User login
Transition Expansion
Thousands of Michigan residents will have a better chance of avoiding readmission to the hospital thanks to a groundbreaking new collaboration between three of the state’s healthcare leaders.
Based on SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) model, the collaborative program will be managed by the University of Michigan in collaboration with Blue Cross Blue Shield of Michigan. The Michigan Blues provide and administer health benefits to 4.7 million Michigan residents.
Project BOOST helps hospitals reduce readmission rates by providing them with proven resources and expert mentoring to optimize the discharge transition process, enhance patient and family education practices, and improve the flow of information between inpatient and outpatient providers. Project BOOST was developed through a grant from the John A. Hartford Foundation. Earlier in the year, the program recruited 15 Michigan sites to participate. Training begins in May.
Each improvement team will be assigned a mentor to coach them through the process of planning, implementing, and evaluating Project BOOST at their site. Program participants will receive face-to-face training, monthly coaching sessions with their mentors, and a comprehensive toolkit to implement Project BOOST. Sites also participate in an online peer learning and collaboration network.
“This kind of innovative, targeted program benefits both the patient and the healthcare provider by establishing better communication between all parties,” says Scott Flanders, MD, FHM, associate professor and director of hospital medicine at the University of Michigan in Ann Arbor, and SHM president.
To Flanders, it’s no coincidence that hospitalists are taking the lead in improving hospital discharges. “Readmissions are a pervasive but preventable problem,” he says. “Hospitalists are uniquely positioned to provide leadership within the hospital, to promote positive, system-based changes that improve patient satisfaction, and promote collaboration between hospitalists and primary-care physicians.”
In addition to being preventable, readmissions are costly, draining the resources, time, and energy of the patient, PCPs, and hospitals. Research in the April 2009 New England Journal of Medicine indicates that 20% of hospitalized patients are readmitted to the hospital within a month of their discharge.1 Nationally, readmissions cost Medicare $17.4 billion each year.1
Collaborative Partnerships
Prior to the program’s launch in Michigan, SHM recruited and mentored Project BOOST sites independently. However, like many productive relationships in a hospital, Project BOOST in Michigan depends on collaboration between experts.
“Blue Cross Blue Shield of Michigan is confident that this project, like our other Value Partnership programs that focus on robust, statewide, data-driven quality-improvement (QI) partnerships, will have a positive impact on thousands of Michigan lives,” says David Share, MD, MPH, BCBS Michigan’s senior associate medical director of Healthcare Quality. “We look forward to helping hospitals, physicians, and patients work together to assure smooth transitions between inpatient and outpatient care, and to reduce readmissions and improve the patient experience.”
For University of Michigan hospitalist Christopher Kim, MD, MBA, FHM, Project BOOST is a chance to work with a diverse set of groups. “We are grateful for the opportunity to work with not just Blue Cross Blue Shield of Michigan, but also with the other physician organizations across our state to implement and share best-practice ideas in transitions of care,” says Kim, director of the statewide collaborative program on transitions of care.
Results and Reports
Having launched six pilot sites just two years ago, adding 24 additional sites in 2009, Project BOOST is still a relatively young QI program, which makes reliable quantitative data about its effectiveness tough to come by. The expansion into Michigan gives SHM and others the prospect of programwide measurement of how Project BOOST affects discharge and reduces readmissions.
“This is a tremendous opportunity to improve patient safety, reduce readmissions, and study the impact of Project BOOST interventions through patient-level data,” says Mark Williams, MD, FHM, Journal of Hospital Medicine editor, principal investigator for Project BOOST, and former SHM president. “We’re thrilled to be working with the state’s healthcare leaders to implement this critical program.”
Nonetheless, in the absence of comprehensive data, the early reports from Project BOOST sites are promising. At Piedmont Hospital in the Atlanta area, the rate of readmission among patients under the age of 70 participating in BOOST is 8.5%, compared with 25.5% among nonparticipants. The readmission rate among BOOST participants at Piedmont over the age of 70 was 22%, compared with 26% of nonparticipants. When SSM St. Mary’s Medical Center in St. Louis implemented BOOST at its 33-bed hospitalist unit, 30-day readmissions dropped to 7% from 12% within three months.
Patient satisfaction rates also increased markedly, to 68% from 52%. And in 2009, the University of Pennsylvania Health System awarded its annual Operational Quality and Safety Award to the Project BOOST implementation team at the hospital.
BOOST’s Reach Expands
Project BOOST leaders are planning an aggressive expansion in the near future. In addition to the potential for new program sites, SHM has made materials available to hospitalists through the Project BOOST Resource Room at SHM’s newly redesigned Web site (see “The New Face of HospitalMedicine.org,” p. 12), www.hospitalmedicine.org/boost.
In addition to free resources, new BOOST materials are for sale through SHM’s online store. The Project BOOST Implementation Guide—available electronically for free through the resource room—is now available for sale as a hard copy. The online store also features a new Project BOOST instructional DVD for hospitalists, “Using Teach Back to Improve Communication with Patients.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14): 1418-1428.
Thousands of Michigan residents will have a better chance of avoiding readmission to the hospital thanks to a groundbreaking new collaboration between three of the state’s healthcare leaders.
Based on SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) model, the collaborative program will be managed by the University of Michigan in collaboration with Blue Cross Blue Shield of Michigan. The Michigan Blues provide and administer health benefits to 4.7 million Michigan residents.
Project BOOST helps hospitals reduce readmission rates by providing them with proven resources and expert mentoring to optimize the discharge transition process, enhance patient and family education practices, and improve the flow of information between inpatient and outpatient providers. Project BOOST was developed through a grant from the John A. Hartford Foundation. Earlier in the year, the program recruited 15 Michigan sites to participate. Training begins in May.
Each improvement team will be assigned a mentor to coach them through the process of planning, implementing, and evaluating Project BOOST at their site. Program participants will receive face-to-face training, monthly coaching sessions with their mentors, and a comprehensive toolkit to implement Project BOOST. Sites also participate in an online peer learning and collaboration network.
“This kind of innovative, targeted program benefits both the patient and the healthcare provider by establishing better communication between all parties,” says Scott Flanders, MD, FHM, associate professor and director of hospital medicine at the University of Michigan in Ann Arbor, and SHM president.
To Flanders, it’s no coincidence that hospitalists are taking the lead in improving hospital discharges. “Readmissions are a pervasive but preventable problem,” he says. “Hospitalists are uniquely positioned to provide leadership within the hospital, to promote positive, system-based changes that improve patient satisfaction, and promote collaboration between hospitalists and primary-care physicians.”
In addition to being preventable, readmissions are costly, draining the resources, time, and energy of the patient, PCPs, and hospitals. Research in the April 2009 New England Journal of Medicine indicates that 20% of hospitalized patients are readmitted to the hospital within a month of their discharge.1 Nationally, readmissions cost Medicare $17.4 billion each year.1
Collaborative Partnerships
Prior to the program’s launch in Michigan, SHM recruited and mentored Project BOOST sites independently. However, like many productive relationships in a hospital, Project BOOST in Michigan depends on collaboration between experts.
“Blue Cross Blue Shield of Michigan is confident that this project, like our other Value Partnership programs that focus on robust, statewide, data-driven quality-improvement (QI) partnerships, will have a positive impact on thousands of Michigan lives,” says David Share, MD, MPH, BCBS Michigan’s senior associate medical director of Healthcare Quality. “We look forward to helping hospitals, physicians, and patients work together to assure smooth transitions between inpatient and outpatient care, and to reduce readmissions and improve the patient experience.”
For University of Michigan hospitalist Christopher Kim, MD, MBA, FHM, Project BOOST is a chance to work with a diverse set of groups. “We are grateful for the opportunity to work with not just Blue Cross Blue Shield of Michigan, but also with the other physician organizations across our state to implement and share best-practice ideas in transitions of care,” says Kim, director of the statewide collaborative program on transitions of care.
Results and Reports
Having launched six pilot sites just two years ago, adding 24 additional sites in 2009, Project BOOST is still a relatively young QI program, which makes reliable quantitative data about its effectiveness tough to come by. The expansion into Michigan gives SHM and others the prospect of programwide measurement of how Project BOOST affects discharge and reduces readmissions.
“This is a tremendous opportunity to improve patient safety, reduce readmissions, and study the impact of Project BOOST interventions through patient-level data,” says Mark Williams, MD, FHM, Journal of Hospital Medicine editor, principal investigator for Project BOOST, and former SHM president. “We’re thrilled to be working with the state’s healthcare leaders to implement this critical program.”
Nonetheless, in the absence of comprehensive data, the early reports from Project BOOST sites are promising. At Piedmont Hospital in the Atlanta area, the rate of readmission among patients under the age of 70 participating in BOOST is 8.5%, compared with 25.5% among nonparticipants. The readmission rate among BOOST participants at Piedmont over the age of 70 was 22%, compared with 26% of nonparticipants. When SSM St. Mary’s Medical Center in St. Louis implemented BOOST at its 33-bed hospitalist unit, 30-day readmissions dropped to 7% from 12% within three months.
Patient satisfaction rates also increased markedly, to 68% from 52%. And in 2009, the University of Pennsylvania Health System awarded its annual Operational Quality and Safety Award to the Project BOOST implementation team at the hospital.
BOOST’s Reach Expands
Project BOOST leaders are planning an aggressive expansion in the near future. In addition to the potential for new program sites, SHM has made materials available to hospitalists through the Project BOOST Resource Room at SHM’s newly redesigned Web site (see “The New Face of HospitalMedicine.org,” p. 12), www.hospitalmedicine.org/boost.
In addition to free resources, new BOOST materials are for sale through SHM’s online store. The Project BOOST Implementation Guide—available electronically for free through the resource room—is now available for sale as a hard copy. The online store also features a new Project BOOST instructional DVD for hospitalists, “Using Teach Back to Improve Communication with Patients.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14): 1418-1428.
Thousands of Michigan residents will have a better chance of avoiding readmission to the hospital thanks to a groundbreaking new collaboration between three of the state’s healthcare leaders.
Based on SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) model, the collaborative program will be managed by the University of Michigan in collaboration with Blue Cross Blue Shield of Michigan. The Michigan Blues provide and administer health benefits to 4.7 million Michigan residents.
Project BOOST helps hospitals reduce readmission rates by providing them with proven resources and expert mentoring to optimize the discharge transition process, enhance patient and family education practices, and improve the flow of information between inpatient and outpatient providers. Project BOOST was developed through a grant from the John A. Hartford Foundation. Earlier in the year, the program recruited 15 Michigan sites to participate. Training begins in May.
Each improvement team will be assigned a mentor to coach them through the process of planning, implementing, and evaluating Project BOOST at their site. Program participants will receive face-to-face training, monthly coaching sessions with their mentors, and a comprehensive toolkit to implement Project BOOST. Sites also participate in an online peer learning and collaboration network.
“This kind of innovative, targeted program benefits both the patient and the healthcare provider by establishing better communication between all parties,” says Scott Flanders, MD, FHM, associate professor and director of hospital medicine at the University of Michigan in Ann Arbor, and SHM president.
To Flanders, it’s no coincidence that hospitalists are taking the lead in improving hospital discharges. “Readmissions are a pervasive but preventable problem,” he says. “Hospitalists are uniquely positioned to provide leadership within the hospital, to promote positive, system-based changes that improve patient satisfaction, and promote collaboration between hospitalists and primary-care physicians.”
In addition to being preventable, readmissions are costly, draining the resources, time, and energy of the patient, PCPs, and hospitals. Research in the April 2009 New England Journal of Medicine indicates that 20% of hospitalized patients are readmitted to the hospital within a month of their discharge.1 Nationally, readmissions cost Medicare $17.4 billion each year.1
Collaborative Partnerships
Prior to the program’s launch in Michigan, SHM recruited and mentored Project BOOST sites independently. However, like many productive relationships in a hospital, Project BOOST in Michigan depends on collaboration between experts.
“Blue Cross Blue Shield of Michigan is confident that this project, like our other Value Partnership programs that focus on robust, statewide, data-driven quality-improvement (QI) partnerships, will have a positive impact on thousands of Michigan lives,” says David Share, MD, MPH, BCBS Michigan’s senior associate medical director of Healthcare Quality. “We look forward to helping hospitals, physicians, and patients work together to assure smooth transitions between inpatient and outpatient care, and to reduce readmissions and improve the patient experience.”
For University of Michigan hospitalist Christopher Kim, MD, MBA, FHM, Project BOOST is a chance to work with a diverse set of groups. “We are grateful for the opportunity to work with not just Blue Cross Blue Shield of Michigan, but also with the other physician organizations across our state to implement and share best-practice ideas in transitions of care,” says Kim, director of the statewide collaborative program on transitions of care.
Results and Reports
Having launched six pilot sites just two years ago, adding 24 additional sites in 2009, Project BOOST is still a relatively young QI program, which makes reliable quantitative data about its effectiveness tough to come by. The expansion into Michigan gives SHM and others the prospect of programwide measurement of how Project BOOST affects discharge and reduces readmissions.
“This is a tremendous opportunity to improve patient safety, reduce readmissions, and study the impact of Project BOOST interventions through patient-level data,” says Mark Williams, MD, FHM, Journal of Hospital Medicine editor, principal investigator for Project BOOST, and former SHM president. “We’re thrilled to be working with the state’s healthcare leaders to implement this critical program.”
Nonetheless, in the absence of comprehensive data, the early reports from Project BOOST sites are promising. At Piedmont Hospital in the Atlanta area, the rate of readmission among patients under the age of 70 participating in BOOST is 8.5%, compared with 25.5% among nonparticipants. The readmission rate among BOOST participants at Piedmont over the age of 70 was 22%, compared with 26% of nonparticipants. When SSM St. Mary’s Medical Center in St. Louis implemented BOOST at its 33-bed hospitalist unit, 30-day readmissions dropped to 7% from 12% within three months.
Patient satisfaction rates also increased markedly, to 68% from 52%. And in 2009, the University of Pennsylvania Health System awarded its annual Operational Quality and Safety Award to the Project BOOST implementation team at the hospital.
BOOST’s Reach Expands
Project BOOST leaders are planning an aggressive expansion in the near future. In addition to the potential for new program sites, SHM has made materials available to hospitalists through the Project BOOST Resource Room at SHM’s newly redesigned Web site (see “The New Face of HospitalMedicine.org,” p. 12), www.hospitalmedicine.org/boost.
In addition to free resources, new BOOST materials are for sale through SHM’s online store. The Project BOOST Implementation Guide—available electronically for free through the resource room—is now available for sale as a hard copy. The online store also features a new Project BOOST instructional DVD for hospitalists, “Using Teach Back to Improve Communication with Patients.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14): 1418-1428.
Attention to Detail
Hospitalists will be essential players in helping their institutions prepare for the Recovery Audit Contractor (RAC) program, now being rolled out nationwide by the Centers for Medicare & Medicaid Services (CMS). The program is part of CMS’ arsenal to ferret out improper payments and prevent fraud, waste, and abuse in the Medicare system.
All providers who bill Medicare fee-for-service are fair game for an RAC audit, which scrutinizes medical records to validate diagnosis-related groups (DRGs), coding, and the necessity of care provided by hospitals. Hospitalists are being asked to document their diagnosis and treatment decisions more precisely and thoroughly than ever, ensuring that DRG coding is appropriate, medical necessity is watertight, and hospitals are defended from costly overpayment recovery.
Specificity of documentation is the hospitalist’s most potent weapon against this new layer of federal audits.
In a three-year demonstration of the RAC program that ended in March 2008, one-third of all medical records audited resulted in an overpayment finding and collection. RACs collected more than $900 million in overpayments and returned nearly $38 million in underpayments. One-third of provider appeals (physician, hospital, and other providers) were successful during the demo program, according to a June 2008 CMS evaluation report. (Download a copy of the report at www.cms.hhs.gov/RAC/Downloads/RAC_Demonstration_Evaluation_Report.pdf.)
How the Audits Work

Listen to an interview with Dr. Pinson
Out of concern that the Medicare Trust Fund might not be adequately protected against improper payments by existing error detection and prevention efforts, Congress directed CMS to use RACs to identify and recoup Medicare overpayments under Section 306 of the Medicare Modernization Act of 2003, and directed CMS to make the program permanent by 2010 under Section 302 of the Tax Relief and Health Care Act of 2006. According to CMS, RACs were implemented so that physicians and other providers could avoid submitting claims that do not comply with Medicare rules, CMS could lower its error rate, and taxpayers and future Medicare beneficiaries would be protected.1
CMS has contracted with four regional RACs for the national program, and each will use proprietary auditing software to review paid claims from Medicare Part A and Part B providers to ensure that they meet Medicare’s statutory, regulatory, and policy requirements and regulations.
The RACs use automated review for claims that clearly contain errors that resulted in improper payments (e.g., claims for duplicate or uncovered services, claims that violate a written Medicare policy or sanctioned coding guideline), in which case the RAC notifies the provider of the overpayment. For cases in which there is a high probability—but not certainty—that the claim contains an overpayment, the RAC requests medical records from the provider (including imaged medical records on CD or DVD) to conduct a complex review and make a determination as to whether payment of the claim was correct, or whether there was an over- or underpayment.
CMS uses a Web-based data warehouse to ensure that RACs do not review claims that have previously been reviewed by another entity, such as a Medicare carrier, fiscal intermediary, the Office of Inspector General, or a quality-improvement organization (QIO).
The four regional RACs are ramping up their claim review activities in all states, says Connie Leonard, director of CMS’ Division of Recovery Audit Operations. When overpayments are confirmed, the RACs issue letters demanding providers to repay their Medicare carrier or intermediary within 30 days. For confirmed underpayments, RACs inform the provider’s Medicare contractor or fiscal intermediary, which then forwards the additional payment, Leonard says.
Providers can repay an overpayment by check or installment plan on or before 30 days after receiving the RAC demand letter. The Medicare contractors use recoupment—reducing present or future Medicare payments—on day 41. Providers who wish to dispute overpayment charges can take their case through the usual Medicare claims appeal process. RACs also offer a “discussion period”—from the date the provider gets a “Detailed Review Results” letter until the date of recoupment—to discuss with the RAC an improper payment determination outside the normal appeal process, Leonard says.

—Kathy DeVault, RHIA, CCS, CCS-P, manager, Professional Practice Resources, American Health Information Management Association, Chicago
If providers disagree with the RAC’s determination, Leonard says, they should either 1) pay by check by day 30 and file for appeal by day 120 of the demand letter; 2) allow recoupment on day 41 and file for appeal by day 120; 3) stop the recoupment by filing an appeal by day 30; or 4) request an extended payment plan and appeal by day 120.
Some physicians in the demonstration project regarded the third-party RAC companies as “bounty hunters” operating without sufficient CMS oversight, imposing undue administrative burdens on physician practices, and lacking the clinical expertise to adjudicate claims appropriately, according to Michael Schweitz, MD, a rheumatologist from West Palm Beach, Fla., who testified before a Congressional committee in 2008 about RAC activities.
In response, CMS has modified the program (see “Refinements in Permanent RAC Program,” p. 8) in several ways to address those flaws and ensure a fair and smooth auditing process, Leonard says. (Listen to an audio interview with Ms. Leonard)
All About the Details
Because RACs focus on coding and documentation that fails to support DRG designations, hospitalists who focus on accurate and precise documentation that can be coded properly will greatly help their hospitals defend against RAC audits, as well as yield better payment and improved quality scores, says Richard D. Pinson, MD, FACP, CCS, principal of HCQ Consulting in Nashville, Tenn. Pinson will present “Documentation Tips Your Hospital Will Love You For” at HM10 in Washington, D.C., this month. A video/audio download of the presentation will be available on SHM’s Web site in May.
“Coding rules and terminology often don’t match what we’re used to writing in the record, so hospitalists need to learn what these connections are and use them in their medical record documentation,” Pinson says. “This is a core skill for hospitalists: being able to translate clinical terminology into the correct coding terminology for hospitals and coders.”
For example, if a hospitalist sees that a pre-operative patient has severe congestive heart failure, that condition cannot be coded as a complication of the patient’s care or considered as such in the DRG assignment, Pinson explains. If the hospitalist says the patient has an acute exacerbation of systolic heart failure, then that is a major comorbidity and ought to be documented as such. The average value of a major comorbidity in a surgical case could be as much as $20,000 per case, Pinson notes. If the DRG assignment included acute exacerbation but the medical chart only said severe congestive heart failure, the hospital would face recoupment of payment from an RAC audit.
“If we’re inconsistent or ambiguous in how we apply our terms, we can end up inadvertently upcoding. The key is: Learn to use the right terms that correspond to the right codes, based on what your patient actually has, and then be consistent throughout the record in your use of those terms,” Pinson says. For example, “we may admit a patient and say at the very beginning that the patient probably has aspiration pneumonia. We then treat the patient for aspiration pneumonia but leave it out of the discharge summary. The coder may code aspiration pneumonia, but the RAC auditor may point out that it was only mentioned in the patient’s record once, as possible, and may recoup any payment for treatment beyond simple pneumonia.”
Level of care and symptom-based DRG designations are red flags for RAC recovery, Pinson says. When the auditor sees a DRG based on symptoms rather than diagnoses (e.g., chest pain, syncope, transient ischemic attack, dehydration) and it is billed as inpatient status instead of observation status, that’s a target. Those symptoms, he says, often don’t meet the medical necessity criteria for inpatient status.
Pinson advises hospitalists to ask their institution’s case-management department, or hire an external consultant, to abstract key criteria for patient status designation, and to consider starting a patient as observation status until a precise diagnosis can be made that warrants hospital admission. Hospitalists should then describe the patient’s situation more precisely in the medical record as a diagnosis, not just as symptoms—e.g., syncope suspected due to cardiac arrhythmia, or chest pain suspected to be angina.
“For inpatient billing, those uncertain diagnoses, described that way, count as if they were established conditions. They don’t go into symptom DRGs,” Pinson says. “If you’re doing these things to protect the validity of you hospital’s billing, you’ll be protecting yourself at the same time, and it’s unlikely that RACs will single you out at all for auditing.”
Hospitalists can be valuable participants on their institutions’ RAC response team, providing clinical clarification on cases and helping to draft appeal letters.
There are several other red flags that RACs zero in on and hospitalists should watch out for, says Kathy DeVault, RHIA, CCS, CCS-P, manager of Professional Practice Resources for the American Health Information Management Association (AHIMA). Specificity in the medical record makes all the difference. For example, by identifying incorrect coding for excisional debridement (removal of infected tissue), RACs collected nearly $18 million in overpayments in fiscal-year 2006 because medical record documentation omitted such details as the word “excisional” (e.g., sharp debridement coded as excisional debridement), whether it was performed in the operating room or not, instruments used, the extent and depth of the procedure, and if the cutting of tissue was outside or beyond the wound margin.
DeVault warns that “RACs are targeting confusion between septicemia and urosepsis.” According to CMS, if the hospital reports a patient’s principal diagnosis as septicemia (03.89) but the medical record indicates the diagnosis of urosepsis, the RAC will bump the diagnosis code down to urinary tract infection (599.0), a lower-payment DRG, and demand recoupment.1
Urosepsis does not have a specific ICD-9-CM diagnosis code, and defaults to a simple UTI code, as referenced in ICD-9-CM. Unless the physician states in his or her documentation that the patient’s condition was systemic sepsis or septicemia, urosepsis would be coded as a UTI. RACS also denied some respiratory-failure claims for incorrect sequencing of principal diagnosis (e.g., respiratory failure vs. sepsis). The American Hospital Association has issued a regulatory advisory about these issues (web.mhanet.com/userdocs/articles/RAC/AHA_RAC_Coding Advisory_071608.pdf).
DeVault highlights three additional RAC targets that might impact HM:
- Documentation for transbronchial biopsy (a surgical DRG) in which the medical record only shows pathology of bronchus tissue (which RACs regard as nonsurgical);
- Failure to document the severity of a patient’s anemia as such to meet the medical necessity requirement of a blood transfusion (e.g., a chronic blood loss anemia or a pernicious anemia); and
- Documentation of treatments performed by intensivists in an ICU. By the time a patient’s attending physician sees their patient out of the ICU, DeVault says, their acute renal failure could be turned around but the attending might not document what happened in the ICU. The intensivist must see to it that the documentation allows the appropriate DRG assignment for the level of care the patient received.
AHIMA has published a 65-page RAC Audit Toolkit that describes the audit process, outlines preparations and procedures, and offers concrete guidance for appeals. Download a copy at www.ahima.org/infocenter/documents/RACToolkitFINAL.pdf. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
Reference
- The Medicare Recovery Audit Contractor (RAC) program: an evaluation of the 3-year demonstration. CMS Web site. Available at: www.cms.hhs.gov/RAC/Downloads/RACEvaluationReport.pdf. Accessed March 3, 2010.
Hospitalists will be essential players in helping their institutions prepare for the Recovery Audit Contractor (RAC) program, now being rolled out nationwide by the Centers for Medicare & Medicaid Services (CMS). The program is part of CMS’ arsenal to ferret out improper payments and prevent fraud, waste, and abuse in the Medicare system.
All providers who bill Medicare fee-for-service are fair game for an RAC audit, which scrutinizes medical records to validate diagnosis-related groups (DRGs), coding, and the necessity of care provided by hospitals. Hospitalists are being asked to document their diagnosis and treatment decisions more precisely and thoroughly than ever, ensuring that DRG coding is appropriate, medical necessity is watertight, and hospitals are defended from costly overpayment recovery.
Specificity of documentation is the hospitalist’s most potent weapon against this new layer of federal audits.
In a three-year demonstration of the RAC program that ended in March 2008, one-third of all medical records audited resulted in an overpayment finding and collection. RACs collected more than $900 million in overpayments and returned nearly $38 million in underpayments. One-third of provider appeals (physician, hospital, and other providers) were successful during the demo program, according to a June 2008 CMS evaluation report. (Download a copy of the report at www.cms.hhs.gov/RAC/Downloads/RAC_Demonstration_Evaluation_Report.pdf.)
How the Audits Work

Listen to an interview with Dr. Pinson
Out of concern that the Medicare Trust Fund might not be adequately protected against improper payments by existing error detection and prevention efforts, Congress directed CMS to use RACs to identify and recoup Medicare overpayments under Section 306 of the Medicare Modernization Act of 2003, and directed CMS to make the program permanent by 2010 under Section 302 of the Tax Relief and Health Care Act of 2006. According to CMS, RACs were implemented so that physicians and other providers could avoid submitting claims that do not comply with Medicare rules, CMS could lower its error rate, and taxpayers and future Medicare beneficiaries would be protected.1
CMS has contracted with four regional RACs for the national program, and each will use proprietary auditing software to review paid claims from Medicare Part A and Part B providers to ensure that they meet Medicare’s statutory, regulatory, and policy requirements and regulations.
The RACs use automated review for claims that clearly contain errors that resulted in improper payments (e.g., claims for duplicate or uncovered services, claims that violate a written Medicare policy or sanctioned coding guideline), in which case the RAC notifies the provider of the overpayment. For cases in which there is a high probability—but not certainty—that the claim contains an overpayment, the RAC requests medical records from the provider (including imaged medical records on CD or DVD) to conduct a complex review and make a determination as to whether payment of the claim was correct, or whether there was an over- or underpayment.
CMS uses a Web-based data warehouse to ensure that RACs do not review claims that have previously been reviewed by another entity, such as a Medicare carrier, fiscal intermediary, the Office of Inspector General, or a quality-improvement organization (QIO).
The four regional RACs are ramping up their claim review activities in all states, says Connie Leonard, director of CMS’ Division of Recovery Audit Operations. When overpayments are confirmed, the RACs issue letters demanding providers to repay their Medicare carrier or intermediary within 30 days. For confirmed underpayments, RACs inform the provider’s Medicare contractor or fiscal intermediary, which then forwards the additional payment, Leonard says.
Providers can repay an overpayment by check or installment plan on or before 30 days after receiving the RAC demand letter. The Medicare contractors use recoupment—reducing present or future Medicare payments—on day 41. Providers who wish to dispute overpayment charges can take their case through the usual Medicare claims appeal process. RACs also offer a “discussion period”—from the date the provider gets a “Detailed Review Results” letter until the date of recoupment—to discuss with the RAC an improper payment determination outside the normal appeal process, Leonard says.

—Kathy DeVault, RHIA, CCS, CCS-P, manager, Professional Practice Resources, American Health Information Management Association, Chicago
If providers disagree with the RAC’s determination, Leonard says, they should either 1) pay by check by day 30 and file for appeal by day 120 of the demand letter; 2) allow recoupment on day 41 and file for appeal by day 120; 3) stop the recoupment by filing an appeal by day 30; or 4) request an extended payment plan and appeal by day 120.
Some physicians in the demonstration project regarded the third-party RAC companies as “bounty hunters” operating without sufficient CMS oversight, imposing undue administrative burdens on physician practices, and lacking the clinical expertise to adjudicate claims appropriately, according to Michael Schweitz, MD, a rheumatologist from West Palm Beach, Fla., who testified before a Congressional committee in 2008 about RAC activities.
In response, CMS has modified the program (see “Refinements in Permanent RAC Program,” p. 8) in several ways to address those flaws and ensure a fair and smooth auditing process, Leonard says. (Listen to an audio interview with Ms. Leonard)
All About the Details
Because RACs focus on coding and documentation that fails to support DRG designations, hospitalists who focus on accurate and precise documentation that can be coded properly will greatly help their hospitals defend against RAC audits, as well as yield better payment and improved quality scores, says Richard D. Pinson, MD, FACP, CCS, principal of HCQ Consulting in Nashville, Tenn. Pinson will present “Documentation Tips Your Hospital Will Love You For” at HM10 in Washington, D.C., this month. A video/audio download of the presentation will be available on SHM’s Web site in May.
“Coding rules and terminology often don’t match what we’re used to writing in the record, so hospitalists need to learn what these connections are and use them in their medical record documentation,” Pinson says. “This is a core skill for hospitalists: being able to translate clinical terminology into the correct coding terminology for hospitals and coders.”
For example, if a hospitalist sees that a pre-operative patient has severe congestive heart failure, that condition cannot be coded as a complication of the patient’s care or considered as such in the DRG assignment, Pinson explains. If the hospitalist says the patient has an acute exacerbation of systolic heart failure, then that is a major comorbidity and ought to be documented as such. The average value of a major comorbidity in a surgical case could be as much as $20,000 per case, Pinson notes. If the DRG assignment included acute exacerbation but the medical chart only said severe congestive heart failure, the hospital would face recoupment of payment from an RAC audit.
“If we’re inconsistent or ambiguous in how we apply our terms, we can end up inadvertently upcoding. The key is: Learn to use the right terms that correspond to the right codes, based on what your patient actually has, and then be consistent throughout the record in your use of those terms,” Pinson says. For example, “we may admit a patient and say at the very beginning that the patient probably has aspiration pneumonia. We then treat the patient for aspiration pneumonia but leave it out of the discharge summary. The coder may code aspiration pneumonia, but the RAC auditor may point out that it was only mentioned in the patient’s record once, as possible, and may recoup any payment for treatment beyond simple pneumonia.”
Level of care and symptom-based DRG designations are red flags for RAC recovery, Pinson says. When the auditor sees a DRG based on symptoms rather than diagnoses (e.g., chest pain, syncope, transient ischemic attack, dehydration) and it is billed as inpatient status instead of observation status, that’s a target. Those symptoms, he says, often don’t meet the medical necessity criteria for inpatient status.
Pinson advises hospitalists to ask their institution’s case-management department, or hire an external consultant, to abstract key criteria for patient status designation, and to consider starting a patient as observation status until a precise diagnosis can be made that warrants hospital admission. Hospitalists should then describe the patient’s situation more precisely in the medical record as a diagnosis, not just as symptoms—e.g., syncope suspected due to cardiac arrhythmia, or chest pain suspected to be angina.
“For inpatient billing, those uncertain diagnoses, described that way, count as if they were established conditions. They don’t go into symptom DRGs,” Pinson says. “If you’re doing these things to protect the validity of you hospital’s billing, you’ll be protecting yourself at the same time, and it’s unlikely that RACs will single you out at all for auditing.”
Hospitalists can be valuable participants on their institutions’ RAC response team, providing clinical clarification on cases and helping to draft appeal letters.
There are several other red flags that RACs zero in on and hospitalists should watch out for, says Kathy DeVault, RHIA, CCS, CCS-P, manager of Professional Practice Resources for the American Health Information Management Association (AHIMA). Specificity in the medical record makes all the difference. For example, by identifying incorrect coding for excisional debridement (removal of infected tissue), RACs collected nearly $18 million in overpayments in fiscal-year 2006 because medical record documentation omitted such details as the word “excisional” (e.g., sharp debridement coded as excisional debridement), whether it was performed in the operating room or not, instruments used, the extent and depth of the procedure, and if the cutting of tissue was outside or beyond the wound margin.
DeVault warns that “RACs are targeting confusion between septicemia and urosepsis.” According to CMS, if the hospital reports a patient’s principal diagnosis as septicemia (03.89) but the medical record indicates the diagnosis of urosepsis, the RAC will bump the diagnosis code down to urinary tract infection (599.0), a lower-payment DRG, and demand recoupment.1
Urosepsis does not have a specific ICD-9-CM diagnosis code, and defaults to a simple UTI code, as referenced in ICD-9-CM. Unless the physician states in his or her documentation that the patient’s condition was systemic sepsis or septicemia, urosepsis would be coded as a UTI. RACS also denied some respiratory-failure claims for incorrect sequencing of principal diagnosis (e.g., respiratory failure vs. sepsis). The American Hospital Association has issued a regulatory advisory about these issues (web.mhanet.com/userdocs/articles/RAC/AHA_RAC_Coding Advisory_071608.pdf).
DeVault highlights three additional RAC targets that might impact HM:
- Documentation for transbronchial biopsy (a surgical DRG) in which the medical record only shows pathology of bronchus tissue (which RACs regard as nonsurgical);
- Failure to document the severity of a patient’s anemia as such to meet the medical necessity requirement of a blood transfusion (e.g., a chronic blood loss anemia or a pernicious anemia); and
- Documentation of treatments performed by intensivists in an ICU. By the time a patient’s attending physician sees their patient out of the ICU, DeVault says, their acute renal failure could be turned around but the attending might not document what happened in the ICU. The intensivist must see to it that the documentation allows the appropriate DRG assignment for the level of care the patient received.
AHIMA has published a 65-page RAC Audit Toolkit that describes the audit process, outlines preparations and procedures, and offers concrete guidance for appeals. Download a copy at www.ahima.org/infocenter/documents/RACToolkitFINAL.pdf. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
Reference
- The Medicare Recovery Audit Contractor (RAC) program: an evaluation of the 3-year demonstration. CMS Web site. Available at: www.cms.hhs.gov/RAC/Downloads/RACEvaluationReport.pdf. Accessed March 3, 2010.
Hospitalists will be essential players in helping their institutions prepare for the Recovery Audit Contractor (RAC) program, now being rolled out nationwide by the Centers for Medicare & Medicaid Services (CMS). The program is part of CMS’ arsenal to ferret out improper payments and prevent fraud, waste, and abuse in the Medicare system.
All providers who bill Medicare fee-for-service are fair game for an RAC audit, which scrutinizes medical records to validate diagnosis-related groups (DRGs), coding, and the necessity of care provided by hospitals. Hospitalists are being asked to document their diagnosis and treatment decisions more precisely and thoroughly than ever, ensuring that DRG coding is appropriate, medical necessity is watertight, and hospitals are defended from costly overpayment recovery.
Specificity of documentation is the hospitalist’s most potent weapon against this new layer of federal audits.
In a three-year demonstration of the RAC program that ended in March 2008, one-third of all medical records audited resulted in an overpayment finding and collection. RACs collected more than $900 million in overpayments and returned nearly $38 million in underpayments. One-third of provider appeals (physician, hospital, and other providers) were successful during the demo program, according to a June 2008 CMS evaluation report. (Download a copy of the report at www.cms.hhs.gov/RAC/Downloads/RAC_Demonstration_Evaluation_Report.pdf.)
How the Audits Work

Listen to an interview with Dr. Pinson
Out of concern that the Medicare Trust Fund might not be adequately protected against improper payments by existing error detection and prevention efforts, Congress directed CMS to use RACs to identify and recoup Medicare overpayments under Section 306 of the Medicare Modernization Act of 2003, and directed CMS to make the program permanent by 2010 under Section 302 of the Tax Relief and Health Care Act of 2006. According to CMS, RACs were implemented so that physicians and other providers could avoid submitting claims that do not comply with Medicare rules, CMS could lower its error rate, and taxpayers and future Medicare beneficiaries would be protected.1
CMS has contracted with four regional RACs for the national program, and each will use proprietary auditing software to review paid claims from Medicare Part A and Part B providers to ensure that they meet Medicare’s statutory, regulatory, and policy requirements and regulations.
The RACs use automated review for claims that clearly contain errors that resulted in improper payments (e.g., claims for duplicate or uncovered services, claims that violate a written Medicare policy or sanctioned coding guideline), in which case the RAC notifies the provider of the overpayment. For cases in which there is a high probability—but not certainty—that the claim contains an overpayment, the RAC requests medical records from the provider (including imaged medical records on CD or DVD) to conduct a complex review and make a determination as to whether payment of the claim was correct, or whether there was an over- or underpayment.
CMS uses a Web-based data warehouse to ensure that RACs do not review claims that have previously been reviewed by another entity, such as a Medicare carrier, fiscal intermediary, the Office of Inspector General, or a quality-improvement organization (QIO).
The four regional RACs are ramping up their claim review activities in all states, says Connie Leonard, director of CMS’ Division of Recovery Audit Operations. When overpayments are confirmed, the RACs issue letters demanding providers to repay their Medicare carrier or intermediary within 30 days. For confirmed underpayments, RACs inform the provider’s Medicare contractor or fiscal intermediary, which then forwards the additional payment, Leonard says.
Providers can repay an overpayment by check or installment plan on or before 30 days after receiving the RAC demand letter. The Medicare contractors use recoupment—reducing present or future Medicare payments—on day 41. Providers who wish to dispute overpayment charges can take their case through the usual Medicare claims appeal process. RACs also offer a “discussion period”—from the date the provider gets a “Detailed Review Results” letter until the date of recoupment—to discuss with the RAC an improper payment determination outside the normal appeal process, Leonard says.

—Kathy DeVault, RHIA, CCS, CCS-P, manager, Professional Practice Resources, American Health Information Management Association, Chicago
If providers disagree with the RAC’s determination, Leonard says, they should either 1) pay by check by day 30 and file for appeal by day 120 of the demand letter; 2) allow recoupment on day 41 and file for appeal by day 120; 3) stop the recoupment by filing an appeal by day 30; or 4) request an extended payment plan and appeal by day 120.
Some physicians in the demonstration project regarded the third-party RAC companies as “bounty hunters” operating without sufficient CMS oversight, imposing undue administrative burdens on physician practices, and lacking the clinical expertise to adjudicate claims appropriately, according to Michael Schweitz, MD, a rheumatologist from West Palm Beach, Fla., who testified before a Congressional committee in 2008 about RAC activities.
In response, CMS has modified the program (see “Refinements in Permanent RAC Program,” p. 8) in several ways to address those flaws and ensure a fair and smooth auditing process, Leonard says. (Listen to an audio interview with Ms. Leonard)
All About the Details
Because RACs focus on coding and documentation that fails to support DRG designations, hospitalists who focus on accurate and precise documentation that can be coded properly will greatly help their hospitals defend against RAC audits, as well as yield better payment and improved quality scores, says Richard D. Pinson, MD, FACP, CCS, principal of HCQ Consulting in Nashville, Tenn. Pinson will present “Documentation Tips Your Hospital Will Love You For” at HM10 in Washington, D.C., this month. A video/audio download of the presentation will be available on SHM’s Web site in May.
“Coding rules and terminology often don’t match what we’re used to writing in the record, so hospitalists need to learn what these connections are and use them in their medical record documentation,” Pinson says. “This is a core skill for hospitalists: being able to translate clinical terminology into the correct coding terminology for hospitals and coders.”
For example, if a hospitalist sees that a pre-operative patient has severe congestive heart failure, that condition cannot be coded as a complication of the patient’s care or considered as such in the DRG assignment, Pinson explains. If the hospitalist says the patient has an acute exacerbation of systolic heart failure, then that is a major comorbidity and ought to be documented as such. The average value of a major comorbidity in a surgical case could be as much as $20,000 per case, Pinson notes. If the DRG assignment included acute exacerbation but the medical chart only said severe congestive heart failure, the hospital would face recoupment of payment from an RAC audit.
“If we’re inconsistent or ambiguous in how we apply our terms, we can end up inadvertently upcoding. The key is: Learn to use the right terms that correspond to the right codes, based on what your patient actually has, and then be consistent throughout the record in your use of those terms,” Pinson says. For example, “we may admit a patient and say at the very beginning that the patient probably has aspiration pneumonia. We then treat the patient for aspiration pneumonia but leave it out of the discharge summary. The coder may code aspiration pneumonia, but the RAC auditor may point out that it was only mentioned in the patient’s record once, as possible, and may recoup any payment for treatment beyond simple pneumonia.”
Level of care and symptom-based DRG designations are red flags for RAC recovery, Pinson says. When the auditor sees a DRG based on symptoms rather than diagnoses (e.g., chest pain, syncope, transient ischemic attack, dehydration) and it is billed as inpatient status instead of observation status, that’s a target. Those symptoms, he says, often don’t meet the medical necessity criteria for inpatient status.
Pinson advises hospitalists to ask their institution’s case-management department, or hire an external consultant, to abstract key criteria for patient status designation, and to consider starting a patient as observation status until a precise diagnosis can be made that warrants hospital admission. Hospitalists should then describe the patient’s situation more precisely in the medical record as a diagnosis, not just as symptoms—e.g., syncope suspected due to cardiac arrhythmia, or chest pain suspected to be angina.
“For inpatient billing, those uncertain diagnoses, described that way, count as if they were established conditions. They don’t go into symptom DRGs,” Pinson says. “If you’re doing these things to protect the validity of you hospital’s billing, you’ll be protecting yourself at the same time, and it’s unlikely that RACs will single you out at all for auditing.”
Hospitalists can be valuable participants on their institutions’ RAC response team, providing clinical clarification on cases and helping to draft appeal letters.
There are several other red flags that RACs zero in on and hospitalists should watch out for, says Kathy DeVault, RHIA, CCS, CCS-P, manager of Professional Practice Resources for the American Health Information Management Association (AHIMA). Specificity in the medical record makes all the difference. For example, by identifying incorrect coding for excisional debridement (removal of infected tissue), RACs collected nearly $18 million in overpayments in fiscal-year 2006 because medical record documentation omitted such details as the word “excisional” (e.g., sharp debridement coded as excisional debridement), whether it was performed in the operating room or not, instruments used, the extent and depth of the procedure, and if the cutting of tissue was outside or beyond the wound margin.
DeVault warns that “RACs are targeting confusion between septicemia and urosepsis.” According to CMS, if the hospital reports a patient’s principal diagnosis as septicemia (03.89) but the medical record indicates the diagnosis of urosepsis, the RAC will bump the diagnosis code down to urinary tract infection (599.0), a lower-payment DRG, and demand recoupment.1
Urosepsis does not have a specific ICD-9-CM diagnosis code, and defaults to a simple UTI code, as referenced in ICD-9-CM. Unless the physician states in his or her documentation that the patient’s condition was systemic sepsis or septicemia, urosepsis would be coded as a UTI. RACS also denied some respiratory-failure claims for incorrect sequencing of principal diagnosis (e.g., respiratory failure vs. sepsis). The American Hospital Association has issued a regulatory advisory about these issues (web.mhanet.com/userdocs/articles/RAC/AHA_RAC_Coding Advisory_071608.pdf).
DeVault highlights three additional RAC targets that might impact HM:
- Documentation for transbronchial biopsy (a surgical DRG) in which the medical record only shows pathology of bronchus tissue (which RACs regard as nonsurgical);
- Failure to document the severity of a patient’s anemia as such to meet the medical necessity requirement of a blood transfusion (e.g., a chronic blood loss anemia or a pernicious anemia); and
- Documentation of treatments performed by intensivists in an ICU. By the time a patient’s attending physician sees their patient out of the ICU, DeVault says, their acute renal failure could be turned around but the attending might not document what happened in the ICU. The intensivist must see to it that the documentation allows the appropriate DRG assignment for the level of care the patient received.
AHIMA has published a 65-page RAC Audit Toolkit that describes the audit process, outlines preparations and procedures, and offers concrete guidance for appeals. Download a copy at www.ahima.org/infocenter/documents/RACToolkitFINAL.pdf. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
Reference
- The Medicare Recovery Audit Contractor (RAC) program: an evaluation of the 3-year demonstration. CMS Web site. Available at: www.cms.hhs.gov/RAC/Downloads/RACEvaluationReport.pdf. Accessed March 3, 2010.
Hospitalists in Haiti
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
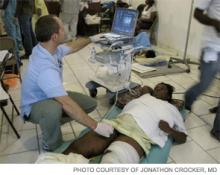
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
Denileukin diftitox has significant, durable responses in CTCL
The recombinant fusion protein denileukin diftitox (DD) produced a significant and durable overall response rate at 2 dose levels as compared to placebo in patients with cutaneous T-cell lymphoma (CTCL).
These phase 3 results confirm the efficacy, safety, and clinical benefit of DD in CD25-positive, stage IA to III CTCL. An earlier trial included more heavily pretreated late-stage patients.
Andres Negro-Vilar, MD, PhD, and colleagues reported the current results March 8 ahead of print in the Journal of Clinical Oncology.
The investigators randomized 144 patients—44 to placebo, 45 to 9 mg/kg/day DD, and 55 to 18 m/kg/day DD. Patients received the treatment on days 1 through 5 of each 21-day course for up to 8 courses.
Patients were a median age of 59 years, two thirds had disease stage IIA or earlier, and 94% had received 3 or fewer prior therapies. Prior therapies included phototherapy (48%), interferon alfa (20%), electron beam readiotherapy (48%), system cytotoxic chemotherapy (26%), topical chemotherapy (25%), and other therapies (30%).
Most patients (85%) had mycosis fungoides, 6.3% had Sézary syndrome, and 8.3% had other cutaneous lymphomas.
After a median of 6 treatment courses, patients receiving either dose of DD had a statistically significant overall response rate (ORR) compared to placebo.
Patients in the 18 µg DD group had a 49% ORR, including 9% complete response (CR) or clinical complete response (CCR). This compared to an ORR of 16% for placebo patients (P=0.0015).
Patients in the 9 µg group had a 37.8% ORR, including 11% CR/CCR. This ORR was also significantly better compared to placebo (P=0.0297).
About half of the placebo patients experienced progressive disease compared with 21% of the DD-treated patients.
Progression-free survival (PFS) was significantly longer in the DD-treated patients. The 18 µg-arm had a median PFS of 971 days, the 9 µg-arm had a median PFS of 794 days, and the placebo patients had a median PFS of 124 days.
DD-treated patients in both dose groups experienced significantly superior duration of response, time to response, and time to treatment failure compared to the placebo patients.
DD-treated patients reported more adverse events and serious adverse events than the placebo patients. The investigators observed that the AEs occurred most frequently during the first 2 or 3 treatment courses and then declined to placebo levels.
DD combines the diphtheria toxin with human interleukin-2 (IL-2). DD binds to and is internalized by the IL-2 receptor. Therefore, it is most efficient at killing cells that express the intermediate- or high-affinity IL-2 receptor.
The investigators suggest that the 18 µg/kg/day dose may improve the response rate without increasing toxicity. The higher dose “provides more benefit, such as a higher ORR and statistically significant improvements in several supportive end points . . . DD may represent an important treatment option for many patients with these challenging diseases,” they said. ![]()

The recombinant fusion protein denileukin diftitox (DD) produced a significant and durable overall response rate at 2 dose levels as compared to placebo in patients with cutaneous T-cell lymphoma (CTCL).
These phase 3 results confirm the efficacy, safety, and clinical benefit of DD in CD25-positive, stage IA to III CTCL. An earlier trial included more heavily pretreated late-stage patients.
Andres Negro-Vilar, MD, PhD, and colleagues reported the current results March 8 ahead of print in the Journal of Clinical Oncology.
The investigators randomized 144 patients—44 to placebo, 45 to 9 mg/kg/day DD, and 55 to 18 m/kg/day DD. Patients received the treatment on days 1 through 5 of each 21-day course for up to 8 courses.
Patients were a median age of 59 years, two thirds had disease stage IIA or earlier, and 94% had received 3 or fewer prior therapies. Prior therapies included phototherapy (48%), interferon alfa (20%), electron beam readiotherapy (48%), system cytotoxic chemotherapy (26%), topical chemotherapy (25%), and other therapies (30%).
Most patients (85%) had mycosis fungoides, 6.3% had Sézary syndrome, and 8.3% had other cutaneous lymphomas.
After a median of 6 treatment courses, patients receiving either dose of DD had a statistically significant overall response rate (ORR) compared to placebo.
Patients in the 18 µg DD group had a 49% ORR, including 9% complete response (CR) or clinical complete response (CCR). This compared to an ORR of 16% for placebo patients (P=0.0015).
Patients in the 9 µg group had a 37.8% ORR, including 11% CR/CCR. This ORR was also significantly better compared to placebo (P=0.0297).
About half of the placebo patients experienced progressive disease compared with 21% of the DD-treated patients.
Progression-free survival (PFS) was significantly longer in the DD-treated patients. The 18 µg-arm had a median PFS of 971 days, the 9 µg-arm had a median PFS of 794 days, and the placebo patients had a median PFS of 124 days.
DD-treated patients in both dose groups experienced significantly superior duration of response, time to response, and time to treatment failure compared to the placebo patients.
DD-treated patients reported more adverse events and serious adverse events than the placebo patients. The investigators observed that the AEs occurred most frequently during the first 2 or 3 treatment courses and then declined to placebo levels.
DD combines the diphtheria toxin with human interleukin-2 (IL-2). DD binds to and is internalized by the IL-2 receptor. Therefore, it is most efficient at killing cells that express the intermediate- or high-affinity IL-2 receptor.
The investigators suggest that the 18 µg/kg/day dose may improve the response rate without increasing toxicity. The higher dose “provides more benefit, such as a higher ORR and statistically significant improvements in several supportive end points . . . DD may represent an important treatment option for many patients with these challenging diseases,” they said. ![]()

The recombinant fusion protein denileukin diftitox (DD) produced a significant and durable overall response rate at 2 dose levels as compared to placebo in patients with cutaneous T-cell lymphoma (CTCL).
These phase 3 results confirm the efficacy, safety, and clinical benefit of DD in CD25-positive, stage IA to III CTCL. An earlier trial included more heavily pretreated late-stage patients.
Andres Negro-Vilar, MD, PhD, and colleagues reported the current results March 8 ahead of print in the Journal of Clinical Oncology.
The investigators randomized 144 patients—44 to placebo, 45 to 9 mg/kg/day DD, and 55 to 18 m/kg/day DD. Patients received the treatment on days 1 through 5 of each 21-day course for up to 8 courses.
Patients were a median age of 59 years, two thirds had disease stage IIA or earlier, and 94% had received 3 or fewer prior therapies. Prior therapies included phototherapy (48%), interferon alfa (20%), electron beam readiotherapy (48%), system cytotoxic chemotherapy (26%), topical chemotherapy (25%), and other therapies (30%).
Most patients (85%) had mycosis fungoides, 6.3% had Sézary syndrome, and 8.3% had other cutaneous lymphomas.
After a median of 6 treatment courses, patients receiving either dose of DD had a statistically significant overall response rate (ORR) compared to placebo.
Patients in the 18 µg DD group had a 49% ORR, including 9% complete response (CR) or clinical complete response (CCR). This compared to an ORR of 16% for placebo patients (P=0.0015).
Patients in the 9 µg group had a 37.8% ORR, including 11% CR/CCR. This ORR was also significantly better compared to placebo (P=0.0297).
About half of the placebo patients experienced progressive disease compared with 21% of the DD-treated patients.
Progression-free survival (PFS) was significantly longer in the DD-treated patients. The 18 µg-arm had a median PFS of 971 days, the 9 µg-arm had a median PFS of 794 days, and the placebo patients had a median PFS of 124 days.
DD-treated patients in both dose groups experienced significantly superior duration of response, time to response, and time to treatment failure compared to the placebo patients.
DD-treated patients reported more adverse events and serious adverse events than the placebo patients. The investigators observed that the AEs occurred most frequently during the first 2 or 3 treatment courses and then declined to placebo levels.
DD combines the diphtheria toxin with human interleukin-2 (IL-2). DD binds to and is internalized by the IL-2 receptor. Therefore, it is most efficient at killing cells that express the intermediate- or high-affinity IL-2 receptor.
The investigators suggest that the 18 µg/kg/day dose may improve the response rate without increasing toxicity. The higher dose “provides more benefit, such as a higher ORR and statistically significant improvements in several supportive end points . . . DD may represent an important treatment option for many patients with these challenging diseases,” they said. ![]()
ONLINE EXCLUSIVE: Audio interviews with Medicare audit program experts
The dawn of a new era: Transforming our domestic response to hepatitis B & C
Our understanding of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections has improved in recent years. Safe and effective vaccines for HBV as well as effective antiviral therapies for HBV and HCV infections are now available. However, current approaches to the prevention and control of chronic HBV and HCV infections have fallen short, resulting in a major public health problem. The prevalence of chronic HBV and HCV infections is expected to increase in the United States, as is the burden of hepatitis-associated cirrhosis, end-stage liver disease, and liver cancer. The time to develop new strategies to prevent, screen, and treat chronic viral hepatitis is now.
Our understanding of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections has improved in recent years. Safe and effective vaccines for HBV as well as effective antiviral therapies for HBV and HCV infections are now available. However, current approaches to the prevention and control of chronic HBV and HCV infections have fallen short, resulting in a major public health problem. The prevalence of chronic HBV and HCV infections is expected to increase in the United States, as is the burden of hepatitis-associated cirrhosis, end-stage liver disease, and liver cancer. The time to develop new strategies to prevent, screen, and treat chronic viral hepatitis is now.
Our understanding of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections has improved in recent years. Safe and effective vaccines for HBV as well as effective antiviral therapies for HBV and HCV infections are now available. However, current approaches to the prevention and control of chronic HBV and HCV infections have fallen short, resulting in a major public health problem. The prevalence of chronic HBV and HCV infections is expected to increase in the United States, as is the burden of hepatitis-associated cirrhosis, end-stage liver disease, and liver cancer. The time to develop new strategies to prevent, screen, and treat chronic viral hepatitis is now.
New tools for detecting occult monoclonal gammopathy, a cause of secondary osteoporosis
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
KEY POINTS
- Minor back pain can be a symptom of spinal compression fracture.
- Rapidly declining bone density or a low Z score on dual-energy x-ray absorptiometry suggests that osteoporosis is secondary to another condition.
- The evidence to date supports the use of bone turnover markers in conjunction with density measurements to ascertain early on whether osteoporosis is responding to treatment, but the use of biochemical markers by themselves to screen for osteoporosis is not encouraged.
- Standard tests may fail to detect myeloma in the presence of worsening bone density.
- While serum and urine protein electrophoreses are still the standard screening tests for multiple myeloma, additional testing with serum free light chain analysis should be considered if the suspicion is high.
Vaccine update 2010: Keeping up with the changes
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.
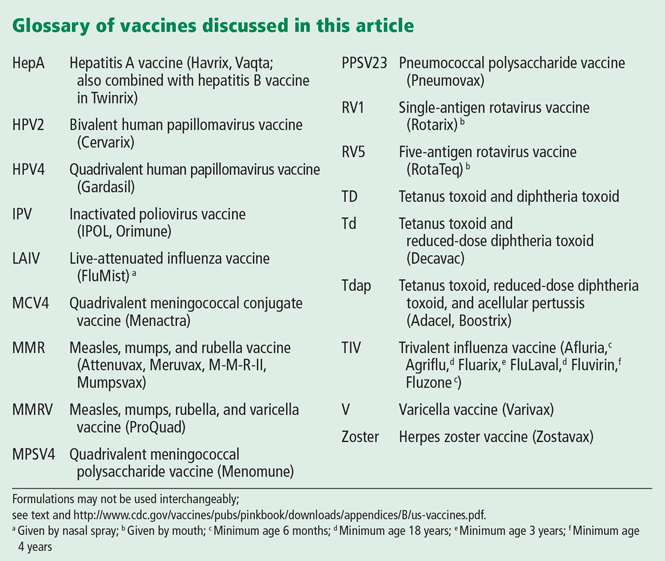
VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
KEY POINTS
- New recommendations for infants and children:
- Rotavirus vaccination for infants
- Seasonal influenza vaccine yearly at ages 5–18
- Hepatitis A vaccine at age 12–23 months
- Varicella vaccine at 12–15 months and again at 4–6 years, with catch-up for others.
- New recommendations for adolescents:
- Meningococcus quadrivalent conjugate vaccine for all at age 11 or 12 and catch-up through age 18
- A shot of tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis vaccine (Tdap) at age 11 or 12 and catch-up through age 18
- Human papillomavirus vaccine (three doses) for girls at age 11 or 12 and catch-up through age 26.
- New recommendations for adults:
- One dose of Tdap instead of the next tetanus-diphtheria booster
- Herpes zoster vaccine at age 60 or older
- Pneumococcal vaccination extended to smokers and people with asthma, with a second dose 5 years after the first for people who have immune suppression, sickle cell disease, or asplenia.
Bony bridge of a bifid rib
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
Giant nodules on the hands
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.