User login
Hand‐Carried Ultrasound Use
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
Hospitalist Physician Leadership Skills
Physicians assume myriad leadership roles within medical institutions. Clinically‐oriented leadership roles can range from managing a small group of providers, to leading entire health systems, to heading up national quality improvement initiatives. While often competent in the practice of medicine, many physicians have not pursued structured management or administrative training. In a survey of Medicine Department Chairs at academic medical centers, none had advanced management degrees despite spending an average of 55% of their time on administrative duties. It is not uncommon for physicians to attend leadership development programs or management seminars, as evidenced by the increasing demand for education.1 Various methods for skill enhancement have been described24; however, the most effective approaches have yet to be determined.
Miller and Dollard5 and Bandura6, 7 have explained that behavioral contracts have evolved from social cognitive theory principles. These contracts are formal written agreements, often negotiated between 2 individuals, to facilitate behavior change. Typically, they involve a clear definition of expected behaviors with specific consequences (usually positive reinforcement).810 Their use in modifying physician behavior, particularly those related to leadership, has not been studied.
Hospitalist physicians represent the fastest growing specialty in the United States.11, 12 Among other responsibilities, they have taken on roles as leaders in hospital administration, education, quality improvement, and public health.1315 The Society of Hospital Medicine (SHM), the largest US organization committed to the practice of hospital medicine,16 has established Leadership Academies to prepare hospitalists for these duties. The goal of this study was to assess how hospitalist physicians' commitment to grow as leaders was expressed using behavioral contacts as a vehicle to clarify their intentions and whether behavioral change occurred over time.
Methods
Study Design
A qualitative study design was selected to explore how current and future hospitalist leaders planned to modify their behaviors after participating in a hospitalist leadership training course. Participants were encouraged to complete a behavioral contract highlighting their personal goals.
Approximately 12 months later, follow‐up data were collected. Participants were sent copies of their behavioral contracts and surveyed about the extent to which they have realized their personal goals.
Subjects
Hospitalist leaders participating in the 4‐day level I or II leadership courses of the SHM Leadership Academy were studied.
Data Collection
In the final sessions of the 2007‐2008 Leadership Academy courses, participants completed an optional behavioral contract exercise in which they partnered with a colleague and were asked to identify 4 action plans they intended to implement upon their return home. These were written down and signed. Selected demographic information was also collected.
Follow‐up surveys were sent by mail and electronically to a subset of participants with completed behavioral contracts. A 5‐point Likert scale (strongly agree . . . strongly disagree) was used to assess the extent of adherence to the goals listed in the behavioral contracts.
Data Analysis
Transcripts were analyzed using an editing organizing style, a qualitative analysis technique to find meaningful units or segments of text that both stand on their own and relate to the purpose of the study.12 With this method, the coding template emerges from the data. Two investigators independently analyzed the transcripts and created a coding template based on common themes identified among the participants. In cases of discrepant coding, the 2 investigators had discussions to reach consensus. The authors agreed on representative quotes for each theme. Triangulation was established through sharing results of the analysis with a subset of participants.
Follow‐up survey data was summarized descriptively showing proportion data.
Results
Response Rate and Participant Demographics
Out of 264 people who completed the course, 120 decided to participate in the optional behavioral contract exercise. The median age of participants was 38 years (Table 1). The majority were male (84; 70.0%), and hospitalist leaders (76; 63.3%). The median time in practice as a hospitalist was 4 years. Fewer than one‐half held an academic appointment (40; 33.3%) with most being at the rank of Assistant Professor (14; 11.7%). Most of the participants worked in a private hospital (80; 66.7%).
| Characteristic | |
|---|---|
| |
| Age in years [median (SD)] | 38 (8) |
| Male [n (%)] | 84 (70.0) |
| Years in practice as hospitalist [median (SD)] | 4 (13) |
| Leader of hospitalist program [n (%)] | 76 (63.3) |
| Academic affiliation [n (%)] | 40 (33.3) |
| Academic rank [n (%)] | |
| Instructor | 9 (7.5) |
| Assistant professor | 14 (11.7) |
| Associate professor | 13 (10.8) |
| Hospital type [n (%)] | |
| Private | 80 (66.7) |
| University | 15 (12.5) |
| Government | 2 (1.7) |
| Veterans administration | 0 (0.0) |
| Other | 1 (0.1) |
Results of Qualitative Analysis of Behavioral Contracts
From the analyses of the behavioral contracts, themes emerged related to ways in which participants hoped to develop and improve. The themes and the frequencies with which they were recorded in the behavioral contracts are shown in Table 2.
| Theme | Total Number of Times Theme Mentioned in All Behavioral Contracts | Number of Respondents Referring to Theme [n (%)] |
|---|---|---|
| ||
| Improving communication and interpersonal skills | 132 | 70 (58.3) |
| Refinement of vision, goals, and strategic planning | 115 | 62 (51.7) |
| Improve intrapersonal development | 65 | 36 (30.0) |
| Enhance negotiation skills | 65 | 44 (36.7) |
| Commit to organizational change | 53 | 32 (26.7) |
| Understanding business drivers | 38 | 28 (23.3) |
| Setting performance and clinical metrics | 34 | 26 (21.7) |
| Strengthen interdepartmental relations | 32 | 26 (21.7) |
Improving Communication and Interpersonal Skills
A desire to improve communication and listening skills, particularly in the context of conflict resolution, was mentioned repeatedly. Heightened awareness about different personality types to allow for improved interpersonal relationships was another concept that was emphasized.
One female Instructor from an academic medical center described her intentions:
I will try to do a better job at assessing the behavioral tendencies of my partners and adjust my own style for more effective communication.
Refinement of Vision, Goals, and Strategic Planning
Physicians were committed to returning to their home institutions and embarking on initiatives to advance vision and goals of their groups within the context of strategic planning. Participants were interested in creating hospitalist‐specific mission statements, developing specific goals that take advantage of strengths and opportunities while minimizing internal weaknesses and considering external threats. They described wanting to align the interests of members of their hospitalist groups around a common goal.
A female hospitalist leader in private practice wished to:
Clearly define a group vision and commit to re‐evaluation on a regular basis to ensure we are on track . . . and conduct a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis to set future goals.
Improve Intrapersonal Development
Participants expressed desire to improve their leadership skills. Proposed goals included: (1) recognizing their weaknesses and soliciting feedback from colleagues, (2) minimizing emotional response to stress, (3) sharing their knowledge and skills for the benefit of peers, (4) delegating work more effectively to others, (5) reading suggested books on leadership, (6) serving as a positive role model and mentor, and (7) managing meetings and difficult coworkers more skillfully.
One female Assistant Professor from an academic medical center outlined:
I want to be able to: (1) manage up better and effectively negotiate with the administration on behalf of my group; (2) become better at leadership skills by using the tools offered at the Academy; and (3) effectively support my group members to develop their skills to become successful in their chosen niches. I will . . . improve the poor morale in my group.
Enhance Negotiation Skills
Many physician leaders identified negotiation principles and techniques as foundations for improvement for interactions within their own groups, as well as with the hospital administration.
A male private hospitalist leader working for 4 years as a hospitalist described plans to utilize negotiation skills within and outside the group:
Negotiate with my team of hospitalists to make them more compliant with the rules and regulations of the group, and negotiate an excellent contract with hospital administration. . . .
Commit to Organizational Change
The hospitalist respondents described their ability to influence organizational change given their unique position at the interface between patient care delivery and hospital administration. To realize organizational change, commonly cited ideas included recruitment and retention of clinically excellent practitioners, and developing standard protocols to facilitate quality improvement initiatives.
A male Instructor of Medicine listed select areas in which to become more involved:
Participation with the Chief Executive Officer of the company in quality improvement projects, calls to the primary care practitioners upon discharge, and the handoff process.
Other Themes
The final 3 themes included are: understanding business drivers; the establishment of better metrics to assess performance; and the strengthening of interdepartmental relations.
Follow‐up Data About Adherence to Plans Delineated in Behavioral Contracts
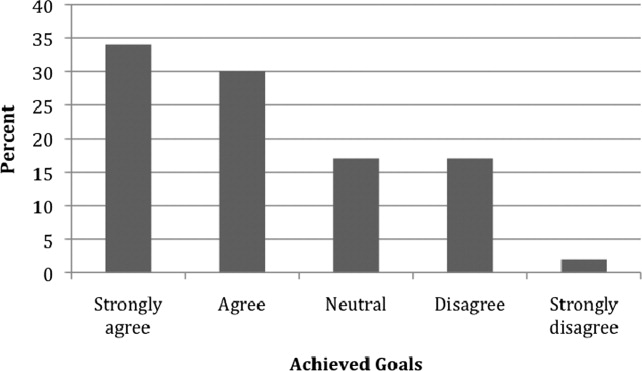
Out of 65 completed behavioral contracts from the 2007 Level I participants, 32 returned a follow‐up survey (response rate 49.3%). Figure 1 shows the extent to which respondents believed that they were compliant with their proposed plans for change or improvement. Degree of adherence was displayed as a proportion of total goals. Out of those who returned a follow‐up survey, all but 1 respondent either strongly agreed or agreed that they adhered to at least one of their goals (96.9%).

Select representative comments that illustrate the physicians' appreciation of using behavioral contracts include:
my approach to problems is a bit more analytical.
simple changes in how I approach people and interact with them has greatly improved my skills as a leader and allowed me to accomplish my goals with much less effort.
Discussion
Through the qualitative analysis of the behavioral contracts completed by participants of a Leadership Academy for hospitalists, we characterized the ways that hospitalist practitioners hoped to evolve as leaders. The major themes that emerged relate not only to their own growth and development but also their pledge to advance the success of the group or division. The level of commitment and impact of the behavioral contracts appear to be reinforced by an overwhelmingly positive response to adherence to personal goals one year after course participation. Communication and interpersonal development were most frequently cited in the behavioral contracts as areas for which the hospitalist leaders acknowledged a desire to grow. In a study of academic department of medicine chairs, communication skills were identified as being vital for effective leadership.3 The Chairs also recognized other proficiencies required for leading that were consistent with those outlined in the behavioral contracts: strategic planning, change management, team building, personnel management, and systems thinking. McDade et al.17 examined the effects of participation in an executive leadership program developed for female academic faculty in medical and dental schools in the United States and Canada. They noted increased self‐assessed leadership capabilities at 18 months after attending the program, across 10 leadership constructs taught in the classes. These leadership constructs resonate with the themes found in the plans for change described by our informants.
Hospitalists are assuming leadership roles in an increasing number and with greater scope; however, until now their perspectives on what skill sets are required to be successful have not been well documented. Significant time, effort, and money are invested into the development of hospitalists as leaders.4 The behavioral contract appears to be a tool acceptable to hospitalist physicians; perhaps it can be used as part annual reviews with hospitalists aspiring to be leaders.
Several limitations of the study shall be considered. First, not all participants attending the Leadership Academy opted to fill out the behavioral contracts. Second, this qualitative study is limited to those practitioners who are genuinely interested in growing as leaders as evidenced by their willingness to invest in going to the course. Third, follow‐up surveys relied on self‐assessment and it is not known whether actual realization of these goals occurred or the extent to which behavioral contracts were responsible. Further, follow‐up data were only completed by 49% percent of those targeted. However, hospitalists may be fairly resistant to being surveyed as evidenced by the fact that SHM's 2005‐2006 membership survey yielded a response rate of only 26%.18 Finally, many of the thematic goals were described by fewer than 50% of informants. However, it is important to note that the elements included on each person's behavioral contract emerged spontaneously. If subjects were specifically asked about each theme, the number of comments related to each would certainly be much higher. Qualitative analysis does not really allow us to know whether one theme is more important than another merely because it was mentioned more frequently.
Hospitalist leaders appear to be committed to professional growth and they have reported realization of goals delineated in their behavioral contracts. While varied methods are being used as part of physician leadership training programs, behavioral contracts may enhance promise for change.
Acknowledgements
The authors thank Regina Hess for assistance in data preparation and Laurence Wellikson, MD, FHM, Russell Holman, MD and Erica Pearson (all from the SHM) for data collection.
Physicians assume myriad leadership roles within medical institutions. Clinically‐oriented leadership roles can range from managing a small group of providers, to leading entire health systems, to heading up national quality improvement initiatives. While often competent in the practice of medicine, many physicians have not pursued structured management or administrative training. In a survey of Medicine Department Chairs at academic medical centers, none had advanced management degrees despite spending an average of 55% of their time on administrative duties. It is not uncommon for physicians to attend leadership development programs or management seminars, as evidenced by the increasing demand for education.1 Various methods for skill enhancement have been described24; however, the most effective approaches have yet to be determined.
Miller and Dollard5 and Bandura6, 7 have explained that behavioral contracts have evolved from social cognitive theory principles. These contracts are formal written agreements, often negotiated between 2 individuals, to facilitate behavior change. Typically, they involve a clear definition of expected behaviors with specific consequences (usually positive reinforcement).810 Their use in modifying physician behavior, particularly those related to leadership, has not been studied.
Hospitalist physicians represent the fastest growing specialty in the United States.11, 12 Among other responsibilities, they have taken on roles as leaders in hospital administration, education, quality improvement, and public health.1315 The Society of Hospital Medicine (SHM), the largest US organization committed to the practice of hospital medicine,16 has established Leadership Academies to prepare hospitalists for these duties. The goal of this study was to assess how hospitalist physicians' commitment to grow as leaders was expressed using behavioral contacts as a vehicle to clarify their intentions and whether behavioral change occurred over time.
Methods
Study Design
A qualitative study design was selected to explore how current and future hospitalist leaders planned to modify their behaviors after participating in a hospitalist leadership training course. Participants were encouraged to complete a behavioral contract highlighting their personal goals.
Approximately 12 months later, follow‐up data were collected. Participants were sent copies of their behavioral contracts and surveyed about the extent to which they have realized their personal goals.
Subjects
Hospitalist leaders participating in the 4‐day level I or II leadership courses of the SHM Leadership Academy were studied.
Data Collection
In the final sessions of the 2007‐2008 Leadership Academy courses, participants completed an optional behavioral contract exercise in which they partnered with a colleague and were asked to identify 4 action plans they intended to implement upon their return home. These were written down and signed. Selected demographic information was also collected.
Follow‐up surveys were sent by mail and electronically to a subset of participants with completed behavioral contracts. A 5‐point Likert scale (strongly agree . . . strongly disagree) was used to assess the extent of adherence to the goals listed in the behavioral contracts.
Data Analysis
Transcripts were analyzed using an editing organizing style, a qualitative analysis technique to find meaningful units or segments of text that both stand on their own and relate to the purpose of the study.12 With this method, the coding template emerges from the data. Two investigators independently analyzed the transcripts and created a coding template based on common themes identified among the participants. In cases of discrepant coding, the 2 investigators had discussions to reach consensus. The authors agreed on representative quotes for each theme. Triangulation was established through sharing results of the analysis with a subset of participants.
Follow‐up survey data was summarized descriptively showing proportion data.
Results
Response Rate and Participant Demographics
Out of 264 people who completed the course, 120 decided to participate in the optional behavioral contract exercise. The median age of participants was 38 years (Table 1). The majority were male (84; 70.0%), and hospitalist leaders (76; 63.3%). The median time in practice as a hospitalist was 4 years. Fewer than one‐half held an academic appointment (40; 33.3%) with most being at the rank of Assistant Professor (14; 11.7%). Most of the participants worked in a private hospital (80; 66.7%).
| Characteristic | |
|---|---|
| |
| Age in years [median (SD)] | 38 (8) |
| Male [n (%)] | 84 (70.0) |
| Years in practice as hospitalist [median (SD)] | 4 (13) |
| Leader of hospitalist program [n (%)] | 76 (63.3) |
| Academic affiliation [n (%)] | 40 (33.3) |
| Academic rank [n (%)] | |
| Instructor | 9 (7.5) |
| Assistant professor | 14 (11.7) |
| Associate professor | 13 (10.8) |
| Hospital type [n (%)] | |
| Private | 80 (66.7) |
| University | 15 (12.5) |
| Government | 2 (1.7) |
| Veterans administration | 0 (0.0) |
| Other | 1 (0.1) |
Results of Qualitative Analysis of Behavioral Contracts
From the analyses of the behavioral contracts, themes emerged related to ways in which participants hoped to develop and improve. The themes and the frequencies with which they were recorded in the behavioral contracts are shown in Table 2.
| Theme | Total Number of Times Theme Mentioned in All Behavioral Contracts | Number of Respondents Referring to Theme [n (%)] |
|---|---|---|
| ||
| Improving communication and interpersonal skills | 132 | 70 (58.3) |
| Refinement of vision, goals, and strategic planning | 115 | 62 (51.7) |
| Improve intrapersonal development | 65 | 36 (30.0) |
| Enhance negotiation skills | 65 | 44 (36.7) |
| Commit to organizational change | 53 | 32 (26.7) |
| Understanding business drivers | 38 | 28 (23.3) |
| Setting performance and clinical metrics | 34 | 26 (21.7) |
| Strengthen interdepartmental relations | 32 | 26 (21.7) |
Improving Communication and Interpersonal Skills
A desire to improve communication and listening skills, particularly in the context of conflict resolution, was mentioned repeatedly. Heightened awareness about different personality types to allow for improved interpersonal relationships was another concept that was emphasized.
One female Instructor from an academic medical center described her intentions:
I will try to do a better job at assessing the behavioral tendencies of my partners and adjust my own style for more effective communication.
Refinement of Vision, Goals, and Strategic Planning
Physicians were committed to returning to their home institutions and embarking on initiatives to advance vision and goals of their groups within the context of strategic planning. Participants were interested in creating hospitalist‐specific mission statements, developing specific goals that take advantage of strengths and opportunities while minimizing internal weaknesses and considering external threats. They described wanting to align the interests of members of their hospitalist groups around a common goal.
A female hospitalist leader in private practice wished to:
Clearly define a group vision and commit to re‐evaluation on a regular basis to ensure we are on track . . . and conduct a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis to set future goals.
Improve Intrapersonal Development
Participants expressed desire to improve their leadership skills. Proposed goals included: (1) recognizing their weaknesses and soliciting feedback from colleagues, (2) minimizing emotional response to stress, (3) sharing their knowledge and skills for the benefit of peers, (4) delegating work more effectively to others, (5) reading suggested books on leadership, (6) serving as a positive role model and mentor, and (7) managing meetings and difficult coworkers more skillfully.
One female Assistant Professor from an academic medical center outlined:
I want to be able to: (1) manage up better and effectively negotiate with the administration on behalf of my group; (2) become better at leadership skills by using the tools offered at the Academy; and (3) effectively support my group members to develop their skills to become successful in their chosen niches. I will . . . improve the poor morale in my group.
Enhance Negotiation Skills
Many physician leaders identified negotiation principles and techniques as foundations for improvement for interactions within their own groups, as well as with the hospital administration.
A male private hospitalist leader working for 4 years as a hospitalist described plans to utilize negotiation skills within and outside the group:
Negotiate with my team of hospitalists to make them more compliant with the rules and regulations of the group, and negotiate an excellent contract with hospital administration. . . .
Commit to Organizational Change
The hospitalist respondents described their ability to influence organizational change given their unique position at the interface between patient care delivery and hospital administration. To realize organizational change, commonly cited ideas included recruitment and retention of clinically excellent practitioners, and developing standard protocols to facilitate quality improvement initiatives.
A male Instructor of Medicine listed select areas in which to become more involved:
Participation with the Chief Executive Officer of the company in quality improvement projects, calls to the primary care practitioners upon discharge, and the handoff process.
Other Themes
The final 3 themes included are: understanding business drivers; the establishment of better metrics to assess performance; and the strengthening of interdepartmental relations.
Follow‐up Data About Adherence to Plans Delineated in Behavioral Contracts
Out of 65 completed behavioral contracts from the 2007 Level I participants, 32 returned a follow‐up survey (response rate 49.3%). Figure 1 shows the extent to which respondents believed that they were compliant with their proposed plans for change or improvement. Degree of adherence was displayed as a proportion of total goals. Out of those who returned a follow‐up survey, all but 1 respondent either strongly agreed or agreed that they adhered to at least one of their goals (96.9%).

Select representative comments that illustrate the physicians' appreciation of using behavioral contracts include:
my approach to problems is a bit more analytical.
simple changes in how I approach people and interact with them has greatly improved my skills as a leader and allowed me to accomplish my goals with much less effort.
Discussion
Through the qualitative analysis of the behavioral contracts completed by participants of a Leadership Academy for hospitalists, we characterized the ways that hospitalist practitioners hoped to evolve as leaders. The major themes that emerged relate not only to their own growth and development but also their pledge to advance the success of the group or division. The level of commitment and impact of the behavioral contracts appear to be reinforced by an overwhelmingly positive response to adherence to personal goals one year after course participation. Communication and interpersonal development were most frequently cited in the behavioral contracts as areas for which the hospitalist leaders acknowledged a desire to grow. In a study of academic department of medicine chairs, communication skills were identified as being vital for effective leadership.3 The Chairs also recognized other proficiencies required for leading that were consistent with those outlined in the behavioral contracts: strategic planning, change management, team building, personnel management, and systems thinking. McDade et al.17 examined the effects of participation in an executive leadership program developed for female academic faculty in medical and dental schools in the United States and Canada. They noted increased self‐assessed leadership capabilities at 18 months after attending the program, across 10 leadership constructs taught in the classes. These leadership constructs resonate with the themes found in the plans for change described by our informants.
Hospitalists are assuming leadership roles in an increasing number and with greater scope; however, until now their perspectives on what skill sets are required to be successful have not been well documented. Significant time, effort, and money are invested into the development of hospitalists as leaders.4 The behavioral contract appears to be a tool acceptable to hospitalist physicians; perhaps it can be used as part annual reviews with hospitalists aspiring to be leaders.
Several limitations of the study shall be considered. First, not all participants attending the Leadership Academy opted to fill out the behavioral contracts. Second, this qualitative study is limited to those practitioners who are genuinely interested in growing as leaders as evidenced by their willingness to invest in going to the course. Third, follow‐up surveys relied on self‐assessment and it is not known whether actual realization of these goals occurred or the extent to which behavioral contracts were responsible. Further, follow‐up data were only completed by 49% percent of those targeted. However, hospitalists may be fairly resistant to being surveyed as evidenced by the fact that SHM's 2005‐2006 membership survey yielded a response rate of only 26%.18 Finally, many of the thematic goals were described by fewer than 50% of informants. However, it is important to note that the elements included on each person's behavioral contract emerged spontaneously. If subjects were specifically asked about each theme, the number of comments related to each would certainly be much higher. Qualitative analysis does not really allow us to know whether one theme is more important than another merely because it was mentioned more frequently.
Hospitalist leaders appear to be committed to professional growth and they have reported realization of goals delineated in their behavioral contracts. While varied methods are being used as part of physician leadership training programs, behavioral contracts may enhance promise for change.
Acknowledgements
The authors thank Regina Hess for assistance in data preparation and Laurence Wellikson, MD, FHM, Russell Holman, MD and Erica Pearson (all from the SHM) for data collection.
Physicians assume myriad leadership roles within medical institutions. Clinically‐oriented leadership roles can range from managing a small group of providers, to leading entire health systems, to heading up national quality improvement initiatives. While often competent in the practice of medicine, many physicians have not pursued structured management or administrative training. In a survey of Medicine Department Chairs at academic medical centers, none had advanced management degrees despite spending an average of 55% of their time on administrative duties. It is not uncommon for physicians to attend leadership development programs or management seminars, as evidenced by the increasing demand for education.1 Various methods for skill enhancement have been described24; however, the most effective approaches have yet to be determined.
Miller and Dollard5 and Bandura6, 7 have explained that behavioral contracts have evolved from social cognitive theory principles. These contracts are formal written agreements, often negotiated between 2 individuals, to facilitate behavior change. Typically, they involve a clear definition of expected behaviors with specific consequences (usually positive reinforcement).810 Their use in modifying physician behavior, particularly those related to leadership, has not been studied.
Hospitalist physicians represent the fastest growing specialty in the United States.11, 12 Among other responsibilities, they have taken on roles as leaders in hospital administration, education, quality improvement, and public health.1315 The Society of Hospital Medicine (SHM), the largest US organization committed to the practice of hospital medicine,16 has established Leadership Academies to prepare hospitalists for these duties. The goal of this study was to assess how hospitalist physicians' commitment to grow as leaders was expressed using behavioral contacts as a vehicle to clarify their intentions and whether behavioral change occurred over time.
Methods
Study Design
A qualitative study design was selected to explore how current and future hospitalist leaders planned to modify their behaviors after participating in a hospitalist leadership training course. Participants were encouraged to complete a behavioral contract highlighting their personal goals.
Approximately 12 months later, follow‐up data were collected. Participants were sent copies of their behavioral contracts and surveyed about the extent to which they have realized their personal goals.
Subjects
Hospitalist leaders participating in the 4‐day level I or II leadership courses of the SHM Leadership Academy were studied.
Data Collection
In the final sessions of the 2007‐2008 Leadership Academy courses, participants completed an optional behavioral contract exercise in which they partnered with a colleague and were asked to identify 4 action plans they intended to implement upon their return home. These were written down and signed. Selected demographic information was also collected.
Follow‐up surveys were sent by mail and electronically to a subset of participants with completed behavioral contracts. A 5‐point Likert scale (strongly agree . . . strongly disagree) was used to assess the extent of adherence to the goals listed in the behavioral contracts.
Data Analysis
Transcripts were analyzed using an editing organizing style, a qualitative analysis technique to find meaningful units or segments of text that both stand on their own and relate to the purpose of the study.12 With this method, the coding template emerges from the data. Two investigators independently analyzed the transcripts and created a coding template based on common themes identified among the participants. In cases of discrepant coding, the 2 investigators had discussions to reach consensus. The authors agreed on representative quotes for each theme. Triangulation was established through sharing results of the analysis with a subset of participants.
Follow‐up survey data was summarized descriptively showing proportion data.
Results
Response Rate and Participant Demographics
Out of 264 people who completed the course, 120 decided to participate in the optional behavioral contract exercise. The median age of participants was 38 years (Table 1). The majority were male (84; 70.0%), and hospitalist leaders (76; 63.3%). The median time in practice as a hospitalist was 4 years. Fewer than one‐half held an academic appointment (40; 33.3%) with most being at the rank of Assistant Professor (14; 11.7%). Most of the participants worked in a private hospital (80; 66.7%).
| Characteristic | |
|---|---|
| |
| Age in years [median (SD)] | 38 (8) |
| Male [n (%)] | 84 (70.0) |
| Years in practice as hospitalist [median (SD)] | 4 (13) |
| Leader of hospitalist program [n (%)] | 76 (63.3) |
| Academic affiliation [n (%)] | 40 (33.3) |
| Academic rank [n (%)] | |
| Instructor | 9 (7.5) |
| Assistant professor | 14 (11.7) |
| Associate professor | 13 (10.8) |
| Hospital type [n (%)] | |
| Private | 80 (66.7) |
| University | 15 (12.5) |
| Government | 2 (1.7) |
| Veterans administration | 0 (0.0) |
| Other | 1 (0.1) |
Results of Qualitative Analysis of Behavioral Contracts
From the analyses of the behavioral contracts, themes emerged related to ways in which participants hoped to develop and improve. The themes and the frequencies with which they were recorded in the behavioral contracts are shown in Table 2.
| Theme | Total Number of Times Theme Mentioned in All Behavioral Contracts | Number of Respondents Referring to Theme [n (%)] |
|---|---|---|
| ||
| Improving communication and interpersonal skills | 132 | 70 (58.3) |
| Refinement of vision, goals, and strategic planning | 115 | 62 (51.7) |
| Improve intrapersonal development | 65 | 36 (30.0) |
| Enhance negotiation skills | 65 | 44 (36.7) |
| Commit to organizational change | 53 | 32 (26.7) |
| Understanding business drivers | 38 | 28 (23.3) |
| Setting performance and clinical metrics | 34 | 26 (21.7) |
| Strengthen interdepartmental relations | 32 | 26 (21.7) |
Improving Communication and Interpersonal Skills
A desire to improve communication and listening skills, particularly in the context of conflict resolution, was mentioned repeatedly. Heightened awareness about different personality types to allow for improved interpersonal relationships was another concept that was emphasized.
One female Instructor from an academic medical center described her intentions:
I will try to do a better job at assessing the behavioral tendencies of my partners and adjust my own style for more effective communication.
Refinement of Vision, Goals, and Strategic Planning
Physicians were committed to returning to their home institutions and embarking on initiatives to advance vision and goals of their groups within the context of strategic planning. Participants were interested in creating hospitalist‐specific mission statements, developing specific goals that take advantage of strengths and opportunities while minimizing internal weaknesses and considering external threats. They described wanting to align the interests of members of their hospitalist groups around a common goal.
A female hospitalist leader in private practice wished to:
Clearly define a group vision and commit to re‐evaluation on a regular basis to ensure we are on track . . . and conduct a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis to set future goals.
Improve Intrapersonal Development
Participants expressed desire to improve their leadership skills. Proposed goals included: (1) recognizing their weaknesses and soliciting feedback from colleagues, (2) minimizing emotional response to stress, (3) sharing their knowledge and skills for the benefit of peers, (4) delegating work more effectively to others, (5) reading suggested books on leadership, (6) serving as a positive role model and mentor, and (7) managing meetings and difficult coworkers more skillfully.
One female Assistant Professor from an academic medical center outlined:
I want to be able to: (1) manage up better and effectively negotiate with the administration on behalf of my group; (2) become better at leadership skills by using the tools offered at the Academy; and (3) effectively support my group members to develop their skills to become successful in their chosen niches. I will . . . improve the poor morale in my group.
Enhance Negotiation Skills
Many physician leaders identified negotiation principles and techniques as foundations for improvement for interactions within their own groups, as well as with the hospital administration.
A male private hospitalist leader working for 4 years as a hospitalist described plans to utilize negotiation skills within and outside the group:
Negotiate with my team of hospitalists to make them more compliant with the rules and regulations of the group, and negotiate an excellent contract with hospital administration. . . .
Commit to Organizational Change
The hospitalist respondents described their ability to influence organizational change given their unique position at the interface between patient care delivery and hospital administration. To realize organizational change, commonly cited ideas included recruitment and retention of clinically excellent practitioners, and developing standard protocols to facilitate quality improvement initiatives.
A male Instructor of Medicine listed select areas in which to become more involved:
Participation with the Chief Executive Officer of the company in quality improvement projects, calls to the primary care practitioners upon discharge, and the handoff process.
Other Themes
The final 3 themes included are: understanding business drivers; the establishment of better metrics to assess performance; and the strengthening of interdepartmental relations.
Follow‐up Data About Adherence to Plans Delineated in Behavioral Contracts
Out of 65 completed behavioral contracts from the 2007 Level I participants, 32 returned a follow‐up survey (response rate 49.3%). Figure 1 shows the extent to which respondents believed that they were compliant with their proposed plans for change or improvement. Degree of adherence was displayed as a proportion of total goals. Out of those who returned a follow‐up survey, all but 1 respondent either strongly agreed or agreed that they adhered to at least one of their goals (96.9%).

Select representative comments that illustrate the physicians' appreciation of using behavioral contracts include:
my approach to problems is a bit more analytical.
simple changes in how I approach people and interact with them has greatly improved my skills as a leader and allowed me to accomplish my goals with much less effort.
Discussion
Through the qualitative analysis of the behavioral contracts completed by participants of a Leadership Academy for hospitalists, we characterized the ways that hospitalist practitioners hoped to evolve as leaders. The major themes that emerged relate not only to their own growth and development but also their pledge to advance the success of the group or division. The level of commitment and impact of the behavioral contracts appear to be reinforced by an overwhelmingly positive response to adherence to personal goals one year after course participation. Communication and interpersonal development were most frequently cited in the behavioral contracts as areas for which the hospitalist leaders acknowledged a desire to grow. In a study of academic department of medicine chairs, communication skills were identified as being vital for effective leadership.3 The Chairs also recognized other proficiencies required for leading that were consistent with those outlined in the behavioral contracts: strategic planning, change management, team building, personnel management, and systems thinking. McDade et al.17 examined the effects of participation in an executive leadership program developed for female academic faculty in medical and dental schools in the United States and Canada. They noted increased self‐assessed leadership capabilities at 18 months after attending the program, across 10 leadership constructs taught in the classes. These leadership constructs resonate with the themes found in the plans for change described by our informants.
Hospitalists are assuming leadership roles in an increasing number and with greater scope; however, until now their perspectives on what skill sets are required to be successful have not been well documented. Significant time, effort, and money are invested into the development of hospitalists as leaders.4 The behavioral contract appears to be a tool acceptable to hospitalist physicians; perhaps it can be used as part annual reviews with hospitalists aspiring to be leaders.
Several limitations of the study shall be considered. First, not all participants attending the Leadership Academy opted to fill out the behavioral contracts. Second, this qualitative study is limited to those practitioners who are genuinely interested in growing as leaders as evidenced by their willingness to invest in going to the course. Third, follow‐up surveys relied on self‐assessment and it is not known whether actual realization of these goals occurred or the extent to which behavioral contracts were responsible. Further, follow‐up data were only completed by 49% percent of those targeted. However, hospitalists may be fairly resistant to being surveyed as evidenced by the fact that SHM's 2005‐2006 membership survey yielded a response rate of only 26%.18 Finally, many of the thematic goals were described by fewer than 50% of informants. However, it is important to note that the elements included on each person's behavioral contract emerged spontaneously. If subjects were specifically asked about each theme, the number of comments related to each would certainly be much higher. Qualitative analysis does not really allow us to know whether one theme is more important than another merely because it was mentioned more frequently.
Hospitalist leaders appear to be committed to professional growth and they have reported realization of goals delineated in their behavioral contracts. While varied methods are being used as part of physician leadership training programs, behavioral contracts may enhance promise for change.
Acknowledgements
The authors thank Regina Hess for assistance in data preparation and Laurence Wellikson, MD, FHM, Russell Holman, MD and Erica Pearson (all from the SHM) for data collection.
Copyright © 2010 Society of Hospital Medicine
Hospitalist Use of HCU
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.
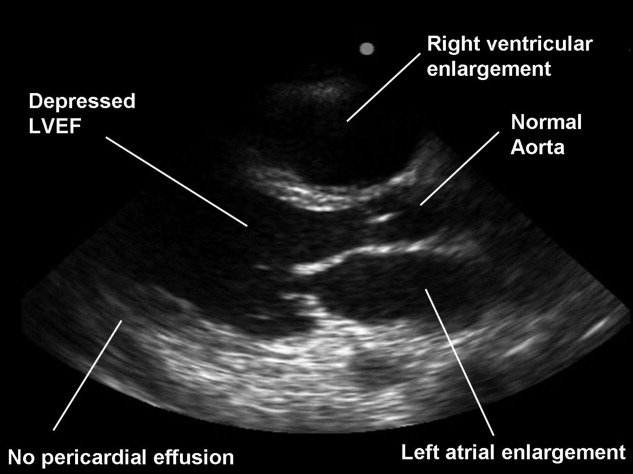
Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
In sight but out of mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, 9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).
Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, 9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, 9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
Drumstick Digits
A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).
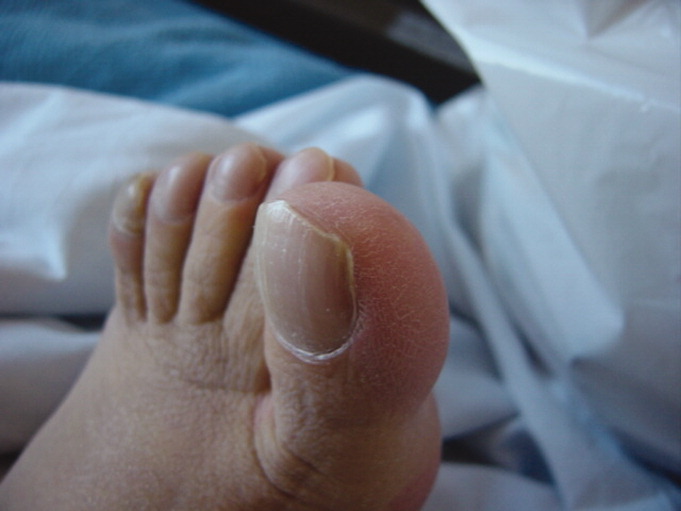
Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).


Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).


Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
Hyponatremia: SIADH or CSW?
An 83‐year‐old man admitted for weakness, lethargy, and mental status changes was found to have human immunodeficiency virus (HIV) disease and cryptococcal meningitis. His hospital course was complicated by worsening hyponatremia (sodium 136 mEq/L). By hospital day 6, the patient's serum sodium had declined to 127 mEq/L from his admission level of 133 mEq/L. The initial impression was that the patient had syndrome of inappropriate antidiuretic hormone (SIADH) and fluid restriction to less than 1500 mL per day was initiated. By hospital day 11, serum sodium continued to decline, to 123 mEq/L, despite fluid restriction.
The past medical history was remarkable for coronary artery disease, hypertension, hyperlipidemia, and anemia, but by self‐report he had not been taking any medications. His review of systems was positive for intermittent bouts of diarrhea.
Vital signs on day 11 included a temperature of 37.3C, blood pressure (BP) of 105/55 mm Hg, and pulse of 90 beats per minute. The BP on admission had been 145/86 mm Hg but had steadily declined with fluid restriction. On physical examination, he appeared thin and cachetic with no evidence of jugular venous distention, rales, or peripheral edema to suggest volume overload. He had been receiving 2 to 4 L of isotonic saline daily for 5 days before the fluid restriction was initiated. The urine output continuously exceeded his intake by at least 500 mL per day throughout his hospital course. His only inpatient medications were amphotericin B and flucytosine. For nutritional supplementation, he was receiving a high‐calorie supplement with free‐water flushes via a nasogastric tube.
Laboratory results revealed a serum sodium concentration of 123 mEq/L, serum potassium of 4.4 mEq/L, serum creatinine of 0.6 mg/dL, urine sodium of 139 mEq/L, serum osmolality of 272 mOsm/kg, and urine osmolality of 598 mOsm/kg (see Table 1). Urinalysis revealed a specific gravity of 1.030. A random serum cortisol level was 11.1 g/dL. A thyroid‐stimulating hormone (TSH) level was 1.32 IU/mL. Brain natriuretic peptide (BNP) was elevated, at 686 pg/mL. A fractional excretion of uric acid was also elevated, at 83.8%.
| Parameters | Day 1 | Fluid Restriction Initiated: Day 6 | Day 8 | Fluid Resuscitation Initiated: Day 11 | Day 13 | Day 15 | Day 26 | Day 37* | Day 40 | Day 44 |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Na (mEq/L) | 133 | 127 | 126 | 123 | 131 | 119 | 140 | 131 | 132 | 135 |
| K (mEq/L) | 4.2 | 4.2 | ||||||||
| BUN (mg/dL) | 39 | 36 | ||||||||
| Cr (mg/dL) | 1.1 | 0.9 | ||||||||
| UNa (mEq/L) | 139 | 86 | 154 | 138 | ||||||
| UOsm (mOsm/kg) | 598 | 362 | 376 | |||||||
| SOsm (mOsm/kg) | 272 | 273 | 279 | |||||||
| BNP (pg/mL) | 686 | 900 | 222 | |||||||
| SUA (mg/dL) | 1.7 | 2.6 | 1.6 | |||||||
| UUA (mg/dL) | 38 | 11 | ||||||||
| FEUA (%) | 83.82 | 28.21 | ||||||||
| FENa (%) | 3.94 | 7.33 | ||||||||
| BP (mm Hg) | 147 | 136 | 122 | 105 | 101 | 90 | 125 | 132 | 140 | |
| Total input (mL) | 700 | NR | 1285 | 3320 | NR | 3040 | 4030 | 4240 | 3120 | 1900 |
| Urine output (mL) | 500 | NR | 2400 | 6501 | NR | 3150 | 3380 | 2950 | 1900 | 950 |
The clinical assessment was volume depletion given the high urine specific gravity, decreasing BP, and a negative fluid balance. The hyponatremia was determined to be due to sodium loss rather than dilution from inappropriate antidiuretic hormone secretion. Intravenous fluid (IVF) hydration with isotonic saline was initiated with a goal to keep the patient in positive fluid balance. The serum sodium level gradually improved to 140 mEq/L over the next 10 days. Attempts to decrease the rate of IVF resulted in a fall in serum sodium and improved when isotonic saline was increased. Eventually, the patient was placed on fludrocortisone, which normalized his urine output and serum sodium.
The response to the treatment regimen supported our diagnosis of cerebral salt wasting (CSW). The patient's serum sodium concentration upon discharge was 135 mEq/L.
Discussion
Our case illustrates the diagnostic challenge presented to physicians when they manage hyponatremia in the setting of a central nervous system (CNS) event. Hyponatremia (sodium 136 mEq/L) has been associated with confusion, lethargy, seizures, coma, and even death.1 Hyponatremia has been reported to occur in up to 30% of the patients with subarachnoid hemorrhage.2, 3
SIADH is frequently the cause of hyponatremia in a patient with a concurrent intracranial process. However, CSW is an important diagnosis to consider and differentiate from SIADH. In a retrospective review of 316 patients with subarachnoid hemorrhage and hyponatremia, 69% were determined to be due to SIADH while 6.5% were from CSW.4 Both CSW and SIADH have been reported to occur in the setting of head trauma, intracranial or metastatic neoplasm, carcinomatous or infectious meningitis, subarachnoid hemorrhage, and CNS surgery. Cryptococcal meningitis as an etiology of CSW has not been previously reported.
The main differentiating feature between SIADH and CSW is that CSW is a dysfunction of renal sodium absorption whereas in SIADH renal sodium handling is intact. This also leads to a difference in the extracellular volume status. SIADH is associated with an increased to normal volume status whereas CSW is a volume‐depleted state. Our patient exhibited a low serum osmolality and a high urine osmolality in the context of hyponatremia, which is present in both CSW and SIADH. However, the clinical course and presentation suggested volume loss, specifically the diarrhea, high urine specific gravity, declining BP, and a negative fluid balance. Some other features that are helpful in determining the volume status may include orthostatic changes, tachycardia, and skin turgor.
Our patient had a low serum uric acid, which is also present in both SIADH and CSW. The key difference between the 2 is that while uric acid will improve with resolution of hyponatremia in SIADH, it will remain low in CSW, as in our patient's uric acid levels, which remained low after normalization of the serum sodium.
Finally, the hyponatremia improved with isotonic fluid repletion, which would not occur in SIADH. The majority of the CSW patients will respond to volume repletion alone, as CSW is a transient condition that will usually resolve in 3 to 4 weeks.3 However, a few patients may require fludrocortisone, as was needed in our patient.
The renal wasting of sodium in CSW is poorly understood. Some postulated mechanisms cite the disruption of sympathetic neural input to the kidney and natriuresis induced by natriuretic peptides. Natriuretic peptides, in particular BNP, have been reported to be elevated in patients with CSW.5, 6 Natriuretic peptides cause salt wasting by inhibition of sodium reabsorption in renal tubule and intramedullary collecting.5, 6 Renin and aldosterone release can also inhibited by the natriuretic peptides. BNP levels were elevated in our patient despite volume loss and no signs of congestive heart failure. Cardiac congestion is a possible etiology for the elevated BNP levels, which peaked to 900 pg/mL on hospital day 37. However, 3 days later the BNP levels declined to 222 pg/mL despite the fact that he was continually in positive fluid balance, suggesting that the BNP elevation was due to CSW and not heart failure.
Conclusions
Our case illustrates the diagnostic and management challenge of hyponatremia in the setting of a CNS event. Both SIADH and CSW are possible etiologies but it is important to make a differentiation. Levels of natriuretic peptides and changes in fractional excretion of uric acid may help differentiate between the 2 conditions.6 The key difference mechanistically is that CSW is due to sodium‐handling deficits, whereas in SIADH sodium‐handling is intact. It is essential to establish volume status since SIADH is a euvolemic to mildly hypervolemic state vs. CSW, which is a volume‐depleted state.7
CSW is well recognized in the neurosurgical arena. The hospitalist will encounter neurosurgical patients with increasing frequency, and thus having an understanding of this disorder, including its diagnosis and treatment, is key.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- .Hyponatremia in acute brain disease: the cerebral salt wasting syndrome.Eur J Intern Med.2002;13(1):9–14.
- .Cerebral salt wasting syndrome: a review.Neurosurgery.1996;38(1):152–160.
- ,,, et al.The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage.Clin Endocrinol (Oxf).2006;64(3):250–254.
- ,,.Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage. Clinical and TCD correlations.Stroke.2000;31(1):118–122.
- ,,, et al.Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage.Lancet.1997;349(9047):245–249.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83(5):905–908.
An 83‐year‐old man admitted for weakness, lethargy, and mental status changes was found to have human immunodeficiency virus (HIV) disease and cryptococcal meningitis. His hospital course was complicated by worsening hyponatremia (sodium 136 mEq/L). By hospital day 6, the patient's serum sodium had declined to 127 mEq/L from his admission level of 133 mEq/L. The initial impression was that the patient had syndrome of inappropriate antidiuretic hormone (SIADH) and fluid restriction to less than 1500 mL per day was initiated. By hospital day 11, serum sodium continued to decline, to 123 mEq/L, despite fluid restriction.
The past medical history was remarkable for coronary artery disease, hypertension, hyperlipidemia, and anemia, but by self‐report he had not been taking any medications. His review of systems was positive for intermittent bouts of diarrhea.
Vital signs on day 11 included a temperature of 37.3C, blood pressure (BP) of 105/55 mm Hg, and pulse of 90 beats per minute. The BP on admission had been 145/86 mm Hg but had steadily declined with fluid restriction. On physical examination, he appeared thin and cachetic with no evidence of jugular venous distention, rales, or peripheral edema to suggest volume overload. He had been receiving 2 to 4 L of isotonic saline daily for 5 days before the fluid restriction was initiated. The urine output continuously exceeded his intake by at least 500 mL per day throughout his hospital course. His only inpatient medications were amphotericin B and flucytosine. For nutritional supplementation, he was receiving a high‐calorie supplement with free‐water flushes via a nasogastric tube.
Laboratory results revealed a serum sodium concentration of 123 mEq/L, serum potassium of 4.4 mEq/L, serum creatinine of 0.6 mg/dL, urine sodium of 139 mEq/L, serum osmolality of 272 mOsm/kg, and urine osmolality of 598 mOsm/kg (see Table 1). Urinalysis revealed a specific gravity of 1.030. A random serum cortisol level was 11.1 g/dL. A thyroid‐stimulating hormone (TSH) level was 1.32 IU/mL. Brain natriuretic peptide (BNP) was elevated, at 686 pg/mL. A fractional excretion of uric acid was also elevated, at 83.8%.
| Parameters | Day 1 | Fluid Restriction Initiated: Day 6 | Day 8 | Fluid Resuscitation Initiated: Day 11 | Day 13 | Day 15 | Day 26 | Day 37* | Day 40 | Day 44 |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Na (mEq/L) | 133 | 127 | 126 | 123 | 131 | 119 | 140 | 131 | 132 | 135 |
| K (mEq/L) | 4.2 | 4.2 | ||||||||
| BUN (mg/dL) | 39 | 36 | ||||||||
| Cr (mg/dL) | 1.1 | 0.9 | ||||||||
| UNa (mEq/L) | 139 | 86 | 154 | 138 | ||||||
| UOsm (mOsm/kg) | 598 | 362 | 376 | |||||||
| SOsm (mOsm/kg) | 272 | 273 | 279 | |||||||
| BNP (pg/mL) | 686 | 900 | 222 | |||||||
| SUA (mg/dL) | 1.7 | 2.6 | 1.6 | |||||||
| UUA (mg/dL) | 38 | 11 | ||||||||
| FEUA (%) | 83.82 | 28.21 | ||||||||
| FENa (%) | 3.94 | 7.33 | ||||||||
| BP (mm Hg) | 147 | 136 | 122 | 105 | 101 | 90 | 125 | 132 | 140 | |
| Total input (mL) | 700 | NR | 1285 | 3320 | NR | 3040 | 4030 | 4240 | 3120 | 1900 |
| Urine output (mL) | 500 | NR | 2400 | 6501 | NR | 3150 | 3380 | 2950 | 1900 | 950 |
The clinical assessment was volume depletion given the high urine specific gravity, decreasing BP, and a negative fluid balance. The hyponatremia was determined to be due to sodium loss rather than dilution from inappropriate antidiuretic hormone secretion. Intravenous fluid (IVF) hydration with isotonic saline was initiated with a goal to keep the patient in positive fluid balance. The serum sodium level gradually improved to 140 mEq/L over the next 10 days. Attempts to decrease the rate of IVF resulted in a fall in serum sodium and improved when isotonic saline was increased. Eventually, the patient was placed on fludrocortisone, which normalized his urine output and serum sodium.
The response to the treatment regimen supported our diagnosis of cerebral salt wasting (CSW). The patient's serum sodium concentration upon discharge was 135 mEq/L.
Discussion
Our case illustrates the diagnostic challenge presented to physicians when they manage hyponatremia in the setting of a central nervous system (CNS) event. Hyponatremia (sodium 136 mEq/L) has been associated with confusion, lethargy, seizures, coma, and even death.1 Hyponatremia has been reported to occur in up to 30% of the patients with subarachnoid hemorrhage.2, 3
SIADH is frequently the cause of hyponatremia in a patient with a concurrent intracranial process. However, CSW is an important diagnosis to consider and differentiate from SIADH. In a retrospective review of 316 patients with subarachnoid hemorrhage and hyponatremia, 69% were determined to be due to SIADH while 6.5% were from CSW.4 Both CSW and SIADH have been reported to occur in the setting of head trauma, intracranial or metastatic neoplasm, carcinomatous or infectious meningitis, subarachnoid hemorrhage, and CNS surgery. Cryptococcal meningitis as an etiology of CSW has not been previously reported.
The main differentiating feature between SIADH and CSW is that CSW is a dysfunction of renal sodium absorption whereas in SIADH renal sodium handling is intact. This also leads to a difference in the extracellular volume status. SIADH is associated with an increased to normal volume status whereas CSW is a volume‐depleted state. Our patient exhibited a low serum osmolality and a high urine osmolality in the context of hyponatremia, which is present in both CSW and SIADH. However, the clinical course and presentation suggested volume loss, specifically the diarrhea, high urine specific gravity, declining BP, and a negative fluid balance. Some other features that are helpful in determining the volume status may include orthostatic changes, tachycardia, and skin turgor.
Our patient had a low serum uric acid, which is also present in both SIADH and CSW. The key difference between the 2 is that while uric acid will improve with resolution of hyponatremia in SIADH, it will remain low in CSW, as in our patient's uric acid levels, which remained low after normalization of the serum sodium.
Finally, the hyponatremia improved with isotonic fluid repletion, which would not occur in SIADH. The majority of the CSW patients will respond to volume repletion alone, as CSW is a transient condition that will usually resolve in 3 to 4 weeks.3 However, a few patients may require fludrocortisone, as was needed in our patient.
The renal wasting of sodium in CSW is poorly understood. Some postulated mechanisms cite the disruption of sympathetic neural input to the kidney and natriuresis induced by natriuretic peptides. Natriuretic peptides, in particular BNP, have been reported to be elevated in patients with CSW.5, 6 Natriuretic peptides cause salt wasting by inhibition of sodium reabsorption in renal tubule and intramedullary collecting.5, 6 Renin and aldosterone release can also inhibited by the natriuretic peptides. BNP levels were elevated in our patient despite volume loss and no signs of congestive heart failure. Cardiac congestion is a possible etiology for the elevated BNP levels, which peaked to 900 pg/mL on hospital day 37. However, 3 days later the BNP levels declined to 222 pg/mL despite the fact that he was continually in positive fluid balance, suggesting that the BNP elevation was due to CSW and not heart failure.
Conclusions
Our case illustrates the diagnostic and management challenge of hyponatremia in the setting of a CNS event. Both SIADH and CSW are possible etiologies but it is important to make a differentiation. Levels of natriuretic peptides and changes in fractional excretion of uric acid may help differentiate between the 2 conditions.6 The key difference mechanistically is that CSW is due to sodium‐handling deficits, whereas in SIADH sodium‐handling is intact. It is essential to establish volume status since SIADH is a euvolemic to mildly hypervolemic state vs. CSW, which is a volume‐depleted state.7
CSW is well recognized in the neurosurgical arena. The hospitalist will encounter neurosurgical patients with increasing frequency, and thus having an understanding of this disorder, including its diagnosis and treatment, is key.
An 83‐year‐old man admitted for weakness, lethargy, and mental status changes was found to have human immunodeficiency virus (HIV) disease and cryptococcal meningitis. His hospital course was complicated by worsening hyponatremia (sodium 136 mEq/L). By hospital day 6, the patient's serum sodium had declined to 127 mEq/L from his admission level of 133 mEq/L. The initial impression was that the patient had syndrome of inappropriate antidiuretic hormone (SIADH) and fluid restriction to less than 1500 mL per day was initiated. By hospital day 11, serum sodium continued to decline, to 123 mEq/L, despite fluid restriction.
The past medical history was remarkable for coronary artery disease, hypertension, hyperlipidemia, and anemia, but by self‐report he had not been taking any medications. His review of systems was positive for intermittent bouts of diarrhea.
Vital signs on day 11 included a temperature of 37.3C, blood pressure (BP) of 105/55 mm Hg, and pulse of 90 beats per minute. The BP on admission had been 145/86 mm Hg but had steadily declined with fluid restriction. On physical examination, he appeared thin and cachetic with no evidence of jugular venous distention, rales, or peripheral edema to suggest volume overload. He had been receiving 2 to 4 L of isotonic saline daily for 5 days before the fluid restriction was initiated. The urine output continuously exceeded his intake by at least 500 mL per day throughout his hospital course. His only inpatient medications were amphotericin B and flucytosine. For nutritional supplementation, he was receiving a high‐calorie supplement with free‐water flushes via a nasogastric tube.
Laboratory results revealed a serum sodium concentration of 123 mEq/L, serum potassium of 4.4 mEq/L, serum creatinine of 0.6 mg/dL, urine sodium of 139 mEq/L, serum osmolality of 272 mOsm/kg, and urine osmolality of 598 mOsm/kg (see Table 1). Urinalysis revealed a specific gravity of 1.030. A random serum cortisol level was 11.1 g/dL. A thyroid‐stimulating hormone (TSH) level was 1.32 IU/mL. Brain natriuretic peptide (BNP) was elevated, at 686 pg/mL. A fractional excretion of uric acid was also elevated, at 83.8%.
| Parameters | Day 1 | Fluid Restriction Initiated: Day 6 | Day 8 | Fluid Resuscitation Initiated: Day 11 | Day 13 | Day 15 | Day 26 | Day 37* | Day 40 | Day 44 |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Na (mEq/L) | 133 | 127 | 126 | 123 | 131 | 119 | 140 | 131 | 132 | 135 |
| K (mEq/L) | 4.2 | 4.2 | ||||||||
| BUN (mg/dL) | 39 | 36 | ||||||||
| Cr (mg/dL) | 1.1 | 0.9 | ||||||||
| UNa (mEq/L) | 139 | 86 | 154 | 138 | ||||||
| UOsm (mOsm/kg) | 598 | 362 | 376 | |||||||
| SOsm (mOsm/kg) | 272 | 273 | 279 | |||||||
| BNP (pg/mL) | 686 | 900 | 222 | |||||||
| SUA (mg/dL) | 1.7 | 2.6 | 1.6 | |||||||
| UUA (mg/dL) | 38 | 11 | ||||||||
| FEUA (%) | 83.82 | 28.21 | ||||||||
| FENa (%) | 3.94 | 7.33 | ||||||||
| BP (mm Hg) | 147 | 136 | 122 | 105 | 101 | 90 | 125 | 132 | 140 | |
| Total input (mL) | 700 | NR | 1285 | 3320 | NR | 3040 | 4030 | 4240 | 3120 | 1900 |
| Urine output (mL) | 500 | NR | 2400 | 6501 | NR | 3150 | 3380 | 2950 | 1900 | 950 |
The clinical assessment was volume depletion given the high urine specific gravity, decreasing BP, and a negative fluid balance. The hyponatremia was determined to be due to sodium loss rather than dilution from inappropriate antidiuretic hormone secretion. Intravenous fluid (IVF) hydration with isotonic saline was initiated with a goal to keep the patient in positive fluid balance. The serum sodium level gradually improved to 140 mEq/L over the next 10 days. Attempts to decrease the rate of IVF resulted in a fall in serum sodium and improved when isotonic saline was increased. Eventually, the patient was placed on fludrocortisone, which normalized his urine output and serum sodium.
The response to the treatment regimen supported our diagnosis of cerebral salt wasting (CSW). The patient's serum sodium concentration upon discharge was 135 mEq/L.
Discussion
Our case illustrates the diagnostic challenge presented to physicians when they manage hyponatremia in the setting of a central nervous system (CNS) event. Hyponatremia (sodium 136 mEq/L) has been associated with confusion, lethargy, seizures, coma, and even death.1 Hyponatremia has been reported to occur in up to 30% of the patients with subarachnoid hemorrhage.2, 3
SIADH is frequently the cause of hyponatremia in a patient with a concurrent intracranial process. However, CSW is an important diagnosis to consider and differentiate from SIADH. In a retrospective review of 316 patients with subarachnoid hemorrhage and hyponatremia, 69% were determined to be due to SIADH while 6.5% were from CSW.4 Both CSW and SIADH have been reported to occur in the setting of head trauma, intracranial or metastatic neoplasm, carcinomatous or infectious meningitis, subarachnoid hemorrhage, and CNS surgery. Cryptococcal meningitis as an etiology of CSW has not been previously reported.
The main differentiating feature between SIADH and CSW is that CSW is a dysfunction of renal sodium absorption whereas in SIADH renal sodium handling is intact. This also leads to a difference in the extracellular volume status. SIADH is associated with an increased to normal volume status whereas CSW is a volume‐depleted state. Our patient exhibited a low serum osmolality and a high urine osmolality in the context of hyponatremia, which is present in both CSW and SIADH. However, the clinical course and presentation suggested volume loss, specifically the diarrhea, high urine specific gravity, declining BP, and a negative fluid balance. Some other features that are helpful in determining the volume status may include orthostatic changes, tachycardia, and skin turgor.
Our patient had a low serum uric acid, which is also present in both SIADH and CSW. The key difference between the 2 is that while uric acid will improve with resolution of hyponatremia in SIADH, it will remain low in CSW, as in our patient's uric acid levels, which remained low after normalization of the serum sodium.
Finally, the hyponatremia improved with isotonic fluid repletion, which would not occur in SIADH. The majority of the CSW patients will respond to volume repletion alone, as CSW is a transient condition that will usually resolve in 3 to 4 weeks.3 However, a few patients may require fludrocortisone, as was needed in our patient.
The renal wasting of sodium in CSW is poorly understood. Some postulated mechanisms cite the disruption of sympathetic neural input to the kidney and natriuresis induced by natriuretic peptides. Natriuretic peptides, in particular BNP, have been reported to be elevated in patients with CSW.5, 6 Natriuretic peptides cause salt wasting by inhibition of sodium reabsorption in renal tubule and intramedullary collecting.5, 6 Renin and aldosterone release can also inhibited by the natriuretic peptides. BNP levels were elevated in our patient despite volume loss and no signs of congestive heart failure. Cardiac congestion is a possible etiology for the elevated BNP levels, which peaked to 900 pg/mL on hospital day 37. However, 3 days later the BNP levels declined to 222 pg/mL despite the fact that he was continually in positive fluid balance, suggesting that the BNP elevation was due to CSW and not heart failure.
Conclusions
Our case illustrates the diagnostic and management challenge of hyponatremia in the setting of a CNS event. Both SIADH and CSW are possible etiologies but it is important to make a differentiation. Levels of natriuretic peptides and changes in fractional excretion of uric acid may help differentiate between the 2 conditions.6 The key difference mechanistically is that CSW is due to sodium‐handling deficits, whereas in SIADH sodium‐handling is intact. It is essential to establish volume status since SIADH is a euvolemic to mildly hypervolemic state vs. CSW, which is a volume‐depleted state.7
CSW is well recognized in the neurosurgical arena. The hospitalist will encounter neurosurgical patients with increasing frequency, and thus having an understanding of this disorder, including its diagnosis and treatment, is key.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- .Hyponatremia in acute brain disease: the cerebral salt wasting syndrome.Eur J Intern Med.2002;13(1):9–14.
- .Cerebral salt wasting syndrome: a review.Neurosurgery.1996;38(1):152–160.
- ,,, et al.The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage.Clin Endocrinol (Oxf).2006;64(3):250–254.
- ,,.Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage. Clinical and TCD correlations.Stroke.2000;31(1):118–122.
- ,,, et al.Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage.Lancet.1997;349(9047):245–249.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83(5):905–908.
- ,.Hyponatremia.N Engl J Med.2000;342(21):1581–1589.
- .Hyponatremia in acute brain disease: the cerebral salt wasting syndrome.Eur J Intern Med.2002;13(1):9–14.
- .Cerebral salt wasting syndrome: a review.Neurosurgery.1996;38(1):152–160.
- ,,, et al.The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage.Clin Endocrinol (Oxf).2006;64(3):250–254.
- ,,.Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage. Clinical and TCD correlations.Stroke.2000;31(1):118–122.
- ,,, et al.Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage.Lancet.1997;349(9047):245–249.
- ,,,.Clinical assessment of extracellular fluid volume in hyponatremia.Am J Med.1987;83(5):905–908.
Ethics of the Hospitalist Model
Wachter and Goldman1 first described hospitalists in 1996 as a new breed of physicians who devote blocks of time exclusively to the care of hospitalized patients. Since its definition, the hospitalist model has prompted 2 major debates. First, does the hospitalist system improve clinical efficiency, quality of care, cost effectiveness, and patient satisfaction? A series of large and small randomized trials have all but definitively proven the hospitalist model's advantage. Yet whether the hospitalist model is good for patient care has proven to remain contentious, as most recently demonstrated by the discussion between Williams2 and Centor3 and others like it.4, 5 What is clear in these exchanges is that the debate has shifted to the second great debate: does the hospitalist model pose inherent conflicts in clinical ethics? What are the implications of the purposeful discontinuity in care, the autonomy issues raised by mandatory hospitalist use, and the structural management issues that potentially pit hospitalists against patients in fiduciary and financial conflicts of interest? These important issues are certainly not new, and the hospitalist model has made much effort to address some of them.6, 7 This work aims to serve as a review of these important ethical concerns, demonstrating how some questions have been answered, while some remain unanswered.
The Hospitalist Model's Founding Premise
A growing threshold for hospital admission in the last 3 decades caused primary care physicians (PCPs) to see a diminishing number of inpatients. A survey in 1978 found that PCPs spent 40% of their time in the hospital, rounding on 10 patients per day.8 By 2001, PCPs spent 10% of their time in the hospital on average, and most PCPs rounded on fewer than 2 inpatients per day.9 The cost of inefficiencies associated with primary coordination of care in the hospital increasingly outweighed the tradeoff of preserving the patient‐PCP relationship in the hospital. Converging with increasing attention on cost controls through the restructuring of service provision, the hospitalist was born. Wachter10 argued that the hospitalist model could alleviate inpatient demands placed on PCPs while improving the outcomes and lowering the cost of care for hospitalized patients.
Early on there were setbacks to proving Wachter's10 case. Small studies found hospitalists to have higher hospital charges and longer length of stays.11 A survey of PCPs found only 56% were satisfied with communication with hospitalists and that most believed that patients generally preferred to be cared for in the hospital by their regular physician.12, 13 Meltzer and Herthko14 found 70% of people sampled said they would prefer care by their own physician to that of a hospitalist if they were hospitalized for a general medical condition. Yet this study found in a national random‐digit phone survey that only 10% of the respondents would pay $750 for their PCP to follow them to the hospital, the cost savings of the hospitalist system proven by the only 2 randomized trials performed at the time.15, 16 To 90% of respondents, the value of the PCP at the bedside was not worth the cost tradeoff to keep them there.
The meteoric rise in the number of hospitalists reflects the many studies and reviews that affirmed the premise that hospitalists improved inpatient efficiency without harmful effects on quality of care.17, 18 In a large retrospective cohort study of over 75,000 patients in 45 hospitals across the country, Lindenauer et al.19 found that hospitalists had a $268 lower cost when compared to internists, $125 lower cost when compared to family physicians, and a shorter hospital stay by about one‐half day when compared to both groups. The group found no significant difference in rates of death or readmission rates. While called modest in the text, these savings over time and volume add up for hospitals. Patients benefit from hospitalist care, researchers hypothesize, because of their familiarity with hospital systems, their increased availability to patients, and their experience with common hospital problems. Though the Lindenauer et al.19 study was criticized for design flaws, it prompted the editorialist McMahon20 to assert that the question was sufficiently answered, and it was time to move on away from the studies focusing on cost and comparing outcomes. As Wachter21 wrote, the demand for hospitalists is now relatively de‐linked from the field's original premiseefficiency advantagesand is now both more diversified and more robust. The model has become an accepted mode of care for hospitalized patients, with up to 20,000 hospitalists currently practicing in 29% of all hospitals and in over one‐half of hospitals with over 200 beds in the United States.22, 23
The Patient‐Physician Relationship
Purposeful discontinuity of care in the hospitalist system has the potential to diminish the doctor‐patient relationship.12 This relationship is built on a bond of loyalty, confidentiality, and trust. Handing off care to a hospitalist when the patient is most vulnerable can be viewed as a violation of this covenant. According to Meltzer,24 the hospitalist model pits Franicis Peabody's25 intimate personal relationship between patient and physician against Adam Smith et al.'s11 benefits of specialization. Peabody25 observed that physicians' lack of understanding of their patients as persons is especially acute in the hospital, where
one gets in the habit of using the oil immersion lamp instead of the low power, and focuses too intently in the center of the field. . . . The institutional eye tends to become focused on the lung, and it forgets that the lung is only one member of the body.
This movement toward patient‐centered medicine fits into an ever‐growing sentiment to value the social as well as the physiological, a holistic approach to the patient as a person. This emphasis was the original justification for PCPs to coordinate increasingly specialized hospital care and translate recommendations suitable to patients. Can the long‐term relationship between patient and PCP be replaced by the hospital generalist, or would hospitalists be inherently deficient in their abilities to coordinate care appropriate for patients? Hospitalized patients are frequently in no position to make complex decisions regarding their care.26 Lo7 argues that PCPs who know patients over extended periods of time are in a better position to respect patient wishes by individualizing discussions with patients and checking that patients' decisions are consistent with their core values. The long‐term relationship is also critical for designing a complex discharge plan suitable to the patients' ability and resources. Information about long‐term patient compliance with medications is much more available to PCPs. Patients trust physicians to keep promises made concerning end‐of‐life issues, and these assurances are vulnerable during handoffs of care. Pantilat et al.6 provide a case study of an outpatient Do‐Not‐Resuscitate order ineffective in the hospital. These scenarios occur because most written advance directives are unavailable in acute situations, and when they are, hospitalists unfamiliar with the patient's wishes may hesitate to act on directives not specific enough to answer the acute clinical question.27
Hospitalists' broadened responsibility to systematically improve the care of patients may potentially improve end‐of‐life care. Patient values can be better communicated to hospitalists by encouraging inpatients to complete advance directive surveys and then asking hospitalists to discuss those directives with their patients.6 Significantly, Auerbach and Pantilat28 found that end‐of‐life care was improved with hospitalist care. This chart review found hospitalists more likely to have discussions with patients and their families regarding care and providing comfort care more frequently at the time of death than community‐based physicians. The authors hypothesize that hospitalists may have better communication with dying patients and their families because they spend more time in the hospital each day, using frequent meetings to better understand the preferences of patients. These preferences often require clarification and often change after admission, making previous discussions about end‐of‐life care with PCPs moot. Greater expertise in hospital care may also allow hospitalists to better recognize patients who are nearing death and may explain the fewer symptoms documented by Auerbach and Pantilat28 at the end of life among patients cared for by hospitalists compared to community‐based physicians.
Hospital medicine has taken continuity of care issues seriously, and responded by making pragmatic recommendations to preserve the patient‐PCP relationship in the hospital and assuage the perception that patients have been dropped. Harlan et al.29 identify important issues around good communication between pediatric hospitalists and PCPs including the content and timing of communication beneficial to the patient. Hospitalists can use a standard script for introducing themselves to patients, explaining their role, and their continued coordination with the PCP.30 PCPs can still be involved in the care of their patients in hospitals through continuity visits or phone calls with patients and through better communication with hospitalists.31 Generally, reimbursing PCPs for their increased role in the hospitalist system can encourage better communication with hospitalists.19 Potential disagreements between PCPs and hospitalist regarding the care of the patient can be resolved through explicit conflict resolution procedures within the hospitalist system.6
These procedural solutions are only as successful as they are used. A large review by Kripalani et al.32 found communication between hospitalists and PCPs occurred infrequently (3%‐20%), affecting the quality of care in approximately 25% of follow‐up visits and contributing to PCP dissatisfaction. Sharma et al.33 found that continuity visits decreased from 50.5% in 1996 to 39.8% in 2006. In a survey of patients cared for in a hospitalist system, Hruby et al.34 found that 33% of hospitalized patients had some contact with their PCP directly and 66% of patients were satisfied with the contact they or their relative had with their PCP. When probed, patient satisfaction is too vague a measurement to assess the complex value of the patient‐physician relationship. Studying these issues may require relying more on individualized narratives rather than generalized statistics, or may require years of follow‐up. As Centor3 argues, we need this broader perspective of the patient's experience in order to understand the effects of the hospitalist model on patient trust in their PCP and in their overall care. Studies by Davis et al.35 and Halpert et al.36 assert that rising quality of care and patient satisfaction with the hospitalist system rebuts coordination of care concerns. Yet we need more studies investigating the relationship between improved communication and patient outcomes, as evidence is currently conflicting on this subject.32, 37, 38
The Journal of Hospital Medicine has pursued this research agenda; the April 2009 issue presents several studies describing best practices in the discharging of hospitalized patients. Manning et al.39 describe a tool to assess patient mobility after discharge, and O'Leary et al.40 used electronic health records to create a better discharge summary. Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) has shown improvements in discharge transition procedures41 and the use of transition coaches for vulnerable older patients has been proven cost‐effective and has been scaled up to more than 100 healthcare organizations.42, 43
Inpatient care handoff to PCPs is not entirely novel, as surgeons, oncologists, cardiologists, and other specialists have always grappled with continuity of care. It would be prudent to investigate what can be learned from these efforts, and which practices can be best applied to the hospitalist model. More longitudinal studies need to investigate the prevalence and success of the procedural recommendations to preserve the patient‐physician relationship. We need to know more about what works and what does not. How have hospitals found novel ways in implementing these approaches, and how can they be applied to a diversity of hospital settings? We need a better outcome measurement than patient or physician satisfaction for probing the subtleties of the patient‐physician relationship. There is a sizeable population that does not have a PCP to care for them before hospitalization or after discharge, and discussions about continuity of care must address these patients. Last, these best practices and patient centered values need to be incorporated into the core competencies of residencies and fellowships for a new generation of hospitalists.
Maintaining the continuity of the physician‐patient relationship is an integral part of the original premise of the hospitalist model. Importantly, Meltzer24 found that this discontinuity within the hospital has the potential to eliminate the savings of the hospitalist system. Yet concerns about continuity of care do not sufficiently encompass the complexand at times fragilerelationship between physician and patient. The survival of the physician‐patient relationship depends on the hospitalist model's affirmation of the values of coordination and Peabody's25 approach to patient‐centered care. If the hospitalist model is to thrive, it needs to emphasize its duty as steward of the PCP‐patient relationship as much as it focuses on efficiency and cost‐effectiveness.
Patient Autonomy
The mandatory transfer of patients into the hospitalist model raises serious ethical issues. A survey in 2000 of PCPs found that 23% were required to use hospitalists for all admissions.44 Other surveys found this prevalence to be as low as 2%.12 Nevertheless, several high profile cases of Health Maintenance Organizations (HMOs)Prudential HealthCareSouth Florida, Prudential, Humana, and Cigna Corporationall using mandatory hospitalists, prompted protests from professional organizations and there were even legislative efforts to ban the practice of the mandatory use of hospitalists in 2000 and 2001.45 Today, most insurance plans, as well as the Society of Hospital Medicine (SHM), support voluntary rather than mandatory hospitalist use.46 Yet while not mandatory, the hospitalist is the default provider in many settings, giving a de facto mandate for hospitalist care. As Royo et al.47 point out, the rise in physician employment by hospitals has facilitated a self‐selecting progression toward a structural network that closely resembles the mandatory model.
While PCPs and internists contested mandatory hospitalist plans as infringements on their autonomy, they overlooked the harm to the patient's autonomy. When healthy in the ambulatory setting, the patient has the opportunity to choose his or her doctor to provide longitudinal care. When the patient is admitted acutely to a hospital, the patient does not have the freedom to choose a physician; the patient is assigned to the hospitalist on duty that night. This call for patient autonomy is of utmost importance in the hospitalized patient, where patients are increasingly sicker, their diseases under a high‐powered lens, and their options diminished. This freedom of choice is integral to the patient‐physician partnership. Yet this freedom of choice is largely hindered by the employer's choice in the health plan for their employees or an individual's ability to pay for a health plan. These represent some of the many barriers to choice facing patients in the American model of health insurance.
As the hospitalist system grows to become the accepted mode of hospital care, more patients need to be informed about the transition of care to another physician and what steps are taken to ensure appropriate continuity of care. Transfers of patients from PCPs to hospitalists must be voluntary, with the decision left to patient care preferences.48 Educating patients in the outpatient setting about the hospitalist model, its benefits, risks, and alternatives, is necessary for them to make informed decisions about hospital care. This will require the collaboration of PCPs and hospitalists together. The continued success of the model depends on the nurturance of the partnership between the PCP, the hospitalist, and the patient.
Meltzer and Herthko14 have proposed that patients pay a premium for the option to choose a PCP that is not mandated to transfer their care to a hospitalist, in order to offset cost savings with the hospitalist system. Yet Meltzer and Herthko's14 study suggests that many patients could not afford to pay this premium and, in effect, patient autonomy would be preserved for the affluent. This raises the oft‐neglected professional ethic of justice for low‐income patients. Alexander and Lantos49 were resigned to see this infringement on patient autonomy as an inevitable consequence of balancing the desires of patients with the drive to lower cost and improve outcomes. If the hospitalist model grows to be the predominant mode of care, it is unclear if patient choice can survive. Investigators need to test whether the advantages of hospitalist care can coexist with voluntary programs. If it proves that they indeed cannot, then the hospitalist system will need to respond to concerned patients with honest answers and find pragmatic solutions to diminishing patient choice.
Conflict of Interest
The hospitalist system's main benefit of cost‐savings prompted Pantilat et al.6 to wonder whether hospitalists would face a conflict of interest between what is best for the patient and what financial incentives and utilization review encourage or require them to do. The financial support provided by many hospitals to meet the operating expenses of hospitalist programs is often associated with explicit or implicit incentives to reduce the length of hospital stay and costs.50 With hospitals employing hospitalists and increasingly pressuring them to decrease length of stay and discharge patients quickly, patients may have no advocate to protect them from discharge planners. Many hospitalists supplement their income by supervising discharge planners, and a dispute would put the hospitalist in the uncomfortable position of advocating for his patient against his employer and colleagues. While conflicts of interests occur in many managed care arrangements, they may be more acute in hospitalist systems. A weakened patient‐physician relationship may put the patients' best interest inferior to the employer's interests. Hospitalists do not immediately deal with adverse consequences of premature discharges in the outpatient setting and virtually no malpractice case law considers the obligations and practices of hospitalists in these settings.51
The SHM identified a core competency of hospitalists to
recommend treatment options that optimize patient care, include consideration of resource utilization, and are formulated without regard to financial incentives or other conflicts of interest.52
Ethical issues concerning conflict of interest remain unanswered, largely because no information about organizational features such as explicit incentives for reductions in length of stay is available to researchers or to patients. This is the wrong approach and only feeds the fear that hospitalists may weigh patients' best interest with financial incentives. Abbo and Volandes53 have argued that ambivalence to cost considerations is hazardous. If the hospitalist model cannot be forthright with the active considerations of costs in daily clinical practice, it is unlikely to truly make strides at cost savings, and may even raise the cost of care in the long run.
Jonsen et al.54 provide ethical standards for considering costs in clinical decisions. First, a physician's first priority should be to provide patient‐centered care that focuses on medical indications and patient preferences. Second, quality care does not mean all available care; quality care reflects what is not only diagnostically sound and technically correct, but also appropriate. Third, conflicts of interest are most vulnerable when there is a failing of the patient‐physician relationship. Health care organizations should expect physicians to argue for policies that provide all services that have a reasonable likelihood of benefiting the patient. Fourth, patient and physician autonomy and freedom of choice should be maximized within the limits of the system. Persons should be fully informed of the constraints of the system before choosing it. Plans need to disclose any financial incentive arrangements that exist between the plan and the physician. And incentive arrangements should be based on quality of care rather than on underutilization of care services. Fifth, the system should reflect principles of just distribution, ensuring that all who have a fair claim to service should receive it without discrimination. Last, capitation plans should share risks among physicians, not patients, while incentives are provided for improvements in access, prevention, and patient satisfaction.
Conflicts of interest have been a concern for as long as physicians have been paid for services. Fears about interference into the doctor‐patient relationship, whether they are from government or business, continue to stall real efforts to lower skyrocketing medical costs. The hospitalist model rebuts conflict of interest claims with improved outcomes, efficiency, and quality of care in the many reviews cited above. These arguments do prove that the hospitalist model's emphasis on medically indicated and appropriate care does address Jonsen et al.'s54 first and second standards. Yet, as Jonsen et al.54 point out, without strongly emphasizing the patient‐physician relationship and patient autonomy, it leaves itself vulnerable to creating conflicts of interest. Hospitalist systems need to be forthright about their explicit or implicit incentive structures and disclose this information to patients in a timely manner for them to make informed decisions. These incentives should be linked to quality of care and patient satisfaction, not cost savings. Last, hospitalist training programs should make ethical cost considerations a core competency of their curriculum.
Conclusions
Hospitalism was founded on the premise that it could improve the quality and reduce the cost of hospital care. Many randomized studies have all but definitively proven this original assertion. It is now time for the model to prove that these gains are not to the detriment of the patient‐physician relationship. Hospitalism must define itself as the steward of this relationship, valuing it as much as it values outcomes and costs. This is of particular concern in the United States as Medicare Part A (payment for inpatient care) is scheduled to go bankrupt in 2019, leading to potentially reasonable fears of hospital‐motivated cost containment.57
Investigators must find an outcome that encompasses the complexity of the patient‐physician relationship, and methods to improve it must be studied and improved upon. Preserving the patient‐physician relationship is a systemic issue, and full‐time hospitalists may be in the best position to implement systemic reforms to improve communication and continuity of care. Pham's56 case study of a hospitalist piecing together disparate parts of the patient's story illustrates this point. This should include more investigation into the prevalence of use and success of methods aimed at protecting the patient‐physician relationship at critical points in the handover of care. When proven successful, The SHM should propose new standards and safeguards to insure that these methods become standard practice in patient care. This effort, led by Snow et al.,57 is currently underway.
A hospitalist model that does not emphasize mitigating the effects of the diminishing patient‐physician relationship leaves itself exposed to further infringements on autonomy and choice. It is unclear whether patient autonomy and choice can coexist in a successful hospitalist system. The consequences of these unanswered ethical questions need to be explored. The professions of primary care need to be more proactive in educating patients about choice of care in hospitals, and hospitalists need to provide that choice, allowing voluntary programs in hospital care when feasible.
When combined, a wounded patient‐physician relationship and impaired patient autonomy leave the hospitalist model vulnerable to claims of financial and fiduciary conflict of interest. These concerns need not be inherent to the hospitalist systems, but hospitalists will need to be forthright and honest about incentives structures, and link them to quality of care and patient satisfaction, not to efficiency and cost savings.
It is indeed time for hospitalism to move onaway from proving its founding premise, and toward addressing these lingering ethical issues. Hospitalism's continued growth and success depends on it.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists and the hospital medicine system of care are good for patient care.Arch Intern Med.2008;168(12):1254–1256, discussion 1259–1260.
- .A hospitalist inpatient system does not improve patient care outcomes.Arch Intern Med.2008;168(12):1257–1258, discussion 1259–1260.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: Yes.Can Fam Physician.2008;54(8):1100–1101,1104–1106.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: No.Can Fam Physician.2008;54(8):1101–1103,1105–1107.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282:171–174.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48:281–290.
- Robert Wood Johnson Foundation.Medical Practice in the United States.Princeton, NJ:The Robert Wood Johnson Foundation;1981.
- .Response to David Meltzer's paper “Hospitalists and the doctor‐patient relationship.”J Legal Stud2001;30:615–623.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- ,,.Primary care family physicians and 2 hospitalists models: comparison of outcomes, processes, and costs.J Fam Prac.2002;51:1021–1027.
- ,,, et al.Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48(4):218–229.
- ,,, et al.Physician attitudes towards and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,.Patients' willingness to pay for hospital care by their primary care physician versus hospitalists: results of a national survey. [Society of General Internal Medicine 23rd annual meeting. Boston, Massachusetts, USA. May 4–6, 2000. Abstracts.]J Gen Intern Med.2000;15(suppl 1):135.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560.
- ,,, et al.Effects of hospitalist physicians on an academic general medicine service: results of a randomized trial. [22nd Annual meeting of The Society of General Internal Medicine. San Francisco, California, USA. April 29‐May 1, 1999. Abstracts.]J Gen Intern Med.1999;14(suppl 2):112.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Economic and healthcare forces of hospitalist movement.Mt Sinai J Med.2008;75(5):424–429.
- ,,, et al.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- .The hospitalist movement—time to move on.N Engl J Med.2007;357:2627–2629.
- .Today's New England Journal Hospitalist Study. Weblog Entry.Wachter's World: The Hospitalist.2007. Available at: http://www.the‐hospitalist.org/blogs/wachters_world/archive/2007/12/20/today‐s‐new‐england‐journal‐hospitalist‐study.aspx. Accessed July 2009.
- ,,, et al.The Rise of the Hospitalist in California.Oakland, CA:California Health Care Foundation;2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Hospitalists and the doctor patient relationship.J Legal Stud.2001;2:615–623.
- .Landmark article March 19, 1927: the care of the patient. By Francis W. Peabody.JAMA.1984;252:813–818.
- .The Practice of Autonomy: Patients, Doctors, and Medical Decisions.New York, NY:Oxford University Press;1998.
- ,,, et al.A prospective study of advance directives for life‐sustaining care.N Engl J Med.1991;324:882–888.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,, et al.Pediatric hospitalists and primary care providers: a communication needs assessment.J Hosp Med.2009;4(3):187–193.
- .What should you say after “Hello”?Today's Hospitalist Apr2008. Available at: http://www.todayshospitalist.com/index.php?b=articles_read48:267–272.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,,, et al.Effects of hospitalists on cost, outcomes and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24:381–386.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- ,,.Home alone: mobility independence before discharge.J Hosp Med.2009;4:252–254.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4:219–225.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org. Accessed July2009.
- ,,,.The care transitions intervention: results for a randomized control trial.Arch Intern Med.2006;166:1822–1828.
- Care Transitions Program. Available at: http://www.caretransitions.org. Accessed July2009.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160(19):2902–2908.
- .Use of mandatory hospitalists blasted.ACP‐ASIM Observer, May1999. Available at: http://www.acpinternist.org/archives/1999/05/hosps.htm. Accessed July 2009.
- .Hospitalists: the next big thing?Trustee Magazine, May2005. Available at: http://www.trusteemag.com/trusteemag_app/jsp/articledisplay.jsp?dcrpath=TRUSTEEMAG/PubsNewsArticleGen/data/2005/0505TRU_FEA_CoverStory. Accessed July 2009.
- ,,.Hospitalist medicine: voluntary or mandatory?Virtual Mentor.2008;10(12):813–816.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- ,.The doctor‐patient relationship in the post‐managed care era.Am J Bioeth2006;6(1):29–32.
- ,,, et al.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- Society of Hospital Medicine.Professionalism and medical ethics.J Hosp Med.2006;1:90–91.
- ,.Teaching residents to consider costs in medical decision making.Am J Bioeth2006;6(4):33–34.
- ,,.Clinical Ethics: A Practical Approach to Ethical Decision in Clinical Medicine.6th ed.New York, NY:McGraw‐Hill Medical;2006.
- ,. The 2004 Medicare and Social Security trustees reports. National Center for Policy Analysis, Study No. 266.2004. Available at: http://www.ncpa.org/pub/st/st266. Accessed July 2009.
- .Dismantling Rube Goldberg: cutting through the chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–70. [http://dx.doi.org/10.1002/jhm.510]
Wachter and Goldman1 first described hospitalists in 1996 as a new breed of physicians who devote blocks of time exclusively to the care of hospitalized patients. Since its definition, the hospitalist model has prompted 2 major debates. First, does the hospitalist system improve clinical efficiency, quality of care, cost effectiveness, and patient satisfaction? A series of large and small randomized trials have all but definitively proven the hospitalist model's advantage. Yet whether the hospitalist model is good for patient care has proven to remain contentious, as most recently demonstrated by the discussion between Williams2 and Centor3 and others like it.4, 5 What is clear in these exchanges is that the debate has shifted to the second great debate: does the hospitalist model pose inherent conflicts in clinical ethics? What are the implications of the purposeful discontinuity in care, the autonomy issues raised by mandatory hospitalist use, and the structural management issues that potentially pit hospitalists against patients in fiduciary and financial conflicts of interest? These important issues are certainly not new, and the hospitalist model has made much effort to address some of them.6, 7 This work aims to serve as a review of these important ethical concerns, demonstrating how some questions have been answered, while some remain unanswered.
The Hospitalist Model's Founding Premise
A growing threshold for hospital admission in the last 3 decades caused primary care physicians (PCPs) to see a diminishing number of inpatients. A survey in 1978 found that PCPs spent 40% of their time in the hospital, rounding on 10 patients per day.8 By 2001, PCPs spent 10% of their time in the hospital on average, and most PCPs rounded on fewer than 2 inpatients per day.9 The cost of inefficiencies associated with primary coordination of care in the hospital increasingly outweighed the tradeoff of preserving the patient‐PCP relationship in the hospital. Converging with increasing attention on cost controls through the restructuring of service provision, the hospitalist was born. Wachter10 argued that the hospitalist model could alleviate inpatient demands placed on PCPs while improving the outcomes and lowering the cost of care for hospitalized patients.
Early on there were setbacks to proving Wachter's10 case. Small studies found hospitalists to have higher hospital charges and longer length of stays.11 A survey of PCPs found only 56% were satisfied with communication with hospitalists and that most believed that patients generally preferred to be cared for in the hospital by their regular physician.12, 13 Meltzer and Herthko14 found 70% of people sampled said they would prefer care by their own physician to that of a hospitalist if they were hospitalized for a general medical condition. Yet this study found in a national random‐digit phone survey that only 10% of the respondents would pay $750 for their PCP to follow them to the hospital, the cost savings of the hospitalist system proven by the only 2 randomized trials performed at the time.15, 16 To 90% of respondents, the value of the PCP at the bedside was not worth the cost tradeoff to keep them there.
The meteoric rise in the number of hospitalists reflects the many studies and reviews that affirmed the premise that hospitalists improved inpatient efficiency without harmful effects on quality of care.17, 18 In a large retrospective cohort study of over 75,000 patients in 45 hospitals across the country, Lindenauer et al.19 found that hospitalists had a $268 lower cost when compared to internists, $125 lower cost when compared to family physicians, and a shorter hospital stay by about one‐half day when compared to both groups. The group found no significant difference in rates of death or readmission rates. While called modest in the text, these savings over time and volume add up for hospitals. Patients benefit from hospitalist care, researchers hypothesize, because of their familiarity with hospital systems, their increased availability to patients, and their experience with common hospital problems. Though the Lindenauer et al.19 study was criticized for design flaws, it prompted the editorialist McMahon20 to assert that the question was sufficiently answered, and it was time to move on away from the studies focusing on cost and comparing outcomes. As Wachter21 wrote, the demand for hospitalists is now relatively de‐linked from the field's original premiseefficiency advantagesand is now both more diversified and more robust. The model has become an accepted mode of care for hospitalized patients, with up to 20,000 hospitalists currently practicing in 29% of all hospitals and in over one‐half of hospitals with over 200 beds in the United States.22, 23
The Patient‐Physician Relationship
Purposeful discontinuity of care in the hospitalist system has the potential to diminish the doctor‐patient relationship.12 This relationship is built on a bond of loyalty, confidentiality, and trust. Handing off care to a hospitalist when the patient is most vulnerable can be viewed as a violation of this covenant. According to Meltzer,24 the hospitalist model pits Franicis Peabody's25 intimate personal relationship between patient and physician against Adam Smith et al.'s11 benefits of specialization. Peabody25 observed that physicians' lack of understanding of their patients as persons is especially acute in the hospital, where
one gets in the habit of using the oil immersion lamp instead of the low power, and focuses too intently in the center of the field. . . . The institutional eye tends to become focused on the lung, and it forgets that the lung is only one member of the body.
This movement toward patient‐centered medicine fits into an ever‐growing sentiment to value the social as well as the physiological, a holistic approach to the patient as a person. This emphasis was the original justification for PCPs to coordinate increasingly specialized hospital care and translate recommendations suitable to patients. Can the long‐term relationship between patient and PCP be replaced by the hospital generalist, or would hospitalists be inherently deficient in their abilities to coordinate care appropriate for patients? Hospitalized patients are frequently in no position to make complex decisions regarding their care.26 Lo7 argues that PCPs who know patients over extended periods of time are in a better position to respect patient wishes by individualizing discussions with patients and checking that patients' decisions are consistent with their core values. The long‐term relationship is also critical for designing a complex discharge plan suitable to the patients' ability and resources. Information about long‐term patient compliance with medications is much more available to PCPs. Patients trust physicians to keep promises made concerning end‐of‐life issues, and these assurances are vulnerable during handoffs of care. Pantilat et al.6 provide a case study of an outpatient Do‐Not‐Resuscitate order ineffective in the hospital. These scenarios occur because most written advance directives are unavailable in acute situations, and when they are, hospitalists unfamiliar with the patient's wishes may hesitate to act on directives not specific enough to answer the acute clinical question.27
Hospitalists' broadened responsibility to systematically improve the care of patients may potentially improve end‐of‐life care. Patient values can be better communicated to hospitalists by encouraging inpatients to complete advance directive surveys and then asking hospitalists to discuss those directives with their patients.6 Significantly, Auerbach and Pantilat28 found that end‐of‐life care was improved with hospitalist care. This chart review found hospitalists more likely to have discussions with patients and their families regarding care and providing comfort care more frequently at the time of death than community‐based physicians. The authors hypothesize that hospitalists may have better communication with dying patients and their families because they spend more time in the hospital each day, using frequent meetings to better understand the preferences of patients. These preferences often require clarification and often change after admission, making previous discussions about end‐of‐life care with PCPs moot. Greater expertise in hospital care may also allow hospitalists to better recognize patients who are nearing death and may explain the fewer symptoms documented by Auerbach and Pantilat28 at the end of life among patients cared for by hospitalists compared to community‐based physicians.
Hospital medicine has taken continuity of care issues seriously, and responded by making pragmatic recommendations to preserve the patient‐PCP relationship in the hospital and assuage the perception that patients have been dropped. Harlan et al.29 identify important issues around good communication between pediatric hospitalists and PCPs including the content and timing of communication beneficial to the patient. Hospitalists can use a standard script for introducing themselves to patients, explaining their role, and their continued coordination with the PCP.30 PCPs can still be involved in the care of their patients in hospitals through continuity visits or phone calls with patients and through better communication with hospitalists.31 Generally, reimbursing PCPs for their increased role in the hospitalist system can encourage better communication with hospitalists.19 Potential disagreements between PCPs and hospitalist regarding the care of the patient can be resolved through explicit conflict resolution procedures within the hospitalist system.6
These procedural solutions are only as successful as they are used. A large review by Kripalani et al.32 found communication between hospitalists and PCPs occurred infrequently (3%‐20%), affecting the quality of care in approximately 25% of follow‐up visits and contributing to PCP dissatisfaction. Sharma et al.33 found that continuity visits decreased from 50.5% in 1996 to 39.8% in 2006. In a survey of patients cared for in a hospitalist system, Hruby et al.34 found that 33% of hospitalized patients had some contact with their PCP directly and 66% of patients were satisfied with the contact they or their relative had with their PCP. When probed, patient satisfaction is too vague a measurement to assess the complex value of the patient‐physician relationship. Studying these issues may require relying more on individualized narratives rather than generalized statistics, or may require years of follow‐up. As Centor3 argues, we need this broader perspective of the patient's experience in order to understand the effects of the hospitalist model on patient trust in their PCP and in their overall care. Studies by Davis et al.35 and Halpert et al.36 assert that rising quality of care and patient satisfaction with the hospitalist system rebuts coordination of care concerns. Yet we need more studies investigating the relationship between improved communication and patient outcomes, as evidence is currently conflicting on this subject.32, 37, 38
The Journal of Hospital Medicine has pursued this research agenda; the April 2009 issue presents several studies describing best practices in the discharging of hospitalized patients. Manning et al.39 describe a tool to assess patient mobility after discharge, and O'Leary et al.40 used electronic health records to create a better discharge summary. Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) has shown improvements in discharge transition procedures41 and the use of transition coaches for vulnerable older patients has been proven cost‐effective and has been scaled up to more than 100 healthcare organizations.42, 43
Inpatient care handoff to PCPs is not entirely novel, as surgeons, oncologists, cardiologists, and other specialists have always grappled with continuity of care. It would be prudent to investigate what can be learned from these efforts, and which practices can be best applied to the hospitalist model. More longitudinal studies need to investigate the prevalence and success of the procedural recommendations to preserve the patient‐physician relationship. We need to know more about what works and what does not. How have hospitals found novel ways in implementing these approaches, and how can they be applied to a diversity of hospital settings? We need a better outcome measurement than patient or physician satisfaction for probing the subtleties of the patient‐physician relationship. There is a sizeable population that does not have a PCP to care for them before hospitalization or after discharge, and discussions about continuity of care must address these patients. Last, these best practices and patient centered values need to be incorporated into the core competencies of residencies and fellowships for a new generation of hospitalists.
Maintaining the continuity of the physician‐patient relationship is an integral part of the original premise of the hospitalist model. Importantly, Meltzer24 found that this discontinuity within the hospital has the potential to eliminate the savings of the hospitalist system. Yet concerns about continuity of care do not sufficiently encompass the complexand at times fragilerelationship between physician and patient. The survival of the physician‐patient relationship depends on the hospitalist model's affirmation of the values of coordination and Peabody's25 approach to patient‐centered care. If the hospitalist model is to thrive, it needs to emphasize its duty as steward of the PCP‐patient relationship as much as it focuses on efficiency and cost‐effectiveness.
Patient Autonomy
The mandatory transfer of patients into the hospitalist model raises serious ethical issues. A survey in 2000 of PCPs found that 23% were required to use hospitalists for all admissions.44 Other surveys found this prevalence to be as low as 2%.12 Nevertheless, several high profile cases of Health Maintenance Organizations (HMOs)Prudential HealthCareSouth Florida, Prudential, Humana, and Cigna Corporationall using mandatory hospitalists, prompted protests from professional organizations and there were even legislative efforts to ban the practice of the mandatory use of hospitalists in 2000 and 2001.45 Today, most insurance plans, as well as the Society of Hospital Medicine (SHM), support voluntary rather than mandatory hospitalist use.46 Yet while not mandatory, the hospitalist is the default provider in many settings, giving a de facto mandate for hospitalist care. As Royo et al.47 point out, the rise in physician employment by hospitals has facilitated a self‐selecting progression toward a structural network that closely resembles the mandatory model.
While PCPs and internists contested mandatory hospitalist plans as infringements on their autonomy, they overlooked the harm to the patient's autonomy. When healthy in the ambulatory setting, the patient has the opportunity to choose his or her doctor to provide longitudinal care. When the patient is admitted acutely to a hospital, the patient does not have the freedom to choose a physician; the patient is assigned to the hospitalist on duty that night. This call for patient autonomy is of utmost importance in the hospitalized patient, where patients are increasingly sicker, their diseases under a high‐powered lens, and their options diminished. This freedom of choice is integral to the patient‐physician partnership. Yet this freedom of choice is largely hindered by the employer's choice in the health plan for their employees or an individual's ability to pay for a health plan. These represent some of the many barriers to choice facing patients in the American model of health insurance.
As the hospitalist system grows to become the accepted mode of hospital care, more patients need to be informed about the transition of care to another physician and what steps are taken to ensure appropriate continuity of care. Transfers of patients from PCPs to hospitalists must be voluntary, with the decision left to patient care preferences.48 Educating patients in the outpatient setting about the hospitalist model, its benefits, risks, and alternatives, is necessary for them to make informed decisions about hospital care. This will require the collaboration of PCPs and hospitalists together. The continued success of the model depends on the nurturance of the partnership between the PCP, the hospitalist, and the patient.
Meltzer and Herthko14 have proposed that patients pay a premium for the option to choose a PCP that is not mandated to transfer their care to a hospitalist, in order to offset cost savings with the hospitalist system. Yet Meltzer and Herthko's14 study suggests that many patients could not afford to pay this premium and, in effect, patient autonomy would be preserved for the affluent. This raises the oft‐neglected professional ethic of justice for low‐income patients. Alexander and Lantos49 were resigned to see this infringement on patient autonomy as an inevitable consequence of balancing the desires of patients with the drive to lower cost and improve outcomes. If the hospitalist model grows to be the predominant mode of care, it is unclear if patient choice can survive. Investigators need to test whether the advantages of hospitalist care can coexist with voluntary programs. If it proves that they indeed cannot, then the hospitalist system will need to respond to concerned patients with honest answers and find pragmatic solutions to diminishing patient choice.
Conflict of Interest
The hospitalist system's main benefit of cost‐savings prompted Pantilat et al.6 to wonder whether hospitalists would face a conflict of interest between what is best for the patient and what financial incentives and utilization review encourage or require them to do. The financial support provided by many hospitals to meet the operating expenses of hospitalist programs is often associated with explicit or implicit incentives to reduce the length of hospital stay and costs.50 With hospitals employing hospitalists and increasingly pressuring them to decrease length of stay and discharge patients quickly, patients may have no advocate to protect them from discharge planners. Many hospitalists supplement their income by supervising discharge planners, and a dispute would put the hospitalist in the uncomfortable position of advocating for his patient against his employer and colleagues. While conflicts of interests occur in many managed care arrangements, they may be more acute in hospitalist systems. A weakened patient‐physician relationship may put the patients' best interest inferior to the employer's interests. Hospitalists do not immediately deal with adverse consequences of premature discharges in the outpatient setting and virtually no malpractice case law considers the obligations and practices of hospitalists in these settings.51
The SHM identified a core competency of hospitalists to
recommend treatment options that optimize patient care, include consideration of resource utilization, and are formulated without regard to financial incentives or other conflicts of interest.52
Ethical issues concerning conflict of interest remain unanswered, largely because no information about organizational features such as explicit incentives for reductions in length of stay is available to researchers or to patients. This is the wrong approach and only feeds the fear that hospitalists may weigh patients' best interest with financial incentives. Abbo and Volandes53 have argued that ambivalence to cost considerations is hazardous. If the hospitalist model cannot be forthright with the active considerations of costs in daily clinical practice, it is unlikely to truly make strides at cost savings, and may even raise the cost of care in the long run.
Jonsen et al.54 provide ethical standards for considering costs in clinical decisions. First, a physician's first priority should be to provide patient‐centered care that focuses on medical indications and patient preferences. Second, quality care does not mean all available care; quality care reflects what is not only diagnostically sound and technically correct, but also appropriate. Third, conflicts of interest are most vulnerable when there is a failing of the patient‐physician relationship. Health care organizations should expect physicians to argue for policies that provide all services that have a reasonable likelihood of benefiting the patient. Fourth, patient and physician autonomy and freedom of choice should be maximized within the limits of the system. Persons should be fully informed of the constraints of the system before choosing it. Plans need to disclose any financial incentive arrangements that exist between the plan and the physician. And incentive arrangements should be based on quality of care rather than on underutilization of care services. Fifth, the system should reflect principles of just distribution, ensuring that all who have a fair claim to service should receive it without discrimination. Last, capitation plans should share risks among physicians, not patients, while incentives are provided for improvements in access, prevention, and patient satisfaction.
Conflicts of interest have been a concern for as long as physicians have been paid for services. Fears about interference into the doctor‐patient relationship, whether they are from government or business, continue to stall real efforts to lower skyrocketing medical costs. The hospitalist model rebuts conflict of interest claims with improved outcomes, efficiency, and quality of care in the many reviews cited above. These arguments do prove that the hospitalist model's emphasis on medically indicated and appropriate care does address Jonsen et al.'s54 first and second standards. Yet, as Jonsen et al.54 point out, without strongly emphasizing the patient‐physician relationship and patient autonomy, it leaves itself vulnerable to creating conflicts of interest. Hospitalist systems need to be forthright about their explicit or implicit incentive structures and disclose this information to patients in a timely manner for them to make informed decisions. These incentives should be linked to quality of care and patient satisfaction, not cost savings. Last, hospitalist training programs should make ethical cost considerations a core competency of their curriculum.
Conclusions
Hospitalism was founded on the premise that it could improve the quality and reduce the cost of hospital care. Many randomized studies have all but definitively proven this original assertion. It is now time for the model to prove that these gains are not to the detriment of the patient‐physician relationship. Hospitalism must define itself as the steward of this relationship, valuing it as much as it values outcomes and costs. This is of particular concern in the United States as Medicare Part A (payment for inpatient care) is scheduled to go bankrupt in 2019, leading to potentially reasonable fears of hospital‐motivated cost containment.57
Investigators must find an outcome that encompasses the complexity of the patient‐physician relationship, and methods to improve it must be studied and improved upon. Preserving the patient‐physician relationship is a systemic issue, and full‐time hospitalists may be in the best position to implement systemic reforms to improve communication and continuity of care. Pham's56 case study of a hospitalist piecing together disparate parts of the patient's story illustrates this point. This should include more investigation into the prevalence of use and success of methods aimed at protecting the patient‐physician relationship at critical points in the handover of care. When proven successful, The SHM should propose new standards and safeguards to insure that these methods become standard practice in patient care. This effort, led by Snow et al.,57 is currently underway.
A hospitalist model that does not emphasize mitigating the effects of the diminishing patient‐physician relationship leaves itself exposed to further infringements on autonomy and choice. It is unclear whether patient autonomy and choice can coexist in a successful hospitalist system. The consequences of these unanswered ethical questions need to be explored. The professions of primary care need to be more proactive in educating patients about choice of care in hospitals, and hospitalists need to provide that choice, allowing voluntary programs in hospital care when feasible.
When combined, a wounded patient‐physician relationship and impaired patient autonomy leave the hospitalist model vulnerable to claims of financial and fiduciary conflict of interest. These concerns need not be inherent to the hospitalist systems, but hospitalists will need to be forthright and honest about incentives structures, and link them to quality of care and patient satisfaction, not to efficiency and cost savings.
It is indeed time for hospitalism to move onaway from proving its founding premise, and toward addressing these lingering ethical issues. Hospitalism's continued growth and success depends on it.
Wachter and Goldman1 first described hospitalists in 1996 as a new breed of physicians who devote blocks of time exclusively to the care of hospitalized patients. Since its definition, the hospitalist model has prompted 2 major debates. First, does the hospitalist system improve clinical efficiency, quality of care, cost effectiveness, and patient satisfaction? A series of large and small randomized trials have all but definitively proven the hospitalist model's advantage. Yet whether the hospitalist model is good for patient care has proven to remain contentious, as most recently demonstrated by the discussion between Williams2 and Centor3 and others like it.4, 5 What is clear in these exchanges is that the debate has shifted to the second great debate: does the hospitalist model pose inherent conflicts in clinical ethics? What are the implications of the purposeful discontinuity in care, the autonomy issues raised by mandatory hospitalist use, and the structural management issues that potentially pit hospitalists against patients in fiduciary and financial conflicts of interest? These important issues are certainly not new, and the hospitalist model has made much effort to address some of them.6, 7 This work aims to serve as a review of these important ethical concerns, demonstrating how some questions have been answered, while some remain unanswered.
The Hospitalist Model's Founding Premise
A growing threshold for hospital admission in the last 3 decades caused primary care physicians (PCPs) to see a diminishing number of inpatients. A survey in 1978 found that PCPs spent 40% of their time in the hospital, rounding on 10 patients per day.8 By 2001, PCPs spent 10% of their time in the hospital on average, and most PCPs rounded on fewer than 2 inpatients per day.9 The cost of inefficiencies associated with primary coordination of care in the hospital increasingly outweighed the tradeoff of preserving the patient‐PCP relationship in the hospital. Converging with increasing attention on cost controls through the restructuring of service provision, the hospitalist was born. Wachter10 argued that the hospitalist model could alleviate inpatient demands placed on PCPs while improving the outcomes and lowering the cost of care for hospitalized patients.
Early on there were setbacks to proving Wachter's10 case. Small studies found hospitalists to have higher hospital charges and longer length of stays.11 A survey of PCPs found only 56% were satisfied with communication with hospitalists and that most believed that patients generally preferred to be cared for in the hospital by their regular physician.12, 13 Meltzer and Herthko14 found 70% of people sampled said they would prefer care by their own physician to that of a hospitalist if they were hospitalized for a general medical condition. Yet this study found in a national random‐digit phone survey that only 10% of the respondents would pay $750 for their PCP to follow them to the hospital, the cost savings of the hospitalist system proven by the only 2 randomized trials performed at the time.15, 16 To 90% of respondents, the value of the PCP at the bedside was not worth the cost tradeoff to keep them there.
The meteoric rise in the number of hospitalists reflects the many studies and reviews that affirmed the premise that hospitalists improved inpatient efficiency without harmful effects on quality of care.17, 18 In a large retrospective cohort study of over 75,000 patients in 45 hospitals across the country, Lindenauer et al.19 found that hospitalists had a $268 lower cost when compared to internists, $125 lower cost when compared to family physicians, and a shorter hospital stay by about one‐half day when compared to both groups. The group found no significant difference in rates of death or readmission rates. While called modest in the text, these savings over time and volume add up for hospitals. Patients benefit from hospitalist care, researchers hypothesize, because of their familiarity with hospital systems, their increased availability to patients, and their experience with common hospital problems. Though the Lindenauer et al.19 study was criticized for design flaws, it prompted the editorialist McMahon20 to assert that the question was sufficiently answered, and it was time to move on away from the studies focusing on cost and comparing outcomes. As Wachter21 wrote, the demand for hospitalists is now relatively de‐linked from the field's original premiseefficiency advantagesand is now both more diversified and more robust. The model has become an accepted mode of care for hospitalized patients, with up to 20,000 hospitalists currently practicing in 29% of all hospitals and in over one‐half of hospitals with over 200 beds in the United States.22, 23
The Patient‐Physician Relationship
Purposeful discontinuity of care in the hospitalist system has the potential to diminish the doctor‐patient relationship.12 This relationship is built on a bond of loyalty, confidentiality, and trust. Handing off care to a hospitalist when the patient is most vulnerable can be viewed as a violation of this covenant. According to Meltzer,24 the hospitalist model pits Franicis Peabody's25 intimate personal relationship between patient and physician against Adam Smith et al.'s11 benefits of specialization. Peabody25 observed that physicians' lack of understanding of their patients as persons is especially acute in the hospital, where
one gets in the habit of using the oil immersion lamp instead of the low power, and focuses too intently in the center of the field. . . . The institutional eye tends to become focused on the lung, and it forgets that the lung is only one member of the body.
This movement toward patient‐centered medicine fits into an ever‐growing sentiment to value the social as well as the physiological, a holistic approach to the patient as a person. This emphasis was the original justification for PCPs to coordinate increasingly specialized hospital care and translate recommendations suitable to patients. Can the long‐term relationship between patient and PCP be replaced by the hospital generalist, or would hospitalists be inherently deficient in their abilities to coordinate care appropriate for patients? Hospitalized patients are frequently in no position to make complex decisions regarding their care.26 Lo7 argues that PCPs who know patients over extended periods of time are in a better position to respect patient wishes by individualizing discussions with patients and checking that patients' decisions are consistent with their core values. The long‐term relationship is also critical for designing a complex discharge plan suitable to the patients' ability and resources. Information about long‐term patient compliance with medications is much more available to PCPs. Patients trust physicians to keep promises made concerning end‐of‐life issues, and these assurances are vulnerable during handoffs of care. Pantilat et al.6 provide a case study of an outpatient Do‐Not‐Resuscitate order ineffective in the hospital. These scenarios occur because most written advance directives are unavailable in acute situations, and when they are, hospitalists unfamiliar with the patient's wishes may hesitate to act on directives not specific enough to answer the acute clinical question.27
Hospitalists' broadened responsibility to systematically improve the care of patients may potentially improve end‐of‐life care. Patient values can be better communicated to hospitalists by encouraging inpatients to complete advance directive surveys and then asking hospitalists to discuss those directives with their patients.6 Significantly, Auerbach and Pantilat28 found that end‐of‐life care was improved with hospitalist care. This chart review found hospitalists more likely to have discussions with patients and their families regarding care and providing comfort care more frequently at the time of death than community‐based physicians. The authors hypothesize that hospitalists may have better communication with dying patients and their families because they spend more time in the hospital each day, using frequent meetings to better understand the preferences of patients. These preferences often require clarification and often change after admission, making previous discussions about end‐of‐life care with PCPs moot. Greater expertise in hospital care may also allow hospitalists to better recognize patients who are nearing death and may explain the fewer symptoms documented by Auerbach and Pantilat28 at the end of life among patients cared for by hospitalists compared to community‐based physicians.
Hospital medicine has taken continuity of care issues seriously, and responded by making pragmatic recommendations to preserve the patient‐PCP relationship in the hospital and assuage the perception that patients have been dropped. Harlan et al.29 identify important issues around good communication between pediatric hospitalists and PCPs including the content and timing of communication beneficial to the patient. Hospitalists can use a standard script for introducing themselves to patients, explaining their role, and their continued coordination with the PCP.30 PCPs can still be involved in the care of their patients in hospitals through continuity visits or phone calls with patients and through better communication with hospitalists.31 Generally, reimbursing PCPs for their increased role in the hospitalist system can encourage better communication with hospitalists.19 Potential disagreements between PCPs and hospitalist regarding the care of the patient can be resolved through explicit conflict resolution procedures within the hospitalist system.6
These procedural solutions are only as successful as they are used. A large review by Kripalani et al.32 found communication between hospitalists and PCPs occurred infrequently (3%‐20%), affecting the quality of care in approximately 25% of follow‐up visits and contributing to PCP dissatisfaction. Sharma et al.33 found that continuity visits decreased from 50.5% in 1996 to 39.8% in 2006. In a survey of patients cared for in a hospitalist system, Hruby et al.34 found that 33% of hospitalized patients had some contact with their PCP directly and 66% of patients were satisfied with the contact they or their relative had with their PCP. When probed, patient satisfaction is too vague a measurement to assess the complex value of the patient‐physician relationship. Studying these issues may require relying more on individualized narratives rather than generalized statistics, or may require years of follow‐up. As Centor3 argues, we need this broader perspective of the patient's experience in order to understand the effects of the hospitalist model on patient trust in their PCP and in their overall care. Studies by Davis et al.35 and Halpert et al.36 assert that rising quality of care and patient satisfaction with the hospitalist system rebuts coordination of care concerns. Yet we need more studies investigating the relationship between improved communication and patient outcomes, as evidence is currently conflicting on this subject.32, 37, 38
The Journal of Hospital Medicine has pursued this research agenda; the April 2009 issue presents several studies describing best practices in the discharging of hospitalized patients. Manning et al.39 describe a tool to assess patient mobility after discharge, and O'Leary et al.40 used electronic health records to create a better discharge summary. Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) has shown improvements in discharge transition procedures41 and the use of transition coaches for vulnerable older patients has been proven cost‐effective and has been scaled up to more than 100 healthcare organizations.42, 43
Inpatient care handoff to PCPs is not entirely novel, as surgeons, oncologists, cardiologists, and other specialists have always grappled with continuity of care. It would be prudent to investigate what can be learned from these efforts, and which practices can be best applied to the hospitalist model. More longitudinal studies need to investigate the prevalence and success of the procedural recommendations to preserve the patient‐physician relationship. We need to know more about what works and what does not. How have hospitals found novel ways in implementing these approaches, and how can they be applied to a diversity of hospital settings? We need a better outcome measurement than patient or physician satisfaction for probing the subtleties of the patient‐physician relationship. There is a sizeable population that does not have a PCP to care for them before hospitalization or after discharge, and discussions about continuity of care must address these patients. Last, these best practices and patient centered values need to be incorporated into the core competencies of residencies and fellowships for a new generation of hospitalists.
Maintaining the continuity of the physician‐patient relationship is an integral part of the original premise of the hospitalist model. Importantly, Meltzer24 found that this discontinuity within the hospital has the potential to eliminate the savings of the hospitalist system. Yet concerns about continuity of care do not sufficiently encompass the complexand at times fragilerelationship between physician and patient. The survival of the physician‐patient relationship depends on the hospitalist model's affirmation of the values of coordination and Peabody's25 approach to patient‐centered care. If the hospitalist model is to thrive, it needs to emphasize its duty as steward of the PCP‐patient relationship as much as it focuses on efficiency and cost‐effectiveness.
Patient Autonomy
The mandatory transfer of patients into the hospitalist model raises serious ethical issues. A survey in 2000 of PCPs found that 23% were required to use hospitalists for all admissions.44 Other surveys found this prevalence to be as low as 2%.12 Nevertheless, several high profile cases of Health Maintenance Organizations (HMOs)Prudential HealthCareSouth Florida, Prudential, Humana, and Cigna Corporationall using mandatory hospitalists, prompted protests from professional organizations and there were even legislative efforts to ban the practice of the mandatory use of hospitalists in 2000 and 2001.45 Today, most insurance plans, as well as the Society of Hospital Medicine (SHM), support voluntary rather than mandatory hospitalist use.46 Yet while not mandatory, the hospitalist is the default provider in many settings, giving a de facto mandate for hospitalist care. As Royo et al.47 point out, the rise in physician employment by hospitals has facilitated a self‐selecting progression toward a structural network that closely resembles the mandatory model.
While PCPs and internists contested mandatory hospitalist plans as infringements on their autonomy, they overlooked the harm to the patient's autonomy. When healthy in the ambulatory setting, the patient has the opportunity to choose his or her doctor to provide longitudinal care. When the patient is admitted acutely to a hospital, the patient does not have the freedom to choose a physician; the patient is assigned to the hospitalist on duty that night. This call for patient autonomy is of utmost importance in the hospitalized patient, where patients are increasingly sicker, their diseases under a high‐powered lens, and their options diminished. This freedom of choice is integral to the patient‐physician partnership. Yet this freedom of choice is largely hindered by the employer's choice in the health plan for their employees or an individual's ability to pay for a health plan. These represent some of the many barriers to choice facing patients in the American model of health insurance.
As the hospitalist system grows to become the accepted mode of hospital care, more patients need to be informed about the transition of care to another physician and what steps are taken to ensure appropriate continuity of care. Transfers of patients from PCPs to hospitalists must be voluntary, with the decision left to patient care preferences.48 Educating patients in the outpatient setting about the hospitalist model, its benefits, risks, and alternatives, is necessary for them to make informed decisions about hospital care. This will require the collaboration of PCPs and hospitalists together. The continued success of the model depends on the nurturance of the partnership between the PCP, the hospitalist, and the patient.
Meltzer and Herthko14 have proposed that patients pay a premium for the option to choose a PCP that is not mandated to transfer their care to a hospitalist, in order to offset cost savings with the hospitalist system. Yet Meltzer and Herthko's14 study suggests that many patients could not afford to pay this premium and, in effect, patient autonomy would be preserved for the affluent. This raises the oft‐neglected professional ethic of justice for low‐income patients. Alexander and Lantos49 were resigned to see this infringement on patient autonomy as an inevitable consequence of balancing the desires of patients with the drive to lower cost and improve outcomes. If the hospitalist model grows to be the predominant mode of care, it is unclear if patient choice can survive. Investigators need to test whether the advantages of hospitalist care can coexist with voluntary programs. If it proves that they indeed cannot, then the hospitalist system will need to respond to concerned patients with honest answers and find pragmatic solutions to diminishing patient choice.
Conflict of Interest
The hospitalist system's main benefit of cost‐savings prompted Pantilat et al.6 to wonder whether hospitalists would face a conflict of interest between what is best for the patient and what financial incentives and utilization review encourage or require them to do. The financial support provided by many hospitals to meet the operating expenses of hospitalist programs is often associated with explicit or implicit incentives to reduce the length of hospital stay and costs.50 With hospitals employing hospitalists and increasingly pressuring them to decrease length of stay and discharge patients quickly, patients may have no advocate to protect them from discharge planners. Many hospitalists supplement their income by supervising discharge planners, and a dispute would put the hospitalist in the uncomfortable position of advocating for his patient against his employer and colleagues. While conflicts of interests occur in many managed care arrangements, they may be more acute in hospitalist systems. A weakened patient‐physician relationship may put the patients' best interest inferior to the employer's interests. Hospitalists do not immediately deal with adverse consequences of premature discharges in the outpatient setting and virtually no malpractice case law considers the obligations and practices of hospitalists in these settings.51
The SHM identified a core competency of hospitalists to
recommend treatment options that optimize patient care, include consideration of resource utilization, and are formulated without regard to financial incentives or other conflicts of interest.52
Ethical issues concerning conflict of interest remain unanswered, largely because no information about organizational features such as explicit incentives for reductions in length of stay is available to researchers or to patients. This is the wrong approach and only feeds the fear that hospitalists may weigh patients' best interest with financial incentives. Abbo and Volandes53 have argued that ambivalence to cost considerations is hazardous. If the hospitalist model cannot be forthright with the active considerations of costs in daily clinical practice, it is unlikely to truly make strides at cost savings, and may even raise the cost of care in the long run.
Jonsen et al.54 provide ethical standards for considering costs in clinical decisions. First, a physician's first priority should be to provide patient‐centered care that focuses on medical indications and patient preferences. Second, quality care does not mean all available care; quality care reflects what is not only diagnostically sound and technically correct, but also appropriate. Third, conflicts of interest are most vulnerable when there is a failing of the patient‐physician relationship. Health care organizations should expect physicians to argue for policies that provide all services that have a reasonable likelihood of benefiting the patient. Fourth, patient and physician autonomy and freedom of choice should be maximized within the limits of the system. Persons should be fully informed of the constraints of the system before choosing it. Plans need to disclose any financial incentive arrangements that exist between the plan and the physician. And incentive arrangements should be based on quality of care rather than on underutilization of care services. Fifth, the system should reflect principles of just distribution, ensuring that all who have a fair claim to service should receive it without discrimination. Last, capitation plans should share risks among physicians, not patients, while incentives are provided for improvements in access, prevention, and patient satisfaction.
Conflicts of interest have been a concern for as long as physicians have been paid for services. Fears about interference into the doctor‐patient relationship, whether they are from government or business, continue to stall real efforts to lower skyrocketing medical costs. The hospitalist model rebuts conflict of interest claims with improved outcomes, efficiency, and quality of care in the many reviews cited above. These arguments do prove that the hospitalist model's emphasis on medically indicated and appropriate care does address Jonsen et al.'s54 first and second standards. Yet, as Jonsen et al.54 point out, without strongly emphasizing the patient‐physician relationship and patient autonomy, it leaves itself vulnerable to creating conflicts of interest. Hospitalist systems need to be forthright about their explicit or implicit incentive structures and disclose this information to patients in a timely manner for them to make informed decisions. These incentives should be linked to quality of care and patient satisfaction, not cost savings. Last, hospitalist training programs should make ethical cost considerations a core competency of their curriculum.
Conclusions
Hospitalism was founded on the premise that it could improve the quality and reduce the cost of hospital care. Many randomized studies have all but definitively proven this original assertion. It is now time for the model to prove that these gains are not to the detriment of the patient‐physician relationship. Hospitalism must define itself as the steward of this relationship, valuing it as much as it values outcomes and costs. This is of particular concern in the United States as Medicare Part A (payment for inpatient care) is scheduled to go bankrupt in 2019, leading to potentially reasonable fears of hospital‐motivated cost containment.57
Investigators must find an outcome that encompasses the complexity of the patient‐physician relationship, and methods to improve it must be studied and improved upon. Preserving the patient‐physician relationship is a systemic issue, and full‐time hospitalists may be in the best position to implement systemic reforms to improve communication and continuity of care. Pham's56 case study of a hospitalist piecing together disparate parts of the patient's story illustrates this point. This should include more investigation into the prevalence of use and success of methods aimed at protecting the patient‐physician relationship at critical points in the handover of care. When proven successful, The SHM should propose new standards and safeguards to insure that these methods become standard practice in patient care. This effort, led by Snow et al.,57 is currently underway.
A hospitalist model that does not emphasize mitigating the effects of the diminishing patient‐physician relationship leaves itself exposed to further infringements on autonomy and choice. It is unclear whether patient autonomy and choice can coexist in a successful hospitalist system. The consequences of these unanswered ethical questions need to be explored. The professions of primary care need to be more proactive in educating patients about choice of care in hospitals, and hospitalists need to provide that choice, allowing voluntary programs in hospital care when feasible.
When combined, a wounded patient‐physician relationship and impaired patient autonomy leave the hospitalist model vulnerable to claims of financial and fiduciary conflict of interest. These concerns need not be inherent to the hospitalist systems, but hospitalists will need to be forthright and honest about incentives structures, and link them to quality of care and patient satisfaction, not to efficiency and cost savings.
It is indeed time for hospitalism to move onaway from proving its founding premise, and toward addressing these lingering ethical issues. Hospitalism's continued growth and success depends on it.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists and the hospital medicine system of care are good for patient care.Arch Intern Med.2008;168(12):1254–1256, discussion 1259–1260.
- .A hospitalist inpatient system does not improve patient care outcomes.Arch Intern Med.2008;168(12):1257–1258, discussion 1259–1260.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: Yes.Can Fam Physician.2008;54(8):1100–1101,1104–1106.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: No.Can Fam Physician.2008;54(8):1101–1103,1105–1107.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282:171–174.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48:281–290.
- Robert Wood Johnson Foundation.Medical Practice in the United States.Princeton, NJ:The Robert Wood Johnson Foundation;1981.
- .Response to David Meltzer's paper “Hospitalists and the doctor‐patient relationship.”J Legal Stud2001;30:615–623.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- ,,.Primary care family physicians and 2 hospitalists models: comparison of outcomes, processes, and costs.J Fam Prac.2002;51:1021–1027.
- ,,, et al.Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48(4):218–229.
- ,,, et al.Physician attitudes towards and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,.Patients' willingness to pay for hospital care by their primary care physician versus hospitalists: results of a national survey. [Society of General Internal Medicine 23rd annual meeting. Boston, Massachusetts, USA. May 4–6, 2000. Abstracts.]J Gen Intern Med.2000;15(suppl 1):135.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560.
- ,,, et al.Effects of hospitalist physicians on an academic general medicine service: results of a randomized trial. [22nd Annual meeting of The Society of General Internal Medicine. San Francisco, California, USA. April 29‐May 1, 1999. Abstracts.]J Gen Intern Med.1999;14(suppl 2):112.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Economic and healthcare forces of hospitalist movement.Mt Sinai J Med.2008;75(5):424–429.
- ,,, et al.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- .The hospitalist movement—time to move on.N Engl J Med.2007;357:2627–2629.
- .Today's New England Journal Hospitalist Study. Weblog Entry.Wachter's World: The Hospitalist.2007. Available at: http://www.the‐hospitalist.org/blogs/wachters_world/archive/2007/12/20/today‐s‐new‐england‐journal‐hospitalist‐study.aspx. Accessed July 2009.
- ,,, et al.The Rise of the Hospitalist in California.Oakland, CA:California Health Care Foundation;2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Hospitalists and the doctor patient relationship.J Legal Stud.2001;2:615–623.
- .Landmark article March 19, 1927: the care of the patient. By Francis W. Peabody.JAMA.1984;252:813–818.
- .The Practice of Autonomy: Patients, Doctors, and Medical Decisions.New York, NY:Oxford University Press;1998.
- ,,, et al.A prospective study of advance directives for life‐sustaining care.N Engl J Med.1991;324:882–888.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,, et al.Pediatric hospitalists and primary care providers: a communication needs assessment.J Hosp Med.2009;4(3):187–193.
- .What should you say after “Hello”?Today's Hospitalist Apr2008. Available at: http://www.todayshospitalist.com/index.php?b=articles_read48:267–272.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,,, et al.Effects of hospitalists on cost, outcomes and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24:381–386.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- ,,.Home alone: mobility independence before discharge.J Hosp Med.2009;4:252–254.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4:219–225.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org. Accessed July2009.
- ,,,.The care transitions intervention: results for a randomized control trial.Arch Intern Med.2006;166:1822–1828.
- Care Transitions Program. Available at: http://www.caretransitions.org. Accessed July2009.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160(19):2902–2908.
- .Use of mandatory hospitalists blasted.ACP‐ASIM Observer, May1999. Available at: http://www.acpinternist.org/archives/1999/05/hosps.htm. Accessed July 2009.
- .Hospitalists: the next big thing?Trustee Magazine, May2005. Available at: http://www.trusteemag.com/trusteemag_app/jsp/articledisplay.jsp?dcrpath=TRUSTEEMAG/PubsNewsArticleGen/data/2005/0505TRU_FEA_CoverStory. Accessed July 2009.
- ,,.Hospitalist medicine: voluntary or mandatory?Virtual Mentor.2008;10(12):813–816.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- ,.The doctor‐patient relationship in the post‐managed care era.Am J Bioeth2006;6(1):29–32.
- ,,, et al.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- Society of Hospital Medicine.Professionalism and medical ethics.J Hosp Med.2006;1:90–91.
- ,.Teaching residents to consider costs in medical decision making.Am J Bioeth2006;6(4):33–34.
- ,,.Clinical Ethics: A Practical Approach to Ethical Decision in Clinical Medicine.6th ed.New York, NY:McGraw‐Hill Medical;2006.
- ,. The 2004 Medicare and Social Security trustees reports. National Center for Policy Analysis, Study No. 266.2004. Available at: http://www.ncpa.org/pub/st/st266. Accessed July 2009.
- .Dismantling Rube Goldberg: cutting through the chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–70. [http://dx.doi.org/10.1002/jhm.510]
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists and the hospital medicine system of care are good for patient care.Arch Intern Med.2008;168(12):1254–1256, discussion 1259–1260.
- .A hospitalist inpatient system does not improve patient care outcomes.Arch Intern Med.2008;168(12):1257–1258, discussion 1259–1260.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: Yes.Can Fam Physician.2008;54(8):1100–1101,1104–1106.
- .Are inpatients' needs better served by hospitalists than by their family doctors?: No.Can Fam Physician.2008;54(8):1101–1103,1105–1107.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282:171–174.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48:281–290.
- Robert Wood Johnson Foundation.Medical Practice in the United States.Princeton, NJ:The Robert Wood Johnson Foundation;1981.
- .Response to David Meltzer's paper “Hospitalists and the doctor‐patient relationship.”J Legal Stud2001;30:615–623.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- ,,.Primary care family physicians and 2 hospitalists models: comparison of outcomes, processes, and costs.J Fam Prac.2002;51:1021–1027.
- ,,, et al.Primary care physician attitudes regarding communication with hospitalists.Dis Mon.2002;48(4):218–229.
- ,,, et al.Physician attitudes towards and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,.Patients' willingness to pay for hospital care by their primary care physician versus hospitalists: results of a national survey. [Society of General Internal Medicine 23rd annual meeting. Boston, Massachusetts, USA. May 4–6, 2000. Abstracts.]J Gen Intern Med.2000;15(suppl 1):135.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560.
- ,,, et al.Effects of hospitalist physicians on an academic general medicine service: results of a randomized trial. [22nd Annual meeting of The Society of General Internal Medicine. San Francisco, California, USA. April 29‐May 1, 1999. Abstracts.]J Gen Intern Med.1999;14(suppl 2):112.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.Economic and healthcare forces of hospitalist movement.Mt Sinai J Med.2008;75(5):424–429.
- ,,, et al.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- .The hospitalist movement—time to move on.N Engl J Med.2007;357:2627–2629.
- .Today's New England Journal Hospitalist Study. Weblog Entry.Wachter's World: The Hospitalist.2007. Available at: http://www.the‐hospitalist.org/blogs/wachters_world/archive/2007/12/20/today‐s‐new‐england‐journal‐hospitalist‐study.aspx. Accessed July 2009.
- ,,, et al.The Rise of the Hospitalist in California.Oakland, CA:California Health Care Foundation;2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Hospitalists and the doctor patient relationship.J Legal Stud.2001;2:615–623.
- .Landmark article March 19, 1927: the care of the patient. By Francis W. Peabody.JAMA.1984;252:813–818.
- .The Practice of Autonomy: Patients, Doctors, and Medical Decisions.New York, NY:Oxford University Press;1998.
- ,,, et al.A prospective study of advance directives for life‐sustaining care.N Engl J Med.1991;324:882–888.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,, et al.Pediatric hospitalists and primary care providers: a communication needs assessment.J Hosp Med.2009;4(3):187–193.
- .What should you say after “Hello”?Today's Hospitalist Apr2008. Available at: http://www.todayshospitalist.com/index.php?b=articles_read48:267–272.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,,, et al.Effects of hospitalists on cost, outcomes and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24:381–386.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- ,,.Home alone: mobility independence before discharge.J Hosp Med.2009;4:252–254.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4:219–225.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org. Accessed July2009.
- ,,,.The care transitions intervention: results for a randomized control trial.Arch Intern Med.2006;166:1822–1828.
- Care Transitions Program. Available at: http://www.caretransitions.org. Accessed July2009.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160(19):2902–2908.
- .Use of mandatory hospitalists blasted.ACP‐ASIM Observer, May1999. Available at: http://www.acpinternist.org/archives/1999/05/hosps.htm. Accessed July 2009.
- .Hospitalists: the next big thing?Trustee Magazine, May2005. Available at: http://www.trusteemag.com/trusteemag_app/jsp/articledisplay.jsp?dcrpath=TRUSTEEMAG/PubsNewsArticleGen/data/2005/0505TRU_FEA_CoverStory. Accessed July 2009.
- ,,.Hospitalist medicine: voluntary or mandatory?Virtual Mentor.2008;10(12):813–816.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- ,.The doctor‐patient relationship in the post‐managed care era.Am J Bioeth2006;6(1):29–32.
- ,,, et al.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- Society of Hospital Medicine.Professionalism and medical ethics.J Hosp Med.2006;1:90–91.
- ,.Teaching residents to consider costs in medical decision making.Am J Bioeth2006;6(4):33–34.
- ,,.Clinical Ethics: A Practical Approach to Ethical Decision in Clinical Medicine.6th ed.New York, NY:McGraw‐Hill Medical;2006.
- ,. The 2004 Medicare and Social Security trustees reports. National Center for Policy Analysis, Study No. 266.2004. Available at: http://www.ncpa.org/pub/st/st266. Accessed July 2009.
- .Dismantling Rube Goldberg: cutting through the chaos to achieve coordinated care.J Hosp Med.2009;4(4):259–260.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–70. [http://dx.doi.org/10.1002/jhm.510]
Patient Hospital Financial Burden
Hospitalizations often impose a tremendous financial burden on patients and their families, adding to the stress and long‐term impact from medical illnesses. It is widely underappreciated that physicians can play an important role in substantially reducing patients' out‐of‐pocket expenses by participating in hospital‐based case review and utilization management. These topics are not a focus of most formal training curricula and unfortunately are typically viewed by medical staff as intrusive, time consuming, or only in terms of enhancing the facility's profitability. In reality, with strict rules governing insurance benefits the facility's interests are typically aligned with those of the patient.
One of the greatest impacts on a patient's financial liability is whether an admission is classified in observation vs. inpatient status, and is subject to much confusion. It is a common misperception that these are time‐based designations. Instead, they revolve around stringent medical necessity guidelines that examine the severity of the illness and the intensity of services provided.1 Inpatient stays may be brief, even a single day, if justified by medical need (although these short durations are closely scrutinized by the payors) or if involving a short list of procedures automatically triggering that status (ie, defibrillator placement).2 Conversely, observation status, although usually up to only 48 hours, can extend longer if inpatient criteria are never met and are then apt to generate large bills.
The key concept for the financial liability of patients in observation status is that their billing structure revolves around being categorized as outpatients, even though they stay overnight and are physically housed and cared for in the expensive hospital setting.3 This nonintuitive classification can culminate in unexpectedly high charges for which the patient is liable (Table 1): medications at inflated hospital pharmacy prices, especially when expensive antibiotics or immunosuppressive agents are administered (since outpatient prescriptions are not often covered by policies); ancillary services, radiology or laboratory tests with a high patient share of cost; and an hourly room charge that can easily exceed $30 per hour. The latter can be especially burdensome, as most insurance plans only cover the first 48 hours of observation. During that period the patient would be liable for just their copayment, but afterward they could be billed for the full amount. Hospitalizations well beyond the 48 hours can thus present tremendous hardships to those patients who never meet the stringent criteria for categorization as inpatients, and whose status thus must remain outpatient‐observation. Keeping patients over a weekend for procedures that are not available at the facility until the following Monday can put these individuals beyond the 48 hour observation interval and cause unintentional rapidly escalating out‐of‐pocket expenses. Other strategies to reduce the patient's financial liability include allowing patients to take their own medications from home (with pharmacy supervision and verification, per hospital guidelines), and limiting evaluations to just the admitting diagnosis (ie, pursuing other issues after discharge). In addition, an observation stay can never be ordered ahead of time for an outpatient procedure, as that type of admission is reserved for those individuals who unexpectedly need further care at the conclusion of the recovery period (typically 4 to 6 hours). Thus, the not uncommon practice of doing a patient a favor by letting them stay overnight after an outpatient procedure thereby can be a great disservice by dramatically increasing patient liability. One can well imagine how these scenarios lead to lay press exposs of the patient receiving a bill for a $25 aspirin and a night's stay 4 times more expensive than a luxury hotel. This is not to say that going home is the best or safest plan for a particular patient, but rather that the hospital is often an unnecessarily expensive (and in that sense inappropriate) location when there are alternatives. It is up to the individual hospital to determine how to handle rapidly escalating charges related to the admission status and the timeliness of a discharge. Many centers in effect write off highly select bills that are considered either uncollectible (ie, from indigent patients) or the fault of the facility's inefficiencies. So as not to have inconsistent billing policies across different insurers and patients, however, facilities are obligated to have uniform protocols for attempting to collect chargesa scenario that can be quite harsh for those individuals with significant and discoverable monetary resources.
| Observation (Outpatient Status) (Medicare Part B) | Inpatient (Medicare Part A) | |
|---|---|---|
| ||
| Room and board | Medicare deductible: $1068 per admission (waived if readmission in 60 days) | |
| 48 hours | 20% of allowable charge (APC) | |
| >48 hours | 100% hospital charges | |
| Medications | 100% hospital charges | |
| Supplies | Up to 100% hospital charges | |
| Surgical | ||
| Operating room | Typically 20% copay of APC | |
| Recovery room | Typically 20% copay of APC | |
| Diagnostic | ||
| Laboratory | 20% copay of allowable charges | |
| Radiology | 20% copay of allowable charges | |
| Ancillary | ||
| Physical therapy | 20% copay of allowable charges | |
| Occupational therapy | 20% copay of allowable charges | |
| Speech therapy | 20% copay of allowable charges | |
Working with the physician for a timely discharge, hospital case managers and social workers are likely to arrive at creative solutions in the patient's best financial interest (ie, taxicab coupons and inexpensive hotels). As many patients simply do not have the resources to cope with unplanned overnight charges, it behooves the physician to make every effort to start outpatient procedures early in the day so as to minimize the chance of logistic problems triggering a potentially expensive overnight hospital stay.
Compare the observation patient's liability to that of the typically much‐preferred status of inpatient (Table 1) in which all expenses are rolled into one diagnosis‐related group (DRG) prospective payment.3 In the case of Medicare, the patient's bill would be the inpatient deductible, and this might be covered in its entirety by a supplemental policy. One absolutely cannot, however, simply avoid using the observation status and instead make all admissions inpatients; this would cause unnecessary resource utilization and expose the hospital to denial of payment for the entire episode of care. To prevent this situation, there are nationally‐recognized guidelines that strictly define when a hospitalization warrants an inpatient level of care. Integral to the individual qualifying for their policy's inpatient benefit, however, is that the chart must reflect not just the severity of illness but also intensity of services ordered by the physician.1 Similarly, changing a patient's status (ie, from observation to inpatient) must follow rigorous guidelines wherein the justification and timing are fully described in the body of the chart to an extent that would withstand audit.
Consider the example (Table 2) of a patient with a leg fracture admitted for pain due to edema and early compartment syndrome: a scenario appropriate for inpatient status, liability of just the $1092 Medicare deductible, and eligibility for postdischarge skilled nursing facility care. Had the charting erroneously only indicated pain and need for a new cast, then observation status would have yielded a bill for $3426, plus out of pocket nursing home expenses of over $150/day.
| Patient Liability for Observation Status (Medicare Part B) | Inpatient Charges (Covered by Medicare Part A Deductible) | |
|---|---|---|
| ||
| Room and board | $1788 | $1030 |
| Medications | $755 | $1196 |
| Supplies | $106 | $528 |
| Procedures and emergency room | $229 | $1145 |
| Diagnostic | ||
| Laboratory | $72 | $359 |
| Radiology | $159 | $795 |
| Ancillary | ||
| Electrocardiogram | $22 | $110 |
| Physical therapy | $295 | $1475 |
| Patient liability for hospitalization | $3426 | $1068 deductible for total charges of $6638 |
| Patient liability for subsequent skilled nursing facility | $159 per day* | Small daily co‐pay* |
Not only does the physician need to accurately chart the reasons for admission, but it is also extremely helpful to specifically document why the patient is not amenable to outpatient therapy. Examples include a clearly articulated history of failed attempts at home or emergency room treatment, or the need for close monitoring (ie, telemetry). In this regard case managers also provide a fresh set of eyes to evaluate the clarity and completeness of medical charting. What seems like obvious decision‐making to a physician may require expanded detailed notes to satisfy a third‐party review.
The work design of the case managers and utilization review team varies between facilities. Ideally, cases are reviewed upon admission (or within the first 24 hours), and then periodically thereafter. Many medical centers have this process computerized, wherein inpatient criteria are available online and status issues can be tracked daily. This nearly real‐time information serves as the basis for interacting with the attending physician, and is necessary because the chart documentation may not be amended after discharge. Having a robust database for all admissions is also immensely helpful in those hospitals which employ a Physician Advisor (PA) as a liaison and educator to the medical staff. This newly and now nationally recognized PA position serves an important role in educating the providers not just about these patient advocacy topics, but also other issues such as length‐of‐stay. Interestingly, having the infrastructure of a criteria‐driven database to follow the intensity of inpatient services on a daily basis gives case managers an objective perspective of when a patient requires less care and is ready for transfer to a lower acuity facility or discharge home. Physician participation is important when the patient thus runs out of intensities, since there will need to be early coordination of efforts for home health or skilled nursing care, durable medical equipment supplies, or outpatient infusions. It is important that physicians not view these activities as an inappropriate rush for discharge. In our experience most patients are in fact much happier to be out of the hospital and receiving home or skilled nursing care. Those in need of physical or occupational therapy may in fact have superior care in facilities dedicated to those activities. In addition, unnecessarily prolonged hospitalizations carry their own risks, such as hospital‐acquired infections and deep venous thromboses. An additional motivator for discharge is that, just as there are insurance plan limits for outpatient benefits, there can also be caps for inpatient services. Physicians thus have a role in preserving the limited and precious number of covered inpatient days of care, beyond which time the patient would be financially totally responsible. For example, most states limit the number of inpatient days covered by Medicaid. In Florida there is a cap of only 45 days per year (unless the patient is pediatric or within the first year of a transplant4). Similarly, there have been patients and families shocked and ill‐prepared to discover that all their Medicare hospital benefits were exhausted5: a not well‐publicized possibility, as in the setting of expensive intensive care units, transplantation, or chemotherapy. Timely discharges and careful resource utilization by physicians thus not only help the hospital but also are important for the patient.
In summary, physicians need to be aware that there can be tremendous financial hardship to patients caused by inappropriate or unnecessarily long observation stays, especially in cases where an inpatient designation would have been justified by appropriate documentation. Case managers, although employed by the facility, can thus assist physicians in this regard and together play an important role as patient advocates.
- Interqual® Level of Care Criteria: Acute Care Adult.Newton, MA:McKesson Health Solutions;2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Final changes to the hospital outpatient prospective payment system and CY 2009. Available at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/list.asp. Accessed September 2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Medicare Program; Changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/IPPS/itemdetail.asp. Accessed September 2009.
- Florida Medicaid covered services. Available at: http://www.fdhc.state. fl.us/Medicaid/MediPass/pdf/HealthyOutcomesCoveredServicesFlyerEnglish 0606.pdf. Accessed September 2009.
- Medicare Benefit Policy Manual: Chapter 5. Lifetime reserve days. Available at: http://www.cms.hhs.gov/manuals/Downloads/bp102c05.pdf. Accessed September 2009.
Hospitalizations often impose a tremendous financial burden on patients and their families, adding to the stress and long‐term impact from medical illnesses. It is widely underappreciated that physicians can play an important role in substantially reducing patients' out‐of‐pocket expenses by participating in hospital‐based case review and utilization management. These topics are not a focus of most formal training curricula and unfortunately are typically viewed by medical staff as intrusive, time consuming, or only in terms of enhancing the facility's profitability. In reality, with strict rules governing insurance benefits the facility's interests are typically aligned with those of the patient.
One of the greatest impacts on a patient's financial liability is whether an admission is classified in observation vs. inpatient status, and is subject to much confusion. It is a common misperception that these are time‐based designations. Instead, they revolve around stringent medical necessity guidelines that examine the severity of the illness and the intensity of services provided.1 Inpatient stays may be brief, even a single day, if justified by medical need (although these short durations are closely scrutinized by the payors) or if involving a short list of procedures automatically triggering that status (ie, defibrillator placement).2 Conversely, observation status, although usually up to only 48 hours, can extend longer if inpatient criteria are never met and are then apt to generate large bills.
The key concept for the financial liability of patients in observation status is that their billing structure revolves around being categorized as outpatients, even though they stay overnight and are physically housed and cared for in the expensive hospital setting.3 This nonintuitive classification can culminate in unexpectedly high charges for which the patient is liable (Table 1): medications at inflated hospital pharmacy prices, especially when expensive antibiotics or immunosuppressive agents are administered (since outpatient prescriptions are not often covered by policies); ancillary services, radiology or laboratory tests with a high patient share of cost; and an hourly room charge that can easily exceed $30 per hour. The latter can be especially burdensome, as most insurance plans only cover the first 48 hours of observation. During that period the patient would be liable for just their copayment, but afterward they could be billed for the full amount. Hospitalizations well beyond the 48 hours can thus present tremendous hardships to those patients who never meet the stringent criteria for categorization as inpatients, and whose status thus must remain outpatient‐observation. Keeping patients over a weekend for procedures that are not available at the facility until the following Monday can put these individuals beyond the 48 hour observation interval and cause unintentional rapidly escalating out‐of‐pocket expenses. Other strategies to reduce the patient's financial liability include allowing patients to take their own medications from home (with pharmacy supervision and verification, per hospital guidelines), and limiting evaluations to just the admitting diagnosis (ie, pursuing other issues after discharge). In addition, an observation stay can never be ordered ahead of time for an outpatient procedure, as that type of admission is reserved for those individuals who unexpectedly need further care at the conclusion of the recovery period (typically 4 to 6 hours). Thus, the not uncommon practice of doing a patient a favor by letting them stay overnight after an outpatient procedure thereby can be a great disservice by dramatically increasing patient liability. One can well imagine how these scenarios lead to lay press exposs of the patient receiving a bill for a $25 aspirin and a night's stay 4 times more expensive than a luxury hotel. This is not to say that going home is the best or safest plan for a particular patient, but rather that the hospital is often an unnecessarily expensive (and in that sense inappropriate) location when there are alternatives. It is up to the individual hospital to determine how to handle rapidly escalating charges related to the admission status and the timeliness of a discharge. Many centers in effect write off highly select bills that are considered either uncollectible (ie, from indigent patients) or the fault of the facility's inefficiencies. So as not to have inconsistent billing policies across different insurers and patients, however, facilities are obligated to have uniform protocols for attempting to collect chargesa scenario that can be quite harsh for those individuals with significant and discoverable monetary resources.
| Observation (Outpatient Status) (Medicare Part B) | Inpatient (Medicare Part A) | |
|---|---|---|
| ||
| Room and board | Medicare deductible: $1068 per admission (waived if readmission in 60 days) | |
| 48 hours | 20% of allowable charge (APC) | |
| >48 hours | 100% hospital charges | |
| Medications | 100% hospital charges | |
| Supplies | Up to 100% hospital charges | |
| Surgical | ||
| Operating room | Typically 20% copay of APC | |
| Recovery room | Typically 20% copay of APC | |
| Diagnostic | ||
| Laboratory | 20% copay of allowable charges | |
| Radiology | 20% copay of allowable charges | |
| Ancillary | ||
| Physical therapy | 20% copay of allowable charges | |
| Occupational therapy | 20% copay of allowable charges | |
| Speech therapy | 20% copay of allowable charges | |
Working with the physician for a timely discharge, hospital case managers and social workers are likely to arrive at creative solutions in the patient's best financial interest (ie, taxicab coupons and inexpensive hotels). As many patients simply do not have the resources to cope with unplanned overnight charges, it behooves the physician to make every effort to start outpatient procedures early in the day so as to minimize the chance of logistic problems triggering a potentially expensive overnight hospital stay.
Compare the observation patient's liability to that of the typically much‐preferred status of inpatient (Table 1) in which all expenses are rolled into one diagnosis‐related group (DRG) prospective payment.3 In the case of Medicare, the patient's bill would be the inpatient deductible, and this might be covered in its entirety by a supplemental policy. One absolutely cannot, however, simply avoid using the observation status and instead make all admissions inpatients; this would cause unnecessary resource utilization and expose the hospital to denial of payment for the entire episode of care. To prevent this situation, there are nationally‐recognized guidelines that strictly define when a hospitalization warrants an inpatient level of care. Integral to the individual qualifying for their policy's inpatient benefit, however, is that the chart must reflect not just the severity of illness but also intensity of services ordered by the physician.1 Similarly, changing a patient's status (ie, from observation to inpatient) must follow rigorous guidelines wherein the justification and timing are fully described in the body of the chart to an extent that would withstand audit.
Consider the example (Table 2) of a patient with a leg fracture admitted for pain due to edema and early compartment syndrome: a scenario appropriate for inpatient status, liability of just the $1092 Medicare deductible, and eligibility for postdischarge skilled nursing facility care. Had the charting erroneously only indicated pain and need for a new cast, then observation status would have yielded a bill for $3426, plus out of pocket nursing home expenses of over $150/day.
| Patient Liability for Observation Status (Medicare Part B) | Inpatient Charges (Covered by Medicare Part A Deductible) | |
|---|---|---|
| ||
| Room and board | $1788 | $1030 |
| Medications | $755 | $1196 |
| Supplies | $106 | $528 |
| Procedures and emergency room | $229 | $1145 |
| Diagnostic | ||
| Laboratory | $72 | $359 |
| Radiology | $159 | $795 |
| Ancillary | ||
| Electrocardiogram | $22 | $110 |
| Physical therapy | $295 | $1475 |
| Patient liability for hospitalization | $3426 | $1068 deductible for total charges of $6638 |
| Patient liability for subsequent skilled nursing facility | $159 per day* | Small daily co‐pay* |
Not only does the physician need to accurately chart the reasons for admission, but it is also extremely helpful to specifically document why the patient is not amenable to outpatient therapy. Examples include a clearly articulated history of failed attempts at home or emergency room treatment, or the need for close monitoring (ie, telemetry). In this regard case managers also provide a fresh set of eyes to evaluate the clarity and completeness of medical charting. What seems like obvious decision‐making to a physician may require expanded detailed notes to satisfy a third‐party review.
The work design of the case managers and utilization review team varies between facilities. Ideally, cases are reviewed upon admission (or within the first 24 hours), and then periodically thereafter. Many medical centers have this process computerized, wherein inpatient criteria are available online and status issues can be tracked daily. This nearly real‐time information serves as the basis for interacting with the attending physician, and is necessary because the chart documentation may not be amended after discharge. Having a robust database for all admissions is also immensely helpful in those hospitals which employ a Physician Advisor (PA) as a liaison and educator to the medical staff. This newly and now nationally recognized PA position serves an important role in educating the providers not just about these patient advocacy topics, but also other issues such as length‐of‐stay. Interestingly, having the infrastructure of a criteria‐driven database to follow the intensity of inpatient services on a daily basis gives case managers an objective perspective of when a patient requires less care and is ready for transfer to a lower acuity facility or discharge home. Physician participation is important when the patient thus runs out of intensities, since there will need to be early coordination of efforts for home health or skilled nursing care, durable medical equipment supplies, or outpatient infusions. It is important that physicians not view these activities as an inappropriate rush for discharge. In our experience most patients are in fact much happier to be out of the hospital and receiving home or skilled nursing care. Those in need of physical or occupational therapy may in fact have superior care in facilities dedicated to those activities. In addition, unnecessarily prolonged hospitalizations carry their own risks, such as hospital‐acquired infections and deep venous thromboses. An additional motivator for discharge is that, just as there are insurance plan limits for outpatient benefits, there can also be caps for inpatient services. Physicians thus have a role in preserving the limited and precious number of covered inpatient days of care, beyond which time the patient would be financially totally responsible. For example, most states limit the number of inpatient days covered by Medicaid. In Florida there is a cap of only 45 days per year (unless the patient is pediatric or within the first year of a transplant4). Similarly, there have been patients and families shocked and ill‐prepared to discover that all their Medicare hospital benefits were exhausted5: a not well‐publicized possibility, as in the setting of expensive intensive care units, transplantation, or chemotherapy. Timely discharges and careful resource utilization by physicians thus not only help the hospital but also are important for the patient.
In summary, physicians need to be aware that there can be tremendous financial hardship to patients caused by inappropriate or unnecessarily long observation stays, especially in cases where an inpatient designation would have been justified by appropriate documentation. Case managers, although employed by the facility, can thus assist physicians in this regard and together play an important role as patient advocates.
Hospitalizations often impose a tremendous financial burden on patients and their families, adding to the stress and long‐term impact from medical illnesses. It is widely underappreciated that physicians can play an important role in substantially reducing patients' out‐of‐pocket expenses by participating in hospital‐based case review and utilization management. These topics are not a focus of most formal training curricula and unfortunately are typically viewed by medical staff as intrusive, time consuming, or only in terms of enhancing the facility's profitability. In reality, with strict rules governing insurance benefits the facility's interests are typically aligned with those of the patient.
One of the greatest impacts on a patient's financial liability is whether an admission is classified in observation vs. inpatient status, and is subject to much confusion. It is a common misperception that these are time‐based designations. Instead, they revolve around stringent medical necessity guidelines that examine the severity of the illness and the intensity of services provided.1 Inpatient stays may be brief, even a single day, if justified by medical need (although these short durations are closely scrutinized by the payors) or if involving a short list of procedures automatically triggering that status (ie, defibrillator placement).2 Conversely, observation status, although usually up to only 48 hours, can extend longer if inpatient criteria are never met and are then apt to generate large bills.
The key concept for the financial liability of patients in observation status is that their billing structure revolves around being categorized as outpatients, even though they stay overnight and are physically housed and cared for in the expensive hospital setting.3 This nonintuitive classification can culminate in unexpectedly high charges for which the patient is liable (Table 1): medications at inflated hospital pharmacy prices, especially when expensive antibiotics or immunosuppressive agents are administered (since outpatient prescriptions are not often covered by policies); ancillary services, radiology or laboratory tests with a high patient share of cost; and an hourly room charge that can easily exceed $30 per hour. The latter can be especially burdensome, as most insurance plans only cover the first 48 hours of observation. During that period the patient would be liable for just their copayment, but afterward they could be billed for the full amount. Hospitalizations well beyond the 48 hours can thus present tremendous hardships to those patients who never meet the stringent criteria for categorization as inpatients, and whose status thus must remain outpatient‐observation. Keeping patients over a weekend for procedures that are not available at the facility until the following Monday can put these individuals beyond the 48 hour observation interval and cause unintentional rapidly escalating out‐of‐pocket expenses. Other strategies to reduce the patient's financial liability include allowing patients to take their own medications from home (with pharmacy supervision and verification, per hospital guidelines), and limiting evaluations to just the admitting diagnosis (ie, pursuing other issues after discharge). In addition, an observation stay can never be ordered ahead of time for an outpatient procedure, as that type of admission is reserved for those individuals who unexpectedly need further care at the conclusion of the recovery period (typically 4 to 6 hours). Thus, the not uncommon practice of doing a patient a favor by letting them stay overnight after an outpatient procedure thereby can be a great disservice by dramatically increasing patient liability. One can well imagine how these scenarios lead to lay press exposs of the patient receiving a bill for a $25 aspirin and a night's stay 4 times more expensive than a luxury hotel. This is not to say that going home is the best or safest plan for a particular patient, but rather that the hospital is often an unnecessarily expensive (and in that sense inappropriate) location when there are alternatives. It is up to the individual hospital to determine how to handle rapidly escalating charges related to the admission status and the timeliness of a discharge. Many centers in effect write off highly select bills that are considered either uncollectible (ie, from indigent patients) or the fault of the facility's inefficiencies. So as not to have inconsistent billing policies across different insurers and patients, however, facilities are obligated to have uniform protocols for attempting to collect chargesa scenario that can be quite harsh for those individuals with significant and discoverable monetary resources.
| Observation (Outpatient Status) (Medicare Part B) | Inpatient (Medicare Part A) | |
|---|---|---|
| ||
| Room and board | Medicare deductible: $1068 per admission (waived if readmission in 60 days) | |
| 48 hours | 20% of allowable charge (APC) | |
| >48 hours | 100% hospital charges | |
| Medications | 100% hospital charges | |
| Supplies | Up to 100% hospital charges | |
| Surgical | ||
| Operating room | Typically 20% copay of APC | |
| Recovery room | Typically 20% copay of APC | |
| Diagnostic | ||
| Laboratory | 20% copay of allowable charges | |
| Radiology | 20% copay of allowable charges | |
| Ancillary | ||
| Physical therapy | 20% copay of allowable charges | |
| Occupational therapy | 20% copay of allowable charges | |
| Speech therapy | 20% copay of allowable charges | |
Working with the physician for a timely discharge, hospital case managers and social workers are likely to arrive at creative solutions in the patient's best financial interest (ie, taxicab coupons and inexpensive hotels). As many patients simply do not have the resources to cope with unplanned overnight charges, it behooves the physician to make every effort to start outpatient procedures early in the day so as to minimize the chance of logistic problems triggering a potentially expensive overnight hospital stay.
Compare the observation patient's liability to that of the typically much‐preferred status of inpatient (Table 1) in which all expenses are rolled into one diagnosis‐related group (DRG) prospective payment.3 In the case of Medicare, the patient's bill would be the inpatient deductible, and this might be covered in its entirety by a supplemental policy. One absolutely cannot, however, simply avoid using the observation status and instead make all admissions inpatients; this would cause unnecessary resource utilization and expose the hospital to denial of payment for the entire episode of care. To prevent this situation, there are nationally‐recognized guidelines that strictly define when a hospitalization warrants an inpatient level of care. Integral to the individual qualifying for their policy's inpatient benefit, however, is that the chart must reflect not just the severity of illness but also intensity of services ordered by the physician.1 Similarly, changing a patient's status (ie, from observation to inpatient) must follow rigorous guidelines wherein the justification and timing are fully described in the body of the chart to an extent that would withstand audit.
Consider the example (Table 2) of a patient with a leg fracture admitted for pain due to edema and early compartment syndrome: a scenario appropriate for inpatient status, liability of just the $1092 Medicare deductible, and eligibility for postdischarge skilled nursing facility care. Had the charting erroneously only indicated pain and need for a new cast, then observation status would have yielded a bill for $3426, plus out of pocket nursing home expenses of over $150/day.
| Patient Liability for Observation Status (Medicare Part B) | Inpatient Charges (Covered by Medicare Part A Deductible) | |
|---|---|---|
| ||
| Room and board | $1788 | $1030 |
| Medications | $755 | $1196 |
| Supplies | $106 | $528 |
| Procedures and emergency room | $229 | $1145 |
| Diagnostic | ||
| Laboratory | $72 | $359 |
| Radiology | $159 | $795 |
| Ancillary | ||
| Electrocardiogram | $22 | $110 |
| Physical therapy | $295 | $1475 |
| Patient liability for hospitalization | $3426 | $1068 deductible for total charges of $6638 |
| Patient liability for subsequent skilled nursing facility | $159 per day* | Small daily co‐pay* |
Not only does the physician need to accurately chart the reasons for admission, but it is also extremely helpful to specifically document why the patient is not amenable to outpatient therapy. Examples include a clearly articulated history of failed attempts at home or emergency room treatment, or the need for close monitoring (ie, telemetry). In this regard case managers also provide a fresh set of eyes to evaluate the clarity and completeness of medical charting. What seems like obvious decision‐making to a physician may require expanded detailed notes to satisfy a third‐party review.
The work design of the case managers and utilization review team varies between facilities. Ideally, cases are reviewed upon admission (or within the first 24 hours), and then periodically thereafter. Many medical centers have this process computerized, wherein inpatient criteria are available online and status issues can be tracked daily. This nearly real‐time information serves as the basis for interacting with the attending physician, and is necessary because the chart documentation may not be amended after discharge. Having a robust database for all admissions is also immensely helpful in those hospitals which employ a Physician Advisor (PA) as a liaison and educator to the medical staff. This newly and now nationally recognized PA position serves an important role in educating the providers not just about these patient advocacy topics, but also other issues such as length‐of‐stay. Interestingly, having the infrastructure of a criteria‐driven database to follow the intensity of inpatient services on a daily basis gives case managers an objective perspective of when a patient requires less care and is ready for transfer to a lower acuity facility or discharge home. Physician participation is important when the patient thus runs out of intensities, since there will need to be early coordination of efforts for home health or skilled nursing care, durable medical equipment supplies, or outpatient infusions. It is important that physicians not view these activities as an inappropriate rush for discharge. In our experience most patients are in fact much happier to be out of the hospital and receiving home or skilled nursing care. Those in need of physical or occupational therapy may in fact have superior care in facilities dedicated to those activities. In addition, unnecessarily prolonged hospitalizations carry their own risks, such as hospital‐acquired infections and deep venous thromboses. An additional motivator for discharge is that, just as there are insurance plan limits for outpatient benefits, there can also be caps for inpatient services. Physicians thus have a role in preserving the limited and precious number of covered inpatient days of care, beyond which time the patient would be financially totally responsible. For example, most states limit the number of inpatient days covered by Medicaid. In Florida there is a cap of only 45 days per year (unless the patient is pediatric or within the first year of a transplant4). Similarly, there have been patients and families shocked and ill‐prepared to discover that all their Medicare hospital benefits were exhausted5: a not well‐publicized possibility, as in the setting of expensive intensive care units, transplantation, or chemotherapy. Timely discharges and careful resource utilization by physicians thus not only help the hospital but also are important for the patient.
In summary, physicians need to be aware that there can be tremendous financial hardship to patients caused by inappropriate or unnecessarily long observation stays, especially in cases where an inpatient designation would have been justified by appropriate documentation. Case managers, although employed by the facility, can thus assist physicians in this regard and together play an important role as patient advocates.
- Interqual® Level of Care Criteria: Acute Care Adult.Newton, MA:McKesson Health Solutions;2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Final changes to the hospital outpatient prospective payment system and CY 2009. Available at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/list.asp. Accessed September 2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Medicare Program; Changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/IPPS/itemdetail.asp. Accessed September 2009.
- Florida Medicaid covered services. Available at: http://www.fdhc.state. fl.us/Medicaid/MediPass/pdf/HealthyOutcomesCoveredServicesFlyerEnglish 0606.pdf. Accessed September 2009.
- Medicare Benefit Policy Manual: Chapter 5. Lifetime reserve days. Available at: http://www.cms.hhs.gov/manuals/Downloads/bp102c05.pdf. Accessed September 2009.
- Interqual® Level of Care Criteria: Acute Care Adult.Newton, MA:McKesson Health Solutions;2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Final changes to the hospital outpatient prospective payment system and CY 2009. Available at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/list.asp. Accessed September 2009.
- Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS).2008. Medicare Program; Changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/IPPS/itemdetail.asp. Accessed September 2009.
- Florida Medicaid covered services. Available at: http://www.fdhc.state. fl.us/Medicaid/MediPass/pdf/HealthyOutcomesCoveredServicesFlyerEnglish 0606.pdf. Accessed September 2009.
- Medicare Benefit Policy Manual: Chapter 5. Lifetime reserve days. Available at: http://www.cms.hhs.gov/manuals/Downloads/bp102c05.pdf. Accessed September 2009.
Myelofibrosis with Hepatosplenomegaly
An 83‐year‐old man with a 7‐year history of myelofibrosis presented to the hospital with progressive weakness and fatigue, which resulted in him tripping and falling onto his left hand and arm 1 day prior to admission. His past medical history was significant for transfusion‐dependent anemia and hypertension. His current treatment regimen for myelofibrosis included thalidomide and darbopoetin alfa.
Physical examination revealed a pale and edematous man who was holding his injured arm to his chest, but in no distress. He had massive hepatosplenomegaly (Figure 1) and pitting edema of the lower extremities that extended to his abdomen.

Laboratory studies showed a white blood count of 5000, hematocrit of 29%, and platelets of 218,000. The peripheral blood smear (Figure 2) showed marked anisocytosis, poikilocytosis, and teardrop cells (Figure 2; arrow).
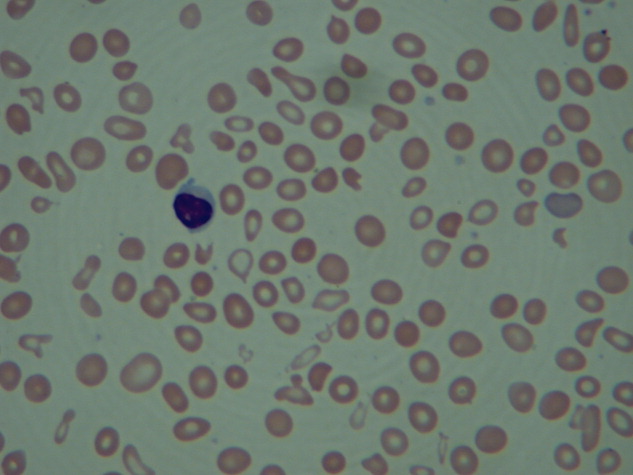
Imaging of the left arm and hand was significant for a third metacarpal fracture and first phalanx fracture. Of note, these x‐rays also revealed numerous round lucencies within the osseous structures of the left hand, wrist, and forearm (Figure 3; arrow).
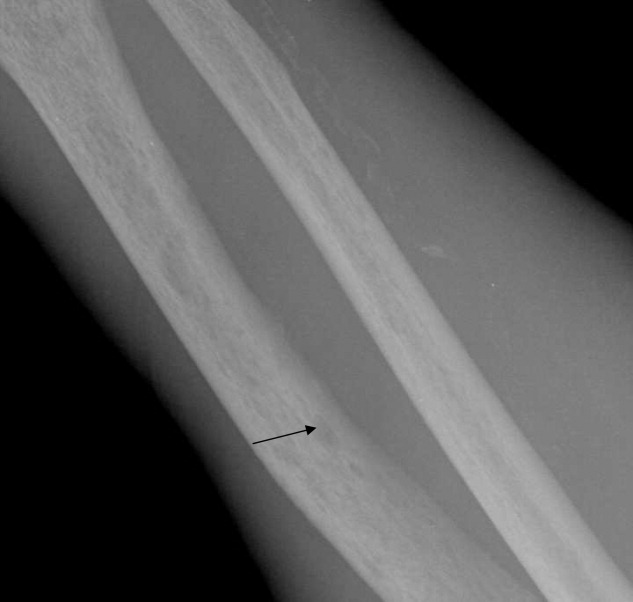
The patient's hospital course was uncomplicated and included casting of the left arm, treatment of his lower extremity edema, and transfusion for a slowly declining hematocrit. He was discharged home after several days but died 1 month later.
Primary myelofibrosis is a myeloproliferative disease that consists of 2 phases. The first phase is the growth and proliferation of abnormal bone marrow stem cells, which leads to ineffective erythropoiesis. This is followed by reactive myelofibrosis and extramedullary hematopoiesis.1 These 2 phases of the disease can lead to a constellation of findings, as illustrated in these images. The median length of survival from diagnosis is 3 to 5 years, with the main causes of death being infection, hemorrhage, cardiac failure, and leukemic transformation.1 Presenting signs, symptoms, and laboratory results may include cachexia, splenomegaly, anemia, an increased or decreased white blood cell count and/or platelet count, and an increase in lactate dehydrogenase. Radiographically, the most common findings are marked splenomegaly and osteosclerosis.2
Osteosclerotic lesions are found in 30% to 70% of patients with myelofibrosis and are a result of marrow fibrosis, which leads to the appearance of diffuse, patchy increases in bone density.2 Osteolytic lesions, as seen in this case, are much less common. They appear in the literature in case reports, but are not considered to be a typical finding. They are usually painful and have been reported as a poor prognostic indicator.3, 4
- Myelofibrosis with myeloid metaplasia.N Engl J Med.2000;342(17):1255‒1265.
- ,,,,.Imaging findings in patients with myelofibrosis.Eur Radiol.1999;9:1366‒1375.
- ,,, et al.Unusual radiological findings in a case of myelofibrosis secondary to polycythemia vera.Ann Hematol.2006;85:555‒556.
- ,,.Osteolytic bone lesions in a patient with idiopathic myelofibrosis and bronchial carcinoma.J Clin Pathol.1995;48:867‒868.
An 83‐year‐old man with a 7‐year history of myelofibrosis presented to the hospital with progressive weakness and fatigue, which resulted in him tripping and falling onto his left hand and arm 1 day prior to admission. His past medical history was significant for transfusion‐dependent anemia and hypertension. His current treatment regimen for myelofibrosis included thalidomide and darbopoetin alfa.
Physical examination revealed a pale and edematous man who was holding his injured arm to his chest, but in no distress. He had massive hepatosplenomegaly (Figure 1) and pitting edema of the lower extremities that extended to his abdomen.

Laboratory studies showed a white blood count of 5000, hematocrit of 29%, and platelets of 218,000. The peripheral blood smear (Figure 2) showed marked anisocytosis, poikilocytosis, and teardrop cells (Figure 2; arrow).

Imaging of the left arm and hand was significant for a third metacarpal fracture and first phalanx fracture. Of note, these x‐rays also revealed numerous round lucencies within the osseous structures of the left hand, wrist, and forearm (Figure 3; arrow).

The patient's hospital course was uncomplicated and included casting of the left arm, treatment of his lower extremity edema, and transfusion for a slowly declining hematocrit. He was discharged home after several days but died 1 month later.
Primary myelofibrosis is a myeloproliferative disease that consists of 2 phases. The first phase is the growth and proliferation of abnormal bone marrow stem cells, which leads to ineffective erythropoiesis. This is followed by reactive myelofibrosis and extramedullary hematopoiesis.1 These 2 phases of the disease can lead to a constellation of findings, as illustrated in these images. The median length of survival from diagnosis is 3 to 5 years, with the main causes of death being infection, hemorrhage, cardiac failure, and leukemic transformation.1 Presenting signs, symptoms, and laboratory results may include cachexia, splenomegaly, anemia, an increased or decreased white blood cell count and/or platelet count, and an increase in lactate dehydrogenase. Radiographically, the most common findings are marked splenomegaly and osteosclerosis.2
Osteosclerotic lesions are found in 30% to 70% of patients with myelofibrosis and are a result of marrow fibrosis, which leads to the appearance of diffuse, patchy increases in bone density.2 Osteolytic lesions, as seen in this case, are much less common. They appear in the literature in case reports, but are not considered to be a typical finding. They are usually painful and have been reported as a poor prognostic indicator.3, 4
An 83‐year‐old man with a 7‐year history of myelofibrosis presented to the hospital with progressive weakness and fatigue, which resulted in him tripping and falling onto his left hand and arm 1 day prior to admission. His past medical history was significant for transfusion‐dependent anemia and hypertension. His current treatment regimen for myelofibrosis included thalidomide and darbopoetin alfa.
Physical examination revealed a pale and edematous man who was holding his injured arm to his chest, but in no distress. He had massive hepatosplenomegaly (Figure 1) and pitting edema of the lower extremities that extended to his abdomen.

Laboratory studies showed a white blood count of 5000, hematocrit of 29%, and platelets of 218,000. The peripheral blood smear (Figure 2) showed marked anisocytosis, poikilocytosis, and teardrop cells (Figure 2; arrow).

Imaging of the left arm and hand was significant for a third metacarpal fracture and first phalanx fracture. Of note, these x‐rays also revealed numerous round lucencies within the osseous structures of the left hand, wrist, and forearm (Figure 3; arrow).

The patient's hospital course was uncomplicated and included casting of the left arm, treatment of his lower extremity edema, and transfusion for a slowly declining hematocrit. He was discharged home after several days but died 1 month later.
Primary myelofibrosis is a myeloproliferative disease that consists of 2 phases. The first phase is the growth and proliferation of abnormal bone marrow stem cells, which leads to ineffective erythropoiesis. This is followed by reactive myelofibrosis and extramedullary hematopoiesis.1 These 2 phases of the disease can lead to a constellation of findings, as illustrated in these images. The median length of survival from diagnosis is 3 to 5 years, with the main causes of death being infection, hemorrhage, cardiac failure, and leukemic transformation.1 Presenting signs, symptoms, and laboratory results may include cachexia, splenomegaly, anemia, an increased or decreased white blood cell count and/or platelet count, and an increase in lactate dehydrogenase. Radiographically, the most common findings are marked splenomegaly and osteosclerosis.2
Osteosclerotic lesions are found in 30% to 70% of patients with myelofibrosis and are a result of marrow fibrosis, which leads to the appearance of diffuse, patchy increases in bone density.2 Osteolytic lesions, as seen in this case, are much less common. They appear in the literature in case reports, but are not considered to be a typical finding. They are usually painful and have been reported as a poor prognostic indicator.3, 4
- Myelofibrosis with myeloid metaplasia.N Engl J Med.2000;342(17):1255‒1265.
- ,,,,.Imaging findings in patients with myelofibrosis.Eur Radiol.1999;9:1366‒1375.
- ,,, et al.Unusual radiological findings in a case of myelofibrosis secondary to polycythemia vera.Ann Hematol.2006;85:555‒556.
- ,,.Osteolytic bone lesions in a patient with idiopathic myelofibrosis and bronchial carcinoma.J Clin Pathol.1995;48:867‒868.
- Myelofibrosis with myeloid metaplasia.N Engl J Med.2000;342(17):1255‒1265.
- ,,,,.Imaging findings in patients with myelofibrosis.Eur Radiol.1999;9:1366‒1375.
- ,,, et al.Unusual radiological findings in a case of myelofibrosis secondary to polycythemia vera.Ann Hematol.2006;85:555‒556.
- ,,.Osteolytic bone lesions in a patient with idiopathic myelofibrosis and bronchial carcinoma.J Clin Pathol.1995;48:867‒868.