User login
Cost‐Related Medication Underuse
The affordability of prescription medications continues to be one of the most pressing public health issues in the United States. Many patients reduce their prescribed doses to make medications last longer or do not fill prescriptions because of cost.1 Cost‐related medication underuse affects patients with and without drug insurance coverage,2 and is likely to become even more problematic as employers scale back on drug benefits3 and drug prices continue to increase.4 The landmark Patient Protection and Affordability Act passed in March 2010 does little to address this issue.5
Existing estimates of cost‐related medication underuse come largely from surveys of ambulatory patients. For example, using data from the Medicare Current Beneficiary Survey, Maden et al. estimated that 11% to 15% of patients reduced medication use in the past year because of cost.6 Tseng and colleagues found very similar rates of cost‐related underuse in managed care beneficiaries with diabetes.7
Hospitalized patients, who have a high burden of disease and tend to use more medications than their ambulatory counterparts, may be particularly vulnerable to cost‐related underuse but, thus far, have been subject to little investigation. New medications, which are frequently prescribed at the time of discharge, may exacerbate these issues further and contribute to preventable readmissions. Accordingly, we surveyed a cohort of medical inpatients at a large academic medical center to estimate the prevalence and predictors of cost‐related medication underuse for hospitalized managed care patients, and to identify strategies that patients perceive as helpful to make medications more affordable.
METHODS
Study Sample
We identified consecutive patients newly admitted to the general medicine, cardiology, or oncology services at Brigham and Women's Hospital from November 2008 to December 2009. For our survey, we included only those patients who received medical benefits through 1 of 3 large insurers with whom our hospital has pay‐for‐performance contracts. Annually, there are approximately 4000 patients covered by these insurers admitted to the 3 clinical services we evaluated, We focused on patients who had a primary care physician at one of the hospital's outpatient practices because of the existence of an automated infrastructure to identify these managed care beneficiaries of these insurers who are newly hospitalized, and because patients covered by commercial insurance plans likely represent a conservative lower‐bound of cost‐related medication underuse among hospitalized patients.
Patients were surveyed on the first non‐holiday weekday after admission. We excluded patients who had been discharged prior to the daily admission list being generated, or who, on a previous admission, had completed our survey or declined to be surveyed. We also excluded several patients who were not beneficiaries of the target insurers and were erroneously included on the managed care admission roster.
Potentially eligible patients were approached on the hospital ward by 1 of 3 study care coordinators (2 nurses and 1 pharmacist) and were asked if they were willing to participate in a research project about medication use that involved a short verbally delivered in‐person (inpatient) survey, a brief postdischarge telephone call, and a review of their electronic health record. The Institutional Review Board of Brigham and Women's Hospital approved this study.
Inpatient Survey
Our survey instrument was developed iteratively and pilot‐tested to improve face validity. Questions about cost‐related underuse were based on validated measures.8, 9 Specifically, we asked whether in the past year patients had: (1) not filled a prescription because it was too expensive, (2) skipped doses to make medicines last longer, (3) took less medicine than prescribed to make the medicine last longer, or (4) split pills to make the medication last longer.
Questions about strategies to improve medication affordability assessed whether patients thought it would be helpful to: (1) discuss medication affordability with healthcare workers (inpatient doctors, outpatient doctors, nurses, pharmacists, or social workers); (2) have their medications reviewed by a nurse or pharmacist; (3) receive information about lower cost but equally effective medication options, or about programs that provide medications at reduced costs; and/or (4) have their copayments/coinsurance lowered. Possible responses to all of these questions were binary, ie, yes or no.
In addition, patients were asked about the nature of their drug insurance coverage, the prescription medications that they currently use, whether they know their copayment levels (for generic and brand‐name medications), and, if so, what these amounts were, their annual household income, and their self‐identified race. Information on patient age, gender, and the primary reason for hospitalization was obtained from the electronic health record. This source was also used to verify the accuracy of the self‐reported preadmission medication list. When there were discrepancies between preadmission medications reported by patients and those recorded in their chart, the later was used because our hospital reconciles and records all medications at the time of hospital admission for all patients.
Postdischarge Survey
Within 3 days of discharge, patients were contacted by telephone and asked about new medications they were prescribed on discharge, if any. The discharge summary was used to verify the accuracy of the information provided by patients. The interviewers clarified any apparent discrepancies between the 2 sources of information with the patient. Patients who had been prescribed a new medication were asked whether or not they had filled their prescription. For patients who had, we asked whether: (1) they knew how much they would have to pay prior to going to the pharmacy, (2) they had discussed less expensive options with their pharmacist, and (3) they had discussed medication costs with their inpatient or outpatient physicians.
Data Analysis
We used descriptive statistics to summarize the characteristics of our respondents and our overall survey results. We generated univariate and multivariable logistic regression models to identify whether prehospitalization cost‐related medication underuse was influenced by patient age, gender, income, race, and the number of medications patients used on a regular basis. For the purpose of these analyses, we classified patients as reporting cost‐related underuse if they responded yes to any of the 4 strategies described above (ie, not filling medications, skipping doses, taking less medication, or splitting pills to make medicines last longer). Patients whose incomes were above the median level in our cohort were categorized as being of high‐income. Our multivariable model had a c‐statistic of 0.75, suggesting good discriminative ability.
RESULTS
During the study period, 483 potentially‐eligible patients were admitted to the general medicine, cardiology, and oncology services. We excluded 167 because they had been discharged prior to being identified, had been surveyed or already declined participation on a prior admission, or were not managed care enrollees (see Appendix A). Of the remaining 316 subjects, 130 participated in the inpatient survey (response rate = 41%); 93 (75%) of these patients were reached by telephone after hospital discharge and completed the postdischarge survey. The baseline characteristics of our respondents are presented in Table 1. Patients had a mean age of 52 years, were 50% male and two‐thirds of white race, represented a range of household incomes, and almost all had employer‐sponsored prescription coverage. Prior to admission, patients took an average of 5 prescription medications and paid an average copayment of $10.80 and $21.60 for each generic and brand‐name prescription, respectively.
| Characteristic | N = 130 |
|---|---|
| |
| Age, mean years (SD) | 52 (11.2) |
| Male, % | 65 (50.0) |
| Race/ethnicity,* n (%) | |
| Caucasian/white | 84 (67.2) |
| Black/African American | 20 (16.0) |
| Latino/Hispanic | 13 (10.4) |
| Asian | 3 (2.4) |
| American Indian or Alaska Native | 1 (0.8) |
| Other | 4 (3.2) |
| Annual household income,* n (%) | |
| <$30,000 | 15 (12.8) |
| $30,000‐$75,000 | 49 (41.9) |
| >$75,000 | 53 (45.3) |
| Insurance coverage for outpatient prescription drugs,* n (%) | |
| Employer or spouse's employer | 123 (96.0) |
| Independent | 5 (3.9) |
| Medication copayments,* mean $ (SD) | |
| Brand‐name medications | 21.6 (14.2) |
| Generic medications | 10.8 (6.0) |
| No. of medications prior to admission, mean (SD) | 5.5 (4.3) |
| Category of discharge diagnosis, n (%) | |
| Cardiovascular | 40 (30.8) |
| Gastrointestinal | 23 (17.7) |
| Pulmonary | 23 (17.7) |
| Infectious | 13 (10.0) |
| Oncology | 5 (3.8) |
| Renal | 6 (4.6) |
| Psychiatric | 3 (2.3) |
| Hematologic | 4 (3.1) |
| Neurologic | 5 (3.8) |
| Musculoskeletal | 5 (3.8) |
| Respiratory | 2 (1.5) |
| Endocrine | 1 (0.8) |
Cost‐Related Medication Underuse
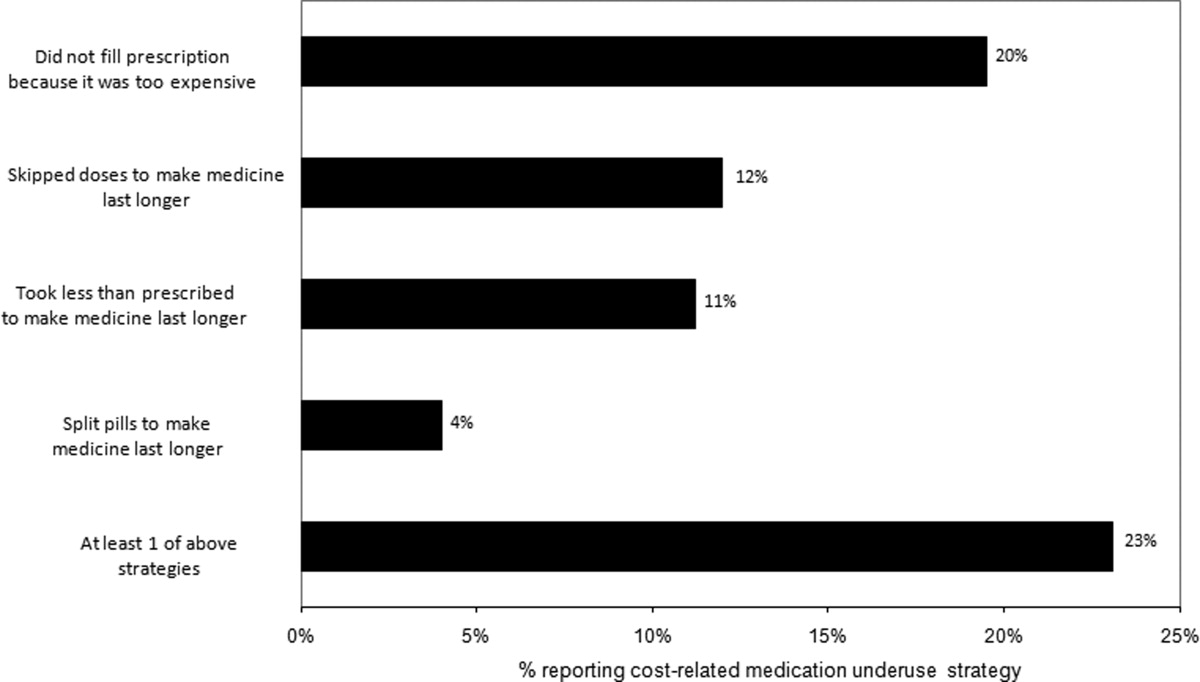
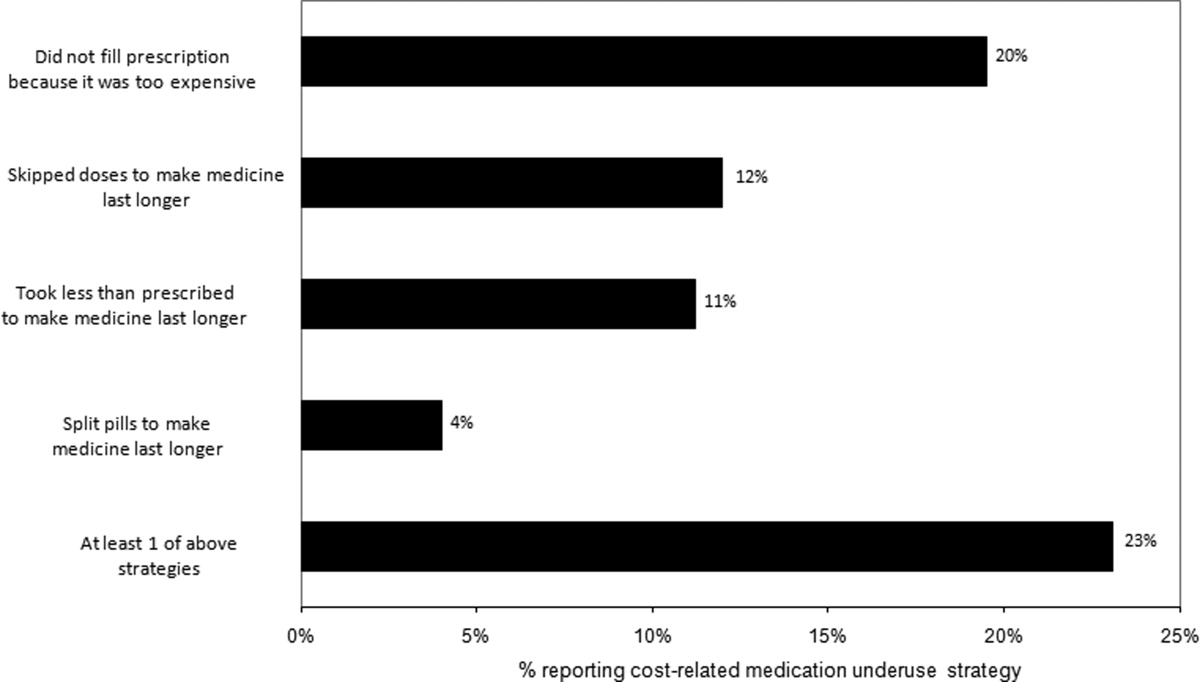
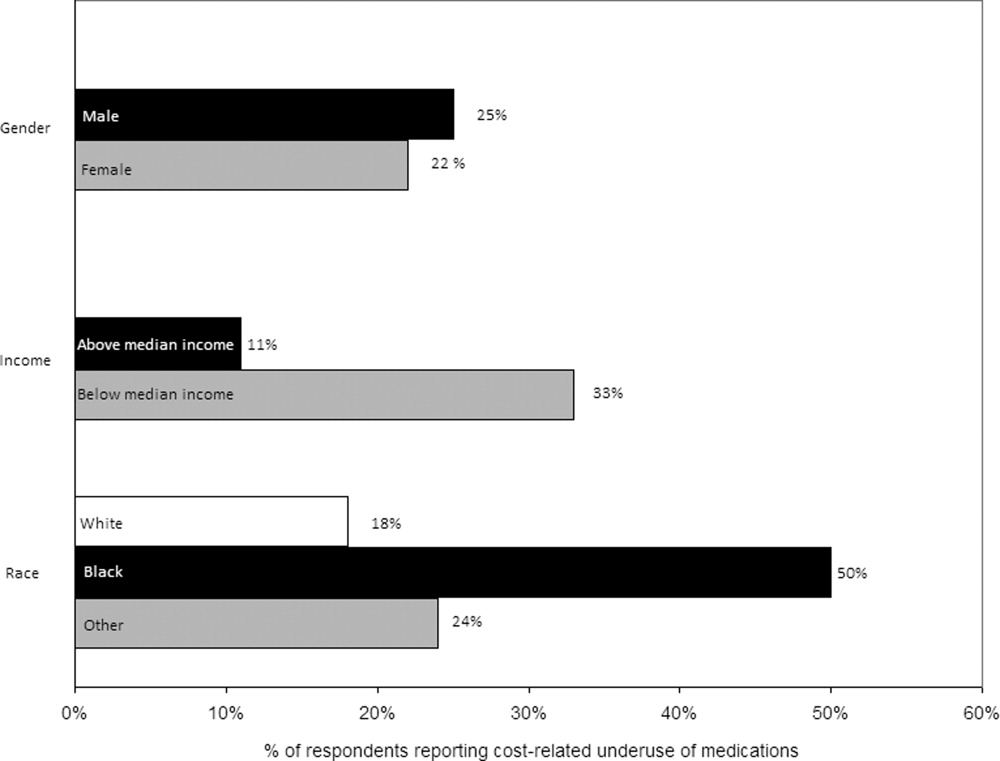
Thirty (23%) of the survey respondents reported at least 1 cost‐related medication underuse strategy in the year prior to their hospital admission (Figure 1), most commonly not filling a prescription at all because of cost (n = 26; 20%). Rates of cost‐related underuse were highest for patients of black race, low income, and women (Figure 2).
In unadjusted analyses, black respondents had 4.60 (95% confidence interval [CI], 1.63 to 13.0) times the odds of reporting cost‐related underuse than non‐Hispanic white respondents (Table 2). The association of black race and cost‐related underuse appears to be confounded, in part, by income (adjusted odds ratio for black race was 4.16; 95% CI, 1.34 to 12.86) and the number of medications patients used on a regular basis (adjusted odds ratio for black race was 4.14; 95% CI, 1.44 to 11.96). After controlling for these variables, as well as age and gender, the relationship between race and cost‐related underuse remained statistically significant (adjusted odds ratio 3.39; 95% CI, 1.05 to 11.02) (Table 2).
| Predictor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| ||
| Age (per additional year) | 0.98 (0.941.02) | 0.97 (0.931.01) |
| Male (vs female) | 0.84 (0.371.90) | 1.03 (0.432.48) |
| Race (vs white race) | ||
| Black | 4.60 (1.6313.0) | 3.39 (1.0511.02) |
| Other | 1.10 (0.363.37) | 0.77 (0.202.99) |
| No. of medications (per additional medication) | 1.10 (1.001.20) | 1.10 (1.001.22) |
| High income (vs low income) | 0.62 (0.271.42) | 0.71 (0.242.07) |
Strategies to Help Make Medications More Affordable
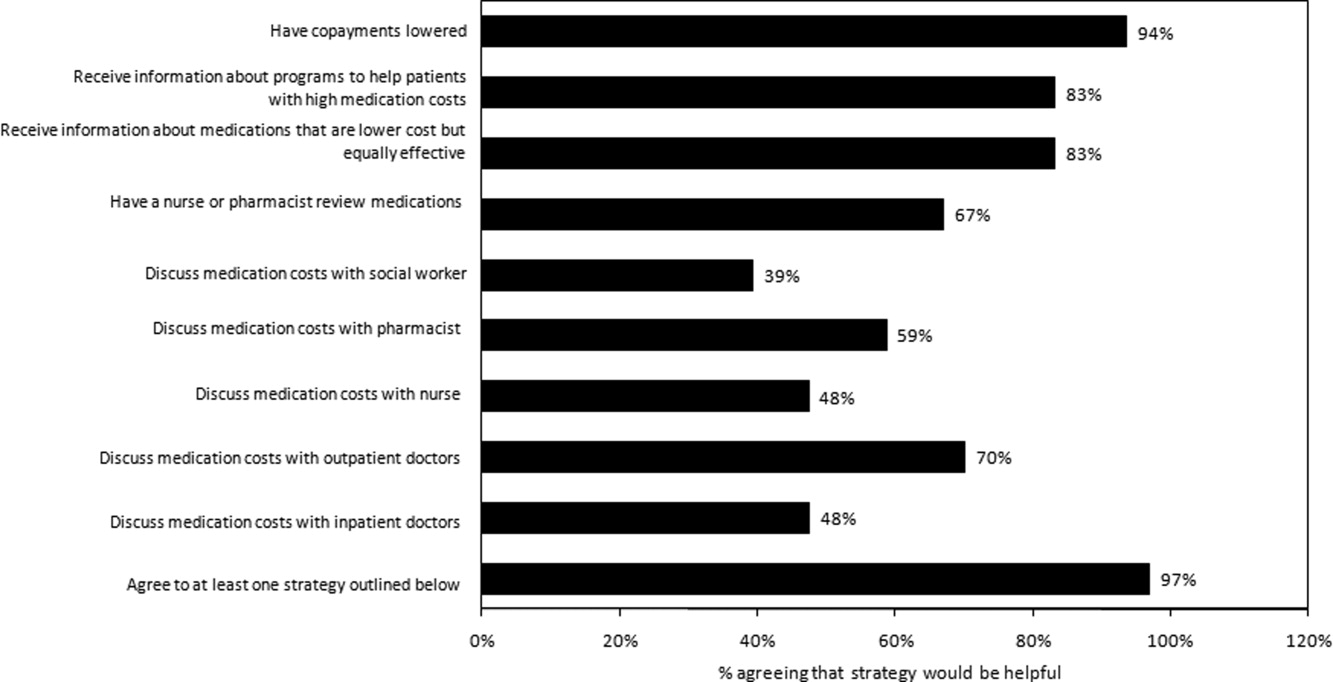
Virtually all respondents (n = 123; 95%) endorsed at least one of the proposed strategies to make medications more affordable (Figure 3). A majority felt that lowering cost sharing (94%), or receiving information about lower‐cost medication options (83%) or programs to subsidize medication costs (83%) would be helpful. Approximately 70% of patients stated that speaking to their outpatient physicians might be helpful, although only 14% reported actually speaking with their primary care provider about medication costs in the past year. Results were mixed for other strategies, including speaking with their inpatient physicians.
Postdischarge Medication Use
Seventy‐six (82%) respondents to the outpatient survey were prescribed a new medication at the time of hospital discharge, and virtually all (95%) had filled prescriptions for these medications by the time of the follow‐up survey. Patients paid an average of $27.63 (standard deviation $39.24) in out‐of‐pocket costs for these medications. Few (16%) patients knew how much they would have to pay before they had gone to the pharmacy to fill their prescription (see Appendix B). Even fewer patients asked, or were spoken to by their pharmacist, about less expensive medication options (7%), and almost none had spoken to their inpatient (4%) or outpatient providers (2%) about the cost of their newly prescribed drugs.
DISCUSSION
Almost a quarter of the medical inpatients we surveyed had not filled a medication because of cost, or had skipped doses, reduced dosages, or split pills to make their medicines last longer in the prior year. This amount is larger than that found in many prior studies, conducted in outpatient settings, in which 11% to 19% of patients report cost‐related underuse.68, 10, 11 Our results are particularly striking considering that our study cohort consisted exclusively of patients with commercial health insurance, the vast majority of whom also had employer‐sponsored drug coverage. Cost‐related medication underuse may be even more prevalent among hospitalized patients with less generous benefits, including the uninsured and perhaps even beneficiaries of Medicare Part D.
Reductions in medication use because of cost were particularly high among black patients, whose odds of reporting cost‐related underuse were more than 3 times higher than that of patients of non‐Hispanic white race. Race‐related differences in cost‐related underuse have been observed in outpatient studies,68, 12 and may be an important contributor to racial disparities in evidence‐based medication use.1315 These differences may, in part, reflect racial variations in socioeconomic status; lower income patients, who are more likely to be from a racial or ethnic minority, are more sensitive to cost sharing than higher income individuals.16 Consistent with this, the relationship between race and cost‐related underuse in our study was smaller but still highly significant in multivariable models that adjusted for income.
Not surprisingly, the underuse of effective prescription medications is associated with adverse clinical and economic consequences.17 Heisler et al. found that patients who had restricted medications because of cost were 76% more likely to report a decline in their health status than those who had not.18 The health effects of cost‐related underuse are likely to be particularly significant for hospitalized patients, given their high burden of disease and the frequency with which they are prescribed medications at discharge to treat the condition that led to their initial hospitalization. Thus, targeting efforts to address cost‐related underuse patients who are hospitalized may be an efficient method of improving patient health and reducing preventable readmissions. This is consistent with efforts that address, in the inpatient setting, other health issues that are commonly encountered in the ambulatory arena, such as immunizations and smoking cessation.19
Our survey respondents endorsed numerous strategies as being potentially helpful. Predictably, support for lowering copayments was extremely high. While this may not be practical or even desirable for some medications, lowering copayments for highly effective medications, such as statins and antihypertensives, in the context of value‐based insurance design, is an increasingly adopted strategy that has the potential to simultaneously improve clinical outcomes and reduce overall health spending.20, 21
While the majority of patients felt that talking to their outpatient physicians or pharmacists about medication costs might be helpful, the effectiveness of this strategy is unclear. Consistent with prior results,22, 23 the vast majority of the patients we surveyed had not discussed medication costs prior to their admission or after filling newly prescribed medications. Further, although physicians could help reduce drug expenditures in a variety of ways, including the increased ordering of generic drugs,24 many physicians are uncomfortable talking to their patients about costs,25 have limited knowledge about their patients' out‐of‐pocket expenditures, feel that addressing this issue is not their responsibility,26 or do not have resources, such as electronic formulary information, that could facilitate these discussions in an efficient manner.
An alternative strategy may be to provide patients with better education about medication costs. Virtually none of the patients we surveyed knew how much they would pay for their new prescriptions before visiting the pharmacy. These findings are similar to those observed in the outpatient setting,27 and suggest an opportunity to provide patients with information about the cost of their newly and previously prescribed drugs, and to facilitate discussions between patients and inpatient providers about predischarge prescribing decisions, in the same spirit as other predischarge patient education.28 Of course, issues related to transitions of care between the hospital and community setting, and coordination between inpatient and outpatient providers, must be adequately addressed for this strategy to be effective.
Our study has several notable limitations. It had a relatively small sample size and low response rate. Respondents may have differed systematically from non‐respondents, and we were unable to compare the characteristics of both populations. Further, we studied commercially insured inpatients on internal medicine services at an academic medical center, and thus our results may not be generalizable to patients hospitalized in other settings, or with different types of insurance coverage, including the uninsured. The primary outcome of our study was to determine self‐reported cost‐related underuse. While we used validated measures,8 it is possible that patients who reported reducing their medication use in response to cost may not have actually done so. We did not collect information on education or health literacy, nor did we have access to detailed information about our respondents' pharmacy benefit design structures; these important factors may have confounded our analyses, and/or may have been mediators of our observed results, and should be evaluated further in future studies. We did not have adequate statistical power to evaluate whether patients using specific classes of medications were particularly prone to cost‐related underuse.
Despite these limitations, our study is the first, to our knowledge, to evaluate the impact of medication costs on use in a cohort of hospitalized individuals. The high levels of cost‐related underuse that we observed is concerning. Our results support calls for the further development of interventions to address high medication costs and for the consideration of novel approaches to assist patients around the time of hospital discharge.
APPENDICES
APPENDIX A. Survey response flow diagram.
APPENDIX B. Behaviors to address the cost of medications prescribed at hospital discharge.
- USA Today/Kaiser Family Foundation/Harvard School of Public Health.The Public on Prescription Drugs and Pharmaceutical Companies.2008. Available at: http://www.kff.org/kaiserpolls/pomr030408pkg.cfm. Accessed September 5, 2008.
- ,,, et al.Pharmacy benefits and the use of drugs by the chronically ill.JAMA.2004;291(19):2344–2350.
- Kaiser Family Foundation and Health Research and Educational Trust.Employer Health Benefits Annual Survey,2009.year="2009"2009. Available at: http://ehbs.kff.org/pdf/2009/7936.pdf. Accessed May 5,year="2010"2010.
- Kaiser Family Foundation.Prescription Drug Trends.2007. Available at: http://www.kff.org/rxdrugs/upload/3057_06.pdf. Accessed December 5,year="2007"2007.
- The Patient Protection and Affordable Care Act, H.R. 3590, Section 2713 (c).Washington, DC:111 Congress;2010.
- ,,, et al.Cost‐related medication nonadherence and spending on basic needs following implementation of Medicare Part D.JAMA.2008;299(16):1922–1928.
- ,,, et al.Race/ethnicity and economic differences in cost‐related medication underuse among insured adults with diabetes: the Translating Research Into Action for Diabetes Study.Diabetes Care.2008;31(2):261–266.
- ,,, et al.Cost‐related medication nonadherence among elderly and disabled Medicare beneficiaries: a national survey 1 year before the Medicare drug benefit.Arch Intern Med.2006;166(17):1829–1835.
- ,,, et al.Prescription drug coverage and seniors: findings from a 2003 national survey.Health Aff (Millwood). Jan‐Jun 2005;Suppl Web Exclusives: W5‐152‐W155‐166.
- ,,.Cost‐related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk.Am J Public Health.2004;94(10):1782–1787.
- ,,.Problems paying out‐of‐pocket medication costs among older adults with diabetes.Diabetes Care.2004;27(2):384–391.
- ,,.Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study.J Gen Intern Med.2007;22(11):1572–1578.
- ,,,,,.Long‐term persistence in use of statin therapy in elderly patients.JAMA.2002;288(4):455–461.
- ,,, et al.Predictors of adherence with antihypertensive and lipid‐lowering therapy.Arch Intern Med.2005;165(10):1147–1152.
- ,,,.Racial disparities in the quality of medication use in older adults: baseline findings from a longitudinal study.J Gen Intern Med.2010;25(3)228–234.
- ,,,,,.Effects of increased patient cost sharing on socioeconomic disparities in health care.J Gen Intern Med.2008;23(8):1131–1136.
- .Relationship between high cost sharing and adverse outcomes: a truism that's tough to prove.Am J Manag Care.2010;16(4):287–289.
- ,,,,,.The health effects of restricting prescription medication use because of cost.Med Care.2004;42(7):626–634.
- ,.Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial.Can Med Assoc J.2009;180(13):1297–1303.
- .Copayment levels and medication adherence: less is more.Circulation.2009;119(3):365–367.
- ,,,,.Cost‐effectiveness of providing full drug coverage to increase medication adherence in post‐myocardial infarction Medicare beneficiaries.Circulation.2008;117(10):1261–1268.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164(16):1749–1755.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,,,.Patients' perceptions of generic medications.Health Aff (Millwood).2009;28(2):546–556.
- ,,.Physician strategies to reduce patients' out‐of‐pocket prescription costs.Arch Intern Med.2005;165(6):633–636.
- ,,, et al.Physicians' perceived knowledge of and responsibility for managing patients' out‐of‐pocket costs for prescription drugs.Ann Pharmacother.2006;40(9):1534–1540.
- ,,, et al.The effect of pharmacy benefit design on patient‐physician communication about costs.J Gen Intern Med.2006;21(4):334–339.
- ,,,.Discharge education improves clinical outcomes in patients with chronic heart failure.Circulation.2005;111(2):179–185.
The affordability of prescription medications continues to be one of the most pressing public health issues in the United States. Many patients reduce their prescribed doses to make medications last longer or do not fill prescriptions because of cost.1 Cost‐related medication underuse affects patients with and without drug insurance coverage,2 and is likely to become even more problematic as employers scale back on drug benefits3 and drug prices continue to increase.4 The landmark Patient Protection and Affordability Act passed in March 2010 does little to address this issue.5
Existing estimates of cost‐related medication underuse come largely from surveys of ambulatory patients. For example, using data from the Medicare Current Beneficiary Survey, Maden et al. estimated that 11% to 15% of patients reduced medication use in the past year because of cost.6 Tseng and colleagues found very similar rates of cost‐related underuse in managed care beneficiaries with diabetes.7
Hospitalized patients, who have a high burden of disease and tend to use more medications than their ambulatory counterparts, may be particularly vulnerable to cost‐related underuse but, thus far, have been subject to little investigation. New medications, which are frequently prescribed at the time of discharge, may exacerbate these issues further and contribute to preventable readmissions. Accordingly, we surveyed a cohort of medical inpatients at a large academic medical center to estimate the prevalence and predictors of cost‐related medication underuse for hospitalized managed care patients, and to identify strategies that patients perceive as helpful to make medications more affordable.
METHODS
Study Sample
We identified consecutive patients newly admitted to the general medicine, cardiology, or oncology services at Brigham and Women's Hospital from November 2008 to December 2009. For our survey, we included only those patients who received medical benefits through 1 of 3 large insurers with whom our hospital has pay‐for‐performance contracts. Annually, there are approximately 4000 patients covered by these insurers admitted to the 3 clinical services we evaluated, We focused on patients who had a primary care physician at one of the hospital's outpatient practices because of the existence of an automated infrastructure to identify these managed care beneficiaries of these insurers who are newly hospitalized, and because patients covered by commercial insurance plans likely represent a conservative lower‐bound of cost‐related medication underuse among hospitalized patients.
Patients were surveyed on the first non‐holiday weekday after admission. We excluded patients who had been discharged prior to the daily admission list being generated, or who, on a previous admission, had completed our survey or declined to be surveyed. We also excluded several patients who were not beneficiaries of the target insurers and were erroneously included on the managed care admission roster.
Potentially eligible patients were approached on the hospital ward by 1 of 3 study care coordinators (2 nurses and 1 pharmacist) and were asked if they were willing to participate in a research project about medication use that involved a short verbally delivered in‐person (inpatient) survey, a brief postdischarge telephone call, and a review of their electronic health record. The Institutional Review Board of Brigham and Women's Hospital approved this study.
Inpatient Survey
Our survey instrument was developed iteratively and pilot‐tested to improve face validity. Questions about cost‐related underuse were based on validated measures.8, 9 Specifically, we asked whether in the past year patients had: (1) not filled a prescription because it was too expensive, (2) skipped doses to make medicines last longer, (3) took less medicine than prescribed to make the medicine last longer, or (4) split pills to make the medication last longer.
Questions about strategies to improve medication affordability assessed whether patients thought it would be helpful to: (1) discuss medication affordability with healthcare workers (inpatient doctors, outpatient doctors, nurses, pharmacists, or social workers); (2) have their medications reviewed by a nurse or pharmacist; (3) receive information about lower cost but equally effective medication options, or about programs that provide medications at reduced costs; and/or (4) have their copayments/coinsurance lowered. Possible responses to all of these questions were binary, ie, yes or no.
In addition, patients were asked about the nature of their drug insurance coverage, the prescription medications that they currently use, whether they know their copayment levels (for generic and brand‐name medications), and, if so, what these amounts were, their annual household income, and their self‐identified race. Information on patient age, gender, and the primary reason for hospitalization was obtained from the electronic health record. This source was also used to verify the accuracy of the self‐reported preadmission medication list. When there were discrepancies between preadmission medications reported by patients and those recorded in their chart, the later was used because our hospital reconciles and records all medications at the time of hospital admission for all patients.
Postdischarge Survey
Within 3 days of discharge, patients were contacted by telephone and asked about new medications they were prescribed on discharge, if any. The discharge summary was used to verify the accuracy of the information provided by patients. The interviewers clarified any apparent discrepancies between the 2 sources of information with the patient. Patients who had been prescribed a new medication were asked whether or not they had filled their prescription. For patients who had, we asked whether: (1) they knew how much they would have to pay prior to going to the pharmacy, (2) they had discussed less expensive options with their pharmacist, and (3) they had discussed medication costs with their inpatient or outpatient physicians.
Data Analysis
We used descriptive statistics to summarize the characteristics of our respondents and our overall survey results. We generated univariate and multivariable logistic regression models to identify whether prehospitalization cost‐related medication underuse was influenced by patient age, gender, income, race, and the number of medications patients used on a regular basis. For the purpose of these analyses, we classified patients as reporting cost‐related underuse if they responded yes to any of the 4 strategies described above (ie, not filling medications, skipping doses, taking less medication, or splitting pills to make medicines last longer). Patients whose incomes were above the median level in our cohort were categorized as being of high‐income. Our multivariable model had a c‐statistic of 0.75, suggesting good discriminative ability.
RESULTS
During the study period, 483 potentially‐eligible patients were admitted to the general medicine, cardiology, and oncology services. We excluded 167 because they had been discharged prior to being identified, had been surveyed or already declined participation on a prior admission, or were not managed care enrollees (see Appendix A). Of the remaining 316 subjects, 130 participated in the inpatient survey (response rate = 41%); 93 (75%) of these patients were reached by telephone after hospital discharge and completed the postdischarge survey. The baseline characteristics of our respondents are presented in Table 1. Patients had a mean age of 52 years, were 50% male and two‐thirds of white race, represented a range of household incomes, and almost all had employer‐sponsored prescription coverage. Prior to admission, patients took an average of 5 prescription medications and paid an average copayment of $10.80 and $21.60 for each generic and brand‐name prescription, respectively.
| Characteristic | N = 130 |
|---|---|
| |
| Age, mean years (SD) | 52 (11.2) |
| Male, % | 65 (50.0) |
| Race/ethnicity,* n (%) | |
| Caucasian/white | 84 (67.2) |
| Black/African American | 20 (16.0) |
| Latino/Hispanic | 13 (10.4) |
| Asian | 3 (2.4) |
| American Indian or Alaska Native | 1 (0.8) |
| Other | 4 (3.2) |
| Annual household income,* n (%) | |
| <$30,000 | 15 (12.8) |
| $30,000‐$75,000 | 49 (41.9) |
| >$75,000 | 53 (45.3) |
| Insurance coverage for outpatient prescription drugs,* n (%) | |
| Employer or spouse's employer | 123 (96.0) |
| Independent | 5 (3.9) |
| Medication copayments,* mean $ (SD) | |
| Brand‐name medications | 21.6 (14.2) |
| Generic medications | 10.8 (6.0) |
| No. of medications prior to admission, mean (SD) | 5.5 (4.3) |
| Category of discharge diagnosis, n (%) | |
| Cardiovascular | 40 (30.8) |
| Gastrointestinal | 23 (17.7) |
| Pulmonary | 23 (17.7) |
| Infectious | 13 (10.0) |
| Oncology | 5 (3.8) |
| Renal | 6 (4.6) |
| Psychiatric | 3 (2.3) |
| Hematologic | 4 (3.1) |
| Neurologic | 5 (3.8) |
| Musculoskeletal | 5 (3.8) |
| Respiratory | 2 (1.5) |
| Endocrine | 1 (0.8) |
Cost‐Related Medication Underuse
Thirty (23%) of the survey respondents reported at least 1 cost‐related medication underuse strategy in the year prior to their hospital admission (Figure 1), most commonly not filling a prescription at all because of cost (n = 26; 20%). Rates of cost‐related underuse were highest for patients of black race, low income, and women (Figure 2).


In unadjusted analyses, black respondents had 4.60 (95% confidence interval [CI], 1.63 to 13.0) times the odds of reporting cost‐related underuse than non‐Hispanic white respondents (Table 2). The association of black race and cost‐related underuse appears to be confounded, in part, by income (adjusted odds ratio for black race was 4.16; 95% CI, 1.34 to 12.86) and the number of medications patients used on a regular basis (adjusted odds ratio for black race was 4.14; 95% CI, 1.44 to 11.96). After controlling for these variables, as well as age and gender, the relationship between race and cost‐related underuse remained statistically significant (adjusted odds ratio 3.39; 95% CI, 1.05 to 11.02) (Table 2).
| Predictor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| ||
| Age (per additional year) | 0.98 (0.941.02) | 0.97 (0.931.01) |
| Male (vs female) | 0.84 (0.371.90) | 1.03 (0.432.48) |
| Race (vs white race) | ||
| Black | 4.60 (1.6313.0) | 3.39 (1.0511.02) |
| Other | 1.10 (0.363.37) | 0.77 (0.202.99) |
| No. of medications (per additional medication) | 1.10 (1.001.20) | 1.10 (1.001.22) |
| High income (vs low income) | 0.62 (0.271.42) | 0.71 (0.242.07) |
Strategies to Help Make Medications More Affordable
Virtually all respondents (n = 123; 95%) endorsed at least one of the proposed strategies to make medications more affordable (Figure 3). A majority felt that lowering cost sharing (94%), or receiving information about lower‐cost medication options (83%) or programs to subsidize medication costs (83%) would be helpful. Approximately 70% of patients stated that speaking to their outpatient physicians might be helpful, although only 14% reported actually speaking with their primary care provider about medication costs in the past year. Results were mixed for other strategies, including speaking with their inpatient physicians.

Postdischarge Medication Use
Seventy‐six (82%) respondents to the outpatient survey were prescribed a new medication at the time of hospital discharge, and virtually all (95%) had filled prescriptions for these medications by the time of the follow‐up survey. Patients paid an average of $27.63 (standard deviation $39.24) in out‐of‐pocket costs for these medications. Few (16%) patients knew how much they would have to pay before they had gone to the pharmacy to fill their prescription (see Appendix B). Even fewer patients asked, or were spoken to by their pharmacist, about less expensive medication options (7%), and almost none had spoken to their inpatient (4%) or outpatient providers (2%) about the cost of their newly prescribed drugs.
DISCUSSION
Almost a quarter of the medical inpatients we surveyed had not filled a medication because of cost, or had skipped doses, reduced dosages, or split pills to make their medicines last longer in the prior year. This amount is larger than that found in many prior studies, conducted in outpatient settings, in which 11% to 19% of patients report cost‐related underuse.68, 10, 11 Our results are particularly striking considering that our study cohort consisted exclusively of patients with commercial health insurance, the vast majority of whom also had employer‐sponsored drug coverage. Cost‐related medication underuse may be even more prevalent among hospitalized patients with less generous benefits, including the uninsured and perhaps even beneficiaries of Medicare Part D.
Reductions in medication use because of cost were particularly high among black patients, whose odds of reporting cost‐related underuse were more than 3 times higher than that of patients of non‐Hispanic white race. Race‐related differences in cost‐related underuse have been observed in outpatient studies,68, 12 and may be an important contributor to racial disparities in evidence‐based medication use.1315 These differences may, in part, reflect racial variations in socioeconomic status; lower income patients, who are more likely to be from a racial or ethnic minority, are more sensitive to cost sharing than higher income individuals.16 Consistent with this, the relationship between race and cost‐related underuse in our study was smaller but still highly significant in multivariable models that adjusted for income.
Not surprisingly, the underuse of effective prescription medications is associated with adverse clinical and economic consequences.17 Heisler et al. found that patients who had restricted medications because of cost were 76% more likely to report a decline in their health status than those who had not.18 The health effects of cost‐related underuse are likely to be particularly significant for hospitalized patients, given their high burden of disease and the frequency with which they are prescribed medications at discharge to treat the condition that led to their initial hospitalization. Thus, targeting efforts to address cost‐related underuse patients who are hospitalized may be an efficient method of improving patient health and reducing preventable readmissions. This is consistent with efforts that address, in the inpatient setting, other health issues that are commonly encountered in the ambulatory arena, such as immunizations and smoking cessation.19
Our survey respondents endorsed numerous strategies as being potentially helpful. Predictably, support for lowering copayments was extremely high. While this may not be practical or even desirable for some medications, lowering copayments for highly effective medications, such as statins and antihypertensives, in the context of value‐based insurance design, is an increasingly adopted strategy that has the potential to simultaneously improve clinical outcomes and reduce overall health spending.20, 21
While the majority of patients felt that talking to their outpatient physicians or pharmacists about medication costs might be helpful, the effectiveness of this strategy is unclear. Consistent with prior results,22, 23 the vast majority of the patients we surveyed had not discussed medication costs prior to their admission or after filling newly prescribed medications. Further, although physicians could help reduce drug expenditures in a variety of ways, including the increased ordering of generic drugs,24 many physicians are uncomfortable talking to their patients about costs,25 have limited knowledge about their patients' out‐of‐pocket expenditures, feel that addressing this issue is not their responsibility,26 or do not have resources, such as electronic formulary information, that could facilitate these discussions in an efficient manner.
An alternative strategy may be to provide patients with better education about medication costs. Virtually none of the patients we surveyed knew how much they would pay for their new prescriptions before visiting the pharmacy. These findings are similar to those observed in the outpatient setting,27 and suggest an opportunity to provide patients with information about the cost of their newly and previously prescribed drugs, and to facilitate discussions between patients and inpatient providers about predischarge prescribing decisions, in the same spirit as other predischarge patient education.28 Of course, issues related to transitions of care between the hospital and community setting, and coordination between inpatient and outpatient providers, must be adequately addressed for this strategy to be effective.
Our study has several notable limitations. It had a relatively small sample size and low response rate. Respondents may have differed systematically from non‐respondents, and we were unable to compare the characteristics of both populations. Further, we studied commercially insured inpatients on internal medicine services at an academic medical center, and thus our results may not be generalizable to patients hospitalized in other settings, or with different types of insurance coverage, including the uninsured. The primary outcome of our study was to determine self‐reported cost‐related underuse. While we used validated measures,8 it is possible that patients who reported reducing their medication use in response to cost may not have actually done so. We did not collect information on education or health literacy, nor did we have access to detailed information about our respondents' pharmacy benefit design structures; these important factors may have confounded our analyses, and/or may have been mediators of our observed results, and should be evaluated further in future studies. We did not have adequate statistical power to evaluate whether patients using specific classes of medications were particularly prone to cost‐related underuse.
Despite these limitations, our study is the first, to our knowledge, to evaluate the impact of medication costs on use in a cohort of hospitalized individuals. The high levels of cost‐related underuse that we observed is concerning. Our results support calls for the further development of interventions to address high medication costs and for the consideration of novel approaches to assist patients around the time of hospital discharge.
APPENDICES
APPENDIX A. Survey response flow diagram.
APPENDIX B. Behaviors to address the cost of medications prescribed at hospital discharge.
The affordability of prescription medications continues to be one of the most pressing public health issues in the United States. Many patients reduce their prescribed doses to make medications last longer or do not fill prescriptions because of cost.1 Cost‐related medication underuse affects patients with and without drug insurance coverage,2 and is likely to become even more problematic as employers scale back on drug benefits3 and drug prices continue to increase.4 The landmark Patient Protection and Affordability Act passed in March 2010 does little to address this issue.5
Existing estimates of cost‐related medication underuse come largely from surveys of ambulatory patients. For example, using data from the Medicare Current Beneficiary Survey, Maden et al. estimated that 11% to 15% of patients reduced medication use in the past year because of cost.6 Tseng and colleagues found very similar rates of cost‐related underuse in managed care beneficiaries with diabetes.7
Hospitalized patients, who have a high burden of disease and tend to use more medications than their ambulatory counterparts, may be particularly vulnerable to cost‐related underuse but, thus far, have been subject to little investigation. New medications, which are frequently prescribed at the time of discharge, may exacerbate these issues further and contribute to preventable readmissions. Accordingly, we surveyed a cohort of medical inpatients at a large academic medical center to estimate the prevalence and predictors of cost‐related medication underuse for hospitalized managed care patients, and to identify strategies that patients perceive as helpful to make medications more affordable.
METHODS
Study Sample
We identified consecutive patients newly admitted to the general medicine, cardiology, or oncology services at Brigham and Women's Hospital from November 2008 to December 2009. For our survey, we included only those patients who received medical benefits through 1 of 3 large insurers with whom our hospital has pay‐for‐performance contracts. Annually, there are approximately 4000 patients covered by these insurers admitted to the 3 clinical services we evaluated, We focused on patients who had a primary care physician at one of the hospital's outpatient practices because of the existence of an automated infrastructure to identify these managed care beneficiaries of these insurers who are newly hospitalized, and because patients covered by commercial insurance plans likely represent a conservative lower‐bound of cost‐related medication underuse among hospitalized patients.
Patients were surveyed on the first non‐holiday weekday after admission. We excluded patients who had been discharged prior to the daily admission list being generated, or who, on a previous admission, had completed our survey or declined to be surveyed. We also excluded several patients who were not beneficiaries of the target insurers and were erroneously included on the managed care admission roster.
Potentially eligible patients were approached on the hospital ward by 1 of 3 study care coordinators (2 nurses and 1 pharmacist) and were asked if they were willing to participate in a research project about medication use that involved a short verbally delivered in‐person (inpatient) survey, a brief postdischarge telephone call, and a review of their electronic health record. The Institutional Review Board of Brigham and Women's Hospital approved this study.
Inpatient Survey
Our survey instrument was developed iteratively and pilot‐tested to improve face validity. Questions about cost‐related underuse were based on validated measures.8, 9 Specifically, we asked whether in the past year patients had: (1) not filled a prescription because it was too expensive, (2) skipped doses to make medicines last longer, (3) took less medicine than prescribed to make the medicine last longer, or (4) split pills to make the medication last longer.
Questions about strategies to improve medication affordability assessed whether patients thought it would be helpful to: (1) discuss medication affordability with healthcare workers (inpatient doctors, outpatient doctors, nurses, pharmacists, or social workers); (2) have their medications reviewed by a nurse or pharmacist; (3) receive information about lower cost but equally effective medication options, or about programs that provide medications at reduced costs; and/or (4) have their copayments/coinsurance lowered. Possible responses to all of these questions were binary, ie, yes or no.
In addition, patients were asked about the nature of their drug insurance coverage, the prescription medications that they currently use, whether they know their copayment levels (for generic and brand‐name medications), and, if so, what these amounts were, their annual household income, and their self‐identified race. Information on patient age, gender, and the primary reason for hospitalization was obtained from the electronic health record. This source was also used to verify the accuracy of the self‐reported preadmission medication list. When there were discrepancies between preadmission medications reported by patients and those recorded in their chart, the later was used because our hospital reconciles and records all medications at the time of hospital admission for all patients.
Postdischarge Survey
Within 3 days of discharge, patients were contacted by telephone and asked about new medications they were prescribed on discharge, if any. The discharge summary was used to verify the accuracy of the information provided by patients. The interviewers clarified any apparent discrepancies between the 2 sources of information with the patient. Patients who had been prescribed a new medication were asked whether or not they had filled their prescription. For patients who had, we asked whether: (1) they knew how much they would have to pay prior to going to the pharmacy, (2) they had discussed less expensive options with their pharmacist, and (3) they had discussed medication costs with their inpatient or outpatient physicians.
Data Analysis
We used descriptive statistics to summarize the characteristics of our respondents and our overall survey results. We generated univariate and multivariable logistic regression models to identify whether prehospitalization cost‐related medication underuse was influenced by patient age, gender, income, race, and the number of medications patients used on a regular basis. For the purpose of these analyses, we classified patients as reporting cost‐related underuse if they responded yes to any of the 4 strategies described above (ie, not filling medications, skipping doses, taking less medication, or splitting pills to make medicines last longer). Patients whose incomes were above the median level in our cohort were categorized as being of high‐income. Our multivariable model had a c‐statistic of 0.75, suggesting good discriminative ability.
RESULTS
During the study period, 483 potentially‐eligible patients were admitted to the general medicine, cardiology, and oncology services. We excluded 167 because they had been discharged prior to being identified, had been surveyed or already declined participation on a prior admission, or were not managed care enrollees (see Appendix A). Of the remaining 316 subjects, 130 participated in the inpatient survey (response rate = 41%); 93 (75%) of these patients were reached by telephone after hospital discharge and completed the postdischarge survey. The baseline characteristics of our respondents are presented in Table 1. Patients had a mean age of 52 years, were 50% male and two‐thirds of white race, represented a range of household incomes, and almost all had employer‐sponsored prescription coverage. Prior to admission, patients took an average of 5 prescription medications and paid an average copayment of $10.80 and $21.60 for each generic and brand‐name prescription, respectively.
| Characteristic | N = 130 |
|---|---|
| |
| Age, mean years (SD) | 52 (11.2) |
| Male, % | 65 (50.0) |
| Race/ethnicity,* n (%) | |
| Caucasian/white | 84 (67.2) |
| Black/African American | 20 (16.0) |
| Latino/Hispanic | 13 (10.4) |
| Asian | 3 (2.4) |
| American Indian or Alaska Native | 1 (0.8) |
| Other | 4 (3.2) |
| Annual household income,* n (%) | |
| <$30,000 | 15 (12.8) |
| $30,000‐$75,000 | 49 (41.9) |
| >$75,000 | 53 (45.3) |
| Insurance coverage for outpatient prescription drugs,* n (%) | |
| Employer or spouse's employer | 123 (96.0) |
| Independent | 5 (3.9) |
| Medication copayments,* mean $ (SD) | |
| Brand‐name medications | 21.6 (14.2) |
| Generic medications | 10.8 (6.0) |
| No. of medications prior to admission, mean (SD) | 5.5 (4.3) |
| Category of discharge diagnosis, n (%) | |
| Cardiovascular | 40 (30.8) |
| Gastrointestinal | 23 (17.7) |
| Pulmonary | 23 (17.7) |
| Infectious | 13 (10.0) |
| Oncology | 5 (3.8) |
| Renal | 6 (4.6) |
| Psychiatric | 3 (2.3) |
| Hematologic | 4 (3.1) |
| Neurologic | 5 (3.8) |
| Musculoskeletal | 5 (3.8) |
| Respiratory | 2 (1.5) |
| Endocrine | 1 (0.8) |
Cost‐Related Medication Underuse
Thirty (23%) of the survey respondents reported at least 1 cost‐related medication underuse strategy in the year prior to their hospital admission (Figure 1), most commonly not filling a prescription at all because of cost (n = 26; 20%). Rates of cost‐related underuse were highest for patients of black race, low income, and women (Figure 2).


In unadjusted analyses, black respondents had 4.60 (95% confidence interval [CI], 1.63 to 13.0) times the odds of reporting cost‐related underuse than non‐Hispanic white respondents (Table 2). The association of black race and cost‐related underuse appears to be confounded, in part, by income (adjusted odds ratio for black race was 4.16; 95% CI, 1.34 to 12.86) and the number of medications patients used on a regular basis (adjusted odds ratio for black race was 4.14; 95% CI, 1.44 to 11.96). After controlling for these variables, as well as age and gender, the relationship between race and cost‐related underuse remained statistically significant (adjusted odds ratio 3.39; 95% CI, 1.05 to 11.02) (Table 2).
| Predictor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| ||
| Age (per additional year) | 0.98 (0.941.02) | 0.97 (0.931.01) |
| Male (vs female) | 0.84 (0.371.90) | 1.03 (0.432.48) |
| Race (vs white race) | ||
| Black | 4.60 (1.6313.0) | 3.39 (1.0511.02) |
| Other | 1.10 (0.363.37) | 0.77 (0.202.99) |
| No. of medications (per additional medication) | 1.10 (1.001.20) | 1.10 (1.001.22) |
| High income (vs low income) | 0.62 (0.271.42) | 0.71 (0.242.07) |
Strategies to Help Make Medications More Affordable
Virtually all respondents (n = 123; 95%) endorsed at least one of the proposed strategies to make medications more affordable (Figure 3). A majority felt that lowering cost sharing (94%), or receiving information about lower‐cost medication options (83%) or programs to subsidize medication costs (83%) would be helpful. Approximately 70% of patients stated that speaking to their outpatient physicians might be helpful, although only 14% reported actually speaking with their primary care provider about medication costs in the past year. Results were mixed for other strategies, including speaking with their inpatient physicians.

Postdischarge Medication Use
Seventy‐six (82%) respondents to the outpatient survey were prescribed a new medication at the time of hospital discharge, and virtually all (95%) had filled prescriptions for these medications by the time of the follow‐up survey. Patients paid an average of $27.63 (standard deviation $39.24) in out‐of‐pocket costs for these medications. Few (16%) patients knew how much they would have to pay before they had gone to the pharmacy to fill their prescription (see Appendix B). Even fewer patients asked, or were spoken to by their pharmacist, about less expensive medication options (7%), and almost none had spoken to their inpatient (4%) or outpatient providers (2%) about the cost of their newly prescribed drugs.
DISCUSSION
Almost a quarter of the medical inpatients we surveyed had not filled a medication because of cost, or had skipped doses, reduced dosages, or split pills to make their medicines last longer in the prior year. This amount is larger than that found in many prior studies, conducted in outpatient settings, in which 11% to 19% of patients report cost‐related underuse.68, 10, 11 Our results are particularly striking considering that our study cohort consisted exclusively of patients with commercial health insurance, the vast majority of whom also had employer‐sponsored drug coverage. Cost‐related medication underuse may be even more prevalent among hospitalized patients with less generous benefits, including the uninsured and perhaps even beneficiaries of Medicare Part D.
Reductions in medication use because of cost were particularly high among black patients, whose odds of reporting cost‐related underuse were more than 3 times higher than that of patients of non‐Hispanic white race. Race‐related differences in cost‐related underuse have been observed in outpatient studies,68, 12 and may be an important contributor to racial disparities in evidence‐based medication use.1315 These differences may, in part, reflect racial variations in socioeconomic status; lower income patients, who are more likely to be from a racial or ethnic minority, are more sensitive to cost sharing than higher income individuals.16 Consistent with this, the relationship between race and cost‐related underuse in our study was smaller but still highly significant in multivariable models that adjusted for income.
Not surprisingly, the underuse of effective prescription medications is associated with adverse clinical and economic consequences.17 Heisler et al. found that patients who had restricted medications because of cost were 76% more likely to report a decline in their health status than those who had not.18 The health effects of cost‐related underuse are likely to be particularly significant for hospitalized patients, given their high burden of disease and the frequency with which they are prescribed medications at discharge to treat the condition that led to their initial hospitalization. Thus, targeting efforts to address cost‐related underuse patients who are hospitalized may be an efficient method of improving patient health and reducing preventable readmissions. This is consistent with efforts that address, in the inpatient setting, other health issues that are commonly encountered in the ambulatory arena, such as immunizations and smoking cessation.19
Our survey respondents endorsed numerous strategies as being potentially helpful. Predictably, support for lowering copayments was extremely high. While this may not be practical or even desirable for some medications, lowering copayments for highly effective medications, such as statins and antihypertensives, in the context of value‐based insurance design, is an increasingly adopted strategy that has the potential to simultaneously improve clinical outcomes and reduce overall health spending.20, 21
While the majority of patients felt that talking to their outpatient physicians or pharmacists about medication costs might be helpful, the effectiveness of this strategy is unclear. Consistent with prior results,22, 23 the vast majority of the patients we surveyed had not discussed medication costs prior to their admission or after filling newly prescribed medications. Further, although physicians could help reduce drug expenditures in a variety of ways, including the increased ordering of generic drugs,24 many physicians are uncomfortable talking to their patients about costs,25 have limited knowledge about their patients' out‐of‐pocket expenditures, feel that addressing this issue is not their responsibility,26 or do not have resources, such as electronic formulary information, that could facilitate these discussions in an efficient manner.
An alternative strategy may be to provide patients with better education about medication costs. Virtually none of the patients we surveyed knew how much they would pay for their new prescriptions before visiting the pharmacy. These findings are similar to those observed in the outpatient setting,27 and suggest an opportunity to provide patients with information about the cost of their newly and previously prescribed drugs, and to facilitate discussions between patients and inpatient providers about predischarge prescribing decisions, in the same spirit as other predischarge patient education.28 Of course, issues related to transitions of care between the hospital and community setting, and coordination between inpatient and outpatient providers, must be adequately addressed for this strategy to be effective.
Our study has several notable limitations. It had a relatively small sample size and low response rate. Respondents may have differed systematically from non‐respondents, and we were unable to compare the characteristics of both populations. Further, we studied commercially insured inpatients on internal medicine services at an academic medical center, and thus our results may not be generalizable to patients hospitalized in other settings, or with different types of insurance coverage, including the uninsured. The primary outcome of our study was to determine self‐reported cost‐related underuse. While we used validated measures,8 it is possible that patients who reported reducing their medication use in response to cost may not have actually done so. We did not collect information on education or health literacy, nor did we have access to detailed information about our respondents' pharmacy benefit design structures; these important factors may have confounded our analyses, and/or may have been mediators of our observed results, and should be evaluated further in future studies. We did not have adequate statistical power to evaluate whether patients using specific classes of medications were particularly prone to cost‐related underuse.
Despite these limitations, our study is the first, to our knowledge, to evaluate the impact of medication costs on use in a cohort of hospitalized individuals. The high levels of cost‐related underuse that we observed is concerning. Our results support calls for the further development of interventions to address high medication costs and for the consideration of novel approaches to assist patients around the time of hospital discharge.
APPENDICES
APPENDIX A. Survey response flow diagram.
APPENDIX B. Behaviors to address the cost of medications prescribed at hospital discharge.
- USA Today/Kaiser Family Foundation/Harvard School of Public Health.The Public on Prescription Drugs and Pharmaceutical Companies.2008. Available at: http://www.kff.org/kaiserpolls/pomr030408pkg.cfm. Accessed September 5, 2008.
- ,,, et al.Pharmacy benefits and the use of drugs by the chronically ill.JAMA.2004;291(19):2344–2350.
- Kaiser Family Foundation and Health Research and Educational Trust.Employer Health Benefits Annual Survey,2009.year="2009"2009. Available at: http://ehbs.kff.org/pdf/2009/7936.pdf. Accessed May 5,year="2010"2010.
- Kaiser Family Foundation.Prescription Drug Trends.2007. Available at: http://www.kff.org/rxdrugs/upload/3057_06.pdf. Accessed December 5,year="2007"2007.
- The Patient Protection and Affordable Care Act, H.R. 3590, Section 2713 (c).Washington, DC:111 Congress;2010.
- ,,, et al.Cost‐related medication nonadherence and spending on basic needs following implementation of Medicare Part D.JAMA.2008;299(16):1922–1928.
- ,,, et al.Race/ethnicity and economic differences in cost‐related medication underuse among insured adults with diabetes: the Translating Research Into Action for Diabetes Study.Diabetes Care.2008;31(2):261–266.
- ,,, et al.Cost‐related medication nonadherence among elderly and disabled Medicare beneficiaries: a national survey 1 year before the Medicare drug benefit.Arch Intern Med.2006;166(17):1829–1835.
- ,,, et al.Prescription drug coverage and seniors: findings from a 2003 national survey.Health Aff (Millwood). Jan‐Jun 2005;Suppl Web Exclusives: W5‐152‐W155‐166.
- ,,.Cost‐related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk.Am J Public Health.2004;94(10):1782–1787.
- ,,.Problems paying out‐of‐pocket medication costs among older adults with diabetes.Diabetes Care.2004;27(2):384–391.
- ,,.Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study.J Gen Intern Med.2007;22(11):1572–1578.
- ,,,,,.Long‐term persistence in use of statin therapy in elderly patients.JAMA.2002;288(4):455–461.
- ,,, et al.Predictors of adherence with antihypertensive and lipid‐lowering therapy.Arch Intern Med.2005;165(10):1147–1152.
- ,,,.Racial disparities in the quality of medication use in older adults: baseline findings from a longitudinal study.J Gen Intern Med.2010;25(3)228–234.
- ,,,,,.Effects of increased patient cost sharing on socioeconomic disparities in health care.J Gen Intern Med.2008;23(8):1131–1136.
- .Relationship between high cost sharing and adverse outcomes: a truism that's tough to prove.Am J Manag Care.2010;16(4):287–289.
- ,,,,,.The health effects of restricting prescription medication use because of cost.Med Care.2004;42(7):626–634.
- ,.Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial.Can Med Assoc J.2009;180(13):1297–1303.
- .Copayment levels and medication adherence: less is more.Circulation.2009;119(3):365–367.
- ,,,,.Cost‐effectiveness of providing full drug coverage to increase medication adherence in post‐myocardial infarction Medicare beneficiaries.Circulation.2008;117(10):1261–1268.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164(16):1749–1755.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,,,.Patients' perceptions of generic medications.Health Aff (Millwood).2009;28(2):546–556.
- ,,.Physician strategies to reduce patients' out‐of‐pocket prescription costs.Arch Intern Med.2005;165(6):633–636.
- ,,, et al.Physicians' perceived knowledge of and responsibility for managing patients' out‐of‐pocket costs for prescription drugs.Ann Pharmacother.2006;40(9):1534–1540.
- ,,, et al.The effect of pharmacy benefit design on patient‐physician communication about costs.J Gen Intern Med.2006;21(4):334–339.
- ,,,.Discharge education improves clinical outcomes in patients with chronic heart failure.Circulation.2005;111(2):179–185.
- USA Today/Kaiser Family Foundation/Harvard School of Public Health.The Public on Prescription Drugs and Pharmaceutical Companies.2008. Available at: http://www.kff.org/kaiserpolls/pomr030408pkg.cfm. Accessed September 5, 2008.
- ,,, et al.Pharmacy benefits and the use of drugs by the chronically ill.JAMA.2004;291(19):2344–2350.
- Kaiser Family Foundation and Health Research and Educational Trust.Employer Health Benefits Annual Survey,2009.year="2009"2009. Available at: http://ehbs.kff.org/pdf/2009/7936.pdf. Accessed May 5,year="2010"2010.
- Kaiser Family Foundation.Prescription Drug Trends.2007. Available at: http://www.kff.org/rxdrugs/upload/3057_06.pdf. Accessed December 5,year="2007"2007.
- The Patient Protection and Affordable Care Act, H.R. 3590, Section 2713 (c).Washington, DC:111 Congress;2010.
- ,,, et al.Cost‐related medication nonadherence and spending on basic needs following implementation of Medicare Part D.JAMA.2008;299(16):1922–1928.
- ,,, et al.Race/ethnicity and economic differences in cost‐related medication underuse among insured adults with diabetes: the Translating Research Into Action for Diabetes Study.Diabetes Care.2008;31(2):261–266.
- ,,, et al.Cost‐related medication nonadherence among elderly and disabled Medicare beneficiaries: a national survey 1 year before the Medicare drug benefit.Arch Intern Med.2006;166(17):1829–1835.
- ,,, et al.Prescription drug coverage and seniors: findings from a 2003 national survey.Health Aff (Millwood). Jan‐Jun 2005;Suppl Web Exclusives: W5‐152‐W155‐166.
- ,,.Cost‐related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk.Am J Public Health.2004;94(10):1782–1787.
- ,,.Problems paying out‐of‐pocket medication costs among older adults with diabetes.Diabetes Care.2004;27(2):384–391.
- ,,.Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study.J Gen Intern Med.2007;22(11):1572–1578.
- ,,,,,.Long‐term persistence in use of statin therapy in elderly patients.JAMA.2002;288(4):455–461.
- ,,, et al.Predictors of adherence with antihypertensive and lipid‐lowering therapy.Arch Intern Med.2005;165(10):1147–1152.
- ,,,.Racial disparities in the quality of medication use in older adults: baseline findings from a longitudinal study.J Gen Intern Med.2010;25(3)228–234.
- ,,,,,.Effects of increased patient cost sharing on socioeconomic disparities in health care.J Gen Intern Med.2008;23(8):1131–1136.
- .Relationship between high cost sharing and adverse outcomes: a truism that's tough to prove.Am J Manag Care.2010;16(4):287–289.
- ,,,,,.The health effects of restricting prescription medication use because of cost.Med Care.2004;42(7):626–634.
- ,.Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial.Can Med Assoc J.2009;180(13):1297–1303.
- .Copayment levels and medication adherence: less is more.Circulation.2009;119(3):365–367.
- ,,,,.Cost‐effectiveness of providing full drug coverage to increase medication adherence in post‐myocardial infarction Medicare beneficiaries.Circulation.2008;117(10):1261–1268.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164(16):1749–1755.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,,,.Patients' perceptions of generic medications.Health Aff (Millwood).2009;28(2):546–556.
- ,,.Physician strategies to reduce patients' out‐of‐pocket prescription costs.Arch Intern Med.2005;165(6):633–636.
- ,,, et al.Physicians' perceived knowledge of and responsibility for managing patients' out‐of‐pocket costs for prescription drugs.Ann Pharmacother.2006;40(9):1534–1540.
- ,,, et al.The effect of pharmacy benefit design on patient‐physician communication about costs.J Gen Intern Med.2006;21(4):334–339.
- ,,,.Discharge education improves clinical outcomes in patients with chronic heart failure.Circulation.2005;111(2):179–185.
Copyright © 2011 Society of Hospital Medicine
Hospitalist Versus Traditional Systems
In the United States, general medical inpatient care is increasingly provided by hospital‐based physicians, also called hospitalists.1 The field of pediatrics is no exception, and by 2005 there were an estimated 1000 pediatric hospitalists in the workforce.2 Current numbers are likely to be greater than 2500, as the need for pediatric hospitalists has grown considerably.
At the same time, the quality of care delivered by the United States health system has come under increased scrutiny. In 2001, the Institute of Medicine, in its report on the quality of healthcare in America, concluded that between the care we have and what we could have lies not just a gap but a chasm.3 Meanwhile, the cost of healthcare delivery continues to increase. The pressure to deliver cost‐effective, high quality care is among the more important forces driving the proliferation of hospitalists.4
Over the last decade, data supporting the role of hospitalists in improving quality of care for adult patients has continued to accumulate.58 A 2007 retrospective cohort study by Lindenaur et al.7 included nearly 77,000 adult patients and found small reductions in length of stay without adverse effects on mortality or readmission rates, and a 2009 systematic review by Peterson6 included 33 studies and concluded that in general inpatient care of general medical patients by hospitalist physicians leads to decreased hospital cost and length of stay. A 2002 study by Meltzer et al.8 is also interesting, suggesting that improvements in costs and short‐term mortality are related to the disease‐specific experience of hospitalists.
Similar data for pediatric hospitalists has been slower to emerge. A systematic review of the literature by Landrigan et al., which included studies through 2004, concluded that [R]esearch suggests that pediatric hospitalists decrease costs and length of stay . The quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking.9 Since the publication of that review, there have been multiple studies which have sought to evaluate the quality of pediatric hospitalist systems. This review was undertaken to synthesize this new information, and to determine the effect of pediatric hospitalist systems on quality of care.
METHODS
A review of the available English language literature on the Medline database was undertaken in November of 2010 to answer the question, What are the differences in quality of care and outcomes of inpatient medical care provided by hospitalists versus non‐hospitalists in the pediatric population? Care metrics of interest were categorized according to the Society of Hospital Medicine's recommendations for measuring hospital performance.10
Search terms used (with additional medical subject headings [MeSH] terms in parenthesis) were hospital medicine (hospitalist), pediatrics (child health, child welfare), cost (cost and cost analysis), quality (quality indicators, healthcare), outcomes (outcome assessment, healthcare; outcomes and process assessment, healthcare); volume, patient satisfaction, length of stay, productivity (efficiency), provider satisfaction (attitude of health personnel, job satisfaction), mortality, and readmission rate (patient readmission). The citing articles search tool was used to identify other articles that potentially could meet criteria. Finally, references cited in the selected articles, as well as in excluded literature reviews, were searched for additional articles.
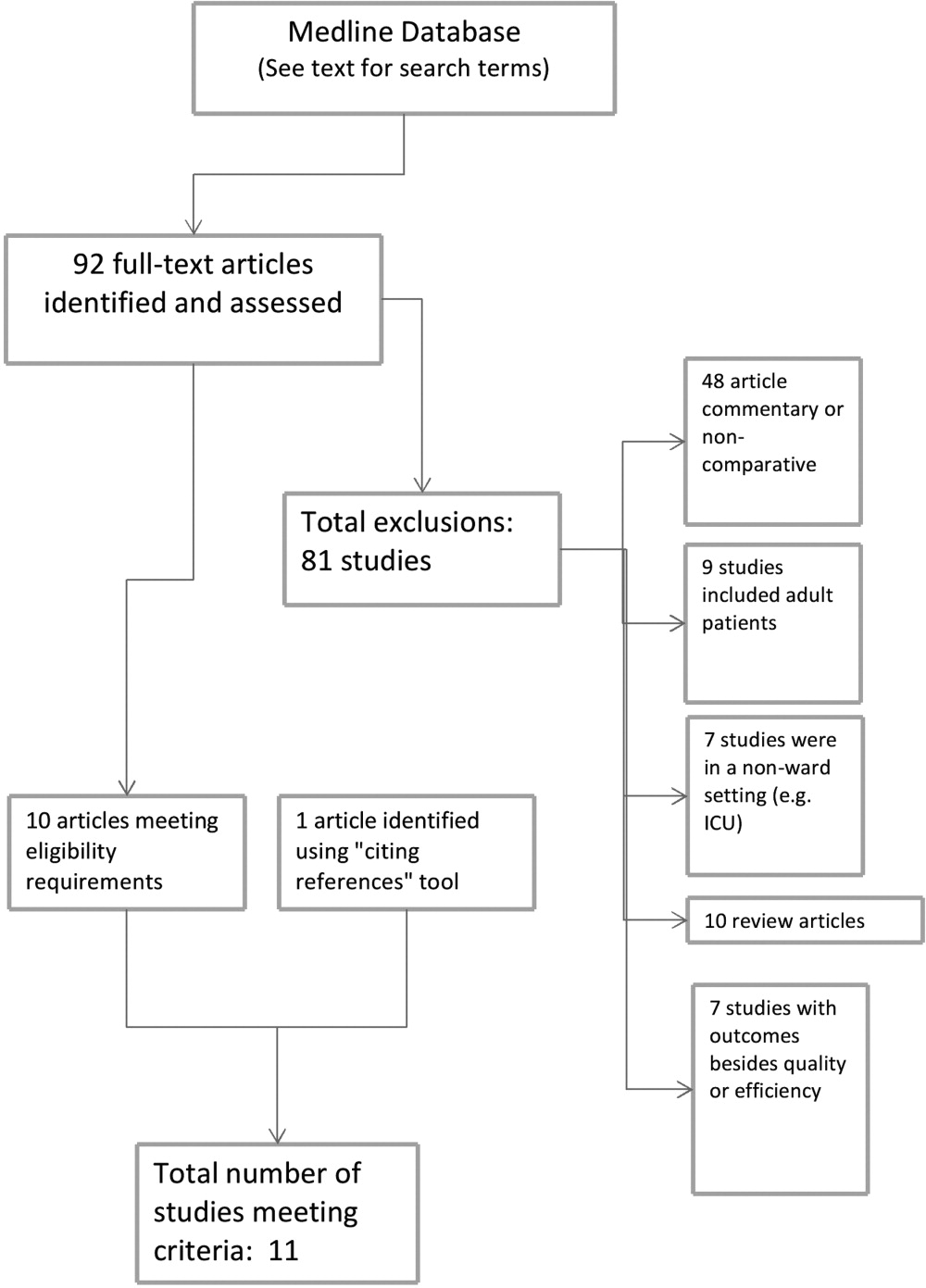
Articles were deemed eligible if they were published in a peer‐reviewed journal, if they had a comparative experimental design for hospitalists versus non‐hospitalists, and if they dealt exclusively with pediatric hospitalists. Noncomparative studies were excluded, as were studies that pertained to settings besides that of an inpatient pediatrics ward, such as pediatric intensive care units or emergency rooms. The search algorithm is diagrammed in Figure 1.
The selected articles were reviewed for the relevant outcome measures. The quality of each article was assessed using the Oxford Centre for Evidence‐Based Medicine levels of evidence,11 a widely accepted standard for critical analysis of studies. Levels of evidence are assigned to studies, from 1a (systematic reviews of randomized controlled trials) to 5 (expert opinion only). Well‐conducted prospective cohort studies receive a rating of 2c; those with wide confidence intervals due to small sample size receive a minus () modifier. This system does not specifically address survey studies, which were therefore not assigned a level of evidence.
RESULTS
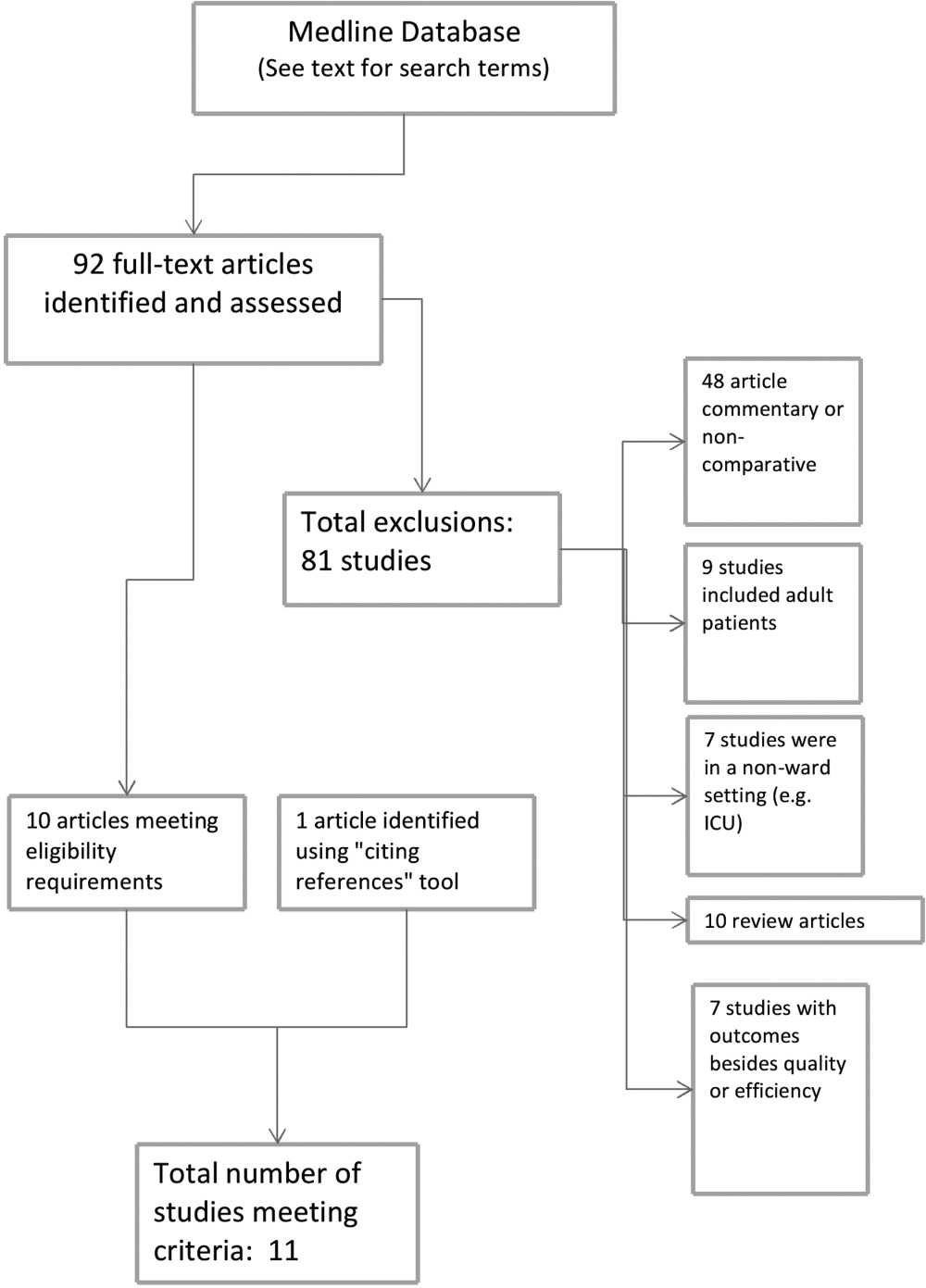
The screening process yielded 92 possible relevant articles, which were then reviewed individually (by G.M.M.) by title and abstract. A total of 81 articles were excluded, including 48 studies that were either noncomparative or descriptive in nature. Ten of the identified articles were reviews and did not contain primary data. Nine studies were not restricted to the pediatric population. Also excluded were 7 studies that did not have outcomes related to quality (eg, billing performance), and 7 studies of hospitalists in settings besides general pediatric wards (eg, pediatric intensive care units). Ten studies were thus identified. The cited reference tool was used to identify an additional article which met criteria, yielding 11 total articles that were included in the review.
Five of the identified studies published prior to 2005 were previously reviewed by Landrigan et al.9 Since then, 6 additional studies of similar nature have been published and were included here. Articles that met criteria but appeared in an earlier review are included in Table 1; new articles appear in Table 2. The results of all 11 articles were included for this discussion.
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Bellet and Whitaker13 (2000) | Cincinnati Children's Hospital Medical Center, Cincinnati, OH | 1440 general pediatric patients | LOS, costs (2c) | LOS shorter (2.4 vs 2.7 days) |
| Retrospective cohort study | Readmission rate, subspecialty consultations, mortality (2c, low power) | Costs lower ($2720 vs $3002) | ||
| Readmissions higher for hospitalists (1% vs 3%) | ||||
| No differences in consultations | ||||
| No mortality in study | ||||
| Ogershok et al.16 (2001) | West Virginia University Children's Hospitals, Morgantown, WV | 2177 general pediatric patients | LOS, cost (2c) | No difference in LOS |
| Retrospective cohort study | Readmission rate, patient satisfaction, mortality (2c, low power) | Costs lower ($1238 vs $1421) | ||
| Lab and radiology tests ordered less often | ||||
| No difference in mortality or readmission rates | ||||
| No difference in satisfaction scores | ||||
| Wells et al.15 (2001) | Valley Children's Hospital, Madera, CA | 182 general pediatric patients | LOS, cost, patient satisfaction, follow‐up rate (2c, low power) | LOS shorter (45.2 vs 66.8 hr; P = 0.01) |
| Prospective cohort study | No LOS or cost benefit for patients with bronchiolitis, gastroenteritis, or pneumonia | |||
| Costs lower ($2701 vs $4854; P = 0.005) for patients with asthma | ||||
| No difference in outpatient follow‐up rate | ||||
| Landrigan et al.14 (2002) | Boston Children's Hospital, Boston, MA | 17,873 general pediatric patients | LOS, cost (2c) | LOS shorter (2.2 vs 2.5 days) |
| Retrospective cohort study | Readmission rate, follow‐up rate, mortality (2c, low power) | Costs lower ($1139 vs $1356) | ||
| No difference in follow‐up rate | ||||
| No mortality in study | ||||
| Dwight et al.12 (2004) | Hospital for Sick Children, Toronto, Ontario, Canada | 3807 general pediatric patients | LOS (2c) | LOS shorter (from 2.9 to 2.5 days; P = 0.04) |
| Retrospective cohort study | Subspecialty consultations, readmission rate, mortality (2c, low power) | No difference in readmission rates | ||
| No difference in mortality | ||||
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Boyd et al.21 (2006) | St Joseph's Hospital and Medical Center, Phoenix, AZ | 1009 patients with 11 most common DRGs (3 groups) | Cost, LOS, and readmission rate (2c, low power) | LOS longer (2.6 2.0 vs 3.1 2.6 vs 2.9 2.3, mean SD) |
| Retrospective cohort study | Costs higher ($1781 $1449 (faculty) vs $1954 $1212 (hospitalist group 1) vs $1964 $1495 (hospitalist group 2) | |||
| No difference in readmission rates | ||||
| Conway et al.22 (2006) | National provider survey | 213 hospitalists and 352 community pediatrician survey responses | Self‐reported evidence‐based medicine use (descriptive study, no assignable level) | Hospitalists more likely to follow EBG for following: VCUG and RUS after first UTI, albuterol and ipratropium in first 24 hr for asthma |
| Descriptive study | Hospitalists less likely to use the following unproven therapies: levalbuterol and inhaled or oral steroids for bronchiolitis, stool culture or rotavirus testing for gastroenteritis, or ipratropium after 24 hr for asthma | |||
| Srivastava et al.17 (2007) | University of Utah Health Sciences Center, Salt Lake City, UT | 1970 patients with asthma, dehydration, or viral illness | LOS, cost (2c, no confidence intervals reported) | LOS shorter for asthma (0.23 days, 13%) and for dehydration (0.19 days, 11%) |
| Retrospective cohort study | No LOS difference for patients with viral illness | |||
| Costs lower for asthma ($105.51, 9.3%) and for dehydration ($86.22, 7.8%) | ||||
| Simon et al.19 (2007) | Children's Hospital of Denver, Denver, CO | 759 patients undergoing spinal fusion before and after availability of hospitalist consultation | LOS (4, unaccounted confounding factors) | LOS shorter, 6.5 (6.26.7) days to 4.8 (4.55.1) |
| Retrospective cohort study | ||||
| Bekmezian et al.18 (2008) | UCLA Hospital and Medical Center, Los Angeles, CA | 925 subspecialty patients on GI and Heme/Onc services vs hospitalist service | LOS, cost, readmission rate, mortality (2c, low power) | LOS shorter (38%, P < 0.01) |
| Retrospective cohort study | Cost lower (29%, P < 0.05) | |||
| Readmissions lower (36 for faculty vs none for hospitalists, P = 0.02) | ||||
| No difference in mortality | ||||
| Conway and Keren20 (2009) | Multicenter, 25 children's hospitals | 20,892 patients identified with UTI admissions in PHIS database | LOS, cost, evidence‐based medicine use (2c) | No difference in LOS |
| Retrospective cohort study | No difference in cost | |||
| No difference in performance of EBM guideline (VCUG and RUS for first UTI) | ||||
Effect on Length of Stay, Cost, and Resource Utilization
Ten articles addressed length of stay as an outcome measure, and 8 included cost as well. Five have been previously reported9 (see Table 1). Of these, Dwight et al.,12, Bellet and Whitaker,13 and Landrigan et al.14 found decreased length of stay (LOS) and cost for all patients. Wells et al.15 found significantly decreased LOS and cost for asthma patients but not for all diagnoses taken together, and Ogershok et al.16 found lower hospital costs but not length of stay. Five of the 6 new studies, listed in Table 2, reported on length of stay and cost. Three showed some benefits for length of stay: Srivastava et al.17 reported improvement in length of stay and cost for asthma and dehydration, but not for all diagnoses together; Bekmezian et al.18 reported improved length of stay and cost for pediatric hospitalists for patients on a hematology and gastroenterology service; and Simon et al.19 attributes a generalized decrease in length of stay on a surgical service to implementation of hospitalist comanagement of their most complex patients, though hospitalists only comanaged 12% of the patients in the study. A multicentered study in 2009 by Conway and Keren20 reported no significant difference in length of stay for general pediatric patients with urinary tract infections.
Of the 4 total studies that showed significant advantage in length of stay for hospitalist groups, improvement ranged from 11% to 38%. All attempted to adjust for diagnosis and severity using diagnosis‐related groups (DRGs) or other methods. Dwight et al.,12 Bellet and Whitaker,13 and Bekmezian et al.18 used retrospective or historical comparison alone, while Landrigan et al.14 had both concurrent and historical comparison groups.
In contrast to the other studies, Boyd et al.21 in 2006 found significant advantages, in both length of stay and cost, for a faculty/resident service in comparison to a hospitalist service. This nonrandomized, retrospective cohort study included 1009 pediatric patients, with the 11 most common DRGs, admitted during the same time period to either a traditional faculty/resident team or 1 of 2 private practice hospitalist groups at an academic medical center. The 8 general pediatric faculty practice attendings were dedicated to inpatient care while on service, and rotated bimonthly. The authors found that the faculty group patients had significantly shorter lengths of stay and total direct patient costs.
Cost‐comparison results were reported by 7 of the studies. Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 and Bekmezian et al.18 reported reductions in cost for all patients varying from 9% to 29%, while Wells et al.15 and Srivastava et al.17 found reductions in cost only for patients with certain diagnoses. Srivastava et al.17 analyzed 1970 patients, admitted with primary diagnoses of asthma, dehydration, or viral illness, over a 5‐year period from 1993 to 1997. Cost‐per‐patient was reduced between 9.3% for asthma and 7.8% for dehydrations, but when combined with the viral illness group, the difference was not statistically significant. Wells et al.15 studied 182 admissions over a 1‐year period, and found significant reductions in cost of 44% (P < 0.005) for patients with asthma but not for bronchiolitis, gastroenteritis, or pneumonia. In 2009, Conway and Keren20 studied a multicentered cohort of 20,892 children hospitalized for urinary tract infection, and found no significant difference in hospitalization costs between hospitalist services and more traditional models.
Other Quality Measures
Though financial outcomes (length of stay, cost, and resource utilization) were the primary area of emphasis for most of the selected articles, other parameters with more of a focus on quality were examined as well. The studies by Dwight et al.,12 Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 Bekmezian et al.,18 and Boyd et al.21 examined mortality and readmission rate. None of these studies reported differences in mortality rate, though none were powered to do so. When studying readmission rate, Bellet and Whitaker13 reported a statistically significant lower rate of readmission for a traditionally staffed service versus the hospitalist service (1% vs 3%; P = 0.006). In contrast, Bekmezian et al.18 found a lower readmission rate for the hospitalist service (4.4% vs 0%; P = 0.02). The studies by Dwight et al.,12 Landrigan et al.,14 Ogershok et al.,16 and Boyd et al.21 did not detect differences in readmission rates.
Two studies measured patient satisfaction.15, 16 Ogershok et al.16 utilized hospital‐generated patient satisfaction surveys, completed at discharge, for comparison and found no differences between the hospitalist and non‐hospitalist ward services. Wells et al.15 utilized a standardized patient satisfaction assessment tool, given at discharge, followed by a telephone interview after 1 month. At discharge, parents rated hospitalist physicians higher in courtesy (P < 0.05) and friendliness (P < 0.005), though this difference was not detected in the telephone interviews 1 month later. However, at that time, parents did indicate that they received better explanations about their child's illness if their child was seen by their primary care physician rather than a hospitalist.
In 2006, a study by Conway et al.22 reported on the use of evidence‐based therapies and tests by hospitalists as compared to community pediatricians. The survey identified evidence‐based therapies and tests for asthma, bronchiolitis, gastroenteritis, and first‐time urinary tract infection (UTI) diagnosis. A total of 213 hospitalists and 228 community pediatricians met the inclusion criteria by returning the completed survey. After multivariate regression analysis, hospitalists were found to be more likely to use 4 of 5 evidence‐based therapies and recommended tests, and were less likely to use 6 of 7 therapies and tests of unproven benefit. In 2009, Conway and Clancy23 again studied the use of evidence‐based therapies, this time using more objective measures. In this report, the Pediatric Health Information System (PHIS) was examined for a cohort of 20,892 patients. After multivariable regression analysis, there was no statistical difference in the performance of evidence‐based imaging following a first UTI between hospitals staffed primarily by community pediatricians versus those with pediatric hospitalist systems. However, it should be noted that the evidence base for UTI‐related imaging has been debated in the literature over the past decade.
DISCUSSION
Of the 11 studies selected for this review, 10 measured length of stay as an outcome, with the majority favoring hospitalists but with mixed results. Three of these studies, those by Dwight et al.,12 Bellet and Whitaker,13 and Landrigan et al.,14 demonstrated 11% to 14% improvement for hospitalist services compared to community pediatricians. Boyd et al.,21 however, found exactly the opposite result, and 2 studies by Conway and Keren20 and Ogershok et al.16 found no difference in length of stay. Two more studies found benefits restricted to certain conditions: Wells et al.15 found 32% shorter lengths of stay for asthma, but not for other conditions; Srivastava et al.17 found a 13% reduction in length of stay for asthma and 11% for dehydration, but none for viral illnesses or when all conditions were combined. Bekmezian et al.18 found shorter lengths of stay on a hospitalist service for hematology and gastroenterology patients, and Simon et al.19 attribute a general trend of decreasing lengths of stay on a surgical service to the implementation of hospital comanagement for a small percentage of patients.
The most common quality measures studied were patient satisfaction, readmission rates, and mortality. Only 1 study by Ogershok et al.16 reported on patient satisfaction and found few differences between hospitalists and community pediatricians. Readmission rate were reported by 6 studies. Bellet and Whitaker13 found a higher readmission rate for pediatric hospitalists, Bekmezian et al.18 found a lower rate but on a subspecialty service. The study with the greatest power for this analysis, by Landrigan et al.14 with nearly 18,000 patients, found no difference, and neither did another 3 studies. Unsurprisingly, no study detected differences in mortality; it would be extremely difficult to adequately power a study to do so in the general pediatric setting, where mortality is rare.
The effect of relative experience of hospitalist physicians is uncertain. Boyd et al.21 speculated that 1 possible cause for the decreased lengths of stay and costs associated with their faculty group compared to hospitalists may have been due to the increased experience of the faculty group. Unfortunately, they were unable to generate statistical significance due to the small numbers of physicians in the study. In contrast, the hospitalists in the report by Dwight et al.12 had decreased lengths of stay but were less experienced. In the adult literature, the study by Meltzer et al.8 suggests that improved outcomes from hospitalist systems may not become apparent for 1 or more years after implementation, but none of the pediatric studies included in our review specifically address this issue. This leaves the possibility open that the hospitalist systems evaluated in some studies had insufficient time in which to develop increased efficiencies.
There were several limitations to our studies. First, due to the heterogeneity and methodological variations among the included studies, we were unable to perform a meta‐analysis. Second, the overall quality of evidence is limited due to the lack of randomized control trials. Third, a lack of agreement on appropriate quality markers has limited the study of quality of care. Published reports continue to focus on financial measures, such as length of stay, despite the recommendation in the previous review by Landrigan et al.9 that such studies would be of limited value. Finally, the current variability of hospitalist models and lack of study of factors that might influence outcomes makes comparisons difficult.
Despite these limitations, several interesting trends emerge from these studies. One such trend is that the more recent studies highlight that simple classification of hospitalist system versus traditional system fails to measure the complexity and nuance of care delivery. The 2006 study by Boyd et al.21 is especially notable because it showed the opposite effect of previous studies, namely, an increase in length of stay and costs for hospitalists at St Joseph's Medical Center in Phoenix, Arizona. In this study, the traditional faculty group was employed by the hospital, and the hospitalist group was a private practice model. The authors suggest that their faculty physicians were therefore operating like hospitalists in that almost all of their time was focused on inpatient care while they were on service. They also had a limited number of general pediatricians, who attended in the inpatient setting, who were more experienced than the private practice groups. Also, the authors theorize that their faculty may have had a closer working relationship with their residents due to additional service responsibilities and locations of the faculty group onsite. Further study of the care models utilized by faculty and hospitalist practices at St Joseph's and other hospitals may reveal important insights about improving the quality and efficiency of inpatient pediatric care in general.
Though there is a clear trend in the adult literature indicating that the use of hospitalists results in superior quality of care, there is less evidence for pediatric systems. The aforementioned previous review by Landrigan et al.9, in 2006 concluded that emerging research suggests that pediatric hospitalist systems decrease cost and length of stay, but also the quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking. Data from the 6 additional studies presented here lend limited support to the first hypothesis, and the presence of only 1 negative study is not sufficient to undermine it.
While data on quality markers such as readmission rate or mortality remain elusive, the 2 studies by Conway et al.20, 22 attempt to evaluate quality by comparing the use of evidence‐based therapies by hospitalists and community pediatricians. Though the use of objective PHIS data for UTI in 2009 did not confirm the conclusion suggested by the 2006 provider survey study, the attempt to find measurable outcomes such as the use of evidence‐based therapies is a start but we need more metrics, including rigorous patient outcome metrics, to define the quality of our care systems. Before the effect of hospitalist systems on quality is fully understood, more work will need to be done defining metrics for comparison.
Unfortunately, over 5 years since the previous review by Landrigan et al.9 called for increased focus on inpatient quality and understanding how to improve, the sophistication of our measurement of pediatric inpatient quality and understanding of the mechanisms underlying improvement is still in its infancy. We propose a solution at multiple levels.
First, the investment in research comparing system‐level interventions (eg, discharge process A vs discharge process B) must be increased. This investment increased significantly due to the over $1 billion in Recovery Act funding for comparative effectiveness research.23 However, the future investment in comparative effectiveness research, often called patient‐centered outcomes research, and proportion of investment focused on delivery system interventions is unclear. We propose that the investment in comparing delivery system interventions is essential to improving not only hospital medicine systems, but, more importantly, the healthcare system broadly. In addition, research investment needs to focus on reliably implementing proven interventions in systems of care, and evaluating both the effects on patient outcomes and cost, and the contextual factors associated with successful implementation.24 A hospital medicine example would be the comparison of the implementation of a guideline for a common disease across a set of hospitals. One could perform a prospective observational design, in which one compares high intensity versus low intensity intervention and assesses the baseline characteristics of the hospital systems, to understand their association with successful implementation and, ultimately, patient outcomes. One could also perform a clustered randomized design.
Second, the development and implementation of pediatric quality of care measures, including in the inpatient setting, needs to increase rapidly. The Children's Health Insurance Program (CHIP) and its focus on an initial core set of quality measures that expands over time, through an investment in measure development and validation, is an opportunity for pediatric hospital medicine. Inpatient measures should be a focus of measure development and implementation. We must move beyond a limited set of inpatient measures to a broader set focused on issues such as patient safety, hospital‐acquired infections, outcomes for common illnesses, and transitions of care. We also need better measures for important pediatric populations, such as children with complex medical conditions.25
Third, our understanding of the mechanisms leading to improvement in hospital medicine systems needs to be developed. Studies of hospital medicine systems should move past simple binary comparisons of hospitalist systems versus traditional systems to understand the effect on patient outcomes and cost of factors such as years of experience, volume of patients seen overall and with a specific condition, staffing model, training, quality improvement knowledge and application, and health information systems. These factors may be additive or multiplicative to the performance of inpatient systems once put into place, but these hypotheses need to be tested.
Fourth, individual hospitalists and their groups must focus on quality measurement and improvement in quality and value delivered. At Cincinnati, we have a portfolio of quality and value projects derived from our strategic objectives, illustrated in Figure 2. The projects have leaders and teams to drive improvement and measure results. Increasingly, we are able to publish these results in peer‐reviewed journals. On a quarterly basis, we review the portfolio via a dashboard and/or run and control charts. We establish new projects and set new goals on at least an annual basis. It is important to note that at the beginning of the 2010‐2011 fiscal year, almost all initiatives identified as priorities were yellow or red. Our group is now planning new initiatives and goals for next year. This is one method applicable to our setting, but a focus on quality and value and measuring results needs to be part of every hospital medicine program. As payer focus on value increases, this will be essential to demonstrate how a hospitalist group improves outcomes and adds value.
CONCLUSION
This review suggests that the use of hospitalists can improve the quality of inpatient care in the pediatric population, but this is not a universal finding and, most importantly, the mechanisms of improvement are poorly understood. We propose 4 components to address these issues so that a systematic review 5 years from now would be much more robust. These are: 1) increased investment in research comparing system‐level interventions and reliable implementation; 2) further development and implementation of pediatric quality of care measures in the inpatient setting; 3) understanding the mechanisms and factors leading to improvement in hospital medicine systems; and 4) an increased focus on quality measurement, and improvement in quality and value delivered by all individual hospitalists and their groups.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists.Mayo Clin Proc.2009;84(3):248–254.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;375(25):2589–2600.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–875.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- Society of Hospital Medicine. Measuring hospitalist performance: metrics, reports, and dashboards. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Publications; April2007.
- Oxford Centre for Evidence‐Based Medicine levels of evidence. Updated March 2009. Available at: http://www.cebm.net/index.aspx?o=1025. Accessed March 14,2011.
- ,,,.Evaluation of a staff‐only hospitalist system in a tertiary care, academic children's hospital.Pediatrics.2004;114(6):1545–1549.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105(3 pt 1):478–484.
- ,,, et al.Impact of a health maintenance organization hospitalist system in academic pediatrics.Pediatrics.2002;110(4):720–728.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual.2001;16(5):174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila).2001;40(12):653–662.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,.Staff‐only pediatric hospitalist care of patients with medically complex subspecialty conditions in a major teaching hospital.Arch Pediatr Adolesc Med.2008;162(10):975–980.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2(1):23–30.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154(6):789–796.
- ,,,,.Comparison of outcome measures for a traditional pediatric faculty service and nonfaculty hospitalist services in a community teaching hospital.Pediatrics.2006;118(4):1327–1331.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,.Comparative‐effectiveness research—implications of the federal coordinating council's report.N Engl J Med.2009;361(4):328–330.
- ,.Charting a path from comparative effectiveness funding to improved patient‐centered health care.JAMA.2010;303(10):985–986.
- ,,, et al.Children with medical complexity: an emerging population for clinical and research initiatives.Pediatrics.2011;127(3):529–538.
In the United States, general medical inpatient care is increasingly provided by hospital‐based physicians, also called hospitalists.1 The field of pediatrics is no exception, and by 2005 there were an estimated 1000 pediatric hospitalists in the workforce.2 Current numbers are likely to be greater than 2500, as the need for pediatric hospitalists has grown considerably.
At the same time, the quality of care delivered by the United States health system has come under increased scrutiny. In 2001, the Institute of Medicine, in its report on the quality of healthcare in America, concluded that between the care we have and what we could have lies not just a gap but a chasm.3 Meanwhile, the cost of healthcare delivery continues to increase. The pressure to deliver cost‐effective, high quality care is among the more important forces driving the proliferation of hospitalists.4
Over the last decade, data supporting the role of hospitalists in improving quality of care for adult patients has continued to accumulate.58 A 2007 retrospective cohort study by Lindenaur et al.7 included nearly 77,000 adult patients and found small reductions in length of stay without adverse effects on mortality or readmission rates, and a 2009 systematic review by Peterson6 included 33 studies and concluded that in general inpatient care of general medical patients by hospitalist physicians leads to decreased hospital cost and length of stay. A 2002 study by Meltzer et al.8 is also interesting, suggesting that improvements in costs and short‐term mortality are related to the disease‐specific experience of hospitalists.
Similar data for pediatric hospitalists has been slower to emerge. A systematic review of the literature by Landrigan et al., which included studies through 2004, concluded that [R]esearch suggests that pediatric hospitalists decrease costs and length of stay . The quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking.9 Since the publication of that review, there have been multiple studies which have sought to evaluate the quality of pediatric hospitalist systems. This review was undertaken to synthesize this new information, and to determine the effect of pediatric hospitalist systems on quality of care.
METHODS
A review of the available English language literature on the Medline database was undertaken in November of 2010 to answer the question, What are the differences in quality of care and outcomes of inpatient medical care provided by hospitalists versus non‐hospitalists in the pediatric population? Care metrics of interest were categorized according to the Society of Hospital Medicine's recommendations for measuring hospital performance.10
Search terms used (with additional medical subject headings [MeSH] terms in parenthesis) were hospital medicine (hospitalist), pediatrics (child health, child welfare), cost (cost and cost analysis), quality (quality indicators, healthcare), outcomes (outcome assessment, healthcare; outcomes and process assessment, healthcare); volume, patient satisfaction, length of stay, productivity (efficiency), provider satisfaction (attitude of health personnel, job satisfaction), mortality, and readmission rate (patient readmission). The citing articles search tool was used to identify other articles that potentially could meet criteria. Finally, references cited in the selected articles, as well as in excluded literature reviews, were searched for additional articles.
Articles were deemed eligible if they were published in a peer‐reviewed journal, if they had a comparative experimental design for hospitalists versus non‐hospitalists, and if they dealt exclusively with pediatric hospitalists. Noncomparative studies were excluded, as were studies that pertained to settings besides that of an inpatient pediatrics ward, such as pediatric intensive care units or emergency rooms. The search algorithm is diagrammed in Figure 1.

The selected articles were reviewed for the relevant outcome measures. The quality of each article was assessed using the Oxford Centre for Evidence‐Based Medicine levels of evidence,11 a widely accepted standard for critical analysis of studies. Levels of evidence are assigned to studies, from 1a (systematic reviews of randomized controlled trials) to 5 (expert opinion only). Well‐conducted prospective cohort studies receive a rating of 2c; those with wide confidence intervals due to small sample size receive a minus () modifier. This system does not specifically address survey studies, which were therefore not assigned a level of evidence.
RESULTS
The screening process yielded 92 possible relevant articles, which were then reviewed individually (by G.M.M.) by title and abstract. A total of 81 articles were excluded, including 48 studies that were either noncomparative or descriptive in nature. Ten of the identified articles were reviews and did not contain primary data. Nine studies were not restricted to the pediatric population. Also excluded were 7 studies that did not have outcomes related to quality (eg, billing performance), and 7 studies of hospitalists in settings besides general pediatric wards (eg, pediatric intensive care units). Ten studies were thus identified. The cited reference tool was used to identify an additional article which met criteria, yielding 11 total articles that were included in the review.
Five of the identified studies published prior to 2005 were previously reviewed by Landrigan et al.9 Since then, 6 additional studies of similar nature have been published and were included here. Articles that met criteria but appeared in an earlier review are included in Table 1; new articles appear in Table 2. The results of all 11 articles were included for this discussion.
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Bellet and Whitaker13 (2000) | Cincinnati Children's Hospital Medical Center, Cincinnati, OH | 1440 general pediatric patients | LOS, costs (2c) | LOS shorter (2.4 vs 2.7 days) |
| Retrospective cohort study | Readmission rate, subspecialty consultations, mortality (2c, low power) | Costs lower ($2720 vs $3002) | ||
| Readmissions higher for hospitalists (1% vs 3%) | ||||
| No differences in consultations | ||||
| No mortality in study | ||||
| Ogershok et al.16 (2001) | West Virginia University Children's Hospitals, Morgantown, WV | 2177 general pediatric patients | LOS, cost (2c) | No difference in LOS |
| Retrospective cohort study | Readmission rate, patient satisfaction, mortality (2c, low power) | Costs lower ($1238 vs $1421) | ||
| Lab and radiology tests ordered less often | ||||
| No difference in mortality or readmission rates | ||||
| No difference in satisfaction scores | ||||
| Wells et al.15 (2001) | Valley Children's Hospital, Madera, CA | 182 general pediatric patients | LOS, cost, patient satisfaction, follow‐up rate (2c, low power) | LOS shorter (45.2 vs 66.8 hr; P = 0.01) |
| Prospective cohort study | No LOS or cost benefit for patients with bronchiolitis, gastroenteritis, or pneumonia | |||
| Costs lower ($2701 vs $4854; P = 0.005) for patients with asthma | ||||
| No difference in outpatient follow‐up rate | ||||
| Landrigan et al.14 (2002) | Boston Children's Hospital, Boston, MA | 17,873 general pediatric patients | LOS, cost (2c) | LOS shorter (2.2 vs 2.5 days) |
| Retrospective cohort study | Readmission rate, follow‐up rate, mortality (2c, low power) | Costs lower ($1139 vs $1356) | ||
| No difference in follow‐up rate | ||||
| No mortality in study | ||||
| Dwight et al.12 (2004) | Hospital for Sick Children, Toronto, Ontario, Canada | 3807 general pediatric patients | LOS (2c) | LOS shorter (from 2.9 to 2.5 days; P = 0.04) |
| Retrospective cohort study | Subspecialty consultations, readmission rate, mortality (2c, low power) | No difference in readmission rates | ||
| No difference in mortality | ||||
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Boyd et al.21 (2006) | St Joseph's Hospital and Medical Center, Phoenix, AZ | 1009 patients with 11 most common DRGs (3 groups) | Cost, LOS, and readmission rate (2c, low power) | LOS longer (2.6 2.0 vs 3.1 2.6 vs 2.9 2.3, mean SD) |
| Retrospective cohort study | Costs higher ($1781 $1449 (faculty) vs $1954 $1212 (hospitalist group 1) vs $1964 $1495 (hospitalist group 2) | |||
| No difference in readmission rates | ||||
| Conway et al.22 (2006) | National provider survey | 213 hospitalists and 352 community pediatrician survey responses | Self‐reported evidence‐based medicine use (descriptive study, no assignable level) | Hospitalists more likely to follow EBG for following: VCUG and RUS after first UTI, albuterol and ipratropium in first 24 hr for asthma |
| Descriptive study | Hospitalists less likely to use the following unproven therapies: levalbuterol and inhaled or oral steroids for bronchiolitis, stool culture or rotavirus testing for gastroenteritis, or ipratropium after 24 hr for asthma | |||
| Srivastava et al.17 (2007) | University of Utah Health Sciences Center, Salt Lake City, UT | 1970 patients with asthma, dehydration, or viral illness | LOS, cost (2c, no confidence intervals reported) | LOS shorter for asthma (0.23 days, 13%) and for dehydration (0.19 days, 11%) |
| Retrospective cohort study | No LOS difference for patients with viral illness | |||
| Costs lower for asthma ($105.51, 9.3%) and for dehydration ($86.22, 7.8%) | ||||
| Simon et al.19 (2007) | Children's Hospital of Denver, Denver, CO | 759 patients undergoing spinal fusion before and after availability of hospitalist consultation | LOS (4, unaccounted confounding factors) | LOS shorter, 6.5 (6.26.7) days to 4.8 (4.55.1) |
| Retrospective cohort study | ||||
| Bekmezian et al.18 (2008) | UCLA Hospital and Medical Center, Los Angeles, CA | 925 subspecialty patients on GI and Heme/Onc services vs hospitalist service | LOS, cost, readmission rate, mortality (2c, low power) | LOS shorter (38%, P < 0.01) |
| Retrospective cohort study | Cost lower (29%, P < 0.05) | |||
| Readmissions lower (36 for faculty vs none for hospitalists, P = 0.02) | ||||
| No difference in mortality | ||||
| Conway and Keren20 (2009) | Multicenter, 25 children's hospitals | 20,892 patients identified with UTI admissions in PHIS database | LOS, cost, evidence‐based medicine use (2c) | No difference in LOS |
| Retrospective cohort study | No difference in cost | |||
| No difference in performance of EBM guideline (VCUG and RUS for first UTI) | ||||
Effect on Length of Stay, Cost, and Resource Utilization
Ten articles addressed length of stay as an outcome measure, and 8 included cost as well. Five have been previously reported9 (see Table 1). Of these, Dwight et al.,12, Bellet and Whitaker,13 and Landrigan et al.14 found decreased length of stay (LOS) and cost for all patients. Wells et al.15 found significantly decreased LOS and cost for asthma patients but not for all diagnoses taken together, and Ogershok et al.16 found lower hospital costs but not length of stay. Five of the 6 new studies, listed in Table 2, reported on length of stay and cost. Three showed some benefits for length of stay: Srivastava et al.17 reported improvement in length of stay and cost for asthma and dehydration, but not for all diagnoses together; Bekmezian et al.18 reported improved length of stay and cost for pediatric hospitalists for patients on a hematology and gastroenterology service; and Simon et al.19 attributes a generalized decrease in length of stay on a surgical service to implementation of hospitalist comanagement of their most complex patients, though hospitalists only comanaged 12% of the patients in the study. A multicentered study in 2009 by Conway and Keren20 reported no significant difference in length of stay for general pediatric patients with urinary tract infections.
Of the 4 total studies that showed significant advantage in length of stay for hospitalist groups, improvement ranged from 11% to 38%. All attempted to adjust for diagnosis and severity using diagnosis‐related groups (DRGs) or other methods. Dwight et al.,12 Bellet and Whitaker,13 and Bekmezian et al.18 used retrospective or historical comparison alone, while Landrigan et al.14 had both concurrent and historical comparison groups.
In contrast to the other studies, Boyd et al.21 in 2006 found significant advantages, in both length of stay and cost, for a faculty/resident service in comparison to a hospitalist service. This nonrandomized, retrospective cohort study included 1009 pediatric patients, with the 11 most common DRGs, admitted during the same time period to either a traditional faculty/resident team or 1 of 2 private practice hospitalist groups at an academic medical center. The 8 general pediatric faculty practice attendings were dedicated to inpatient care while on service, and rotated bimonthly. The authors found that the faculty group patients had significantly shorter lengths of stay and total direct patient costs.
Cost‐comparison results were reported by 7 of the studies. Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 and Bekmezian et al.18 reported reductions in cost for all patients varying from 9% to 29%, while Wells et al.15 and Srivastava et al.17 found reductions in cost only for patients with certain diagnoses. Srivastava et al.17 analyzed 1970 patients, admitted with primary diagnoses of asthma, dehydration, or viral illness, over a 5‐year period from 1993 to 1997. Cost‐per‐patient was reduced between 9.3% for asthma and 7.8% for dehydrations, but when combined with the viral illness group, the difference was not statistically significant. Wells et al.15 studied 182 admissions over a 1‐year period, and found significant reductions in cost of 44% (P < 0.005) for patients with asthma but not for bronchiolitis, gastroenteritis, or pneumonia. In 2009, Conway and Keren20 studied a multicentered cohort of 20,892 children hospitalized for urinary tract infection, and found no significant difference in hospitalization costs between hospitalist services and more traditional models.
Other Quality Measures
Though financial outcomes (length of stay, cost, and resource utilization) were the primary area of emphasis for most of the selected articles, other parameters with more of a focus on quality were examined as well. The studies by Dwight et al.,12 Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 Bekmezian et al.,18 and Boyd et al.21 examined mortality and readmission rate. None of these studies reported differences in mortality rate, though none were powered to do so. When studying readmission rate, Bellet and Whitaker13 reported a statistically significant lower rate of readmission for a traditionally staffed service versus the hospitalist service (1% vs 3%; P = 0.006). In contrast, Bekmezian et al.18 found a lower readmission rate for the hospitalist service (4.4% vs 0%; P = 0.02). The studies by Dwight et al.,12 Landrigan et al.,14 Ogershok et al.,16 and Boyd et al.21 did not detect differences in readmission rates.
Two studies measured patient satisfaction.15, 16 Ogershok et al.16 utilized hospital‐generated patient satisfaction surveys, completed at discharge, for comparison and found no differences between the hospitalist and non‐hospitalist ward services. Wells et al.15 utilized a standardized patient satisfaction assessment tool, given at discharge, followed by a telephone interview after 1 month. At discharge, parents rated hospitalist physicians higher in courtesy (P < 0.05) and friendliness (P < 0.005), though this difference was not detected in the telephone interviews 1 month later. However, at that time, parents did indicate that they received better explanations about their child's illness if their child was seen by their primary care physician rather than a hospitalist.
In 2006, a study by Conway et al.22 reported on the use of evidence‐based therapies and tests by hospitalists as compared to community pediatricians. The survey identified evidence‐based therapies and tests for asthma, bronchiolitis, gastroenteritis, and first‐time urinary tract infection (UTI) diagnosis. A total of 213 hospitalists and 228 community pediatricians met the inclusion criteria by returning the completed survey. After multivariate regression analysis, hospitalists were found to be more likely to use 4 of 5 evidence‐based therapies and recommended tests, and were less likely to use 6 of 7 therapies and tests of unproven benefit. In 2009, Conway and Clancy23 again studied the use of evidence‐based therapies, this time using more objective measures. In this report, the Pediatric Health Information System (PHIS) was examined for a cohort of 20,892 patients. After multivariable regression analysis, there was no statistical difference in the performance of evidence‐based imaging following a first UTI between hospitals staffed primarily by community pediatricians versus those with pediatric hospitalist systems. However, it should be noted that the evidence base for UTI‐related imaging has been debated in the literature over the past decade.
DISCUSSION
Of the 11 studies selected for this review, 10 measured length of stay as an outcome, with the majority favoring hospitalists but with mixed results. Three of these studies, those by Dwight et al.,12 Bellet and Whitaker,13 and Landrigan et al.,14 demonstrated 11% to 14% improvement for hospitalist services compared to community pediatricians. Boyd et al.,21 however, found exactly the opposite result, and 2 studies by Conway and Keren20 and Ogershok et al.16 found no difference in length of stay. Two more studies found benefits restricted to certain conditions: Wells et al.15 found 32% shorter lengths of stay for asthma, but not for other conditions; Srivastava et al.17 found a 13% reduction in length of stay for asthma and 11% for dehydration, but none for viral illnesses or when all conditions were combined. Bekmezian et al.18 found shorter lengths of stay on a hospitalist service for hematology and gastroenterology patients, and Simon et al.19 attribute a general trend of decreasing lengths of stay on a surgical service to the implementation of hospital comanagement for a small percentage of patients.
The most common quality measures studied were patient satisfaction, readmission rates, and mortality. Only 1 study by Ogershok et al.16 reported on patient satisfaction and found few differences between hospitalists and community pediatricians. Readmission rate were reported by 6 studies. Bellet and Whitaker13 found a higher readmission rate for pediatric hospitalists, Bekmezian et al.18 found a lower rate but on a subspecialty service. The study with the greatest power for this analysis, by Landrigan et al.14 with nearly 18,000 patients, found no difference, and neither did another 3 studies. Unsurprisingly, no study detected differences in mortality; it would be extremely difficult to adequately power a study to do so in the general pediatric setting, where mortality is rare.
The effect of relative experience of hospitalist physicians is uncertain. Boyd et al.21 speculated that 1 possible cause for the decreased lengths of stay and costs associated with their faculty group compared to hospitalists may have been due to the increased experience of the faculty group. Unfortunately, they were unable to generate statistical significance due to the small numbers of physicians in the study. In contrast, the hospitalists in the report by Dwight et al.12 had decreased lengths of stay but were less experienced. In the adult literature, the study by Meltzer et al.8 suggests that improved outcomes from hospitalist systems may not become apparent for 1 or more years after implementation, but none of the pediatric studies included in our review specifically address this issue. This leaves the possibility open that the hospitalist systems evaluated in some studies had insufficient time in which to develop increased efficiencies.
There were several limitations to our studies. First, due to the heterogeneity and methodological variations among the included studies, we were unable to perform a meta‐analysis. Second, the overall quality of evidence is limited due to the lack of randomized control trials. Third, a lack of agreement on appropriate quality markers has limited the study of quality of care. Published reports continue to focus on financial measures, such as length of stay, despite the recommendation in the previous review by Landrigan et al.9 that such studies would be of limited value. Finally, the current variability of hospitalist models and lack of study of factors that might influence outcomes makes comparisons difficult.
Despite these limitations, several interesting trends emerge from these studies. One such trend is that the more recent studies highlight that simple classification of hospitalist system versus traditional system fails to measure the complexity and nuance of care delivery. The 2006 study by Boyd et al.21 is especially notable because it showed the opposite effect of previous studies, namely, an increase in length of stay and costs for hospitalists at St Joseph's Medical Center in Phoenix, Arizona. In this study, the traditional faculty group was employed by the hospital, and the hospitalist group was a private practice model. The authors suggest that their faculty physicians were therefore operating like hospitalists in that almost all of their time was focused on inpatient care while they were on service. They also had a limited number of general pediatricians, who attended in the inpatient setting, who were more experienced than the private practice groups. Also, the authors theorize that their faculty may have had a closer working relationship with their residents due to additional service responsibilities and locations of the faculty group onsite. Further study of the care models utilized by faculty and hospitalist practices at St Joseph's and other hospitals may reveal important insights about improving the quality and efficiency of inpatient pediatric care in general.
Though there is a clear trend in the adult literature indicating that the use of hospitalists results in superior quality of care, there is less evidence for pediatric systems. The aforementioned previous review by Landrigan et al.9, in 2006 concluded that emerging research suggests that pediatric hospitalist systems decrease cost and length of stay, but also the quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking. Data from the 6 additional studies presented here lend limited support to the first hypothesis, and the presence of only 1 negative study is not sufficient to undermine it.
While data on quality markers such as readmission rate or mortality remain elusive, the 2 studies by Conway et al.20, 22 attempt to evaluate quality by comparing the use of evidence‐based therapies by hospitalists and community pediatricians. Though the use of objective PHIS data for UTI in 2009 did not confirm the conclusion suggested by the 2006 provider survey study, the attempt to find measurable outcomes such as the use of evidence‐based therapies is a start but we need more metrics, including rigorous patient outcome metrics, to define the quality of our care systems. Before the effect of hospitalist systems on quality is fully understood, more work will need to be done defining metrics for comparison.
Unfortunately, over 5 years since the previous review by Landrigan et al.9 called for increased focus on inpatient quality and understanding how to improve, the sophistication of our measurement of pediatric inpatient quality and understanding of the mechanisms underlying improvement is still in its infancy. We propose a solution at multiple levels.
First, the investment in research comparing system‐level interventions (eg, discharge process A vs discharge process B) must be increased. This investment increased significantly due to the over $1 billion in Recovery Act funding for comparative effectiveness research.23 However, the future investment in comparative effectiveness research, often called patient‐centered outcomes research, and proportion of investment focused on delivery system interventions is unclear. We propose that the investment in comparing delivery system interventions is essential to improving not only hospital medicine systems, but, more importantly, the healthcare system broadly. In addition, research investment needs to focus on reliably implementing proven interventions in systems of care, and evaluating both the effects on patient outcomes and cost, and the contextual factors associated with successful implementation.24 A hospital medicine example would be the comparison of the implementation of a guideline for a common disease across a set of hospitals. One could perform a prospective observational design, in which one compares high intensity versus low intensity intervention and assesses the baseline characteristics of the hospital systems, to understand their association with successful implementation and, ultimately, patient outcomes. One could also perform a clustered randomized design.
Second, the development and implementation of pediatric quality of care measures, including in the inpatient setting, needs to increase rapidly. The Children's Health Insurance Program (CHIP) and its focus on an initial core set of quality measures that expands over time, through an investment in measure development and validation, is an opportunity for pediatric hospital medicine. Inpatient measures should be a focus of measure development and implementation. We must move beyond a limited set of inpatient measures to a broader set focused on issues such as patient safety, hospital‐acquired infections, outcomes for common illnesses, and transitions of care. We also need better measures for important pediatric populations, such as children with complex medical conditions.25
Third, our understanding of the mechanisms leading to improvement in hospital medicine systems needs to be developed. Studies of hospital medicine systems should move past simple binary comparisons of hospitalist systems versus traditional systems to understand the effect on patient outcomes and cost of factors such as years of experience, volume of patients seen overall and with a specific condition, staffing model, training, quality improvement knowledge and application, and health information systems. These factors may be additive or multiplicative to the performance of inpatient systems once put into place, but these hypotheses need to be tested.
Fourth, individual hospitalists and their groups must focus on quality measurement and improvement in quality and value delivered. At Cincinnati, we have a portfolio of quality and value projects derived from our strategic objectives, illustrated in Figure 2. The projects have leaders and teams to drive improvement and measure results. Increasingly, we are able to publish these results in peer‐reviewed journals. On a quarterly basis, we review the portfolio via a dashboard and/or run and control charts. We establish new projects and set new goals on at least an annual basis. It is important to note that at the beginning of the 2010‐2011 fiscal year, almost all initiatives identified as priorities were yellow or red. Our group is now planning new initiatives and goals for next year. This is one method applicable to our setting, but a focus on quality and value and measuring results needs to be part of every hospital medicine program. As payer focus on value increases, this will be essential to demonstrate how a hospitalist group improves outcomes and adds value.

CONCLUSION
This review suggests that the use of hospitalists can improve the quality of inpatient care in the pediatric population, but this is not a universal finding and, most importantly, the mechanisms of improvement are poorly understood. We propose 4 components to address these issues so that a systematic review 5 years from now would be much more robust. These are: 1) increased investment in research comparing system‐level interventions and reliable implementation; 2) further development and implementation of pediatric quality of care measures in the inpatient setting; 3) understanding the mechanisms and factors leading to improvement in hospital medicine systems; and 4) an increased focus on quality measurement, and improvement in quality and value delivered by all individual hospitalists and their groups.
In the United States, general medical inpatient care is increasingly provided by hospital‐based physicians, also called hospitalists.1 The field of pediatrics is no exception, and by 2005 there were an estimated 1000 pediatric hospitalists in the workforce.2 Current numbers are likely to be greater than 2500, as the need for pediatric hospitalists has grown considerably.
At the same time, the quality of care delivered by the United States health system has come under increased scrutiny. In 2001, the Institute of Medicine, in its report on the quality of healthcare in America, concluded that between the care we have and what we could have lies not just a gap but a chasm.3 Meanwhile, the cost of healthcare delivery continues to increase. The pressure to deliver cost‐effective, high quality care is among the more important forces driving the proliferation of hospitalists.4
Over the last decade, data supporting the role of hospitalists in improving quality of care for adult patients has continued to accumulate.58 A 2007 retrospective cohort study by Lindenaur et al.7 included nearly 77,000 adult patients and found small reductions in length of stay without adverse effects on mortality or readmission rates, and a 2009 systematic review by Peterson6 included 33 studies and concluded that in general inpatient care of general medical patients by hospitalist physicians leads to decreased hospital cost and length of stay. A 2002 study by Meltzer et al.8 is also interesting, suggesting that improvements in costs and short‐term mortality are related to the disease‐specific experience of hospitalists.
Similar data for pediatric hospitalists has been slower to emerge. A systematic review of the literature by Landrigan et al., which included studies through 2004, concluded that [R]esearch suggests that pediatric hospitalists decrease costs and length of stay . The quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking.9 Since the publication of that review, there have been multiple studies which have sought to evaluate the quality of pediatric hospitalist systems. This review was undertaken to synthesize this new information, and to determine the effect of pediatric hospitalist systems on quality of care.
METHODS
A review of the available English language literature on the Medline database was undertaken in November of 2010 to answer the question, What are the differences in quality of care and outcomes of inpatient medical care provided by hospitalists versus non‐hospitalists in the pediatric population? Care metrics of interest were categorized according to the Society of Hospital Medicine's recommendations for measuring hospital performance.10
Search terms used (with additional medical subject headings [MeSH] terms in parenthesis) were hospital medicine (hospitalist), pediatrics (child health, child welfare), cost (cost and cost analysis), quality (quality indicators, healthcare), outcomes (outcome assessment, healthcare; outcomes and process assessment, healthcare); volume, patient satisfaction, length of stay, productivity (efficiency), provider satisfaction (attitude of health personnel, job satisfaction), mortality, and readmission rate (patient readmission). The citing articles search tool was used to identify other articles that potentially could meet criteria. Finally, references cited in the selected articles, as well as in excluded literature reviews, were searched for additional articles.
Articles were deemed eligible if they were published in a peer‐reviewed journal, if they had a comparative experimental design for hospitalists versus non‐hospitalists, and if they dealt exclusively with pediatric hospitalists. Noncomparative studies were excluded, as were studies that pertained to settings besides that of an inpatient pediatrics ward, such as pediatric intensive care units or emergency rooms. The search algorithm is diagrammed in Figure 1.

The selected articles were reviewed for the relevant outcome measures. The quality of each article was assessed using the Oxford Centre for Evidence‐Based Medicine levels of evidence,11 a widely accepted standard for critical analysis of studies. Levels of evidence are assigned to studies, from 1a (systematic reviews of randomized controlled trials) to 5 (expert opinion only). Well‐conducted prospective cohort studies receive a rating of 2c; those with wide confidence intervals due to small sample size receive a minus () modifier. This system does not specifically address survey studies, which were therefore not assigned a level of evidence.
RESULTS
The screening process yielded 92 possible relevant articles, which were then reviewed individually (by G.M.M.) by title and abstract. A total of 81 articles were excluded, including 48 studies that were either noncomparative or descriptive in nature. Ten of the identified articles were reviews and did not contain primary data. Nine studies were not restricted to the pediatric population. Also excluded were 7 studies that did not have outcomes related to quality (eg, billing performance), and 7 studies of hospitalists in settings besides general pediatric wards (eg, pediatric intensive care units). Ten studies were thus identified. The cited reference tool was used to identify an additional article which met criteria, yielding 11 total articles that were included in the review.
Five of the identified studies published prior to 2005 were previously reviewed by Landrigan et al.9 Since then, 6 additional studies of similar nature have been published and were included here. Articles that met criteria but appeared in an earlier review are included in Table 1; new articles appear in Table 2. The results of all 11 articles were included for this discussion.
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Bellet and Whitaker13 (2000) | Cincinnati Children's Hospital Medical Center, Cincinnati, OH | 1440 general pediatric patients | LOS, costs (2c) | LOS shorter (2.4 vs 2.7 days) |
| Retrospective cohort study | Readmission rate, subspecialty consultations, mortality (2c, low power) | Costs lower ($2720 vs $3002) | ||
| Readmissions higher for hospitalists (1% vs 3%) | ||||
| No differences in consultations | ||||
| No mortality in study | ||||
| Ogershok et al.16 (2001) | West Virginia University Children's Hospitals, Morgantown, WV | 2177 general pediatric patients | LOS, cost (2c) | No difference in LOS |
| Retrospective cohort study | Readmission rate, patient satisfaction, mortality (2c, low power) | Costs lower ($1238 vs $1421) | ||
| Lab and radiology tests ordered less often | ||||
| No difference in mortality or readmission rates | ||||
| No difference in satisfaction scores | ||||
| Wells et al.15 (2001) | Valley Children's Hospital, Madera, CA | 182 general pediatric patients | LOS, cost, patient satisfaction, follow‐up rate (2c, low power) | LOS shorter (45.2 vs 66.8 hr; P = 0.01) |
| Prospective cohort study | No LOS or cost benefit for patients with bronchiolitis, gastroenteritis, or pneumonia | |||
| Costs lower ($2701 vs $4854; P = 0.005) for patients with asthma | ||||
| No difference in outpatient follow‐up rate | ||||
| Landrigan et al.14 (2002) | Boston Children's Hospital, Boston, MA | 17,873 general pediatric patients | LOS, cost (2c) | LOS shorter (2.2 vs 2.5 days) |
| Retrospective cohort study | Readmission rate, follow‐up rate, mortality (2c, low power) | Costs lower ($1139 vs $1356) | ||
| No difference in follow‐up rate | ||||
| No mortality in study | ||||
| Dwight et al.12 (2004) | Hospital for Sick Children, Toronto, Ontario, Canada | 3807 general pediatric patients | LOS (2c) | LOS shorter (from 2.9 to 2.5 days; P = 0.04) |
| Retrospective cohort study | Subspecialty consultations, readmission rate, mortality (2c, low power) | No difference in readmission rates | ||
| No difference in mortality | ||||
| Source | Site | Study Design | Outcomes Measured (Oxford Level of Evidence) | Results for Hospitalists |
|---|---|---|---|---|
| ||||
| Boyd et al.21 (2006) | St Joseph's Hospital and Medical Center, Phoenix, AZ | 1009 patients with 11 most common DRGs (3 groups) | Cost, LOS, and readmission rate (2c, low power) | LOS longer (2.6 2.0 vs 3.1 2.6 vs 2.9 2.3, mean SD) |
| Retrospective cohort study | Costs higher ($1781 $1449 (faculty) vs $1954 $1212 (hospitalist group 1) vs $1964 $1495 (hospitalist group 2) | |||
| No difference in readmission rates | ||||
| Conway et al.22 (2006) | National provider survey | 213 hospitalists and 352 community pediatrician survey responses | Self‐reported evidence‐based medicine use (descriptive study, no assignable level) | Hospitalists more likely to follow EBG for following: VCUG and RUS after first UTI, albuterol and ipratropium in first 24 hr for asthma |
| Descriptive study | Hospitalists less likely to use the following unproven therapies: levalbuterol and inhaled or oral steroids for bronchiolitis, stool culture or rotavirus testing for gastroenteritis, or ipratropium after 24 hr for asthma | |||
| Srivastava et al.17 (2007) | University of Utah Health Sciences Center, Salt Lake City, UT | 1970 patients with asthma, dehydration, or viral illness | LOS, cost (2c, no confidence intervals reported) | LOS shorter for asthma (0.23 days, 13%) and for dehydration (0.19 days, 11%) |
| Retrospective cohort study | No LOS difference for patients with viral illness | |||
| Costs lower for asthma ($105.51, 9.3%) and for dehydration ($86.22, 7.8%) | ||||
| Simon et al.19 (2007) | Children's Hospital of Denver, Denver, CO | 759 patients undergoing spinal fusion before and after availability of hospitalist consultation | LOS (4, unaccounted confounding factors) | LOS shorter, 6.5 (6.26.7) days to 4.8 (4.55.1) |
| Retrospective cohort study | ||||
| Bekmezian et al.18 (2008) | UCLA Hospital and Medical Center, Los Angeles, CA | 925 subspecialty patients on GI and Heme/Onc services vs hospitalist service | LOS, cost, readmission rate, mortality (2c, low power) | LOS shorter (38%, P < 0.01) |
| Retrospective cohort study | Cost lower (29%, P < 0.05) | |||
| Readmissions lower (36 for faculty vs none for hospitalists, P = 0.02) | ||||
| No difference in mortality | ||||
| Conway and Keren20 (2009) | Multicenter, 25 children's hospitals | 20,892 patients identified with UTI admissions in PHIS database | LOS, cost, evidence‐based medicine use (2c) | No difference in LOS |
| Retrospective cohort study | No difference in cost | |||
| No difference in performance of EBM guideline (VCUG and RUS for first UTI) | ||||
Effect on Length of Stay, Cost, and Resource Utilization
Ten articles addressed length of stay as an outcome measure, and 8 included cost as well. Five have been previously reported9 (see Table 1). Of these, Dwight et al.,12, Bellet and Whitaker,13 and Landrigan et al.14 found decreased length of stay (LOS) and cost for all patients. Wells et al.15 found significantly decreased LOS and cost for asthma patients but not for all diagnoses taken together, and Ogershok et al.16 found lower hospital costs but not length of stay. Five of the 6 new studies, listed in Table 2, reported on length of stay and cost. Three showed some benefits for length of stay: Srivastava et al.17 reported improvement in length of stay and cost for asthma and dehydration, but not for all diagnoses together; Bekmezian et al.18 reported improved length of stay and cost for pediatric hospitalists for patients on a hematology and gastroenterology service; and Simon et al.19 attributes a generalized decrease in length of stay on a surgical service to implementation of hospitalist comanagement of their most complex patients, though hospitalists only comanaged 12% of the patients in the study. A multicentered study in 2009 by Conway and Keren20 reported no significant difference in length of stay for general pediatric patients with urinary tract infections.
Of the 4 total studies that showed significant advantage in length of stay for hospitalist groups, improvement ranged from 11% to 38%. All attempted to adjust for diagnosis and severity using diagnosis‐related groups (DRGs) or other methods. Dwight et al.,12 Bellet and Whitaker,13 and Bekmezian et al.18 used retrospective or historical comparison alone, while Landrigan et al.14 had both concurrent and historical comparison groups.
In contrast to the other studies, Boyd et al.21 in 2006 found significant advantages, in both length of stay and cost, for a faculty/resident service in comparison to a hospitalist service. This nonrandomized, retrospective cohort study included 1009 pediatric patients, with the 11 most common DRGs, admitted during the same time period to either a traditional faculty/resident team or 1 of 2 private practice hospitalist groups at an academic medical center. The 8 general pediatric faculty practice attendings were dedicated to inpatient care while on service, and rotated bimonthly. The authors found that the faculty group patients had significantly shorter lengths of stay and total direct patient costs.
Cost‐comparison results were reported by 7 of the studies. Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 and Bekmezian et al.18 reported reductions in cost for all patients varying from 9% to 29%, while Wells et al.15 and Srivastava et al.17 found reductions in cost only for patients with certain diagnoses. Srivastava et al.17 analyzed 1970 patients, admitted with primary diagnoses of asthma, dehydration, or viral illness, over a 5‐year period from 1993 to 1997. Cost‐per‐patient was reduced between 9.3% for asthma and 7.8% for dehydrations, but when combined with the viral illness group, the difference was not statistically significant. Wells et al.15 studied 182 admissions over a 1‐year period, and found significant reductions in cost of 44% (P < 0.005) for patients with asthma but not for bronchiolitis, gastroenteritis, or pneumonia. In 2009, Conway and Keren20 studied a multicentered cohort of 20,892 children hospitalized for urinary tract infection, and found no significant difference in hospitalization costs between hospitalist services and more traditional models.
Other Quality Measures
Though financial outcomes (length of stay, cost, and resource utilization) were the primary area of emphasis for most of the selected articles, other parameters with more of a focus on quality were examined as well. The studies by Dwight et al.,12 Bellet and Whitaker,13 Landrigan et al.,14 Ogershok et al.,16 Bekmezian et al.,18 and Boyd et al.21 examined mortality and readmission rate. None of these studies reported differences in mortality rate, though none were powered to do so. When studying readmission rate, Bellet and Whitaker13 reported a statistically significant lower rate of readmission for a traditionally staffed service versus the hospitalist service (1% vs 3%; P = 0.006). In contrast, Bekmezian et al.18 found a lower readmission rate for the hospitalist service (4.4% vs 0%; P = 0.02). The studies by Dwight et al.,12 Landrigan et al.,14 Ogershok et al.,16 and Boyd et al.21 did not detect differences in readmission rates.
Two studies measured patient satisfaction.15, 16 Ogershok et al.16 utilized hospital‐generated patient satisfaction surveys, completed at discharge, for comparison and found no differences between the hospitalist and non‐hospitalist ward services. Wells et al.15 utilized a standardized patient satisfaction assessment tool, given at discharge, followed by a telephone interview after 1 month. At discharge, parents rated hospitalist physicians higher in courtesy (P < 0.05) and friendliness (P < 0.005), though this difference was not detected in the telephone interviews 1 month later. However, at that time, parents did indicate that they received better explanations about their child's illness if their child was seen by their primary care physician rather than a hospitalist.
In 2006, a study by Conway et al.22 reported on the use of evidence‐based therapies and tests by hospitalists as compared to community pediatricians. The survey identified evidence‐based therapies and tests for asthma, bronchiolitis, gastroenteritis, and first‐time urinary tract infection (UTI) diagnosis. A total of 213 hospitalists and 228 community pediatricians met the inclusion criteria by returning the completed survey. After multivariate regression analysis, hospitalists were found to be more likely to use 4 of 5 evidence‐based therapies and recommended tests, and were less likely to use 6 of 7 therapies and tests of unproven benefit. In 2009, Conway and Clancy23 again studied the use of evidence‐based therapies, this time using more objective measures. In this report, the Pediatric Health Information System (PHIS) was examined for a cohort of 20,892 patients. After multivariable regression analysis, there was no statistical difference in the performance of evidence‐based imaging following a first UTI between hospitals staffed primarily by community pediatricians versus those with pediatric hospitalist systems. However, it should be noted that the evidence base for UTI‐related imaging has been debated in the literature over the past decade.
DISCUSSION
Of the 11 studies selected for this review, 10 measured length of stay as an outcome, with the majority favoring hospitalists but with mixed results. Three of these studies, those by Dwight et al.,12 Bellet and Whitaker,13 and Landrigan et al.,14 demonstrated 11% to 14% improvement for hospitalist services compared to community pediatricians. Boyd et al.,21 however, found exactly the opposite result, and 2 studies by Conway and Keren20 and Ogershok et al.16 found no difference in length of stay. Two more studies found benefits restricted to certain conditions: Wells et al.15 found 32% shorter lengths of stay for asthma, but not for other conditions; Srivastava et al.17 found a 13% reduction in length of stay for asthma and 11% for dehydration, but none for viral illnesses or when all conditions were combined. Bekmezian et al.18 found shorter lengths of stay on a hospitalist service for hematology and gastroenterology patients, and Simon et al.19 attribute a general trend of decreasing lengths of stay on a surgical service to the implementation of hospital comanagement for a small percentage of patients.
The most common quality measures studied were patient satisfaction, readmission rates, and mortality. Only 1 study by Ogershok et al.16 reported on patient satisfaction and found few differences between hospitalists and community pediatricians. Readmission rate were reported by 6 studies. Bellet and Whitaker13 found a higher readmission rate for pediatric hospitalists, Bekmezian et al.18 found a lower rate but on a subspecialty service. The study with the greatest power for this analysis, by Landrigan et al.14 with nearly 18,000 patients, found no difference, and neither did another 3 studies. Unsurprisingly, no study detected differences in mortality; it would be extremely difficult to adequately power a study to do so in the general pediatric setting, where mortality is rare.
The effect of relative experience of hospitalist physicians is uncertain. Boyd et al.21 speculated that 1 possible cause for the decreased lengths of stay and costs associated with their faculty group compared to hospitalists may have been due to the increased experience of the faculty group. Unfortunately, they were unable to generate statistical significance due to the small numbers of physicians in the study. In contrast, the hospitalists in the report by Dwight et al.12 had decreased lengths of stay but were less experienced. In the adult literature, the study by Meltzer et al.8 suggests that improved outcomes from hospitalist systems may not become apparent for 1 or more years after implementation, but none of the pediatric studies included in our review specifically address this issue. This leaves the possibility open that the hospitalist systems evaluated in some studies had insufficient time in which to develop increased efficiencies.
There were several limitations to our studies. First, due to the heterogeneity and methodological variations among the included studies, we were unable to perform a meta‐analysis. Second, the overall quality of evidence is limited due to the lack of randomized control trials. Third, a lack of agreement on appropriate quality markers has limited the study of quality of care. Published reports continue to focus on financial measures, such as length of stay, despite the recommendation in the previous review by Landrigan et al.9 that such studies would be of limited value. Finally, the current variability of hospitalist models and lack of study of factors that might influence outcomes makes comparisons difficult.
Despite these limitations, several interesting trends emerge from these studies. One such trend is that the more recent studies highlight that simple classification of hospitalist system versus traditional system fails to measure the complexity and nuance of care delivery. The 2006 study by Boyd et al.21 is especially notable because it showed the opposite effect of previous studies, namely, an increase in length of stay and costs for hospitalists at St Joseph's Medical Center in Phoenix, Arizona. In this study, the traditional faculty group was employed by the hospital, and the hospitalist group was a private practice model. The authors suggest that their faculty physicians were therefore operating like hospitalists in that almost all of their time was focused on inpatient care while they were on service. They also had a limited number of general pediatricians, who attended in the inpatient setting, who were more experienced than the private practice groups. Also, the authors theorize that their faculty may have had a closer working relationship with their residents due to additional service responsibilities and locations of the faculty group onsite. Further study of the care models utilized by faculty and hospitalist practices at St Joseph's and other hospitals may reveal important insights about improving the quality and efficiency of inpatient pediatric care in general.
Though there is a clear trend in the adult literature indicating that the use of hospitalists results in superior quality of care, there is less evidence for pediatric systems. The aforementioned previous review by Landrigan et al.9, in 2006 concluded that emerging research suggests that pediatric hospitalist systems decrease cost and length of stay, but also the quality of care in pediatric hospitalist systems is unclear, because rigorous metrics to evaluate quality are lacking. Data from the 6 additional studies presented here lend limited support to the first hypothesis, and the presence of only 1 negative study is not sufficient to undermine it.
While data on quality markers such as readmission rate or mortality remain elusive, the 2 studies by Conway et al.20, 22 attempt to evaluate quality by comparing the use of evidence‐based therapies by hospitalists and community pediatricians. Though the use of objective PHIS data for UTI in 2009 did not confirm the conclusion suggested by the 2006 provider survey study, the attempt to find measurable outcomes such as the use of evidence‐based therapies is a start but we need more metrics, including rigorous patient outcome metrics, to define the quality of our care systems. Before the effect of hospitalist systems on quality is fully understood, more work will need to be done defining metrics for comparison.
Unfortunately, over 5 years since the previous review by Landrigan et al.9 called for increased focus on inpatient quality and understanding how to improve, the sophistication of our measurement of pediatric inpatient quality and understanding of the mechanisms underlying improvement is still in its infancy. We propose a solution at multiple levels.
First, the investment in research comparing system‐level interventions (eg, discharge process A vs discharge process B) must be increased. This investment increased significantly due to the over $1 billion in Recovery Act funding for comparative effectiveness research.23 However, the future investment in comparative effectiveness research, often called patient‐centered outcomes research, and proportion of investment focused on delivery system interventions is unclear. We propose that the investment in comparing delivery system interventions is essential to improving not only hospital medicine systems, but, more importantly, the healthcare system broadly. In addition, research investment needs to focus on reliably implementing proven interventions in systems of care, and evaluating both the effects on patient outcomes and cost, and the contextual factors associated with successful implementation.24 A hospital medicine example would be the comparison of the implementation of a guideline for a common disease across a set of hospitals. One could perform a prospective observational design, in which one compares high intensity versus low intensity intervention and assesses the baseline characteristics of the hospital systems, to understand their association with successful implementation and, ultimately, patient outcomes. One could also perform a clustered randomized design.
Second, the development and implementation of pediatric quality of care measures, including in the inpatient setting, needs to increase rapidly. The Children's Health Insurance Program (CHIP) and its focus on an initial core set of quality measures that expands over time, through an investment in measure development and validation, is an opportunity for pediatric hospital medicine. Inpatient measures should be a focus of measure development and implementation. We must move beyond a limited set of inpatient measures to a broader set focused on issues such as patient safety, hospital‐acquired infections, outcomes for common illnesses, and transitions of care. We also need better measures for important pediatric populations, such as children with complex medical conditions.25
Third, our understanding of the mechanisms leading to improvement in hospital medicine systems needs to be developed. Studies of hospital medicine systems should move past simple binary comparisons of hospitalist systems versus traditional systems to understand the effect on patient outcomes and cost of factors such as years of experience, volume of patients seen overall and with a specific condition, staffing model, training, quality improvement knowledge and application, and health information systems. These factors may be additive or multiplicative to the performance of inpatient systems once put into place, but these hypotheses need to be tested.
Fourth, individual hospitalists and their groups must focus on quality measurement and improvement in quality and value delivered. At Cincinnati, we have a portfolio of quality and value projects derived from our strategic objectives, illustrated in Figure 2. The projects have leaders and teams to drive improvement and measure results. Increasingly, we are able to publish these results in peer‐reviewed journals. On a quarterly basis, we review the portfolio via a dashboard and/or run and control charts. We establish new projects and set new goals on at least an annual basis. It is important to note that at the beginning of the 2010‐2011 fiscal year, almost all initiatives identified as priorities were yellow or red. Our group is now planning new initiatives and goals for next year. This is one method applicable to our setting, but a focus on quality and value and measuring results needs to be part of every hospital medicine program. As payer focus on value increases, this will be essential to demonstrate how a hospitalist group improves outcomes and adds value.

CONCLUSION
This review suggests that the use of hospitalists can improve the quality of inpatient care in the pediatric population, but this is not a universal finding and, most importantly, the mechanisms of improvement are poorly understood. We propose 4 components to address these issues so that a systematic review 5 years from now would be much more robust. These are: 1) increased investment in research comparing system‐level interventions and reliable implementation; 2) further development and implementation of pediatric quality of care measures in the inpatient setting; 3) understanding the mechanisms and factors leading to improvement in hospital medicine systems; and 4) an increased focus on quality measurement, and improvement in quality and value delivered by all individual hospitalists and their groups.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists.Mayo Clin Proc.2009;84(3):248–254.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;375(25):2589–2600.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–875.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- Society of Hospital Medicine. Measuring hospitalist performance: metrics, reports, and dashboards. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Publications; April2007.
- Oxford Centre for Evidence‐Based Medicine levels of evidence. Updated March 2009. Available at: http://www.cebm.net/index.aspx?o=1025. Accessed March 14,2011.
- ,,,.Evaluation of a staff‐only hospitalist system in a tertiary care, academic children's hospital.Pediatrics.2004;114(6):1545–1549.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105(3 pt 1):478–484.
- ,,, et al.Impact of a health maintenance organization hospitalist system in academic pediatrics.Pediatrics.2002;110(4):720–728.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual.2001;16(5):174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila).2001;40(12):653–662.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,.Staff‐only pediatric hospitalist care of patients with medically complex subspecialty conditions in a major teaching hospital.Arch Pediatr Adolesc Med.2008;162(10):975–980.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2(1):23–30.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154(6):789–796.
- ,,,,.Comparison of outcome measures for a traditional pediatric faculty service and nonfaculty hospitalist services in a community teaching hospital.Pediatrics.2006;118(4):1327–1331.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,.Comparative‐effectiveness research—implications of the federal coordinating council's report.N Engl J Med.2009;361(4):328–330.
- ,.Charting a path from comparative effectiveness funding to improved patient‐centered health care.JAMA.2010;303(10):985–986.
- ,,, et al.Children with medical complexity: an emerging population for clinical and research initiatives.Pediatrics.2011;127(3):529–538.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists.Mayo Clin Proc.2009;84(3):248–254.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;375(25):2589–2600.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–875.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- Society of Hospital Medicine. Measuring hospitalist performance: metrics, reports, and dashboards. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Publications; April2007.
- Oxford Centre for Evidence‐Based Medicine levels of evidence. Updated March 2009. Available at: http://www.cebm.net/index.aspx?o=1025. Accessed March 14,2011.
- ,,,.Evaluation of a staff‐only hospitalist system in a tertiary care, academic children's hospital.Pediatrics.2004;114(6):1545–1549.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105(3 pt 1):478–484.
- ,,, et al.Impact of a health maintenance organization hospitalist system in academic pediatrics.Pediatrics.2002;110(4):720–728.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual.2001;16(5):174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila).2001;40(12):653–662.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,.Staff‐only pediatric hospitalist care of patients with medically complex subspecialty conditions in a major teaching hospital.Arch Pediatr Adolesc Med.2008;162(10):975–980.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2(1):23–30.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154(6):789–796.
- ,,,,.Comparison of outcome measures for a traditional pediatric faculty service and nonfaculty hospitalist services in a community teaching hospital.Pediatrics.2006;118(4):1327–1331.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,.Comparative‐effectiveness research—implications of the federal coordinating council's report.N Engl J Med.2009;361(4):328–330.
- ,.Charting a path from comparative effectiveness funding to improved patient‐centered health care.JAMA.2010;303(10):985–986.
- ,,, et al.Children with medical complexity: an emerging population for clinical and research initiatives.Pediatrics.2011;127(3):529–538.
Insurance and LOS for Children With CAP
Disparities in patterns of care and outcomes for ambulatory‐care sensitive conditions remain a persistent problem for children.19 Many studies have focused on disparities in hospitalization rates and length of stay (LOS) related to asthma, however, few studies have focused on community‐acquired pneumonia (CAP) despite the fact that pneumonia is the most common, preventable, and potentially serious infection in childhood.10 Providers, payers, and families have a common interest in minimizing hospital LOS for different reasons (eg, minimizing costs, lost wages, exposure to antibiotic‐resistant bacteria), however, this interest is balanced against the potentially greater risk of readmission and adverse outcomes if LOS is inappropriately short. To date, the relationship between insurance status and LOS for CAP remains unexplored.
As in other conditions, substantial variation exists with respect to patterns of care and outcomes for children hospitalized with CAP.11 For example, children hospitalized in rural settings have a shorter LOS for pneumonia than those hospitalized in large urban settings.12 Children from racial/ethnic minorities tend to have higher rates of CAP‐associated complications, including death.11 Decades of prior studies have documented that uninsured children are less likely than insured children to make preventive care visits and obtain prescription medications, but differences in LOS or hospitalization rates between insured and uninsured children with CAP have not been studied.6, 8, 13, 14 Though imperfect, insurance status is 1 proxy for healthcare access, and current healthcare reform efforts aim to improve healthcare access and decrease socioeconomic gradients in health by increasing the number of insured American children. Nonetheless, quantifying the relationship between insurance status on LOS for children hospitalized with CAP is a first step towards understanding the influence of ambulatory care access on hospitalization for ambulatory‐care sensitive conditions.
The purpose of this study was to investigate the influence of insurance status and type on LOS for children hospitalized with CAP. In addition, we sought to determine if there were consistent trends over time in the association between insurance status and type with LOS for children hospitalized with CAP.
METHODS
Study Design and Data Source
This retrospective cross‐sectional study used data from the 1997, 2000, 2003, and 2006 Kids' Inpatient Database (KID). The KID is part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality (AHRQ). It is the only dataset on hospital use and outcomes specifically designed to study children's use of hospital services in the United States. The KID samples pediatric discharges from all community non‐rehabilitation hospitals in states participating in the Healthcare Cost and Utilization Project, using a complex stratification system, across pediatric discharge type and hospital characteristics. Community hospitals in the KID are defined as all non‐federal, short‐term, general and other specialty hospitals, including academic medical centers, obstetrics‐gynecology, otolaryngology, orthopedic, and children's hospitals. Federal hospitals, long‐term hospitals, psychiatric hospitals, alcohol/chemical dependency treatment facilities and hospitals units within institutions are excluded. Discharge‐level weights assigned to discharges within the stratum permit calculation of national estimates. Datasets, which each contain approximately 3 million discharges (unweighted), are released every 3 years beginning with 1997. The 2006 KID is the most recently available dataset and contains hospital administrative data from 38 states, representing 88.8% of the estimated US population.15 This study was considered exempt from review by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia.
Study Participants
Patients 18 years of age and younger were eligible for inclusion if they required hospitalization for CAP in 1997, 2000, 2003, or 2006. Using a previously validated algorithm, patients were considered as having CAP if they met 1 of 2 criteria: 1) International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9 CM) primary diagnosis code indicating pneumonia (480‐483, 485‐486), empyema (510), or pleurisy (511.0‐1, 511.9); or 2) primary diagnosis of pneumonia‐related symptom (eg, cough, fever, tachypnea) and secondary diagnosis of pneumonia, empyema or pleurisy. Pneumonia‐related symptoms included fever, respiratory abnormality unspecified, shortness of breath, tachypnea, wheezing, cough, hemoptysis, abnormal sputum, chest pain, and abnormal chest sounds.16 Because there is no specific ICD‐9 code for nosocomial pneumonia, this previously validated approach minimized such misclassification16 (eg, a child hospitalized following traumatic injury who then develops ventilator‐associated pneumonia is likely to have trauma, rather than pneumonia or a pneumonia‐related symptom, listed as the primary diagnosis). Patients with the following comorbid conditions (identified by KID data elements and ICD‐9 CM codes) were excluded as these comorbidities are characterized by risk factors not reflective of the general pediatric population: acquired and congenital immunologic disorders, malignancy, collagen vascular disease, sickle cell disease, cystic fibrosis, organ transplant, congenital heart defects, and heart failure. Patients identified as in‐hospital births were excluded to minimize the inclusion of perinatally acquired and nosocomial infections occurring in neonates. Patients with a secondary diagnosis code indicating trauma were also excluded, as a diagnosis of pneumonia in this population likely reflects nosocomial etiology. CAP‐related complications (eg, effusion, abscess; for complete list, see Supporting Appendix A in the online version of this article) were identified using ICD‐9 CM diagnosis and procedure codes. Asthma‐related hospitalizations were identified using ICD‐9 CM diagnosis code 493 in any secondary diagnosis field.
Primary Exposure
The primary exposure was insurance type, categorized as private, public, uninsured, or other (eg, Civilian Health and Medical Program Uniform Service (CHAMPUS), worker's compensation, union‐based insurance, but definition varies by state precluding categorization as purely public or private).
Primary Outcome
The primary outcome was the hospital LOS calculated in days.
Statistical Analysis
Consistent with prior work,12 subjects were characterized by age, race, sex, the presence or absence of a pneumonia‐associated complication, discharge status (discharge from hospital vs in‐hospital death), hospital type (rural, urban non‐teaching, urban teaching non‐children's, urban teaching children's), and hospital region (Northeast, Midwest, South, West). Age groups for analysis were defined as <1 year (infant), 1 to 5 years (preschool age), 6 to 11 years (school‐age), and 12 to 18 years old (adolescent). Race was recorded as a single variable (white, black, other, and missing). Patient information for race was missing from 32% of discharges in 1997, 18% in 2000, 29% in 2003, and 26% in 2006. Patients with missing race data were included to preserve the integrity of our estimates. Categorical variables were summarized by frequencies and percents. Continuous variables were summarized by mean and standard deviation values.
All analyses accounted for the complex sampling design with the survey commands included in STATA, version 10 (College Station, TX) to produce weighted estimates. To determine the adjusted impact of patient and hospital‐level characteristics in our cohort, we constructed multivariable negative binomial regression models using all available covariates for LOS because of its rightward‐skewed distribution. The negative binomial model produced an incident rate ratio (IRR) for LOS (IRR >1 indicates that the risk factor is associated with a longer length of stay). As recommended in the AHRQ technical documentation, variance estimates for each model accounted for the clustering of data at the hospital level. To address the impact of missing race data on outcome, we constructed additional multivariable negative binomial regression models while varying the underlying assumptions about race classification. In these secondary analyses, children with race coded as missing were sequentially excluded, assumed to be white, and assumed to be black. These analyses were repeated after excluding insurance from the multivariable model.
RESULTS
The more than 10.5 million children sampled (unweighted) in KID during these 4 time periods (1997, 2000, 2003, and 2006) are representative of the more than 28.9 million children hospitalized in the United States. In each of these sample years, there were approximately 150,000 children hospitalized with pneumonia across the United States (Table 1). Of those hospitalized, 23% to 28% had a concomitant diagnosis of asthma; 6% to 8% had a pneumonia‐associated complication; and mortality was <0.01% in each sample year for patients hospitalized with pneumonia. In all years, among those with racial/ethnic data, the sample population was predominantly white boys less than 6 years old. The greatest proportion of children were hospitalized in urban non‐teaching settings, and also those children living in the southern regions of the United States.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| N = 148,702 | N = 157,847 | N = 157,743 | N = 156,810 | |
| ||||
| Race | ||||
| White | 56,348 (38) | 68,643 (44) | 54,903 (35) | 56,108 (36) |
| Black | 22,864 (15) | 22,580 (14) | 17,960 (11) | 18,800 (12) |
| Other | 22,203 (15) | 38,448 (24) | 39,138 (25) | 40,803 (26) |
| Missing | 47,287 (32) | 28,175 (18) | 45,588 (29) | 41,099 (26) |
| Age category | ||||
| <1 year | 43,851 (29) | 44,470 (28) | 37,798 (24) | 37,705 (24) |
| 1 through 5 years | 75,033 (50) | 76,385 (48) | 77,530 (49) | 79,519 (51) |
| 6 through 11 years | 19,372 (13) | 21,403 (14) | 23,126 (15) | 23,494 (15) |
| >12 years | 10,446 (7) | 15,589 (9) | 19,289 (12) | 16,092 (10) |
| Hospital type | ||||
| Urban non‐teaching | 52,756 (35) | 50,718 (32) | 52,552 (34) | 50,718 (32) |
| Rural | 47,910 (32) | 41,715 (27) | 39,605 (26) | 31,947 (21) |
| Urban teaching non‐children's | 20,378 (14) | 30,981 (20) | 28,432 (18) | 30,194 (20) |
| Urban teaching children's | 27,658 (19) | 34,021 (22) | 34,454 (22) | 41,035 (27) |
| Male sex | 83,291 (56) | 8,783 (56) | 86,034 (55) | 85,508 (55) |
| Region* | ||||
| Northeast | 19,750 (13) | 26,092 (17) | 23,867 (15) | 23,832 (15) |
| Midwest | 33,053 (22) | 30,706 (19) | 35,714 (23) | 35,900 (23) |
| South | 68,958 (46) | 68,663 (44) | 65,994 (42) | 65,460 (42) |
| West | 26,741 (18) | 32,385 (21) | 32,169 (20) | 31,618 (20) |
| Asthma | 26,971 (24) | 31,746 (28) | 27,729 (24) | 26,822 (23) |
| Pneumonia‐associated complication | 8,831 (6) | 11,084 (7) | 12,005 (8) | 11,724 (7) |
| Died | 334 (0.002) | 394 (0.002) | 270 (0.002) | 193 (0.001) |
| Insurance | ||||
| Private | 65,428 (44) | 73,528 (47) | 68,720 (44) | 63,997 (41) |
| Public | 68,024 (46) | 71,698 (45) | 76,779 (49) | 80,226 (51) |
| Uninsured | 9,922 (7) | 8,336 (5) | 6,381 (4) | 6,912 (4) |
| Other | 4,964 (3) | 4,285 (3) | 5,391 (3) | 5,283 (3) |
There was little variation in the insurance status of children hospitalized with CAP between 1997 and 2006. In each of the sampled years, at least 40% of sampled children were privately insured, at least 40% were publicly insured, and approximately 5% were uninsured (Table 1). In all years, there were significant racial/ethnic disparities in insurance coverage such that whites were 4 to 6 times more likely to have private insurance than blacks, however, the large amount of missing race/ethnicity data warrant caution in interpreting this finding (Table 2; also see Supporting Information Appendix B in the online version of this article). We also found that children less than 1 year old were the most likely to be publicly insured in all years (see Supporting Appendix C in the online version of this article). There were also regional differences related to insurance coverage such that a greater proportion of children hospitalized in facilities located in the southern part of the United States were publicly insured. Notably, there were no significant differences in CAP‐associated mortality or asthma related to insurance coverage (Table 2). In 2006, CAP‐associated complications occurred in 8.5% of children with private insurance, 6.5% of children with public insurance, and 7.7% of uninsured children; the relative distribution of complications by insurance type were similar in previous years of the KID survey.
| Private | Public | Uninsured | Other Insurance | P | |
|---|---|---|---|---|---|
| |||||
| No. of children (%) | 63,997 (41) | 80,226 (51) | 6,912 (4) | 5,283 (3) | |
| Male sex | 34,639 (41) | 44,140 (52) | 3,727 (4) | 2,808 (3) | 0.092 |
| Race | |||||
| White | 30,707 (55) | 21,282 (38) | 2,241 (4) | 1,774 (3) | <0.001 |
| Black* | 5,112 (27) | 12,239 (65) | 988 (5) | 426 (3) | |
| Other | 11,033 (27) | 26,489 (65) | 2,112 (5) | 1,076 (3) | |
| Missing | 17,145 (42) | 20,216 (49) | 1,572 (4) | 2,007 (4) | |
| Age category | |||||
| <1 year | 10,788 (29) | 24,762 (65) | 1,164 (3) | 880 (3) | <0.001 |
| 1 through 5 years | 33,664 (42) | 39,531 (50) | 3,442 (4) | 2,673 (3) | |
| 6 through 11 years | 11,660 (50) | 9,684 (41) | 1,085 (5) | 1,015 (4) | |
| >12 years | 7,885 (49) | 6,249 (39) | 1,221 (8) | 714 (4) | |
| Hospital type | |||||
| Urban non‐teaching | 22,429 (44) | 24,241 (49) | 2,440 (5) | 1,555 (2) | <0.001 |
| Rural | 10,880 (34) | 18,396 (58) | 1,290 (4) | 1,109 (3) | |
| Urban teaching non‐children's | 13,130 (44) | 14,542 (48) | 1,721 (6) | 750 (2) | |
| Urban teaching children's | 16,591 (40) | 21,544 (53) | 1,417 (3) | 1,465 (4) | |
| Region | |||||
| Northeast | 12,364 (52) | 9,620 (40) | 1,466 (6) | 377 (2) | <0.001 |
| Midwest∥ | 17,891 (50) | 15,573 (43) | 1,160 (3) | 1,215 (3) | |
| South∥ | 21,479 (33) | 38,112 (58) | 3,108 (5) | 2,495 (4) | |
| West∥ | 12,263 (39) | 16,921 (44) | 1,178 (5) | 1,195 (5) | |
| Asthma | 10,829 (41) | 13,923 (52) | 1,119 (4) | 866 (3) | 0.193 |
| Pneumonia‐associated complication | 5,416 (46) | 5,206 (45) | 532 (4) | 556 (5) | <0.001 |
| Died | 66 (34) | 115 (60) | 3 (1) | 8 (5) | 0.131 |
After examining the general and demographic characteristics, we then examined mean LOS for all children with CAP in each sample year (Table 3). The mean LOS for children with CAP was 3.44 days in 1997, with marginal decreases in subsequent years to a mean LOS of 3.18 days in 2006. The distribution of LOS for children with CAP revealed that nearly 70% of children were hospitalized for fewer than 3 days, another 22% to 28% were hospitalized for less than 1 week, and only 3% were hospitalized for more than 1 week. This distribution did not change substantially between 1997 and 2006. Next, we compared mean LOS by insurance type and race/ethnicity in unadjusted analyses. In each sample year, publicly insured children hospitalized with CAP had significantly longer LOS than privately insured children (P < 0.001). Similarly, in all years excepting 1997, uninsured children hospitalized with CAP had significantly shorter LOS than privately insured children. There were also significant racial differences in LOS for children with CAP, such that black children had longer LOS than white children with CAP. However, the large amount of missing data for race/ethnicity limited the robustness of this finding, and subsequent sensitivity analyses demonstrated that there were no consistent racial/ethnic disparities in LOS (see Supporting Appendix B in the online version of this article). These sensitivity analyses for missing race data did not alter our primary finding of shorter LOS for uninsured versus publicly or privately insured children.
| 1997 | P | 2000 | P | 2003 | P | 2006 | P | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Overall | 3.44 (0.04) | 3.35 (0.05) | 3.27 (0.05) | 3.18 (0.04) | ||||
| Insurance type | ||||||||
| Private | 3.21 (0.04) | 3.19 (0.04) | 3.09 (0.04) | 3.00 (0.03) | ||||
| Public | 3.71 (0.06) | <0.001 | 3.57 (0.06) | <0.001 | 3.44 (0.06) | <0.001 | 3.34 (0.05) | <0.001 |
| Uninsured | 3.18 (0.14) | 0.792 | 2.92 (0.07) | <0.001 | 2.80 (0.05) | <0.001 | 2.82 (0.05) | <0.001 |
| Other | 3.32 (0.11) | 0.319 | 3.55 (0.14) | 0.0134 | 3.54 (0.21) | 0.037 | 3.42 (0.13) | 0.001 |
| Race | ||||||||
| White | 3.31 (0.05) | 3.18 (0.04) | 3.19 (0.05) | 3.10 (0.04) | ||||
| Black | 3.61 (0.08) | <0.001 | 3.32 (0.07) | <0.001 | 3.36 (0.08) | <0.001 | 3.31 (0.07) | <0.001 |
| Other | 3.96 (0.11) | <0.001 | 3.81 (0.09) | <0.001 | 3.67 (0.10) | <0.001 | 3.56 (0.08) | <0.001 |
| Missing | 3.27 (0.08) | 0.645 | 3.18 (0.08) | 0.926 | 2.99 (0.06) | 0.0134 | 2.86 (0.04) | <0.001 |
After controlling for child age, race/ethnicity, gender, hospital type, transfer status, and presence of asthma or pneumonia‐associated complications, our multivariable analyses examining the relationship between insurance coverage and hospital LOS yielded the following results (Table 4). First, publicly insured children had significantly longer hospital stays than privately insured children, and uninsured children had significantly shorter hospital stays than privately insured children in all years except 1997. Second, children admitted with CAP at urban teaching children's hospitals had significantly longer LOS than those admitted to urban non‐teaching hospitals, and, in 2003, children admitted with CAP to rural hospitals had significantly shorter LOS than those admitted to urban non‐teaching hospitals. Third, children older than 1 year consistently had shorter hospital stays than infants less than 1 year old. Finally, though concomitant diagnosis of asthma did not consistently influence LOS, children who developed any complications had significantly longer LOS than those who did not. The cumulative impact of seemingly small differences in LOS is great. For example, in 2006, our model suggests that, for every 1000 children hospitalized with CAP in a given year, after adjusting for differences in sex, age, race, hospital‐type, region, transfer status, and diagnosis of asthma or complications, publicly insured children spend 90 to 130 more days in the hospital than privately insured children, whereas uninsured children spend between 40 to 90 fewer days in the hospital than privately insured children.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| Variable | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| ||||
| Age category | ||||
| <1 year | ||||
| 15 years | 0.82 (0.81, 0.84) | 0.83 (0.88, 0.95) | 0.86 (0.85, 0.88) | 0.87 (0.86, 0.89) |
| 611 years | 0.91 (0.87, 0.95) | 0.91 (0.88, 0.94) | 0.93 (0.91, 0.95) | 0.93 (0.90, 0.95) |
| >12 years | 1.03 (0.99, 1.07) | 1.17 (1.11, 1.22) | 1.09 (1.06, 1.13) | 1.13 (1.09, 1.16) |
| Race | ||||
| White | ||||
| Black | 1.04 (0.99, 1.08) | 1.00 (0.95, 1.03) | 1.00 (0.98, 1.03) | 1.02 (0.98, 1.06) |
| Other | 1.09 (1.05, 1.13) | 1.11 (1.08, 1.15) | 1.09 (1.06, 1.12) | 1.08 (1.05, 1.11) |
| Missing | 1.00 (0.94, 1.06) | 1.01 (0.96, 1.06) | 0.95 (0.92, 0.99)* | 0.96 (0.93, 0.99) |
| Sex | ||||
| Female | 1.02 (0.94, 1.06) | 1.01 (0.99, 1.02) | 1.01(0.93, 100) | 1.01 (1.00, 1.02) |
| Insurance type | ||||
| Private | ||||
| Public | 1.13 (1.11, 1.16) | 1.11 (1.09, 1.14) | 1.11 (1.09, 1.13) | 1.11 (1.09, 1.13) |
| Uninsured | 1.01 (0.91, 1.11) | 0.93 (0.89, 0.96) | 0.92 (0.90, 0.96) | 0.94 (0.91, 0.96) |
| Other | 1.01 (0.96, 1.06) | 1.10 (1.03, 1.18) | 1.10 (1.02, 1.19)* | 1.07 (1.02, 1.13) |
| Hospital type | ||||
| Urban non‐teaching | ||||
| Rural | 0.98 (0.92, 1.04) | 0.96 (0.92, 1.00) | 0.97 (0.94, 1.00) | 0.97 (0.93, 1.00) |
| Urban teaching (non‐children's) | 0.99 (0.95, 1.04) | 1.06 (1.02, 1.10) | 1.06 (1.02, 1.10) | 1.03 (0.99, 1.07) |
| Urban teaching children's | 1.2 (1.14, 1.26) | 1.23 (1.16, 1.30) | 1.28 (1.21, 1.37) | 1.25 (1.19, 1.31) |
| Region | ||||
| Northeast | ||||
| Midwest | 0.93 (0.88, 0.98)* | 0.96 (0.92, 1.00) | 0.95 (0.91, 0.99)* | 0.95 (0.91, 0.99)* |
| South | 0.98 (0.94, 1.02) | 1.06 (1.02, 1.10)* | 1.04 (1.00, 1.09) | 1.03 (0.98, 1.08) |
| West | 0.97 (0.92, 1.01) | 1.22 (1.16, 1.30)* | 1.02 (0.97, 1.08) | 1.06 (1.00, 1.12)* |
| Transfer status | ||||
| Transfer | 1.35 (1.25, 1.46) | 1.39 (1.27, 1.52) | 1.31 (1.23, 1.37 ) | 1.16 (1.10, 1.23) |
| Asthma | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99) | 0.98 (0.96, 1.00) | 0.98 (0.97, 1.00)* |
| Pneumonia Complications | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99)* | 0.98 (0.96, 1.0) | 0.98 (0.97, 1.00)* |
| Any complication | 2.20 (2.07, 2.34) | 2.23 (2.07, 2.40) | 2.22 (2.22, 2.44) | 2.37 (2.27, 2.47) |
DISCUSSION
In this nationally representative sample selected over the past 10 years, we found that publicly insured children hospitalized with CAP have significantly longer LOS than those who are privately insured, and that, since 2000, uninsured children hospitalized with CAP have significantly shorter LOS than those who are privately insured. Though these observed differences are small, they are consistent across all 4 sampled years and, because CAP is one of the most common pediatric inpatient diagnoses, the cumulative impact of the observed differences on hospital LOS is great. Insurance status is often considered a proxy for access to preventive and ambulatory healthcare services or socioeconomic status. However, the underlying mechanisms relating insurance status to healthcare access, utilization, and ultimately, health outcomes are highly complex and difficult to elucidate.17 The observed variation in this study raises questions about the potential influence of insurance status on hospital discharge practices. Additional research is necessary to understand whether there are differences in processes of care (eg, performance of blood cultures or chest radiographs), quality of care, or other outcomes, such as readmissions, related to CAP inpatient management for children with different insurance coverage.
Apart from differences in hospital discharge practices, another possible explanation for uninsured children with CAP having shorter LOS is that these children have less severe disease than privately insured. This may occur if uninsured children with CAP are evaluated in the emergency department rather than the office setting, because emergency department providers may be more likely to admit children with CAP who lack a consistent access to ambulatory primary care services. Countering this alternative, prior studies have shown that uninsured groups are more likely to have greater disease severity than privately insured groups at the time of hospital admission.18, 19 In this study, we attempted to identify children with greater severity of disease using ICD‐9 codes for CAP‐associated complications. Though this is a relatively crude method that might lead to an underestimate of the total number of children with complications, we found that there were no significant differences in the prevalence of CAP‐associated complications between uninsured and insured groups in all sampled years.
On the other hand, uninsured patients may be released earlier by providers in order to reduce the amount of uncompensated care provided, or possibly because parents may urge providers to discharge their children, given their inability to pay forthcoming hospital bills and/or avoid further lost wages due to work absence.20, 21 In California, Bindman et al. demonstrated that decreasing the frequency of Medicaid recertification, and consequently increasing the likelihood of continuous insurance coverage, was associated with a decreased risk of hospitalization for ambulatory‐care sensitive conditions.5
We also found that children admitted to urban teaching children's hospitals with CAP had significantly longer LOS than those admitted to urban non‐teaching hospitals, whereas children in rural hospitals had significantly shorter LOS than those in urban non‐teaching hospitals in 2003. These findings are consistent with prior data from 1996 to1998 demonstrating that children admitted to rural hospitals in New York and Pennsylvania had significantly shorter LOS than large urban hospitals for 19 medical and 9 surgical conditions, including pneumonia.12 These findings may reflect underlying differences in between rural and urban hospital transfer practices, whereby rural hospitals may be more likely than urban hospitals to transfer children with relatively more severe illness to urban referral centers and retain children with less severe illness, leading to shorter LOS.12 Though our empiric understanding of differences in LOS between teaching and non‐teaching hospitals is currently limited, clinical experience supports the notion that there may be decreases in efficiency that occur in teaching hospitals, and are a result of the supervision required for care provided by trainees. It is also possible that, despite our exclusion of comorbid conditions, some children with complex or chronic medical conditions were included in this study. These children are often cared for at teaching hospitals, regardless of the primary cause for admission, and are more likely to have public insurance than other children, thus confounding the relationship between hospital type, insurance type and status, and LOS for children with CAP. The limitations of this dataset preclude further examination of this issue.
There are some limitations to this study. First, the KID data are cross‐sectional and causal inferences are limited. However, our results demonstrating that uninsured children hospitalized with CAP had shorter LOS than privately insured children were quite consistent in each sample year, suggesting that our results are a true association. Additionally, insurance status in KID is typically collected at admission, however, it is not possible to determine whether specific changes to insurance status that occurred during the hospitalization were applied to the data. The impact of this limitation would depend on the type of insurance obtained by the patient. If uninsured patients obtained public insurance, our study would underestimate the increased LOS for publicly insured patients, compared with privately insured patients, but have no effect on the difference in LOS between uninsured and privately insured patients. In the unlikely event that uninsured patients obtained private insurance, then our study would underestimate the difference for uninsured patients, compared with privately insured patients, biasing our current study results towards the null. Second, a substantial proportion of sampled children had missing data for race/ethnicity. To assess the impact of the missing race/ethnicity data on our results, we conducted sensitivity analyses and found that, though difficult to make any definitive conclusions about the relationship between race/ethnicity and LOS for children with CAP, there were no changes to our primary findings regarding differences in LOS between children with different insurance status and type. Third, KID does not include data about other unmeasured confounders (eg, parent income, parent education, regular source of care) that might be related to LOS, as well as a broad spectrum of pediatric outcomes. Serious consideration of expanding KID to include these variables is warranted. Fourth, the other category of insurance is not uniformly coded across states in the KID database. While some states use this category to classify public insurance options other than Medicare and Medicaid, other states include private insurance options in this group. Thus, it is possible that some patients with public insurance are misclassified as having other insurance. We would expect such misclassification to bias our findings towards the null hypothesis. Finally, we focused on the relationship between child health insurance status and CAP, only 1 ambulatory care‐sensitive condition. Additional research examining the relationship between insurance type and other ambulatory care‐sensitive conditions is warranted.
In summary, we found that, after multivariable adjustment, uninsured children hospitalized with community‐acquired pneumonia had significantly shorter LOS than privately insured children, and publicly insured children had a significantly longer hospital stay than privately insured children in these 4 nationally representative samples from 1997 to 2006. Current federal and state efforts to increase enrollment of children into insurance programs are a first step in reducing healthcare disparities. However, insurance coverage alone does not guarantee access to healthcare, thus, these efforts in isolation will likely be insufficient to achieve optimal health for the children of our country. As healthcare reform legislation is implemented, these findings provide hospitals and policy makers additional impetus to develop ways to achieve the ideal length of stay for every child; this ideal state will be achieved when clinical status and course, rather than nonclinical factors such as insurance type or provider's unease with ambulatory follow‐up, determine the duration of hospitalization for every child.
- ,,,,,.Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials.JAMA.2007;298:179–186.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154:789–796.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124:e1081–e1087.
- ,,.Medicaid re‐enrollment policies and children's risk of hospitalizations for ambulatory care sensitive conditions.Med Care.2008;46:1049–1054.
- ,.Differences associated with age, transfer status, and insurance coverage in end‐of‐life hospital care for children.J Hosp Med.2008;3:376–383.
- ,,,,,.Health care for children and youth in the United States: annual report on patterns of coverage, utilization, quality, and expenditures by a county level of urban influence.Ambul Pediatr.2006;6:241–264.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Variation in hospital discharges for ambulatory care‐sensitive conditions among children.Pediatrics.2000;106:942–948.
- ,,,,,.Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007.Pediatrics.2011;127:411–418.
- ,,,,.Patterns of hospital‐based pediatric care across diverse ethnicities: the case of pneumonia.J Health Care Poor Underserved.2004;15:462–473.
- ,,,,.Equivalent lengths of stay of pediatric patients hospitalized in rural and nonrural hospitals.Pediatrics.2004;114:e400–e408.
- ,.Effect of Child Health Insurance Plan enrollment on the utilization of health care services by children using a public safety net system.Pediatrics.2002;110:940–945.
- ,,,,,.Relationships between welfare status, health insurance status, and health and medical care among children with asthma.Am J Public Health.2002;92:1446–1452.
- HCUP Kids' Inpatient Database (KID). Healthcare Cost and Utilization Project (HCUP), 1997, 2000, 2003, 2006. Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed May 17,2010.
- ,,, et al.Community‐acquired pneumonia: can it be defined with claims data?Am J Med Qual.1997;12:187–193.
- .Sicker and poorer—the consequences of being uninsured: a review of the research on the relationship between health insurance, medical care use, health, work, and income.Med Care Res Rev.2003;60:3S–75S; discussion76S–112S.
- ,,,,,.Socioeconomic variation in asthma hospitalization: excess utilization or greater need?Pediatrics.1999;103:e75.
- ,,, et al.Analysis of 23 million US hospitalizations: uninsured children have higher all‐cause in‐hospital mortality.J Public Health (Oxf).2010;32(2)236–244.
- ,.The impact of welfare reform on parents' ability to care for their children's health.Am J Public Health.1999;89:502–505.
- ,,.Knowledge of welfare reform program provisions among families of children with chronic conditions.Am J Public Health.2002;92:228–230.
Disparities in patterns of care and outcomes for ambulatory‐care sensitive conditions remain a persistent problem for children.19 Many studies have focused on disparities in hospitalization rates and length of stay (LOS) related to asthma, however, few studies have focused on community‐acquired pneumonia (CAP) despite the fact that pneumonia is the most common, preventable, and potentially serious infection in childhood.10 Providers, payers, and families have a common interest in minimizing hospital LOS for different reasons (eg, minimizing costs, lost wages, exposure to antibiotic‐resistant bacteria), however, this interest is balanced against the potentially greater risk of readmission and adverse outcomes if LOS is inappropriately short. To date, the relationship between insurance status and LOS for CAP remains unexplored.
As in other conditions, substantial variation exists with respect to patterns of care and outcomes for children hospitalized with CAP.11 For example, children hospitalized in rural settings have a shorter LOS for pneumonia than those hospitalized in large urban settings.12 Children from racial/ethnic minorities tend to have higher rates of CAP‐associated complications, including death.11 Decades of prior studies have documented that uninsured children are less likely than insured children to make preventive care visits and obtain prescription medications, but differences in LOS or hospitalization rates between insured and uninsured children with CAP have not been studied.6, 8, 13, 14 Though imperfect, insurance status is 1 proxy for healthcare access, and current healthcare reform efforts aim to improve healthcare access and decrease socioeconomic gradients in health by increasing the number of insured American children. Nonetheless, quantifying the relationship between insurance status on LOS for children hospitalized with CAP is a first step towards understanding the influence of ambulatory care access on hospitalization for ambulatory‐care sensitive conditions.
The purpose of this study was to investigate the influence of insurance status and type on LOS for children hospitalized with CAP. In addition, we sought to determine if there were consistent trends over time in the association between insurance status and type with LOS for children hospitalized with CAP.
METHODS
Study Design and Data Source
This retrospective cross‐sectional study used data from the 1997, 2000, 2003, and 2006 Kids' Inpatient Database (KID). The KID is part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality (AHRQ). It is the only dataset on hospital use and outcomes specifically designed to study children's use of hospital services in the United States. The KID samples pediatric discharges from all community non‐rehabilitation hospitals in states participating in the Healthcare Cost and Utilization Project, using a complex stratification system, across pediatric discharge type and hospital characteristics. Community hospitals in the KID are defined as all non‐federal, short‐term, general and other specialty hospitals, including academic medical centers, obstetrics‐gynecology, otolaryngology, orthopedic, and children's hospitals. Federal hospitals, long‐term hospitals, psychiatric hospitals, alcohol/chemical dependency treatment facilities and hospitals units within institutions are excluded. Discharge‐level weights assigned to discharges within the stratum permit calculation of national estimates. Datasets, which each contain approximately 3 million discharges (unweighted), are released every 3 years beginning with 1997. The 2006 KID is the most recently available dataset and contains hospital administrative data from 38 states, representing 88.8% of the estimated US population.15 This study was considered exempt from review by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia.
Study Participants
Patients 18 years of age and younger were eligible for inclusion if they required hospitalization for CAP in 1997, 2000, 2003, or 2006. Using a previously validated algorithm, patients were considered as having CAP if they met 1 of 2 criteria: 1) International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9 CM) primary diagnosis code indicating pneumonia (480‐483, 485‐486), empyema (510), or pleurisy (511.0‐1, 511.9); or 2) primary diagnosis of pneumonia‐related symptom (eg, cough, fever, tachypnea) and secondary diagnosis of pneumonia, empyema or pleurisy. Pneumonia‐related symptoms included fever, respiratory abnormality unspecified, shortness of breath, tachypnea, wheezing, cough, hemoptysis, abnormal sputum, chest pain, and abnormal chest sounds.16 Because there is no specific ICD‐9 code for nosocomial pneumonia, this previously validated approach minimized such misclassification16 (eg, a child hospitalized following traumatic injury who then develops ventilator‐associated pneumonia is likely to have trauma, rather than pneumonia or a pneumonia‐related symptom, listed as the primary diagnosis). Patients with the following comorbid conditions (identified by KID data elements and ICD‐9 CM codes) were excluded as these comorbidities are characterized by risk factors not reflective of the general pediatric population: acquired and congenital immunologic disorders, malignancy, collagen vascular disease, sickle cell disease, cystic fibrosis, organ transplant, congenital heart defects, and heart failure. Patients identified as in‐hospital births were excluded to minimize the inclusion of perinatally acquired and nosocomial infections occurring in neonates. Patients with a secondary diagnosis code indicating trauma were also excluded, as a diagnosis of pneumonia in this population likely reflects nosocomial etiology. CAP‐related complications (eg, effusion, abscess; for complete list, see Supporting Appendix A in the online version of this article) were identified using ICD‐9 CM diagnosis and procedure codes. Asthma‐related hospitalizations were identified using ICD‐9 CM diagnosis code 493 in any secondary diagnosis field.
Primary Exposure
The primary exposure was insurance type, categorized as private, public, uninsured, or other (eg, Civilian Health and Medical Program Uniform Service (CHAMPUS), worker's compensation, union‐based insurance, but definition varies by state precluding categorization as purely public or private).
Primary Outcome
The primary outcome was the hospital LOS calculated in days.
Statistical Analysis
Consistent with prior work,12 subjects were characterized by age, race, sex, the presence or absence of a pneumonia‐associated complication, discharge status (discharge from hospital vs in‐hospital death), hospital type (rural, urban non‐teaching, urban teaching non‐children's, urban teaching children's), and hospital region (Northeast, Midwest, South, West). Age groups for analysis were defined as <1 year (infant), 1 to 5 years (preschool age), 6 to 11 years (school‐age), and 12 to 18 years old (adolescent). Race was recorded as a single variable (white, black, other, and missing). Patient information for race was missing from 32% of discharges in 1997, 18% in 2000, 29% in 2003, and 26% in 2006. Patients with missing race data were included to preserve the integrity of our estimates. Categorical variables were summarized by frequencies and percents. Continuous variables were summarized by mean and standard deviation values.
All analyses accounted for the complex sampling design with the survey commands included in STATA, version 10 (College Station, TX) to produce weighted estimates. To determine the adjusted impact of patient and hospital‐level characteristics in our cohort, we constructed multivariable negative binomial regression models using all available covariates for LOS because of its rightward‐skewed distribution. The negative binomial model produced an incident rate ratio (IRR) for LOS (IRR >1 indicates that the risk factor is associated with a longer length of stay). As recommended in the AHRQ technical documentation, variance estimates for each model accounted for the clustering of data at the hospital level. To address the impact of missing race data on outcome, we constructed additional multivariable negative binomial regression models while varying the underlying assumptions about race classification. In these secondary analyses, children with race coded as missing were sequentially excluded, assumed to be white, and assumed to be black. These analyses were repeated after excluding insurance from the multivariable model.
RESULTS
The more than 10.5 million children sampled (unweighted) in KID during these 4 time periods (1997, 2000, 2003, and 2006) are representative of the more than 28.9 million children hospitalized in the United States. In each of these sample years, there were approximately 150,000 children hospitalized with pneumonia across the United States (Table 1). Of those hospitalized, 23% to 28% had a concomitant diagnosis of asthma; 6% to 8% had a pneumonia‐associated complication; and mortality was <0.01% in each sample year for patients hospitalized with pneumonia. In all years, among those with racial/ethnic data, the sample population was predominantly white boys less than 6 years old. The greatest proportion of children were hospitalized in urban non‐teaching settings, and also those children living in the southern regions of the United States.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| N = 148,702 | N = 157,847 | N = 157,743 | N = 156,810 | |
| ||||
| Race | ||||
| White | 56,348 (38) | 68,643 (44) | 54,903 (35) | 56,108 (36) |
| Black | 22,864 (15) | 22,580 (14) | 17,960 (11) | 18,800 (12) |
| Other | 22,203 (15) | 38,448 (24) | 39,138 (25) | 40,803 (26) |
| Missing | 47,287 (32) | 28,175 (18) | 45,588 (29) | 41,099 (26) |
| Age category | ||||
| <1 year | 43,851 (29) | 44,470 (28) | 37,798 (24) | 37,705 (24) |
| 1 through 5 years | 75,033 (50) | 76,385 (48) | 77,530 (49) | 79,519 (51) |
| 6 through 11 years | 19,372 (13) | 21,403 (14) | 23,126 (15) | 23,494 (15) |
| >12 years | 10,446 (7) | 15,589 (9) | 19,289 (12) | 16,092 (10) |
| Hospital type | ||||
| Urban non‐teaching | 52,756 (35) | 50,718 (32) | 52,552 (34) | 50,718 (32) |
| Rural | 47,910 (32) | 41,715 (27) | 39,605 (26) | 31,947 (21) |
| Urban teaching non‐children's | 20,378 (14) | 30,981 (20) | 28,432 (18) | 30,194 (20) |
| Urban teaching children's | 27,658 (19) | 34,021 (22) | 34,454 (22) | 41,035 (27) |
| Male sex | 83,291 (56) | 8,783 (56) | 86,034 (55) | 85,508 (55) |
| Region* | ||||
| Northeast | 19,750 (13) | 26,092 (17) | 23,867 (15) | 23,832 (15) |
| Midwest | 33,053 (22) | 30,706 (19) | 35,714 (23) | 35,900 (23) |
| South | 68,958 (46) | 68,663 (44) | 65,994 (42) | 65,460 (42) |
| West | 26,741 (18) | 32,385 (21) | 32,169 (20) | 31,618 (20) |
| Asthma | 26,971 (24) | 31,746 (28) | 27,729 (24) | 26,822 (23) |
| Pneumonia‐associated complication | 8,831 (6) | 11,084 (7) | 12,005 (8) | 11,724 (7) |
| Died | 334 (0.002) | 394 (0.002) | 270 (0.002) | 193 (0.001) |
| Insurance | ||||
| Private | 65,428 (44) | 73,528 (47) | 68,720 (44) | 63,997 (41) |
| Public | 68,024 (46) | 71,698 (45) | 76,779 (49) | 80,226 (51) |
| Uninsured | 9,922 (7) | 8,336 (5) | 6,381 (4) | 6,912 (4) |
| Other | 4,964 (3) | 4,285 (3) | 5,391 (3) | 5,283 (3) |
There was little variation in the insurance status of children hospitalized with CAP between 1997 and 2006. In each of the sampled years, at least 40% of sampled children were privately insured, at least 40% were publicly insured, and approximately 5% were uninsured (Table 1). In all years, there were significant racial/ethnic disparities in insurance coverage such that whites were 4 to 6 times more likely to have private insurance than blacks, however, the large amount of missing race/ethnicity data warrant caution in interpreting this finding (Table 2; also see Supporting Information Appendix B in the online version of this article). We also found that children less than 1 year old were the most likely to be publicly insured in all years (see Supporting Appendix C in the online version of this article). There were also regional differences related to insurance coverage such that a greater proportion of children hospitalized in facilities located in the southern part of the United States were publicly insured. Notably, there were no significant differences in CAP‐associated mortality or asthma related to insurance coverage (Table 2). In 2006, CAP‐associated complications occurred in 8.5% of children with private insurance, 6.5% of children with public insurance, and 7.7% of uninsured children; the relative distribution of complications by insurance type were similar in previous years of the KID survey.
| Private | Public | Uninsured | Other Insurance | P | |
|---|---|---|---|---|---|
| |||||
| No. of children (%) | 63,997 (41) | 80,226 (51) | 6,912 (4) | 5,283 (3) | |
| Male sex | 34,639 (41) | 44,140 (52) | 3,727 (4) | 2,808 (3) | 0.092 |
| Race | |||||
| White | 30,707 (55) | 21,282 (38) | 2,241 (4) | 1,774 (3) | <0.001 |
| Black* | 5,112 (27) | 12,239 (65) | 988 (5) | 426 (3) | |
| Other | 11,033 (27) | 26,489 (65) | 2,112 (5) | 1,076 (3) | |
| Missing | 17,145 (42) | 20,216 (49) | 1,572 (4) | 2,007 (4) | |
| Age category | |||||
| <1 year | 10,788 (29) | 24,762 (65) | 1,164 (3) | 880 (3) | <0.001 |
| 1 through 5 years | 33,664 (42) | 39,531 (50) | 3,442 (4) | 2,673 (3) | |
| 6 through 11 years | 11,660 (50) | 9,684 (41) | 1,085 (5) | 1,015 (4) | |
| >12 years | 7,885 (49) | 6,249 (39) | 1,221 (8) | 714 (4) | |
| Hospital type | |||||
| Urban non‐teaching | 22,429 (44) | 24,241 (49) | 2,440 (5) | 1,555 (2) | <0.001 |
| Rural | 10,880 (34) | 18,396 (58) | 1,290 (4) | 1,109 (3) | |
| Urban teaching non‐children's | 13,130 (44) | 14,542 (48) | 1,721 (6) | 750 (2) | |
| Urban teaching children's | 16,591 (40) | 21,544 (53) | 1,417 (3) | 1,465 (4) | |
| Region | |||||
| Northeast | 12,364 (52) | 9,620 (40) | 1,466 (6) | 377 (2) | <0.001 |
| Midwest∥ | 17,891 (50) | 15,573 (43) | 1,160 (3) | 1,215 (3) | |
| South∥ | 21,479 (33) | 38,112 (58) | 3,108 (5) | 2,495 (4) | |
| West∥ | 12,263 (39) | 16,921 (44) | 1,178 (5) | 1,195 (5) | |
| Asthma | 10,829 (41) | 13,923 (52) | 1,119 (4) | 866 (3) | 0.193 |
| Pneumonia‐associated complication | 5,416 (46) | 5,206 (45) | 532 (4) | 556 (5) | <0.001 |
| Died | 66 (34) | 115 (60) | 3 (1) | 8 (5) | 0.131 |
After examining the general and demographic characteristics, we then examined mean LOS for all children with CAP in each sample year (Table 3). The mean LOS for children with CAP was 3.44 days in 1997, with marginal decreases in subsequent years to a mean LOS of 3.18 days in 2006. The distribution of LOS for children with CAP revealed that nearly 70% of children were hospitalized for fewer than 3 days, another 22% to 28% were hospitalized for less than 1 week, and only 3% were hospitalized for more than 1 week. This distribution did not change substantially between 1997 and 2006. Next, we compared mean LOS by insurance type and race/ethnicity in unadjusted analyses. In each sample year, publicly insured children hospitalized with CAP had significantly longer LOS than privately insured children (P < 0.001). Similarly, in all years excepting 1997, uninsured children hospitalized with CAP had significantly shorter LOS than privately insured children. There were also significant racial differences in LOS for children with CAP, such that black children had longer LOS than white children with CAP. However, the large amount of missing data for race/ethnicity limited the robustness of this finding, and subsequent sensitivity analyses demonstrated that there were no consistent racial/ethnic disparities in LOS (see Supporting Appendix B in the online version of this article). These sensitivity analyses for missing race data did not alter our primary finding of shorter LOS for uninsured versus publicly or privately insured children.
| 1997 | P | 2000 | P | 2003 | P | 2006 | P | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Overall | 3.44 (0.04) | 3.35 (0.05) | 3.27 (0.05) | 3.18 (0.04) | ||||
| Insurance type | ||||||||
| Private | 3.21 (0.04) | 3.19 (0.04) | 3.09 (0.04) | 3.00 (0.03) | ||||
| Public | 3.71 (0.06) | <0.001 | 3.57 (0.06) | <0.001 | 3.44 (0.06) | <0.001 | 3.34 (0.05) | <0.001 |
| Uninsured | 3.18 (0.14) | 0.792 | 2.92 (0.07) | <0.001 | 2.80 (0.05) | <0.001 | 2.82 (0.05) | <0.001 |
| Other | 3.32 (0.11) | 0.319 | 3.55 (0.14) | 0.0134 | 3.54 (0.21) | 0.037 | 3.42 (0.13) | 0.001 |
| Race | ||||||||
| White | 3.31 (0.05) | 3.18 (0.04) | 3.19 (0.05) | 3.10 (0.04) | ||||
| Black | 3.61 (0.08) | <0.001 | 3.32 (0.07) | <0.001 | 3.36 (0.08) | <0.001 | 3.31 (0.07) | <0.001 |
| Other | 3.96 (0.11) | <0.001 | 3.81 (0.09) | <0.001 | 3.67 (0.10) | <0.001 | 3.56 (0.08) | <0.001 |
| Missing | 3.27 (0.08) | 0.645 | 3.18 (0.08) | 0.926 | 2.99 (0.06) | 0.0134 | 2.86 (0.04) | <0.001 |
After controlling for child age, race/ethnicity, gender, hospital type, transfer status, and presence of asthma or pneumonia‐associated complications, our multivariable analyses examining the relationship between insurance coverage and hospital LOS yielded the following results (Table 4). First, publicly insured children had significantly longer hospital stays than privately insured children, and uninsured children had significantly shorter hospital stays than privately insured children in all years except 1997. Second, children admitted with CAP at urban teaching children's hospitals had significantly longer LOS than those admitted to urban non‐teaching hospitals, and, in 2003, children admitted with CAP to rural hospitals had significantly shorter LOS than those admitted to urban non‐teaching hospitals. Third, children older than 1 year consistently had shorter hospital stays than infants less than 1 year old. Finally, though concomitant diagnosis of asthma did not consistently influence LOS, children who developed any complications had significantly longer LOS than those who did not. The cumulative impact of seemingly small differences in LOS is great. For example, in 2006, our model suggests that, for every 1000 children hospitalized with CAP in a given year, after adjusting for differences in sex, age, race, hospital‐type, region, transfer status, and diagnosis of asthma or complications, publicly insured children spend 90 to 130 more days in the hospital than privately insured children, whereas uninsured children spend between 40 to 90 fewer days in the hospital than privately insured children.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| Variable | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| ||||
| Age category | ||||
| <1 year | ||||
| 15 years | 0.82 (0.81, 0.84) | 0.83 (0.88, 0.95) | 0.86 (0.85, 0.88) | 0.87 (0.86, 0.89) |
| 611 years | 0.91 (0.87, 0.95) | 0.91 (0.88, 0.94) | 0.93 (0.91, 0.95) | 0.93 (0.90, 0.95) |
| >12 years | 1.03 (0.99, 1.07) | 1.17 (1.11, 1.22) | 1.09 (1.06, 1.13) | 1.13 (1.09, 1.16) |
| Race | ||||
| White | ||||
| Black | 1.04 (0.99, 1.08) | 1.00 (0.95, 1.03) | 1.00 (0.98, 1.03) | 1.02 (0.98, 1.06) |
| Other | 1.09 (1.05, 1.13) | 1.11 (1.08, 1.15) | 1.09 (1.06, 1.12) | 1.08 (1.05, 1.11) |
| Missing | 1.00 (0.94, 1.06) | 1.01 (0.96, 1.06) | 0.95 (0.92, 0.99)* | 0.96 (0.93, 0.99) |
| Sex | ||||
| Female | 1.02 (0.94, 1.06) | 1.01 (0.99, 1.02) | 1.01(0.93, 100) | 1.01 (1.00, 1.02) |
| Insurance type | ||||
| Private | ||||
| Public | 1.13 (1.11, 1.16) | 1.11 (1.09, 1.14) | 1.11 (1.09, 1.13) | 1.11 (1.09, 1.13) |
| Uninsured | 1.01 (0.91, 1.11) | 0.93 (0.89, 0.96) | 0.92 (0.90, 0.96) | 0.94 (0.91, 0.96) |
| Other | 1.01 (0.96, 1.06) | 1.10 (1.03, 1.18) | 1.10 (1.02, 1.19)* | 1.07 (1.02, 1.13) |
| Hospital type | ||||
| Urban non‐teaching | ||||
| Rural | 0.98 (0.92, 1.04) | 0.96 (0.92, 1.00) | 0.97 (0.94, 1.00) | 0.97 (0.93, 1.00) |
| Urban teaching (non‐children's) | 0.99 (0.95, 1.04) | 1.06 (1.02, 1.10) | 1.06 (1.02, 1.10) | 1.03 (0.99, 1.07) |
| Urban teaching children's | 1.2 (1.14, 1.26) | 1.23 (1.16, 1.30) | 1.28 (1.21, 1.37) | 1.25 (1.19, 1.31) |
| Region | ||||
| Northeast | ||||
| Midwest | 0.93 (0.88, 0.98)* | 0.96 (0.92, 1.00) | 0.95 (0.91, 0.99)* | 0.95 (0.91, 0.99)* |
| South | 0.98 (0.94, 1.02) | 1.06 (1.02, 1.10)* | 1.04 (1.00, 1.09) | 1.03 (0.98, 1.08) |
| West | 0.97 (0.92, 1.01) | 1.22 (1.16, 1.30)* | 1.02 (0.97, 1.08) | 1.06 (1.00, 1.12)* |
| Transfer status | ||||
| Transfer | 1.35 (1.25, 1.46) | 1.39 (1.27, 1.52) | 1.31 (1.23, 1.37 ) | 1.16 (1.10, 1.23) |
| Asthma | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99) | 0.98 (0.96, 1.00) | 0.98 (0.97, 1.00)* |
| Pneumonia Complications | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99)* | 0.98 (0.96, 1.0) | 0.98 (0.97, 1.00)* |
| Any complication | 2.20 (2.07, 2.34) | 2.23 (2.07, 2.40) | 2.22 (2.22, 2.44) | 2.37 (2.27, 2.47) |
DISCUSSION
In this nationally representative sample selected over the past 10 years, we found that publicly insured children hospitalized with CAP have significantly longer LOS than those who are privately insured, and that, since 2000, uninsured children hospitalized with CAP have significantly shorter LOS than those who are privately insured. Though these observed differences are small, they are consistent across all 4 sampled years and, because CAP is one of the most common pediatric inpatient diagnoses, the cumulative impact of the observed differences on hospital LOS is great. Insurance status is often considered a proxy for access to preventive and ambulatory healthcare services or socioeconomic status. However, the underlying mechanisms relating insurance status to healthcare access, utilization, and ultimately, health outcomes are highly complex and difficult to elucidate.17 The observed variation in this study raises questions about the potential influence of insurance status on hospital discharge practices. Additional research is necessary to understand whether there are differences in processes of care (eg, performance of blood cultures or chest radiographs), quality of care, or other outcomes, such as readmissions, related to CAP inpatient management for children with different insurance coverage.
Apart from differences in hospital discharge practices, another possible explanation for uninsured children with CAP having shorter LOS is that these children have less severe disease than privately insured. This may occur if uninsured children with CAP are evaluated in the emergency department rather than the office setting, because emergency department providers may be more likely to admit children with CAP who lack a consistent access to ambulatory primary care services. Countering this alternative, prior studies have shown that uninsured groups are more likely to have greater disease severity than privately insured groups at the time of hospital admission.18, 19 In this study, we attempted to identify children with greater severity of disease using ICD‐9 codes for CAP‐associated complications. Though this is a relatively crude method that might lead to an underestimate of the total number of children with complications, we found that there were no significant differences in the prevalence of CAP‐associated complications between uninsured and insured groups in all sampled years.
On the other hand, uninsured patients may be released earlier by providers in order to reduce the amount of uncompensated care provided, or possibly because parents may urge providers to discharge their children, given their inability to pay forthcoming hospital bills and/or avoid further lost wages due to work absence.20, 21 In California, Bindman et al. demonstrated that decreasing the frequency of Medicaid recertification, and consequently increasing the likelihood of continuous insurance coverage, was associated with a decreased risk of hospitalization for ambulatory‐care sensitive conditions.5
We also found that children admitted to urban teaching children's hospitals with CAP had significantly longer LOS than those admitted to urban non‐teaching hospitals, whereas children in rural hospitals had significantly shorter LOS than those in urban non‐teaching hospitals in 2003. These findings are consistent with prior data from 1996 to1998 demonstrating that children admitted to rural hospitals in New York and Pennsylvania had significantly shorter LOS than large urban hospitals for 19 medical and 9 surgical conditions, including pneumonia.12 These findings may reflect underlying differences in between rural and urban hospital transfer practices, whereby rural hospitals may be more likely than urban hospitals to transfer children with relatively more severe illness to urban referral centers and retain children with less severe illness, leading to shorter LOS.12 Though our empiric understanding of differences in LOS between teaching and non‐teaching hospitals is currently limited, clinical experience supports the notion that there may be decreases in efficiency that occur in teaching hospitals, and are a result of the supervision required for care provided by trainees. It is also possible that, despite our exclusion of comorbid conditions, some children with complex or chronic medical conditions were included in this study. These children are often cared for at teaching hospitals, regardless of the primary cause for admission, and are more likely to have public insurance than other children, thus confounding the relationship between hospital type, insurance type and status, and LOS for children with CAP. The limitations of this dataset preclude further examination of this issue.
There are some limitations to this study. First, the KID data are cross‐sectional and causal inferences are limited. However, our results demonstrating that uninsured children hospitalized with CAP had shorter LOS than privately insured children were quite consistent in each sample year, suggesting that our results are a true association. Additionally, insurance status in KID is typically collected at admission, however, it is not possible to determine whether specific changes to insurance status that occurred during the hospitalization were applied to the data. The impact of this limitation would depend on the type of insurance obtained by the patient. If uninsured patients obtained public insurance, our study would underestimate the increased LOS for publicly insured patients, compared with privately insured patients, but have no effect on the difference in LOS between uninsured and privately insured patients. In the unlikely event that uninsured patients obtained private insurance, then our study would underestimate the difference for uninsured patients, compared with privately insured patients, biasing our current study results towards the null. Second, a substantial proportion of sampled children had missing data for race/ethnicity. To assess the impact of the missing race/ethnicity data on our results, we conducted sensitivity analyses and found that, though difficult to make any definitive conclusions about the relationship between race/ethnicity and LOS for children with CAP, there were no changes to our primary findings regarding differences in LOS between children with different insurance status and type. Third, KID does not include data about other unmeasured confounders (eg, parent income, parent education, regular source of care) that might be related to LOS, as well as a broad spectrum of pediatric outcomes. Serious consideration of expanding KID to include these variables is warranted. Fourth, the other category of insurance is not uniformly coded across states in the KID database. While some states use this category to classify public insurance options other than Medicare and Medicaid, other states include private insurance options in this group. Thus, it is possible that some patients with public insurance are misclassified as having other insurance. We would expect such misclassification to bias our findings towards the null hypothesis. Finally, we focused on the relationship between child health insurance status and CAP, only 1 ambulatory care‐sensitive condition. Additional research examining the relationship between insurance type and other ambulatory care‐sensitive conditions is warranted.
In summary, we found that, after multivariable adjustment, uninsured children hospitalized with community‐acquired pneumonia had significantly shorter LOS than privately insured children, and publicly insured children had a significantly longer hospital stay than privately insured children in these 4 nationally representative samples from 1997 to 2006. Current federal and state efforts to increase enrollment of children into insurance programs are a first step in reducing healthcare disparities. However, insurance coverage alone does not guarantee access to healthcare, thus, these efforts in isolation will likely be insufficient to achieve optimal health for the children of our country. As healthcare reform legislation is implemented, these findings provide hospitals and policy makers additional impetus to develop ways to achieve the ideal length of stay for every child; this ideal state will be achieved when clinical status and course, rather than nonclinical factors such as insurance type or provider's unease with ambulatory follow‐up, determine the duration of hospitalization for every child.
Disparities in patterns of care and outcomes for ambulatory‐care sensitive conditions remain a persistent problem for children.19 Many studies have focused on disparities in hospitalization rates and length of stay (LOS) related to asthma, however, few studies have focused on community‐acquired pneumonia (CAP) despite the fact that pneumonia is the most common, preventable, and potentially serious infection in childhood.10 Providers, payers, and families have a common interest in minimizing hospital LOS for different reasons (eg, minimizing costs, lost wages, exposure to antibiotic‐resistant bacteria), however, this interest is balanced against the potentially greater risk of readmission and adverse outcomes if LOS is inappropriately short. To date, the relationship between insurance status and LOS for CAP remains unexplored.
As in other conditions, substantial variation exists with respect to patterns of care and outcomes for children hospitalized with CAP.11 For example, children hospitalized in rural settings have a shorter LOS for pneumonia than those hospitalized in large urban settings.12 Children from racial/ethnic minorities tend to have higher rates of CAP‐associated complications, including death.11 Decades of prior studies have documented that uninsured children are less likely than insured children to make preventive care visits and obtain prescription medications, but differences in LOS or hospitalization rates between insured and uninsured children with CAP have not been studied.6, 8, 13, 14 Though imperfect, insurance status is 1 proxy for healthcare access, and current healthcare reform efforts aim to improve healthcare access and decrease socioeconomic gradients in health by increasing the number of insured American children. Nonetheless, quantifying the relationship between insurance status on LOS for children hospitalized with CAP is a first step towards understanding the influence of ambulatory care access on hospitalization for ambulatory‐care sensitive conditions.
The purpose of this study was to investigate the influence of insurance status and type on LOS for children hospitalized with CAP. In addition, we sought to determine if there were consistent trends over time in the association between insurance status and type with LOS for children hospitalized with CAP.
METHODS
Study Design and Data Source
This retrospective cross‐sectional study used data from the 1997, 2000, 2003, and 2006 Kids' Inpatient Database (KID). The KID is part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality (AHRQ). It is the only dataset on hospital use and outcomes specifically designed to study children's use of hospital services in the United States. The KID samples pediatric discharges from all community non‐rehabilitation hospitals in states participating in the Healthcare Cost and Utilization Project, using a complex stratification system, across pediatric discharge type and hospital characteristics. Community hospitals in the KID are defined as all non‐federal, short‐term, general and other specialty hospitals, including academic medical centers, obstetrics‐gynecology, otolaryngology, orthopedic, and children's hospitals. Federal hospitals, long‐term hospitals, psychiatric hospitals, alcohol/chemical dependency treatment facilities and hospitals units within institutions are excluded. Discharge‐level weights assigned to discharges within the stratum permit calculation of national estimates. Datasets, which each contain approximately 3 million discharges (unweighted), are released every 3 years beginning with 1997. The 2006 KID is the most recently available dataset and contains hospital administrative data from 38 states, representing 88.8% of the estimated US population.15 This study was considered exempt from review by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia.
Study Participants
Patients 18 years of age and younger were eligible for inclusion if they required hospitalization for CAP in 1997, 2000, 2003, or 2006. Using a previously validated algorithm, patients were considered as having CAP if they met 1 of 2 criteria: 1) International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9 CM) primary diagnosis code indicating pneumonia (480‐483, 485‐486), empyema (510), or pleurisy (511.0‐1, 511.9); or 2) primary diagnosis of pneumonia‐related symptom (eg, cough, fever, tachypnea) and secondary diagnosis of pneumonia, empyema or pleurisy. Pneumonia‐related symptoms included fever, respiratory abnormality unspecified, shortness of breath, tachypnea, wheezing, cough, hemoptysis, abnormal sputum, chest pain, and abnormal chest sounds.16 Because there is no specific ICD‐9 code for nosocomial pneumonia, this previously validated approach minimized such misclassification16 (eg, a child hospitalized following traumatic injury who then develops ventilator‐associated pneumonia is likely to have trauma, rather than pneumonia or a pneumonia‐related symptom, listed as the primary diagnosis). Patients with the following comorbid conditions (identified by KID data elements and ICD‐9 CM codes) were excluded as these comorbidities are characterized by risk factors not reflective of the general pediatric population: acquired and congenital immunologic disorders, malignancy, collagen vascular disease, sickle cell disease, cystic fibrosis, organ transplant, congenital heart defects, and heart failure. Patients identified as in‐hospital births were excluded to minimize the inclusion of perinatally acquired and nosocomial infections occurring in neonates. Patients with a secondary diagnosis code indicating trauma were also excluded, as a diagnosis of pneumonia in this population likely reflects nosocomial etiology. CAP‐related complications (eg, effusion, abscess; for complete list, see Supporting Appendix A in the online version of this article) were identified using ICD‐9 CM diagnosis and procedure codes. Asthma‐related hospitalizations were identified using ICD‐9 CM diagnosis code 493 in any secondary diagnosis field.
Primary Exposure
The primary exposure was insurance type, categorized as private, public, uninsured, or other (eg, Civilian Health and Medical Program Uniform Service (CHAMPUS), worker's compensation, union‐based insurance, but definition varies by state precluding categorization as purely public or private).
Primary Outcome
The primary outcome was the hospital LOS calculated in days.
Statistical Analysis
Consistent with prior work,12 subjects were characterized by age, race, sex, the presence or absence of a pneumonia‐associated complication, discharge status (discharge from hospital vs in‐hospital death), hospital type (rural, urban non‐teaching, urban teaching non‐children's, urban teaching children's), and hospital region (Northeast, Midwest, South, West). Age groups for analysis were defined as <1 year (infant), 1 to 5 years (preschool age), 6 to 11 years (school‐age), and 12 to 18 years old (adolescent). Race was recorded as a single variable (white, black, other, and missing). Patient information for race was missing from 32% of discharges in 1997, 18% in 2000, 29% in 2003, and 26% in 2006. Patients with missing race data were included to preserve the integrity of our estimates. Categorical variables were summarized by frequencies and percents. Continuous variables were summarized by mean and standard deviation values.
All analyses accounted for the complex sampling design with the survey commands included in STATA, version 10 (College Station, TX) to produce weighted estimates. To determine the adjusted impact of patient and hospital‐level characteristics in our cohort, we constructed multivariable negative binomial regression models using all available covariates for LOS because of its rightward‐skewed distribution. The negative binomial model produced an incident rate ratio (IRR) for LOS (IRR >1 indicates that the risk factor is associated with a longer length of stay). As recommended in the AHRQ technical documentation, variance estimates for each model accounted for the clustering of data at the hospital level. To address the impact of missing race data on outcome, we constructed additional multivariable negative binomial regression models while varying the underlying assumptions about race classification. In these secondary analyses, children with race coded as missing were sequentially excluded, assumed to be white, and assumed to be black. These analyses were repeated after excluding insurance from the multivariable model.
RESULTS
The more than 10.5 million children sampled (unweighted) in KID during these 4 time periods (1997, 2000, 2003, and 2006) are representative of the more than 28.9 million children hospitalized in the United States. In each of these sample years, there were approximately 150,000 children hospitalized with pneumonia across the United States (Table 1). Of those hospitalized, 23% to 28% had a concomitant diagnosis of asthma; 6% to 8% had a pneumonia‐associated complication; and mortality was <0.01% in each sample year for patients hospitalized with pneumonia. In all years, among those with racial/ethnic data, the sample population was predominantly white boys less than 6 years old. The greatest proportion of children were hospitalized in urban non‐teaching settings, and also those children living in the southern regions of the United States.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| N = 148,702 | N = 157,847 | N = 157,743 | N = 156,810 | |
| ||||
| Race | ||||
| White | 56,348 (38) | 68,643 (44) | 54,903 (35) | 56,108 (36) |
| Black | 22,864 (15) | 22,580 (14) | 17,960 (11) | 18,800 (12) |
| Other | 22,203 (15) | 38,448 (24) | 39,138 (25) | 40,803 (26) |
| Missing | 47,287 (32) | 28,175 (18) | 45,588 (29) | 41,099 (26) |
| Age category | ||||
| <1 year | 43,851 (29) | 44,470 (28) | 37,798 (24) | 37,705 (24) |
| 1 through 5 years | 75,033 (50) | 76,385 (48) | 77,530 (49) | 79,519 (51) |
| 6 through 11 years | 19,372 (13) | 21,403 (14) | 23,126 (15) | 23,494 (15) |
| >12 years | 10,446 (7) | 15,589 (9) | 19,289 (12) | 16,092 (10) |
| Hospital type | ||||
| Urban non‐teaching | 52,756 (35) | 50,718 (32) | 52,552 (34) | 50,718 (32) |
| Rural | 47,910 (32) | 41,715 (27) | 39,605 (26) | 31,947 (21) |
| Urban teaching non‐children's | 20,378 (14) | 30,981 (20) | 28,432 (18) | 30,194 (20) |
| Urban teaching children's | 27,658 (19) | 34,021 (22) | 34,454 (22) | 41,035 (27) |
| Male sex | 83,291 (56) | 8,783 (56) | 86,034 (55) | 85,508 (55) |
| Region* | ||||
| Northeast | 19,750 (13) | 26,092 (17) | 23,867 (15) | 23,832 (15) |
| Midwest | 33,053 (22) | 30,706 (19) | 35,714 (23) | 35,900 (23) |
| South | 68,958 (46) | 68,663 (44) | 65,994 (42) | 65,460 (42) |
| West | 26,741 (18) | 32,385 (21) | 32,169 (20) | 31,618 (20) |
| Asthma | 26,971 (24) | 31,746 (28) | 27,729 (24) | 26,822 (23) |
| Pneumonia‐associated complication | 8,831 (6) | 11,084 (7) | 12,005 (8) | 11,724 (7) |
| Died | 334 (0.002) | 394 (0.002) | 270 (0.002) | 193 (0.001) |
| Insurance | ||||
| Private | 65,428 (44) | 73,528 (47) | 68,720 (44) | 63,997 (41) |
| Public | 68,024 (46) | 71,698 (45) | 76,779 (49) | 80,226 (51) |
| Uninsured | 9,922 (7) | 8,336 (5) | 6,381 (4) | 6,912 (4) |
| Other | 4,964 (3) | 4,285 (3) | 5,391 (3) | 5,283 (3) |
There was little variation in the insurance status of children hospitalized with CAP between 1997 and 2006. In each of the sampled years, at least 40% of sampled children were privately insured, at least 40% were publicly insured, and approximately 5% were uninsured (Table 1). In all years, there were significant racial/ethnic disparities in insurance coverage such that whites were 4 to 6 times more likely to have private insurance than blacks, however, the large amount of missing race/ethnicity data warrant caution in interpreting this finding (Table 2; also see Supporting Information Appendix B in the online version of this article). We also found that children less than 1 year old were the most likely to be publicly insured in all years (see Supporting Appendix C in the online version of this article). There were also regional differences related to insurance coverage such that a greater proportion of children hospitalized in facilities located in the southern part of the United States were publicly insured. Notably, there were no significant differences in CAP‐associated mortality or asthma related to insurance coverage (Table 2). In 2006, CAP‐associated complications occurred in 8.5% of children with private insurance, 6.5% of children with public insurance, and 7.7% of uninsured children; the relative distribution of complications by insurance type were similar in previous years of the KID survey.
| Private | Public | Uninsured | Other Insurance | P | |
|---|---|---|---|---|---|
| |||||
| No. of children (%) | 63,997 (41) | 80,226 (51) | 6,912 (4) | 5,283 (3) | |
| Male sex | 34,639 (41) | 44,140 (52) | 3,727 (4) | 2,808 (3) | 0.092 |
| Race | |||||
| White | 30,707 (55) | 21,282 (38) | 2,241 (4) | 1,774 (3) | <0.001 |
| Black* | 5,112 (27) | 12,239 (65) | 988 (5) | 426 (3) | |
| Other | 11,033 (27) | 26,489 (65) | 2,112 (5) | 1,076 (3) | |
| Missing | 17,145 (42) | 20,216 (49) | 1,572 (4) | 2,007 (4) | |
| Age category | |||||
| <1 year | 10,788 (29) | 24,762 (65) | 1,164 (3) | 880 (3) | <0.001 |
| 1 through 5 years | 33,664 (42) | 39,531 (50) | 3,442 (4) | 2,673 (3) | |
| 6 through 11 years | 11,660 (50) | 9,684 (41) | 1,085 (5) | 1,015 (4) | |
| >12 years | 7,885 (49) | 6,249 (39) | 1,221 (8) | 714 (4) | |
| Hospital type | |||||
| Urban non‐teaching | 22,429 (44) | 24,241 (49) | 2,440 (5) | 1,555 (2) | <0.001 |
| Rural | 10,880 (34) | 18,396 (58) | 1,290 (4) | 1,109 (3) | |
| Urban teaching non‐children's | 13,130 (44) | 14,542 (48) | 1,721 (6) | 750 (2) | |
| Urban teaching children's | 16,591 (40) | 21,544 (53) | 1,417 (3) | 1,465 (4) | |
| Region | |||||
| Northeast | 12,364 (52) | 9,620 (40) | 1,466 (6) | 377 (2) | <0.001 |
| Midwest∥ | 17,891 (50) | 15,573 (43) | 1,160 (3) | 1,215 (3) | |
| South∥ | 21,479 (33) | 38,112 (58) | 3,108 (5) | 2,495 (4) | |
| West∥ | 12,263 (39) | 16,921 (44) | 1,178 (5) | 1,195 (5) | |
| Asthma | 10,829 (41) | 13,923 (52) | 1,119 (4) | 866 (3) | 0.193 |
| Pneumonia‐associated complication | 5,416 (46) | 5,206 (45) | 532 (4) | 556 (5) | <0.001 |
| Died | 66 (34) | 115 (60) | 3 (1) | 8 (5) | 0.131 |
After examining the general and demographic characteristics, we then examined mean LOS for all children with CAP in each sample year (Table 3). The mean LOS for children with CAP was 3.44 days in 1997, with marginal decreases in subsequent years to a mean LOS of 3.18 days in 2006. The distribution of LOS for children with CAP revealed that nearly 70% of children were hospitalized for fewer than 3 days, another 22% to 28% were hospitalized for less than 1 week, and only 3% were hospitalized for more than 1 week. This distribution did not change substantially between 1997 and 2006. Next, we compared mean LOS by insurance type and race/ethnicity in unadjusted analyses. In each sample year, publicly insured children hospitalized with CAP had significantly longer LOS than privately insured children (P < 0.001). Similarly, in all years excepting 1997, uninsured children hospitalized with CAP had significantly shorter LOS than privately insured children. There were also significant racial differences in LOS for children with CAP, such that black children had longer LOS than white children with CAP. However, the large amount of missing data for race/ethnicity limited the robustness of this finding, and subsequent sensitivity analyses demonstrated that there were no consistent racial/ethnic disparities in LOS (see Supporting Appendix B in the online version of this article). These sensitivity analyses for missing race data did not alter our primary finding of shorter LOS for uninsured versus publicly or privately insured children.
| 1997 | P | 2000 | P | 2003 | P | 2006 | P | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Overall | 3.44 (0.04) | 3.35 (0.05) | 3.27 (0.05) | 3.18 (0.04) | ||||
| Insurance type | ||||||||
| Private | 3.21 (0.04) | 3.19 (0.04) | 3.09 (0.04) | 3.00 (0.03) | ||||
| Public | 3.71 (0.06) | <0.001 | 3.57 (0.06) | <0.001 | 3.44 (0.06) | <0.001 | 3.34 (0.05) | <0.001 |
| Uninsured | 3.18 (0.14) | 0.792 | 2.92 (0.07) | <0.001 | 2.80 (0.05) | <0.001 | 2.82 (0.05) | <0.001 |
| Other | 3.32 (0.11) | 0.319 | 3.55 (0.14) | 0.0134 | 3.54 (0.21) | 0.037 | 3.42 (0.13) | 0.001 |
| Race | ||||||||
| White | 3.31 (0.05) | 3.18 (0.04) | 3.19 (0.05) | 3.10 (0.04) | ||||
| Black | 3.61 (0.08) | <0.001 | 3.32 (0.07) | <0.001 | 3.36 (0.08) | <0.001 | 3.31 (0.07) | <0.001 |
| Other | 3.96 (0.11) | <0.001 | 3.81 (0.09) | <0.001 | 3.67 (0.10) | <0.001 | 3.56 (0.08) | <0.001 |
| Missing | 3.27 (0.08) | 0.645 | 3.18 (0.08) | 0.926 | 2.99 (0.06) | 0.0134 | 2.86 (0.04) | <0.001 |
After controlling for child age, race/ethnicity, gender, hospital type, transfer status, and presence of asthma or pneumonia‐associated complications, our multivariable analyses examining the relationship between insurance coverage and hospital LOS yielded the following results (Table 4). First, publicly insured children had significantly longer hospital stays than privately insured children, and uninsured children had significantly shorter hospital stays than privately insured children in all years except 1997. Second, children admitted with CAP at urban teaching children's hospitals had significantly longer LOS than those admitted to urban non‐teaching hospitals, and, in 2003, children admitted with CAP to rural hospitals had significantly shorter LOS than those admitted to urban non‐teaching hospitals. Third, children older than 1 year consistently had shorter hospital stays than infants less than 1 year old. Finally, though concomitant diagnosis of asthma did not consistently influence LOS, children who developed any complications had significantly longer LOS than those who did not. The cumulative impact of seemingly small differences in LOS is great. For example, in 2006, our model suggests that, for every 1000 children hospitalized with CAP in a given year, after adjusting for differences in sex, age, race, hospital‐type, region, transfer status, and diagnosis of asthma or complications, publicly insured children spend 90 to 130 more days in the hospital than privately insured children, whereas uninsured children spend between 40 to 90 fewer days in the hospital than privately insured children.
| 1997 | 2000 | 2003 | 2006 | |
|---|---|---|---|---|
| Variable | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| ||||
| Age category | ||||
| <1 year | ||||
| 15 years | 0.82 (0.81, 0.84) | 0.83 (0.88, 0.95) | 0.86 (0.85, 0.88) | 0.87 (0.86, 0.89) |
| 611 years | 0.91 (0.87, 0.95) | 0.91 (0.88, 0.94) | 0.93 (0.91, 0.95) | 0.93 (0.90, 0.95) |
| >12 years | 1.03 (0.99, 1.07) | 1.17 (1.11, 1.22) | 1.09 (1.06, 1.13) | 1.13 (1.09, 1.16) |
| Race | ||||
| White | ||||
| Black | 1.04 (0.99, 1.08) | 1.00 (0.95, 1.03) | 1.00 (0.98, 1.03) | 1.02 (0.98, 1.06) |
| Other | 1.09 (1.05, 1.13) | 1.11 (1.08, 1.15) | 1.09 (1.06, 1.12) | 1.08 (1.05, 1.11) |
| Missing | 1.00 (0.94, 1.06) | 1.01 (0.96, 1.06) | 0.95 (0.92, 0.99)* | 0.96 (0.93, 0.99) |
| Sex | ||||
| Female | 1.02 (0.94, 1.06) | 1.01 (0.99, 1.02) | 1.01(0.93, 100) | 1.01 (1.00, 1.02) |
| Insurance type | ||||
| Private | ||||
| Public | 1.13 (1.11, 1.16) | 1.11 (1.09, 1.14) | 1.11 (1.09, 1.13) | 1.11 (1.09, 1.13) |
| Uninsured | 1.01 (0.91, 1.11) | 0.93 (0.89, 0.96) | 0.92 (0.90, 0.96) | 0.94 (0.91, 0.96) |
| Other | 1.01 (0.96, 1.06) | 1.10 (1.03, 1.18) | 1.10 (1.02, 1.19)* | 1.07 (1.02, 1.13) |
| Hospital type | ||||
| Urban non‐teaching | ||||
| Rural | 0.98 (0.92, 1.04) | 0.96 (0.92, 1.00) | 0.97 (0.94, 1.00) | 0.97 (0.93, 1.00) |
| Urban teaching (non‐children's) | 0.99 (0.95, 1.04) | 1.06 (1.02, 1.10) | 1.06 (1.02, 1.10) | 1.03 (0.99, 1.07) |
| Urban teaching children's | 1.2 (1.14, 1.26) | 1.23 (1.16, 1.30) | 1.28 (1.21, 1.37) | 1.25 (1.19, 1.31) |
| Region | ||||
| Northeast | ||||
| Midwest | 0.93 (0.88, 0.98)* | 0.96 (0.92, 1.00) | 0.95 (0.91, 0.99)* | 0.95 (0.91, 0.99)* |
| South | 0.98 (0.94, 1.02) | 1.06 (1.02, 1.10)* | 1.04 (1.00, 1.09) | 1.03 (0.98, 1.08) |
| West | 0.97 (0.92, 1.01) | 1.22 (1.16, 1.30)* | 1.02 (0.97, 1.08) | 1.06 (1.00, 1.12)* |
| Transfer status | ||||
| Transfer | 1.35 (1.25, 1.46) | 1.39 (1.27, 1.52) | 1.31 (1.23, 1.37 ) | 1.16 (1.10, 1.23) |
| Asthma | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99) | 0.98 (0.96, 1.00) | 0.98 (0.97, 1.00)* |
| Pneumonia Complications | 0.99 (0.96, 1.03) | 0.97 (0.95, 0.99)* | 0.98 (0.96, 1.0) | 0.98 (0.97, 1.00)* |
| Any complication | 2.20 (2.07, 2.34) | 2.23 (2.07, 2.40) | 2.22 (2.22, 2.44) | 2.37 (2.27, 2.47) |
DISCUSSION
In this nationally representative sample selected over the past 10 years, we found that publicly insured children hospitalized with CAP have significantly longer LOS than those who are privately insured, and that, since 2000, uninsured children hospitalized with CAP have significantly shorter LOS than those who are privately insured. Though these observed differences are small, they are consistent across all 4 sampled years and, because CAP is one of the most common pediatric inpatient diagnoses, the cumulative impact of the observed differences on hospital LOS is great. Insurance status is often considered a proxy for access to preventive and ambulatory healthcare services or socioeconomic status. However, the underlying mechanisms relating insurance status to healthcare access, utilization, and ultimately, health outcomes are highly complex and difficult to elucidate.17 The observed variation in this study raises questions about the potential influence of insurance status on hospital discharge practices. Additional research is necessary to understand whether there are differences in processes of care (eg, performance of blood cultures or chest radiographs), quality of care, or other outcomes, such as readmissions, related to CAP inpatient management for children with different insurance coverage.
Apart from differences in hospital discharge practices, another possible explanation for uninsured children with CAP having shorter LOS is that these children have less severe disease than privately insured. This may occur if uninsured children with CAP are evaluated in the emergency department rather than the office setting, because emergency department providers may be more likely to admit children with CAP who lack a consistent access to ambulatory primary care services. Countering this alternative, prior studies have shown that uninsured groups are more likely to have greater disease severity than privately insured groups at the time of hospital admission.18, 19 In this study, we attempted to identify children with greater severity of disease using ICD‐9 codes for CAP‐associated complications. Though this is a relatively crude method that might lead to an underestimate of the total number of children with complications, we found that there were no significant differences in the prevalence of CAP‐associated complications between uninsured and insured groups in all sampled years.
On the other hand, uninsured patients may be released earlier by providers in order to reduce the amount of uncompensated care provided, or possibly because parents may urge providers to discharge their children, given their inability to pay forthcoming hospital bills and/or avoid further lost wages due to work absence.20, 21 In California, Bindman et al. demonstrated that decreasing the frequency of Medicaid recertification, and consequently increasing the likelihood of continuous insurance coverage, was associated with a decreased risk of hospitalization for ambulatory‐care sensitive conditions.5
We also found that children admitted to urban teaching children's hospitals with CAP had significantly longer LOS than those admitted to urban non‐teaching hospitals, whereas children in rural hospitals had significantly shorter LOS than those in urban non‐teaching hospitals in 2003. These findings are consistent with prior data from 1996 to1998 demonstrating that children admitted to rural hospitals in New York and Pennsylvania had significantly shorter LOS than large urban hospitals for 19 medical and 9 surgical conditions, including pneumonia.12 These findings may reflect underlying differences in between rural and urban hospital transfer practices, whereby rural hospitals may be more likely than urban hospitals to transfer children with relatively more severe illness to urban referral centers and retain children with less severe illness, leading to shorter LOS.12 Though our empiric understanding of differences in LOS between teaching and non‐teaching hospitals is currently limited, clinical experience supports the notion that there may be decreases in efficiency that occur in teaching hospitals, and are a result of the supervision required for care provided by trainees. It is also possible that, despite our exclusion of comorbid conditions, some children with complex or chronic medical conditions were included in this study. These children are often cared for at teaching hospitals, regardless of the primary cause for admission, and are more likely to have public insurance than other children, thus confounding the relationship between hospital type, insurance type and status, and LOS for children with CAP. The limitations of this dataset preclude further examination of this issue.
There are some limitations to this study. First, the KID data are cross‐sectional and causal inferences are limited. However, our results demonstrating that uninsured children hospitalized with CAP had shorter LOS than privately insured children were quite consistent in each sample year, suggesting that our results are a true association. Additionally, insurance status in KID is typically collected at admission, however, it is not possible to determine whether specific changes to insurance status that occurred during the hospitalization were applied to the data. The impact of this limitation would depend on the type of insurance obtained by the patient. If uninsured patients obtained public insurance, our study would underestimate the increased LOS for publicly insured patients, compared with privately insured patients, but have no effect on the difference in LOS between uninsured and privately insured patients. In the unlikely event that uninsured patients obtained private insurance, then our study would underestimate the difference for uninsured patients, compared with privately insured patients, biasing our current study results towards the null. Second, a substantial proportion of sampled children had missing data for race/ethnicity. To assess the impact of the missing race/ethnicity data on our results, we conducted sensitivity analyses and found that, though difficult to make any definitive conclusions about the relationship between race/ethnicity and LOS for children with CAP, there were no changes to our primary findings regarding differences in LOS between children with different insurance status and type. Third, KID does not include data about other unmeasured confounders (eg, parent income, parent education, regular source of care) that might be related to LOS, as well as a broad spectrum of pediatric outcomes. Serious consideration of expanding KID to include these variables is warranted. Fourth, the other category of insurance is not uniformly coded across states in the KID database. While some states use this category to classify public insurance options other than Medicare and Medicaid, other states include private insurance options in this group. Thus, it is possible that some patients with public insurance are misclassified as having other insurance. We would expect such misclassification to bias our findings towards the null hypothesis. Finally, we focused on the relationship between child health insurance status and CAP, only 1 ambulatory care‐sensitive condition. Additional research examining the relationship between insurance type and other ambulatory care‐sensitive conditions is warranted.
In summary, we found that, after multivariable adjustment, uninsured children hospitalized with community‐acquired pneumonia had significantly shorter LOS than privately insured children, and publicly insured children had a significantly longer hospital stay than privately insured children in these 4 nationally representative samples from 1997 to 2006. Current federal and state efforts to increase enrollment of children into insurance programs are a first step in reducing healthcare disparities. However, insurance coverage alone does not guarantee access to healthcare, thus, these efforts in isolation will likely be insufficient to achieve optimal health for the children of our country. As healthcare reform legislation is implemented, these findings provide hospitals and policy makers additional impetus to develop ways to achieve the ideal length of stay for every child; this ideal state will be achieved when clinical status and course, rather than nonclinical factors such as insurance type or provider's unease with ambulatory follow‐up, determine the duration of hospitalization for every child.
- ,,,,,.Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials.JAMA.2007;298:179–186.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154:789–796.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124:e1081–e1087.
- ,,.Medicaid re‐enrollment policies and children's risk of hospitalizations for ambulatory care sensitive conditions.Med Care.2008;46:1049–1054.
- ,.Differences associated with age, transfer status, and insurance coverage in end‐of‐life hospital care for children.J Hosp Med.2008;3:376–383.
- ,,,,,.Health care for children and youth in the United States: annual report on patterns of coverage, utilization, quality, and expenditures by a county level of urban influence.Ambul Pediatr.2006;6:241–264.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Variation in hospital discharges for ambulatory care‐sensitive conditions among children.Pediatrics.2000;106:942–948.
- ,,,,,.Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007.Pediatrics.2011;127:411–418.
- ,,,,.Patterns of hospital‐based pediatric care across diverse ethnicities: the case of pneumonia.J Health Care Poor Underserved.2004;15:462–473.
- ,,,,.Equivalent lengths of stay of pediatric patients hospitalized in rural and nonrural hospitals.Pediatrics.2004;114:e400–e408.
- ,.Effect of Child Health Insurance Plan enrollment on the utilization of health care services by children using a public safety net system.Pediatrics.2002;110:940–945.
- ,,,,,.Relationships between welfare status, health insurance status, and health and medical care among children with asthma.Am J Public Health.2002;92:1446–1452.
- HCUP Kids' Inpatient Database (KID). Healthcare Cost and Utilization Project (HCUP), 1997, 2000, 2003, 2006. Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed May 17,2010.
- ,,, et al.Community‐acquired pneumonia: can it be defined with claims data?Am J Med Qual.1997;12:187–193.
- .Sicker and poorer—the consequences of being uninsured: a review of the research on the relationship between health insurance, medical care use, health, work, and income.Med Care Res Rev.2003;60:3S–75S; discussion76S–112S.
- ,,,,,.Socioeconomic variation in asthma hospitalization: excess utilization or greater need?Pediatrics.1999;103:e75.
- ,,, et al.Analysis of 23 million US hospitalizations: uninsured children have higher all‐cause in‐hospital mortality.J Public Health (Oxf).2010;32(2)236–244.
- ,.The impact of welfare reform on parents' ability to care for their children's health.Am J Public Health.1999;89:502–505.
- ,,.Knowledge of welfare reform program provisions among families of children with chronic conditions.Am J Public Health.2002;92:228–230.
- ,,,,,.Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials.JAMA.2007;298:179–186.
- ,.Factors associated with variability in outcomes for children hospitalized with urinary tract infection.J Pediatr.2009;154:789–796.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124:e1081–e1087.
- ,,.Medicaid re‐enrollment policies and children's risk of hospitalizations for ambulatory care sensitive conditions.Med Care.2008;46:1049–1054.
- ,.Differences associated with age, transfer status, and insurance coverage in end‐of‐life hospital care for children.J Hosp Med.2008;3:376–383.
- ,,,,,.Health care for children and youth in the United States: annual report on patterns of coverage, utilization, quality, and expenditures by a county level of urban influence.Ambul Pediatr.2006;6:241–264.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Variation in hospital discharges for ambulatory care‐sensitive conditions among children.Pediatrics.2000;106:942–948.
- ,,,,,.Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007.Pediatrics.2011;127:411–418.
- ,,,,.Patterns of hospital‐based pediatric care across diverse ethnicities: the case of pneumonia.J Health Care Poor Underserved.2004;15:462–473.
- ,,,,.Equivalent lengths of stay of pediatric patients hospitalized in rural and nonrural hospitals.Pediatrics.2004;114:e400–e408.
- ,.Effect of Child Health Insurance Plan enrollment on the utilization of health care services by children using a public safety net system.Pediatrics.2002;110:940–945.
- ,,,,,.Relationships between welfare status, health insurance status, and health and medical care among children with asthma.Am J Public Health.2002;92:1446–1452.
- HCUP Kids' Inpatient Database (KID). Healthcare Cost and Utilization Project (HCUP), 1997, 2000, 2003, 2006. Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed May 17,2010.
- ,,, et al.Community‐acquired pneumonia: can it be defined with claims data?Am J Med Qual.1997;12:187–193.
- .Sicker and poorer—the consequences of being uninsured: a review of the research on the relationship between health insurance, medical care use, health, work, and income.Med Care Res Rev.2003;60:3S–75S; discussion76S–112S.
- ,,,,,.Socioeconomic variation in asthma hospitalization: excess utilization or greater need?Pediatrics.1999;103:e75.
- ,,, et al.Analysis of 23 million US hospitalizations: uninsured children have higher all‐cause in‐hospital mortality.J Public Health (Oxf).2010;32(2)236–244.
- ,.The impact of welfare reform on parents' ability to care for their children's health.Am J Public Health.1999;89:502–505.
- ,,.Knowledge of welfare reform program provisions among families of children with chronic conditions.Am J Public Health.2002;92:228–230.
Copyright © 2011 Society of Hospital Medicine
Student Care Transitions Curriculum
There is increasing evidence that the transfer of medically complex patients across different settings can be associated with poor communication and patient dissatisfaction with the care received, potentially leading to negative clinical outcomes. While medical schools are beginning to introduce curricula on these transitions of care, few have been evaluated and subjected to peer review with the purpose of finding the most effective teaching and training methods.
Older adults and those with multiple chronic diseases frequently require medical care that spans multiple locations, and thus are most at risk for poor clinical outcomes during care transitions.1, 2 Medication errors and adverse drug reactions after hospital discharge are common.3, 4 Unsuccessful care transitions may also result in nonelective readmission after discharge, and there is evidence that readmissions may be a quality indicator for hospital care.57 Poor communication between patients and their healthcare providers is another element of poorly executed care transitions. Qualitative studies show that patients are frequently dissatisfied with the discharge process and are often unprepared to assume responsibility for their own care when they leave the hospital.8 Communication among providers can also be suboptimal. One meta‐analysis found that hospital physicians and primary care providers communicated infrequently and the availability of discharge summaries at the postdischarge visit was low, which may have affected the quality of care.9
Knowing that these gaps are common, there have been signs of increased emphasis on improving communication and working in teams as part of health professions training. The Institute of Medicine, in its 2003 report Health Professions Education: A Bridge to Quality,10 stressed education on management of chronic diseases, working in interdisciplinary teams, as well as a focus on quality improvement. In addition, the American Association of Medical Colleges (AAMC) encouraged training medical students on preparing safe discharge plans in its 2007 geriatrics competencies.11
Some medical schools have introduced care transitions curricula, though few have published data on their effectiveness. A search for teaching products using care transitions or transitional care on the online educational portals POGOe (Portal of Geriatric Online Education) and MedEdPortal yielded a total of 7 unique sets of teaching materials on care transitions for medical students.1218 However, a search on PubMed in July 2010 for peer‐reviewed articles on care transitions curricula developed for medical students which contained evaluation data only yielded 3 articles.1921 These 3 curricula, written by Bray‐Hall et al., Lai et al., and Ouchida et al., respectively, all trained third‐year medical students on diverse aspects of the discharge process using methods such as lectures, workshops, and patient visits, and showed favorable skill and knowledge outcomes.
Recognizing the importance of care transitions in medical education, a new curriculum addressing this topic was developed and introduced for fourth‐year medical students at the Emory University School of Medicine in 2009. The broad goal for this module was to develop a course concentrating on concrete skills that would train students to perform better care transitions while minimizing the time they had to spend away from a busy Internal Medicine sub‐internship. This curriculum used a mixed approach that included face‐to‐face teaching with faculty, online didactic instruction and interaction, and direct patient care. The course objectives were for students to develop a working fund of knowledge on care transitions, to learn to write a complete discharge summary, and to communicate the elements of a safe discharge plan. This article will describe the implementation of this curriculum and its evaluation.
METHODS
The Emory Care Transitions Curriculum started in August 2009 with fourth‐year medical students at the Emory University School of Medicine. This section will describe the details of the implementation of this curriculum, as well as the evaluation methodology and results.
Overview
This module was offered to Emory medical students participating in a required Senior Medicine rotation during their fourth year. The study population consisted of the 121 fourth‐year Emory medical students who participated in this rotation during the academic year that started in August 2009 and ended in April 2010. Students participated in the rotation at 1 of 3 teaching sites: Grady Memorial Hospital (GMH), Emory University Hospital (EUH), and the Atlanta VA Medical Center (AVAMC); 98 students completed their rotation at GMH, 12 at EUH, and 11 at AVAMC. For all online activities, students used the Blackboard platform software, available to them at
Course Description
The course consisted of 3 components, each associated with specific student assignments: a slide presentation on care transitions with an associated case discussion, training on discharge summaries, and the execution of a postdischarge phone call. Figure 1 describes the course delivery schedule.
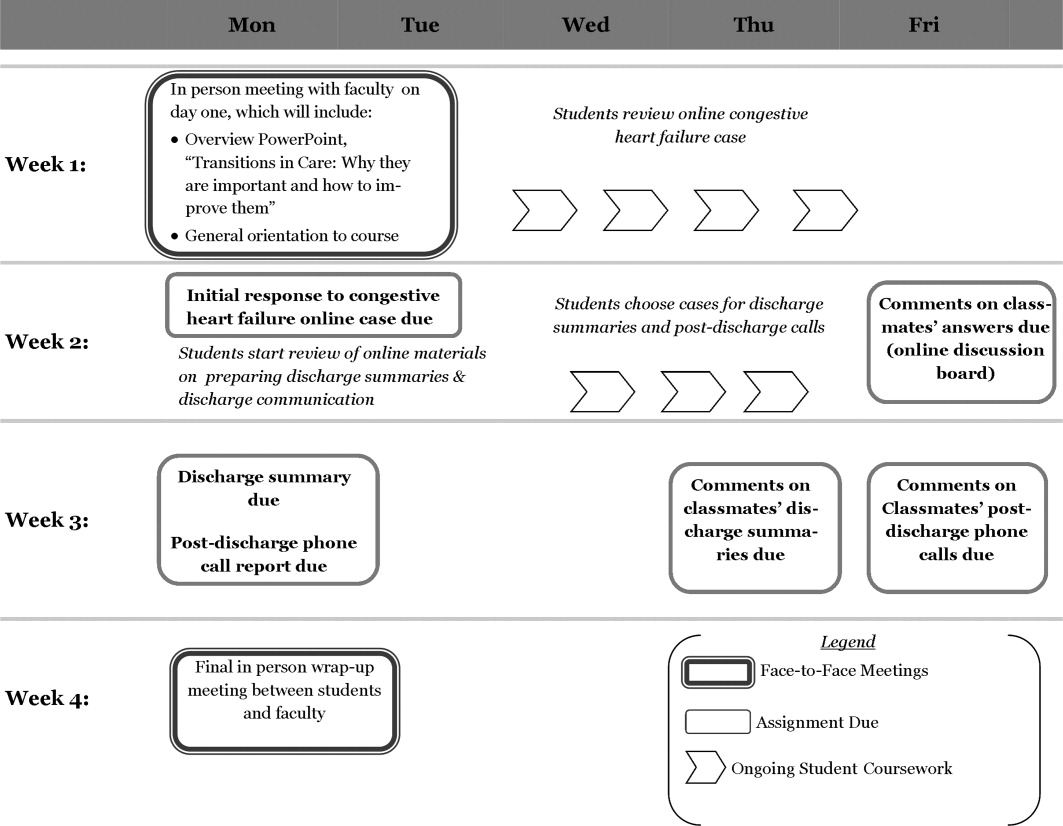
Slide Presentation and Case Discussion
This section started on day 2 of the clerkship, with a face‐to‐face lecture titled Transitions of Care: Why They Are Important, and How to Improve Them. It included the following components: definition of the different posthospital discharge options, explanation of the reasons for the complexity of care transitions in high‐risk patient populations, and an enumeration of methods to improve the safety of care transitions. Students also read a review article on the topic to further add to their fund of knowledge.23
The second part of the section involved discussion of a case posted on Blackboard (a discussion board) designed to highlight some of the challenges associated with care transitions. The case included 2 successive discharge summaries for an elderly patient with congestive heart failure: 1 for the initial exacerbation, and the other for a readmission. Using an online discussion board, students were asked to report the strong points and shortcomings of the patient's management, as well as those of the discharge summaries. Then the students were asked to post responses to at least 2 of their classmates' reports on the discussion board.
Training on Discharge Summaries
During the module, students received training on how to prepare a complete and informative discharge summary. This online training consisted of a lecture prepared by a faculty member (M.A.E.) and the use of a discharge summary template based on a guide prepared by the Boston Association of Academic Hospitalists (BAAHM), which is part of a toolkit available from the Society of Hospital Medicine.24 After reading the lecture, each student selected 1 of the patients they cared for during their rotation, and wrote a discharge summary. They posted it to a Blackboard discussion board, and were then asked to comment on one of their classmates' reports on the same forum. Faculty (M.A.E. and R.C.) also gave online feedback to each student about their discharge summary.
Postdischarge Phone Call
Students were also assigned to communicate with the patient for whom they prepared a discharge summary by performing a postdischarge phone call within a week of the patient's departure from the hospital. They reviewed a discharge checklist adapted from Ideal Discharge for an Elderly Patient: A Hospitalist Checklist, issued by the Society of Hospital Medicine.25 This document contains the necessary elements of a safe discharge plan, and used these points as the basis of the patient phone interview. The goal of the call and the use of the checklist was to reinforce the main elements of communication with patients that need to occur before they leave the hospital.
Students then used the checklist as the basis for a short (<400 words) report discussing the strong points and shortcomings of their patient's discharge, and posted it on a Blackboard discussion board. They were also asked to comment on at least one of their classmates' reports on the board. Faculty (M.A.E. and R.C.) also participated in the discussion board, commenting at least once on all students' reports.
Evaluation
The course was evaluated in order to assess changes in skills, knowledge and attitudes, as well as satisfaction with the course.
Evaluation Components
In order to assess the outcomes described above, questionnaires were utilized, and objective criteria were used to evaluate students' work. Students completed a pretest before the first face‐to‐face session, and a posttest after the second in‐person discussion. Pretest items were identical to those in the posttest, except that the posttest also contained 6 satisfaction questions. The components that were included in both pre‐ and posttests were:
Five multiple choice questions measuring students' confidence in their own skills regarding discharge summaries and transitional care (pre‐ and postsurvey). These 5 questions were adapted from the questionnaire developed by Lai et al.20 Confidence questionnaire items are detailed in Table 1.
Five multiple choice questions assessing students' attitudes regarding the importance of different components of the care transitions process (pre‐ and postsurvey). Attitude questionnaire items are detailed in Table 1.
Ten multiple choice questions in which each had one right answer, assessing students' knowledge base on transitional care issues (pre‐and postsurvey). Knowledge questions and their correct answers are detailed in Table 2.
| Mean Likert Scores* | P Value | ||
|---|---|---|---|
| Pre‐Course | Post‐Course | ||
| |||
| Confidence items | |||
| 1. I am confident in my ability to involve patients in making a plan for their care. | 3.8 | 4.2 | <0.001 |
| 2. I am confident in my ability to review patients' medications and side effects. | 3.4 | 4.1 | <0.001 |
| 3. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.4 | 4.3 | <0.001 |
| 4. I am confident in my ability to prepare a complete discharge summary. | 3.0 | 4.2 | <0.001 |
| 5. I can identify the different types of places that may serve as a setting for discharge from the inpatient setting. | 3.1 | 3.9 | <0.001 |
| Total confidence score (out of 25) | 16.7 | 20.7 | <0.001 |
| Attitude items | |||
| 1. A hospital physician should always communicate with a patient's primary care physician before that patient is discharged from the hospital, in order to ensure a smooth transition of care. | 4.0 | 4.1 | 0.78 |
| 2. Before a patient is discharged from the hospital, a physician (not just the nurse or case manager) should always meet with the patient to discuss his medications, and goals of care. | 4.4 | 4.4 | 0.50 |
| 3. It is critical for a primary care physician to have access to a discharge summary when seeing a patient for the first time after leaving the hospital. | 4.6 | 4.7 | 0.25 |
| 4. The main reason patients often don't take their medications properly after discharge is that they are confused by the instructions given to them at the hospital. | 3.7 | 3.8 | 0.65 |
| 5. Avoiding rehospitalization should be a top priority for physicians in the process of discharge from the hospital. | 4.1 | 4.3 | 0.95 |
| Total attitude score (out of 25) | 20.8 | 21.3 | 0.07 |
| Percent Correct | |||
|---|---|---|---|
| Question (Correct Answer in Parenthesis) | Pre‐Course | Post‐Course | P Value |
| |||
| 1. When a patient is discharged with home health care, which of the following services is usually not part of the package? (A caregiver to sit with the patient and supervise them most of the day.) | 70 | 83 | 0.020* |
| 2. When a patient is discharged from the hospital to a skilled nursing facility (SNF) for further care, which of these is a service that is typically provided? (Physical therapy.) | 78 | 75 | 0.649 |
| 3. Which of the following rehabilitation activities is more likely to be in the job description of an occupational therapist? (Training of strength in upper extremities.) | 24 | 51 | <0.001* |
| 4. Which of these is least likely to be a cause of poor patient outcomes after hospital discharge? (The discharging of patients to skilled nursing facilities.) | 93 | 97 | 0.166 |
| 5. Which of these is more likely to be an indicator of poor outcomes after hospital discharge? (Having had 3 hospitalizations in the last 6 months.) | 93 | 96 | 0.287 |
| 6. Which of these data is the least likely to be an indicator that the patient is too sick to be discharged from the hospital? (Hemoglobin concentration of 9.5 g/dl.) | 45 | 74 | <0.001* |
| 7. Which of the following medications would merit the most time spent on communication with patients, family members, and receiving physicians? (Furosemide.) | 58 | 96 | <0.001* |
| 8. You are caring for an 89‐year‐old man who is being treated in the hospital for an exacerbation of his congestive heart failure (CHF). He is doing well, ambulating 100 feet without shortness of breath, and is showing understanding of the need for all his different medications. However, he is not yet back to his functional baseline. Which of the following is the LEAST appropriate setting for discharge? (Hospice care.) | 74 | 70 | 0.458 |
| 9. Which of the following is true about skilled nursing facility (SNF) care? (Patients can be admitted for treatment with IV antibiotics for several weeks.) | 52 | 79 | <0.001* |
| 10. Educating patients at discharge about their illness and medication has been found to help decrease readmission rates. (True.) | 97 | 100 | 0.045 |
| Percentage of total questions correct | 68 | 82 | <0.001* |
The questionnaire items were developed by study faculty (M.A.E. and J.M.F.) and were edited in consultation with clinical faculty members from outside Emory with experience developing care transitions curricula: Dr. Karin Ouchida of Montefiore Medical Center in New York City, and Dr. William Lyons of the University of Nebraska Medical Center.
The 6 posttest items addressed student satisfaction with individual course components, which were: the heart failure online case, training on preparing discharge summaries, initial in‐person slide presentation, postdischarge phone call, overall online discussion across all items, and finally, satisfaction with the overall course. Questionnaire items on comfort, attitudes, and satisfaction all used a five‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The 10 multiple‐choice questions on knowledge each had one right answer.
Each student completed one discharge summary during the course. For it to be deemed satisfactory, it had to have the 5 following components, all present in the BAAHM template24:
A documented discharge medication list with specific dosing schedules.
Lists of admission medications and/or a list of medication changes during hospitalization.
A discharge plan that specifies the next setting of care, as well as the planned follow‐up.
A hospital course organized by system and/or specific chronology.
A physical exam, laboratory tests, and diagnostic studies performed on admission.
Student reports of their postdischarge phone call were also evaluated by study faculty (M.A.E. and R.C.). For the report of the interview to be considered satisfactory, it had to contain at least the following 2 elements:
A discussion of the patient's medication list, including documentation of a discussion of hazardous medications (e.g., furosemide, warfarin, digoxin, insulin) if applicable.
Documentation of a discussion on follow‐up plans with a primary physician or specialist.
Data Analysis
Outcomes were evaluated based on the results of pre‐ and posttest questionnaires, in addition to the satisfactoriness of discharge summaries and postdischarge phone call reports. As for the questionnaires, 4 types of scores were analyzed based on students' questionnaire responses:
Skills Confidence Score: The sum of Likert scores for confidence items on the pre‐ and posttest was the confidence score, with a highest possible score of 25, in which the highest scores were associated with the most confidence in executing the discharge process.
Attitude Score: The sum of Likert scores for attitude items on the pre‐ and posttest was the attitude score, with a highest possible score of 25, in which the highest scores were associated with student attitudes ascribing the most importance to a safe discharge process.
Knowledge Score: The percentage of total correct answers on knowledge questions on the pre‐ and posttest were used to obtain the knowledge score, in which a score of 100 was highest.
Satisfaction Score: Satisfaction questions on the posttest questionnaire were analyzed separately to assess satisfaction with each component of the curriculum, ranging from poor (score = 1) to excellent (score = 5). We also determined the percentage of students who rated each portion of the course good or better.
In addition to questionnaire scores, students' performance in the preparation of discharge summaries and the postdischarge phone interview were evaluated. Discharge summaries and postdischarge phone interviews were classified as satisfactory or unsatisfactory based on the criteria outlined in the previous section on outcome evaluation.
Quantitative and Qualitative Analysis
Skills confidence, attitude, and knowledge scores were compared between pre‐ and posttest. Paired t tests were used to calculate statistical significance. A P value below 0.05 was considered statistically significant, using two‐tailed tests.
We also analyzed whether there were any differences in changes in confidence, attitude, and knowledge scores according to the time of year in which the course was taken by students. For this, we divided the nine‐month course into 3 trimesters (AugustOctober, NovemberJanuary, and FebruaryApril). In order to determine whether 3 were any differences in score changes among the different periods, we used a one‐way analysis of variance (ANOVA), in which a P value below 0.05 would indicate a statistically significant difference among the periods. All statistical analyses were performed using SPSS 17.0 for Windows.
Statistical tests were not utilized for the satisfaction scores, but the overall goal was for their mean to be 3 (good) or above. Also, the percentages of satisfactory discharge summaries and postdischarge phone interviews were measured. The goal was for both tasks to have a percentage of satisfactory evaluations of 80% or above.
RESULTS
The 121 students who took the module completed both the pre‐ and posttests. Table 1 details the mean pre‐ and posttest Likert scores for all confidence and attitude questions, as well as the changes in the 25‐point total confidence and attitude score from pre‐ to posttest. The change in confidence scores among survey participants was statistically significant (P < 0.001), while the change in attitude score was not (P = 0.07). Table 2 compares the percentage of correct answers before and after the course for individual knowledge questions, as well as for the entire knowledge quiz. Changes in total knowledge scores were statistically significant: the mean percentage of correct answers out of 10 questions was 68% on the pretest, and 82% on the posttest (P < 0.001).
Table 3 measures the changes in confidence, attitude, and knowledge scores by the period of the year in which students took the course. One‐way ANOVA tests for each of the 3 domains did not find statistically significant changes in confidence, attitude, or knowledge scores among the 3 trimesters in which we divided the module's calendar.
| Section of Questionnaire | Total for Year | Period of Year | F Value | P Value | ||
|---|---|---|---|---|---|---|
| AugustOctober | NovemberJanuary | FebruaryApril | ||||
| Confidence | ||||||
| Mean pre‐course score | 16.7 | 16.5 | 16.5 | 17.0 | ||
| Mean post‐course score | 20.7 | 21.0 | 19.9 | 21.0 | ||
| Mean change in score | 4.0 | 4.5 | 3.4 | 4.0 | 0.92 | 0.40 |
| Attitude | ||||||
| Mean pre‐course score | 20.8 | 20.9 | 20.5 | 20.6 | ||
| Mean post‐course score | 21.3 | 21.3 | 21.3 | 21.1 | ||
| Mean change in score | 0.5 | 0.4 | 0.8 | 0.5 | 0.13 | 0.88 |
| Knowledge | ||||||
| Mean pre‐course percentage correct | 68 | 71.3 | 67.4 | 66.4 | ||
| Mean post‐course percentage correct | 82 | 82.5 | 80.9 | 82.1 | ||
| Mean change in score | 14 | 11.2 | 13.5 | 15.7 | 0.60 | 0.55 |
| No. of participants | 121 | 40 | 34 | 47 | ||
Table 4 shows satisfaction scores on the posttest. The overall Likert rating for the course was 3.9, with 97.5% of students rating it good or better. The highest‐rated individual component of the course by Likert score was the training on discharge summaries, with a rating of 4.1. The lowest‐rated by this parameter was the congestive heart failure case, with a rating of 3.6. The online discussion across all topics had the lowest percentage of students rating it good or above, at 83.5%.
| Curriculum Section | Mean Likert Rating (Out of 5) | Percentage Rated Good or Above |
|---|---|---|
| Congestive heart failure case | 3.6 | 95.0 |
| Discharge summaries | 4.1 | 96.7 |
| Initial in‐person slide presentation | 4.0 | 97.5 |
| Postdischarge phone call | 3.7 | 95.0 |
| Online discussion for all topics | 3.7 | 83.5 |
| Overall curriculum rating | 3.9 | 97.5 |
As for student discharge summaries, 109 out of 121 (90.1%) met all the criteria in order to be deemed satisfactory; 109 out of 121 (90.1%) of postdischarge phone call reports met both required components. Both these results exceeded the goal of 80% set before the course started.
DISCUSSION
The Emory Care Transitions Curriculum for fourth‐year medical students started in the 20092010 academic year with the main goal of teaching students transferable skills that would ultimately lead to their participating in safer hospital discharges in their future practice as physicians. At the end of this course, students exhibited greater confidence in managing the discharge process, improved overall fund of knowledge relating to care transitions, and a demonstration of appropriate skills related to preparing discharge summaries and communicating with patients at discharge. This was all executed with a delivery method that students found engaging.
Analyzing the results, it is noteworthy that confidence improved, while attitudes did not. Even though confidence in performing a task does not necessarily reflect one's ability to perform it, our students' confidence scores may serve as a proxy for their ability to manage tasks related to the discharge process, like managing medications and preparing discharge summaries. Thus, while some studies suggest that self‐assessment among physicians may not always relate well to competence,26, 27 in our study, students did demonstrate skills in discharge summary preparation and in identifying the most relevant aspects of patient communication at hospital discharge. As for the absence of attitude change, this may have partly been a function of the fact that students in our group started with attitude scores that were already quite high, with a mean pretest attitude score of 20.7 out of 25.
Changes in student confidence, attitudes, and knowledge from pre‐ to posttest did not vary significantly across the academic year. Thus, one could interpret from our findings that more experienced students who took the module close to graduation benefited similarly from the course to those who completed it earlier, at least according to those rubrics. Another possible source of variation in student experience was the hospital in which students rotated: the demographics of GMH, with its large uninsured population; AVAMC, with more elderly patients; and EUH, with a more affluent profile, are certainly different. However, the number of students rotating at EUH and AVAMC were comparatively too small to attempt to draw any conclusions about how rotation site affected student experiences.
The use of a blended approach that integrated face‐to‐face didactics, patient care, and online learning offered some advantages. Curricular goals were achieved through a course that required only 2 hours of in‐person faculty time with students. This is significant, considering the time demands that academic medical faculty usually face. This approach also permitted students who were participating in a busy clinical rotation, and had limited opportunities to meet as a group, the ability to do coursework at their own pace. Another strength of the study is that all students who participated in the rotation were able to complete their surveys.
As for limitations, it is worth noting that in a course with a blended curriculum, the online discussion had the lowest percentage of students rating it good or better. Part of the perceived difficulty may have resulted from the fact that there are no other courses in the Emory medical curriculum that utilize discussion boards or distance learning methods as teaching tools. Despite this generation of students' technological savvy, this new mode of discussion may have proven difficult to pick up when they were in the midst of a busy clinical rotation. This serves as a reminder that while online curricula have proven successful in this and other settings, each element needs to be tailored to the audience. One other factor to be considered while interpreting this study's results is that this study utilized some survey instruments that have not been previously validated, even though they were developed in consultation with experts in the field of care transitions education.
We used a dichotomous, criteria‐based system to rate students' discharge summaries and reports of postdischarge phone calls. While this quantitative approach allowed us to more objectively define the quality of students' work, it did offer some disadvantages. First, even though we based the rating system for discharge summaries on a BAAHM template, it was not subjected to more extensive validation. Moreover, the quantitative approach diverted us from finding themes and other qualitative data from students' write‐ups, which could potentially have given us a fuller picture of their work.
This study contributes to the small, but growing, literature on care transitions education. The studies by Bray‐Hall et al., Lai et al., and Ouchida et al.,1921 used different methodologies, but all were directed at third‐year students using curricula with classroom and clinical learning, and showed favorable outcomes in knowledge and skills. The present study also showed positive results in students' knowledge and skills, but targeted it toward graduating medical students and included a focus on concrete skills, such as discharge summary preparation. It also utilized a nontraditional delivery approach which reached its objectives while also limiting the demands on faculty and students' face time during busy clinical rotations, which is especially important when considering that students were dispersed at multiple sites.
It is likely that medical schools will implement more care transitions curricula in coming years, as organizations like the AAMC11 and the Institute of Medicine10 increase the pressure to train future doctors to better address the needs of older and chronically ill patients, who require care from professionals of multiple disciplines, in disparate care settings. Moreover, the 2010 Patient Protection and Affordable Care Act28 contains provisions with financial incentives for hospitals to decrease readmissions. Once these become widely implemented, there will be a greater impetus to train medical practitioners to discharge patients more safely. When that occurs, medical schools will have additional compelling reasons to offer courses that teach students skills to execute better care transitions. The hoped‐for outcome of these curricula will ultimately be safer and more effective patient care.
Acknowledgements
The authors thank Dr. Ted Johnson at the Emory University School of Medicine for editorial review. They also express their gratitude to Dr. Karin Ouchida at Montefiore Medical Center and Dr. William Lyons at University of Nebraska Medical Center for their technical support in preparation of the curriculum. Disclosures: None of the authors has any relevant conflicts of interest. All coauthors have seen and agree with the contents of this manuscript. The authors are responsible for the integrity of the data described in this study. The research in this manuscript has not been submitted or accepted for publication in another journal.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170(3):345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,.Adverse drug events in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.1999;33:1147–1153.
- ,,,,.The association between the quality of inpatient care and early readmission: A meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,.Hospital readmissions as a measure of quality of health care: Advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians.JAMA.2007;297:831–841.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington, DC:National Academy Press;2003.
- American Association of Medical Colleges. Geriatric Competencies for Medical Students: Recommendations of the July 2007 Geriatrics Consensus Conference.2008. Available at: http://www.aamc.org/newsroom/press kits/competencies.pdf. Accessed February 25, 2009.
- Eskildsen M, Price T, Tenover JL. Computer‐based geriatrics workbooks for resident teaching. MedEdPortal. 2007. Available at http://services. aamc.org/30/mededportal/servlet/s/segment/mededportal/ find_resources/ browse/?subid=640.
- .Care transitions in the older adult.MedEdPortal.2008. Available at http://services.aamc.org/30/mededportal/servlet/s/segment/mededportal/find_resources/browse/?subid=678. Accessed March 19, 2011.
- ,,, et al.Web‐based module to train and assess competency in systems‐based practice.Portal of Online Geriatric Education.2009. Product ID #20002. Available at: http://www. pogoe.org/node/407. Accessed March 19, 2011.
- .CHAMP (Curriculum for the Hospitalized Aging Medical Patient): The ideal hospital discharge.Portal of Online Geriatric Education.2009. Product ID #18995. Available at: http://www. pogoe.org/productid/18995. Accessed March 19, 2011.
- .M1 care transitions.Portal of Online Geriatric Education.2009. Product ID #20450. Available at: http://www. pogoe.org/node/660. Accessed March 19, 2011.
- .Transitional care.Portal of Online Geriatric Education.2007. Product ID #18991. Available at: http://www.pogoe.org/node/262. Accessed March 19, 2011.
- .Discharge summary feedback.Portal of Online Geriatric Education.2009. Product ID #20546. Available at: http://www.pogoe.org/node/788. Accessed March 19, 2011.
- ,,.Toward safe hospital discharge: A transitions in care curriculum for medical students.J Gen Intern Med.25(8):878–881.
- ,,,,.Postdischarge follow‐up visits for medical and pharmacy students on an inpatient medicine clerkship.J Hosp Med.2008;3(1):20–27.
- ,,,,.Fast forward rounds: An effective method for teaching medical students to transition patients safely across care settings.J Am Geriatr Soc.2009;57:910–917.
- .Fourth‐year medical student care transitions curriculum (free login required).Portal of Online Geriatric Education.2010. Available at: http://www.pogoe.org/node/867. Accessed July 16, 2010.
- .Falling through the cracks: Challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- Society of Hospital Medicine. Quality initiatives for patient care—BOOSTing care transitions resource room.2010. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_Care Transitions/html_CC/12Clinical Tools/01_Toolkits.cfm. Accessed November 16, 2010.
- Society of Hospital Medicine. Ideal discharge for an elderly patient: A hospitalist checklist.2005. Available at: http://www.hospitalmedicine. org/AM/Template.cfm?Section=QI_Clinical_Tools8(2):105–110.
- ,,.Difficulties in recognizing one's own incompetence: Novice physicians who are unskilled and unaware of it.Acad Med.2001;76(10 suppl):S87–S89.
- Patient Protection and Affordable Care Act, H.R. 3590.ENR, 111th Congress, 2010.
There is increasing evidence that the transfer of medically complex patients across different settings can be associated with poor communication and patient dissatisfaction with the care received, potentially leading to negative clinical outcomes. While medical schools are beginning to introduce curricula on these transitions of care, few have been evaluated and subjected to peer review with the purpose of finding the most effective teaching and training methods.
Older adults and those with multiple chronic diseases frequently require medical care that spans multiple locations, and thus are most at risk for poor clinical outcomes during care transitions.1, 2 Medication errors and adverse drug reactions after hospital discharge are common.3, 4 Unsuccessful care transitions may also result in nonelective readmission after discharge, and there is evidence that readmissions may be a quality indicator for hospital care.57 Poor communication between patients and their healthcare providers is another element of poorly executed care transitions. Qualitative studies show that patients are frequently dissatisfied with the discharge process and are often unprepared to assume responsibility for their own care when they leave the hospital.8 Communication among providers can also be suboptimal. One meta‐analysis found that hospital physicians and primary care providers communicated infrequently and the availability of discharge summaries at the postdischarge visit was low, which may have affected the quality of care.9
Knowing that these gaps are common, there have been signs of increased emphasis on improving communication and working in teams as part of health professions training. The Institute of Medicine, in its 2003 report Health Professions Education: A Bridge to Quality,10 stressed education on management of chronic diseases, working in interdisciplinary teams, as well as a focus on quality improvement. In addition, the American Association of Medical Colleges (AAMC) encouraged training medical students on preparing safe discharge plans in its 2007 geriatrics competencies.11
Some medical schools have introduced care transitions curricula, though few have published data on their effectiveness. A search for teaching products using care transitions or transitional care on the online educational portals POGOe (Portal of Geriatric Online Education) and MedEdPortal yielded a total of 7 unique sets of teaching materials on care transitions for medical students.1218 However, a search on PubMed in July 2010 for peer‐reviewed articles on care transitions curricula developed for medical students which contained evaluation data only yielded 3 articles.1921 These 3 curricula, written by Bray‐Hall et al., Lai et al., and Ouchida et al., respectively, all trained third‐year medical students on diverse aspects of the discharge process using methods such as lectures, workshops, and patient visits, and showed favorable skill and knowledge outcomes.
Recognizing the importance of care transitions in medical education, a new curriculum addressing this topic was developed and introduced for fourth‐year medical students at the Emory University School of Medicine in 2009. The broad goal for this module was to develop a course concentrating on concrete skills that would train students to perform better care transitions while minimizing the time they had to spend away from a busy Internal Medicine sub‐internship. This curriculum used a mixed approach that included face‐to‐face teaching with faculty, online didactic instruction and interaction, and direct patient care. The course objectives were for students to develop a working fund of knowledge on care transitions, to learn to write a complete discharge summary, and to communicate the elements of a safe discharge plan. This article will describe the implementation of this curriculum and its evaluation.
METHODS
The Emory Care Transitions Curriculum started in August 2009 with fourth‐year medical students at the Emory University School of Medicine. This section will describe the details of the implementation of this curriculum, as well as the evaluation methodology and results.
Overview
This module was offered to Emory medical students participating in a required Senior Medicine rotation during their fourth year. The study population consisted of the 121 fourth‐year Emory medical students who participated in this rotation during the academic year that started in August 2009 and ended in April 2010. Students participated in the rotation at 1 of 3 teaching sites: Grady Memorial Hospital (GMH), Emory University Hospital (EUH), and the Atlanta VA Medical Center (AVAMC); 98 students completed their rotation at GMH, 12 at EUH, and 11 at AVAMC. For all online activities, students used the Blackboard platform software, available to them at
Course Description
The course consisted of 3 components, each associated with specific student assignments: a slide presentation on care transitions with an associated case discussion, training on discharge summaries, and the execution of a postdischarge phone call. Figure 1 describes the course delivery schedule.

Slide Presentation and Case Discussion
This section started on day 2 of the clerkship, with a face‐to‐face lecture titled Transitions of Care: Why They Are Important, and How to Improve Them. It included the following components: definition of the different posthospital discharge options, explanation of the reasons for the complexity of care transitions in high‐risk patient populations, and an enumeration of methods to improve the safety of care transitions. Students also read a review article on the topic to further add to their fund of knowledge.23
The second part of the section involved discussion of a case posted on Blackboard (a discussion board) designed to highlight some of the challenges associated with care transitions. The case included 2 successive discharge summaries for an elderly patient with congestive heart failure: 1 for the initial exacerbation, and the other for a readmission. Using an online discussion board, students were asked to report the strong points and shortcomings of the patient's management, as well as those of the discharge summaries. Then the students were asked to post responses to at least 2 of their classmates' reports on the discussion board.
Training on Discharge Summaries
During the module, students received training on how to prepare a complete and informative discharge summary. This online training consisted of a lecture prepared by a faculty member (M.A.E.) and the use of a discharge summary template based on a guide prepared by the Boston Association of Academic Hospitalists (BAAHM), which is part of a toolkit available from the Society of Hospital Medicine.24 After reading the lecture, each student selected 1 of the patients they cared for during their rotation, and wrote a discharge summary. They posted it to a Blackboard discussion board, and were then asked to comment on one of their classmates' reports on the same forum. Faculty (M.A.E. and R.C.) also gave online feedback to each student about their discharge summary.
Postdischarge Phone Call
Students were also assigned to communicate with the patient for whom they prepared a discharge summary by performing a postdischarge phone call within a week of the patient's departure from the hospital. They reviewed a discharge checklist adapted from Ideal Discharge for an Elderly Patient: A Hospitalist Checklist, issued by the Society of Hospital Medicine.25 This document contains the necessary elements of a safe discharge plan, and used these points as the basis of the patient phone interview. The goal of the call and the use of the checklist was to reinforce the main elements of communication with patients that need to occur before they leave the hospital.
Students then used the checklist as the basis for a short (<400 words) report discussing the strong points and shortcomings of their patient's discharge, and posted it on a Blackboard discussion board. They were also asked to comment on at least one of their classmates' reports on the board. Faculty (M.A.E. and R.C.) also participated in the discussion board, commenting at least once on all students' reports.
Evaluation
The course was evaluated in order to assess changes in skills, knowledge and attitudes, as well as satisfaction with the course.
Evaluation Components
In order to assess the outcomes described above, questionnaires were utilized, and objective criteria were used to evaluate students' work. Students completed a pretest before the first face‐to‐face session, and a posttest after the second in‐person discussion. Pretest items were identical to those in the posttest, except that the posttest also contained 6 satisfaction questions. The components that were included in both pre‐ and posttests were:
Five multiple choice questions measuring students' confidence in their own skills regarding discharge summaries and transitional care (pre‐ and postsurvey). These 5 questions were adapted from the questionnaire developed by Lai et al.20 Confidence questionnaire items are detailed in Table 1.
Five multiple choice questions assessing students' attitudes regarding the importance of different components of the care transitions process (pre‐ and postsurvey). Attitude questionnaire items are detailed in Table 1.
Ten multiple choice questions in which each had one right answer, assessing students' knowledge base on transitional care issues (pre‐and postsurvey). Knowledge questions and their correct answers are detailed in Table 2.
| Mean Likert Scores* | P Value | ||
|---|---|---|---|
| Pre‐Course | Post‐Course | ||
| |||
| Confidence items | |||
| 1. I am confident in my ability to involve patients in making a plan for their care. | 3.8 | 4.2 | <0.001 |
| 2. I am confident in my ability to review patients' medications and side effects. | 3.4 | 4.1 | <0.001 |
| 3. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.4 | 4.3 | <0.001 |
| 4. I am confident in my ability to prepare a complete discharge summary. | 3.0 | 4.2 | <0.001 |
| 5. I can identify the different types of places that may serve as a setting for discharge from the inpatient setting. | 3.1 | 3.9 | <0.001 |
| Total confidence score (out of 25) | 16.7 | 20.7 | <0.001 |
| Attitude items | |||
| 1. A hospital physician should always communicate with a patient's primary care physician before that patient is discharged from the hospital, in order to ensure a smooth transition of care. | 4.0 | 4.1 | 0.78 |
| 2. Before a patient is discharged from the hospital, a physician (not just the nurse or case manager) should always meet with the patient to discuss his medications, and goals of care. | 4.4 | 4.4 | 0.50 |
| 3. It is critical for a primary care physician to have access to a discharge summary when seeing a patient for the first time after leaving the hospital. | 4.6 | 4.7 | 0.25 |
| 4. The main reason patients often don't take their medications properly after discharge is that they are confused by the instructions given to them at the hospital. | 3.7 | 3.8 | 0.65 |
| 5. Avoiding rehospitalization should be a top priority for physicians in the process of discharge from the hospital. | 4.1 | 4.3 | 0.95 |
| Total attitude score (out of 25) | 20.8 | 21.3 | 0.07 |
| Percent Correct | |||
|---|---|---|---|
| Question (Correct Answer in Parenthesis) | Pre‐Course | Post‐Course | P Value |
| |||
| 1. When a patient is discharged with home health care, which of the following services is usually not part of the package? (A caregiver to sit with the patient and supervise them most of the day.) | 70 | 83 | 0.020* |
| 2. When a patient is discharged from the hospital to a skilled nursing facility (SNF) for further care, which of these is a service that is typically provided? (Physical therapy.) | 78 | 75 | 0.649 |
| 3. Which of the following rehabilitation activities is more likely to be in the job description of an occupational therapist? (Training of strength in upper extremities.) | 24 | 51 | <0.001* |
| 4. Which of these is least likely to be a cause of poor patient outcomes after hospital discharge? (The discharging of patients to skilled nursing facilities.) | 93 | 97 | 0.166 |
| 5. Which of these is more likely to be an indicator of poor outcomes after hospital discharge? (Having had 3 hospitalizations in the last 6 months.) | 93 | 96 | 0.287 |
| 6. Which of these data is the least likely to be an indicator that the patient is too sick to be discharged from the hospital? (Hemoglobin concentration of 9.5 g/dl.) | 45 | 74 | <0.001* |
| 7. Which of the following medications would merit the most time spent on communication with patients, family members, and receiving physicians? (Furosemide.) | 58 | 96 | <0.001* |
| 8. You are caring for an 89‐year‐old man who is being treated in the hospital for an exacerbation of his congestive heart failure (CHF). He is doing well, ambulating 100 feet without shortness of breath, and is showing understanding of the need for all his different medications. However, he is not yet back to his functional baseline. Which of the following is the LEAST appropriate setting for discharge? (Hospice care.) | 74 | 70 | 0.458 |
| 9. Which of the following is true about skilled nursing facility (SNF) care? (Patients can be admitted for treatment with IV antibiotics for several weeks.) | 52 | 79 | <0.001* |
| 10. Educating patients at discharge about their illness and medication has been found to help decrease readmission rates. (True.) | 97 | 100 | 0.045 |
| Percentage of total questions correct | 68 | 82 | <0.001* |
The questionnaire items were developed by study faculty (M.A.E. and J.M.F.) and were edited in consultation with clinical faculty members from outside Emory with experience developing care transitions curricula: Dr. Karin Ouchida of Montefiore Medical Center in New York City, and Dr. William Lyons of the University of Nebraska Medical Center.
The 6 posttest items addressed student satisfaction with individual course components, which were: the heart failure online case, training on preparing discharge summaries, initial in‐person slide presentation, postdischarge phone call, overall online discussion across all items, and finally, satisfaction with the overall course. Questionnaire items on comfort, attitudes, and satisfaction all used a five‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The 10 multiple‐choice questions on knowledge each had one right answer.
Each student completed one discharge summary during the course. For it to be deemed satisfactory, it had to have the 5 following components, all present in the BAAHM template24:
A documented discharge medication list with specific dosing schedules.
Lists of admission medications and/or a list of medication changes during hospitalization.
A discharge plan that specifies the next setting of care, as well as the planned follow‐up.
A hospital course organized by system and/or specific chronology.
A physical exam, laboratory tests, and diagnostic studies performed on admission.
Student reports of their postdischarge phone call were also evaluated by study faculty (M.A.E. and R.C.). For the report of the interview to be considered satisfactory, it had to contain at least the following 2 elements:
A discussion of the patient's medication list, including documentation of a discussion of hazardous medications (e.g., furosemide, warfarin, digoxin, insulin) if applicable.
Documentation of a discussion on follow‐up plans with a primary physician or specialist.
Data Analysis
Outcomes were evaluated based on the results of pre‐ and posttest questionnaires, in addition to the satisfactoriness of discharge summaries and postdischarge phone call reports. As for the questionnaires, 4 types of scores were analyzed based on students' questionnaire responses:
Skills Confidence Score: The sum of Likert scores for confidence items on the pre‐ and posttest was the confidence score, with a highest possible score of 25, in which the highest scores were associated with the most confidence in executing the discharge process.
Attitude Score: The sum of Likert scores for attitude items on the pre‐ and posttest was the attitude score, with a highest possible score of 25, in which the highest scores were associated with student attitudes ascribing the most importance to a safe discharge process.
Knowledge Score: The percentage of total correct answers on knowledge questions on the pre‐ and posttest were used to obtain the knowledge score, in which a score of 100 was highest.
Satisfaction Score: Satisfaction questions on the posttest questionnaire were analyzed separately to assess satisfaction with each component of the curriculum, ranging from poor (score = 1) to excellent (score = 5). We also determined the percentage of students who rated each portion of the course good or better.
In addition to questionnaire scores, students' performance in the preparation of discharge summaries and the postdischarge phone interview were evaluated. Discharge summaries and postdischarge phone interviews were classified as satisfactory or unsatisfactory based on the criteria outlined in the previous section on outcome evaluation.
Quantitative and Qualitative Analysis
Skills confidence, attitude, and knowledge scores were compared between pre‐ and posttest. Paired t tests were used to calculate statistical significance. A P value below 0.05 was considered statistically significant, using two‐tailed tests.
We also analyzed whether there were any differences in changes in confidence, attitude, and knowledge scores according to the time of year in which the course was taken by students. For this, we divided the nine‐month course into 3 trimesters (AugustOctober, NovemberJanuary, and FebruaryApril). In order to determine whether 3 were any differences in score changes among the different periods, we used a one‐way analysis of variance (ANOVA), in which a P value below 0.05 would indicate a statistically significant difference among the periods. All statistical analyses were performed using SPSS 17.0 for Windows.
Statistical tests were not utilized for the satisfaction scores, but the overall goal was for their mean to be 3 (good) or above. Also, the percentages of satisfactory discharge summaries and postdischarge phone interviews were measured. The goal was for both tasks to have a percentage of satisfactory evaluations of 80% or above.
RESULTS
The 121 students who took the module completed both the pre‐ and posttests. Table 1 details the mean pre‐ and posttest Likert scores for all confidence and attitude questions, as well as the changes in the 25‐point total confidence and attitude score from pre‐ to posttest. The change in confidence scores among survey participants was statistically significant (P < 0.001), while the change in attitude score was not (P = 0.07). Table 2 compares the percentage of correct answers before and after the course for individual knowledge questions, as well as for the entire knowledge quiz. Changes in total knowledge scores were statistically significant: the mean percentage of correct answers out of 10 questions was 68% on the pretest, and 82% on the posttest (P < 0.001).
Table 3 measures the changes in confidence, attitude, and knowledge scores by the period of the year in which students took the course. One‐way ANOVA tests for each of the 3 domains did not find statistically significant changes in confidence, attitude, or knowledge scores among the 3 trimesters in which we divided the module's calendar.
| Section of Questionnaire | Total for Year | Period of Year | F Value | P Value | ||
|---|---|---|---|---|---|---|
| AugustOctober | NovemberJanuary | FebruaryApril | ||||
| Confidence | ||||||
| Mean pre‐course score | 16.7 | 16.5 | 16.5 | 17.0 | ||
| Mean post‐course score | 20.7 | 21.0 | 19.9 | 21.0 | ||
| Mean change in score | 4.0 | 4.5 | 3.4 | 4.0 | 0.92 | 0.40 |
| Attitude | ||||||
| Mean pre‐course score | 20.8 | 20.9 | 20.5 | 20.6 | ||
| Mean post‐course score | 21.3 | 21.3 | 21.3 | 21.1 | ||
| Mean change in score | 0.5 | 0.4 | 0.8 | 0.5 | 0.13 | 0.88 |
| Knowledge | ||||||
| Mean pre‐course percentage correct | 68 | 71.3 | 67.4 | 66.4 | ||
| Mean post‐course percentage correct | 82 | 82.5 | 80.9 | 82.1 | ||
| Mean change in score | 14 | 11.2 | 13.5 | 15.7 | 0.60 | 0.55 |
| No. of participants | 121 | 40 | 34 | 47 | ||
Table 4 shows satisfaction scores on the posttest. The overall Likert rating for the course was 3.9, with 97.5% of students rating it good or better. The highest‐rated individual component of the course by Likert score was the training on discharge summaries, with a rating of 4.1. The lowest‐rated by this parameter was the congestive heart failure case, with a rating of 3.6. The online discussion across all topics had the lowest percentage of students rating it good or above, at 83.5%.
| Curriculum Section | Mean Likert Rating (Out of 5) | Percentage Rated Good or Above |
|---|---|---|
| Congestive heart failure case | 3.6 | 95.0 |
| Discharge summaries | 4.1 | 96.7 |
| Initial in‐person slide presentation | 4.0 | 97.5 |
| Postdischarge phone call | 3.7 | 95.0 |
| Online discussion for all topics | 3.7 | 83.5 |
| Overall curriculum rating | 3.9 | 97.5 |
As for student discharge summaries, 109 out of 121 (90.1%) met all the criteria in order to be deemed satisfactory; 109 out of 121 (90.1%) of postdischarge phone call reports met both required components. Both these results exceeded the goal of 80% set before the course started.
DISCUSSION
The Emory Care Transitions Curriculum for fourth‐year medical students started in the 20092010 academic year with the main goal of teaching students transferable skills that would ultimately lead to their participating in safer hospital discharges in their future practice as physicians. At the end of this course, students exhibited greater confidence in managing the discharge process, improved overall fund of knowledge relating to care transitions, and a demonstration of appropriate skills related to preparing discharge summaries and communicating with patients at discharge. This was all executed with a delivery method that students found engaging.
Analyzing the results, it is noteworthy that confidence improved, while attitudes did not. Even though confidence in performing a task does not necessarily reflect one's ability to perform it, our students' confidence scores may serve as a proxy for their ability to manage tasks related to the discharge process, like managing medications and preparing discharge summaries. Thus, while some studies suggest that self‐assessment among physicians may not always relate well to competence,26, 27 in our study, students did demonstrate skills in discharge summary preparation and in identifying the most relevant aspects of patient communication at hospital discharge. As for the absence of attitude change, this may have partly been a function of the fact that students in our group started with attitude scores that were already quite high, with a mean pretest attitude score of 20.7 out of 25.
Changes in student confidence, attitudes, and knowledge from pre‐ to posttest did not vary significantly across the academic year. Thus, one could interpret from our findings that more experienced students who took the module close to graduation benefited similarly from the course to those who completed it earlier, at least according to those rubrics. Another possible source of variation in student experience was the hospital in which students rotated: the demographics of GMH, with its large uninsured population; AVAMC, with more elderly patients; and EUH, with a more affluent profile, are certainly different. However, the number of students rotating at EUH and AVAMC were comparatively too small to attempt to draw any conclusions about how rotation site affected student experiences.
The use of a blended approach that integrated face‐to‐face didactics, patient care, and online learning offered some advantages. Curricular goals were achieved through a course that required only 2 hours of in‐person faculty time with students. This is significant, considering the time demands that academic medical faculty usually face. This approach also permitted students who were participating in a busy clinical rotation, and had limited opportunities to meet as a group, the ability to do coursework at their own pace. Another strength of the study is that all students who participated in the rotation were able to complete their surveys.
As for limitations, it is worth noting that in a course with a blended curriculum, the online discussion had the lowest percentage of students rating it good or better. Part of the perceived difficulty may have resulted from the fact that there are no other courses in the Emory medical curriculum that utilize discussion boards or distance learning methods as teaching tools. Despite this generation of students' technological savvy, this new mode of discussion may have proven difficult to pick up when they were in the midst of a busy clinical rotation. This serves as a reminder that while online curricula have proven successful in this and other settings, each element needs to be tailored to the audience. One other factor to be considered while interpreting this study's results is that this study utilized some survey instruments that have not been previously validated, even though they were developed in consultation with experts in the field of care transitions education.
We used a dichotomous, criteria‐based system to rate students' discharge summaries and reports of postdischarge phone calls. While this quantitative approach allowed us to more objectively define the quality of students' work, it did offer some disadvantages. First, even though we based the rating system for discharge summaries on a BAAHM template, it was not subjected to more extensive validation. Moreover, the quantitative approach diverted us from finding themes and other qualitative data from students' write‐ups, which could potentially have given us a fuller picture of their work.
This study contributes to the small, but growing, literature on care transitions education. The studies by Bray‐Hall et al., Lai et al., and Ouchida et al.,1921 used different methodologies, but all were directed at third‐year students using curricula with classroom and clinical learning, and showed favorable outcomes in knowledge and skills. The present study also showed positive results in students' knowledge and skills, but targeted it toward graduating medical students and included a focus on concrete skills, such as discharge summary preparation. It also utilized a nontraditional delivery approach which reached its objectives while also limiting the demands on faculty and students' face time during busy clinical rotations, which is especially important when considering that students were dispersed at multiple sites.
It is likely that medical schools will implement more care transitions curricula in coming years, as organizations like the AAMC11 and the Institute of Medicine10 increase the pressure to train future doctors to better address the needs of older and chronically ill patients, who require care from professionals of multiple disciplines, in disparate care settings. Moreover, the 2010 Patient Protection and Affordable Care Act28 contains provisions with financial incentives for hospitals to decrease readmissions. Once these become widely implemented, there will be a greater impetus to train medical practitioners to discharge patients more safely. When that occurs, medical schools will have additional compelling reasons to offer courses that teach students skills to execute better care transitions. The hoped‐for outcome of these curricula will ultimately be safer and more effective patient care.
Acknowledgements
The authors thank Dr. Ted Johnson at the Emory University School of Medicine for editorial review. They also express their gratitude to Dr. Karin Ouchida at Montefiore Medical Center and Dr. William Lyons at University of Nebraska Medical Center for their technical support in preparation of the curriculum. Disclosures: None of the authors has any relevant conflicts of interest. All coauthors have seen and agree with the contents of this manuscript. The authors are responsible for the integrity of the data described in this study. The research in this manuscript has not been submitted or accepted for publication in another journal.
There is increasing evidence that the transfer of medically complex patients across different settings can be associated with poor communication and patient dissatisfaction with the care received, potentially leading to negative clinical outcomes. While medical schools are beginning to introduce curricula on these transitions of care, few have been evaluated and subjected to peer review with the purpose of finding the most effective teaching and training methods.
Older adults and those with multiple chronic diseases frequently require medical care that spans multiple locations, and thus are most at risk for poor clinical outcomes during care transitions.1, 2 Medication errors and adverse drug reactions after hospital discharge are common.3, 4 Unsuccessful care transitions may also result in nonelective readmission after discharge, and there is evidence that readmissions may be a quality indicator for hospital care.57 Poor communication between patients and their healthcare providers is another element of poorly executed care transitions. Qualitative studies show that patients are frequently dissatisfied with the discharge process and are often unprepared to assume responsibility for their own care when they leave the hospital.8 Communication among providers can also be suboptimal. One meta‐analysis found that hospital physicians and primary care providers communicated infrequently and the availability of discharge summaries at the postdischarge visit was low, which may have affected the quality of care.9
Knowing that these gaps are common, there have been signs of increased emphasis on improving communication and working in teams as part of health professions training. The Institute of Medicine, in its 2003 report Health Professions Education: A Bridge to Quality,10 stressed education on management of chronic diseases, working in interdisciplinary teams, as well as a focus on quality improvement. In addition, the American Association of Medical Colleges (AAMC) encouraged training medical students on preparing safe discharge plans in its 2007 geriatrics competencies.11
Some medical schools have introduced care transitions curricula, though few have published data on their effectiveness. A search for teaching products using care transitions or transitional care on the online educational portals POGOe (Portal of Geriatric Online Education) and MedEdPortal yielded a total of 7 unique sets of teaching materials on care transitions for medical students.1218 However, a search on PubMed in July 2010 for peer‐reviewed articles on care transitions curricula developed for medical students which contained evaluation data only yielded 3 articles.1921 These 3 curricula, written by Bray‐Hall et al., Lai et al., and Ouchida et al., respectively, all trained third‐year medical students on diverse aspects of the discharge process using methods such as lectures, workshops, and patient visits, and showed favorable skill and knowledge outcomes.
Recognizing the importance of care transitions in medical education, a new curriculum addressing this topic was developed and introduced for fourth‐year medical students at the Emory University School of Medicine in 2009. The broad goal for this module was to develop a course concentrating on concrete skills that would train students to perform better care transitions while minimizing the time they had to spend away from a busy Internal Medicine sub‐internship. This curriculum used a mixed approach that included face‐to‐face teaching with faculty, online didactic instruction and interaction, and direct patient care. The course objectives were for students to develop a working fund of knowledge on care transitions, to learn to write a complete discharge summary, and to communicate the elements of a safe discharge plan. This article will describe the implementation of this curriculum and its evaluation.
METHODS
The Emory Care Transitions Curriculum started in August 2009 with fourth‐year medical students at the Emory University School of Medicine. This section will describe the details of the implementation of this curriculum, as well as the evaluation methodology and results.
Overview
This module was offered to Emory medical students participating in a required Senior Medicine rotation during their fourth year. The study population consisted of the 121 fourth‐year Emory medical students who participated in this rotation during the academic year that started in August 2009 and ended in April 2010. Students participated in the rotation at 1 of 3 teaching sites: Grady Memorial Hospital (GMH), Emory University Hospital (EUH), and the Atlanta VA Medical Center (AVAMC); 98 students completed their rotation at GMH, 12 at EUH, and 11 at AVAMC. For all online activities, students used the Blackboard platform software, available to them at
Course Description
The course consisted of 3 components, each associated with specific student assignments: a slide presentation on care transitions with an associated case discussion, training on discharge summaries, and the execution of a postdischarge phone call. Figure 1 describes the course delivery schedule.

Slide Presentation and Case Discussion
This section started on day 2 of the clerkship, with a face‐to‐face lecture titled Transitions of Care: Why They Are Important, and How to Improve Them. It included the following components: definition of the different posthospital discharge options, explanation of the reasons for the complexity of care transitions in high‐risk patient populations, and an enumeration of methods to improve the safety of care transitions. Students also read a review article on the topic to further add to their fund of knowledge.23
The second part of the section involved discussion of a case posted on Blackboard (a discussion board) designed to highlight some of the challenges associated with care transitions. The case included 2 successive discharge summaries for an elderly patient with congestive heart failure: 1 for the initial exacerbation, and the other for a readmission. Using an online discussion board, students were asked to report the strong points and shortcomings of the patient's management, as well as those of the discharge summaries. Then the students were asked to post responses to at least 2 of their classmates' reports on the discussion board.
Training on Discharge Summaries
During the module, students received training on how to prepare a complete and informative discharge summary. This online training consisted of a lecture prepared by a faculty member (M.A.E.) and the use of a discharge summary template based on a guide prepared by the Boston Association of Academic Hospitalists (BAAHM), which is part of a toolkit available from the Society of Hospital Medicine.24 After reading the lecture, each student selected 1 of the patients they cared for during their rotation, and wrote a discharge summary. They posted it to a Blackboard discussion board, and were then asked to comment on one of their classmates' reports on the same forum. Faculty (M.A.E. and R.C.) also gave online feedback to each student about their discharge summary.
Postdischarge Phone Call
Students were also assigned to communicate with the patient for whom they prepared a discharge summary by performing a postdischarge phone call within a week of the patient's departure from the hospital. They reviewed a discharge checklist adapted from Ideal Discharge for an Elderly Patient: A Hospitalist Checklist, issued by the Society of Hospital Medicine.25 This document contains the necessary elements of a safe discharge plan, and used these points as the basis of the patient phone interview. The goal of the call and the use of the checklist was to reinforce the main elements of communication with patients that need to occur before they leave the hospital.
Students then used the checklist as the basis for a short (<400 words) report discussing the strong points and shortcomings of their patient's discharge, and posted it on a Blackboard discussion board. They were also asked to comment on at least one of their classmates' reports on the board. Faculty (M.A.E. and R.C.) also participated in the discussion board, commenting at least once on all students' reports.
Evaluation
The course was evaluated in order to assess changes in skills, knowledge and attitudes, as well as satisfaction with the course.
Evaluation Components
In order to assess the outcomes described above, questionnaires were utilized, and objective criteria were used to evaluate students' work. Students completed a pretest before the first face‐to‐face session, and a posttest after the second in‐person discussion. Pretest items were identical to those in the posttest, except that the posttest also contained 6 satisfaction questions. The components that were included in both pre‐ and posttests were:
Five multiple choice questions measuring students' confidence in their own skills regarding discharge summaries and transitional care (pre‐ and postsurvey). These 5 questions were adapted from the questionnaire developed by Lai et al.20 Confidence questionnaire items are detailed in Table 1.
Five multiple choice questions assessing students' attitudes regarding the importance of different components of the care transitions process (pre‐ and postsurvey). Attitude questionnaire items are detailed in Table 1.
Ten multiple choice questions in which each had one right answer, assessing students' knowledge base on transitional care issues (pre‐and postsurvey). Knowledge questions and their correct answers are detailed in Table 2.
| Mean Likert Scores* | P Value | ||
|---|---|---|---|
| Pre‐Course | Post‐Course | ||
| |||
| Confidence items | |||
| 1. I am confident in my ability to involve patients in making a plan for their care. | 3.8 | 4.2 | <0.001 |
| 2. I am confident in my ability to review patients' medications and side effects. | 3.4 | 4.1 | <0.001 |
| 3. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.4 | 4.3 | <0.001 |
| 4. I am confident in my ability to prepare a complete discharge summary. | 3.0 | 4.2 | <0.001 |
| 5. I can identify the different types of places that may serve as a setting for discharge from the inpatient setting. | 3.1 | 3.9 | <0.001 |
| Total confidence score (out of 25) | 16.7 | 20.7 | <0.001 |
| Attitude items | |||
| 1. A hospital physician should always communicate with a patient's primary care physician before that patient is discharged from the hospital, in order to ensure a smooth transition of care. | 4.0 | 4.1 | 0.78 |
| 2. Before a patient is discharged from the hospital, a physician (not just the nurse or case manager) should always meet with the patient to discuss his medications, and goals of care. | 4.4 | 4.4 | 0.50 |
| 3. It is critical for a primary care physician to have access to a discharge summary when seeing a patient for the first time after leaving the hospital. | 4.6 | 4.7 | 0.25 |
| 4. The main reason patients often don't take their medications properly after discharge is that they are confused by the instructions given to them at the hospital. | 3.7 | 3.8 | 0.65 |
| 5. Avoiding rehospitalization should be a top priority for physicians in the process of discharge from the hospital. | 4.1 | 4.3 | 0.95 |
| Total attitude score (out of 25) | 20.8 | 21.3 | 0.07 |
| Percent Correct | |||
|---|---|---|---|
| Question (Correct Answer in Parenthesis) | Pre‐Course | Post‐Course | P Value |
| |||
| 1. When a patient is discharged with home health care, which of the following services is usually not part of the package? (A caregiver to sit with the patient and supervise them most of the day.) | 70 | 83 | 0.020* |
| 2. When a patient is discharged from the hospital to a skilled nursing facility (SNF) for further care, which of these is a service that is typically provided? (Physical therapy.) | 78 | 75 | 0.649 |
| 3. Which of the following rehabilitation activities is more likely to be in the job description of an occupational therapist? (Training of strength in upper extremities.) | 24 | 51 | <0.001* |
| 4. Which of these is least likely to be a cause of poor patient outcomes after hospital discharge? (The discharging of patients to skilled nursing facilities.) | 93 | 97 | 0.166 |
| 5. Which of these is more likely to be an indicator of poor outcomes after hospital discharge? (Having had 3 hospitalizations in the last 6 months.) | 93 | 96 | 0.287 |
| 6. Which of these data is the least likely to be an indicator that the patient is too sick to be discharged from the hospital? (Hemoglobin concentration of 9.5 g/dl.) | 45 | 74 | <0.001* |
| 7. Which of the following medications would merit the most time spent on communication with patients, family members, and receiving physicians? (Furosemide.) | 58 | 96 | <0.001* |
| 8. You are caring for an 89‐year‐old man who is being treated in the hospital for an exacerbation of his congestive heart failure (CHF). He is doing well, ambulating 100 feet without shortness of breath, and is showing understanding of the need for all his different medications. However, he is not yet back to his functional baseline. Which of the following is the LEAST appropriate setting for discharge? (Hospice care.) | 74 | 70 | 0.458 |
| 9. Which of the following is true about skilled nursing facility (SNF) care? (Patients can be admitted for treatment with IV antibiotics for several weeks.) | 52 | 79 | <0.001* |
| 10. Educating patients at discharge about their illness and medication has been found to help decrease readmission rates. (True.) | 97 | 100 | 0.045 |
| Percentage of total questions correct | 68 | 82 | <0.001* |
The questionnaire items were developed by study faculty (M.A.E. and J.M.F.) and were edited in consultation with clinical faculty members from outside Emory with experience developing care transitions curricula: Dr. Karin Ouchida of Montefiore Medical Center in New York City, and Dr. William Lyons of the University of Nebraska Medical Center.
The 6 posttest items addressed student satisfaction with individual course components, which were: the heart failure online case, training on preparing discharge summaries, initial in‐person slide presentation, postdischarge phone call, overall online discussion across all items, and finally, satisfaction with the overall course. Questionnaire items on comfort, attitudes, and satisfaction all used a five‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The 10 multiple‐choice questions on knowledge each had one right answer.
Each student completed one discharge summary during the course. For it to be deemed satisfactory, it had to have the 5 following components, all present in the BAAHM template24:
A documented discharge medication list with specific dosing schedules.
Lists of admission medications and/or a list of medication changes during hospitalization.
A discharge plan that specifies the next setting of care, as well as the planned follow‐up.
A hospital course organized by system and/or specific chronology.
A physical exam, laboratory tests, and diagnostic studies performed on admission.
Student reports of their postdischarge phone call were also evaluated by study faculty (M.A.E. and R.C.). For the report of the interview to be considered satisfactory, it had to contain at least the following 2 elements:
A discussion of the patient's medication list, including documentation of a discussion of hazardous medications (e.g., furosemide, warfarin, digoxin, insulin) if applicable.
Documentation of a discussion on follow‐up plans with a primary physician or specialist.
Data Analysis
Outcomes were evaluated based on the results of pre‐ and posttest questionnaires, in addition to the satisfactoriness of discharge summaries and postdischarge phone call reports. As for the questionnaires, 4 types of scores were analyzed based on students' questionnaire responses:
Skills Confidence Score: The sum of Likert scores for confidence items on the pre‐ and posttest was the confidence score, with a highest possible score of 25, in which the highest scores were associated with the most confidence in executing the discharge process.
Attitude Score: The sum of Likert scores for attitude items on the pre‐ and posttest was the attitude score, with a highest possible score of 25, in which the highest scores were associated with student attitudes ascribing the most importance to a safe discharge process.
Knowledge Score: The percentage of total correct answers on knowledge questions on the pre‐ and posttest were used to obtain the knowledge score, in which a score of 100 was highest.
Satisfaction Score: Satisfaction questions on the posttest questionnaire were analyzed separately to assess satisfaction with each component of the curriculum, ranging from poor (score = 1) to excellent (score = 5). We also determined the percentage of students who rated each portion of the course good or better.
In addition to questionnaire scores, students' performance in the preparation of discharge summaries and the postdischarge phone interview were evaluated. Discharge summaries and postdischarge phone interviews were classified as satisfactory or unsatisfactory based on the criteria outlined in the previous section on outcome evaluation.
Quantitative and Qualitative Analysis
Skills confidence, attitude, and knowledge scores were compared between pre‐ and posttest. Paired t tests were used to calculate statistical significance. A P value below 0.05 was considered statistically significant, using two‐tailed tests.
We also analyzed whether there were any differences in changes in confidence, attitude, and knowledge scores according to the time of year in which the course was taken by students. For this, we divided the nine‐month course into 3 trimesters (AugustOctober, NovemberJanuary, and FebruaryApril). In order to determine whether 3 were any differences in score changes among the different periods, we used a one‐way analysis of variance (ANOVA), in which a P value below 0.05 would indicate a statistically significant difference among the periods. All statistical analyses were performed using SPSS 17.0 for Windows.
Statistical tests were not utilized for the satisfaction scores, but the overall goal was for their mean to be 3 (good) or above. Also, the percentages of satisfactory discharge summaries and postdischarge phone interviews were measured. The goal was for both tasks to have a percentage of satisfactory evaluations of 80% or above.
RESULTS
The 121 students who took the module completed both the pre‐ and posttests. Table 1 details the mean pre‐ and posttest Likert scores for all confidence and attitude questions, as well as the changes in the 25‐point total confidence and attitude score from pre‐ to posttest. The change in confidence scores among survey participants was statistically significant (P < 0.001), while the change in attitude score was not (P = 0.07). Table 2 compares the percentage of correct answers before and after the course for individual knowledge questions, as well as for the entire knowledge quiz. Changes in total knowledge scores were statistically significant: the mean percentage of correct answers out of 10 questions was 68% on the pretest, and 82% on the posttest (P < 0.001).
Table 3 measures the changes in confidence, attitude, and knowledge scores by the period of the year in which students took the course. One‐way ANOVA tests for each of the 3 domains did not find statistically significant changes in confidence, attitude, or knowledge scores among the 3 trimesters in which we divided the module's calendar.
| Section of Questionnaire | Total for Year | Period of Year | F Value | P Value | ||
|---|---|---|---|---|---|---|
| AugustOctober | NovemberJanuary | FebruaryApril | ||||
| Confidence | ||||||
| Mean pre‐course score | 16.7 | 16.5 | 16.5 | 17.0 | ||
| Mean post‐course score | 20.7 | 21.0 | 19.9 | 21.0 | ||
| Mean change in score | 4.0 | 4.5 | 3.4 | 4.0 | 0.92 | 0.40 |
| Attitude | ||||||
| Mean pre‐course score | 20.8 | 20.9 | 20.5 | 20.6 | ||
| Mean post‐course score | 21.3 | 21.3 | 21.3 | 21.1 | ||
| Mean change in score | 0.5 | 0.4 | 0.8 | 0.5 | 0.13 | 0.88 |
| Knowledge | ||||||
| Mean pre‐course percentage correct | 68 | 71.3 | 67.4 | 66.4 | ||
| Mean post‐course percentage correct | 82 | 82.5 | 80.9 | 82.1 | ||
| Mean change in score | 14 | 11.2 | 13.5 | 15.7 | 0.60 | 0.55 |
| No. of participants | 121 | 40 | 34 | 47 | ||
Table 4 shows satisfaction scores on the posttest. The overall Likert rating for the course was 3.9, with 97.5% of students rating it good or better. The highest‐rated individual component of the course by Likert score was the training on discharge summaries, with a rating of 4.1. The lowest‐rated by this parameter was the congestive heart failure case, with a rating of 3.6. The online discussion across all topics had the lowest percentage of students rating it good or above, at 83.5%.
| Curriculum Section | Mean Likert Rating (Out of 5) | Percentage Rated Good or Above |
|---|---|---|
| Congestive heart failure case | 3.6 | 95.0 |
| Discharge summaries | 4.1 | 96.7 |
| Initial in‐person slide presentation | 4.0 | 97.5 |
| Postdischarge phone call | 3.7 | 95.0 |
| Online discussion for all topics | 3.7 | 83.5 |
| Overall curriculum rating | 3.9 | 97.5 |
As for student discharge summaries, 109 out of 121 (90.1%) met all the criteria in order to be deemed satisfactory; 109 out of 121 (90.1%) of postdischarge phone call reports met both required components. Both these results exceeded the goal of 80% set before the course started.
DISCUSSION
The Emory Care Transitions Curriculum for fourth‐year medical students started in the 20092010 academic year with the main goal of teaching students transferable skills that would ultimately lead to their participating in safer hospital discharges in their future practice as physicians. At the end of this course, students exhibited greater confidence in managing the discharge process, improved overall fund of knowledge relating to care transitions, and a demonstration of appropriate skills related to preparing discharge summaries and communicating with patients at discharge. This was all executed with a delivery method that students found engaging.
Analyzing the results, it is noteworthy that confidence improved, while attitudes did not. Even though confidence in performing a task does not necessarily reflect one's ability to perform it, our students' confidence scores may serve as a proxy for their ability to manage tasks related to the discharge process, like managing medications and preparing discharge summaries. Thus, while some studies suggest that self‐assessment among physicians may not always relate well to competence,26, 27 in our study, students did demonstrate skills in discharge summary preparation and in identifying the most relevant aspects of patient communication at hospital discharge. As for the absence of attitude change, this may have partly been a function of the fact that students in our group started with attitude scores that were already quite high, with a mean pretest attitude score of 20.7 out of 25.
Changes in student confidence, attitudes, and knowledge from pre‐ to posttest did not vary significantly across the academic year. Thus, one could interpret from our findings that more experienced students who took the module close to graduation benefited similarly from the course to those who completed it earlier, at least according to those rubrics. Another possible source of variation in student experience was the hospital in which students rotated: the demographics of GMH, with its large uninsured population; AVAMC, with more elderly patients; and EUH, with a more affluent profile, are certainly different. However, the number of students rotating at EUH and AVAMC were comparatively too small to attempt to draw any conclusions about how rotation site affected student experiences.
The use of a blended approach that integrated face‐to‐face didactics, patient care, and online learning offered some advantages. Curricular goals were achieved through a course that required only 2 hours of in‐person faculty time with students. This is significant, considering the time demands that academic medical faculty usually face. This approach also permitted students who were participating in a busy clinical rotation, and had limited opportunities to meet as a group, the ability to do coursework at their own pace. Another strength of the study is that all students who participated in the rotation were able to complete their surveys.
As for limitations, it is worth noting that in a course with a blended curriculum, the online discussion had the lowest percentage of students rating it good or better. Part of the perceived difficulty may have resulted from the fact that there are no other courses in the Emory medical curriculum that utilize discussion boards or distance learning methods as teaching tools. Despite this generation of students' technological savvy, this new mode of discussion may have proven difficult to pick up when they were in the midst of a busy clinical rotation. This serves as a reminder that while online curricula have proven successful in this and other settings, each element needs to be tailored to the audience. One other factor to be considered while interpreting this study's results is that this study utilized some survey instruments that have not been previously validated, even though they were developed in consultation with experts in the field of care transitions education.
We used a dichotomous, criteria‐based system to rate students' discharge summaries and reports of postdischarge phone calls. While this quantitative approach allowed us to more objectively define the quality of students' work, it did offer some disadvantages. First, even though we based the rating system for discharge summaries on a BAAHM template, it was not subjected to more extensive validation. Moreover, the quantitative approach diverted us from finding themes and other qualitative data from students' write‐ups, which could potentially have given us a fuller picture of their work.
This study contributes to the small, but growing, literature on care transitions education. The studies by Bray‐Hall et al., Lai et al., and Ouchida et al.,1921 used different methodologies, but all were directed at third‐year students using curricula with classroom and clinical learning, and showed favorable outcomes in knowledge and skills. The present study also showed positive results in students' knowledge and skills, but targeted it toward graduating medical students and included a focus on concrete skills, such as discharge summary preparation. It also utilized a nontraditional delivery approach which reached its objectives while also limiting the demands on faculty and students' face time during busy clinical rotations, which is especially important when considering that students were dispersed at multiple sites.
It is likely that medical schools will implement more care transitions curricula in coming years, as organizations like the AAMC11 and the Institute of Medicine10 increase the pressure to train future doctors to better address the needs of older and chronically ill patients, who require care from professionals of multiple disciplines, in disparate care settings. Moreover, the 2010 Patient Protection and Affordable Care Act28 contains provisions with financial incentives for hospitals to decrease readmissions. Once these become widely implemented, there will be a greater impetus to train medical practitioners to discharge patients more safely. When that occurs, medical schools will have additional compelling reasons to offer courses that teach students skills to execute better care transitions. The hoped‐for outcome of these curricula will ultimately be safer and more effective patient care.
Acknowledgements
The authors thank Dr. Ted Johnson at the Emory University School of Medicine for editorial review. They also express their gratitude to Dr. Karin Ouchida at Montefiore Medical Center and Dr. William Lyons at University of Nebraska Medical Center for their technical support in preparation of the curriculum. Disclosures: None of the authors has any relevant conflicts of interest. All coauthors have seen and agree with the contents of this manuscript. The authors are responsible for the integrity of the data described in this study. The research in this manuscript has not been submitted or accepted for publication in another journal.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170(3):345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,.Adverse drug events in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.1999;33:1147–1153.
- ,,,,.The association between the quality of inpatient care and early readmission: A meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,.Hospital readmissions as a measure of quality of health care: Advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians.JAMA.2007;297:831–841.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington, DC:National Academy Press;2003.
- American Association of Medical Colleges. Geriatric Competencies for Medical Students: Recommendations of the July 2007 Geriatrics Consensus Conference.2008. Available at: http://www.aamc.org/newsroom/press kits/competencies.pdf. Accessed February 25, 2009.
- Eskildsen M, Price T, Tenover JL. Computer‐based geriatrics workbooks for resident teaching. MedEdPortal. 2007. Available at http://services. aamc.org/30/mededportal/servlet/s/segment/mededportal/ find_resources/ browse/?subid=640.
- .Care transitions in the older adult.MedEdPortal.2008. Available at http://services.aamc.org/30/mededportal/servlet/s/segment/mededportal/find_resources/browse/?subid=678. Accessed March 19, 2011.
- ,,, et al.Web‐based module to train and assess competency in systems‐based practice.Portal of Online Geriatric Education.2009. Product ID #20002. Available at: http://www. pogoe.org/node/407. Accessed March 19, 2011.
- .CHAMP (Curriculum for the Hospitalized Aging Medical Patient): The ideal hospital discharge.Portal of Online Geriatric Education.2009. Product ID #18995. Available at: http://www. pogoe.org/productid/18995. Accessed March 19, 2011.
- .M1 care transitions.Portal of Online Geriatric Education.2009. Product ID #20450. Available at: http://www. pogoe.org/node/660. Accessed March 19, 2011.
- .Transitional care.Portal of Online Geriatric Education.2007. Product ID #18991. Available at: http://www.pogoe.org/node/262. Accessed March 19, 2011.
- .Discharge summary feedback.Portal of Online Geriatric Education.2009. Product ID #20546. Available at: http://www.pogoe.org/node/788. Accessed March 19, 2011.
- ,,.Toward safe hospital discharge: A transitions in care curriculum for medical students.J Gen Intern Med.25(8):878–881.
- ,,,,.Postdischarge follow‐up visits for medical and pharmacy students on an inpatient medicine clerkship.J Hosp Med.2008;3(1):20–27.
- ,,,,.Fast forward rounds: An effective method for teaching medical students to transition patients safely across care settings.J Am Geriatr Soc.2009;57:910–917.
- .Fourth‐year medical student care transitions curriculum (free login required).Portal of Online Geriatric Education.2010. Available at: http://www.pogoe.org/node/867. Accessed July 16, 2010.
- .Falling through the cracks: Challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- Society of Hospital Medicine. Quality initiatives for patient care—BOOSTing care transitions resource room.2010. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_Care Transitions/html_CC/12Clinical Tools/01_Toolkits.cfm. Accessed November 16, 2010.
- Society of Hospital Medicine. Ideal discharge for an elderly patient: A hospitalist checklist.2005. Available at: http://www.hospitalmedicine. org/AM/Template.cfm?Section=QI_Clinical_Tools8(2):105–110.
- ,,.Difficulties in recognizing one's own incompetence: Novice physicians who are unskilled and unaware of it.Acad Med.2001;76(10 suppl):S87–S89.
- Patient Protection and Affordable Care Act, H.R. 3590.ENR, 111th Congress, 2010.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170(3):345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,.Adverse drug events in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.1999;33:1147–1153.
- ,,,,.The association between the quality of inpatient care and early readmission: A meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,.Hospital readmissions as a measure of quality of health care: Advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians.JAMA.2007;297:831–841.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington, DC:National Academy Press;2003.
- American Association of Medical Colleges. Geriatric Competencies for Medical Students: Recommendations of the July 2007 Geriatrics Consensus Conference.2008. Available at: http://www.aamc.org/newsroom/press kits/competencies.pdf. Accessed February 25, 2009.
- Eskildsen M, Price T, Tenover JL. Computer‐based geriatrics workbooks for resident teaching. MedEdPortal. 2007. Available at http://services. aamc.org/30/mededportal/servlet/s/segment/mededportal/ find_resources/ browse/?subid=640.
- .Care transitions in the older adult.MedEdPortal.2008. Available at http://services.aamc.org/30/mededportal/servlet/s/segment/mededportal/find_resources/browse/?subid=678. Accessed March 19, 2011.
- ,,, et al.Web‐based module to train and assess competency in systems‐based practice.Portal of Online Geriatric Education.2009. Product ID #20002. Available at: http://www. pogoe.org/node/407. Accessed March 19, 2011.
- .CHAMP (Curriculum for the Hospitalized Aging Medical Patient): The ideal hospital discharge.Portal of Online Geriatric Education.2009. Product ID #18995. Available at: http://www. pogoe.org/productid/18995. Accessed March 19, 2011.
- .M1 care transitions.Portal of Online Geriatric Education.2009. Product ID #20450. Available at: http://www. pogoe.org/node/660. Accessed March 19, 2011.
- .Transitional care.Portal of Online Geriatric Education.2007. Product ID #18991. Available at: http://www.pogoe.org/node/262. Accessed March 19, 2011.
- .Discharge summary feedback.Portal of Online Geriatric Education.2009. Product ID #20546. Available at: http://www.pogoe.org/node/788. Accessed March 19, 2011.
- ,,.Toward safe hospital discharge: A transitions in care curriculum for medical students.J Gen Intern Med.25(8):878–881.
- ,,,,.Postdischarge follow‐up visits for medical and pharmacy students on an inpatient medicine clerkship.J Hosp Med.2008;3(1):20–27.
- ,,,,.Fast forward rounds: An effective method for teaching medical students to transition patients safely across care settings.J Am Geriatr Soc.2009;57:910–917.
- .Fourth‐year medical student care transitions curriculum (free login required).Portal of Online Geriatric Education.2010. Available at: http://www.pogoe.org/node/867. Accessed July 16, 2010.
- .Falling through the cracks: Challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- Society of Hospital Medicine. Quality initiatives for patient care—BOOSTing care transitions resource room.2010. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_Care Transitions/html_CC/12Clinical Tools/01_Toolkits.cfm. Accessed November 16, 2010.
- Society of Hospital Medicine. Ideal discharge for an elderly patient: A hospitalist checklist.2005. Available at: http://www.hospitalmedicine. org/AM/Template.cfm?Section=QI_Clinical_Tools8(2):105–110.
- ,,.Difficulties in recognizing one's own incompetence: Novice physicians who are unskilled and unaware of it.Acad Med.2001;76(10 suppl):S87–S89.
- Patient Protection and Affordable Care Act, H.R. 3590.ENR, 111th Congress, 2010.
Copyright © 2011 Society of Hospital Medicine
ONLINE EXCLUSIVE: Listen to hospitalists Greg Misky and Tosha Wetterneck discuss career satisfaction
Click here to listen to Dr. Misky.
Click here to listen to Dr. Wetterneck.
Click here to listen to Dr. Misky.
Click here to listen to Dr. Wetterneck.
Click here to listen to Dr. Misky.
Click here to listen to Dr. Wetterneck.
Pediatric HM Literature
Clinical question: What is the incidence of kernicterus over the past few decades?
Background: Recent guidelines for hyperbilirubinemia have recommended a systematic approach to management in order to prevent the occurrence of severe hyperbilirubinemia and, potentially, kernicterus. There has been concern that rates of kernicterus might have increased in the 1990s, when a “kindler, gentler” approach to hyperbilirubinemia was advocated by earlier guidelines.
Study design: Retrospective observational study.
Setting: California registry of developmental services enrollees.
Synopsis: Of 64,346 children born from 1988 to 1997 who received services from the California Department of Developmental Services (DDS) from 1988 to 2002, 25 met a strict definition of kernicterus. The time trend of incidence remained stable during the study years at 0.44 (95% confidence interval [CI]: 0.28-0.65) per 100,000 live births. There were no significant differences in rates before and after 1994.
Data from a national database of death certificates revealed a similar stable trend in deaths attributed to kernicterus.
A primary limitation of this study is the lack of clarity surrounding enrollment in California’s DDS by children with kernicterus. Although all children with developmental disabilities are eligible, the exact enrollment rate likely is unknown. However, this is one of the first studies to put a denominator on kernicterus in this country.
Updated guidelines on the management of hyperbilirubinemia in 2004 advocated a safer, more systematic approach to management, in part because of concerns that there had been a resurgence of kernicterus. This now seems less likely and this article adds to a body of literature that raises questions about whether a large population of patients with hyperbilirubinemia who are at extremely low risk for kernicterus are being overtreated.
Bottom line: Kernicterus rates remained unchanged in the 1990s.
Citation: Brooks JC, Fisher-Owens SA, Wu YW, Strauss DJ, Newman TB. Evidence suggests there was not a “resurgence” of kernicterus in the 1990s. Pediatrics. 2011;127:672-679.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the incidence of kernicterus over the past few decades?
Background: Recent guidelines for hyperbilirubinemia have recommended a systematic approach to management in order to prevent the occurrence of severe hyperbilirubinemia and, potentially, kernicterus. There has been concern that rates of kernicterus might have increased in the 1990s, when a “kindler, gentler” approach to hyperbilirubinemia was advocated by earlier guidelines.
Study design: Retrospective observational study.
Setting: California registry of developmental services enrollees.
Synopsis: Of 64,346 children born from 1988 to 1997 who received services from the California Department of Developmental Services (DDS) from 1988 to 2002, 25 met a strict definition of kernicterus. The time trend of incidence remained stable during the study years at 0.44 (95% confidence interval [CI]: 0.28-0.65) per 100,000 live births. There were no significant differences in rates before and after 1994.
Data from a national database of death certificates revealed a similar stable trend in deaths attributed to kernicterus.
A primary limitation of this study is the lack of clarity surrounding enrollment in California’s DDS by children with kernicterus. Although all children with developmental disabilities are eligible, the exact enrollment rate likely is unknown. However, this is one of the first studies to put a denominator on kernicterus in this country.
Updated guidelines on the management of hyperbilirubinemia in 2004 advocated a safer, more systematic approach to management, in part because of concerns that there had been a resurgence of kernicterus. This now seems less likely and this article adds to a body of literature that raises questions about whether a large population of patients with hyperbilirubinemia who are at extremely low risk for kernicterus are being overtreated.
Bottom line: Kernicterus rates remained unchanged in the 1990s.
Citation: Brooks JC, Fisher-Owens SA, Wu YW, Strauss DJ, Newman TB. Evidence suggests there was not a “resurgence” of kernicterus in the 1990s. Pediatrics. 2011;127:672-679.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the incidence of kernicterus over the past few decades?
Background: Recent guidelines for hyperbilirubinemia have recommended a systematic approach to management in order to prevent the occurrence of severe hyperbilirubinemia and, potentially, kernicterus. There has been concern that rates of kernicterus might have increased in the 1990s, when a “kindler, gentler” approach to hyperbilirubinemia was advocated by earlier guidelines.
Study design: Retrospective observational study.
Setting: California registry of developmental services enrollees.
Synopsis: Of 64,346 children born from 1988 to 1997 who received services from the California Department of Developmental Services (DDS) from 1988 to 2002, 25 met a strict definition of kernicterus. The time trend of incidence remained stable during the study years at 0.44 (95% confidence interval [CI]: 0.28-0.65) per 100,000 live births. There were no significant differences in rates before and after 1994.
Data from a national database of death certificates revealed a similar stable trend in deaths attributed to kernicterus.
A primary limitation of this study is the lack of clarity surrounding enrollment in California’s DDS by children with kernicterus. Although all children with developmental disabilities are eligible, the exact enrollment rate likely is unknown. However, this is one of the first studies to put a denominator on kernicterus in this country.
Updated guidelines on the management of hyperbilirubinemia in 2004 advocated a safer, more systematic approach to management, in part because of concerns that there had been a resurgence of kernicterus. This now seems less likely and this article adds to a body of literature that raises questions about whether a large population of patients with hyperbilirubinemia who are at extremely low risk for kernicterus are being overtreated.
Bottom line: Kernicterus rates remained unchanged in the 1990s.
Citation: Brooks JC, Fisher-Owens SA, Wu YW, Strauss DJ, Newman TB. Evidence suggests there was not a “resurgence” of kernicterus in the 1990s. Pediatrics. 2011;127:672-679.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
The Earlier, the Better
Every morning at 8 a.m., a multidisciplinary team at Wayne Memorial Hospital in Honesdale, Pa., a rural pocket of about 5,000 people about 30 miles northeast of Scranton, gathers to discuss discharge planning. Representatives from social services, home health, nursing, physical therapy, pharmacy, and the HM group attend the meeting. Each stakeholder weighs in, listens to others, and voices concerns when applicable.
“We go through each patient in the morning, briefly, and go through the plan so that when there’s a discharge coming, everybody is on the same page and can try to get everything organized,” says Louis O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine, the hospitalist program contracted by Wayne Memorial, which has 98 acute-care beds. “The hospital has reminded us to be cognizant of getting that early discharge, and it’s become almost so rote now that we don’t even have to worry about it. It’s just a thing we do.”
Better bed management is a new mantra for hospitalists nationwide, because fewer open beds means fewer dollars for both the physician and the hospital. Better bed management also means improved patient satisfaction scores, as most patients would rather be at home (and those scores in the coming years will factor into Medicare reimbursement). And better bed management means reduced backlogs across the hospital, particularly “boarders” in the ED.
“The pressure really is on the hospital for a number of reasons,” says Ken Simone, DO, SFHM, president of Hospitalist and Practice Solutions in Veazie, Maine, and a member of Team Hospitalist. “In terms of reimbursement, the sooner they can get a patient out of the hospital, it opens bed space for patients in the emergency department. It eases up bottlenecks because the patient in the ED may not need the bed that is being opened, but they may need an ICU bed, and the ICU patient is stable enough to be transferred to that medical bed that you’re opening up. So it’s a domino effect, and it certainly helps with creating a better flow within the hospital.”
It sounds simple, of course: Discharge inpatients early in the day and fill that bed with another patient, akin to a busy restaurant flipping tables to reduce the line stretching out the front door. The more customers, the more money made—both for the restaurant (i.e. hospital) and the servers (i.e. providers). And the less potential customers wait, the happier they are with their service.
But adding new beds, at nearly $1 million per bed inclusive of the space, infrastructure, and technology, is unacceptable math for most U.S. hospitals struggling to make ends meet in a tough economy.1 By contrast, an aggressive bed-management approach creates virtual bed capacity that creates more revenue-generating opportunities without those costs. And as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys tie patient satisfaction more directly to compensation, the more attention that will be focused on the discharge, as it will be the last process the patient experiences, and the one they likely will remember the most.
So if everyone agrees that discharging inpatients earlier in the day is a good idea, what’s the holdup? Interviews with more than a half-dozen hospitalists show a handful of factors that are present in all hospitals, large and small, academic and community:
- Downstream complications. HM is only one piece of the discharge puzzle. Hospitalists might be ready to discharge, but without that last test, or the ability to reach a pharmacist, the process slows. Even when discharge is complete, the room needs to be cleared and cleaned.
- Rounding protocols. Hospital-ists intuitively round on the sickest patients first, but that time-honored tradition has the byproduct of pushing those patients most likely to be sent home to the end of the line, automatically delaying discharges.
- Shift flexibility. Many hospitalist groups have morning shifts that begin at 8 a.m. Given the time it takes to craft discharge orders and deal with inevitable wrinkles in the process, that almost guarantees discharges will be pushed to later in the day.
- Hospital infrastructure. Insti-tutional bed management begins at the top, with a commitment across departments that discharge procedures are a shared priority. Without such across-the-board buy-in, the best hospitalists can do is fight against the tide. For example, a room could be vacated at 10 a.m., but housekeeping isn’t notified (or prepared) to clean the room for two hours because there is no institutional procedure in place to govern that decision.
“It’s harder than you think,” Dr. O’Boyle admits. “There are always extraneous factors that can delay the hospitalists from getting [discharges] done.”
Continued below...
No Consensus
Although a variety of techniques can help improve early day discharge, all have hurdles. Two of the most common suggestions are geographic rounding and discharge lounges. A third is the active bed-management (ABM) model that hospitalist Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center’s HM division, wrote about in the Annals of Internal Medicine in December 2008.2
Geographic rounding, also dubbed unit-based setups, can help improve bed management because all participants are co-located; however, the gains likely are not enough to motivate an institution to implement the model without demonstrated improvements to other systems as well, says John Nelson, MD, FACP, MHM, cofounder and past president of SHM and a principal in the practice management firm Nelson Flores Hospital Medicine Consultants.
Discharge lounges—areas usually administered by a nurse and billed as a place for patients to gather after they’ve been formally discharged but before they have arranged a ride to physically leave the hospital—have been adopted by many hospitals. Dr. Simone and others question the liability issues associated with keeping discharged patients under the watch of hospital staff and also wonder whether the setup can have a negative impact on patient satisfaction. (For more on discharge lounges, check out “Solution of Problem,” at www.the-hospitalist.org.)

—David Bachman, MD, senior medical director for transitions of care, MaineHealth Clinical Integration, Portland, Maine
David Bachman, MD, senior medical director for transitions of care at MaineHealth Clinical Integration in Portland, Maine, and a former hospital administrator in New England, sees hospitalists as a lynchpin to the discharge process, but he also urges them to get the hospital to see them as “change agents” who need institutional support to make significant improvements.
“You’re trying to run cases through and it’s all dependent on downstream activity,” Dr. Bachman says. “If the hospitalist can push back and get this recognized as a hospital issue, that’s the only time when this problem can be solved. Hospitalists are a piece of the puzzle, but it’s not just them.”
Ideas to Chew On
Mitchell Wilson, MD, SFHM, chief medical officer for Eagle Hospital Physicians in Atlanta, agrees that reprioritizing physician rounds to encourage discharges would push patients out earlier, but he wants to see more physician assistants and nurse practitioners (PAs and NPs) blended into those rounds. The partnership would be a relatively simple and direct way for physicians to pass off nonclinical or less-intensive duties that afford them more time to focus on discharge planning. A dedicated nurse for HM service and the use of telemedicine could be folded into HM practices to help.
Each of the techniques would serve to get patients out earlier on what is arguably the most costly day of their stay. “Hospitals generally lose money on the last day of a patient’s stay,” Dr. Wilson says. “When appropriate from a patient care standpoint, discharging your patient and getting the bed ready for the next patient sooner is definitely an advantage for the hospital, and for the next patient.”
Dr. Bachman says one of the main hurdles to that process is no single provider “has clear responsibility and oversight. … It’s this diffuse responsibility.” That’s where Dr. Howell and colleagues thought ABM would work well. At Hopkins Bayview, hospitalists staffed an active bed-management program that rounded twice daily in ICUs and visited the ED regularly. The hospitalist on the 12-hour shift had no other duties, a luxury that HM pioneer Robert Wachter, MD, MHM, described at the time as “freeing him or her up to act as a full-time air traffic controller for all medical patients.”
The intervention reduced ED throughput for admitted patients by 98 minutes, to 360 minutes from 458 minutes. It also cut the amount of time the ED diverted ambulances because of overcrowding—the so-called “yellow alert”—by 6%, and the amount of time ambulances were diverted due to a lack of ICU beds—“red alert”—by 27%. Dr. Howell, an SHM board member, says the results showed how hospitalists can lead throughput change through institutions but that more work needs to be done to focus on early-day discharge.
“The hospital medicine side may be incentivized for early discharges,” he says, “but the hospital systems may not.”
Dr. Howell pushes for “2-by-10,” shorthand for identifying two patients daily who could be discharged by 10 a.m. because “the ED doesn’t necessarily need more beds for 24 hours. They need more beds early in the day.” But in keeping with the ABM model, Dr. Howell believes fiscal and personnel resources have to be dedicated to the problem to expect results. In the Hopkins Bayview intervention, Dr. Wachter, professor and associate chairman of the Department of Medicine at the University of California at San Francisco, chief of the division of hospital medicine, and chief of the medical service at UCSF Medical Center, estimated the annual costs of ABM at close to $1 million a year, given the likely need for four to six full-time equivalent hospitalists, according to a post on his Wachter’s World blog (www.wachtersworld. com) after the report was published.
One idea Dr. Howell suggests to push earlier discharges is restructuring physician workweeks, setting aside certain days for admission and certain days for follow-ups. If two shifts of follow-up days are scheduled after two days of admissions, it’s likely a hospitalist could follow a patient through their entire stay, he says. “You have to structure the doctor’s day to focus on discharges first,” he adds.
Dr. Howell also believes multidisciplinary rounds are key to earlier discharges. At Wayne Memorial Hospital and other places that have instituted such teams, discharge usually is just one byproduct of a construct ultimately aimed at quality improvement. Wayne Memorial’s Dr. O’Boyle says that since the team approach was initiated in September 2009, the hospital’s LOS has dropped by 0.75 days and patient satisfaction scores have risen about 25%. Those metrics will be key data points in the years to come as discharges and readmissions become tied to reimbursement via healthcare reform (see “Value-Based Purchasing Raises the Stakes,” May 2011).
“One of the biggest factors for readmissions are things like pharmacy errors, and lack of follow-up, and other loose ends that, if you’re in too much of a hurry to get people out and you don’t have the whole team approach and make sure all your I’s are dotted and T’s are crossed, then they have an increased chance of coming back,” Dr. O’Boyle says. “So we focus on patient satisfaction, and we focus on the discharge day and the discharge time to prevent readmissions and to maximize patient satisfaction. That’s the bottom line for the hospital…It’s interesting how the bottom line seems to follow quality.”
Continued below...
Inherent Conflicts?
Early-day discharge actually can be a bad thing in some cases, Dr. Nelson says. Think of a case in which a patient might be ready for discharge in the late evening or during an overnight. To wait until the morning to send that patient home might not be the best approach.

—Louis O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, Honesdale, Pa.
“The place that manages length of stay most efficiently probably has plenty of late-day discharge,” he says.
Another potential conflict getting in the way of early-day discharge is what Dr. Wilson calls “admission competition.” For example, a hospitalist is working on discharge papers early in the morning but is then called away for a consult on an acute-care case in the ED or elsewhere. Each of the duties is important, but conflicting duties leave the hospitalist having to make choices.
“It’s not all straightforward,” Dr. Nelson says.
Emergency Nurses Association President AnnMarie Papa, DNP, RN, CEN, NE-BC, FAEN, says that collaboration between nurses and physicians is an answer to such competition. Calling the problem a “wrinkle across the system,” Papa says that without hospital administrators taking point and declaring the issue of discharge a priority, little wholesale improvement will be made. Even then, physicians and nurses—as the two main groups interacting with the patient—have to work together, she adds.
“Hospitalists have to partner with nurses,” Papa says, imploring physicians and nurses to work together on discharge decisions. “If the physicians and nurses collaborate on the decision and plans of care for the patients and the care they’re giving them and the discharge instructions, then it’s a win-win for everybody.”
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Litvak E, Bisognano M. More patients, less payment: increasing hospital efficiency in the aftermath of health reform. Health Affairs. 2011;30(1): 76-80.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Int Med. 2008;149(11):804-810.
Every morning at 8 a.m., a multidisciplinary team at Wayne Memorial Hospital in Honesdale, Pa., a rural pocket of about 5,000 people about 30 miles northeast of Scranton, gathers to discuss discharge planning. Representatives from social services, home health, nursing, physical therapy, pharmacy, and the HM group attend the meeting. Each stakeholder weighs in, listens to others, and voices concerns when applicable.
“We go through each patient in the morning, briefly, and go through the plan so that when there’s a discharge coming, everybody is on the same page and can try to get everything organized,” says Louis O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine, the hospitalist program contracted by Wayne Memorial, which has 98 acute-care beds. “The hospital has reminded us to be cognizant of getting that early discharge, and it’s become almost so rote now that we don’t even have to worry about it. It’s just a thing we do.”
Better bed management is a new mantra for hospitalists nationwide, because fewer open beds means fewer dollars for both the physician and the hospital. Better bed management also means improved patient satisfaction scores, as most patients would rather be at home (and those scores in the coming years will factor into Medicare reimbursement). And better bed management means reduced backlogs across the hospital, particularly “boarders” in the ED.
“The pressure really is on the hospital for a number of reasons,” says Ken Simone, DO, SFHM, president of Hospitalist and Practice Solutions in Veazie, Maine, and a member of Team Hospitalist. “In terms of reimbursement, the sooner they can get a patient out of the hospital, it opens bed space for patients in the emergency department. It eases up bottlenecks because the patient in the ED may not need the bed that is being opened, but they may need an ICU bed, and the ICU patient is stable enough to be transferred to that medical bed that you’re opening up. So it’s a domino effect, and it certainly helps with creating a better flow within the hospital.”
It sounds simple, of course: Discharge inpatients early in the day and fill that bed with another patient, akin to a busy restaurant flipping tables to reduce the line stretching out the front door. The more customers, the more money made—both for the restaurant (i.e. hospital) and the servers (i.e. providers). And the less potential customers wait, the happier they are with their service.
But adding new beds, at nearly $1 million per bed inclusive of the space, infrastructure, and technology, is unacceptable math for most U.S. hospitals struggling to make ends meet in a tough economy.1 By contrast, an aggressive bed-management approach creates virtual bed capacity that creates more revenue-generating opportunities without those costs. And as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys tie patient satisfaction more directly to compensation, the more attention that will be focused on the discharge, as it will be the last process the patient experiences, and the one they likely will remember the most.
So if everyone agrees that discharging inpatients earlier in the day is a good idea, what’s the holdup? Interviews with more than a half-dozen hospitalists show a handful of factors that are present in all hospitals, large and small, academic and community:
- Downstream complications. HM is only one piece of the discharge puzzle. Hospitalists might be ready to discharge, but without that last test, or the ability to reach a pharmacist, the process slows. Even when discharge is complete, the room needs to be cleared and cleaned.
- Rounding protocols. Hospital-ists intuitively round on the sickest patients first, but that time-honored tradition has the byproduct of pushing those patients most likely to be sent home to the end of the line, automatically delaying discharges.
- Shift flexibility. Many hospitalist groups have morning shifts that begin at 8 a.m. Given the time it takes to craft discharge orders and deal with inevitable wrinkles in the process, that almost guarantees discharges will be pushed to later in the day.
- Hospital infrastructure. Insti-tutional bed management begins at the top, with a commitment across departments that discharge procedures are a shared priority. Without such across-the-board buy-in, the best hospitalists can do is fight against the tide. For example, a room could be vacated at 10 a.m., but housekeeping isn’t notified (or prepared) to clean the room for two hours because there is no institutional procedure in place to govern that decision.
“It’s harder than you think,” Dr. O’Boyle admits. “There are always extraneous factors that can delay the hospitalists from getting [discharges] done.”
Continued below...
No Consensus
Although a variety of techniques can help improve early day discharge, all have hurdles. Two of the most common suggestions are geographic rounding and discharge lounges. A third is the active bed-management (ABM) model that hospitalist Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center’s HM division, wrote about in the Annals of Internal Medicine in December 2008.2
Geographic rounding, also dubbed unit-based setups, can help improve bed management because all participants are co-located; however, the gains likely are not enough to motivate an institution to implement the model without demonstrated improvements to other systems as well, says John Nelson, MD, FACP, MHM, cofounder and past president of SHM and a principal in the practice management firm Nelson Flores Hospital Medicine Consultants.
Discharge lounges—areas usually administered by a nurse and billed as a place for patients to gather after they’ve been formally discharged but before they have arranged a ride to physically leave the hospital—have been adopted by many hospitals. Dr. Simone and others question the liability issues associated with keeping discharged patients under the watch of hospital staff and also wonder whether the setup can have a negative impact on patient satisfaction. (For more on discharge lounges, check out “Solution of Problem,” at www.the-hospitalist.org.)

—David Bachman, MD, senior medical director for transitions of care, MaineHealth Clinical Integration, Portland, Maine
David Bachman, MD, senior medical director for transitions of care at MaineHealth Clinical Integration in Portland, Maine, and a former hospital administrator in New England, sees hospitalists as a lynchpin to the discharge process, but he also urges them to get the hospital to see them as “change agents” who need institutional support to make significant improvements.
“You’re trying to run cases through and it’s all dependent on downstream activity,” Dr. Bachman says. “If the hospitalist can push back and get this recognized as a hospital issue, that’s the only time when this problem can be solved. Hospitalists are a piece of the puzzle, but it’s not just them.”
Ideas to Chew On
Mitchell Wilson, MD, SFHM, chief medical officer for Eagle Hospital Physicians in Atlanta, agrees that reprioritizing physician rounds to encourage discharges would push patients out earlier, but he wants to see more physician assistants and nurse practitioners (PAs and NPs) blended into those rounds. The partnership would be a relatively simple and direct way for physicians to pass off nonclinical or less-intensive duties that afford them more time to focus on discharge planning. A dedicated nurse for HM service and the use of telemedicine could be folded into HM practices to help.
Each of the techniques would serve to get patients out earlier on what is arguably the most costly day of their stay. “Hospitals generally lose money on the last day of a patient’s stay,” Dr. Wilson says. “When appropriate from a patient care standpoint, discharging your patient and getting the bed ready for the next patient sooner is definitely an advantage for the hospital, and for the next patient.”
Dr. Bachman says one of the main hurdles to that process is no single provider “has clear responsibility and oversight. … It’s this diffuse responsibility.” That’s where Dr. Howell and colleagues thought ABM would work well. At Hopkins Bayview, hospitalists staffed an active bed-management program that rounded twice daily in ICUs and visited the ED regularly. The hospitalist on the 12-hour shift had no other duties, a luxury that HM pioneer Robert Wachter, MD, MHM, described at the time as “freeing him or her up to act as a full-time air traffic controller for all medical patients.”
The intervention reduced ED throughput for admitted patients by 98 minutes, to 360 minutes from 458 minutes. It also cut the amount of time the ED diverted ambulances because of overcrowding—the so-called “yellow alert”—by 6%, and the amount of time ambulances were diverted due to a lack of ICU beds—“red alert”—by 27%. Dr. Howell, an SHM board member, says the results showed how hospitalists can lead throughput change through institutions but that more work needs to be done to focus on early-day discharge.
“The hospital medicine side may be incentivized for early discharges,” he says, “but the hospital systems may not.”
Dr. Howell pushes for “2-by-10,” shorthand for identifying two patients daily who could be discharged by 10 a.m. because “the ED doesn’t necessarily need more beds for 24 hours. They need more beds early in the day.” But in keeping with the ABM model, Dr. Howell believes fiscal and personnel resources have to be dedicated to the problem to expect results. In the Hopkins Bayview intervention, Dr. Wachter, professor and associate chairman of the Department of Medicine at the University of California at San Francisco, chief of the division of hospital medicine, and chief of the medical service at UCSF Medical Center, estimated the annual costs of ABM at close to $1 million a year, given the likely need for four to six full-time equivalent hospitalists, according to a post on his Wachter’s World blog (www.wachtersworld. com) after the report was published.
One idea Dr. Howell suggests to push earlier discharges is restructuring physician workweeks, setting aside certain days for admission and certain days for follow-ups. If two shifts of follow-up days are scheduled after two days of admissions, it’s likely a hospitalist could follow a patient through their entire stay, he says. “You have to structure the doctor’s day to focus on discharges first,” he adds.
Dr. Howell also believes multidisciplinary rounds are key to earlier discharges. At Wayne Memorial Hospital and other places that have instituted such teams, discharge usually is just one byproduct of a construct ultimately aimed at quality improvement. Wayne Memorial’s Dr. O’Boyle says that since the team approach was initiated in September 2009, the hospital’s LOS has dropped by 0.75 days and patient satisfaction scores have risen about 25%. Those metrics will be key data points in the years to come as discharges and readmissions become tied to reimbursement via healthcare reform (see “Value-Based Purchasing Raises the Stakes,” May 2011).
“One of the biggest factors for readmissions are things like pharmacy errors, and lack of follow-up, and other loose ends that, if you’re in too much of a hurry to get people out and you don’t have the whole team approach and make sure all your I’s are dotted and T’s are crossed, then they have an increased chance of coming back,” Dr. O’Boyle says. “So we focus on patient satisfaction, and we focus on the discharge day and the discharge time to prevent readmissions and to maximize patient satisfaction. That’s the bottom line for the hospital…It’s interesting how the bottom line seems to follow quality.”
Continued below...
Inherent Conflicts?
Early-day discharge actually can be a bad thing in some cases, Dr. Nelson says. Think of a case in which a patient might be ready for discharge in the late evening or during an overnight. To wait until the morning to send that patient home might not be the best approach.

—Louis O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, Honesdale, Pa.
“The place that manages length of stay most efficiently probably has plenty of late-day discharge,” he says.
Another potential conflict getting in the way of early-day discharge is what Dr. Wilson calls “admission competition.” For example, a hospitalist is working on discharge papers early in the morning but is then called away for a consult on an acute-care case in the ED or elsewhere. Each of the duties is important, but conflicting duties leave the hospitalist having to make choices.
“It’s not all straightforward,” Dr. Nelson says.
Emergency Nurses Association President AnnMarie Papa, DNP, RN, CEN, NE-BC, FAEN, says that collaboration between nurses and physicians is an answer to such competition. Calling the problem a “wrinkle across the system,” Papa says that without hospital administrators taking point and declaring the issue of discharge a priority, little wholesale improvement will be made. Even then, physicians and nurses—as the two main groups interacting with the patient—have to work together, she adds.
“Hospitalists have to partner with nurses,” Papa says, imploring physicians and nurses to work together on discharge decisions. “If the physicians and nurses collaborate on the decision and plans of care for the patients and the care they’re giving them and the discharge instructions, then it’s a win-win for everybody.”
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Litvak E, Bisognano M. More patients, less payment: increasing hospital efficiency in the aftermath of health reform. Health Affairs. 2011;30(1): 76-80.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Int Med. 2008;149(11):804-810.
Every morning at 8 a.m., a multidisciplinary team at Wayne Memorial Hospital in Honesdale, Pa., a rural pocket of about 5,000 people about 30 miles northeast of Scranton, gathers to discuss discharge planning. Representatives from social services, home health, nursing, physical therapy, pharmacy, and the HM group attend the meeting. Each stakeholder weighs in, listens to others, and voices concerns when applicable.
“We go through each patient in the morning, briefly, and go through the plan so that when there’s a discharge coming, everybody is on the same page and can try to get everything organized,” says Louis O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine, the hospitalist program contracted by Wayne Memorial, which has 98 acute-care beds. “The hospital has reminded us to be cognizant of getting that early discharge, and it’s become almost so rote now that we don’t even have to worry about it. It’s just a thing we do.”
Better bed management is a new mantra for hospitalists nationwide, because fewer open beds means fewer dollars for both the physician and the hospital. Better bed management also means improved patient satisfaction scores, as most patients would rather be at home (and those scores in the coming years will factor into Medicare reimbursement). And better bed management means reduced backlogs across the hospital, particularly “boarders” in the ED.
“The pressure really is on the hospital for a number of reasons,” says Ken Simone, DO, SFHM, president of Hospitalist and Practice Solutions in Veazie, Maine, and a member of Team Hospitalist. “In terms of reimbursement, the sooner they can get a patient out of the hospital, it opens bed space for patients in the emergency department. It eases up bottlenecks because the patient in the ED may not need the bed that is being opened, but they may need an ICU bed, and the ICU patient is stable enough to be transferred to that medical bed that you’re opening up. So it’s a domino effect, and it certainly helps with creating a better flow within the hospital.”
It sounds simple, of course: Discharge inpatients early in the day and fill that bed with another patient, akin to a busy restaurant flipping tables to reduce the line stretching out the front door. The more customers, the more money made—both for the restaurant (i.e. hospital) and the servers (i.e. providers). And the less potential customers wait, the happier they are with their service.
But adding new beds, at nearly $1 million per bed inclusive of the space, infrastructure, and technology, is unacceptable math for most U.S. hospitals struggling to make ends meet in a tough economy.1 By contrast, an aggressive bed-management approach creates virtual bed capacity that creates more revenue-generating opportunities without those costs. And as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys tie patient satisfaction more directly to compensation, the more attention that will be focused on the discharge, as it will be the last process the patient experiences, and the one they likely will remember the most.
So if everyone agrees that discharging inpatients earlier in the day is a good idea, what’s the holdup? Interviews with more than a half-dozen hospitalists show a handful of factors that are present in all hospitals, large and small, academic and community:
- Downstream complications. HM is only one piece of the discharge puzzle. Hospitalists might be ready to discharge, but without that last test, or the ability to reach a pharmacist, the process slows. Even when discharge is complete, the room needs to be cleared and cleaned.
- Rounding protocols. Hospital-ists intuitively round on the sickest patients first, but that time-honored tradition has the byproduct of pushing those patients most likely to be sent home to the end of the line, automatically delaying discharges.
- Shift flexibility. Many hospitalist groups have morning shifts that begin at 8 a.m. Given the time it takes to craft discharge orders and deal with inevitable wrinkles in the process, that almost guarantees discharges will be pushed to later in the day.
- Hospital infrastructure. Insti-tutional bed management begins at the top, with a commitment across departments that discharge procedures are a shared priority. Without such across-the-board buy-in, the best hospitalists can do is fight against the tide. For example, a room could be vacated at 10 a.m., but housekeeping isn’t notified (or prepared) to clean the room for two hours because there is no institutional procedure in place to govern that decision.
“It’s harder than you think,” Dr. O’Boyle admits. “There are always extraneous factors that can delay the hospitalists from getting [discharges] done.”
Continued below...
No Consensus
Although a variety of techniques can help improve early day discharge, all have hurdles. Two of the most common suggestions are geographic rounding and discharge lounges. A third is the active bed-management (ABM) model that hospitalist Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center’s HM division, wrote about in the Annals of Internal Medicine in December 2008.2
Geographic rounding, also dubbed unit-based setups, can help improve bed management because all participants are co-located; however, the gains likely are not enough to motivate an institution to implement the model without demonstrated improvements to other systems as well, says John Nelson, MD, FACP, MHM, cofounder and past president of SHM and a principal in the practice management firm Nelson Flores Hospital Medicine Consultants.
Discharge lounges—areas usually administered by a nurse and billed as a place for patients to gather after they’ve been formally discharged but before they have arranged a ride to physically leave the hospital—have been adopted by many hospitals. Dr. Simone and others question the liability issues associated with keeping discharged patients under the watch of hospital staff and also wonder whether the setup can have a negative impact on patient satisfaction. (For more on discharge lounges, check out “Solution of Problem,” at www.the-hospitalist.org.)

—David Bachman, MD, senior medical director for transitions of care, MaineHealth Clinical Integration, Portland, Maine
David Bachman, MD, senior medical director for transitions of care at MaineHealth Clinical Integration in Portland, Maine, and a former hospital administrator in New England, sees hospitalists as a lynchpin to the discharge process, but he also urges them to get the hospital to see them as “change agents” who need institutional support to make significant improvements.
“You’re trying to run cases through and it’s all dependent on downstream activity,” Dr. Bachman says. “If the hospitalist can push back and get this recognized as a hospital issue, that’s the only time when this problem can be solved. Hospitalists are a piece of the puzzle, but it’s not just them.”
Ideas to Chew On
Mitchell Wilson, MD, SFHM, chief medical officer for Eagle Hospital Physicians in Atlanta, agrees that reprioritizing physician rounds to encourage discharges would push patients out earlier, but he wants to see more physician assistants and nurse practitioners (PAs and NPs) blended into those rounds. The partnership would be a relatively simple and direct way for physicians to pass off nonclinical or less-intensive duties that afford them more time to focus on discharge planning. A dedicated nurse for HM service and the use of telemedicine could be folded into HM practices to help.
Each of the techniques would serve to get patients out earlier on what is arguably the most costly day of their stay. “Hospitals generally lose money on the last day of a patient’s stay,” Dr. Wilson says. “When appropriate from a patient care standpoint, discharging your patient and getting the bed ready for the next patient sooner is definitely an advantage for the hospital, and for the next patient.”
Dr. Bachman says one of the main hurdles to that process is no single provider “has clear responsibility and oversight. … It’s this diffuse responsibility.” That’s where Dr. Howell and colleagues thought ABM would work well. At Hopkins Bayview, hospitalists staffed an active bed-management program that rounded twice daily in ICUs and visited the ED regularly. The hospitalist on the 12-hour shift had no other duties, a luxury that HM pioneer Robert Wachter, MD, MHM, described at the time as “freeing him or her up to act as a full-time air traffic controller for all medical patients.”
The intervention reduced ED throughput for admitted patients by 98 minutes, to 360 minutes from 458 minutes. It also cut the amount of time the ED diverted ambulances because of overcrowding—the so-called “yellow alert”—by 6%, and the amount of time ambulances were diverted due to a lack of ICU beds—“red alert”—by 27%. Dr. Howell, an SHM board member, says the results showed how hospitalists can lead throughput change through institutions but that more work needs to be done to focus on early-day discharge.
“The hospital medicine side may be incentivized for early discharges,” he says, “but the hospital systems may not.”
Dr. Howell pushes for “2-by-10,” shorthand for identifying two patients daily who could be discharged by 10 a.m. because “the ED doesn’t necessarily need more beds for 24 hours. They need more beds early in the day.” But in keeping with the ABM model, Dr. Howell believes fiscal and personnel resources have to be dedicated to the problem to expect results. In the Hopkins Bayview intervention, Dr. Wachter, professor and associate chairman of the Department of Medicine at the University of California at San Francisco, chief of the division of hospital medicine, and chief of the medical service at UCSF Medical Center, estimated the annual costs of ABM at close to $1 million a year, given the likely need for four to six full-time equivalent hospitalists, according to a post on his Wachter’s World blog (www.wachtersworld. com) after the report was published.
One idea Dr. Howell suggests to push earlier discharges is restructuring physician workweeks, setting aside certain days for admission and certain days for follow-ups. If two shifts of follow-up days are scheduled after two days of admissions, it’s likely a hospitalist could follow a patient through their entire stay, he says. “You have to structure the doctor’s day to focus on discharges first,” he adds.
Dr. Howell also believes multidisciplinary rounds are key to earlier discharges. At Wayne Memorial Hospital and other places that have instituted such teams, discharge usually is just one byproduct of a construct ultimately aimed at quality improvement. Wayne Memorial’s Dr. O’Boyle says that since the team approach was initiated in September 2009, the hospital’s LOS has dropped by 0.75 days and patient satisfaction scores have risen about 25%. Those metrics will be key data points in the years to come as discharges and readmissions become tied to reimbursement via healthcare reform (see “Value-Based Purchasing Raises the Stakes,” May 2011).
“One of the biggest factors for readmissions are things like pharmacy errors, and lack of follow-up, and other loose ends that, if you’re in too much of a hurry to get people out and you don’t have the whole team approach and make sure all your I’s are dotted and T’s are crossed, then they have an increased chance of coming back,” Dr. O’Boyle says. “So we focus on patient satisfaction, and we focus on the discharge day and the discharge time to prevent readmissions and to maximize patient satisfaction. That’s the bottom line for the hospital…It’s interesting how the bottom line seems to follow quality.”
Continued below...
Inherent Conflicts?
Early-day discharge actually can be a bad thing in some cases, Dr. Nelson says. Think of a case in which a patient might be ready for discharge in the late evening or during an overnight. To wait until the morning to send that patient home might not be the best approach.

—Louis O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, Honesdale, Pa.
“The place that manages length of stay most efficiently probably has plenty of late-day discharge,” he says.
Another potential conflict getting in the way of early-day discharge is what Dr. Wilson calls “admission competition.” For example, a hospitalist is working on discharge papers early in the morning but is then called away for a consult on an acute-care case in the ED or elsewhere. Each of the duties is important, but conflicting duties leave the hospitalist having to make choices.
“It’s not all straightforward,” Dr. Nelson says.
Emergency Nurses Association President AnnMarie Papa, DNP, RN, CEN, NE-BC, FAEN, says that collaboration between nurses and physicians is an answer to such competition. Calling the problem a “wrinkle across the system,” Papa says that without hospital administrators taking point and declaring the issue of discharge a priority, little wholesale improvement will be made. Even then, physicians and nurses—as the two main groups interacting with the patient—have to work together, she adds.
“Hospitalists have to partner with nurses,” Papa says, imploring physicians and nurses to work together on discharge decisions. “If the physicians and nurses collaborate on the decision and plans of care for the patients and the care they’re giving them and the discharge instructions, then it’s a win-win for everybody.”
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Litvak E, Bisognano M. More patients, less payment: increasing hospital efficiency in the aftermath of health reform. Health Affairs. 2011;30(1): 76-80.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Int Med. 2008;149(11):804-810.
HM@15 - Is Hospital Medicine a Good Bet for Improving Patient Satisfaction?
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
Mark Your Calendar
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
Survey Insights
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference