User login
HM10 PREVIEW: The Last Word
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
HM10 PREVIEW: Crystal Ball
He’s not a hospitalist. He’s not even a doctor. In fact, less than a decade ago, he was executive director of a Massachusetts water resources board and best known for his views on how to best clean up Boston Bay. But from his perch as president and CEO of Beth Israel Deaconess Medical Center in Boston, Paul Levy is a leading voice in contemporary healthcare, quality measures, and transparency.
HM10’s keynote speaker is most well-known in both medical and management circles for launching a blog in 2006 about the daily operations of his institution, aptly found at www.runningahospital.blogspot.com. Levy, whose address is at 9 a.m. Friday, April 9, spoke to The Hospitalist about his views on the role HM practitioners can play in quality improvement (QI), the importance of communication in medicine, and what he hopes hospitalists can learn from the experiences of his hospital.
Question: What made you accept the offer to be the keynote speaker at HM10?
Answer: I have tremendous respect for the hospital medicine industry. They are positioned to be an exceptionally important part of the care delivery system. In terms of working alongside them, they’re also interesting people. I enjoy working with them.
Q: Why are you looking forward to speaking?
A: I’d like to share our experience with qualitative care improvements and process improvements. The hospitalists, because of their position within the hospital and their relationships (with specialists and with administrators in the C-suite), are on the vanguard of being able to truly improve how we deliver care.
Q: Your address is titled “The Hospitalist’s Role in the Hospital of the Future.” Can you provide an overview of the topics you plan to talk about?
A: It's a classic discussion on how you do process improvement. How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals. They are in a position to make meaningful impacts on multiple floors.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Q: How do you encourage your HM physicians to do that?
A: I don’t have to. They do it. We have found it very valuable, and in our place, it’s led to better outcomes. That means better patient care.
Q: Can you give an example of that value to the institution?
A: We initiated a rapid-response program several years ago we call “Triggers.” When a patient displays certain symptoms—changes in heart rate, blood pressure, oxygen saturation, etc.—there is an automatic trigger that calls in a senior nurse and a senior attending physician. We have already demonstrated a reduction in fatalities and a reduction in mortality rates because of this. We have so few codes right now on our on floors that we had to move our codes to our simulators because residents were not getting enough training. It’s a good problem to have.
Q: Do you see more physicians, hospitalists particularly, embracing technology in the hopes of improving care delivery and process administration?
A: Sure. We have a group of residents who created some type of Wiki with their work. I’m not even sure how it works; it just happens. I know there are some hospitals out there preventing this from happening. I think that’s just stupid. It’s a fear that something proprietary or private will be posted for everyone to see. I don’t particularly think it’s founded. We’ve all heard stories about someone who leaves a folder full of files on the subway. Are we going to stop people from riding the subway?
Q: What do you think HM’s outlook is?
A: I think it’s bright. I’m kind of new to medicine. I’ve only been here about eight years. I kind of wonder what used to happen before hospitalists. I guess primary-care physicians came in early in the morning and late at night. That doesn’t sound like an efficient system to deliver care.
Q: What do you want conference attendees to take away from your words?
A: I just hope the stories I tell about process improvements help everyone in their institutions. It’s up to them.
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
He’s not a hospitalist. He’s not even a doctor. In fact, less than a decade ago, he was executive director of a Massachusetts water resources board and best known for his views on how to best clean up Boston Bay. But from his perch as president and CEO of Beth Israel Deaconess Medical Center in Boston, Paul Levy is a leading voice in contemporary healthcare, quality measures, and transparency.
HM10’s keynote speaker is most well-known in both medical and management circles for launching a blog in 2006 about the daily operations of his institution, aptly found at www.runningahospital.blogspot.com. Levy, whose address is at 9 a.m. Friday, April 9, spoke to The Hospitalist about his views on the role HM practitioners can play in quality improvement (QI), the importance of communication in medicine, and what he hopes hospitalists can learn from the experiences of his hospital.
Question: What made you accept the offer to be the keynote speaker at HM10?
Answer: I have tremendous respect for the hospital medicine industry. They are positioned to be an exceptionally important part of the care delivery system. In terms of working alongside them, they’re also interesting people. I enjoy working with them.
Q: Why are you looking forward to speaking?
A: I’d like to share our experience with qualitative care improvements and process improvements. The hospitalists, because of their position within the hospital and their relationships (with specialists and with administrators in the C-suite), are on the vanguard of being able to truly improve how we deliver care.
Q: Your address is titled “The Hospitalist’s Role in the Hospital of the Future.” Can you provide an overview of the topics you plan to talk about?
A: It's a classic discussion on how you do process improvement. How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals. They are in a position to make meaningful impacts on multiple floors.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Q: How do you encourage your HM physicians to do that?
A: I don’t have to. They do it. We have found it very valuable, and in our place, it’s led to better outcomes. That means better patient care.
Q: Can you give an example of that value to the institution?
A: We initiated a rapid-response program several years ago we call “Triggers.” When a patient displays certain symptoms—changes in heart rate, blood pressure, oxygen saturation, etc.—there is an automatic trigger that calls in a senior nurse and a senior attending physician. We have already demonstrated a reduction in fatalities and a reduction in mortality rates because of this. We have so few codes right now on our on floors that we had to move our codes to our simulators because residents were not getting enough training. It’s a good problem to have.
Q: Do you see more physicians, hospitalists particularly, embracing technology in the hopes of improving care delivery and process administration?
A: Sure. We have a group of residents who created some type of Wiki with their work. I’m not even sure how it works; it just happens. I know there are some hospitals out there preventing this from happening. I think that’s just stupid. It’s a fear that something proprietary or private will be posted for everyone to see. I don’t particularly think it’s founded. We’ve all heard stories about someone who leaves a folder full of files on the subway. Are we going to stop people from riding the subway?
Q: What do you think HM’s outlook is?
A: I think it’s bright. I’m kind of new to medicine. I’ve only been here about eight years. I kind of wonder what used to happen before hospitalists. I guess primary-care physicians came in early in the morning and late at night. That doesn’t sound like an efficient system to deliver care.
Q: What do you want conference attendees to take away from your words?
A: I just hope the stories I tell about process improvements help everyone in their institutions. It’s up to them.
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
He’s not a hospitalist. He’s not even a doctor. In fact, less than a decade ago, he was executive director of a Massachusetts water resources board and best known for his views on how to best clean up Boston Bay. But from his perch as president and CEO of Beth Israel Deaconess Medical Center in Boston, Paul Levy is a leading voice in contemporary healthcare, quality measures, and transparency.
HM10’s keynote speaker is most well-known in both medical and management circles for launching a blog in 2006 about the daily operations of his institution, aptly found at www.runningahospital.blogspot.com. Levy, whose address is at 9 a.m. Friday, April 9, spoke to The Hospitalist about his views on the role HM practitioners can play in quality improvement (QI), the importance of communication in medicine, and what he hopes hospitalists can learn from the experiences of his hospital.
Question: What made you accept the offer to be the keynote speaker at HM10?
Answer: I have tremendous respect for the hospital medicine industry. They are positioned to be an exceptionally important part of the care delivery system. In terms of working alongside them, they’re also interesting people. I enjoy working with them.
Q: Why are you looking forward to speaking?
A: I’d like to share our experience with qualitative care improvements and process improvements. The hospitalists, because of their position within the hospital and their relationships (with specialists and with administrators in the C-suite), are on the vanguard of being able to truly improve how we deliver care.
Q: Your address is titled “The Hospitalist’s Role in the Hospital of the Future.” Can you provide an overview of the topics you plan to talk about?
A: It's a classic discussion on how you do process improvement. How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals. They are in a position to make meaningful impacts on multiple floors.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Q: How do you encourage your HM physicians to do that?
A: I don’t have to. They do it. We have found it very valuable, and in our place, it’s led to better outcomes. That means better patient care.
Q: Can you give an example of that value to the institution?
A: We initiated a rapid-response program several years ago we call “Triggers.” When a patient displays certain symptoms—changes in heart rate, blood pressure, oxygen saturation, etc.—there is an automatic trigger that calls in a senior nurse and a senior attending physician. We have already demonstrated a reduction in fatalities and a reduction in mortality rates because of this. We have so few codes right now on our on floors that we had to move our codes to our simulators because residents were not getting enough training. It’s a good problem to have.
Q: Do you see more physicians, hospitalists particularly, embracing technology in the hopes of improving care delivery and process administration?
A: Sure. We have a group of residents who created some type of Wiki with their work. I’m not even sure how it works; it just happens. I know there are some hospitals out there preventing this from happening. I think that’s just stupid. It’s a fear that something proprietary or private will be posted for everyone to see. I don’t particularly think it’s founded. We’ve all heard stories about someone who leaves a folder full of files on the subway. Are we going to stop people from riding the subway?
Q: What do you think HM’s outlook is?
A: I think it’s bright. I’m kind of new to medicine. I’ve only been here about eight years. I kind of wonder what used to happen before hospitalists. I guess primary-care physicians came in early in the morning and late at night. That doesn’t sound like an efficient system to deliver care.
Q: What do you want conference attendees to take away from your words?
A: I just hope the stories I tell about process improvements help everyone in their institutions. It’s up to them.
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
HM10 PREVIEW: Bigger & Better
If a medical meeting of the minds is only as good as its CME options, then HM10 will be the best SHM annual meeting yet.
A pair of new pre-courses—“Essential Neurology for the Hospitalist” and “Early Career Hospitalist: Skills for Success”—will debut at HM10, which kicks off April 8 at the Gaylord National Resort & Convention Center in National Harbor, Md., outside of Washington, D.C. The new learning sessions boost to 20 the number of Category 1 credits physicians can earn toward the American Medical Association’s (AMA) Physician Recognition Award. Last year, hospitalists could earn a maximum of 15 credits.
While both of the new pre-courses drew heavy interest among early registrants, the latter was particularly intriguing, says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine in Florida.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“It’s really going to allow our early-career hospitalists to acquire some of the skills they don’t necessarily acquire during residency, such as billing and coding and understanding of quality measures,” Dr. Jaffer boasts, “as well as how best to communicate with consultants, patients, and families. They’ll have a better understanding of how important some of these issues are. … They form the background, as well as the foundation, for one’s career as a hospitalist.”
Also new this year is the added attention being paid to the American Board of Internal Medicine (ABIM) Maintenance of Certification (MOC) learning session, which led all pre-courses in early registration. The class is in its second year but is more appealing this year because of the new Recognition of Focused Practice in Hospital Medicine via MOC. Working in conjunction with ABIM, the pre-course offers the opportunity for ABIM-certified physicians the chance to earn 20 points toward the Self-Evaluation of Medical Knowledge requirement of the MOC program. Two modules will be presented: Internal Medicine-Office Based and Internal Medicine-Hospital Based.
“There’s an updated module,” says Geri Barnes, SHM senior director of education and meetings. “(Attendees) should get really good information there.”
Also new this year is a series of interactive workshops SHM launched to provide topic-specific information from experts. Spread over three days, the 18 workshops include “Improving Care for the Hospitalized Elder,” “Ultrasound Imaging Skills for Invasive Beside Procedures,” and “Blood Management: Hospitalists in a New Role.”
The workshops, which are limited to 100 participants, are an attempt to engage a new class of HM devotees by recruiting a new class of speakers and leaders. “In previous years, we were not getting all our membership involved with the annual meeting,” Dr. Jaffer says. “We were really handpicking our speakers on experience, expertise, and how well they were known in their field and how good of speakers they were. This year, we really reached out.”
HM10 also features “special-interest forums” at 4:45 p.m. Friday, April 9. The informal sessions—on 18 topics ranging from “Information Technology” to “Women in HM”—afford hospitalists direct access to SHM board members, committee members, and staff. Barnes says the information gathered in last year’s sessions was discussed by SHM. For instance, the “Early Career Hospitalist” forum was the inspiration for one of the new pre-courses.
“We’re not real good at getting that message across, but I would say that those (forums) are extremely valuable,” Barnes says. “The same thing goes for the town hall (1 p.m. Sunday, April 11). That’s an opportunity to meet with the executive committee and board members, and ask them anything you want to.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
If a medical meeting of the minds is only as good as its CME options, then HM10 will be the best SHM annual meeting yet.
A pair of new pre-courses—“Essential Neurology for the Hospitalist” and “Early Career Hospitalist: Skills for Success”—will debut at HM10, which kicks off April 8 at the Gaylord National Resort & Convention Center in National Harbor, Md., outside of Washington, D.C. The new learning sessions boost to 20 the number of Category 1 credits physicians can earn toward the American Medical Association’s (AMA) Physician Recognition Award. Last year, hospitalists could earn a maximum of 15 credits.
While both of the new pre-courses drew heavy interest among early registrants, the latter was particularly intriguing, says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine in Florida.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“It’s really going to allow our early-career hospitalists to acquire some of the skills they don’t necessarily acquire during residency, such as billing and coding and understanding of quality measures,” Dr. Jaffer boasts, “as well as how best to communicate with consultants, patients, and families. They’ll have a better understanding of how important some of these issues are. … They form the background, as well as the foundation, for one’s career as a hospitalist.”
Also new this year is the added attention being paid to the American Board of Internal Medicine (ABIM) Maintenance of Certification (MOC) learning session, which led all pre-courses in early registration. The class is in its second year but is more appealing this year because of the new Recognition of Focused Practice in Hospital Medicine via MOC. Working in conjunction with ABIM, the pre-course offers the opportunity for ABIM-certified physicians the chance to earn 20 points toward the Self-Evaluation of Medical Knowledge requirement of the MOC program. Two modules will be presented: Internal Medicine-Office Based and Internal Medicine-Hospital Based.
“There’s an updated module,” says Geri Barnes, SHM senior director of education and meetings. “(Attendees) should get really good information there.”
Also new this year is a series of interactive workshops SHM launched to provide topic-specific information from experts. Spread over three days, the 18 workshops include “Improving Care for the Hospitalized Elder,” “Ultrasound Imaging Skills for Invasive Beside Procedures,” and “Blood Management: Hospitalists in a New Role.”
The workshops, which are limited to 100 participants, are an attempt to engage a new class of HM devotees by recruiting a new class of speakers and leaders. “In previous years, we were not getting all our membership involved with the annual meeting,” Dr. Jaffer says. “We were really handpicking our speakers on experience, expertise, and how well they were known in their field and how good of speakers they were. This year, we really reached out.”
HM10 also features “special-interest forums” at 4:45 p.m. Friday, April 9. The informal sessions—on 18 topics ranging from “Information Technology” to “Women in HM”—afford hospitalists direct access to SHM board members, committee members, and staff. Barnes says the information gathered in last year’s sessions was discussed by SHM. For instance, the “Early Career Hospitalist” forum was the inspiration for one of the new pre-courses.
“We’re not real good at getting that message across, but I would say that those (forums) are extremely valuable,” Barnes says. “The same thing goes for the town hall (1 p.m. Sunday, April 11). That’s an opportunity to meet with the executive committee and board members, and ask them anything you want to.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
If a medical meeting of the minds is only as good as its CME options, then HM10 will be the best SHM annual meeting yet.
A pair of new pre-courses—“Essential Neurology for the Hospitalist” and “Early Career Hospitalist: Skills for Success”—will debut at HM10, which kicks off April 8 at the Gaylord National Resort & Convention Center in National Harbor, Md., outside of Washington, D.C. The new learning sessions boost to 20 the number of Category 1 credits physicians can earn toward the American Medical Association’s (AMA) Physician Recognition Award. Last year, hospitalists could earn a maximum of 15 credits.
While both of the new pre-courses drew heavy interest among early registrants, the latter was particularly intriguing, says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine in Florida.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“It’s really going to allow our early-career hospitalists to acquire some of the skills they don’t necessarily acquire during residency, such as billing and coding and understanding of quality measures,” Dr. Jaffer boasts, “as well as how best to communicate with consultants, patients, and families. They’ll have a better understanding of how important some of these issues are. … They form the background, as well as the foundation, for one’s career as a hospitalist.”
Also new this year is the added attention being paid to the American Board of Internal Medicine (ABIM) Maintenance of Certification (MOC) learning session, which led all pre-courses in early registration. The class is in its second year but is more appealing this year because of the new Recognition of Focused Practice in Hospital Medicine via MOC. Working in conjunction with ABIM, the pre-course offers the opportunity for ABIM-certified physicians the chance to earn 20 points toward the Self-Evaluation of Medical Knowledge requirement of the MOC program. Two modules will be presented: Internal Medicine-Office Based and Internal Medicine-Hospital Based.
“There’s an updated module,” says Geri Barnes, SHM senior director of education and meetings. “(Attendees) should get really good information there.”
Also new this year is a series of interactive workshops SHM launched to provide topic-specific information from experts. Spread over three days, the 18 workshops include “Improving Care for the Hospitalized Elder,” “Ultrasound Imaging Skills for Invasive Beside Procedures,” and “Blood Management: Hospitalists in a New Role.”
The workshops, which are limited to 100 participants, are an attempt to engage a new class of HM devotees by recruiting a new class of speakers and leaders. “In previous years, we were not getting all our membership involved with the annual meeting,” Dr. Jaffer says. “We were really handpicking our speakers on experience, expertise, and how well they were known in their field and how good of speakers they were. This year, we really reached out.”
HM10 also features “special-interest forums” at 4:45 p.m. Friday, April 9. The informal sessions—on 18 topics ranging from “Information Technology” to “Women in HM”—afford hospitalists direct access to SHM board members, committee members, and staff. Barnes says the information gathered in last year’s sessions was discussed by SHM. For instance, the “Early Career Hospitalist” forum was the inspiration for one of the new pre-courses.
“We’re not real good at getting that message across, but I would say that those (forums) are extremely valuable,” Barnes says. “The same thing goes for the town hall (1 p.m. Sunday, April 11). That’s an opportunity to meet with the executive committee and board members, and ask them anything you want to.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
HM10 PREVIEW: Divide & Conquer
The National Association of Inpatient Physicians (NAIP)—now known as SHM—first held an annual meeting in the spring of 1998 in San Diego. Some 100 hospitalists attended the largest gathering of the nascent field. A little more than a decade later, 2,000-plus hospitalists crowded together in Chicago for HM09.
That exponential growth is tied to a host of factors, mostly the swelling ranks of hospitalists. But in terms of conventioneers, the year-over-year spikes in attendance mean that more and more first-timers are attending SHM’s annual gathering. In fact, SHM estimates that 30% of attendees are first-timers.
“Over the last five years, we’ve seen a significant increase in attendance over each year, with the culmination being that 2009 sold out,” says Geri Barnes, SHM senior director of education and meetings. “There are those who return year after year, and new members. It’s primarily new members.”
The growth in the annual meeting attendance confirms that HM is growing in numbers and influence. More and more physicians are making HM a career, and the annual meeting is the perfect place to learn more about the specialty and all it has to offer.
“Sometimes new physicians become hospitalists thinking that it might be a transition time for them. By going to these meetings, they can really cement in their minds the excitement and enthusiasm for being a hospital medicine physician,” says Brian Bossard, MD, FACP, FHM, founder of Inpatient Physicians Associations, a Lincoln, Neb.-based operator of three HM groups. “If they were thinking about transitioning out of HM into a fellowship, this might really encourage them to think twice about that.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
A four-day conference with more than 90 educational sessions can be intimidating to the first-time attendee. How does a rookie navigate all that “excitement and enthusiasm”? Which sessions does one attend? How does a young hospitalist get matched up with a mentor? What should a veteran hospitalist be learning that they can relay to their colleagues back home?
“First and foremost, they get to interact with colleagues from all over the country, as well as we now have hospitalists from other parts of the world actually coming to our annual meeting,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “So one is the ability to interact and talk about the fast-changing field of hospital medicine. Two is that they get to learn about both clinical topics as well as other areas in HM that are more practical, either related to operations, quality improvement, patient safety, research.
“The third thing is that you get a chance to present your work, whether you’re a researcher, a QI guru, [or] you’re an educator. You get a chance to present your work in the form of a poster or an oral presentation. Fourth, you also get a chance to see a lot of the work that our colleagues from all across the country and the world are doing in HM to actually change it.”
The key to extracting all that information is preparation, according to those who have attended the meeting in the past. That includes checking out the pre-course schedule online, prioritizing meetings that relate to one’s day-to-day responsibilities, picking the educational tracks that appeal most, and taking advantage of the exhibit hall, which runs the length of the conference.
Femi Adewunmi, MD, MBA, FHM, has been to five SHM annual meetings, and has attended three pre-courses in that time. As medical director of the hospitalist service at Johnston Medical Center in Smithfield, N.C., he has found the learning sessions on practice management and billing and coding to be the most helpful. He recommends both to first-timers looking to accumulate real-world tips they can apply to their HM practices.
There is “a constant battle that we face trying to justify why you’re asking for more resources,” Dr. Adewunmi says. “To be able to do that convincingly, you need to be able to demonstrate your worth. … For people who have never gone to any of the pre-courses, any of them are a great tool. The amount of knowledge you come away with is pretty phenomenal. You’re given the little nuggets you need to do whatever you need to do.”
Dr. Bossard, a member of Team Hospitalist, estimates he’s been to 10 of the 13 annual meetings. He usually travels with colleagues and makes sure to coordinate educational tracks before the conference begins so that the group avoids redundancy by splitting up sessions.
“Divide and conquer,” Dr. Bossard adds. “We always bring it back to our group in smaller, bullet-type fashion. We all get a taste of sessions we weren’t able to attend.”
Barnes agrees that planning ahead is the key to success. “Do it based on what your primary role is,” she says. “Is your primary role research? Are you an academic hospitalist? Do you have an important role leading quality initiatives? Are you a group leader? At the end of the day, you can follow a track all the way down or you can jump across tracks—whatever is appealing.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
The National Association of Inpatient Physicians (NAIP)—now known as SHM—first held an annual meeting in the spring of 1998 in San Diego. Some 100 hospitalists attended the largest gathering of the nascent field. A little more than a decade later, 2,000-plus hospitalists crowded together in Chicago for HM09.
That exponential growth is tied to a host of factors, mostly the swelling ranks of hospitalists. But in terms of conventioneers, the year-over-year spikes in attendance mean that more and more first-timers are attending SHM’s annual gathering. In fact, SHM estimates that 30% of attendees are first-timers.
“Over the last five years, we’ve seen a significant increase in attendance over each year, with the culmination being that 2009 sold out,” says Geri Barnes, SHM senior director of education and meetings. “There are those who return year after year, and new members. It’s primarily new members.”
The growth in the annual meeting attendance confirms that HM is growing in numbers and influence. More and more physicians are making HM a career, and the annual meeting is the perfect place to learn more about the specialty and all it has to offer.
“Sometimes new physicians become hospitalists thinking that it might be a transition time for them. By going to these meetings, they can really cement in their minds the excitement and enthusiasm for being a hospital medicine physician,” says Brian Bossard, MD, FACP, FHM, founder of Inpatient Physicians Associations, a Lincoln, Neb.-based operator of three HM groups. “If they were thinking about transitioning out of HM into a fellowship, this might really encourage them to think twice about that.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
A four-day conference with more than 90 educational sessions can be intimidating to the first-time attendee. How does a rookie navigate all that “excitement and enthusiasm”? Which sessions does one attend? How does a young hospitalist get matched up with a mentor? What should a veteran hospitalist be learning that they can relay to their colleagues back home?
“First and foremost, they get to interact with colleagues from all over the country, as well as we now have hospitalists from other parts of the world actually coming to our annual meeting,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “So one is the ability to interact and talk about the fast-changing field of hospital medicine. Two is that they get to learn about both clinical topics as well as other areas in HM that are more practical, either related to operations, quality improvement, patient safety, research.
“The third thing is that you get a chance to present your work, whether you’re a researcher, a QI guru, [or] you’re an educator. You get a chance to present your work in the form of a poster or an oral presentation. Fourth, you also get a chance to see a lot of the work that our colleagues from all across the country and the world are doing in HM to actually change it.”
The key to extracting all that information is preparation, according to those who have attended the meeting in the past. That includes checking out the pre-course schedule online, prioritizing meetings that relate to one’s day-to-day responsibilities, picking the educational tracks that appeal most, and taking advantage of the exhibit hall, which runs the length of the conference.
Femi Adewunmi, MD, MBA, FHM, has been to five SHM annual meetings, and has attended three pre-courses in that time. As medical director of the hospitalist service at Johnston Medical Center in Smithfield, N.C., he has found the learning sessions on practice management and billing and coding to be the most helpful. He recommends both to first-timers looking to accumulate real-world tips they can apply to their HM practices.
There is “a constant battle that we face trying to justify why you’re asking for more resources,” Dr. Adewunmi says. “To be able to do that convincingly, you need to be able to demonstrate your worth. … For people who have never gone to any of the pre-courses, any of them are a great tool. The amount of knowledge you come away with is pretty phenomenal. You’re given the little nuggets you need to do whatever you need to do.”
Dr. Bossard, a member of Team Hospitalist, estimates he’s been to 10 of the 13 annual meetings. He usually travels with colleagues and makes sure to coordinate educational tracks before the conference begins so that the group avoids redundancy by splitting up sessions.
“Divide and conquer,” Dr. Bossard adds. “We always bring it back to our group in smaller, bullet-type fashion. We all get a taste of sessions we weren’t able to attend.”
Barnes agrees that planning ahead is the key to success. “Do it based on what your primary role is,” she says. “Is your primary role research? Are you an academic hospitalist? Do you have an important role leading quality initiatives? Are you a group leader? At the end of the day, you can follow a track all the way down or you can jump across tracks—whatever is appealing.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
The National Association of Inpatient Physicians (NAIP)—now known as SHM—first held an annual meeting in the spring of 1998 in San Diego. Some 100 hospitalists attended the largest gathering of the nascent field. A little more than a decade later, 2,000-plus hospitalists crowded together in Chicago for HM09.
That exponential growth is tied to a host of factors, mostly the swelling ranks of hospitalists. But in terms of conventioneers, the year-over-year spikes in attendance mean that more and more first-timers are attending SHM’s annual gathering. In fact, SHM estimates that 30% of attendees are first-timers.
“Over the last five years, we’ve seen a significant increase in attendance over each year, with the culmination being that 2009 sold out,” says Geri Barnes, SHM senior director of education and meetings. “There are those who return year after year, and new members. It’s primarily new members.”
The growth in the annual meeting attendance confirms that HM is growing in numbers and influence. More and more physicians are making HM a career, and the annual meeting is the perfect place to learn more about the specialty and all it has to offer.
“Sometimes new physicians become hospitalists thinking that it might be a transition time for them. By going to these meetings, they can really cement in their minds the excitement and enthusiasm for being a hospital medicine physician,” says Brian Bossard, MD, FACP, FHM, founder of Inpatient Physicians Associations, a Lincoln, Neb.-based operator of three HM groups. “If they were thinking about transitioning out of HM into a fellowship, this might really encourage them to think twice about that.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
A four-day conference with more than 90 educational sessions can be intimidating to the first-time attendee. How does a rookie navigate all that “excitement and enthusiasm”? Which sessions does one attend? How does a young hospitalist get matched up with a mentor? What should a veteran hospitalist be learning that they can relay to their colleagues back home?
“First and foremost, they get to interact with colleagues from all over the country, as well as we now have hospitalists from other parts of the world actually coming to our annual meeting,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “So one is the ability to interact and talk about the fast-changing field of hospital medicine. Two is that they get to learn about both clinical topics as well as other areas in HM that are more practical, either related to operations, quality improvement, patient safety, research.
“The third thing is that you get a chance to present your work, whether you’re a researcher, a QI guru, [or] you’re an educator. You get a chance to present your work in the form of a poster or an oral presentation. Fourth, you also get a chance to see a lot of the work that our colleagues from all across the country and the world are doing in HM to actually change it.”
The key to extracting all that information is preparation, according to those who have attended the meeting in the past. That includes checking out the pre-course schedule online, prioritizing meetings that relate to one’s day-to-day responsibilities, picking the educational tracks that appeal most, and taking advantage of the exhibit hall, which runs the length of the conference.
Femi Adewunmi, MD, MBA, FHM, has been to five SHM annual meetings, and has attended three pre-courses in that time. As medical director of the hospitalist service at Johnston Medical Center in Smithfield, N.C., he has found the learning sessions on practice management and billing and coding to be the most helpful. He recommends both to first-timers looking to accumulate real-world tips they can apply to their HM practices.
There is “a constant battle that we face trying to justify why you’re asking for more resources,” Dr. Adewunmi says. “To be able to do that convincingly, you need to be able to demonstrate your worth. … For people who have never gone to any of the pre-courses, any of them are a great tool. The amount of knowledge you come away with is pretty phenomenal. You’re given the little nuggets you need to do whatever you need to do.”
Dr. Bossard, a member of Team Hospitalist, estimates he’s been to 10 of the 13 annual meetings. He usually travels with colleagues and makes sure to coordinate educational tracks before the conference begins so that the group avoids redundancy by splitting up sessions.
“Divide and conquer,” Dr. Bossard adds. “We always bring it back to our group in smaller, bullet-type fashion. We all get a taste of sessions we weren’t able to attend.”
Barnes agrees that planning ahead is the key to success. “Do it based on what your primary role is,” she says. “Is your primary role research? Are you an academic hospitalist? Do you have an important role leading quality initiatives? Are you a group leader? At the end of the day, you can follow a track all the way down or you can jump across tracks—whatever is appealing.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Hospital-Acquired Conditions & The Hospitalist
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
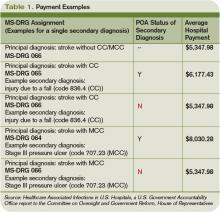
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
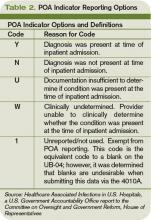
POA Indicators
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
POA Indicators
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
POA Indicators
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
HM10 PREVIEW: Center Stage
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Perioperative Medicine Summit 2010
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Bone and Soft-Tissue Sarcomas
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Clinical presentation and imaging of bone and soft-tissue sarcomas
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.