68 (21.5%) | 81 (25.7%) |
| 9 to 12 | 82 (25.9%) | 84 (26.7%) |
| 13 or more | 69 (21.8%) | 73 (23.2%) |
| Insurance or payor | ||
| Medicare, age less than 65 years | 18 (5.7%) | 13 (4.1%) |
| Medicare, age 65 years and older | 56 (17.7%) | 40 (12.7%) |
| Medicaid, age less than 65 years | 98 (31.0%) | 130 (41.3%) |
| Medicaid, age 65 years and older | 17 (5.4%) | 20 (6.3%) |
| Commercial or veteran | 85 (26.9%) | 61 (19.4%) |
| Self‐pay | 42 (13.3%) | 51 (16.2%) |
| Religious participation | ||
| Never | 159 (50.3%) | 164 (52.1%) |
| 1‐24 times per year | 55 (17.4%) | 51 (16.2%) |
| 1‐7 times per week | 102 (32.3%) | 100 (31.7%) |
| Volunteer activity, 1 or more hour/month | 31 (9.8%) | 39 (12.4%) |
| Employment status | ||
| Not working | 229 (72.5%) | 233 (74.4%)* |
| Part‐time (<37.5 hours/week) | 30 (9.5%) | 25 (8.0%)* |
| Full‐time (at least 37.5 hour/week) | 57 (18.0%) | 55 (17.6%)* |
| Number of discharge medications, mean (SD) | 10.5 (4.8) | 9.9 (5.1) |
| Severity of illness, mean (SD) | 1.8 (1.2) | 1.6 (1.3) |
| Charlson‐Deyo comorbidity, mean (SD) | 1.7 (1.4) | 1.6 (1.9) |
| Index hospital length of stay, days, mean (SD) | 3.9 (3.5) | 3.5 (3.5) |
| Blood urea nitrogen, mean (SD) | 17.9 (12.9) | 19.1 (12.9) |
| Probability of repeat admission, Pra, mean (SD) | 0.486 (0.072) | 0.495 (0.076) |
We asked outpatient physicians about their receipt of discharge communication from hospital physicians. The text of the question was, How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? We mailed the question 10 days after discharge to outpatient physicians designated by patients enrolled in the study. Among patients in the discharge software group, 75.0% (237/316) of their outpatient physicians responded to the question. The response rate was 80.6% (254/315) from physicians who followed patients in the usual care group. Respondents from the discharge software group said within 1‐2 days or within a week for 56.0% (177/316) of patients. Respondents from the usual care group said within 1‐2 days or within a week for 57.1% (180/315) of patients. The difference between the intervention groups, 1.1% (95% CI, 9.2% to 6.9%), was not significant.
The primary, prespecified, outcome of the study was the proportion of patients with at least 1 readmission to the hospital. After intervention with discharge software versus usual care, there was no significant difference in readmission rates (Table 2) or time to first readmission (Figure 2). We screened all baseline variables in Table 1 and sought predictors of readmission to employ in adjusted models. For example, we evaluated physician level of training because we wondered if experience or seniority affected readmission when hospital physicians used the discharge software or usual care discharge. The candidate variable, physician level of training, did not correlate with readmission (rho = 0.066; P = 0.100), so it was dropped from subsequent analyses. After screening all variables in Table 1, we found 4 independent predictors of readmission: previous hospitalizations, previous emergency department visits, heart failure, and physical function. Generalized estimating equations for readmission that adjusted for predictor variables confirmed a negligible parameter estimate for the discharge intervention variable coefficient (Table 2).

| Outcome | Discharge Software, n (%) | Usual Care, n (%) | Parameter Estimate Without Cluster Correction Intervention Coefficient (95% CI) | P Value | Parameter Estimate With Cluster Correction Intervention Coefficient (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Readmitted within 6 months | 117 (37.0%) | 119 (37.8%) | 0.005* (0.076, 0.067) | 0.897 | 0.005* (0.074, 0.065) | 0.894 |
| Emergency department visit within 6 months | 112 (35.4%) | 128 (40.6%) | 0.052 (0.128, 0.024) | 0.179 | 0.052 (0.115, 0.011) | 0.108 |
| Adverse event within 1 month | 23 (7.3%) | 23 (7.3%) | 0.003 (0.037, 0.043) | 0.886 | 0.003 (0.037, 0.043) | 0.884 |
We evaluated emergency department visits that were unrelated to readmission as secondary, prespecified, outcomes. The results were similar to readmission results. While the proportion of patients with at least 1 emergency department visit was lower for the discharge software intervention, the difference with usual care was not significant (Table 2). There was no significant difference between interventions for time to first emergency department visit (Figure 3).

Postdischarge adverse events were secondary, prespecified, outcomes. Data for adverse event adjudication were available for 98% (309/316) of discharge software patients and 97% (307/315) of usual care patients. Within 1 month after discharge, 46 patients had adverse events probably or definitely related to medical management. Two patients had 2 events and 1 patient had 3 events. For analysis, we randomly selected 1 event per patient. When comparing patients assigned to discharge software versus usual care, there were no differences in adverse events related to medical management (Table 2). Most of the events were possible adverse drug events (74%; 34/46). The adverse event severity was several days of symptoms or nonpermanent disability for 76% (35/46) of the adverse events. Adjudicators rated 26% (12/46) of the adverse events as preventable and 46% (21/46) as ameliorable. The absolute numbers of events were small. There were no differences between discharge software and usual care patients within adverse event strata defined by type, severity, preventable, or ameliorable (Table 3). For most of the patients with adverse events, the adjudicators could not identify a system problem or preventability category (Table 3). When a deficiency was evident, there was no pattern to suggest a significant difference between discharge software patients versus usual care patients.
| Discharge Software (n) | Usual Care Discharge (n) | |
|---|---|---|
| ||
| At least 1 adverse event | 23 | 23 |
| Preventable adverse event | 7 | 5 |
| Ameliorable adverse event | 9 | 12 |
| Adverse event severity | ||
| Serious laboratory abnormality only or 1 day of symptoms | 5 | 5 |
| Several days of symptoms or nonpermanent disability | 18 | 17 |
| Permanent disability or death | 0 | 1 |
| Adverse event by type | ||
| Possible adverse drug event | 17 | 17 |
| Procedure‐related injury | 2 | 1 |
| Therapeutic error | 4 | 4 |
| Diagnostic error | 0 | 1 |
| System problems associated with preventable or ameliorable adverse events | ||
| Inadequate patient education regarding the medical condition or its treatment | 0 | 1 |
| Poor communication between patient and physician | 2 | 1 |
| Poor communication between hospital and community physicians | 0 | 0 |
| Inadequate monitoring of the patient's illness after discharge | 0 | 6 |
| Inadequate monitoring of the patient's treatment after discharge | 2 | 6 |
| No emergency contact number given to patient to call about problems | 0 | 0 |
| Patient with problems getting prescribed medications immediately | 1 | 0 |
| Inadequate home services | 0 | 0 |
| Delayed follow‐up care | 0 | 3 |
| Premature hospital discharge | 1 | 2 |
| Adverse drug event (ADE) preventability categories | ||
| Drug involved in the ADE inappropriate for the clinical condition | 2 | 4 |
| Dose, route, or frequency inappropriate for age, weight, creatinine clearance, or disease | 1 | 2 |
| Failure to obtain required lab tests and/or drug levels | 1 | 2 |
| Prior history of an adverse event or allergy to the drug | 1 | 2 |
| Drug‐drug interaction involved in the ADE | 2 | 0 |
| Toxic serum drug level documented | 0 | 0 |
| Noncompliance involved in the ADE | 0 | 1 |
When we designed the trial, we assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for readmissions, emergency department visits, and adverse events. For all of these outcomes, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on confidence intervals for intervention coefficients (Table 2).
We performed an exploratory stratified analysis. We evaluated the intervention effect on readmission within subgroups defined by covariates that predicted readmission (Table 4). When the intervention groups were compared within baseline categories of previous hospitalizations, previous emergency department visits, heart failure, and physical functioning, there was a consistent pattern with no differential effect by intervention assignment. None of the intervention coefficients were statistically significant (Table 4).
| Subgroup | Discharge Software Readmitted n/n (%) | Usual Care Readmitted n/n (%) | Adjusted Parameter Estimate Intervention Coefficient (95% CI) |
|---|---|---|---|
| |||
| Hospital admissions during year prior to index admission | |||
| 0 or 1 | 77/247 (31.2) | 73/224 (32.6) | 0.025 (0.095, 0.045)* |
| 2 or more | 40/69 (58.0) | 46/91 (50.5) | 0.059 (0.090, 0.208)* |
| Emergency department visits during 6 months before index admission | |||
| 0 or 1 | 64/194 (33.0) | 45/168 (26.8) | 0.033 (0.047, 0.113) |
| 2 or more | 53/122 (43.4) | 74/147 (50.3) | 0.071 (0.188, 0.046) |
| Heart failure | |||
| Present | 40/80 (50.0) | 36/67 (53.7) | 0.024 (0.224, 0.177) |
| Absent | 77/236 (32.6) | 83/248 (33.5) | 0.000 (0.076, 0.075) |
| Physical functioning from SF‐36 | |||
| Lowest third | 55/128 (43.0) | 59/121 (48.8) | 0.032 (0.161, 0.096) |
| Upper two‐thirds | 62/188 (33.0) | 60/194 (30.9) | 0.012 (0.071, 0.095) |
Assessment of the Success of the Blind
We evaluated the adequacy of the blind for outcome assessors who interviewed patients or adjudicated adverse events. The guesses of outcomes assessors were unrelated to true intervention assignment (all P values >0.097). We interpreted the blind as adequate for outcome assessors who recorded readmissions, emergency department visits, and adverse events.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software versus usual care handwritten discharge. The discharge software with CPOE implemented elements of high‐quality discharge planning and communication endorsed by the National Quality Forum and systematic reviews.6, 38 Despite theoretical benefits, our discharge software intervention did not reduce readmissions or emergency department visits. What were potential explanations for our results? We assumed an association between postdischarge adverse events and readmissions or emergency department visits.1 Our failure to reduce adverse events might explain the failure to reduce readmissions or emergency department visits. Another potential explanation was related to adverse drug events. Other investigators showed most postdischarge adverse events were adverse drug events and our data confirmed previous studies.1, 2 Medication reconciliation at discharge was a potential mechanism for adverse drug event reduction.14 Medication reconciliation was the standard at the study hospital, so it was unethical to deny reconciliation to patients assigned to either intervention.39 Required medication reconciliation in both groups, by its known effect on preventable adverse drug events, might have reduced the event rates in both groups.14 This possibility is supported by the low rate of adverse events observed in our study compared with other studies.1 We speculate that the low background rate of adverse events at the study hospital may have minimized events in both the discharge software and usual care groups and prevented detection of software benefits, if present.39
One limitation of our study may have been the discharge software. The automated decision support in our software lacked features that might have improved outcomes. For example, the software did not generate a list of diagnostic test results that were pending at the time of discharge. Our software relied on prompts to the physician user that did not specify which tests were pending. The software did not perform error checks on the discharge orders to warn physicians about drug‐drug interactions, therapeutic duplications, or missing items (eg, immunizations, drugs, education). The absence of these software enhancements made our discharge process vulnerable to the lapses and slips of the physician user. Whether or not such enhancements affect clinically relevant outcomes remains a testable hypothesis for future studies.
Another limitation of our study was the outpatient physician response. Discharge software did not increase the proportion of outpatient physicians who said they received communication within 7 days after hospital discharge. Our intervention addressed the sending partner but not the receiving partner in the communication dyad. Our discharge software was not designed to change information flow within the outpatient physician office. We do not know if discharge communication arrived and remained unnoticed until the patient called or visited the outpatient clinic. Future studies of discharge communication should consider a closed loop design to assure receipt and comprehension.
When we designed our study, we expected at least some variance between patient clusters attributable to the physician who performed the discharge. Our analysis of intracluster correlation revealed negligible variance. We speculate the highly‐standardized discharge process implemented by discharge software and usual care at our hospital resulted in minimal variance. Future studies of discharge interventions may consider designs that avoid cluster randomization.
In conclusion, a discharge software application of CPOE did not affect readmissions, emergency department visits, or adverse events after discharge.
Acknowledgements
The authors thank Howard S. Cohen, MD, for his review of the trial protocol and the manuscript.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170(3):345–349.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2(2):58–68.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14(2):109–119.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139(1):31–39.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,,,.Compliance with post‐hospitalization follow‐up visits: rationing by inconvenience?Ethn Dis.1999;9(3):387–395.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345(13):965–970.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,.Closing the loop of patient care—a clinical trial of a computerized discharge medication program.Proc Annu Symp Comput Appl Med Care.1994:841–845.
- ,,,.A comprehensive inpatient discharge system.Proc AMIA Annu Fall Symp.1996:699–703.
- Agency for Healthcare Research and Quality. Making health care safer: a critical analysis of patient safety practices, subchapter 42.3. Discharge summaries and follow‐up. Available at: http://www.ahrq.gov/clinic/ptsafety/chap42b. htm#42.3. Accessed January 2009.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43(4):374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45(5):614–617.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3(6):446–454.
- .SF‐36 health survey update.Spine.2000;25(24):3130–3139.
- ,,,,,.Development of a method to identify seniors at high risk for high hospital utilization.Med Care.2002;40(9):782–793.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: arandomized clinical trial.JAMA.1999;281(7):613–620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,.Identification of factors associated with hospital readmission and development of a predictive model.Health Serv Res.1992;27(1):81–101.
- ,.Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool?Health Serv Res.2000;34(7):1469–1489.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,.The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit.Am J Manag Care.2000;6(8):925–933.
- ,,,.Clinical Epidemiology: A Basic Science for Clinical Medicine.2nd ed.Boston:Little, Brown;1991.
- ,,,,.Identifying clinically significant preventable adverse drug events through a hospital's database of adverse drug reaction reports.Am J Health Syst Pharm.2002;59(18):1742–1749.
- ,,,,,.A pharmacy discharge plan for hospitalized elderly patients—a randomized controlled trial.Age Ageing.2001;30(1):33–40.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16(3):289–293.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Can readmission after stroke be prevented? Results of a randomized clinical study: a postdischarge follow‐up service for stroke survivors.Stroke.2000;31(5):1038–1045.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–147.
- ,,.Indexes and boundaries for “quantitative significance” in statistical decisions.J Clin Epidemiol.1990;43(12):1273–1284.
- National Quality Forum. Safe Practices for Better Healthcare 2006 Update, A Consensus Report, Safe Practice 11: Discharge Systems. Available at: http://qualityforum.org/pdf/reports/safe_practices/txsppublic.pdf. Accessed January 2009.
- ,.OSF Healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170(3):345–349.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2(2):58–68.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14(2):109–119.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139(1):31–39.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,,,.Compliance with post‐hospitalization follow‐up visits: rationing by inconvenience?Ethn Dis.1999;9(3):387–395.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345(13):965–970.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,.Closing the loop of patient care—a clinical trial of a computerized discharge medication program.Proc Annu Symp Comput Appl Med Care.1994:841–845.
- ,,,.A comprehensive inpatient discharge system.Proc AMIA Annu Fall Symp.1996:699–703.
- Agency for Healthcare Research and Quality. Making health care safer: a critical analysis of patient safety practices, subchapter 42.3. Discharge summaries and follow‐up. Available at: http://www.ahrq.gov/clinic/ptsafety/chap42b. htm#42.3. Accessed January 2009.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43(4):374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45(5):614–617.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3(6):446–454.
- .SF‐36 health survey update.Spine.2000;25(24):3130–3139.
- ,,,,,.Development of a method to identify seniors at high risk for high hospital utilization.Med Care.2002;40(9):782–793.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: arandomized clinical trial.JAMA.1999;281(7):613–620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,.Identification of factors associated with hospital readmission and development of a predictive model.Health Serv Res.1992;27(1):81–101.
- ,.Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool?Health Serv Res.2000;34(7):1469–1489.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,.The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit.Am J Manag Care.2000;6(8):925–933.
- ,,,.Clinical Epidemiology: A Basic Science for Clinical Medicine.2nd ed.Boston:Little, Brown;1991.
- ,,,,.Identifying clinically significant preventable adverse drug events through a hospital's database of adverse drug reaction reports.Am J Health Syst Pharm.2002;59(18):1742–1749.
- ,,,,,.A pharmacy discharge plan for hospitalized elderly patients—a randomized controlled trial.Age Ageing.2001;30(1):33–40.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16(3):289–293.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Can readmission after stroke be prevented? Results of a randomized clinical study: a postdischarge follow‐up service for stroke survivors.Stroke.2000;31(5):1038–1045.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–147.
- ,,.Indexes and boundaries for “quantitative significance” in statistical decisions.J Clin Epidemiol.1990;43(12):1273–1284.
- National Quality Forum. Safe Practices for Better Healthcare 2006 Update, A Consensus Report, Safe Practice 11: Discharge Systems. Available at: http://qualityforum.org/pdf/reports/safe_practices/txsppublic.pdf. Accessed January 2009.
- ,.OSF Healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
Copyright © 2009 Society of Hospital Medicine
Prepared for Discharge Scale
Patients are vulnerable to adverse events when they transition from the hospital to outpatient care.13 Approximately 19%‐23% of patients experience adverse events within 4 weeks after acute care hospitalization.3, 4 One cause of postdischarge adverse events is ineffective discharge planning1, 2, 5, 6 Efforts to study and improve the hospital discharge‐planning processes require appropriate and valid measurement instruments. These instruments must assess the discharge process from multiple perspectives. One of the important perspectives is the patient's.7, 8
The PREPARED Patient Questionnaire is a comprehensive quality improvement tool to assess hospital discharge‐planning processes and outcomes from the patient's perspective.9, 10 The PREPARED acronym describes the content of this tool used to investigate the following phenomena: 1) prescriptions, 2) ready to reenter community, 3) education, 4) placement, 5) assurance of safety, 6) realistic expectations, 7) empowerment, and 8) directed to appropriate services.9 The PREPARED questionnaire was developed for, modified for, and validated with patients at least 65 years old. When administered to elderly patients 1 week after hospital discharge, the PREPARED has face, content, and construct validity.9
We considered the PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention. We sought a survey questionnaire to assess the patients' perceptions after the discharge intervention. In 2004, we found noother validated questionnaires except the PREPARED. We also noted some limitations of the PREPARED. The validated population for the PREPARED was patients older than 65 years. In our clinical trial, we planned to enroll adults of all ages. Another limitation was the PREPARED response scoring system that assigned missing data values to patients who took no medicines, needed no services, or needed no equipment.10 We were concerned about the potential for unacceptably large numbers of patients with nonignorable missing data. We decided to address the above limitations with a validation study in our patient population and with a revised response scoring system.
In the present article, we describe item reduction and validation for the Brief PREPARED (B‐PREPARED) scale to measure patients' perceptions of their preparedness for hospital discharge. When we designed B‐PREPARED, we asked the following question: Does a subset of PREPARED items with a revised scoring system have internal consistency, construct validity, and predictive validity in a population of adult patients with broad age range? We also wanted a brief scale with acceptable, defined statistical properties for multiple users. One user class included clinicians who guide and assess discharge‐planning processes. Other users would be researchers like us who measure differences between treatment groups after discharge process interventions.
METHODS
The Peoria Institutional Review Board approved the protocol for human research. The patient sample for scale analysis was a prospective cohort. Follow‐up was 1 month after patient's discharge from a 730‐bed acute‐care teaching hospital in central Illinois. The patients were enrolled in an ongoing cluster randomized clinical trial with blinded outcome assessment. Willing patients or their proxies provided written consent for study participation. Enrollment occurred between December 2004 and July 2006.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to the patient's home by internal medicine hospitalists. Patient inclusion in our cluster randomized trial required a probability of repeat admission (Pra) score of at least 0.40.11, 12 Hence, the patients in the scale analysis cohort had the same high probability for repeat admission. The research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. The Pra score came from a logistic model of age, sex, prior hospitalizations, prior doctor visits, self‐rated health status, informal caregiver, and comorbid coronary heart disease and diabetes mellitus.11, 12
Patient Exclusion Criteria
We excluded patients if the discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. Because follow‐up occurred via interview, patients without telephones or English‐ or Spanish‐language skills were excluded. Patients with cognitive impairment could participate with consent from a legally authorized representative and with a proxy whospent a minimum of 3 hours daily with the patient and was willing to answer postdischarge interviews.
Baseline Assessment
During the index hospital admission, trained data abstractors recorded baseline patient data: age, sex, race, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, and coronary heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospital admission. We recorded the availability of an informal caregiver in response to the question Is there a friend, relative, or neighbor who would take care of you for a few days if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, or excellent.
Discharge and Postdischarge Procedures
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge‐planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aide providers, and others. After discharge, trained research personnel conducted 2 telephone interviews with the patient or the patient's proxy. The first interview occurred 1 week after discharge. Interviewers read verbatim items from the PREPARED10 and the Satisfaction with Information about Medicines Scale (SIMS).13 During the second telephone interview 30 10 days after discharge, interviewers recorded if patients had experienced at least 1 emergency department visit during the month after discharge.
The purpose of the PREPARED items was to have a bank of items and responses that could be used to generate the B‐PREPARED scale. The PREPARED questionnaire was originally developed to provide feedback to hospital ward staff about the quality of discharge‐planning activities that occurred during hospitalization.9 Discriminant factor analysis on the original 16 process questions revealed 4 factors that explained 57% of the total variance in patient/caregiver responses. The PREPARED domains included information exchange on community services and equipment, management of medication, the process of preparing to cope after discharge, and having control over one's discharge circumstances.9 The purpose of the SIMS was as a construct to compare with the B‐PREPARED scale. The derivation and validity of the SIMS have been described extensively elsewhere.13 In summary, the SIMS items were derived from recommendations of the Association of the British Pharmaceutical Industry. The intent of the SIMS was to determine if a patient's medication information needs were met and to allow comparison between patients or groups. Respondents selected 1 of 5 options for each of the 17 items. The sum of scores for each of the SIMS items yielded a total score that ranged from 0 to 17. Patients with high total SIMS scores had high satisfaction with the amount of medication information they received. Validation samples included inpatients and outpatients with a variety of diseases and characteristics. SIMS demonstrated adequate internal consistency, test‐retest reliability, and criterion‐related validity.13
Item Selection and Scoring of the B‐PREPARED Instrument
We selected an initial pool of items from the PREPARED instrument.10 The goal was a parsimonious, comprehensive, and valid instrument for use in clinical and research environments. When we retained or deleted items, our decision process was conservative, conceptual, and statistical. We performed item reduction in the following steps defined a priori. First, we agreed on items consistent with domains in the prepared for discharge construct as defined by expert consensus.9 Second, we excluded items that assessed qualities of the discharge process that were imperceptible to the patient on the day of discharge. Third, we excluded items that elicited open‐ended responses unsuitable for quantitative scale development and analysis. Fourth, we assessed reliability as defined by the Cronbach's alpha statistic. We excluded items that substantially decreased Cronbach's alpha.
Measures of Construct Validity
We used 2 measures of construct validity in our assessment of B‐PREPARED. One construct was patient worry. During the interview 1 week after discharge, research personnel asked, Now that you have been out of the hospital for a while, has anything been worrying you about managing at home? Response options for the dichotomous worry item were no or yes. We anticipated worried patients would have lower B‐PREPARED scale values. The other construct, SIMS, evaluated patient preparedness related to medication information exchange. The hypothesis was a positive correlation between SIMS and B‐PREPARED scale values.
Measure of Predictive Validity
We asked if B‐PREPARED predicted and discriminated groups of patients who did or did not visit emergency departments after hospital discharge. Emergency department visits were relevant adverse outcomes because of their association with postdischarge adverse events due to inpatient treatment.4 Emergency department visits reflected new or worsening symptoms after discharge. In our scale analysis, the hypothesis was patients with at least 1 emergency department visit would have lower B‐PREPARED scale values.
Analysis
Analyses were performed with SPSS PC (Version 14.0.2, SPSS Inc, Chicago, IL). We reported descriptive statistics as means, standard deviations, and range for interval variables and percentages for nominal variables. To determine the internal consistency of the scale, we calculated Cronbach's alpha. We assessed the distribution of the B‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered important. Statistical inference tests were the Mann‐Whitney U for median differences between 2 groups and the Spearman correlation for associations. We reported medians with 25th and 75th percentiles. Differences between 2 correlations were tested using Fischer z transformations. The accepted level of significance was P < 0.05.
RESULTS
Description of Cohort
We approached 5124 patients during the index hospital admission. After applying exclusion criteria, we obtained consent and enrolled 491 patients. The reasons for exclusion were low Pra score for 34.9% of ineligible patients, discharge to nursing home for 12.8%, declined consent for 10.8%, nonparticipating hospitalist service for 9.1%, discharged during screen for 8.5%, previously enrolled in study for 5.6%, and declined screening for 2.3%. Each of the other exclusion criteria accounted for less than 4% of the ineligible patients. After subtracting 6% of eligible patients (31 of 491) who died, withdrew, or were lost during the first month, there were 460 patients available for analysis. Table 1 describes the patients' characteristics. Most of the patients, 75.2% (346 of 460), were less than 65 years old, and the mean age was 53.9 15.5 years. Many patients had chronic diseases including diabetes mellitus, coronary heart disease, heart failure, and chronic obstructive pulmonary disease. Most patients, 81.5% (375 of 460), rated their health as poor or fair, and 53.5% (246/460) had 1 or more hospital admissions during the year before their index admission. Cohort patients had a high probability of repeat admission: mean Pra 0.49 0.07 (range 0.400.70).
| Characteristic | n (%) |
|---|---|
| Sex (male) | 193 (42.0%) |
| Age (years) | |
| 1930 | 35 (7.6%) |
| 3164 | 311 (67.6%) |
| 6598 | 114 (24.8%) |
| Race | |
| White | 275 (59.8%) |
| Black | 124 (27.0%) |
| Other | 61 (13.3%) |
| Self‐rated health status | |
| Poor | 139 (30.2%) |
| Fair | 236 (51.3%) |
| Good | 70 (15.2%) |
| Very good | 13 (2.8%) |
| Excellent | 2 (0.4%) |
| Diabetes mellitus | 259 (56.3%) |
| Chronic obstructive pulmonary disease | 79 (17.2%) |
| Coronary heart disease | 188 (40.9%) |
| Heart failure (n = 456) | 100 (21.7%) |
| Informal caregiver available (yes) | 459 (99.1%) |
| Hospital admissions during year prior to index admission | |
| 0 | 214 (46.5%) |
| 1 | 131 (28.5%) |
| 2 | 47 (10.2%) |
| 3 or 4 | 35 (7.6%) |
| 515 | 33 (7.2%) |
Item Reduction, Internal Consistency, and Score Distributions
Item reduction resulted in 12 items that fulfilled conceptual criteria. Table 2 shows the items and the distribution of responses. One of the 12 items, delays on the day you left the hospital (item 12, Table 2), was deleted because the item depressed the Cronbach's alpha. The B‐PREPARED with 11 items had acceptable internal consistency for the full cohort (Cronbach's alpha = 0.76).
| Item Text | Descriptor for Score 0 | Descriptor for Score 1 | Descriptor for Score 2 | |
|---|---|---|---|---|
| ||||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | None (40, 8.7%) | Some, but not enough (95, 20.7%) | As much as I needed; or Not taking any medications (325, 70.7%) |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | None (198, 43.0%) | Some, but not enough (54, 11.7%) | As much as I needed; or Not taking any medications (208, 45.2%) |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | No written instructions and no time spent (116, 25.2%) | Yes, received written instructions but no time spent (49, 10.7%) | Yes, received written instructions and yes, time spent; or, Not taking any medications (291, 63.3%) |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | None (55, 12.0%) | Some, but not enough (90, 19.6%) | As much as I needed (315, 68.5%) |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | None (89, 19.3%) | Some, but not enough (40, 8.7%) | As much as I needed; or No services needed (331, 72.0%) |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | None (49, 10.7%) | Some, but not enough (22, 4.8%) | As much as I needed; or No equipment needed (389, 84.6%) |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | No (42, 9.1%) | Yes; or No one needed to arrange because services were already in place or no services needed (418, 90.9%) | |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | No (16, 3.5%) | Yes; or No one needed to because equipment already in place or no equipment needed (444, 96.5%) | |
| 9 | Before you were discharged from hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | No (116, 25.2%) | Yes (344, 74.8%) | |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | Not confident (25, 5.4%) | Unsure (103, 22.4%) | Confident (332, 72.2%) |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | Unprepared (39, 8.5%) | Moderately prepared (132, 28.7%) | Very prepared (288, 62.6%) |
| 12 | After you were told you could leave the hospital, were there any delays on the day you left the hospital? | Yes (122, 26.5%) | No (338, 73.5%) | |
For an individual patient, the sum of the scores for each item yielded a B‐PREPARED scale value. In the 460‐patient cohort, B‐PREPARED scale values had a mean of 17.3 4.3 and a negatively skewed distribution. A high scale value reflected high perception of discharge preparedness. Each of the 11 items correlated significantly with the B‐PREPARED scale value (P < 0.001, 2‐tailed).
There were substantial ceiling effects with individual items but not in the B‐PREPARED total score. Five of the 9 items with 3 response options had a ceiling effect above 70%. Three items had a dichotomous response option (items 7, 8, and 9). In 2 of these 3 items, more than 90% of respondents selected the response indicating higher preparedness. The total B‐PREPARED did not have noteworthy floor or ceiling effects. In this sample's total B‐PREPARED scores, 0.2% of respondents had the lowest score of 3, and 20% had the highest score of 22.
Principal Component Analysis
In the component analysis, we evaluated the correlation matrix of the 11 items in the B‐PREPARED scale. A Kaiser‐Meyer‐Olkin statistic of 0.76 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 54.2% of the variance associated with the 11‐item B‐PREPARED scale. After inspection of scree plots, we determined that 3 components were extracted before the eigenvalue fell below 1. The pattern matrix for the promax rotation was inspected, and the factor loading of each item appears in Table 3. The item content identified the first component as self‐care information for medications and activities. The second component was equipment and services. The third component was confidence. All B‐PREPARED items loaded primarily on 1 of the 3 components (Table 3).
| Item text | Component | |||
|---|---|---|---|---|
| Self‐care Information for Medications and Activity | Equipment and Services | Confidence | ||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | 0.749 | 0.032 | 0.019 |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | 0.778 | .008 | 0.003 |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | 0.758 | 0.030 | 0.084 |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | 0.581 | 0.101 | 0.195 |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | 0.158 | 0.639 | 0.124 |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | 0.183 | 0.701 | ‐0.152 |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | 0.081 | 0.654 | 0.199 |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | 0.138 | 0.655 | 0.095 |
| 9 | Before you were discharged from the hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | 0.181 | 0.211 | 0.369 |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | 0.036 | 0.058 | 0.876 |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | 0.018 | 0.032 | 0.875 |
Construct Validity
We assessed 2 constructs: worry and satisfaction with medication information (SIMS). In the cohort, 25% of patients (115 of 460) reported worry about managing at home. Worried patients had significantly lower B‐PREPARED scale values (median [25%, 75%] = 14 [10, 16]) than patients who did not worry (median [25%, 75%] = 17 [14, 20], P < .001). We calculated SIMS and then correlated SIMS with B‐PREPARED and components. In the cohort, the mean SIMS was 12.1 4.7 (range 0‐17). Patients with greater satisfaction on the SIMS also had higher B‐PREPARED scale values (rho = 0.45, P < 0.001). There was a significant positive correlation between SIMS and the B‐PREPARED component called self‐care information for medications and activities (rho = 0.46, P < 0.001). The other 2 B‐PREPARED components, equipment/services and confidence, were positively correlated with SIMS at much lower levels (rho = 0.18 and rho = 0.24, respectively, both Fischer z transformations P < .001). The B‐PREPARED scale demonstrated validity with the constructs of worry and satisfaction with medication information.
Predictive Validity
We assessed the capacity of the B‐PREPARED to predict and discriminate groups of patients who did or did not visit emergency departments. Within 1 month of hospital discharge, 16.5% of the cohort (76 of 460 patients) had at least 1 visit to an emergency department. B‐PREPARED scale values were lower for those patients who visited emergency departments (median [25%, 75%] = 14 [12, 18]) than those who did not (median [25%, 75%] = 16 [13, 19], P = 0.011). The B‐PREPARED scale analysis supported the hypothesized relationship with emergency department visits.
Correlations between B‐PREPARED and Baseline Characteristics
We evaluated the correlations between a patient's B‐PREPARED scale value and baseline characteristics, shown in Table 1. There was a weak positive correlation with self‐rated health status (rho 0.17, P < .001). Patients who perceived better health status had higher B‐PREPARED scale values than those with poorer status. The other baseline characteristics in Table 1 were not associated with B‐PREPARED scale values.
DISCUSSION
The B‐PREPARED scale measures patients' perceptions of their preparedness for hospital discharge home. Scale items came from the PREPARED, a survey with validated psychometric properties in elderly patients. We assessed the B‐PREPARED in a cohort of young and elderly adult patients. We examined the B‐PREPARED instrument for internal consistency, construct validity, and predictive validity. In comparison with the domains identified in the full PREPARED instrument,9 the abbreviated B‐PREPARED scale identified similar domains. Some differences were anticipated because we limited items to those the respondents would be able to perceive before leaving the hospital.
The results of our study should be interpreted in the context of strengths and limitations. One of the strengths was the validity of the PREPARED, from which the B‐PREPARED was derived.7 The conceptually rigorous process used to develop the PREPARED questionnaire allowed us to draw from a bank of concise, well‐worded items.9 The B‐PREPARED extends validity to a population of adults of all ages with high risk for readmission. The other strength of the B‐PREPARED was the association with the clinically relevant constructs worry and satisfaction with medication information. The B‐PREPARED also discriminated between patients who did and those who did not return to emergency departments after discharge. Although the patient population for the B‐PREPARED validation was one of the strengths of this study, it is also a limitation. Our cohort lacked diversity with respect to readmission risk. The results of our study may not generalize to patients with low risk for repeated admission. Furthermore, all our patients were discharged home. The exclusion of other discharge destinations helped us to enroll a cohort with homogenous risk for readmission. However, our exclusion criteria did not allow us to validate the B‐PREPARED in patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities.
Another limitation related to outpatient visits after discharge. We did not analyze outpatient sites other than emergency departments. For all of our study patients, the discharging hospitalist scheduled at least 1 outpatient visit with the primary care practitioner. For some patients, the hospitalist also scheduled postdischarge visits for diagnostic evaluations like cardiac stress tests, endoscopies, radiographs, or other laboratory tests. When these visits occurred, they represented successful execution of the discharge plan. Sometimes patients arrived for planned or unplanned outpatient visits with exacerbated symptoms or adverse events. These latter visits might represent failures of the discharge plan. Our data collection did not allow us to distinguish outpatient visits as successes or failures of the discharge plan. When we counted only emergency department visits, we may have underestimated the number of patients with adverse events who sought and received successful treatment in outpatient clinics. Future studies should consider ascertainment of planned and unplanned outpatient visits for exacerbated symptoms and adverse events.
After our study began enrollment, other investigators published the Readiness for Hospital Discharge Scale14 and Care Transitions Measure.15 The design and validation of these sampling instruments differed with each other and with the B‐PREPARED. The differences made the 3 scales complementary but not interchangeable. For example, investigators administered the 21‐item Readiness for Hospital Discharge Scale on the day of discharge to adult medical‐surgical patients, postpartum mothers, and parents of hospitalized children. In contrast, we administered the B‐PREPARED 1 week after discharge to adult internal medicine patients or their proxies. The Readiness for Hospital Discharge subscales were personal status, knowledge, coping ability, and expected support. These subscales were similar to the components of the B‐PREPARED. The Readiness for Hospital Discharge Scale demonstrated internal consistency and construct validity but did not predict patients who returned to emergency departments after hospital discharge.14 Future users of the Readiness for Hospital Discharge Scale or the B‐PREPARED should consider their patient populations and the date of administration when selecting 1 scale versus another. If brevity is important to a clinician or researcher, then the 11‐item B‐PREPARED scale may be considered.
The Care Transitions Measure also differed from the B‐PREPARED. The 15‐item Care Transitions Measure evaluated an adult population with a broad age range and with chronic obstructive pulmonary disease, heart failure, stroke, or hip fracture.15 The diseases represented in the population for the Care Transitions Measure were similar to those in the B‐PREPARED cohort, although the distribution of the diseases differed. When validating the Care Transitions Measure, investigators administered questionnaires 6 to 12 weeks after discharge. The Care Transitions Measure had 4 factors: critical understanding, preferences important, management preparation, and care plan. The factors of the Care Transitions Measure were comparable to the components of the B‐PREPARED, and both scales assessed medication self‐management. However, the Care Transitions Measure addressed patient preferences with specific items, whereas the B‐PREPARED used the scoring system to quantify patient preferences. Both the Care Transitions Measure and the B‐PREPARED demonstrated internal consistency and discriminated between patients who did and those who did not return to emergency departments after hospital discharge.15 When selecting a scale, future users should consider the B‐PREPARED only for assessments 1 week post discharge and should consider the Care Transitions Measure for later assessments.
There are applications of the B‐PREPARED scale in hospital quality improvement efforts. Hospitals have multiple motivations to pursue quality improvement projects related to discharge processes: satisfaction of patients, reduction in adverse events, relation with referring physicians, and accreditation by regulators.6, 16 When hospital‐based clinicians survey patients, they may wish to use a brief, reliable, and validated instrument like the B‐PREPARED questionnaire.
CONCLUSIONS
The B‐PREPARED provided a reliable and valid measure of patients' perceptions of their preparedness for hospital discharge home. Clinicians and researchers may find the B‐PREPARED useful to guide, assess, and compare discharge‐planning interventions.
Acknowledgements
The authors thank Dr. Karen Grimmer‐Somers, PhD, for permission to use the PREPARED instrument and for her thoughtful comments on the draft manuscript.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Review of the literature on survey instruments used to collect data on hospital patients' perceptions of care.Health Serv Res.2005;40:1996–2017.
- ,.A patient‐centered model of care for hospital discharge.Clin Nurs Res.2004;13:117–136.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- PREPARED Patient Questionnaire. Available at: http://www.unisa.edu.au/cahe/pubs/Patient%20scoring.pdf. Accessed June 14,2007.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,.The Satisfaction with Information about Medicines Scales (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,.Psychometric properties of the Readiness for Hospital Discharge Scale.J Nurs Meas.2006;14:163–180.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed June 14,2007.
Patients are vulnerable to adverse events when they transition from the hospital to outpatient care.13 Approximately 19%‐23% of patients experience adverse events within 4 weeks after acute care hospitalization.3, 4 One cause of postdischarge adverse events is ineffective discharge planning1, 2, 5, 6 Efforts to study and improve the hospital discharge‐planning processes require appropriate and valid measurement instruments. These instruments must assess the discharge process from multiple perspectives. One of the important perspectives is the patient's.7, 8
The PREPARED Patient Questionnaire is a comprehensive quality improvement tool to assess hospital discharge‐planning processes and outcomes from the patient's perspective.9, 10 The PREPARED acronym describes the content of this tool used to investigate the following phenomena: 1) prescriptions, 2) ready to reenter community, 3) education, 4) placement, 5) assurance of safety, 6) realistic expectations, 7) empowerment, and 8) directed to appropriate services.9 The PREPARED questionnaire was developed for, modified for, and validated with patients at least 65 years old. When administered to elderly patients 1 week after hospital discharge, the PREPARED has face, content, and construct validity.9
We considered the PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention. We sought a survey questionnaire to assess the patients' perceptions after the discharge intervention. In 2004, we found noother validated questionnaires except the PREPARED. We also noted some limitations of the PREPARED. The validated population for the PREPARED was patients older than 65 years. In our clinical trial, we planned to enroll adults of all ages. Another limitation was the PREPARED response scoring system that assigned missing data values to patients who took no medicines, needed no services, or needed no equipment.10 We were concerned about the potential for unacceptably large numbers of patients with nonignorable missing data. We decided to address the above limitations with a validation study in our patient population and with a revised response scoring system.
In the present article, we describe item reduction and validation for the Brief PREPARED (B‐PREPARED) scale to measure patients' perceptions of their preparedness for hospital discharge. When we designed B‐PREPARED, we asked the following question: Does a subset of PREPARED items with a revised scoring system have internal consistency, construct validity, and predictive validity in a population of adult patients with broad age range? We also wanted a brief scale with acceptable, defined statistical properties for multiple users. One user class included clinicians who guide and assess discharge‐planning processes. Other users would be researchers like us who measure differences between treatment groups after discharge process interventions.
METHODS
The Peoria Institutional Review Board approved the protocol for human research. The patient sample for scale analysis was a prospective cohort. Follow‐up was 1 month after patient's discharge from a 730‐bed acute‐care teaching hospital in central Illinois. The patients were enrolled in an ongoing cluster randomized clinical trial with blinded outcome assessment. Willing patients or their proxies provided written consent for study participation. Enrollment occurred between December 2004 and July 2006.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to the patient's home by internal medicine hospitalists. Patient inclusion in our cluster randomized trial required a probability of repeat admission (Pra) score of at least 0.40.11, 12 Hence, the patients in the scale analysis cohort had the same high probability for repeat admission. The research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. The Pra score came from a logistic model of age, sex, prior hospitalizations, prior doctor visits, self‐rated health status, informal caregiver, and comorbid coronary heart disease and diabetes mellitus.11, 12
Patient Exclusion Criteria
We excluded patients if the discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. Because follow‐up occurred via interview, patients without telephones or English‐ or Spanish‐language skills were excluded. Patients with cognitive impairment could participate with consent from a legally authorized representative and with a proxy whospent a minimum of 3 hours daily with the patient and was willing to answer postdischarge interviews.
Baseline Assessment
During the index hospital admission, trained data abstractors recorded baseline patient data: age, sex, race, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, and coronary heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospital admission. We recorded the availability of an informal caregiver in response to the question Is there a friend, relative, or neighbor who would take care of you for a few days if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, or excellent.
Discharge and Postdischarge Procedures
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge‐planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aide providers, and others. After discharge, trained research personnel conducted 2 telephone interviews with the patient or the patient's proxy. The first interview occurred 1 week after discharge. Interviewers read verbatim items from the PREPARED10 and the Satisfaction with Information about Medicines Scale (SIMS).13 During the second telephone interview 30 10 days after discharge, interviewers recorded if patients had experienced at least 1 emergency department visit during the month after discharge.
The purpose of the PREPARED items was to have a bank of items and responses that could be used to generate the B‐PREPARED scale. The PREPARED questionnaire was originally developed to provide feedback to hospital ward staff about the quality of discharge‐planning activities that occurred during hospitalization.9 Discriminant factor analysis on the original 16 process questions revealed 4 factors that explained 57% of the total variance in patient/caregiver responses. The PREPARED domains included information exchange on community services and equipment, management of medication, the process of preparing to cope after discharge, and having control over one's discharge circumstances.9 The purpose of the SIMS was as a construct to compare with the B‐PREPARED scale. The derivation and validity of the SIMS have been described extensively elsewhere.13 In summary, the SIMS items were derived from recommendations of the Association of the British Pharmaceutical Industry. The intent of the SIMS was to determine if a patient's medication information needs were met and to allow comparison between patients or groups. Respondents selected 1 of 5 options for each of the 17 items. The sum of scores for each of the SIMS items yielded a total score that ranged from 0 to 17. Patients with high total SIMS scores had high satisfaction with the amount of medication information they received. Validation samples included inpatients and outpatients with a variety of diseases and characteristics. SIMS demonstrated adequate internal consistency, test‐retest reliability, and criterion‐related validity.13
Item Selection and Scoring of the B‐PREPARED Instrument
We selected an initial pool of items from the PREPARED instrument.10 The goal was a parsimonious, comprehensive, and valid instrument for use in clinical and research environments. When we retained or deleted items, our decision process was conservative, conceptual, and statistical. We performed item reduction in the following steps defined a priori. First, we agreed on items consistent with domains in the prepared for discharge construct as defined by expert consensus.9 Second, we excluded items that assessed qualities of the discharge process that were imperceptible to the patient on the day of discharge. Third, we excluded items that elicited open‐ended responses unsuitable for quantitative scale development and analysis. Fourth, we assessed reliability as defined by the Cronbach's alpha statistic. We excluded items that substantially decreased Cronbach's alpha.
Measures of Construct Validity
We used 2 measures of construct validity in our assessment of B‐PREPARED. One construct was patient worry. During the interview 1 week after discharge, research personnel asked, Now that you have been out of the hospital for a while, has anything been worrying you about managing at home? Response options for the dichotomous worry item were no or yes. We anticipated worried patients would have lower B‐PREPARED scale values. The other construct, SIMS, evaluated patient preparedness related to medication information exchange. The hypothesis was a positive correlation between SIMS and B‐PREPARED scale values.
Measure of Predictive Validity
We asked if B‐PREPARED predicted and discriminated groups of patients who did or did not visit emergency departments after hospital discharge. Emergency department visits were relevant adverse outcomes because of their association with postdischarge adverse events due to inpatient treatment.4 Emergency department visits reflected new or worsening symptoms after discharge. In our scale analysis, the hypothesis was patients with at least 1 emergency department visit would have lower B‐PREPARED scale values.
Analysis
Analyses were performed with SPSS PC (Version 14.0.2, SPSS Inc, Chicago, IL). We reported descriptive statistics as means, standard deviations, and range for interval variables and percentages for nominal variables. To determine the internal consistency of the scale, we calculated Cronbach's alpha. We assessed the distribution of the B‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered important. Statistical inference tests were the Mann‐Whitney U for median differences between 2 groups and the Spearman correlation for associations. We reported medians with 25th and 75th percentiles. Differences between 2 correlations were tested using Fischer z transformations. The accepted level of significance was P < 0.05.
RESULTS
Description of Cohort
We approached 5124 patients during the index hospital admission. After applying exclusion criteria, we obtained consent and enrolled 491 patients. The reasons for exclusion were low Pra score for 34.9% of ineligible patients, discharge to nursing home for 12.8%, declined consent for 10.8%, nonparticipating hospitalist service for 9.1%, discharged during screen for 8.5%, previously enrolled in study for 5.6%, and declined screening for 2.3%. Each of the other exclusion criteria accounted for less than 4% of the ineligible patients. After subtracting 6% of eligible patients (31 of 491) who died, withdrew, or were lost during the first month, there were 460 patients available for analysis. Table 1 describes the patients' characteristics. Most of the patients, 75.2% (346 of 460), were less than 65 years old, and the mean age was 53.9 15.5 years. Many patients had chronic diseases including diabetes mellitus, coronary heart disease, heart failure, and chronic obstructive pulmonary disease. Most patients, 81.5% (375 of 460), rated their health as poor or fair, and 53.5% (246/460) had 1 or more hospital admissions during the year before their index admission. Cohort patients had a high probability of repeat admission: mean Pra 0.49 0.07 (range 0.400.70).
| Characteristic | n (%) |
|---|---|
| Sex (male) | 193 (42.0%) |
| Age (years) | |
| 1930 | 35 (7.6%) |
| 3164 | 311 (67.6%) |
| 6598 | 114 (24.8%) |
| Race | |
| White | 275 (59.8%) |
| Black | 124 (27.0%) |
| Other | 61 (13.3%) |
| Self‐rated health status | |
| Poor | 139 (30.2%) |
| Fair | 236 (51.3%) |
| Good | 70 (15.2%) |
| Very good | 13 (2.8%) |
| Excellent | 2 (0.4%) |
| Diabetes mellitus | 259 (56.3%) |
| Chronic obstructive pulmonary disease | 79 (17.2%) |
| Coronary heart disease | 188 (40.9%) |
| Heart failure (n = 456) | 100 (21.7%) |
| Informal caregiver available (yes) | 459 (99.1%) |
| Hospital admissions during year prior to index admission | |
| 0 | 214 (46.5%) |
| 1 | 131 (28.5%) |
| 2 | 47 (10.2%) |
| 3 or 4 | 35 (7.6%) |
| 515 | 33 (7.2%) |
Item Reduction, Internal Consistency, and Score Distributions
Item reduction resulted in 12 items that fulfilled conceptual criteria. Table 2 shows the items and the distribution of responses. One of the 12 items, delays on the day you left the hospital (item 12, Table 2), was deleted because the item depressed the Cronbach's alpha. The B‐PREPARED with 11 items had acceptable internal consistency for the full cohort (Cronbach's alpha = 0.76).
| Item Text | Descriptor for Score 0 | Descriptor for Score 1 | Descriptor for Score 2 | |
|---|---|---|---|---|
| ||||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | None (40, 8.7%) | Some, but not enough (95, 20.7%) | As much as I needed; or Not taking any medications (325, 70.7%) |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | None (198, 43.0%) | Some, but not enough (54, 11.7%) | As much as I needed; or Not taking any medications (208, 45.2%) |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | No written instructions and no time spent (116, 25.2%) | Yes, received written instructions but no time spent (49, 10.7%) | Yes, received written instructions and yes, time spent; or, Not taking any medications (291, 63.3%) |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | None (55, 12.0%) | Some, but not enough (90, 19.6%) | As much as I needed (315, 68.5%) |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | None (89, 19.3%) | Some, but not enough (40, 8.7%) | As much as I needed; or No services needed (331, 72.0%) |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | None (49, 10.7%) | Some, but not enough (22, 4.8%) | As much as I needed; or No equipment needed (389, 84.6%) |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | No (42, 9.1%) | Yes; or No one needed to arrange because services were already in place or no services needed (418, 90.9%) | |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | No (16, 3.5%) | Yes; or No one needed to because equipment already in place or no equipment needed (444, 96.5%) | |
| 9 | Before you were discharged from hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | No (116, 25.2%) | Yes (344, 74.8%) | |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | Not confident (25, 5.4%) | Unsure (103, 22.4%) | Confident (332, 72.2%) |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | Unprepared (39, 8.5%) | Moderately prepared (132, 28.7%) | Very prepared (288, 62.6%) |
| 12 | After you were told you could leave the hospital, were there any delays on the day you left the hospital? | Yes (122, 26.5%) | No (338, 73.5%) | |
For an individual patient, the sum of the scores for each item yielded a B‐PREPARED scale value. In the 460‐patient cohort, B‐PREPARED scale values had a mean of 17.3 4.3 and a negatively skewed distribution. A high scale value reflected high perception of discharge preparedness. Each of the 11 items correlated significantly with the B‐PREPARED scale value (P < 0.001, 2‐tailed).
There were substantial ceiling effects with individual items but not in the B‐PREPARED total score. Five of the 9 items with 3 response options had a ceiling effect above 70%. Three items had a dichotomous response option (items 7, 8, and 9). In 2 of these 3 items, more than 90% of respondents selected the response indicating higher preparedness. The total B‐PREPARED did not have noteworthy floor or ceiling effects. In this sample's total B‐PREPARED scores, 0.2% of respondents had the lowest score of 3, and 20% had the highest score of 22.
Principal Component Analysis
In the component analysis, we evaluated the correlation matrix of the 11 items in the B‐PREPARED scale. A Kaiser‐Meyer‐Olkin statistic of 0.76 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 54.2% of the variance associated with the 11‐item B‐PREPARED scale. After inspection of scree plots, we determined that 3 components were extracted before the eigenvalue fell below 1. The pattern matrix for the promax rotation was inspected, and the factor loading of each item appears in Table 3. The item content identified the first component as self‐care information for medications and activities. The second component was equipment and services. The third component was confidence. All B‐PREPARED items loaded primarily on 1 of the 3 components (Table 3).
| Item text | Component | |||
|---|---|---|---|---|
| Self‐care Information for Medications and Activity | Equipment and Services | Confidence | ||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | 0.749 | 0.032 | 0.019 |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | 0.778 | .008 | 0.003 |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | 0.758 | 0.030 | 0.084 |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | 0.581 | 0.101 | 0.195 |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | 0.158 | 0.639 | 0.124 |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | 0.183 | 0.701 | ‐0.152 |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | 0.081 | 0.654 | 0.199 |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | 0.138 | 0.655 | 0.095 |
| 9 | Before you were discharged from the hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | 0.181 | 0.211 | 0.369 |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | 0.036 | 0.058 | 0.876 |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | 0.018 | 0.032 | 0.875 |
Construct Validity
We assessed 2 constructs: worry and satisfaction with medication information (SIMS). In the cohort, 25% of patients (115 of 460) reported worry about managing at home. Worried patients had significantly lower B‐PREPARED scale values (median [25%, 75%] = 14 [10, 16]) than patients who did not worry (median [25%, 75%] = 17 [14, 20], P < .001). We calculated SIMS and then correlated SIMS with B‐PREPARED and components. In the cohort, the mean SIMS was 12.1 4.7 (range 0‐17). Patients with greater satisfaction on the SIMS also had higher B‐PREPARED scale values (rho = 0.45, P < 0.001). There was a significant positive correlation between SIMS and the B‐PREPARED component called self‐care information for medications and activities (rho = 0.46, P < 0.001). The other 2 B‐PREPARED components, equipment/services and confidence, were positively correlated with SIMS at much lower levels (rho = 0.18 and rho = 0.24, respectively, both Fischer z transformations P < .001). The B‐PREPARED scale demonstrated validity with the constructs of worry and satisfaction with medication information.
Predictive Validity
We assessed the capacity of the B‐PREPARED to predict and discriminate groups of patients who did or did not visit emergency departments. Within 1 month of hospital discharge, 16.5% of the cohort (76 of 460 patients) had at least 1 visit to an emergency department. B‐PREPARED scale values were lower for those patients who visited emergency departments (median [25%, 75%] = 14 [12, 18]) than those who did not (median [25%, 75%] = 16 [13, 19], P = 0.011). The B‐PREPARED scale analysis supported the hypothesized relationship with emergency department visits.
Correlations between B‐PREPARED and Baseline Characteristics
We evaluated the correlations between a patient's B‐PREPARED scale value and baseline characteristics, shown in Table 1. There was a weak positive correlation with self‐rated health status (rho 0.17, P < .001). Patients who perceived better health status had higher B‐PREPARED scale values than those with poorer status. The other baseline characteristics in Table 1 were not associated with B‐PREPARED scale values.
DISCUSSION
The B‐PREPARED scale measures patients' perceptions of their preparedness for hospital discharge home. Scale items came from the PREPARED, a survey with validated psychometric properties in elderly patients. We assessed the B‐PREPARED in a cohort of young and elderly adult patients. We examined the B‐PREPARED instrument for internal consistency, construct validity, and predictive validity. In comparison with the domains identified in the full PREPARED instrument,9 the abbreviated B‐PREPARED scale identified similar domains. Some differences were anticipated because we limited items to those the respondents would be able to perceive before leaving the hospital.
The results of our study should be interpreted in the context of strengths and limitations. One of the strengths was the validity of the PREPARED, from which the B‐PREPARED was derived.7 The conceptually rigorous process used to develop the PREPARED questionnaire allowed us to draw from a bank of concise, well‐worded items.9 The B‐PREPARED extends validity to a population of adults of all ages with high risk for readmission. The other strength of the B‐PREPARED was the association with the clinically relevant constructs worry and satisfaction with medication information. The B‐PREPARED also discriminated between patients who did and those who did not return to emergency departments after discharge. Although the patient population for the B‐PREPARED validation was one of the strengths of this study, it is also a limitation. Our cohort lacked diversity with respect to readmission risk. The results of our study may not generalize to patients with low risk for repeated admission. Furthermore, all our patients were discharged home. The exclusion of other discharge destinations helped us to enroll a cohort with homogenous risk for readmission. However, our exclusion criteria did not allow us to validate the B‐PREPARED in patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities.
Another limitation related to outpatient visits after discharge. We did not analyze outpatient sites other than emergency departments. For all of our study patients, the discharging hospitalist scheduled at least 1 outpatient visit with the primary care practitioner. For some patients, the hospitalist also scheduled postdischarge visits for diagnostic evaluations like cardiac stress tests, endoscopies, radiographs, or other laboratory tests. When these visits occurred, they represented successful execution of the discharge plan. Sometimes patients arrived for planned or unplanned outpatient visits with exacerbated symptoms or adverse events. These latter visits might represent failures of the discharge plan. Our data collection did not allow us to distinguish outpatient visits as successes or failures of the discharge plan. When we counted only emergency department visits, we may have underestimated the number of patients with adverse events who sought and received successful treatment in outpatient clinics. Future studies should consider ascertainment of planned and unplanned outpatient visits for exacerbated symptoms and adverse events.
After our study began enrollment, other investigators published the Readiness for Hospital Discharge Scale14 and Care Transitions Measure.15 The design and validation of these sampling instruments differed with each other and with the B‐PREPARED. The differences made the 3 scales complementary but not interchangeable. For example, investigators administered the 21‐item Readiness for Hospital Discharge Scale on the day of discharge to adult medical‐surgical patients, postpartum mothers, and parents of hospitalized children. In contrast, we administered the B‐PREPARED 1 week after discharge to adult internal medicine patients or their proxies. The Readiness for Hospital Discharge subscales were personal status, knowledge, coping ability, and expected support. These subscales were similar to the components of the B‐PREPARED. The Readiness for Hospital Discharge Scale demonstrated internal consistency and construct validity but did not predict patients who returned to emergency departments after hospital discharge.14 Future users of the Readiness for Hospital Discharge Scale or the B‐PREPARED should consider their patient populations and the date of administration when selecting 1 scale versus another. If brevity is important to a clinician or researcher, then the 11‐item B‐PREPARED scale may be considered.
The Care Transitions Measure also differed from the B‐PREPARED. The 15‐item Care Transitions Measure evaluated an adult population with a broad age range and with chronic obstructive pulmonary disease, heart failure, stroke, or hip fracture.15 The diseases represented in the population for the Care Transitions Measure were similar to those in the B‐PREPARED cohort, although the distribution of the diseases differed. When validating the Care Transitions Measure, investigators administered questionnaires 6 to 12 weeks after discharge. The Care Transitions Measure had 4 factors: critical understanding, preferences important, management preparation, and care plan. The factors of the Care Transitions Measure were comparable to the components of the B‐PREPARED, and both scales assessed medication self‐management. However, the Care Transitions Measure addressed patient preferences with specific items, whereas the B‐PREPARED used the scoring system to quantify patient preferences. Both the Care Transitions Measure and the B‐PREPARED demonstrated internal consistency and discriminated between patients who did and those who did not return to emergency departments after hospital discharge.15 When selecting a scale, future users should consider the B‐PREPARED only for assessments 1 week post discharge and should consider the Care Transitions Measure for later assessments.
There are applications of the B‐PREPARED scale in hospital quality improvement efforts. Hospitals have multiple motivations to pursue quality improvement projects related to discharge processes: satisfaction of patients, reduction in adverse events, relation with referring physicians, and accreditation by regulators.6, 16 When hospital‐based clinicians survey patients, they may wish to use a brief, reliable, and validated instrument like the B‐PREPARED questionnaire.
CONCLUSIONS
The B‐PREPARED provided a reliable and valid measure of patients' perceptions of their preparedness for hospital discharge home. Clinicians and researchers may find the B‐PREPARED useful to guide, assess, and compare discharge‐planning interventions.
Acknowledgements
The authors thank Dr. Karen Grimmer‐Somers, PhD, for permission to use the PREPARED instrument and for her thoughtful comments on the draft manuscript.
Patients are vulnerable to adverse events when they transition from the hospital to outpatient care.13 Approximately 19%‐23% of patients experience adverse events within 4 weeks after acute care hospitalization.3, 4 One cause of postdischarge adverse events is ineffective discharge planning1, 2, 5, 6 Efforts to study and improve the hospital discharge‐planning processes require appropriate and valid measurement instruments. These instruments must assess the discharge process from multiple perspectives. One of the important perspectives is the patient's.7, 8
The PREPARED Patient Questionnaire is a comprehensive quality improvement tool to assess hospital discharge‐planning processes and outcomes from the patient's perspective.9, 10 The PREPARED acronym describes the content of this tool used to investigate the following phenomena: 1) prescriptions, 2) ready to reenter community, 3) education, 4) placement, 5) assurance of safety, 6) realistic expectations, 7) empowerment, and 8) directed to appropriate services.9 The PREPARED questionnaire was developed for, modified for, and validated with patients at least 65 years old. When administered to elderly patients 1 week after hospital discharge, the PREPARED has face, content, and construct validity.9
We considered the PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention. We sought a survey questionnaire to assess the patients' perceptions after the discharge intervention. In 2004, we found noother validated questionnaires except the PREPARED. We also noted some limitations of the PREPARED. The validated population for the PREPARED was patients older than 65 years. In our clinical trial, we planned to enroll adults of all ages. Another limitation was the PREPARED response scoring system that assigned missing data values to patients who took no medicines, needed no services, or needed no equipment.10 We were concerned about the potential for unacceptably large numbers of patients with nonignorable missing data. We decided to address the above limitations with a validation study in our patient population and with a revised response scoring system.
In the present article, we describe item reduction and validation for the Brief PREPARED (B‐PREPARED) scale to measure patients' perceptions of their preparedness for hospital discharge. When we designed B‐PREPARED, we asked the following question: Does a subset of PREPARED items with a revised scoring system have internal consistency, construct validity, and predictive validity in a population of adult patients with broad age range? We also wanted a brief scale with acceptable, defined statistical properties for multiple users. One user class included clinicians who guide and assess discharge‐planning processes. Other users would be researchers like us who measure differences between treatment groups after discharge process interventions.
METHODS
The Peoria Institutional Review Board approved the protocol for human research. The patient sample for scale analysis was a prospective cohort. Follow‐up was 1 month after patient's discharge from a 730‐bed acute‐care teaching hospital in central Illinois. The patients were enrolled in an ongoing cluster randomized clinical trial with blinded outcome assessment. Willing patients or their proxies provided written consent for study participation. Enrollment occurred between December 2004 and July 2006.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to the patient's home by internal medicine hospitalists. Patient inclusion in our cluster randomized trial required a probability of repeat admission (Pra) score of at least 0.40.11, 12 Hence, the patients in the scale analysis cohort had the same high probability for repeat admission. The research coordinators calculated the Pra within 2 days before discharge from the index hospitalization. The Pra score came from a logistic model of age, sex, prior hospitalizations, prior doctor visits, self‐rated health status, informal caregiver, and comorbid coronary heart disease and diabetes mellitus.11, 12
Patient Exclusion Criteria
We excluded patients if the discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. Because follow‐up occurred via interview, patients without telephones or English‐ or Spanish‐language skills were excluded. Patients with cognitive impairment could participate with consent from a legally authorized representative and with a proxy whospent a minimum of 3 hours daily with the patient and was willing to answer postdischarge interviews.
Baseline Assessment
During the index hospital admission, trained data abstractors recorded baseline patient data: age, sex, race, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, and coronary heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospital admission. We recorded the availability of an informal caregiver in response to the question Is there a friend, relative, or neighbor who would take care of you for a few days if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, or excellent.
Discharge and Postdischarge Procedures
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge‐planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aide providers, and others. After discharge, trained research personnel conducted 2 telephone interviews with the patient or the patient's proxy. The first interview occurred 1 week after discharge. Interviewers read verbatim items from the PREPARED10 and the Satisfaction with Information about Medicines Scale (SIMS).13 During the second telephone interview 30 10 days after discharge, interviewers recorded if patients had experienced at least 1 emergency department visit during the month after discharge.
The purpose of the PREPARED items was to have a bank of items and responses that could be used to generate the B‐PREPARED scale. The PREPARED questionnaire was originally developed to provide feedback to hospital ward staff about the quality of discharge‐planning activities that occurred during hospitalization.9 Discriminant factor analysis on the original 16 process questions revealed 4 factors that explained 57% of the total variance in patient/caregiver responses. The PREPARED domains included information exchange on community services and equipment, management of medication, the process of preparing to cope after discharge, and having control over one's discharge circumstances.9 The purpose of the SIMS was as a construct to compare with the B‐PREPARED scale. The derivation and validity of the SIMS have been described extensively elsewhere.13 In summary, the SIMS items were derived from recommendations of the Association of the British Pharmaceutical Industry. The intent of the SIMS was to determine if a patient's medication information needs were met and to allow comparison between patients or groups. Respondents selected 1 of 5 options for each of the 17 items. The sum of scores for each of the SIMS items yielded a total score that ranged from 0 to 17. Patients with high total SIMS scores had high satisfaction with the amount of medication information they received. Validation samples included inpatients and outpatients with a variety of diseases and characteristics. SIMS demonstrated adequate internal consistency, test‐retest reliability, and criterion‐related validity.13
Item Selection and Scoring of the B‐PREPARED Instrument
We selected an initial pool of items from the PREPARED instrument.10 The goal was a parsimonious, comprehensive, and valid instrument for use in clinical and research environments. When we retained or deleted items, our decision process was conservative, conceptual, and statistical. We performed item reduction in the following steps defined a priori. First, we agreed on items consistent with domains in the prepared for discharge construct as defined by expert consensus.9 Second, we excluded items that assessed qualities of the discharge process that were imperceptible to the patient on the day of discharge. Third, we excluded items that elicited open‐ended responses unsuitable for quantitative scale development and analysis. Fourth, we assessed reliability as defined by the Cronbach's alpha statistic. We excluded items that substantially decreased Cronbach's alpha.
Measures of Construct Validity
We used 2 measures of construct validity in our assessment of B‐PREPARED. One construct was patient worry. During the interview 1 week after discharge, research personnel asked, Now that you have been out of the hospital for a while, has anything been worrying you about managing at home? Response options for the dichotomous worry item were no or yes. We anticipated worried patients would have lower B‐PREPARED scale values. The other construct, SIMS, evaluated patient preparedness related to medication information exchange. The hypothesis was a positive correlation between SIMS and B‐PREPARED scale values.
Measure of Predictive Validity
We asked if B‐PREPARED predicted and discriminated groups of patients who did or did not visit emergency departments after hospital discharge. Emergency department visits were relevant adverse outcomes because of their association with postdischarge adverse events due to inpatient treatment.4 Emergency department visits reflected new or worsening symptoms after discharge. In our scale analysis, the hypothesis was patients with at least 1 emergency department visit would have lower B‐PREPARED scale values.
Analysis
Analyses were performed with SPSS PC (Version 14.0.2, SPSS Inc, Chicago, IL). We reported descriptive statistics as means, standard deviations, and range for interval variables and percentages for nominal variables. To determine the internal consistency of the scale, we calculated Cronbach's alpha. We assessed the distribution of the B‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered important. Statistical inference tests were the Mann‐Whitney U for median differences between 2 groups and the Spearman correlation for associations. We reported medians with 25th and 75th percentiles. Differences between 2 correlations were tested using Fischer z transformations. The accepted level of significance was P < 0.05.
RESULTS
Description of Cohort
We approached 5124 patients during the index hospital admission. After applying exclusion criteria, we obtained consent and enrolled 491 patients. The reasons for exclusion were low Pra score for 34.9% of ineligible patients, discharge to nursing home for 12.8%, declined consent for 10.8%, nonparticipating hospitalist service for 9.1%, discharged during screen for 8.5%, previously enrolled in study for 5.6%, and declined screening for 2.3%. Each of the other exclusion criteria accounted for less than 4% of the ineligible patients. After subtracting 6% of eligible patients (31 of 491) who died, withdrew, or were lost during the first month, there were 460 patients available for analysis. Table 1 describes the patients' characteristics. Most of the patients, 75.2% (346 of 460), were less than 65 years old, and the mean age was 53.9 15.5 years. Many patients had chronic diseases including diabetes mellitus, coronary heart disease, heart failure, and chronic obstructive pulmonary disease. Most patients, 81.5% (375 of 460), rated their health as poor or fair, and 53.5% (246/460) had 1 or more hospital admissions during the year before their index admission. Cohort patients had a high probability of repeat admission: mean Pra 0.49 0.07 (range 0.400.70).
| Characteristic | n (%) |
|---|---|
| Sex (male) | 193 (42.0%) |
| Age (years) | |
| 1930 | 35 (7.6%) |
| 3164 | 311 (67.6%) |
| 6598 | 114 (24.8%) |
| Race | |
| White | 275 (59.8%) |
| Black | 124 (27.0%) |
| Other | 61 (13.3%) |
| Self‐rated health status | |
| Poor | 139 (30.2%) |
| Fair | 236 (51.3%) |
| Good | 70 (15.2%) |
| Very good | 13 (2.8%) |
| Excellent | 2 (0.4%) |
| Diabetes mellitus | 259 (56.3%) |
| Chronic obstructive pulmonary disease | 79 (17.2%) |
| Coronary heart disease | 188 (40.9%) |
| Heart failure (n = 456) | 100 (21.7%) |
| Informal caregiver available (yes) | 459 (99.1%) |
| Hospital admissions during year prior to index admission | |
| 0 | 214 (46.5%) |
| 1 | 131 (28.5%) |
| 2 | 47 (10.2%) |
| 3 or 4 | 35 (7.6%) |
| 515 | 33 (7.2%) |
Item Reduction, Internal Consistency, and Score Distributions
Item reduction resulted in 12 items that fulfilled conceptual criteria. Table 2 shows the items and the distribution of responses. One of the 12 items, delays on the day you left the hospital (item 12, Table 2), was deleted because the item depressed the Cronbach's alpha. The B‐PREPARED with 11 items had acceptable internal consistency for the full cohort (Cronbach's alpha = 0.76).
| Item Text | Descriptor for Score 0 | Descriptor for Score 1 | Descriptor for Score 2 | |
|---|---|---|---|---|
| ||||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | None (40, 8.7%) | Some, but not enough (95, 20.7%) | As much as I needed; or Not taking any medications (325, 70.7%) |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | None (198, 43.0%) | Some, but not enough (54, 11.7%) | As much as I needed; or Not taking any medications (208, 45.2%) |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | No written instructions and no time spent (116, 25.2%) | Yes, received written instructions but no time spent (49, 10.7%) | Yes, received written instructions and yes, time spent; or, Not taking any medications (291, 63.3%) |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | None (55, 12.0%) | Some, but not enough (90, 19.6%) | As much as I needed (315, 68.5%) |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | None (89, 19.3%) | Some, but not enough (40, 8.7%) | As much as I needed; or No services needed (331, 72.0%) |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | None (49, 10.7%) | Some, but not enough (22, 4.8%) | As much as I needed; or No equipment needed (389, 84.6%) |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | No (42, 9.1%) | Yes; or No one needed to arrange because services were already in place or no services needed (418, 90.9%) | |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | No (16, 3.5%) | Yes; or No one needed to because equipment already in place or no equipment needed (444, 96.5%) | |
| 9 | Before you were discharged from hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | No (116, 25.2%) | Yes (344, 74.8%) | |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | Not confident (25, 5.4%) | Unsure (103, 22.4%) | Confident (332, 72.2%) |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | Unprepared (39, 8.5%) | Moderately prepared (132, 28.7%) | Very prepared (288, 62.6%) |
| 12 | After you were told you could leave the hospital, were there any delays on the day you left the hospital? | Yes (122, 26.5%) | No (338, 73.5%) | |
For an individual patient, the sum of the scores for each item yielded a B‐PREPARED scale value. In the 460‐patient cohort, B‐PREPARED scale values had a mean of 17.3 4.3 and a negatively skewed distribution. A high scale value reflected high perception of discharge preparedness. Each of the 11 items correlated significantly with the B‐PREPARED scale value (P < 0.001, 2‐tailed).
There were substantial ceiling effects with individual items but not in the B‐PREPARED total score. Five of the 9 items with 3 response options had a ceiling effect above 70%. Three items had a dichotomous response option (items 7, 8, and 9). In 2 of these 3 items, more than 90% of respondents selected the response indicating higher preparedness. The total B‐PREPARED did not have noteworthy floor or ceiling effects. In this sample's total B‐PREPARED scores, 0.2% of respondents had the lowest score of 3, and 20% had the highest score of 22.
Principal Component Analysis
In the component analysis, we evaluated the correlation matrix of the 11 items in the B‐PREPARED scale. A Kaiser‐Meyer‐Olkin statistic of 0.76 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 54.2% of the variance associated with the 11‐item B‐PREPARED scale. After inspection of scree plots, we determined that 3 components were extracted before the eigenvalue fell below 1. The pattern matrix for the promax rotation was inspected, and the factor loading of each item appears in Table 3. The item content identified the first component as self‐care information for medications and activities. The second component was equipment and services. The third component was confidence. All B‐PREPARED items loaded primarily on 1 of the 3 components (Table 3).
| Item text | Component | |||
|---|---|---|---|---|
| Self‐care Information for Medications and Activity | Equipment and Services | Confidence | ||
| 1 | While you were in the hospital, how much information did you receive about the medications that you were to take at home? | 0.749 | 0.032 | 0.019 |
| 2 | While you were in the hospital, how much information did you receive about the side effects of the medications that you were to take at home? | 0.778 | .008 | 0.003 |
| 3 | While you were in the hospital, were you given written instructions about your medications? If yes, did someone spend time explaining the written instructions? | 0.758 | 0.030 | 0.084 |
| 4 | While you were in the hospital, how much information did you receive on how you would manage your usual activities when you went home? | 0.581 | 0.101 | 0.195 |
| 5 | While you were in the hospital, how much information did you receive on community services you might use once you went home? | 0.158 | 0.639 | 0.124 |
| 6 | While you were in the hospital, how much information did you receive on equipment you might need once you went home? | 0.183 | 0.701 | ‐0.152 |
| 7 | Before you were discharged from the hospital, did anyone arrange community services for you to use at home? | 0.081 | 0.654 | 0.199 |
| 8 | Before you were discharged from the hospital, did anyone arrange equipment for you? | 0.138 | 0.655 | 0.095 |
| 9 | Before you were discharged from the hospital, was there any other information you would have liked while you were in the hospital to prepare you for coping at home? | 0.181 | 0.211 | 0.369 |
| 10 | After you were told you could leave the hospital, how confident did you feel about managing at home? | 0.036 | 0.058 | 0.876 |
| 11 | Looking back to the time you left the hospital, overall, how prepared did you feel for returning home? | 0.018 | 0.032 | 0.875 |
Construct Validity
We assessed 2 constructs: worry and satisfaction with medication information (SIMS). In the cohort, 25% of patients (115 of 460) reported worry about managing at home. Worried patients had significantly lower B‐PREPARED scale values (median [25%, 75%] = 14 [10, 16]) than patients who did not worry (median [25%, 75%] = 17 [14, 20], P < .001). We calculated SIMS and then correlated SIMS with B‐PREPARED and components. In the cohort, the mean SIMS was 12.1 4.7 (range 0‐17). Patients with greater satisfaction on the SIMS also had higher B‐PREPARED scale values (rho = 0.45, P < 0.001). There was a significant positive correlation between SIMS and the B‐PREPARED component called self‐care information for medications and activities (rho = 0.46, P < 0.001). The other 2 B‐PREPARED components, equipment/services and confidence, were positively correlated with SIMS at much lower levels (rho = 0.18 and rho = 0.24, respectively, both Fischer z transformations P < .001). The B‐PREPARED scale demonstrated validity with the constructs of worry and satisfaction with medication information.
Predictive Validity
We assessed the capacity of the B‐PREPARED to predict and discriminate groups of patients who did or did not visit emergency departments. Within 1 month of hospital discharge, 16.5% of the cohort (76 of 460 patients) had at least 1 visit to an emergency department. B‐PREPARED scale values were lower for those patients who visited emergency departments (median [25%, 75%] = 14 [12, 18]) than those who did not (median [25%, 75%] = 16 [13, 19], P = 0.011). The B‐PREPARED scale analysis supported the hypothesized relationship with emergency department visits.
Correlations between B‐PREPARED and Baseline Characteristics
We evaluated the correlations between a patient's B‐PREPARED scale value and baseline characteristics, shown in Table 1. There was a weak positive correlation with self‐rated health status (rho 0.17, P < .001). Patients who perceived better health status had higher B‐PREPARED scale values than those with poorer status. The other baseline characteristics in Table 1 were not associated with B‐PREPARED scale values.
DISCUSSION
The B‐PREPARED scale measures patients' perceptions of their preparedness for hospital discharge home. Scale items came from the PREPARED, a survey with validated psychometric properties in elderly patients. We assessed the B‐PREPARED in a cohort of young and elderly adult patients. We examined the B‐PREPARED instrument for internal consistency, construct validity, and predictive validity. In comparison with the domains identified in the full PREPARED instrument,9 the abbreviated B‐PREPARED scale identified similar domains. Some differences were anticipated because we limited items to those the respondents would be able to perceive before leaving the hospital.
The results of our study should be interpreted in the context of strengths and limitations. One of the strengths was the validity of the PREPARED, from which the B‐PREPARED was derived.7 The conceptually rigorous process used to develop the PREPARED questionnaire allowed us to draw from a bank of concise, well‐worded items.9 The B‐PREPARED extends validity to a population of adults of all ages with high risk for readmission. The other strength of the B‐PREPARED was the association with the clinically relevant constructs worry and satisfaction with medication information. The B‐PREPARED also discriminated between patients who did and those who did not return to emergency departments after discharge. Although the patient population for the B‐PREPARED validation was one of the strengths of this study, it is also a limitation. Our cohort lacked diversity with respect to readmission risk. The results of our study may not generalize to patients with low risk for repeated admission. Furthermore, all our patients were discharged home. The exclusion of other discharge destinations helped us to enroll a cohort with homogenous risk for readmission. However, our exclusion criteria did not allow us to validate the B‐PREPARED in patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities.
Another limitation related to outpatient visits after discharge. We did not analyze outpatient sites other than emergency departments. For all of our study patients, the discharging hospitalist scheduled at least 1 outpatient visit with the primary care practitioner. For some patients, the hospitalist also scheduled postdischarge visits for diagnostic evaluations like cardiac stress tests, endoscopies, radiographs, or other laboratory tests. When these visits occurred, they represented successful execution of the discharge plan. Sometimes patients arrived for planned or unplanned outpatient visits with exacerbated symptoms or adverse events. These latter visits might represent failures of the discharge plan. Our data collection did not allow us to distinguish outpatient visits as successes or failures of the discharge plan. When we counted only emergency department visits, we may have underestimated the number of patients with adverse events who sought and received successful treatment in outpatient clinics. Future studies should consider ascertainment of planned and unplanned outpatient visits for exacerbated symptoms and adverse events.
After our study began enrollment, other investigators published the Readiness for Hospital Discharge Scale14 and Care Transitions Measure.15 The design and validation of these sampling instruments differed with each other and with the B‐PREPARED. The differences made the 3 scales complementary but not interchangeable. For example, investigators administered the 21‐item Readiness for Hospital Discharge Scale on the day of discharge to adult medical‐surgical patients, postpartum mothers, and parents of hospitalized children. In contrast, we administered the B‐PREPARED 1 week after discharge to adult internal medicine patients or their proxies. The Readiness for Hospital Discharge subscales were personal status, knowledge, coping ability, and expected support. These subscales were similar to the components of the B‐PREPARED. The Readiness for Hospital Discharge Scale demonstrated internal consistency and construct validity but did not predict patients who returned to emergency departments after hospital discharge.14 Future users of the Readiness for Hospital Discharge Scale or the B‐PREPARED should consider their patient populations and the date of administration when selecting 1 scale versus another. If brevity is important to a clinician or researcher, then the 11‐item B‐PREPARED scale may be considered.
The Care Transitions Measure also differed from the B‐PREPARED. The 15‐item Care Transitions Measure evaluated an adult population with a broad age range and with chronic obstructive pulmonary disease, heart failure, stroke, or hip fracture.15 The diseases represented in the population for the Care Transitions Measure were similar to those in the B‐PREPARED cohort, although the distribution of the diseases differed. When validating the Care Transitions Measure, investigators administered questionnaires 6 to 12 weeks after discharge. The Care Transitions Measure had 4 factors: critical understanding, preferences important, management preparation, and care plan. The factors of the Care Transitions Measure were comparable to the components of the B‐PREPARED, and both scales assessed medication self‐management. However, the Care Transitions Measure addressed patient preferences with specific items, whereas the B‐PREPARED used the scoring system to quantify patient preferences. Both the Care Transitions Measure and the B‐PREPARED demonstrated internal consistency and discriminated between patients who did and those who did not return to emergency departments after hospital discharge.15 When selecting a scale, future users should consider the B‐PREPARED only for assessments 1 week post discharge and should consider the Care Transitions Measure for later assessments.
There are applications of the B‐PREPARED scale in hospital quality improvement efforts. Hospitals have multiple motivations to pursue quality improvement projects related to discharge processes: satisfaction of patients, reduction in adverse events, relation with referring physicians, and accreditation by regulators.6, 16 When hospital‐based clinicians survey patients, they may wish to use a brief, reliable, and validated instrument like the B‐PREPARED questionnaire.
CONCLUSIONS
The B‐PREPARED provided a reliable and valid measure of patients' perceptions of their preparedness for hospital discharge home. Clinicians and researchers may find the B‐PREPARED useful to guide, assess, and compare discharge‐planning interventions.
Acknowledgements
The authors thank Dr. Karen Grimmer‐Somers, PhD, for permission to use the PREPARED instrument and for her thoughtful comments on the draft manuscript.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Review of the literature on survey instruments used to collect data on hospital patients' perceptions of care.Health Serv Res.2005;40:1996–2017.
- ,.A patient‐centered model of care for hospital discharge.Clin Nurs Res.2004;13:117–136.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- PREPARED Patient Questionnaire. Available at: http://www.unisa.edu.au/cahe/pubs/Patient%20scoring.pdf. Accessed June 14,2007.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,.The Satisfaction with Information about Medicines Scales (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,.Psychometric properties of the Readiness for Hospital Discharge Scale.J Nurs Meas.2006;14:163–180.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed June 14,2007.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Review of the literature on survey instruments used to collect data on hospital patients' perceptions of care.Health Serv Res.2005;40:1996–2017.
- ,.A patient‐centered model of care for hospital discharge.Clin Nurs Res.2004;13:117–136.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- PREPARED Patient Questionnaire. Available at: http://www.unisa.edu.au/cahe/pubs/Patient%20scoring.pdf. Accessed June 14,2007.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,.The Satisfaction with Information about Medicines Scales (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,.Psychometric properties of the Readiness for Hospital Discharge Scale.J Nurs Meas.2006;14:163–180.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed June 14,2007.
Copyright © 2008 Society of Hospital Medicine
Discharge Planning Scale
Preventable adverse events occur when patients transition from hospital to outpatient care.1, 2 The most common cause for postdischarge adverse events is poor communication between inpatient healthcare providers and outpatient primary care physicians.1 Adverse events also occur because of inadequate processes to communicate unresolved problems, monitor drug therapies, or monitor the patient's overall condition.1 Efforts to reduce adverse events logically focus on effective discharge planning and communication.
Systematic reviews have evaluated clinical trials to improve discharge planning and communication.36 Reviewers often reported inconclusive results because of a shortage of high‐quality trials with validated outcome measures.35 Reviewers recommended future studies to develop and validate outcome measures that assessed the discharge process from various perspectives.4 One important perspective was the assessment by the outpatient, primary care physician who was responsible for patient care after discharge.79
One of the authors (K.G.S.) developed the Physician‐PREPARED questionnaire to measure perceptions of outpatient physicians about the quality of hospital discharge. Item content came from studies in Australia that investigated barriers to best practice in discharge planning for older patients.1013 Fifteen items asked community physicians about their awareness of discharge planning processes for their patients. Items also assessed the adequacy of information provided about discharge plans. The Physician‐PREPARED items underwent assessment in Australia. Evaluation revealed well‐worded text, unambiguous response options, face validity, and content validity.
We reconsidered the Physician‐PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention in the United States. Our goal was a comprehensive survey instrument and scale to measure the perceptions of outpatient physicians after the discharge intervention. We found no other appropriate, validated questionnaires except the Physician‐PREPARED. However, we recognized some limitations to the Physician‐PREPARED. The items were developed for Australian physicians who treated elderly patients. We wanted to assess North American physicians who cared for a broad age range of adults. The Physician‐PREPARED did not have a scale with validated, psychometric performance characteristics in our population. We decided to address the above limitations with a scale development and validation study in the United States.
In the present work, we describe item development for the Physician‐PREPARED that occurred in Australia. Then we present item reduction and validation for the Modified Physician‐PREPARED that occurred in the United States. Our primary objective was to validate a scale to measure perceptions of outpatient physicians about qualities of discharge planning and communication. The secondary objectives were to quantify the scale's internal consistency and construct validity. Our goal was a brief scale with acceptable, defined statistical properties for clinicians and researchers.
PATIENTS AND METHODS
Item Development for the Physician‐PREPARED
Australian investigators designed the Physician‐PREPARED survey instrument to measure the quality of discharge planning activities and communication. The investigators developed the survey with the following process that was not published previously. First, a literature review identified survey content germane to outpatient practitioners.10 Investigators conducted interviews, focus groups, and pilot surveys to prioritize items for the survey instrument. The volunteer subjects for item development were general medical practitioners in Adelaide and Sydney, the capital cities of two states in Australia. The draft instrument was circulated to a small group of general medical practitioners for comment on layout, wording, and question intent. After feedback, minor modifications were made to item content and response categories. The result of development in Australia was a survey instrument with 15 items (see Appendix). The items reflected the following key areas of discharge quality: timeliness of communication, patient health status at discharge, adequacy of discharge support services, discharge medication information, and reasons for medication changes. These areas were congruent with the results of other investigators who assessed the quality of discharge planning and communication.14, 15
Validation of the Modified Physician‐PREPARED
The validation study for the Modified Physician‐PREPARED occurred in Illinois. The Peoria Institutional Review Board approved and monitored the human research. The patient sample for validation was a prospective cohort from a cluster randomized clinical trial. Willing patients or their proxies provided written consent for study participation. Patient enrollment occurred between December 2004 and August 2006. The subjects for scale analysis were the outpatient primary care physicians or practitioners designated by patients in the cohort. Outpatient physicians and practitioners gave implied consent when they completed and returned questionnaires. Follow‐up was 10 or more days after the patient's discharge from an acute care, 730‐bed, teaching hospital.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to home by internal medicine hospitalist physicians. Patient inclusion in the cluster‐randomized trial required a probability of repeat admission (Pra) score greater than or equal to 0.40.16, 17 Consequently, the patients in the scale analysis cohort had the same high probability for repeat admission. The Pra score came from patient or proxy responses to questions about age, prior hospitalizations, prior doctor visits, self‐rated health status, and other health‐related questions.16, 17 In previous validation studies with elderly outpatients, a Pra score above 0.5 predicted that patients would have 1 hospital admission per person‐year of survival.16 In other validation studies with inpatients aged 18 to 101 years, the Pra items predicted nonroutine discharge planning needs.18
Exclusion Criteria
The exclusion criteria were designed to enroll a cohort with homogeneous risk for readmission. We excluded patients if their discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Hospice patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. We also used exclusion criteria to avoid illogical enrollments. If the designated outpatient primary care physician or practitioner also managed the patient during the index hospitalization, then there was no perceived barrier to communication and the patient was excluded. Cognitive impairment was a conditional exclusion criterion. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.19 A patient with cognitive impairment could participate with consent from a legally authorized representative. Before we enrolled a cognitively impaired patient, we required a proxy who spent a minimum of 3 hours daily with the patient and who agreed to answer interview questions.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient data to calculate the Pra: age, gender, diabetes mellitus, and ischemic heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospitalization. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative or neighbor who would take care of you for a few days, if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, and excellent. In addition, we recorded heart failure and chronic obstructive pulmonary disease because of their possible association with readmission.20, 21 Information about outpatient physicians or practitioners came from the hospital's administrative database and was limited to specialty training.
Discharge Process
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aid providers, and others. Patients designated an outpatient primary care physician or nurse practitioner or physician assistant to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated outpatient primary care professional.
Item Reduction and Scoring
To develop a scale, we selected items from the Physician‐PREPARED survey instrument (see Appendix). Our goal was a parsimonious, comprehensive, and valid scale for use in clinical and research environments. We applied item reduction techniques according to the following steps that were defined a priori. First, we deleted items with nominal response categories that lacked graded or ordinal characteristics. This exclusion criterion caused us to delete the following items from the questionnaire in the appendix: (1a) Who made you aware of the admission, (2a) Who made you aware of the patient's discharge, and (5a) How did you receive this information? We deleted open‐ended questions, such as: (13) Have you any suggestions how the patient's discharge could have been improved? Next, we excluded items with a large proportion of missing responses because respondents checked Not applicable. Only item 12 from the Physician‐PREPARED fulfilled the latter criterion (see Appendix). Question 12 asked, Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? Among 403 respondents, 52% answered question 12 as Not applicable.
Measures of Construct Validity
We used 3 measures of construct validity in our assessment of the Modified Physician‐PREPARED scale. The first construct item asked the outpatient practitioner, Were you involved at all in planning the patient's discharge? The first construct was relevant because involvement by outpatient physicians improves the quality of hospital discharges.22 The second construct item asked, Are you aware of any community support services that are involved in providing assistance to the patient since discharge? For the third construct, we asked (Appendix item 11), Has the patient voiced any concerns that they have not been coping since discharge? We chose community support services and patient coping because these are clinically relevant and correlated with patients' perceptions of discharge preparedness.23 When we assessed construct validity, our hypotheses were significantly higher Modified Physician‐PREPARED scale values for respondents who answered yes to the construct questions about involvement and awareness and answered no to the question about patient‐voiced concerns.
Analysis
Analyses were performed with SPSS PC (version 14.0.2; SPSS Inc, Chicago, Illinois). We reported descriptive statistics as means, standard deviations (SDs), and range for interval variables; median and range for ordinal variables; and percentages for nominal variables. While developing the scale, the unit of analysis was the physician response to a unique patient. Specific descriptive analyses used the unique respondent as the unit of analysis. To determine the internal consistency of the scale, we calculated Cronbach's alpha with SPSS RELIABILITY. We assessed the distribution of the Modified Physician‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered for interpretation.24 Statistical inference tests were the Mann‐Whitney U for median differences for 2 groups, the Kruskal‐Wallis for more than 2 groups, and Spearman correlation for associations. The accepted level of significance was P < 0.05.
RESULTS
Description of Validation Cohort for the Modified Physician‐PREPARED
We sent questionnaires to the primary care physician, nurse practitioner, or physician assistant designated by 549 patients. The survey response rate was 76% (417/549). If a respondent failed to check any response option for 2 or more scale items, then the questionnaire was excluded from analysis. We excluded 3% (14/549) of questionnaires for failure to respond to items. The responses from the remaining 403 questionnaires were analyzed. We did not exclude questionnaires from respondents who followed homebound patients or other patients who failed to come to the clinic for postdischarge visits. Our analysis included 90 questionnaires (22%) from respondents who had no contact with the patient after discharge.
The patient characteristics appear in Table 1. Most of the patients were less than 65 years old (77%, 310/403). Many patients had chronic diseases including diabetes mellitus, ischemic heart disease, heart failure, or chronic obstructive pulmonary disease. Most patients, 81% (327/403), rated their health as poor or fair and 55% (223/403) had 1 or more hospital admissions during the year before their index admission. The questionnaire respondents were primary care physicians who practiced internal medicine (41%, 167/403), medicine‐pediatrics (27%, 108/403), family practice (24%, 97/403), or other specialties (3%, 10/403). Nurse practitioners or physician assistants completed 5% (21/403) of questionnaires.
| Characteristic | Number (%) |
|---|---|
| |
| Gender, female | 235 (58.3%) |
| Race | |
| White | 284 (70.5%) |
| Black | 116 (28.8%) |
| Other | 3 (0.7%) |
| Self‐rated health status | |
| Poor | 125 (31.0%) |
| Fair | 202 (50.1%) |
| Good | 61 (15.1%) |
| Very good | 13 (3.2%) |
| Excellent | 2 (0.5%) |
| Diabetes mellitus | 226 (56.1%) |
| Chronic obstructive pulmonary disease | 76 (18.9%) |
| Ischemic heart disease | 165 (40.9%) |
| Heart failure | 90 (22.3%) |
| Hospital admissions during prior year (includes index admission) | 2.2 (2.0) [0‐15]* |
| Age (years) | 53.6 (15.1) [19‐98]* |
| Pra score | 0.49 (0.07) [0.40‐0.70]* |
We conducted descriptive analyses that treated the respondent as the unit of analysis. There were 172 unique respondents. The number of questionnaires per respondent ranged from 1 to 20 with a median of 1 questionnaire. Respondents varied in the time to return a questionnaire. We measured response time as the difference between the date we received the questionnaire and the date of discharge. The response time ranged from 10 to 90 days with a median of 21 days after discharge.
Modified Physician‐PREPARED: Item Reduction, Internal Consistency, and Score Distributions
The questionnaire items appear in the Appendix. After item reduction, there were 8 items included in the Modified Physician‐PREPARED scale analysis (Table 2). None of the 8 items caused substantive reduction in Cronbach's alpha, so all were retained. The 8‐item scale had acceptable internal consistency (Cronbach's alpha = 0.86). For an individual questionnaire, the sum of the scores for eight items yielded the Modified Physician‐PREPARED scale value. High scale values reflected high perceptions of discharge quality. Each of the 8 items correlated significantly and positively with the scale value (P < 0.001, 2‐tailed).
| Item Text | Descriptor for Score = 1 | Descriptor for Score = 2 | Descriptor for Score = 3 | No Score | |
|---|---|---|---|---|---|
| 1. | When were you made aware that this patient had been admitted to hospital? | Not at all; 55 (13.6%) | After patient was discharged; 65 (16.1%) | Prior to hospitalization; while patient was in hospital; or on the day of discharge; 281 (69.7%) | Missing response; 2 (0.5%) |
| 2. | When were you made aware that the patient was going to be discharged? | Not at all; 115 (28.5%) | Within a week after discharge; or longer than a week after discharge; 61 (15.1%) | While patient was still in hospital; or on day of discharge; or within 1‐2 days after discharge; 225 (55.8%) | Missing response; 2 (0.5%) |
| 3. | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Longer than a week; or not received; or other 115 (28.5%) | Within a week; 186 (46.2%) | Within 1‐2 days; 101 (25.1%) | Missing response; 1 (0.2%) |
| 4. | Was this sufficient notice to address this patient's postdischarge needs? | Less than sufficient; 98 (24.3%) | Sufficient; 246 (61.0%) | More than sufficient; 46 (11.4%) | Missing response; 13 (3.2%) |
| 5. | Have you received adequate information about this patient's discharge health status? | No; 103 (25.6%) | Yes; 295 (73.2%) | Missing response; 5 (1.2%) | |
| 6. | Have you received adequate written information about the patient's medicines and medication management? | Less than adequate; or no information at all; 103 (25.6%) | Adequate; 262 (65.0%) | More than adequate; 38 (9.4%) | Missing response; 0 (0%) |
| 7. | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Less than sufficient; or no information at all; 129 (32.0%) | Sufficient; or not applicable (there was no change in medications); 240 (59.6%) | More than sufficient; 29 (7.2%) | Missing response; 5 (1.2%) |
| 8. | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | Less than adequate; or no discharge plans; 82 (20.3%) | Adequate; 276 (68.5%) | More than adequate; 32 (7.9%) | Missing response; 13 (3.2%) |
Table 2 shows the distribution of responses to each item in the Modified Physician‐PREPARED questionnaire. There were substantial ceiling effects for 2 individual items. One of the 7 items with 3 response options had ceiling effects approaching 70% (item 1). One item had 2 response options and 73% responded yes (item 5). The distribution of Modified Physician‐PREPARED scale values for 403 questionnaires had mean 16.6 4.0 SD and skew 0.6 (standard error of skew = 0.1). When scale values of patients 64 years and younger were compared with those of 65 and older, there were no significant differences (P = 0.606). The scale values did not have noteworthy floor or ceiling effects. The distribution of scale values showed 1.2% (5/403) of respondents had the lowest score of 8 and 1.7% (7/403) had the highest score of 24.
Modified Physician‐PREPARED: Principal Component Analysis
The purpose of the principal component analysis was to evaluate the relationships between the items and domains. In the component analysis, we evaluated the correlation matrix of the 8 items in the Modified Physician‐PREPARED scale. The Kaiser‐Meyer‐Olkin statistic of 0.89 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 66% of the variance associated with the 8‐item scale. After inspection of scree plots, we determined that 2 components were extracted before the eigenvalue fell substantially below 1. The pattern matrix for the promax rotation was inspected and the factor loading for each item appears in Table 3. The item content identified 1 component as timeliness of communication. The other component was adequacy of discharge plan/transmission. Within the adequacy component, the item content addressed patient health status, medication information, and reasons for medication changes. All items loaded primarily on 1 of the components; except item 3, which loaded on both components.
| Item Text | Component | ||
|---|---|---|---|
| Adequacy of Discharge Plan/Transmission | Timeliness of Communication | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why one type of medication is used in preference to another?) | 0.900 | 0.132 |
| 6 | Have you received adequate written information about the patient's medicines and medication management? | 0.849 | 0.056 |
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | 0.796 | 0.050 |
| 5 | Have you received adequate information about this patient's discharge health status? | 0.774 | 0.012 |
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | 0.744 | 0.132 |
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | 0.403 | 0.373 |
| 1 | When were you made aware that this patient had been admitted to hospital? | 0.154 | 0.964 |
| 2 | When were you made aware that the patient was going to be discharged? | 0.123 | 0.779 |
Modified Physician‐PREPARED: Construct Validity
We compared Modified Physician‐PREPARED scale values between dichotomous groups defined by construct variables. When considering the discharge planning and communication for a specific patient, outpatient primary care practitioners reported higher scale values when they were involved in the discharge planning (median [25%, 75%] = 19 [19, 20.5]) than when they were not involved (17 [12.4, 19], P < 0.001). In addition, outpatient practitioners responded with higher scale values when they were aware of community support services (18 [16, 20]) than when they were unaware (17 [12, 19], P = 0.002). There was a nonsignificant trend to higher scale values if patients voiced no concern about coping after discharge (18 [15, 19]) versus concern (17 [12, 19], P = 0.059). For all 3 constructs, the analysis revealed higher Modified Physician‐PREPARED scale values that were in the same direction as hypothesized. We approximated the construct analysis with subscales defined by the principal components (data not shown). The subscale analysis confirmed the direction and significance of the analysis with the full, 8‐item, Modified Physician‐PREPARED scale.
Modified Physician‐PREPARED: Correlations with Baseline Characteristics
We evaluated the correlations between a patient's Modified Physician‐PREPARED scale value and baseline characteristics in Table 1. Patient characteristics were not associated with scale values. We also assessed the median differences between the scale values by practitioner specialty and found no significant differences.
DISCUSSION
The Modified Physician‐PREPARED scale measured the quality of discharge planning and communication from the perspective of the outpatient primary care physician or practitioner. We described the derivation of the scale items. We demonstrated the reliability and validity of the scale among physicians and practitioners who provided postdischarge care to patients at high risk for readmission to the hospital. The item content included timeliness, adequacy, patient health status, medication information, and reasons for medication changes.
According to expert consensus guidelines for hospital discharge care, the communication with the outpatient primary care physician should occur as soon as possible after discharge.25 Recommended data elements in the communication include condition at discharge, diagnoses, medications added, medications discontinued, and medications changed.25 We found the Modified Physician‐PREPARED scale items included content that was consistent with expert consensus guidelines. The items also assessed timeliness and adequacy, 2 domains important to outpatient physicians.14, 26
The Modified Physician‐PREPARED is one of several questionnaires developed to measure qualities of discharge processes from the perspective of outpatient physicians.8, 15, 2733 Previous questionnaires did not report psychometrics except 1 that assessed the quality of discharge summaries and measured test‐retest reliability.33 We are not aware of other physician questionnaires with reliable or valid scales besides the Modified Physician‐PREPARED.
We believe 1 application of the Modified Physician‐PREPARED questionnaire is in quality improvement efforts within hospitals. Most hospitals and inpatient physicians rely on discharge letters or summaries to communicate information about the hospitalization to outpatient practitioners.6 However, systematic problems with generation and transmission of letters and summaries make them sometimes unreliable as sources of consistent, timely, accurate, or important information.6 When patients arrive for their posthospital visits, their outpatient physicians have received no discharge letter for 16% to 53% of patients and no discharge summary for 66% to 88%.6 Among outpatient physicians, 41% attribute preventable adverse events for at least 1 of their patients to inadequate discharge communication.34 One hospital accreditation organization includes discharge communication improvement as a national patient safety goal in the United States.35 Hospitals have multiple motivations to pursue quality improvement projects related to discharge communication: reduction in adverse events, relation with referring physicians, and accreditation by regulators. When surveying physicians, hospital personnel may wish to use a reliable and validated instrument like the Modified Physician‐PREPARED questionnaire.
Another application of the Modified Physician‐PREPARED scale is in research. An example is our randomized, controlled trial to measure the value of a discharge intervention. We published the rationale and design for our intervention.36 In the future, we will analyze the results of our trial and we will need validated scales. One of the trial outcomes is the perspective of the outpatient physician. We expect to compare the scores on the Modified Physician‐PREPARED scale values from community practitioners who treated test patients versus control patients. The statistical properties of the Modified Physician‐PREPARED scale that we validated in the current work will allow us to estimate the precision of between‐group differences and to perform tests of inference.
The results of our study should be interpreted in the context of strengths and limitations. We were able to generalize the validity of the Modified Physician‐PREPARED to North American primary care physicians who treated adult outpatients with a broad age range. We minimized biases with the high survey response rate and low proportion of missing responses. During validation, we asked physicians to evaluate patient transitions from hospital to home. Consequently, the Modified Physician‐PREPARED scale may not apply when doctors follow patients after discharge to nursing homes or other acute care facilities. We excluded patients with low probability of repeat admission: hospice patients and patients with low Pra scores. The purpose of our exclusion criteria was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. We recognize that the Modified Physician‐PREPARED may not generalize to physicians who treat hospice patients or patients with low probability for readmission.
Additional limitations relate to test‐retest reliability and to the clinical meaning of small changes in scale values. In our study, physician respondents returned questionnaires approximately 3 weeks after hospital discharge. We did not ask physicians to complete the questionnaire again after they returned the first questionnaire. Therefore, the test‐retest reliability for the Modified Physician‐PREPARED is unknown. Our protocol was not designed to detect the minimum important difference in the scale values. Consequently, small changes in scale values have uncertain clinical relevance. Future studies are necessary to assess the minimum important difference in the scale values.
CONCLUSION
The Modified Physician‐PREPARED scale was a reliable and valid measure of outpatient physician perceptions of quality and communication after hospital discharge. Clinicians and researchers may find the scale useful to guide, assess, and compare discharge‐planning activities.
APPENDIX
PHYSICIAN‐PREPARED QUESTIONNAIRE
| Item | Question | Response Options |
|---|---|---|
| 1 | When were you made aware that this patient had been admitted to hospital? | Prior to hospitalization |
| While patient was in hospital | ||
| On the day of discharge | ||
| After patient was discharged | ||
| Not at all | ||
| 1a | Who made you aware of the admission? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Ambulance | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 2 | When were you made aware that the patient was going to be discharged? | While patient was still in hospital |
| On day of discharge | ||
| Within 1‐2 days after discharge | ||
| Within a week after discharge | ||
| Longer than a week after discharge | ||
| Not at all | ||
| 2a | Who made you aware of the patient's discharge? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Within 1‐2 days |
| Within a week | ||
| Longer than a week | ||
| Not received | ||
| Other, please specify _________________________ | ||
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | More than sufficient |
| Sufficient | ||
| Less than sufficient | ||
| 5 | Have you received adequate information about this patient's discharge health status? | Yes |
| No | ||
| 5a | How did you receive this information? (Check all that apply) | Telephone call |
| Fax | ||
| Electronic mail system | ||
| Written/typed letter | ||
| 6 | Have you received adequate written information about the patient's medicines and medication management? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No information at all | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Not applicable (there was no change in medications) |
| More than sufficient | ||
| Sufficient | ||
| Less than sufficient | ||
| No information at all | ||
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No discharge plans | ||
| 9 | Were you involved at all in planning the patient's discharge? | Yes |
| No | ||
| 10 | Are you aware of any community support services that are involved in providing assistance to the patient since discharge? | Yes |
| No | ||
| 11 | Has the patient voiced any concerns that they have not been coping since discharge? | Yes |
| No | ||
| Not applicable (no contact with patient since discharge) | ||
| 12 | Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? | Not applicable (no caretaker) |
| Yes | ||
| No | ||
| Not applicable (no contact with caretaker since discharge) | ||
| 13 | Have you any suggestions how the patient's discharge could have been improved? | __________________________________________ |
| __________________________________________ | ||
| __________________________________________ |
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,.Care home versus hospital and own home environments for rehabilitation of older people.Cochrane Database Syst Rev.2003;(2):CD003164.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Effects of a multidisciplinary, post‐discharge continuance of care intervention on quality of life, discharge satisfaction, and hospital length of stay: a randomized controlled trial.Int J Qual Health Care.2005;17:43–51.
- .Hospitalists and family physicians: understanding opportunities and risks.J Fam Pract.2004;53:473–481.
- ,,,.performance indicators for discharge planning: a focused review of the literature.Aust J Adv Nurs.1999;16:20–28.
- Informing discharge plans. Assessments of elderly patients in Australian public hospitals: a field study. Available at: http://ijahsp.nova.edu/articles/Vol2number3/Grimmer‐Discharge_Plans.htm. Accessed January2008.
- ,,.Experiences of elderly patients regarding independent community living after discharge from hospital: a longitudinal study.Int J Qual Health Care.2004;16:465–472.
- ,,.Life Post‐Discharge: Longitudinal Qualitative and Quantitative Study of 100 Elderly People Post Discharge from Hospital. Technical Report Produced for South Australian Department of Human Services. South Australia: South Australian Department of Human Services;2002.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,,.Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care?Br J Gen Pract.1997;47:563–566.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,,,,.Prospective evaluation of a screen for complex discharge planning in hospitalized adults.J Am Geriatr Soc.2003;51:678–682.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613– 620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16:289–293.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,.Factor analysis in the development and refinement of clinical assessment instruments.Psychol Assess.1995;7:286–299.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,.Home from hospital: a survey of hospital discharge arrangements in Northamptonshire.J Public Health Med.1992;14:145–150.
- ,,.Electronic clinical communications implementation (ECCI) in Scotland: a mixed‐methods programme evaluation.J Eval Clin Pract.2004;10:11–20.
- ,.General practitioner response to elderly patients discharged from hospital.BMJ.1990;300:159–161.
- ,,,,.Information about patients' deaths: general practitioners' current practice and views on receiving a death register.Br J Gen Pract.1994;44:315–316.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,,,.The value of inpatient pharmaceutical counselling to elderly patients prior to discharge.Br J Clin Pharmacol.2002;54:657–664.
- ,.Usefulness of letters from hospitals to general practitioners.Br Med J (Clin Res Ed).1984;288:1813–1814.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed January2008.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
Preventable adverse events occur when patients transition from hospital to outpatient care.1, 2 The most common cause for postdischarge adverse events is poor communication between inpatient healthcare providers and outpatient primary care physicians.1 Adverse events also occur because of inadequate processes to communicate unresolved problems, monitor drug therapies, or monitor the patient's overall condition.1 Efforts to reduce adverse events logically focus on effective discharge planning and communication.
Systematic reviews have evaluated clinical trials to improve discharge planning and communication.36 Reviewers often reported inconclusive results because of a shortage of high‐quality trials with validated outcome measures.35 Reviewers recommended future studies to develop and validate outcome measures that assessed the discharge process from various perspectives.4 One important perspective was the assessment by the outpatient, primary care physician who was responsible for patient care after discharge.79
One of the authors (K.G.S.) developed the Physician‐PREPARED questionnaire to measure perceptions of outpatient physicians about the quality of hospital discharge. Item content came from studies in Australia that investigated barriers to best practice in discharge planning for older patients.1013 Fifteen items asked community physicians about their awareness of discharge planning processes for their patients. Items also assessed the adequacy of information provided about discharge plans. The Physician‐PREPARED items underwent assessment in Australia. Evaluation revealed well‐worded text, unambiguous response options, face validity, and content validity.
We reconsidered the Physician‐PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention in the United States. Our goal was a comprehensive survey instrument and scale to measure the perceptions of outpatient physicians after the discharge intervention. We found no other appropriate, validated questionnaires except the Physician‐PREPARED. However, we recognized some limitations to the Physician‐PREPARED. The items were developed for Australian physicians who treated elderly patients. We wanted to assess North American physicians who cared for a broad age range of adults. The Physician‐PREPARED did not have a scale with validated, psychometric performance characteristics in our population. We decided to address the above limitations with a scale development and validation study in the United States.
In the present work, we describe item development for the Physician‐PREPARED that occurred in Australia. Then we present item reduction and validation for the Modified Physician‐PREPARED that occurred in the United States. Our primary objective was to validate a scale to measure perceptions of outpatient physicians about qualities of discharge planning and communication. The secondary objectives were to quantify the scale's internal consistency and construct validity. Our goal was a brief scale with acceptable, defined statistical properties for clinicians and researchers.
PATIENTS AND METHODS
Item Development for the Physician‐PREPARED
Australian investigators designed the Physician‐PREPARED survey instrument to measure the quality of discharge planning activities and communication. The investigators developed the survey with the following process that was not published previously. First, a literature review identified survey content germane to outpatient practitioners.10 Investigators conducted interviews, focus groups, and pilot surveys to prioritize items for the survey instrument. The volunteer subjects for item development were general medical practitioners in Adelaide and Sydney, the capital cities of two states in Australia. The draft instrument was circulated to a small group of general medical practitioners for comment on layout, wording, and question intent. After feedback, minor modifications were made to item content and response categories. The result of development in Australia was a survey instrument with 15 items (see Appendix). The items reflected the following key areas of discharge quality: timeliness of communication, patient health status at discharge, adequacy of discharge support services, discharge medication information, and reasons for medication changes. These areas were congruent with the results of other investigators who assessed the quality of discharge planning and communication.14, 15
Validation of the Modified Physician‐PREPARED
The validation study for the Modified Physician‐PREPARED occurred in Illinois. The Peoria Institutional Review Board approved and monitored the human research. The patient sample for validation was a prospective cohort from a cluster randomized clinical trial. Willing patients or their proxies provided written consent for study participation. Patient enrollment occurred between December 2004 and August 2006. The subjects for scale analysis were the outpatient primary care physicians or practitioners designated by patients in the cohort. Outpatient physicians and practitioners gave implied consent when they completed and returned questionnaires. Follow‐up was 10 or more days after the patient's discharge from an acute care, 730‐bed, teaching hospital.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to home by internal medicine hospitalist physicians. Patient inclusion in the cluster‐randomized trial required a probability of repeat admission (Pra) score greater than or equal to 0.40.16, 17 Consequently, the patients in the scale analysis cohort had the same high probability for repeat admission. The Pra score came from patient or proxy responses to questions about age, prior hospitalizations, prior doctor visits, self‐rated health status, and other health‐related questions.16, 17 In previous validation studies with elderly outpatients, a Pra score above 0.5 predicted that patients would have 1 hospital admission per person‐year of survival.16 In other validation studies with inpatients aged 18 to 101 years, the Pra items predicted nonroutine discharge planning needs.18
Exclusion Criteria
The exclusion criteria were designed to enroll a cohort with homogeneous risk for readmission. We excluded patients if their discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Hospice patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. We also used exclusion criteria to avoid illogical enrollments. If the designated outpatient primary care physician or practitioner also managed the patient during the index hospitalization, then there was no perceived barrier to communication and the patient was excluded. Cognitive impairment was a conditional exclusion criterion. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.19 A patient with cognitive impairment could participate with consent from a legally authorized representative. Before we enrolled a cognitively impaired patient, we required a proxy who spent a minimum of 3 hours daily with the patient and who agreed to answer interview questions.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient data to calculate the Pra: age, gender, diabetes mellitus, and ischemic heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospitalization. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative or neighbor who would take care of you for a few days, if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, and excellent. In addition, we recorded heart failure and chronic obstructive pulmonary disease because of their possible association with readmission.20, 21 Information about outpatient physicians or practitioners came from the hospital's administrative database and was limited to specialty training.
Discharge Process
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aid providers, and others. Patients designated an outpatient primary care physician or nurse practitioner or physician assistant to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated outpatient primary care professional.
Item Reduction and Scoring
To develop a scale, we selected items from the Physician‐PREPARED survey instrument (see Appendix). Our goal was a parsimonious, comprehensive, and valid scale for use in clinical and research environments. We applied item reduction techniques according to the following steps that were defined a priori. First, we deleted items with nominal response categories that lacked graded or ordinal characteristics. This exclusion criterion caused us to delete the following items from the questionnaire in the appendix: (1a) Who made you aware of the admission, (2a) Who made you aware of the patient's discharge, and (5a) How did you receive this information? We deleted open‐ended questions, such as: (13) Have you any suggestions how the patient's discharge could have been improved? Next, we excluded items with a large proportion of missing responses because respondents checked Not applicable. Only item 12 from the Physician‐PREPARED fulfilled the latter criterion (see Appendix). Question 12 asked, Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? Among 403 respondents, 52% answered question 12 as Not applicable.
Measures of Construct Validity
We used 3 measures of construct validity in our assessment of the Modified Physician‐PREPARED scale. The first construct item asked the outpatient practitioner, Were you involved at all in planning the patient's discharge? The first construct was relevant because involvement by outpatient physicians improves the quality of hospital discharges.22 The second construct item asked, Are you aware of any community support services that are involved in providing assistance to the patient since discharge? For the third construct, we asked (Appendix item 11), Has the patient voiced any concerns that they have not been coping since discharge? We chose community support services and patient coping because these are clinically relevant and correlated with patients' perceptions of discharge preparedness.23 When we assessed construct validity, our hypotheses were significantly higher Modified Physician‐PREPARED scale values for respondents who answered yes to the construct questions about involvement and awareness and answered no to the question about patient‐voiced concerns.
Analysis
Analyses were performed with SPSS PC (version 14.0.2; SPSS Inc, Chicago, Illinois). We reported descriptive statistics as means, standard deviations (SDs), and range for interval variables; median and range for ordinal variables; and percentages for nominal variables. While developing the scale, the unit of analysis was the physician response to a unique patient. Specific descriptive analyses used the unique respondent as the unit of analysis. To determine the internal consistency of the scale, we calculated Cronbach's alpha with SPSS RELIABILITY. We assessed the distribution of the Modified Physician‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered for interpretation.24 Statistical inference tests were the Mann‐Whitney U for median differences for 2 groups, the Kruskal‐Wallis for more than 2 groups, and Spearman correlation for associations. The accepted level of significance was P < 0.05.
RESULTS
Description of Validation Cohort for the Modified Physician‐PREPARED
We sent questionnaires to the primary care physician, nurse practitioner, or physician assistant designated by 549 patients. The survey response rate was 76% (417/549). If a respondent failed to check any response option for 2 or more scale items, then the questionnaire was excluded from analysis. We excluded 3% (14/549) of questionnaires for failure to respond to items. The responses from the remaining 403 questionnaires were analyzed. We did not exclude questionnaires from respondents who followed homebound patients or other patients who failed to come to the clinic for postdischarge visits. Our analysis included 90 questionnaires (22%) from respondents who had no contact with the patient after discharge.
The patient characteristics appear in Table 1. Most of the patients were less than 65 years old (77%, 310/403). Many patients had chronic diseases including diabetes mellitus, ischemic heart disease, heart failure, or chronic obstructive pulmonary disease. Most patients, 81% (327/403), rated their health as poor or fair and 55% (223/403) had 1 or more hospital admissions during the year before their index admission. The questionnaire respondents were primary care physicians who practiced internal medicine (41%, 167/403), medicine‐pediatrics (27%, 108/403), family practice (24%, 97/403), or other specialties (3%, 10/403). Nurse practitioners or physician assistants completed 5% (21/403) of questionnaires.
| Characteristic | Number (%) |
|---|---|
| |
| Gender, female | 235 (58.3%) |
| Race | |
| White | 284 (70.5%) |
| Black | 116 (28.8%) |
| Other | 3 (0.7%) |
| Self‐rated health status | |
| Poor | 125 (31.0%) |
| Fair | 202 (50.1%) |
| Good | 61 (15.1%) |
| Very good | 13 (3.2%) |
| Excellent | 2 (0.5%) |
| Diabetes mellitus | 226 (56.1%) |
| Chronic obstructive pulmonary disease | 76 (18.9%) |
| Ischemic heart disease | 165 (40.9%) |
| Heart failure | 90 (22.3%) |
| Hospital admissions during prior year (includes index admission) | 2.2 (2.0) [0‐15]* |
| Age (years) | 53.6 (15.1) [19‐98]* |
| Pra score | 0.49 (0.07) [0.40‐0.70]* |
We conducted descriptive analyses that treated the respondent as the unit of analysis. There were 172 unique respondents. The number of questionnaires per respondent ranged from 1 to 20 with a median of 1 questionnaire. Respondents varied in the time to return a questionnaire. We measured response time as the difference between the date we received the questionnaire and the date of discharge. The response time ranged from 10 to 90 days with a median of 21 days after discharge.
Modified Physician‐PREPARED: Item Reduction, Internal Consistency, and Score Distributions
The questionnaire items appear in the Appendix. After item reduction, there were 8 items included in the Modified Physician‐PREPARED scale analysis (Table 2). None of the 8 items caused substantive reduction in Cronbach's alpha, so all were retained. The 8‐item scale had acceptable internal consistency (Cronbach's alpha = 0.86). For an individual questionnaire, the sum of the scores for eight items yielded the Modified Physician‐PREPARED scale value. High scale values reflected high perceptions of discharge quality. Each of the 8 items correlated significantly and positively with the scale value (P < 0.001, 2‐tailed).
| Item Text | Descriptor for Score = 1 | Descriptor for Score = 2 | Descriptor for Score = 3 | No Score | |
|---|---|---|---|---|---|
| 1. | When were you made aware that this patient had been admitted to hospital? | Not at all; 55 (13.6%) | After patient was discharged; 65 (16.1%) | Prior to hospitalization; while patient was in hospital; or on the day of discharge; 281 (69.7%) | Missing response; 2 (0.5%) |
| 2. | When were you made aware that the patient was going to be discharged? | Not at all; 115 (28.5%) | Within a week after discharge; or longer than a week after discharge; 61 (15.1%) | While patient was still in hospital; or on day of discharge; or within 1‐2 days after discharge; 225 (55.8%) | Missing response; 2 (0.5%) |
| 3. | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Longer than a week; or not received; or other 115 (28.5%) | Within a week; 186 (46.2%) | Within 1‐2 days; 101 (25.1%) | Missing response; 1 (0.2%) |
| 4. | Was this sufficient notice to address this patient's postdischarge needs? | Less than sufficient; 98 (24.3%) | Sufficient; 246 (61.0%) | More than sufficient; 46 (11.4%) | Missing response; 13 (3.2%) |
| 5. | Have you received adequate information about this patient's discharge health status? | No; 103 (25.6%) | Yes; 295 (73.2%) | Missing response; 5 (1.2%) | |
| 6. | Have you received adequate written information about the patient's medicines and medication management? | Less than adequate; or no information at all; 103 (25.6%) | Adequate; 262 (65.0%) | More than adequate; 38 (9.4%) | Missing response; 0 (0%) |
| 7. | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Less than sufficient; or no information at all; 129 (32.0%) | Sufficient; or not applicable (there was no change in medications); 240 (59.6%) | More than sufficient; 29 (7.2%) | Missing response; 5 (1.2%) |
| 8. | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | Less than adequate; or no discharge plans; 82 (20.3%) | Adequate; 276 (68.5%) | More than adequate; 32 (7.9%) | Missing response; 13 (3.2%) |
Table 2 shows the distribution of responses to each item in the Modified Physician‐PREPARED questionnaire. There were substantial ceiling effects for 2 individual items. One of the 7 items with 3 response options had ceiling effects approaching 70% (item 1). One item had 2 response options and 73% responded yes (item 5). The distribution of Modified Physician‐PREPARED scale values for 403 questionnaires had mean 16.6 4.0 SD and skew 0.6 (standard error of skew = 0.1). When scale values of patients 64 years and younger were compared with those of 65 and older, there were no significant differences (P = 0.606). The scale values did not have noteworthy floor or ceiling effects. The distribution of scale values showed 1.2% (5/403) of respondents had the lowest score of 8 and 1.7% (7/403) had the highest score of 24.
Modified Physician‐PREPARED: Principal Component Analysis
The purpose of the principal component analysis was to evaluate the relationships between the items and domains. In the component analysis, we evaluated the correlation matrix of the 8 items in the Modified Physician‐PREPARED scale. The Kaiser‐Meyer‐Olkin statistic of 0.89 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 66% of the variance associated with the 8‐item scale. After inspection of scree plots, we determined that 2 components were extracted before the eigenvalue fell substantially below 1. The pattern matrix for the promax rotation was inspected and the factor loading for each item appears in Table 3. The item content identified 1 component as timeliness of communication. The other component was adequacy of discharge plan/transmission. Within the adequacy component, the item content addressed patient health status, medication information, and reasons for medication changes. All items loaded primarily on 1 of the components; except item 3, which loaded on both components.
| Item Text | Component | ||
|---|---|---|---|
| Adequacy of Discharge Plan/Transmission | Timeliness of Communication | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why one type of medication is used in preference to another?) | 0.900 | 0.132 |
| 6 | Have you received adequate written information about the patient's medicines and medication management? | 0.849 | 0.056 |
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | 0.796 | 0.050 |
| 5 | Have you received adequate information about this patient's discharge health status? | 0.774 | 0.012 |
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | 0.744 | 0.132 |
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | 0.403 | 0.373 |
| 1 | When were you made aware that this patient had been admitted to hospital? | 0.154 | 0.964 |
| 2 | When were you made aware that the patient was going to be discharged? | 0.123 | 0.779 |
Modified Physician‐PREPARED: Construct Validity
We compared Modified Physician‐PREPARED scale values between dichotomous groups defined by construct variables. When considering the discharge planning and communication for a specific patient, outpatient primary care practitioners reported higher scale values when they were involved in the discharge planning (median [25%, 75%] = 19 [19, 20.5]) than when they were not involved (17 [12.4, 19], P < 0.001). In addition, outpatient practitioners responded with higher scale values when they were aware of community support services (18 [16, 20]) than when they were unaware (17 [12, 19], P = 0.002). There was a nonsignificant trend to higher scale values if patients voiced no concern about coping after discharge (18 [15, 19]) versus concern (17 [12, 19], P = 0.059). For all 3 constructs, the analysis revealed higher Modified Physician‐PREPARED scale values that were in the same direction as hypothesized. We approximated the construct analysis with subscales defined by the principal components (data not shown). The subscale analysis confirmed the direction and significance of the analysis with the full, 8‐item, Modified Physician‐PREPARED scale.
Modified Physician‐PREPARED: Correlations with Baseline Characteristics
We evaluated the correlations between a patient's Modified Physician‐PREPARED scale value and baseline characteristics in Table 1. Patient characteristics were not associated with scale values. We also assessed the median differences between the scale values by practitioner specialty and found no significant differences.
DISCUSSION
The Modified Physician‐PREPARED scale measured the quality of discharge planning and communication from the perspective of the outpatient primary care physician or practitioner. We described the derivation of the scale items. We demonstrated the reliability and validity of the scale among physicians and practitioners who provided postdischarge care to patients at high risk for readmission to the hospital. The item content included timeliness, adequacy, patient health status, medication information, and reasons for medication changes.
According to expert consensus guidelines for hospital discharge care, the communication with the outpatient primary care physician should occur as soon as possible after discharge.25 Recommended data elements in the communication include condition at discharge, diagnoses, medications added, medications discontinued, and medications changed.25 We found the Modified Physician‐PREPARED scale items included content that was consistent with expert consensus guidelines. The items also assessed timeliness and adequacy, 2 domains important to outpatient physicians.14, 26
The Modified Physician‐PREPARED is one of several questionnaires developed to measure qualities of discharge processes from the perspective of outpatient physicians.8, 15, 2733 Previous questionnaires did not report psychometrics except 1 that assessed the quality of discharge summaries and measured test‐retest reliability.33 We are not aware of other physician questionnaires with reliable or valid scales besides the Modified Physician‐PREPARED.
We believe 1 application of the Modified Physician‐PREPARED questionnaire is in quality improvement efforts within hospitals. Most hospitals and inpatient physicians rely on discharge letters or summaries to communicate information about the hospitalization to outpatient practitioners.6 However, systematic problems with generation and transmission of letters and summaries make them sometimes unreliable as sources of consistent, timely, accurate, or important information.6 When patients arrive for their posthospital visits, their outpatient physicians have received no discharge letter for 16% to 53% of patients and no discharge summary for 66% to 88%.6 Among outpatient physicians, 41% attribute preventable adverse events for at least 1 of their patients to inadequate discharge communication.34 One hospital accreditation organization includes discharge communication improvement as a national patient safety goal in the United States.35 Hospitals have multiple motivations to pursue quality improvement projects related to discharge communication: reduction in adverse events, relation with referring physicians, and accreditation by regulators. When surveying physicians, hospital personnel may wish to use a reliable and validated instrument like the Modified Physician‐PREPARED questionnaire.
Another application of the Modified Physician‐PREPARED scale is in research. An example is our randomized, controlled trial to measure the value of a discharge intervention. We published the rationale and design for our intervention.36 In the future, we will analyze the results of our trial and we will need validated scales. One of the trial outcomes is the perspective of the outpatient physician. We expect to compare the scores on the Modified Physician‐PREPARED scale values from community practitioners who treated test patients versus control patients. The statistical properties of the Modified Physician‐PREPARED scale that we validated in the current work will allow us to estimate the precision of between‐group differences and to perform tests of inference.
The results of our study should be interpreted in the context of strengths and limitations. We were able to generalize the validity of the Modified Physician‐PREPARED to North American primary care physicians who treated adult outpatients with a broad age range. We minimized biases with the high survey response rate and low proportion of missing responses. During validation, we asked physicians to evaluate patient transitions from hospital to home. Consequently, the Modified Physician‐PREPARED scale may not apply when doctors follow patients after discharge to nursing homes or other acute care facilities. We excluded patients with low probability of repeat admission: hospice patients and patients with low Pra scores. The purpose of our exclusion criteria was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. We recognize that the Modified Physician‐PREPARED may not generalize to physicians who treat hospice patients or patients with low probability for readmission.
Additional limitations relate to test‐retest reliability and to the clinical meaning of small changes in scale values. In our study, physician respondents returned questionnaires approximately 3 weeks after hospital discharge. We did not ask physicians to complete the questionnaire again after they returned the first questionnaire. Therefore, the test‐retest reliability for the Modified Physician‐PREPARED is unknown. Our protocol was not designed to detect the minimum important difference in the scale values. Consequently, small changes in scale values have uncertain clinical relevance. Future studies are necessary to assess the minimum important difference in the scale values.
CONCLUSION
The Modified Physician‐PREPARED scale was a reliable and valid measure of outpatient physician perceptions of quality and communication after hospital discharge. Clinicians and researchers may find the scale useful to guide, assess, and compare discharge‐planning activities.
APPENDIX
PHYSICIAN‐PREPARED QUESTIONNAIRE
| Item | Question | Response Options |
|---|---|---|
| 1 | When were you made aware that this patient had been admitted to hospital? | Prior to hospitalization |
| While patient was in hospital | ||
| On the day of discharge | ||
| After patient was discharged | ||
| Not at all | ||
| 1a | Who made you aware of the admission? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Ambulance | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 2 | When were you made aware that the patient was going to be discharged? | While patient was still in hospital |
| On day of discharge | ||
| Within 1‐2 days after discharge | ||
| Within a week after discharge | ||
| Longer than a week after discharge | ||
| Not at all | ||
| 2a | Who made you aware of the patient's discharge? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Within 1‐2 days |
| Within a week | ||
| Longer than a week | ||
| Not received | ||
| Other, please specify _________________________ | ||
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | More than sufficient |
| Sufficient | ||
| Less than sufficient | ||
| 5 | Have you received adequate information about this patient's discharge health status? | Yes |
| No | ||
| 5a | How did you receive this information? (Check all that apply) | Telephone call |
| Fax | ||
| Electronic mail system | ||
| Written/typed letter | ||
| 6 | Have you received adequate written information about the patient's medicines and medication management? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No information at all | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Not applicable (there was no change in medications) |
| More than sufficient | ||
| Sufficient | ||
| Less than sufficient | ||
| No information at all | ||
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No discharge plans | ||
| 9 | Were you involved at all in planning the patient's discharge? | Yes |
| No | ||
| 10 | Are you aware of any community support services that are involved in providing assistance to the patient since discharge? | Yes |
| No | ||
| 11 | Has the patient voiced any concerns that they have not been coping since discharge? | Yes |
| No | ||
| Not applicable (no contact with patient since discharge) | ||
| 12 | Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? | Not applicable (no caretaker) |
| Yes | ||
| No | ||
| Not applicable (no contact with caretaker since discharge) | ||
| 13 | Have you any suggestions how the patient's discharge could have been improved? | __________________________________________ |
| __________________________________________ | ||
| __________________________________________ |
Preventable adverse events occur when patients transition from hospital to outpatient care.1, 2 The most common cause for postdischarge adverse events is poor communication between inpatient healthcare providers and outpatient primary care physicians.1 Adverse events also occur because of inadequate processes to communicate unresolved problems, monitor drug therapies, or monitor the patient's overall condition.1 Efforts to reduce adverse events logically focus on effective discharge planning and communication.
Systematic reviews have evaluated clinical trials to improve discharge planning and communication.36 Reviewers often reported inconclusive results because of a shortage of high‐quality trials with validated outcome measures.35 Reviewers recommended future studies to develop and validate outcome measures that assessed the discharge process from various perspectives.4 One important perspective was the assessment by the outpatient, primary care physician who was responsible for patient care after discharge.79
One of the authors (K.G.S.) developed the Physician‐PREPARED questionnaire to measure perceptions of outpatient physicians about the quality of hospital discharge. Item content came from studies in Australia that investigated barriers to best practice in discharge planning for older patients.1013 Fifteen items asked community physicians about their awareness of discharge planning processes for their patients. Items also assessed the adequacy of information provided about discharge plans. The Physician‐PREPARED items underwent assessment in Australia. Evaluation revealed well‐worded text, unambiguous response options, face validity, and content validity.
We reconsidered the Physician‐PREPARED questionnaire when we designed a clinical trial to assess the value of a discharge intervention in the United States. Our goal was a comprehensive survey instrument and scale to measure the perceptions of outpatient physicians after the discharge intervention. We found no other appropriate, validated questionnaires except the Physician‐PREPARED. However, we recognized some limitations to the Physician‐PREPARED. The items were developed for Australian physicians who treated elderly patients. We wanted to assess North American physicians who cared for a broad age range of adults. The Physician‐PREPARED did not have a scale with validated, psychometric performance characteristics in our population. We decided to address the above limitations with a scale development and validation study in the United States.
In the present work, we describe item development for the Physician‐PREPARED that occurred in Australia. Then we present item reduction and validation for the Modified Physician‐PREPARED that occurred in the United States. Our primary objective was to validate a scale to measure perceptions of outpatient physicians about qualities of discharge planning and communication. The secondary objectives were to quantify the scale's internal consistency and construct validity. Our goal was a brief scale with acceptable, defined statistical properties for clinicians and researchers.
PATIENTS AND METHODS
Item Development for the Physician‐PREPARED
Australian investigators designed the Physician‐PREPARED survey instrument to measure the quality of discharge planning activities and communication. The investigators developed the survey with the following process that was not published previously. First, a literature review identified survey content germane to outpatient practitioners.10 Investigators conducted interviews, focus groups, and pilot surveys to prioritize items for the survey instrument. The volunteer subjects for item development were general medical practitioners in Adelaide and Sydney, the capital cities of two states in Australia. The draft instrument was circulated to a small group of general medical practitioners for comment on layout, wording, and question intent. After feedback, minor modifications were made to item content and response categories. The result of development in Australia was a survey instrument with 15 items (see Appendix). The items reflected the following key areas of discharge quality: timeliness of communication, patient health status at discharge, adequacy of discharge support services, discharge medication information, and reasons for medication changes. These areas were congruent with the results of other investigators who assessed the quality of discharge planning and communication.14, 15
Validation of the Modified Physician‐PREPARED
The validation study for the Modified Physician‐PREPARED occurred in Illinois. The Peoria Institutional Review Board approved and monitored the human research. The patient sample for validation was a prospective cohort from a cluster randomized clinical trial. Willing patients or their proxies provided written consent for study participation. Patient enrollment occurred between December 2004 and August 2006. The subjects for scale analysis were the outpatient primary care physicians or practitioners designated by patients in the cohort. Outpatient physicians and practitioners gave implied consent when they completed and returned questionnaires. Follow‐up was 10 or more days after the patient's discharge from an acute care, 730‐bed, teaching hospital.
Patient Inclusion Criteria
Trained research coordinators identified all consecutive adult inpatients who were discharged to home by internal medicine hospitalist physicians. Patient inclusion in the cluster‐randomized trial required a probability of repeat admission (Pra) score greater than or equal to 0.40.16, 17 Consequently, the patients in the scale analysis cohort had the same high probability for repeat admission. The Pra score came from patient or proxy responses to questions about age, prior hospitalizations, prior doctor visits, self‐rated health status, and other health‐related questions.16, 17 In previous validation studies with elderly outpatients, a Pra score above 0.5 predicted that patients would have 1 hospital admission per person‐year of survival.16 In other validation studies with inpatients aged 18 to 101 years, the Pra items predicted nonroutine discharge planning needs.18
Exclusion Criteria
The exclusion criteria were designed to enroll a cohort with homogeneous risk for readmission. We excluded patients if their discharge destination was a nursing home, another acute care hospital, or an inpatient rehabilitation unit. Hospice patients were excluded if life expectancy was less than 6 months as estimated by the hospitalist. We also used exclusion criteria to avoid illogical enrollments. If the designated outpatient primary care physician or practitioner also managed the patient during the index hospitalization, then there was no perceived barrier to communication and the patient was excluded. Cognitive impairment was a conditional exclusion criterion. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.19 A patient with cognitive impairment could participate with consent from a legally authorized representative. Before we enrolled a cognitively impaired patient, we required a proxy who spent a minimum of 3 hours daily with the patient and who agreed to answer interview questions.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient data to calculate the Pra: age, gender, diabetes mellitus, and ischemic heart disease. Patients or proxies provided the number of hospital admissions and doctor visits during the year before the index hospitalization. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative or neighbor who would take care of you for a few days, if necessary? Patients rated their health status on the following scale: poor, fair, good, very good, and excellent. In addition, we recorded heart failure and chronic obstructive pulmonary disease because of their possible association with readmission.20, 21 Information about outpatient physicians or practitioners came from the hospital's administrative database and was limited to specialty training.
Discharge Process
At the end of the index hospitalization, hospitalists and ward nurses used standardized forms for discharge diagnoses, prescriptions, instructions, and appointments. Discharge planning nurses or social workers consulted with hospitalists and ward nurses and then coordinated service providers including home health nurses, physical therapists, home health aides, homemaker service providers, durable medical equipment vendors, home oxygen vendors, home infusion pharmacists, social workers, rehabilitation service providers, legal aid providers, and others. Patients designated an outpatient primary care physician or nurse practitioner or physician assistant to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated outpatient primary care professional.
Item Reduction and Scoring
To develop a scale, we selected items from the Physician‐PREPARED survey instrument (see Appendix). Our goal was a parsimonious, comprehensive, and valid scale for use in clinical and research environments. We applied item reduction techniques according to the following steps that were defined a priori. First, we deleted items with nominal response categories that lacked graded or ordinal characteristics. This exclusion criterion caused us to delete the following items from the questionnaire in the appendix: (1a) Who made you aware of the admission, (2a) Who made you aware of the patient's discharge, and (5a) How did you receive this information? We deleted open‐ended questions, such as: (13) Have you any suggestions how the patient's discharge could have been improved? Next, we excluded items with a large proportion of missing responses because respondents checked Not applicable. Only item 12 from the Physician‐PREPARED fulfilled the latter criterion (see Appendix). Question 12 asked, Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? Among 403 respondents, 52% answered question 12 as Not applicable.
Measures of Construct Validity
We used 3 measures of construct validity in our assessment of the Modified Physician‐PREPARED scale. The first construct item asked the outpatient practitioner, Were you involved at all in planning the patient's discharge? The first construct was relevant because involvement by outpatient physicians improves the quality of hospital discharges.22 The second construct item asked, Are you aware of any community support services that are involved in providing assistance to the patient since discharge? For the third construct, we asked (Appendix item 11), Has the patient voiced any concerns that they have not been coping since discharge? We chose community support services and patient coping because these are clinically relevant and correlated with patients' perceptions of discharge preparedness.23 When we assessed construct validity, our hypotheses were significantly higher Modified Physician‐PREPARED scale values for respondents who answered yes to the construct questions about involvement and awareness and answered no to the question about patient‐voiced concerns.
Analysis
Analyses were performed with SPSS PC (version 14.0.2; SPSS Inc, Chicago, Illinois). We reported descriptive statistics as means, standard deviations (SDs), and range for interval variables; median and range for ordinal variables; and percentages for nominal variables. While developing the scale, the unit of analysis was the physician response to a unique patient. Specific descriptive analyses used the unique respondent as the unit of analysis. To determine the internal consistency of the scale, we calculated Cronbach's alpha with SPSS RELIABILITY. We assessed the distribution of the Modified Physician‐PREPARED scale with visual and statistical tests for skewness. While using the SPSS FACTOR program, we performed principal components extractions and then rotated components using the oblique promax technique. Component scores were saved using the regression score procedure. Component loadings above 0.30 were considered for interpretation.24 Statistical inference tests were the Mann‐Whitney U for median differences for 2 groups, the Kruskal‐Wallis for more than 2 groups, and Spearman correlation for associations. The accepted level of significance was P < 0.05.
RESULTS
Description of Validation Cohort for the Modified Physician‐PREPARED
We sent questionnaires to the primary care physician, nurse practitioner, or physician assistant designated by 549 patients. The survey response rate was 76% (417/549). If a respondent failed to check any response option for 2 or more scale items, then the questionnaire was excluded from analysis. We excluded 3% (14/549) of questionnaires for failure to respond to items. The responses from the remaining 403 questionnaires were analyzed. We did not exclude questionnaires from respondents who followed homebound patients or other patients who failed to come to the clinic for postdischarge visits. Our analysis included 90 questionnaires (22%) from respondents who had no contact with the patient after discharge.
The patient characteristics appear in Table 1. Most of the patients were less than 65 years old (77%, 310/403). Many patients had chronic diseases including diabetes mellitus, ischemic heart disease, heart failure, or chronic obstructive pulmonary disease. Most patients, 81% (327/403), rated their health as poor or fair and 55% (223/403) had 1 or more hospital admissions during the year before their index admission. The questionnaire respondents were primary care physicians who practiced internal medicine (41%, 167/403), medicine‐pediatrics (27%, 108/403), family practice (24%, 97/403), or other specialties (3%, 10/403). Nurse practitioners or physician assistants completed 5% (21/403) of questionnaires.
| Characteristic | Number (%) |
|---|---|
| |
| Gender, female | 235 (58.3%) |
| Race | |
| White | 284 (70.5%) |
| Black | 116 (28.8%) |
| Other | 3 (0.7%) |
| Self‐rated health status | |
| Poor | 125 (31.0%) |
| Fair | 202 (50.1%) |
| Good | 61 (15.1%) |
| Very good | 13 (3.2%) |
| Excellent | 2 (0.5%) |
| Diabetes mellitus | 226 (56.1%) |
| Chronic obstructive pulmonary disease | 76 (18.9%) |
| Ischemic heart disease | 165 (40.9%) |
| Heart failure | 90 (22.3%) |
| Hospital admissions during prior year (includes index admission) | 2.2 (2.0) [0‐15]* |
| Age (years) | 53.6 (15.1) [19‐98]* |
| Pra score | 0.49 (0.07) [0.40‐0.70]* |
We conducted descriptive analyses that treated the respondent as the unit of analysis. There were 172 unique respondents. The number of questionnaires per respondent ranged from 1 to 20 with a median of 1 questionnaire. Respondents varied in the time to return a questionnaire. We measured response time as the difference between the date we received the questionnaire and the date of discharge. The response time ranged from 10 to 90 days with a median of 21 days after discharge.
Modified Physician‐PREPARED: Item Reduction, Internal Consistency, and Score Distributions
The questionnaire items appear in the Appendix. After item reduction, there were 8 items included in the Modified Physician‐PREPARED scale analysis (Table 2). None of the 8 items caused substantive reduction in Cronbach's alpha, so all were retained. The 8‐item scale had acceptable internal consistency (Cronbach's alpha = 0.86). For an individual questionnaire, the sum of the scores for eight items yielded the Modified Physician‐PREPARED scale value. High scale values reflected high perceptions of discharge quality. Each of the 8 items correlated significantly and positively with the scale value (P < 0.001, 2‐tailed).
| Item Text | Descriptor for Score = 1 | Descriptor for Score = 2 | Descriptor for Score = 3 | No Score | |
|---|---|---|---|---|---|
| 1. | When were you made aware that this patient had been admitted to hospital? | Not at all; 55 (13.6%) | After patient was discharged; 65 (16.1%) | Prior to hospitalization; while patient was in hospital; or on the day of discharge; 281 (69.7%) | Missing response; 2 (0.5%) |
| 2. | When were you made aware that the patient was going to be discharged? | Not at all; 115 (28.5%) | Within a week after discharge; or longer than a week after discharge; 61 (15.1%) | While patient was still in hospital; or on day of discharge; or within 1‐2 days after discharge; 225 (55.8%) | Missing response; 2 (0.5%) |
| 3. | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Longer than a week; or not received; or other 115 (28.5%) | Within a week; 186 (46.2%) | Within 1‐2 days; 101 (25.1%) | Missing response; 1 (0.2%) |
| 4. | Was this sufficient notice to address this patient's postdischarge needs? | Less than sufficient; 98 (24.3%) | Sufficient; 246 (61.0%) | More than sufficient; 46 (11.4%) | Missing response; 13 (3.2%) |
| 5. | Have you received adequate information about this patient's discharge health status? | No; 103 (25.6%) | Yes; 295 (73.2%) | Missing response; 5 (1.2%) | |
| 6. | Have you received adequate written information about the patient's medicines and medication management? | Less than adequate; or no information at all; 103 (25.6%) | Adequate; 262 (65.0%) | More than adequate; 38 (9.4%) | Missing response; 0 (0%) |
| 7. | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Less than sufficient; or no information at all; 129 (32.0%) | Sufficient; or not applicable (there was no change in medications); 240 (59.6%) | More than sufficient; 29 (7.2%) | Missing response; 5 (1.2%) |
| 8. | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | Less than adequate; or no discharge plans; 82 (20.3%) | Adequate; 276 (68.5%) | More than adequate; 32 (7.9%) | Missing response; 13 (3.2%) |
Table 2 shows the distribution of responses to each item in the Modified Physician‐PREPARED questionnaire. There were substantial ceiling effects for 2 individual items. One of the 7 items with 3 response options had ceiling effects approaching 70% (item 1). One item had 2 response options and 73% responded yes (item 5). The distribution of Modified Physician‐PREPARED scale values for 403 questionnaires had mean 16.6 4.0 SD and skew 0.6 (standard error of skew = 0.1). When scale values of patients 64 years and younger were compared with those of 65 and older, there were no significant differences (P = 0.606). The scale values did not have noteworthy floor or ceiling effects. The distribution of scale values showed 1.2% (5/403) of respondents had the lowest score of 8 and 1.7% (7/403) had the highest score of 24.
Modified Physician‐PREPARED: Principal Component Analysis
The purpose of the principal component analysis was to evaluate the relationships between the items and domains. In the component analysis, we evaluated the correlation matrix of the 8 items in the Modified Physician‐PREPARED scale. The Kaiser‐Meyer‐Olkin statistic of 0.89 indicated sufficient sampling adequacy to extract components from the matrix. Principal components extracted 66% of the variance associated with the 8‐item scale. After inspection of scree plots, we determined that 2 components were extracted before the eigenvalue fell substantially below 1. The pattern matrix for the promax rotation was inspected and the factor loading for each item appears in Table 3. The item content identified 1 component as timeliness of communication. The other component was adequacy of discharge plan/transmission. Within the adequacy component, the item content addressed patient health status, medication information, and reasons for medication changes. All items loaded primarily on 1 of the components; except item 3, which loaded on both components.
| Item Text | Component | ||
|---|---|---|---|
| Adequacy of Discharge Plan/Transmission | Timeliness of Communication | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why one type of medication is used in preference to another?) | 0.900 | 0.132 |
| 6 | Have you received adequate written information about the patient's medicines and medication management? | 0.849 | 0.056 |
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | 0.796 | 0.050 |
| 5 | Have you received adequate information about this patient's discharge health status? | 0.774 | 0.012 |
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | 0.744 | 0.132 |
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | 0.403 | 0.373 |
| 1 | When were you made aware that this patient had been admitted to hospital? | 0.154 | 0.964 |
| 2 | When were you made aware that the patient was going to be discharged? | 0.123 | 0.779 |
Modified Physician‐PREPARED: Construct Validity
We compared Modified Physician‐PREPARED scale values between dichotomous groups defined by construct variables. When considering the discharge planning and communication for a specific patient, outpatient primary care practitioners reported higher scale values when they were involved in the discharge planning (median [25%, 75%] = 19 [19, 20.5]) than when they were not involved (17 [12.4, 19], P < 0.001). In addition, outpatient practitioners responded with higher scale values when they were aware of community support services (18 [16, 20]) than when they were unaware (17 [12, 19], P = 0.002). There was a nonsignificant trend to higher scale values if patients voiced no concern about coping after discharge (18 [15, 19]) versus concern (17 [12, 19], P = 0.059). For all 3 constructs, the analysis revealed higher Modified Physician‐PREPARED scale values that were in the same direction as hypothesized. We approximated the construct analysis with subscales defined by the principal components (data not shown). The subscale analysis confirmed the direction and significance of the analysis with the full, 8‐item, Modified Physician‐PREPARED scale.
Modified Physician‐PREPARED: Correlations with Baseline Characteristics
We evaluated the correlations between a patient's Modified Physician‐PREPARED scale value and baseline characteristics in Table 1. Patient characteristics were not associated with scale values. We also assessed the median differences between the scale values by practitioner specialty and found no significant differences.
DISCUSSION
The Modified Physician‐PREPARED scale measured the quality of discharge planning and communication from the perspective of the outpatient primary care physician or practitioner. We described the derivation of the scale items. We demonstrated the reliability and validity of the scale among physicians and practitioners who provided postdischarge care to patients at high risk for readmission to the hospital. The item content included timeliness, adequacy, patient health status, medication information, and reasons for medication changes.
According to expert consensus guidelines for hospital discharge care, the communication with the outpatient primary care physician should occur as soon as possible after discharge.25 Recommended data elements in the communication include condition at discharge, diagnoses, medications added, medications discontinued, and medications changed.25 We found the Modified Physician‐PREPARED scale items included content that was consistent with expert consensus guidelines. The items also assessed timeliness and adequacy, 2 domains important to outpatient physicians.14, 26
The Modified Physician‐PREPARED is one of several questionnaires developed to measure qualities of discharge processes from the perspective of outpatient physicians.8, 15, 2733 Previous questionnaires did not report psychometrics except 1 that assessed the quality of discharge summaries and measured test‐retest reliability.33 We are not aware of other physician questionnaires with reliable or valid scales besides the Modified Physician‐PREPARED.
We believe 1 application of the Modified Physician‐PREPARED questionnaire is in quality improvement efforts within hospitals. Most hospitals and inpatient physicians rely on discharge letters or summaries to communicate information about the hospitalization to outpatient practitioners.6 However, systematic problems with generation and transmission of letters and summaries make them sometimes unreliable as sources of consistent, timely, accurate, or important information.6 When patients arrive for their posthospital visits, their outpatient physicians have received no discharge letter for 16% to 53% of patients and no discharge summary for 66% to 88%.6 Among outpatient physicians, 41% attribute preventable adverse events for at least 1 of their patients to inadequate discharge communication.34 One hospital accreditation organization includes discharge communication improvement as a national patient safety goal in the United States.35 Hospitals have multiple motivations to pursue quality improvement projects related to discharge communication: reduction in adverse events, relation with referring physicians, and accreditation by regulators. When surveying physicians, hospital personnel may wish to use a reliable and validated instrument like the Modified Physician‐PREPARED questionnaire.
Another application of the Modified Physician‐PREPARED scale is in research. An example is our randomized, controlled trial to measure the value of a discharge intervention. We published the rationale and design for our intervention.36 In the future, we will analyze the results of our trial and we will need validated scales. One of the trial outcomes is the perspective of the outpatient physician. We expect to compare the scores on the Modified Physician‐PREPARED scale values from community practitioners who treated test patients versus control patients. The statistical properties of the Modified Physician‐PREPARED scale that we validated in the current work will allow us to estimate the precision of between‐group differences and to perform tests of inference.
The results of our study should be interpreted in the context of strengths and limitations. We were able to generalize the validity of the Modified Physician‐PREPARED to North American primary care physicians who treated adult outpatients with a broad age range. We minimized biases with the high survey response rate and low proportion of missing responses. During validation, we asked physicians to evaluate patient transitions from hospital to home. Consequently, the Modified Physician‐PREPARED scale may not apply when doctors follow patients after discharge to nursing homes or other acute care facilities. We excluded patients with low probability of repeat admission: hospice patients and patients with low Pra scores. The purpose of our exclusion criteria was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. We recognize that the Modified Physician‐PREPARED may not generalize to physicians who treat hospice patients or patients with low probability for readmission.
Additional limitations relate to test‐retest reliability and to the clinical meaning of small changes in scale values. In our study, physician respondents returned questionnaires approximately 3 weeks after hospital discharge. We did not ask physicians to complete the questionnaire again after they returned the first questionnaire. Therefore, the test‐retest reliability for the Modified Physician‐PREPARED is unknown. Our protocol was not designed to detect the minimum important difference in the scale values. Consequently, small changes in scale values have uncertain clinical relevance. Future studies are necessary to assess the minimum important difference in the scale values.
CONCLUSION
The Modified Physician‐PREPARED scale was a reliable and valid measure of outpatient physician perceptions of quality and communication after hospital discharge. Clinicians and researchers may find the scale useful to guide, assess, and compare discharge‐planning activities.
APPENDIX
PHYSICIAN‐PREPARED QUESTIONNAIRE
| Item | Question | Response Options |
|---|---|---|
| 1 | When were you made aware that this patient had been admitted to hospital? | Prior to hospitalization |
| While patient was in hospital | ||
| On the day of discharge | ||
| After patient was discharged | ||
| Not at all | ||
| 1a | Who made you aware of the admission? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Ambulance | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 2 | When were you made aware that the patient was going to be discharged? | While patient was still in hospital |
| On day of discharge | ||
| Within 1‐2 days after discharge | ||
| Within a week after discharge | ||
| Longer than a week after discharge | ||
| Not at all | ||
| 2a | Who made you aware of the patient's discharge? | Hospital ward staff |
| Discharge planner | ||
| Hospital medical staff | ||
| Patient | ||
| Patient's family/friends | ||
| Other, please specify _________________________ | ||
| 3 | How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | Within 1‐2 days |
| Within a week | ||
| Longer than a week | ||
| Not received | ||
| Other, please specify _________________________ | ||
| 4 | Was this sufficient notice to address this patient's postdischarge needs? | More than sufficient |
| Sufficient | ||
| Less than sufficient | ||
| 5 | Have you received adequate information about this patient's discharge health status? | Yes |
| No | ||
| 5a | How did you receive this information? (Check all that apply) | Telephone call |
| Fax | ||
| Electronic mail system | ||
| Written/typed letter | ||
| 6 | Have you received adequate written information about the patient's medicines and medication management? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No information at all | ||
| 7 | Did you receive sufficient reasons for changes in medication? (For example, why 1 type of medication is used in preference to another?) | Not applicable (there was no change in medications) |
| More than sufficient | ||
| Sufficient | ||
| Less than sufficient | ||
| No information at all | ||
| 8 | In your opinion, how adequate were the discharge plans to assist this patient to assume safe, independent community living? | More than adequate |
| Adequate | ||
| Less than adequate | ||
| No discharge plans | ||
| 9 | Were you involved at all in planning the patient's discharge? | Yes |
| No | ||
| 10 | Are you aware of any community support services that are involved in providing assistance to the patient since discharge? | Yes |
| No | ||
| 11 | Has the patient voiced any concerns that they have not been coping since discharge? | Yes |
| No | ||
| Not applicable (no contact with patient since discharge) | ||
| 12 | Has the patient's caretaker voiced any concerns that they have not been coping since the patient was discharged? | Not applicable (no caretaker) |
| Yes | ||
| No | ||
| Not applicable (no contact with caretaker since discharge) | ||
| 13 | Have you any suggestions how the patient's discharge could have been improved? | __________________________________________ |
| __________________________________________ | ||
| __________________________________________ |
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,.Care home versus hospital and own home environments for rehabilitation of older people.Cochrane Database Syst Rev.2003;(2):CD003164.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Effects of a multidisciplinary, post‐discharge continuance of care intervention on quality of life, discharge satisfaction, and hospital length of stay: a randomized controlled trial.Int J Qual Health Care.2005;17:43–51.
- .Hospitalists and family physicians: understanding opportunities and risks.J Fam Pract.2004;53:473–481.
- ,,,.performance indicators for discharge planning: a focused review of the literature.Aust J Adv Nurs.1999;16:20–28.
- Informing discharge plans. Assessments of elderly patients in Australian public hospitals: a field study. Available at: http://ijahsp.nova.edu/articles/Vol2number3/Grimmer‐Discharge_Plans.htm. Accessed January2008.
- ,,.Experiences of elderly patients regarding independent community living after discharge from hospital: a longitudinal study.Int J Qual Health Care.2004;16:465–472.
- ,,.Life Post‐Discharge: Longitudinal Qualitative and Quantitative Study of 100 Elderly People Post Discharge from Hospital. Technical Report Produced for South Australian Department of Human Services. South Australia: South Australian Department of Human Services;2002.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,,.Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care?Br J Gen Pract.1997;47:563–566.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,,,,.Prospective evaluation of a screen for complex discharge planning in hospitalized adults.J Am Geriatr Soc.2003;51:678–682.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613– 620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16:289–293.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,.Factor analysis in the development and refinement of clinical assessment instruments.Psychol Assess.1995;7:286–299.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,.Home from hospital: a survey of hospital discharge arrangements in Northamptonshire.J Public Health Med.1992;14:145–150.
- ,,.Electronic clinical communications implementation (ECCI) in Scotland: a mixed‐methods programme evaluation.J Eval Clin Pract.2004;10:11–20.
- ,.General practitioner response to elderly patients discharged from hospital.BMJ.1990;300:159–161.
- ,,,,.Information about patients' deaths: general practitioners' current practice and views on receiving a death register.Br J Gen Pract.1994;44:315–316.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,,,.The value of inpatient pharmaceutical counselling to elderly patients prior to discharge.Br J Clin Pharmacol.2002;54:657–664.
- ,.Usefulness of letters from hospitals to general practitioners.Br Med J (Clin Res Ed).1984;288:1813–1814.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed January2008.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;(4):CD003716.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,,.Care home versus hospital and own home environments for rehabilitation of older people.Cochrane Database Syst Rev.2003;(2):CD003164.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Effects of a multidisciplinary, post‐discharge continuance of care intervention on quality of life, discharge satisfaction, and hospital length of stay: a randomized controlled trial.Int J Qual Health Care.2005;17:43–51.
- .Hospitalists and family physicians: understanding opportunities and risks.J Fam Pract.2004;53:473–481.
- ,,,.performance indicators for discharge planning: a focused review of the literature.Aust J Adv Nurs.1999;16:20–28.
- Informing discharge plans. Assessments of elderly patients in Australian public hospitals: a field study. Available at: http://ijahsp.nova.edu/articles/Vol2number3/Grimmer‐Discharge_Plans.htm. Accessed January2008.
- ,,.Experiences of elderly patients regarding independent community living after discharge from hospital: a longitudinal study.Int J Qual Health Care.2004;16:465–472.
- ,,.Life Post‐Discharge: Longitudinal Qualitative and Quantitative Study of 100 Elderly People Post Discharge from Hospital. Technical Report Produced for South Australian Department of Human Services. South Australia: South Australian Department of Human Services;2002.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,,.Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care?Br J Gen Pract.1997;47:563–566.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,,,.Predictive validity of the Pra instrument among older recipients of managed care.J Am Geriatr Soc.1997;45:614–617.
- ,,,,,.Prospective evaluation of a screen for complex discharge planning in hospitalized adults.J Am Geriatr Soc.2003;51:678–682.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613– 620.
- ,,, et al.Predicting non‐elective hospital readmissions: a multi‐site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions.J Clin Epidemiol.2000;53:1113–1118.
- ,,,,.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial.Fam Pract.1999;16:289–293.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,.Factor analysis in the development and refinement of clinical assessment instruments.Psychol Assess.1995;7:286–299.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,.Home from hospital: a survey of hospital discharge arrangements in Northamptonshire.J Public Health Med.1992;14:145–150.
- ,,.Electronic clinical communications implementation (ECCI) in Scotland: a mixed‐methods programme evaluation.J Eval Clin Pract.2004;10:11–20.
- ,.General practitioner response to elderly patients discharged from hospital.BMJ.1990;300:159–161.
- ,,,,.Information about patients' deaths: general practitioners' current practice and views on receiving a death register.Br J Gen Pract.1994;44:315–316.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,,,.The value of inpatient pharmaceutical counselling to elderly patients prior to discharge.Br J Clin Pharmacol.2002;54:657–664.
- ,.Usefulness of letters from hospitals to general practitioners.Br Med J (Clin Res Ed).1984;288:1813–1814.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
- 2007 Hospital/critical access hospital national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed January2008.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
Copyright © 2008 Society of Hospital Medicine