User login
Heart Failure Program Readmissions
Congestive heart failure (CHF) is a common disease with high mortality and morbidity.1, 2 Better physiological understanding has led to significant advances in therapy in recent years, with synthesis of this evidence into widely available treatment guidelines.3, 4 However, patients who have had an acute hospitalization with heart failure continue to have a high rate of symptomatic relapse, with up to 25% readmitted within 3 months.2 One of the major challenges in heart failure therapy is to avert these relapses to prevent hospital readmission.
Angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers, and spironolactone have promised a reduction in hospitalization rates as well as mortality; however, suboptimal prescribing5 and adherence to therapy6, 7 may limit their anticipated benefits. This has led to interest in improved systems of care to reduce hospital utilization. Such approaches have included improved systems for optimizing medications,68 comprehensive discharge planning and postdischarge support,914 and self‐management and case management strategies1517 to enhance patient participation in care.
Combinations of these strategies are known as disease management programs (DMPs), and trials of such combination strategies to improve patient outcomes have been promising.1823 Recognized features4 include skilled multidisciplinary team care; individualized guideline‐based treatment plans that may include dietary and exercise programs as well as optimal pharmacological therapy; patient education and self‐management strategies; improved integration between hospital and community care providers; vigilant follow‐up including prompt review after hospitalization; ready access to expert assessment in the event of deterioration; and regular monitoring with expert titration of therapy, through clinics, home visits, or telemonitoring. Several randomized controlled trials have suggested that DMPs may reduce heart failure‐related9, 1517 and all‐cause9, 10 readmissions. Meta‐analyses12, 1823 have demonstrated reduction in risk of all‐cause readmission of 12% to 25% as well as a reduction in mortality of 14% to 25%.
Trials of DMPs have generally involved careful participant selection, and differences in methods and outcome reporting have led some reviewers to be circumspect in their interpretation of the impact of these programs on readmission rates.23 A large, real‐world quality improvement program conducted as part of the Royal Australasian College of Physicians Clinical Support Systems Project provided an opportunity to measure whether a multifaceted program targeting a representative group of patients with CHF and their healthcare providers could reduce readmission rates. As previously published, this program delivered measurable improvements in processes of care including evidence‐based prescribing, adherence, multidisciplinary involvement, and discharge communication, associated with a reduction in 12‐month mortality.24
Objective
The Brisbane Cardiac Consortium sought to improve processes of care for patients with CHF by using evidence‐based strategies targeting patients and their healthcare providers to optimize uptake of management guidelines, improve discharge processes between hospital and primary care, and increase patient participation in care. We hypothesized that the program would reduce hospital readmissions in the intervention patients in the first 12 months following discharge.
Methods
Setting
The program was conducted in 3 metropolitan public teaching hospitals in Brisbane, Australia (Royal Brisbane, Princess Alexandra, and Queen Elizabeth II Hospitals) and their associated Divisions of General Practice, targeting the hospital and posthospital care of patients with CHF.
Design
The study was a prospective time series study. Consecutive participants were enrolled continuously between October 1, 2000 and August 31, 2002. Interventions were introduced progressively as systems matured. For evaluation purposes, we predefined a baseline cohort (October 1, 2000 to April 17, 2001) who were admitted prior to implementation of any interventions, and an intervention cohort (February 15, 2002 to August 31, 2002) who were admitted after all interventions were mature. The study was approved by the Ethics Committees of all participating institutions.
Participants
All patients with a recorded clinical diagnosis of CHF within 48 hours of hospital presentation, and evidence of at least 2 supporting clinical signs (raised jugular venous pressure, third or fourth heart sounds, bilateral chest crackles, dependent edema, or cardiomegaly and/or pulmonary edema on chest x‐ray) were identified prospectively by trained research nurses. Patients were ineligible for reevaluation if they had already been enrolled in the study. Detailed data were abstracted from the medical record including demographics, illness characteristics, and comorbid conditions.
Interventions
Provider‐directed Interventions
Provider‐directed interventions aimed to improve clinician compliance with agreed management guidelines using decision support tools, reminders, education and academic detailing, and regular performance feedback. These interventions were delivered by project staff and local clinical leaders and were directed toward both hospital clinicians (internists and cardiologists) and general practitioners providing community care.
Patient‐directed Interventions
Patient‐directed interventions included written evidence‐based patient education, pharmacist discharge medication review and inpatient education, and patient diaries. Comprehensive discharge summaries including target‐directed management plans were provided to the general practitioner and community pharmacist.
Participants were considered suitable for more intensive posthospital intervention and follow‐up if they: (1) did not have cognitive impairment or psychiatric illness which would preclude participation in self‐care; (2) did not have a life expectancy due to comorbidities estimated to be less than 6 months; (3) had a stable residence in the community where they could be contacted by telephone; (4) attended a general practitioner within the greater Brisbane area; and (5) consented to more detailed follow‐up. In the baseline phase, this intensive group was contacted by nursing staff at 1, 3, 6, and 12 months for data collection purposes; in the intervention phase, these participants received enhanced predischarge pharmacist education; postdischarge pharmacist telephone follow‐up of medication understanding and adherence; telephone reminders from project nursing staff at 1, 3, 6, and 12 months to attend their general practitioner; and individualized, written, guideline‐based reminders sent to participating general practitioners.
Measures and Analysis
The primary outcome measure was all‐cause hospital readmission over 12 months. Secondary outcomes included 12‐month all‐cause mortality, 12‐month readmissions due to CHF, total hospital days, and the combined endpoint of death or readmission (ie, readmission‐free survival) at 12 months.
Readmission data were obtained from the Queensland Health Information Centre by matching patient data with the Queensland Hospital Admitted Patient Data Collection. Admission to any Queensland hospital is captured in this database. Readmission was defined as due to CHF (same‐cause) if a principal diagnosis code from ICD‐10‐AM code chapter I50 was assigned. Mortality data were obtained from the Australian Institute of Health and Welfare (AIHW) National Death Index.
Processes of inpatient care were collected by trained research nurses using a standardized structured chart abstraction tool. Data items were based on guideline recommendations for patient assessment, investigation, and management.
All analyses were conducted using SAS version for Windows 9.1 (SAS Institute, Cary, NC). Baseline and intervention patient characteristics were compared using independent samples t test for continuous variables and contingency tables with chi‐square tests for proportions.
Logistic regression models adjusted for hospital and posthospital intensity (considered to be significant potential confounders) were used to test the strength of association between the intervention and readmission (or death and readmission); Cox proportional hazards model was used to assess the time to first readmission or death. A Wilcoxon 2‐sample test was used to compare total number of days in hospital over the 12‐month follow‐up period, as these data were highly positively skewed; means rather than medians are reported, as the median was 0 in each group and hence uninformative. Frequency of readmission was compared using Poisson regression adjusted for hospital. A P value of 0.05 was considered significant in all analyses.
Preliminary analysis revealed a number of differences in baseline clinical characteristics between the 2 groups. To account for measured differences other than hospital and intervention intensity, propensity scores (the conditional probability of assignment to a particular treatment group given a vector of observed covariates) were developed using a logistic model with the control or intervention group as the dependent variable and baseline patient characteristic variables with P < 0.2 (as shown in Table 1) as the independent variables. The equation obtained from this model was used to estimate a propensity score for each patient. These scores along with hospital and intervention intensity were then used to provide estimates adjusted for baseline differences between the control and intervention groups.25
| Characteristic | Baseline (n = 197) | Intervention (n = 219) | P Value |
|---|---|---|---|
| |||
| Hospital, n (%) | 0.001 | ||
| 1 | 75 (38) | 100 (46) | |
| 2 | 40 (20) | 17 (8) | |
| 3 | 82 (42) | 102 (46) | |
| Age (years), mean (range) | 75 (24‐100) | 78 (32‐102) | 0.059 |
| Female, n (%) | 103 (52) | 118 (54) | 0.74 |
| Hostel resident, n (%) | 15 (8) | 38 (17) | <0.01 |
| Previous CHF admission, n (%) | 52 (26) | 26 (12) | <0.01 |
| Contributing factors, n (%) | |||
| Hypertension | 104 (53) | 139 (63) | 0.027 |
| Coronary disease | 107 (54) | 118 (54) | 0.93 |
| Valvular disease | 20 (10) | 45 (21) | <0.01 |
| Cardiomyopathy | 29 (15) | 33 (15) | 0.92 |
| NYHA class III/IV, n (%) | 143 (73) | 155 (71) | 0.68 |
| Atrial fibrillation, n (%) | 65 (33) | 78 (36) | 0.57 |
| LVEF % (mean) | 24 | 28 | 0.10 |
| Cardiologist care, n (%) | 42 (21) | 61 (28) | 0.12 |
| Comorbidity score | 2.6 (1,8) | 2.7 (1,10) | 0.52 |
Results
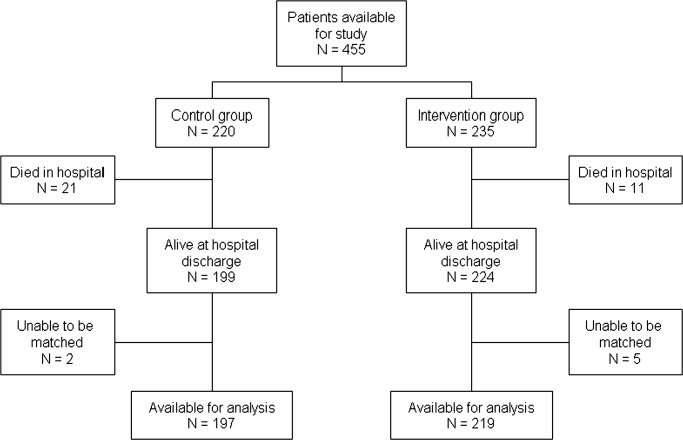
There were 220 patients identified with a clinical diagnosis of CHF during the baseline period, and 235 during the intervention period. Figure 1 shows ascertainment, in‐hospital mortality, and eligibility rates for the 2 cohorts. Eighty‐nine (45%) of baseline patients and 76 (35%) of intervention patients received intensive posthospital follow‐up as described above. Information on readmission was available for 197 baseline patients and 219 intervention patients discharged alive; this is the sample used for all analyses in this report. Table 1 shows the demographic and clinical characteristics of these patients. Table 2 summarizes the previously reported improvements in processes of care.
| Process indicator | Baseline (n = 220) [n (%)>] | Intervention (n = 235) [n (%)] | P Value |
|---|---|---|---|
| |||
| Assessment of reversible triggers | 166 (75) | 211 (90) | <0.001 |
| DVT prophylaxis | 57 (26) | 148 (63) | <0.001 |
| Imaging of left ventricular function | 135 (61) | 164 (70) | 0.002 |
| Scheduled outpatient visit within 30 days | 87 (46)* | 130 (59) | 0.005 |
| ACE inhibitor prescription at discharge | 136 (71)* | 163 (74) | 0.46 |
| Beta‐blocker prescription at discharge | 61 (32)* | 113 (52) | <0.001 |
| Avoid deleterious agents at discharge | 180 (94)* | 214 (98) | 0.79 |
Duing the 12‐month follow‐up, 107 (49%) of intervention patients were readmitted to the hospital compared to 71 (36%) of control patients, representing a 1.7‐fold increase in the adjusted probability of readmission in the intervention group (odds ratio [OR] = 1.71, 95% confidence interval [CI] = 1.14‐2.56; P = 0.009). As shown in Table 3, this was partly balanced by a trend toward reduced post‐hospital mortality, such that no significant difference was seen in readmission‐free survival.
| Baseline (%) | Intervention (%) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| ||||
| Readmitted within 12 months | 71/197 (36) | 107/219 (49) | 1.71* (1.14, 2.56); 1.90 (1.24, 2.91) | 0.009; 0.004 |
| Death within 12 months | 59/197 (30) | 53/219 (24) | 0.68* (0.44, 1.07) | 0.099 |
| Death or readmission within 12 months | 104/197 (53) | 133/219 (61) | 1.30* (0.87, 1.93); 1.36 (0.89, 2.08) | 0.20; 0.15 |
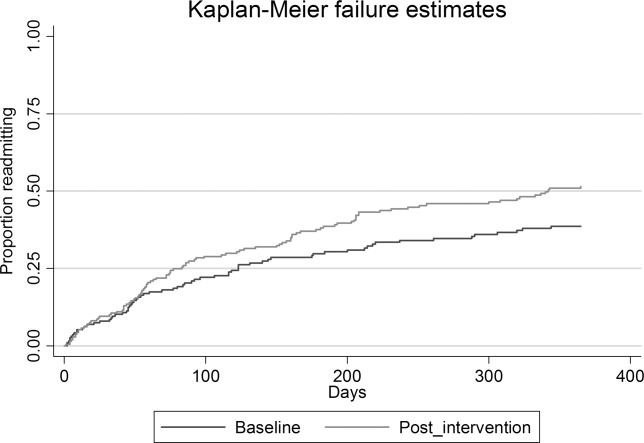
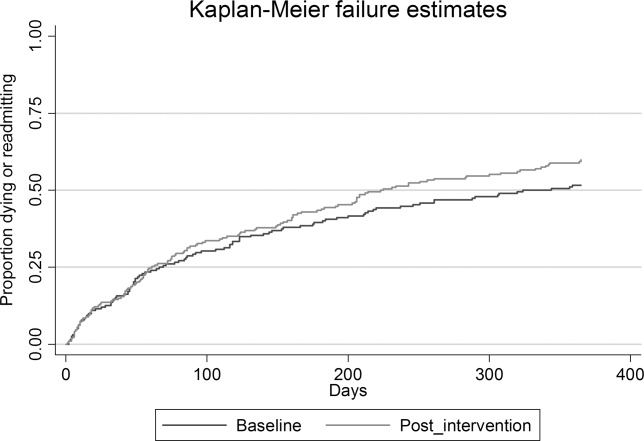
Time‐to‐event analysis (Figures 2 and 3) demonstrated similar findings, with a significant reduction in time to first readmission in the intervention group (adjusted hazard ratio [HR] = 1.43; 95% CI = 1.04‐1.97; P = 0.046) but no difference in time to death or first readmission (adjusted HR = 1.14; 95% CI = 0.86‐1.46; P = 0.36).
There was a trend to increased readmissions attributed to heart failure: 47 (21.5%) of intervention patients compared to 33 (16.7%) in the baseline group (OR = 1.30; 95% CI = 0.87‐1.93; P = 0.20). No significant difference was demonstrated in the frequency of readmissions (average 0.75 admission per participant per year in baseline, compared to 0.93 intervention; P = 0.32) nor the mean number of days in hospital in 12 months subsequent to the index admission (5.9 in the baseline group compared to 6.5 in the intervention group; P = 0.1).
Subgroup analysis by intervention intensity showed similar results, with 42 of 76 (55.3%) intensive group participants in the intervention group and 36 of 89 (40.4%) in the baseline group requiring hospital readmission within 12 months. The HR for death or readmission was estimated to be 1.27 (95% CI = 0.85‐1.9).
Discussion
In this study, heart failure patients who received a multidisciplinary intervention (including inpatient education, self‐management support, improved timely medical follow‐up, and better integration between hospital and primary care) showed a trend to improved 1‐year post‐hospital survival, but this appeared to be at the cost of increased readmissions among survivors. This occurred despite our previously reported improved optimization of pharmacological therapy both in‐hospital and posthospital with this program.18
There are a number of potential explanations for this finding, which have important implications for adoption of disease management programs. First, the intervention may not have been of sufficient intensity. Programs primarily aimed at educating providers and patients in evidence‐based guidelines, without structured postdischarge support, have not always improved clinical outcomes.26 In our study, general practitioners were supported to provide improved postdischarge care to their CHF patients, but direct postdischarge patient support was only provided to consenting patients and was limited in scope. There is still some debate about which elements of successful DMPs are most important for efficacy. Most authorities support the central importance of medication optimization, intensive education, and self‐care support. Taylor et al.23 found stronger evidence for programs using individual case management or outreach rather than clinic‐based interventions. Yu et al.27 concluded that outpatient drug titration and ready access to specialist review were factors contributing to success. In our program, even the more intensive intervention did not include regular clinical review by specialist nurses, a system for rapid review in the event of deterioration or supervised drug titration protocols. Furthermore, strategies which prompted more frequent primary care review and improved patient, carer, and general practitioner recognition of disease deterioration may have provided more opportunities to initiate readmission, especially in the absence of an alternative care pathway such as rapid‐access clinics or outreach services.28
Second, this study may reflect the reality of generalizing randomized controlled trial data to an unselected population. Many trials enrolled patients with high anticipated event rates but excluded patients with complex comorbidities, poor life expectancy, and cognitive impairment. Such studies enrolled a high‐risk population (10%‐48% of screened patients randomized) who had a relatively high readmission rate (50%‐60% at 6‐12 months) compared to our unselected population. These studies may overstate the benefits of applying heart failure DMPs in an unselected population. Galbreath et al.29 enrolled a self‐selected community sample of heart failure patients into a disease management program incorporating education, self‐management, telephone support, and advice to primary care providers and home health providers. Like our model, they demonstrated a survival benefit in the intervention group but no reduction in hospital or other healthcare utilization.
Third, only about one‐half of the readmissions were due to heart failure, again reflecting the complexity of this real‐world patient group. Interventions that focus on a single disease in patients with complex comorbidities might be expected to have only limited impact on their subsequent healthcare needs.
Fourth, findings may reflect differences in patient characteristics between the 2 cohorts. While statistical adjustment for measured differences did not have any significant impact on results, unmeasured patient characteristics may have introduced bias. The beforeafter nature of the study also raises the possibility that temporal trends in care practices influenced patient outcomes, such as changing patterns of drug and device therapies. There is conflicting evidence in the literature regarding trends in CHF readmission rates,3032 but it is possible that health system factors external to the study contributed to a higher readmission rate in the later cohort.
Finally, there was a trend toward reduction in mortality within the intervention cohort. These additional survivors might be expected to have more advanced heart failure or other comorbid disease, and therefore may have been more susceptible to deterioration and the need for inpatient care.
Conclusions
We acknowledge the weaknesses inherent in this nonrandomized study design, including convenience sampling, measured and unmeasured confounders and temporal trends in processes and systems of care. Nonetheless, this real world study suggests a note of caution in the widespread enthusiasm for chronic disease management programs. A complex bundle of interventions that resulted in measurable improvements in adherence to evidence‐based guidelines, discharge processes, integration between care providers, and patient education appeared to prolong life expectancy but increase hospital utilization. Mortality reduction in an incurable chronic disease such as heart failure will increase the burden of disease (and therefore treatment costs) unless treatments concurrently reduce disability and the frequency of symptomatic relapse.33 Whether this balance is achieved will depend on patient selection and the intensity and/or components of the intervention. These factors have not been fully defined in the literature to date.
Our study suggests that a widely applied, discharge‐focused intervention which primarily augmented the CHF management knowledge of care providers and patients, and enhanced attendance within the existing care model of primary care and internal medicine/cardiology outpatient services, improved the quality of care and may have reduced mortality at the cost of higher hospital utilization. It raises questions about whether a disease management service can achieve the uncertain promise of reduced readmissions in a cost‐effective manner outside of a high‐risk experimental population.
Acknowledgements
The authors acknowledge the contribution of the advisory and working groups of the Brisbane Cardiac Consortium. The authors appreciate the support of clinicians from the Internal Medicine, Cardiology, and Pharmacy Departments of the participating hospitals as well as staff from the Brisbane North and Brisbane Inner South Divisions of General Practice. The authors are grateful for the efforts of the staff of the PAH Clinical Services Evaluation Unit and the RBWH Internal Medicine Research Unit for data collection and data management; and the Queensland Health Information Centre and Australian Institute of Health and Welfare (AIHW) National Death Index for data matching.
- ,,,,.More ‘malignant’ than cancer? Five‐year survival following a first admission with heart failure.Eur J Heart Fail.2001;3:315–322.
- ,,, et al.;Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe.Part 1: patient characteristics and diagnosis.Eur Heart J.2003;24(5):442–463.
- National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Chronic Heart Failure Clinical Practice Guidelines Writing Panel.Guidelines for management of patients with chronic heart failure in Australia.Med J Aust.2001;174:459–466.
- ,,.Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(11):1115–1140.
- ,,, et al.Quality of care of patients hospitalized with congestive heart failure.Intern Med J.2003;33(4):140–151.
- ,,, et al.Improvements in 1‐year cardiovascular clinical outcomes associated with a hospital‐based discharge medication program.Ann Intern Med.2004;141(6):446–453.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team.Arch Intern Med.1999;159(16):1939–1945.
- .Impact of pharmacist interventions on hospital readmissions for heart failure.Am J Health Syst Pharm.1999;56:1339–1342.
- ,,.Effects of a multidisciplinary, home‐based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study.Lancet.1999;354:1077–1083.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,.Home‐based intervention in congestive heart failure. Long‐term implications on readmission and survival.Circulation.2002;105(24):2861–2866.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders. A randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Quality of life of individuals with heart failure. A randomized trial of the effectiveness of two models of hospital‐to‐home transition.Med Care.2002;40(4):271–282.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,,,,.Effect of a standardised nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,,,.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:83–89.
- ,,,.A systematic review of randomized trials of disease management programs in heart failure.Am J Med.2001;2001(110):378–384.
- ,,,.Multidisciplinary strategies for the management of heart failure patients at high risk for admission.J Am Coll Cardiol.2004;44(4):810–819.
- ,,,,.Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta‐analysis.Eur J Heart Fail.2005;7:1133–1144.
- ,,,.The effectiveness of disease management programmes in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports.Eur Heart J.2004;25:1570–1595.
- ,,,,,.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;91:899–906.
- ,,, et al.Clinical service organisation for heart failure.Cochrane Database Syst Rev.2005(2):CD002752.pub2.
- ,,, et al.Achieving better in‐hospital and after‐hospital care of patients with acute cardiac disease.Med J Aust.2004;180:S83–S88.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,,,,,.The results of a randomized trial of a quality improvement intervention in the care of patients with heart failure.Am J Med.2000;109(6):443–449.
- ,,.Disease management programmes for older people with heart failure: crucial characteristics which improve post‐discharge outcomes.Eur Heart J.2006;27:596–612.
- ,,.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Long‐term healthcare and cost outcomes of disease management in a large, randomized, community‐based population with heart failure.Circulation.2004;110(23):3518–3526.
- ,,,.Trends in postdischarge mortality and readmissions. Has length of stay declined too far?Arch Intern Med.2004;164:538–544.
- ,,,.Is the prognosis of heart failure improving?Eur J Heart Fail.1999;1(3):229–241.
- ,,, et al.Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000.Am J Med.2004;116(9):581–589.
- ,,.Repeated hospitalization for the same disease: a multiplier of national health costs.Milbank Mem Fund Q.1908;58(3):454–471.
Congestive heart failure (CHF) is a common disease with high mortality and morbidity.1, 2 Better physiological understanding has led to significant advances in therapy in recent years, with synthesis of this evidence into widely available treatment guidelines.3, 4 However, patients who have had an acute hospitalization with heart failure continue to have a high rate of symptomatic relapse, with up to 25% readmitted within 3 months.2 One of the major challenges in heart failure therapy is to avert these relapses to prevent hospital readmission.
Angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers, and spironolactone have promised a reduction in hospitalization rates as well as mortality; however, suboptimal prescribing5 and adherence to therapy6, 7 may limit their anticipated benefits. This has led to interest in improved systems of care to reduce hospital utilization. Such approaches have included improved systems for optimizing medications,68 comprehensive discharge planning and postdischarge support,914 and self‐management and case management strategies1517 to enhance patient participation in care.
Combinations of these strategies are known as disease management programs (DMPs), and trials of such combination strategies to improve patient outcomes have been promising.1823 Recognized features4 include skilled multidisciplinary team care; individualized guideline‐based treatment plans that may include dietary and exercise programs as well as optimal pharmacological therapy; patient education and self‐management strategies; improved integration between hospital and community care providers; vigilant follow‐up including prompt review after hospitalization; ready access to expert assessment in the event of deterioration; and regular monitoring with expert titration of therapy, through clinics, home visits, or telemonitoring. Several randomized controlled trials have suggested that DMPs may reduce heart failure‐related9, 1517 and all‐cause9, 10 readmissions. Meta‐analyses12, 1823 have demonstrated reduction in risk of all‐cause readmission of 12% to 25% as well as a reduction in mortality of 14% to 25%.
Trials of DMPs have generally involved careful participant selection, and differences in methods and outcome reporting have led some reviewers to be circumspect in their interpretation of the impact of these programs on readmission rates.23 A large, real‐world quality improvement program conducted as part of the Royal Australasian College of Physicians Clinical Support Systems Project provided an opportunity to measure whether a multifaceted program targeting a representative group of patients with CHF and their healthcare providers could reduce readmission rates. As previously published, this program delivered measurable improvements in processes of care including evidence‐based prescribing, adherence, multidisciplinary involvement, and discharge communication, associated with a reduction in 12‐month mortality.24
Objective
The Brisbane Cardiac Consortium sought to improve processes of care for patients with CHF by using evidence‐based strategies targeting patients and their healthcare providers to optimize uptake of management guidelines, improve discharge processes between hospital and primary care, and increase patient participation in care. We hypothesized that the program would reduce hospital readmissions in the intervention patients in the first 12 months following discharge.
Methods
Setting
The program was conducted in 3 metropolitan public teaching hospitals in Brisbane, Australia (Royal Brisbane, Princess Alexandra, and Queen Elizabeth II Hospitals) and their associated Divisions of General Practice, targeting the hospital and posthospital care of patients with CHF.
Design
The study was a prospective time series study. Consecutive participants were enrolled continuously between October 1, 2000 and August 31, 2002. Interventions were introduced progressively as systems matured. For evaluation purposes, we predefined a baseline cohort (October 1, 2000 to April 17, 2001) who were admitted prior to implementation of any interventions, and an intervention cohort (February 15, 2002 to August 31, 2002) who were admitted after all interventions were mature. The study was approved by the Ethics Committees of all participating institutions.
Participants
All patients with a recorded clinical diagnosis of CHF within 48 hours of hospital presentation, and evidence of at least 2 supporting clinical signs (raised jugular venous pressure, third or fourth heart sounds, bilateral chest crackles, dependent edema, or cardiomegaly and/or pulmonary edema on chest x‐ray) were identified prospectively by trained research nurses. Patients were ineligible for reevaluation if they had already been enrolled in the study. Detailed data were abstracted from the medical record including demographics, illness characteristics, and comorbid conditions.
Interventions
Provider‐directed Interventions
Provider‐directed interventions aimed to improve clinician compliance with agreed management guidelines using decision support tools, reminders, education and academic detailing, and regular performance feedback. These interventions were delivered by project staff and local clinical leaders and were directed toward both hospital clinicians (internists and cardiologists) and general practitioners providing community care.
Patient‐directed Interventions
Patient‐directed interventions included written evidence‐based patient education, pharmacist discharge medication review and inpatient education, and patient diaries. Comprehensive discharge summaries including target‐directed management plans were provided to the general practitioner and community pharmacist.
Participants were considered suitable for more intensive posthospital intervention and follow‐up if they: (1) did not have cognitive impairment or psychiatric illness which would preclude participation in self‐care; (2) did not have a life expectancy due to comorbidities estimated to be less than 6 months; (3) had a stable residence in the community where they could be contacted by telephone; (4) attended a general practitioner within the greater Brisbane area; and (5) consented to more detailed follow‐up. In the baseline phase, this intensive group was contacted by nursing staff at 1, 3, 6, and 12 months for data collection purposes; in the intervention phase, these participants received enhanced predischarge pharmacist education; postdischarge pharmacist telephone follow‐up of medication understanding and adherence; telephone reminders from project nursing staff at 1, 3, 6, and 12 months to attend their general practitioner; and individualized, written, guideline‐based reminders sent to participating general practitioners.
Measures and Analysis
The primary outcome measure was all‐cause hospital readmission over 12 months. Secondary outcomes included 12‐month all‐cause mortality, 12‐month readmissions due to CHF, total hospital days, and the combined endpoint of death or readmission (ie, readmission‐free survival) at 12 months.
Readmission data were obtained from the Queensland Health Information Centre by matching patient data with the Queensland Hospital Admitted Patient Data Collection. Admission to any Queensland hospital is captured in this database. Readmission was defined as due to CHF (same‐cause) if a principal diagnosis code from ICD‐10‐AM code chapter I50 was assigned. Mortality data were obtained from the Australian Institute of Health and Welfare (AIHW) National Death Index.
Processes of inpatient care were collected by trained research nurses using a standardized structured chart abstraction tool. Data items were based on guideline recommendations for patient assessment, investigation, and management.
All analyses were conducted using SAS version for Windows 9.1 (SAS Institute, Cary, NC). Baseline and intervention patient characteristics were compared using independent samples t test for continuous variables and contingency tables with chi‐square tests for proportions.
Logistic regression models adjusted for hospital and posthospital intensity (considered to be significant potential confounders) were used to test the strength of association between the intervention and readmission (or death and readmission); Cox proportional hazards model was used to assess the time to first readmission or death. A Wilcoxon 2‐sample test was used to compare total number of days in hospital over the 12‐month follow‐up period, as these data were highly positively skewed; means rather than medians are reported, as the median was 0 in each group and hence uninformative. Frequency of readmission was compared using Poisson regression adjusted for hospital. A P value of 0.05 was considered significant in all analyses.
Preliminary analysis revealed a number of differences in baseline clinical characteristics between the 2 groups. To account for measured differences other than hospital and intervention intensity, propensity scores (the conditional probability of assignment to a particular treatment group given a vector of observed covariates) were developed using a logistic model with the control or intervention group as the dependent variable and baseline patient characteristic variables with P < 0.2 (as shown in Table 1) as the independent variables. The equation obtained from this model was used to estimate a propensity score for each patient. These scores along with hospital and intervention intensity were then used to provide estimates adjusted for baseline differences between the control and intervention groups.25
| Characteristic | Baseline (n = 197) | Intervention (n = 219) | P Value |
|---|---|---|---|
| |||
| Hospital, n (%) | 0.001 | ||
| 1 | 75 (38) | 100 (46) | |
| 2 | 40 (20) | 17 (8) | |
| 3 | 82 (42) | 102 (46) | |
| Age (years), mean (range) | 75 (24‐100) | 78 (32‐102) | 0.059 |
| Female, n (%) | 103 (52) | 118 (54) | 0.74 |
| Hostel resident, n (%) | 15 (8) | 38 (17) | <0.01 |
| Previous CHF admission, n (%) | 52 (26) | 26 (12) | <0.01 |
| Contributing factors, n (%) | |||
| Hypertension | 104 (53) | 139 (63) | 0.027 |
| Coronary disease | 107 (54) | 118 (54) | 0.93 |
| Valvular disease | 20 (10) | 45 (21) | <0.01 |
| Cardiomyopathy | 29 (15) | 33 (15) | 0.92 |
| NYHA class III/IV, n (%) | 143 (73) | 155 (71) | 0.68 |
| Atrial fibrillation, n (%) | 65 (33) | 78 (36) | 0.57 |
| LVEF % (mean) | 24 | 28 | 0.10 |
| Cardiologist care, n (%) | 42 (21) | 61 (28) | 0.12 |
| Comorbidity score | 2.6 (1,8) | 2.7 (1,10) | 0.52 |
Results
There were 220 patients identified with a clinical diagnosis of CHF during the baseline period, and 235 during the intervention period. Figure 1 shows ascertainment, in‐hospital mortality, and eligibility rates for the 2 cohorts. Eighty‐nine (45%) of baseline patients and 76 (35%) of intervention patients received intensive posthospital follow‐up as described above. Information on readmission was available for 197 baseline patients and 219 intervention patients discharged alive; this is the sample used for all analyses in this report. Table 1 shows the demographic and clinical characteristics of these patients. Table 2 summarizes the previously reported improvements in processes of care.

| Process indicator | Baseline (n = 220) [n (%)>] | Intervention (n = 235) [n (%)] | P Value |
|---|---|---|---|
| |||
| Assessment of reversible triggers | 166 (75) | 211 (90) | <0.001 |
| DVT prophylaxis | 57 (26) | 148 (63) | <0.001 |
| Imaging of left ventricular function | 135 (61) | 164 (70) | 0.002 |
| Scheduled outpatient visit within 30 days | 87 (46)* | 130 (59) | 0.005 |
| ACE inhibitor prescription at discharge | 136 (71)* | 163 (74) | 0.46 |
| Beta‐blocker prescription at discharge | 61 (32)* | 113 (52) | <0.001 |
| Avoid deleterious agents at discharge | 180 (94)* | 214 (98) | 0.79 |
Duing the 12‐month follow‐up, 107 (49%) of intervention patients were readmitted to the hospital compared to 71 (36%) of control patients, representing a 1.7‐fold increase in the adjusted probability of readmission in the intervention group (odds ratio [OR] = 1.71, 95% confidence interval [CI] = 1.14‐2.56; P = 0.009). As shown in Table 3, this was partly balanced by a trend toward reduced post‐hospital mortality, such that no significant difference was seen in readmission‐free survival.
| Baseline (%) | Intervention (%) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| ||||
| Readmitted within 12 months | 71/197 (36) | 107/219 (49) | 1.71* (1.14, 2.56); 1.90 (1.24, 2.91) | 0.009; 0.004 |
| Death within 12 months | 59/197 (30) | 53/219 (24) | 0.68* (0.44, 1.07) | 0.099 |
| Death or readmission within 12 months | 104/197 (53) | 133/219 (61) | 1.30* (0.87, 1.93); 1.36 (0.89, 2.08) | 0.20; 0.15 |
Time‐to‐event analysis (Figures 2 and 3) demonstrated similar findings, with a significant reduction in time to first readmission in the intervention group (adjusted hazard ratio [HR] = 1.43; 95% CI = 1.04‐1.97; P = 0.046) but no difference in time to death or first readmission (adjusted HR = 1.14; 95% CI = 0.86‐1.46; P = 0.36).


There was a trend to increased readmissions attributed to heart failure: 47 (21.5%) of intervention patients compared to 33 (16.7%) in the baseline group (OR = 1.30; 95% CI = 0.87‐1.93; P = 0.20). No significant difference was demonstrated in the frequency of readmissions (average 0.75 admission per participant per year in baseline, compared to 0.93 intervention; P = 0.32) nor the mean number of days in hospital in 12 months subsequent to the index admission (5.9 in the baseline group compared to 6.5 in the intervention group; P = 0.1).
Subgroup analysis by intervention intensity showed similar results, with 42 of 76 (55.3%) intensive group participants in the intervention group and 36 of 89 (40.4%) in the baseline group requiring hospital readmission within 12 months. The HR for death or readmission was estimated to be 1.27 (95% CI = 0.85‐1.9).
Discussion
In this study, heart failure patients who received a multidisciplinary intervention (including inpatient education, self‐management support, improved timely medical follow‐up, and better integration between hospital and primary care) showed a trend to improved 1‐year post‐hospital survival, but this appeared to be at the cost of increased readmissions among survivors. This occurred despite our previously reported improved optimization of pharmacological therapy both in‐hospital and posthospital with this program.18
There are a number of potential explanations for this finding, which have important implications for adoption of disease management programs. First, the intervention may not have been of sufficient intensity. Programs primarily aimed at educating providers and patients in evidence‐based guidelines, without structured postdischarge support, have not always improved clinical outcomes.26 In our study, general practitioners were supported to provide improved postdischarge care to their CHF patients, but direct postdischarge patient support was only provided to consenting patients and was limited in scope. There is still some debate about which elements of successful DMPs are most important for efficacy. Most authorities support the central importance of medication optimization, intensive education, and self‐care support. Taylor et al.23 found stronger evidence for programs using individual case management or outreach rather than clinic‐based interventions. Yu et al.27 concluded that outpatient drug titration and ready access to specialist review were factors contributing to success. In our program, even the more intensive intervention did not include regular clinical review by specialist nurses, a system for rapid review in the event of deterioration or supervised drug titration protocols. Furthermore, strategies which prompted more frequent primary care review and improved patient, carer, and general practitioner recognition of disease deterioration may have provided more opportunities to initiate readmission, especially in the absence of an alternative care pathway such as rapid‐access clinics or outreach services.28
Second, this study may reflect the reality of generalizing randomized controlled trial data to an unselected population. Many trials enrolled patients with high anticipated event rates but excluded patients with complex comorbidities, poor life expectancy, and cognitive impairment. Such studies enrolled a high‐risk population (10%‐48% of screened patients randomized) who had a relatively high readmission rate (50%‐60% at 6‐12 months) compared to our unselected population. These studies may overstate the benefits of applying heart failure DMPs in an unselected population. Galbreath et al.29 enrolled a self‐selected community sample of heart failure patients into a disease management program incorporating education, self‐management, telephone support, and advice to primary care providers and home health providers. Like our model, they demonstrated a survival benefit in the intervention group but no reduction in hospital or other healthcare utilization.
Third, only about one‐half of the readmissions were due to heart failure, again reflecting the complexity of this real‐world patient group. Interventions that focus on a single disease in patients with complex comorbidities might be expected to have only limited impact on their subsequent healthcare needs.
Fourth, findings may reflect differences in patient characteristics between the 2 cohorts. While statistical adjustment for measured differences did not have any significant impact on results, unmeasured patient characteristics may have introduced bias. The beforeafter nature of the study also raises the possibility that temporal trends in care practices influenced patient outcomes, such as changing patterns of drug and device therapies. There is conflicting evidence in the literature regarding trends in CHF readmission rates,3032 but it is possible that health system factors external to the study contributed to a higher readmission rate in the later cohort.
Finally, there was a trend toward reduction in mortality within the intervention cohort. These additional survivors might be expected to have more advanced heart failure or other comorbid disease, and therefore may have been more susceptible to deterioration and the need for inpatient care.
Conclusions
We acknowledge the weaknesses inherent in this nonrandomized study design, including convenience sampling, measured and unmeasured confounders and temporal trends in processes and systems of care. Nonetheless, this real world study suggests a note of caution in the widespread enthusiasm for chronic disease management programs. A complex bundle of interventions that resulted in measurable improvements in adherence to evidence‐based guidelines, discharge processes, integration between care providers, and patient education appeared to prolong life expectancy but increase hospital utilization. Mortality reduction in an incurable chronic disease such as heart failure will increase the burden of disease (and therefore treatment costs) unless treatments concurrently reduce disability and the frequency of symptomatic relapse.33 Whether this balance is achieved will depend on patient selection and the intensity and/or components of the intervention. These factors have not been fully defined in the literature to date.
Our study suggests that a widely applied, discharge‐focused intervention which primarily augmented the CHF management knowledge of care providers and patients, and enhanced attendance within the existing care model of primary care and internal medicine/cardiology outpatient services, improved the quality of care and may have reduced mortality at the cost of higher hospital utilization. It raises questions about whether a disease management service can achieve the uncertain promise of reduced readmissions in a cost‐effective manner outside of a high‐risk experimental population.
Acknowledgements
The authors acknowledge the contribution of the advisory and working groups of the Brisbane Cardiac Consortium. The authors appreciate the support of clinicians from the Internal Medicine, Cardiology, and Pharmacy Departments of the participating hospitals as well as staff from the Brisbane North and Brisbane Inner South Divisions of General Practice. The authors are grateful for the efforts of the staff of the PAH Clinical Services Evaluation Unit and the RBWH Internal Medicine Research Unit for data collection and data management; and the Queensland Health Information Centre and Australian Institute of Health and Welfare (AIHW) National Death Index for data matching.
Congestive heart failure (CHF) is a common disease with high mortality and morbidity.1, 2 Better physiological understanding has led to significant advances in therapy in recent years, with synthesis of this evidence into widely available treatment guidelines.3, 4 However, patients who have had an acute hospitalization with heart failure continue to have a high rate of symptomatic relapse, with up to 25% readmitted within 3 months.2 One of the major challenges in heart failure therapy is to avert these relapses to prevent hospital readmission.
Angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers, and spironolactone have promised a reduction in hospitalization rates as well as mortality; however, suboptimal prescribing5 and adherence to therapy6, 7 may limit their anticipated benefits. This has led to interest in improved systems of care to reduce hospital utilization. Such approaches have included improved systems for optimizing medications,68 comprehensive discharge planning and postdischarge support,914 and self‐management and case management strategies1517 to enhance patient participation in care.
Combinations of these strategies are known as disease management programs (DMPs), and trials of such combination strategies to improve patient outcomes have been promising.1823 Recognized features4 include skilled multidisciplinary team care; individualized guideline‐based treatment plans that may include dietary and exercise programs as well as optimal pharmacological therapy; patient education and self‐management strategies; improved integration between hospital and community care providers; vigilant follow‐up including prompt review after hospitalization; ready access to expert assessment in the event of deterioration; and regular monitoring with expert titration of therapy, through clinics, home visits, or telemonitoring. Several randomized controlled trials have suggested that DMPs may reduce heart failure‐related9, 1517 and all‐cause9, 10 readmissions. Meta‐analyses12, 1823 have demonstrated reduction in risk of all‐cause readmission of 12% to 25% as well as a reduction in mortality of 14% to 25%.
Trials of DMPs have generally involved careful participant selection, and differences in methods and outcome reporting have led some reviewers to be circumspect in their interpretation of the impact of these programs on readmission rates.23 A large, real‐world quality improvement program conducted as part of the Royal Australasian College of Physicians Clinical Support Systems Project provided an opportunity to measure whether a multifaceted program targeting a representative group of patients with CHF and their healthcare providers could reduce readmission rates. As previously published, this program delivered measurable improvements in processes of care including evidence‐based prescribing, adherence, multidisciplinary involvement, and discharge communication, associated with a reduction in 12‐month mortality.24
Objective
The Brisbane Cardiac Consortium sought to improve processes of care for patients with CHF by using evidence‐based strategies targeting patients and their healthcare providers to optimize uptake of management guidelines, improve discharge processes between hospital and primary care, and increase patient participation in care. We hypothesized that the program would reduce hospital readmissions in the intervention patients in the first 12 months following discharge.
Methods
Setting
The program was conducted in 3 metropolitan public teaching hospitals in Brisbane, Australia (Royal Brisbane, Princess Alexandra, and Queen Elizabeth II Hospitals) and their associated Divisions of General Practice, targeting the hospital and posthospital care of patients with CHF.
Design
The study was a prospective time series study. Consecutive participants were enrolled continuously between October 1, 2000 and August 31, 2002. Interventions were introduced progressively as systems matured. For evaluation purposes, we predefined a baseline cohort (October 1, 2000 to April 17, 2001) who were admitted prior to implementation of any interventions, and an intervention cohort (February 15, 2002 to August 31, 2002) who were admitted after all interventions were mature. The study was approved by the Ethics Committees of all participating institutions.
Participants
All patients with a recorded clinical diagnosis of CHF within 48 hours of hospital presentation, and evidence of at least 2 supporting clinical signs (raised jugular venous pressure, third or fourth heart sounds, bilateral chest crackles, dependent edema, or cardiomegaly and/or pulmonary edema on chest x‐ray) were identified prospectively by trained research nurses. Patients were ineligible for reevaluation if they had already been enrolled in the study. Detailed data were abstracted from the medical record including demographics, illness characteristics, and comorbid conditions.
Interventions
Provider‐directed Interventions
Provider‐directed interventions aimed to improve clinician compliance with agreed management guidelines using decision support tools, reminders, education and academic detailing, and regular performance feedback. These interventions were delivered by project staff and local clinical leaders and were directed toward both hospital clinicians (internists and cardiologists) and general practitioners providing community care.
Patient‐directed Interventions
Patient‐directed interventions included written evidence‐based patient education, pharmacist discharge medication review and inpatient education, and patient diaries. Comprehensive discharge summaries including target‐directed management plans were provided to the general practitioner and community pharmacist.
Participants were considered suitable for more intensive posthospital intervention and follow‐up if they: (1) did not have cognitive impairment or psychiatric illness which would preclude participation in self‐care; (2) did not have a life expectancy due to comorbidities estimated to be less than 6 months; (3) had a stable residence in the community where they could be contacted by telephone; (4) attended a general practitioner within the greater Brisbane area; and (5) consented to more detailed follow‐up. In the baseline phase, this intensive group was contacted by nursing staff at 1, 3, 6, and 12 months for data collection purposes; in the intervention phase, these participants received enhanced predischarge pharmacist education; postdischarge pharmacist telephone follow‐up of medication understanding and adherence; telephone reminders from project nursing staff at 1, 3, 6, and 12 months to attend their general practitioner; and individualized, written, guideline‐based reminders sent to participating general practitioners.
Measures and Analysis
The primary outcome measure was all‐cause hospital readmission over 12 months. Secondary outcomes included 12‐month all‐cause mortality, 12‐month readmissions due to CHF, total hospital days, and the combined endpoint of death or readmission (ie, readmission‐free survival) at 12 months.
Readmission data were obtained from the Queensland Health Information Centre by matching patient data with the Queensland Hospital Admitted Patient Data Collection. Admission to any Queensland hospital is captured in this database. Readmission was defined as due to CHF (same‐cause) if a principal diagnosis code from ICD‐10‐AM code chapter I50 was assigned. Mortality data were obtained from the Australian Institute of Health and Welfare (AIHW) National Death Index.
Processes of inpatient care were collected by trained research nurses using a standardized structured chart abstraction tool. Data items were based on guideline recommendations for patient assessment, investigation, and management.
All analyses were conducted using SAS version for Windows 9.1 (SAS Institute, Cary, NC). Baseline and intervention patient characteristics were compared using independent samples t test for continuous variables and contingency tables with chi‐square tests for proportions.
Logistic regression models adjusted for hospital and posthospital intensity (considered to be significant potential confounders) were used to test the strength of association between the intervention and readmission (or death and readmission); Cox proportional hazards model was used to assess the time to first readmission or death. A Wilcoxon 2‐sample test was used to compare total number of days in hospital over the 12‐month follow‐up period, as these data were highly positively skewed; means rather than medians are reported, as the median was 0 in each group and hence uninformative. Frequency of readmission was compared using Poisson regression adjusted for hospital. A P value of 0.05 was considered significant in all analyses.
Preliminary analysis revealed a number of differences in baseline clinical characteristics between the 2 groups. To account for measured differences other than hospital and intervention intensity, propensity scores (the conditional probability of assignment to a particular treatment group given a vector of observed covariates) were developed using a logistic model with the control or intervention group as the dependent variable and baseline patient characteristic variables with P < 0.2 (as shown in Table 1) as the independent variables. The equation obtained from this model was used to estimate a propensity score for each patient. These scores along with hospital and intervention intensity were then used to provide estimates adjusted for baseline differences between the control and intervention groups.25
| Characteristic | Baseline (n = 197) | Intervention (n = 219) | P Value |
|---|---|---|---|
| |||
| Hospital, n (%) | 0.001 | ||
| 1 | 75 (38) | 100 (46) | |
| 2 | 40 (20) | 17 (8) | |
| 3 | 82 (42) | 102 (46) | |
| Age (years), mean (range) | 75 (24‐100) | 78 (32‐102) | 0.059 |
| Female, n (%) | 103 (52) | 118 (54) | 0.74 |
| Hostel resident, n (%) | 15 (8) | 38 (17) | <0.01 |
| Previous CHF admission, n (%) | 52 (26) | 26 (12) | <0.01 |
| Contributing factors, n (%) | |||
| Hypertension | 104 (53) | 139 (63) | 0.027 |
| Coronary disease | 107 (54) | 118 (54) | 0.93 |
| Valvular disease | 20 (10) | 45 (21) | <0.01 |
| Cardiomyopathy | 29 (15) | 33 (15) | 0.92 |
| NYHA class III/IV, n (%) | 143 (73) | 155 (71) | 0.68 |
| Atrial fibrillation, n (%) | 65 (33) | 78 (36) | 0.57 |
| LVEF % (mean) | 24 | 28 | 0.10 |
| Cardiologist care, n (%) | 42 (21) | 61 (28) | 0.12 |
| Comorbidity score | 2.6 (1,8) | 2.7 (1,10) | 0.52 |
Results
There were 220 patients identified with a clinical diagnosis of CHF during the baseline period, and 235 during the intervention period. Figure 1 shows ascertainment, in‐hospital mortality, and eligibility rates for the 2 cohorts. Eighty‐nine (45%) of baseline patients and 76 (35%) of intervention patients received intensive posthospital follow‐up as described above. Information on readmission was available for 197 baseline patients and 219 intervention patients discharged alive; this is the sample used for all analyses in this report. Table 1 shows the demographic and clinical characteristics of these patients. Table 2 summarizes the previously reported improvements in processes of care.

| Process indicator | Baseline (n = 220) [n (%)>] | Intervention (n = 235) [n (%)] | P Value |
|---|---|---|---|
| |||
| Assessment of reversible triggers | 166 (75) | 211 (90) | <0.001 |
| DVT prophylaxis | 57 (26) | 148 (63) | <0.001 |
| Imaging of left ventricular function | 135 (61) | 164 (70) | 0.002 |
| Scheduled outpatient visit within 30 days | 87 (46)* | 130 (59) | 0.005 |
| ACE inhibitor prescription at discharge | 136 (71)* | 163 (74) | 0.46 |
| Beta‐blocker prescription at discharge | 61 (32)* | 113 (52) | <0.001 |
| Avoid deleterious agents at discharge | 180 (94)* | 214 (98) | 0.79 |
Duing the 12‐month follow‐up, 107 (49%) of intervention patients were readmitted to the hospital compared to 71 (36%) of control patients, representing a 1.7‐fold increase in the adjusted probability of readmission in the intervention group (odds ratio [OR] = 1.71, 95% confidence interval [CI] = 1.14‐2.56; P = 0.009). As shown in Table 3, this was partly balanced by a trend toward reduced post‐hospital mortality, such that no significant difference was seen in readmission‐free survival.
| Baseline (%) | Intervention (%) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| ||||
| Readmitted within 12 months | 71/197 (36) | 107/219 (49) | 1.71* (1.14, 2.56); 1.90 (1.24, 2.91) | 0.009; 0.004 |
| Death within 12 months | 59/197 (30) | 53/219 (24) | 0.68* (0.44, 1.07) | 0.099 |
| Death or readmission within 12 months | 104/197 (53) | 133/219 (61) | 1.30* (0.87, 1.93); 1.36 (0.89, 2.08) | 0.20; 0.15 |
Time‐to‐event analysis (Figures 2 and 3) demonstrated similar findings, with a significant reduction in time to first readmission in the intervention group (adjusted hazard ratio [HR] = 1.43; 95% CI = 1.04‐1.97; P = 0.046) but no difference in time to death or first readmission (adjusted HR = 1.14; 95% CI = 0.86‐1.46; P = 0.36).


There was a trend to increased readmissions attributed to heart failure: 47 (21.5%) of intervention patients compared to 33 (16.7%) in the baseline group (OR = 1.30; 95% CI = 0.87‐1.93; P = 0.20). No significant difference was demonstrated in the frequency of readmissions (average 0.75 admission per participant per year in baseline, compared to 0.93 intervention; P = 0.32) nor the mean number of days in hospital in 12 months subsequent to the index admission (5.9 in the baseline group compared to 6.5 in the intervention group; P = 0.1).
Subgroup analysis by intervention intensity showed similar results, with 42 of 76 (55.3%) intensive group participants in the intervention group and 36 of 89 (40.4%) in the baseline group requiring hospital readmission within 12 months. The HR for death or readmission was estimated to be 1.27 (95% CI = 0.85‐1.9).
Discussion
In this study, heart failure patients who received a multidisciplinary intervention (including inpatient education, self‐management support, improved timely medical follow‐up, and better integration between hospital and primary care) showed a trend to improved 1‐year post‐hospital survival, but this appeared to be at the cost of increased readmissions among survivors. This occurred despite our previously reported improved optimization of pharmacological therapy both in‐hospital and posthospital with this program.18
There are a number of potential explanations for this finding, which have important implications for adoption of disease management programs. First, the intervention may not have been of sufficient intensity. Programs primarily aimed at educating providers and patients in evidence‐based guidelines, without structured postdischarge support, have not always improved clinical outcomes.26 In our study, general practitioners were supported to provide improved postdischarge care to their CHF patients, but direct postdischarge patient support was only provided to consenting patients and was limited in scope. There is still some debate about which elements of successful DMPs are most important for efficacy. Most authorities support the central importance of medication optimization, intensive education, and self‐care support. Taylor et al.23 found stronger evidence for programs using individual case management or outreach rather than clinic‐based interventions. Yu et al.27 concluded that outpatient drug titration and ready access to specialist review were factors contributing to success. In our program, even the more intensive intervention did not include regular clinical review by specialist nurses, a system for rapid review in the event of deterioration or supervised drug titration protocols. Furthermore, strategies which prompted more frequent primary care review and improved patient, carer, and general practitioner recognition of disease deterioration may have provided more opportunities to initiate readmission, especially in the absence of an alternative care pathway such as rapid‐access clinics or outreach services.28
Second, this study may reflect the reality of generalizing randomized controlled trial data to an unselected population. Many trials enrolled patients with high anticipated event rates but excluded patients with complex comorbidities, poor life expectancy, and cognitive impairment. Such studies enrolled a high‐risk population (10%‐48% of screened patients randomized) who had a relatively high readmission rate (50%‐60% at 6‐12 months) compared to our unselected population. These studies may overstate the benefits of applying heart failure DMPs in an unselected population. Galbreath et al.29 enrolled a self‐selected community sample of heart failure patients into a disease management program incorporating education, self‐management, telephone support, and advice to primary care providers and home health providers. Like our model, they demonstrated a survival benefit in the intervention group but no reduction in hospital or other healthcare utilization.
Third, only about one‐half of the readmissions were due to heart failure, again reflecting the complexity of this real‐world patient group. Interventions that focus on a single disease in patients with complex comorbidities might be expected to have only limited impact on their subsequent healthcare needs.
Fourth, findings may reflect differences in patient characteristics between the 2 cohorts. While statistical adjustment for measured differences did not have any significant impact on results, unmeasured patient characteristics may have introduced bias. The beforeafter nature of the study also raises the possibility that temporal trends in care practices influenced patient outcomes, such as changing patterns of drug and device therapies. There is conflicting evidence in the literature regarding trends in CHF readmission rates,3032 but it is possible that health system factors external to the study contributed to a higher readmission rate in the later cohort.
Finally, there was a trend toward reduction in mortality within the intervention cohort. These additional survivors might be expected to have more advanced heart failure or other comorbid disease, and therefore may have been more susceptible to deterioration and the need for inpatient care.
Conclusions
We acknowledge the weaknesses inherent in this nonrandomized study design, including convenience sampling, measured and unmeasured confounders and temporal trends in processes and systems of care. Nonetheless, this real world study suggests a note of caution in the widespread enthusiasm for chronic disease management programs. A complex bundle of interventions that resulted in measurable improvements in adherence to evidence‐based guidelines, discharge processes, integration between care providers, and patient education appeared to prolong life expectancy but increase hospital utilization. Mortality reduction in an incurable chronic disease such as heart failure will increase the burden of disease (and therefore treatment costs) unless treatments concurrently reduce disability and the frequency of symptomatic relapse.33 Whether this balance is achieved will depend on patient selection and the intensity and/or components of the intervention. These factors have not been fully defined in the literature to date.
Our study suggests that a widely applied, discharge‐focused intervention which primarily augmented the CHF management knowledge of care providers and patients, and enhanced attendance within the existing care model of primary care and internal medicine/cardiology outpatient services, improved the quality of care and may have reduced mortality at the cost of higher hospital utilization. It raises questions about whether a disease management service can achieve the uncertain promise of reduced readmissions in a cost‐effective manner outside of a high‐risk experimental population.
Acknowledgements
The authors acknowledge the contribution of the advisory and working groups of the Brisbane Cardiac Consortium. The authors appreciate the support of clinicians from the Internal Medicine, Cardiology, and Pharmacy Departments of the participating hospitals as well as staff from the Brisbane North and Brisbane Inner South Divisions of General Practice. The authors are grateful for the efforts of the staff of the PAH Clinical Services Evaluation Unit and the RBWH Internal Medicine Research Unit for data collection and data management; and the Queensland Health Information Centre and Australian Institute of Health and Welfare (AIHW) National Death Index for data matching.
- ,,,,.More ‘malignant’ than cancer? Five‐year survival following a first admission with heart failure.Eur J Heart Fail.2001;3:315–322.
- ,,, et al.;Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe.Part 1: patient characteristics and diagnosis.Eur Heart J.2003;24(5):442–463.
- National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Chronic Heart Failure Clinical Practice Guidelines Writing Panel.Guidelines for management of patients with chronic heart failure in Australia.Med J Aust.2001;174:459–466.
- ,,.Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(11):1115–1140.
- ,,, et al.Quality of care of patients hospitalized with congestive heart failure.Intern Med J.2003;33(4):140–151.
- ,,, et al.Improvements in 1‐year cardiovascular clinical outcomes associated with a hospital‐based discharge medication program.Ann Intern Med.2004;141(6):446–453.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team.Arch Intern Med.1999;159(16):1939–1945.
- .Impact of pharmacist interventions on hospital readmissions for heart failure.Am J Health Syst Pharm.1999;56:1339–1342.
- ,,.Effects of a multidisciplinary, home‐based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study.Lancet.1999;354:1077–1083.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,.Home‐based intervention in congestive heart failure. Long‐term implications on readmission and survival.Circulation.2002;105(24):2861–2866.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders. A randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Quality of life of individuals with heart failure. A randomized trial of the effectiveness of two models of hospital‐to‐home transition.Med Care.2002;40(4):271–282.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,,,,.Effect of a standardised nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,,,.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:83–89.
- ,,,.A systematic review of randomized trials of disease management programs in heart failure.Am J Med.2001;2001(110):378–384.
- ,,,.Multidisciplinary strategies for the management of heart failure patients at high risk for admission.J Am Coll Cardiol.2004;44(4):810–819.
- ,,,,.Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta‐analysis.Eur J Heart Fail.2005;7:1133–1144.
- ,,,.The effectiveness of disease management programmes in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports.Eur Heart J.2004;25:1570–1595.
- ,,,,,.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;91:899–906.
- ,,, et al.Clinical service organisation for heart failure.Cochrane Database Syst Rev.2005(2):CD002752.pub2.
- ,,, et al.Achieving better in‐hospital and after‐hospital care of patients with acute cardiac disease.Med J Aust.2004;180:S83–S88.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,,,,,.The results of a randomized trial of a quality improvement intervention in the care of patients with heart failure.Am J Med.2000;109(6):443–449.
- ,,.Disease management programmes for older people with heart failure: crucial characteristics which improve post‐discharge outcomes.Eur Heart J.2006;27:596–612.
- ,,.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Long‐term healthcare and cost outcomes of disease management in a large, randomized, community‐based population with heart failure.Circulation.2004;110(23):3518–3526.
- ,,,.Trends in postdischarge mortality and readmissions. Has length of stay declined too far?Arch Intern Med.2004;164:538–544.
- ,,,.Is the prognosis of heart failure improving?Eur J Heart Fail.1999;1(3):229–241.
- ,,, et al.Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000.Am J Med.2004;116(9):581–589.
- ,,.Repeated hospitalization for the same disease: a multiplier of national health costs.Milbank Mem Fund Q.1908;58(3):454–471.
- ,,,,.More ‘malignant’ than cancer? Five‐year survival following a first admission with heart failure.Eur J Heart Fail.2001;3:315–322.
- ,,, et al.;Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe.Part 1: patient characteristics and diagnosis.Eur Heart J.2003;24(5):442–463.
- National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Chronic Heart Failure Clinical Practice Guidelines Writing Panel.Guidelines for management of patients with chronic heart failure in Australia.Med J Aust.2001;174:459–466.
- ,,.Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(11):1115–1140.
- ,,, et al.Quality of care of patients hospitalized with congestive heart failure.Intern Med J.2003;33(4):140–151.
- ,,, et al.Improvements in 1‐year cardiovascular clinical outcomes associated with a hospital‐based discharge medication program.Ann Intern Med.2004;141(6):446–453.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team.Arch Intern Med.1999;159(16):1939–1945.
- .Impact of pharmacist interventions on hospital readmissions for heart failure.Am J Health Syst Pharm.1999;56:1339–1342.
- ,,.Effects of a multidisciplinary, home‐based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study.Lancet.1999;354:1077–1083.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,.Home‐based intervention in congestive heart failure. Long‐term implications on readmission and survival.Circulation.2002;105(24):2861–2866.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders. A randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Quality of life of individuals with heart failure. A randomized trial of the effectiveness of two models of hospital‐to‐home transition.Med Care.2002;40(4):271–282.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,,,,.Effect of a standardised nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,,,.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:83–89.
- ,,,.A systematic review of randomized trials of disease management programs in heart failure.Am J Med.2001;2001(110):378–384.
- ,,,.Multidisciplinary strategies for the management of heart failure patients at high risk for admission.J Am Coll Cardiol.2004;44(4):810–819.
- ,,,,.Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta‐analysis.Eur J Heart Fail.2005;7:1133–1144.
- ,,,.The effectiveness of disease management programmes in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports.Eur Heart J.2004;25:1570–1595.
- ,,,,,.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;91:899–906.
- ,,, et al.Clinical service organisation for heart failure.Cochrane Database Syst Rev.2005(2):CD002752.pub2.
- ,,, et al.Achieving better in‐hospital and after‐hospital care of patients with acute cardiac disease.Med J Aust.2004;180:S83–S88.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,,,,,.The results of a randomized trial of a quality improvement intervention in the care of patients with heart failure.Am J Med.2000;109(6):443–449.
- ,,.Disease management programmes for older people with heart failure: crucial characteristics which improve post‐discharge outcomes.Eur Heart J.2006;27:596–612.
- ,,.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Long‐term healthcare and cost outcomes of disease management in a large, randomized, community‐based population with heart failure.Circulation.2004;110(23):3518–3526.
- ,,,.Trends in postdischarge mortality and readmissions. Has length of stay declined too far?Arch Intern Med.2004;164:538–544.
- ,,,.Is the prognosis of heart failure improving?Eur J Heart Fail.1999;1(3):229–241.
- ,,, et al.Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000.Am J Med.2004;116(9):581–589.
- ,,.Repeated hospitalization for the same disease: a multiplier of national health costs.Milbank Mem Fund Q.1908;58(3):454–471.
Copyright © 2010 Society of Hospital Medicine