User login
Teachable Moments
With World Stroke Day scheduled for Saturday, a frequent speaker for the National Stroke Association (NSA) wants to remind hospitalists to push their patients to know their risk factors.
"They have an excellent opportunity to be an educator, particularly because of that captive audience," says David Willis, MD, a primary-care physician in Ocala, Fla., who frequently holds educational events for the NSA.
Dr. Willis cites data from a 2010 survey (PDF) compiled by NSA and Boehringer Ingelheim Pharmaceuticals that shows while more than 75% of healthcare providers reported talking to patients about atrial fibrillation (AF) and stroke, nearly half don't recall the conversation. And just 40% of patients initiate the discussion.
Dr. Willis, who served on the steering committee that interpreted the survey results, says that hospitalists dealing with AF patients can "quarterback" care plans and help improve communication with post-discharge physicians, be they primary care or specialists.
"We may not be getting that thought across as well as we think we are," he says.
Improved communication and transitions will become more important as unnecessary readmissions related to AF or stroke financially impact physicians because the government may reduce reimbursements for repeated hospital visits. Dr. Willis suggests that hospitalists take the reins of integrating their patient education efforts into checklists, health information technology, or some formalized process.
"My experience is, if you create protocols, they usually work better than educating people at a provider level," he says.
With World Stroke Day scheduled for Saturday, a frequent speaker for the National Stroke Association (NSA) wants to remind hospitalists to push their patients to know their risk factors.
"They have an excellent opportunity to be an educator, particularly because of that captive audience," says David Willis, MD, a primary-care physician in Ocala, Fla., who frequently holds educational events for the NSA.
Dr. Willis cites data from a 2010 survey (PDF) compiled by NSA and Boehringer Ingelheim Pharmaceuticals that shows while more than 75% of healthcare providers reported talking to patients about atrial fibrillation (AF) and stroke, nearly half don't recall the conversation. And just 40% of patients initiate the discussion.
Dr. Willis, who served on the steering committee that interpreted the survey results, says that hospitalists dealing with AF patients can "quarterback" care plans and help improve communication with post-discharge physicians, be they primary care or specialists.
"We may not be getting that thought across as well as we think we are," he says.
Improved communication and transitions will become more important as unnecessary readmissions related to AF or stroke financially impact physicians because the government may reduce reimbursements for repeated hospital visits. Dr. Willis suggests that hospitalists take the reins of integrating their patient education efforts into checklists, health information technology, or some formalized process.
"My experience is, if you create protocols, they usually work better than educating people at a provider level," he says.
With World Stroke Day scheduled for Saturday, a frequent speaker for the National Stroke Association (NSA) wants to remind hospitalists to push their patients to know their risk factors.
"They have an excellent opportunity to be an educator, particularly because of that captive audience," says David Willis, MD, a primary-care physician in Ocala, Fla., who frequently holds educational events for the NSA.
Dr. Willis cites data from a 2010 survey (PDF) compiled by NSA and Boehringer Ingelheim Pharmaceuticals that shows while more than 75% of healthcare providers reported talking to patients about atrial fibrillation (AF) and stroke, nearly half don't recall the conversation. And just 40% of patients initiate the discussion.
Dr. Willis, who served on the steering committee that interpreted the survey results, says that hospitalists dealing with AF patients can "quarterback" care plans and help improve communication with post-discharge physicians, be they primary care or specialists.
"We may not be getting that thought across as well as we think we are," he says.
Improved communication and transitions will become more important as unnecessary readmissions related to AF or stroke financially impact physicians because the government may reduce reimbursements for repeated hospital visits. Dr. Willis suggests that hospitalists take the reins of integrating their patient education efforts into checklists, health information technology, or some formalized process.
"My experience is, if you create protocols, they usually work better than educating people at a provider level," he says.
Specialty Hospitalists to Meet in Vegas
Medical professionals from across the country will attend the first national meeting on the topic of specialty hospitalists Nov. 4 at the Mandalay Bay Resort and Casino in Las Vegas. Sponsored by SHM, the American Hospital Association, the Neurohospitalist Society, and OBGynHospitalist.com, the gathering is for anyone interested in adopting a hospital-focused model of practice, including physician and nonphysician clinicians, as well as those in medical support industries, such as insurance carriers, policymakers, and healthcare media.
According to organizers, the one-day meeting will be structured to encourage networking and exchange of ideas among attendees, and will include presentations, panel discussions, and Q&A sessions.
"This is less 'Come hear from people who have this all figured out' … it's 'Come hear from people who are thinking about this a lot.' But the attendees are a big part of the knowledge base," says John Nelson, MD, MHM, hospitalist medical director at Overlake Hospital in Bellevue, Wash.
Dr. Nelson, cofounder and past president of SHM as well as the Nov. 4 meeting director, says he hopes to bring together healthcare leaders from diverse backgrounds to share their experiences and insights. Since this movement is growing organically rather than descending from a central agency, organizers expect to centralize the sharing of ideas and best practices.
Nearly 60 interested parties have pre-registered for the meeting, according to SHM. Attendees will take what they have learned back to their own hospitals or businesses, Dr. Nelson says, and continue the conversation with their colleagues.
The cost to attend the meeting is $350 and seats remain available; register by phone, 800-843-3360, or via the SHM website.
Medical professionals from across the country will attend the first national meeting on the topic of specialty hospitalists Nov. 4 at the Mandalay Bay Resort and Casino in Las Vegas. Sponsored by SHM, the American Hospital Association, the Neurohospitalist Society, and OBGynHospitalist.com, the gathering is for anyone interested in adopting a hospital-focused model of practice, including physician and nonphysician clinicians, as well as those in medical support industries, such as insurance carriers, policymakers, and healthcare media.
According to organizers, the one-day meeting will be structured to encourage networking and exchange of ideas among attendees, and will include presentations, panel discussions, and Q&A sessions.
"This is less 'Come hear from people who have this all figured out' … it's 'Come hear from people who are thinking about this a lot.' But the attendees are a big part of the knowledge base," says John Nelson, MD, MHM, hospitalist medical director at Overlake Hospital in Bellevue, Wash.
Dr. Nelson, cofounder and past president of SHM as well as the Nov. 4 meeting director, says he hopes to bring together healthcare leaders from diverse backgrounds to share their experiences and insights. Since this movement is growing organically rather than descending from a central agency, organizers expect to centralize the sharing of ideas and best practices.
Nearly 60 interested parties have pre-registered for the meeting, according to SHM. Attendees will take what they have learned back to their own hospitals or businesses, Dr. Nelson says, and continue the conversation with their colleagues.
The cost to attend the meeting is $350 and seats remain available; register by phone, 800-843-3360, or via the SHM website.
Medical professionals from across the country will attend the first national meeting on the topic of specialty hospitalists Nov. 4 at the Mandalay Bay Resort and Casino in Las Vegas. Sponsored by SHM, the American Hospital Association, the Neurohospitalist Society, and OBGynHospitalist.com, the gathering is for anyone interested in adopting a hospital-focused model of practice, including physician and nonphysician clinicians, as well as those in medical support industries, such as insurance carriers, policymakers, and healthcare media.
According to organizers, the one-day meeting will be structured to encourage networking and exchange of ideas among attendees, and will include presentations, panel discussions, and Q&A sessions.
"This is less 'Come hear from people who have this all figured out' … it's 'Come hear from people who are thinking about this a lot.' But the attendees are a big part of the knowledge base," says John Nelson, MD, MHM, hospitalist medical director at Overlake Hospital in Bellevue, Wash.
Dr. Nelson, cofounder and past president of SHM as well as the Nov. 4 meeting director, says he hopes to bring together healthcare leaders from diverse backgrounds to share their experiences and insights. Since this movement is growing organically rather than descending from a central agency, organizers expect to centralize the sharing of ideas and best practices.
Nearly 60 interested parties have pre-registered for the meeting, according to SHM. Attendees will take what they have learned back to their own hospitals or businesses, Dr. Nelson says, and continue the conversation with their colleagues.
The cost to attend the meeting is $350 and seats remain available; register by phone, 800-843-3360, or via the SHM website.
Mortality Among Elders With Pneumonia
Pneumonia occurs more commonly among older persons.1 With advancing age, the frequency of hospitalizations and mortality for pneumonia are higher.2 Among the tools developed to predict short‐term mortality is the pneumonia severity index (PSI), which is the best known among severity of illness indices for pneumonia.3 Its ability to predict short‐term mortality for CAP, particularly in identifying those at low risk was previously demonstrated.4 More recently, the extension of its utility in predicting 30‐day mortality for healthcare‐associated pneumonia (HCAP) was demonstrated.5
Severity of illness is one of several risk factors for adverse outcomes among older persons with acute illness. Besides comorbidity, other factors include functional impairment and atypical presentation. Information on physical functioning had equal importance as laboratory data in prognostication of in‐hospital mortality.6 In addition, walking impairment was 1 of 5 components of a risk adjustment index developed to predict 1‐year mortality for hospitalized older persons.7 Atypical presentations of illness, such as delirium and falls, independently predicted poor outcomes among hospitalized older patients.8
Specifically for pneumonia, functional status has also been shown to be an independent predictor of short‐term mortality among older patients hospitalized with CAP.913 Among atypical presentations, only absence of chills was an independent prognostic factor for CAP.9 Bacteremia was an independent factor related to death among adults with CAP, albeit for severe disease resulting in intensive care unit admission.14 It was also included in a severity assessment score; its higher scores were associated with early mortality.15 However, blood culture results are only available 2 to 3 days into the hospital episode. Therefore, bacteremia is a potential risk factor for mortality that is not identifiable at the start of hospitalization.
While PSI is a comprehensive collection of demographic, clinical, and investigative measures, it does not include items on functional status or atypical presentation. Neither does it account for recent hospitalization or comorbid conditions of significance to older persons, such as dementia and depression. It is plausible that at least some of these factors hold added prognostic value.
With all these in mind, we conducted a study with the following objectives: 1) to determine whether functional impairment, recent hospitalization, comorbid conditions of particular significance with advancing age, and atypical presentation are significantly associated with short‐term mortality among older patients hospitalized for CAP and HCAP, after taking into account PSI; and 2) if so, to estimate the magnitude of increased mortality risk with these factors. We tested our null hypotheses that, after adjustment for PSI class, 1) recent hospitalization, 2) pre‐morbid functional impairment, 3) dementia and depression, and 4) atypical presentation of illness have no association with 30‐day mortality for older persons hospitalized for CAP and HCAP, both combined and alone.
PATIENTS AND METHODS
Design and Setting
This was a retrospective cohort study that employed secondary analyses of chart and administrative data. The setting was 3 acute care public hospitals of the National Healthcare Group (NHG) cluster in Singapore. We merged data from hospital charts, the NHG Operations Data Store administrative database, and the national death registry. The local Institution Review Board (IRB) approved waiver of consent, and all other study procedures were consistent with the principles of the Helsinki Declaration.
Patient Population
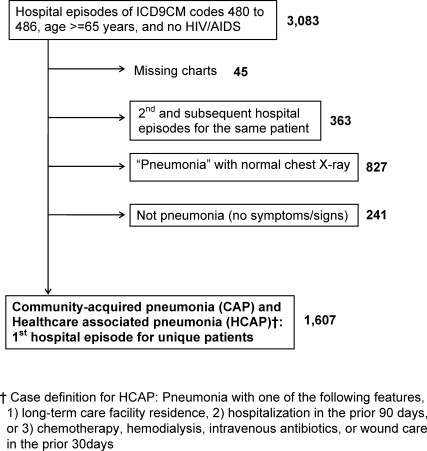
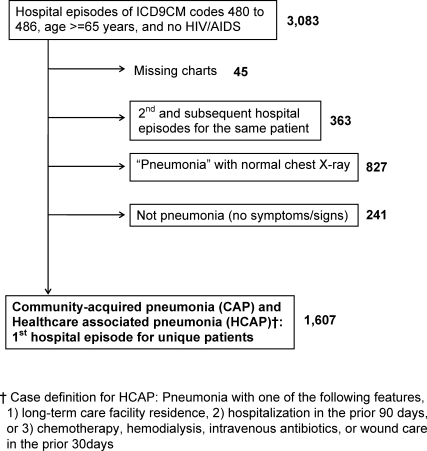
We included first hospital episodes of adults aged 65 years or older with the principal diagnosis of pneumonia in 2007. These episodes were identified by their primary International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM) codes of 480 to 486 in the administrative data. Next, we applied our study definition of pneumonia, which required the presence of acute symptoms or signs of pneumonia at the point of hospital admission, and a chest radiograph with features consistent with pneumonia that was obtained during the period from 24 hours before, to 48 hours after, hospital admission. In doing so, we included patients with community‐acquired pneumonia (CAP)16 and healthcare‐associated pneumonia (HCAP),17 but not hospital‐acquired pneumonia (HAP). We excluded patients whose charts were not accessible for review because of human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) and those whose charts were unavailable for other reasons. The study flow diagram is shown in Figure 1.
We assigned the diagnosis of HCAP to patients who were admitted to an acute care hospital for 2 or more days in the prior 90 days, resided in a nursing home or long‐term care facility, or received of intravenous antibiotic therapy, chemotherapy, wound care, or hemodialysis in the prior 30 days.18 Remaining patients were assigned CAP.
Data Collection
Trained research nurses used an abstraction protocol to collect demographic and clinical information from the charts, and to extract laboratory results and chest radiograph reports from the computerized clinical records. Where radiological reports were equivocal with respect to features of pneumonia, we obtained the opinion of one of our respiratory physician investigators whose decision was final. A researcher with bio‐informatics expertise extracted admission‐related information from the administrative data. Chart, administrative, and mortality data were merged to assemble the study database.
Outcome and Explanatory Variables
The outcome (dependent) variable was 30‐day all‐cause mortality. The following explanatory (independent) variables were examined:
Pneumonia severity index (PSI): We used PSI class as specified in the original studies.4
Recent hospitalization: Hospitalization in the prior 90 days and 30 days were explored.
Atypical presentation of illness: Acute geriatric syndromes (falls or acute impairment of mobility), and absence of cough and purulent sputum were examined. Delirium was not one of the syndromes because PSI includes altered mental state as an item.4
Functional impairment: Pre‐morbid ambulation impairment and feeding impairment were examined. Impairment was defined as needing assistance or being totally dependent.
Additional comorbid conditions: We selected dementia and depression, as they may have impact on mortality in older persons but were not included in PSI.
We did not include bacteremia, because its presence cannot be determined at the time of illness presentation.
From previous experience, we anticipated missing values for functional status measures in up to 5% of charts. Where values were missing, we used the simple imputation strategy of assigning no ambulation or feeding impairment.
Sample Size Calculation
With a sample size of 1400 patients and a 30‐day mortality rate of 25%, 350 cases of death were expected. Using the rule of thumb of at least 10 cases per independent variable,19 we were able to work with 35 candidate explanatory variables in logistic regression for the entire group. Assuming that the subpopulations of CAP and HCAP consist of 700 patients each, with mortality rates of 20% and 30%, respectively, then 14 could be explored for CAP and 21 candidate variables for HCAP.
Data Analyses
Pre‐morbid ambulation impairment and feeding impairment probably represent different points along the continuum of functional impairment. During preliminary analyses when both variables were adjusted for each other in logistic regression, pre‐morbid ambulation impairment (odds ratio [OR] 4.94, 95% confidence interval [CI] 3.79 to 6.43) was associated with 30‐day mortality, whereas pre‐morbid feeding impairment was not (OR 0.82, 95% CI 0.61 to 1.09). As such, pre‐morbid ambulation impairment was selected as the variable to represent functional impairment. Hospitalization in the prior 30 days was more strongly associated with 30‐day mortality (OR 2.38, 95% CI 1.77 to 3.21) than was hospitalization in the prior 90 days (OR 1.90, 95% CI 1.49 to 2.41). Therefore, hospitalization in the prior 30 days was selected as the variable to reflect recent hospitalization.
We used logistic regression analysis and regressed 30‐day mortality on PSI class and other explanatory variables. OR estimates and their 95% CI were used to quantify the strength of associations of the explanatory variables with mortality, and to test their statistical significance. In addition, we explored the possibility of interactions between PSI class and the patient factors. To this end, we constructed additional regression models that included appropriate interaction terms and tested their statistical significance. As a form of sensitivity analysis, we repeated the regression analyses only for hospital episodes with complete functional data and observed the extent to which OR estimates changed. Furthermore, we performed 2‐level hierarchical modeling to account for clustering at the hospital level and re‐examined the OR and 95% CI for the patient factors. We conducted these analyses for the entire group, and repeated them separately for CAP and HCAP. Finally, to estimate the extent to which the patient factors would increase predicted 30‐day mortality, we performed marginal effects analyses for the entire group to quantify the increased risk when individual factors were present.
We used STATA version 9.2 (Stata Corp, College Station, TX) for all statistical analyses. Hierarchical modeling was performed using the xtlogit command. STATA post‐estimation commands mfx and prvalue were employed to estimate marginal effects and predicted probabilities, respectively. The unit of analysis was patients. Statistical significance was defined by P values of less than 0.05.
RESULTS
Among 1607 patients included, 890 (55.4%) had CAP and 717 (44.6%) had HCAP. Baseline patient characteristics of patients with CAP and HCAP are shown in Table 1. The 30‐day mortality rate was 28.1% for the entire group, and 20.6% and 37.4% for patients with CAP and HCAP, respectively. When stratified according to PSI classes 2, 3, 4, and 5, this rate was 0%, 8.2%, 24.4%, and 56.0%, respectively. Because there were no deaths among those with PSI class 2, this category was merged with class 3 for the regression analyses. Missing data on pre‐morbid ambulation impairment and feeding impairment occurred for 39 (2.4%) and 69 (4.6%) patients, respectively.
| Whole Study Population (n = 1607) | Those With CAP (n = 890) | Those With HCAP (n = 717) | |
|---|---|---|---|
| |||
| Median age, years (IQR) | 80 (7487) | 79 (7385) | 82 (7588) |
| Male, n (%) | 876 (54.5) | 477 (53.6) | 399 (55.7) |
| Median pneumonia severity index (PSI) score, (IQR) | 109 (87134) | 100 (82121) | 120 (99144) |
| PSI class: | |||
| 2 | 98 (6.1) | 84 (9.4) | 14 (2.0) |
| 3 | 353 (22.0) | 260 (29.2) | 93 (13.0) |
| 4 | 713 (44.4) | 386 (43.4) | 327 (45.6) |
| 5 | 443 (27.6) | 160 (18.0) | 283 (39.5) |
| Pre‐morbid ambulation impairment, n (%) | 798 (49.7) | 287 (32.3) | 511 (71.3) |
| Pre‐morbid feeding impairment, n (%) | 298 (18.5) | 74 (8.3) | 224 (31.2) |
| Hospitalization in prior 30 days, n (%) | 209 (13.0) | 0 (0) | 209 (29.2) |
| Nursing home residence, n (%) | 362 (22.5) | 0 (0) | 362 (50.5) |
| Acute geriatric syndromes, n (%) | 442 (27.5) | 241 (27.1) | 201 (28.0) |
| Absence of both cough and purulent sputum, n (%) | 559 (34.8) | 226 (25.4) | 333 (46.4) |
| Dementia, n (%) | 307 (19.1) | 121 (13.6) | 178 (25.8) |
| Depression, n (%) | 165 (10.3) | 53 (6.0) | 109 (15.8) |
| Neoplastic disease, n (%) | 108 (6.7) | 33 (3.7) | 75 (10.5) |
| Liver disease, n (%) | 48 (3.0) | 25 (2.8) | 23 (3.2) |
| Congestive heart failure, n (%) | 257 (16.0) | 129 (14.5) | 128 (17.9) |
| Stroke, n (%) | 490 (30.5) | 215 (24.2) | 275 (38.4) |
| Renal failure, n (%) | 220 (13.7) | 97 (10.9) | 123 (17.2) |
| Chronic lung disease, n (%) | 316 (19.7) | 177 (19.9) | 139 (19.4) |
| Diabetes mellitus, n (%) | 515 (32.1) | 273 (30.7) | 242 (33.8) |
| Emergency department diagnosis of pneumonia, n (%) | 857 (53.3) | 494 (55.5) | 363 (50.6) |
For CAP and HCAP together, pre‐morbid ambulation impairment was associated with increased 30‐day mortality (339/798 [42.5%] vs 112/809 [13.8%], unadjusted OR 4.60, 95% CI 3.60 to 5.87, P < 0.01), as was hospitalization in the prior 30 days (94/209 [45.0%] vs 357/1398 [25.5%], unadjusted OR 2.38, 95% CI 1.77 to 3.21, P = 0.02). This was also the case for dementia (118/307 [38.4%] vs 333/1300 [25.6%], unadjusted OR 1.81, 95% CI 1.40 to 2.35, P < 0.01), acute geriatric syndromes (163/442 [36.9%] vs 288/1165 [24.7%], unadjusted OR 1.78, 95% CI 1.41 to 2.25, P < 0.01), and absence of cough and purulent sputum (226/559 [40.4%] vs 225/1048 [21.5%], unadjusted OR 2.48, 95% CI 1.98 to 3.11, P < 0.01). However, depression was not significantly associated with 30‐day mortality (57/165 [34.6%] vs 394/1442 [27.3%], unadjusted OR 1.40, 95% CI 1.00 to 1.97, P = 0.05).
Table 2 summarizes the results of logistic regression. It shows that pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were all independently associated with 30‐day mortality after adjustment for PSI score for the entire group. These associations remained statistically significant when CAP and HCAP were examined separately. Because none of those with CAP could have hospitalization in the prior 30 days, this factor was not included in the CAP model. The strength of association for the same patient factor varied across the pneumonia sub‐type. This was markedly so for pre‐morbid ambulation impairment, with the OR estimate being almost 3‐fold higher for CAP than for HCAP. Dementia, depression, and acute geriatric syndromes were not associated with 30‐day mortality. When the analyses were repeated after excluding hospital episodes with missing values for pre‐morbid ambulation impairment, the same 3 variables were significantly associated with 30‐day mortality, with trivial differences in strength of association compared to when imputation was performed. The OR estimates for pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were 2.82 (95% CI 2.12 to 3.76), 1.83 (95% CI 1.42 to 2.83), and 1.47 (95% CI 1.14 to 1.91).
| Baseline Patient Factors | Adjusted Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| All Patients (n = 1607) | Patients With CAP (n = 890) | Patients With HCAP (n = 717) | |
| |||
| Pneumonia severity index (PSI) class (reference: PSI classes 2 and 3 combined): | |||
| 4 | 3.37* (2.20 to 5.17) | 4.02* (2.29 to 7.08) | 2.69* (1.38 to 5.26) |
| 5 | 11.19* (7.14 to 17.55) | 13.03* (7.00 to 24.24) | 9.73* (4.86 to 19.46) |
| Pre‐morbid ambulation impairment | 2.61* (1.98 to 3.45) | 4.56* (3.06 to 6.78) | 1.60* (1.06 to 2.42) |
| Hospitalization in the prior 30 days | 1.93* (1.38 to 2.71) | 2.13* (1.47 to 3.09) | |
| Dementia | 1.00 (0.74 to 1.37) | 0.82 (0.49 to 1.38) | 1.15 (0.78 to 1.69) |
| Depression | 0.83 (0.56 to 1.23) | 1.03 (0.48 to 2.18) | 0.83 (0.53 to 1.31) |
| Acute geriatric syndromes | 0.96 (0.72 to 1.26) | 1.26 (0.83 to 1.92) | 0.74 (0.50 to 1.08) |
| Absence of cough and purulent sputum | 1.47* (1.14 to 1.90) | 1.64* (1.08 to 2.46) | 1.45* (1.04 to 2.03) |
Two‐level hierarchical modeling to account for clustering at the hospital level obtained negligible change in OR estimates of the patient factors and their 95% CI. There were no statistically significant interactions between PSI class and the 3 patient factors (results not shown).
The model‐predicted increase in mortality risk with presence of individual patient factors for the entire group is shown in Table 3. Across the 3 factors, 30‐day mortality increased by 1.9% to 6.1% for those with PSI class 2 and 3, and by 9.0% to 23.2% for those with PSI class 5. The upper end of these ranges represented the effect of pre‐morbid ambulation impairment, while the lower end was that for absence of cough and purulent sputum. With reference to the predicted mortality rates for PSI class which are listed in the footnotes of Table 3, the adverse prognosis conferred by individual patient factors amounted to relative risk inflation of 27% to 145% depending on the specific factor and PSI class.
| Predicted Increase in 30‐Day Mortality With Presence of Single Baseline Patient Factors, % (95% Confidence Interval) | |||
|---|---|---|---|
| PSI Classes 2 and 3 (n = 449) | PSI Class 4 (n = 700) | PSI Class 5 (n = 413) | |
| |||
| Pre‐morbid ambulation impairment | 6.1 (3.2 to 9.0) | 15.0 (10.2 to 19.7) | 23.2 (16.8 to 29.7) |
| Hospitalization in the prior 30 days | 3.6 (0.9 to 6.3) | 9.3 (3.6 to 15.1) | 15.7 (7.3 to 24.2) |
| Absence of cough and purulent sputum | 1.9 (0.4 to 3.4) | 5.0 (1.4 to 8.6) | 9.0 (3.0 to 15.0) |
DISCUSSION
After accounting for PSI class, we found 3 additional patient factors that were independently associated with 30‐day mortality among older persons hospitalized for pneumonia. Firstly, our study confirms that impaired physical function reflected by pre‐morbid ambulation impairment increases mortality risk, as previously demonstrated by Torres et al.10 It is likely that impaired function reflects an underlying vulnerability for adverse outcomes that is seen across primary diagnoses.7 Secondly, recent hospitalization often indicates clinical, functional, and social complexities, as well as increased likelihood of infection by more virulent organisms commonly associated with healthcare‐related infections. Together, these 2 factors could increase mortality risk. Thirdly, atypical presentations may be associated with increased mortality, because these often occur in frail older persons who are vulnerable to adverse outcomes8 due to diseases suffered and treatment received. Atypical presentations may also result in delayed diagnosis and treatment of pneumonia.
Pilotto et al. found that a multidimensional index comprising functional status, comorbidity burden, mental status, and nutritional assessment, among others, had a higher predictive accuracy for 30‐day mortality than did PSI.20 While there was a previous attempt to combine PSI with independent predictors to identify low‐risk older patients with CAP,21 we could not find similar work on the range of patient factors examined in this study. Indeed, the most important contribution that our study brings to the growing body of literature on short‐term mortality, among older persons hospitalized for pneumonia, is the prognostic importance of these 3 additional patient factors over and above severity of illness measured by PSI. With reference to the baseline predicted risk for different PSI class categories shown in Table 3, we have demonstrated that the predicted increase in mortality risk with the presence of these 3 factors is often not trivial, particularly for those with more severe pneumonia.
These 3 patient factors retained prognostic significance after accounting for PSI class for HCAP. However, only 2 factors were associated with mortality for CAP, because by definition recent hospitalization does not occur. A relevant discussion point is whether CAP and HCAP should be grouped together or classified separately. It is pertinent to reflect that the utility of making a distinction between CAP and HCAP appears to lie largely in the domain of therapeutics regarding the initial choice of antibiotics,18, 2225 although there has been some debate on this point.26 Moreover, the major features of HCAP, namely recent hospitalization (albeit in the prior 30 days, rather than 90 days) and nursing home residence (an item in PSI) were included in our regression analyses. Therefore, it seems reasonable to consider CAP and HCAP as a single group for risk stratification at the clinical frontline. We also argue that combining CAP and HCAP for risk adjustment will result in larger sample sizes that can minimize uncertainty around treatment effect estimates, when comparing across different interventions or providers. The same approach of analyzing CAP and HCAP together was adopted in a recent study that compared US hospitals on their risk‐adjusted performance for pneumonia among Medicare beneficiaries.27
The 30‐day mortality rates in this study are higher than those in the original PSI studies, even when stratified according to PSI class. However, more recent studies also registered relatively high mortality rates ranging from 18% to 19%.12, 28 There are a number of possible reasons for the higher mortality rates observed in our study. Firstly, we included both CAP and HCAP, whereas some other studies focused only on CAP. Secondly, the original PSI studies excluded patients with previous hospitalization within 7 days of admission, while we included them. Thirdly, our study population was relatively old (median age: 80 years) and had a higher proportion from nursing homes (22%). Although age and nursing home residence are variables in the PSI, the weights assigned to these 2 items may not adequately reflect the magnitude of mortality risk they confer. Finally, our understanding is that the study population comprises a relatively high proportion of patients who have do‐not‐resuscitate (DNR) instructions, though this was not measured. All these patient characteristics are likely to be associated with higher mortality risk.
The major strength of this study relates to its real world setting, where there were no major exclusion criteria except for HIV/AIDS. In addition, the clinical data at our disposal allowed selection from a relatively wide range of patient factors, beyond that commonly available in administrative data alone.
However, a few important limitations need to be acknowledged. Firstly, the retrospective nature of the study restricted data to those routinely collected, rather than that specifically acquired for research. Important unmeasured factors include inflammatory markers such as C‐reactive protein (CRP) or procalcitonin levels which have been shown to have prognostic value.29 Others include frailty, socioeconomic status, and social support.20 Secondly, increased likelihood of measurement error associated with retrospectively collected data could result in bias with uncertain direction. Thirdly, our strategy of assuming no functional impairment in the absence of documentation raises the possibility of underidentification and consequent bias in the direction of underestimation of the strength of association between pre‐morbid ambulation impairment and mortality. If so, the true association could even be stronger. Finally, we did not capture do‐not‐resuscitate (DNR) decisions because these were not consistently documented in the charts. We concede that DNR status is expected to be associated with short‐term mortality30 and therefore remains an unobserved factor that may explain a proportion of the mortality risk attributed to other factors in our study, such as pre‐morbid ambulation impairment.
Where do we proceed from here? Given our findings, further work that examines the unmeasured factors mentioned should be done. CRP and procalcitonin levels can be extracted from the laboratory results database when they are measured. However, specification of the other 3 factors is more challenging, given that these represent clinical or social constructs wherein optimal measurement is less certain. It would be important to estimate how much these factors improve the prediction of short‐term mortality beyond that achieved by PSI and the patient factors we have identified.
Nonetheless, the clinical implications of our work are clear. While PSI class is a time‐tested tool, addition of pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum can further improve risk stratification for short‐term mortality, when older persons present initially with clinical and radiological features of pneumonia. Information on these factors should be available in routine clinical care and, therefore, their use in risk stratification should be considered. For more valid and credible risk adjustment, these 3 factors could be considered in addition to severity of illness indices where data availability permits.
CONCLUSION
Recent hospitalization, pre‐morbid ambulation impairment, and atypical clinical presentation were independently associated with higher 30‐day mortality among older persons hospitalized for pneumonia, after adjusting for severity of illness with PSI class. These factors could be considered in addition to PSI, when performing risk stratification and adjustment in this setting.
Acknowledgements
The authors thank Clinical Associate Professor Sin Fai Lam for his assistance in the study, and the medical board chairmen of the 3 study hospitals for their support and encouragement.
- .Community‐acquired pneumonia in the elderly.Clin Infect Dis.2000;31:1066–1078.
- ,,,,,.Hospitalized community‐acquired pneumonia in the elderly—age‐ and sex‐related patterns of care and outcome in the United States.Am J Respir Crit Care Med.2002;165:766–772.
- ,,,,.Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database.Am J Med.1993;94:153–159.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Application and comparison of scoring indices to predict outcomes in patients with healthcare‐associated pneumonia.Critical Care.2011;15:R32.
- ,,,,,.Predicting in‐hospital mortality: the importance of functional status information.Med Care.1995;33:906–921.
- ,,, et al.Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments.Med Care.2003;41:70–83.
- ,,,,.Illness presentation in elderly patients.Arch Intern Med.1995;155:1060–1064.
- ,,, et al.Community‐acquired pneumonia in the elderly: Spanish multicentre study.Eur Respir J.2003;21:294–302.
- ,,, et al.Outcome predictors of pneumonia in elderly patients: importance of functional assessment.J Am Geriatr Soc.2004;52:1603–1609.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127;1260–1270.
- ,,.Assessment of pneumonia in older adults: effect of functional status.J Am Geriatr Soc.2006;54:1062–1067.
- ,,, et al.Only severely limited, premorbid functional status is associated with short‐ and longterm mortality in patients with pneumonia who are critically ill: a prospective observational study.Chest.2011;139:88–94.
- ,,, et al.Severe community‐acquired pneumonia: assessment of microbial aetiology as mortality factor.Eur Respir J.2004;24:779–785.
- ,,,,,.PIRO score for community‐acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community‐acquired pneumonia.Crit Care Med.2009;37:456–462.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,,,,.Epidemiology and outcomes of health‐care–associated pneumonia—results from a large US database of culture‐positive pneumonia.Chest.2005;128:3854–3862.
- American Thoracic Society and Infectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,.Conceptual and practical issues in developing risk‐adjustment methods. In: Iezzoni LI, editor.Risk Adjustment for Measuring Health Care Outcomes.3rd ed.Chicago, IL:Health Administration Press;2003:179–205.
- ,,, et al.The multidimensional prognostic index predicts short‐ and long‐term mortality in hospitalized geriatric patients with pneumonia.J Gerontol A Biol Sci Med Sci.2009;64A:880–887.
- ,,, et al.A validation and potential modification of the pneumonia severity index in elderly patients with community‐acquired pneumonia.J Am Geriatr Soc.2006;54:1212–1219.
- ,.Health care‐associated pneumonia—a new therapeutic paradigm.Chest.2005;128:3784–3786.
- ,,, et al.Health care‐associated pneumonia requiring hospital admission.Arch Intern Med.2007;167:1393–1399.
- ,,, et al.Health care‐associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes—Proceedings of the HCAP Summit.Clin Infect Dis.2008;46(suppl 4):S296–S334.
- ,,,,;for the Study Group of the Italian Society of Internal Medicine.Outcomes of patients hospitalized with community‐acquired, health care‐associated, and hospital‐acquired pneumonia.Ann Intern Med.2009;150:19–26.
- ,.Healthcare‐associated pneumonia is a heterogeneous disease, and all patients do not need the same broad‐spectrum antibiotic therapy as complex nosocomial pneumonia.Curr Opin Infect Dis.2009;22:316–325.
- ,,, et al.The performance of US hospitals as reflected in risk‐standardized 30‐day mortality and readmission rates for Medicare beneficiaries with pneumonia.J Hosp Med.2010;5:E12–E18.
- ,,, et al.Temporal trends in outcomes of older patients with pneumonia.Arch Intern Med.2000;160:3385–3391.
- ,.Clinical review: the role of biomarkers in the diagnosis and management of community‐acquired pneumonia.Critical Care.2010;14:203.
- ,,, et al.Community‐acquired pneumonia and do‐not‐resuscitate orders.J Am Geriatr Soc.2002;50:290–299.
Pneumonia occurs more commonly among older persons.1 With advancing age, the frequency of hospitalizations and mortality for pneumonia are higher.2 Among the tools developed to predict short‐term mortality is the pneumonia severity index (PSI), which is the best known among severity of illness indices for pneumonia.3 Its ability to predict short‐term mortality for CAP, particularly in identifying those at low risk was previously demonstrated.4 More recently, the extension of its utility in predicting 30‐day mortality for healthcare‐associated pneumonia (HCAP) was demonstrated.5
Severity of illness is one of several risk factors for adverse outcomes among older persons with acute illness. Besides comorbidity, other factors include functional impairment and atypical presentation. Information on physical functioning had equal importance as laboratory data in prognostication of in‐hospital mortality.6 In addition, walking impairment was 1 of 5 components of a risk adjustment index developed to predict 1‐year mortality for hospitalized older persons.7 Atypical presentations of illness, such as delirium and falls, independently predicted poor outcomes among hospitalized older patients.8
Specifically for pneumonia, functional status has also been shown to be an independent predictor of short‐term mortality among older patients hospitalized with CAP.913 Among atypical presentations, only absence of chills was an independent prognostic factor for CAP.9 Bacteremia was an independent factor related to death among adults with CAP, albeit for severe disease resulting in intensive care unit admission.14 It was also included in a severity assessment score; its higher scores were associated with early mortality.15 However, blood culture results are only available 2 to 3 days into the hospital episode. Therefore, bacteremia is a potential risk factor for mortality that is not identifiable at the start of hospitalization.
While PSI is a comprehensive collection of demographic, clinical, and investigative measures, it does not include items on functional status or atypical presentation. Neither does it account for recent hospitalization or comorbid conditions of significance to older persons, such as dementia and depression. It is plausible that at least some of these factors hold added prognostic value.
With all these in mind, we conducted a study with the following objectives: 1) to determine whether functional impairment, recent hospitalization, comorbid conditions of particular significance with advancing age, and atypical presentation are significantly associated with short‐term mortality among older patients hospitalized for CAP and HCAP, after taking into account PSI; and 2) if so, to estimate the magnitude of increased mortality risk with these factors. We tested our null hypotheses that, after adjustment for PSI class, 1) recent hospitalization, 2) pre‐morbid functional impairment, 3) dementia and depression, and 4) atypical presentation of illness have no association with 30‐day mortality for older persons hospitalized for CAP and HCAP, both combined and alone.
PATIENTS AND METHODS
Design and Setting
This was a retrospective cohort study that employed secondary analyses of chart and administrative data. The setting was 3 acute care public hospitals of the National Healthcare Group (NHG) cluster in Singapore. We merged data from hospital charts, the NHG Operations Data Store administrative database, and the national death registry. The local Institution Review Board (IRB) approved waiver of consent, and all other study procedures were consistent with the principles of the Helsinki Declaration.
Patient Population
We included first hospital episodes of adults aged 65 years or older with the principal diagnosis of pneumonia in 2007. These episodes were identified by their primary International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM) codes of 480 to 486 in the administrative data. Next, we applied our study definition of pneumonia, which required the presence of acute symptoms or signs of pneumonia at the point of hospital admission, and a chest radiograph with features consistent with pneumonia that was obtained during the period from 24 hours before, to 48 hours after, hospital admission. In doing so, we included patients with community‐acquired pneumonia (CAP)16 and healthcare‐associated pneumonia (HCAP),17 but not hospital‐acquired pneumonia (HAP). We excluded patients whose charts were not accessible for review because of human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) and those whose charts were unavailable for other reasons. The study flow diagram is shown in Figure 1.

We assigned the diagnosis of HCAP to patients who were admitted to an acute care hospital for 2 or more days in the prior 90 days, resided in a nursing home or long‐term care facility, or received of intravenous antibiotic therapy, chemotherapy, wound care, or hemodialysis in the prior 30 days.18 Remaining patients were assigned CAP.
Data Collection
Trained research nurses used an abstraction protocol to collect demographic and clinical information from the charts, and to extract laboratory results and chest radiograph reports from the computerized clinical records. Where radiological reports were equivocal with respect to features of pneumonia, we obtained the opinion of one of our respiratory physician investigators whose decision was final. A researcher with bio‐informatics expertise extracted admission‐related information from the administrative data. Chart, administrative, and mortality data were merged to assemble the study database.
Outcome and Explanatory Variables
The outcome (dependent) variable was 30‐day all‐cause mortality. The following explanatory (independent) variables were examined:
Pneumonia severity index (PSI): We used PSI class as specified in the original studies.4
Recent hospitalization: Hospitalization in the prior 90 days and 30 days were explored.
Atypical presentation of illness: Acute geriatric syndromes (falls or acute impairment of mobility), and absence of cough and purulent sputum were examined. Delirium was not one of the syndromes because PSI includes altered mental state as an item.4
Functional impairment: Pre‐morbid ambulation impairment and feeding impairment were examined. Impairment was defined as needing assistance or being totally dependent.
Additional comorbid conditions: We selected dementia and depression, as they may have impact on mortality in older persons but were not included in PSI.
We did not include bacteremia, because its presence cannot be determined at the time of illness presentation.
From previous experience, we anticipated missing values for functional status measures in up to 5% of charts. Where values were missing, we used the simple imputation strategy of assigning no ambulation or feeding impairment.
Sample Size Calculation
With a sample size of 1400 patients and a 30‐day mortality rate of 25%, 350 cases of death were expected. Using the rule of thumb of at least 10 cases per independent variable,19 we were able to work with 35 candidate explanatory variables in logistic regression for the entire group. Assuming that the subpopulations of CAP and HCAP consist of 700 patients each, with mortality rates of 20% and 30%, respectively, then 14 could be explored for CAP and 21 candidate variables for HCAP.
Data Analyses
Pre‐morbid ambulation impairment and feeding impairment probably represent different points along the continuum of functional impairment. During preliminary analyses when both variables were adjusted for each other in logistic regression, pre‐morbid ambulation impairment (odds ratio [OR] 4.94, 95% confidence interval [CI] 3.79 to 6.43) was associated with 30‐day mortality, whereas pre‐morbid feeding impairment was not (OR 0.82, 95% CI 0.61 to 1.09). As such, pre‐morbid ambulation impairment was selected as the variable to represent functional impairment. Hospitalization in the prior 30 days was more strongly associated with 30‐day mortality (OR 2.38, 95% CI 1.77 to 3.21) than was hospitalization in the prior 90 days (OR 1.90, 95% CI 1.49 to 2.41). Therefore, hospitalization in the prior 30 days was selected as the variable to reflect recent hospitalization.
We used logistic regression analysis and regressed 30‐day mortality on PSI class and other explanatory variables. OR estimates and their 95% CI were used to quantify the strength of associations of the explanatory variables with mortality, and to test their statistical significance. In addition, we explored the possibility of interactions between PSI class and the patient factors. To this end, we constructed additional regression models that included appropriate interaction terms and tested their statistical significance. As a form of sensitivity analysis, we repeated the regression analyses only for hospital episodes with complete functional data and observed the extent to which OR estimates changed. Furthermore, we performed 2‐level hierarchical modeling to account for clustering at the hospital level and re‐examined the OR and 95% CI for the patient factors. We conducted these analyses for the entire group, and repeated them separately for CAP and HCAP. Finally, to estimate the extent to which the patient factors would increase predicted 30‐day mortality, we performed marginal effects analyses for the entire group to quantify the increased risk when individual factors were present.
We used STATA version 9.2 (Stata Corp, College Station, TX) for all statistical analyses. Hierarchical modeling was performed using the xtlogit command. STATA post‐estimation commands mfx and prvalue were employed to estimate marginal effects and predicted probabilities, respectively. The unit of analysis was patients. Statistical significance was defined by P values of less than 0.05.
RESULTS
Among 1607 patients included, 890 (55.4%) had CAP and 717 (44.6%) had HCAP. Baseline patient characteristics of patients with CAP and HCAP are shown in Table 1. The 30‐day mortality rate was 28.1% for the entire group, and 20.6% and 37.4% for patients with CAP and HCAP, respectively. When stratified according to PSI classes 2, 3, 4, and 5, this rate was 0%, 8.2%, 24.4%, and 56.0%, respectively. Because there were no deaths among those with PSI class 2, this category was merged with class 3 for the regression analyses. Missing data on pre‐morbid ambulation impairment and feeding impairment occurred for 39 (2.4%) and 69 (4.6%) patients, respectively.
| Whole Study Population (n = 1607) | Those With CAP (n = 890) | Those With HCAP (n = 717) | |
|---|---|---|---|
| |||
| Median age, years (IQR) | 80 (7487) | 79 (7385) | 82 (7588) |
| Male, n (%) | 876 (54.5) | 477 (53.6) | 399 (55.7) |
| Median pneumonia severity index (PSI) score, (IQR) | 109 (87134) | 100 (82121) | 120 (99144) |
| PSI class: | |||
| 2 | 98 (6.1) | 84 (9.4) | 14 (2.0) |
| 3 | 353 (22.0) | 260 (29.2) | 93 (13.0) |
| 4 | 713 (44.4) | 386 (43.4) | 327 (45.6) |
| 5 | 443 (27.6) | 160 (18.0) | 283 (39.5) |
| Pre‐morbid ambulation impairment, n (%) | 798 (49.7) | 287 (32.3) | 511 (71.3) |
| Pre‐morbid feeding impairment, n (%) | 298 (18.5) | 74 (8.3) | 224 (31.2) |
| Hospitalization in prior 30 days, n (%) | 209 (13.0) | 0 (0) | 209 (29.2) |
| Nursing home residence, n (%) | 362 (22.5) | 0 (0) | 362 (50.5) |
| Acute geriatric syndromes, n (%) | 442 (27.5) | 241 (27.1) | 201 (28.0) |
| Absence of both cough and purulent sputum, n (%) | 559 (34.8) | 226 (25.4) | 333 (46.4) |
| Dementia, n (%) | 307 (19.1) | 121 (13.6) | 178 (25.8) |
| Depression, n (%) | 165 (10.3) | 53 (6.0) | 109 (15.8) |
| Neoplastic disease, n (%) | 108 (6.7) | 33 (3.7) | 75 (10.5) |
| Liver disease, n (%) | 48 (3.0) | 25 (2.8) | 23 (3.2) |
| Congestive heart failure, n (%) | 257 (16.0) | 129 (14.5) | 128 (17.9) |
| Stroke, n (%) | 490 (30.5) | 215 (24.2) | 275 (38.4) |
| Renal failure, n (%) | 220 (13.7) | 97 (10.9) | 123 (17.2) |
| Chronic lung disease, n (%) | 316 (19.7) | 177 (19.9) | 139 (19.4) |
| Diabetes mellitus, n (%) | 515 (32.1) | 273 (30.7) | 242 (33.8) |
| Emergency department diagnosis of pneumonia, n (%) | 857 (53.3) | 494 (55.5) | 363 (50.6) |
For CAP and HCAP together, pre‐morbid ambulation impairment was associated with increased 30‐day mortality (339/798 [42.5%] vs 112/809 [13.8%], unadjusted OR 4.60, 95% CI 3.60 to 5.87, P < 0.01), as was hospitalization in the prior 30 days (94/209 [45.0%] vs 357/1398 [25.5%], unadjusted OR 2.38, 95% CI 1.77 to 3.21, P = 0.02). This was also the case for dementia (118/307 [38.4%] vs 333/1300 [25.6%], unadjusted OR 1.81, 95% CI 1.40 to 2.35, P < 0.01), acute geriatric syndromes (163/442 [36.9%] vs 288/1165 [24.7%], unadjusted OR 1.78, 95% CI 1.41 to 2.25, P < 0.01), and absence of cough and purulent sputum (226/559 [40.4%] vs 225/1048 [21.5%], unadjusted OR 2.48, 95% CI 1.98 to 3.11, P < 0.01). However, depression was not significantly associated with 30‐day mortality (57/165 [34.6%] vs 394/1442 [27.3%], unadjusted OR 1.40, 95% CI 1.00 to 1.97, P = 0.05).
Table 2 summarizes the results of logistic regression. It shows that pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were all independently associated with 30‐day mortality after adjustment for PSI score for the entire group. These associations remained statistically significant when CAP and HCAP were examined separately. Because none of those with CAP could have hospitalization in the prior 30 days, this factor was not included in the CAP model. The strength of association for the same patient factor varied across the pneumonia sub‐type. This was markedly so for pre‐morbid ambulation impairment, with the OR estimate being almost 3‐fold higher for CAP than for HCAP. Dementia, depression, and acute geriatric syndromes were not associated with 30‐day mortality. When the analyses were repeated after excluding hospital episodes with missing values for pre‐morbid ambulation impairment, the same 3 variables were significantly associated with 30‐day mortality, with trivial differences in strength of association compared to when imputation was performed. The OR estimates for pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were 2.82 (95% CI 2.12 to 3.76), 1.83 (95% CI 1.42 to 2.83), and 1.47 (95% CI 1.14 to 1.91).
| Baseline Patient Factors | Adjusted Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| All Patients (n = 1607) | Patients With CAP (n = 890) | Patients With HCAP (n = 717) | |
| |||
| Pneumonia severity index (PSI) class (reference: PSI classes 2 and 3 combined): | |||
| 4 | 3.37* (2.20 to 5.17) | 4.02* (2.29 to 7.08) | 2.69* (1.38 to 5.26) |
| 5 | 11.19* (7.14 to 17.55) | 13.03* (7.00 to 24.24) | 9.73* (4.86 to 19.46) |
| Pre‐morbid ambulation impairment | 2.61* (1.98 to 3.45) | 4.56* (3.06 to 6.78) | 1.60* (1.06 to 2.42) |
| Hospitalization in the prior 30 days | 1.93* (1.38 to 2.71) | 2.13* (1.47 to 3.09) | |
| Dementia | 1.00 (0.74 to 1.37) | 0.82 (0.49 to 1.38) | 1.15 (0.78 to 1.69) |
| Depression | 0.83 (0.56 to 1.23) | 1.03 (0.48 to 2.18) | 0.83 (0.53 to 1.31) |
| Acute geriatric syndromes | 0.96 (0.72 to 1.26) | 1.26 (0.83 to 1.92) | 0.74 (0.50 to 1.08) |
| Absence of cough and purulent sputum | 1.47* (1.14 to 1.90) | 1.64* (1.08 to 2.46) | 1.45* (1.04 to 2.03) |
Two‐level hierarchical modeling to account for clustering at the hospital level obtained negligible change in OR estimates of the patient factors and their 95% CI. There were no statistically significant interactions between PSI class and the 3 patient factors (results not shown).
The model‐predicted increase in mortality risk with presence of individual patient factors for the entire group is shown in Table 3. Across the 3 factors, 30‐day mortality increased by 1.9% to 6.1% for those with PSI class 2 and 3, and by 9.0% to 23.2% for those with PSI class 5. The upper end of these ranges represented the effect of pre‐morbid ambulation impairment, while the lower end was that for absence of cough and purulent sputum. With reference to the predicted mortality rates for PSI class which are listed in the footnotes of Table 3, the adverse prognosis conferred by individual patient factors amounted to relative risk inflation of 27% to 145% depending on the specific factor and PSI class.
| Predicted Increase in 30‐Day Mortality With Presence of Single Baseline Patient Factors, % (95% Confidence Interval) | |||
|---|---|---|---|
| PSI Classes 2 and 3 (n = 449) | PSI Class 4 (n = 700) | PSI Class 5 (n = 413) | |
| |||
| Pre‐morbid ambulation impairment | 6.1 (3.2 to 9.0) | 15.0 (10.2 to 19.7) | 23.2 (16.8 to 29.7) |
| Hospitalization in the prior 30 days | 3.6 (0.9 to 6.3) | 9.3 (3.6 to 15.1) | 15.7 (7.3 to 24.2) |
| Absence of cough and purulent sputum | 1.9 (0.4 to 3.4) | 5.0 (1.4 to 8.6) | 9.0 (3.0 to 15.0) |
DISCUSSION
After accounting for PSI class, we found 3 additional patient factors that were independently associated with 30‐day mortality among older persons hospitalized for pneumonia. Firstly, our study confirms that impaired physical function reflected by pre‐morbid ambulation impairment increases mortality risk, as previously demonstrated by Torres et al.10 It is likely that impaired function reflects an underlying vulnerability for adverse outcomes that is seen across primary diagnoses.7 Secondly, recent hospitalization often indicates clinical, functional, and social complexities, as well as increased likelihood of infection by more virulent organisms commonly associated with healthcare‐related infections. Together, these 2 factors could increase mortality risk. Thirdly, atypical presentations may be associated with increased mortality, because these often occur in frail older persons who are vulnerable to adverse outcomes8 due to diseases suffered and treatment received. Atypical presentations may also result in delayed diagnosis and treatment of pneumonia.
Pilotto et al. found that a multidimensional index comprising functional status, comorbidity burden, mental status, and nutritional assessment, among others, had a higher predictive accuracy for 30‐day mortality than did PSI.20 While there was a previous attempt to combine PSI with independent predictors to identify low‐risk older patients with CAP,21 we could not find similar work on the range of patient factors examined in this study. Indeed, the most important contribution that our study brings to the growing body of literature on short‐term mortality, among older persons hospitalized for pneumonia, is the prognostic importance of these 3 additional patient factors over and above severity of illness measured by PSI. With reference to the baseline predicted risk for different PSI class categories shown in Table 3, we have demonstrated that the predicted increase in mortality risk with the presence of these 3 factors is often not trivial, particularly for those with more severe pneumonia.
These 3 patient factors retained prognostic significance after accounting for PSI class for HCAP. However, only 2 factors were associated with mortality for CAP, because by definition recent hospitalization does not occur. A relevant discussion point is whether CAP and HCAP should be grouped together or classified separately. It is pertinent to reflect that the utility of making a distinction between CAP and HCAP appears to lie largely in the domain of therapeutics regarding the initial choice of antibiotics,18, 2225 although there has been some debate on this point.26 Moreover, the major features of HCAP, namely recent hospitalization (albeit in the prior 30 days, rather than 90 days) and nursing home residence (an item in PSI) were included in our regression analyses. Therefore, it seems reasonable to consider CAP and HCAP as a single group for risk stratification at the clinical frontline. We also argue that combining CAP and HCAP for risk adjustment will result in larger sample sizes that can minimize uncertainty around treatment effect estimates, when comparing across different interventions or providers. The same approach of analyzing CAP and HCAP together was adopted in a recent study that compared US hospitals on their risk‐adjusted performance for pneumonia among Medicare beneficiaries.27
The 30‐day mortality rates in this study are higher than those in the original PSI studies, even when stratified according to PSI class. However, more recent studies also registered relatively high mortality rates ranging from 18% to 19%.12, 28 There are a number of possible reasons for the higher mortality rates observed in our study. Firstly, we included both CAP and HCAP, whereas some other studies focused only on CAP. Secondly, the original PSI studies excluded patients with previous hospitalization within 7 days of admission, while we included them. Thirdly, our study population was relatively old (median age: 80 years) and had a higher proportion from nursing homes (22%). Although age and nursing home residence are variables in the PSI, the weights assigned to these 2 items may not adequately reflect the magnitude of mortality risk they confer. Finally, our understanding is that the study population comprises a relatively high proportion of patients who have do‐not‐resuscitate (DNR) instructions, though this was not measured. All these patient characteristics are likely to be associated with higher mortality risk.
The major strength of this study relates to its real world setting, where there were no major exclusion criteria except for HIV/AIDS. In addition, the clinical data at our disposal allowed selection from a relatively wide range of patient factors, beyond that commonly available in administrative data alone.
However, a few important limitations need to be acknowledged. Firstly, the retrospective nature of the study restricted data to those routinely collected, rather than that specifically acquired for research. Important unmeasured factors include inflammatory markers such as C‐reactive protein (CRP) or procalcitonin levels which have been shown to have prognostic value.29 Others include frailty, socioeconomic status, and social support.20 Secondly, increased likelihood of measurement error associated with retrospectively collected data could result in bias with uncertain direction. Thirdly, our strategy of assuming no functional impairment in the absence of documentation raises the possibility of underidentification and consequent bias in the direction of underestimation of the strength of association between pre‐morbid ambulation impairment and mortality. If so, the true association could even be stronger. Finally, we did not capture do‐not‐resuscitate (DNR) decisions because these were not consistently documented in the charts. We concede that DNR status is expected to be associated with short‐term mortality30 and therefore remains an unobserved factor that may explain a proportion of the mortality risk attributed to other factors in our study, such as pre‐morbid ambulation impairment.
Where do we proceed from here? Given our findings, further work that examines the unmeasured factors mentioned should be done. CRP and procalcitonin levels can be extracted from the laboratory results database when they are measured. However, specification of the other 3 factors is more challenging, given that these represent clinical or social constructs wherein optimal measurement is less certain. It would be important to estimate how much these factors improve the prediction of short‐term mortality beyond that achieved by PSI and the patient factors we have identified.
Nonetheless, the clinical implications of our work are clear. While PSI class is a time‐tested tool, addition of pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum can further improve risk stratification for short‐term mortality, when older persons present initially with clinical and radiological features of pneumonia. Information on these factors should be available in routine clinical care and, therefore, their use in risk stratification should be considered. For more valid and credible risk adjustment, these 3 factors could be considered in addition to severity of illness indices where data availability permits.
CONCLUSION
Recent hospitalization, pre‐morbid ambulation impairment, and atypical clinical presentation were independently associated with higher 30‐day mortality among older persons hospitalized for pneumonia, after adjusting for severity of illness with PSI class. These factors could be considered in addition to PSI, when performing risk stratification and adjustment in this setting.
Acknowledgements
The authors thank Clinical Associate Professor Sin Fai Lam for his assistance in the study, and the medical board chairmen of the 3 study hospitals for their support and encouragement.
Pneumonia occurs more commonly among older persons.1 With advancing age, the frequency of hospitalizations and mortality for pneumonia are higher.2 Among the tools developed to predict short‐term mortality is the pneumonia severity index (PSI), which is the best known among severity of illness indices for pneumonia.3 Its ability to predict short‐term mortality for CAP, particularly in identifying those at low risk was previously demonstrated.4 More recently, the extension of its utility in predicting 30‐day mortality for healthcare‐associated pneumonia (HCAP) was demonstrated.5
Severity of illness is one of several risk factors for adverse outcomes among older persons with acute illness. Besides comorbidity, other factors include functional impairment and atypical presentation. Information on physical functioning had equal importance as laboratory data in prognostication of in‐hospital mortality.6 In addition, walking impairment was 1 of 5 components of a risk adjustment index developed to predict 1‐year mortality for hospitalized older persons.7 Atypical presentations of illness, such as delirium and falls, independently predicted poor outcomes among hospitalized older patients.8
Specifically for pneumonia, functional status has also been shown to be an independent predictor of short‐term mortality among older patients hospitalized with CAP.913 Among atypical presentations, only absence of chills was an independent prognostic factor for CAP.9 Bacteremia was an independent factor related to death among adults with CAP, albeit for severe disease resulting in intensive care unit admission.14 It was also included in a severity assessment score; its higher scores were associated with early mortality.15 However, blood culture results are only available 2 to 3 days into the hospital episode. Therefore, bacteremia is a potential risk factor for mortality that is not identifiable at the start of hospitalization.
While PSI is a comprehensive collection of demographic, clinical, and investigative measures, it does not include items on functional status or atypical presentation. Neither does it account for recent hospitalization or comorbid conditions of significance to older persons, such as dementia and depression. It is plausible that at least some of these factors hold added prognostic value.
With all these in mind, we conducted a study with the following objectives: 1) to determine whether functional impairment, recent hospitalization, comorbid conditions of particular significance with advancing age, and atypical presentation are significantly associated with short‐term mortality among older patients hospitalized for CAP and HCAP, after taking into account PSI; and 2) if so, to estimate the magnitude of increased mortality risk with these factors. We tested our null hypotheses that, after adjustment for PSI class, 1) recent hospitalization, 2) pre‐morbid functional impairment, 3) dementia and depression, and 4) atypical presentation of illness have no association with 30‐day mortality for older persons hospitalized for CAP and HCAP, both combined and alone.
PATIENTS AND METHODS
Design and Setting
This was a retrospective cohort study that employed secondary analyses of chart and administrative data. The setting was 3 acute care public hospitals of the National Healthcare Group (NHG) cluster in Singapore. We merged data from hospital charts, the NHG Operations Data Store administrative database, and the national death registry. The local Institution Review Board (IRB) approved waiver of consent, and all other study procedures were consistent with the principles of the Helsinki Declaration.
Patient Population
We included first hospital episodes of adults aged 65 years or older with the principal diagnosis of pneumonia in 2007. These episodes were identified by their primary International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM) codes of 480 to 486 in the administrative data. Next, we applied our study definition of pneumonia, which required the presence of acute symptoms or signs of pneumonia at the point of hospital admission, and a chest radiograph with features consistent with pneumonia that was obtained during the period from 24 hours before, to 48 hours after, hospital admission. In doing so, we included patients with community‐acquired pneumonia (CAP)16 and healthcare‐associated pneumonia (HCAP),17 but not hospital‐acquired pneumonia (HAP). We excluded patients whose charts were not accessible for review because of human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) and those whose charts were unavailable for other reasons. The study flow diagram is shown in Figure 1.

We assigned the diagnosis of HCAP to patients who were admitted to an acute care hospital for 2 or more days in the prior 90 days, resided in a nursing home or long‐term care facility, or received of intravenous antibiotic therapy, chemotherapy, wound care, or hemodialysis in the prior 30 days.18 Remaining patients were assigned CAP.
Data Collection
Trained research nurses used an abstraction protocol to collect demographic and clinical information from the charts, and to extract laboratory results and chest radiograph reports from the computerized clinical records. Where radiological reports were equivocal with respect to features of pneumonia, we obtained the opinion of one of our respiratory physician investigators whose decision was final. A researcher with bio‐informatics expertise extracted admission‐related information from the administrative data. Chart, administrative, and mortality data were merged to assemble the study database.
Outcome and Explanatory Variables
The outcome (dependent) variable was 30‐day all‐cause mortality. The following explanatory (independent) variables were examined:
Pneumonia severity index (PSI): We used PSI class as specified in the original studies.4
Recent hospitalization: Hospitalization in the prior 90 days and 30 days were explored.
Atypical presentation of illness: Acute geriatric syndromes (falls or acute impairment of mobility), and absence of cough and purulent sputum were examined. Delirium was not one of the syndromes because PSI includes altered mental state as an item.4
Functional impairment: Pre‐morbid ambulation impairment and feeding impairment were examined. Impairment was defined as needing assistance or being totally dependent.
Additional comorbid conditions: We selected dementia and depression, as they may have impact on mortality in older persons but were not included in PSI.
We did not include bacteremia, because its presence cannot be determined at the time of illness presentation.
From previous experience, we anticipated missing values for functional status measures in up to 5% of charts. Where values were missing, we used the simple imputation strategy of assigning no ambulation or feeding impairment.
Sample Size Calculation
With a sample size of 1400 patients and a 30‐day mortality rate of 25%, 350 cases of death were expected. Using the rule of thumb of at least 10 cases per independent variable,19 we were able to work with 35 candidate explanatory variables in logistic regression for the entire group. Assuming that the subpopulations of CAP and HCAP consist of 700 patients each, with mortality rates of 20% and 30%, respectively, then 14 could be explored for CAP and 21 candidate variables for HCAP.
Data Analyses
Pre‐morbid ambulation impairment and feeding impairment probably represent different points along the continuum of functional impairment. During preliminary analyses when both variables were adjusted for each other in logistic regression, pre‐morbid ambulation impairment (odds ratio [OR] 4.94, 95% confidence interval [CI] 3.79 to 6.43) was associated with 30‐day mortality, whereas pre‐morbid feeding impairment was not (OR 0.82, 95% CI 0.61 to 1.09). As such, pre‐morbid ambulation impairment was selected as the variable to represent functional impairment. Hospitalization in the prior 30 days was more strongly associated with 30‐day mortality (OR 2.38, 95% CI 1.77 to 3.21) than was hospitalization in the prior 90 days (OR 1.90, 95% CI 1.49 to 2.41). Therefore, hospitalization in the prior 30 days was selected as the variable to reflect recent hospitalization.
We used logistic regression analysis and regressed 30‐day mortality on PSI class and other explanatory variables. OR estimates and their 95% CI were used to quantify the strength of associations of the explanatory variables with mortality, and to test their statistical significance. In addition, we explored the possibility of interactions between PSI class and the patient factors. To this end, we constructed additional regression models that included appropriate interaction terms and tested their statistical significance. As a form of sensitivity analysis, we repeated the regression analyses only for hospital episodes with complete functional data and observed the extent to which OR estimates changed. Furthermore, we performed 2‐level hierarchical modeling to account for clustering at the hospital level and re‐examined the OR and 95% CI for the patient factors. We conducted these analyses for the entire group, and repeated them separately for CAP and HCAP. Finally, to estimate the extent to which the patient factors would increase predicted 30‐day mortality, we performed marginal effects analyses for the entire group to quantify the increased risk when individual factors were present.
We used STATA version 9.2 (Stata Corp, College Station, TX) for all statistical analyses. Hierarchical modeling was performed using the xtlogit command. STATA post‐estimation commands mfx and prvalue were employed to estimate marginal effects and predicted probabilities, respectively. The unit of analysis was patients. Statistical significance was defined by P values of less than 0.05.
RESULTS
Among 1607 patients included, 890 (55.4%) had CAP and 717 (44.6%) had HCAP. Baseline patient characteristics of patients with CAP and HCAP are shown in Table 1. The 30‐day mortality rate was 28.1% for the entire group, and 20.6% and 37.4% for patients with CAP and HCAP, respectively. When stratified according to PSI classes 2, 3, 4, and 5, this rate was 0%, 8.2%, 24.4%, and 56.0%, respectively. Because there were no deaths among those with PSI class 2, this category was merged with class 3 for the regression analyses. Missing data on pre‐morbid ambulation impairment and feeding impairment occurred for 39 (2.4%) and 69 (4.6%) patients, respectively.
| Whole Study Population (n = 1607) | Those With CAP (n = 890) | Those With HCAP (n = 717) | |
|---|---|---|---|
| |||
| Median age, years (IQR) | 80 (7487) | 79 (7385) | 82 (7588) |
| Male, n (%) | 876 (54.5) | 477 (53.6) | 399 (55.7) |
| Median pneumonia severity index (PSI) score, (IQR) | 109 (87134) | 100 (82121) | 120 (99144) |
| PSI class: | |||
| 2 | 98 (6.1) | 84 (9.4) | 14 (2.0) |
| 3 | 353 (22.0) | 260 (29.2) | 93 (13.0) |
| 4 | 713 (44.4) | 386 (43.4) | 327 (45.6) |
| 5 | 443 (27.6) | 160 (18.0) | 283 (39.5) |
| Pre‐morbid ambulation impairment, n (%) | 798 (49.7) | 287 (32.3) | 511 (71.3) |
| Pre‐morbid feeding impairment, n (%) | 298 (18.5) | 74 (8.3) | 224 (31.2) |
| Hospitalization in prior 30 days, n (%) | 209 (13.0) | 0 (0) | 209 (29.2) |
| Nursing home residence, n (%) | 362 (22.5) | 0 (0) | 362 (50.5) |
| Acute geriatric syndromes, n (%) | 442 (27.5) | 241 (27.1) | 201 (28.0) |
| Absence of both cough and purulent sputum, n (%) | 559 (34.8) | 226 (25.4) | 333 (46.4) |
| Dementia, n (%) | 307 (19.1) | 121 (13.6) | 178 (25.8) |
| Depression, n (%) | 165 (10.3) | 53 (6.0) | 109 (15.8) |
| Neoplastic disease, n (%) | 108 (6.7) | 33 (3.7) | 75 (10.5) |
| Liver disease, n (%) | 48 (3.0) | 25 (2.8) | 23 (3.2) |
| Congestive heart failure, n (%) | 257 (16.0) | 129 (14.5) | 128 (17.9) |
| Stroke, n (%) | 490 (30.5) | 215 (24.2) | 275 (38.4) |
| Renal failure, n (%) | 220 (13.7) | 97 (10.9) | 123 (17.2) |
| Chronic lung disease, n (%) | 316 (19.7) | 177 (19.9) | 139 (19.4) |
| Diabetes mellitus, n (%) | 515 (32.1) | 273 (30.7) | 242 (33.8) |
| Emergency department diagnosis of pneumonia, n (%) | 857 (53.3) | 494 (55.5) | 363 (50.6) |
For CAP and HCAP together, pre‐morbid ambulation impairment was associated with increased 30‐day mortality (339/798 [42.5%] vs 112/809 [13.8%], unadjusted OR 4.60, 95% CI 3.60 to 5.87, P < 0.01), as was hospitalization in the prior 30 days (94/209 [45.0%] vs 357/1398 [25.5%], unadjusted OR 2.38, 95% CI 1.77 to 3.21, P = 0.02). This was also the case for dementia (118/307 [38.4%] vs 333/1300 [25.6%], unadjusted OR 1.81, 95% CI 1.40 to 2.35, P < 0.01), acute geriatric syndromes (163/442 [36.9%] vs 288/1165 [24.7%], unadjusted OR 1.78, 95% CI 1.41 to 2.25, P < 0.01), and absence of cough and purulent sputum (226/559 [40.4%] vs 225/1048 [21.5%], unadjusted OR 2.48, 95% CI 1.98 to 3.11, P < 0.01). However, depression was not significantly associated with 30‐day mortality (57/165 [34.6%] vs 394/1442 [27.3%], unadjusted OR 1.40, 95% CI 1.00 to 1.97, P = 0.05).
Table 2 summarizes the results of logistic regression. It shows that pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were all independently associated with 30‐day mortality after adjustment for PSI score for the entire group. These associations remained statistically significant when CAP and HCAP were examined separately. Because none of those with CAP could have hospitalization in the prior 30 days, this factor was not included in the CAP model. The strength of association for the same patient factor varied across the pneumonia sub‐type. This was markedly so for pre‐morbid ambulation impairment, with the OR estimate being almost 3‐fold higher for CAP than for HCAP. Dementia, depression, and acute geriatric syndromes were not associated with 30‐day mortality. When the analyses were repeated after excluding hospital episodes with missing values for pre‐morbid ambulation impairment, the same 3 variables were significantly associated with 30‐day mortality, with trivial differences in strength of association compared to when imputation was performed. The OR estimates for pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum were 2.82 (95% CI 2.12 to 3.76), 1.83 (95% CI 1.42 to 2.83), and 1.47 (95% CI 1.14 to 1.91).
| Baseline Patient Factors | Adjusted Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| All Patients (n = 1607) | Patients With CAP (n = 890) | Patients With HCAP (n = 717) | |
| |||
| Pneumonia severity index (PSI) class (reference: PSI classes 2 and 3 combined): | |||
| 4 | 3.37* (2.20 to 5.17) | 4.02* (2.29 to 7.08) | 2.69* (1.38 to 5.26) |
| 5 | 11.19* (7.14 to 17.55) | 13.03* (7.00 to 24.24) | 9.73* (4.86 to 19.46) |
| Pre‐morbid ambulation impairment | 2.61* (1.98 to 3.45) | 4.56* (3.06 to 6.78) | 1.60* (1.06 to 2.42) |
| Hospitalization in the prior 30 days | 1.93* (1.38 to 2.71) | 2.13* (1.47 to 3.09) | |
| Dementia | 1.00 (0.74 to 1.37) | 0.82 (0.49 to 1.38) | 1.15 (0.78 to 1.69) |
| Depression | 0.83 (0.56 to 1.23) | 1.03 (0.48 to 2.18) | 0.83 (0.53 to 1.31) |
| Acute geriatric syndromes | 0.96 (0.72 to 1.26) | 1.26 (0.83 to 1.92) | 0.74 (0.50 to 1.08) |
| Absence of cough and purulent sputum | 1.47* (1.14 to 1.90) | 1.64* (1.08 to 2.46) | 1.45* (1.04 to 2.03) |
Two‐level hierarchical modeling to account for clustering at the hospital level obtained negligible change in OR estimates of the patient factors and their 95% CI. There were no statistically significant interactions between PSI class and the 3 patient factors (results not shown).
The model‐predicted increase in mortality risk with presence of individual patient factors for the entire group is shown in Table 3. Across the 3 factors, 30‐day mortality increased by 1.9% to 6.1% for those with PSI class 2 and 3, and by 9.0% to 23.2% for those with PSI class 5. The upper end of these ranges represented the effect of pre‐morbid ambulation impairment, while the lower end was that for absence of cough and purulent sputum. With reference to the predicted mortality rates for PSI class which are listed in the footnotes of Table 3, the adverse prognosis conferred by individual patient factors amounted to relative risk inflation of 27% to 145% depending on the specific factor and PSI class.
| Predicted Increase in 30‐Day Mortality With Presence of Single Baseline Patient Factors, % (95% Confidence Interval) | |||
|---|---|---|---|
| PSI Classes 2 and 3 (n = 449) | PSI Class 4 (n = 700) | PSI Class 5 (n = 413) | |
| |||
| Pre‐morbid ambulation impairment | 6.1 (3.2 to 9.0) | 15.0 (10.2 to 19.7) | 23.2 (16.8 to 29.7) |
| Hospitalization in the prior 30 days | 3.6 (0.9 to 6.3) | 9.3 (3.6 to 15.1) | 15.7 (7.3 to 24.2) |
| Absence of cough and purulent sputum | 1.9 (0.4 to 3.4) | 5.0 (1.4 to 8.6) | 9.0 (3.0 to 15.0) |
DISCUSSION
After accounting for PSI class, we found 3 additional patient factors that were independently associated with 30‐day mortality among older persons hospitalized for pneumonia. Firstly, our study confirms that impaired physical function reflected by pre‐morbid ambulation impairment increases mortality risk, as previously demonstrated by Torres et al.10 It is likely that impaired function reflects an underlying vulnerability for adverse outcomes that is seen across primary diagnoses.7 Secondly, recent hospitalization often indicates clinical, functional, and social complexities, as well as increased likelihood of infection by more virulent organisms commonly associated with healthcare‐related infections. Together, these 2 factors could increase mortality risk. Thirdly, atypical presentations may be associated with increased mortality, because these often occur in frail older persons who are vulnerable to adverse outcomes8 due to diseases suffered and treatment received. Atypical presentations may also result in delayed diagnosis and treatment of pneumonia.
Pilotto et al. found that a multidimensional index comprising functional status, comorbidity burden, mental status, and nutritional assessment, among others, had a higher predictive accuracy for 30‐day mortality than did PSI.20 While there was a previous attempt to combine PSI with independent predictors to identify low‐risk older patients with CAP,21 we could not find similar work on the range of patient factors examined in this study. Indeed, the most important contribution that our study brings to the growing body of literature on short‐term mortality, among older persons hospitalized for pneumonia, is the prognostic importance of these 3 additional patient factors over and above severity of illness measured by PSI. With reference to the baseline predicted risk for different PSI class categories shown in Table 3, we have demonstrated that the predicted increase in mortality risk with the presence of these 3 factors is often not trivial, particularly for those with more severe pneumonia.
These 3 patient factors retained prognostic significance after accounting for PSI class for HCAP. However, only 2 factors were associated with mortality for CAP, because by definition recent hospitalization does not occur. A relevant discussion point is whether CAP and HCAP should be grouped together or classified separately. It is pertinent to reflect that the utility of making a distinction between CAP and HCAP appears to lie largely in the domain of therapeutics regarding the initial choice of antibiotics,18, 2225 although there has been some debate on this point.26 Moreover, the major features of HCAP, namely recent hospitalization (albeit in the prior 30 days, rather than 90 days) and nursing home residence (an item in PSI) were included in our regression analyses. Therefore, it seems reasonable to consider CAP and HCAP as a single group for risk stratification at the clinical frontline. We also argue that combining CAP and HCAP for risk adjustment will result in larger sample sizes that can minimize uncertainty around treatment effect estimates, when comparing across different interventions or providers. The same approach of analyzing CAP and HCAP together was adopted in a recent study that compared US hospitals on their risk‐adjusted performance for pneumonia among Medicare beneficiaries.27
The 30‐day mortality rates in this study are higher than those in the original PSI studies, even when stratified according to PSI class. However, more recent studies also registered relatively high mortality rates ranging from 18% to 19%.12, 28 There are a number of possible reasons for the higher mortality rates observed in our study. Firstly, we included both CAP and HCAP, whereas some other studies focused only on CAP. Secondly, the original PSI studies excluded patients with previous hospitalization within 7 days of admission, while we included them. Thirdly, our study population was relatively old (median age: 80 years) and had a higher proportion from nursing homes (22%). Although age and nursing home residence are variables in the PSI, the weights assigned to these 2 items may not adequately reflect the magnitude of mortality risk they confer. Finally, our understanding is that the study population comprises a relatively high proportion of patients who have do‐not‐resuscitate (DNR) instructions, though this was not measured. All these patient characteristics are likely to be associated with higher mortality risk.
The major strength of this study relates to its real world setting, where there were no major exclusion criteria except for HIV/AIDS. In addition, the clinical data at our disposal allowed selection from a relatively wide range of patient factors, beyond that commonly available in administrative data alone.
However, a few important limitations need to be acknowledged. Firstly, the retrospective nature of the study restricted data to those routinely collected, rather than that specifically acquired for research. Important unmeasured factors include inflammatory markers such as C‐reactive protein (CRP) or procalcitonin levels which have been shown to have prognostic value.29 Others include frailty, socioeconomic status, and social support.20 Secondly, increased likelihood of measurement error associated with retrospectively collected data could result in bias with uncertain direction. Thirdly, our strategy of assuming no functional impairment in the absence of documentation raises the possibility of underidentification and consequent bias in the direction of underestimation of the strength of association between pre‐morbid ambulation impairment and mortality. If so, the true association could even be stronger. Finally, we did not capture do‐not‐resuscitate (DNR) decisions because these were not consistently documented in the charts. We concede that DNR status is expected to be associated with short‐term mortality30 and therefore remains an unobserved factor that may explain a proportion of the mortality risk attributed to other factors in our study, such as pre‐morbid ambulation impairment.
Where do we proceed from here? Given our findings, further work that examines the unmeasured factors mentioned should be done. CRP and procalcitonin levels can be extracted from the laboratory results database when they are measured. However, specification of the other 3 factors is more challenging, given that these represent clinical or social constructs wherein optimal measurement is less certain. It would be important to estimate how much these factors improve the prediction of short‐term mortality beyond that achieved by PSI and the patient factors we have identified.
Nonetheless, the clinical implications of our work are clear. While PSI class is a time‐tested tool, addition of pre‐morbid ambulation impairment, hospitalization in the prior 30 days, and absence of cough and purulent sputum can further improve risk stratification for short‐term mortality, when older persons present initially with clinical and radiological features of pneumonia. Information on these factors should be available in routine clinical care and, therefore, their use in risk stratification should be considered. For more valid and credible risk adjustment, these 3 factors could be considered in addition to severity of illness indices where data availability permits.
CONCLUSION
Recent hospitalization, pre‐morbid ambulation impairment, and atypical clinical presentation were independently associated with higher 30‐day mortality among older persons hospitalized for pneumonia, after adjusting for severity of illness with PSI class. These factors could be considered in addition to PSI, when performing risk stratification and adjustment in this setting.
Acknowledgements
The authors thank Clinical Associate Professor Sin Fai Lam for his assistance in the study, and the medical board chairmen of the 3 study hospitals for their support and encouragement.
- .Community‐acquired pneumonia in the elderly.Clin Infect Dis.2000;31:1066–1078.
- ,,,,,.Hospitalized community‐acquired pneumonia in the elderly—age‐ and sex‐related patterns of care and outcome in the United States.Am J Respir Crit Care Med.2002;165:766–772.
- ,,,,.Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database.Am J Med.1993;94:153–159.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Application and comparison of scoring indices to predict outcomes in patients with healthcare‐associated pneumonia.Critical Care.2011;15:R32.
- ,,,,,.Predicting in‐hospital mortality: the importance of functional status information.Med Care.1995;33:906–921.
- ,,, et al.Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments.Med Care.2003;41:70–83.
- ,,,,.Illness presentation in elderly patients.Arch Intern Med.1995;155:1060–1064.
- ,,, et al.Community‐acquired pneumonia in the elderly: Spanish multicentre study.Eur Respir J.2003;21:294–302.
- ,,, et al.Outcome predictors of pneumonia in elderly patients: importance of functional assessment.J Am Geriatr Soc.2004;52:1603–1609.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127;1260–1270.
- ,,.Assessment of pneumonia in older adults: effect of functional status.J Am Geriatr Soc.2006;54:1062–1067.
- ,,, et al.Only severely limited, premorbid functional status is associated with short‐ and longterm mortality in patients with pneumonia who are critically ill: a prospective observational study.Chest.2011;139:88–94.
- ,,, et al.Severe community‐acquired pneumonia: assessment of microbial aetiology as mortality factor.Eur Respir J.2004;24:779–785.
- ,,,,,.PIRO score for community‐acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community‐acquired pneumonia.Crit Care Med.2009;37:456–462.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,,,,.Epidemiology and outcomes of health‐care–associated pneumonia—results from a large US database of culture‐positive pneumonia.Chest.2005;128:3854–3862.
- American Thoracic Society and Infectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,.Conceptual and practical issues in developing risk‐adjustment methods. In: Iezzoni LI, editor.Risk Adjustment for Measuring Health Care Outcomes.3rd ed.Chicago, IL:Health Administration Press;2003:179–205.
- ,,, et al.The multidimensional prognostic index predicts short‐ and long‐term mortality in hospitalized geriatric patients with pneumonia.J Gerontol A Biol Sci Med Sci.2009;64A:880–887.
- ,,, et al.A validation and potential modification of the pneumonia severity index in elderly patients with community‐acquired pneumonia.J Am Geriatr Soc.2006;54:1212–1219.
- ,.Health care‐associated pneumonia—a new therapeutic paradigm.Chest.2005;128:3784–3786.
- ,,, et al.Health care‐associated pneumonia requiring hospital admission.Arch Intern Med.2007;167:1393–1399.
- ,,, et al.Health care‐associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes—Proceedings of the HCAP Summit.Clin Infect Dis.2008;46(suppl 4):S296–S334.
- ,,,,;for the Study Group of the Italian Society of Internal Medicine.Outcomes of patients hospitalized with community‐acquired, health care‐associated, and hospital‐acquired pneumonia.Ann Intern Med.2009;150:19–26.
- ,.Healthcare‐associated pneumonia is a heterogeneous disease, and all patients do not need the same broad‐spectrum antibiotic therapy as complex nosocomial pneumonia.Curr Opin Infect Dis.2009;22:316–325.
- ,,, et al.The performance of US hospitals as reflected in risk‐standardized 30‐day mortality and readmission rates for Medicare beneficiaries with pneumonia.J Hosp Med.2010;5:E12–E18.
- ,,, et al.Temporal trends in outcomes of older patients with pneumonia.Arch Intern Med.2000;160:3385–3391.
- ,.Clinical review: the role of biomarkers in the diagnosis and management of community‐acquired pneumonia.Critical Care.2010;14:203.
- ,,, et al.Community‐acquired pneumonia and do‐not‐resuscitate orders.J Am Geriatr Soc.2002;50:290–299.
- .Community‐acquired pneumonia in the elderly.Clin Infect Dis.2000;31:1066–1078.
- ,,,,,.Hospitalized community‐acquired pneumonia in the elderly—age‐ and sex‐related patterns of care and outcome in the United States.Am J Respir Crit Care Med.2002;165:766–772.
- ,,,,.Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database.Am J Med.1993;94:153–159.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Application and comparison of scoring indices to predict outcomes in patients with healthcare‐associated pneumonia.Critical Care.2011;15:R32.
- ,,,,,.Predicting in‐hospital mortality: the importance of functional status information.Med Care.1995;33:906–921.
- ,,, et al.Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments.Med Care.2003;41:70–83.
- ,,,,.Illness presentation in elderly patients.Arch Intern Med.1995;155:1060–1064.
- ,,, et al.Community‐acquired pneumonia in the elderly: Spanish multicentre study.Eur Respir J.2003;21:294–302.
- ,,, et al.Outcome predictors of pneumonia in elderly patients: importance of functional assessment.J Am Geriatr Soc.2004;52:1603–1609.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127;1260–1270.
- ,,.Assessment of pneumonia in older adults: effect of functional status.J Am Geriatr Soc.2006;54:1062–1067.
- ,,, et al.Only severely limited, premorbid functional status is associated with short‐ and longterm mortality in patients with pneumonia who are critically ill: a prospective observational study.Chest.2011;139:88–94.
- ,,, et al.Severe community‐acquired pneumonia: assessment of microbial aetiology as mortality factor.Eur Respir J.2004;24:779–785.
- ,,,,,.PIRO score for community‐acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community‐acquired pneumonia.Crit Care Med.2009;37:456–462.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,,,,.Epidemiology and outcomes of health‐care–associated pneumonia—results from a large US database of culture‐positive pneumonia.Chest.2005;128:3854–3862.
- American Thoracic Society and Infectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,.Conceptual and practical issues in developing risk‐adjustment methods. In: Iezzoni LI, editor.Risk Adjustment for Measuring Health Care Outcomes.3rd ed.Chicago, IL:Health Administration Press;2003:179–205.
- ,,, et al.The multidimensional prognostic index predicts short‐ and long‐term mortality in hospitalized geriatric patients with pneumonia.J Gerontol A Biol Sci Med Sci.2009;64A:880–887.
- ,,, et al.A validation and potential modification of the pneumonia severity index in elderly patients with community‐acquired pneumonia.J Am Geriatr Soc.2006;54:1212–1219.
- ,.Health care‐associated pneumonia—a new therapeutic paradigm.Chest.2005;128:3784–3786.
- ,,, et al.Health care‐associated pneumonia requiring hospital admission.Arch Intern Med.2007;167:1393–1399.
- ,,, et al.Health care‐associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes—Proceedings of the HCAP Summit.Clin Infect Dis.2008;46(suppl 4):S296–S334.
- ,,,,;for the Study Group of the Italian Society of Internal Medicine.Outcomes of patients hospitalized with community‐acquired, health care‐associated, and hospital‐acquired pneumonia.Ann Intern Med.2009;150:19–26.
- ,.Healthcare‐associated pneumonia is a heterogeneous disease, and all patients do not need the same broad‐spectrum antibiotic therapy as complex nosocomial pneumonia.Curr Opin Infect Dis.2009;22:316–325.
- ,,, et al.The performance of US hospitals as reflected in risk‐standardized 30‐day mortality and readmission rates for Medicare beneficiaries with pneumonia.J Hosp Med.2010;5:E12–E18.
- ,,, et al.Temporal trends in outcomes of older patients with pneumonia.Arch Intern Med.2000;160:3385–3391.
- ,.Clinical review: the role of biomarkers in the diagnosis and management of community‐acquired pneumonia.Critical Care.2010;14:203.
- ,,, et al.Community‐acquired pneumonia and do‐not‐resuscitate orders.J Am Geriatr Soc.2002;50:290–299.
Copyright © 2011 Society of Hospital Medicine
Observation Care in Children's Hospitals
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.
| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.
Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.

| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.

Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.

| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.

Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
Copyright © 2011 Society of Hospital Medicine
Refined Risk Stratification Guides Leukemia Transplant Decisions
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
EXPERT ANALYSIS FROM THE ANNUAL ONCOLOGY CONGRESS
Measuring Quality of Care
The measurement of quality of care has been the mantra of health policy care for the past decade, and has become as American as apple pie and Chevrolet. Yet there have been few data showing that the institution of quality of care guidelines has had any impact on mortality or morbidity.
Despite this lack of data, hospitals are being financially rewarded or penalized based on their ability to meet guidelines established by the Center for Medicare and Medicaid Services in conjunction with the American College of Cardiology and the American Heart Association. Two recent reports provide insight on the progress we have achieved with guidelines in heart failure and in instituting the shortening of the door-to-balloon time (D2B) for percutaneous coronary artery intervention (PCI) in ST-segment elevation MI.
Decreasing heart failure readmission within 30 days, which occurs in approximately one-third of hospitalized patients, has become a target for the quality improvement process. Using the "Get With the Guidelines Heart Failure" registry, a recent analysis indicates that there is a very poor correlation between the achievement or those standards and the 30 day mortality and readmission rate (Circulation 2011;124:712-9).
The guidelines include measurement of cardiac function, application of the usual heart failure medications, and discharge instructions. Data were collected in almost 20,000 patients in 153 hospitals during 2005. Adherence to these guidelines was quite good and was achieved in more than 75% of the hospitals, yet it was unrelated to the 30 day mortality or hospital readmission.
The authors emphasized that the factors that affect survival and readmission are very heterogeneous. Basing pay-for-performance standards on a single measure (such as readmission rates) may penalize institutions that face impediments that are unrelated to performance measurements. Penalizing hospitals that have high readmission rates as a result of a large populations of vulnerable patients may penalize institutions that actually could benefit from more resources in order to achieve better outcomes.
The effectiveness of PCI, when it is performed in less than 90 minutes in STEMI patients, has been supported by clinical data from selected cardiac centers. The application to the larger patient population of the guideline to shorten D2B time to less than 90 minutes has been championed by the ACC, which launched the D2B Alliance in 2006 and by the AHA in 2007 with its Mission: Lifeline program.
The success of these efforts was reported in August (Circulation 2011;124:1038-45) and indicates that in a selected group of CMS-reporting hospitals, D2B time decreased from 96 minutes in 2005 to 64 minutes in 2010. In addition, the percentage of patients with a D2B time of less than 90 minutes increased from 44% to 91%, and that of patients with D2B of less than 75 minutes rose from 27% to 70%. The success of this effort is to be applauded, but the report is striking for its absence of any information regarding outcomes of the shortened D2B time. Unfortunately, there is little outcome information available, with the exception of data from Michigan on all Medicare providers in that state, which indicates that although D2B time decreased by 90 minutes, there was no significant benefit.
Measurement of quality remains elusive, in spite of the good intentions of physicians and health planners to use a variety of seemingly beneficial criteria for its definition.
As consumers, we know that quality is not easy to measure. Most of us can compare the quality of American automobiles vs. their foreign competitors by "kicking the tires," that is, by doing a little research. But even with this knowledge, we are not always sure that the particular car we buy will be better or last longer. Health care faces the same problem. Establishing quality care measurements will require a great deal of further research before we can reward or penalize hospitals and physicians for their performance.
It is possible that in our zeal to measure what we can, we are confusing process with content. How to put a number on the performance that leads to quality remains uncertain using our current methodology.-
Dr. Sidney Goldstein is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The measurement of quality of care has been the mantra of health policy care for the past decade, and has become as American as apple pie and Chevrolet. Yet there have been few data showing that the institution of quality of care guidelines has had any impact on mortality or morbidity.
Despite this lack of data, hospitals are being financially rewarded or penalized based on their ability to meet guidelines established by the Center for Medicare and Medicaid Services in conjunction with the American College of Cardiology and the American Heart Association. Two recent reports provide insight on the progress we have achieved with guidelines in heart failure and in instituting the shortening of the door-to-balloon time (D2B) for percutaneous coronary artery intervention (PCI) in ST-segment elevation MI.
Decreasing heart failure readmission within 30 days, which occurs in approximately one-third of hospitalized patients, has become a target for the quality improvement process. Using the "Get With the Guidelines Heart Failure" registry, a recent analysis indicates that there is a very poor correlation between the achievement or those standards and the 30 day mortality and readmission rate (Circulation 2011;124:712-9).
The guidelines include measurement of cardiac function, application of the usual heart failure medications, and discharge instructions. Data were collected in almost 20,000 patients in 153 hospitals during 2005. Adherence to these guidelines was quite good and was achieved in more than 75% of the hospitals, yet it was unrelated to the 30 day mortality or hospital readmission.
The authors emphasized that the factors that affect survival and readmission are very heterogeneous. Basing pay-for-performance standards on a single measure (such as readmission rates) may penalize institutions that face impediments that are unrelated to performance measurements. Penalizing hospitals that have high readmission rates as a result of a large populations of vulnerable patients may penalize institutions that actually could benefit from more resources in order to achieve better outcomes.
The effectiveness of PCI, when it is performed in less than 90 minutes in STEMI patients, has been supported by clinical data from selected cardiac centers. The application to the larger patient population of the guideline to shorten D2B time to less than 90 minutes has been championed by the ACC, which launched the D2B Alliance in 2006 and by the AHA in 2007 with its Mission: Lifeline program.
The success of these efforts was reported in August (Circulation 2011;124:1038-45) and indicates that in a selected group of CMS-reporting hospitals, D2B time decreased from 96 minutes in 2005 to 64 minutes in 2010. In addition, the percentage of patients with a D2B time of less than 90 minutes increased from 44% to 91%, and that of patients with D2B of less than 75 minutes rose from 27% to 70%. The success of this effort is to be applauded, but the report is striking for its absence of any information regarding outcomes of the shortened D2B time. Unfortunately, there is little outcome information available, with the exception of data from Michigan on all Medicare providers in that state, which indicates that although D2B time decreased by 90 minutes, there was no significant benefit.
Measurement of quality remains elusive, in spite of the good intentions of physicians and health planners to use a variety of seemingly beneficial criteria for its definition.
As consumers, we know that quality is not easy to measure. Most of us can compare the quality of American automobiles vs. their foreign competitors by "kicking the tires," that is, by doing a little research. But even with this knowledge, we are not always sure that the particular car we buy will be better or last longer. Health care faces the same problem. Establishing quality care measurements will require a great deal of further research before we can reward or penalize hospitals and physicians for their performance.
It is possible that in our zeal to measure what we can, we are confusing process with content. How to put a number on the performance that leads to quality remains uncertain using our current methodology.-
Dr. Sidney Goldstein is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The measurement of quality of care has been the mantra of health policy care for the past decade, and has become as American as apple pie and Chevrolet. Yet there have been few data showing that the institution of quality of care guidelines has had any impact on mortality or morbidity.
Despite this lack of data, hospitals are being financially rewarded or penalized based on their ability to meet guidelines established by the Center for Medicare and Medicaid Services in conjunction with the American College of Cardiology and the American Heart Association. Two recent reports provide insight on the progress we have achieved with guidelines in heart failure and in instituting the shortening of the door-to-balloon time (D2B) for percutaneous coronary artery intervention (PCI) in ST-segment elevation MI.
Decreasing heart failure readmission within 30 days, which occurs in approximately one-third of hospitalized patients, has become a target for the quality improvement process. Using the "Get With the Guidelines Heart Failure" registry, a recent analysis indicates that there is a very poor correlation between the achievement or those standards and the 30 day mortality and readmission rate (Circulation 2011;124:712-9).
The guidelines include measurement of cardiac function, application of the usual heart failure medications, and discharge instructions. Data were collected in almost 20,000 patients in 153 hospitals during 2005. Adherence to these guidelines was quite good and was achieved in more than 75% of the hospitals, yet it was unrelated to the 30 day mortality or hospital readmission.
The authors emphasized that the factors that affect survival and readmission are very heterogeneous. Basing pay-for-performance standards on a single measure (such as readmission rates) may penalize institutions that face impediments that are unrelated to performance measurements. Penalizing hospitals that have high readmission rates as a result of a large populations of vulnerable patients may penalize institutions that actually could benefit from more resources in order to achieve better outcomes.
The effectiveness of PCI, when it is performed in less than 90 minutes in STEMI patients, has been supported by clinical data from selected cardiac centers. The application to the larger patient population of the guideline to shorten D2B time to less than 90 minutes has been championed by the ACC, which launched the D2B Alliance in 2006 and by the AHA in 2007 with its Mission: Lifeline program.
The success of these efforts was reported in August (Circulation 2011;124:1038-45) and indicates that in a selected group of CMS-reporting hospitals, D2B time decreased from 96 minutes in 2005 to 64 minutes in 2010. In addition, the percentage of patients with a D2B time of less than 90 minutes increased from 44% to 91%, and that of patients with D2B of less than 75 minutes rose from 27% to 70%. The success of this effort is to be applauded, but the report is striking for its absence of any information regarding outcomes of the shortened D2B time. Unfortunately, there is little outcome information available, with the exception of data from Michigan on all Medicare providers in that state, which indicates that although D2B time decreased by 90 minutes, there was no significant benefit.
Measurement of quality remains elusive, in spite of the good intentions of physicians and health planners to use a variety of seemingly beneficial criteria for its definition.
As consumers, we know that quality is not easy to measure. Most of us can compare the quality of American automobiles vs. their foreign competitors by "kicking the tires," that is, by doing a little research. But even with this knowledge, we are not always sure that the particular car we buy will be better or last longer. Health care faces the same problem. Establishing quality care measurements will require a great deal of further research before we can reward or penalize hospitals and physicians for their performance.
It is possible that in our zeal to measure what we can, we are confusing process with content. How to put a number on the performance that leads to quality remains uncertain using our current methodology.-
Dr. Sidney Goldstein is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Dispel Myths to Recognize Child Abuse
SAN FRANCISCO – The color of a bruise indicates its age. You’ll almost always see bruising when a child has a fracture. Sexual abuse leaves behind physical exam findings.
These are all myths that can get in the way of physicians recognizing abuse of an infant or child. Physicians are required by law to report all suspicions of nonaccidental trauma, a catch-all term for child abuse, shaken baby syndrome, and battered-child syndrome.
Physicians can meet that obligation by ignoring these myths, recognizing red flags for nonaccidental trauma, and being familiar with signs of accidental trauma or medical conditions that can mimic the physical findings of nonaccidental trauma, Dr. Maureen D. McCollough said at the annual meeting of the American College of Emergency Physicians.
Myth: The age of bruises can be accurately determined by their color – red, purple, yellow, green, or brown. In reality, there is no predictable order or chronology of color in bruising, and even in the same person bruises of similar ages may have different colors, said Dr. McCollough of the University of Southern California, Los Angeles, and director of pediatric emergency medicine at Los Angeles County USC Medical Center.
Studies have shown poor interobserver reliability in assessing bruise coloring and poor physician accuracy in characterizing coloring.
Red flags of suspicion should go up if you see multiple bruises or lacerations, or see them in unusual locations. Accidental toddler tumbles can produce multiple bruises, but generally these are on bony prominences. Unusual locations for pediatric bruising include the lower back, buttocks, cheeks, ears, or neck. Bruising anywhere in an infant who is not yet mobile is suspicious.
"Remember, if you don’t cruise, you don’t bruise," she said.
Be suspicious if the pattern of the marks, bruises, or lacerations remind you of an object like a hand, hairbrush, belt, or buckle. Bruises around wrists or extremities may be from the child being tied up. Tight elastic socks can leave a mark around an infant’s leg that mimics this, in which case the parent should be able to provide a sock with dimensions that match the bruising.
Visible injuries around a baby’s mouth or frenulum should raise a red flag for forced feeding. Genital injuries may indicate forced toilet training. Hair pulling produces characteristic marks of traumatic alopecia – an incompletely bald child with diffuse alopecia, broken hairs, and no loose hairs at the periphery.
A wide variety of problems can mimic the visual signs of nonaccidental bruising, including dermal melanosis, vitamin K deficiency, leukemia, hemophilia, millipede secretions, Ehlers-Danlos syndrome, dermatitis, lice, and more.
An equally impressive array of events can mimic the look of abusive burns, bullae, and erythema. These include the cultural practices of coining, cupping, spooning, or moxibustion, skin infections, allergic reactions, herpes or varicella infection, diaper dermatitis, impetigo, and more.
Accidental burns usually have a typical "splash" pattern if liquid is involved, or a child who grasps something hot will have burns on the volar aspect of the fingers and palm. Accidental cigarette burns usually have a streaky appearance.
If there are no splash marks, or there is a sharp line of demarcation, or burns are limited to the perineum, consider that the child may have been forcibly immersed in something hot. Intentional cigarette burns tend to be similar in size – often 5-mm circles – and create injuries from bullae to deep craters that scab over. These usually are on the palms or soles but can be anywhere on the body. Again, be suspicious if you see a burn mark that looks like an object, such as a radiator or an iron.
Myth: Fractures usually are associated with overlying bruising. In fact, children with inflicted skeletal fractures often have no associated bruising. Bruising is present in only 43% of skull fractures and less than 20% of lower extremity fractures in cases of abuse, Dr. McCollough said.
Infants who can’t walk shouldn’t fracture. Spiral fractures caused by the twisting of a long bone such as the femur suggest nonaccidental trauma. Toddler spiral fractures of the tibia, on the other hand, are very common, caused when a leg is trapped under the body during a fall, such as getting a leg caught in a couch. "This is not abuse," she said.
Raise the red flags when you see swelling of a body part that is out of proportion to a described injury; this may indicate an underlying fracture. A diaphyseal (midshaft) fracture in a child less than 3 years old is suspect, and metaphyseal or epiphyseal fractures beyond the newborn period (also called corner fractures or bucket handle fractures) are virtually diagnostic of abuse.
The posterior ribs are the most common area of nonaccidental rib fractures.
Suspect head injuries and possible abuse if the child has unexplained seizures, vomiting, changes in neurological or mental status, or large scalp hematomas. Be suspicious if the parents’ explanation changes over time, if there is intracranial bleeds after "minimal" trauma, or if you find retinal hemorrhages outside of the newborn period, she said.
Myth: Sexual abuse leaves physical findings. More myths: A colposcope is needed to detect sexual abuse, and some girls are born without hymens.
Although hymens come in a wide variety of shapes and sizes, a study of more than 1,100 newborn girls showed that all of them had one, she noted. Reviews of cases of sexual abuse show that physical exam findings of pediatric sexual abuse are rare because the tissue is very elastic and heals quickly.
Physical evidence will be more likely if force was used, if the child resisted, if there are great differences in the sizes and ages of the perpetrator and victim, and if a foreign object was forced into the mouth, vagina, or anus. Bruising or bite marks on a child’s penis may suggest nonaccidental trauma from forced toilet training.
When you see visible clues to what may be abuse, photograph or draw what you see and include something in the image to show size or scale. Don’t just rely on written notes, she said.
SAN FRANCISCO – The color of a bruise indicates its age. You’ll almost always see bruising when a child has a fracture. Sexual abuse leaves behind physical exam findings.
These are all myths that can get in the way of physicians recognizing abuse of an infant or child. Physicians are required by law to report all suspicions of nonaccidental trauma, a catch-all term for child abuse, shaken baby syndrome, and battered-child syndrome.
Physicians can meet that obligation by ignoring these myths, recognizing red flags for nonaccidental trauma, and being familiar with signs of accidental trauma or medical conditions that can mimic the physical findings of nonaccidental trauma, Dr. Maureen D. McCollough said at the annual meeting of the American College of Emergency Physicians.
Myth: The age of bruises can be accurately determined by their color – red, purple, yellow, green, or brown. In reality, there is no predictable order or chronology of color in bruising, and even in the same person bruises of similar ages may have different colors, said Dr. McCollough of the University of Southern California, Los Angeles, and director of pediatric emergency medicine at Los Angeles County USC Medical Center.
Studies have shown poor interobserver reliability in assessing bruise coloring and poor physician accuracy in characterizing coloring.
Red flags of suspicion should go up if you see multiple bruises or lacerations, or see them in unusual locations. Accidental toddler tumbles can produce multiple bruises, but generally these are on bony prominences. Unusual locations for pediatric bruising include the lower back, buttocks, cheeks, ears, or neck. Bruising anywhere in an infant who is not yet mobile is suspicious.
"Remember, if you don’t cruise, you don’t bruise," she said.
Be suspicious if the pattern of the marks, bruises, or lacerations remind you of an object like a hand, hairbrush, belt, or buckle. Bruises around wrists or extremities may be from the child being tied up. Tight elastic socks can leave a mark around an infant’s leg that mimics this, in which case the parent should be able to provide a sock with dimensions that match the bruising.
Visible injuries around a baby’s mouth or frenulum should raise a red flag for forced feeding. Genital injuries may indicate forced toilet training. Hair pulling produces characteristic marks of traumatic alopecia – an incompletely bald child with diffuse alopecia, broken hairs, and no loose hairs at the periphery.
A wide variety of problems can mimic the visual signs of nonaccidental bruising, including dermal melanosis, vitamin K deficiency, leukemia, hemophilia, millipede secretions, Ehlers-Danlos syndrome, dermatitis, lice, and more.
An equally impressive array of events can mimic the look of abusive burns, bullae, and erythema. These include the cultural practices of coining, cupping, spooning, or moxibustion, skin infections, allergic reactions, herpes or varicella infection, diaper dermatitis, impetigo, and more.
Accidental burns usually have a typical "splash" pattern if liquid is involved, or a child who grasps something hot will have burns on the volar aspect of the fingers and palm. Accidental cigarette burns usually have a streaky appearance.
If there are no splash marks, or there is a sharp line of demarcation, or burns are limited to the perineum, consider that the child may have been forcibly immersed in something hot. Intentional cigarette burns tend to be similar in size – often 5-mm circles – and create injuries from bullae to deep craters that scab over. These usually are on the palms or soles but can be anywhere on the body. Again, be suspicious if you see a burn mark that looks like an object, such as a radiator or an iron.
Myth: Fractures usually are associated with overlying bruising. In fact, children with inflicted skeletal fractures often have no associated bruising. Bruising is present in only 43% of skull fractures and less than 20% of lower extremity fractures in cases of abuse, Dr. McCollough said.
Infants who can’t walk shouldn’t fracture. Spiral fractures caused by the twisting of a long bone such as the femur suggest nonaccidental trauma. Toddler spiral fractures of the tibia, on the other hand, are very common, caused when a leg is trapped under the body during a fall, such as getting a leg caught in a couch. "This is not abuse," she said.
Raise the red flags when you see swelling of a body part that is out of proportion to a described injury; this may indicate an underlying fracture. A diaphyseal (midshaft) fracture in a child less than 3 years old is suspect, and metaphyseal or epiphyseal fractures beyond the newborn period (also called corner fractures or bucket handle fractures) are virtually diagnostic of abuse.
The posterior ribs are the most common area of nonaccidental rib fractures.
Suspect head injuries and possible abuse if the child has unexplained seizures, vomiting, changes in neurological or mental status, or large scalp hematomas. Be suspicious if the parents’ explanation changes over time, if there is intracranial bleeds after "minimal" trauma, or if you find retinal hemorrhages outside of the newborn period, she said.
Myth: Sexual abuse leaves physical findings. More myths: A colposcope is needed to detect sexual abuse, and some girls are born without hymens.
Although hymens come in a wide variety of shapes and sizes, a study of more than 1,100 newborn girls showed that all of them had one, she noted. Reviews of cases of sexual abuse show that physical exam findings of pediatric sexual abuse are rare because the tissue is very elastic and heals quickly.
Physical evidence will be more likely if force was used, if the child resisted, if there are great differences in the sizes and ages of the perpetrator and victim, and if a foreign object was forced into the mouth, vagina, or anus. Bruising or bite marks on a child’s penis may suggest nonaccidental trauma from forced toilet training.
When you see visible clues to what may be abuse, photograph or draw what you see and include something in the image to show size or scale. Don’t just rely on written notes, she said.
SAN FRANCISCO – The color of a bruise indicates its age. You’ll almost always see bruising when a child has a fracture. Sexual abuse leaves behind physical exam findings.
These are all myths that can get in the way of physicians recognizing abuse of an infant or child. Physicians are required by law to report all suspicions of nonaccidental trauma, a catch-all term for child abuse, shaken baby syndrome, and battered-child syndrome.
Physicians can meet that obligation by ignoring these myths, recognizing red flags for nonaccidental trauma, and being familiar with signs of accidental trauma or medical conditions that can mimic the physical findings of nonaccidental trauma, Dr. Maureen D. McCollough said at the annual meeting of the American College of Emergency Physicians.
Myth: The age of bruises can be accurately determined by their color – red, purple, yellow, green, or brown. In reality, there is no predictable order or chronology of color in bruising, and even in the same person bruises of similar ages may have different colors, said Dr. McCollough of the University of Southern California, Los Angeles, and director of pediatric emergency medicine at Los Angeles County USC Medical Center.
Studies have shown poor interobserver reliability in assessing bruise coloring and poor physician accuracy in characterizing coloring.
Red flags of suspicion should go up if you see multiple bruises or lacerations, or see them in unusual locations. Accidental toddler tumbles can produce multiple bruises, but generally these are on bony prominences. Unusual locations for pediatric bruising include the lower back, buttocks, cheeks, ears, or neck. Bruising anywhere in an infant who is not yet mobile is suspicious.
"Remember, if you don’t cruise, you don’t bruise," she said.
Be suspicious if the pattern of the marks, bruises, or lacerations remind you of an object like a hand, hairbrush, belt, or buckle. Bruises around wrists or extremities may be from the child being tied up. Tight elastic socks can leave a mark around an infant’s leg that mimics this, in which case the parent should be able to provide a sock with dimensions that match the bruising.
Visible injuries around a baby’s mouth or frenulum should raise a red flag for forced feeding. Genital injuries may indicate forced toilet training. Hair pulling produces characteristic marks of traumatic alopecia – an incompletely bald child with diffuse alopecia, broken hairs, and no loose hairs at the periphery.
A wide variety of problems can mimic the visual signs of nonaccidental bruising, including dermal melanosis, vitamin K deficiency, leukemia, hemophilia, millipede secretions, Ehlers-Danlos syndrome, dermatitis, lice, and more.
An equally impressive array of events can mimic the look of abusive burns, bullae, and erythema. These include the cultural practices of coining, cupping, spooning, or moxibustion, skin infections, allergic reactions, herpes or varicella infection, diaper dermatitis, impetigo, and more.
Accidental burns usually have a typical "splash" pattern if liquid is involved, or a child who grasps something hot will have burns on the volar aspect of the fingers and palm. Accidental cigarette burns usually have a streaky appearance.
If there are no splash marks, or there is a sharp line of demarcation, or burns are limited to the perineum, consider that the child may have been forcibly immersed in something hot. Intentional cigarette burns tend to be similar in size – often 5-mm circles – and create injuries from bullae to deep craters that scab over. These usually are on the palms or soles but can be anywhere on the body. Again, be suspicious if you see a burn mark that looks like an object, such as a radiator or an iron.
Myth: Fractures usually are associated with overlying bruising. In fact, children with inflicted skeletal fractures often have no associated bruising. Bruising is present in only 43% of skull fractures and less than 20% of lower extremity fractures in cases of abuse, Dr. McCollough said.
Infants who can’t walk shouldn’t fracture. Spiral fractures caused by the twisting of a long bone such as the femur suggest nonaccidental trauma. Toddler spiral fractures of the tibia, on the other hand, are very common, caused when a leg is trapped under the body during a fall, such as getting a leg caught in a couch. "This is not abuse," she said.
Raise the red flags when you see swelling of a body part that is out of proportion to a described injury; this may indicate an underlying fracture. A diaphyseal (midshaft) fracture in a child less than 3 years old is suspect, and metaphyseal or epiphyseal fractures beyond the newborn period (also called corner fractures or bucket handle fractures) are virtually diagnostic of abuse.
The posterior ribs are the most common area of nonaccidental rib fractures.
Suspect head injuries and possible abuse if the child has unexplained seizures, vomiting, changes in neurological or mental status, or large scalp hematomas. Be suspicious if the parents’ explanation changes over time, if there is intracranial bleeds after "minimal" trauma, or if you find retinal hemorrhages outside of the newborn period, she said.
Myth: Sexual abuse leaves physical findings. More myths: A colposcope is needed to detect sexual abuse, and some girls are born without hymens.
Although hymens come in a wide variety of shapes and sizes, a study of more than 1,100 newborn girls showed that all of them had one, she noted. Reviews of cases of sexual abuse show that physical exam findings of pediatric sexual abuse are rare because the tissue is very elastic and heals quickly.
Physical evidence will be more likely if force was used, if the child resisted, if there are great differences in the sizes and ages of the perpetrator and victim, and if a foreign object was forced into the mouth, vagina, or anus. Bruising or bite marks on a child’s penis may suggest nonaccidental trauma from forced toilet training.
When you see visible clues to what may be abuse, photograph or draw what you see and include something in the image to show size or scale. Don’t just rely on written notes, she said.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF EMERGENCY PHYSICIANS
The Child With Short Stature
Growth is a terrific biomarker for general health, and a slowing of growth may be a sign of underlying disease. So which children deserve an evaluation?
Short stature is defined as growth below the third percentile. In addition to these children, a child who is crossing one percentile line on the growth chart also deserves evaluation. The sole exception is an otherwise healthy child developing well who may, in the second year of life, adjust to genetics (for example, a big baby born to short parents).
The key is to identify the short child by monitoring the growth pattern, evaluating him to find a specific diagnosis, and then targeting the clinical intervention.
Consistent measurement of a child’s height at every health care encounter is the most important strategy to identify a child with short stature. Some children do not go for regular well-child visits once they have most of their immunizations completed and may show up for sick visits only. In many cases, only weight but not height is measured during these acute care visits. For example, in my pediatric endocrinology practice, it is not unusual to see children who are 12 years old without a height measurement for the previous 7 years because the family did not present to the primary care physician for well care.
The benefits of these routine measurements go beyond identification of short stature. Any child with poor growth needs to be evaluated by a specialist who can go through an extensive differential diagnosis.
Helpful guidelines include the 2009 "Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children" from clinicians at Blanks Children’s Hospital in Des Moines, Iowa, and "Development of an Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children"’ (J. Pediatr. Nursing 2011;26:312-24).
Sometimes I hear families or primary care physicians say, "Let’s just wait and see." It is advisable to see a child back in 6 months to monitor growth velocity, but watching poor linear growth year after year will not optimize the height outcome. The problem with later intervention is that the older child with short stature does not have enough "catch up" time. Therefore, additional evaluation is warranted if you diagnose short stature and you remain concerned after 6 months.
For a child who warrants this additional evaluation, a bone age x-ray is helpful (although not diagnostic of a specific condition). Other recommended studies include a complete blood count; chemistry panel; free thyroxine (free T4) with thyroid stimulating hormone (TSH); insulinlike growth factor 1 (IGF-1), C-reactive protein, urinalysis, and a celiac panel (IgG and IgA class of anti–tissue transglutaminase [anti-tTG]; antiendomysial antibodies, IgA class [EMA-IgA]; and quantitative IgA). In addition, for girls, a karyotype can rule out Turner’s syndrome.
Obtaining the correct test can sometimes be a problem. For example, IGF-1 is similar to many other test names on a laboratory test list. The odds of a lab technician performing the right test are low, because on their alphabetical test list, IGF BP 1 appears at the top (and this test is not useful at all!). This pitfall can be avoided by including the lab specific test code for IGF-1, which your local pediatric endocrinologist can help you find.
Other testing may be warranted, based on history and physical findings. For example, if a child has a history of pneumonia and frequent sinusitis, I would order a sweat chloride test to rule out cystic fibrosis.
If there is no clear explanation, and the slowed growth does not respond to your intervention, refer the patient to a specialist.
The growth chart will help guide the type of referral. If linear growth is poor and weight gain is appropriate (that is, their body mass index is normal), consider referral to a pediatric endocrinologist.
If linear growth is poor, but weight gain is more strikingly affected (that is, BMI is low for age), consider referral instead to a pediatric gastroenterologist.
If testing reveals electrolyte abnormalities, consider referral to pediatric nephrology.
If the child has congenital anomalies or a developmental delay in addition to short stature, then referral to a geneticist becomes appropriate.
Once a short stature diagnosis is established, a targeted approach to optimization of growth can be planned. Human growth hormone therapy, for example, typically is ordered by a pediatric endocrinologist for a number of diagnoses. Indications include growth hormone deficiency, Turner’s syndrome, Noonan’s syndrome, Prader-Willi syndrome, and children born small for gestational age who fail to catch up. A pediatric nephrologist also might prescribe this therapy for a child with renal failure who is not growing.
Dr. Counts is an associate professor of pediatrics and chief of the division of pediatric endocrinology at the University of Maryland, Baltimore. She works on multiple research studies with funding to the University of Maryland, Baltimore, from Eli Lilly, Pfizer, and Novo Nordisk.
Growth is a terrific biomarker for general health, and a slowing of growth may be a sign of underlying disease. So which children deserve an evaluation?
Short stature is defined as growth below the third percentile. In addition to these children, a child who is crossing one percentile line on the growth chart also deserves evaluation. The sole exception is an otherwise healthy child developing well who may, in the second year of life, adjust to genetics (for example, a big baby born to short parents).
The key is to identify the short child by monitoring the growth pattern, evaluating him to find a specific diagnosis, and then targeting the clinical intervention.
Consistent measurement of a child’s height at every health care encounter is the most important strategy to identify a child with short stature. Some children do not go for regular well-child visits once they have most of their immunizations completed and may show up for sick visits only. In many cases, only weight but not height is measured during these acute care visits. For example, in my pediatric endocrinology practice, it is not unusual to see children who are 12 years old without a height measurement for the previous 7 years because the family did not present to the primary care physician for well care.
The benefits of these routine measurements go beyond identification of short stature. Any child with poor growth needs to be evaluated by a specialist who can go through an extensive differential diagnosis.
Helpful guidelines include the 2009 "Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children" from clinicians at Blanks Children’s Hospital in Des Moines, Iowa, and "Development of an Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children"’ (J. Pediatr. Nursing 2011;26:312-24).
Sometimes I hear families or primary care physicians say, "Let’s just wait and see." It is advisable to see a child back in 6 months to monitor growth velocity, but watching poor linear growth year after year will not optimize the height outcome. The problem with later intervention is that the older child with short stature does not have enough "catch up" time. Therefore, additional evaluation is warranted if you diagnose short stature and you remain concerned after 6 months.
For a child who warrants this additional evaluation, a bone age x-ray is helpful (although not diagnostic of a specific condition). Other recommended studies include a complete blood count; chemistry panel; free thyroxine (free T4) with thyroid stimulating hormone (TSH); insulinlike growth factor 1 (IGF-1), C-reactive protein, urinalysis, and a celiac panel (IgG and IgA class of anti–tissue transglutaminase [anti-tTG]; antiendomysial antibodies, IgA class [EMA-IgA]; and quantitative IgA). In addition, for girls, a karyotype can rule out Turner’s syndrome.
Obtaining the correct test can sometimes be a problem. For example, IGF-1 is similar to many other test names on a laboratory test list. The odds of a lab technician performing the right test are low, because on their alphabetical test list, IGF BP 1 appears at the top (and this test is not useful at all!). This pitfall can be avoided by including the lab specific test code for IGF-1, which your local pediatric endocrinologist can help you find.
Other testing may be warranted, based on history and physical findings. For example, if a child has a history of pneumonia and frequent sinusitis, I would order a sweat chloride test to rule out cystic fibrosis.
If there is no clear explanation, and the slowed growth does not respond to your intervention, refer the patient to a specialist.
The growth chart will help guide the type of referral. If linear growth is poor and weight gain is appropriate (that is, their body mass index is normal), consider referral to a pediatric endocrinologist.
If linear growth is poor, but weight gain is more strikingly affected (that is, BMI is low for age), consider referral instead to a pediatric gastroenterologist.
If testing reveals electrolyte abnormalities, consider referral to pediatric nephrology.
If the child has congenital anomalies or a developmental delay in addition to short stature, then referral to a geneticist becomes appropriate.
Once a short stature diagnosis is established, a targeted approach to optimization of growth can be planned. Human growth hormone therapy, for example, typically is ordered by a pediatric endocrinologist for a number of diagnoses. Indications include growth hormone deficiency, Turner’s syndrome, Noonan’s syndrome, Prader-Willi syndrome, and children born small for gestational age who fail to catch up. A pediatric nephrologist also might prescribe this therapy for a child with renal failure who is not growing.
Dr. Counts is an associate professor of pediatrics and chief of the division of pediatric endocrinology at the University of Maryland, Baltimore. She works on multiple research studies with funding to the University of Maryland, Baltimore, from Eli Lilly, Pfizer, and Novo Nordisk.
Growth is a terrific biomarker for general health, and a slowing of growth may be a sign of underlying disease. So which children deserve an evaluation?
Short stature is defined as growth below the third percentile. In addition to these children, a child who is crossing one percentile line on the growth chart also deserves evaluation. The sole exception is an otherwise healthy child developing well who may, in the second year of life, adjust to genetics (for example, a big baby born to short parents).
The key is to identify the short child by monitoring the growth pattern, evaluating him to find a specific diagnosis, and then targeting the clinical intervention.
Consistent measurement of a child’s height at every health care encounter is the most important strategy to identify a child with short stature. Some children do not go for regular well-child visits once they have most of their immunizations completed and may show up for sick visits only. In many cases, only weight but not height is measured during these acute care visits. For example, in my pediatric endocrinology practice, it is not unusual to see children who are 12 years old without a height measurement for the previous 7 years because the family did not present to the primary care physician for well care.
The benefits of these routine measurements go beyond identification of short stature. Any child with poor growth needs to be evaluated by a specialist who can go through an extensive differential diagnosis.
Helpful guidelines include the 2009 "Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children" from clinicians at Blanks Children’s Hospital in Des Moines, Iowa, and "Development of an Evidence-Based Clinical Practice Guideline on Linear Growth Measurement of Children"’ (J. Pediatr. Nursing 2011;26:312-24).
Sometimes I hear families or primary care physicians say, "Let’s just wait and see." It is advisable to see a child back in 6 months to monitor growth velocity, but watching poor linear growth year after year will not optimize the height outcome. The problem with later intervention is that the older child with short stature does not have enough "catch up" time. Therefore, additional evaluation is warranted if you diagnose short stature and you remain concerned after 6 months.
For a child who warrants this additional evaluation, a bone age x-ray is helpful (although not diagnostic of a specific condition). Other recommended studies include a complete blood count; chemistry panel; free thyroxine (free T4) with thyroid stimulating hormone (TSH); insulinlike growth factor 1 (IGF-1), C-reactive protein, urinalysis, and a celiac panel (IgG and IgA class of anti–tissue transglutaminase [anti-tTG]; antiendomysial antibodies, IgA class [EMA-IgA]; and quantitative IgA). In addition, for girls, a karyotype can rule out Turner’s syndrome.
Obtaining the correct test can sometimes be a problem. For example, IGF-1 is similar to many other test names on a laboratory test list. The odds of a lab technician performing the right test are low, because on their alphabetical test list, IGF BP 1 appears at the top (and this test is not useful at all!). This pitfall can be avoided by including the lab specific test code for IGF-1, which your local pediatric endocrinologist can help you find.
Other testing may be warranted, based on history and physical findings. For example, if a child has a history of pneumonia and frequent sinusitis, I would order a sweat chloride test to rule out cystic fibrosis.
If there is no clear explanation, and the slowed growth does not respond to your intervention, refer the patient to a specialist.
The growth chart will help guide the type of referral. If linear growth is poor and weight gain is appropriate (that is, their body mass index is normal), consider referral to a pediatric endocrinologist.
If linear growth is poor, but weight gain is more strikingly affected (that is, BMI is low for age), consider referral instead to a pediatric gastroenterologist.
If testing reveals electrolyte abnormalities, consider referral to pediatric nephrology.
If the child has congenital anomalies or a developmental delay in addition to short stature, then referral to a geneticist becomes appropriate.
Once a short stature diagnosis is established, a targeted approach to optimization of growth can be planned. Human growth hormone therapy, for example, typically is ordered by a pediatric endocrinologist for a number of diagnoses. Indications include growth hormone deficiency, Turner’s syndrome, Noonan’s syndrome, Prader-Willi syndrome, and children born small for gestational age who fail to catch up. A pediatric nephrologist also might prescribe this therapy for a child with renal failure who is not growing.
Dr. Counts is an associate professor of pediatrics and chief of the division of pediatric endocrinology at the University of Maryland, Baltimore. She works on multiple research studies with funding to the University of Maryland, Baltimore, from Eli Lilly, Pfizer, and Novo Nordisk.
Fear Not the Switch from ICD-9 to ICD-10
Some people adopt a “Chicken Little” mentality when faced with making big changes, says Kathy DeVault, RHIS, CCS, CCS-P, manager of professional practice resources for the American Health Information Management Association (AHIMA). The change she’s referring to is the switch from the current version of the International Statistical Classification of Diseases coding system (ICD-9-CM) to the ICD-10-CM/ICD-10-PCS, which must be effective in hospitals by Oct. 1, 2013.
Hospitalist Jeffrey Farber, MD, assistant professor of geriatrics and palliative medicine and director of the Mobile ACE Service at Mount Sinai Hospital in New York City, also is director of the Clinical Documentation Improvement Department at Mount Sinai. He already is intimately involved with his hospital’s ICD-10 implementation process.
“For hospitals, this is a very big deal,” Dr. Farber says, “because it affects not just the coding department, but quality, compliance, and public reporting. On the physician side, there will be major changes in clinical documentation. Hospitalists who also do procedures, even bedside procedures, need to understand what is required.”
Why the Change?
Surprisingly, the U.S. is the last industrialized country in the world to upgrade to the ICD-10 system. The older system, in use since 1979, does not reflect three decades of change in medicine. “ICD-10 allows for a much better capture of specific types of treated diagnoses, provided services, and performed procedures,” Dr. Farber says, “and allows a lot more room to grow for the future.”
At first glance, the sheer numbers of new codes appear daunting. For example, procedures codes will increase from the current 4,000 to approximately 87,000. Hospitalists who perform procedures must include more description in their notes, including devices used and anatomical location of device placement.
Even if you’re not doing procedures, you may not relish the prospect of going from the current 14,000 ICD-9-CM diagnoses codes to nearly 70,000 ICD-10 codes. But, Dr. Farber explains, many of the increased descriptors have to do with laterality, which previously was not captured. To note a diagnosis of stroke, you will have to write not only whether it occurred in the posterior cerebral blood vessel, but also whether it was right or left posterior cerebral.
Ultimately, he believes, this type of specificity will relieve a burden on hospitalists, because providing more specific documentation should reduce queries from coders.
Common-Sense Approach
The October 2013 deadline allows plenty of time for physician training, says DeVault, who has been training coders through AHIMA’s ICD-10 Academy the past two years. Breaking the process down into manageable steps is helpful, she says.
—Jeffrey Farber, MD, assistant professor, geriatrics and palliative medicine, director, Clinical Documentation Improvement Department, Mount Sinai Hospital, New York City
“Look at your group’s most common, acute conditions, for example, and ask, ‘What is missing in the documentation?’ Especially if you can make bridges with your health information management (HIM) department, you will find that there are many opportunities to teach each other,” she says.
Hospitalists can do several things to ready their group for ICD-10, Dr. Farber says. Take a proactive stance, he advises, and select your group’s top 25 diagnoses. Then work with coding staff to map them from ICD-9 to ICD-10. On a macro level, understand what your hospital’s timeline is for the change. DeVault says that HIM departments are eager to collaborate with physician champions.
The good news: The sky isn’t really falling, according to DeVault. And the change to ICD-10 actually offers lots of opportunities for collaborations between hospitalists and health information departments.
Gretchen Henkel is a freelance writer based in California.
Watch Out for GEMs
Physicians often are encouraged to use general equivalence maps (GEMs) to acquaint themselves with the differences between coding sets. Relying solely on GEMs, however, is not a good idea for the long term, DeVault cautions. “GEMs are meant to serve as a transition tool but are not designed to code from,” she says. “It’s imperative that coders—and providers—actually learn the new system and that they not rely on GEMs for coding.”—GH
Resources for Physicians
- The AHIMA ICD-10 website offers a timeline for assessment and implementation of ICD-10 under the “Physician Office Role-Based Model” heading.
- The AMA’s resources include a physician timeline for ICD-10 implementation (PDF).
- SHM’s website offers a wealth of resources on documentation billing and coding for hospitalists.
Some people adopt a “Chicken Little” mentality when faced with making big changes, says Kathy DeVault, RHIS, CCS, CCS-P, manager of professional practice resources for the American Health Information Management Association (AHIMA). The change she’s referring to is the switch from the current version of the International Statistical Classification of Diseases coding system (ICD-9-CM) to the ICD-10-CM/ICD-10-PCS, which must be effective in hospitals by Oct. 1, 2013.
Hospitalist Jeffrey Farber, MD, assistant professor of geriatrics and palliative medicine and director of the Mobile ACE Service at Mount Sinai Hospital in New York City, also is director of the Clinical Documentation Improvement Department at Mount Sinai. He already is intimately involved with his hospital’s ICD-10 implementation process.
“For hospitals, this is a very big deal,” Dr. Farber says, “because it affects not just the coding department, but quality, compliance, and public reporting. On the physician side, there will be major changes in clinical documentation. Hospitalists who also do procedures, even bedside procedures, need to understand what is required.”
Why the Change?
Surprisingly, the U.S. is the last industrialized country in the world to upgrade to the ICD-10 system. The older system, in use since 1979, does not reflect three decades of change in medicine. “ICD-10 allows for a much better capture of specific types of treated diagnoses, provided services, and performed procedures,” Dr. Farber says, “and allows a lot more room to grow for the future.”
At first glance, the sheer numbers of new codes appear daunting. For example, procedures codes will increase from the current 4,000 to approximately 87,000. Hospitalists who perform procedures must include more description in their notes, including devices used and anatomical location of device placement.
Even if you’re not doing procedures, you may not relish the prospect of going from the current 14,000 ICD-9-CM diagnoses codes to nearly 70,000 ICD-10 codes. But, Dr. Farber explains, many of the increased descriptors have to do with laterality, which previously was not captured. To note a diagnosis of stroke, you will have to write not only whether it occurred in the posterior cerebral blood vessel, but also whether it was right or left posterior cerebral.
Ultimately, he believes, this type of specificity will relieve a burden on hospitalists, because providing more specific documentation should reduce queries from coders.
Common-Sense Approach
The October 2013 deadline allows plenty of time for physician training, says DeVault, who has been training coders through AHIMA’s ICD-10 Academy the past two years. Breaking the process down into manageable steps is helpful, she says.
—Jeffrey Farber, MD, assistant professor, geriatrics and palliative medicine, director, Clinical Documentation Improvement Department, Mount Sinai Hospital, New York City
“Look at your group’s most common, acute conditions, for example, and ask, ‘What is missing in the documentation?’ Especially if you can make bridges with your health information management (HIM) department, you will find that there are many opportunities to teach each other,” she says.
Hospitalists can do several things to ready their group for ICD-10, Dr. Farber says. Take a proactive stance, he advises, and select your group’s top 25 diagnoses. Then work with coding staff to map them from ICD-9 to ICD-10. On a macro level, understand what your hospital’s timeline is for the change. DeVault says that HIM departments are eager to collaborate with physician champions.
The good news: The sky isn’t really falling, according to DeVault. And the change to ICD-10 actually offers lots of opportunities for collaborations between hospitalists and health information departments.
Gretchen Henkel is a freelance writer based in California.
Watch Out for GEMs
Physicians often are encouraged to use general equivalence maps (GEMs) to acquaint themselves with the differences between coding sets. Relying solely on GEMs, however, is not a good idea for the long term, DeVault cautions. “GEMs are meant to serve as a transition tool but are not designed to code from,” she says. “It’s imperative that coders—and providers—actually learn the new system and that they not rely on GEMs for coding.”—GH
Resources for Physicians
- The AHIMA ICD-10 website offers a timeline for assessment and implementation of ICD-10 under the “Physician Office Role-Based Model” heading.
- The AMA’s resources include a physician timeline for ICD-10 implementation (PDF).
- SHM’s website offers a wealth of resources on documentation billing and coding for hospitalists.
Some people adopt a “Chicken Little” mentality when faced with making big changes, says Kathy DeVault, RHIS, CCS, CCS-P, manager of professional practice resources for the American Health Information Management Association (AHIMA). The change she’s referring to is the switch from the current version of the International Statistical Classification of Diseases coding system (ICD-9-CM) to the ICD-10-CM/ICD-10-PCS, which must be effective in hospitals by Oct. 1, 2013.
Hospitalist Jeffrey Farber, MD, assistant professor of geriatrics and palliative medicine and director of the Mobile ACE Service at Mount Sinai Hospital in New York City, also is director of the Clinical Documentation Improvement Department at Mount Sinai. He already is intimately involved with his hospital’s ICD-10 implementation process.
“For hospitals, this is a very big deal,” Dr. Farber says, “because it affects not just the coding department, but quality, compliance, and public reporting. On the physician side, there will be major changes in clinical documentation. Hospitalists who also do procedures, even bedside procedures, need to understand what is required.”
Why the Change?
Surprisingly, the U.S. is the last industrialized country in the world to upgrade to the ICD-10 system. The older system, in use since 1979, does not reflect three decades of change in medicine. “ICD-10 allows for a much better capture of specific types of treated diagnoses, provided services, and performed procedures,” Dr. Farber says, “and allows a lot more room to grow for the future.”
At first glance, the sheer numbers of new codes appear daunting. For example, procedures codes will increase from the current 4,000 to approximately 87,000. Hospitalists who perform procedures must include more description in their notes, including devices used and anatomical location of device placement.
Even if you’re not doing procedures, you may not relish the prospect of going from the current 14,000 ICD-9-CM diagnoses codes to nearly 70,000 ICD-10 codes. But, Dr. Farber explains, many of the increased descriptors have to do with laterality, which previously was not captured. To note a diagnosis of stroke, you will have to write not only whether it occurred in the posterior cerebral blood vessel, but also whether it was right or left posterior cerebral.
Ultimately, he believes, this type of specificity will relieve a burden on hospitalists, because providing more specific documentation should reduce queries from coders.
Common-Sense Approach
The October 2013 deadline allows plenty of time for physician training, says DeVault, who has been training coders through AHIMA’s ICD-10 Academy the past two years. Breaking the process down into manageable steps is helpful, she says.
—Jeffrey Farber, MD, assistant professor, geriatrics and palliative medicine, director, Clinical Documentation Improvement Department, Mount Sinai Hospital, New York City
“Look at your group’s most common, acute conditions, for example, and ask, ‘What is missing in the documentation?’ Especially if you can make bridges with your health information management (HIM) department, you will find that there are many opportunities to teach each other,” she says.
Hospitalists can do several things to ready their group for ICD-10, Dr. Farber says. Take a proactive stance, he advises, and select your group’s top 25 diagnoses. Then work with coding staff to map them from ICD-9 to ICD-10. On a macro level, understand what your hospital’s timeline is for the change. DeVault says that HIM departments are eager to collaborate with physician champions.
The good news: The sky isn’t really falling, according to DeVault. And the change to ICD-10 actually offers lots of opportunities for collaborations between hospitalists and health information departments.
Gretchen Henkel is a freelance writer based in California.
Watch Out for GEMs
Physicians often are encouraged to use general equivalence maps (GEMs) to acquaint themselves with the differences between coding sets. Relying solely on GEMs, however, is not a good idea for the long term, DeVault cautions. “GEMs are meant to serve as a transition tool but are not designed to code from,” she says. “It’s imperative that coders—and providers—actually learn the new system and that they not rely on GEMs for coding.”—GH
Resources for Physicians
- The AHIMA ICD-10 website offers a timeline for assessment and implementation of ICD-10 under the “Physician Office Role-Based Model” heading.
- The AMA’s resources include a physician timeline for ICD-10 implementation (PDF).
- SHM’s website offers a wealth of resources on documentation billing and coding for hospitalists.
Vaccine candidate reduces malaria risk

Credit: St Jude Children’s
Research Hospital
First results from a phase 3 trial of the vaccine candidate RTS,S/AS01 indicate it provides young African children with protection against clinical and severe malaria.
The researchers also said RTS,S/AS01 has an acceptable safety and tolerability profile.
These results were announced October 19 at the Malaria Forum, hosted by the Bill & Melinda Gates Foundation in Seattle, Washington.
The findings were also published online in The New England Journal of Medicine.
“The publication of the first results in children aged 5 to 17 months marks an important milestone in the development of RTS,S/AS01,” said Irving Hoffman, PA, MPH, co-principal investigator at a study site in Lilongwe, Malawi.
The trial is still ongoing, being conducted at 11 sites in 7 countries across sub-Saharan Africa. But the researchers have performed an initial analysis of results in the first 6000 children enrolled, who were aged 5 months to 17 months at the time of enrollment.
The children received 3 doses of RTS,S/AS01 and were followed for a 12-month period. RTS,S/AS01 reduced the risk of clinical malaria in these children by 56% and the risk of severe malaria by 47%.
“These results confirm findings from previous phase 2 studies and support ongoing efforts to advance the development of this malaria vaccine candidate,” Hoffman said.
Efficacy and safety results in 6- to 12-week-old infants are expected by the end of 2012, according to the investigators. However, they have performed an analysis of severe malaria episodes reported thus far in all 15,460 children enrolled in the trial, ranging from 6 weeks to 17 months of age.
The analysis showed that RTS,S/AS01 had 35% efficacy over a follow-up period ranging between 0 months and 22 months (average, 11.5 months). Further information about the longer-term effects of RTS,S/AS01—30 months after the third dose—should be available by the end of 2014, the researchers said.
The overall incidence of serious adverse events in this trial was comparable between RTS,S/AS01 recipients (18%) and those receiving a control vaccine (22%)
There were differences in the rates of certain serious adverse events between the vaccine groups. Seizures and meningitis were both more frequent in the RTS,S/AS01 group. Seizures were linked to fever, and meningitis was considered unlikely to be vaccine-related.
RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative, together with African research centers. The partners are all represented on the Clinical Trials Partnership Committee, which is responsible for the conduct of the trial.
Major funding for clinical development comes from a grant by the Bill & Melinda Gates Foundation. ![]()

Credit: St Jude Children’s
Research Hospital
First results from a phase 3 trial of the vaccine candidate RTS,S/AS01 indicate it provides young African children with protection against clinical and severe malaria.
The researchers also said RTS,S/AS01 has an acceptable safety and tolerability profile.
These results were announced October 19 at the Malaria Forum, hosted by the Bill & Melinda Gates Foundation in Seattle, Washington.
The findings were also published online in The New England Journal of Medicine.
“The publication of the first results in children aged 5 to 17 months marks an important milestone in the development of RTS,S/AS01,” said Irving Hoffman, PA, MPH, co-principal investigator at a study site in Lilongwe, Malawi.
The trial is still ongoing, being conducted at 11 sites in 7 countries across sub-Saharan Africa. But the researchers have performed an initial analysis of results in the first 6000 children enrolled, who were aged 5 months to 17 months at the time of enrollment.
The children received 3 doses of RTS,S/AS01 and were followed for a 12-month period. RTS,S/AS01 reduced the risk of clinical malaria in these children by 56% and the risk of severe malaria by 47%.
“These results confirm findings from previous phase 2 studies and support ongoing efforts to advance the development of this malaria vaccine candidate,” Hoffman said.
Efficacy and safety results in 6- to 12-week-old infants are expected by the end of 2012, according to the investigators. However, they have performed an analysis of severe malaria episodes reported thus far in all 15,460 children enrolled in the trial, ranging from 6 weeks to 17 months of age.
The analysis showed that RTS,S/AS01 had 35% efficacy over a follow-up period ranging between 0 months and 22 months (average, 11.5 months). Further information about the longer-term effects of RTS,S/AS01—30 months after the third dose—should be available by the end of 2014, the researchers said.
The overall incidence of serious adverse events in this trial was comparable between RTS,S/AS01 recipients (18%) and those receiving a control vaccine (22%)
There were differences in the rates of certain serious adverse events between the vaccine groups. Seizures and meningitis were both more frequent in the RTS,S/AS01 group. Seizures were linked to fever, and meningitis was considered unlikely to be vaccine-related.
RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative, together with African research centers. The partners are all represented on the Clinical Trials Partnership Committee, which is responsible for the conduct of the trial.
Major funding for clinical development comes from a grant by the Bill & Melinda Gates Foundation. ![]()

Credit: St Jude Children’s
Research Hospital
First results from a phase 3 trial of the vaccine candidate RTS,S/AS01 indicate it provides young African children with protection against clinical and severe malaria.
The researchers also said RTS,S/AS01 has an acceptable safety and tolerability profile.
These results were announced October 19 at the Malaria Forum, hosted by the Bill & Melinda Gates Foundation in Seattle, Washington.
The findings were also published online in The New England Journal of Medicine.
“The publication of the first results in children aged 5 to 17 months marks an important milestone in the development of RTS,S/AS01,” said Irving Hoffman, PA, MPH, co-principal investigator at a study site in Lilongwe, Malawi.
The trial is still ongoing, being conducted at 11 sites in 7 countries across sub-Saharan Africa. But the researchers have performed an initial analysis of results in the first 6000 children enrolled, who were aged 5 months to 17 months at the time of enrollment.
The children received 3 doses of RTS,S/AS01 and were followed for a 12-month period. RTS,S/AS01 reduced the risk of clinical malaria in these children by 56% and the risk of severe malaria by 47%.
“These results confirm findings from previous phase 2 studies and support ongoing efforts to advance the development of this malaria vaccine candidate,” Hoffman said.
Efficacy and safety results in 6- to 12-week-old infants are expected by the end of 2012, according to the investigators. However, they have performed an analysis of severe malaria episodes reported thus far in all 15,460 children enrolled in the trial, ranging from 6 weeks to 17 months of age.
The analysis showed that RTS,S/AS01 had 35% efficacy over a follow-up period ranging between 0 months and 22 months (average, 11.5 months). Further information about the longer-term effects of RTS,S/AS01—30 months after the third dose—should be available by the end of 2014, the researchers said.
The overall incidence of serious adverse events in this trial was comparable between RTS,S/AS01 recipients (18%) and those receiving a control vaccine (22%)
There were differences in the rates of certain serious adverse events between the vaccine groups. Seizures and meningitis were both more frequent in the RTS,S/AS01 group. Seizures were linked to fever, and meningitis was considered unlikely to be vaccine-related.
RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative, together with African research centers. The partners are all represented on the Clinical Trials Partnership Committee, which is responsible for the conduct of the trial.
Major funding for clinical development comes from a grant by the Bill & Melinda Gates Foundation. ![]()