User login
Tackling Readmissions Together
A new report in the Journal of Hospital Medicine suggests that more than 60% of 30-day readmissions are viewed as potentially preventable by hospitalists.
The study, “Hospitalists Assess the Causes of Early Hospital Readmissions,” found that of 298 cases reviewed, 15% were deemed “preventable” by a team of 17 hospitalist reviewers at Providence Health & Services in Portland, Ore. (DOI: 10.1002/jhm.909). Another 40% were tagged as “possibly preventable,” the authors reported.
When the reviewers analyzed cases to determine what interventions could have prevented readmissions, team-based approaches were the answer much of the time, says lead author and hospitalist Douglas Koekkoek, MD. Those potential answers included earlier follow-up conversations with primary-care physicians (PCPs), palliative care-consults, and more education about home-care management.
“When a physician looks at just his locus of control, they tend to be a little pessimistic,” Dr. Koekkoek says. “When they take a systems approach ... then they gain that optimism.”
The report found that in 23% of the cases, hospitalists found that extending length of stay by a day or two could have prevented a readmission. Dr. Koekkoek says that inherent conflict?balancing the cost of every hospitalized day against keeping patients long enough to prevent readmission?is “the bread and butter of hospitalist care.”
“There is going to be a natural conflict of ‘I can’t keep people in the hospital forever,’” he adds.
Given that the federal government is likely to start reducing reimbursements for unnecessary readmissions, Dr. Koekkoek says HM groups have to view their work as one front in the battle to keep patients from returning. And that has to include outpatient physicians, patient education, and other techniques.
“The take-home is we are part of a care team,” he adds. “And the team extends beyond the hospital’s walls.”
A new report in the Journal of Hospital Medicine suggests that more than 60% of 30-day readmissions are viewed as potentially preventable by hospitalists.
The study, “Hospitalists Assess the Causes of Early Hospital Readmissions,” found that of 298 cases reviewed, 15% were deemed “preventable” by a team of 17 hospitalist reviewers at Providence Health & Services in Portland, Ore. (DOI: 10.1002/jhm.909). Another 40% were tagged as “possibly preventable,” the authors reported.
When the reviewers analyzed cases to determine what interventions could have prevented readmissions, team-based approaches were the answer much of the time, says lead author and hospitalist Douglas Koekkoek, MD. Those potential answers included earlier follow-up conversations with primary-care physicians (PCPs), palliative care-consults, and more education about home-care management.
“When a physician looks at just his locus of control, they tend to be a little pessimistic,” Dr. Koekkoek says. “When they take a systems approach ... then they gain that optimism.”
The report found that in 23% of the cases, hospitalists found that extending length of stay by a day or two could have prevented a readmission. Dr. Koekkoek says that inherent conflict?balancing the cost of every hospitalized day against keeping patients long enough to prevent readmission?is “the bread and butter of hospitalist care.”
“There is going to be a natural conflict of ‘I can’t keep people in the hospital forever,’” he adds.
Given that the federal government is likely to start reducing reimbursements for unnecessary readmissions, Dr. Koekkoek says HM groups have to view their work as one front in the battle to keep patients from returning. And that has to include outpatient physicians, patient education, and other techniques.
“The take-home is we are part of a care team,” he adds. “And the team extends beyond the hospital’s walls.”
A new report in the Journal of Hospital Medicine suggests that more than 60% of 30-day readmissions are viewed as potentially preventable by hospitalists.
The study, “Hospitalists Assess the Causes of Early Hospital Readmissions,” found that of 298 cases reviewed, 15% were deemed “preventable” by a team of 17 hospitalist reviewers at Providence Health & Services in Portland, Ore. (DOI: 10.1002/jhm.909). Another 40% were tagged as “possibly preventable,” the authors reported.
When the reviewers analyzed cases to determine what interventions could have prevented readmissions, team-based approaches were the answer much of the time, says lead author and hospitalist Douglas Koekkoek, MD. Those potential answers included earlier follow-up conversations with primary-care physicians (PCPs), palliative care-consults, and more education about home-care management.
“When a physician looks at just his locus of control, they tend to be a little pessimistic,” Dr. Koekkoek says. “When they take a systems approach ... then they gain that optimism.”
The report found that in 23% of the cases, hospitalists found that extending length of stay by a day or two could have prevented a readmission. Dr. Koekkoek says that inherent conflict?balancing the cost of every hospitalized day against keeping patients long enough to prevent readmission?is “the bread and butter of hospitalist care.”
“There is going to be a natural conflict of ‘I can’t keep people in the hospital forever,’” he adds.
Given that the federal government is likely to start reducing reimbursements for unnecessary readmissions, Dr. Koekkoek says HM groups have to view their work as one front in the battle to keep patients from returning. And that has to include outpatient physicians, patient education, and other techniques.
“The take-home is we are part of a care team,” he adds. “And the team extends beyond the hospital’s walls.”
Dr. Wachter Named ABIM's Chair-Elect
In a career with many HM “firsts," Robert M. Wachter, MD, MHM, is in line to become the first hospitalist to chair the American Board of Internal Medicine (ABIM), which provides certification for the majority of working hospitalists. Dr. Wachter, chief of the 50-faculty Division of Hospital Medicine at the University of California at San Francisco, was elected chair-elect by ABIM’s board on July 1, and is expected to become chair in July 2012.
“Bob Wachter becoming chair-elect of the ABIM is certainly an important milestone for our field,” says Scott Flanders, MD, SFHM, a hospitalist at the University of Michigan Health System and past president of SHM. “The ABIM recognized the importance of hospital medicine several years ago when they decided to add a hospitalist to the board. It should come as no surprise that they reached out to the man who is viewed as the father of hospital medicine.”
Christine K. Cassel, MD, ABIM’s president and CEO, says the election is less about recognition for the specialty and more about recognizing Dr. Wachter's transformational leadership in a number of areas, including hospital medicine.
“He has done the same kind of thing in the areas of patient safety and medical errors, and is now focusing on diagnostic accuracy,” she adds. According to Dr. Cassel, Dr. Wachter has helped ABIM focus on the need for transparency in physician performance information and report cards. “But it does indicate the maturity of the [HM] field that you have leaders like Bob—and he’s not the only one. Many emerging national leaders in healthcare are hospitalists,” she says.
ABIM sets standards and certifies physicians practicing in internal medicine and its 19 subspecialties. The board, whose members are all board-certified and represent those various subspecialties, guides the overall mission and direction in improving healthcare quality by the way it sets standards for certification, Dr. Cassel says. “The chair’s specific power and responsibility is to make sure the board runs effectively,” she adds.
In the first year of a Maintenance of Certification (MOC) program for physicians focused in hospital medicine offered by ABIM, 93 hospitalists completed the requirement and earned that designation.
In a career with many HM “firsts," Robert M. Wachter, MD, MHM, is in line to become the first hospitalist to chair the American Board of Internal Medicine (ABIM), which provides certification for the majority of working hospitalists. Dr. Wachter, chief of the 50-faculty Division of Hospital Medicine at the University of California at San Francisco, was elected chair-elect by ABIM’s board on July 1, and is expected to become chair in July 2012.
“Bob Wachter becoming chair-elect of the ABIM is certainly an important milestone for our field,” says Scott Flanders, MD, SFHM, a hospitalist at the University of Michigan Health System and past president of SHM. “The ABIM recognized the importance of hospital medicine several years ago when they decided to add a hospitalist to the board. It should come as no surprise that they reached out to the man who is viewed as the father of hospital medicine.”
Christine K. Cassel, MD, ABIM’s president and CEO, says the election is less about recognition for the specialty and more about recognizing Dr. Wachter's transformational leadership in a number of areas, including hospital medicine.
“He has done the same kind of thing in the areas of patient safety and medical errors, and is now focusing on diagnostic accuracy,” she adds. According to Dr. Cassel, Dr. Wachter has helped ABIM focus on the need for transparency in physician performance information and report cards. “But it does indicate the maturity of the [HM] field that you have leaders like Bob—and he’s not the only one. Many emerging national leaders in healthcare are hospitalists,” she says.
ABIM sets standards and certifies physicians practicing in internal medicine and its 19 subspecialties. The board, whose members are all board-certified and represent those various subspecialties, guides the overall mission and direction in improving healthcare quality by the way it sets standards for certification, Dr. Cassel says. “The chair’s specific power and responsibility is to make sure the board runs effectively,” she adds.
In the first year of a Maintenance of Certification (MOC) program for physicians focused in hospital medicine offered by ABIM, 93 hospitalists completed the requirement and earned that designation.
In a career with many HM “firsts," Robert M. Wachter, MD, MHM, is in line to become the first hospitalist to chair the American Board of Internal Medicine (ABIM), which provides certification for the majority of working hospitalists. Dr. Wachter, chief of the 50-faculty Division of Hospital Medicine at the University of California at San Francisco, was elected chair-elect by ABIM’s board on July 1, and is expected to become chair in July 2012.
“Bob Wachter becoming chair-elect of the ABIM is certainly an important milestone for our field,” says Scott Flanders, MD, SFHM, a hospitalist at the University of Michigan Health System and past president of SHM. “The ABIM recognized the importance of hospital medicine several years ago when they decided to add a hospitalist to the board. It should come as no surprise that they reached out to the man who is viewed as the father of hospital medicine.”
Christine K. Cassel, MD, ABIM’s president and CEO, says the election is less about recognition for the specialty and more about recognizing Dr. Wachter's transformational leadership in a number of areas, including hospital medicine.
“He has done the same kind of thing in the areas of patient safety and medical errors, and is now focusing on diagnostic accuracy,” she adds. According to Dr. Cassel, Dr. Wachter has helped ABIM focus on the need for transparency in physician performance information and report cards. “But it does indicate the maturity of the [HM] field that you have leaders like Bob—and he’s not the only one. Many emerging national leaders in healthcare are hospitalists,” she says.
ABIM sets standards and certifies physicians practicing in internal medicine and its 19 subspecialties. The board, whose members are all board-certified and represent those various subspecialties, guides the overall mission and direction in improving healthcare quality by the way it sets standards for certification, Dr. Cassel says. “The chair’s specific power and responsibility is to make sure the board runs effectively,” she adds.
In the first year of a Maintenance of Certification (MOC) program for physicians focused in hospital medicine offered by ABIM, 93 hospitalists completed the requirement and earned that designation.
Vorinostat Fails as Second-Line Therapy for Mesothelioma
STOCKHOLM – The results of the largest clinical trial to date in malignant pleural mesothelioma have left patients still without a standard second-line-treatment for this deadly tumor in the chest lining.
"Vorinostat [Zolinza] did not improve survival compared with placebo," Dr. Lee M. Krug said at the European Multidisciplinary Cancer Congress, where he reported outcomes of the disappointing phase III VANTAGE 014 trial.
Overall survival was not significantly different with a median of 31 weeks in patients on vorinostat and 27 weeks in those on placebo (hazard ratio, 0.98; P = 0.858). Planned analyses found no subgroups had any advantage in overall survival from vorinostat, said Dr. Krug of Memorial Sloan-Kettering Cancer Center in New York City.
Discussant Dr. Rolf A. Stahel of the University Hospital Zurich lamented, "This has been the largest study ever in mesothelioma. ... Despite this huge effort, the result is negative."
Patients were included if they had a diagnosis of malignant pleural mesothelioma with a pleural lesion at least 1 cm in thickness. They could have received up to two prior systemic regimens with pemetrexed (Alimta) and a platinum. They had to have a Karnofsky performance status of at least 70 and adequate organ function.
Vorinostat is a histone deacetylase (HDAC) inhibitor. In all, 660 patients were randomized to vorinostat (300 mg) or placebo. Both were given orally twice daily for 3 days out of 7 days in a 3-week cycle. The population was predominantly male, with a slightly greater percentage in the vorinostat arm (86% vs. 81%). Almost all patients (90%) had stage III-IV disease.
Researchers were puzzled by a change in survival rates in patients who were measured at the time of the third interim analysis, compared with those who were enrolled after the third interim analysis, said Dr. Krug.
At the third interim analysis, the hazard ratio for overall survival was 0.86, "which was just shy of the 0.83 hazard ratio required for this to be a positive trial." After the interim analysis – which occurred halfway through the study – the hazard ratio was 1.32.
The test of interaction between survival effect and time of enrollment, suggested that there was a less-than-2% chance that this switch occurred randomly. "As yet, we have not identified any causes," Dr. Krug said.
Median progression-free survival (determined by independent radiologic review) was significantly improved statistically in the vorinostat arm but not in a clinically-significant way: 6.3 weeks for vorinostat vs. 6.1 weeks for placebo (HR, 0.75; P less than 0.001).
Secondary end points (overall objective response rate, the dyspnea score on the Lung Cancer Symptom Scale as modified for mesothelioma, and forced vital capacity – were no better with vorinostat than with placebo. There were two confirmed radiologic responses, one in each arm.
"The adverse events were comparable between the two arms. ... [S]erious adverse events were slightly increased for some toxicities that you might expect to see with vorinostat," said Dr. Krug. These included fatigue, nausea, and dehydration. Tumor pain was greater in the placebo arm.
Despite the negative results, just by its sheer size, the trial "provides an excellent source of information with regards to this patient population, such as data on their pulmonary function, [symptomatology], serum markers, and also the large tumor bank that was collected," Dr. Krug told attendees at the joint congress of the European Cancer Organization (ECCO), the European Society For Medical Oncology (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO).
The study was supported by Merck Laboratories. No conflicts were reported at the meeting. Dr. Krug previously reported relationships with numerous companies, including receiving research funding from Merck.
STOCKHOLM – The results of the largest clinical trial to date in malignant pleural mesothelioma have left patients still without a standard second-line-treatment for this deadly tumor in the chest lining.
"Vorinostat [Zolinza] did not improve survival compared with placebo," Dr. Lee M. Krug said at the European Multidisciplinary Cancer Congress, where he reported outcomes of the disappointing phase III VANTAGE 014 trial.
Overall survival was not significantly different with a median of 31 weeks in patients on vorinostat and 27 weeks in those on placebo (hazard ratio, 0.98; P = 0.858). Planned analyses found no subgroups had any advantage in overall survival from vorinostat, said Dr. Krug of Memorial Sloan-Kettering Cancer Center in New York City.
Discussant Dr. Rolf A. Stahel of the University Hospital Zurich lamented, "This has been the largest study ever in mesothelioma. ... Despite this huge effort, the result is negative."
Patients were included if they had a diagnosis of malignant pleural mesothelioma with a pleural lesion at least 1 cm in thickness. They could have received up to two prior systemic regimens with pemetrexed (Alimta) and a platinum. They had to have a Karnofsky performance status of at least 70 and adequate organ function.
Vorinostat is a histone deacetylase (HDAC) inhibitor. In all, 660 patients were randomized to vorinostat (300 mg) or placebo. Both were given orally twice daily for 3 days out of 7 days in a 3-week cycle. The population was predominantly male, with a slightly greater percentage in the vorinostat arm (86% vs. 81%). Almost all patients (90%) had stage III-IV disease.
Researchers were puzzled by a change in survival rates in patients who were measured at the time of the third interim analysis, compared with those who were enrolled after the third interim analysis, said Dr. Krug.
At the third interim analysis, the hazard ratio for overall survival was 0.86, "which was just shy of the 0.83 hazard ratio required for this to be a positive trial." After the interim analysis – which occurred halfway through the study – the hazard ratio was 1.32.
The test of interaction between survival effect and time of enrollment, suggested that there was a less-than-2% chance that this switch occurred randomly. "As yet, we have not identified any causes," Dr. Krug said.
Median progression-free survival (determined by independent radiologic review) was significantly improved statistically in the vorinostat arm but not in a clinically-significant way: 6.3 weeks for vorinostat vs. 6.1 weeks for placebo (HR, 0.75; P less than 0.001).
Secondary end points (overall objective response rate, the dyspnea score on the Lung Cancer Symptom Scale as modified for mesothelioma, and forced vital capacity – were no better with vorinostat than with placebo. There were two confirmed radiologic responses, one in each arm.
"The adverse events were comparable between the two arms. ... [S]erious adverse events were slightly increased for some toxicities that you might expect to see with vorinostat," said Dr. Krug. These included fatigue, nausea, and dehydration. Tumor pain was greater in the placebo arm.
Despite the negative results, just by its sheer size, the trial "provides an excellent source of information with regards to this patient population, such as data on their pulmonary function, [symptomatology], serum markers, and also the large tumor bank that was collected," Dr. Krug told attendees at the joint congress of the European Cancer Organization (ECCO), the European Society For Medical Oncology (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO).
The study was supported by Merck Laboratories. No conflicts were reported at the meeting. Dr. Krug previously reported relationships with numerous companies, including receiving research funding from Merck.
STOCKHOLM – The results of the largest clinical trial to date in malignant pleural mesothelioma have left patients still without a standard second-line-treatment for this deadly tumor in the chest lining.
"Vorinostat [Zolinza] did not improve survival compared with placebo," Dr. Lee M. Krug said at the European Multidisciplinary Cancer Congress, where he reported outcomes of the disappointing phase III VANTAGE 014 trial.
Overall survival was not significantly different with a median of 31 weeks in patients on vorinostat and 27 weeks in those on placebo (hazard ratio, 0.98; P = 0.858). Planned analyses found no subgroups had any advantage in overall survival from vorinostat, said Dr. Krug of Memorial Sloan-Kettering Cancer Center in New York City.
Discussant Dr. Rolf A. Stahel of the University Hospital Zurich lamented, "This has been the largest study ever in mesothelioma. ... Despite this huge effort, the result is negative."
Patients were included if they had a diagnosis of malignant pleural mesothelioma with a pleural lesion at least 1 cm in thickness. They could have received up to two prior systemic regimens with pemetrexed (Alimta) and a platinum. They had to have a Karnofsky performance status of at least 70 and adequate organ function.
Vorinostat is a histone deacetylase (HDAC) inhibitor. In all, 660 patients were randomized to vorinostat (300 mg) or placebo. Both were given orally twice daily for 3 days out of 7 days in a 3-week cycle. The population was predominantly male, with a slightly greater percentage in the vorinostat arm (86% vs. 81%). Almost all patients (90%) had stage III-IV disease.
Researchers were puzzled by a change in survival rates in patients who were measured at the time of the third interim analysis, compared with those who were enrolled after the third interim analysis, said Dr. Krug.
At the third interim analysis, the hazard ratio for overall survival was 0.86, "which was just shy of the 0.83 hazard ratio required for this to be a positive trial." After the interim analysis – which occurred halfway through the study – the hazard ratio was 1.32.
The test of interaction between survival effect and time of enrollment, suggested that there was a less-than-2% chance that this switch occurred randomly. "As yet, we have not identified any causes," Dr. Krug said.
Median progression-free survival (determined by independent radiologic review) was significantly improved statistically in the vorinostat arm but not in a clinically-significant way: 6.3 weeks for vorinostat vs. 6.1 weeks for placebo (HR, 0.75; P less than 0.001).
Secondary end points (overall objective response rate, the dyspnea score on the Lung Cancer Symptom Scale as modified for mesothelioma, and forced vital capacity – were no better with vorinostat than with placebo. There were two confirmed radiologic responses, one in each arm.
"The adverse events were comparable between the two arms. ... [S]erious adverse events were slightly increased for some toxicities that you might expect to see with vorinostat," said Dr. Krug. These included fatigue, nausea, and dehydration. Tumor pain was greater in the placebo arm.
Despite the negative results, just by its sheer size, the trial "provides an excellent source of information with regards to this patient population, such as data on their pulmonary function, [symptomatology], serum markers, and also the large tumor bank that was collected," Dr. Krug told attendees at the joint congress of the European Cancer Organization (ECCO), the European Society For Medical Oncology (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO).
The study was supported by Merck Laboratories. No conflicts were reported at the meeting. Dr. Krug previously reported relationships with numerous companies, including receiving research funding from Merck.
FROM THE EUROPEAN MULTIDISCIPLINARY CANCER CONGRESS
Finding: Overall survival was not significantly different at a median of 31 weeks with vorinostat and 27 weeks with placebo (HR, 0.98; P = .858).
Source: A phase III trial of 660 patients with mesothelioma who were randomized to receive vorinostat or placebo as second-line therapy.
Disclosures: The study was supported by Merck Laboratories. No conflicts were reported at the meeting. Dr. Krug previously reported relationships with numerous companies, including receiving research funding from Merck.
Guiding Patients Facing Decisions about “Futile” Chemotherapy
Volume 9, Issue 5, September-October 2011, Pages 184-187
| doi:10.1016/j.suponc.2011.04.001 |
| Permissions & Reprints |
How We Do It
Received 4 April 2011; Accepted 16 December 2011. Available online 24 September 2011.
Case Presentation
Ms. G is a 71-year-old woman with metastatic gastric adenocarcinoma recently diagnosed after an extensive surgical resection for a small bowel obstruction (SBO). She was admitted from the surgery clinic with intractable nausea and vomiting. An abdominal computerized tomographic (CT) scan revealed a partial SBO and peritoneal carcinomatosis. Given her recent surgery, the extent of her disease, and high likelihood of recurrent SBO, the surgical team decided that Ms. G was no longer a surgical candidate. When her symptoms did not improve with conservative measures, both oncology and palliative medicine were consulted to assist with symptom management and goals of care. The oncology team stated that Ms. G was still a chemotherapy candidate and suggested that she attend her new patient evaluation in oncology clinic the following week. The palliative medicine team then met with the patient to discuss management options and her preferences for care. The palliative care team explained ways to control her nausea and vomiting without using a nasogastric tube, and the patient agreed to transfer to their service for symptom management. The palliative team explained that her cancer was incurable but that chemotherapy options existed to help control her disease and possibly prolong her life. They also explained that the chemotherapy has side effects and that the patient would need to decide if she wanted to undergo treatment and accept potential side effects for the possibility of prolonging her life by weeks to months and improving her symptoms. As an alternative, she was told that she could focus solely on symptom control with medications and allow her disease to take its natural course. Ms. G was asked to think about how she wanted to spend the time she had left. Prior to discharge, as her symptoms improved, Ms. G was evaluated by another oncologist, who, after consulting the expert gastrointestinal cancer team, explained to her that the current chemotherapy options available for metastatic gastric cancer were rarely, if ever, successful at reversing malignant obstruction. With this information, the patient decided to be discharged home with hospice and spend time with her family. She died peacefully at her home approximately two weeks later.
Article Outline
- Futile Is as Futile Does
- Palliative Care: It's Not Just Giving Up on People
- Oncology: Palliative Care Is Giving Up
- Take-Home Messages
- Acknowledgments
- References
Futile Is as Futile Does
When deciding whether or not chemotherapy is “futile,” the concept of medical futility must be explored.[1] Though it remains difficult to adequately define, the qualitative and quantitative descriptions offered by Schneiderman et al[2] are widely used. Qualitatively, futile treatment “merely preserves permanent unconsciousness or cannot end dependence on intensive medical care.” More precisely, it is a medical treatment “that in the last 100 cases … has been useless.”[2] A useful, albeit imprecise, definition of futile chemotherapy is that in which the burdens and risks outweigh the benefits. As an example, studies on chemotherapy for advanced non-small-cell lung cancer (NSCLC) have shown that patients with poor performance status or chemotherapy-unresponsive disease receive little benefit in terms of response rates and survival. [3] and [4] A retrospective analysis by Massarelli et al3 showed dismal response rates for third- and fourth-line NSCLC chemotherapy of 2.3% and 0%, respectively. Additionally, an observational study by Zietemann and Duell[4] showed that 40% and 50% of patients receiving second- and third-line chemotherapy for NSCLC die during or soon after treatment, respectively, and that over 20% receive chemotherapy within 14 days of death. Neither study commented on quality of life experienced by patients. However, a recent study by Temel et al[5] demonstrated that NSCLC patients receiving concurrent palliative care and standard oncologic care had better quality of life and even longer survival than patients receiving only standard oncologic care, despite being less likely to receive aggressive end-of-life care. Though limited to patients with NSCLC, these studies illustrate that chemotherapy in advanced cancer is often futile, especially when less aggressive care can improve quality of life as well as survival.
Addressing the futility of chemotherapy with patients is challenging for most oncologists. Although defining treatments as “futile” is suitable in the medical literature, it is a word that may carry negative connotations, such as hopelessness or abandonment, to patients. A more descriptive and less negative term, “nonbeneficial,” may be used when discussing futile chemotherapy with patients. The point when chemotherapy becomes nonbeneficial, and thus futile, is different for each patient and might even change over time. Addressing the patient's definition of nonbeneficial chemotherapy regularly during treatment ensures that the patient's goals are clear and allows the oncologist to direct conversation toward alternative options, such as palliative and hospice care, when chemotherapy cannot provide the benefits sought by the patient. This can be as simple as asking the patient, “Do you think the chemotherapy is giving you enough benefit to continue?”
Palliative Care: It's Not Just Giving Up on People
Both the physician and the patient face several decisions when considering whether or not to pursue chemotherapy for advanced cancer. First of all, the patient must decide how much information he or she wants from the oncologist. If the patient is the decision maker, he or she must choose to accept chemotherapy that is palliative, not curative. After a frank discussion about the anticipated outcomes and symptoms associated with chemotherapy, the patient must consider whether he or she can accept the burden of treatment for the potential of prolonging life by days, weeks, or months. On the other hand, the oncologist must decide if chemotherapy should even be offered, based on patient performance status, known therapeutic outcomes, and patient values and goals. The oncologist can reassure patients that the best available data show that patients who use hospice for even one day actually live longer than those who do not.[6] Once informed about what palliative care and hospice offer, the patient may determine whether or not alternatives to chemotherapy are more favorable. If the patient qualifies for clinical trials, he or she must decide to accept treatment with uncertain outcome. When reflecting upon such difficult issues, both the patient and oncologist should involve others to help guide decision making. Oncologists can consult trusted colleagues for their expertise and to ensure that they are using the best information available. Patients should involve loved ones whom they trust to help make decisions in their best interest. Table 1 provides key questions that the oncologist faces when making these decisions and how to approach them.
| Table 1: Questions to discuss with the patient when chemotherapy may be futile | ||
| Question | Leading prompts | Comment |
| What is the patient’s current understanding of the disease? | How much do you know about your cancer at this point?
How much do you want to know? | Be sure the patient is ready to discuss this issue and that you have enough time for discussion.
Ask if there are others who should receive this information simultaneously, afterwards, or instead of the patient. |
| What are the patient’s goals? | Knowing that we can’t cure your cancer, what are your goals, wishes, or hopes for the future? | Treatment decisions may be impacted greatly by a patient’s personal goals (e.g. patient wants to live to child’s graduation, or patient wants to be as comfortable as possible) |
| If chemotherapy is an option and the patient is interested, is he/she aware of potential risks and benefits? | Although everyone responds differently, these are the likely side effects and outcomes of this treatment… | Be specific in terms of likelihood of response, type of response (palliation instead of cure, extent of life prolongation expected, symptom relief, etc.) and how likely it is that treatment will help achieve patient’s goals.
Discuss potential symptom burden from treatment in detail.
Patient needs to be able to make informed decision about risks vs. benefits involved in potential treatment. |
| If the patient declines chemotherapy, treatment is not indicated, or treatment fails, what other options are available? | Let’s talk about options to make sure that you are comfortable and enjoy the highest quality of life possible in the time that you have left. | Focus on pain and symptom management. Discuss hospice options (home vs. inpatient) and make referrals when appropriate.
Stress that you will continue your relationship with the patient (possibly as their hospice provider) and that you will ensure that their symptoms are managed, either directly or through hospice nurses. |
As an alternative to addressing the above issues with the patient independently, oncologists may involve a palliative care specialist to facilitate this conversation.[7] Particularly in cases where the oncologist decides that chemotherapy is no longer a viable option, it may be easier, from both the patient and the provider perspectives, for the palliative care specialist to have this discussion. In a recent survey of patients on our oncology ward, the great majority did not want to discuss advance directives (ADs) with their oncologist—these patients thought ADs were important and should be discussed but were more comfortable discussing them with the admitting provider than the oncologist.[8] Patients may feel that they are disappointing their oncologist by being unable to take further treatment or by admitting that treatment has failed them. Similarly, oncologists might view having this discussion as an admission of their failure as a provider. The palliative care specialist, on the other hand, has no responsibility for chemotherapy and possibly no prior relationship with the patient, thus alleviating this type of emotional association between provider and patient. Furthermore, the conversation about nonbeneficial chemotherapy provides a segue for the palliative care provider to discuss with patients what he or she does best: establishing goals of care, managing symptoms, and maintaining comfort. For the palliative care specialist, providing symptom management and the best possible quality of life for patients are the fundamental goals. Death is generally not viewed with a sense of failure when palliation is the focus of care.
Oncology: Palliative Care Is Giving Up
We still hear from oncologists like ourselves the dreaded words “What do you want me to do, give up on the patient?” or, to the patient, “What, are you giving up? I thought you'd keep fighting!” We would argue that current best practices include knowing when the risks and harms of chemotherapy outweigh any potential chance of benefit. Physicians and patients should follow current National Comprehensive Cancer Network (NCCN) guidelines for solid tumors such as breast9 and lung10 cancer and stop chemotherapy when the chance of success is minimal. If the doctor cannot describe a specific, substantial benefit that outweighs the toxicity, he or she should not recommend it.[11] And all the relevant guidelines call for considering a switch to nonchemotherapy palliative care when the patient's performance status is Eastern Cooperative Oncology Group (ECOG) ≥3, defined as “3 = Capable of only limited self-care, confined to bed or chair more than 50% of waking hours.”[12] Such a simple threshold could dramatically reduce the use of chemotherapy at the end of life and lessen downstream toxicities.
Oncologists can implement several strategies to help facilitate the transition from aggressive care to comfort care (Table 2). For patients with incurable cancer, oncologists can hold early discussions about palliative and hospice options that will need to be implemented when chemotherapy is no longer able to control their disease. This discussion introduces palliative medicine as part of the care plan for incurable disease and allows the patient to anticipate such a transition. Oncologists can also provide reassurance that they will continue to be involved in their patient's care and to support them, even if the patient does not undergo further chemotherapy. There are at least four studies that show equal[13] or better[6] survival, smoother transitions to hospice when death is inevitable, less intensive end-of-life care, and superior patient and family outcomes with concurrent palliative care. [14] and [15] By helping patients establish legal documents, such as ADs and power of attorney, oncologists and palliative care specialists can alleviate some of the stress related to the end of life and make the transition to comfort care easier. Finally, oncologists can review guidelines such as those from the NCCN and American Society of Clinical Oncology, which call for a switch to palliative care when the cancer has grown on three regimens or the patient's ECOG performance status is three or above. [11] and [12]
| Table 2: Things that help ONCOLOGISTS and their patients | ||
| Item | How it helps | Comments |
| Early discussion of palliative and hospice care when chemotherapy may no longer help. | Hospice (and eventual death) will not come as a complete surprise. | “We will do our best to help you with this cancer, but at some point there may not be any treatments known to help….” “Remember the conversation we had when we first met?...” |
| Reassurance that the oncologist will not abandon the patient if concurrent care is given. | This major fear may keep oncology patients at the same practice they have known for years – it is familiar – when they would be better served by transition. | There are now at least 4 randomized trials showing that most patients will accept concurrent palliative care if offered, and that outcomes are equal or better, at less cost.6,13,14,15 |
| Legal documents such as Advance Medical Directives, Durable Medical Power of Attorney | Reinforces the seriousness and “now” aspect of care. | These are readily available in all states at no cost. They are not the final word on how to live one’s remaining time, but will get the conversation started. |
| Best nationally recognized information showing that further chemotherapy will not help due to 3 prior failures, or is not indicated due to poor performance status.9,10 | The oncologist can point to the right page and say “The best national guidelines call for a switch away from chemo…because it will do no good and will cause harmful side effects.” | Readily accessed from the Internet. |
| Use decision aids, similar to Adjuvant!. | Increases the amount of truthful information given, even when the news is bad, and helps with transition points. | An increasing number of these are available[i],[ii],[iii],[iv] and will soon be offered as smart phone applications (aps). |
Communication tools, such as the National Cancer Institute's Oncotalk and EPEC-O, are useful for oncologists seeking to further enhance their communication skills.
Take-Home Messages
Guiding patients in making decisions about nonbeneficial, or futile, chemotherapy presents a challenge for many oncologists as well as their patients and families. Though futility is difficult to define, oncologists and their patients can decide through regular, open discussion if the burdens of chemotherapy outweigh the benefits and whether or not chemotherapy can achieve the reasonable benefits desired by the patient. “Your cancer is advancing despite our best efforts to keep it from growing. Let's talk about what options we have at this point and see what will work best for you.” To make such decisions, oncologists must obtain the most current information and convey it to patients (or their designated decision makers) as clearly as possible. “Based on the latest evidence, there is a 20% chance that the cancer will shrink or stay the same size with this treatment and an 80% chance that it will continue to grow despite treatment.” Both oncologists and their patients should involve those whom they trust to help with decision making. In cases where chemotherapy is nonbeneficial, oncologists may prefer to involve palliative and hospice care specialists to discuss the transition to comfort care with the patient. “At this time, I do not have any treatments that are likely to help you live longer or more comfortably, but I want to make sure that we get the most out of the rest of your life. I have asked a palliative care specialist to help us make this possible.” In order to ease the transition from aggressive or curative care to comfort care, oncologists can employ approaches such as early discussion of palliative and hospice care, assuring the patient of continued involvement in their care, and helping patients with ADs. These approaches not only benefit patients and their families but also strengthen the relationship between the oncologist and the patients and their families.
Acknowledgments
This research was supported by grants GO8 LM0095259 from the National Library of Medicine and R01CA116227-01 (both to T. J. S.) from the National Cancer Institute.
References [PubMed ID in brackets]
1 P.R. Helft, M. Siegler and J. Lantos, The rise and fall of the medical futility movement, . N Engl J Med, 343 (2000), pp. 293–296 [10911014].
2 L.J. Schneiderman, N.S. Jecker and A.R. Jonsen, Medical futility: its meaning and ethical implications, . Ann Intern Med, 112 (1990), pp. 949–954 [2187394].
3 E. Massarelli, F. Andre, D.D. Liu, J.J. Lee, M. Wolf, A. Fandi, J. Ochs, T. Le Chevalier, F. Fossella and R.S. Herbst, A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer, . Lung Cancer, 39 1 (2003), pp. 55–61 [12499095].
4 V. Zietemann and T. Duell, Every-day clinical practice in patients with advanced non-small-cell lung cancer, . Lung Cancer, 68 2 (2010), pp. 273–277 [19632737].
5 J.S. Temel, J.A. Greer, A. Muzikansky, E.R. Gallagher, S. Admane, V.A. Jackson, C.M. Dahlin, C.D. Blinderman, J. Jacobsen, W.F. Pirl, J.A. Billings and T.J. Lynch, Early palliative care for patients with metastatic non-small-cell lung cancer, . N Engl J Med, 363 (2010), pp. 733–742 [20818875].
6 S.R. Connor, B. Pyenson, K. Fitch, C. Spence and K. Iwasaki, Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage, 33 3 (2007), pp. 238–246.
7 S.E. Harrington and T.J. Smith, The role of chemotherapy at the end of life: “when is enough, enough?”. JAMA, 299 22 (2008), pp. 2667–2678.
8 L. Dow, R. Matsuyama, L. Kuhn, L. Lyckholm, E.B. Lamont and T.J. Smith, Paradoxes in advance care planning. J Clin Oncol, 28 2 (2010), pp. 299–304.
9 NCCN Breast Cancer Clinical Practice Guidelines Panel, R.W. Carlson, D.C. Allred, B.O. Anderson, H.J. Burstein, W.B. Carter, S.B. Edge, J.K. Erban, W.B. Farrar, L.J. Goldstein, W.J. Gradishar, D.F. Hayes, C.A. Hudis, M. Jahanzeb, K. Kiel, B.M. Ljung, P.K. Marcom, I.A. Mayer, B. McCormick, L.M. Nabell, L.J. Pierce, E.C. Reed, M.L. Smith, G. Somlo, R.L. Theriault, N.S. Topham, J.H. Ward, E.P. Winer and A.C. Wolff, Breast cancer. J Natl Compr Canc Netw, 7 2 (2009), pp. 122–192.
10 NCCN Non-Small Cell Lung Cancer Panel Members, D.S. Ettinger, W. Akerley, G. Bepler, M.G. Blum, A. Chang, R.T. Cheney, L.R. Chirieac, T.A. D'Amico, T.L. Demmy, A.K. Ganti, R. Govindan, F.W. Grannis, T. Jahan, M. Jahanzeb, D.H. Johnson, A. Kessinger, R. Komaki, F.M. Kong, M.G. Kris, L.M. Krug, Q.T. Le, I.T. Lennes, R. Martins, J. O'Malley, R.U. Osarogiagbon, G.A. Otterson, J.D. Patel, K.M. Pisters, K. Reckamp, G.J. Riely, E. Rohren, G.R. Simon, S.J. Swanson, D.E. Wood and S.C. Yang, Non-small cell lung cancer. J Natl Compr Canc Netw, 8 7 (2010), pp. 740–801.
11 American Society of Clinical Oncology Outcomes Working Group, Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol, 14 (1996), pp. 671–679.
12 Eastern Cooperative Oncology Group, ECOG Performance Status, http://ecog.dfci.harvard.edu/general/perf_stat.html Accessed November 30, 2010.
13 J. Finn, K. Pienta and J. Parzuchowski, Bridging cancer treatment and hospice care. Proc Am Soc Clin Oncol, 21 (2002), p. 1452.
14 G. Gade, I. Venohr, D. Conner, K. McGrady, J. Beane, R.H. Richardson, M.P. Williams, M. Liberson, M. Blum and R. Della Penna, Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med, 11 2 (2008), pp. 180–190.
Copyright © 2011 Elsevier Inc. All rights reserved.
Volume 9, Issue 5, September-October 2011, Pages 184-187
| doi:10.1016/j.suponc.2011.04.001 |
| Permissions & Reprints |
How We Do It
Received 4 April 2011; Accepted 16 December 2011. Available online 24 September 2011.
Case Presentation
Ms. G is a 71-year-old woman with metastatic gastric adenocarcinoma recently diagnosed after an extensive surgical resection for a small bowel obstruction (SBO). She was admitted from the surgery clinic with intractable nausea and vomiting. An abdominal computerized tomographic (CT) scan revealed a partial SBO and peritoneal carcinomatosis. Given her recent surgery, the extent of her disease, and high likelihood of recurrent SBO, the surgical team decided that Ms. G was no longer a surgical candidate. When her symptoms did not improve with conservative measures, both oncology and palliative medicine were consulted to assist with symptom management and goals of care. The oncology team stated that Ms. G was still a chemotherapy candidate and suggested that she attend her new patient evaluation in oncology clinic the following week. The palliative medicine team then met with the patient to discuss management options and her preferences for care. The palliative care team explained ways to control her nausea and vomiting without using a nasogastric tube, and the patient agreed to transfer to their service for symptom management. The palliative team explained that her cancer was incurable but that chemotherapy options existed to help control her disease and possibly prolong her life. They also explained that the chemotherapy has side effects and that the patient would need to decide if she wanted to undergo treatment and accept potential side effects for the possibility of prolonging her life by weeks to months and improving her symptoms. As an alternative, she was told that she could focus solely on symptom control with medications and allow her disease to take its natural course. Ms. G was asked to think about how she wanted to spend the time she had left. Prior to discharge, as her symptoms improved, Ms. G was evaluated by another oncologist, who, after consulting the expert gastrointestinal cancer team, explained to her that the current chemotherapy options available for metastatic gastric cancer were rarely, if ever, successful at reversing malignant obstruction. With this information, the patient decided to be discharged home with hospice and spend time with her family. She died peacefully at her home approximately two weeks later.
Article Outline
- Futile Is as Futile Does
- Palliative Care: It's Not Just Giving Up on People
- Oncology: Palliative Care Is Giving Up
- Take-Home Messages
- Acknowledgments
- References
Futile Is as Futile Does
When deciding whether or not chemotherapy is “futile,” the concept of medical futility must be explored.[1] Though it remains difficult to adequately define, the qualitative and quantitative descriptions offered by Schneiderman et al[2] are widely used. Qualitatively, futile treatment “merely preserves permanent unconsciousness or cannot end dependence on intensive medical care.” More precisely, it is a medical treatment “that in the last 100 cases … has been useless.”[2] A useful, albeit imprecise, definition of futile chemotherapy is that in which the burdens and risks outweigh the benefits. As an example, studies on chemotherapy for advanced non-small-cell lung cancer (NSCLC) have shown that patients with poor performance status or chemotherapy-unresponsive disease receive little benefit in terms of response rates and survival. [3] and [4] A retrospective analysis by Massarelli et al3 showed dismal response rates for third- and fourth-line NSCLC chemotherapy of 2.3% and 0%, respectively. Additionally, an observational study by Zietemann and Duell[4] showed that 40% and 50% of patients receiving second- and third-line chemotherapy for NSCLC die during or soon after treatment, respectively, and that over 20% receive chemotherapy within 14 days of death. Neither study commented on quality of life experienced by patients. However, a recent study by Temel et al[5] demonstrated that NSCLC patients receiving concurrent palliative care and standard oncologic care had better quality of life and even longer survival than patients receiving only standard oncologic care, despite being less likely to receive aggressive end-of-life care. Though limited to patients with NSCLC, these studies illustrate that chemotherapy in advanced cancer is often futile, especially when less aggressive care can improve quality of life as well as survival.
Addressing the futility of chemotherapy with patients is challenging for most oncologists. Although defining treatments as “futile” is suitable in the medical literature, it is a word that may carry negative connotations, such as hopelessness or abandonment, to patients. A more descriptive and less negative term, “nonbeneficial,” may be used when discussing futile chemotherapy with patients. The point when chemotherapy becomes nonbeneficial, and thus futile, is different for each patient and might even change over time. Addressing the patient's definition of nonbeneficial chemotherapy regularly during treatment ensures that the patient's goals are clear and allows the oncologist to direct conversation toward alternative options, such as palliative and hospice care, when chemotherapy cannot provide the benefits sought by the patient. This can be as simple as asking the patient, “Do you think the chemotherapy is giving you enough benefit to continue?”
Palliative Care: It's Not Just Giving Up on People
Both the physician and the patient face several decisions when considering whether or not to pursue chemotherapy for advanced cancer. First of all, the patient must decide how much information he or she wants from the oncologist. If the patient is the decision maker, he or she must choose to accept chemotherapy that is palliative, not curative. After a frank discussion about the anticipated outcomes and symptoms associated with chemotherapy, the patient must consider whether he or she can accept the burden of treatment for the potential of prolonging life by days, weeks, or months. On the other hand, the oncologist must decide if chemotherapy should even be offered, based on patient performance status, known therapeutic outcomes, and patient values and goals. The oncologist can reassure patients that the best available data show that patients who use hospice for even one day actually live longer than those who do not.[6] Once informed about what palliative care and hospice offer, the patient may determine whether or not alternatives to chemotherapy are more favorable. If the patient qualifies for clinical trials, he or she must decide to accept treatment with uncertain outcome. When reflecting upon such difficult issues, both the patient and oncologist should involve others to help guide decision making. Oncologists can consult trusted colleagues for their expertise and to ensure that they are using the best information available. Patients should involve loved ones whom they trust to help make decisions in their best interest. Table 1 provides key questions that the oncologist faces when making these decisions and how to approach them.
| Table 1: Questions to discuss with the patient when chemotherapy may be futile | ||
| Question | Leading prompts | Comment |
| What is the patient’s current understanding of the disease? | How much do you know about your cancer at this point?
How much do you want to know? | Be sure the patient is ready to discuss this issue and that you have enough time for discussion.
Ask if there are others who should receive this information simultaneously, afterwards, or instead of the patient. |
| What are the patient’s goals? | Knowing that we can’t cure your cancer, what are your goals, wishes, or hopes for the future? | Treatment decisions may be impacted greatly by a patient’s personal goals (e.g. patient wants to live to child’s graduation, or patient wants to be as comfortable as possible) |
| If chemotherapy is an option and the patient is interested, is he/she aware of potential risks and benefits? | Although everyone responds differently, these are the likely side effects and outcomes of this treatment… | Be specific in terms of likelihood of response, type of response (palliation instead of cure, extent of life prolongation expected, symptom relief, etc.) and how likely it is that treatment will help achieve patient’s goals.
Discuss potential symptom burden from treatment in detail.
Patient needs to be able to make informed decision about risks vs. benefits involved in potential treatment. |
| If the patient declines chemotherapy, treatment is not indicated, or treatment fails, what other options are available? | Let’s talk about options to make sure that you are comfortable and enjoy the highest quality of life possible in the time that you have left. | Focus on pain and symptom management. Discuss hospice options (home vs. inpatient) and make referrals when appropriate.
Stress that you will continue your relationship with the patient (possibly as their hospice provider) and that you will ensure that their symptoms are managed, either directly or through hospice nurses. |
As an alternative to addressing the above issues with the patient independently, oncologists may involve a palliative care specialist to facilitate this conversation.[7] Particularly in cases where the oncologist decides that chemotherapy is no longer a viable option, it may be easier, from both the patient and the provider perspectives, for the palliative care specialist to have this discussion. In a recent survey of patients on our oncology ward, the great majority did not want to discuss advance directives (ADs) with their oncologist—these patients thought ADs were important and should be discussed but were more comfortable discussing them with the admitting provider than the oncologist.[8] Patients may feel that they are disappointing their oncologist by being unable to take further treatment or by admitting that treatment has failed them. Similarly, oncologists might view having this discussion as an admission of their failure as a provider. The palliative care specialist, on the other hand, has no responsibility for chemotherapy and possibly no prior relationship with the patient, thus alleviating this type of emotional association between provider and patient. Furthermore, the conversation about nonbeneficial chemotherapy provides a segue for the palliative care provider to discuss with patients what he or she does best: establishing goals of care, managing symptoms, and maintaining comfort. For the palliative care specialist, providing symptom management and the best possible quality of life for patients are the fundamental goals. Death is generally not viewed with a sense of failure when palliation is the focus of care.
Oncology: Palliative Care Is Giving Up
We still hear from oncologists like ourselves the dreaded words “What do you want me to do, give up on the patient?” or, to the patient, “What, are you giving up? I thought you'd keep fighting!” We would argue that current best practices include knowing when the risks and harms of chemotherapy outweigh any potential chance of benefit. Physicians and patients should follow current National Comprehensive Cancer Network (NCCN) guidelines for solid tumors such as breast9 and lung10 cancer and stop chemotherapy when the chance of success is minimal. If the doctor cannot describe a specific, substantial benefit that outweighs the toxicity, he or she should not recommend it.[11] And all the relevant guidelines call for considering a switch to nonchemotherapy palliative care when the patient's performance status is Eastern Cooperative Oncology Group (ECOG) ≥3, defined as “3 = Capable of only limited self-care, confined to bed or chair more than 50% of waking hours.”[12] Such a simple threshold could dramatically reduce the use of chemotherapy at the end of life and lessen downstream toxicities.
Oncologists can implement several strategies to help facilitate the transition from aggressive care to comfort care (Table 2). For patients with incurable cancer, oncologists can hold early discussions about palliative and hospice options that will need to be implemented when chemotherapy is no longer able to control their disease. This discussion introduces palliative medicine as part of the care plan for incurable disease and allows the patient to anticipate such a transition. Oncologists can also provide reassurance that they will continue to be involved in their patient's care and to support them, even if the patient does not undergo further chemotherapy. There are at least four studies that show equal[13] or better[6] survival, smoother transitions to hospice when death is inevitable, less intensive end-of-life care, and superior patient and family outcomes with concurrent palliative care. [14] and [15] By helping patients establish legal documents, such as ADs and power of attorney, oncologists and palliative care specialists can alleviate some of the stress related to the end of life and make the transition to comfort care easier. Finally, oncologists can review guidelines such as those from the NCCN and American Society of Clinical Oncology, which call for a switch to palliative care when the cancer has grown on three regimens or the patient's ECOG performance status is three or above. [11] and [12]
| Table 2: Things that help ONCOLOGISTS and their patients | ||
| Item | How it helps | Comments |
| Early discussion of palliative and hospice care when chemotherapy may no longer help. | Hospice (and eventual death) will not come as a complete surprise. | “We will do our best to help you with this cancer, but at some point there may not be any treatments known to help….” “Remember the conversation we had when we first met?...” |
| Reassurance that the oncologist will not abandon the patient if concurrent care is given. | This major fear may keep oncology patients at the same practice they have known for years – it is familiar – when they would be better served by transition. | There are now at least 4 randomized trials showing that most patients will accept concurrent palliative care if offered, and that outcomes are equal or better, at less cost.6,13,14,15 |
| Legal documents such as Advance Medical Directives, Durable Medical Power of Attorney | Reinforces the seriousness and “now” aspect of care. | These are readily available in all states at no cost. They are not the final word on how to live one’s remaining time, but will get the conversation started. |
| Best nationally recognized information showing that further chemotherapy will not help due to 3 prior failures, or is not indicated due to poor performance status.9,10 | The oncologist can point to the right page and say “The best national guidelines call for a switch away from chemo…because it will do no good and will cause harmful side effects.” | Readily accessed from the Internet. |
| Use decision aids, similar to Adjuvant!. | Increases the amount of truthful information given, even when the news is bad, and helps with transition points. | An increasing number of these are available[i],[ii],[iii],[iv] and will soon be offered as smart phone applications (aps). |
Communication tools, such as the National Cancer Institute's Oncotalk and EPEC-O, are useful for oncologists seeking to further enhance their communication skills.
Take-Home Messages
Guiding patients in making decisions about nonbeneficial, or futile, chemotherapy presents a challenge for many oncologists as well as their patients and families. Though futility is difficult to define, oncologists and their patients can decide through regular, open discussion if the burdens of chemotherapy outweigh the benefits and whether or not chemotherapy can achieve the reasonable benefits desired by the patient. “Your cancer is advancing despite our best efforts to keep it from growing. Let's talk about what options we have at this point and see what will work best for you.” To make such decisions, oncologists must obtain the most current information and convey it to patients (or their designated decision makers) as clearly as possible. “Based on the latest evidence, there is a 20% chance that the cancer will shrink or stay the same size with this treatment and an 80% chance that it will continue to grow despite treatment.” Both oncologists and their patients should involve those whom they trust to help with decision making. In cases where chemotherapy is nonbeneficial, oncologists may prefer to involve palliative and hospice care specialists to discuss the transition to comfort care with the patient. “At this time, I do not have any treatments that are likely to help you live longer or more comfortably, but I want to make sure that we get the most out of the rest of your life. I have asked a palliative care specialist to help us make this possible.” In order to ease the transition from aggressive or curative care to comfort care, oncologists can employ approaches such as early discussion of palliative and hospice care, assuring the patient of continued involvement in their care, and helping patients with ADs. These approaches not only benefit patients and their families but also strengthen the relationship between the oncologist and the patients and their families.
Acknowledgments
This research was supported by grants GO8 LM0095259 from the National Library of Medicine and R01CA116227-01 (both to T. J. S.) from the National Cancer Institute.
References [PubMed ID in brackets]
1 P.R. Helft, M. Siegler and J. Lantos, The rise and fall of the medical futility movement, . N Engl J Med, 343 (2000), pp. 293–296 [10911014].
2 L.J. Schneiderman, N.S. Jecker and A.R. Jonsen, Medical futility: its meaning and ethical implications, . Ann Intern Med, 112 (1990), pp. 949–954 [2187394].
3 E. Massarelli, F. Andre, D.D. Liu, J.J. Lee, M. Wolf, A. Fandi, J. Ochs, T. Le Chevalier, F. Fossella and R.S. Herbst, A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer, . Lung Cancer, 39 1 (2003), pp. 55–61 [12499095].
4 V. Zietemann and T. Duell, Every-day clinical practice in patients with advanced non-small-cell lung cancer, . Lung Cancer, 68 2 (2010), pp. 273–277 [19632737].
5 J.S. Temel, J.A. Greer, A. Muzikansky, E.R. Gallagher, S. Admane, V.A. Jackson, C.M. Dahlin, C.D. Blinderman, J. Jacobsen, W.F. Pirl, J.A. Billings and T.J. Lynch, Early palliative care for patients with metastatic non-small-cell lung cancer, . N Engl J Med, 363 (2010), pp. 733–742 [20818875].
6 S.R. Connor, B. Pyenson, K. Fitch, C. Spence and K. Iwasaki, Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage, 33 3 (2007), pp. 238–246.
7 S.E. Harrington and T.J. Smith, The role of chemotherapy at the end of life: “when is enough, enough?”. JAMA, 299 22 (2008), pp. 2667–2678.
8 L. Dow, R. Matsuyama, L. Kuhn, L. Lyckholm, E.B. Lamont and T.J. Smith, Paradoxes in advance care planning. J Clin Oncol, 28 2 (2010), pp. 299–304.
9 NCCN Breast Cancer Clinical Practice Guidelines Panel, R.W. Carlson, D.C. Allred, B.O. Anderson, H.J. Burstein, W.B. Carter, S.B. Edge, J.K. Erban, W.B. Farrar, L.J. Goldstein, W.J. Gradishar, D.F. Hayes, C.A. Hudis, M. Jahanzeb, K. Kiel, B.M. Ljung, P.K. Marcom, I.A. Mayer, B. McCormick, L.M. Nabell, L.J. Pierce, E.C. Reed, M.L. Smith, G. Somlo, R.L. Theriault, N.S. Topham, J.H. Ward, E.P. Winer and A.C. Wolff, Breast cancer. J Natl Compr Canc Netw, 7 2 (2009), pp. 122–192.
10 NCCN Non-Small Cell Lung Cancer Panel Members, D.S. Ettinger, W. Akerley, G. Bepler, M.G. Blum, A. Chang, R.T. Cheney, L.R. Chirieac, T.A. D'Amico, T.L. Demmy, A.K. Ganti, R. Govindan, F.W. Grannis, T. Jahan, M. Jahanzeb, D.H. Johnson, A. Kessinger, R. Komaki, F.M. Kong, M.G. Kris, L.M. Krug, Q.T. Le, I.T. Lennes, R. Martins, J. O'Malley, R.U. Osarogiagbon, G.A. Otterson, J.D. Patel, K.M. Pisters, K. Reckamp, G.J. Riely, E. Rohren, G.R. Simon, S.J. Swanson, D.E. Wood and S.C. Yang, Non-small cell lung cancer. J Natl Compr Canc Netw, 8 7 (2010), pp. 740–801.
11 American Society of Clinical Oncology Outcomes Working Group, Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol, 14 (1996), pp. 671–679.
12 Eastern Cooperative Oncology Group, ECOG Performance Status, http://ecog.dfci.harvard.edu/general/perf_stat.html Accessed November 30, 2010.
13 J. Finn, K. Pienta and J. Parzuchowski, Bridging cancer treatment and hospice care. Proc Am Soc Clin Oncol, 21 (2002), p. 1452.
14 G. Gade, I. Venohr, D. Conner, K. McGrady, J. Beane, R.H. Richardson, M.P. Williams, M. Liberson, M. Blum and R. Della Penna, Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med, 11 2 (2008), pp. 180–190.
Copyright © 2011 Elsevier Inc. All rights reserved.
Volume 9, Issue 5, September-October 2011, Pages 184-187
| doi:10.1016/j.suponc.2011.04.001 |
| Permissions & Reprints |
How We Do It
Received 4 April 2011; Accepted 16 December 2011. Available online 24 September 2011.
Case Presentation
Ms. G is a 71-year-old woman with metastatic gastric adenocarcinoma recently diagnosed after an extensive surgical resection for a small bowel obstruction (SBO). She was admitted from the surgery clinic with intractable nausea and vomiting. An abdominal computerized tomographic (CT) scan revealed a partial SBO and peritoneal carcinomatosis. Given her recent surgery, the extent of her disease, and high likelihood of recurrent SBO, the surgical team decided that Ms. G was no longer a surgical candidate. When her symptoms did not improve with conservative measures, both oncology and palliative medicine were consulted to assist with symptom management and goals of care. The oncology team stated that Ms. G was still a chemotherapy candidate and suggested that she attend her new patient evaluation in oncology clinic the following week. The palliative medicine team then met with the patient to discuss management options and her preferences for care. The palliative care team explained ways to control her nausea and vomiting without using a nasogastric tube, and the patient agreed to transfer to their service for symptom management. The palliative team explained that her cancer was incurable but that chemotherapy options existed to help control her disease and possibly prolong her life. They also explained that the chemotherapy has side effects and that the patient would need to decide if she wanted to undergo treatment and accept potential side effects for the possibility of prolonging her life by weeks to months and improving her symptoms. As an alternative, she was told that she could focus solely on symptom control with medications and allow her disease to take its natural course. Ms. G was asked to think about how she wanted to spend the time she had left. Prior to discharge, as her symptoms improved, Ms. G was evaluated by another oncologist, who, after consulting the expert gastrointestinal cancer team, explained to her that the current chemotherapy options available for metastatic gastric cancer were rarely, if ever, successful at reversing malignant obstruction. With this information, the patient decided to be discharged home with hospice and spend time with her family. She died peacefully at her home approximately two weeks later.
Article Outline
- Futile Is as Futile Does
- Palliative Care: It's Not Just Giving Up on People
- Oncology: Palliative Care Is Giving Up
- Take-Home Messages
- Acknowledgments
- References
Futile Is as Futile Does
When deciding whether or not chemotherapy is “futile,” the concept of medical futility must be explored.[1] Though it remains difficult to adequately define, the qualitative and quantitative descriptions offered by Schneiderman et al[2] are widely used. Qualitatively, futile treatment “merely preserves permanent unconsciousness or cannot end dependence on intensive medical care.” More precisely, it is a medical treatment “that in the last 100 cases … has been useless.”[2] A useful, albeit imprecise, definition of futile chemotherapy is that in which the burdens and risks outweigh the benefits. As an example, studies on chemotherapy for advanced non-small-cell lung cancer (NSCLC) have shown that patients with poor performance status or chemotherapy-unresponsive disease receive little benefit in terms of response rates and survival. [3] and [4] A retrospective analysis by Massarelli et al3 showed dismal response rates for third- and fourth-line NSCLC chemotherapy of 2.3% and 0%, respectively. Additionally, an observational study by Zietemann and Duell[4] showed that 40% and 50% of patients receiving second- and third-line chemotherapy for NSCLC die during or soon after treatment, respectively, and that over 20% receive chemotherapy within 14 days of death. Neither study commented on quality of life experienced by patients. However, a recent study by Temel et al[5] demonstrated that NSCLC patients receiving concurrent palliative care and standard oncologic care had better quality of life and even longer survival than patients receiving only standard oncologic care, despite being less likely to receive aggressive end-of-life care. Though limited to patients with NSCLC, these studies illustrate that chemotherapy in advanced cancer is often futile, especially when less aggressive care can improve quality of life as well as survival.
Addressing the futility of chemotherapy with patients is challenging for most oncologists. Although defining treatments as “futile” is suitable in the medical literature, it is a word that may carry negative connotations, such as hopelessness or abandonment, to patients. A more descriptive and less negative term, “nonbeneficial,” may be used when discussing futile chemotherapy with patients. The point when chemotherapy becomes nonbeneficial, and thus futile, is different for each patient and might even change over time. Addressing the patient's definition of nonbeneficial chemotherapy regularly during treatment ensures that the patient's goals are clear and allows the oncologist to direct conversation toward alternative options, such as palliative and hospice care, when chemotherapy cannot provide the benefits sought by the patient. This can be as simple as asking the patient, “Do you think the chemotherapy is giving you enough benefit to continue?”
Palliative Care: It's Not Just Giving Up on People
Both the physician and the patient face several decisions when considering whether or not to pursue chemotherapy for advanced cancer. First of all, the patient must decide how much information he or she wants from the oncologist. If the patient is the decision maker, he or she must choose to accept chemotherapy that is palliative, not curative. After a frank discussion about the anticipated outcomes and symptoms associated with chemotherapy, the patient must consider whether he or she can accept the burden of treatment for the potential of prolonging life by days, weeks, or months. On the other hand, the oncologist must decide if chemotherapy should even be offered, based on patient performance status, known therapeutic outcomes, and patient values and goals. The oncologist can reassure patients that the best available data show that patients who use hospice for even one day actually live longer than those who do not.[6] Once informed about what palliative care and hospice offer, the patient may determine whether or not alternatives to chemotherapy are more favorable. If the patient qualifies for clinical trials, he or she must decide to accept treatment with uncertain outcome. When reflecting upon such difficult issues, both the patient and oncologist should involve others to help guide decision making. Oncologists can consult trusted colleagues for their expertise and to ensure that they are using the best information available. Patients should involve loved ones whom they trust to help make decisions in their best interest. Table 1 provides key questions that the oncologist faces when making these decisions and how to approach them.
| Table 1: Questions to discuss with the patient when chemotherapy may be futile | ||
| Question | Leading prompts | Comment |
| What is the patient’s current understanding of the disease? | How much do you know about your cancer at this point?
How much do you want to know? | Be sure the patient is ready to discuss this issue and that you have enough time for discussion.
Ask if there are others who should receive this information simultaneously, afterwards, or instead of the patient. |
| What are the patient’s goals? | Knowing that we can’t cure your cancer, what are your goals, wishes, or hopes for the future? | Treatment decisions may be impacted greatly by a patient’s personal goals (e.g. patient wants to live to child’s graduation, or patient wants to be as comfortable as possible) |
| If chemotherapy is an option and the patient is interested, is he/she aware of potential risks and benefits? | Although everyone responds differently, these are the likely side effects and outcomes of this treatment… | Be specific in terms of likelihood of response, type of response (palliation instead of cure, extent of life prolongation expected, symptom relief, etc.) and how likely it is that treatment will help achieve patient’s goals.
Discuss potential symptom burden from treatment in detail.
Patient needs to be able to make informed decision about risks vs. benefits involved in potential treatment. |
| If the patient declines chemotherapy, treatment is not indicated, or treatment fails, what other options are available? | Let’s talk about options to make sure that you are comfortable and enjoy the highest quality of life possible in the time that you have left. | Focus on pain and symptom management. Discuss hospice options (home vs. inpatient) and make referrals when appropriate.
Stress that you will continue your relationship with the patient (possibly as their hospice provider) and that you will ensure that their symptoms are managed, either directly or through hospice nurses. |
As an alternative to addressing the above issues with the patient independently, oncologists may involve a palliative care specialist to facilitate this conversation.[7] Particularly in cases where the oncologist decides that chemotherapy is no longer a viable option, it may be easier, from both the patient and the provider perspectives, for the palliative care specialist to have this discussion. In a recent survey of patients on our oncology ward, the great majority did not want to discuss advance directives (ADs) with their oncologist—these patients thought ADs were important and should be discussed but were more comfortable discussing them with the admitting provider than the oncologist.[8] Patients may feel that they are disappointing their oncologist by being unable to take further treatment or by admitting that treatment has failed them. Similarly, oncologists might view having this discussion as an admission of their failure as a provider. The palliative care specialist, on the other hand, has no responsibility for chemotherapy and possibly no prior relationship with the patient, thus alleviating this type of emotional association between provider and patient. Furthermore, the conversation about nonbeneficial chemotherapy provides a segue for the palliative care provider to discuss with patients what he or she does best: establishing goals of care, managing symptoms, and maintaining comfort. For the palliative care specialist, providing symptom management and the best possible quality of life for patients are the fundamental goals. Death is generally not viewed with a sense of failure when palliation is the focus of care.
Oncology: Palliative Care Is Giving Up
We still hear from oncologists like ourselves the dreaded words “What do you want me to do, give up on the patient?” or, to the patient, “What, are you giving up? I thought you'd keep fighting!” We would argue that current best practices include knowing when the risks and harms of chemotherapy outweigh any potential chance of benefit. Physicians and patients should follow current National Comprehensive Cancer Network (NCCN) guidelines for solid tumors such as breast9 and lung10 cancer and stop chemotherapy when the chance of success is minimal. If the doctor cannot describe a specific, substantial benefit that outweighs the toxicity, he or she should not recommend it.[11] And all the relevant guidelines call for considering a switch to nonchemotherapy palliative care when the patient's performance status is Eastern Cooperative Oncology Group (ECOG) ≥3, defined as “3 = Capable of only limited self-care, confined to bed or chair more than 50% of waking hours.”[12] Such a simple threshold could dramatically reduce the use of chemotherapy at the end of life and lessen downstream toxicities.
Oncologists can implement several strategies to help facilitate the transition from aggressive care to comfort care (Table 2). For patients with incurable cancer, oncologists can hold early discussions about palliative and hospice options that will need to be implemented when chemotherapy is no longer able to control their disease. This discussion introduces palliative medicine as part of the care plan for incurable disease and allows the patient to anticipate such a transition. Oncologists can also provide reassurance that they will continue to be involved in their patient's care and to support them, even if the patient does not undergo further chemotherapy. There are at least four studies that show equal[13] or better[6] survival, smoother transitions to hospice when death is inevitable, less intensive end-of-life care, and superior patient and family outcomes with concurrent palliative care. [14] and [15] By helping patients establish legal documents, such as ADs and power of attorney, oncologists and palliative care specialists can alleviate some of the stress related to the end of life and make the transition to comfort care easier. Finally, oncologists can review guidelines such as those from the NCCN and American Society of Clinical Oncology, which call for a switch to palliative care when the cancer has grown on three regimens or the patient's ECOG performance status is three or above. [11] and [12]
| Table 2: Things that help ONCOLOGISTS and their patients | ||
| Item | How it helps | Comments |
| Early discussion of palliative and hospice care when chemotherapy may no longer help. | Hospice (and eventual death) will not come as a complete surprise. | “We will do our best to help you with this cancer, but at some point there may not be any treatments known to help….” “Remember the conversation we had when we first met?...” |
| Reassurance that the oncologist will not abandon the patient if concurrent care is given. | This major fear may keep oncology patients at the same practice they have known for years – it is familiar – when they would be better served by transition. | There are now at least 4 randomized trials showing that most patients will accept concurrent palliative care if offered, and that outcomes are equal or better, at less cost.6,13,14,15 |
| Legal documents such as Advance Medical Directives, Durable Medical Power of Attorney | Reinforces the seriousness and “now” aspect of care. | These are readily available in all states at no cost. They are not the final word on how to live one’s remaining time, but will get the conversation started. |
| Best nationally recognized information showing that further chemotherapy will not help due to 3 prior failures, or is not indicated due to poor performance status.9,10 | The oncologist can point to the right page and say “The best national guidelines call for a switch away from chemo…because it will do no good and will cause harmful side effects.” | Readily accessed from the Internet. |
| Use decision aids, similar to Adjuvant!. | Increases the amount of truthful information given, even when the news is bad, and helps with transition points. | An increasing number of these are available[i],[ii],[iii],[iv] and will soon be offered as smart phone applications (aps). |
Communication tools, such as the National Cancer Institute's Oncotalk and EPEC-O, are useful for oncologists seeking to further enhance their communication skills.
Take-Home Messages
Guiding patients in making decisions about nonbeneficial, or futile, chemotherapy presents a challenge for many oncologists as well as their patients and families. Though futility is difficult to define, oncologists and their patients can decide through regular, open discussion if the burdens of chemotherapy outweigh the benefits and whether or not chemotherapy can achieve the reasonable benefits desired by the patient. “Your cancer is advancing despite our best efforts to keep it from growing. Let's talk about what options we have at this point and see what will work best for you.” To make such decisions, oncologists must obtain the most current information and convey it to patients (or their designated decision makers) as clearly as possible. “Based on the latest evidence, there is a 20% chance that the cancer will shrink or stay the same size with this treatment and an 80% chance that it will continue to grow despite treatment.” Both oncologists and their patients should involve those whom they trust to help with decision making. In cases where chemotherapy is nonbeneficial, oncologists may prefer to involve palliative and hospice care specialists to discuss the transition to comfort care with the patient. “At this time, I do not have any treatments that are likely to help you live longer or more comfortably, but I want to make sure that we get the most out of the rest of your life. I have asked a palliative care specialist to help us make this possible.” In order to ease the transition from aggressive or curative care to comfort care, oncologists can employ approaches such as early discussion of palliative and hospice care, assuring the patient of continued involvement in their care, and helping patients with ADs. These approaches not only benefit patients and their families but also strengthen the relationship between the oncologist and the patients and their families.
Acknowledgments
This research was supported by grants GO8 LM0095259 from the National Library of Medicine and R01CA116227-01 (both to T. J. S.) from the National Cancer Institute.
References [PubMed ID in brackets]
1 P.R. Helft, M. Siegler and J. Lantos, The rise and fall of the medical futility movement, . N Engl J Med, 343 (2000), pp. 293–296 [10911014].
2 L.J. Schneiderman, N.S. Jecker and A.R. Jonsen, Medical futility: its meaning and ethical implications, . Ann Intern Med, 112 (1990), pp. 949–954 [2187394].
3 E. Massarelli, F. Andre, D.D. Liu, J.J. Lee, M. Wolf, A. Fandi, J. Ochs, T. Le Chevalier, F. Fossella and R.S. Herbst, A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer, . Lung Cancer, 39 1 (2003), pp. 55–61 [12499095].
4 V. Zietemann and T. Duell, Every-day clinical practice in patients with advanced non-small-cell lung cancer, . Lung Cancer, 68 2 (2010), pp. 273–277 [19632737].
5 J.S. Temel, J.A. Greer, A. Muzikansky, E.R. Gallagher, S. Admane, V.A. Jackson, C.M. Dahlin, C.D. Blinderman, J. Jacobsen, W.F. Pirl, J.A. Billings and T.J. Lynch, Early palliative care for patients with metastatic non-small-cell lung cancer, . N Engl J Med, 363 (2010), pp. 733–742 [20818875].
6 S.R. Connor, B. Pyenson, K. Fitch, C. Spence and K. Iwasaki, Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage, 33 3 (2007), pp. 238–246.
7 S.E. Harrington and T.J. Smith, The role of chemotherapy at the end of life: “when is enough, enough?”. JAMA, 299 22 (2008), pp. 2667–2678.
8 L. Dow, R. Matsuyama, L. Kuhn, L. Lyckholm, E.B. Lamont and T.J. Smith, Paradoxes in advance care planning. J Clin Oncol, 28 2 (2010), pp. 299–304.
9 NCCN Breast Cancer Clinical Practice Guidelines Panel, R.W. Carlson, D.C. Allred, B.O. Anderson, H.J. Burstein, W.B. Carter, S.B. Edge, J.K. Erban, W.B. Farrar, L.J. Goldstein, W.J. Gradishar, D.F. Hayes, C.A. Hudis, M. Jahanzeb, K. Kiel, B.M. Ljung, P.K. Marcom, I.A. Mayer, B. McCormick, L.M. Nabell, L.J. Pierce, E.C. Reed, M.L. Smith, G. Somlo, R.L. Theriault, N.S. Topham, J.H. Ward, E.P. Winer and A.C. Wolff, Breast cancer. J Natl Compr Canc Netw, 7 2 (2009), pp. 122–192.
10 NCCN Non-Small Cell Lung Cancer Panel Members, D.S. Ettinger, W. Akerley, G. Bepler, M.G. Blum, A. Chang, R.T. Cheney, L.R. Chirieac, T.A. D'Amico, T.L. Demmy, A.K. Ganti, R. Govindan, F.W. Grannis, T. Jahan, M. Jahanzeb, D.H. Johnson, A. Kessinger, R. Komaki, F.M. Kong, M.G. Kris, L.M. Krug, Q.T. Le, I.T. Lennes, R. Martins, J. O'Malley, R.U. Osarogiagbon, G.A. Otterson, J.D. Patel, K.M. Pisters, K. Reckamp, G.J. Riely, E. Rohren, G.R. Simon, S.J. Swanson, D.E. Wood and S.C. Yang, Non-small cell lung cancer. J Natl Compr Canc Netw, 8 7 (2010), pp. 740–801.
11 American Society of Clinical Oncology Outcomes Working Group, Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol, 14 (1996), pp. 671–679.
12 Eastern Cooperative Oncology Group, ECOG Performance Status, http://ecog.dfci.harvard.edu/general/perf_stat.html Accessed November 30, 2010.
13 J. Finn, K. Pienta and J. Parzuchowski, Bridging cancer treatment and hospice care. Proc Am Soc Clin Oncol, 21 (2002), p. 1452.
14 G. Gade, I. Venohr, D. Conner, K. McGrady, J. Beane, R.H. Richardson, M.P. Williams, M. Liberson, M. Blum and R. Della Penna, Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med, 11 2 (2008), pp. 180–190.
Copyright © 2011 Elsevier Inc. All rights reserved.
Daughters and Sons
Editors’ Note: Patients are part of family systems, and understanding these systems can help psychiatrists advance treatment. That’s why we’re launching a new column we are calling Families in Psychiatry. In this column, Dr. Alison M. Heru will examine issues faced by psychiatrists who are involved in family therapy and psychoeducation. She will also look at family research and at the impact that caring for patients with mental illness has on caregivers. Often, she will also offer a global perspective on these issues. If you have an idea for Dr. Heru, e-mail her at [email protected].
At age 7, Maggie Jarry watched her mother "walk around the apartment trying to catch her eyes because she believed they had floated out of her face." Her mother often locked herself in the bathroom and talked to herself in the mirror because she believed that had telepathic powers.
"We lived like this for a year, until a babysitter and her mother figured out what was going on" and got professional help for Maggie’s mother. Her mother was diagnosed with schizoaffective disorder, and after she died in 2007, Maggie Jarry felt free to share her experience (Psychiatr. Serv. 2009;60:1587-8). "During these years, no one asked me about my experiences of living with my mom while she was ill. I was expected to just go play and be a child while she was in the hospital," Maggie wrote.
Maggie, now a community organizer, is part of a new consumer organization that seeks to provide support and resources to those who have a parent with mental illness. In the organization’s blog, called "Daughters and Sons," one writer expressed the burden of children living with parents with mental illness this way:
"People can be told that your family member is ‘sick’ but until they have concrete examples of how a day becomes an eternity as a child sits unknowingly, waiting for a storm to pass that has no time limit, they really have no idea what it is like. Until people ‘get it,’ they won’t be inclined to help change it and deal with it on a societal level. Unlike the adults who can get away from dysfunction, the child growing up in a crooked house has no escape."
In Maggie’s case, when her mother was doing well, the two had a good relationship. The bond that existed between the two underscores the essential human role that parenting can have in helping patients with mental illness reach wellness.
Many resources are available for children of parents with mental illness, although there are more in Europe, Australia, and New Zealand than in the United States. In the Netherlands, an online group course for Parents With Mental Illness has been piloted, and in Finland, clinician training has been studied. In the United States, the National Research Council and the Institute of Medicine produced a report on Depression in Parents, Parenting and Children: Opportunities to Improve Identification, Treatment, and Prevention. In addition, excellent web resources are listed at end of the column.
What can individual adult psychiatrists do? We can include children in family meetings about the parent’s illness. We can answer their questions about psychiatric illness. Children can also provide great insight into family functioning – strengths and weaknesses. We can provide age-appropriate literature when they visit the hospital or come with their parent to our office. We can ask our patients, their spouses, and other caregiving adults about their children. We can ask if they need help with parenting, and provide appropriate resources. We can reassure our patients that we want to help them become better parents, not remove their children! Families can be referred for help and support. If you see your patient as being part of a family, then you have a family or systems perspective of health care.
There are many family psychiatrists and many of us are members of the Association of Family Psychiatrists, which is an organization allied with the American Psychiatric Association. We have a website and a newsletter. Family psychiatrists are found in diverse settings, such as child and adolescent inpatient units, geriatric clinics, and psychosomatic medicine services. Those of us in outpatient practice may use family therapy as a single modality. Most of us, however, incorporate a family approach in our care of the patient. We use medication, individual therapy, and family interventions.
Several international family psychiatry resources are available online. Among them are the "Mental Health and Growing Up" leaflets, the "Children of Parents With a Mental Illness" website, and the Effective Family Programme.
I look forward to bringing you updates on family psychiatry, the latest in evidence-based family interventions, and other information aimed at helping you keep an updated family systems approach in your practice. Let me hear from you.
Editors’ Note: Patients are part of family systems, and understanding these systems can help psychiatrists advance treatment. That’s why we’re launching a new column we are calling Families in Psychiatry. In this column, Dr. Alison M. Heru will examine issues faced by psychiatrists who are involved in family therapy and psychoeducation. She will also look at family research and at the impact that caring for patients with mental illness has on caregivers. Often, she will also offer a global perspective on these issues. If you have an idea for Dr. Heru, e-mail her at [email protected].
At age 7, Maggie Jarry watched her mother "walk around the apartment trying to catch her eyes because she believed they had floated out of her face." Her mother often locked herself in the bathroom and talked to herself in the mirror because she believed that had telepathic powers.
"We lived like this for a year, until a babysitter and her mother figured out what was going on" and got professional help for Maggie’s mother. Her mother was diagnosed with schizoaffective disorder, and after she died in 2007, Maggie Jarry felt free to share her experience (Psychiatr. Serv. 2009;60:1587-8). "During these years, no one asked me about my experiences of living with my mom while she was ill. I was expected to just go play and be a child while she was in the hospital," Maggie wrote.
Maggie, now a community organizer, is part of a new consumer organization that seeks to provide support and resources to those who have a parent with mental illness. In the organization’s blog, called "Daughters and Sons," one writer expressed the burden of children living with parents with mental illness this way:
"People can be told that your family member is ‘sick’ but until they have concrete examples of how a day becomes an eternity as a child sits unknowingly, waiting for a storm to pass that has no time limit, they really have no idea what it is like. Until people ‘get it,’ they won’t be inclined to help change it and deal with it on a societal level. Unlike the adults who can get away from dysfunction, the child growing up in a crooked house has no escape."
In Maggie’s case, when her mother was doing well, the two had a good relationship. The bond that existed between the two underscores the essential human role that parenting can have in helping patients with mental illness reach wellness.
Many resources are available for children of parents with mental illness, although there are more in Europe, Australia, and New Zealand than in the United States. In the Netherlands, an online group course for Parents With Mental Illness has been piloted, and in Finland, clinician training has been studied. In the United States, the National Research Council and the Institute of Medicine produced a report on Depression in Parents, Parenting and Children: Opportunities to Improve Identification, Treatment, and Prevention. In addition, excellent web resources are listed at end of the column.
What can individual adult psychiatrists do? We can include children in family meetings about the parent’s illness. We can answer their questions about psychiatric illness. Children can also provide great insight into family functioning – strengths and weaknesses. We can provide age-appropriate literature when they visit the hospital or come with their parent to our office. We can ask our patients, their spouses, and other caregiving adults about their children. We can ask if they need help with parenting, and provide appropriate resources. We can reassure our patients that we want to help them become better parents, not remove their children! Families can be referred for help and support. If you see your patient as being part of a family, then you have a family or systems perspective of health care.
There are many family psychiatrists and many of us are members of the Association of Family Psychiatrists, which is an organization allied with the American Psychiatric Association. We have a website and a newsletter. Family psychiatrists are found in diverse settings, such as child and adolescent inpatient units, geriatric clinics, and psychosomatic medicine services. Those of us in outpatient practice may use family therapy as a single modality. Most of us, however, incorporate a family approach in our care of the patient. We use medication, individual therapy, and family interventions.
Several international family psychiatry resources are available online. Among them are the "Mental Health and Growing Up" leaflets, the "Children of Parents With a Mental Illness" website, and the Effective Family Programme.
I look forward to bringing you updates on family psychiatry, the latest in evidence-based family interventions, and other information aimed at helping you keep an updated family systems approach in your practice. Let me hear from you.
Editors’ Note: Patients are part of family systems, and understanding these systems can help psychiatrists advance treatment. That’s why we’re launching a new column we are calling Families in Psychiatry. In this column, Dr. Alison M. Heru will examine issues faced by psychiatrists who are involved in family therapy and psychoeducation. She will also look at family research and at the impact that caring for patients with mental illness has on caregivers. Often, she will also offer a global perspective on these issues. If you have an idea for Dr. Heru, e-mail her at [email protected].
At age 7, Maggie Jarry watched her mother "walk around the apartment trying to catch her eyes because she believed they had floated out of her face." Her mother often locked herself in the bathroom and talked to herself in the mirror because she believed that had telepathic powers.
"We lived like this for a year, until a babysitter and her mother figured out what was going on" and got professional help for Maggie’s mother. Her mother was diagnosed with schizoaffective disorder, and after she died in 2007, Maggie Jarry felt free to share her experience (Psychiatr. Serv. 2009;60:1587-8). "During these years, no one asked me about my experiences of living with my mom while she was ill. I was expected to just go play and be a child while she was in the hospital," Maggie wrote.
Maggie, now a community organizer, is part of a new consumer organization that seeks to provide support and resources to those who have a parent with mental illness. In the organization’s blog, called "Daughters and Sons," one writer expressed the burden of children living with parents with mental illness this way:
"People can be told that your family member is ‘sick’ but until they have concrete examples of how a day becomes an eternity as a child sits unknowingly, waiting for a storm to pass that has no time limit, they really have no idea what it is like. Until people ‘get it,’ they won’t be inclined to help change it and deal with it on a societal level. Unlike the adults who can get away from dysfunction, the child growing up in a crooked house has no escape."
In Maggie’s case, when her mother was doing well, the two had a good relationship. The bond that existed between the two underscores the essential human role that parenting can have in helping patients with mental illness reach wellness.
Many resources are available for children of parents with mental illness, although there are more in Europe, Australia, and New Zealand than in the United States. In the Netherlands, an online group course for Parents With Mental Illness has been piloted, and in Finland, clinician training has been studied. In the United States, the National Research Council and the Institute of Medicine produced a report on Depression in Parents, Parenting and Children: Opportunities to Improve Identification, Treatment, and Prevention. In addition, excellent web resources are listed at end of the column.
What can individual adult psychiatrists do? We can include children in family meetings about the parent’s illness. We can answer their questions about psychiatric illness. Children can also provide great insight into family functioning – strengths and weaknesses. We can provide age-appropriate literature when they visit the hospital or come with their parent to our office. We can ask our patients, their spouses, and other caregiving adults about their children. We can ask if they need help with parenting, and provide appropriate resources. We can reassure our patients that we want to help them become better parents, not remove their children! Families can be referred for help and support. If you see your patient as being part of a family, then you have a family or systems perspective of health care.
There are many family psychiatrists and many of us are members of the Association of Family Psychiatrists, which is an organization allied with the American Psychiatric Association. We have a website and a newsletter. Family psychiatrists are found in diverse settings, such as child and adolescent inpatient units, geriatric clinics, and psychosomatic medicine services. Those of us in outpatient practice may use family therapy as a single modality. Most of us, however, incorporate a family approach in our care of the patient. We use medication, individual therapy, and family interventions.
Several international family psychiatry resources are available online. Among them are the "Mental Health and Growing Up" leaflets, the "Children of Parents With a Mental Illness" website, and the Effective Family Programme.
I look forward to bringing you updates on family psychiatry, the latest in evidence-based family interventions, and other information aimed at helping you keep an updated family systems approach in your practice. Let me hear from you.
Pemetrexed/Bevacizumab Maintenance Combo Stalls Lung Cancer
STOCKHOLM – Adding pemetrexed to bevacizumab maintenance therapy cut the relative risk of disease progression for patients with advanced nonsquamous non–small cell lung cancer in a phase III clinical trial.
Patients on the combination had a median progression-free survival of 10.2 months from the start of first-line induction therapy vs. 6.6 months with solo bevacizumab maintenance in the randomized open label study (hazard ratio, 0.50; P less than.001).
The same measure from randomization to maintenance therapy was twice as long with the combination therapy as with bevacizumab alone – 7.4 months vs. 3.7 months (HR = 0.48; P less than.001).
"First-line cisplatin/pemetrexed/bevacizumab followed by continuation maintenance with bevacizumab and pemetrexed achieved a patient PFS [progression-free survival] benefit of unprecedented magnitude," said Dr. Fabrice Barlesi, who presented the results of the AVAPERL trial at the European Multidisciplinary Cancer Congress.
The researchers recruited patients with previously untreated stage IIIB-IV advanced nonsquamous non–small cell lung cancer (nsNSCLC). All patients received four 3-week cycles of first-line induction with bevacizumab, pemetrexed (Alimta), and cisplatin.
Patients with complete response, partial response, or stable disease at the end of this treatment were randomized to continuation maintenance with bevacizumab or bevacizumab and pemetrexed in 3-week cycles until disease progression. Progression-free survival was assessed from the beginning of induction therapy to first progressive disease or death from any cause.
A total of 376 patients started first-line induction therapy; 123 were not eligible for randomization due to disease progression. Of the remainder, 253 patients were randomized to maintenance therapy with bevacizumab alone (125) or bevacizumab plus pemetrexed (128). Three patients did not receive maintenance treatment.
Median follow-up was 11 months for this analysis presented at the joint congress of the European Cancer Organization (ECCO), the European Society for Medical Oncology (ESMO), and the European Society for Radiotherapy and Oncology (ESTRO).
Overall survival from induction was 15.7 months with bevacizumab alone but has not been reached yet with the combination maintenance therapy, according to Dr. Barlesi of the multidisciplinary oncology and therapeutic innovations department at the Assistance Publique Hôpitaux de Marseille, France.
First-line therapy with cisplatin, pemetrexed, and bevacizumab was well tolerated with no new or unexpected toxicities.
Notably, grade 3-5 hematologic adverse events were greater with the bevacizumab plus pemetrexed arm vs. the control group (10% vs. 0%). Grade 3-5 nonhematologic events also were greater with the combination maintenance treatment (31% vs. 22%).
Pemetrexed is approved in the United States for maintenance treatment of nonsquamous locally advanced or metastatic non–small cell lung cancer that has not progressed after four cycles of platinum-based chemotherapy. AVAPERL was the first phase III trial to investigate the combination of pemetrexed and bevacizumab as maintenance therapy in this disease.
The study was funded by Hoffman-La Roche. Dr. Barlesi reported that he has been a consultant for and received research funding from Roche and Lilly. One of the study authors is an employee for Hoffman-La Roche.
STOCKHOLM – Adding pemetrexed to bevacizumab maintenance therapy cut the relative risk of disease progression for patients with advanced nonsquamous non–small cell lung cancer in a phase III clinical trial.
Patients on the combination had a median progression-free survival of 10.2 months from the start of first-line induction therapy vs. 6.6 months with solo bevacizumab maintenance in the randomized open label study (hazard ratio, 0.50; P less than.001).
The same measure from randomization to maintenance therapy was twice as long with the combination therapy as with bevacizumab alone – 7.4 months vs. 3.7 months (HR = 0.48; P less than.001).
"First-line cisplatin/pemetrexed/bevacizumab followed by continuation maintenance with bevacizumab and pemetrexed achieved a patient PFS [progression-free survival] benefit of unprecedented magnitude," said Dr. Fabrice Barlesi, who presented the results of the AVAPERL trial at the European Multidisciplinary Cancer Congress.
The researchers recruited patients with previously untreated stage IIIB-IV advanced nonsquamous non–small cell lung cancer (nsNSCLC). All patients received four 3-week cycles of first-line induction with bevacizumab, pemetrexed (Alimta), and cisplatin.
Patients with complete response, partial response, or stable disease at the end of this treatment were randomized to continuation maintenance with bevacizumab or bevacizumab and pemetrexed in 3-week cycles until disease progression. Progression-free survival was assessed from the beginning of induction therapy to first progressive disease or death from any cause.
A total of 376 patients started first-line induction therapy; 123 were not eligible for randomization due to disease progression. Of the remainder, 253 patients were randomized to maintenance therapy with bevacizumab alone (125) or bevacizumab plus pemetrexed (128). Three patients did not receive maintenance treatment.
Median follow-up was 11 months for this analysis presented at the joint congress of the European Cancer Organization (ECCO), the European Society for Medical Oncology (ESMO), and the European Society for Radiotherapy and Oncology (ESTRO).
Overall survival from induction was 15.7 months with bevacizumab alone but has not been reached yet with the combination maintenance therapy, according to Dr. Barlesi of the multidisciplinary oncology and therapeutic innovations department at the Assistance Publique Hôpitaux de Marseille, France.
First-line therapy with cisplatin, pemetrexed, and bevacizumab was well tolerated with no new or unexpected toxicities.
Notably, grade 3-5 hematologic adverse events were greater with the bevacizumab plus pemetrexed arm vs. the control group (10% vs. 0%). Grade 3-5 nonhematologic events also were greater with the combination maintenance treatment (31% vs. 22%).
Pemetrexed is approved in the United States for maintenance treatment of nonsquamous locally advanced or metastatic non–small cell lung cancer that has not progressed after four cycles of platinum-based chemotherapy. AVAPERL was the first phase III trial to investigate the combination of pemetrexed and bevacizumab as maintenance therapy in this disease.
The study was funded by Hoffman-La Roche. Dr. Barlesi reported that he has been a consultant for and received research funding from Roche and Lilly. One of the study authors is an employee for Hoffman-La Roche.
STOCKHOLM – Adding pemetrexed to bevacizumab maintenance therapy cut the relative risk of disease progression for patients with advanced nonsquamous non–small cell lung cancer in a phase III clinical trial.
Patients on the combination had a median progression-free survival of 10.2 months from the start of first-line induction therapy vs. 6.6 months with solo bevacizumab maintenance in the randomized open label study (hazard ratio, 0.50; P less than.001).
The same measure from randomization to maintenance therapy was twice as long with the combination therapy as with bevacizumab alone – 7.4 months vs. 3.7 months (HR = 0.48; P less than.001).
"First-line cisplatin/pemetrexed/bevacizumab followed by continuation maintenance with bevacizumab and pemetrexed achieved a patient PFS [progression-free survival] benefit of unprecedented magnitude," said Dr. Fabrice Barlesi, who presented the results of the AVAPERL trial at the European Multidisciplinary Cancer Congress.
The researchers recruited patients with previously untreated stage IIIB-IV advanced nonsquamous non–small cell lung cancer (nsNSCLC). All patients received four 3-week cycles of first-line induction with bevacizumab, pemetrexed (Alimta), and cisplatin.
Patients with complete response, partial response, or stable disease at the end of this treatment were randomized to continuation maintenance with bevacizumab or bevacizumab and pemetrexed in 3-week cycles until disease progression. Progression-free survival was assessed from the beginning of induction therapy to first progressive disease or death from any cause.
A total of 376 patients started first-line induction therapy; 123 were not eligible for randomization due to disease progression. Of the remainder, 253 patients were randomized to maintenance therapy with bevacizumab alone (125) or bevacizumab plus pemetrexed (128). Three patients did not receive maintenance treatment.
Median follow-up was 11 months for this analysis presented at the joint congress of the European Cancer Organization (ECCO), the European Society for Medical Oncology (ESMO), and the European Society for Radiotherapy and Oncology (ESTRO).
Overall survival from induction was 15.7 months with bevacizumab alone but has not been reached yet with the combination maintenance therapy, according to Dr. Barlesi of the multidisciplinary oncology and therapeutic innovations department at the Assistance Publique Hôpitaux de Marseille, France.
First-line therapy with cisplatin, pemetrexed, and bevacizumab was well tolerated with no new or unexpected toxicities.
Notably, grade 3-5 hematologic adverse events were greater with the bevacizumab plus pemetrexed arm vs. the control group (10% vs. 0%). Grade 3-5 nonhematologic events also were greater with the combination maintenance treatment (31% vs. 22%).
Pemetrexed is approved in the United States for maintenance treatment of nonsquamous locally advanced or metastatic non–small cell lung cancer that has not progressed after four cycles of platinum-based chemotherapy. AVAPERL was the first phase III trial to investigate the combination of pemetrexed and bevacizumab as maintenance therapy in this disease.
The study was funded by Hoffman-La Roche. Dr. Barlesi reported that he has been a consultant for and received research funding from Roche and Lilly. One of the study authors is an employee for Hoffman-La Roche.
FROM THE EUROPEAN MULTIDISCIPLINARY CANCER CONGRESS
Findings: Patients on the combination maintenance therapy had a median progression-free survival of 10.2 months vs. 6.6 months for bevacizumab alone from the start of first-line induction therapy (hazard ratio, 0.50, P less than.001).
Source: Open-label, phase III trial of 376 patients with previously untreated stage IIIB-IV advanced nsNSCLC.
Disclosures: The study was funded by Hoffman-La Roche. Dr. Barlesi reported that he has been a consultant for and received research funding from Roche and Lilly. One of the study authors is an employee for Hoffman-La Roche.
Introducing Our New International Editors
If our readers have any doubt, all they need do is casually scan the program for the recent VAM in Chicago or examine the content and Editorial Board of a current copy of the JVS to realize that the Society for Vascular Surgery is unquestionably an international organization. This sort of globalization, in contrast to the variety that, according to Tom Friedman, makes the world flat instead makes our specialty more vibrant and interesting than ever.
Because of this world change we believe that our journals should better represent the people that write and read our journals, and so, too, this newspaper. For this reason, we introduce in this issue our two newest members of the Vascular Specialist Editorial Board: From England, we have Professor Cliff Shearman, and from Australia, Professor Rob Fitridge. Both have a well-deserved preeminence and will broaden the view and the viewpoint of your SVS newspaper.
Professor Shearman is professor of vascular surgery at the University of Southampton and a consultant vascular surgeon at Southampton University Hospitals NHS Trust. He was on the Council and Chairman of the Training and Education Committee of the Vascular Society of Great Britain and Ireland, and President of the Society (2009-2010) He has a long time interest in training and was appointed head of the Wessex Post Graduate School of Surgery in 2007. He is currently president elect of the Society for Academic Research Surgery and Director of Professional Practice for the Association of Surgeons of Great Britain and Ireland. His main clinical interest is in the vascular complications of diabetes and in particular trying to reduce the rate of amputation in this group.
Professor Fitridge is professor of vascular surgery at the University of Adelaide and Head of Vascular Surgery at The Queen Elizabeth Hospital. He became chairman of the Board of Vascular Surgery in 2002 and during his tenure the online curriculum was developed. In collaboration with Matt Thompson he edited "Mechanisms of Vascular Disease: A Textbook for Vascular Surgeons" published by Cambridge University Press. His research interests include the systemic effects of skeletal muscle reperfusion injury and outcome modelling in aortic surgery. He recently was elected president of the ANZSVS and is president of the World Federation of Vascular Societies.
We welcome these new additions to our editorial team.
George Andros, M.D.
Medical Editor
If our readers have any doubt, all they need do is casually scan the program for the recent VAM in Chicago or examine the content and Editorial Board of a current copy of the JVS to realize that the Society for Vascular Surgery is unquestionably an international organization. This sort of globalization, in contrast to the variety that, according to Tom Friedman, makes the world flat instead makes our specialty more vibrant and interesting than ever.
Because of this world change we believe that our journals should better represent the people that write and read our journals, and so, too, this newspaper. For this reason, we introduce in this issue our two newest members of the Vascular Specialist Editorial Board: From England, we have Professor Cliff Shearman, and from Australia, Professor Rob Fitridge. Both have a well-deserved preeminence and will broaden the view and the viewpoint of your SVS newspaper.
Professor Shearman is professor of vascular surgery at the University of Southampton and a consultant vascular surgeon at Southampton University Hospitals NHS Trust. He was on the Council and Chairman of the Training and Education Committee of the Vascular Society of Great Britain and Ireland, and President of the Society (2009-2010) He has a long time interest in training and was appointed head of the Wessex Post Graduate School of Surgery in 2007. He is currently president elect of the Society for Academic Research Surgery and Director of Professional Practice for the Association of Surgeons of Great Britain and Ireland. His main clinical interest is in the vascular complications of diabetes and in particular trying to reduce the rate of amputation in this group.
Professor Fitridge is professor of vascular surgery at the University of Adelaide and Head of Vascular Surgery at The Queen Elizabeth Hospital. He became chairman of the Board of Vascular Surgery in 2002 and during his tenure the online curriculum was developed. In collaboration with Matt Thompson he edited "Mechanisms of Vascular Disease: A Textbook for Vascular Surgeons" published by Cambridge University Press. His research interests include the systemic effects of skeletal muscle reperfusion injury and outcome modelling in aortic surgery. He recently was elected president of the ANZSVS and is president of the World Federation of Vascular Societies.
We welcome these new additions to our editorial team.
George Andros, M.D.
Medical Editor
If our readers have any doubt, all they need do is casually scan the program for the recent VAM in Chicago or examine the content and Editorial Board of a current copy of the JVS to realize that the Society for Vascular Surgery is unquestionably an international organization. This sort of globalization, in contrast to the variety that, according to Tom Friedman, makes the world flat instead makes our specialty more vibrant and interesting than ever.
Because of this world change we believe that our journals should better represent the people that write and read our journals, and so, too, this newspaper. For this reason, we introduce in this issue our two newest members of the Vascular Specialist Editorial Board: From England, we have Professor Cliff Shearman, and from Australia, Professor Rob Fitridge. Both have a well-deserved preeminence and will broaden the view and the viewpoint of your SVS newspaper.
Professor Shearman is professor of vascular surgery at the University of Southampton and a consultant vascular surgeon at Southampton University Hospitals NHS Trust. He was on the Council and Chairman of the Training and Education Committee of the Vascular Society of Great Britain and Ireland, and President of the Society (2009-2010) He has a long time interest in training and was appointed head of the Wessex Post Graduate School of Surgery in 2007. He is currently president elect of the Society for Academic Research Surgery and Director of Professional Practice for the Association of Surgeons of Great Britain and Ireland. His main clinical interest is in the vascular complications of diabetes and in particular trying to reduce the rate of amputation in this group.
Professor Fitridge is professor of vascular surgery at the University of Adelaide and Head of Vascular Surgery at The Queen Elizabeth Hospital. He became chairman of the Board of Vascular Surgery in 2002 and during his tenure the online curriculum was developed. In collaboration with Matt Thompson he edited "Mechanisms of Vascular Disease: A Textbook for Vascular Surgeons" published by Cambridge University Press. His research interests include the systemic effects of skeletal muscle reperfusion injury and outcome modelling in aortic surgery. He recently was elected president of the ANZSVS and is president of the World Federation of Vascular Societies.
We welcome these new additions to our editorial team.
George Andros, M.D.
Medical Editor
Can RCTs be Misleading and Biased?
Randomized controlled trials (RCTs) constitute level 1 evidence, which is widely considered the best data upon which to base medical practice. This is particularly true when the RCTs are published in leading journals like the New England Journal of Medicine or Lancet. Such trials are viewed by many as the Holy Grail of medicine and thus infallible and inviolate.
However, RCTs can have many flaws that render them obsolete, non-applicable or outright misleading. More importantly RCTs can be misinterpreted or spun by their authors or others so that they exert an effect on practice trends or standards unjustified by their data.
Possible flaws in RCTs are of two types:
1. Timeliness flaws can occur when progress is made in the treatment under evaluation arm or the control arm. Examples would be the early trials of carotid stenting (CAS) vs. carotid endarterectomy (CEA). If progress in CAS technology or patient selection occurs, a trial showing CAS inferiority becomes invalid. In contrast, the landmark trials showing CEA to be superior to medical treatment in preventing strokes have become obsolete because dramatic progress has been made with medical treatment.
2. Many design flaws can impair the validity of RCTs. These include patient selection flaws (e.g. in SAPPHIRE, patients were selected for randomization only if they were high risk for CEA). SAPPHIRE also included 71% asymptomatic patients in whom the high adverse event rates for both CEA and CAS were unjustified. Good medical treatment would have served these patients better. CREST also had patient selection flaws. It was originally designed to compare CAS and CEA only in symptomatic patients. When adequate numbers of patients could not be recruited, asymptomatic patients were added, thereby diluting the power of the study and impairing the statistical significance of some of its results.
Other design flaws include questionable competence of operators in a trial (e.g. the CAS operators in the EVA-3S and ICSS trials); problems with randomization (e.g. SAPPHIRE in which only 10% of eligible patients were randomized); and questionable applicability of RCT results to real world practice (e.g. CAS operators in CREST were highly vetted and more skilled than others performing the procedure).
There are also idiosyncratic flaws, as in the EVAR 2 trial in patients unfit for open repair. Although this trial, published in Lancet, showed EVAR to have similar mortality to no treatment, half the deaths in the group randomized to EVAR occurred from rupture during a lengthy (average 57 days) waiting period before treatment. Had these deaths been prevented by a more timely EVAR, the conclusion of EVAR 2 might have been different.
Inappropriate or questionable primary endpoints in RCTS are another design flaw that can lead to misleading conclusions. An example is the inclusion of minor myocardial infarctions (MIs) with strokes and deaths as a composite endpoint in a CAS vs. CEA trial (e.g. SAPPHIRE and CREST).
The components of the primary endpoint in the CAS and CEA arms of CREST were death, stroke, and myocardial infarction. Total stroke and minor strokes were both significantly different in the two groups in favor of CEA, and death and major strokes, although not significantly different between the two groups were both numerically higher for CAS. (See complete table oline at www. vascularspecialistonline.com)
Although it is arguable, it is hard to understand how minor MIs are the equivalent of strokes and deaths, and only when MIs were included were the adverse event rates in the two groups similar (7.2% for CAS vs 6.8% for CEA, P = .051).
So much for the flaws in RCTs. What about good trials or those with only minor weaknesses? Even these can result in misleading conclusions when the authors reach conclusions unjustified by their own data. SAPPHIRE and CREST are two recent examples.
Despite the flaws in these trials, both of which were reported in the New England Journal of Medicine, the authors concluded that "with high risk patients CAS and CEA are equivalent treatments" (SAPPHIRE) and "among patients with symptomatic and asymptomatic carotid stenosis, the risk of the composite primary end-point ... did not differ significantly in the group undergoing CAS and the group undergoing CEA" (CREST).
Although the CREST authors pointed out the higher incidence of stroke with stenting, others have used the CREST study to claim equivalence of CAS and CEA. Nowhere is this more apparent than in the recent American Heart Association (AHA) Guideline on the management of patients with extracranial carotid and vertebral artery disease.
This important and influential document, which was also approved by 13 other organizations including the SVS, stated that "CAS is indicated as an alternative to CEA for symptomatic patients at average or low risk of complications associated from endovascular interventions...." In Webster’s Dictionary one definition of "alternative" is "a choice between 2 things".
This clearly implies equivalence, and it has been so interpreted by many others, particularly those biased toward catheter based treatment. Of note, the AHA Guideline appears to be based largely on CREST, and did not even consider the findings of the ICSS trial, published in Lancet the same day as the main article reporting CREST.
Although ICSS may also have flaws, it showed, in a large group of only symptomatic patients, that CAS produced significantly more strokes and diffusion weighted MRI defects than did CEA. It is hard to understand why these ICSS results did not have more of an influence on the AHA Guideline.
Although my bias as a CAS enthusiast makes me believe that CAS will ultimately have a major role in the treatment of carotid stenosis patients, that bias is not yet sufficient for me to spin the data and believe we are now there. One has to wonder if bias more intense than mine was involved in the conclusion reached in the AHA Guideline.
Thus, it is apparent that misleading conclusions can be reached in articles reporting RCTs in leading journals. These can be the result of flaws in the RCTs and/or unrecognized author bias. More importantly, the results of even good trials can be further misinterpreted by others to guide practice standards in a way unjustified by the data.
It is important for all to recognize the possible role of bias in these misinterpretations. By recognizing the possible flaws in RCTs and that physicians, like all other people, are influenced by bias, we can exercise the judgment to use RCTs fairly to help us treat individual patients optimally.n
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and professor of surgery and William J. von Liebig Chair in vascular surgery at Case Western Reserve University and The Cleveland Clinic.
Randomized controlled trials (RCTs) constitute level 1 evidence, which is widely considered the best data upon which to base medical practice. This is particularly true when the RCTs are published in leading journals like the New England Journal of Medicine or Lancet. Such trials are viewed by many as the Holy Grail of medicine and thus infallible and inviolate.
However, RCTs can have many flaws that render them obsolete, non-applicable or outright misleading. More importantly RCTs can be misinterpreted or spun by their authors or others so that they exert an effect on practice trends or standards unjustified by their data.
Possible flaws in RCTs are of two types:
1. Timeliness flaws can occur when progress is made in the treatment under evaluation arm or the control arm. Examples would be the early trials of carotid stenting (CAS) vs. carotid endarterectomy (CEA). If progress in CAS technology or patient selection occurs, a trial showing CAS inferiority becomes invalid. In contrast, the landmark trials showing CEA to be superior to medical treatment in preventing strokes have become obsolete because dramatic progress has been made with medical treatment.
2. Many design flaws can impair the validity of RCTs. These include patient selection flaws (e.g. in SAPPHIRE, patients were selected for randomization only if they were high risk for CEA). SAPPHIRE also included 71% asymptomatic patients in whom the high adverse event rates for both CEA and CAS were unjustified. Good medical treatment would have served these patients better. CREST also had patient selection flaws. It was originally designed to compare CAS and CEA only in symptomatic patients. When adequate numbers of patients could not be recruited, asymptomatic patients were added, thereby diluting the power of the study and impairing the statistical significance of some of its results.
Other design flaws include questionable competence of operators in a trial (e.g. the CAS operators in the EVA-3S and ICSS trials); problems with randomization (e.g. SAPPHIRE in which only 10% of eligible patients were randomized); and questionable applicability of RCT results to real world practice (e.g. CAS operators in CREST were highly vetted and more skilled than others performing the procedure).
There are also idiosyncratic flaws, as in the EVAR 2 trial in patients unfit for open repair. Although this trial, published in Lancet, showed EVAR to have similar mortality to no treatment, half the deaths in the group randomized to EVAR occurred from rupture during a lengthy (average 57 days) waiting period before treatment. Had these deaths been prevented by a more timely EVAR, the conclusion of EVAR 2 might have been different.
Inappropriate or questionable primary endpoints in RCTS are another design flaw that can lead to misleading conclusions. An example is the inclusion of minor myocardial infarctions (MIs) with strokes and deaths as a composite endpoint in a CAS vs. CEA trial (e.g. SAPPHIRE and CREST).
The components of the primary endpoint in the CAS and CEA arms of CREST were death, stroke, and myocardial infarction. Total stroke and minor strokes were both significantly different in the two groups in favor of CEA, and death and major strokes, although not significantly different between the two groups were both numerically higher for CAS. (See complete table oline at www. vascularspecialistonline.com)
Although it is arguable, it is hard to understand how minor MIs are the equivalent of strokes and deaths, and only when MIs were included were the adverse event rates in the two groups similar (7.2% for CAS vs 6.8% for CEA, P = .051).
So much for the flaws in RCTs. What about good trials or those with only minor weaknesses? Even these can result in misleading conclusions when the authors reach conclusions unjustified by their own data. SAPPHIRE and CREST are two recent examples.
Despite the flaws in these trials, both of which were reported in the New England Journal of Medicine, the authors concluded that "with high risk patients CAS and CEA are equivalent treatments" (SAPPHIRE) and "among patients with symptomatic and asymptomatic carotid stenosis, the risk of the composite primary end-point ... did not differ significantly in the group undergoing CAS and the group undergoing CEA" (CREST).
Although the CREST authors pointed out the higher incidence of stroke with stenting, others have used the CREST study to claim equivalence of CAS and CEA. Nowhere is this more apparent than in the recent American Heart Association (AHA) Guideline on the management of patients with extracranial carotid and vertebral artery disease.
This important and influential document, which was also approved by 13 other organizations including the SVS, stated that "CAS is indicated as an alternative to CEA for symptomatic patients at average or low risk of complications associated from endovascular interventions...." In Webster’s Dictionary one definition of "alternative" is "a choice between 2 things".
This clearly implies equivalence, and it has been so interpreted by many others, particularly those biased toward catheter based treatment. Of note, the AHA Guideline appears to be based largely on CREST, and did not even consider the findings of the ICSS trial, published in Lancet the same day as the main article reporting CREST.
Although ICSS may also have flaws, it showed, in a large group of only symptomatic patients, that CAS produced significantly more strokes and diffusion weighted MRI defects than did CEA. It is hard to understand why these ICSS results did not have more of an influence on the AHA Guideline.
Although my bias as a CAS enthusiast makes me believe that CAS will ultimately have a major role in the treatment of carotid stenosis patients, that bias is not yet sufficient for me to spin the data and believe we are now there. One has to wonder if bias more intense than mine was involved in the conclusion reached in the AHA Guideline.
Thus, it is apparent that misleading conclusions can be reached in articles reporting RCTs in leading journals. These can be the result of flaws in the RCTs and/or unrecognized author bias. More importantly, the results of even good trials can be further misinterpreted by others to guide practice standards in a way unjustified by the data.
It is important for all to recognize the possible role of bias in these misinterpretations. By recognizing the possible flaws in RCTs and that physicians, like all other people, are influenced by bias, we can exercise the judgment to use RCTs fairly to help us treat individual patients optimally.n
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and professor of surgery and William J. von Liebig Chair in vascular surgery at Case Western Reserve University and The Cleveland Clinic.
Randomized controlled trials (RCTs) constitute level 1 evidence, which is widely considered the best data upon which to base medical practice. This is particularly true when the RCTs are published in leading journals like the New England Journal of Medicine or Lancet. Such trials are viewed by many as the Holy Grail of medicine and thus infallible and inviolate.
However, RCTs can have many flaws that render them obsolete, non-applicable or outright misleading. More importantly RCTs can be misinterpreted or spun by their authors or others so that they exert an effect on practice trends or standards unjustified by their data.
Possible flaws in RCTs are of two types:
1. Timeliness flaws can occur when progress is made in the treatment under evaluation arm or the control arm. Examples would be the early trials of carotid stenting (CAS) vs. carotid endarterectomy (CEA). If progress in CAS technology or patient selection occurs, a trial showing CAS inferiority becomes invalid. In contrast, the landmark trials showing CEA to be superior to medical treatment in preventing strokes have become obsolete because dramatic progress has been made with medical treatment.
2. Many design flaws can impair the validity of RCTs. These include patient selection flaws (e.g. in SAPPHIRE, patients were selected for randomization only if they were high risk for CEA). SAPPHIRE also included 71% asymptomatic patients in whom the high adverse event rates for both CEA and CAS were unjustified. Good medical treatment would have served these patients better. CREST also had patient selection flaws. It was originally designed to compare CAS and CEA only in symptomatic patients. When adequate numbers of patients could not be recruited, asymptomatic patients were added, thereby diluting the power of the study and impairing the statistical significance of some of its results.
Other design flaws include questionable competence of operators in a trial (e.g. the CAS operators in the EVA-3S and ICSS trials); problems with randomization (e.g. SAPPHIRE in which only 10% of eligible patients were randomized); and questionable applicability of RCT results to real world practice (e.g. CAS operators in CREST were highly vetted and more skilled than others performing the procedure).
There are also idiosyncratic flaws, as in the EVAR 2 trial in patients unfit for open repair. Although this trial, published in Lancet, showed EVAR to have similar mortality to no treatment, half the deaths in the group randomized to EVAR occurred from rupture during a lengthy (average 57 days) waiting period before treatment. Had these deaths been prevented by a more timely EVAR, the conclusion of EVAR 2 might have been different.
Inappropriate or questionable primary endpoints in RCTS are another design flaw that can lead to misleading conclusions. An example is the inclusion of minor myocardial infarctions (MIs) with strokes and deaths as a composite endpoint in a CAS vs. CEA trial (e.g. SAPPHIRE and CREST).
The components of the primary endpoint in the CAS and CEA arms of CREST were death, stroke, and myocardial infarction. Total stroke and minor strokes were both significantly different in the two groups in favor of CEA, and death and major strokes, although not significantly different between the two groups were both numerically higher for CAS. (See complete table oline at www. vascularspecialistonline.com)
Although it is arguable, it is hard to understand how minor MIs are the equivalent of strokes and deaths, and only when MIs were included were the adverse event rates in the two groups similar (7.2% for CAS vs 6.8% for CEA, P = .051).
So much for the flaws in RCTs. What about good trials or those with only minor weaknesses? Even these can result in misleading conclusions when the authors reach conclusions unjustified by their own data. SAPPHIRE and CREST are two recent examples.
Despite the flaws in these trials, both of which were reported in the New England Journal of Medicine, the authors concluded that "with high risk patients CAS and CEA are equivalent treatments" (SAPPHIRE) and "among patients with symptomatic and asymptomatic carotid stenosis, the risk of the composite primary end-point ... did not differ significantly in the group undergoing CAS and the group undergoing CEA" (CREST).
Although the CREST authors pointed out the higher incidence of stroke with stenting, others have used the CREST study to claim equivalence of CAS and CEA. Nowhere is this more apparent than in the recent American Heart Association (AHA) Guideline on the management of patients with extracranial carotid and vertebral artery disease.
This important and influential document, which was also approved by 13 other organizations including the SVS, stated that "CAS is indicated as an alternative to CEA for symptomatic patients at average or low risk of complications associated from endovascular interventions...." In Webster’s Dictionary one definition of "alternative" is "a choice between 2 things".
This clearly implies equivalence, and it has been so interpreted by many others, particularly those biased toward catheter based treatment. Of note, the AHA Guideline appears to be based largely on CREST, and did not even consider the findings of the ICSS trial, published in Lancet the same day as the main article reporting CREST.
Although ICSS may also have flaws, it showed, in a large group of only symptomatic patients, that CAS produced significantly more strokes and diffusion weighted MRI defects than did CEA. It is hard to understand why these ICSS results did not have more of an influence on the AHA Guideline.
Although my bias as a CAS enthusiast makes me believe that CAS will ultimately have a major role in the treatment of carotid stenosis patients, that bias is not yet sufficient for me to spin the data and believe we are now there. One has to wonder if bias more intense than mine was involved in the conclusion reached in the AHA Guideline.
Thus, it is apparent that misleading conclusions can be reached in articles reporting RCTs in leading journals. These can be the result of flaws in the RCTs and/or unrecognized author bias. More importantly, the results of even good trials can be further misinterpreted by others to guide practice standards in a way unjustified by the data.
It is important for all to recognize the possible role of bias in these misinterpretations. By recognizing the possible flaws in RCTs and that physicians, like all other people, are influenced by bias, we can exercise the judgment to use RCTs fairly to help us treat individual patients optimally.n
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and professor of surgery and William J. von Liebig Chair in vascular surgery at Case Western Reserve University and The Cleveland Clinic.
Continuous Admission Model Reduces LOS
Smooth and timely hospital patient flow can have multiple positive effects including reduced wait times for services, decreased congestion in the Emergency Department (ED), and increased patient and staff satisfaction.14 One way to improve patient flow is to remove variation along the care pathway.57
For teaching hospitals that provide team‐based care, 1 significant source of variation involves the emergent admission process.8, 9 Typically, for services that admit the majority of their patients from the ED, 1 team is assigned to all admitting duties on a particular day; the on‐call team. While teams rotate between designations of on‐call, post‐call, and pre‐call over the course of the week, only the team designated on‐call accepts new admissions. This bolus call structure creates the need for extensive cross‐coverage, large variations in team admissions, and disparate team workloads.1012 Moreover, the effects of these variations may persist and extend along the care pathway, ultimately impacting timely patient discharge. Therefore, interventions aimed at improving the admission process may be candidates for improved patient flow.
The objective of this study is to evaluate the effect of changing the admission process from a bolus admission system to a trickle system that evenly distributes newly admitted patients to each of the physician‐led care teams. We hypothesize that by removing variation within the team admission process, team workload will be smoothed and ultimately result in patients being discharged by the team in a more uniform pattern. We evaluate this hypothesis by measuring length of stay and daily discharge rate.
METHODS
Setting
This retrospective study was conducted on the General Internal Medicine clinical teaching unit (GIM CTU) at a large academic tertiary care center in Toronto, Canada. GIM provides acute, nonsurgical care to a patient population composed primarily of elderly patients with complex chronic illnesses. GIM receives 98% of its inpatient admissions from the ED. On a daily basis, the ED sees approximately 100 patients, of which nearly 20% are admitted to hospital. GIM constitutes the single largest admitting service in the ED, admitting nearly half of all emergent admissions. Surgical and specialized medical services (eg, Cardiology, Oncology, Nephrology) admit the remaining half.
On March 2, 2009, the GIM CTU underwent a structural change from a bolus admission system to a trickle system of admissions to each care team. Figure 1 depicts a typical pre‐change admission pattern where each of the 4 care teams would admit a bolus of patients on a given day (left panel), and a typical post‐change admission pattern where the variation in daily admissions is smoothed out as a result of the trickle admission system (right panel). No change was made to care team members; each team consisted of an attending physician, 1 senior resident, 2 to 3 junior residents, 1 social worker, 1 physiotherapist, 1 occupational therapist, and 1 pharmacist. The Appendix provides a detailed description of the structural change.
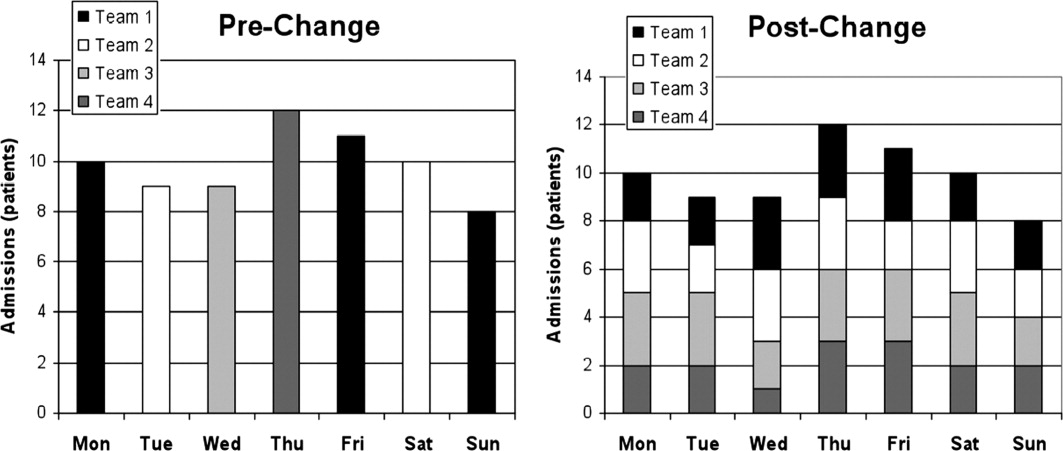
Data Collection
Records were obtained from the hospital's Electronic Patient Record, which contains information on socio‐demographics, diagnosis, length of stay (LOS), patient disposition, attending physician, and date of admission and discharge.
Data were collected for 2 time periods, the pre‐change period (March to August 2008) and the post‐change period (March to August 2009). The new system was implemented on March 2, 2009. The same months of 2 consecutive years were used to account for any seasonal variation in patient volumes and diagnoses. During the pre‐change and post‐change periods, the hospital maintained the same admitting and discharge policies and protocols. Similarly, the authors are unaware of any provincial‐wide government policies that would have impacted only 1 of either the pre‐change or post‐change periods.
Outcomes
Two main outcomes were studied, daily discharge rate (DDR)13 and LOS. DDR was expressed as the number of discharges on a particular day divided by the total patient census on that day. DDR was calculated by team, stratified by their call schedule status (on‐call, post‐call, postpost‐call, pre‐call, or none of these), and then aggregated. A day was defined as a 24‐hour period beginning at 8 AM. This was chosen because it better reflects the period when decisions are made and work is completed. Daily team‐specific patient census was measured at 8 AM. LOS was measured in days, calculated for each patient using the admission and discharge dates.
The DDR calculation included only those patients who were admitted and discharged within the study periods. For analysis of LOS, we also included patients admitted prior to, but discharged during, the study periods.
We included all patients admitted to GIM. Patient discharge dispositions were categorized into 5 groups: discharge home, interfacility transfers (discharged to long‐term care, rehabilitation, chronic care, etc), intrafacility transfers (to other inpatient services within the hospital), death, and left against medical advice. To focus on discharges that may be influenced by the team, for analysis of both DDR and LOS, only patients discharged home and interfacility and intrafacility transfers were included (deaths and patients who left against medical advice were not included).
Statistical Analysis
To assess whether the trickle system smoothed discharge rates, we fitted a logistic regression model and compared the variability in the log‐odds of discharge across the 4 main types of call days (on‐call, post‐call, postpost‐call, pre‐call) in the pre‐change and post‐change periods. The number of discharges on a given day was modeled as a binomial outcome with sample size equal to the census for that day and a log‐odds of discharge that depended on type of call day and a random error component. In this model, the effect of type of call day was allowed to be different in the pre‐change and post‐change periods. To account for the fact that data were collected on 180 consecutive days in each time period, we modeled the error component for each team in each time period as an autoregressive time series. We summarized the smoothness of discharge rates across type of call day in each period by calculating the variance of the corresponding regression parameters (the log‐odds ratios). By comparing the variances in the 2 periods, we were able to compute the probability that there was a reduction in variability, or equivalently, a smoothing of DDR. This model was fitted with Bayesian methods, implemented using Markov chain Monte Carlo (MCMC) techniques in the software WinBUGS.14 Uninformative priors were used for all parameters; model convergence was checked with the Gelman‐Brooks Rubin statistics. Further details are available from the authors on request. Summary estimates of discharge rates on the 4 main types of call day were calculated for the pre‐change and post‐change periods and plotted with 95% credible intervals.
Descriptive statistics were calculated for age, case mix group (CMG), total admission and discharges, and LOS. We chose to report median LOS, rather than the mean, because this modulates the influence of outliers in the samples.
KaplanMeier curves were also plotted for LOS. We tested for equality of the KaplanMeier curves using a weighted log‐rank test (G‐rho), which gave more weight to smaller LOS values (giving weight equal to the proportion of patients not yet discharged). This weighting was performed because an improvement in operational efficiency was more likely to have an effect on patients who could be discharged more quickly (<7 days) than patients whose discharge was delayed by factors outside the hospital's control.
All other statistical analyses were performed using R (version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by The University Health Network Research Ethics Board.
RESULTS
During the 2 study periods, a total of 2734 patients were discharged, 1446 in the pre‐change period (1535 admitted), and 1288 in the post‐change period (1363 admitted). Table 1 presents mean age and primary CMG diagnosis.
| Pre‐Intervention Period (March 3August 29, 2008) 1446 Total Discharges (Mean Age [SD], 66 [18.6]) | Post‐Intervention Period (March 2August 28, 2009) 1288 Total Discharges (Mean Age [SD], 67 [18.8]) | |||
|---|---|---|---|---|
| CMG Rank | CMG Description | N (%) | CMG Description | N (%) |
| ||||
| Pneumonia | 117 (7.4) | Heart failure | 102 (7.4) | |
| 2 | Heart failure | 84 (5.3) | Pneumonia | 65 (4.7) |
| 3 | G.I. hemorrhage | 68 (4.3) | Esoph/gastro/misc digestive disorder | 61 (4.4) |
| 4 | Esoph/gastro/misc digestive disorder | 62 (3.9) | Lower urinary tract infection | 56 (4.1) |
| 5 | Red blood cell disorders | 59 (3.7) | G.I. hemorrhage | 52 (3.8) |
| 6 | Nutrit/misc metabolic disorder | 56 (3.5) | Nutrit/misc metabolic disorder | 47 (3.4) |
| 7 | Reticuloendothelial disorder | 56 (3.5) | Cerebrovascular disorder | 41 (3.0) |
| 8 | Lower urinary tract infection | 50 (3.2) | Red blood cell disorders | 40 (2.9) |
| 9 | Respiratory infect and inflamm | 42 (2.7) | Ungroupable input data | 36 (2.6) |
| 10 | Cerebrovascular disorder | 40 (2.5) | Chronic obstructive pulmonary disease | 33 (2.4) |
Figure 2 shows the estimated average team‐specific DDR's according to call schedule status, along with 95% credible intervals. With the exception of the postpost‐call day, each black point (2009, post‐change period) is closer to the overall average DDR of 9.9% than each corresponding gray point (2008, pre‐change period). In our Bayesian model, there was a 96.9% probability that the variability across call schedule status was reduced in the post‐change period, substantial evidence of smoother discharge rates across different types of call days.
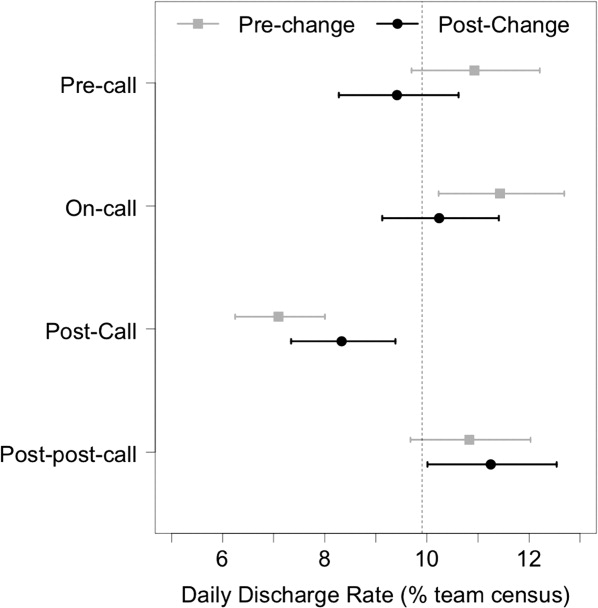
Summary statistics for the LOS for both groups can be seen in Table 2. The median LOS in the post‐change period was statistically significantly shorter than in the pre‐change period (4.8 days vs 5.1 days, P < 0.001).
| Pre‐Change | Post‐Change | ||
|---|---|---|---|
| |||
| N | 1446 | 1288 | t Test comparing means |
| Mean LOS (SD) | 8.7 (15) | 8.8 (16) | P = 0.89 |
| Wilcoxon rank‐sum test | |||
| Median LOS | 5.06 | 4.79 | P = 0.0065 |
Figure 3 shows the estimated KaplanMeier curves of time to discharge (LOS) in both time periods. Differences between the 2 study periods in the proportion of patients that had been discharged at each time point (the vertical distance between the curves) can be observed, particularly in the shorter LOS times.
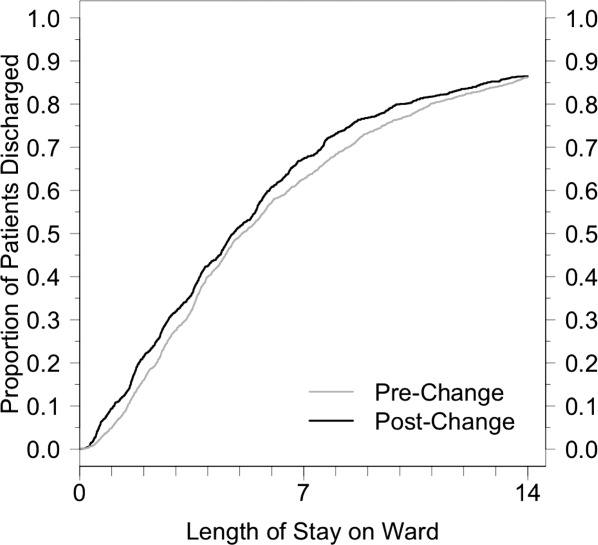
DISCUSSION
Previous studies have suggested that systems become more efficient when every day runs the same way.15 Achieving this for the number of daily discharges from the ward should have a positive effect on the flow of patients through the GIM service.16 Wong et al. showed how the on call schedule of medical personnel had a strong effect on the variation in daily discharges.17 A more recent study by the same authors demonstrated, through a computer simulation model, that smoothing patient discharges over the course of the week decreases the number of ED beds occupied by admitted patients.18 After introducing a structural change to our admission system that made the daily admissions of patients to each care team uniform, we showed a significant reduction in the variation of discharge rates from day to day, and the expected improvement in patient flow as shown by a decrease in the median LOS.
This intervention changed only 1 component of a complex patient care process, of which the resident on‐call schedule is only a small part. Nevertheless, this small change, designed to optimize the doctors' contribution to patient flow, was sufficient in effecting a significant reduction in the variation of the DDR. Inpatients follow a usual course in the hospital, requiring an average LOS of 4 to 5 days. In the bolus system of admissions, we observed what was essentially a cohort effect where the same bolus of patients was discharged on roughly the same day, an average of 4 to 5 days after admission. If the daily variation in discharges were only dependent on the daily variation in admissions, by making the influx of inpatients constant, we should have eliminated this cohort effect. Although the variation in discharges was reduced, it was not completely eliminated, suggesting that elements of the old system are retained. It is possible that the senior resident's management of the patients on the team has a stronger influence than that of other members of the team, and the flow of patients may still be affected by their call schedule.
We observed a significant reduction (0.3 days) in median LOS. By making each day look the same for admissions to each care team, and by making each day look more uniform for discharges from each care team, we were able to improve our unit's operational efficiency. Other benefits of the new system included: less cross‐coverage, since after‐hours there was always a member of each team to look after their own patients; the elimination of the post‐call day for the entire team; and the relatively decreased average daily workload.
The bulk of the reduction in median LOS was attributed to short‐stay patients. The flow of very sick patients who require prolonged inpatient treatment, or those waiting for post‐acute care beds (rehabilitation, long‐term care, convalescence, etc) may be less sensitive to improvements in internal efficiencies.
Although the improvement in LOS was modest, it was certainly no worse than in the older system, and the change was accompanied by the many other benefits already mentioned. In fact, ours is not the only hospital in the city that has made this change. Early results of a qualitative study exploring the perceptions of attending staff, residents, and students of the new systemparticularly its effects on the educational experienceare encouraging, showing overall positive opinions about the change. Further studies aimed at analyzing the barriers to efficient patient discharges may help identify important factors, such as those already mentioned, that this change in structure did not address. Policymakers could address other components of the discharge process, particularly the chronic shortage of post‐acute care beds. Finally, an economic analysis could provide insights about the potential savings that such structural changes could represent.
This study has several limitations. It took place in a single teaching hospital in Canada and, therefore, may not be generalizable to community hospitals or to settings that do not provide single‐payer free public healthcare. Nevertheless, most hospital units are subject to the effects of medical personnel scheduling, and the variation in patient flow processes that this produces. The current resident association collective agreement in Ontario still allows trainees to be scheduled for continuous 24‐hour duty periods. An exact replication of our structure would not be possible in settings with more stringent duty‐hour restrictions. Nevertheless, the goal of the structural change was to make the influx of patients to each care team constant, and this is achievable regardless of the length of the trainee call period. Although there is no reason to suspect a systematic difference in the mix of patients from 2008 to 2009, it would have been preferable to use a propensity score to compare clinical characteristics of the 2 patient groups. We used a relatively new metric, DDR, which was created in our institution and already has been used in several studies. However, it has not yet been validated in other centers.
One of the limitations of a before‐and‐after analysis is our inability to adjust for other changes that may have occurred during the study periods. These known and unknown factors may have had effects on the findings.
CONCLUSIONS
A new admission structure was introduced to the GIM CTU in March 2009, with the intention of changing the admissions to each care team from a bolus to a trickle system. This study was a real‐world demonstration of a concept that had, until this point, only been observed in robust simulation models. When the daily influx of patients to a care team becomes constant, the number of discharges from that team experience less daily variation, and the overall efficiency of the team improves, as measured by a reduction in the median LOS. Standardizing the care processes on the GIM inpatient ward improves overall efficiency and capacity.
- ,.Systematic review of emergency department crowding: causes, effects, and solutions.Ann Emerg Med.2008;52(2):126–136.
- ,,, et al.Fewer intensive care unit refusals and a higher capacity utilization by using a cyclic surgical case schedule.J Crit Care.2008;23(2):222–226.
- .Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction.Am J Med Qual.2009;24(4):344–346.
- ,.Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction.J Healthc Manag.2005;50(5):327–342.
- ,,.Managing variation in demand: lessons from the UK National Health Service.J Healthc Manag.2006;51(5):309–322.
- ,,,,,.The timing of neonatal discharge: an example of unwarranted variation?Pediatrics.2001;107(1):73–77.
- ,,,,.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87(3):216–219.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,,.House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167(1):47–52.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,,,.Evaluation of a redesign initiative in an internal‐medicine residency.N Engl J Med.2010;362(14):1304–1311.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- ,,,,.Real‐time operational feedback: daily discharge rate as a novel hospital efficiency metric.Qual Saf Health Care.2010;19(6):e32.
- ,,,.WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility.Statistics and Computing.2000;10(4):325–337.
- Institute for Healthcare Improvement. Optimizing patient flow: moving patients smoothly through acute care settings;2003. Available at: http://www.ihi.org.
- Canadian Institute for Health Information. Waiting for health care in Canada: what we know and what we don't know;2006. Available at: http://www.cihi.ca.
- ,,, et al.How much do operational processes affect hospital inpatient discharge rates?J Public Health (Oxf).2009;31(4):546–553.
- ,,,,.Smoothing inpatient discharges decreases emergency department congestion: a system dynamics simulation model.Emerg Med J.2010;27(8):593–598.
Smooth and timely hospital patient flow can have multiple positive effects including reduced wait times for services, decreased congestion in the Emergency Department (ED), and increased patient and staff satisfaction.14 One way to improve patient flow is to remove variation along the care pathway.57
For teaching hospitals that provide team‐based care, 1 significant source of variation involves the emergent admission process.8, 9 Typically, for services that admit the majority of their patients from the ED, 1 team is assigned to all admitting duties on a particular day; the on‐call team. While teams rotate between designations of on‐call, post‐call, and pre‐call over the course of the week, only the team designated on‐call accepts new admissions. This bolus call structure creates the need for extensive cross‐coverage, large variations in team admissions, and disparate team workloads.1012 Moreover, the effects of these variations may persist and extend along the care pathway, ultimately impacting timely patient discharge. Therefore, interventions aimed at improving the admission process may be candidates for improved patient flow.
The objective of this study is to evaluate the effect of changing the admission process from a bolus admission system to a trickle system that evenly distributes newly admitted patients to each of the physician‐led care teams. We hypothesize that by removing variation within the team admission process, team workload will be smoothed and ultimately result in patients being discharged by the team in a more uniform pattern. We evaluate this hypothesis by measuring length of stay and daily discharge rate.
METHODS
Setting
This retrospective study was conducted on the General Internal Medicine clinical teaching unit (GIM CTU) at a large academic tertiary care center in Toronto, Canada. GIM provides acute, nonsurgical care to a patient population composed primarily of elderly patients with complex chronic illnesses. GIM receives 98% of its inpatient admissions from the ED. On a daily basis, the ED sees approximately 100 patients, of which nearly 20% are admitted to hospital. GIM constitutes the single largest admitting service in the ED, admitting nearly half of all emergent admissions. Surgical and specialized medical services (eg, Cardiology, Oncology, Nephrology) admit the remaining half.
On March 2, 2009, the GIM CTU underwent a structural change from a bolus admission system to a trickle system of admissions to each care team. Figure 1 depicts a typical pre‐change admission pattern where each of the 4 care teams would admit a bolus of patients on a given day (left panel), and a typical post‐change admission pattern where the variation in daily admissions is smoothed out as a result of the trickle admission system (right panel). No change was made to care team members; each team consisted of an attending physician, 1 senior resident, 2 to 3 junior residents, 1 social worker, 1 physiotherapist, 1 occupational therapist, and 1 pharmacist. The Appendix provides a detailed description of the structural change.

Data Collection
Records were obtained from the hospital's Electronic Patient Record, which contains information on socio‐demographics, diagnosis, length of stay (LOS), patient disposition, attending physician, and date of admission and discharge.
Data were collected for 2 time periods, the pre‐change period (March to August 2008) and the post‐change period (March to August 2009). The new system was implemented on March 2, 2009. The same months of 2 consecutive years were used to account for any seasonal variation in patient volumes and diagnoses. During the pre‐change and post‐change periods, the hospital maintained the same admitting and discharge policies and protocols. Similarly, the authors are unaware of any provincial‐wide government policies that would have impacted only 1 of either the pre‐change or post‐change periods.
Outcomes
Two main outcomes were studied, daily discharge rate (DDR)13 and LOS. DDR was expressed as the number of discharges on a particular day divided by the total patient census on that day. DDR was calculated by team, stratified by their call schedule status (on‐call, post‐call, postpost‐call, pre‐call, or none of these), and then aggregated. A day was defined as a 24‐hour period beginning at 8 AM. This was chosen because it better reflects the period when decisions are made and work is completed. Daily team‐specific patient census was measured at 8 AM. LOS was measured in days, calculated for each patient using the admission and discharge dates.
The DDR calculation included only those patients who were admitted and discharged within the study periods. For analysis of LOS, we also included patients admitted prior to, but discharged during, the study periods.
We included all patients admitted to GIM. Patient discharge dispositions were categorized into 5 groups: discharge home, interfacility transfers (discharged to long‐term care, rehabilitation, chronic care, etc), intrafacility transfers (to other inpatient services within the hospital), death, and left against medical advice. To focus on discharges that may be influenced by the team, for analysis of both DDR and LOS, only patients discharged home and interfacility and intrafacility transfers were included (deaths and patients who left against medical advice were not included).
Statistical Analysis
To assess whether the trickle system smoothed discharge rates, we fitted a logistic regression model and compared the variability in the log‐odds of discharge across the 4 main types of call days (on‐call, post‐call, postpost‐call, pre‐call) in the pre‐change and post‐change periods. The number of discharges on a given day was modeled as a binomial outcome with sample size equal to the census for that day and a log‐odds of discharge that depended on type of call day and a random error component. In this model, the effect of type of call day was allowed to be different in the pre‐change and post‐change periods. To account for the fact that data were collected on 180 consecutive days in each time period, we modeled the error component for each team in each time period as an autoregressive time series. We summarized the smoothness of discharge rates across type of call day in each period by calculating the variance of the corresponding regression parameters (the log‐odds ratios). By comparing the variances in the 2 periods, we were able to compute the probability that there was a reduction in variability, or equivalently, a smoothing of DDR. This model was fitted with Bayesian methods, implemented using Markov chain Monte Carlo (MCMC) techniques in the software WinBUGS.14 Uninformative priors were used for all parameters; model convergence was checked with the Gelman‐Brooks Rubin statistics. Further details are available from the authors on request. Summary estimates of discharge rates on the 4 main types of call day were calculated for the pre‐change and post‐change periods and plotted with 95% credible intervals.
Descriptive statistics were calculated for age, case mix group (CMG), total admission and discharges, and LOS. We chose to report median LOS, rather than the mean, because this modulates the influence of outliers in the samples.
KaplanMeier curves were also plotted for LOS. We tested for equality of the KaplanMeier curves using a weighted log‐rank test (G‐rho), which gave more weight to smaller LOS values (giving weight equal to the proportion of patients not yet discharged). This weighting was performed because an improvement in operational efficiency was more likely to have an effect on patients who could be discharged more quickly (<7 days) than patients whose discharge was delayed by factors outside the hospital's control.
All other statistical analyses were performed using R (version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by The University Health Network Research Ethics Board.
RESULTS
During the 2 study periods, a total of 2734 patients were discharged, 1446 in the pre‐change period (1535 admitted), and 1288 in the post‐change period (1363 admitted). Table 1 presents mean age and primary CMG diagnosis.
| Pre‐Intervention Period (March 3August 29, 2008) 1446 Total Discharges (Mean Age [SD], 66 [18.6]) | Post‐Intervention Period (March 2August 28, 2009) 1288 Total Discharges (Mean Age [SD], 67 [18.8]) | |||
|---|---|---|---|---|
| CMG Rank | CMG Description | N (%) | CMG Description | N (%) |
| ||||
| Pneumonia | 117 (7.4) | Heart failure | 102 (7.4) | |
| 2 | Heart failure | 84 (5.3) | Pneumonia | 65 (4.7) |
| 3 | G.I. hemorrhage | 68 (4.3) | Esoph/gastro/misc digestive disorder | 61 (4.4) |
| 4 | Esoph/gastro/misc digestive disorder | 62 (3.9) | Lower urinary tract infection | 56 (4.1) |
| 5 | Red blood cell disorders | 59 (3.7) | G.I. hemorrhage | 52 (3.8) |
| 6 | Nutrit/misc metabolic disorder | 56 (3.5) | Nutrit/misc metabolic disorder | 47 (3.4) |
| 7 | Reticuloendothelial disorder | 56 (3.5) | Cerebrovascular disorder | 41 (3.0) |
| 8 | Lower urinary tract infection | 50 (3.2) | Red blood cell disorders | 40 (2.9) |
| 9 | Respiratory infect and inflamm | 42 (2.7) | Ungroupable input data | 36 (2.6) |
| 10 | Cerebrovascular disorder | 40 (2.5) | Chronic obstructive pulmonary disease | 33 (2.4) |
Figure 2 shows the estimated average team‐specific DDR's according to call schedule status, along with 95% credible intervals. With the exception of the postpost‐call day, each black point (2009, post‐change period) is closer to the overall average DDR of 9.9% than each corresponding gray point (2008, pre‐change period). In our Bayesian model, there was a 96.9% probability that the variability across call schedule status was reduced in the post‐change period, substantial evidence of smoother discharge rates across different types of call days.

Summary statistics for the LOS for both groups can be seen in Table 2. The median LOS in the post‐change period was statistically significantly shorter than in the pre‐change period (4.8 days vs 5.1 days, P < 0.001).
| Pre‐Change | Post‐Change | ||
|---|---|---|---|
| |||
| N | 1446 | 1288 | t Test comparing means |
| Mean LOS (SD) | 8.7 (15) | 8.8 (16) | P = 0.89 |
| Wilcoxon rank‐sum test | |||
| Median LOS | 5.06 | 4.79 | P = 0.0065 |
Figure 3 shows the estimated KaplanMeier curves of time to discharge (LOS) in both time periods. Differences between the 2 study periods in the proportion of patients that had been discharged at each time point (the vertical distance between the curves) can be observed, particularly in the shorter LOS times.

DISCUSSION
Previous studies have suggested that systems become more efficient when every day runs the same way.15 Achieving this for the number of daily discharges from the ward should have a positive effect on the flow of patients through the GIM service.16 Wong et al. showed how the on call schedule of medical personnel had a strong effect on the variation in daily discharges.17 A more recent study by the same authors demonstrated, through a computer simulation model, that smoothing patient discharges over the course of the week decreases the number of ED beds occupied by admitted patients.18 After introducing a structural change to our admission system that made the daily admissions of patients to each care team uniform, we showed a significant reduction in the variation of discharge rates from day to day, and the expected improvement in patient flow as shown by a decrease in the median LOS.
This intervention changed only 1 component of a complex patient care process, of which the resident on‐call schedule is only a small part. Nevertheless, this small change, designed to optimize the doctors' contribution to patient flow, was sufficient in effecting a significant reduction in the variation of the DDR. Inpatients follow a usual course in the hospital, requiring an average LOS of 4 to 5 days. In the bolus system of admissions, we observed what was essentially a cohort effect where the same bolus of patients was discharged on roughly the same day, an average of 4 to 5 days after admission. If the daily variation in discharges were only dependent on the daily variation in admissions, by making the influx of inpatients constant, we should have eliminated this cohort effect. Although the variation in discharges was reduced, it was not completely eliminated, suggesting that elements of the old system are retained. It is possible that the senior resident's management of the patients on the team has a stronger influence than that of other members of the team, and the flow of patients may still be affected by their call schedule.
We observed a significant reduction (0.3 days) in median LOS. By making each day look the same for admissions to each care team, and by making each day look more uniform for discharges from each care team, we were able to improve our unit's operational efficiency. Other benefits of the new system included: less cross‐coverage, since after‐hours there was always a member of each team to look after their own patients; the elimination of the post‐call day for the entire team; and the relatively decreased average daily workload.
The bulk of the reduction in median LOS was attributed to short‐stay patients. The flow of very sick patients who require prolonged inpatient treatment, or those waiting for post‐acute care beds (rehabilitation, long‐term care, convalescence, etc) may be less sensitive to improvements in internal efficiencies.
Although the improvement in LOS was modest, it was certainly no worse than in the older system, and the change was accompanied by the many other benefits already mentioned. In fact, ours is not the only hospital in the city that has made this change. Early results of a qualitative study exploring the perceptions of attending staff, residents, and students of the new systemparticularly its effects on the educational experienceare encouraging, showing overall positive opinions about the change. Further studies aimed at analyzing the barriers to efficient patient discharges may help identify important factors, such as those already mentioned, that this change in structure did not address. Policymakers could address other components of the discharge process, particularly the chronic shortage of post‐acute care beds. Finally, an economic analysis could provide insights about the potential savings that such structural changes could represent.
This study has several limitations. It took place in a single teaching hospital in Canada and, therefore, may not be generalizable to community hospitals or to settings that do not provide single‐payer free public healthcare. Nevertheless, most hospital units are subject to the effects of medical personnel scheduling, and the variation in patient flow processes that this produces. The current resident association collective agreement in Ontario still allows trainees to be scheduled for continuous 24‐hour duty periods. An exact replication of our structure would not be possible in settings with more stringent duty‐hour restrictions. Nevertheless, the goal of the structural change was to make the influx of patients to each care team constant, and this is achievable regardless of the length of the trainee call period. Although there is no reason to suspect a systematic difference in the mix of patients from 2008 to 2009, it would have been preferable to use a propensity score to compare clinical characteristics of the 2 patient groups. We used a relatively new metric, DDR, which was created in our institution and already has been used in several studies. However, it has not yet been validated in other centers.
One of the limitations of a before‐and‐after analysis is our inability to adjust for other changes that may have occurred during the study periods. These known and unknown factors may have had effects on the findings.
CONCLUSIONS
A new admission structure was introduced to the GIM CTU in March 2009, with the intention of changing the admissions to each care team from a bolus to a trickle system. This study was a real‐world demonstration of a concept that had, until this point, only been observed in robust simulation models. When the daily influx of patients to a care team becomes constant, the number of discharges from that team experience less daily variation, and the overall efficiency of the team improves, as measured by a reduction in the median LOS. Standardizing the care processes on the GIM inpatient ward improves overall efficiency and capacity.
Smooth and timely hospital patient flow can have multiple positive effects including reduced wait times for services, decreased congestion in the Emergency Department (ED), and increased patient and staff satisfaction.14 One way to improve patient flow is to remove variation along the care pathway.57
For teaching hospitals that provide team‐based care, 1 significant source of variation involves the emergent admission process.8, 9 Typically, for services that admit the majority of their patients from the ED, 1 team is assigned to all admitting duties on a particular day; the on‐call team. While teams rotate between designations of on‐call, post‐call, and pre‐call over the course of the week, only the team designated on‐call accepts new admissions. This bolus call structure creates the need for extensive cross‐coverage, large variations in team admissions, and disparate team workloads.1012 Moreover, the effects of these variations may persist and extend along the care pathway, ultimately impacting timely patient discharge. Therefore, interventions aimed at improving the admission process may be candidates for improved patient flow.
The objective of this study is to evaluate the effect of changing the admission process from a bolus admission system to a trickle system that evenly distributes newly admitted patients to each of the physician‐led care teams. We hypothesize that by removing variation within the team admission process, team workload will be smoothed and ultimately result in patients being discharged by the team in a more uniform pattern. We evaluate this hypothesis by measuring length of stay and daily discharge rate.
METHODS
Setting
This retrospective study was conducted on the General Internal Medicine clinical teaching unit (GIM CTU) at a large academic tertiary care center in Toronto, Canada. GIM provides acute, nonsurgical care to a patient population composed primarily of elderly patients with complex chronic illnesses. GIM receives 98% of its inpatient admissions from the ED. On a daily basis, the ED sees approximately 100 patients, of which nearly 20% are admitted to hospital. GIM constitutes the single largest admitting service in the ED, admitting nearly half of all emergent admissions. Surgical and specialized medical services (eg, Cardiology, Oncology, Nephrology) admit the remaining half.
On March 2, 2009, the GIM CTU underwent a structural change from a bolus admission system to a trickle system of admissions to each care team. Figure 1 depicts a typical pre‐change admission pattern where each of the 4 care teams would admit a bolus of patients on a given day (left panel), and a typical post‐change admission pattern where the variation in daily admissions is smoothed out as a result of the trickle admission system (right panel). No change was made to care team members; each team consisted of an attending physician, 1 senior resident, 2 to 3 junior residents, 1 social worker, 1 physiotherapist, 1 occupational therapist, and 1 pharmacist. The Appendix provides a detailed description of the structural change.

Data Collection
Records were obtained from the hospital's Electronic Patient Record, which contains information on socio‐demographics, diagnosis, length of stay (LOS), patient disposition, attending physician, and date of admission and discharge.
Data were collected for 2 time periods, the pre‐change period (March to August 2008) and the post‐change period (March to August 2009). The new system was implemented on March 2, 2009. The same months of 2 consecutive years were used to account for any seasonal variation in patient volumes and diagnoses. During the pre‐change and post‐change periods, the hospital maintained the same admitting and discharge policies and protocols. Similarly, the authors are unaware of any provincial‐wide government policies that would have impacted only 1 of either the pre‐change or post‐change periods.
Outcomes
Two main outcomes were studied, daily discharge rate (DDR)13 and LOS. DDR was expressed as the number of discharges on a particular day divided by the total patient census on that day. DDR was calculated by team, stratified by their call schedule status (on‐call, post‐call, postpost‐call, pre‐call, or none of these), and then aggregated. A day was defined as a 24‐hour period beginning at 8 AM. This was chosen because it better reflects the period when decisions are made and work is completed. Daily team‐specific patient census was measured at 8 AM. LOS was measured in days, calculated for each patient using the admission and discharge dates.
The DDR calculation included only those patients who were admitted and discharged within the study periods. For analysis of LOS, we also included patients admitted prior to, but discharged during, the study periods.
We included all patients admitted to GIM. Patient discharge dispositions were categorized into 5 groups: discharge home, interfacility transfers (discharged to long‐term care, rehabilitation, chronic care, etc), intrafacility transfers (to other inpatient services within the hospital), death, and left against medical advice. To focus on discharges that may be influenced by the team, for analysis of both DDR and LOS, only patients discharged home and interfacility and intrafacility transfers were included (deaths and patients who left against medical advice were not included).
Statistical Analysis
To assess whether the trickle system smoothed discharge rates, we fitted a logistic regression model and compared the variability in the log‐odds of discharge across the 4 main types of call days (on‐call, post‐call, postpost‐call, pre‐call) in the pre‐change and post‐change periods. The number of discharges on a given day was modeled as a binomial outcome with sample size equal to the census for that day and a log‐odds of discharge that depended on type of call day and a random error component. In this model, the effect of type of call day was allowed to be different in the pre‐change and post‐change periods. To account for the fact that data were collected on 180 consecutive days in each time period, we modeled the error component for each team in each time period as an autoregressive time series. We summarized the smoothness of discharge rates across type of call day in each period by calculating the variance of the corresponding regression parameters (the log‐odds ratios). By comparing the variances in the 2 periods, we were able to compute the probability that there was a reduction in variability, or equivalently, a smoothing of DDR. This model was fitted with Bayesian methods, implemented using Markov chain Monte Carlo (MCMC) techniques in the software WinBUGS.14 Uninformative priors were used for all parameters; model convergence was checked with the Gelman‐Brooks Rubin statistics. Further details are available from the authors on request. Summary estimates of discharge rates on the 4 main types of call day were calculated for the pre‐change and post‐change periods and plotted with 95% credible intervals.
Descriptive statistics were calculated for age, case mix group (CMG), total admission and discharges, and LOS. We chose to report median LOS, rather than the mean, because this modulates the influence of outliers in the samples.
KaplanMeier curves were also plotted for LOS. We tested for equality of the KaplanMeier curves using a weighted log‐rank test (G‐rho), which gave more weight to smaller LOS values (giving weight equal to the proportion of patients not yet discharged). This weighting was performed because an improvement in operational efficiency was more likely to have an effect on patients who could be discharged more quickly (<7 days) than patients whose discharge was delayed by factors outside the hospital's control.
All other statistical analyses were performed using R (version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by The University Health Network Research Ethics Board.
RESULTS
During the 2 study periods, a total of 2734 patients were discharged, 1446 in the pre‐change period (1535 admitted), and 1288 in the post‐change period (1363 admitted). Table 1 presents mean age and primary CMG diagnosis.
| Pre‐Intervention Period (March 3August 29, 2008) 1446 Total Discharges (Mean Age [SD], 66 [18.6]) | Post‐Intervention Period (March 2August 28, 2009) 1288 Total Discharges (Mean Age [SD], 67 [18.8]) | |||
|---|---|---|---|---|
| CMG Rank | CMG Description | N (%) | CMG Description | N (%) |
| ||||
| Pneumonia | 117 (7.4) | Heart failure | 102 (7.4) | |
| 2 | Heart failure | 84 (5.3) | Pneumonia | 65 (4.7) |
| 3 | G.I. hemorrhage | 68 (4.3) | Esoph/gastro/misc digestive disorder | 61 (4.4) |
| 4 | Esoph/gastro/misc digestive disorder | 62 (3.9) | Lower urinary tract infection | 56 (4.1) |
| 5 | Red blood cell disorders | 59 (3.7) | G.I. hemorrhage | 52 (3.8) |
| 6 | Nutrit/misc metabolic disorder | 56 (3.5) | Nutrit/misc metabolic disorder | 47 (3.4) |
| 7 | Reticuloendothelial disorder | 56 (3.5) | Cerebrovascular disorder | 41 (3.0) |
| 8 | Lower urinary tract infection | 50 (3.2) | Red blood cell disorders | 40 (2.9) |
| 9 | Respiratory infect and inflamm | 42 (2.7) | Ungroupable input data | 36 (2.6) |
| 10 | Cerebrovascular disorder | 40 (2.5) | Chronic obstructive pulmonary disease | 33 (2.4) |
Figure 2 shows the estimated average team‐specific DDR's according to call schedule status, along with 95% credible intervals. With the exception of the postpost‐call day, each black point (2009, post‐change period) is closer to the overall average DDR of 9.9% than each corresponding gray point (2008, pre‐change period). In our Bayesian model, there was a 96.9% probability that the variability across call schedule status was reduced in the post‐change period, substantial evidence of smoother discharge rates across different types of call days.

Summary statistics for the LOS for both groups can be seen in Table 2. The median LOS in the post‐change period was statistically significantly shorter than in the pre‐change period (4.8 days vs 5.1 days, P < 0.001).
| Pre‐Change | Post‐Change | ||
|---|---|---|---|
| |||
| N | 1446 | 1288 | t Test comparing means |
| Mean LOS (SD) | 8.7 (15) | 8.8 (16) | P = 0.89 |
| Wilcoxon rank‐sum test | |||
| Median LOS | 5.06 | 4.79 | P = 0.0065 |
Figure 3 shows the estimated KaplanMeier curves of time to discharge (LOS) in both time periods. Differences between the 2 study periods in the proportion of patients that had been discharged at each time point (the vertical distance between the curves) can be observed, particularly in the shorter LOS times.

DISCUSSION
Previous studies have suggested that systems become more efficient when every day runs the same way.15 Achieving this for the number of daily discharges from the ward should have a positive effect on the flow of patients through the GIM service.16 Wong et al. showed how the on call schedule of medical personnel had a strong effect on the variation in daily discharges.17 A more recent study by the same authors demonstrated, through a computer simulation model, that smoothing patient discharges over the course of the week decreases the number of ED beds occupied by admitted patients.18 After introducing a structural change to our admission system that made the daily admissions of patients to each care team uniform, we showed a significant reduction in the variation of discharge rates from day to day, and the expected improvement in patient flow as shown by a decrease in the median LOS.
This intervention changed only 1 component of a complex patient care process, of which the resident on‐call schedule is only a small part. Nevertheless, this small change, designed to optimize the doctors' contribution to patient flow, was sufficient in effecting a significant reduction in the variation of the DDR. Inpatients follow a usual course in the hospital, requiring an average LOS of 4 to 5 days. In the bolus system of admissions, we observed what was essentially a cohort effect where the same bolus of patients was discharged on roughly the same day, an average of 4 to 5 days after admission. If the daily variation in discharges were only dependent on the daily variation in admissions, by making the influx of inpatients constant, we should have eliminated this cohort effect. Although the variation in discharges was reduced, it was not completely eliminated, suggesting that elements of the old system are retained. It is possible that the senior resident's management of the patients on the team has a stronger influence than that of other members of the team, and the flow of patients may still be affected by their call schedule.
We observed a significant reduction (0.3 days) in median LOS. By making each day look the same for admissions to each care team, and by making each day look more uniform for discharges from each care team, we were able to improve our unit's operational efficiency. Other benefits of the new system included: less cross‐coverage, since after‐hours there was always a member of each team to look after their own patients; the elimination of the post‐call day for the entire team; and the relatively decreased average daily workload.
The bulk of the reduction in median LOS was attributed to short‐stay patients. The flow of very sick patients who require prolonged inpatient treatment, or those waiting for post‐acute care beds (rehabilitation, long‐term care, convalescence, etc) may be less sensitive to improvements in internal efficiencies.
Although the improvement in LOS was modest, it was certainly no worse than in the older system, and the change was accompanied by the many other benefits already mentioned. In fact, ours is not the only hospital in the city that has made this change. Early results of a qualitative study exploring the perceptions of attending staff, residents, and students of the new systemparticularly its effects on the educational experienceare encouraging, showing overall positive opinions about the change. Further studies aimed at analyzing the barriers to efficient patient discharges may help identify important factors, such as those already mentioned, that this change in structure did not address. Policymakers could address other components of the discharge process, particularly the chronic shortage of post‐acute care beds. Finally, an economic analysis could provide insights about the potential savings that such structural changes could represent.
This study has several limitations. It took place in a single teaching hospital in Canada and, therefore, may not be generalizable to community hospitals or to settings that do not provide single‐payer free public healthcare. Nevertheless, most hospital units are subject to the effects of medical personnel scheduling, and the variation in patient flow processes that this produces. The current resident association collective agreement in Ontario still allows trainees to be scheduled for continuous 24‐hour duty periods. An exact replication of our structure would not be possible in settings with more stringent duty‐hour restrictions. Nevertheless, the goal of the structural change was to make the influx of patients to each care team constant, and this is achievable regardless of the length of the trainee call period. Although there is no reason to suspect a systematic difference in the mix of patients from 2008 to 2009, it would have been preferable to use a propensity score to compare clinical characteristics of the 2 patient groups. We used a relatively new metric, DDR, which was created in our institution and already has been used in several studies. However, it has not yet been validated in other centers.
One of the limitations of a before‐and‐after analysis is our inability to adjust for other changes that may have occurred during the study periods. These known and unknown factors may have had effects on the findings.
CONCLUSIONS
A new admission structure was introduced to the GIM CTU in March 2009, with the intention of changing the admissions to each care team from a bolus to a trickle system. This study was a real‐world demonstration of a concept that had, until this point, only been observed in robust simulation models. When the daily influx of patients to a care team becomes constant, the number of discharges from that team experience less daily variation, and the overall efficiency of the team improves, as measured by a reduction in the median LOS. Standardizing the care processes on the GIM inpatient ward improves overall efficiency and capacity.
- ,.Systematic review of emergency department crowding: causes, effects, and solutions.Ann Emerg Med.2008;52(2):126–136.
- ,,, et al.Fewer intensive care unit refusals and a higher capacity utilization by using a cyclic surgical case schedule.J Crit Care.2008;23(2):222–226.
- .Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction.Am J Med Qual.2009;24(4):344–346.
- ,.Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction.J Healthc Manag.2005;50(5):327–342.
- ,,.Managing variation in demand: lessons from the UK National Health Service.J Healthc Manag.2006;51(5):309–322.
- ,,,,,.The timing of neonatal discharge: an example of unwarranted variation?Pediatrics.2001;107(1):73–77.
- ,,,,.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87(3):216–219.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,,.House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167(1):47–52.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,,,.Evaluation of a redesign initiative in an internal‐medicine residency.N Engl J Med.2010;362(14):1304–1311.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- ,,,,.Real‐time operational feedback: daily discharge rate as a novel hospital efficiency metric.Qual Saf Health Care.2010;19(6):e32.
- ,,,.WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility.Statistics and Computing.2000;10(4):325–337.
- Institute for Healthcare Improvement. Optimizing patient flow: moving patients smoothly through acute care settings;2003. Available at: http://www.ihi.org.
- Canadian Institute for Health Information. Waiting for health care in Canada: what we know and what we don't know;2006. Available at: http://www.cihi.ca.
- ,,, et al.How much do operational processes affect hospital inpatient discharge rates?J Public Health (Oxf).2009;31(4):546–553.
- ,,,,.Smoothing inpatient discharges decreases emergency department congestion: a system dynamics simulation model.Emerg Med J.2010;27(8):593–598.
- ,.Systematic review of emergency department crowding: causes, effects, and solutions.Ann Emerg Med.2008;52(2):126–136.
- ,,, et al.Fewer intensive care unit refusals and a higher capacity utilization by using a cyclic surgical case schedule.J Crit Care.2008;23(2):222–226.
- .Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction.Am J Med Qual.2009;24(4):344–346.
- ,.Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction.J Healthc Manag.2005;50(5):327–342.
- ,,.Managing variation in demand: lessons from the UK National Health Service.J Healthc Manag.2006;51(5):309–322.
- ,,,,,.The timing of neonatal discharge: an example of unwarranted variation?Pediatrics.2001;107(1):73–77.
- ,,,,.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87(3):216–219.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,,.House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167(1):47–52.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,,,.Evaluation of a redesign initiative in an internal‐medicine residency.N Engl J Med.2010;362(14):1304–1311.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- ,,,,.Real‐time operational feedback: daily discharge rate as a novel hospital efficiency metric.Qual Saf Health Care.2010;19(6):e32.
- ,,,.WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility.Statistics and Computing.2000;10(4):325–337.
- Institute for Healthcare Improvement. Optimizing patient flow: moving patients smoothly through acute care settings;2003. Available at: http://www.ihi.org.
- Canadian Institute for Health Information. Waiting for health care in Canada: what we know and what we don't know;2006. Available at: http://www.cihi.ca.
- ,,, et al.How much do operational processes affect hospital inpatient discharge rates?J Public Health (Oxf).2009;31(4):546–553.
- ,,,,.Smoothing inpatient discharges decreases emergency department congestion: a system dynamics simulation model.Emerg Med J.2010;27(8):593–598.
U.S. Diabetes-Free Life Expectancy on the Decline
Americans are living longer – but not healthier – lives, researchers have found.
While average life expectancy is now higher than ever, the number of years Americans can expect to live without type 2 diabetes is decreasing. A prolonged period of morbidity is more likely to characterize people’s later years now than it was in the 1980s.
The difference was driven by a marked increase in the number of Americans who are obese, according to findings published Sept. 23 by Solveig A. Cunningham, Ph.D., an epidemiologist at Emory University in Atlanta, and colleagues. More than one-third of American adults now have a body-mass index of 30 kg/m2 or higher.
"All of the observed reductions in diabetes-free life expectancy at the population level were actually due to increases in diabetes only among obese individuals," Dr. Cunningham and colleagues reported (Diabetes Care 2011;34:2225-30 [doi:10.2337/dc11-0462]).
The fact that obese individuals would be more likely to be diagnosed with diabetes in the 2000s than in the 1980s, they wrote, may be because obese adults were 3.4% heavier in the latter period, or it may reflect better diagnosis of diabetes in this population group, the researchers hypothesized. Another possible explanation "is that diabetes risks are higher with younger age of obesity onset; indeed, the prevalence of obesity has tripled among children since the 1970s."
For other weight groups, including people classified as overweight (BMI, 25-30), the diabetes-free life expectancy actually improved from the 1980s to the 2000s. However, this was largely thanks to overall mortality decreasing. Life expectancy as a whole was 70.6 years for men and 77.4 years for women in the 1980s, and had increased to 74.3 for men and 79 years for women by 2000-2004.
The proportion of obese individuals had grown enough in the same time period to alter estimates of disease-free life expectancy for the population as a whole, the investigators found.
All together, diabetes-free life expectancy for an 18-year-old U.S. man was 1.7 years longer in the 1980s than it was during 2000-2004; for a woman, it was 1.5 years longer in the 1980s. The proportion of 18-year-olds who would be diagnosed with diabetes in their lifetimes increased by almost 50% among women between 1980-1989 and 2000-2004, and almost doubled among men, Dr. Cunningham and colleagues found.
Obese men lost an average of 5.6 years of diabetes-free life between the 1980s and the early 2000s, whereas women lost 2.5 years in the same interval. "In 2000-2004, obese 18-year-old men and women could expect to live 13.7 and 19.1 fewer years without diabetes, respectively, compared with normal/underweight 18-year-old men and women," the investigators wrote.
For their research, Dr. Cunningham and colleagues examined data from the National Vital Statistics System and the National Health Interview Survey, which collects self-reported health information from the noninstitutionalized, nonmilitary population.
The 1980-1989 NHIS surveys analyzed by Dr. Cunningham and colleagues contained responses from 143,765 adults (507 diagnosed diabetic in the previous year), and the 2000-2004 period surveys contained responses from 150,718 (1,366 diagnosed diabetic in the previous year).
Dr. Cunningham and colleagues examined diabetes and obesity incidence in the survey population in the context of changes in national mortality rates and population aging between the time periods.
Limitations of the study included its reliance on self-reported information about diabetes diagnosis, weight, and height, which is susceptible to bias. However, the investigators wrote that "NHIS data are collected via rigorous in-person interviews, and the differences in reported and measured BMI are not large and do not affect health risk estimates, including those associated with diabetes."
The researchers concluded that their results "suggest that in the face of budgetary or logistic constraints, new efforts to prevent diabetes can have the greatest impact among obese individuals," as all other groups were shown to have improved diabetes-free life expectancy over the decades studied.
Dr. Cunningham and colleagues reported no conflicts of interest.
Americans are living longer – but not healthier – lives, researchers have found.
While average life expectancy is now higher than ever, the number of years Americans can expect to live without type 2 diabetes is decreasing. A prolonged period of morbidity is more likely to characterize people’s later years now than it was in the 1980s.
The difference was driven by a marked increase in the number of Americans who are obese, according to findings published Sept. 23 by Solveig A. Cunningham, Ph.D., an epidemiologist at Emory University in Atlanta, and colleagues. More than one-third of American adults now have a body-mass index of 30 kg/m2 or higher.
"All of the observed reductions in diabetes-free life expectancy at the population level were actually due to increases in diabetes only among obese individuals," Dr. Cunningham and colleagues reported (Diabetes Care 2011;34:2225-30 [doi:10.2337/dc11-0462]).
The fact that obese individuals would be more likely to be diagnosed with diabetes in the 2000s than in the 1980s, they wrote, may be because obese adults were 3.4% heavier in the latter period, or it may reflect better diagnosis of diabetes in this population group, the researchers hypothesized. Another possible explanation "is that diabetes risks are higher with younger age of obesity onset; indeed, the prevalence of obesity has tripled among children since the 1970s."
For other weight groups, including people classified as overweight (BMI, 25-30), the diabetes-free life expectancy actually improved from the 1980s to the 2000s. However, this was largely thanks to overall mortality decreasing. Life expectancy as a whole was 70.6 years for men and 77.4 years for women in the 1980s, and had increased to 74.3 for men and 79 years for women by 2000-2004.
The proportion of obese individuals had grown enough in the same time period to alter estimates of disease-free life expectancy for the population as a whole, the investigators found.
All together, diabetes-free life expectancy for an 18-year-old U.S. man was 1.7 years longer in the 1980s than it was during 2000-2004; for a woman, it was 1.5 years longer in the 1980s. The proportion of 18-year-olds who would be diagnosed with diabetes in their lifetimes increased by almost 50% among women between 1980-1989 and 2000-2004, and almost doubled among men, Dr. Cunningham and colleagues found.
Obese men lost an average of 5.6 years of diabetes-free life between the 1980s and the early 2000s, whereas women lost 2.5 years in the same interval. "In 2000-2004, obese 18-year-old men and women could expect to live 13.7 and 19.1 fewer years without diabetes, respectively, compared with normal/underweight 18-year-old men and women," the investigators wrote.
For their research, Dr. Cunningham and colleagues examined data from the National Vital Statistics System and the National Health Interview Survey, which collects self-reported health information from the noninstitutionalized, nonmilitary population.
The 1980-1989 NHIS surveys analyzed by Dr. Cunningham and colleagues contained responses from 143,765 adults (507 diagnosed diabetic in the previous year), and the 2000-2004 period surveys contained responses from 150,718 (1,366 diagnosed diabetic in the previous year).
Dr. Cunningham and colleagues examined diabetes and obesity incidence in the survey population in the context of changes in national mortality rates and population aging between the time periods.
Limitations of the study included its reliance on self-reported information about diabetes diagnosis, weight, and height, which is susceptible to bias. However, the investigators wrote that "NHIS data are collected via rigorous in-person interviews, and the differences in reported and measured BMI are not large and do not affect health risk estimates, including those associated with diabetes."
The researchers concluded that their results "suggest that in the face of budgetary or logistic constraints, new efforts to prevent diabetes can have the greatest impact among obese individuals," as all other groups were shown to have improved diabetes-free life expectancy over the decades studied.
Dr. Cunningham and colleagues reported no conflicts of interest.
Americans are living longer – but not healthier – lives, researchers have found.
While average life expectancy is now higher than ever, the number of years Americans can expect to live without type 2 diabetes is decreasing. A prolonged period of morbidity is more likely to characterize people’s later years now than it was in the 1980s.
The difference was driven by a marked increase in the number of Americans who are obese, according to findings published Sept. 23 by Solveig A. Cunningham, Ph.D., an epidemiologist at Emory University in Atlanta, and colleagues. More than one-third of American adults now have a body-mass index of 30 kg/m2 or higher.
"All of the observed reductions in diabetes-free life expectancy at the population level were actually due to increases in diabetes only among obese individuals," Dr. Cunningham and colleagues reported (Diabetes Care 2011;34:2225-30 [doi:10.2337/dc11-0462]).
The fact that obese individuals would be more likely to be diagnosed with diabetes in the 2000s than in the 1980s, they wrote, may be because obese adults were 3.4% heavier in the latter period, or it may reflect better diagnosis of diabetes in this population group, the researchers hypothesized. Another possible explanation "is that diabetes risks are higher with younger age of obesity onset; indeed, the prevalence of obesity has tripled among children since the 1970s."
For other weight groups, including people classified as overweight (BMI, 25-30), the diabetes-free life expectancy actually improved from the 1980s to the 2000s. However, this was largely thanks to overall mortality decreasing. Life expectancy as a whole was 70.6 years for men and 77.4 years for women in the 1980s, and had increased to 74.3 for men and 79 years for women by 2000-2004.
The proportion of obese individuals had grown enough in the same time period to alter estimates of disease-free life expectancy for the population as a whole, the investigators found.
All together, diabetes-free life expectancy for an 18-year-old U.S. man was 1.7 years longer in the 1980s than it was during 2000-2004; for a woman, it was 1.5 years longer in the 1980s. The proportion of 18-year-olds who would be diagnosed with diabetes in their lifetimes increased by almost 50% among women between 1980-1989 and 2000-2004, and almost doubled among men, Dr. Cunningham and colleagues found.
Obese men lost an average of 5.6 years of diabetes-free life between the 1980s and the early 2000s, whereas women lost 2.5 years in the same interval. "In 2000-2004, obese 18-year-old men and women could expect to live 13.7 and 19.1 fewer years without diabetes, respectively, compared with normal/underweight 18-year-old men and women," the investigators wrote.
For their research, Dr. Cunningham and colleagues examined data from the National Vital Statistics System and the National Health Interview Survey, which collects self-reported health information from the noninstitutionalized, nonmilitary population.
The 1980-1989 NHIS surveys analyzed by Dr. Cunningham and colleagues contained responses from 143,765 adults (507 diagnosed diabetic in the previous year), and the 2000-2004 period surveys contained responses from 150,718 (1,366 diagnosed diabetic in the previous year).
Dr. Cunningham and colleagues examined diabetes and obesity incidence in the survey population in the context of changes in national mortality rates and population aging between the time periods.
Limitations of the study included its reliance on self-reported information about diabetes diagnosis, weight, and height, which is susceptible to bias. However, the investigators wrote that "NHIS data are collected via rigorous in-person interviews, and the differences in reported and measured BMI are not large and do not affect health risk estimates, including those associated with diabetes."
The researchers concluded that their results "suggest that in the face of budgetary or logistic constraints, new efforts to prevent diabetes can have the greatest impact among obese individuals," as all other groups were shown to have improved diabetes-free life expectancy over the decades studied.
Dr. Cunningham and colleagues reported no conflicts of interest.
FROM DIABETES CARE
Major Findings: While life expectancy increased between the 1980s and the early 2000s for Americans, diabetes-free life expectancy shrank by 1.7 years in men and 1.5 years in women overall, and by 5.6 and 2.5 years, respectively, in the obese.
Data Source: Nearly 300,000 national health interview surveys conducted between 1980 and 2004, along with data collected by the National Vital Statistics System.
Disclosures: Dr. Cunningham and colleagues reported no conflicts of interest.