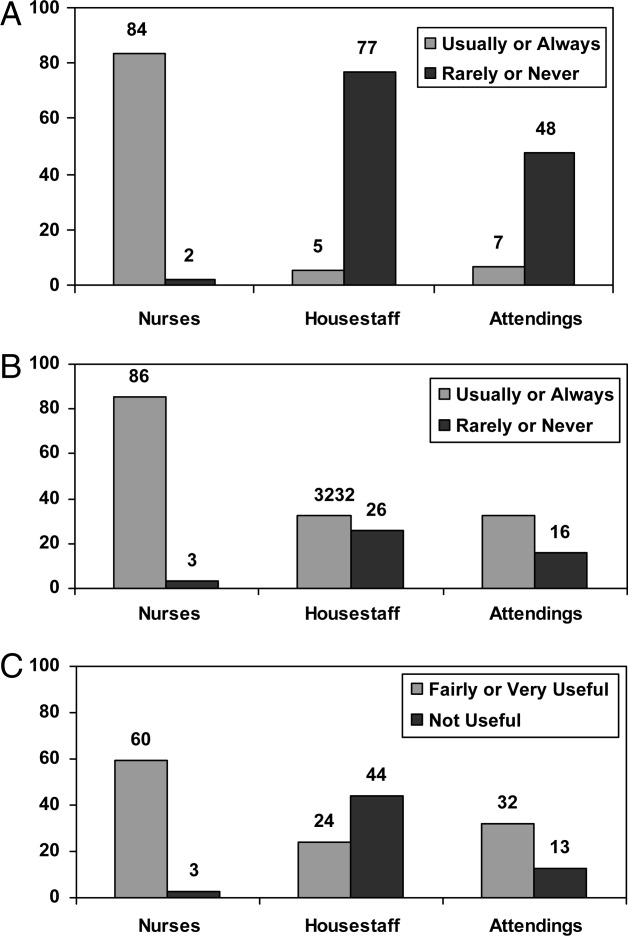
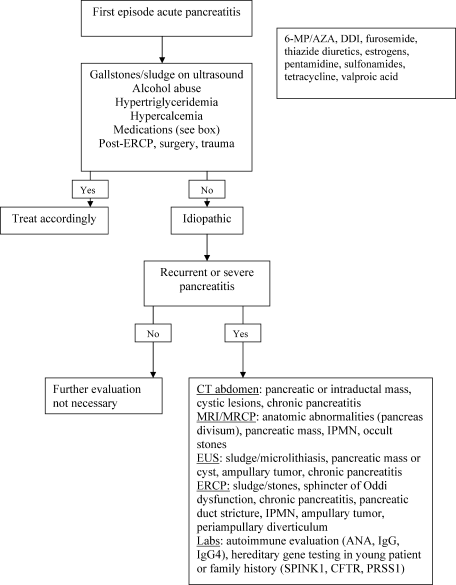
User login
Positional Atrial Flutter?
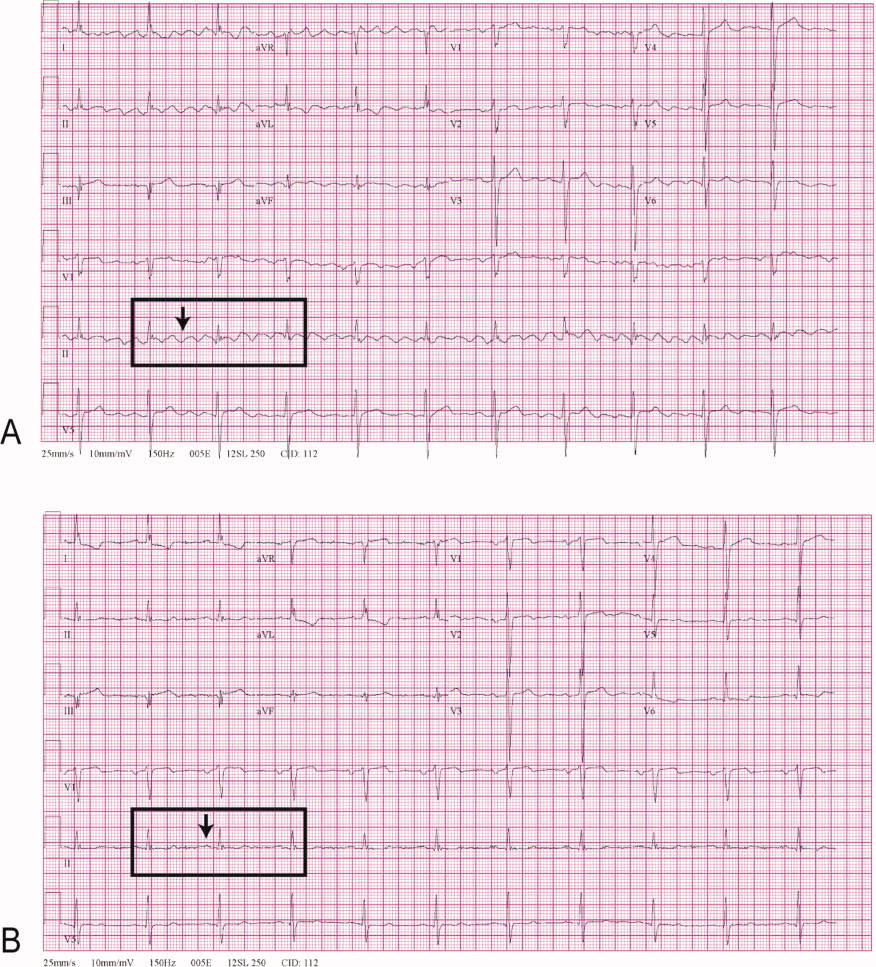
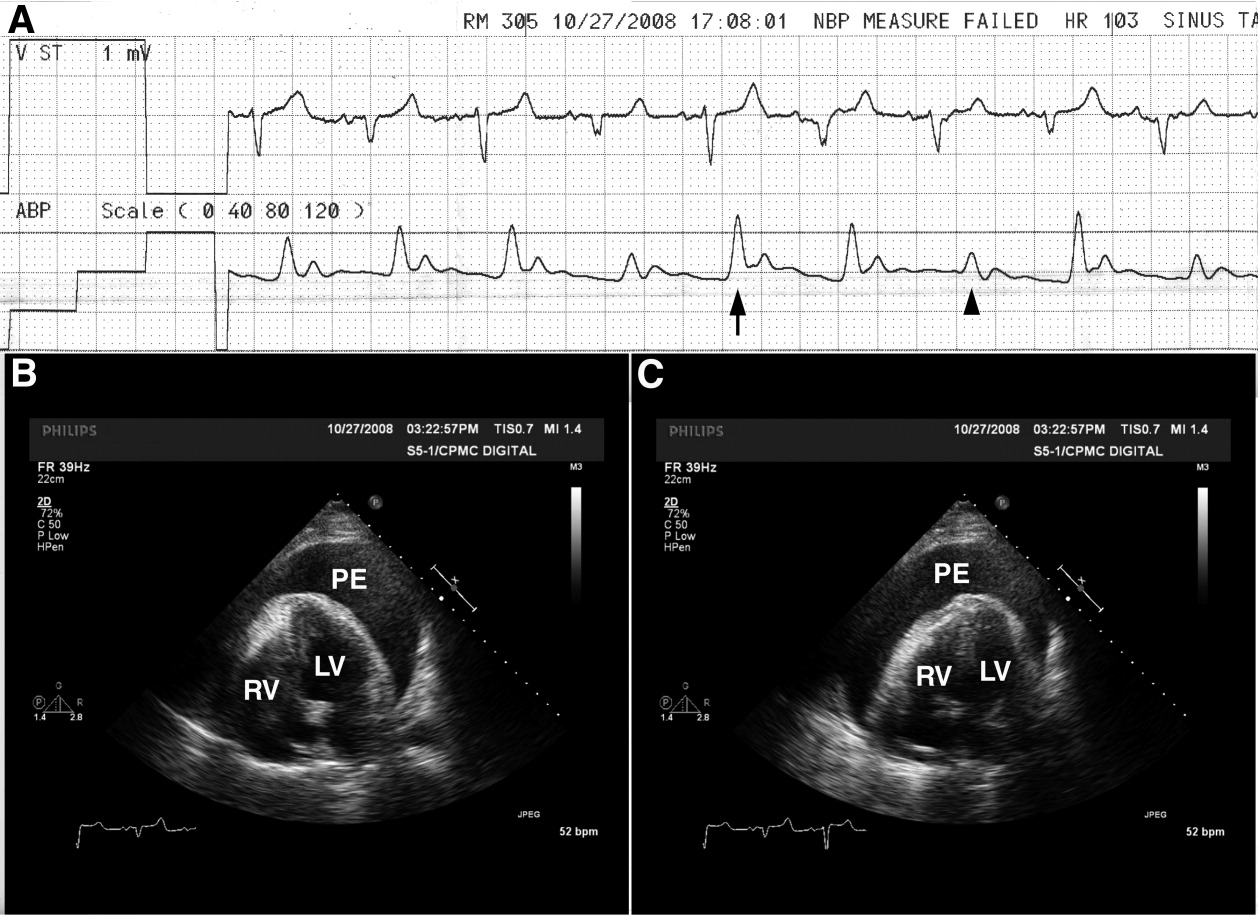
A 68‐year‐old man with a history of congestive heart failure and hypertension presented to the emergency department with fatigue and dyspnea of 3 weeks duration. Physical examination was consistent with heart failure. In addition, a right upper extremity resting tremor was noticed. An electrocardiogram (ECG) revealed an atrial flutter with a conduction ratio of 4:1 (Figure 1A). He denied palpitations or a previous history of atrial flutter/fibrillation. Unlike typical atrial flutter, these flutter like waves were distinctly absent in lead III, the only limb lead not connected to the right arm.

While holding the patient's right arm to control the tremor, a second ECG tracing was obtained. As expected the flutter like waves disappeared (Figure 1B). These ECG findings were attributed to the patient's tremor. A neurological consultation established a clinical diagnosis of Parkinson's disease. His congestive heart failure (CHF) was treated with increasing diuretics and appropriate treatment for Parkinson's disease was initiated.
A 68‐year‐old man with a history of congestive heart failure and hypertension presented to the emergency department with fatigue and dyspnea of 3 weeks duration. Physical examination was consistent with heart failure. In addition, a right upper extremity resting tremor was noticed. An electrocardiogram (ECG) revealed an atrial flutter with a conduction ratio of 4:1 (Figure 1A). He denied palpitations or a previous history of atrial flutter/fibrillation. Unlike typical atrial flutter, these flutter like waves were distinctly absent in lead III, the only limb lead not connected to the right arm.

While holding the patient's right arm to control the tremor, a second ECG tracing was obtained. As expected the flutter like waves disappeared (Figure 1B). These ECG findings were attributed to the patient's tremor. A neurological consultation established a clinical diagnosis of Parkinson's disease. His congestive heart failure (CHF) was treated with increasing diuretics and appropriate treatment for Parkinson's disease was initiated.
A 68‐year‐old man with a history of congestive heart failure and hypertension presented to the emergency department with fatigue and dyspnea of 3 weeks duration. Physical examination was consistent with heart failure. In addition, a right upper extremity resting tremor was noticed. An electrocardiogram (ECG) revealed an atrial flutter with a conduction ratio of 4:1 (Figure 1A). He denied palpitations or a previous history of atrial flutter/fibrillation. Unlike typical atrial flutter, these flutter like waves were distinctly absent in lead III, the only limb lead not connected to the right arm.

While holding the patient's right arm to control the tremor, a second ECG tracing was obtained. As expected the flutter like waves disappeared (Figure 1B). These ECG findings were attributed to the patient's tremor. A neurological consultation established a clinical diagnosis of Parkinson's disease. His congestive heart failure (CHF) was treated with increasing diuretics and appropriate treatment for Parkinson's disease was initiated.
Hospitalists and Quality of Care
Quality of care in US hospitals is inconsistent and often below accepted standards.1 This observation has catalyzed a number of performance measurement initiatives intended to publicize gaps and spur quality improvement.2 As the field has evolved, organizational factors such as teaching status, ownership model, nurse staffing levels, and hospital volume have been found to be associated with performance on quality measures.1, 3‐7 Hospitalists represent a more recent change in the organization of inpatient care8 that may impact hospital‐level performance. In fact, most hospitals provide financial support to hospitalists, not only for hopes of improving efficiency, but also for improving quality and safety.9
Only a few single‐site studies have examined the impact of hospitalists on quality of care for common medical conditions (ie, pneumonia, congestive heart failure, and acute myocardial infarction), and each has focused on patient‐level effects. Rifkin et al.10, 11 did not find differences between hospitalists' and nonhospitalists' patients in terms of pneumonia process measures. Roytman et al.12 found hospitalists more frequently prescribed afterload‐reducing agents for congestive heart failure (CHF), but other studies have shown no differences in care quality for heart failure.13, 14 Importantly, no studies have examined the role of hospitalists in the care of patients with acute myocardial infarction (AMI). In addition, studies have not addressed the effect of hospitalists at the hospital level to understand whether hospitalists have broader system‐level effects reflected by overall hospital performance.
We hypothesized that the presence of hospitalists within a hospital would be associated with improvements in hospital‐level adherence to publicly reported quality process measures, and having a greater percentage of patients admitted by hospitalists would be associated with improved performance. To test these hypotheses, we linked data from a statewide census of hospitalists with data collected as part of a hospital quality‐reporting initiative.
Materials and Methods
Study Sites
We examined the performance of 209 hospitals (63% of all 334 non‐federal facilities in California) participating in the California Hospital Assessment and Reporting Taskforce (CHART) at the time of the survey. CHART is a voluntary quality reporting initiative that began publicly reporting hospital quality data in January 2006.
Hospital‐level Organizational, Case‐mix, and Quality Data
Hospital organizational characteristics (eg, bed size) were obtained from publicly available discharge and utilization data sets from the California Office of Statewide Health Planning and Development (OSHPD). We also linked hospital‐level patient‐mix data (eg, race) from these OSHPD files.
We obtained quality of care data from CHART for January 2006 through June 2007, the time period corresponding to the survey. Quality metrics included 16 measures collected by the Center for Medicare and Medicaid Services (www.cms.hhs.gov) and extensively used in quality research.1, 4, 13, 15‐17 Rather than define a single measure, we examined multiple process measures, anticipating differential impacts of hospitalists on various processes of care for AMI, CHF, and pneumonia. Measures were further divided among those that are usually measured upon initial presentation to the hospital and those that are measured throughout the entire hospitalization and discharge. This division reflects the division of care in the hospital, where emergency room physicians are likely to have a more critical role for admission processes.
Survey Process
We surveyed all nonfederal, acute care hospitals in California that participated in CHART.2 We first identified contacts at each site via professional society mailing lists. We then sent web‐based surveys to all with available email addresses and a fax/paper survey to the remainder. We surveyed individuals between October 2006 and April 2007 and repeated the process at intervals of 1 to 3 weeks. For remaining nonrespondents, we placed a direct call unless consent to survey had been specifically refused. We contacted the following persons in sequence: (1) hospital executives or administrative leaders; (2) hospital medicine department leaders; (3) admitting emergency room personnel or medical staff officers; and (4) hospital website information. In the case of multiple responses with disagreement, the hospital/hospitalist leader's response was treated as the primary source. At each step, respondents were asked to answer questions only if they had a direct working knowledge of their hospitalist services.
Survey Data
Our key survey question to all respondents included whether the respondents could confirm their hospitals had at least one hospitalist medicine group. Hospital leaders were also asked to participate in a more comprehensive survey of their organizational and clinical characteristics. Within the comprehensive survey, leaders also provided estimates of the percent of general medical patients admitted by hospitalists. This measure, used in prior surveys of hospital leaders,9 was intended to be an easily understood approximation of the intensity of hospitalist utilization in any given hospital. A more rigorous, direct measure was not feasible due to the complexity of obtaining admission data over such a large, diverse set of hospitals.
Process Performance Measures
AMI measures assessed at admission included aspirin and ‐blocker administration within 24 hours of arrival. AMI measures assessed at discharge included aspirin administration, ‐blocker administration, angiotensin converting enzyme inhibitor (ACE‐I) (or angiotensin receptor blocker [ARB]) administration for left ventricular (LV) dysfunction, and smoking cessation counseling. There were no CHF admission measures. CHF discharge measures included assessment of LV function, the use of an ACE‐I or ARB for LV dysfunction, and smoking cessation counseling. Pneumonia admission measures included the drawing of blood cultures prior to the receipt of antibiotics, timely administration of initial antibiotics (<8 hours), and antibiotics consistent with recommendations. Pneumonia discharge measures included pneumococcal vaccination, flu vaccination, and smoking cessation counseling.
For each performance measure, we quantified the percentage of missed quality opportunities, defined as the number of patients who did not receive a care process divided by the number of eligible patients, multiplied by 100. In addition, we calculated composite scores for admission and discharge measures across each condition. We summed the numerators and denominators of individual performance measures to generate a disease‐specific composite numerator and denominator. Both individual and composite scores were produced using methodology outlined by the Center for Medicare & Medicaid Services.18 In order to retain as representative a sample of hospitals as possible, we calculated composite scores for hospitals that had a minimum of 25 observations in at least 2 of the quality indicators that made up each composite score.
Statistical Analysis
We used chi‐square tests, Student t tests, and Mann‐Whitney tests, where appropriate, to compare hospital‐level characteristics of hospitals that utilized hospitalists vs. those that did not. Similar analyses were performed among the subset of hospitals that utilized hospitalists. Among this subgroup of hospitals, we compared hospital‐level characteristics between hospitals that provided information regarding the percent of patients admitted by hospitalists vs. those who did not provide this information.
We used multivariable, generalized linear regression models to assess the relationship between having at least 1 hospitalist group and the percentage of missed quality of care measures. Because percentages were not normally distributed (ie, a majority of hospitals had few missed opportunities, while a minority had many), multivariable models employed log‐link functions with a gamma distribution.19, 20 Coefficients for our key predictor (presence of hospitalists) were transformed back to the original units (percentage of missed quality opportunities) so that a positive coefficient represented a higher number of quality measures missed relative to hospitals without hospitalists. Models were adjusted for factors previously reported to be associated with care quality. Hospital organizational characteristics included the number of beds, teaching status, registered nursing (RN) hours per adjusted patient day, and hospital ownership (for‐profit vs. not‐for‐profit). Hospital patient mix factors included annual percentage of admissions by insurance status (Medicare, Medicaid, other), annual percentage of admissions by race (white vs. nonwhite), annual percentage of do‐not‐resuscitate status at admission, and mean diagnosis‐related group‐based case‐mix index.21 We additionally adjusted for the number of cardiac catheterizations, a measure that moderately correlates with the number of cardiologists and technology utilization.22‐24 In our subset analysis among those hospitals with hospitalists, our key predictor for regression analyses was the percentage of patients admitted by hospitalists. For ease of interpretation, the percentage of patients admitted by hospitalists was centered on the mean across all respondent hospitals, and we report the effect of increasing by 10% the percentage of patients admitted by hospitalists. Models were adjusted for the same hospital organizational characteristics listed above. For those models, a positive coefficient also meant a higher number of measures missed.
For both sets of predictors, we additionally tested for the presence of interactions between the predictors and hospital bed size (both continuous as well as dichotomized at 150 beds) in composite measure performance, given the possibility that any hospitalist effect may be greater among smaller, resource‐limited hospitals. Tests for interaction were performed with the likelihood ratio test. In addition, to minimize any potential bias or loss of power that might result from limiting the analysis to hospitals with complete data, we used the multivariate imputation by chained equations method, as implemented in STATA 9.2 (StataCorp, College Station, TX), to create 10 imputed datasets.25 Imputation of missing values was restricted to confounding variables. Standard methods were then used to combine results over the 10 imputed datasets. We also applied Bonferroni corrections to composite measure tests based on the number of composites generated (n = 5). Thus, for the 5 inpatient composites created, standard definitions of significance (P 0.05) were corrected by dividing composite P values by 5, requiring P 0.01 for significance. The institutional review board of the University of California, San Francisco, approved the study. All analyses were performed using STATA 9.2.
Results
Characteristics of Participating Sites
There were 209 eligible hospitals. All 209 (100%) hospitals provided data about the presence or absence of hospitalists via at least 1 of our survey strategies. The majority of identification of hospitalist utilization was via contact with either hospital or hospitalist leaders, n = 147 (70.3%). Web‐sites informed hospitalist prevalence in only 3 (1.4%) hospitals. There were 8 (3.8%) occurrences of disagreement between sources, all of which had available hospital/hospitalist leader responses. Only 1 (0.5%) hospital did not have the minimum 25 patients eligible for any disease‐specific quality measures during the data reporting period. Collectively, the remaining 208 hospitals accounted for 81% of California's acute care hospital population.
Comparisons of Sites With Hospitalists and Those Without
A total of 170 hospitals (82%) participating in CHART used hospitalists. Hospitals with and without hospitalists differed by a variety of characteristics (Table 1). Sites with hospitalists were larger, less likely to be for‐profit, had more registered nursing hours per day, and performed more cardiac catheterizations.
| Characteristic | Hospitals Without Hospitalists (n = 38) | Hospitals With Hospitalists (n = 170) | P Value* |
|---|---|---|---|
| |||
| Number of beds, n (% of hospitals) | <0.001 | ||
| 0‐99 | 16 (42.1) | 14 (8.2) | |
| 100‐199 | 8 (21.1) | 44 (25.9) | |
| 200‐299 | 7 (18.4) | 42 (24.7) | |
| 300+ | 7 (18.4) | 70 (41.2) | |
| For profit, n (% of hospitals) | 9 (23.7) | 18 (10.6) | 0.03 |
| Teaching hospital, n (% of hospitals) | 7 (18.4) | 55 (32.4) | 0.09 |
| RN hours per adjusted patient day, number of hours (IQR) | 7.4 (5.7‐8.6) | 8.5 (7.4‐9.9) | <0.001 |
| Annual cardiac catheterizations, n (IQR) | 0 (0‐356) | 210 (0‐813) | 0.007 |
| Hospital total census days, n (IQR) | 37161 (14910‐59750) | 60626 (34402‐87950) | <0.001 |
| ICU total census, n (IQR) | 2193 (1132‐4289) | 3855 (2489‐6379) | <0.001 |
| Medicare insurance, % patients (IQR) | 36.9 (28.5‐48.0) | 35.3(28.2‐44.3) | 0.95 |
| Medicaid insurance, % patients (IQR) | 21.0 (12.7‐48.3) | 16.6 (5.6‐27.6) | 0.02 |
| Race, white, % patients (IQR) | 53.7 (26.0‐82.7) | 59.1 (45.6‐74.3) | 0.73 |
| DNR at admission, % patients (IQR) | 3.6 (2.0‐6.4) | 4.4 (2.7‐7.1) | 0.12 |
| Case‐mix index, index (IQR) | 1.05 (0.90‐1.21) | 1.13 (1.01‐1.26) | 0.11 |
Relationship Between Hospitalist Group Utilization and the Percentage of Missed Quality Opportunities
Table 2 shows the frequency of missed quality opportunities in sites with hospitalists compared to those without. In general, for both individual and composite measures of quality, multivariable adjustment modestly attenuated the observed differences between the 2 groups of hospitals. We present only the more conservative adjusted estimates.
| Quality Measure | Number of Hospitals | Adjusted Mean % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative % Change | P Value | |
|---|---|---|---|---|---|---|
| Hospitals Without Hospitalists | Hospitals With Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 193 | 3.7 (2.4‐5.1) | 3.4 (2.3‐4.4) | 0.3 | 10.0 | 0.44 |
| Beta‐blocker at admission | 186 | 7.8 (4.7‐10.9) | 6.4 (4.4‐8.3) | 1.4 | 18.3 | 0.19 |
| AMI admission composite | 186 | 5.5 (3.6‐7.5) | 4.8 (3.4‐6.1) | 0.7 | 14.3 | 0.26 |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 173 | 7.5 (4.5‐10.4) | 5.2 (3.4‐6.9) | 2.3 | 31.0 | 0.02 |
| Beta‐blocker at discharge | 179 | 6.6 (3.8‐9.4) | 5.9 (3.6‐8.2) | 0.7 | 9.6 | 0.54 |
| ACE‐I/ARB at discharge | 119 | 20.7 (9.5‐31.8) | 11.8 (6.6‐17.0) | 8.9 | 43.0 | 0.006 |
| Smoking cessation counseling | 193 | 3.8 (2.4‐5.1) | 3.4 (2.4‐4.4) | 0.4 | 10.0 | 0.44 |
| AMI hospital/discharge composite | 179 | 6.4 (4.1‐8.6) | 5.3 (3.7‐6.8) | 1.1 | 17.6 | 0.16 |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 208 | 12.6 (7.7‐17.6) | 6.5 (4.6‐8.4) | 6.1 | 48.2 | <0.001 |
| ACE‐I/ARB at discharge | 201 | 14.7 (10.0‐19.4) | 12.9 (9.8‐16.1) | 1.8 | 12.1 | 0.31 |
| Smoking cessation counseling | 168 | 9.1 (2.9‐15.4) | 9.0 (4.2‐13.8) | 0.1 | 1.8 | 0.98 |
| CHF hospital/discharge composite | 201 | 12.2 (7.9‐16.5) | 8.2 (6.2‐10.2) | 4.0 | 33.1 | 0.006* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Blood culture before antibiotics | 206 | 12.0 (9.1‐14.9) | 10.9 (8.8‐13.0) | 1.1 | 9.1 | 0.29 |
| Timing of antibiotics <8 hours | 208 | 5.8 (4.1‐7.5) | 6.2 (4.7‐7.7) | 0.4 | 6.9 | 0.56 |
| Initial antibiotic consistent with recommendations | 207 | 15.0 (11.6‐18.6) | 13.8 (10.9‐16.8) | 1.2 | 8.1 | 0.27 |
| Pneumonia admission composite | 207 | 10.5 (8.5‐12.5) | 9.9 (8.3‐11.5) | 0.6 | 5.9 | 0.37 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 208 | 29.4 (19.5‐39.2) | 27.1 (19.9‐34.3) | 2.3 | 7.7 | 0.54 |
| Influenza vaccine | 207 | 36.9 (25.4‐48.4) | 35.0 (27.0‐43.1) | 1.9 | 5.2 | 0.67 |
| Smoking cessation counseling | 196 | 15.4 (7.8‐23.1) | 13.9 (8.9‐18.9) | 1.5 | 10.2 | 0.59 |
| Pneumonia hospital/discharge composite | 207 | 29.6 (20.5‐38.7) | 27.3 (20.9‐33.6) | 2.3 | 7.8 | 0.51 |
Compared to hospitals without hospitalists, those with hospitalists did not have any statistically significant differences in the individual and composite admission measures for each of the disease processes. In contrast, there were statistically significant differences between hospitalist and nonhospitalist sites for many individual cardiac processes of care that typically occur after admission from the emergency room (ie, LV function assessment for CHF) or those that occurred at discharge (ie, aspirin and ACE‐I/ARB at discharge for AMI). Similarly, the composite discharge scores for AMI and CHF revealed better overall process measure performance at sites with hospitalists, although the AMI composite did not meet statistical significance. There were no statistically significant differences between groups for the pneumonia process measures assessed at discharge. In addition, for composite measures there were no statistically significant interactions between hospitalist prevalence and bed size, although there was a trend (P = 0.06) for the CHF discharge composite, with a larger effect of hospitalists among smaller hospitals.
Percent of Patients Admitted by Hospitalists
Of the 171 hospitals with hospitalists, 71 (42%) estimated the percent of patients admitted by their hospitalist physicians. Among the respondents, the mean and median percentages of medical patients admitted by hospitalists were 51% (SD = 25%) and 49% (IQR = 30‐70%), respectively. Thirty hospitals were above the sample mean. Compared to nonrespondent sites, respondent hospitals took care of more white patients; otherwise, respondent and nonrespondent hospitals were similar in terms of bed size, location, performance across each measure, and other observable characteristics (Supporting Information, Appendix 1).
Relationship Between the Estimated Percentages of Medical Patients Admitted by Hospitalists and Missed Quality Opportunities
Table 3 displays the change in missed quality measures associated with each additional 10% of patients estimated to be admitted by hospitalists. A higher estimated percentage of patients admitted by hospitalists was associated with statistically significant improvements in quality of care across a majority of individual measures and for all composite discharge measures regardless of condition. For example, every 10% increase in the mean estimated number of patients admitted by hospitalists was associated with a mean of 0.6% (P < 0.001), 0.5% (P = 0.004), and 1.5% (P = 0.006) fewer missed quality opportunities for AMI, CHF, and pneumonia discharge process measures composites, respectively. In addition, for these composite measures, there were no statistically significant interactions between the estimated percentage of patients admitted by hospitalists and bed size (dichotomized at 150 beds), although there was a trend (P = 0.09) for the AMI discharge composite, with a larger effect of hospitalists among smaller hospitals.
| Quality Measure | Number of Hospitals | Adjusted % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative Percent Change | P Value | |
|---|---|---|---|---|---|---|
| Among Hospitals With Mean % of Patients Admitted by Hospitalists | Among Hospitals With Mean + 10% of Patients Admitted by Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐3.1) | 0.3 | 10.2 | 0.001 |
| Beta‐blocker at admission | 65 | 5.8 (3.4‐8.2) | 5.1 (3.0‐7.3) | 0.7 | 11.9 | <0.001 |
| AMI admission composite | 65 | 4.5 (2.9‐6.1) | 4.0 (2.6‐5.5) | 0.5 | 11.1 | <0.001* |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 62 | 5.1 (3.3‐6.9) | 4.6 (3.1‐6.2) | 0.5 | 9.0 | 0.03 |
| Beta‐blocker at discharge | 63 | 5.1 (2.9‐7.2) | 4.3 (2.5‐6.0) | 0.8 | 15.4 | <0.001 |
| ACE‐I/ARB at discharge | 44 | 11.4 (6.2‐16.6) | 10.3 (5.4‐15.1) | 1.1 | 10.0 | 0.02 |
| Smoking cessation counseling | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐4.1) | 0.3 | 10.2 | 0.001 |
| AMI hospital/discharge composite | 63 | 5.0 (3.3‐6.7) | 4.4 (3.0‐5.8) | 0.6 | 11.3 | 0.001* |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 71 | 5.9 (4.1‐7.6) | 5.6 (3.9‐7.2) | 0.3 | 2.9 | 0.07 |
| ACE‐I/ARB at discharge | 70 | 12.3 (8.6‐16.0) | 11.4 (7.9‐15.0) | 0.9 | 7.1 | 0.008* |
| Smoking cessation counseling | 56 | 8.4 (4.1‐12.6) | 8.2 (4.2‐12.3) | 0.2 | 1.7 | 0.67 |
| CHF hospital/discharge composite | 70 | 7.7 (5.8‐9.6) | 7.2 (5.4‐9.0) | 0.5 | 6.0 | 0.004* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Timing of antibiotics <8 hours | 71 | 5.9 (4.2‐7.6) | 5.9 (4.1‐7.7) | 0.0 | 0.0 | 0.98 |
| Blood culture before antibiotics | 71 | 10.0 (8.0‐12.0) | 9.8 (7.7‐11.8) | 0.2 | 2.6 | 0.18 |
| Initial antibiotic consistent with recommendations | 71 | 13.3 (10.4‐16.2) | 12.9 (9.9‐15.9) | 0.4 | 2.8 | 0.20 |
| Pneumonia admission composite | 71 | 9.4 (7.7‐11.1) | 9.2 (7.6‐10.9) | 0.2 | 1.8 | 0.23 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 71 | 27.0 (19.2‐34.8) | 24.7 (17.2‐32.2) | 2.3 | 8.4 | 0.006 |
| Influenza vaccine | 71 | 34.1 (25.9‐42.2) | 32.6 (24.7‐40.5) | 1.5 | 4.3 | 0.03 |
| Smoking cessation counseling | 67 | 15.2 (9.8‐20.7) | 15.0 (9.6‐20.4) | 0.2 | 2.0 | 0.56 |
| Pneumonia hospital/discharge composite | 71 | 26.7 (20.3‐33.1) | 25.2 (19.0‐31.3) | 1.5 | 5.8 | 0.006* |
In order to test the robustness of our results, we carried out 2 secondary analyses. First, we used multivariable models to generate a propensity score representing the predicted probability of being assigned to a hospital with hospitalists. We then used the propensity score as an additional covariate in subsequent multivariable models. In addition, we performed a complete‐case analysis (including only hospitals with complete data, n = 204) as a check on the sensitivity of our results to missing data. Neither analysis produced results substantially different from those presented.
Discussion
In this cross‐sectional analysis of hospitals participating in a voluntary quality reporting initiative, hospitals with at least 1 hospitalist group had fewer missed discharge care process measures for CHF, even after adjusting for hospital‐level characteristics. In addition, as the estimated percentage of patients admitted by hospitalists increased, the percentage of missed quality opportunities decreased across all measures. The observed relationships were most apparent for measures that could be completed at any time during the hospitalization and at discharge. While it is likely that hospitalists are a marker of a hospital's ability to invest in systems (and as a result, care improvement initiatives), the presence of a potential dose‐response relationship suggests that hospitalists themselves may have a role in improving processes of care.
Our study suggests a generally positive, but mixed, picture of hospitalists' effects on quality process measure performance. Lack of uniformity across measures may depend on the timing of the process measure (eg, whether or not the process is measured at admission or discharge). For example, in contrast to admission process measures, we more commonly observed a positive association between hospitalists and care quality on process measures targeting processes that generally took place later in hospitalization or at discharge. Many admission process measures (eg, door to antibiotic time, blood cultures, and appropriate initial antibiotics) likely occurred prior to hospitalist involvement in most cases and were instead under the direction of emergency medicine physicians. Performance on these measures would not be expected to relate to use of hospitalists, and that is what we observed.
In addition to the timing of when a process was measured or took place, associations between hospitalists and care quality vary by disease. The apparent variation in impact of hospitalists by disease (more impact for cardiac conditions, less for pneumonia) may relate primarily to the characteristics of the processes of care that were measured for each condition. For example, one‐half of the pneumonia process measures related to care occurring within a few hours of admission, while the other one‐half (smoking cessation advice and streptococcal and influenza vaccines) were often administered per protocol or by nonphysician providers.26‐29 However, more of the cardiac measures required physician action (eg, prescription of an ACE‐I at discharge). Alternatively, unmeasured confounders important in the delivery of cardiac care might play an important role in the relationship between hospitalists and cardiac process measure performance.
Our approach to defining hospitalists bears mention as well. While a dichotomous measure of having hospitalists available was only statistically significant for the single CHF discharge composite measure, our measure of hospitalist availabilitythe percentage of patients admitted by hospitalistswas more strongly associated with a larger number of quality measures. Contrast between the dichotomous and continuous measures may have statistical explanations (the power to see differences between 2 groups is more limited with use of a binary predictor, which itself can be subject to bias),30 but may also indicate a dose‐response relationship. A larger number of admissions to hospitalists may help standardize practices, as care is concentrated in a smaller number of physicians' hands. Moreover, larger hospitalist programs may be more likely to have implemented care standardization or quality improvement processes or to have been incorporated into (or lead) hospitals' quality infrastructures. Finally, presence of larger hospitalist groups may be a marker for a hospital's capacity to make hospital‐wide investments in improvement. However, the association between the percentage of patients admitted by hospitalists and care quality persisted even after adjustment for many measures plausibly associated with ability to invest in care quality.
Our study has several limitations. First, although we used a widely accepted definition of hospitalists endorsed by the Society of Hospital Medicine, there are no gold standard definitions for a hospitalist's job description or skill set. As a result, it is possible that a model utilizing rotating internists (from a multispecialty group) might have been misidentified as a hospitalist model. Second, our findings represent a convenience sample of hospitals in a voluntary reporting initiative (CHART) and may not be applicable to hospitals that are less able to participate in such an endeavor. CHART hospitals are recognized to be better performers than the overall California population of hospitals, potentially decreasing variability in our quality of care measures.2 Third, there were significant differences between our comparison groups within the CHART hospitals, including sample size. Although we attempted to adjust our analyses for many important potential confounders and applied conservative measures to assess statistical significance, given the baseline differences, we cannot rule out the possibility of residual confounding by unmeasured factors. Fourth, as described above, this observational study cannot provide robust evidence to support conclusions regarding causality. Fifth, the estimation of the percent of patients admitted by hospitalists is unvalidated and based upon self‐reported and incomplete (41% of respondents) data. We are somewhat reassured by the fact that respondents and nonresponders were similar across all hospital characteristics, as well as outcomes. Sixth, misclassification of the estimated percentage of patients admitted by hospitalists may have influenced our results. Although possible, misclassification often biases results toward the null, potentially weakening any observed association. Given that our respondents were not aware of our hypotheses, there is no reason to expect recall issues to bias the results one way or the other. Finally, for many performance measures, overall performance was excellent among all hospitals (eg, aspirin at admission) with limited variability, thus limiting the ability to assess for differences.
In summary, in a large, cross‐sectional study of California hospitals participating in a voluntary quality reporting initiative, the presence of hospitalists was associated with modest improvements in hospital‐level performance of quality process measures. In addition, we found a relationship between the percentage of patients admitted by hospitalists and improved process measure adherence. Although we cannot determine causality, our data support the hypothesis that dedicated hospital physicians can positively affect the quality of care. Future research should examine this relationship in other settings and should address causality using broader measures of quality including both processes and outcomes.
Acknowledgements
The authors acknowledge Teresa Chipps, BS, Center for Health Services Research, Division of General Internal Medicine and Public Health, Department of Medicine, Vanderbilt University, Nashville, TN, for her administrative and editorial assistance in the preparation of this manuscript.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- CalHospitalCompare.org: online report card simplifies the search for quality hospital care. Available at: http://www.chcf.org/topics/hospitals/index.cfm?itemID=131387. Accessed September 2009.
- ,,, et al.Hospital characteristics and quality of care.JAMA.1992;268:1709–1714.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,, et al.A systematic review and meta‐analysis of studies comparing mortality rates of private for‐profit and private not‐for‐profit hospitals.CMAJ.2002;166:1399–1406.
- ,.Teaching hospitals and quality of care: a review of the literature.Milbank Q.2002;80:569–593.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23:1399–1406.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.The inverse relationship between mortality rates and performance in the Hospital Quality Alliance measures.Health Aff.2007;26:1104–1110.
- ,,,,.Does the Leapfrog program help identify high‐quality hospitals?Jt Comm J Qual Patient Saf.2008;34:318–325.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- CMS HQI demonstration project—composite quality score methodology overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed September 2009.
- ,,.Modeling risk using generalized linear models.J Health Econ.1999;18:153–171.
- ,,.Generalized modeling approaches to risk adjustment of skewed outcomes data.J Health Econ.2005;24:465–488.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,, et al.The Dartmouth Atlas of Cardiovascular Health Care.Chicago:AHA Press;1999. Current data from the Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH. Available at: http://www.dartmouthatlas.org/atlases/atlas_ series.shtm. Accessed September 2009.
- ,,.Differences in per capita rates of revascularization and in choice of revascularization procedure for eleven states.BMC Health Serv Res.2006;6:35.
- ,,.The relationship between physician supply, cardiovascular health service use and cardiac disease burden in Ontario: supply‐need mismatch.Can J Card.2008;24:187.
- .Multiple imputation: a primer.Stat Methods Med Res.1999;8:3–15.
- .Nursing intervention and smoking cessation: Meta‐analysis update.Heart Lung.2006;35:147–163.
- .Ten‐year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high‐risk adults.Am J Med.1998;105:385–392.
- ,,, et al.Role of student pharmacist interns in hospital‐based standing orders pneumococcal vaccination program.J Am Pharm Assoc.2007;47:404–409.
- ,,,.Effect of a pharmacist‐managed program of pneumococcal and influenza immunization on vaccination rates among adult inpatients.Am J Health Syst Pharm.2003;60:1767–1771.
- ,,.Dichotomizing continuous predictors in multiple regression: a bad idea.Stat Med.2006;25:127–141.
Quality of care in US hospitals is inconsistent and often below accepted standards.1 This observation has catalyzed a number of performance measurement initiatives intended to publicize gaps and spur quality improvement.2 As the field has evolved, organizational factors such as teaching status, ownership model, nurse staffing levels, and hospital volume have been found to be associated with performance on quality measures.1, 3‐7 Hospitalists represent a more recent change in the organization of inpatient care8 that may impact hospital‐level performance. In fact, most hospitals provide financial support to hospitalists, not only for hopes of improving efficiency, but also for improving quality and safety.9
Only a few single‐site studies have examined the impact of hospitalists on quality of care for common medical conditions (ie, pneumonia, congestive heart failure, and acute myocardial infarction), and each has focused on patient‐level effects. Rifkin et al.10, 11 did not find differences between hospitalists' and nonhospitalists' patients in terms of pneumonia process measures. Roytman et al.12 found hospitalists more frequently prescribed afterload‐reducing agents for congestive heart failure (CHF), but other studies have shown no differences in care quality for heart failure.13, 14 Importantly, no studies have examined the role of hospitalists in the care of patients with acute myocardial infarction (AMI). In addition, studies have not addressed the effect of hospitalists at the hospital level to understand whether hospitalists have broader system‐level effects reflected by overall hospital performance.
We hypothesized that the presence of hospitalists within a hospital would be associated with improvements in hospital‐level adherence to publicly reported quality process measures, and having a greater percentage of patients admitted by hospitalists would be associated with improved performance. To test these hypotheses, we linked data from a statewide census of hospitalists with data collected as part of a hospital quality‐reporting initiative.
Materials and Methods
Study Sites
We examined the performance of 209 hospitals (63% of all 334 non‐federal facilities in California) participating in the California Hospital Assessment and Reporting Taskforce (CHART) at the time of the survey. CHART is a voluntary quality reporting initiative that began publicly reporting hospital quality data in January 2006.
Hospital‐level Organizational, Case‐mix, and Quality Data
Hospital organizational characteristics (eg, bed size) were obtained from publicly available discharge and utilization data sets from the California Office of Statewide Health Planning and Development (OSHPD). We also linked hospital‐level patient‐mix data (eg, race) from these OSHPD files.
We obtained quality of care data from CHART for January 2006 through June 2007, the time period corresponding to the survey. Quality metrics included 16 measures collected by the Center for Medicare and Medicaid Services (www.cms.hhs.gov) and extensively used in quality research.1, 4, 13, 15‐17 Rather than define a single measure, we examined multiple process measures, anticipating differential impacts of hospitalists on various processes of care for AMI, CHF, and pneumonia. Measures were further divided among those that are usually measured upon initial presentation to the hospital and those that are measured throughout the entire hospitalization and discharge. This division reflects the division of care in the hospital, where emergency room physicians are likely to have a more critical role for admission processes.
Survey Process
We surveyed all nonfederal, acute care hospitals in California that participated in CHART.2 We first identified contacts at each site via professional society mailing lists. We then sent web‐based surveys to all with available email addresses and a fax/paper survey to the remainder. We surveyed individuals between October 2006 and April 2007 and repeated the process at intervals of 1 to 3 weeks. For remaining nonrespondents, we placed a direct call unless consent to survey had been specifically refused. We contacted the following persons in sequence: (1) hospital executives or administrative leaders; (2) hospital medicine department leaders; (3) admitting emergency room personnel or medical staff officers; and (4) hospital website information. In the case of multiple responses with disagreement, the hospital/hospitalist leader's response was treated as the primary source. At each step, respondents were asked to answer questions only if they had a direct working knowledge of their hospitalist services.
Survey Data
Our key survey question to all respondents included whether the respondents could confirm their hospitals had at least one hospitalist medicine group. Hospital leaders were also asked to participate in a more comprehensive survey of their organizational and clinical characteristics. Within the comprehensive survey, leaders also provided estimates of the percent of general medical patients admitted by hospitalists. This measure, used in prior surveys of hospital leaders,9 was intended to be an easily understood approximation of the intensity of hospitalist utilization in any given hospital. A more rigorous, direct measure was not feasible due to the complexity of obtaining admission data over such a large, diverse set of hospitals.
Process Performance Measures
AMI measures assessed at admission included aspirin and ‐blocker administration within 24 hours of arrival. AMI measures assessed at discharge included aspirin administration, ‐blocker administration, angiotensin converting enzyme inhibitor (ACE‐I) (or angiotensin receptor blocker [ARB]) administration for left ventricular (LV) dysfunction, and smoking cessation counseling. There were no CHF admission measures. CHF discharge measures included assessment of LV function, the use of an ACE‐I or ARB for LV dysfunction, and smoking cessation counseling. Pneumonia admission measures included the drawing of blood cultures prior to the receipt of antibiotics, timely administration of initial antibiotics (<8 hours), and antibiotics consistent with recommendations. Pneumonia discharge measures included pneumococcal vaccination, flu vaccination, and smoking cessation counseling.
For each performance measure, we quantified the percentage of missed quality opportunities, defined as the number of patients who did not receive a care process divided by the number of eligible patients, multiplied by 100. In addition, we calculated composite scores for admission and discharge measures across each condition. We summed the numerators and denominators of individual performance measures to generate a disease‐specific composite numerator and denominator. Both individual and composite scores were produced using methodology outlined by the Center for Medicare & Medicaid Services.18 In order to retain as representative a sample of hospitals as possible, we calculated composite scores for hospitals that had a minimum of 25 observations in at least 2 of the quality indicators that made up each composite score.
Statistical Analysis
We used chi‐square tests, Student t tests, and Mann‐Whitney tests, where appropriate, to compare hospital‐level characteristics of hospitals that utilized hospitalists vs. those that did not. Similar analyses were performed among the subset of hospitals that utilized hospitalists. Among this subgroup of hospitals, we compared hospital‐level characteristics between hospitals that provided information regarding the percent of patients admitted by hospitalists vs. those who did not provide this information.
We used multivariable, generalized linear regression models to assess the relationship between having at least 1 hospitalist group and the percentage of missed quality of care measures. Because percentages were not normally distributed (ie, a majority of hospitals had few missed opportunities, while a minority had many), multivariable models employed log‐link functions with a gamma distribution.19, 20 Coefficients for our key predictor (presence of hospitalists) were transformed back to the original units (percentage of missed quality opportunities) so that a positive coefficient represented a higher number of quality measures missed relative to hospitals without hospitalists. Models were adjusted for factors previously reported to be associated with care quality. Hospital organizational characteristics included the number of beds, teaching status, registered nursing (RN) hours per adjusted patient day, and hospital ownership (for‐profit vs. not‐for‐profit). Hospital patient mix factors included annual percentage of admissions by insurance status (Medicare, Medicaid, other), annual percentage of admissions by race (white vs. nonwhite), annual percentage of do‐not‐resuscitate status at admission, and mean diagnosis‐related group‐based case‐mix index.21 We additionally adjusted for the number of cardiac catheterizations, a measure that moderately correlates with the number of cardiologists and technology utilization.22‐24 In our subset analysis among those hospitals with hospitalists, our key predictor for regression analyses was the percentage of patients admitted by hospitalists. For ease of interpretation, the percentage of patients admitted by hospitalists was centered on the mean across all respondent hospitals, and we report the effect of increasing by 10% the percentage of patients admitted by hospitalists. Models were adjusted for the same hospital organizational characteristics listed above. For those models, a positive coefficient also meant a higher number of measures missed.
For both sets of predictors, we additionally tested for the presence of interactions between the predictors and hospital bed size (both continuous as well as dichotomized at 150 beds) in composite measure performance, given the possibility that any hospitalist effect may be greater among smaller, resource‐limited hospitals. Tests for interaction were performed with the likelihood ratio test. In addition, to minimize any potential bias or loss of power that might result from limiting the analysis to hospitals with complete data, we used the multivariate imputation by chained equations method, as implemented in STATA 9.2 (StataCorp, College Station, TX), to create 10 imputed datasets.25 Imputation of missing values was restricted to confounding variables. Standard methods were then used to combine results over the 10 imputed datasets. We also applied Bonferroni corrections to composite measure tests based on the number of composites generated (n = 5). Thus, for the 5 inpatient composites created, standard definitions of significance (P 0.05) were corrected by dividing composite P values by 5, requiring P 0.01 for significance. The institutional review board of the University of California, San Francisco, approved the study. All analyses were performed using STATA 9.2.
Results
Characteristics of Participating Sites
There were 209 eligible hospitals. All 209 (100%) hospitals provided data about the presence or absence of hospitalists via at least 1 of our survey strategies. The majority of identification of hospitalist utilization was via contact with either hospital or hospitalist leaders, n = 147 (70.3%). Web‐sites informed hospitalist prevalence in only 3 (1.4%) hospitals. There were 8 (3.8%) occurrences of disagreement between sources, all of which had available hospital/hospitalist leader responses. Only 1 (0.5%) hospital did not have the minimum 25 patients eligible for any disease‐specific quality measures during the data reporting period. Collectively, the remaining 208 hospitals accounted for 81% of California's acute care hospital population.
Comparisons of Sites With Hospitalists and Those Without
A total of 170 hospitals (82%) participating in CHART used hospitalists. Hospitals with and without hospitalists differed by a variety of characteristics (Table 1). Sites with hospitalists were larger, less likely to be for‐profit, had more registered nursing hours per day, and performed more cardiac catheterizations.
| Characteristic | Hospitals Without Hospitalists (n = 38) | Hospitals With Hospitalists (n = 170) | P Value* |
|---|---|---|---|
| |||
| Number of beds, n (% of hospitals) | <0.001 | ||
| 0‐99 | 16 (42.1) | 14 (8.2) | |
| 100‐199 | 8 (21.1) | 44 (25.9) | |
| 200‐299 | 7 (18.4) | 42 (24.7) | |
| 300+ | 7 (18.4) | 70 (41.2) | |
| For profit, n (% of hospitals) | 9 (23.7) | 18 (10.6) | 0.03 |
| Teaching hospital, n (% of hospitals) | 7 (18.4) | 55 (32.4) | 0.09 |
| RN hours per adjusted patient day, number of hours (IQR) | 7.4 (5.7‐8.6) | 8.5 (7.4‐9.9) | <0.001 |
| Annual cardiac catheterizations, n (IQR) | 0 (0‐356) | 210 (0‐813) | 0.007 |
| Hospital total census days, n (IQR) | 37161 (14910‐59750) | 60626 (34402‐87950) | <0.001 |
| ICU total census, n (IQR) | 2193 (1132‐4289) | 3855 (2489‐6379) | <0.001 |
| Medicare insurance, % patients (IQR) | 36.9 (28.5‐48.0) | 35.3(28.2‐44.3) | 0.95 |
| Medicaid insurance, % patients (IQR) | 21.0 (12.7‐48.3) | 16.6 (5.6‐27.6) | 0.02 |
| Race, white, % patients (IQR) | 53.7 (26.0‐82.7) | 59.1 (45.6‐74.3) | 0.73 |
| DNR at admission, % patients (IQR) | 3.6 (2.0‐6.4) | 4.4 (2.7‐7.1) | 0.12 |
| Case‐mix index, index (IQR) | 1.05 (0.90‐1.21) | 1.13 (1.01‐1.26) | 0.11 |
Relationship Between Hospitalist Group Utilization and the Percentage of Missed Quality Opportunities
Table 2 shows the frequency of missed quality opportunities in sites with hospitalists compared to those without. In general, for both individual and composite measures of quality, multivariable adjustment modestly attenuated the observed differences between the 2 groups of hospitals. We present only the more conservative adjusted estimates.
| Quality Measure | Number of Hospitals | Adjusted Mean % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative % Change | P Value | |
|---|---|---|---|---|---|---|
| Hospitals Without Hospitalists | Hospitals With Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 193 | 3.7 (2.4‐5.1) | 3.4 (2.3‐4.4) | 0.3 | 10.0 | 0.44 |
| Beta‐blocker at admission | 186 | 7.8 (4.7‐10.9) | 6.4 (4.4‐8.3) | 1.4 | 18.3 | 0.19 |
| AMI admission composite | 186 | 5.5 (3.6‐7.5) | 4.8 (3.4‐6.1) | 0.7 | 14.3 | 0.26 |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 173 | 7.5 (4.5‐10.4) | 5.2 (3.4‐6.9) | 2.3 | 31.0 | 0.02 |
| Beta‐blocker at discharge | 179 | 6.6 (3.8‐9.4) | 5.9 (3.6‐8.2) | 0.7 | 9.6 | 0.54 |
| ACE‐I/ARB at discharge | 119 | 20.7 (9.5‐31.8) | 11.8 (6.6‐17.0) | 8.9 | 43.0 | 0.006 |
| Smoking cessation counseling | 193 | 3.8 (2.4‐5.1) | 3.4 (2.4‐4.4) | 0.4 | 10.0 | 0.44 |
| AMI hospital/discharge composite | 179 | 6.4 (4.1‐8.6) | 5.3 (3.7‐6.8) | 1.1 | 17.6 | 0.16 |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 208 | 12.6 (7.7‐17.6) | 6.5 (4.6‐8.4) | 6.1 | 48.2 | <0.001 |
| ACE‐I/ARB at discharge | 201 | 14.7 (10.0‐19.4) | 12.9 (9.8‐16.1) | 1.8 | 12.1 | 0.31 |
| Smoking cessation counseling | 168 | 9.1 (2.9‐15.4) | 9.0 (4.2‐13.8) | 0.1 | 1.8 | 0.98 |
| CHF hospital/discharge composite | 201 | 12.2 (7.9‐16.5) | 8.2 (6.2‐10.2) | 4.0 | 33.1 | 0.006* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Blood culture before antibiotics | 206 | 12.0 (9.1‐14.9) | 10.9 (8.8‐13.0) | 1.1 | 9.1 | 0.29 |
| Timing of antibiotics <8 hours | 208 | 5.8 (4.1‐7.5) | 6.2 (4.7‐7.7) | 0.4 | 6.9 | 0.56 |
| Initial antibiotic consistent with recommendations | 207 | 15.0 (11.6‐18.6) | 13.8 (10.9‐16.8) | 1.2 | 8.1 | 0.27 |
| Pneumonia admission composite | 207 | 10.5 (8.5‐12.5) | 9.9 (8.3‐11.5) | 0.6 | 5.9 | 0.37 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 208 | 29.4 (19.5‐39.2) | 27.1 (19.9‐34.3) | 2.3 | 7.7 | 0.54 |
| Influenza vaccine | 207 | 36.9 (25.4‐48.4) | 35.0 (27.0‐43.1) | 1.9 | 5.2 | 0.67 |
| Smoking cessation counseling | 196 | 15.4 (7.8‐23.1) | 13.9 (8.9‐18.9) | 1.5 | 10.2 | 0.59 |
| Pneumonia hospital/discharge composite | 207 | 29.6 (20.5‐38.7) | 27.3 (20.9‐33.6) | 2.3 | 7.8 | 0.51 |
Compared to hospitals without hospitalists, those with hospitalists did not have any statistically significant differences in the individual and composite admission measures for each of the disease processes. In contrast, there were statistically significant differences between hospitalist and nonhospitalist sites for many individual cardiac processes of care that typically occur after admission from the emergency room (ie, LV function assessment for CHF) or those that occurred at discharge (ie, aspirin and ACE‐I/ARB at discharge for AMI). Similarly, the composite discharge scores for AMI and CHF revealed better overall process measure performance at sites with hospitalists, although the AMI composite did not meet statistical significance. There were no statistically significant differences between groups for the pneumonia process measures assessed at discharge. In addition, for composite measures there were no statistically significant interactions between hospitalist prevalence and bed size, although there was a trend (P = 0.06) for the CHF discharge composite, with a larger effect of hospitalists among smaller hospitals.
Percent of Patients Admitted by Hospitalists
Of the 171 hospitals with hospitalists, 71 (42%) estimated the percent of patients admitted by their hospitalist physicians. Among the respondents, the mean and median percentages of medical patients admitted by hospitalists were 51% (SD = 25%) and 49% (IQR = 30‐70%), respectively. Thirty hospitals were above the sample mean. Compared to nonrespondent sites, respondent hospitals took care of more white patients; otherwise, respondent and nonrespondent hospitals were similar in terms of bed size, location, performance across each measure, and other observable characteristics (Supporting Information, Appendix 1).
Relationship Between the Estimated Percentages of Medical Patients Admitted by Hospitalists and Missed Quality Opportunities
Table 3 displays the change in missed quality measures associated with each additional 10% of patients estimated to be admitted by hospitalists. A higher estimated percentage of patients admitted by hospitalists was associated with statistically significant improvements in quality of care across a majority of individual measures and for all composite discharge measures regardless of condition. For example, every 10% increase in the mean estimated number of patients admitted by hospitalists was associated with a mean of 0.6% (P < 0.001), 0.5% (P = 0.004), and 1.5% (P = 0.006) fewer missed quality opportunities for AMI, CHF, and pneumonia discharge process measures composites, respectively. In addition, for these composite measures, there were no statistically significant interactions between the estimated percentage of patients admitted by hospitalists and bed size (dichotomized at 150 beds), although there was a trend (P = 0.09) for the AMI discharge composite, with a larger effect of hospitalists among smaller hospitals.
| Quality Measure | Number of Hospitals | Adjusted % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative Percent Change | P Value | |
|---|---|---|---|---|---|---|
| Among Hospitals With Mean % of Patients Admitted by Hospitalists | Among Hospitals With Mean + 10% of Patients Admitted by Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐3.1) | 0.3 | 10.2 | 0.001 |
| Beta‐blocker at admission | 65 | 5.8 (3.4‐8.2) | 5.1 (3.0‐7.3) | 0.7 | 11.9 | <0.001 |
| AMI admission composite | 65 | 4.5 (2.9‐6.1) | 4.0 (2.6‐5.5) | 0.5 | 11.1 | <0.001* |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 62 | 5.1 (3.3‐6.9) | 4.6 (3.1‐6.2) | 0.5 | 9.0 | 0.03 |
| Beta‐blocker at discharge | 63 | 5.1 (2.9‐7.2) | 4.3 (2.5‐6.0) | 0.8 | 15.4 | <0.001 |
| ACE‐I/ARB at discharge | 44 | 11.4 (6.2‐16.6) | 10.3 (5.4‐15.1) | 1.1 | 10.0 | 0.02 |
| Smoking cessation counseling | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐4.1) | 0.3 | 10.2 | 0.001 |
| AMI hospital/discharge composite | 63 | 5.0 (3.3‐6.7) | 4.4 (3.0‐5.8) | 0.6 | 11.3 | 0.001* |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 71 | 5.9 (4.1‐7.6) | 5.6 (3.9‐7.2) | 0.3 | 2.9 | 0.07 |
| ACE‐I/ARB at discharge | 70 | 12.3 (8.6‐16.0) | 11.4 (7.9‐15.0) | 0.9 | 7.1 | 0.008* |
| Smoking cessation counseling | 56 | 8.4 (4.1‐12.6) | 8.2 (4.2‐12.3) | 0.2 | 1.7 | 0.67 |
| CHF hospital/discharge composite | 70 | 7.7 (5.8‐9.6) | 7.2 (5.4‐9.0) | 0.5 | 6.0 | 0.004* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Timing of antibiotics <8 hours | 71 | 5.9 (4.2‐7.6) | 5.9 (4.1‐7.7) | 0.0 | 0.0 | 0.98 |
| Blood culture before antibiotics | 71 | 10.0 (8.0‐12.0) | 9.8 (7.7‐11.8) | 0.2 | 2.6 | 0.18 |
| Initial antibiotic consistent with recommendations | 71 | 13.3 (10.4‐16.2) | 12.9 (9.9‐15.9) | 0.4 | 2.8 | 0.20 |
| Pneumonia admission composite | 71 | 9.4 (7.7‐11.1) | 9.2 (7.6‐10.9) | 0.2 | 1.8 | 0.23 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 71 | 27.0 (19.2‐34.8) | 24.7 (17.2‐32.2) | 2.3 | 8.4 | 0.006 |
| Influenza vaccine | 71 | 34.1 (25.9‐42.2) | 32.6 (24.7‐40.5) | 1.5 | 4.3 | 0.03 |
| Smoking cessation counseling | 67 | 15.2 (9.8‐20.7) | 15.0 (9.6‐20.4) | 0.2 | 2.0 | 0.56 |
| Pneumonia hospital/discharge composite | 71 | 26.7 (20.3‐33.1) | 25.2 (19.0‐31.3) | 1.5 | 5.8 | 0.006* |
In order to test the robustness of our results, we carried out 2 secondary analyses. First, we used multivariable models to generate a propensity score representing the predicted probability of being assigned to a hospital with hospitalists. We then used the propensity score as an additional covariate in subsequent multivariable models. In addition, we performed a complete‐case analysis (including only hospitals with complete data, n = 204) as a check on the sensitivity of our results to missing data. Neither analysis produced results substantially different from those presented.
Discussion
In this cross‐sectional analysis of hospitals participating in a voluntary quality reporting initiative, hospitals with at least 1 hospitalist group had fewer missed discharge care process measures for CHF, even after adjusting for hospital‐level characteristics. In addition, as the estimated percentage of patients admitted by hospitalists increased, the percentage of missed quality opportunities decreased across all measures. The observed relationships were most apparent for measures that could be completed at any time during the hospitalization and at discharge. While it is likely that hospitalists are a marker of a hospital's ability to invest in systems (and as a result, care improvement initiatives), the presence of a potential dose‐response relationship suggests that hospitalists themselves may have a role in improving processes of care.
Our study suggests a generally positive, but mixed, picture of hospitalists' effects on quality process measure performance. Lack of uniformity across measures may depend on the timing of the process measure (eg, whether or not the process is measured at admission or discharge). For example, in contrast to admission process measures, we more commonly observed a positive association between hospitalists and care quality on process measures targeting processes that generally took place later in hospitalization or at discharge. Many admission process measures (eg, door to antibiotic time, blood cultures, and appropriate initial antibiotics) likely occurred prior to hospitalist involvement in most cases and were instead under the direction of emergency medicine physicians. Performance on these measures would not be expected to relate to use of hospitalists, and that is what we observed.
In addition to the timing of when a process was measured or took place, associations between hospitalists and care quality vary by disease. The apparent variation in impact of hospitalists by disease (more impact for cardiac conditions, less for pneumonia) may relate primarily to the characteristics of the processes of care that were measured for each condition. For example, one‐half of the pneumonia process measures related to care occurring within a few hours of admission, while the other one‐half (smoking cessation advice and streptococcal and influenza vaccines) were often administered per protocol or by nonphysician providers.26‐29 However, more of the cardiac measures required physician action (eg, prescription of an ACE‐I at discharge). Alternatively, unmeasured confounders important in the delivery of cardiac care might play an important role in the relationship between hospitalists and cardiac process measure performance.
Our approach to defining hospitalists bears mention as well. While a dichotomous measure of having hospitalists available was only statistically significant for the single CHF discharge composite measure, our measure of hospitalist availabilitythe percentage of patients admitted by hospitalistswas more strongly associated with a larger number of quality measures. Contrast between the dichotomous and continuous measures may have statistical explanations (the power to see differences between 2 groups is more limited with use of a binary predictor, which itself can be subject to bias),30 but may also indicate a dose‐response relationship. A larger number of admissions to hospitalists may help standardize practices, as care is concentrated in a smaller number of physicians' hands. Moreover, larger hospitalist programs may be more likely to have implemented care standardization or quality improvement processes or to have been incorporated into (or lead) hospitals' quality infrastructures. Finally, presence of larger hospitalist groups may be a marker for a hospital's capacity to make hospital‐wide investments in improvement. However, the association between the percentage of patients admitted by hospitalists and care quality persisted even after adjustment for many measures plausibly associated with ability to invest in care quality.
Our study has several limitations. First, although we used a widely accepted definition of hospitalists endorsed by the Society of Hospital Medicine, there are no gold standard definitions for a hospitalist's job description or skill set. As a result, it is possible that a model utilizing rotating internists (from a multispecialty group) might have been misidentified as a hospitalist model. Second, our findings represent a convenience sample of hospitals in a voluntary reporting initiative (CHART) and may not be applicable to hospitals that are less able to participate in such an endeavor. CHART hospitals are recognized to be better performers than the overall California population of hospitals, potentially decreasing variability in our quality of care measures.2 Third, there were significant differences between our comparison groups within the CHART hospitals, including sample size. Although we attempted to adjust our analyses for many important potential confounders and applied conservative measures to assess statistical significance, given the baseline differences, we cannot rule out the possibility of residual confounding by unmeasured factors. Fourth, as described above, this observational study cannot provide robust evidence to support conclusions regarding causality. Fifth, the estimation of the percent of patients admitted by hospitalists is unvalidated and based upon self‐reported and incomplete (41% of respondents) data. We are somewhat reassured by the fact that respondents and nonresponders were similar across all hospital characteristics, as well as outcomes. Sixth, misclassification of the estimated percentage of patients admitted by hospitalists may have influenced our results. Although possible, misclassification often biases results toward the null, potentially weakening any observed association. Given that our respondents were not aware of our hypotheses, there is no reason to expect recall issues to bias the results one way or the other. Finally, for many performance measures, overall performance was excellent among all hospitals (eg, aspirin at admission) with limited variability, thus limiting the ability to assess for differences.
In summary, in a large, cross‐sectional study of California hospitals participating in a voluntary quality reporting initiative, the presence of hospitalists was associated with modest improvements in hospital‐level performance of quality process measures. In addition, we found a relationship between the percentage of patients admitted by hospitalists and improved process measure adherence. Although we cannot determine causality, our data support the hypothesis that dedicated hospital physicians can positively affect the quality of care. Future research should examine this relationship in other settings and should address causality using broader measures of quality including both processes and outcomes.
Acknowledgements
The authors acknowledge Teresa Chipps, BS, Center for Health Services Research, Division of General Internal Medicine and Public Health, Department of Medicine, Vanderbilt University, Nashville, TN, for her administrative and editorial assistance in the preparation of this manuscript.
Quality of care in US hospitals is inconsistent and often below accepted standards.1 This observation has catalyzed a number of performance measurement initiatives intended to publicize gaps and spur quality improvement.2 As the field has evolved, organizational factors such as teaching status, ownership model, nurse staffing levels, and hospital volume have been found to be associated with performance on quality measures.1, 3‐7 Hospitalists represent a more recent change in the organization of inpatient care8 that may impact hospital‐level performance. In fact, most hospitals provide financial support to hospitalists, not only for hopes of improving efficiency, but also for improving quality and safety.9
Only a few single‐site studies have examined the impact of hospitalists on quality of care for common medical conditions (ie, pneumonia, congestive heart failure, and acute myocardial infarction), and each has focused on patient‐level effects. Rifkin et al.10, 11 did not find differences between hospitalists' and nonhospitalists' patients in terms of pneumonia process measures. Roytman et al.12 found hospitalists more frequently prescribed afterload‐reducing agents for congestive heart failure (CHF), but other studies have shown no differences in care quality for heart failure.13, 14 Importantly, no studies have examined the role of hospitalists in the care of patients with acute myocardial infarction (AMI). In addition, studies have not addressed the effect of hospitalists at the hospital level to understand whether hospitalists have broader system‐level effects reflected by overall hospital performance.
We hypothesized that the presence of hospitalists within a hospital would be associated with improvements in hospital‐level adherence to publicly reported quality process measures, and having a greater percentage of patients admitted by hospitalists would be associated with improved performance. To test these hypotheses, we linked data from a statewide census of hospitalists with data collected as part of a hospital quality‐reporting initiative.
Materials and Methods
Study Sites
We examined the performance of 209 hospitals (63% of all 334 non‐federal facilities in California) participating in the California Hospital Assessment and Reporting Taskforce (CHART) at the time of the survey. CHART is a voluntary quality reporting initiative that began publicly reporting hospital quality data in January 2006.
Hospital‐level Organizational, Case‐mix, and Quality Data
Hospital organizational characteristics (eg, bed size) were obtained from publicly available discharge and utilization data sets from the California Office of Statewide Health Planning and Development (OSHPD). We also linked hospital‐level patient‐mix data (eg, race) from these OSHPD files.
We obtained quality of care data from CHART for January 2006 through June 2007, the time period corresponding to the survey. Quality metrics included 16 measures collected by the Center for Medicare and Medicaid Services (www.cms.hhs.gov) and extensively used in quality research.1, 4, 13, 15‐17 Rather than define a single measure, we examined multiple process measures, anticipating differential impacts of hospitalists on various processes of care for AMI, CHF, and pneumonia. Measures were further divided among those that are usually measured upon initial presentation to the hospital and those that are measured throughout the entire hospitalization and discharge. This division reflects the division of care in the hospital, where emergency room physicians are likely to have a more critical role for admission processes.
Survey Process
We surveyed all nonfederal, acute care hospitals in California that participated in CHART.2 We first identified contacts at each site via professional society mailing lists. We then sent web‐based surveys to all with available email addresses and a fax/paper survey to the remainder. We surveyed individuals between October 2006 and April 2007 and repeated the process at intervals of 1 to 3 weeks. For remaining nonrespondents, we placed a direct call unless consent to survey had been specifically refused. We contacted the following persons in sequence: (1) hospital executives or administrative leaders; (2) hospital medicine department leaders; (3) admitting emergency room personnel or medical staff officers; and (4) hospital website information. In the case of multiple responses with disagreement, the hospital/hospitalist leader's response was treated as the primary source. At each step, respondents were asked to answer questions only if they had a direct working knowledge of their hospitalist services.
Survey Data
Our key survey question to all respondents included whether the respondents could confirm their hospitals had at least one hospitalist medicine group. Hospital leaders were also asked to participate in a more comprehensive survey of their organizational and clinical characteristics. Within the comprehensive survey, leaders also provided estimates of the percent of general medical patients admitted by hospitalists. This measure, used in prior surveys of hospital leaders,9 was intended to be an easily understood approximation of the intensity of hospitalist utilization in any given hospital. A more rigorous, direct measure was not feasible due to the complexity of obtaining admission data over such a large, diverse set of hospitals.
Process Performance Measures
AMI measures assessed at admission included aspirin and ‐blocker administration within 24 hours of arrival. AMI measures assessed at discharge included aspirin administration, ‐blocker administration, angiotensin converting enzyme inhibitor (ACE‐I) (or angiotensin receptor blocker [ARB]) administration for left ventricular (LV) dysfunction, and smoking cessation counseling. There were no CHF admission measures. CHF discharge measures included assessment of LV function, the use of an ACE‐I or ARB for LV dysfunction, and smoking cessation counseling. Pneumonia admission measures included the drawing of blood cultures prior to the receipt of antibiotics, timely administration of initial antibiotics (<8 hours), and antibiotics consistent with recommendations. Pneumonia discharge measures included pneumococcal vaccination, flu vaccination, and smoking cessation counseling.
For each performance measure, we quantified the percentage of missed quality opportunities, defined as the number of patients who did not receive a care process divided by the number of eligible patients, multiplied by 100. In addition, we calculated composite scores for admission and discharge measures across each condition. We summed the numerators and denominators of individual performance measures to generate a disease‐specific composite numerator and denominator. Both individual and composite scores were produced using methodology outlined by the Center for Medicare & Medicaid Services.18 In order to retain as representative a sample of hospitals as possible, we calculated composite scores for hospitals that had a minimum of 25 observations in at least 2 of the quality indicators that made up each composite score.
Statistical Analysis
We used chi‐square tests, Student t tests, and Mann‐Whitney tests, where appropriate, to compare hospital‐level characteristics of hospitals that utilized hospitalists vs. those that did not. Similar analyses were performed among the subset of hospitals that utilized hospitalists. Among this subgroup of hospitals, we compared hospital‐level characteristics between hospitals that provided information regarding the percent of patients admitted by hospitalists vs. those who did not provide this information.
We used multivariable, generalized linear regression models to assess the relationship between having at least 1 hospitalist group and the percentage of missed quality of care measures. Because percentages were not normally distributed (ie, a majority of hospitals had few missed opportunities, while a minority had many), multivariable models employed log‐link functions with a gamma distribution.19, 20 Coefficients for our key predictor (presence of hospitalists) were transformed back to the original units (percentage of missed quality opportunities) so that a positive coefficient represented a higher number of quality measures missed relative to hospitals without hospitalists. Models were adjusted for factors previously reported to be associated with care quality. Hospital organizational characteristics included the number of beds, teaching status, registered nursing (RN) hours per adjusted patient day, and hospital ownership (for‐profit vs. not‐for‐profit). Hospital patient mix factors included annual percentage of admissions by insurance status (Medicare, Medicaid, other), annual percentage of admissions by race (white vs. nonwhite), annual percentage of do‐not‐resuscitate status at admission, and mean diagnosis‐related group‐based case‐mix index.21 We additionally adjusted for the number of cardiac catheterizations, a measure that moderately correlates with the number of cardiologists and technology utilization.22‐24 In our subset analysis among those hospitals with hospitalists, our key predictor for regression analyses was the percentage of patients admitted by hospitalists. For ease of interpretation, the percentage of patients admitted by hospitalists was centered on the mean across all respondent hospitals, and we report the effect of increasing by 10% the percentage of patients admitted by hospitalists. Models were adjusted for the same hospital organizational characteristics listed above. For those models, a positive coefficient also meant a higher number of measures missed.
For both sets of predictors, we additionally tested for the presence of interactions between the predictors and hospital bed size (both continuous as well as dichotomized at 150 beds) in composite measure performance, given the possibility that any hospitalist effect may be greater among smaller, resource‐limited hospitals. Tests for interaction were performed with the likelihood ratio test. In addition, to minimize any potential bias or loss of power that might result from limiting the analysis to hospitals with complete data, we used the multivariate imputation by chained equations method, as implemented in STATA 9.2 (StataCorp, College Station, TX), to create 10 imputed datasets.25 Imputation of missing values was restricted to confounding variables. Standard methods were then used to combine results over the 10 imputed datasets. We also applied Bonferroni corrections to composite measure tests based on the number of composites generated (n = 5). Thus, for the 5 inpatient composites created, standard definitions of significance (P 0.05) were corrected by dividing composite P values by 5, requiring P 0.01 for significance. The institutional review board of the University of California, San Francisco, approved the study. All analyses were performed using STATA 9.2.
Results
Characteristics of Participating Sites
There were 209 eligible hospitals. All 209 (100%) hospitals provided data about the presence or absence of hospitalists via at least 1 of our survey strategies. The majority of identification of hospitalist utilization was via contact with either hospital or hospitalist leaders, n = 147 (70.3%). Web‐sites informed hospitalist prevalence in only 3 (1.4%) hospitals. There were 8 (3.8%) occurrences of disagreement between sources, all of which had available hospital/hospitalist leader responses. Only 1 (0.5%) hospital did not have the minimum 25 patients eligible for any disease‐specific quality measures during the data reporting period. Collectively, the remaining 208 hospitals accounted for 81% of California's acute care hospital population.
Comparisons of Sites With Hospitalists and Those Without
A total of 170 hospitals (82%) participating in CHART used hospitalists. Hospitals with and without hospitalists differed by a variety of characteristics (Table 1). Sites with hospitalists were larger, less likely to be for‐profit, had more registered nursing hours per day, and performed more cardiac catheterizations.
| Characteristic | Hospitals Without Hospitalists (n = 38) | Hospitals With Hospitalists (n = 170) | P Value* |
|---|---|---|---|
| |||
| Number of beds, n (% of hospitals) | <0.001 | ||
| 0‐99 | 16 (42.1) | 14 (8.2) | |
| 100‐199 | 8 (21.1) | 44 (25.9) | |
| 200‐299 | 7 (18.4) | 42 (24.7) | |
| 300+ | 7 (18.4) | 70 (41.2) | |
| For profit, n (% of hospitals) | 9 (23.7) | 18 (10.6) | 0.03 |
| Teaching hospital, n (% of hospitals) | 7 (18.4) | 55 (32.4) | 0.09 |
| RN hours per adjusted patient day, number of hours (IQR) | 7.4 (5.7‐8.6) | 8.5 (7.4‐9.9) | <0.001 |
| Annual cardiac catheterizations, n (IQR) | 0 (0‐356) | 210 (0‐813) | 0.007 |
| Hospital total census days, n (IQR) | 37161 (14910‐59750) | 60626 (34402‐87950) | <0.001 |
| ICU total census, n (IQR) | 2193 (1132‐4289) | 3855 (2489‐6379) | <0.001 |
| Medicare insurance, % patients (IQR) | 36.9 (28.5‐48.0) | 35.3(28.2‐44.3) | 0.95 |
| Medicaid insurance, % patients (IQR) | 21.0 (12.7‐48.3) | 16.6 (5.6‐27.6) | 0.02 |
| Race, white, % patients (IQR) | 53.7 (26.0‐82.7) | 59.1 (45.6‐74.3) | 0.73 |
| DNR at admission, % patients (IQR) | 3.6 (2.0‐6.4) | 4.4 (2.7‐7.1) | 0.12 |
| Case‐mix index, index (IQR) | 1.05 (0.90‐1.21) | 1.13 (1.01‐1.26) | 0.11 |
Relationship Between Hospitalist Group Utilization and the Percentage of Missed Quality Opportunities
Table 2 shows the frequency of missed quality opportunities in sites with hospitalists compared to those without. In general, for both individual and composite measures of quality, multivariable adjustment modestly attenuated the observed differences between the 2 groups of hospitals. We present only the more conservative adjusted estimates.
| Quality Measure | Number of Hospitals | Adjusted Mean % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative % Change | P Value | |
|---|---|---|---|---|---|---|
| Hospitals Without Hospitalists | Hospitals With Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 193 | 3.7 (2.4‐5.1) | 3.4 (2.3‐4.4) | 0.3 | 10.0 | 0.44 |
| Beta‐blocker at admission | 186 | 7.8 (4.7‐10.9) | 6.4 (4.4‐8.3) | 1.4 | 18.3 | 0.19 |
| AMI admission composite | 186 | 5.5 (3.6‐7.5) | 4.8 (3.4‐6.1) | 0.7 | 14.3 | 0.26 |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 173 | 7.5 (4.5‐10.4) | 5.2 (3.4‐6.9) | 2.3 | 31.0 | 0.02 |
| Beta‐blocker at discharge | 179 | 6.6 (3.8‐9.4) | 5.9 (3.6‐8.2) | 0.7 | 9.6 | 0.54 |
| ACE‐I/ARB at discharge | 119 | 20.7 (9.5‐31.8) | 11.8 (6.6‐17.0) | 8.9 | 43.0 | 0.006 |
| Smoking cessation counseling | 193 | 3.8 (2.4‐5.1) | 3.4 (2.4‐4.4) | 0.4 | 10.0 | 0.44 |
| AMI hospital/discharge composite | 179 | 6.4 (4.1‐8.6) | 5.3 (3.7‐6.8) | 1.1 | 17.6 | 0.16 |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 208 | 12.6 (7.7‐17.6) | 6.5 (4.6‐8.4) | 6.1 | 48.2 | <0.001 |
| ACE‐I/ARB at discharge | 201 | 14.7 (10.0‐19.4) | 12.9 (9.8‐16.1) | 1.8 | 12.1 | 0.31 |
| Smoking cessation counseling | 168 | 9.1 (2.9‐15.4) | 9.0 (4.2‐13.8) | 0.1 | 1.8 | 0.98 |
| CHF hospital/discharge composite | 201 | 12.2 (7.9‐16.5) | 8.2 (6.2‐10.2) | 4.0 | 33.1 | 0.006* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Blood culture before antibiotics | 206 | 12.0 (9.1‐14.9) | 10.9 (8.8‐13.0) | 1.1 | 9.1 | 0.29 |
| Timing of antibiotics <8 hours | 208 | 5.8 (4.1‐7.5) | 6.2 (4.7‐7.7) | 0.4 | 6.9 | 0.56 |
| Initial antibiotic consistent with recommendations | 207 | 15.0 (11.6‐18.6) | 13.8 (10.9‐16.8) | 1.2 | 8.1 | 0.27 |
| Pneumonia admission composite | 207 | 10.5 (8.5‐12.5) | 9.9 (8.3‐11.5) | 0.6 | 5.9 | 0.37 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 208 | 29.4 (19.5‐39.2) | 27.1 (19.9‐34.3) | 2.3 | 7.7 | 0.54 |
| Influenza vaccine | 207 | 36.9 (25.4‐48.4) | 35.0 (27.0‐43.1) | 1.9 | 5.2 | 0.67 |
| Smoking cessation counseling | 196 | 15.4 (7.8‐23.1) | 13.9 (8.9‐18.9) | 1.5 | 10.2 | 0.59 |
| Pneumonia hospital/discharge composite | 207 | 29.6 (20.5‐38.7) | 27.3 (20.9‐33.6) | 2.3 | 7.8 | 0.51 |
Compared to hospitals without hospitalists, those with hospitalists did not have any statistically significant differences in the individual and composite admission measures for each of the disease processes. In contrast, there were statistically significant differences between hospitalist and nonhospitalist sites for many individual cardiac processes of care that typically occur after admission from the emergency room (ie, LV function assessment for CHF) or those that occurred at discharge (ie, aspirin and ACE‐I/ARB at discharge for AMI). Similarly, the composite discharge scores for AMI and CHF revealed better overall process measure performance at sites with hospitalists, although the AMI composite did not meet statistical significance. There were no statistically significant differences between groups for the pneumonia process measures assessed at discharge. In addition, for composite measures there were no statistically significant interactions between hospitalist prevalence and bed size, although there was a trend (P = 0.06) for the CHF discharge composite, with a larger effect of hospitalists among smaller hospitals.
Percent of Patients Admitted by Hospitalists
Of the 171 hospitals with hospitalists, 71 (42%) estimated the percent of patients admitted by their hospitalist physicians. Among the respondents, the mean and median percentages of medical patients admitted by hospitalists were 51% (SD = 25%) and 49% (IQR = 30‐70%), respectively. Thirty hospitals were above the sample mean. Compared to nonrespondent sites, respondent hospitals took care of more white patients; otherwise, respondent and nonrespondent hospitals were similar in terms of bed size, location, performance across each measure, and other observable characteristics (Supporting Information, Appendix 1).
Relationship Between the Estimated Percentages of Medical Patients Admitted by Hospitalists and Missed Quality Opportunities
Table 3 displays the change in missed quality measures associated with each additional 10% of patients estimated to be admitted by hospitalists. A higher estimated percentage of patients admitted by hospitalists was associated with statistically significant improvements in quality of care across a majority of individual measures and for all composite discharge measures regardless of condition. For example, every 10% increase in the mean estimated number of patients admitted by hospitalists was associated with a mean of 0.6% (P < 0.001), 0.5% (P = 0.004), and 1.5% (P = 0.006) fewer missed quality opportunities for AMI, CHF, and pneumonia discharge process measures composites, respectively. In addition, for these composite measures, there were no statistically significant interactions between the estimated percentage of patients admitted by hospitalists and bed size (dichotomized at 150 beds), although there was a trend (P = 0.09) for the AMI discharge composite, with a larger effect of hospitalists among smaller hospitals.
| Quality Measure | Number of Hospitals | Adjusted % Missed Quality Opportunities (95% CI) | Difference With Hospitalists | Relative Percent Change | P Value | |
|---|---|---|---|---|---|---|
| Among Hospitals With Mean % of Patients Admitted by Hospitalists | Among Hospitals With Mean + 10% of Patients Admitted by Hospitalists | |||||
| ||||||
| Acute myocardial infarction | ||||||
| Admission measures | ||||||
| Aspirin at admission | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐3.1) | 0.3 | 10.2 | 0.001 |
| Beta‐blocker at admission | 65 | 5.8 (3.4‐8.2) | 5.1 (3.0‐7.3) | 0.7 | 11.9 | <0.001 |
| AMI admission composite | 65 | 4.5 (2.9‐6.1) | 4.0 (2.6‐5.5) | 0.5 | 11.1 | <0.001* |
| Hospital/discharge measures | ||||||
| Aspirin at discharge | 62 | 5.1 (3.3‐6.9) | 4.6 (3.1‐6.2) | 0.5 | 9.0 | 0.03 |
| Beta‐blocker at discharge | 63 | 5.1 (2.9‐7.2) | 4.3 (2.5‐6.0) | 0.8 | 15.4 | <0.001 |
| ACE‐I/ARB at discharge | 44 | 11.4 (6.2‐16.6) | 10.3 (5.4‐15.1) | 1.1 | 10.0 | 0.02 |
| Smoking cessation counseling | 70 | 3.4 (2.3‐4.6) | 3.1 (2.0‐4.1) | 0.3 | 10.2 | 0.001 |
| AMI hospital/discharge composite | 63 | 5.0 (3.3‐6.7) | 4.4 (3.0‐5.8) | 0.6 | 11.3 | 0.001* |
| Congestive heart failure | ||||||
| Hospital/discharge measures | ||||||
| Ejection fraction assessment | 71 | 5.9 (4.1‐7.6) | 5.6 (3.9‐7.2) | 0.3 | 2.9 | 0.07 |
| ACE‐I/ARB at discharge | 70 | 12.3 (8.6‐16.0) | 11.4 (7.9‐15.0) | 0.9 | 7.1 | 0.008* |
| Smoking cessation counseling | 56 | 8.4 (4.1‐12.6) | 8.2 (4.2‐12.3) | 0.2 | 1.7 | 0.67 |
| CHF hospital/discharge composite | 70 | 7.7 (5.8‐9.6) | 7.2 (5.4‐9.0) | 0.5 | 6.0 | 0.004* |
| Pneumonia | ||||||
| Admission measures | ||||||
| Timing of antibiotics <8 hours | 71 | 5.9 (4.2‐7.6) | 5.9 (4.1‐7.7) | 0.0 | 0.0 | 0.98 |
| Blood culture before antibiotics | 71 | 10.0 (8.0‐12.0) | 9.8 (7.7‐11.8) | 0.2 | 2.6 | 0.18 |
| Initial antibiotic consistent with recommendations | 71 | 13.3 (10.4‐16.2) | 12.9 (9.9‐15.9) | 0.4 | 2.8 | 0.20 |
| Pneumonia admission composite | 71 | 9.4 (7.7‐11.1) | 9.2 (7.6‐10.9) | 0.2 | 1.8 | 0.23 |
| Hospital/discharge measures | ||||||
| Pneumonia vaccine | 71 | 27.0 (19.2‐34.8) | 24.7 (17.2‐32.2) | 2.3 | 8.4 | 0.006 |
| Influenza vaccine | 71 | 34.1 (25.9‐42.2) | 32.6 (24.7‐40.5) | 1.5 | 4.3 | 0.03 |
| Smoking cessation counseling | 67 | 15.2 (9.8‐20.7) | 15.0 (9.6‐20.4) | 0.2 | 2.0 | 0.56 |
| Pneumonia hospital/discharge composite | 71 | 26.7 (20.3‐33.1) | 25.2 (19.0‐31.3) | 1.5 | 5.8 | 0.006* |
In order to test the robustness of our results, we carried out 2 secondary analyses. First, we used multivariable models to generate a propensity score representing the predicted probability of being assigned to a hospital with hospitalists. We then used the propensity score as an additional covariate in subsequent multivariable models. In addition, we performed a complete‐case analysis (including only hospitals with complete data, n = 204) as a check on the sensitivity of our results to missing data. Neither analysis produced results substantially different from those presented.
Discussion
In this cross‐sectional analysis of hospitals participating in a voluntary quality reporting initiative, hospitals with at least 1 hospitalist group had fewer missed discharge care process measures for CHF, even after adjusting for hospital‐level characteristics. In addition, as the estimated percentage of patients admitted by hospitalists increased, the percentage of missed quality opportunities decreased across all measures. The observed relationships were most apparent for measures that could be completed at any time during the hospitalization and at discharge. While it is likely that hospitalists are a marker of a hospital's ability to invest in systems (and as a result, care improvement initiatives), the presence of a potential dose‐response relationship suggests that hospitalists themselves may have a role in improving processes of care.
Our study suggests a generally positive, but mixed, picture of hospitalists' effects on quality process measure performance. Lack of uniformity across measures may depend on the timing of the process measure (eg, whether or not the process is measured at admission or discharge). For example, in contrast to admission process measures, we more commonly observed a positive association between hospitalists and care quality on process measures targeting processes that generally took place later in hospitalization or at discharge. Many admission process measures (eg, door to antibiotic time, blood cultures, and appropriate initial antibiotics) likely occurred prior to hospitalist involvement in most cases and were instead under the direction of emergency medicine physicians. Performance on these measures would not be expected to relate to use of hospitalists, and that is what we observed.
In addition to the timing of when a process was measured or took place, associations between hospitalists and care quality vary by disease. The apparent variation in impact of hospitalists by disease (more impact for cardiac conditions, less for pneumonia) may relate primarily to the characteristics of the processes of care that were measured for each condition. For example, one‐half of the pneumonia process measures related to care occurring within a few hours of admission, while the other one‐half (smoking cessation advice and streptococcal and influenza vaccines) were often administered per protocol or by nonphysician providers.26‐29 However, more of the cardiac measures required physician action (eg, prescription of an ACE‐I at discharge). Alternatively, unmeasured confounders important in the delivery of cardiac care might play an important role in the relationship between hospitalists and cardiac process measure performance.
Our approach to defining hospitalists bears mention as well. While a dichotomous measure of having hospitalists available was only statistically significant for the single CHF discharge composite measure, our measure of hospitalist availabilitythe percentage of patients admitted by hospitalistswas more strongly associated with a larger number of quality measures. Contrast between the dichotomous and continuous measures may have statistical explanations (the power to see differences between 2 groups is more limited with use of a binary predictor, which itself can be subject to bias),30 but may also indicate a dose‐response relationship. A larger number of admissions to hospitalists may help standardize practices, as care is concentrated in a smaller number of physicians' hands. Moreover, larger hospitalist programs may be more likely to have implemented care standardization or quality improvement processes or to have been incorporated into (or lead) hospitals' quality infrastructures. Finally, presence of larger hospitalist groups may be a marker for a hospital's capacity to make hospital‐wide investments in improvement. However, the association between the percentage of patients admitted by hospitalists and care quality persisted even after adjustment for many measures plausibly associated with ability to invest in care quality.
Our study has several limitations. First, although we used a widely accepted definition of hospitalists endorsed by the Society of Hospital Medicine, there are no gold standard definitions for a hospitalist's job description or skill set. As a result, it is possible that a model utilizing rotating internists (from a multispecialty group) might have been misidentified as a hospitalist model. Second, our findings represent a convenience sample of hospitals in a voluntary reporting initiative (CHART) and may not be applicable to hospitals that are less able to participate in such an endeavor. CHART hospitals are recognized to be better performers than the overall California population of hospitals, potentially decreasing variability in our quality of care measures.2 Third, there were significant differences between our comparison groups within the CHART hospitals, including sample size. Although we attempted to adjust our analyses for many important potential confounders and applied conservative measures to assess statistical significance, given the baseline differences, we cannot rule out the possibility of residual confounding by unmeasured factors. Fourth, as described above, this observational study cannot provide robust evidence to support conclusions regarding causality. Fifth, the estimation of the percent of patients admitted by hospitalists is unvalidated and based upon self‐reported and incomplete (41% of respondents) data. We are somewhat reassured by the fact that respondents and nonresponders were similar across all hospital characteristics, as well as outcomes. Sixth, misclassification of the estimated percentage of patients admitted by hospitalists may have influenced our results. Although possible, misclassification often biases results toward the null, potentially weakening any observed association. Given that our respondents were not aware of our hypotheses, there is no reason to expect recall issues to bias the results one way or the other. Finally, for many performance measures, overall performance was excellent among all hospitals (eg, aspirin at admission) with limited variability, thus limiting the ability to assess for differences.
In summary, in a large, cross‐sectional study of California hospitals participating in a voluntary quality reporting initiative, the presence of hospitalists was associated with modest improvements in hospital‐level performance of quality process measures. In addition, we found a relationship between the percentage of patients admitted by hospitalists and improved process measure adherence. Although we cannot determine causality, our data support the hypothesis that dedicated hospital physicians can positively affect the quality of care. Future research should examine this relationship in other settings and should address causality using broader measures of quality including both processes and outcomes.
Acknowledgements
The authors acknowledge Teresa Chipps, BS, Center for Health Services Research, Division of General Internal Medicine and Public Health, Department of Medicine, Vanderbilt University, Nashville, TN, for her administrative and editorial assistance in the preparation of this manuscript.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- CalHospitalCompare.org: online report card simplifies the search for quality hospital care. Available at: http://www.chcf.org/topics/hospitals/index.cfm?itemID=131387. Accessed September 2009.
- ,,, et al.Hospital characteristics and quality of care.JAMA.1992;268:1709–1714.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,, et al.A systematic review and meta‐analysis of studies comparing mortality rates of private for‐profit and private not‐for‐profit hospitals.CMAJ.2002;166:1399–1406.
- ,.Teaching hospitals and quality of care: a review of the literature.Milbank Q.2002;80:569–593.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23:1399–1406.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.The inverse relationship between mortality rates and performance in the Hospital Quality Alliance measures.Health Aff.2007;26:1104–1110.
- ,,,,.Does the Leapfrog program help identify high‐quality hospitals?Jt Comm J Qual Patient Saf.2008;34:318–325.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- CMS HQI demonstration project—composite quality score methodology overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed September 2009.
- ,,.Modeling risk using generalized linear models.J Health Econ.1999;18:153–171.
- ,,.Generalized modeling approaches to risk adjustment of skewed outcomes data.J Health Econ.2005;24:465–488.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,, et al.The Dartmouth Atlas of Cardiovascular Health Care.Chicago:AHA Press;1999. Current data from the Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH. Available at: http://www.dartmouthatlas.org/atlases/atlas_ series.shtm. Accessed September 2009.
- ,,.Differences in per capita rates of revascularization and in choice of revascularization procedure for eleven states.BMC Health Serv Res.2006;6:35.
- ,,.The relationship between physician supply, cardiovascular health service use and cardiac disease burden in Ontario: supply‐need mismatch.Can J Card.2008;24:187.
- .Multiple imputation: a primer.Stat Methods Med Res.1999;8:3–15.
- .Nursing intervention and smoking cessation: Meta‐analysis update.Heart Lung.2006;35:147–163.
- .Ten‐year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high‐risk adults.Am J Med.1998;105:385–392.
- ,,, et al.Role of student pharmacist interns in hospital‐based standing orders pneumococcal vaccination program.J Am Pharm Assoc.2007;47:404–409.
- ,,,.Effect of a pharmacist‐managed program of pneumococcal and influenza immunization on vaccination rates among adult inpatients.Am J Health Syst Pharm.2003;60:1767–1771.
- ,,.Dichotomizing continuous predictors in multiple regression: a bad idea.Stat Med.2006;25:127–141.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- CalHospitalCompare.org: online report card simplifies the search for quality hospital care. Available at: http://www.chcf.org/topics/hospitals/index.cfm?itemID=131387. Accessed September 2009.
- ,,, et al.Hospital characteristics and quality of care.JAMA.1992;268:1709–1714.
- ,,,,.Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module.Arch Intern Med.2002;162:827–833.
- ,,, et al.A systematic review and meta‐analysis of studies comparing mortality rates of private for‐profit and private not‐for‐profit hospitals.CMAJ.2002;166:1399–1406.
- ,.Teaching hospitals and quality of care: a review of the literature.Milbank Q.2002;80:569–593.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20:101–107.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23:1399–1406.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.The inverse relationship between mortality rates and performance in the Hospital Quality Alliance measures.Health Aff.2007;26:1104–1110.
- ,,,,.Does the Leapfrog program help identify high‐quality hospitals?Jt Comm J Qual Patient Saf.2008;34:318–325.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- CMS HQI demonstration project—composite quality score methodology overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed September 2009.
- ,,.Modeling risk using generalized linear models.J Health Econ.1999;18:153–171.
- ,,.Generalized modeling approaches to risk adjustment of skewed outcomes data.J Health Econ.2005;24:465–488.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,, et al.The Dartmouth Atlas of Cardiovascular Health Care.Chicago:AHA Press;1999. Current data from the Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH. Available at: http://www.dartmouthatlas.org/atlases/atlas_ series.shtm. Accessed September 2009.
- ,,.Differences in per capita rates of revascularization and in choice of revascularization procedure for eleven states.BMC Health Serv Res.2006;6:35.
- ,,.The relationship between physician supply, cardiovascular health service use and cardiac disease burden in Ontario: supply‐need mismatch.Can J Card.2008;24:187.
- .Multiple imputation: a primer.Stat Methods Med Res.1999;8:3–15.
- .Nursing intervention and smoking cessation: Meta‐analysis update.Heart Lung.2006;35:147–163.
- .Ten‐year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high‐risk adults.Am J Med.1998;105:385–392.
- ,,, et al.Role of student pharmacist interns in hospital‐based standing orders pneumococcal vaccination program.J Am Pharm Assoc.2007;47:404–409.
- ,,,.Effect of a pharmacist‐managed program of pneumococcal and influenza immunization on vaccination rates among adult inpatients.Am J Health Syst Pharm.2003;60:1767–1771.
- ,,.Dichotomizing continuous predictors in multiple regression: a bad idea.Stat Med.2006;25:127–141.
Copyright © 2010 Society of Hospital Medicine
Hope
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
Self‐Medication and Intractable Cough
A 60 year old man presented with a 1‐month history of severe, dry cough. His medical history is significant for lymphoma (4 years), bone marrow transplant (8 months), stable graft‐vs.‐host disease, with a negative bleeding history. He self‐medicated his cough with 6 to 8 tablets daily of an analgesic containing 250 mg acetaminophen, 65 mg caffeine, and 250 mg aspirin. He developed severe stabbing pain in his right lower quadrant exacerbated by coughing. Several days later he developed severe pain of his left upper abdomen. The abdominal wall was tender. Laboratory tests revealed a hematocrit of 31%, platelet count of 246,000, international normalized prothrombin ratio of 1.0, and activated partial thromboplastin ratio of 0.8. Abdominal computed tomography (CT) without contrast revealed 2 rectus sheath hematomas. A 4‐cm 2‐cm 2‐cm collection consistent with hematoma was contained within the left rectus abdominis muscle sheath above the level of the umbilicus (white arrow, Figure 1A, top). An additional collection measuring 6 cm 6 cm 4 cm was present below the umbilicus and between the posterior aspect of the right rectus abdominus muscle (black arrows, Figure 1A bottom; B and C) and the anterior surface of the bladder (arrowhead, Figure 1A and C). Aspirin was stopped and he was started on moxifloxacin for pneumonia. He was discharged 3 days later with improved cough resulting in improved pain control.
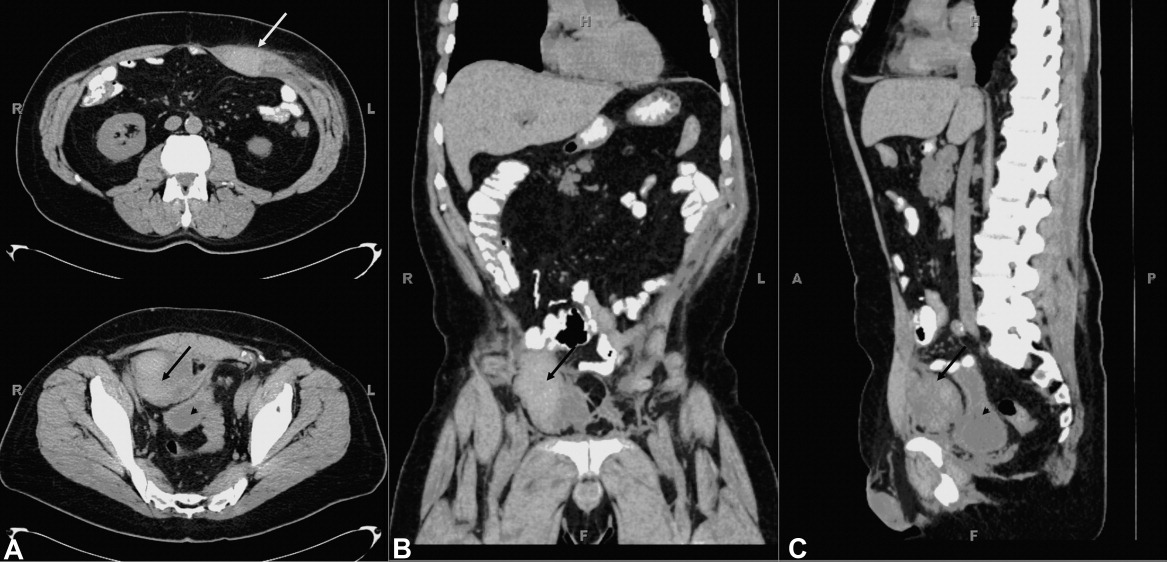
Rectus sheath hematoma may be contained with the fascia when above the arcuate line, located about 5 cm below the umbilicus. Below the arcuate line a rectus sheath hematoma may dissect posteriorly into the prevesicular space of Retzius. Both are seen in this case. Rectus sheath hematoma are usually associated with cough in the setting of coagulopathy, and may require transfusion or surgical evacuation. High dose aspirin, in this case up to 2000 mg per day, may lead to hemorrhage associated with intractable coughing.
A 60 year old man presented with a 1‐month history of severe, dry cough. His medical history is significant for lymphoma (4 years), bone marrow transplant (8 months), stable graft‐vs.‐host disease, with a negative bleeding history. He self‐medicated his cough with 6 to 8 tablets daily of an analgesic containing 250 mg acetaminophen, 65 mg caffeine, and 250 mg aspirin. He developed severe stabbing pain in his right lower quadrant exacerbated by coughing. Several days later he developed severe pain of his left upper abdomen. The abdominal wall was tender. Laboratory tests revealed a hematocrit of 31%, platelet count of 246,000, international normalized prothrombin ratio of 1.0, and activated partial thromboplastin ratio of 0.8. Abdominal computed tomography (CT) without contrast revealed 2 rectus sheath hematomas. A 4‐cm 2‐cm 2‐cm collection consistent with hematoma was contained within the left rectus abdominis muscle sheath above the level of the umbilicus (white arrow, Figure 1A, top). An additional collection measuring 6 cm 6 cm 4 cm was present below the umbilicus and between the posterior aspect of the right rectus abdominus muscle (black arrows, Figure 1A bottom; B and C) and the anterior surface of the bladder (arrowhead, Figure 1A and C). Aspirin was stopped and he was started on moxifloxacin for pneumonia. He was discharged 3 days later with improved cough resulting in improved pain control.

Rectus sheath hematoma may be contained with the fascia when above the arcuate line, located about 5 cm below the umbilicus. Below the arcuate line a rectus sheath hematoma may dissect posteriorly into the prevesicular space of Retzius. Both are seen in this case. Rectus sheath hematoma are usually associated with cough in the setting of coagulopathy, and may require transfusion or surgical evacuation. High dose aspirin, in this case up to 2000 mg per day, may lead to hemorrhage associated with intractable coughing.
A 60 year old man presented with a 1‐month history of severe, dry cough. His medical history is significant for lymphoma (4 years), bone marrow transplant (8 months), stable graft‐vs.‐host disease, with a negative bleeding history. He self‐medicated his cough with 6 to 8 tablets daily of an analgesic containing 250 mg acetaminophen, 65 mg caffeine, and 250 mg aspirin. He developed severe stabbing pain in his right lower quadrant exacerbated by coughing. Several days later he developed severe pain of his left upper abdomen. The abdominal wall was tender. Laboratory tests revealed a hematocrit of 31%, platelet count of 246,000, international normalized prothrombin ratio of 1.0, and activated partial thromboplastin ratio of 0.8. Abdominal computed tomography (CT) without contrast revealed 2 rectus sheath hematomas. A 4‐cm 2‐cm 2‐cm collection consistent with hematoma was contained within the left rectus abdominis muscle sheath above the level of the umbilicus (white arrow, Figure 1A, top). An additional collection measuring 6 cm 6 cm 4 cm was present below the umbilicus and between the posterior aspect of the right rectus abdominus muscle (black arrows, Figure 1A bottom; B and C) and the anterior surface of the bladder (arrowhead, Figure 1A and C). Aspirin was stopped and he was started on moxifloxacin for pneumonia. He was discharged 3 days later with improved cough resulting in improved pain control.

Rectus sheath hematoma may be contained with the fascia when above the arcuate line, located about 5 cm below the umbilicus. Below the arcuate line a rectus sheath hematoma may dissect posteriorly into the prevesicular space of Retzius. Both are seen in this case. Rectus sheath hematoma are usually associated with cough in the setting of coagulopathy, and may require transfusion or surgical evacuation. High dose aspirin, in this case up to 2000 mg per day, may lead to hemorrhage associated with intractable coughing.
Finger Points to the Diagnosis
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin 20 ng/ml).

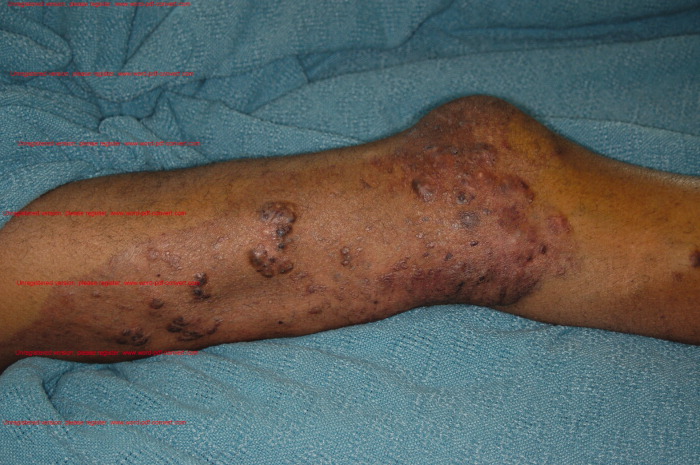
Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin 20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin 20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
Incisional Iliac Hernia
A 61‐year‐old woman presented with 1 month of abdominal pain and bowel irregularity. Physical examination was normal. Computed tomography of the abdomen demonstrated a nonincarcerated hernia of the ascending colon through a 25‐mm bony defect in the superior aspect of the right iliac crest (Figure 1)a defect created 16 years prior when iliac crest bone graft harvest was performed during subtalar fusion. An open surgical approach was employed to reduce the hernia and close the defect with mesh. Her postoperative course was unremarkable. Her symptoms resolved entirely.
The iliac crest is the site utilized most frequently in orthopedic surgery for bone graft harvest. The literature suggests that up to 5% of these procedures may be complicated by symptomatic herniation of abdominal contents.1 Other procedural complications include: donor site pain, nerve injury (commonly the lateral femoral cutaneous nerve, manifesting as meralgia paresthetica), vascular disruption (including superior gluteal and lumbar arteries), hematoma, visible deformity, abnormal gait, and stress fracture.2, 3 Advanced age, obesity, muscular weakness, larger defect size, and full‐thickness harvest have been identified as risk factors.4 The hernia often presents as a soft‐tissue mass originating at the defect site, which may become more pronounced with cough. Auscultation over the area may reveal bowel sounds. The spectrum of associated abdominal symptoms ranges from mild discomfort to colicky pain and distention;3 up to 16% of patients present with acute signs of intestinal obstruction.1 Iliac herniation may be prevented by harvest of a partial thickness graft, whenever possible. Selective removal of bone from the anterior or posterior crest, rather than the middle, may also decrease the risk.4 Clinicians caring for patients with a history of iliac crest bone graft harvest should be familiar with this complication and consider prompt radiologic imaging when investigating new or unexplained abdominal symptoms.
- ,,,.Hernia through iliac crest defects.Int Orthop.1995;19:367–369.
- ,,.Harvesting autogenous iliac bone grafts: a review of complications and techniques.Spine.1989;14:1324–1331.
- ,,,.Hernia through an iliac crest bone graft site: report of a case and review of the literature.Bull Hosp Jt Dis.2006;63:166–168.
- ,.Incisional hernia through iliac crest defects.Arch Orthop Trauma Surg.1989;108:383–385.
A 61‐year‐old woman presented with 1 month of abdominal pain and bowel irregularity. Physical examination was normal. Computed tomography of the abdomen demonstrated a nonincarcerated hernia of the ascending colon through a 25‐mm bony defect in the superior aspect of the right iliac crest (Figure 1)a defect created 16 years prior when iliac crest bone graft harvest was performed during subtalar fusion. An open surgical approach was employed to reduce the hernia and close the defect with mesh. Her postoperative course was unremarkable. Her symptoms resolved entirely.

The iliac crest is the site utilized most frequently in orthopedic surgery for bone graft harvest. The literature suggests that up to 5% of these procedures may be complicated by symptomatic herniation of abdominal contents.1 Other procedural complications include: donor site pain, nerve injury (commonly the lateral femoral cutaneous nerve, manifesting as meralgia paresthetica), vascular disruption (including superior gluteal and lumbar arteries), hematoma, visible deformity, abnormal gait, and stress fracture.2, 3 Advanced age, obesity, muscular weakness, larger defect size, and full‐thickness harvest have been identified as risk factors.4 The hernia often presents as a soft‐tissue mass originating at the defect site, which may become more pronounced with cough. Auscultation over the area may reveal bowel sounds. The spectrum of associated abdominal symptoms ranges from mild discomfort to colicky pain and distention;3 up to 16% of patients present with acute signs of intestinal obstruction.1 Iliac herniation may be prevented by harvest of a partial thickness graft, whenever possible. Selective removal of bone from the anterior or posterior crest, rather than the middle, may also decrease the risk.4 Clinicians caring for patients with a history of iliac crest bone graft harvest should be familiar with this complication and consider prompt radiologic imaging when investigating new or unexplained abdominal symptoms.
A 61‐year‐old woman presented with 1 month of abdominal pain and bowel irregularity. Physical examination was normal. Computed tomography of the abdomen demonstrated a nonincarcerated hernia of the ascending colon through a 25‐mm bony defect in the superior aspect of the right iliac crest (Figure 1)a defect created 16 years prior when iliac crest bone graft harvest was performed during subtalar fusion. An open surgical approach was employed to reduce the hernia and close the defect with mesh. Her postoperative course was unremarkable. Her symptoms resolved entirely.

The iliac crest is the site utilized most frequently in orthopedic surgery for bone graft harvest. The literature suggests that up to 5% of these procedures may be complicated by symptomatic herniation of abdominal contents.1 Other procedural complications include: donor site pain, nerve injury (commonly the lateral femoral cutaneous nerve, manifesting as meralgia paresthetica), vascular disruption (including superior gluteal and lumbar arteries), hematoma, visible deformity, abnormal gait, and stress fracture.2, 3 Advanced age, obesity, muscular weakness, larger defect size, and full‐thickness harvest have been identified as risk factors.4 The hernia often presents as a soft‐tissue mass originating at the defect site, which may become more pronounced with cough. Auscultation over the area may reveal bowel sounds. The spectrum of associated abdominal symptoms ranges from mild discomfort to colicky pain and distention;3 up to 16% of patients present with acute signs of intestinal obstruction.1 Iliac herniation may be prevented by harvest of a partial thickness graft, whenever possible. Selective removal of bone from the anterior or posterior crest, rather than the middle, may also decrease the risk.4 Clinicians caring for patients with a history of iliac crest bone graft harvest should be familiar with this complication and consider prompt radiologic imaging when investigating new or unexplained abdominal symptoms.
- ,,,.Hernia through iliac crest defects.Int Orthop.1995;19:367–369.
- ,,.Harvesting autogenous iliac bone grafts: a review of complications and techniques.Spine.1989;14:1324–1331.
- ,,,.Hernia through an iliac crest bone graft site: report of a case and review of the literature.Bull Hosp Jt Dis.2006;63:166–168.
- ,.Incisional hernia through iliac crest defects.Arch Orthop Trauma Surg.1989;108:383–385.
- ,,,.Hernia through iliac crest defects.Int Orthop.1995;19:367–369.
- ,,.Harvesting autogenous iliac bone grafts: a review of complications and techniques.Spine.1989;14:1324–1331.
- ,,,.Hernia through an iliac crest bone graft site: report of a case and review of the literature.Bull Hosp Jt Dis.2006;63:166–168.
- ,.Incisional hernia through iliac crest defects.Arch Orthop Trauma Surg.1989;108:383–385.
Patient Whiteboards in the Hospital Setting
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).

From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).
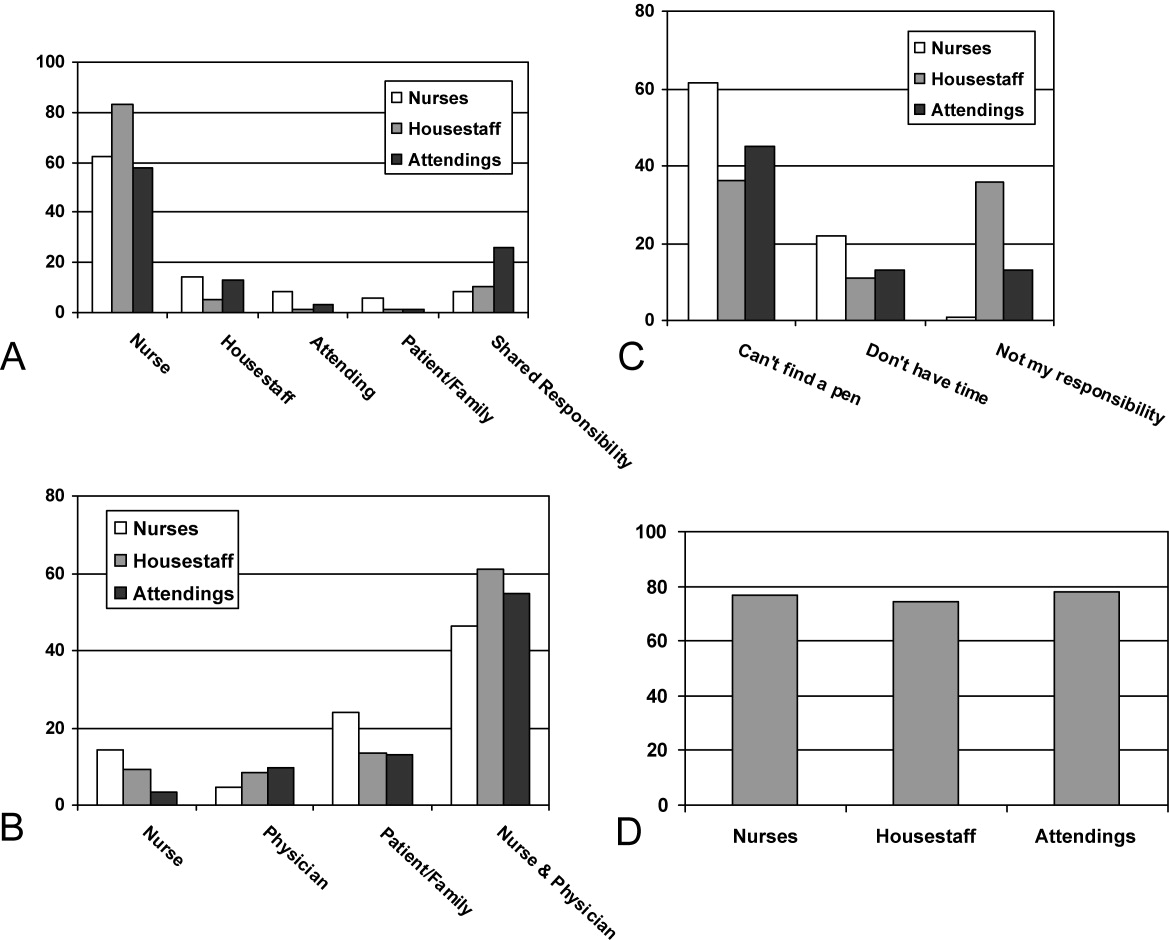
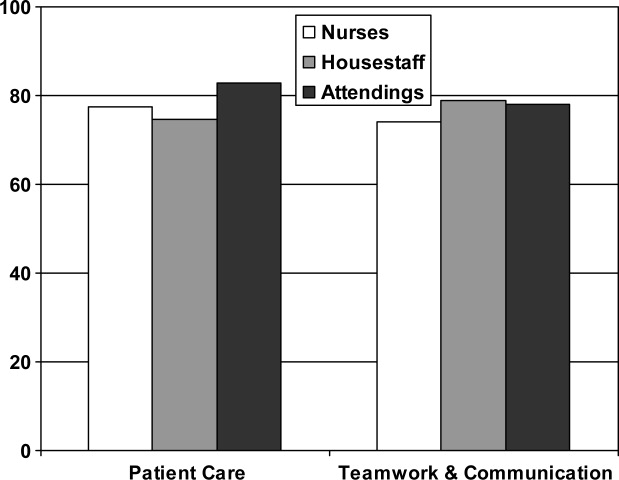
| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).



From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).


| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).



From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).


| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
Acute Pancreatitis
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.
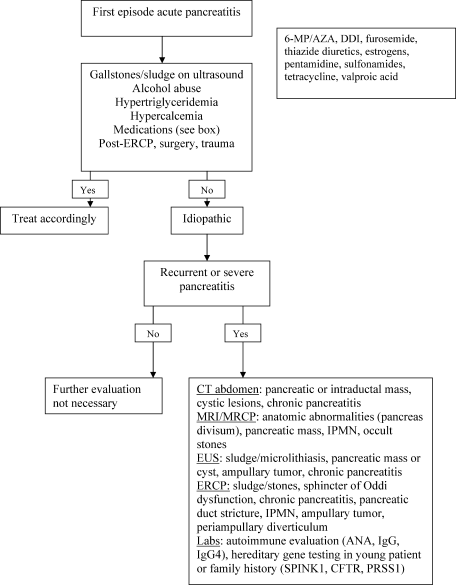
Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | 8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | 60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | 8 mg/dl |
| PaO2 | 60 mm Hg |
| Serum albumin | 3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of 90 mmHg |
| Pulmonary insufficiency | PaO2 60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51
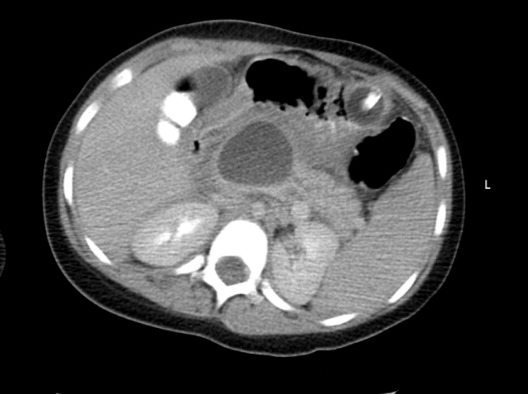
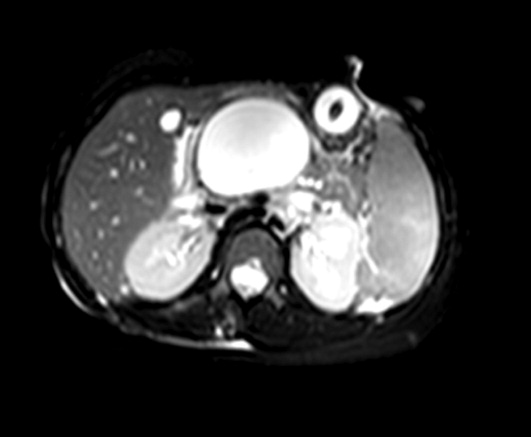
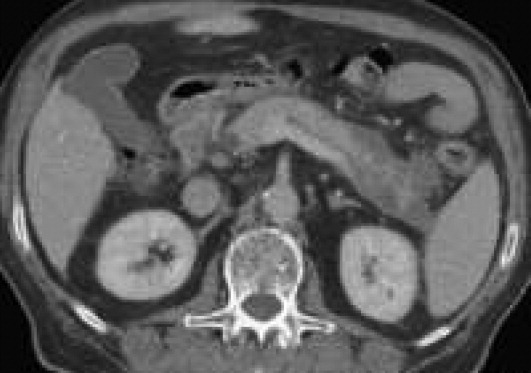
Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.
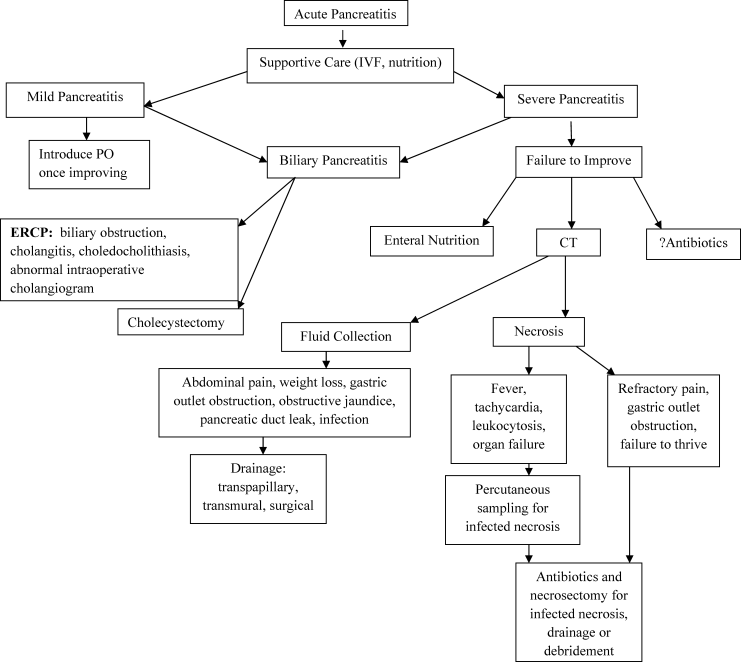
Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.

Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | 8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | 60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | 8 mg/dl |
| PaO2 | 60 mm Hg |
| Serum albumin | 3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of 90 mmHg |
| Pulmonary insufficiency | PaO2 60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51



Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.

Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.

Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | 8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | 60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | 8 mg/dl |
| PaO2 | 60 mm Hg |
| Serum albumin | 3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of 90 mmHg |
| Pulmonary insufficiency | PaO2 60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51



Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.

Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
Electrical Alternans and Pulsus Paradoxus
A 65‐year‐old man with chronic obstructive pulmonary disease and right lung nodule presented with dyspnea. Physical examination revealed a pulse of 130 beats per minute, respiratory rate of 28 times per minute, blood pressure of 100/60 mm Hg, estimated jugular venous pressure of greater than 15 cm above the right atrium at a 45‐degree semirecumbent position, and distant heart sounds. He subsequently developed hypotension and an arterial line was placed. A single‐channel electrocardiogram (Figure 1A; upper tracing) demonstrated electrical alternans. Simultaneous arterial line (Figure 1A; lower tracing) showed decreased systolic blood pressure from 136 mm Hg (Figure 1A; arrow) to 96 mm Hg (Figure 1A; arrowhead) with inspiration, consistent with exaggerated pulsus paradoxus. A transthoracic echocardiogram confirmed a large pericardial effusion with the heart oscillating from side (Figure 1B) to side (Figure 1C) within the pericardial sac. Pericardiocentesis was performed and 1100 mL of bloody pericardial fluid was removed with prompt resolution of hypotension, tachycardia, electrical alternans, and abnormal pulsus paradoxus. Pericardial effusion (PE), right ventricle (RV), and left ventricle (LV) are depicted in Figure 1B, C.
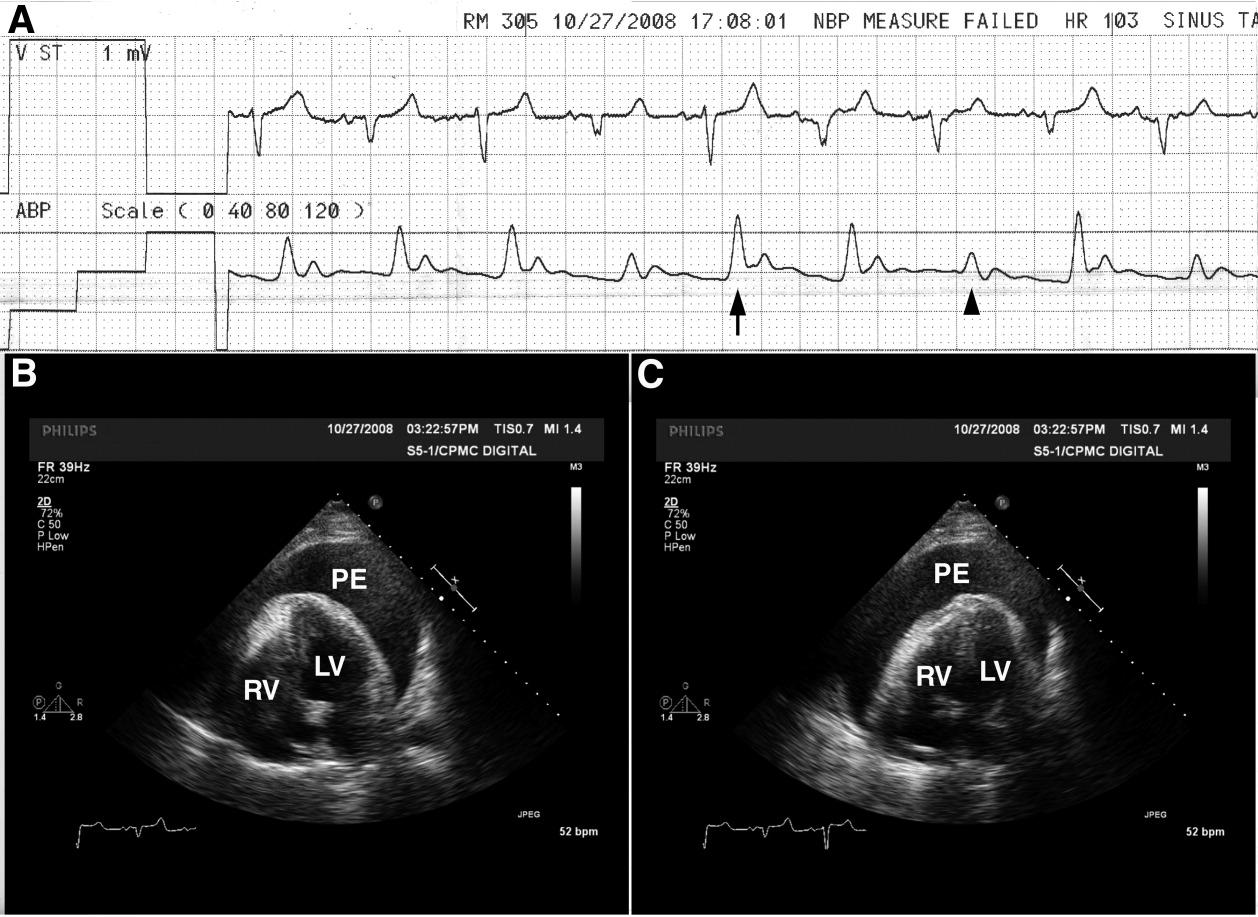
The etiology of this patient's pericardial effusion was felt to be due to metastatic pericardial disease from lung cancer. The mechanism of electrical alternans is felt to be due to motion as the heart oscillates back and forth within the pericardial sac.1 The exaggerated pulsus paradoxus reflects decreased LV filling during inspiration as RV filling increases and compresses the LV, referred to as ventricular interdependence.
- ,,.Quantitative echocardiographic assessment in pericardial disease.Echocardiography.1997;14:207–214.
A 65‐year‐old man with chronic obstructive pulmonary disease and right lung nodule presented with dyspnea. Physical examination revealed a pulse of 130 beats per minute, respiratory rate of 28 times per minute, blood pressure of 100/60 mm Hg, estimated jugular venous pressure of greater than 15 cm above the right atrium at a 45‐degree semirecumbent position, and distant heart sounds. He subsequently developed hypotension and an arterial line was placed. A single‐channel electrocardiogram (Figure 1A; upper tracing) demonstrated electrical alternans. Simultaneous arterial line (Figure 1A; lower tracing) showed decreased systolic blood pressure from 136 mm Hg (Figure 1A; arrow) to 96 mm Hg (Figure 1A; arrowhead) with inspiration, consistent with exaggerated pulsus paradoxus. A transthoracic echocardiogram confirmed a large pericardial effusion with the heart oscillating from side (Figure 1B) to side (Figure 1C) within the pericardial sac. Pericardiocentesis was performed and 1100 mL of bloody pericardial fluid was removed with prompt resolution of hypotension, tachycardia, electrical alternans, and abnormal pulsus paradoxus. Pericardial effusion (PE), right ventricle (RV), and left ventricle (LV) are depicted in Figure 1B, C.

The etiology of this patient's pericardial effusion was felt to be due to metastatic pericardial disease from lung cancer. The mechanism of electrical alternans is felt to be due to motion as the heart oscillates back and forth within the pericardial sac.1 The exaggerated pulsus paradoxus reflects decreased LV filling during inspiration as RV filling increases and compresses the LV, referred to as ventricular interdependence.
A 65‐year‐old man with chronic obstructive pulmonary disease and right lung nodule presented with dyspnea. Physical examination revealed a pulse of 130 beats per minute, respiratory rate of 28 times per minute, blood pressure of 100/60 mm Hg, estimated jugular venous pressure of greater than 15 cm above the right atrium at a 45‐degree semirecumbent position, and distant heart sounds. He subsequently developed hypotension and an arterial line was placed. A single‐channel electrocardiogram (Figure 1A; upper tracing) demonstrated electrical alternans. Simultaneous arterial line (Figure 1A; lower tracing) showed decreased systolic blood pressure from 136 mm Hg (Figure 1A; arrow) to 96 mm Hg (Figure 1A; arrowhead) with inspiration, consistent with exaggerated pulsus paradoxus. A transthoracic echocardiogram confirmed a large pericardial effusion with the heart oscillating from side (Figure 1B) to side (Figure 1C) within the pericardial sac. Pericardiocentesis was performed and 1100 mL of bloody pericardial fluid was removed with prompt resolution of hypotension, tachycardia, electrical alternans, and abnormal pulsus paradoxus. Pericardial effusion (PE), right ventricle (RV), and left ventricle (LV) are depicted in Figure 1B, C.

The etiology of this patient's pericardial effusion was felt to be due to metastatic pericardial disease from lung cancer. The mechanism of electrical alternans is felt to be due to motion as the heart oscillates back and forth within the pericardial sac.1 The exaggerated pulsus paradoxus reflects decreased LV filling during inspiration as RV filling increases and compresses the LV, referred to as ventricular interdependence.
- ,,.Quantitative echocardiographic assessment in pericardial disease.Echocardiography.1997;14:207–214.
- ,,.Quantitative echocardiographic assessment in pericardial disease.Echocardiography.1997;14:207–214.
Significance of Bacteriuria
Staphylococcus aureus (SA) infection can cause a wide range of clinical syndromes, from folliculitis to life‐threatening endocarditis. Further, SA is second only to S. epidermidis as a cause of bacteremia in hospitalized patients.1, 2 Recent single‐institution studies suggests that SA could be the most frequent cause of nosocomial bacteremia,3, 4 but this needs to be validated in multicenter studies. SA bacteremia (SAB) is often complicated by hematogenous seeding into deep tissues or prosthetic material. The association of future hardware infection following SAB is well documented.5, 6 One study showed that SAB can precede and be associated with prosthetic joint infections in up to 34% of cases.6 Intravascular cardiac devices can also be infected by SAB, with rates from 28% to 75% depending on how early the bacteremia occurred in relation to the implantation of the device.5 Risk stratification for these complications is a clinical challenge. Fowler et al.7 postulated some clinical identifiers of complicated SAB; however, predicting which patients will develop a complication from SAB remains very difficult. Muder et al.8 demonstrated that the presence of SA bacteriuria (SABU) correlates with subsequent SAB, but a possible association of SABU with complicated bacteremia was not examined. A more recent study from Huggan et al.9 has suggested a possible association between SABU and poor clinical outcomes in adults with SAB.
We hypothesized that the presence of SABU would identify those patients at increased risk of complications from SAB. SABU may be a practical, economical, and readily available predictor of complicated SAB. Those patients at higher risk for complications may require a more aggressive diagnostic and therapeutic approach.
Methods
We conducted a retrospective cohort study of SAB patients with and without SA in the urine to investigate the association between SABU and the outcomes of the complications and mortality.
The study was conducted at Miami Valley Hospital (MVH, Dayton, OH), an 848‐bed, level 1 trauma center with 69 intensive care unit (ICU) beds. MVH is a community teaching hospital affiliated with Wright State University Boonshoft School of Medicine and averages 35,000 admissions per year. The same microbiology laboratory (Compunet Clinical Laboratories) processed all the blood and urine culture specimens of the patients in this study.
The inclusion criteria were as follows: 1) admission to MVH between January 1, 2004 and December 31, 2007 with a documented episode of SAB (at least 1 positive blood culture); and 2) a documented urine culture within 7 days of the episode of SAB. Patients without a documented urine culture or with inadequate/emncomplete treatment for SAB were excluded. A total of 118 patients were included based on the presence of a positive blood culture for SA and the presence of a documented urine culture. Patient electronic and paper records were reviewed by 3 of the investigators (E.V.P.‐J., S.D.B., and W.B.B.). Patients subsequently admitted to MVH and to MVH's companion medical center in Dayton, Good Samaritan Hospital, were followed through the electronic medical record common to both institutions.
Study patients were divided into 2 cohorts. One cohort included the patients with a urine culture that grew SA, either methicillin‐resistant SA (MRSA) or methicillin‐susceptible SA (MSSA). The other cohort included patients who had either a negative urine culture or a positive urine culture with organisms other than SA. The age, sex, date of admission, length of stay, and duration of follow‐up were recorded for each patient. Clinical variables included blood culture and urine culture results, presence of intravenous catheters, antibiotic therapy and duration, presence of comorbidities, and clinical outcomes (complications and death).
The primary outcome was complications during hospital admission. The 8 complications investigated were as follows: endocarditis, osteomyelitis, septic arthritis, thrombophlebitis, septic shock, septic embolism/abscess, persistent SAB (lasting more than 5 days after starting adequate SA treatment), and recurrent SAB. In addition, the 2 groups were compared on: 1) any complication, 2) average complications, 3) early complications (ie, within the current hospital admission), and 4) delayed complications (ie, complications diagnosed on subsequent admissions).
Statistical Methods
Means standard deviations (SDs) are reported for continuous variables while frequencies and percents are reported for categorical variables. The independent samples t test for continuous variables and the chi square test or Fisher's exact test for categorical variables were used to compare the two cohorts. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons. SPSS 11.0 software (SPSS, Inc., Chicago, IL) was used for all analyses.
Results
Of the 118 patients, 58 were female (49.2%) and 60 male (50.8%). The age of the patients was 63.3 16.7 years (mean SD). The length of hospital stay was 19.3 17.0 days, and the duration of follow up was 8.3 5.7 months. MRSA was isolated in 75 patients (63.6%) and MSSA in 43 patients (36.4%). In the 28 patients with SA in urine cultures, MRSA was found more frequently than MSSA (20 vs. 8 patients). The acquisition of SAB was equally divided among outpatient (35.6%), healthcare‐associated (30.5%), and hospital‐acquired (33.9%) settings.
Table 1 shows that the group with SABU did not differ from the group without SABU in age (66 years vs. 62 years; P = 0.29), sex (43% male vs. 53% male; P = 0.33), length of hospital stay (18 days vs. 20 days; P = 0.59), and duration of follow‐up (6.6 months vs. 8.8 months; P = 0.064). The 2 cohorts also did not differ on the proportion with MRSA bacteremia (71% vs. 61%; P = 0.32), origin of SAB (P = 0.12), and the presence of comorbidities (diabetes mellitus, cardiomyopathy/congestive heart failure, malignancy, renal disease, and immunosuppression) (all P values > 0.30).
| Characteristic | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Age (years) (mean SD) | 66.3 16.3 | 62.4 16.8 | 0.29 |
| Male sex (n [%[) | 12 (42.9) | 48 (53.3) | 0.33 |
| Length of stay (days) (mean SD) | 17.8 16.1 | 19.7 17.3 | 0.59 |
| Follow‐up (months) (mean SD) | 6.6 5.3 | 8.8 5.7 | 0.064 |
| Blood culture (n [%]) | |||
| MRSA | 20 (71.4) | 55 (61.1) | 0.32 |
| MSSA | 8 (28.6) | 35 (38.9) | |
| Origin of the bacteremia [n (%)] | 0.12 | ||
| Community‐acquired | 13 (46.4) | 29 (32.2) | |
| Healthcare‐acquired | 10 (35.7) | 26 (28.9) | |
| Hospital‐acquired | 5 (17.9) | 35 (38.9) | |
| Comorbidities (n [%]) | |||
| DM | 11 (39.3) | 38 (42.2) | 0.78 |
| CHF | 5 (17.9) | 20 (22.2) | 0.62 |
| Cancer | 7 (25.0) | 15 (16.7) | 0.32 |
| ESRD | 4 (14.3) | 12 (13.3) | 1.00 |
| Immunosuppression | 6 (21.4) | 15 (16.7) | 0.58 |
| Patients lost to follow‐up (n [%]) | 5 (17.8) | 8 (8.8) | 0.19 |
Table 2 shows that patients in the SABU group were nearly twice as likely to have a complication as the group without SABU (64% vs. 33%; P = 0.004) and had a higher mean number of complications (0.89 vs. 0.48; P = 0.016). Patients in the SABU group also were more likely to have early complications (64% vs. 23%; P < 0.001) but no more likely to have a delayed complication (14% vs. 12%; P = 0.75). Of the 8 specific complications evaluated, the 2 groups differed only on the presence of septic shock, with the SABU group having 3 times more patients with this complication (21% vs. 7%; P = 0.035). Also, a higher proportion of patients died in the SABU group (32.1% vs. 14.4%; P = 0.036).
| Outcome | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Any complication (n [%]) | 18 (64.3) | 30 (33.3) | 0.004 |
| Average complications (mean SD) | 0.89 0.83 | 0.48 0.77 | 0.016 |
| Timing of complication (n [%]) | |||
| Early | 18 (64.3) | 21 (23.3) | <0.001 |
| Delayed | 4 (14.3) | 11 (12.2) | 0.75 |
| By specific complication, n (%) | |||
| Endocarditis | 1 (3.6) | 5 (5.6) | 1.00 |
| Osteomyelitis | 3 (10.7) | 5 (5.6) | 0.39 |
| Septic arthritis | 2 (7.1) | 3 (3.3) | 0.59 |
| Thrombophlebitis | 1 (3.6) | 3 (3.3) | 1.00 |
| Septic shock | 6 (21.4) | 6 (6.7) | 0.035 |
| Septic embolism/abscess | 6 (21.4) | 10 (11.1) | 0.21 |
| Persistent SAB | 3 (10.7) | 3 (3.3) | 0.14 |
| Recurrent SAB | 3 (10.7) | 8 (8.9) | 0.72 |
| Death (n [%]) | 9 (32.1) | 13 (14.4) | 0.036 |
Patients with MRSA (n = 75) and those with MSSA (n = 43) did not differ on any complication, average complications, early or late complications, or 7 of the specific complications (data not shown). Only with thrombophlebitis did the 2 groups differ; the MSSA group had 4 (9.3%) patients with this complication while none in the MRSA group were affected (P = 0.016).
Discussion
In our retrospective analysis, SAB with concomitant SABU was associated with more severe disease, complications, and death. Compared to SAB patients without SA in the urine, those with SAB and SA in the urine had more total complications and more early complications, especially septic shock. Further, the proportion of deaths in the SABU cohort was more than twice as high (32% vs. 14%). Therefore, the presence of SABU in patients with SAB could potentially be a useful predictor of complicated SAB and death.
The relationship between SABU and early complications and death remained after excluding the complication of septic shock/need for vasopressors from the analysis (data not shown). The lack of relationship between SABU and delayed complications might have been due to the adequacy of treatment for SAB. Appropriateness of therapy, a criterion for patient inclusion, may have lessened the likelihood of an insufficient treatment plan causing complications. Those patients with MRSA did not differ from those with MSSA on the mean number of complications or early and delayed complications. A greater proportion of MSSA patients had thrombophlebitis than MRSA patients.
Other investigations have identified predictors of mortality or complications from SAB,7, 912 but SABU was not included as a variable in most of these studies. Fowler et al.7 proposed a prognostic model of complicated SAB using the predictors from their study; community acquisition of organisms, persistent bacteremia, persistent fever over 72 hours, and skin examination suggestive of an acute systemic infection. Muder et al.8 reported a relationship between SABU and subsequent SAB, but they did not examine the association between SABU and the risk of complicated SAB. Huggan et al.9 found that concomitant SABU is associated with ICU admission and increased in‐hospital mortality in patients with SAB.
SAB patients with SABU may be at risk for early complications. Consequently, such patients may warrant more aggressive evaluation and treatment. Further, SABU in patients with SAB may be indicative of an endocarditis‐like condition. SA is rarely isolated from the urinary tract as a uropathogen, although it may colonize indwelling catheters and may cause catheter‐related urinary tract infections.13, 14 Thus, when present in urine, SA could be a marker of deep tissue dissemination with the potential to cause complications. Guidelines for the management of intravascular device‐associated bacteremia have been published by the Infectious Diseases Society of America (IDSA) and other organizations,15, 16 and recent studies have demonstrated the effectiveness of newer agents for the management of SAB.17 Nevertheless, there is still controversy regarding some aspects of the management of SAB (eg, duration of therapy, criteria for echocardiographic evaluation, role of combination therapy). The presence of SABU, the marker evaluated in our study, may be an additional factor to consider when deciding upon duration of therapy and whether to obtain echocardiography or other imaging.
Our study was limited by its retrospective nature. Patient records were not always complete. For example, not all patients had echocardiography to evaluate for endocarditis or venous ultrasound to evaluate for septic thrombophlebitis. Also, the presence (or proper removal) of intravascular or urinary catheters could not be documented reliably in all patients. In addition, the 7‐day cutoff for obtaining urine cultures may have been too lenient, leading to underdiagnosis of bacteriuria. Finally, while 13 patients were lost to follow‐up, the 2 groups (SABU and No SABU) did not differ in the proportion lost.
In conclusion, our study found that SABU may be a useful predictor of complicated SAB and death. SAB patients with SABU may be at risk for more and earlier complications. These patients may need closer monitoring due to the higher risk of septic shock and death. Additional therapeutic and management recommendations might include: 1) longer duration of therapy even if a removable source of the bacteremia is identified; 2) more frequent and better supervised follow‐up; and 3) imaging studies including either computed tomography (CT) scans or ultrasound for thorough evaluation of complications. Prospective studies including randomized controlled trials are required before implementing these suggested diagnostic and therapeutic recommendations.
Acknowledgements
The authors thank and acknowledge Logan McCool and Adam Woiwood for their administrative contributions to the study. E.V.P.‐J., as the principal investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- ,,, et al.Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two‐year study in 16 hospitals.Eur J Clin Microbiol Infect Dis.2002;21(12):849–855.
- ,,, et al.Nosocomial bloodstream infections in ICU and non‐ICU patients.Am J Infec Control.2005;33(6):333–340.
- ,,, et al.Age‐ and sex‐associated trends in bloodstream infection: a population‐based study in Olmsted County, Minnesota.Arch Intern Med.2007;167(8):834–839.
- ,,.Bloodstream infections in a geriatric cohort: a population‐based study.Am J Med.2007;120(12):1078–1883.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104(9):1029–1033.
- ,,, et al.Infection of orthopedic prostheses after Staphylococcus aureus bacteremia.Clin Infect Dis.2001;32(4):647–649.
- ,,, et al.Clinical identifiers of complicated Staphylococcus aureus bacteremia.Arch Intern Med.2003;163(17):2066–2072.
- ,,, et al.Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic UTI and subsequent staphylococcal bacteremia.Clin Infect Dis.2006;42(1):46–50.
- ,,, et al.Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteremia.J Hosp Infect.2008;69:345–349.
- ,,, et al.Persistent Staphylococcus aureus bacteremia. An analysis of risk factors and outcomes.Arch Int Med.2007;167(17):1861–1867.
- .Staphylococcus aureus bacteremia in older adults: predictors of 7‐day mortality and infection with a methicillin‐resistant strain.Infect Control Hosp Epidemiol.2006;27(11):1219–1225.
- ,,, et al.Infective endocarditis. Diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation2005;111(23):e394–e434.
- ,,, et al.Antibiotic sensitivity of bacteria associated with community‐acquired urinary tract infection in Britain.J Antimicrob Chemother.1999;44(3):359–365.
- .Antibiotic susceptibility of bacterial strains isolated from patients with community‐acquired urinary tract infections in France.Eur J Clin Microbiol Infect Dis.2000;19(2):112–117.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46(suppl 5):S386–S393.
- ,,, et al.Guidelines for the management of intravascular catheter‐related infections.Clin Infect Dis.2001;32(9):1249–1272.
- ,,.Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006;355(7):653–665.
Staphylococcus aureus (SA) infection can cause a wide range of clinical syndromes, from folliculitis to life‐threatening endocarditis. Further, SA is second only to S. epidermidis as a cause of bacteremia in hospitalized patients.1, 2 Recent single‐institution studies suggests that SA could be the most frequent cause of nosocomial bacteremia,3, 4 but this needs to be validated in multicenter studies. SA bacteremia (SAB) is often complicated by hematogenous seeding into deep tissues or prosthetic material. The association of future hardware infection following SAB is well documented.5, 6 One study showed that SAB can precede and be associated with prosthetic joint infections in up to 34% of cases.6 Intravascular cardiac devices can also be infected by SAB, with rates from 28% to 75% depending on how early the bacteremia occurred in relation to the implantation of the device.5 Risk stratification for these complications is a clinical challenge. Fowler et al.7 postulated some clinical identifiers of complicated SAB; however, predicting which patients will develop a complication from SAB remains very difficult. Muder et al.8 demonstrated that the presence of SA bacteriuria (SABU) correlates with subsequent SAB, but a possible association of SABU with complicated bacteremia was not examined. A more recent study from Huggan et al.9 has suggested a possible association between SABU and poor clinical outcomes in adults with SAB.
We hypothesized that the presence of SABU would identify those patients at increased risk of complications from SAB. SABU may be a practical, economical, and readily available predictor of complicated SAB. Those patients at higher risk for complications may require a more aggressive diagnostic and therapeutic approach.
Methods
We conducted a retrospective cohort study of SAB patients with and without SA in the urine to investigate the association between SABU and the outcomes of the complications and mortality.
The study was conducted at Miami Valley Hospital (MVH, Dayton, OH), an 848‐bed, level 1 trauma center with 69 intensive care unit (ICU) beds. MVH is a community teaching hospital affiliated with Wright State University Boonshoft School of Medicine and averages 35,000 admissions per year. The same microbiology laboratory (Compunet Clinical Laboratories) processed all the blood and urine culture specimens of the patients in this study.
The inclusion criteria were as follows: 1) admission to MVH between January 1, 2004 and December 31, 2007 with a documented episode of SAB (at least 1 positive blood culture); and 2) a documented urine culture within 7 days of the episode of SAB. Patients without a documented urine culture or with inadequate/emncomplete treatment for SAB were excluded. A total of 118 patients were included based on the presence of a positive blood culture for SA and the presence of a documented urine culture. Patient electronic and paper records were reviewed by 3 of the investigators (E.V.P.‐J., S.D.B., and W.B.B.). Patients subsequently admitted to MVH and to MVH's companion medical center in Dayton, Good Samaritan Hospital, were followed through the electronic medical record common to both institutions.
Study patients were divided into 2 cohorts. One cohort included the patients with a urine culture that grew SA, either methicillin‐resistant SA (MRSA) or methicillin‐susceptible SA (MSSA). The other cohort included patients who had either a negative urine culture or a positive urine culture with organisms other than SA. The age, sex, date of admission, length of stay, and duration of follow‐up were recorded for each patient. Clinical variables included blood culture and urine culture results, presence of intravenous catheters, antibiotic therapy and duration, presence of comorbidities, and clinical outcomes (complications and death).
The primary outcome was complications during hospital admission. The 8 complications investigated were as follows: endocarditis, osteomyelitis, septic arthritis, thrombophlebitis, septic shock, septic embolism/abscess, persistent SAB (lasting more than 5 days after starting adequate SA treatment), and recurrent SAB. In addition, the 2 groups were compared on: 1) any complication, 2) average complications, 3) early complications (ie, within the current hospital admission), and 4) delayed complications (ie, complications diagnosed on subsequent admissions).
Statistical Methods
Means standard deviations (SDs) are reported for continuous variables while frequencies and percents are reported for categorical variables. The independent samples t test for continuous variables and the chi square test or Fisher's exact test for categorical variables were used to compare the two cohorts. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons. SPSS 11.0 software (SPSS, Inc., Chicago, IL) was used for all analyses.
Results
Of the 118 patients, 58 were female (49.2%) and 60 male (50.8%). The age of the patients was 63.3 16.7 years (mean SD). The length of hospital stay was 19.3 17.0 days, and the duration of follow up was 8.3 5.7 months. MRSA was isolated in 75 patients (63.6%) and MSSA in 43 patients (36.4%). In the 28 patients with SA in urine cultures, MRSA was found more frequently than MSSA (20 vs. 8 patients). The acquisition of SAB was equally divided among outpatient (35.6%), healthcare‐associated (30.5%), and hospital‐acquired (33.9%) settings.
Table 1 shows that the group with SABU did not differ from the group without SABU in age (66 years vs. 62 years; P = 0.29), sex (43% male vs. 53% male; P = 0.33), length of hospital stay (18 days vs. 20 days; P = 0.59), and duration of follow‐up (6.6 months vs. 8.8 months; P = 0.064). The 2 cohorts also did not differ on the proportion with MRSA bacteremia (71% vs. 61%; P = 0.32), origin of SAB (P = 0.12), and the presence of comorbidities (diabetes mellitus, cardiomyopathy/congestive heart failure, malignancy, renal disease, and immunosuppression) (all P values > 0.30).
| Characteristic | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Age (years) (mean SD) | 66.3 16.3 | 62.4 16.8 | 0.29 |
| Male sex (n [%[) | 12 (42.9) | 48 (53.3) | 0.33 |
| Length of stay (days) (mean SD) | 17.8 16.1 | 19.7 17.3 | 0.59 |
| Follow‐up (months) (mean SD) | 6.6 5.3 | 8.8 5.7 | 0.064 |
| Blood culture (n [%]) | |||
| MRSA | 20 (71.4) | 55 (61.1) | 0.32 |
| MSSA | 8 (28.6) | 35 (38.9) | |
| Origin of the bacteremia [n (%)] | 0.12 | ||
| Community‐acquired | 13 (46.4) | 29 (32.2) | |
| Healthcare‐acquired | 10 (35.7) | 26 (28.9) | |
| Hospital‐acquired | 5 (17.9) | 35 (38.9) | |
| Comorbidities (n [%]) | |||
| DM | 11 (39.3) | 38 (42.2) | 0.78 |
| CHF | 5 (17.9) | 20 (22.2) | 0.62 |
| Cancer | 7 (25.0) | 15 (16.7) | 0.32 |
| ESRD | 4 (14.3) | 12 (13.3) | 1.00 |
| Immunosuppression | 6 (21.4) | 15 (16.7) | 0.58 |
| Patients lost to follow‐up (n [%]) | 5 (17.8) | 8 (8.8) | 0.19 |
Table 2 shows that patients in the SABU group were nearly twice as likely to have a complication as the group without SABU (64% vs. 33%; P = 0.004) and had a higher mean number of complications (0.89 vs. 0.48; P = 0.016). Patients in the SABU group also were more likely to have early complications (64% vs. 23%; P < 0.001) but no more likely to have a delayed complication (14% vs. 12%; P = 0.75). Of the 8 specific complications evaluated, the 2 groups differed only on the presence of septic shock, with the SABU group having 3 times more patients with this complication (21% vs. 7%; P = 0.035). Also, a higher proportion of patients died in the SABU group (32.1% vs. 14.4%; P = 0.036).
| Outcome | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Any complication (n [%]) | 18 (64.3) | 30 (33.3) | 0.004 |
| Average complications (mean SD) | 0.89 0.83 | 0.48 0.77 | 0.016 |
| Timing of complication (n [%]) | |||
| Early | 18 (64.3) | 21 (23.3) | <0.001 |
| Delayed | 4 (14.3) | 11 (12.2) | 0.75 |
| By specific complication, n (%) | |||
| Endocarditis | 1 (3.6) | 5 (5.6) | 1.00 |
| Osteomyelitis | 3 (10.7) | 5 (5.6) | 0.39 |
| Septic arthritis | 2 (7.1) | 3 (3.3) | 0.59 |
| Thrombophlebitis | 1 (3.6) | 3 (3.3) | 1.00 |
| Septic shock | 6 (21.4) | 6 (6.7) | 0.035 |
| Septic embolism/abscess | 6 (21.4) | 10 (11.1) | 0.21 |
| Persistent SAB | 3 (10.7) | 3 (3.3) | 0.14 |
| Recurrent SAB | 3 (10.7) | 8 (8.9) | 0.72 |
| Death (n [%]) | 9 (32.1) | 13 (14.4) | 0.036 |
Patients with MRSA (n = 75) and those with MSSA (n = 43) did not differ on any complication, average complications, early or late complications, or 7 of the specific complications (data not shown). Only with thrombophlebitis did the 2 groups differ; the MSSA group had 4 (9.3%) patients with this complication while none in the MRSA group were affected (P = 0.016).
Discussion
In our retrospective analysis, SAB with concomitant SABU was associated with more severe disease, complications, and death. Compared to SAB patients without SA in the urine, those with SAB and SA in the urine had more total complications and more early complications, especially septic shock. Further, the proportion of deaths in the SABU cohort was more than twice as high (32% vs. 14%). Therefore, the presence of SABU in patients with SAB could potentially be a useful predictor of complicated SAB and death.
The relationship between SABU and early complications and death remained after excluding the complication of septic shock/need for vasopressors from the analysis (data not shown). The lack of relationship between SABU and delayed complications might have been due to the adequacy of treatment for SAB. Appropriateness of therapy, a criterion for patient inclusion, may have lessened the likelihood of an insufficient treatment plan causing complications. Those patients with MRSA did not differ from those with MSSA on the mean number of complications or early and delayed complications. A greater proportion of MSSA patients had thrombophlebitis than MRSA patients.
Other investigations have identified predictors of mortality or complications from SAB,7, 912 but SABU was not included as a variable in most of these studies. Fowler et al.7 proposed a prognostic model of complicated SAB using the predictors from their study; community acquisition of organisms, persistent bacteremia, persistent fever over 72 hours, and skin examination suggestive of an acute systemic infection. Muder et al.8 reported a relationship between SABU and subsequent SAB, but they did not examine the association between SABU and the risk of complicated SAB. Huggan et al.9 found that concomitant SABU is associated with ICU admission and increased in‐hospital mortality in patients with SAB.
SAB patients with SABU may be at risk for early complications. Consequently, such patients may warrant more aggressive evaluation and treatment. Further, SABU in patients with SAB may be indicative of an endocarditis‐like condition. SA is rarely isolated from the urinary tract as a uropathogen, although it may colonize indwelling catheters and may cause catheter‐related urinary tract infections.13, 14 Thus, when present in urine, SA could be a marker of deep tissue dissemination with the potential to cause complications. Guidelines for the management of intravascular device‐associated bacteremia have been published by the Infectious Diseases Society of America (IDSA) and other organizations,15, 16 and recent studies have demonstrated the effectiveness of newer agents for the management of SAB.17 Nevertheless, there is still controversy regarding some aspects of the management of SAB (eg, duration of therapy, criteria for echocardiographic evaluation, role of combination therapy). The presence of SABU, the marker evaluated in our study, may be an additional factor to consider when deciding upon duration of therapy and whether to obtain echocardiography or other imaging.
Our study was limited by its retrospective nature. Patient records were not always complete. For example, not all patients had echocardiography to evaluate for endocarditis or venous ultrasound to evaluate for septic thrombophlebitis. Also, the presence (or proper removal) of intravascular or urinary catheters could not be documented reliably in all patients. In addition, the 7‐day cutoff for obtaining urine cultures may have been too lenient, leading to underdiagnosis of bacteriuria. Finally, while 13 patients were lost to follow‐up, the 2 groups (SABU and No SABU) did not differ in the proportion lost.
In conclusion, our study found that SABU may be a useful predictor of complicated SAB and death. SAB patients with SABU may be at risk for more and earlier complications. These patients may need closer monitoring due to the higher risk of septic shock and death. Additional therapeutic and management recommendations might include: 1) longer duration of therapy even if a removable source of the bacteremia is identified; 2) more frequent and better supervised follow‐up; and 3) imaging studies including either computed tomography (CT) scans or ultrasound for thorough evaluation of complications. Prospective studies including randomized controlled trials are required before implementing these suggested diagnostic and therapeutic recommendations.
Acknowledgements
The authors thank and acknowledge Logan McCool and Adam Woiwood for their administrative contributions to the study. E.V.P.‐J., as the principal investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Staphylococcus aureus (SA) infection can cause a wide range of clinical syndromes, from folliculitis to life‐threatening endocarditis. Further, SA is second only to S. epidermidis as a cause of bacteremia in hospitalized patients.1, 2 Recent single‐institution studies suggests that SA could be the most frequent cause of nosocomial bacteremia,3, 4 but this needs to be validated in multicenter studies. SA bacteremia (SAB) is often complicated by hematogenous seeding into deep tissues or prosthetic material. The association of future hardware infection following SAB is well documented.5, 6 One study showed that SAB can precede and be associated with prosthetic joint infections in up to 34% of cases.6 Intravascular cardiac devices can also be infected by SAB, with rates from 28% to 75% depending on how early the bacteremia occurred in relation to the implantation of the device.5 Risk stratification for these complications is a clinical challenge. Fowler et al.7 postulated some clinical identifiers of complicated SAB; however, predicting which patients will develop a complication from SAB remains very difficult. Muder et al.8 demonstrated that the presence of SA bacteriuria (SABU) correlates with subsequent SAB, but a possible association of SABU with complicated bacteremia was not examined. A more recent study from Huggan et al.9 has suggested a possible association between SABU and poor clinical outcomes in adults with SAB.
We hypothesized that the presence of SABU would identify those patients at increased risk of complications from SAB. SABU may be a practical, economical, and readily available predictor of complicated SAB. Those patients at higher risk for complications may require a more aggressive diagnostic and therapeutic approach.
Methods
We conducted a retrospective cohort study of SAB patients with and without SA in the urine to investigate the association between SABU and the outcomes of the complications and mortality.
The study was conducted at Miami Valley Hospital (MVH, Dayton, OH), an 848‐bed, level 1 trauma center with 69 intensive care unit (ICU) beds. MVH is a community teaching hospital affiliated with Wright State University Boonshoft School of Medicine and averages 35,000 admissions per year. The same microbiology laboratory (Compunet Clinical Laboratories) processed all the blood and urine culture specimens of the patients in this study.
The inclusion criteria were as follows: 1) admission to MVH between January 1, 2004 and December 31, 2007 with a documented episode of SAB (at least 1 positive blood culture); and 2) a documented urine culture within 7 days of the episode of SAB. Patients without a documented urine culture or with inadequate/emncomplete treatment for SAB were excluded. A total of 118 patients were included based on the presence of a positive blood culture for SA and the presence of a documented urine culture. Patient electronic and paper records were reviewed by 3 of the investigators (E.V.P.‐J., S.D.B., and W.B.B.). Patients subsequently admitted to MVH and to MVH's companion medical center in Dayton, Good Samaritan Hospital, were followed through the electronic medical record common to both institutions.
Study patients were divided into 2 cohorts. One cohort included the patients with a urine culture that grew SA, either methicillin‐resistant SA (MRSA) or methicillin‐susceptible SA (MSSA). The other cohort included patients who had either a negative urine culture or a positive urine culture with organisms other than SA. The age, sex, date of admission, length of stay, and duration of follow‐up were recorded for each patient. Clinical variables included blood culture and urine culture results, presence of intravenous catheters, antibiotic therapy and duration, presence of comorbidities, and clinical outcomes (complications and death).
The primary outcome was complications during hospital admission. The 8 complications investigated were as follows: endocarditis, osteomyelitis, septic arthritis, thrombophlebitis, septic shock, septic embolism/abscess, persistent SAB (lasting more than 5 days after starting adequate SA treatment), and recurrent SAB. In addition, the 2 groups were compared on: 1) any complication, 2) average complications, 3) early complications (ie, within the current hospital admission), and 4) delayed complications (ie, complications diagnosed on subsequent admissions).
Statistical Methods
Means standard deviations (SDs) are reported for continuous variables while frequencies and percents are reported for categorical variables. The independent samples t test for continuous variables and the chi square test or Fisher's exact test for categorical variables were used to compare the two cohorts. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons. SPSS 11.0 software (SPSS, Inc., Chicago, IL) was used for all analyses.
Results
Of the 118 patients, 58 were female (49.2%) and 60 male (50.8%). The age of the patients was 63.3 16.7 years (mean SD). The length of hospital stay was 19.3 17.0 days, and the duration of follow up was 8.3 5.7 months. MRSA was isolated in 75 patients (63.6%) and MSSA in 43 patients (36.4%). In the 28 patients with SA in urine cultures, MRSA was found more frequently than MSSA (20 vs. 8 patients). The acquisition of SAB was equally divided among outpatient (35.6%), healthcare‐associated (30.5%), and hospital‐acquired (33.9%) settings.
Table 1 shows that the group with SABU did not differ from the group without SABU in age (66 years vs. 62 years; P = 0.29), sex (43% male vs. 53% male; P = 0.33), length of hospital stay (18 days vs. 20 days; P = 0.59), and duration of follow‐up (6.6 months vs. 8.8 months; P = 0.064). The 2 cohorts also did not differ on the proportion with MRSA bacteremia (71% vs. 61%; P = 0.32), origin of SAB (P = 0.12), and the presence of comorbidities (diabetes mellitus, cardiomyopathy/congestive heart failure, malignancy, renal disease, and immunosuppression) (all P values > 0.30).
| Characteristic | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Age (years) (mean SD) | 66.3 16.3 | 62.4 16.8 | 0.29 |
| Male sex (n [%[) | 12 (42.9) | 48 (53.3) | 0.33 |
| Length of stay (days) (mean SD) | 17.8 16.1 | 19.7 17.3 | 0.59 |
| Follow‐up (months) (mean SD) | 6.6 5.3 | 8.8 5.7 | 0.064 |
| Blood culture (n [%]) | |||
| MRSA | 20 (71.4) | 55 (61.1) | 0.32 |
| MSSA | 8 (28.6) | 35 (38.9) | |
| Origin of the bacteremia [n (%)] | 0.12 | ||
| Community‐acquired | 13 (46.4) | 29 (32.2) | |
| Healthcare‐acquired | 10 (35.7) | 26 (28.9) | |
| Hospital‐acquired | 5 (17.9) | 35 (38.9) | |
| Comorbidities (n [%]) | |||
| DM | 11 (39.3) | 38 (42.2) | 0.78 |
| CHF | 5 (17.9) | 20 (22.2) | 0.62 |
| Cancer | 7 (25.0) | 15 (16.7) | 0.32 |
| ESRD | 4 (14.3) | 12 (13.3) | 1.00 |
| Immunosuppression | 6 (21.4) | 15 (16.7) | 0.58 |
| Patients lost to follow‐up (n [%]) | 5 (17.8) | 8 (8.8) | 0.19 |
Table 2 shows that patients in the SABU group were nearly twice as likely to have a complication as the group without SABU (64% vs. 33%; P = 0.004) and had a higher mean number of complications (0.89 vs. 0.48; P = 0.016). Patients in the SABU group also were more likely to have early complications (64% vs. 23%; P < 0.001) but no more likely to have a delayed complication (14% vs. 12%; P = 0.75). Of the 8 specific complications evaluated, the 2 groups differed only on the presence of septic shock, with the SABU group having 3 times more patients with this complication (21% vs. 7%; P = 0.035). Also, a higher proportion of patients died in the SABU group (32.1% vs. 14.4%; P = 0.036).
| Outcome | S. aureus Bacteriuria (n = 28) | No S. aureus Bacteriuria (n = 90) | P Value* |
|---|---|---|---|
| |||
| Any complication (n [%]) | 18 (64.3) | 30 (33.3) | 0.004 |
| Average complications (mean SD) | 0.89 0.83 | 0.48 0.77 | 0.016 |
| Timing of complication (n [%]) | |||
| Early | 18 (64.3) | 21 (23.3) | <0.001 |
| Delayed | 4 (14.3) | 11 (12.2) | 0.75 |
| By specific complication, n (%) | |||
| Endocarditis | 1 (3.6) | 5 (5.6) | 1.00 |
| Osteomyelitis | 3 (10.7) | 5 (5.6) | 0.39 |
| Septic arthritis | 2 (7.1) | 3 (3.3) | 0.59 |
| Thrombophlebitis | 1 (3.6) | 3 (3.3) | 1.00 |
| Septic shock | 6 (21.4) | 6 (6.7) | 0.035 |
| Septic embolism/abscess | 6 (21.4) | 10 (11.1) | 0.21 |
| Persistent SAB | 3 (10.7) | 3 (3.3) | 0.14 |
| Recurrent SAB | 3 (10.7) | 8 (8.9) | 0.72 |
| Death (n [%]) | 9 (32.1) | 13 (14.4) | 0.036 |
Patients with MRSA (n = 75) and those with MSSA (n = 43) did not differ on any complication, average complications, early or late complications, or 7 of the specific complications (data not shown). Only with thrombophlebitis did the 2 groups differ; the MSSA group had 4 (9.3%) patients with this complication while none in the MRSA group were affected (P = 0.016).
Discussion
In our retrospective analysis, SAB with concomitant SABU was associated with more severe disease, complications, and death. Compared to SAB patients without SA in the urine, those with SAB and SA in the urine had more total complications and more early complications, especially septic shock. Further, the proportion of deaths in the SABU cohort was more than twice as high (32% vs. 14%). Therefore, the presence of SABU in patients with SAB could potentially be a useful predictor of complicated SAB and death.
The relationship between SABU and early complications and death remained after excluding the complication of septic shock/need for vasopressors from the analysis (data not shown). The lack of relationship between SABU and delayed complications might have been due to the adequacy of treatment for SAB. Appropriateness of therapy, a criterion for patient inclusion, may have lessened the likelihood of an insufficient treatment plan causing complications. Those patients with MRSA did not differ from those with MSSA on the mean number of complications or early and delayed complications. A greater proportion of MSSA patients had thrombophlebitis than MRSA patients.
Other investigations have identified predictors of mortality or complications from SAB,7, 912 but SABU was not included as a variable in most of these studies. Fowler et al.7 proposed a prognostic model of complicated SAB using the predictors from their study; community acquisition of organisms, persistent bacteremia, persistent fever over 72 hours, and skin examination suggestive of an acute systemic infection. Muder et al.8 reported a relationship between SABU and subsequent SAB, but they did not examine the association between SABU and the risk of complicated SAB. Huggan et al.9 found that concomitant SABU is associated with ICU admission and increased in‐hospital mortality in patients with SAB.
SAB patients with SABU may be at risk for early complications. Consequently, such patients may warrant more aggressive evaluation and treatment. Further, SABU in patients with SAB may be indicative of an endocarditis‐like condition. SA is rarely isolated from the urinary tract as a uropathogen, although it may colonize indwelling catheters and may cause catheter‐related urinary tract infections.13, 14 Thus, when present in urine, SA could be a marker of deep tissue dissemination with the potential to cause complications. Guidelines for the management of intravascular device‐associated bacteremia have been published by the Infectious Diseases Society of America (IDSA) and other organizations,15, 16 and recent studies have demonstrated the effectiveness of newer agents for the management of SAB.17 Nevertheless, there is still controversy regarding some aspects of the management of SAB (eg, duration of therapy, criteria for echocardiographic evaluation, role of combination therapy). The presence of SABU, the marker evaluated in our study, may be an additional factor to consider when deciding upon duration of therapy and whether to obtain echocardiography or other imaging.
Our study was limited by its retrospective nature. Patient records were not always complete. For example, not all patients had echocardiography to evaluate for endocarditis or venous ultrasound to evaluate for septic thrombophlebitis. Also, the presence (or proper removal) of intravascular or urinary catheters could not be documented reliably in all patients. In addition, the 7‐day cutoff for obtaining urine cultures may have been too lenient, leading to underdiagnosis of bacteriuria. Finally, while 13 patients were lost to follow‐up, the 2 groups (SABU and No SABU) did not differ in the proportion lost.
In conclusion, our study found that SABU may be a useful predictor of complicated SAB and death. SAB patients with SABU may be at risk for more and earlier complications. These patients may need closer monitoring due to the higher risk of septic shock and death. Additional therapeutic and management recommendations might include: 1) longer duration of therapy even if a removable source of the bacteremia is identified; 2) more frequent and better supervised follow‐up; and 3) imaging studies including either computed tomography (CT) scans or ultrasound for thorough evaluation of complications. Prospective studies including randomized controlled trials are required before implementing these suggested diagnostic and therapeutic recommendations.
Acknowledgements
The authors thank and acknowledge Logan McCool and Adam Woiwood for their administrative contributions to the study. E.V.P.‐J., as the principal investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- ,,, et al.Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two‐year study in 16 hospitals.Eur J Clin Microbiol Infect Dis.2002;21(12):849–855.
- ,,, et al.Nosocomial bloodstream infections in ICU and non‐ICU patients.Am J Infec Control.2005;33(6):333–340.
- ,,, et al.Age‐ and sex‐associated trends in bloodstream infection: a population‐based study in Olmsted County, Minnesota.Arch Intern Med.2007;167(8):834–839.
- ,,.Bloodstream infections in a geriatric cohort: a population‐based study.Am J Med.2007;120(12):1078–1883.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104(9):1029–1033.
- ,,, et al.Infection of orthopedic prostheses after Staphylococcus aureus bacteremia.Clin Infect Dis.2001;32(4):647–649.
- ,,, et al.Clinical identifiers of complicated Staphylococcus aureus bacteremia.Arch Intern Med.2003;163(17):2066–2072.
- ,,, et al.Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic UTI and subsequent staphylococcal bacteremia.Clin Infect Dis.2006;42(1):46–50.
- ,,, et al.Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteremia.J Hosp Infect.2008;69:345–349.
- ,,, et al.Persistent Staphylococcus aureus bacteremia. An analysis of risk factors and outcomes.Arch Int Med.2007;167(17):1861–1867.
- .Staphylococcus aureus bacteremia in older adults: predictors of 7‐day mortality and infection with a methicillin‐resistant strain.Infect Control Hosp Epidemiol.2006;27(11):1219–1225.
- ,,, et al.Infective endocarditis. Diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation2005;111(23):e394–e434.
- ,,, et al.Antibiotic sensitivity of bacteria associated with community‐acquired urinary tract infection in Britain.J Antimicrob Chemother.1999;44(3):359–365.
- .Antibiotic susceptibility of bacterial strains isolated from patients with community‐acquired urinary tract infections in France.Eur J Clin Microbiol Infect Dis.2000;19(2):112–117.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46(suppl 5):S386–S393.
- ,,, et al.Guidelines for the management of intravascular catheter‐related infections.Clin Infect Dis.2001;32(9):1249–1272.
- ,,.Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006;355(7):653–665.
- ,,, et al.Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two‐year study in 16 hospitals.Eur J Clin Microbiol Infect Dis.2002;21(12):849–855.
- ,,, et al.Nosocomial bloodstream infections in ICU and non‐ICU patients.Am J Infec Control.2005;33(6):333–340.
- ,,, et al.Age‐ and sex‐associated trends in bloodstream infection: a population‐based study in Olmsted County, Minnesota.Arch Intern Med.2007;167(8):834–839.
- ,,.Bloodstream infections in a geriatric cohort: a population‐based study.Am J Med.2007;120(12):1078–1883.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104(9):1029–1033.
- ,,, et al.Infection of orthopedic prostheses after Staphylococcus aureus bacteremia.Clin Infect Dis.2001;32(4):647–649.
- ,,, et al.Clinical identifiers of complicated Staphylococcus aureus bacteremia.Arch Intern Med.2003;163(17):2066–2072.
- ,,, et al.Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic UTI and subsequent staphylococcal bacteremia.Clin Infect Dis.2006;42(1):46–50.
- ,,, et al.Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteremia.J Hosp Infect.2008;69:345–349.
- ,,, et al.Persistent Staphylococcus aureus bacteremia. An analysis of risk factors and outcomes.Arch Int Med.2007;167(17):1861–1867.
- .Staphylococcus aureus bacteremia in older adults: predictors of 7‐day mortality and infection with a methicillin‐resistant strain.Infect Control Hosp Epidemiol.2006;27(11):1219–1225.
- ,,, et al.Infective endocarditis. Diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation2005;111(23):e394–e434.
- ,,, et al.Antibiotic sensitivity of bacteria associated with community‐acquired urinary tract infection in Britain.J Antimicrob Chemother.1999;44(3):359–365.
- .Antibiotic susceptibility of bacterial strains isolated from patients with community‐acquired urinary tract infections in France.Eur J Clin Microbiol Infect Dis.2000;19(2):112–117.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46(suppl 5):S386–S393.
- ,,, et al.Guidelines for the management of intravascular catheter‐related infections.Clin Infect Dis.2001;32(9):1249–1272.
- ,,.Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus.N Engl J Med.2006;355(7):653–665.
Copyright © 2010 Society of Hospital Medicine