User login
Provider Expectations and Experiences
Comanagement is common in hospital medicine practice. And yet, there is no consensus about how comanagement is different from traditional consultative practice. At its core, hospitalist comanagement is a practice arrangement wherein hospitalists and other specialists manage complex patients collaboratively. Beyond this, Huddleston et al. distinguish comanagement from traditional consultations in the comanaging hospitalists' prerogative to provide direct medical care in addition to consultative advice.1 Siegal focuses on the shared responsibility and authority among partnering providers in the comanagement model.2 Whinney and Michota see comanagement as patient care referral at the onset of a care episode, in contrast to consultations that are activated to address emergent problems.3 In a recent study that found the growing adoption of medical comanagement in Medicare beneficiaries (as much as 40% of surgical hospitalizations in 2006), comanagement was defined as an intensive form of consultation involving a claim for evaluation and management services on greater than 70% of inpatient days.4
In addition to the intensity, frequency, timing, responsibility, and authority of care, comanagement may be described by participating physicians' roles. With recent attention on multidisciplinary teams and an increasing focus on collaborative care, many of the hierarchical relations among healthcare providers are breaking down.5 Several studies of multidisciplinary teams suggest that more egalitarian, rather than hierarchical, problem‐solving and decision‐making among team members are beneficial to patients.67 However, neither the intended nor natural team structure under comanagement is known. We sought to shed some light on provider interactions by characterizing the expectations and experiences of providers of a comanaged service. The findings yielded an opportunity to generate an evolving, but conceptually supported definition of comanagement.
SETTING
We conducted a survey study of providers participating in a comanaged inpatient hepatology service at the University of Chicago Medical Center, a 572‐bed urban teaching hospital. The service was created in 2006, partly to address staffing problems related to housestaff work hour restrictions and partly to improve the care of candidates and recipients of liver transplantation. Nonsurgical floor patients with liver diseases were managed on the service by two collaborating teams of providers. The hepatology team consisted of an attending physician and a fellow, while the hospitalist team consisted of a hospitalist and one or two nonphysician providers (physician assistant or nurse practitioner). The practice model is characterized as comanagement because of the highly interdependent nature of the team's daily tasks and the norms of intensive communication, through formal joint daily rounds and informal direct exchanges of instructions and updates. Hepatologists were mainly responsible for coordinating admissions, managing issues related to liver dysfunction, communicating with transplant surgeons if necessary, and arranging postdischarge care. Hospitalists were responsible for admitting patients, managing routine (eg, ordering daily labs) and urgent issues (eg, responding to critical lab values) during hospitalizations, coordinating with ancillary and consultative staff, and discharging patients. Occasional meetings between the hepatology and hospital medicine groups were used to clarify assignment of responsibilities. Floor nurses received in‐servicing at the commencement of the service. Additional details about the service are described elsewhere.8
DATA COLLECTION AND ANALYSIS
For the purpose of our analysis, we defined interactions between any member of the hospitalist and hepatologist teams as pertinent to comanagement. The hospitalist nonphysician provider (NPP) and hepatologistfellow relationships are governed by the more traditional hierarchical dynamics based on supervision and authority according to laws and regulations. At the beginning of the study period, each participant completed nine items of a Baseline Survey that addressed respondents' expectations and preferences for the management of an ideally comanaged service. Responses were solicited using a 4‐point Likert‐type scale and were dichotomized such that agree and somewhat agree were grouped, while disagree and somewhat disagree were grouped for data analysis. Items were generated to address the salient issues of comanagement after reviewing the pertinent literature.
Subsequently, participants were asked to complete Repeated Surveys immediately before each change in membership of the comanaged team between April and October 2008. The surveys were hand delivered by one of the authors (K.H.) on the last day of each team's rotation and were often completed immediately. The seven items of the Repeated Survey reprised items from the Baseline Survey that were rephrased to allow respondents to report their direct experiences on specific teams. Because all providers rotated on the service more than once during the study period, the average value for each Likert‐type response across multiple surveys completed by a single provider was calculated before being dichotomized at the midpoint (<2.5, agree; 2.5, disagree). We reported proportions of respondents in agreement with survey item statements.
Comparison statistics across providers were generated using the chi‐square test. Differences in proportions between related items of the Baseline and Repeated Surveys were compared using the two‐sample test of proportions. All analyses were conducted using a statistics application (STATA 10.0, College Station, TX) with alpha equal to, or less than, 0.05 considered significant. The Institutional Review Board of the University of Chicago approved this project.
RESULTS
All 43 providers completed the Baseline Survey. During the study period, 32 of these participants rotated on the service and completed 177 of the 233 Repeated Surveys (79%) administered. The responses describe team interactions on the 47 unique combinations of providers comprising the comanaged teams. Details of the response rates are shown in Table 1.
| Baseline Survey, Completed/ Administered (%) | Repeated Surveys, Completed/ Administered (%) | Respondents Completing Repeated Surveys, n | Repeated Surveys Completed per Respondent, Median (IQR) | |
|---|---|---|---|---|
| ||||
| Hospitalists | 18/18 (100) | 36/43 (84) | 15 | 2 (2, 3) |
| NPPs | 5/5 (100) | 92/97 (95) | 5 | 20 (18, 20) |
| Hepatologists | 6/6 (100) | 26/42 (62) | 6 | 7 (3.75, 8) |
| Fellows | 12/12 (100) | 23/42 (55) | 6 | 7 (5.5, 8.5) |
| Total | 43/43 (100) | 177/223 (79) | 32 | 4.5 (2, 8.25) |
As shown in Table 2A, items 13, more members of the hospitalist team preferred to be informed about every management decision compared to members of the hepatologist team. Conversely, more of members of the hepatologist team than the hospitalist team preferred their comanaging partners to participate in every decision. A statistically similar proportion of respondents in each of the professional roles indicated desire for greater influence in directing management decisions (Table 2B, item 1).
| A. Baseline Survey | Hospitalists, % (n = 18) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 12) | P‐value |
|---|---|---|---|---|---|
| |||||
| 1. I prefer to be informed about every decision. | 83 | 100 | 17 | 42 | <0.01 |
| 2. I prefer to participate in every decision. | 67 | 100 | 33 | 50 | 0.11 |
| 3. I prefer that my comanager participate in every decision. | 22 | 20 | 50 | 75 | 0.02 |
| 4. I prefer to have the final say in every decision. | 50 | 80 | 50 | 33 | 0.38 |
| 5. There should be one physician leader to direct the overall management of the patients' hospital course. | 89* | 100 | 67 | 83 | 0.43 |
| 6. Physician consensus should always be sought in every clinical decision. | 22 | 40 | 50 | 67 | 0.11 |
| 7. I have a clear understanding of my role on the comanagement service. | 61 | 80 | 83 | 75 | 0.66 |
| 8. I have as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 61 | 60 | 83 | 50 | 0.60 |
| 9. Comanagement tends to improve patient care. | 94 | 100* | 83 | 100* | 0.47 |
| B. Repeated Surveys | Hospitalists, % (n = 15) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 6) | P‐value |
| 1. I would have liked greater influence in directing the overall management. | 40 | 60 | 0 | 17 | 0.12 |
| 2. I was responsible for work in clinical areas I was not comfortable managing. | 0 | 0 | 0 | 0 | NA |
| 3. There was one physician leader to direct the overall management of the patients' hospital course. | 60* | 80 | 67 | 83 | 0.70 |
| 4. Physician consensus was always sought in every clinical decision. | 40 | 40 | 50 | 67 | 0.72 |
| 5. I (have/had) a clear understanding of my role on the comanagement service. | 73 | 80 | 100 | 83 | 0.57 |
| 6. I had as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 53 | 80 | 100 | 67 | 0.20 |
| 7. Patients on my service received better care than they would have without comanagement. | 93 | 40* | 67 | 50* | 0.06 |
For the majority of surveyed areas, there was concordance between expectations and experiences of providers on comanagement. Most providers, regardless of professional role, agreed that there should be a single physician leader to direct the overall management (Table 2A, item 5). The majority perceived that a single physician directed the overall management of the patients' hospital course, although fewer hospitalists did so compared with baseline expectations (Table 2B, item 3). Many respondents felt at baseline that physician consensus should govern every management decision, and a similar proportion actually experienced consensus‐seeking on service.
We found that the proportion of providers reporting an understanding of their role increased slightly, though not significantly, from before (Table 2A, item 7) to after rotating on the comanaged service (Table 2B, item 5). Although not statistically significant, there was a trend towards hospitalists and gastrointestinal (GI) fellows reporting a lack of patient ownership, both before and after serving on the comanaged service. Finally, nearly all respondents reported that comanagement should improve care quality, although only the attending hospitalist and hepatologist felt that their experience on the comanaged service actually improved patient care (Table 2B, item 7).
DISCUSSION
In this survey of providers participating on a comanaged medical service, most reported understanding their role in the collaborative arrangement and had an initial perception that comanagement should improve patient care quality. We found that hospitalists preferred and were expected to participate in care globally, while hepatologists themselves preferred and were expected not to focus on every management decision. The prevalence of desire for ultimate authority across the professional roles suggests tensions that exist in this care model around how decisions are made. The majority of providers preferred and experienced a single physician leader under comanagement, but many also experienced consensus‐seeking for every management decision.
From these findings, we conclude that decision‐making processes are not uniform under comanagement and that some role ambiguity is present, but there appears to be a pattern of natural roles. This pattern can be defined by focus (general for hospitalists vs specialty‐specific for hepatologists), rather than by responsibilities for managing particular medical problems. The preference among both generalists and specialists for the broader involvement of hospitalist comanagers suggests an implicit recognition of the need for integrated management to overcome the silo‐effect within the comanagement structure.9 Although details about how such integration was achieved are not available in our data, we found that comanagement may be distinct from traditional consultative practice in that the consultants (hospitalists in this case) manage not only general medical problems, such as diabetes or hypertension, but hospitalizations more generally. From a mission‐based standpoint, comanagement may be seen as a collaborative management of complex patients by two or more clinical experts with distinct knowledge, skills, or focus enacted for the purpose of improving care quality.
The focus of comanagement on improving quality is in line with the founding charge of the hospital medicine specialty to raise hospital care quality.10 In fact, the distinction between comanagement and consultation may be meaningful only if comanagers can work with specialists to implement evidence‐based practice, process improvement, and address quality and cost concerns. But as seen in NPPs and fellows' skepticism of improved quality under comanagement, there is still clearly work to be done to validate this model through measurable improvement in patients' experiences and outcomes. Proving the advantages of comanagement as a platform for practice improvement remains future work.11
Collaborative arrangements create natural tensions related to team function.5 This is seen in the similar proportion of hospitalists and hepatologists indicating desire for final decision‐making authority. Although comanagement evokes assumptions about egalitarian provider interactions involving shared decision‐making and responsibility, it seems to function empirically under hierarchical as well as consensus‐seeking forms of decision‐making. Providers at the top of hierarchical teams typically experience their work as interdependent and collaborative, and report more positive interactions with other care providers.12 Based on the fact that no hepatologists wanted more influence over decision‐making, we assume that hepatologists were the physician leaders for most of the studied comanaged teams. Under situations characterized by high levels of complexity and interdependence, a team governed by a single leader may often be more effective than one governed by shared authority.8 However, even under hierarchical models, a more participatory than supervisory leadership can help avoid alienating partners through a pattern of we decide, you carry it out that is often associated with ineffective leadership styles.1314 In fact, this alienating effect on providers in subordinate roles (ie, NPPs and fellows) may have contributed to the negative perception of the team's function on improving patient care.
This study is limited in the following ways. We did not have 100% participation in the Repeated Surveys. Attitudes and experiences of participants in a single comanagement practice are not representative of all comanaging providers. However, the goal of this studyto collect unique survey data from providers themselves to inform an evolving definition of comanagementis modest enough in scope to not require a generalizable sample. Because this study unearthed differences in expectations and experiences within a single site, they may serve as a lower bound for the extent of differences across and within multiple sites. In addition, comanagement enacted for complex medical patients is not as common as the comanagement of surgical patients. Moreover, comanagement models in academic hospitals may have structural features and priorities not found in community settings. Whether or not these disparate models share enough in common to be categorized under a single rubric is a valid question.
Although the teamwork structure and provider roles within comanagement vary, the practice arrangement's preoccupation with quality can be seen as its defining feature. Limited evidence, to date,1, 1519 and the rapid proliferation of the model, suggest that quality and efficiency advantages can be obtained from an effective implementation of comanagement. As in any team‐based care model, a common understanding of roles and expectations are essential to enhancing teamwork. Our interpretation of the mission of comanagement may further enhance teamwork through an explicit articulation of shared goals.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Just because you can, doesn't mean that you should: A call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Surgical comanagement: A natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170(4):363–368.
- .Structure and meaning in multidisciplinary teamwork.Sociol Health Illn.1998;20(6):848–873.
- ,,,,.Human factors and cardiac surgery: A multicenter study.J Thorac Cardiov Surg.2000;119(4):661–670.
- ,,.The effects of a collaborative model of primary care on the mortality and hospital use of community‐dwelling older adults.J Gerontol A‐Biol.2001;56(2):M106–M112.
- ,,,,,.Effects of provider characteristics on care coordination under comanagement.J Hosp Med.2010;5:508–513.
- ,,.Crossing the Quality Chasm: A New Health System for the Twenty‐First Century.Washington, DC:Institute of Medicine;2001.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- .Internal medicine comanagement of surgical patients: Can we afford to do this?Arch Intern Med.2010;170(22):1965–1966.
- ,,, et al.Operating room teamwork among physicians and nurses: Teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- .“We decide, you carry it out”: A social network analysis of multidisciplinary longterm care teams.Soc Sci Med.1997;45(9):1411–1421.
- ,,.Patterns of aggressive behavior in experimentally created social climates.J Soc Psychol.1939;10:271–301.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170(22):2004–2010.
- ,,,,,.Outcomes for older patients with hip fractures: The impact of orthopedic and geriatric medicine cocare.J Orthop Trauma.2006;20(3):172–180.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthop Relat Res.1992(274):213–225.
- ,,,.Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes.Arch Intern Med.2009;169(18):1712–1717.
Comanagement is common in hospital medicine practice. And yet, there is no consensus about how comanagement is different from traditional consultative practice. At its core, hospitalist comanagement is a practice arrangement wherein hospitalists and other specialists manage complex patients collaboratively. Beyond this, Huddleston et al. distinguish comanagement from traditional consultations in the comanaging hospitalists' prerogative to provide direct medical care in addition to consultative advice.1 Siegal focuses on the shared responsibility and authority among partnering providers in the comanagement model.2 Whinney and Michota see comanagement as patient care referral at the onset of a care episode, in contrast to consultations that are activated to address emergent problems.3 In a recent study that found the growing adoption of medical comanagement in Medicare beneficiaries (as much as 40% of surgical hospitalizations in 2006), comanagement was defined as an intensive form of consultation involving a claim for evaluation and management services on greater than 70% of inpatient days.4
In addition to the intensity, frequency, timing, responsibility, and authority of care, comanagement may be described by participating physicians' roles. With recent attention on multidisciplinary teams and an increasing focus on collaborative care, many of the hierarchical relations among healthcare providers are breaking down.5 Several studies of multidisciplinary teams suggest that more egalitarian, rather than hierarchical, problem‐solving and decision‐making among team members are beneficial to patients.67 However, neither the intended nor natural team structure under comanagement is known. We sought to shed some light on provider interactions by characterizing the expectations and experiences of providers of a comanaged service. The findings yielded an opportunity to generate an evolving, but conceptually supported definition of comanagement.
SETTING
We conducted a survey study of providers participating in a comanaged inpatient hepatology service at the University of Chicago Medical Center, a 572‐bed urban teaching hospital. The service was created in 2006, partly to address staffing problems related to housestaff work hour restrictions and partly to improve the care of candidates and recipients of liver transplantation. Nonsurgical floor patients with liver diseases were managed on the service by two collaborating teams of providers. The hepatology team consisted of an attending physician and a fellow, while the hospitalist team consisted of a hospitalist and one or two nonphysician providers (physician assistant or nurse practitioner). The practice model is characterized as comanagement because of the highly interdependent nature of the team's daily tasks and the norms of intensive communication, through formal joint daily rounds and informal direct exchanges of instructions and updates. Hepatologists were mainly responsible for coordinating admissions, managing issues related to liver dysfunction, communicating with transplant surgeons if necessary, and arranging postdischarge care. Hospitalists were responsible for admitting patients, managing routine (eg, ordering daily labs) and urgent issues (eg, responding to critical lab values) during hospitalizations, coordinating with ancillary and consultative staff, and discharging patients. Occasional meetings between the hepatology and hospital medicine groups were used to clarify assignment of responsibilities. Floor nurses received in‐servicing at the commencement of the service. Additional details about the service are described elsewhere.8
DATA COLLECTION AND ANALYSIS
For the purpose of our analysis, we defined interactions between any member of the hospitalist and hepatologist teams as pertinent to comanagement. The hospitalist nonphysician provider (NPP) and hepatologistfellow relationships are governed by the more traditional hierarchical dynamics based on supervision and authority according to laws and regulations. At the beginning of the study period, each participant completed nine items of a Baseline Survey that addressed respondents' expectations and preferences for the management of an ideally comanaged service. Responses were solicited using a 4‐point Likert‐type scale and were dichotomized such that agree and somewhat agree were grouped, while disagree and somewhat disagree were grouped for data analysis. Items were generated to address the salient issues of comanagement after reviewing the pertinent literature.
Subsequently, participants were asked to complete Repeated Surveys immediately before each change in membership of the comanaged team between April and October 2008. The surveys were hand delivered by one of the authors (K.H.) on the last day of each team's rotation and were often completed immediately. The seven items of the Repeated Survey reprised items from the Baseline Survey that were rephrased to allow respondents to report their direct experiences on specific teams. Because all providers rotated on the service more than once during the study period, the average value for each Likert‐type response across multiple surveys completed by a single provider was calculated before being dichotomized at the midpoint (<2.5, agree; 2.5, disagree). We reported proportions of respondents in agreement with survey item statements.
Comparison statistics across providers were generated using the chi‐square test. Differences in proportions between related items of the Baseline and Repeated Surveys were compared using the two‐sample test of proportions. All analyses were conducted using a statistics application (STATA 10.0, College Station, TX) with alpha equal to, or less than, 0.05 considered significant. The Institutional Review Board of the University of Chicago approved this project.
RESULTS
All 43 providers completed the Baseline Survey. During the study period, 32 of these participants rotated on the service and completed 177 of the 233 Repeated Surveys (79%) administered. The responses describe team interactions on the 47 unique combinations of providers comprising the comanaged teams. Details of the response rates are shown in Table 1.
| Baseline Survey, Completed/ Administered (%) | Repeated Surveys, Completed/ Administered (%) | Respondents Completing Repeated Surveys, n | Repeated Surveys Completed per Respondent, Median (IQR) | |
|---|---|---|---|---|
| ||||
| Hospitalists | 18/18 (100) | 36/43 (84) | 15 | 2 (2, 3) |
| NPPs | 5/5 (100) | 92/97 (95) | 5 | 20 (18, 20) |
| Hepatologists | 6/6 (100) | 26/42 (62) | 6 | 7 (3.75, 8) |
| Fellows | 12/12 (100) | 23/42 (55) | 6 | 7 (5.5, 8.5) |
| Total | 43/43 (100) | 177/223 (79) | 32 | 4.5 (2, 8.25) |
As shown in Table 2A, items 13, more members of the hospitalist team preferred to be informed about every management decision compared to members of the hepatologist team. Conversely, more of members of the hepatologist team than the hospitalist team preferred their comanaging partners to participate in every decision. A statistically similar proportion of respondents in each of the professional roles indicated desire for greater influence in directing management decisions (Table 2B, item 1).
| A. Baseline Survey | Hospitalists, % (n = 18) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 12) | P‐value |
|---|---|---|---|---|---|
| |||||
| 1. I prefer to be informed about every decision. | 83 | 100 | 17 | 42 | <0.01 |
| 2. I prefer to participate in every decision. | 67 | 100 | 33 | 50 | 0.11 |
| 3. I prefer that my comanager participate in every decision. | 22 | 20 | 50 | 75 | 0.02 |
| 4. I prefer to have the final say in every decision. | 50 | 80 | 50 | 33 | 0.38 |
| 5. There should be one physician leader to direct the overall management of the patients' hospital course. | 89* | 100 | 67 | 83 | 0.43 |
| 6. Physician consensus should always be sought in every clinical decision. | 22 | 40 | 50 | 67 | 0.11 |
| 7. I have a clear understanding of my role on the comanagement service. | 61 | 80 | 83 | 75 | 0.66 |
| 8. I have as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 61 | 60 | 83 | 50 | 0.60 |
| 9. Comanagement tends to improve patient care. | 94 | 100* | 83 | 100* | 0.47 |
| B. Repeated Surveys | Hospitalists, % (n = 15) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 6) | P‐value |
| 1. I would have liked greater influence in directing the overall management. | 40 | 60 | 0 | 17 | 0.12 |
| 2. I was responsible for work in clinical areas I was not comfortable managing. | 0 | 0 | 0 | 0 | NA |
| 3. There was one physician leader to direct the overall management of the patients' hospital course. | 60* | 80 | 67 | 83 | 0.70 |
| 4. Physician consensus was always sought in every clinical decision. | 40 | 40 | 50 | 67 | 0.72 |
| 5. I (have/had) a clear understanding of my role on the comanagement service. | 73 | 80 | 100 | 83 | 0.57 |
| 6. I had as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 53 | 80 | 100 | 67 | 0.20 |
| 7. Patients on my service received better care than they would have without comanagement. | 93 | 40* | 67 | 50* | 0.06 |
For the majority of surveyed areas, there was concordance between expectations and experiences of providers on comanagement. Most providers, regardless of professional role, agreed that there should be a single physician leader to direct the overall management (Table 2A, item 5). The majority perceived that a single physician directed the overall management of the patients' hospital course, although fewer hospitalists did so compared with baseline expectations (Table 2B, item 3). Many respondents felt at baseline that physician consensus should govern every management decision, and a similar proportion actually experienced consensus‐seeking on service.
We found that the proportion of providers reporting an understanding of their role increased slightly, though not significantly, from before (Table 2A, item 7) to after rotating on the comanaged service (Table 2B, item 5). Although not statistically significant, there was a trend towards hospitalists and gastrointestinal (GI) fellows reporting a lack of patient ownership, both before and after serving on the comanaged service. Finally, nearly all respondents reported that comanagement should improve care quality, although only the attending hospitalist and hepatologist felt that their experience on the comanaged service actually improved patient care (Table 2B, item 7).
DISCUSSION
In this survey of providers participating on a comanaged medical service, most reported understanding their role in the collaborative arrangement and had an initial perception that comanagement should improve patient care quality. We found that hospitalists preferred and were expected to participate in care globally, while hepatologists themselves preferred and were expected not to focus on every management decision. The prevalence of desire for ultimate authority across the professional roles suggests tensions that exist in this care model around how decisions are made. The majority of providers preferred and experienced a single physician leader under comanagement, but many also experienced consensus‐seeking for every management decision.
From these findings, we conclude that decision‐making processes are not uniform under comanagement and that some role ambiguity is present, but there appears to be a pattern of natural roles. This pattern can be defined by focus (general for hospitalists vs specialty‐specific for hepatologists), rather than by responsibilities for managing particular medical problems. The preference among both generalists and specialists for the broader involvement of hospitalist comanagers suggests an implicit recognition of the need for integrated management to overcome the silo‐effect within the comanagement structure.9 Although details about how such integration was achieved are not available in our data, we found that comanagement may be distinct from traditional consultative practice in that the consultants (hospitalists in this case) manage not only general medical problems, such as diabetes or hypertension, but hospitalizations more generally. From a mission‐based standpoint, comanagement may be seen as a collaborative management of complex patients by two or more clinical experts with distinct knowledge, skills, or focus enacted for the purpose of improving care quality.
The focus of comanagement on improving quality is in line with the founding charge of the hospital medicine specialty to raise hospital care quality.10 In fact, the distinction between comanagement and consultation may be meaningful only if comanagers can work with specialists to implement evidence‐based practice, process improvement, and address quality and cost concerns. But as seen in NPPs and fellows' skepticism of improved quality under comanagement, there is still clearly work to be done to validate this model through measurable improvement in patients' experiences and outcomes. Proving the advantages of comanagement as a platform for practice improvement remains future work.11
Collaborative arrangements create natural tensions related to team function.5 This is seen in the similar proportion of hospitalists and hepatologists indicating desire for final decision‐making authority. Although comanagement evokes assumptions about egalitarian provider interactions involving shared decision‐making and responsibility, it seems to function empirically under hierarchical as well as consensus‐seeking forms of decision‐making. Providers at the top of hierarchical teams typically experience their work as interdependent and collaborative, and report more positive interactions with other care providers.12 Based on the fact that no hepatologists wanted more influence over decision‐making, we assume that hepatologists were the physician leaders for most of the studied comanaged teams. Under situations characterized by high levels of complexity and interdependence, a team governed by a single leader may often be more effective than one governed by shared authority.8 However, even under hierarchical models, a more participatory than supervisory leadership can help avoid alienating partners through a pattern of we decide, you carry it out that is often associated with ineffective leadership styles.1314 In fact, this alienating effect on providers in subordinate roles (ie, NPPs and fellows) may have contributed to the negative perception of the team's function on improving patient care.
This study is limited in the following ways. We did not have 100% participation in the Repeated Surveys. Attitudes and experiences of participants in a single comanagement practice are not representative of all comanaging providers. However, the goal of this studyto collect unique survey data from providers themselves to inform an evolving definition of comanagementis modest enough in scope to not require a generalizable sample. Because this study unearthed differences in expectations and experiences within a single site, they may serve as a lower bound for the extent of differences across and within multiple sites. In addition, comanagement enacted for complex medical patients is not as common as the comanagement of surgical patients. Moreover, comanagement models in academic hospitals may have structural features and priorities not found in community settings. Whether or not these disparate models share enough in common to be categorized under a single rubric is a valid question.
Although the teamwork structure and provider roles within comanagement vary, the practice arrangement's preoccupation with quality can be seen as its defining feature. Limited evidence, to date,1, 1519 and the rapid proliferation of the model, suggest that quality and efficiency advantages can be obtained from an effective implementation of comanagement. As in any team‐based care model, a common understanding of roles and expectations are essential to enhancing teamwork. Our interpretation of the mission of comanagement may further enhance teamwork through an explicit articulation of shared goals.
Comanagement is common in hospital medicine practice. And yet, there is no consensus about how comanagement is different from traditional consultative practice. At its core, hospitalist comanagement is a practice arrangement wherein hospitalists and other specialists manage complex patients collaboratively. Beyond this, Huddleston et al. distinguish comanagement from traditional consultations in the comanaging hospitalists' prerogative to provide direct medical care in addition to consultative advice.1 Siegal focuses on the shared responsibility and authority among partnering providers in the comanagement model.2 Whinney and Michota see comanagement as patient care referral at the onset of a care episode, in contrast to consultations that are activated to address emergent problems.3 In a recent study that found the growing adoption of medical comanagement in Medicare beneficiaries (as much as 40% of surgical hospitalizations in 2006), comanagement was defined as an intensive form of consultation involving a claim for evaluation and management services on greater than 70% of inpatient days.4
In addition to the intensity, frequency, timing, responsibility, and authority of care, comanagement may be described by participating physicians' roles. With recent attention on multidisciplinary teams and an increasing focus on collaborative care, many of the hierarchical relations among healthcare providers are breaking down.5 Several studies of multidisciplinary teams suggest that more egalitarian, rather than hierarchical, problem‐solving and decision‐making among team members are beneficial to patients.67 However, neither the intended nor natural team structure under comanagement is known. We sought to shed some light on provider interactions by characterizing the expectations and experiences of providers of a comanaged service. The findings yielded an opportunity to generate an evolving, but conceptually supported definition of comanagement.
SETTING
We conducted a survey study of providers participating in a comanaged inpatient hepatology service at the University of Chicago Medical Center, a 572‐bed urban teaching hospital. The service was created in 2006, partly to address staffing problems related to housestaff work hour restrictions and partly to improve the care of candidates and recipients of liver transplantation. Nonsurgical floor patients with liver diseases were managed on the service by two collaborating teams of providers. The hepatology team consisted of an attending physician and a fellow, while the hospitalist team consisted of a hospitalist and one or two nonphysician providers (physician assistant or nurse practitioner). The practice model is characterized as comanagement because of the highly interdependent nature of the team's daily tasks and the norms of intensive communication, through formal joint daily rounds and informal direct exchanges of instructions and updates. Hepatologists were mainly responsible for coordinating admissions, managing issues related to liver dysfunction, communicating with transplant surgeons if necessary, and arranging postdischarge care. Hospitalists were responsible for admitting patients, managing routine (eg, ordering daily labs) and urgent issues (eg, responding to critical lab values) during hospitalizations, coordinating with ancillary and consultative staff, and discharging patients. Occasional meetings between the hepatology and hospital medicine groups were used to clarify assignment of responsibilities. Floor nurses received in‐servicing at the commencement of the service. Additional details about the service are described elsewhere.8
DATA COLLECTION AND ANALYSIS
For the purpose of our analysis, we defined interactions between any member of the hospitalist and hepatologist teams as pertinent to comanagement. The hospitalist nonphysician provider (NPP) and hepatologistfellow relationships are governed by the more traditional hierarchical dynamics based on supervision and authority according to laws and regulations. At the beginning of the study period, each participant completed nine items of a Baseline Survey that addressed respondents' expectations and preferences for the management of an ideally comanaged service. Responses were solicited using a 4‐point Likert‐type scale and were dichotomized such that agree and somewhat agree were grouped, while disagree and somewhat disagree were grouped for data analysis. Items were generated to address the salient issues of comanagement after reviewing the pertinent literature.
Subsequently, participants were asked to complete Repeated Surveys immediately before each change in membership of the comanaged team between April and October 2008. The surveys were hand delivered by one of the authors (K.H.) on the last day of each team's rotation and were often completed immediately. The seven items of the Repeated Survey reprised items from the Baseline Survey that were rephrased to allow respondents to report their direct experiences on specific teams. Because all providers rotated on the service more than once during the study period, the average value for each Likert‐type response across multiple surveys completed by a single provider was calculated before being dichotomized at the midpoint (<2.5, agree; 2.5, disagree). We reported proportions of respondents in agreement with survey item statements.
Comparison statistics across providers were generated using the chi‐square test. Differences in proportions between related items of the Baseline and Repeated Surveys were compared using the two‐sample test of proportions. All analyses were conducted using a statistics application (STATA 10.0, College Station, TX) with alpha equal to, or less than, 0.05 considered significant. The Institutional Review Board of the University of Chicago approved this project.
RESULTS
All 43 providers completed the Baseline Survey. During the study period, 32 of these participants rotated on the service and completed 177 of the 233 Repeated Surveys (79%) administered. The responses describe team interactions on the 47 unique combinations of providers comprising the comanaged teams. Details of the response rates are shown in Table 1.
| Baseline Survey, Completed/ Administered (%) | Repeated Surveys, Completed/ Administered (%) | Respondents Completing Repeated Surveys, n | Repeated Surveys Completed per Respondent, Median (IQR) | |
|---|---|---|---|---|
| ||||
| Hospitalists | 18/18 (100) | 36/43 (84) | 15 | 2 (2, 3) |
| NPPs | 5/5 (100) | 92/97 (95) | 5 | 20 (18, 20) |
| Hepatologists | 6/6 (100) | 26/42 (62) | 6 | 7 (3.75, 8) |
| Fellows | 12/12 (100) | 23/42 (55) | 6 | 7 (5.5, 8.5) |
| Total | 43/43 (100) | 177/223 (79) | 32 | 4.5 (2, 8.25) |
As shown in Table 2A, items 13, more members of the hospitalist team preferred to be informed about every management decision compared to members of the hepatologist team. Conversely, more of members of the hepatologist team than the hospitalist team preferred their comanaging partners to participate in every decision. A statistically similar proportion of respondents in each of the professional roles indicated desire for greater influence in directing management decisions (Table 2B, item 1).
| A. Baseline Survey | Hospitalists, % (n = 18) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 12) | P‐value |
|---|---|---|---|---|---|
| |||||
| 1. I prefer to be informed about every decision. | 83 | 100 | 17 | 42 | <0.01 |
| 2. I prefer to participate in every decision. | 67 | 100 | 33 | 50 | 0.11 |
| 3. I prefer that my comanager participate in every decision. | 22 | 20 | 50 | 75 | 0.02 |
| 4. I prefer to have the final say in every decision. | 50 | 80 | 50 | 33 | 0.38 |
| 5. There should be one physician leader to direct the overall management of the patients' hospital course. | 89* | 100 | 67 | 83 | 0.43 |
| 6. Physician consensus should always be sought in every clinical decision. | 22 | 40 | 50 | 67 | 0.11 |
| 7. I have a clear understanding of my role on the comanagement service. | 61 | 80 | 83 | 75 | 0.66 |
| 8. I have as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 61 | 60 | 83 | 50 | 0.60 |
| 9. Comanagement tends to improve patient care. | 94 | 100* | 83 | 100* | 0.47 |
| B. Repeated Surveys | Hospitalists, % (n = 15) | NPPs, % (n = 5) | Hepatologists, % (n = 6) | GI Fellows, % (n = 6) | P‐value |
| 1. I would have liked greater influence in directing the overall management. | 40 | 60 | 0 | 17 | 0.12 |
| 2. I was responsible for work in clinical areas I was not comfortable managing. | 0 | 0 | 0 | 0 | NA |
| 3. There was one physician leader to direct the overall management of the patients' hospital course. | 60* | 80 | 67 | 83 | 0.70 |
| 4. Physician consensus was always sought in every clinical decision. | 40 | 40 | 50 | 67 | 0.72 |
| 5. I (have/had) a clear understanding of my role on the comanagement service. | 73 | 80 | 100 | 83 | 0.57 |
| 6. I had as much a sense of ownership of patients on the comanaged service as on a non‐comanaged service. | 53 | 80 | 100 | 67 | 0.20 |
| 7. Patients on my service received better care than they would have without comanagement. | 93 | 40* | 67 | 50* | 0.06 |
For the majority of surveyed areas, there was concordance between expectations and experiences of providers on comanagement. Most providers, regardless of professional role, agreed that there should be a single physician leader to direct the overall management (Table 2A, item 5). The majority perceived that a single physician directed the overall management of the patients' hospital course, although fewer hospitalists did so compared with baseline expectations (Table 2B, item 3). Many respondents felt at baseline that physician consensus should govern every management decision, and a similar proportion actually experienced consensus‐seeking on service.
We found that the proportion of providers reporting an understanding of their role increased slightly, though not significantly, from before (Table 2A, item 7) to after rotating on the comanaged service (Table 2B, item 5). Although not statistically significant, there was a trend towards hospitalists and gastrointestinal (GI) fellows reporting a lack of patient ownership, both before and after serving on the comanaged service. Finally, nearly all respondents reported that comanagement should improve care quality, although only the attending hospitalist and hepatologist felt that their experience on the comanaged service actually improved patient care (Table 2B, item 7).
DISCUSSION
In this survey of providers participating on a comanaged medical service, most reported understanding their role in the collaborative arrangement and had an initial perception that comanagement should improve patient care quality. We found that hospitalists preferred and were expected to participate in care globally, while hepatologists themselves preferred and were expected not to focus on every management decision. The prevalence of desire for ultimate authority across the professional roles suggests tensions that exist in this care model around how decisions are made. The majority of providers preferred and experienced a single physician leader under comanagement, but many also experienced consensus‐seeking for every management decision.
From these findings, we conclude that decision‐making processes are not uniform under comanagement and that some role ambiguity is present, but there appears to be a pattern of natural roles. This pattern can be defined by focus (general for hospitalists vs specialty‐specific for hepatologists), rather than by responsibilities for managing particular medical problems. The preference among both generalists and specialists for the broader involvement of hospitalist comanagers suggests an implicit recognition of the need for integrated management to overcome the silo‐effect within the comanagement structure.9 Although details about how such integration was achieved are not available in our data, we found that comanagement may be distinct from traditional consultative practice in that the consultants (hospitalists in this case) manage not only general medical problems, such as diabetes or hypertension, but hospitalizations more generally. From a mission‐based standpoint, comanagement may be seen as a collaborative management of complex patients by two or more clinical experts with distinct knowledge, skills, or focus enacted for the purpose of improving care quality.
The focus of comanagement on improving quality is in line with the founding charge of the hospital medicine specialty to raise hospital care quality.10 In fact, the distinction between comanagement and consultation may be meaningful only if comanagers can work with specialists to implement evidence‐based practice, process improvement, and address quality and cost concerns. But as seen in NPPs and fellows' skepticism of improved quality under comanagement, there is still clearly work to be done to validate this model through measurable improvement in patients' experiences and outcomes. Proving the advantages of comanagement as a platform for practice improvement remains future work.11
Collaborative arrangements create natural tensions related to team function.5 This is seen in the similar proportion of hospitalists and hepatologists indicating desire for final decision‐making authority. Although comanagement evokes assumptions about egalitarian provider interactions involving shared decision‐making and responsibility, it seems to function empirically under hierarchical as well as consensus‐seeking forms of decision‐making. Providers at the top of hierarchical teams typically experience their work as interdependent and collaborative, and report more positive interactions with other care providers.12 Based on the fact that no hepatologists wanted more influence over decision‐making, we assume that hepatologists were the physician leaders for most of the studied comanaged teams. Under situations characterized by high levels of complexity and interdependence, a team governed by a single leader may often be more effective than one governed by shared authority.8 However, even under hierarchical models, a more participatory than supervisory leadership can help avoid alienating partners through a pattern of we decide, you carry it out that is often associated with ineffective leadership styles.1314 In fact, this alienating effect on providers in subordinate roles (ie, NPPs and fellows) may have contributed to the negative perception of the team's function on improving patient care.
This study is limited in the following ways. We did not have 100% participation in the Repeated Surveys. Attitudes and experiences of participants in a single comanagement practice are not representative of all comanaging providers. However, the goal of this studyto collect unique survey data from providers themselves to inform an evolving definition of comanagementis modest enough in scope to not require a generalizable sample. Because this study unearthed differences in expectations and experiences within a single site, they may serve as a lower bound for the extent of differences across and within multiple sites. In addition, comanagement enacted for complex medical patients is not as common as the comanagement of surgical patients. Moreover, comanagement models in academic hospitals may have structural features and priorities not found in community settings. Whether or not these disparate models share enough in common to be categorized under a single rubric is a valid question.
Although the teamwork structure and provider roles within comanagement vary, the practice arrangement's preoccupation with quality can be seen as its defining feature. Limited evidence, to date,1, 1519 and the rapid proliferation of the model, suggest that quality and efficiency advantages can be obtained from an effective implementation of comanagement. As in any team‐based care model, a common understanding of roles and expectations are essential to enhancing teamwork. Our interpretation of the mission of comanagement may further enhance teamwork through an explicit articulation of shared goals.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Just because you can, doesn't mean that you should: A call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Surgical comanagement: A natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170(4):363–368.
- .Structure and meaning in multidisciplinary teamwork.Sociol Health Illn.1998;20(6):848–873.
- ,,,,.Human factors and cardiac surgery: A multicenter study.J Thorac Cardiov Surg.2000;119(4):661–670.
- ,,.The effects of a collaborative model of primary care on the mortality and hospital use of community‐dwelling older adults.J Gerontol A‐Biol.2001;56(2):M106–M112.
- ,,,,,.Effects of provider characteristics on care coordination under comanagement.J Hosp Med.2010;5:508–513.
- ,,.Crossing the Quality Chasm: A New Health System for the Twenty‐First Century.Washington, DC:Institute of Medicine;2001.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- .Internal medicine comanagement of surgical patients: Can we afford to do this?Arch Intern Med.2010;170(22):1965–1966.
- ,,, et al.Operating room teamwork among physicians and nurses: Teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- .“We decide, you carry it out”: A social network analysis of multidisciplinary longterm care teams.Soc Sci Med.1997;45(9):1411–1421.
- ,,.Patterns of aggressive behavior in experimentally created social climates.J Soc Psychol.1939;10:271–301.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170(22):2004–2010.
- ,,,,,.Outcomes for older patients with hip fractures: The impact of orthopedic and geriatric medicine cocare.J Orthop Trauma.2006;20(3):172–180.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthop Relat Res.1992(274):213–225.
- ,,,.Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes.Arch Intern Med.2009;169(18):1712–1717.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Just because you can, doesn't mean that you should: A call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Surgical comanagement: A natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170(4):363–368.
- .Structure and meaning in multidisciplinary teamwork.Sociol Health Illn.1998;20(6):848–873.
- ,,,,.Human factors and cardiac surgery: A multicenter study.J Thorac Cardiov Surg.2000;119(4):661–670.
- ,,.The effects of a collaborative model of primary care on the mortality and hospital use of community‐dwelling older adults.J Gerontol A‐Biol.2001;56(2):M106–M112.
- ,,,,,.Effects of provider characteristics on care coordination under comanagement.J Hosp Med.2010;5:508–513.
- ,,.Crossing the Quality Chasm: A New Health System for the Twenty‐First Century.Washington, DC:Institute of Medicine;2001.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- .Internal medicine comanagement of surgical patients: Can we afford to do this?Arch Intern Med.2010;170(22):1965–1966.
- ,,, et al.Operating room teamwork among physicians and nurses: Teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- .“We decide, you carry it out”: A social network analysis of multidisciplinary longterm care teams.Soc Sci Med.1997;45(9):1411–1421.
- ,,.Patterns of aggressive behavior in experimentally created social climates.J Soc Psychol.1939;10:271–301.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170(22):2004–2010.
- ,,,,,.Outcomes for older patients with hip fractures: The impact of orthopedic and geriatric medicine cocare.J Orthop Trauma.2006;20(3):172–180.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthop Relat Res.1992(274):213–225.
- ,,,.Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes.Arch Intern Med.2009;169(18):1712–1717.
Copyright © 2011 Society of Hospital Medicine
Successfully Promoted Academic Hospitalists
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
The growth of academic hospital medicine has been driven by multiple factors including expanding clinical needs, housestaff duty hours' limitations, and an increasing focus on quality and patient safety.1 Hospitalists at academic medical centers frequently assume roles that differ substantially from traditional faculty positions. Academic hospitalists may have predominantly clinical positions, and may be involved in quality improvement and patient safety projects.24 Because of these commitments, many academic hospitalists spend less time on research or educational efforts.1, 5 Many have raised concerns that these unique job descriptions might lead to less time to devote to scholarship and academic pursuits, and consequently greater challenges in the promotions process.2, 5
There are little published data on promotion and tenure in academics, and even less specifically focused on the promotion of hospitalists. Theoretically, promotion should recognize an individual's contribution to his or her institution and field. However, each institution has unique criteria though which faculty achieve promotion. Previous articles addressing specific groups, such as part‐time,6 clinical faculty,79 or clinician‐educators10 may be relevant to hospitalists, as hospitalists may be more likely to fall into these categories. These reports suggest general agreement that promotion committees should consider and weigh clinical and educational work (in addition to scholarly publications) in the promotions process, but assessment methods vary across institutions and the contribution of activities, such as quality improvement, remain unclear. The educator's portfolio has gained momentum as a way to document valued teaching in many institutions,11, 12 but academic hospitalist participation in education may be limited.13
Literature related to the development of Divisions of General Internal Medicine is relevant insofar as similar concerns for promotion were expressed with the growth of their faculty.14, 15 However, its applicability may be limited by differences between roles of hospitalists and more traditional general medicine faculty.
To better understand the factors influencing promotion for academic hospitalists, the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force (AHTF) undertook a survey of promoted hospitalists who had successfully reached the rank of Associate Professor or higher.
Methods
Development of the Survey
The AHTF is a group of 18 academic hospitalists representing 15 institutions. Draft survey questions were developed by the group and sent to its members for refinement based on group consensus. Three cycles of refinement were performed, and the final survey (Appendix) was converted into an electronic format distributed through SurveyMonkey (SurveyMonkey.com, Portland, OR).
Identification of Survey Recipients
We identified a convenience sample of hospitalists who had been promoted to Associate or Full Professor of Medicine by querying members of the AHTF, the Society of Hospital Medicine (SHM) Academic Committee, and colleagues of academic medical centers with established hospitalist programs. We identified 33 promoted hospitalists.
Each recipient received an email from the AHTF cochairs in January 2009 asking them to complete the survey. If a response was not received in three weeks, a second email was sent. If a response was again not received, an AHTF task‐force member who knew the recipient asked him or her to complete the survey. All responses were received by March 2009.
Data Analysis
We examined responses using descriptive measures. Responses were analyzed across all respondents, as well as between these two subgroups. Statistical analysis with Fisher's exact test was performed using Stata 9.0 (StataCorp, College Station, TX).
Results
Of the 33 hospitalists who received the survey, 26 responded (response rate of 79%). Of these, 25 completed the survey in its entirely and were included in our analysis; 1 did not submit details regarding specific promotion‐related activities. General information regarding the respondents and their programs at the time of their promotion is contained in Table 1.
| |
| No. of institutions represented | 20 |
| Program age | 5.7 years (range 110) |
| Size of hospitalist program at the time of promotion | 10 (range 128) |
| Size of hospitalist program currently | 25 (range 745) |
| Programs that were separate divisions at the time of respondent promotion | 4 (20%) |
| Programs that are now separate divisions | 8 (40%) |
| Programs with 1‐track* promotion system | 2 (10%) |
| Programs with 2‐track promotion system | 8 (40%) |
| Programs with 3‐track promotion system | 9 (45%) |
| Other type of promotion system | 1 (5%) |
| Tenure track* | 8 (32%) |
| Institutions with tenure and promotion criteria that explicitly recognized hospitalist work | 8 (40%) |
The seven nonrespondents were from seven different institutions; however two of these institutions were represented by respondents. One nonrespondent had achieved a rank of Professor (through general medicine); the rest had been promoted to Associate Professor. One nonrespondent is known by the authors to hold a research position.
Ten respondents identified themselves as clinician‐educators (40%), ten as clinician‐administrators (40%), and five as clinician‐researchers (20%). Seventeen (68%) of the promoted hospitalists were not on a tenure track (as defined by them); they were more likely to have administrative or educational roles than a research appointment. Though the majority of self‐identified researchers were among the earliest to have been promoted, there were no statistically significant differences in self‐defined job description between more and less recently promoted hospitalists.
Promoted hospitalists were involved in a diverse range of activities which supported their promotion, including service (eg, institutional committees), education, research, and quality improvement. Nearly all hospitalists surveyed listed teaching and educational activities, and almost all had disseminated scholarly output and some degree of grant funding. Table 2 lists the specific activities in which respondents reported being engaged in each of these domains.
| Activity | Percent of Respondents Engaged in Activity |
|---|---|
| Service | |
| School of Medicine | 56 |
| Department of Medicine | 84 |
| Hospital | 80 |
| Professional societies | 92 |
| Administration | 67 |
| Education | |
| Medical student | 72 |
| Housestaff lectures | 84 |
| Ward/consult attending | 96 |
| Clinic precepting | 40 |
| Course director/curriculum development | 80 |
| Program director (or associate) | 36 |
| Research | |
| Peer‐reviewed publications | 92 |
| Abstract/poster presentations | 80 |
| Invited speaker | 96 |
| Reviewer/editor | 80 |
| Study section | 24 |
| Federal grants | 32 |
| Nonfederal grants (internal and external) | 72 |
| Quality improvement/patient safety | |
| Project member | 36 |
| Project leader | 52 |
| Institutional leadership | 32 |
| Curriculum development | 32 |
A range of individuals assisted the respondents in the promotion process. Twenty‐three (92%) respondents identified the individuals who supported their promotion, and all listed more than one person. Respondents most commonly credited their Section or Division Chief (43%) with facilitating their promotion, followed by Departmental Chairs or Vice/Associate Chairs (22%). Mentors (13%) or peers (8%) were also named. Four respondents (17%) named themselves as the person providing most guidance through the promotions process.
No consistent themes regarding obstacles emerged from free‐text responses to questions about the promotions process. One respondent felt that high clinical expectations made participation in other academic activities a challenge. The only other barriers noted were not being on the radar screen of the Division Chief of GIM, and difficulty identifying external, senior hospitalists to write letters in support of promotion.
When asked about the most important activities supporting their promotion, 24 respondents listed one to two key activities, detailed in Table 3. The most common response was peer‐reviewed publications (33%). Activities related to education and/or teaching were the next most common response (29%), specifically teaching, educational activities, curriculum design, or program director. Research or research funding represented 26% of responses. Valued activities outside of the respondent's institution included national reputation (21%) and service in professional societies (16%). Service or administrative responsibilities were mentioned by 25% of respondents.
| Category of Activity | Frequency of Response* (%) |
|---|---|
| |
| Research | 14 (58) |
| Peer‐reviewed publications | 8 (33) |
| Research | 4 (16) |
| Research funding | 2 (8) |
| Activities outside institution | 8 (33) |
| National reputation | 5 (21) |
| Professional society membership | 3 (13) |
| Education | 7 (29) |
| Teaching | 3 (13) |
| Educational activities | 2 (8) |
| Residency Director | 1 (4) |
| Curriculum development | 1 (4) |
| Service | 6 (25) |
| Service | 3 (13) |
| Administration/leadership of group | 3 (13) |
Discussion
We conducted a unique and comprehensive survey of academic hospitalists who have been promoted since 1995. We identified the most common and important activities contributing to promotion. Contrary to our expectations, survey respondents generally did not report being a hospitalist was a barrier in the promotions process.
Respondents were engaged in a diverse range of activities, including service, education, and research. Interestingly, no one identified him or herself primarily as a clinician. Teaching appeared to be a core component for all surveyed, regardless of academic appointment. Only one felt that her clinical workload as a hospitalist was an obstacle that prevented her from being engaged in other activities important for promotion. With more programs potentially evolving to separate divisions, the issue of being on the radar screen of a General Internal Medicine Division Chief may become less common over time. We hope that as programs mature and the numbers of associate and full professors increase, there will not be difficulty obtaining outside letters.
Although only 23% self‐identified as clinician‐researchers, nearly all had peer‐reviewed publications and other evidence of disseminated scholarly work. Grant funding, both federal and nonfederal, was also common among this group. This finding is consistent with self‐reported activities of a cohort of junior internal medicine faculty followed over three years who were eventually promoted, though the majority of those participants were classified as having either traditional clinician‐educator or clinician‐researcher positions.16
Despite outlining a seemingly clear pathway to promotion for hospitalists, concerns remain. Most importantly, those surveyed seem to have achieved promotion through relatively traditional academic job descriptions. Obtaining or maintaining these types of positions may be difficult as clinical needs at academic centers increase. According to a recent survey of hospitalist faculty,13 over one‐third spend more than 60% of their time on nonteaching clinical services. In that survey, over half of respondents had little or no protected time for scholarly activities. The contrast between this survey's findings and ours raises the question of whether our promoted sample had positions similar to those of most academic hospitalists. Given that the majority of our respondents noted peer‐reviewed publications and grant funding to be among the most important activities for promotion, there may be a dangerous disconnect for junior academic hospitalists who spend the majority of their time in direct patient care. Moreover, the promoted hospitalists in our survey reported relatively less participation in quality improvement/patient safety activities, in contrast to both anecdotal and survey reports that these activities are a major component of many academic hospitalist positions.5, 17 Most academic medical centers do not yet consider achievements in this area in their promotions criteria, potentially creating a barrier for the ranks of clinician quality improvers.1 Thus, significant obstacles to promotion of academic hospitalists may exist.
Leaders in academic hospital medicine are recognizing these potential barriers. A diverse group from major professional societies recently published a summary of the challenges and opportunities for the field of academic hospital medicine.1 Several needs and areas for intervention were identified, including enhanced faculty development and improved documentation of quality improvement activities. The SGIM, the SHM, and the Association of Chiefs and Leaders of General Internal Medicine (ACLGIM) recently cosponsored an intensive four‐day faculty development course for junior faculty to promote skills necessary for academic hospitalist success. Early reports indicate that this was a success.1820
In addition, the AHTF has developed a Quality Portfolio, paralleling the Educator's Portfolio, that can be used as a tool for documenting quality improvement and patient safety activities in a way that can be useful for career development and promotion.4 Lastly, the Society of Hospital Medicine has hosted the inaugural Academic Hospital Medicine Leadership Summit as part of the national meeting to provide mentorship and professional development opportunities for junior faculty. Our hope is that these opportunities, coupled with the growth of mid‐level and senior leaders in hospital medicine, will provide greater infrastructure for the development and promotion of junior faculty.
Our results may have relevance beyond hospitalist groups. With anticipated further limits on housestaff duty hours, more academic physicians may be asked to fill predominantly clinical roles. In addition, a growing emphasis on quality and patient safety may lead to a more general expansion of academicians who focus on these areas.15
Our survey and methodology have limitations. By including only promoted individuals, we did not survey hospitalists with the most difficulties in the promotions processthose who were not promoted. Thus, we are unable to directly compare successful versus unsuccessful strategies. Identifying nonpromoted academic hospitalists to understand the reasons they were not (or have not yet been) promoted could be a next step in this line of inquiry. Additionally, understanding the attitudes of promotions committees regarding hospitalists, and the clinical and quality improvement roles in which they are engaged, could enhance our current results. Finally, we surveyed a convenience sample of a limited numbers of hospitalists and institutions, and were unable to systematically account for variations in promotions criteria across institutions. However, to our knowledge, this is the most comprehensive study of promotions among academic hospitalists to date. Given the common themes that emerged in terms of activities that supported promotion, mentors, and advice, we believe that our sample was sufficient to identify important themes and advance our understanding of this nascent specialty.
In conclusion, our survey of promoted hospital medicine faculty provides valuable information for junior faculty and hospitalist leaders. Success was found through engaging in a diverse set of activities in the traditional areas of education, service, and scholarship, frequently in conjunction with developing recognition outside of their institutions. While all respondents were clinically active, none described themselves as having purely clinical roles. As academic hospitalist roles evolve, academic leaders will need to provide adequate mentorship, create time for scholarly pursuits, and promote documentation and recognition of nontraditional activities that may nonetheless be worthy of promotion.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
- ,,,,,.Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit.J Gen Intern Med.2009;24(5):636–641.
- ,,.Hospitalist educators: future of inpatient internal medicine training.Mt Sinai J Med.2008;75(5):436–451.
- ,,,.Hospitalists as emerging leaders in patient safety: lessons learned and future directions.J Patient Saf.2009;5(1):3–8.
- Quality Portfolio—Template and Instructions. Available at: http://www.sgim.org/userfiles/file/AHTF%20QP%20WEB%20TEMPLATE%20INS TRUCTIONS.pdf. Accessed on April 24,2010.
- .An innovative approach to support hospitalist physicians toward academic success.J Hosp Med.2008;3:314–318.
- ,,,.Institutional policies of U.S. medical schools regarding tenure, promotion, and benefits for part‐time faculty.Acad Med.2000;75(8):846–849.
- ,,,,,.Revising appointment, promotion, and tenure procedures to incorporate an expanded definition of scholarship: the University of Kentucky College of Medicine experience.Acad Med.2000;75(9)913–924.
- ,,,.Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey.Arch Intern Med.2000;160(17):2625–2629.
- ,,, et al.Promotion criteria for clinician‐educators.J Gen Intern Med.2003;18(9):711–716.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79(8):783–790.
- ,,,.Faculty development: academic opportunities for emergency medicine faculty on education career tracks.Acad Emerg Med.2003;10(10):1113–1117.
- ,,,.Burnout and internal medicine resident work hours restrictions.Arch Intern Med.2005;165(22):2595–2600.
- ,.Development of a Division of General Medicine in a Department of Internal Medicine.J Med Ed.1981;56:390–396.
- .The evolution of departments of medicine.N Engl J Med.1980;303(9):489–496.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,.A time to be promoted. The prospective study of promotion in academia.J Gen Intern Med.2006;21(2):123–129.
- Academic Hospitalist Academy. Available at: http://www.sgim.org/index. cfm?pageId=815. Accessed on April 24,2010.
- .Reflections on the Academic Hospitalist Academy.SGIM Forum.2010;33(1):5.
- .The Academic Hospitalist Academy: Get anchored, equipped, and energized.SGIM Forum.2010;33(1):5–6.
- .Four formative days in the life of an academic hospitalist: the Academic Hospitalist Academy.SGIM Forum.2010;33(1):6.
Copyright © 2011 Society of Hospital Medicine
“July Phenomenon” Revisited
The July Phenomenon is a commonly used term referring to poor hospital‐patient outcomes when inexperienced house‐staff start their postgraduate training in July. In addition to being an interesting observation, the validity of July Phenomenon has policy implications for teaching hospitals and residency training programs.
Twenty‐three published studies have tried to determine whether the arrival of new house‐staff is associated with increased patient mortality (see Supporting Appendix A in the online version of this article).123 While those studies make an important attempt to determine the validity of the July Phenomenon, they have some notable limitations. All but four of these studies2, 4, 6, 16 limited their analysis to patients with a specific diagnosis, within a particular hospital unit, or treated by a particular specialty. Many studies limited data to those from a single hospital.1, 3, 4, 10, 11, 14, 15, 20, 22 Nine studies did not include data from the entire year in their analyses,4, 6, 7, 10, 13, 1517, 23 and one did not include data from multiple years.22 One study conducted its analysis on death counts alone and did not account for the number of hospitalized people at risk.6 Finally, the analysis of several studies controlled for no severity of illness markers,6, 10, 21 whereas that from several other studies contained only crude measures of comorbidity and severity of illness.14
In this study, we analyzed data at our teaching hospital to determine if evidence exists for the July Phenomenon at our center. We used a highly discriminative and well‐calibrated multivariate model to calculate the risk of dying in hospital, and quantify the ratio of observed to expected number of hospital deaths. Using this as our outcome statistic, we determined whether or not our hospital experiences a July Phenomenon.
METHODS
This study was approved by The Ottawa Hospital (TOH) Research Ethics Board.
Study Setting
TOH is a tertiary‐care teaching hospital with two inpatient campuses. The hospital operates within a publicly funded health care system, serves a population of approximately 1.5 million people in Ottawa and Eastern Ontario, treats all major trauma patients for the region, and provides most of the oncological care in the region.
TOH is the primary medical teaching hospital at the University of Ottawa. In 2010, there were 197 residents starting their first year of postgraduate training in one of 29 programs.
Inclusion Criteria
The study period extended from April 15, 2004 to December 31, 2008. We used this start time because our hospital switched to new coding systems for procedures and diagnoses in April 2002. Since these new coding systems contributed to our outcome statistic, we used a very long period (ie, two years) for coding patterns to stabilize to ensure that any changes seen were not a function of coding patterns. We ended our study in December 2008 because this was the last date of complete data at the time we started the analysis.
We included all medical, surgical, and obstetrical patients admitted to TOH during this time except those who were: younger than 15 years old; transferred to or from another acute care hospital; or obstetrical patients hospitalized for routine childbirth. These patients were excluded because they were not part of the multivariate model that we used to calculate risk of death in hospital (discussed below).24 These exclusions accounted for 25.4% of all admissions during the study period (36,820less than 15 years old; 12,931transferred to or from the hospital; and 44,220uncomplicated admission for childbirth).
All data used in this study came from The Ottawa Hospital Data Warehouse (TOHDW). This is a repository of clinical, laboratory, and administrative data originating from the hospital's major operational information systems. TOHDW contains information on patient demographics and diagnoses, as well as procedures and patient transfers between different units or hospital services during the admission.
Primary OutcomeRatio of Observed to Expected Number of Deaths per Week
For each study day, we measured the number of hospital deaths from the patient registration table in TOHDW. This statistic was collated for each week to ensure numeric stability, especially in our subgroup analyses.
We calculated the weekly expected number of hospital deaths using an extension of the Escobar model.24 The Escobar is a logistic regression model that estimated the probability of death in hospital that was derived and internally validated on almost 260,000 hospitalizations at 17 hospitals in the Kaiser Permanente Health Plan. It included six covariates that were measurable at admission including: patient age; patient sex; admission urgency (ie, elective or emergent) and service (ie, medical or surgical); admission diagnosis; severity of acute illness as measured by the Laboratory‐based Acute Physiology Score (LAPS); and chronic comorbidities as measured by the COmorbidity Point Score (COPS). Hospitalizations were grouped by admission diagnosis. The final model had excellent discrimination (c‐statistic 0.88) and calibration (P value of Hosmer Lemeshow statistic for entire cohort 0.66). This model was externally validated in our center with a c‐statistic of 0.901.25
We extended the Escobar model in several ways (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). First, we modified it into a survival (rather than a logistic) model so it could estimate a daily probability of death in hospital. Second, we included the same covariates as Escobar except that we expressed LAPS as a time‐dependent covariate (meaning that the model accounted for changes in its value during the hospitalization). Finally, we included other time‐dependent covariates including: admission to intensive care unit; undergoing significant procedures; and awaiting long‐term care. This model had excellent discrimination (concordance probability of 0.895, 95% confidence interval [CI] 0.8890.902) and calibration.
We used this survival model to estimate the daily risk of death for all patients in the hospital each day. Summing these risks over hospital patients on each day returned the daily number of expected hospital deaths. This was collated per week.
The outcome statistic for this study was the ratio of the observed to expected weekly number of hospital deaths. Ratios exceeding 1 indicate that more deaths were observed than were expected (given the distribution of important covariates in those people during that week). This outcome statistic has several advantages. First, it accounts for the number of patients in the hospital each day. This is important because the number of hospital deaths will increase as the number of people in hospital increase. Second, it accounts for the severity of illness in each patient on each hospital day. This accounts for daily changes in risk of patient death, because calculation of the expected number of deaths per day was done using a multivariate survival model that included time‐dependent covariates. Therefore, each individual's predicted hazard of death (which was summed over the entire hospital to calculate the total expected number of deaths in hospital each day) took into account the latest values of these covariates. Previous analyses only accounted for risk of death at admission.
Expressing Physician Experience
The latent measure26 in all July Phenomenon studies is collective house‐staff physician experience. This is quantified by a surrogate date variable in which July 1the date that new house‐staff start their training in North Americarepresents minimal experience and June 30 represents maximal experience. We expressed collective physician experience on a scale from 0 (minimum experience) on July 1 to 1 (maximum experience) on June 30. A similar approach has been used previously13 and has advantages over the other methods used to capture collective house‐staff experience. In the stratified, incomplete approach,47, 911, 13, 1517 periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to times with experienced house‐staff (eg, May and June), while ignoring all other data. The specification of cut‐points for this stratification is arbitrary and the method ignores large amounts of data. In the stratified, complete approach, periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to all other times of the year.8, 12, 14, 1820, 22 This is potentially less biased because there are no lost data. However, the cut‐point for determining when house‐staff transition from inexperienced to experienced is arbitrary, and the model assumes that the transition is sudden. This is suboptimal because acquisition of experience is a gradual, constant process.
The pattern by which collective physician experience changes between July 1st and June 30th is unknown. We therefore expressed this evolution using five different patterns varying from a linear change to a natural logarithmic change (see Supporting Appendix B in the online version of this article).
Analysis
We first examined for autocorrelation in our outcome variable using Ljung‐Box statistics at lag 6 and 12 in PROC ARIMA (SAS 9.2, Cary, NC). If significant autocorrelation was absent in our data, linear regression modeling was used to associate the ratio of the observed to expected number of weekly deaths (the outcome variable) with the collective first year physician experience (the predictor variable). Time‐series methodology was to be used if significant autocorrelation was present.
In our baseline analysis, we included all hospitalizations together. In stratified analyses, we categorized hospitalizations by admission status (emergent vs elective) and admission service (medicine vs surgery).
RESULTS
Between April 15, 2004 and December 31, 2008, The Ottawa Hospital had a total of 152,017 inpatient admissions and 107,731 same day surgeries (an annual rate of 32,222 and 22,835, respectively; an average daily rate of 88 and 63, respectively) that met our study's inclusion criteria. These 259,748 encounters included 164,318 people. Table 1 provides an overall description of the study population.
| Characteristic | |
|---|---|
| |
| Patients/hospitalizations, n | 164,318/259,748 |
| Deaths in‐hospital, n (%) | 7,679 (3.0) |
| Length of admission in days, median (IQR) | 2 (16) |
| Male, n (%) | 124,848 (48.1) |
| Age at admission, median (IQR) | 60 (4674) |
| Admission type, n (%) | |
| Elective surgical | 136,406 (52.5) |
| Elective nonsurgical | 20,104 (7.7) |
| Emergent surgical | 32,046 (12.3) |
| Emergent nonsurgical | 71,192 (27.4) |
| Elixhauser score, median (IQR) | 0 (04) |
| LAPS at admission, median (IQR) | 0 (015) |
| At least one admission to intensive care unit, n (%) | 7,779 (3.0) |
| At least one alternative level of care episode, n (%) | 6,971 (2.7) |
| At least one PIMR procedure, n (%) | 47,288 (18.2) |
| First PIMR score,* median (IQR) | 2 (52) |
Weekly Deaths: Observed, Expected, and Ratio
Figure 1A presents the observed weekly number of deaths during the study period. There was an average of 31 deaths per week (range 1551). Some large fluctuations in the weekly number of deaths were seen; in 2007, for example, the number of observed deaths went from 21 in week 13 up to 46 in week 15. However, no obvious seasonal trends in the observed weekly number of deaths were seen (Figure 1A, heavy line) nor were trends between years obvious.
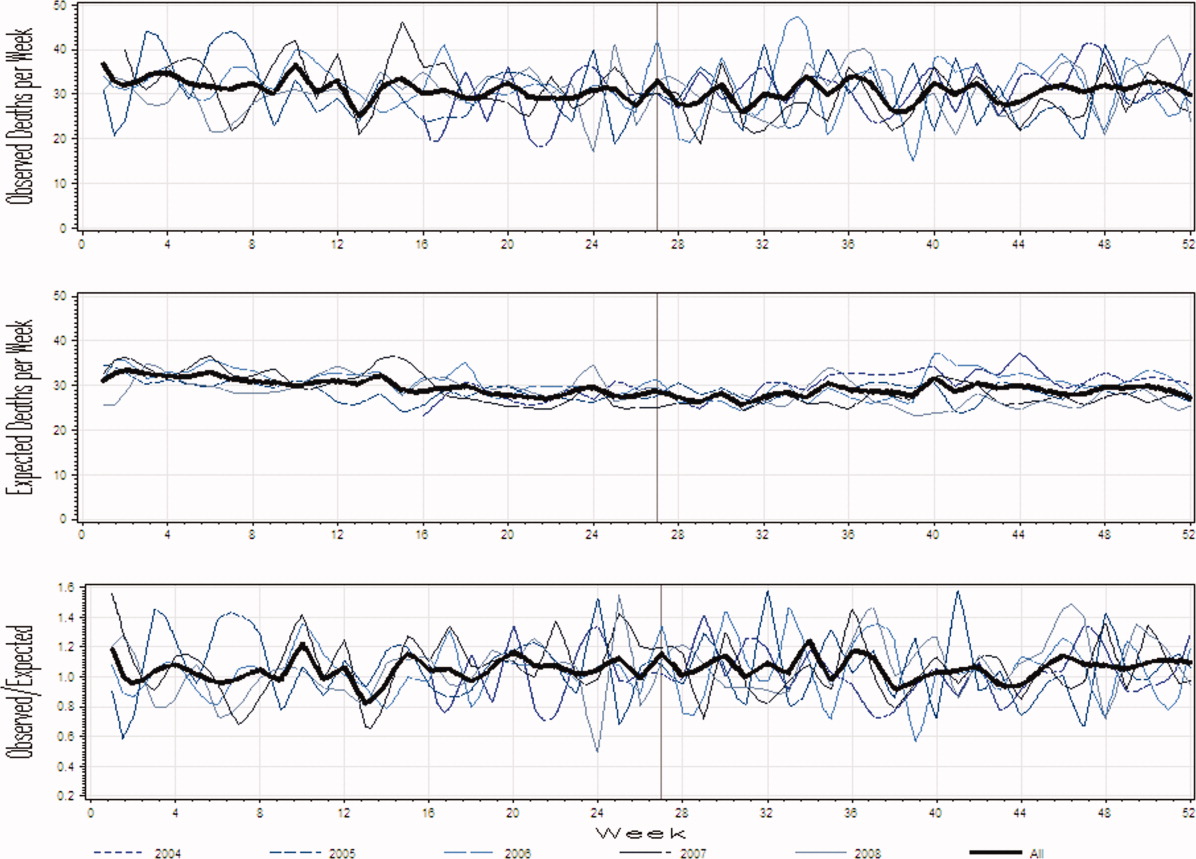
Figure 1B presents the expected weekly number of deaths during the study period. The expected weekly number of deaths averaged 29.6 (range 22.238.7). The expected weekly number of deaths was notably less variable than the observed number of deaths. However, important variations in the expected number of deaths were seen; for example, in 2005, the expected number of deaths increased from 24.1 in week 41 to 29.6 in week 44. Again, we saw no obvious seasonal trends in the expected weekly number of deaths (Figure 1B, heavy line).
Figure 1C illustrates the ratio of observed to the expected weekly number of deaths. The average observed to expected ratio slightly exceeded unity (1.05) and ranged from 0.488 (week 24, in 2008) to 1.821 (week 51, in 2008). We saw no obvious seasonal trends in the ratio of the observed to expected number of weekly deaths. In addition, obvious trends in this ratio were absent over the study period.
Association Between House‐Staff Experience and Death in Hospital
We found no evidence of autocorrelation in the ratio of observed to expected weekly number of deaths. The ratio of observed to expected number of hospital deaths was not significantly associated with house‐staff physician experience (Table 2). This conclusion did not change regardless of which house‐staff physician experience pattern was used in the linear model (Table 2). In addition, our analysis found no significant association between physician experience and patient mortality when analyses were stratified by admission service or admission status (Table 2).
| Patient Population | House‐Staff Experience Pattern (95% CI) | ||||
|---|---|---|---|---|---|
| Linear | Square | Square Root | Cubic | Natural Logarithm | |
| |||||
| All | 0.03 (0.11, 0.06) | 0.02 (0.10, 0.07) | 0.04 (0.15, 0.07) | 0.01 (0.10, 0.08) | 0.05 (0.16, 0.07) |
| Admitting service | |||||
| Medicine | 0.0004 (0.09, 0.10) | 0.01 (0.08, 0.10) | 0.01 (0.13, 0.11) | 0.02 (0.07, 0.11) | 0.03 (0.15, 0.09) |
| Surgery | 0.10 (0.30, 0.10) | 0.11 (0.30, 0.08) | 0.12 (0.37, 0.14) | 0.11 (0.31, 0.08) | 0.09 (0.35, 0.17) |
| Admission status | |||||
| Elective | 0.09 (0.53, 0.35) | 0.10 (0.51, 0.32) | 0.11 (0.66, 0.44) | 0.10 (0.53, 0.33) | 0.11 (0.68, 0.45) |
| Emergent | 0.02 (0.11, 0.07) | 0.01 (0.09, 0.08) | 0.03 (0.14, 0.08) | 0.003 (0.09, 0.09) | 0.04 (0.16, 0.08) |
DISCUSSION
It is natural to suspect that physician experience influences patient outcomes. The commonly discussed July Phenomenon explores changes in teaching‐hospital patient outcomes by time of the academic year. This serves as an ecological surrogate for the latent variable of overall house‐staff experience. Our study used a detailed outcomethe ratio of observed to the expected number of weekly hospital deathsthat adjusted for patient severity of illness. We also modeled collective physician experience using a broad range of patterns. We found no significant variation in mortality rates during the academic year; therefore, the risk of death in hospital does not vary by house‐staff experience at our hospital. This is no evidence of a July Phenomenon for mortality at our center.
We were not surprised that the arrival of inexperienced house‐staff did not significantly change patient mortality for several reasons. First year residents are but one group of treating physicians in a teaching hospital. They are surrounded by many other, more experienced physicians who also contribute to patient care and their outcomes. Given these other physicians, the influence that the relatively smaller number of first year residents have on patient outcomes will be minimized. In addition, the role that these more experienced physicians play in patient care will vary by the experience and ability of residents. The influence of new and inexperienced house‐staff in July will be blunted by an increased role played by staff‐people, fellows, and more experienced house‐staff at that time.
Our study was a methodologically rigorous examination of the July Phenomenon. We used a reliable outcome statisticthe ratio of observed to expected weekly number of hospital deathsthat was created with a validated, discriminative, and well‐calibrated model which predicted risk of death in hospital (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). This statistic is inherently understandable and controlled for patient severity of illness. In addition, our study included a very broad and inclusive group of patients over five years at two hospitals.
Twenty‐three other studies have quantitatively sought a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article). The studies contained a broad assortment of research methodologies, patient populations, and analytical methodologies. Nineteen of these studies (83%) found no evidence of a July Phenomenon for teaching‐hospital mortality. In contrast, two of these studies found notable adjusted odds ratios for death in hospital (1.41 and 1.34) in patients undergoing either general surgery13 or complex cardiovascular surgery,19 respectively. Blumberg22 also found an increased risk of death in surgical patients in July, but used indirect standardized mortality ratios as the outcome statistic and was based on only 139 cases at Maryland teaching hospitals in 1984. Only Jen et al.16 showed an increased risk of hospital death with new house‐staff in a broad patient population. However, this study was restricted to two arbitrarily chosen days (one before and one after house‐staff change‐over) and showed an increased risk of hospital death (adjusted OR 1.05, 95% CI 1.001.15) whose borderline statistical significance could have been driven by the large sample size of the study (n = 299,741).
Therefore, the vast majority of dataincluding those presented in our analysesshow that the risk of teaching‐hospital death does not significantly increase with the arrival of new house‐staff. This prompts the question as to why the July Phenomenon is commonly presented in popular media as a proven fact.2733 We believe this is likely because the concept of the July Phenomenon is understandable and has a rather morbid attraction to people, both inside and outside of the medical profession. Given the large amount of data refuting the true existence of a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article), we believe that this term should only be used only as an example of an interesting idea that is refuted by a proper analysis of the data.
Several limitations of our study are notable. First, our analysis is limited to a single center, albeit with two hospitals. However, ours is one of the largest teaching centers in Canada with many new residents each year. Second, we only examined the association of physician experience on hospital mortality. While it is possible that physician experience significantly influences other patient outcomes, mortality is, obviously, an important and reliably tallied statistic that is used as the primary outcome in most July Phenomenon studies. Third, we excluded approximately a quarter of all hospitalizations from the study. These exclusions were necessary because the Escobar model does not apply to these people and can therefore not be used to predict their risk of death in hospital. However, the vast majority of excluded patients (those less than 15 years old, and women admitted for routine childbirth) have a very low risk of death (the former because they are almost exclusively newborns, and the latter because the risk of maternal death during childbirth is very low). Since these people will contribute very little to either the expected or observed number of deaths, their exclusion will do little to threaten the study's validity. The remaining patients who were transferred to or from other hospitals (n = 12,931) makes a small proportion of the total sampling frame (5% of admissions). Fourth, our study did not identify any significant association between house‐staff experience and patient mortality (Table 2). However, the confidence intervals around our estimates are wide enough, especially in some subgroups such as patients admitted electively, that important changes in patient mortality with house‐staff experience cannot be excluded. For example, whereas our study found that a decrease in the ratio of observed to expected number of deaths exceeding 30% is very unlikely, it is still possible that this decrease is up to 30% (the lower range of the confidence interval in Table 2). However, using this logic, it could also increase by up to 10% (Table 2). Finally, we did not directly measure individual physician experience. New residents can vary extensively in their individual experience and ability. Incorporating individual physician measures of experience and ability would more reliably let us measure the association of new residents with patient outcomes. Without this, we had to rely on an ecological measure of physician experiencenamely calendar date. Again, this method is an industry standard since all studies quantify physician experience ecologically by date (see Supporting Appendix A in the online version of this article).
In summary, our datasimilar to most studies on this topicshow that the risk of death in teaching hospitals does not change with the arrival of new house‐staff.
- ,,.The effects of scheduled intern rotation on the cost and quality of teaching hospital care.Eval Health Prof.1994;17:259–272.
- ,,,.Specialty differences in the “July Phenomenon” for Twin Cities teaching hospitals.Med Care.1993;31:73–83.
- ,,,.The relationship of house staff experience to the cost and quality of inpatient care.JAMA.1990;263:953–957.
- ,,,.Indirect costs for medical education. Is there a July phenomenon?Arch Intern Med.1989;149:765–768.
- ,,,,.The impact of accreditation council for graduate medical education duty hours, the July phenomenon, and hospital teaching status on stroke outcomes.J Stroke Cerebrovasc Dis.2009;18:232–238.
- ,.The killing season—Fact or fiction.BMJ1994;309:1690.
- ,,, et al.The July effect: Impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients.Ann Thorac Surg.2009;88:70–75.
- ,.Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals.J Gen Intern Med.2003;18:639–645.
- ,,,.Neonatal mortality among low birth weight infants during the initial months of the academic year.J Perinatol.2008;28:691–695.
- ,,,,.The “July Phenomenon” and the care of the severely injured patient: Fact or fiction?Surgery.2001;130:346–353.
- ,,, et al.The July effect and cardiac surgery: The effect of the beginning of the academic cycle on outcomes.Am J Surg.2008;196:720–725.
- ,,,.Mortality in Medicare patients undergoing surgery in July in teaching hospitals.Ann Surg.2009;249:871–876.
- ,,, et al.Seasonal variation in surgical outcomes as measured by the American College of Surgeons–National Surgical Quality Improvement Program (ACS‐NSQIP).Ann Surg.2007;246:456–465.
- ,,, et al.Mortality rate and length of stay of patients admitted to the intensive care unit in July.Crit Care Med.2004;32:1161–1165.
- ,,,,,.July—As good a time as any to be injured.J Trauma‐Injury Infect Crit Care.2009;67:1087–1090.
- ,,,,.Early in‐hospital mortality following trainee doctors' first day at work.PLoS ONE.2009;4.
- ,,.Effect of critical care medicine fellows on patient outcome in the intensive care unit.Acad Med.2006;81:S1–S4.
- ,,,,.The “July Phenomenon”: Is trauma the exception?J Am Coll Surg.2009;209:378–384.
- ,,.Impact of cardiothoracic resident turnover on mortality after cardiac surgery: A dynamic human factor.Ann Thorac Surg.2008;86:123–131.
- ,,.Is there a “July Phenomenon” in pediatric neurosurgery at teaching hospitals?J Neurosurg Pediatr.2006;105:169–176.
- ,,,,.Mortality and morbidity by month of birth of neonates admitted to an academic neonatal intensive care unit.Pediatrics.2008;122:E1048–E1052.
- .Measuring surgical quality in Maryland: A model.Health Aff.1988;7:62–78.
- ,,, et al.Complications and death at the start of the new academic year: Is there a July phenomenon?J Trauma‐Injury Infect Crit Care.2010;68(1):19–22.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,,.The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol.2010;63:798–803.
- .Introduction: The logic of latent variables.Latent Class Analysis.Newbury Park, CA:Sage;1987:5–10.
- July Effect. Wikipedia. Available at: http://en.wikipedia.org/wiki/July_effect. Accessed April 1,2011.
- Study proves “killing season” occurs as new doctors start work. September 23,2010. Herald Scotland. Available at: http://www.heraldscotland.com/news/health/study‐proves‐killing‐season‐occurs‐as‐new‐doctors‐start‐work‐1.921632. Accessed April 1, 2011.
- The “July effect”: Worst month for fatal hospital errors, study finds. June 3,2010. ABC News. Available at: http://abcnews.go.com/WN/WellnessNews/july‐month‐fatal‐hospital‐errors‐study‐finds/story?id=10819652. Accessed 1 April, 2011.
- “Deaths rise” with junior doctors. September 22,2010. BBC News. Available at: http://news.bbc.co.uk/2/hi/health/8269729.stm. Accessed April 1, 2011.
- .July: When not to go to the hospital. June 2,2010. Science News. Available at: http://www.sciencenews.org/view/generic/id/59865/title/July_When_not_to_go_to_the_hospital. Accessed April 1, 2011.
- July: A deadly time for hospitals. July 5,2010. National Public Radio. Available at: http://www.npr.org/templates/story/story.php?storyId=128321489. Accessed April 1, 2011.
- .Medical errors and patient safety: Beware the “July effect.” June 4,2010. Better Health. Available at: http://getbetterhealth.com/medical‐errors‐and‐patient‐safety‐beware‐of‐the‐july‐effect/2010.06.04. Accessed April 1, 2011.
The July Phenomenon is a commonly used term referring to poor hospital‐patient outcomes when inexperienced house‐staff start their postgraduate training in July. In addition to being an interesting observation, the validity of July Phenomenon has policy implications for teaching hospitals and residency training programs.
Twenty‐three published studies have tried to determine whether the arrival of new house‐staff is associated with increased patient mortality (see Supporting Appendix A in the online version of this article).123 While those studies make an important attempt to determine the validity of the July Phenomenon, they have some notable limitations. All but four of these studies2, 4, 6, 16 limited their analysis to patients with a specific diagnosis, within a particular hospital unit, or treated by a particular specialty. Many studies limited data to those from a single hospital.1, 3, 4, 10, 11, 14, 15, 20, 22 Nine studies did not include data from the entire year in their analyses,4, 6, 7, 10, 13, 1517, 23 and one did not include data from multiple years.22 One study conducted its analysis on death counts alone and did not account for the number of hospitalized people at risk.6 Finally, the analysis of several studies controlled for no severity of illness markers,6, 10, 21 whereas that from several other studies contained only crude measures of comorbidity and severity of illness.14
In this study, we analyzed data at our teaching hospital to determine if evidence exists for the July Phenomenon at our center. We used a highly discriminative and well‐calibrated multivariate model to calculate the risk of dying in hospital, and quantify the ratio of observed to expected number of hospital deaths. Using this as our outcome statistic, we determined whether or not our hospital experiences a July Phenomenon.
METHODS
This study was approved by The Ottawa Hospital (TOH) Research Ethics Board.
Study Setting
TOH is a tertiary‐care teaching hospital with two inpatient campuses. The hospital operates within a publicly funded health care system, serves a population of approximately 1.5 million people in Ottawa and Eastern Ontario, treats all major trauma patients for the region, and provides most of the oncological care in the region.
TOH is the primary medical teaching hospital at the University of Ottawa. In 2010, there were 197 residents starting their first year of postgraduate training in one of 29 programs.
Inclusion Criteria
The study period extended from April 15, 2004 to December 31, 2008. We used this start time because our hospital switched to new coding systems for procedures and diagnoses in April 2002. Since these new coding systems contributed to our outcome statistic, we used a very long period (ie, two years) for coding patterns to stabilize to ensure that any changes seen were not a function of coding patterns. We ended our study in December 2008 because this was the last date of complete data at the time we started the analysis.
We included all medical, surgical, and obstetrical patients admitted to TOH during this time except those who were: younger than 15 years old; transferred to or from another acute care hospital; or obstetrical patients hospitalized for routine childbirth. These patients were excluded because they were not part of the multivariate model that we used to calculate risk of death in hospital (discussed below).24 These exclusions accounted for 25.4% of all admissions during the study period (36,820less than 15 years old; 12,931transferred to or from the hospital; and 44,220uncomplicated admission for childbirth).
All data used in this study came from The Ottawa Hospital Data Warehouse (TOHDW). This is a repository of clinical, laboratory, and administrative data originating from the hospital's major operational information systems. TOHDW contains information on patient demographics and diagnoses, as well as procedures and patient transfers between different units or hospital services during the admission.
Primary OutcomeRatio of Observed to Expected Number of Deaths per Week
For each study day, we measured the number of hospital deaths from the patient registration table in TOHDW. This statistic was collated for each week to ensure numeric stability, especially in our subgroup analyses.
We calculated the weekly expected number of hospital deaths using an extension of the Escobar model.24 The Escobar is a logistic regression model that estimated the probability of death in hospital that was derived and internally validated on almost 260,000 hospitalizations at 17 hospitals in the Kaiser Permanente Health Plan. It included six covariates that were measurable at admission including: patient age; patient sex; admission urgency (ie, elective or emergent) and service (ie, medical or surgical); admission diagnosis; severity of acute illness as measured by the Laboratory‐based Acute Physiology Score (LAPS); and chronic comorbidities as measured by the COmorbidity Point Score (COPS). Hospitalizations were grouped by admission diagnosis. The final model had excellent discrimination (c‐statistic 0.88) and calibration (P value of Hosmer Lemeshow statistic for entire cohort 0.66). This model was externally validated in our center with a c‐statistic of 0.901.25
We extended the Escobar model in several ways (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). First, we modified it into a survival (rather than a logistic) model so it could estimate a daily probability of death in hospital. Second, we included the same covariates as Escobar except that we expressed LAPS as a time‐dependent covariate (meaning that the model accounted for changes in its value during the hospitalization). Finally, we included other time‐dependent covariates including: admission to intensive care unit; undergoing significant procedures; and awaiting long‐term care. This model had excellent discrimination (concordance probability of 0.895, 95% confidence interval [CI] 0.8890.902) and calibration.
We used this survival model to estimate the daily risk of death for all patients in the hospital each day. Summing these risks over hospital patients on each day returned the daily number of expected hospital deaths. This was collated per week.
The outcome statistic for this study was the ratio of the observed to expected weekly number of hospital deaths. Ratios exceeding 1 indicate that more deaths were observed than were expected (given the distribution of important covariates in those people during that week). This outcome statistic has several advantages. First, it accounts for the number of patients in the hospital each day. This is important because the number of hospital deaths will increase as the number of people in hospital increase. Second, it accounts for the severity of illness in each patient on each hospital day. This accounts for daily changes in risk of patient death, because calculation of the expected number of deaths per day was done using a multivariate survival model that included time‐dependent covariates. Therefore, each individual's predicted hazard of death (which was summed over the entire hospital to calculate the total expected number of deaths in hospital each day) took into account the latest values of these covariates. Previous analyses only accounted for risk of death at admission.
Expressing Physician Experience
The latent measure26 in all July Phenomenon studies is collective house‐staff physician experience. This is quantified by a surrogate date variable in which July 1the date that new house‐staff start their training in North Americarepresents minimal experience and June 30 represents maximal experience. We expressed collective physician experience on a scale from 0 (minimum experience) on July 1 to 1 (maximum experience) on June 30. A similar approach has been used previously13 and has advantages over the other methods used to capture collective house‐staff experience. In the stratified, incomplete approach,47, 911, 13, 1517 periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to times with experienced house‐staff (eg, May and June), while ignoring all other data. The specification of cut‐points for this stratification is arbitrary and the method ignores large amounts of data. In the stratified, complete approach, periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to all other times of the year.8, 12, 14, 1820, 22 This is potentially less biased because there are no lost data. However, the cut‐point for determining when house‐staff transition from inexperienced to experienced is arbitrary, and the model assumes that the transition is sudden. This is suboptimal because acquisition of experience is a gradual, constant process.
The pattern by which collective physician experience changes between July 1st and June 30th is unknown. We therefore expressed this evolution using five different patterns varying from a linear change to a natural logarithmic change (see Supporting Appendix B in the online version of this article).
Analysis
We first examined for autocorrelation in our outcome variable using Ljung‐Box statistics at lag 6 and 12 in PROC ARIMA (SAS 9.2, Cary, NC). If significant autocorrelation was absent in our data, linear regression modeling was used to associate the ratio of the observed to expected number of weekly deaths (the outcome variable) with the collective first year physician experience (the predictor variable). Time‐series methodology was to be used if significant autocorrelation was present.
In our baseline analysis, we included all hospitalizations together. In stratified analyses, we categorized hospitalizations by admission status (emergent vs elective) and admission service (medicine vs surgery).
RESULTS
Between April 15, 2004 and December 31, 2008, The Ottawa Hospital had a total of 152,017 inpatient admissions and 107,731 same day surgeries (an annual rate of 32,222 and 22,835, respectively; an average daily rate of 88 and 63, respectively) that met our study's inclusion criteria. These 259,748 encounters included 164,318 people. Table 1 provides an overall description of the study population.
| Characteristic | |
|---|---|
| |
| Patients/hospitalizations, n | 164,318/259,748 |
| Deaths in‐hospital, n (%) | 7,679 (3.0) |
| Length of admission in days, median (IQR) | 2 (16) |
| Male, n (%) | 124,848 (48.1) |
| Age at admission, median (IQR) | 60 (4674) |
| Admission type, n (%) | |
| Elective surgical | 136,406 (52.5) |
| Elective nonsurgical | 20,104 (7.7) |
| Emergent surgical | 32,046 (12.3) |
| Emergent nonsurgical | 71,192 (27.4) |
| Elixhauser score, median (IQR) | 0 (04) |
| LAPS at admission, median (IQR) | 0 (015) |
| At least one admission to intensive care unit, n (%) | 7,779 (3.0) |
| At least one alternative level of care episode, n (%) | 6,971 (2.7) |
| At least one PIMR procedure, n (%) | 47,288 (18.2) |
| First PIMR score,* median (IQR) | 2 (52) |
Weekly Deaths: Observed, Expected, and Ratio
Figure 1A presents the observed weekly number of deaths during the study period. There was an average of 31 deaths per week (range 1551). Some large fluctuations in the weekly number of deaths were seen; in 2007, for example, the number of observed deaths went from 21 in week 13 up to 46 in week 15. However, no obvious seasonal trends in the observed weekly number of deaths were seen (Figure 1A, heavy line) nor were trends between years obvious.

Figure 1B presents the expected weekly number of deaths during the study period. The expected weekly number of deaths averaged 29.6 (range 22.238.7). The expected weekly number of deaths was notably less variable than the observed number of deaths. However, important variations in the expected number of deaths were seen; for example, in 2005, the expected number of deaths increased from 24.1 in week 41 to 29.6 in week 44. Again, we saw no obvious seasonal trends in the expected weekly number of deaths (Figure 1B, heavy line).
Figure 1C illustrates the ratio of observed to the expected weekly number of deaths. The average observed to expected ratio slightly exceeded unity (1.05) and ranged from 0.488 (week 24, in 2008) to 1.821 (week 51, in 2008). We saw no obvious seasonal trends in the ratio of the observed to expected number of weekly deaths. In addition, obvious trends in this ratio were absent over the study period.
Association Between House‐Staff Experience and Death in Hospital
We found no evidence of autocorrelation in the ratio of observed to expected weekly number of deaths. The ratio of observed to expected number of hospital deaths was not significantly associated with house‐staff physician experience (Table 2). This conclusion did not change regardless of which house‐staff physician experience pattern was used in the linear model (Table 2). In addition, our analysis found no significant association between physician experience and patient mortality when analyses were stratified by admission service or admission status (Table 2).
| Patient Population | House‐Staff Experience Pattern (95% CI) | ||||
|---|---|---|---|---|---|
| Linear | Square | Square Root | Cubic | Natural Logarithm | |
| |||||
| All | 0.03 (0.11, 0.06) | 0.02 (0.10, 0.07) | 0.04 (0.15, 0.07) | 0.01 (0.10, 0.08) | 0.05 (0.16, 0.07) |
| Admitting service | |||||
| Medicine | 0.0004 (0.09, 0.10) | 0.01 (0.08, 0.10) | 0.01 (0.13, 0.11) | 0.02 (0.07, 0.11) | 0.03 (0.15, 0.09) |
| Surgery | 0.10 (0.30, 0.10) | 0.11 (0.30, 0.08) | 0.12 (0.37, 0.14) | 0.11 (0.31, 0.08) | 0.09 (0.35, 0.17) |
| Admission status | |||||
| Elective | 0.09 (0.53, 0.35) | 0.10 (0.51, 0.32) | 0.11 (0.66, 0.44) | 0.10 (0.53, 0.33) | 0.11 (0.68, 0.45) |
| Emergent | 0.02 (0.11, 0.07) | 0.01 (0.09, 0.08) | 0.03 (0.14, 0.08) | 0.003 (0.09, 0.09) | 0.04 (0.16, 0.08) |
DISCUSSION
It is natural to suspect that physician experience influences patient outcomes. The commonly discussed July Phenomenon explores changes in teaching‐hospital patient outcomes by time of the academic year. This serves as an ecological surrogate for the latent variable of overall house‐staff experience. Our study used a detailed outcomethe ratio of observed to the expected number of weekly hospital deathsthat adjusted for patient severity of illness. We also modeled collective physician experience using a broad range of patterns. We found no significant variation in mortality rates during the academic year; therefore, the risk of death in hospital does not vary by house‐staff experience at our hospital. This is no evidence of a July Phenomenon for mortality at our center.
We were not surprised that the arrival of inexperienced house‐staff did not significantly change patient mortality for several reasons. First year residents are but one group of treating physicians in a teaching hospital. They are surrounded by many other, more experienced physicians who also contribute to patient care and their outcomes. Given these other physicians, the influence that the relatively smaller number of first year residents have on patient outcomes will be minimized. In addition, the role that these more experienced physicians play in patient care will vary by the experience and ability of residents. The influence of new and inexperienced house‐staff in July will be blunted by an increased role played by staff‐people, fellows, and more experienced house‐staff at that time.
Our study was a methodologically rigorous examination of the July Phenomenon. We used a reliable outcome statisticthe ratio of observed to expected weekly number of hospital deathsthat was created with a validated, discriminative, and well‐calibrated model which predicted risk of death in hospital (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). This statistic is inherently understandable and controlled for patient severity of illness. In addition, our study included a very broad and inclusive group of patients over five years at two hospitals.
Twenty‐three other studies have quantitatively sought a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article). The studies contained a broad assortment of research methodologies, patient populations, and analytical methodologies. Nineteen of these studies (83%) found no evidence of a July Phenomenon for teaching‐hospital mortality. In contrast, two of these studies found notable adjusted odds ratios for death in hospital (1.41 and 1.34) in patients undergoing either general surgery13 or complex cardiovascular surgery,19 respectively. Blumberg22 also found an increased risk of death in surgical patients in July, but used indirect standardized mortality ratios as the outcome statistic and was based on only 139 cases at Maryland teaching hospitals in 1984. Only Jen et al.16 showed an increased risk of hospital death with new house‐staff in a broad patient population. However, this study was restricted to two arbitrarily chosen days (one before and one after house‐staff change‐over) and showed an increased risk of hospital death (adjusted OR 1.05, 95% CI 1.001.15) whose borderline statistical significance could have been driven by the large sample size of the study (n = 299,741).
Therefore, the vast majority of dataincluding those presented in our analysesshow that the risk of teaching‐hospital death does not significantly increase with the arrival of new house‐staff. This prompts the question as to why the July Phenomenon is commonly presented in popular media as a proven fact.2733 We believe this is likely because the concept of the July Phenomenon is understandable and has a rather morbid attraction to people, both inside and outside of the medical profession. Given the large amount of data refuting the true existence of a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article), we believe that this term should only be used only as an example of an interesting idea that is refuted by a proper analysis of the data.
Several limitations of our study are notable. First, our analysis is limited to a single center, albeit with two hospitals. However, ours is one of the largest teaching centers in Canada with many new residents each year. Second, we only examined the association of physician experience on hospital mortality. While it is possible that physician experience significantly influences other patient outcomes, mortality is, obviously, an important and reliably tallied statistic that is used as the primary outcome in most July Phenomenon studies. Third, we excluded approximately a quarter of all hospitalizations from the study. These exclusions were necessary because the Escobar model does not apply to these people and can therefore not be used to predict their risk of death in hospital. However, the vast majority of excluded patients (those less than 15 years old, and women admitted for routine childbirth) have a very low risk of death (the former because they are almost exclusively newborns, and the latter because the risk of maternal death during childbirth is very low). Since these people will contribute very little to either the expected or observed number of deaths, their exclusion will do little to threaten the study's validity. The remaining patients who were transferred to or from other hospitals (n = 12,931) makes a small proportion of the total sampling frame (5% of admissions). Fourth, our study did not identify any significant association between house‐staff experience and patient mortality (Table 2). However, the confidence intervals around our estimates are wide enough, especially in some subgroups such as patients admitted electively, that important changes in patient mortality with house‐staff experience cannot be excluded. For example, whereas our study found that a decrease in the ratio of observed to expected number of deaths exceeding 30% is very unlikely, it is still possible that this decrease is up to 30% (the lower range of the confidence interval in Table 2). However, using this logic, it could also increase by up to 10% (Table 2). Finally, we did not directly measure individual physician experience. New residents can vary extensively in their individual experience and ability. Incorporating individual physician measures of experience and ability would more reliably let us measure the association of new residents with patient outcomes. Without this, we had to rely on an ecological measure of physician experiencenamely calendar date. Again, this method is an industry standard since all studies quantify physician experience ecologically by date (see Supporting Appendix A in the online version of this article).
In summary, our datasimilar to most studies on this topicshow that the risk of death in teaching hospitals does not change with the arrival of new house‐staff.
The July Phenomenon is a commonly used term referring to poor hospital‐patient outcomes when inexperienced house‐staff start their postgraduate training in July. In addition to being an interesting observation, the validity of July Phenomenon has policy implications for teaching hospitals and residency training programs.
Twenty‐three published studies have tried to determine whether the arrival of new house‐staff is associated with increased patient mortality (see Supporting Appendix A in the online version of this article).123 While those studies make an important attempt to determine the validity of the July Phenomenon, they have some notable limitations. All but four of these studies2, 4, 6, 16 limited their analysis to patients with a specific diagnosis, within a particular hospital unit, or treated by a particular specialty. Many studies limited data to those from a single hospital.1, 3, 4, 10, 11, 14, 15, 20, 22 Nine studies did not include data from the entire year in their analyses,4, 6, 7, 10, 13, 1517, 23 and one did not include data from multiple years.22 One study conducted its analysis on death counts alone and did not account for the number of hospitalized people at risk.6 Finally, the analysis of several studies controlled for no severity of illness markers,6, 10, 21 whereas that from several other studies contained only crude measures of comorbidity and severity of illness.14
In this study, we analyzed data at our teaching hospital to determine if evidence exists for the July Phenomenon at our center. We used a highly discriminative and well‐calibrated multivariate model to calculate the risk of dying in hospital, and quantify the ratio of observed to expected number of hospital deaths. Using this as our outcome statistic, we determined whether or not our hospital experiences a July Phenomenon.
METHODS
This study was approved by The Ottawa Hospital (TOH) Research Ethics Board.
Study Setting
TOH is a tertiary‐care teaching hospital with two inpatient campuses. The hospital operates within a publicly funded health care system, serves a population of approximately 1.5 million people in Ottawa and Eastern Ontario, treats all major trauma patients for the region, and provides most of the oncological care in the region.
TOH is the primary medical teaching hospital at the University of Ottawa. In 2010, there were 197 residents starting their first year of postgraduate training in one of 29 programs.
Inclusion Criteria
The study period extended from April 15, 2004 to December 31, 2008. We used this start time because our hospital switched to new coding systems for procedures and diagnoses in April 2002. Since these new coding systems contributed to our outcome statistic, we used a very long period (ie, two years) for coding patterns to stabilize to ensure that any changes seen were not a function of coding patterns. We ended our study in December 2008 because this was the last date of complete data at the time we started the analysis.
We included all medical, surgical, and obstetrical patients admitted to TOH during this time except those who were: younger than 15 years old; transferred to or from another acute care hospital; or obstetrical patients hospitalized for routine childbirth. These patients were excluded because they were not part of the multivariate model that we used to calculate risk of death in hospital (discussed below).24 These exclusions accounted for 25.4% of all admissions during the study period (36,820less than 15 years old; 12,931transferred to or from the hospital; and 44,220uncomplicated admission for childbirth).
All data used in this study came from The Ottawa Hospital Data Warehouse (TOHDW). This is a repository of clinical, laboratory, and administrative data originating from the hospital's major operational information systems. TOHDW contains information on patient demographics and diagnoses, as well as procedures and patient transfers between different units or hospital services during the admission.
Primary OutcomeRatio of Observed to Expected Number of Deaths per Week
For each study day, we measured the number of hospital deaths from the patient registration table in TOHDW. This statistic was collated for each week to ensure numeric stability, especially in our subgroup analyses.
We calculated the weekly expected number of hospital deaths using an extension of the Escobar model.24 The Escobar is a logistic regression model that estimated the probability of death in hospital that was derived and internally validated on almost 260,000 hospitalizations at 17 hospitals in the Kaiser Permanente Health Plan. It included six covariates that were measurable at admission including: patient age; patient sex; admission urgency (ie, elective or emergent) and service (ie, medical or surgical); admission diagnosis; severity of acute illness as measured by the Laboratory‐based Acute Physiology Score (LAPS); and chronic comorbidities as measured by the COmorbidity Point Score (COPS). Hospitalizations were grouped by admission diagnosis. The final model had excellent discrimination (c‐statistic 0.88) and calibration (P value of Hosmer Lemeshow statistic for entire cohort 0.66). This model was externally validated in our center with a c‐statistic of 0.901.25
We extended the Escobar model in several ways (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). First, we modified it into a survival (rather than a logistic) model so it could estimate a daily probability of death in hospital. Second, we included the same covariates as Escobar except that we expressed LAPS as a time‐dependent covariate (meaning that the model accounted for changes in its value during the hospitalization). Finally, we included other time‐dependent covariates including: admission to intensive care unit; undergoing significant procedures; and awaiting long‐term care. This model had excellent discrimination (concordance probability of 0.895, 95% confidence interval [CI] 0.8890.902) and calibration.
We used this survival model to estimate the daily risk of death for all patients in the hospital each day. Summing these risks over hospital patients on each day returned the daily number of expected hospital deaths. This was collated per week.
The outcome statistic for this study was the ratio of the observed to expected weekly number of hospital deaths. Ratios exceeding 1 indicate that more deaths were observed than were expected (given the distribution of important covariates in those people during that week). This outcome statistic has several advantages. First, it accounts for the number of patients in the hospital each day. This is important because the number of hospital deaths will increase as the number of people in hospital increase. Second, it accounts for the severity of illness in each patient on each hospital day. This accounts for daily changes in risk of patient death, because calculation of the expected number of deaths per day was done using a multivariate survival model that included time‐dependent covariates. Therefore, each individual's predicted hazard of death (which was summed over the entire hospital to calculate the total expected number of deaths in hospital each day) took into account the latest values of these covariates. Previous analyses only accounted for risk of death at admission.
Expressing Physician Experience
The latent measure26 in all July Phenomenon studies is collective house‐staff physician experience. This is quantified by a surrogate date variable in which July 1the date that new house‐staff start their training in North Americarepresents minimal experience and June 30 represents maximal experience. We expressed collective physician experience on a scale from 0 (minimum experience) on July 1 to 1 (maximum experience) on June 30. A similar approach has been used previously13 and has advantages over the other methods used to capture collective house‐staff experience. In the stratified, incomplete approach,47, 911, 13, 1517 periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to times with experienced house‐staff (eg, May and June), while ignoring all other data. The specification of cut‐points for this stratification is arbitrary and the method ignores large amounts of data. In the stratified, complete approach, periods with inexperienced house‐staff (eg, July and August) are grouped together and compared to all other times of the year.8, 12, 14, 1820, 22 This is potentially less biased because there are no lost data. However, the cut‐point for determining when house‐staff transition from inexperienced to experienced is arbitrary, and the model assumes that the transition is sudden. This is suboptimal because acquisition of experience is a gradual, constant process.
The pattern by which collective physician experience changes between July 1st and June 30th is unknown. We therefore expressed this evolution using five different patterns varying from a linear change to a natural logarithmic change (see Supporting Appendix B in the online version of this article).
Analysis
We first examined for autocorrelation in our outcome variable using Ljung‐Box statistics at lag 6 and 12 in PROC ARIMA (SAS 9.2, Cary, NC). If significant autocorrelation was absent in our data, linear regression modeling was used to associate the ratio of the observed to expected number of weekly deaths (the outcome variable) with the collective first year physician experience (the predictor variable). Time‐series methodology was to be used if significant autocorrelation was present.
In our baseline analysis, we included all hospitalizations together. In stratified analyses, we categorized hospitalizations by admission status (emergent vs elective) and admission service (medicine vs surgery).
RESULTS
Between April 15, 2004 and December 31, 2008, The Ottawa Hospital had a total of 152,017 inpatient admissions and 107,731 same day surgeries (an annual rate of 32,222 and 22,835, respectively; an average daily rate of 88 and 63, respectively) that met our study's inclusion criteria. These 259,748 encounters included 164,318 people. Table 1 provides an overall description of the study population.
| Characteristic | |
|---|---|
| |
| Patients/hospitalizations, n | 164,318/259,748 |
| Deaths in‐hospital, n (%) | 7,679 (3.0) |
| Length of admission in days, median (IQR) | 2 (16) |
| Male, n (%) | 124,848 (48.1) |
| Age at admission, median (IQR) | 60 (4674) |
| Admission type, n (%) | |
| Elective surgical | 136,406 (52.5) |
| Elective nonsurgical | 20,104 (7.7) |
| Emergent surgical | 32,046 (12.3) |
| Emergent nonsurgical | 71,192 (27.4) |
| Elixhauser score, median (IQR) | 0 (04) |
| LAPS at admission, median (IQR) | 0 (015) |
| At least one admission to intensive care unit, n (%) | 7,779 (3.0) |
| At least one alternative level of care episode, n (%) | 6,971 (2.7) |
| At least one PIMR procedure, n (%) | 47,288 (18.2) |
| First PIMR score,* median (IQR) | 2 (52) |
Weekly Deaths: Observed, Expected, and Ratio
Figure 1A presents the observed weekly number of deaths during the study period. There was an average of 31 deaths per week (range 1551). Some large fluctuations in the weekly number of deaths were seen; in 2007, for example, the number of observed deaths went from 21 in week 13 up to 46 in week 15. However, no obvious seasonal trends in the observed weekly number of deaths were seen (Figure 1A, heavy line) nor were trends between years obvious.

Figure 1B presents the expected weekly number of deaths during the study period. The expected weekly number of deaths averaged 29.6 (range 22.238.7). The expected weekly number of deaths was notably less variable than the observed number of deaths. However, important variations in the expected number of deaths were seen; for example, in 2005, the expected number of deaths increased from 24.1 in week 41 to 29.6 in week 44. Again, we saw no obvious seasonal trends in the expected weekly number of deaths (Figure 1B, heavy line).
Figure 1C illustrates the ratio of observed to the expected weekly number of deaths. The average observed to expected ratio slightly exceeded unity (1.05) and ranged from 0.488 (week 24, in 2008) to 1.821 (week 51, in 2008). We saw no obvious seasonal trends in the ratio of the observed to expected number of weekly deaths. In addition, obvious trends in this ratio were absent over the study period.
Association Between House‐Staff Experience and Death in Hospital
We found no evidence of autocorrelation in the ratio of observed to expected weekly number of deaths. The ratio of observed to expected number of hospital deaths was not significantly associated with house‐staff physician experience (Table 2). This conclusion did not change regardless of which house‐staff physician experience pattern was used in the linear model (Table 2). In addition, our analysis found no significant association between physician experience and patient mortality when analyses were stratified by admission service or admission status (Table 2).
| Patient Population | House‐Staff Experience Pattern (95% CI) | ||||
|---|---|---|---|---|---|
| Linear | Square | Square Root | Cubic | Natural Logarithm | |
| |||||
| All | 0.03 (0.11, 0.06) | 0.02 (0.10, 0.07) | 0.04 (0.15, 0.07) | 0.01 (0.10, 0.08) | 0.05 (0.16, 0.07) |
| Admitting service | |||||
| Medicine | 0.0004 (0.09, 0.10) | 0.01 (0.08, 0.10) | 0.01 (0.13, 0.11) | 0.02 (0.07, 0.11) | 0.03 (0.15, 0.09) |
| Surgery | 0.10 (0.30, 0.10) | 0.11 (0.30, 0.08) | 0.12 (0.37, 0.14) | 0.11 (0.31, 0.08) | 0.09 (0.35, 0.17) |
| Admission status | |||||
| Elective | 0.09 (0.53, 0.35) | 0.10 (0.51, 0.32) | 0.11 (0.66, 0.44) | 0.10 (0.53, 0.33) | 0.11 (0.68, 0.45) |
| Emergent | 0.02 (0.11, 0.07) | 0.01 (0.09, 0.08) | 0.03 (0.14, 0.08) | 0.003 (0.09, 0.09) | 0.04 (0.16, 0.08) |
DISCUSSION
It is natural to suspect that physician experience influences patient outcomes. The commonly discussed July Phenomenon explores changes in teaching‐hospital patient outcomes by time of the academic year. This serves as an ecological surrogate for the latent variable of overall house‐staff experience. Our study used a detailed outcomethe ratio of observed to the expected number of weekly hospital deathsthat adjusted for patient severity of illness. We also modeled collective physician experience using a broad range of patterns. We found no significant variation in mortality rates during the academic year; therefore, the risk of death in hospital does not vary by house‐staff experience at our hospital. This is no evidence of a July Phenomenon for mortality at our center.
We were not surprised that the arrival of inexperienced house‐staff did not significantly change patient mortality for several reasons. First year residents are but one group of treating physicians in a teaching hospital. They are surrounded by many other, more experienced physicians who also contribute to patient care and their outcomes. Given these other physicians, the influence that the relatively smaller number of first year residents have on patient outcomes will be minimized. In addition, the role that these more experienced physicians play in patient care will vary by the experience and ability of residents. The influence of new and inexperienced house‐staff in July will be blunted by an increased role played by staff‐people, fellows, and more experienced house‐staff at that time.
Our study was a methodologically rigorous examination of the July Phenomenon. We used a reliable outcome statisticthe ratio of observed to expected weekly number of hospital deathsthat was created with a validated, discriminative, and well‐calibrated model which predicted risk of death in hospital (Wong et al., Derivation and validation of a model to predict the daily risk of death in hospital, 2010, unpublished work). This statistic is inherently understandable and controlled for patient severity of illness. In addition, our study included a very broad and inclusive group of patients over five years at two hospitals.
Twenty‐three other studies have quantitatively sought a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article). The studies contained a broad assortment of research methodologies, patient populations, and analytical methodologies. Nineteen of these studies (83%) found no evidence of a July Phenomenon for teaching‐hospital mortality. In contrast, two of these studies found notable adjusted odds ratios for death in hospital (1.41 and 1.34) in patients undergoing either general surgery13 or complex cardiovascular surgery,19 respectively. Blumberg22 also found an increased risk of death in surgical patients in July, but used indirect standardized mortality ratios as the outcome statistic and was based on only 139 cases at Maryland teaching hospitals in 1984. Only Jen et al.16 showed an increased risk of hospital death with new house‐staff in a broad patient population. However, this study was restricted to two arbitrarily chosen days (one before and one after house‐staff change‐over) and showed an increased risk of hospital death (adjusted OR 1.05, 95% CI 1.001.15) whose borderline statistical significance could have been driven by the large sample size of the study (n = 299,741).
Therefore, the vast majority of dataincluding those presented in our analysesshow that the risk of teaching‐hospital death does not significantly increase with the arrival of new house‐staff. This prompts the question as to why the July Phenomenon is commonly presented in popular media as a proven fact.2733 We believe this is likely because the concept of the July Phenomenon is understandable and has a rather morbid attraction to people, both inside and outside of the medical profession. Given the large amount of data refuting the true existence of a July Phenomenon for patient mortality (see Supporting Appendix A in the online version of this article), we believe that this term should only be used only as an example of an interesting idea that is refuted by a proper analysis of the data.
Several limitations of our study are notable. First, our analysis is limited to a single center, albeit with two hospitals. However, ours is one of the largest teaching centers in Canada with many new residents each year. Second, we only examined the association of physician experience on hospital mortality. While it is possible that physician experience significantly influences other patient outcomes, mortality is, obviously, an important and reliably tallied statistic that is used as the primary outcome in most July Phenomenon studies. Third, we excluded approximately a quarter of all hospitalizations from the study. These exclusions were necessary because the Escobar model does not apply to these people and can therefore not be used to predict their risk of death in hospital. However, the vast majority of excluded patients (those less than 15 years old, and women admitted for routine childbirth) have a very low risk of death (the former because they are almost exclusively newborns, and the latter because the risk of maternal death during childbirth is very low). Since these people will contribute very little to either the expected or observed number of deaths, their exclusion will do little to threaten the study's validity. The remaining patients who were transferred to or from other hospitals (n = 12,931) makes a small proportion of the total sampling frame (5% of admissions). Fourth, our study did not identify any significant association between house‐staff experience and patient mortality (Table 2). However, the confidence intervals around our estimates are wide enough, especially in some subgroups such as patients admitted electively, that important changes in patient mortality with house‐staff experience cannot be excluded. For example, whereas our study found that a decrease in the ratio of observed to expected number of deaths exceeding 30% is very unlikely, it is still possible that this decrease is up to 30% (the lower range of the confidence interval in Table 2). However, using this logic, it could also increase by up to 10% (Table 2). Finally, we did not directly measure individual physician experience. New residents can vary extensively in their individual experience and ability. Incorporating individual physician measures of experience and ability would more reliably let us measure the association of new residents with patient outcomes. Without this, we had to rely on an ecological measure of physician experiencenamely calendar date. Again, this method is an industry standard since all studies quantify physician experience ecologically by date (see Supporting Appendix A in the online version of this article).
In summary, our datasimilar to most studies on this topicshow that the risk of death in teaching hospitals does not change with the arrival of new house‐staff.
- ,,.The effects of scheduled intern rotation on the cost and quality of teaching hospital care.Eval Health Prof.1994;17:259–272.
- ,,,.Specialty differences in the “July Phenomenon” for Twin Cities teaching hospitals.Med Care.1993;31:73–83.
- ,,,.The relationship of house staff experience to the cost and quality of inpatient care.JAMA.1990;263:953–957.
- ,,,.Indirect costs for medical education. Is there a July phenomenon?Arch Intern Med.1989;149:765–768.
- ,,,,.The impact of accreditation council for graduate medical education duty hours, the July phenomenon, and hospital teaching status on stroke outcomes.J Stroke Cerebrovasc Dis.2009;18:232–238.
- ,.The killing season—Fact or fiction.BMJ1994;309:1690.
- ,,, et al.The July effect: Impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients.Ann Thorac Surg.2009;88:70–75.
- ,.Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals.J Gen Intern Med.2003;18:639–645.
- ,,,.Neonatal mortality among low birth weight infants during the initial months of the academic year.J Perinatol.2008;28:691–695.
- ,,,,.The “July Phenomenon” and the care of the severely injured patient: Fact or fiction?Surgery.2001;130:346–353.
- ,,, et al.The July effect and cardiac surgery: The effect of the beginning of the academic cycle on outcomes.Am J Surg.2008;196:720–725.
- ,,,.Mortality in Medicare patients undergoing surgery in July in teaching hospitals.Ann Surg.2009;249:871–876.
- ,,, et al.Seasonal variation in surgical outcomes as measured by the American College of Surgeons–National Surgical Quality Improvement Program (ACS‐NSQIP).Ann Surg.2007;246:456–465.
- ,,, et al.Mortality rate and length of stay of patients admitted to the intensive care unit in July.Crit Care Med.2004;32:1161–1165.
- ,,,,,.July—As good a time as any to be injured.J Trauma‐Injury Infect Crit Care.2009;67:1087–1090.
- ,,,,.Early in‐hospital mortality following trainee doctors' first day at work.PLoS ONE.2009;4.
- ,,.Effect of critical care medicine fellows on patient outcome in the intensive care unit.Acad Med.2006;81:S1–S4.
- ,,,,.The “July Phenomenon”: Is trauma the exception?J Am Coll Surg.2009;209:378–384.
- ,,.Impact of cardiothoracic resident turnover on mortality after cardiac surgery: A dynamic human factor.Ann Thorac Surg.2008;86:123–131.
- ,,.Is there a “July Phenomenon” in pediatric neurosurgery at teaching hospitals?J Neurosurg Pediatr.2006;105:169–176.
- ,,,,.Mortality and morbidity by month of birth of neonates admitted to an academic neonatal intensive care unit.Pediatrics.2008;122:E1048–E1052.
- .Measuring surgical quality in Maryland: A model.Health Aff.1988;7:62–78.
- ,,, et al.Complications and death at the start of the new academic year: Is there a July phenomenon?J Trauma‐Injury Infect Crit Care.2010;68(1):19–22.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,,.The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol.2010;63:798–803.
- .Introduction: The logic of latent variables.Latent Class Analysis.Newbury Park, CA:Sage;1987:5–10.
- July Effect. Wikipedia. Available at: http://en.wikipedia.org/wiki/July_effect. Accessed April 1,2011.
- Study proves “killing season” occurs as new doctors start work. September 23,2010. Herald Scotland. Available at: http://www.heraldscotland.com/news/health/study‐proves‐killing‐season‐occurs‐as‐new‐doctors‐start‐work‐1.921632. Accessed April 1, 2011.
- The “July effect”: Worst month for fatal hospital errors, study finds. June 3,2010. ABC News. Available at: http://abcnews.go.com/WN/WellnessNews/july‐month‐fatal‐hospital‐errors‐study‐finds/story?id=10819652. Accessed 1 April, 2011.
- “Deaths rise” with junior doctors. September 22,2010. BBC News. Available at: http://news.bbc.co.uk/2/hi/health/8269729.stm. Accessed April 1, 2011.
- .July: When not to go to the hospital. June 2,2010. Science News. Available at: http://www.sciencenews.org/view/generic/id/59865/title/July_When_not_to_go_to_the_hospital. Accessed April 1, 2011.
- July: A deadly time for hospitals. July 5,2010. National Public Radio. Available at: http://www.npr.org/templates/story/story.php?storyId=128321489. Accessed April 1, 2011.
- .Medical errors and patient safety: Beware the “July effect.” June 4,2010. Better Health. Available at: http://getbetterhealth.com/medical‐errors‐and‐patient‐safety‐beware‐of‐the‐july‐effect/2010.06.04. Accessed April 1, 2011.
- ,,.The effects of scheduled intern rotation on the cost and quality of teaching hospital care.Eval Health Prof.1994;17:259–272.
- ,,,.Specialty differences in the “July Phenomenon” for Twin Cities teaching hospitals.Med Care.1993;31:73–83.
- ,,,.The relationship of house staff experience to the cost and quality of inpatient care.JAMA.1990;263:953–957.
- ,,,.Indirect costs for medical education. Is there a July phenomenon?Arch Intern Med.1989;149:765–768.
- ,,,,.The impact of accreditation council for graduate medical education duty hours, the July phenomenon, and hospital teaching status on stroke outcomes.J Stroke Cerebrovasc Dis.2009;18:232–238.
- ,.The killing season—Fact or fiction.BMJ1994;309:1690.
- ,,, et al.The July effect: Impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients.Ann Thorac Surg.2009;88:70–75.
- ,.Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals.J Gen Intern Med.2003;18:639–645.
- ,,,.Neonatal mortality among low birth weight infants during the initial months of the academic year.J Perinatol.2008;28:691–695.
- ,,,,.The “July Phenomenon” and the care of the severely injured patient: Fact or fiction?Surgery.2001;130:346–353.
- ,,, et al.The July effect and cardiac surgery: The effect of the beginning of the academic cycle on outcomes.Am J Surg.2008;196:720–725.
- ,,,.Mortality in Medicare patients undergoing surgery in July in teaching hospitals.Ann Surg.2009;249:871–876.
- ,,, et al.Seasonal variation in surgical outcomes as measured by the American College of Surgeons–National Surgical Quality Improvement Program (ACS‐NSQIP).Ann Surg.2007;246:456–465.
- ,,, et al.Mortality rate and length of stay of patients admitted to the intensive care unit in July.Crit Care Med.2004;32:1161–1165.
- ,,,,,.July—As good a time as any to be injured.J Trauma‐Injury Infect Crit Care.2009;67:1087–1090.
- ,,,,.Early in‐hospital mortality following trainee doctors' first day at work.PLoS ONE.2009;4.
- ,,.Effect of critical care medicine fellows on patient outcome in the intensive care unit.Acad Med.2006;81:S1–S4.
- ,,,,.The “July Phenomenon”: Is trauma the exception?J Am Coll Surg.2009;209:378–384.
- ,,.Impact of cardiothoracic resident turnover on mortality after cardiac surgery: A dynamic human factor.Ann Thorac Surg.2008;86:123–131.
- ,,.Is there a “July Phenomenon” in pediatric neurosurgery at teaching hospitals?J Neurosurg Pediatr.2006;105:169–176.
- ,,,,.Mortality and morbidity by month of birth of neonates admitted to an academic neonatal intensive care unit.Pediatrics.2008;122:E1048–E1052.
- .Measuring surgical quality in Maryland: A model.Health Aff.1988;7:62–78.
- ,,, et al.Complications and death at the start of the new academic year: Is there a July phenomenon?J Trauma‐Injury Infect Crit Care.2010;68(1):19–22.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,,.The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol.2010;63:798–803.
- .Introduction: The logic of latent variables.Latent Class Analysis.Newbury Park, CA:Sage;1987:5–10.
- July Effect. Wikipedia. Available at: http://en.wikipedia.org/wiki/July_effect. Accessed April 1,2011.
- Study proves “killing season” occurs as new doctors start work. September 23,2010. Herald Scotland. Available at: http://www.heraldscotland.com/news/health/study‐proves‐killing‐season‐occurs‐as‐new‐doctors‐start‐work‐1.921632. Accessed April 1, 2011.
- The “July effect”: Worst month for fatal hospital errors, study finds. June 3,2010. ABC News. Available at: http://abcnews.go.com/WN/WellnessNews/july‐month‐fatal‐hospital‐errors‐study‐finds/story?id=10819652. Accessed 1 April, 2011.
- “Deaths rise” with junior doctors. September 22,2010. BBC News. Available at: http://news.bbc.co.uk/2/hi/health/8269729.stm. Accessed April 1, 2011.
- .July: When not to go to the hospital. June 2,2010. Science News. Available at: http://www.sciencenews.org/view/generic/id/59865/title/July_When_not_to_go_to_the_hospital. Accessed April 1, 2011.
- July: A deadly time for hospitals. July 5,2010. National Public Radio. Available at: http://www.npr.org/templates/story/story.php?storyId=128321489. Accessed April 1, 2011.
- .Medical errors and patient safety: Beware the “July effect.” June 4,2010. Better Health. Available at: http://getbetterhealth.com/medical‐errors‐and‐patient‐safety‐beware‐of‐the‐july‐effect/2010.06.04. Accessed April 1, 2011.
Copyright © 2011 Society of Hospital Medicine
Molecular Profiling Transforming Care of Advanced Lung Cancer
AMSTERDAM – Management of advanced non–small cell lung cancer now demands molecular profiling and personalized treatment. This new era has just begun, but it will quickly transform the field over the next 4 years, Dr. David R. Gandara said in a talk on the state of lung cancer medical oncology.
Increased molecular profiling – Dr. Gandara called for routine molecular profiling for every patient with advanced NSCLC – will mean a "culture change" for the field, and a sharp turn toward "ungrouping" the universe of NSCLC patients into individuals, he told attendees at the World Conference on Lung Cancer, which was sponsored by the International Association for the Study of Lung Cancer.
"We shouldn’t even talk about non–small cell lung cancer" as though it were a single entity, said Dr. Gandara, professor and director of the thoracic oncology program at the University of California, Davis, Cancer Center in Sacramento.
He also recommended new paradigms of drug development to reflect the complex, underlying biology and the inter- and intrapatient heterogeneity of lung cancer. "Transition from empiric to rationally selected and personalized therapy is challenging," Dr. Gandara conceded. But the transition is underway and accelerating.
Until about a year ago, the only lung cancer genes undergoing routine profiling at cancer centers were those for the epidermal growth factor receptor (EGFR) and, at fewer locations, for the oncogene KRAS. Dr. Bruce Johnson, a professor of medicine at Harvard Medical School and the Dana Farber Cancer Institute, both in Boston, takes credit for starting both; his EGFR program began 7 years ago and KRAS testing has been going for 5 years, he said.
Today, testing at several major U.S. cancer centers has added investigational tests for genes such as HER2, PIK3CA, ALK, MET, MEK, BRAF, AKTI, and NRAS. The Lung Cancer Mutation Consortium is in the midst of profiling 10 genes in 1,000 patients.
"Progress has been so dramatic. All but EGFR and KRAS came in the past year," said Marileila Varella Garcia, Ph.D., professor of medical oncology at the University of Colorado in Denver and a leader of the consortium. "At the University of Colorado, it took us 18 months to optimize the test [for 10 mutations], but now we have it and it can only improve. We test for 10 mutations for the same cost as testing for one."
At the Yale Cancer Center, the routine-profiling list stands at 13 genes, said Dr. Roy S. Herbst, chief of medical oncology at Yale in New Haven, Conn. "Right now, the only test that [insurers] pay for is the EGFR mutation test. Once the ALK story is more validated, they will probably pay to find ALK translocations, but with a chip, for the same money you can also test for other mutations for research," Dr. Herbst said.
"In the United States, testing for EGFR mutations is standard of care at most top cancer centers. EGFR is an actionable mutation, with patients considered for erlotinib treatment."
Dr. Varella Garcia, Dr. Johnson, and their collaborators from the consortium reported on the first 516 patients with advanced lung cancer who were tested with the 10-gene panel. The results showed that 280 of the tumors (54%) carried at least one mutation in at least one of the 10 genes that the consortium tested.
KRAS mutations were most prevalent, in 22% of the patients, followed by EGFR mutations in 17%, ALK rearrangement in 10%, MET amplifications in 4%, and a smaller number of genetic changes in each of the other six genes tested. Most mutations were mutually exclusive, with only 3% of tumors having mutations in two different genes, and no tumors with mutations in three or more genes.
"It was surprising that they found actionable mutations in more than half of the tumors they have tested so far. That is very promising," Dr. Gandara said.
Over the next 3-4 years, further studies will likely validate additional genes and mutations, perhaps encompassing about 90% of patients with advanced-stage lung cancer by 2015, Dr. Varella Garcia said in an interview. It’s also likely that a small percentage of these cancers won’t link with any single, identifiable gene mutation and will instead depend on changes in several pathways, something much harder to sort out.
The number of "actionable" gene mutations (mutations that, once found, can receive a targeted treatment) also remains limited but growing. Until recently, the list had a single gene, EGFR. Patients with EGFR mutations are candidates for treatment with erlotinib (Tarceva) or gefitinib (Iressa, which was not approved for routine U.S. use), both drugs from the tyrosine kinase inhibitor class.
A second, recent success story for targeted treatment involves the ALK fusion mutation, a genetic profile of tumors responsive to crizotinib. Results from phase I and II studies showed that crizotinib improved progression-free survival and overall survival in patients with tumors that had an ALK mutation; phase III studies are in progress.
"Patients with tumors that depend on these drivers have significantly better clinical outcomes when treated with specific inhibitors," Dr. Varella Garcia said.
This year’s meeting featured three main themes for patient management, but ultimately all three boil down to molecular biomarkers and molecularly directed treatment, Dr. Gandara said. One main theme – histologic profiles of advanced lung cancer – has been an important focus, but "histology is a transient selection method," he said. "At best, histology is a crude molecular selection device" superseded by molecular profiling itself.
Another important, recent focus has been maintenance therapy, but "the real questions are who gets further treatment after platinum-based induction, and when should they get it," questions best answerable by molecular profiling, he added.
"We have many ‘druggable’ molecular targets," Dr. Gandara noted.
"For almost every mutation [of the 10 genes that] the consortium is testing, we have phase I treatment trials underway," said Dr. Varella Garcia. Patients with tumors that carry KRAS and MEK mutations receive an investigational MEK inhibitor. Patients with tumors that contain HER2 mutations receive trastuzumab (Herceptin) as an investigational agent. Patients with MET mutations receive a MET monoclonal antibody.
Despite success so far, and pervasive optimism that current studies will validate new treatments, researchers cautioned that the management of advanced lung cancer also has some critical, unavoidable limitations.
"We will never cure advanced lung cancer; we can make it a chronic disease," Dr. Varella Garcia said. Effective treatment means that patients’ quality of life improves, and their disease comes under control for several years. But "it is almost universal that these patients with eventually progress again. We cannot cure advanced lung cancer. We can control it with new, targeted treatments that use oral drugs with low toxicity."
Dr. Gandara, Dr. Johnson, and Dr. Herbst disclosed relationships with numerous pharmaceutical and biotechnology companies. Among these, Dr. Johnson listed stock in Celgene and a patent for EGFR testing by Genzyme. Dr. Varella Garcia said that she has been a consultant to Abbott.
AMSTERDAM – Management of advanced non–small cell lung cancer now demands molecular profiling and personalized treatment. This new era has just begun, but it will quickly transform the field over the next 4 years, Dr. David R. Gandara said in a talk on the state of lung cancer medical oncology.
Increased molecular profiling – Dr. Gandara called for routine molecular profiling for every patient with advanced NSCLC – will mean a "culture change" for the field, and a sharp turn toward "ungrouping" the universe of NSCLC patients into individuals, he told attendees at the World Conference on Lung Cancer, which was sponsored by the International Association for the Study of Lung Cancer.
"We shouldn’t even talk about non–small cell lung cancer" as though it were a single entity, said Dr. Gandara, professor and director of the thoracic oncology program at the University of California, Davis, Cancer Center in Sacramento.
He also recommended new paradigms of drug development to reflect the complex, underlying biology and the inter- and intrapatient heterogeneity of lung cancer. "Transition from empiric to rationally selected and personalized therapy is challenging," Dr. Gandara conceded. But the transition is underway and accelerating.
Until about a year ago, the only lung cancer genes undergoing routine profiling at cancer centers were those for the epidermal growth factor receptor (EGFR) and, at fewer locations, for the oncogene KRAS. Dr. Bruce Johnson, a professor of medicine at Harvard Medical School and the Dana Farber Cancer Institute, both in Boston, takes credit for starting both; his EGFR program began 7 years ago and KRAS testing has been going for 5 years, he said.
Today, testing at several major U.S. cancer centers has added investigational tests for genes such as HER2, PIK3CA, ALK, MET, MEK, BRAF, AKTI, and NRAS. The Lung Cancer Mutation Consortium is in the midst of profiling 10 genes in 1,000 patients.
"Progress has been so dramatic. All but EGFR and KRAS came in the past year," said Marileila Varella Garcia, Ph.D., professor of medical oncology at the University of Colorado in Denver and a leader of the consortium. "At the University of Colorado, it took us 18 months to optimize the test [for 10 mutations], but now we have it and it can only improve. We test for 10 mutations for the same cost as testing for one."
At the Yale Cancer Center, the routine-profiling list stands at 13 genes, said Dr. Roy S. Herbst, chief of medical oncology at Yale in New Haven, Conn. "Right now, the only test that [insurers] pay for is the EGFR mutation test. Once the ALK story is more validated, they will probably pay to find ALK translocations, but with a chip, for the same money you can also test for other mutations for research," Dr. Herbst said.
"In the United States, testing for EGFR mutations is standard of care at most top cancer centers. EGFR is an actionable mutation, with patients considered for erlotinib treatment."
Dr. Varella Garcia, Dr. Johnson, and their collaborators from the consortium reported on the first 516 patients with advanced lung cancer who were tested with the 10-gene panel. The results showed that 280 of the tumors (54%) carried at least one mutation in at least one of the 10 genes that the consortium tested.
KRAS mutations were most prevalent, in 22% of the patients, followed by EGFR mutations in 17%, ALK rearrangement in 10%, MET amplifications in 4%, and a smaller number of genetic changes in each of the other six genes tested. Most mutations were mutually exclusive, with only 3% of tumors having mutations in two different genes, and no tumors with mutations in three or more genes.
"It was surprising that they found actionable mutations in more than half of the tumors they have tested so far. That is very promising," Dr. Gandara said.
Over the next 3-4 years, further studies will likely validate additional genes and mutations, perhaps encompassing about 90% of patients with advanced-stage lung cancer by 2015, Dr. Varella Garcia said in an interview. It’s also likely that a small percentage of these cancers won’t link with any single, identifiable gene mutation and will instead depend on changes in several pathways, something much harder to sort out.
The number of "actionable" gene mutations (mutations that, once found, can receive a targeted treatment) also remains limited but growing. Until recently, the list had a single gene, EGFR. Patients with EGFR mutations are candidates for treatment with erlotinib (Tarceva) or gefitinib (Iressa, which was not approved for routine U.S. use), both drugs from the tyrosine kinase inhibitor class.
A second, recent success story for targeted treatment involves the ALK fusion mutation, a genetic profile of tumors responsive to crizotinib. Results from phase I and II studies showed that crizotinib improved progression-free survival and overall survival in patients with tumors that had an ALK mutation; phase III studies are in progress.
"Patients with tumors that depend on these drivers have significantly better clinical outcomes when treated with specific inhibitors," Dr. Varella Garcia said.
This year’s meeting featured three main themes for patient management, but ultimately all three boil down to molecular biomarkers and molecularly directed treatment, Dr. Gandara said. One main theme – histologic profiles of advanced lung cancer – has been an important focus, but "histology is a transient selection method," he said. "At best, histology is a crude molecular selection device" superseded by molecular profiling itself.
Another important, recent focus has been maintenance therapy, but "the real questions are who gets further treatment after platinum-based induction, and when should they get it," questions best answerable by molecular profiling, he added.
"We have many ‘druggable’ molecular targets," Dr. Gandara noted.
"For almost every mutation [of the 10 genes that] the consortium is testing, we have phase I treatment trials underway," said Dr. Varella Garcia. Patients with tumors that carry KRAS and MEK mutations receive an investigational MEK inhibitor. Patients with tumors that contain HER2 mutations receive trastuzumab (Herceptin) as an investigational agent. Patients with MET mutations receive a MET monoclonal antibody.
Despite success so far, and pervasive optimism that current studies will validate new treatments, researchers cautioned that the management of advanced lung cancer also has some critical, unavoidable limitations.
"We will never cure advanced lung cancer; we can make it a chronic disease," Dr. Varella Garcia said. Effective treatment means that patients’ quality of life improves, and their disease comes under control for several years. But "it is almost universal that these patients with eventually progress again. We cannot cure advanced lung cancer. We can control it with new, targeted treatments that use oral drugs with low toxicity."
Dr. Gandara, Dr. Johnson, and Dr. Herbst disclosed relationships with numerous pharmaceutical and biotechnology companies. Among these, Dr. Johnson listed stock in Celgene and a patent for EGFR testing by Genzyme. Dr. Varella Garcia said that she has been a consultant to Abbott.
AMSTERDAM – Management of advanced non–small cell lung cancer now demands molecular profiling and personalized treatment. This new era has just begun, but it will quickly transform the field over the next 4 years, Dr. David R. Gandara said in a talk on the state of lung cancer medical oncology.
Increased molecular profiling – Dr. Gandara called for routine molecular profiling for every patient with advanced NSCLC – will mean a "culture change" for the field, and a sharp turn toward "ungrouping" the universe of NSCLC patients into individuals, he told attendees at the World Conference on Lung Cancer, which was sponsored by the International Association for the Study of Lung Cancer.
"We shouldn’t even talk about non–small cell lung cancer" as though it were a single entity, said Dr. Gandara, professor and director of the thoracic oncology program at the University of California, Davis, Cancer Center in Sacramento.
He also recommended new paradigms of drug development to reflect the complex, underlying biology and the inter- and intrapatient heterogeneity of lung cancer. "Transition from empiric to rationally selected and personalized therapy is challenging," Dr. Gandara conceded. But the transition is underway and accelerating.
Until about a year ago, the only lung cancer genes undergoing routine profiling at cancer centers were those for the epidermal growth factor receptor (EGFR) and, at fewer locations, for the oncogene KRAS. Dr. Bruce Johnson, a professor of medicine at Harvard Medical School and the Dana Farber Cancer Institute, both in Boston, takes credit for starting both; his EGFR program began 7 years ago and KRAS testing has been going for 5 years, he said.
Today, testing at several major U.S. cancer centers has added investigational tests for genes such as HER2, PIK3CA, ALK, MET, MEK, BRAF, AKTI, and NRAS. The Lung Cancer Mutation Consortium is in the midst of profiling 10 genes in 1,000 patients.
"Progress has been so dramatic. All but EGFR and KRAS came in the past year," said Marileila Varella Garcia, Ph.D., professor of medical oncology at the University of Colorado in Denver and a leader of the consortium. "At the University of Colorado, it took us 18 months to optimize the test [for 10 mutations], but now we have it and it can only improve. We test for 10 mutations for the same cost as testing for one."
At the Yale Cancer Center, the routine-profiling list stands at 13 genes, said Dr. Roy S. Herbst, chief of medical oncology at Yale in New Haven, Conn. "Right now, the only test that [insurers] pay for is the EGFR mutation test. Once the ALK story is more validated, they will probably pay to find ALK translocations, but with a chip, for the same money you can also test for other mutations for research," Dr. Herbst said.
"In the United States, testing for EGFR mutations is standard of care at most top cancer centers. EGFR is an actionable mutation, with patients considered for erlotinib treatment."
Dr. Varella Garcia, Dr. Johnson, and their collaborators from the consortium reported on the first 516 patients with advanced lung cancer who were tested with the 10-gene panel. The results showed that 280 of the tumors (54%) carried at least one mutation in at least one of the 10 genes that the consortium tested.
KRAS mutations were most prevalent, in 22% of the patients, followed by EGFR mutations in 17%, ALK rearrangement in 10%, MET amplifications in 4%, and a smaller number of genetic changes in each of the other six genes tested. Most mutations were mutually exclusive, with only 3% of tumors having mutations in two different genes, and no tumors with mutations in three or more genes.
"It was surprising that they found actionable mutations in more than half of the tumors they have tested so far. That is very promising," Dr. Gandara said.
Over the next 3-4 years, further studies will likely validate additional genes and mutations, perhaps encompassing about 90% of patients with advanced-stage lung cancer by 2015, Dr. Varella Garcia said in an interview. It’s also likely that a small percentage of these cancers won’t link with any single, identifiable gene mutation and will instead depend on changes in several pathways, something much harder to sort out.
The number of "actionable" gene mutations (mutations that, once found, can receive a targeted treatment) also remains limited but growing. Until recently, the list had a single gene, EGFR. Patients with EGFR mutations are candidates for treatment with erlotinib (Tarceva) or gefitinib (Iressa, which was not approved for routine U.S. use), both drugs from the tyrosine kinase inhibitor class.
A second, recent success story for targeted treatment involves the ALK fusion mutation, a genetic profile of tumors responsive to crizotinib. Results from phase I and II studies showed that crizotinib improved progression-free survival and overall survival in patients with tumors that had an ALK mutation; phase III studies are in progress.
"Patients with tumors that depend on these drivers have significantly better clinical outcomes when treated with specific inhibitors," Dr. Varella Garcia said.
This year’s meeting featured three main themes for patient management, but ultimately all three boil down to molecular biomarkers and molecularly directed treatment, Dr. Gandara said. One main theme – histologic profiles of advanced lung cancer – has been an important focus, but "histology is a transient selection method," he said. "At best, histology is a crude molecular selection device" superseded by molecular profiling itself.
Another important, recent focus has been maintenance therapy, but "the real questions are who gets further treatment after platinum-based induction, and when should they get it," questions best answerable by molecular profiling, he added.
"We have many ‘druggable’ molecular targets," Dr. Gandara noted.
"For almost every mutation [of the 10 genes that] the consortium is testing, we have phase I treatment trials underway," said Dr. Varella Garcia. Patients with tumors that carry KRAS and MEK mutations receive an investigational MEK inhibitor. Patients with tumors that contain HER2 mutations receive trastuzumab (Herceptin) as an investigational agent. Patients with MET mutations receive a MET monoclonal antibody.
Despite success so far, and pervasive optimism that current studies will validate new treatments, researchers cautioned that the management of advanced lung cancer also has some critical, unavoidable limitations.
"We will never cure advanced lung cancer; we can make it a chronic disease," Dr. Varella Garcia said. Effective treatment means that patients’ quality of life improves, and their disease comes under control for several years. But "it is almost universal that these patients with eventually progress again. We cannot cure advanced lung cancer. We can control it with new, targeted treatments that use oral drugs with low toxicity."
Dr. Gandara, Dr. Johnson, and Dr. Herbst disclosed relationships with numerous pharmaceutical and biotechnology companies. Among these, Dr. Johnson listed stock in Celgene and a patent for EGFR testing by Genzyme. Dr. Varella Garcia said that she has been a consultant to Abbott.
EXPERT ANALYSIS FROM THE WORLD CONFERENCE ON LUNG CANCER
Study supports lifting lifetime ban on MSM

Photo by Juan D. Alfonso
The lifetime ban on blood donation from men who have sex with men (MSM) has been lifted in England, Wales, and Scotland.
Beginning in November, MSM in these countries can donate blood if they have not engaged in sexual activity within the past 12 months. A study published September 8 on bmj.com helped inform this decision.
Several other countries previously lifted the lifetime ban instituted in the 1980s and introduced deferment periods instead. For example, MSM in South Africa must defer blood donation 6 months after sexual activity. And MSM in Australia, Sweden, and Japan must wait 12 months.
The changes to policy in these countries—along with advances in blood screening techniques and knowledge of HIV—prompted calls for Great Britain to revise its blood donor policy.
So Kaye Wellings, of London School of Hygiene and Tropical Medicine, and her colleagues decided to assess the possible effects of revising the policy, as well as past compliance with the lifetime ban. The results of their study were used to inform the policy review conducted by the Advisory Committee on the Safety of Blood, Tissues, and Organs.
Between April 2009 and June 2010, Wellings’s team surveyed 1028 men in Britain who reported having any sexual contact with other men. Of those surveyed, 10.6% reported donating blood since having penetrative sex with a man, and 2.5% had done so in the past 12 months.
The men cited various reasons for not complying with the ban. They believed themselves to be at low risk of having HIV, had confidentiality concerns, did not understand the ban, or thought the ban unfair.
To gain more insight, Wellings and her colleagues conducted interviews with 30 MSMs—19 who had complied with the lifetime ban on blood donation and 11 who had not.
Many of these men considered the lifetime ban to be unfair, discriminatory, and lacking a clear rationale. However, they generally viewed a 1-year deferral rule as feasible and acceptable.
This prompted Wellings and her colleagues to conclude that MSM might be more likely to comply with a 1-year deferral rule than a lifetime ban. And compliance might improve further with better communication, improved confidentiality measures, and clear explanations of the rationale behind the rule. ![]()

Photo by Juan D. Alfonso
The lifetime ban on blood donation from men who have sex with men (MSM) has been lifted in England, Wales, and Scotland.
Beginning in November, MSM in these countries can donate blood if they have not engaged in sexual activity within the past 12 months. A study published September 8 on bmj.com helped inform this decision.
Several other countries previously lifted the lifetime ban instituted in the 1980s and introduced deferment periods instead. For example, MSM in South Africa must defer blood donation 6 months after sexual activity. And MSM in Australia, Sweden, and Japan must wait 12 months.
The changes to policy in these countries—along with advances in blood screening techniques and knowledge of HIV—prompted calls for Great Britain to revise its blood donor policy.
So Kaye Wellings, of London School of Hygiene and Tropical Medicine, and her colleagues decided to assess the possible effects of revising the policy, as well as past compliance with the lifetime ban. The results of their study were used to inform the policy review conducted by the Advisory Committee on the Safety of Blood, Tissues, and Organs.
Between April 2009 and June 2010, Wellings’s team surveyed 1028 men in Britain who reported having any sexual contact with other men. Of those surveyed, 10.6% reported donating blood since having penetrative sex with a man, and 2.5% had done so in the past 12 months.
The men cited various reasons for not complying with the ban. They believed themselves to be at low risk of having HIV, had confidentiality concerns, did not understand the ban, or thought the ban unfair.
To gain more insight, Wellings and her colleagues conducted interviews with 30 MSMs—19 who had complied with the lifetime ban on blood donation and 11 who had not.
Many of these men considered the lifetime ban to be unfair, discriminatory, and lacking a clear rationale. However, they generally viewed a 1-year deferral rule as feasible and acceptable.
This prompted Wellings and her colleagues to conclude that MSM might be more likely to comply with a 1-year deferral rule than a lifetime ban. And compliance might improve further with better communication, improved confidentiality measures, and clear explanations of the rationale behind the rule. ![]()

Photo by Juan D. Alfonso
The lifetime ban on blood donation from men who have sex with men (MSM) has been lifted in England, Wales, and Scotland.
Beginning in November, MSM in these countries can donate blood if they have not engaged in sexual activity within the past 12 months. A study published September 8 on bmj.com helped inform this decision.
Several other countries previously lifted the lifetime ban instituted in the 1980s and introduced deferment periods instead. For example, MSM in South Africa must defer blood donation 6 months after sexual activity. And MSM in Australia, Sweden, and Japan must wait 12 months.
The changes to policy in these countries—along with advances in blood screening techniques and knowledge of HIV—prompted calls for Great Britain to revise its blood donor policy.
So Kaye Wellings, of London School of Hygiene and Tropical Medicine, and her colleagues decided to assess the possible effects of revising the policy, as well as past compliance with the lifetime ban. The results of their study were used to inform the policy review conducted by the Advisory Committee on the Safety of Blood, Tissues, and Organs.
Between April 2009 and June 2010, Wellings’s team surveyed 1028 men in Britain who reported having any sexual contact with other men. Of those surveyed, 10.6% reported donating blood since having penetrative sex with a man, and 2.5% had done so in the past 12 months.
The men cited various reasons for not complying with the ban. They believed themselves to be at low risk of having HIV, had confidentiality concerns, did not understand the ban, or thought the ban unfair.
To gain more insight, Wellings and her colleagues conducted interviews with 30 MSMs—19 who had complied with the lifetime ban on blood donation and 11 who had not.
Many of these men considered the lifetime ban to be unfair, discriminatory, and lacking a clear rationale. However, they generally viewed a 1-year deferral rule as feasible and acceptable.
This prompted Wellings and her colleagues to conclude that MSM might be more likely to comply with a 1-year deferral rule than a lifetime ban. And compliance might improve further with better communication, improved confidentiality measures, and clear explanations of the rationale behind the rule. ![]()
Hospitalists See Value in Palliative Care
HM groups looking for a new revenue stream would be well served to keep an eye on the explosive growth of palliative care, according to a former SHM president who also runs a palliative service.
Steven Pantilat, MD, FACP, SFHM, director of the Palliative Care Leadership Center at the University of California at San Francisco, says data released this summer by the Center to Advance Palliative Care (CAPC) show that 63% of hospitals have palliative-care teams, up from 24.5% in 2000. But growth is lagging in both smaller hospitals and hospitals in the South.
"Hospitals that are looking to improve the systems of care, hospitals that are looking to be more cutting-edge, looking to be adopters of new models of care are going to pursue both hospital medicine and palliative care," Dr. Pantilat says. "That is another way that hospitalists can demonstrate added value."
Dr. Pantilat, who helped create SHM's Palliative-Care Task Force, says hospitalists can provide primary palliative care and should be mindful to identify patients who should be referred to palliative teams. Hospitalists interested in learning more about palliative skills can pursue training programs through CAPC or the American Academy of Hospice and Palliative Medicine.
The growth of HM and palliative care have followed similar tracks in the past decade, and the business case for both services is similar, Dr. Pantilat says. Because demand still outweighs supply in both specialties, many institutions looking for palliative expertise would be pleased to have their HM group take that mantle, particularly as hospitalists are now caring for the majority of inpatients that would benefit from those services, he adds.
"Hospitalists are the ones taking care of those people with advanced, serious, and life-threatening illnesses," Dr. Pantilat says. "De facto, they are already doing this work."
HM groups looking for a new revenue stream would be well served to keep an eye on the explosive growth of palliative care, according to a former SHM president who also runs a palliative service.
Steven Pantilat, MD, FACP, SFHM, director of the Palliative Care Leadership Center at the University of California at San Francisco, says data released this summer by the Center to Advance Palliative Care (CAPC) show that 63% of hospitals have palliative-care teams, up from 24.5% in 2000. But growth is lagging in both smaller hospitals and hospitals in the South.
"Hospitals that are looking to improve the systems of care, hospitals that are looking to be more cutting-edge, looking to be adopters of new models of care are going to pursue both hospital medicine and palliative care," Dr. Pantilat says. "That is another way that hospitalists can demonstrate added value."
Dr. Pantilat, who helped create SHM's Palliative-Care Task Force, says hospitalists can provide primary palliative care and should be mindful to identify patients who should be referred to palliative teams. Hospitalists interested in learning more about palliative skills can pursue training programs through CAPC or the American Academy of Hospice and Palliative Medicine.
The growth of HM and palliative care have followed similar tracks in the past decade, and the business case for both services is similar, Dr. Pantilat says. Because demand still outweighs supply in both specialties, many institutions looking for palliative expertise would be pleased to have their HM group take that mantle, particularly as hospitalists are now caring for the majority of inpatients that would benefit from those services, he adds.
"Hospitalists are the ones taking care of those people with advanced, serious, and life-threatening illnesses," Dr. Pantilat says. "De facto, they are already doing this work."
HM groups looking for a new revenue stream would be well served to keep an eye on the explosive growth of palliative care, according to a former SHM president who also runs a palliative service.
Steven Pantilat, MD, FACP, SFHM, director of the Palliative Care Leadership Center at the University of California at San Francisco, says data released this summer by the Center to Advance Palliative Care (CAPC) show that 63% of hospitals have palliative-care teams, up from 24.5% in 2000. But growth is lagging in both smaller hospitals and hospitals in the South.
"Hospitals that are looking to improve the systems of care, hospitals that are looking to be more cutting-edge, looking to be adopters of new models of care are going to pursue both hospital medicine and palliative care," Dr. Pantilat says. "That is another way that hospitalists can demonstrate added value."
Dr. Pantilat, who helped create SHM's Palliative-Care Task Force, says hospitalists can provide primary palliative care and should be mindful to identify patients who should be referred to palliative teams. Hospitalists interested in learning more about palliative skills can pursue training programs through CAPC or the American Academy of Hospice and Palliative Medicine.
The growth of HM and palliative care have followed similar tracks in the past decade, and the business case for both services is similar, Dr. Pantilat says. Because demand still outweighs supply in both specialties, many institutions looking for palliative expertise would be pleased to have their HM group take that mantle, particularly as hospitalists are now caring for the majority of inpatients that would benefit from those services, he adds.
"Hospitalists are the ones taking care of those people with advanced, serious, and life-threatening illnesses," Dr. Pantilat says. "De facto, they are already doing this work."
To Friend or Not to Friend?
Social networking is nothing new, but with more doctors logging on, it is important to recognize the inherent professional risks.
To help their physicians manage their online reputations, the British Medical Association recently issued social media guidelines reminding their doctors that "ethical and legal duties apply just as much on the Internet as when they are offline." U.S.-based physicians are also encouraged to take precautions.
As a mentor for young hospitalists, Paul Grant, MD, assistant professor at University of Michigan Health System and chair of the SHM Early Career Hospitalist committee, agrees that while there is value in using sites like Facebook and Twitter, it's important to keep the conversation professional.
"While the issue of patient friend requests is probably more common with long-term-care physicians, our group has been encouraged to be aware of our Internet profiles, and to Google ourselves periodically to see what’s out there," he says. Dr. Grant admits he occasionally receives requests from colleagues, but he declines.
"That's the nice thing about Facebook: You treat it like you would any other relationship," says Glenn Lombardi, president of Officite, a Downers Grove, Ill.-based medical website and web-marketing firm that manages more than 1,000 Facebook accounts for medical practices. "You share certain things with certain people, and it's not anything bigger that that."
Lombardi and Dr. Grant offer the following social-networking tips:
- Maintain privacy. Don't accept personal friend requests from patients or colleagues.
- Be proactive. Have a search-engine-optimized website. Make sure your patients know it's the best place to go for information.
- Wait on trends. New social-networking sites, such as Google+, have great potential, Lombardi says, but it is better to see what experts identify as their best and safest purposes before creating a profile.
Social networking is nothing new, but with more doctors logging on, it is important to recognize the inherent professional risks.
To help their physicians manage their online reputations, the British Medical Association recently issued social media guidelines reminding their doctors that "ethical and legal duties apply just as much on the Internet as when they are offline." U.S.-based physicians are also encouraged to take precautions.
As a mentor for young hospitalists, Paul Grant, MD, assistant professor at University of Michigan Health System and chair of the SHM Early Career Hospitalist committee, agrees that while there is value in using sites like Facebook and Twitter, it's important to keep the conversation professional.
"While the issue of patient friend requests is probably more common with long-term-care physicians, our group has been encouraged to be aware of our Internet profiles, and to Google ourselves periodically to see what’s out there," he says. Dr. Grant admits he occasionally receives requests from colleagues, but he declines.
"That's the nice thing about Facebook: You treat it like you would any other relationship," says Glenn Lombardi, president of Officite, a Downers Grove, Ill.-based medical website and web-marketing firm that manages more than 1,000 Facebook accounts for medical practices. "You share certain things with certain people, and it's not anything bigger that that."
Lombardi and Dr. Grant offer the following social-networking tips:
- Maintain privacy. Don't accept personal friend requests from patients or colleagues.
- Be proactive. Have a search-engine-optimized website. Make sure your patients know it's the best place to go for information.
- Wait on trends. New social-networking sites, such as Google+, have great potential, Lombardi says, but it is better to see what experts identify as their best and safest purposes before creating a profile.
Social networking is nothing new, but with more doctors logging on, it is important to recognize the inherent professional risks.
To help their physicians manage their online reputations, the British Medical Association recently issued social media guidelines reminding their doctors that "ethical and legal duties apply just as much on the Internet as when they are offline." U.S.-based physicians are also encouraged to take precautions.
As a mentor for young hospitalists, Paul Grant, MD, assistant professor at University of Michigan Health System and chair of the SHM Early Career Hospitalist committee, agrees that while there is value in using sites like Facebook and Twitter, it's important to keep the conversation professional.
"While the issue of patient friend requests is probably more common with long-term-care physicians, our group has been encouraged to be aware of our Internet profiles, and to Google ourselves periodically to see what’s out there," he says. Dr. Grant admits he occasionally receives requests from colleagues, but he declines.
"That's the nice thing about Facebook: You treat it like you would any other relationship," says Glenn Lombardi, president of Officite, a Downers Grove, Ill.-based medical website and web-marketing firm that manages more than 1,000 Facebook accounts for medical practices. "You share certain things with certain people, and it's not anything bigger that that."
Lombardi and Dr. Grant offer the following social-networking tips:
- Maintain privacy. Don't accept personal friend requests from patients or colleagues.
- Be proactive. Have a search-engine-optimized website. Make sure your patients know it's the best place to go for information.
- Wait on trends. New social-networking sites, such as Google+, have great potential, Lombardi says, but it is better to see what experts identify as their best and safest purposes before creating a profile.
New Beginnings
They both were working the day the planes crashed into the World Trade Center in New York City. They saw the twin towers crash to the ground, the soot and debris covering lower Manhattan, and the puzzled faces of loved ones searching for information in the EDs of their hospitals. And while the memories are vivid and the shock of the terror still resides in them, they have chosen distinctly different paths since the 9/11 attacks 10 years ago.
Born and raised in Queens, Adam Trosterman, MD, grew up looking at the World Trade Center from his apartment window, studied medicine at Albert Einstein Medical Center in Manhattan, and was the intern on call for trauma surgery at NYU Bellevue the day of the attack. Today, he works as a hospitalist in Colorado and plans to spend Sept. 11 biking in the peaceful altitudes of the Rocky Mountains.
“I will probably go for a bike ride with my wife and enjoy some fresh air,” Dr. Trosterman says. “I don’t plan anything special, but I think about [Sept. 11] and I don’t think about October 11.”
A mere 10 blocks south of NYU Bellevue, straight down First Avenue, Dahlia Rizk, DO, was the hospitalist program director at Beth Israel Medical Center and in the middle of grand rounds when she first heard about the attacks on the twin towers. She has since moved to Battery Park, just a few blocks from the construction site for the new World Trade Center, and plans to participate in the 9/11 anniversary ceremony.
“I think that the memorial, the new building, and that whole area is just coming alive again. It is a real testament to the resilience of New Yorkers. Honoring the victims and their families is just so important. It’s such an incredible thing,” Dr. Rizk says. “I’m looking forward to the remembrance and celebrating the human spirit.”
Two physicians, two hospitalists, two human beings: They look back at 9/11 in diverse yet illuminating ways. These are their stories.
The Intern
A self-described New Yorker, Dr. Trosterman remembers Sept. 11, 2001, as a “beautiful, gorgeous morning” in which the sun was high and the temperature was pleasant. He, however, was in poor spirits, as everyone at NYU Bellevue “hated to be on trauma surgery” rotation. He was 29, single, and, as he puts it, “having a very good time” living in Manhattan.
He arrived at work at 6 a.m. and went about the basic duties of every first-year resident on trauma surgery rotation, rounding with two of his colleagues on 15 patients. At about 8:30 a.m., he ran into another surgery intern who informed him there was a “big trauma coming, something to do with a plane, you might want to check it out.”
Big traumas in New York City are a regular occurrence, and after nearly three months on, he says, he was “pretty well versed in how to run the trauma service. You grow up fast.”
“Back in 2001, you grew up really fast,” he adds. “There were no work-hour regulations; I was working 115 hours per week.”
Dr. Trosterman ran to his trauma slots in the ED—they were “acting weird,” he notes—and began setting up the four trauma beds for the unknown mass-casualty incident (MCI). “It took maybe five minutes,” he says. “Then I got a call from one of my colleagues who was a neurosurgery intern. He starts describing to me what happened, and tells me to come up to the ICU.”
—Adam Trosterman, MD, University of Colorado Denver
Bellevue’s ICU is on the 15th floor, with an unobstructed view of lower Manhattan. When he got there, Dr. Trosterman had a perfect view of the horror at the World Trade Center. “I was like, ‘Oh, my God,’ ” he recalls. “There was a humongous hole in the tower. At that point, I almost started laughing to myself. Not really, of course, but … we had to mobilize a whole different system, which, of course, I was a part of. But it was no longer my typical role for trauma.”
The first patient Dr. Trosterman saw that morning was pronounced dead on arrival. Ironically, he says, the patient looked a lot like his best friend’s stepfather, who worked in the World Trade Center. “They were like parents to me,” he says. “I couldn’t get through. It wasn’t until the second day that I could make a call. I don’t think I spoke to them until Sept. 13.” (Fortunately, everyone Dr. Trosterman knew who worked in the towers survived.)
The next patient Dr. Trosterman saw was a police officer who had a dislocated shoulder and a small fracture. He was screaming and it was difficult to tell if his outbursts were pain-related, Dr. Trosterman says. “He was ranting about what had happened—appropriately ranting,” he says. “He was saying, ‘My partner was at my side and I was trying to save him, but I knew I couldn’t get him out and save myself. I just had to run or I would’ve died. I left my partner to die. I left my partner to die.’ It was horrible. He probably still feels guilty about it right now.”
Contrary to some reports, Dr. Trosterman says, Bellevue and other New York City hospitals were overwhelmed with work, if not injured patients. Much of the work following the attacks was moving inpatients to free up space for casualties. The trauma service ballooned by 40 patients. “We saw more people than we ever see,” he says, “and, literally, the same number of doctors. I was, physically, unbelievably busy. I was emotionally worried about my friend and his family, and I hadn’t had contact with anyone for 48 hours. … I was frustrated that all I kept hearing on the radio was that there were no patients. I was like, ‘You need to come visit me and see what I’m doing!’ It was nonstop and nobody was alive.”

—Dahlia Rizk, DO, hospitalist program director, Beth Israel Medical Center, New York City
Dr. Trosterman cared for dozens of patients on 9/11, working into the wee hours of the night (see “The Most Interesting Patient,” below). He was told to go home at 3:30 a.m. but had to return to work at 6 a.m. He says walking out of the hospital that night was like walking through the morgue.
“Manhattan is one of the most happening places, and downtown Manhattan, it doesn’t matter what time of day it is, there’s always somebody in the street and there’s always something open,” he says. “Everything was closed, dead, silent, scary, barren. It was the most surreal thing I can ever remember in my life.”
In the midst of the chaos and confusion, loneliness and isolation replaced communication.
“Everyone was working, working, working, but no one was talking,” Dr. Trosterman says. “When I look back on that day, I feel angry, frustrated, scared, weird....While there weren’t 1,000 people [to save], those 10 or 15 lives that were saved, that were critically ill, were unbelievably important to the doctors who were taking care of them—no one knows about that.”
The Optimist
Ten years ago, Dr. Rizk was director of a three-hospitalist HM service at Beth Israel Medical Center; now the program has 26 FTE hospitalists and 15 physician assistants on staff. She was running late to grand rounds that day, coffee in hand as she passed a television and saw the first news reports of an airplane crashing into the first tower. Moments later, the hospital activated its disaster protocol, and Dr. Rizk rounded up her hospitalists.
“We very rapidly started discharging patients,” she recalls. “I actually went up to the 11th floor of our hospital and could see at that time that the second tower had been hit. It was almost like a dream, like a horrible nightmare. We could see the skyline changing when the first tower dropped. I could hear the sirens and see the smoke that was filling the air.
“We started to create triage stations outside our ED, and we had all the physicians at the hospital available. The ED was pretty chaotic in terms of the throughput. There wasn’t clear instruction; we didn’t know what was happening. ... There was a lot of debris and scratches and fractures that came through our ED.
continued below...
I remember very clearly standing outside of the ED as well, mostly greeting families who were looking for loved ones throughout the course of the day and collecting photographs that we posted on the wall for missing loved ones. And I remember these chilling feelings; there were so few people that were coming in that were in critical condition. I knew that this was not where they would find these patients.”
Beth Israel was not the Level 1 trauma center for lower Manhattan at the time; the now-shuttered St. Vincent’s Hospital was the go-to ED for mass casualty incidents. “They probably got the brunt of those patients, if there were any,” Dr. Rizk says. “I don’t know how many, but I can tell you from the hospitalist standpoint on the inpatient side, there was very little that was done.”
Most of the patients at Beth Israel were wheezing, needing eyewashes, or tending to scrapes and cuts. Dr. Rizk says many of the beds cleared for traumas sat empty. “We were ready, but so little happened in terms of activity on the inpatient side,” she says. “The saddest part really was the faces. I remember a college friend of mine actually coming and looking for his girlfriend’s family member at the time, and I just remember how horrified these family members were going from hospital to hospital throughout the city looking for loved ones.”
In the days and weeks that followed 9/11, Dr. Rizk says, a heavy feeling permeated the city. “Simple things like groceries and shops and restaurants—not that anyone felt like doing that—they just weren’t available,” she says. “Everybody was on foot trying to sort out what happened.”
Her brother-in-law, who worked in the building next to the towers, survived. Others she knew did not. An elementary school friend—a firefighter who rushed into the towers after the attacks—did not make it out. A close friend had an uncle, the head of the Brooklyn fire battalion, who lost his life, too. She attended his funeral.
The months that followed the attacks were “chilling” and “empty,” she says, as the soot covered the community and sorrow pierced those who lived and worked near ground zero.
Since then, Dr. Rizk has watched an “amazing” transformation in lower Manhattan. And it’s not just construction on the new 104-story Freedom Tower or the names of victims etched into the marble fountain walls, but the trees and momentum building for the 10-year anniversary.
“Just to see that renewed hope—it’s exciting,” she says. “I live down there now and am constantly reminded, every day, as I pass ground zero. I am amazed by how resilient the city is. The whole area is coming alive again.”
Dr. Rizk hopes to attend the 9/11 memorial service this month to honor the heroes and applaud New York’s future.
“[It’s] just a symbol of strength and hope for the future of people living together,” she says, “and to recognize that we all have the fundamental human commonality, and we really need to focus on how to move forward as a society—working together as a common goal.”
Jason Carris is editor of The Hospitalist.
They both were working the day the planes crashed into the World Trade Center in New York City. They saw the twin towers crash to the ground, the soot and debris covering lower Manhattan, and the puzzled faces of loved ones searching for information in the EDs of their hospitals. And while the memories are vivid and the shock of the terror still resides in them, they have chosen distinctly different paths since the 9/11 attacks 10 years ago.
Born and raised in Queens, Adam Trosterman, MD, grew up looking at the World Trade Center from his apartment window, studied medicine at Albert Einstein Medical Center in Manhattan, and was the intern on call for trauma surgery at NYU Bellevue the day of the attack. Today, he works as a hospitalist in Colorado and plans to spend Sept. 11 biking in the peaceful altitudes of the Rocky Mountains.
“I will probably go for a bike ride with my wife and enjoy some fresh air,” Dr. Trosterman says. “I don’t plan anything special, but I think about [Sept. 11] and I don’t think about October 11.”
A mere 10 blocks south of NYU Bellevue, straight down First Avenue, Dahlia Rizk, DO, was the hospitalist program director at Beth Israel Medical Center and in the middle of grand rounds when she first heard about the attacks on the twin towers. She has since moved to Battery Park, just a few blocks from the construction site for the new World Trade Center, and plans to participate in the 9/11 anniversary ceremony.
“I think that the memorial, the new building, and that whole area is just coming alive again. It is a real testament to the resilience of New Yorkers. Honoring the victims and their families is just so important. It’s such an incredible thing,” Dr. Rizk says. “I’m looking forward to the remembrance and celebrating the human spirit.”
Two physicians, two hospitalists, two human beings: They look back at 9/11 in diverse yet illuminating ways. These are their stories.
The Intern
A self-described New Yorker, Dr. Trosterman remembers Sept. 11, 2001, as a “beautiful, gorgeous morning” in which the sun was high and the temperature was pleasant. He, however, was in poor spirits, as everyone at NYU Bellevue “hated to be on trauma surgery” rotation. He was 29, single, and, as he puts it, “having a very good time” living in Manhattan.
He arrived at work at 6 a.m. and went about the basic duties of every first-year resident on trauma surgery rotation, rounding with two of his colleagues on 15 patients. At about 8:30 a.m., he ran into another surgery intern who informed him there was a “big trauma coming, something to do with a plane, you might want to check it out.”
Big traumas in New York City are a regular occurrence, and after nearly three months on, he says, he was “pretty well versed in how to run the trauma service. You grow up fast.”
“Back in 2001, you grew up really fast,” he adds. “There were no work-hour regulations; I was working 115 hours per week.”
Dr. Trosterman ran to his trauma slots in the ED—they were “acting weird,” he notes—and began setting up the four trauma beds for the unknown mass-casualty incident (MCI). “It took maybe five minutes,” he says. “Then I got a call from one of my colleagues who was a neurosurgery intern. He starts describing to me what happened, and tells me to come up to the ICU.”
—Adam Trosterman, MD, University of Colorado Denver
Bellevue’s ICU is on the 15th floor, with an unobstructed view of lower Manhattan. When he got there, Dr. Trosterman had a perfect view of the horror at the World Trade Center. “I was like, ‘Oh, my God,’ ” he recalls. “There was a humongous hole in the tower. At that point, I almost started laughing to myself. Not really, of course, but … we had to mobilize a whole different system, which, of course, I was a part of. But it was no longer my typical role for trauma.”
The first patient Dr. Trosterman saw that morning was pronounced dead on arrival. Ironically, he says, the patient looked a lot like his best friend’s stepfather, who worked in the World Trade Center. “They were like parents to me,” he says. “I couldn’t get through. It wasn’t until the second day that I could make a call. I don’t think I spoke to them until Sept. 13.” (Fortunately, everyone Dr. Trosterman knew who worked in the towers survived.)
The next patient Dr. Trosterman saw was a police officer who had a dislocated shoulder and a small fracture. He was screaming and it was difficult to tell if his outbursts were pain-related, Dr. Trosterman says. “He was ranting about what had happened—appropriately ranting,” he says. “He was saying, ‘My partner was at my side and I was trying to save him, but I knew I couldn’t get him out and save myself. I just had to run or I would’ve died. I left my partner to die. I left my partner to die.’ It was horrible. He probably still feels guilty about it right now.”
Contrary to some reports, Dr. Trosterman says, Bellevue and other New York City hospitals were overwhelmed with work, if not injured patients. Much of the work following the attacks was moving inpatients to free up space for casualties. The trauma service ballooned by 40 patients. “We saw more people than we ever see,” he says, “and, literally, the same number of doctors. I was, physically, unbelievably busy. I was emotionally worried about my friend and his family, and I hadn’t had contact with anyone for 48 hours. … I was frustrated that all I kept hearing on the radio was that there were no patients. I was like, ‘You need to come visit me and see what I’m doing!’ It was nonstop and nobody was alive.”

—Dahlia Rizk, DO, hospitalist program director, Beth Israel Medical Center, New York City
Dr. Trosterman cared for dozens of patients on 9/11, working into the wee hours of the night (see “The Most Interesting Patient,” below). He was told to go home at 3:30 a.m. but had to return to work at 6 a.m. He says walking out of the hospital that night was like walking through the morgue.
“Manhattan is one of the most happening places, and downtown Manhattan, it doesn’t matter what time of day it is, there’s always somebody in the street and there’s always something open,” he says. “Everything was closed, dead, silent, scary, barren. It was the most surreal thing I can ever remember in my life.”
In the midst of the chaos and confusion, loneliness and isolation replaced communication.
“Everyone was working, working, working, but no one was talking,” Dr. Trosterman says. “When I look back on that day, I feel angry, frustrated, scared, weird....While there weren’t 1,000 people [to save], those 10 or 15 lives that were saved, that were critically ill, were unbelievably important to the doctors who were taking care of them—no one knows about that.”
The Optimist
Ten years ago, Dr. Rizk was director of a three-hospitalist HM service at Beth Israel Medical Center; now the program has 26 FTE hospitalists and 15 physician assistants on staff. She was running late to grand rounds that day, coffee in hand as she passed a television and saw the first news reports of an airplane crashing into the first tower. Moments later, the hospital activated its disaster protocol, and Dr. Rizk rounded up her hospitalists.
“We very rapidly started discharging patients,” she recalls. “I actually went up to the 11th floor of our hospital and could see at that time that the second tower had been hit. It was almost like a dream, like a horrible nightmare. We could see the skyline changing when the first tower dropped. I could hear the sirens and see the smoke that was filling the air.
“We started to create triage stations outside our ED, and we had all the physicians at the hospital available. The ED was pretty chaotic in terms of the throughput. There wasn’t clear instruction; we didn’t know what was happening. ... There was a lot of debris and scratches and fractures that came through our ED.
continued below...
I remember very clearly standing outside of the ED as well, mostly greeting families who were looking for loved ones throughout the course of the day and collecting photographs that we posted on the wall for missing loved ones. And I remember these chilling feelings; there were so few people that were coming in that were in critical condition. I knew that this was not where they would find these patients.”
Beth Israel was not the Level 1 trauma center for lower Manhattan at the time; the now-shuttered St. Vincent’s Hospital was the go-to ED for mass casualty incidents. “They probably got the brunt of those patients, if there were any,” Dr. Rizk says. “I don’t know how many, but I can tell you from the hospitalist standpoint on the inpatient side, there was very little that was done.”
Most of the patients at Beth Israel were wheezing, needing eyewashes, or tending to scrapes and cuts. Dr. Rizk says many of the beds cleared for traumas sat empty. “We were ready, but so little happened in terms of activity on the inpatient side,” she says. “The saddest part really was the faces. I remember a college friend of mine actually coming and looking for his girlfriend’s family member at the time, and I just remember how horrified these family members were going from hospital to hospital throughout the city looking for loved ones.”
In the days and weeks that followed 9/11, Dr. Rizk says, a heavy feeling permeated the city. “Simple things like groceries and shops and restaurants—not that anyone felt like doing that—they just weren’t available,” she says. “Everybody was on foot trying to sort out what happened.”
Her brother-in-law, who worked in the building next to the towers, survived. Others she knew did not. An elementary school friend—a firefighter who rushed into the towers after the attacks—did not make it out. A close friend had an uncle, the head of the Brooklyn fire battalion, who lost his life, too. She attended his funeral.
The months that followed the attacks were “chilling” and “empty,” she says, as the soot covered the community and sorrow pierced those who lived and worked near ground zero.
Since then, Dr. Rizk has watched an “amazing” transformation in lower Manhattan. And it’s not just construction on the new 104-story Freedom Tower or the names of victims etched into the marble fountain walls, but the trees and momentum building for the 10-year anniversary.
“Just to see that renewed hope—it’s exciting,” she says. “I live down there now and am constantly reminded, every day, as I pass ground zero. I am amazed by how resilient the city is. The whole area is coming alive again.”
Dr. Rizk hopes to attend the 9/11 memorial service this month to honor the heroes and applaud New York’s future.
“[It’s] just a symbol of strength and hope for the future of people living together,” she says, “and to recognize that we all have the fundamental human commonality, and we really need to focus on how to move forward as a society—working together as a common goal.”
Jason Carris is editor of The Hospitalist.
They both were working the day the planes crashed into the World Trade Center in New York City. They saw the twin towers crash to the ground, the soot and debris covering lower Manhattan, and the puzzled faces of loved ones searching for information in the EDs of their hospitals. And while the memories are vivid and the shock of the terror still resides in them, they have chosen distinctly different paths since the 9/11 attacks 10 years ago.
Born and raised in Queens, Adam Trosterman, MD, grew up looking at the World Trade Center from his apartment window, studied medicine at Albert Einstein Medical Center in Manhattan, and was the intern on call for trauma surgery at NYU Bellevue the day of the attack. Today, he works as a hospitalist in Colorado and plans to spend Sept. 11 biking in the peaceful altitudes of the Rocky Mountains.
“I will probably go for a bike ride with my wife and enjoy some fresh air,” Dr. Trosterman says. “I don’t plan anything special, but I think about [Sept. 11] and I don’t think about October 11.”
A mere 10 blocks south of NYU Bellevue, straight down First Avenue, Dahlia Rizk, DO, was the hospitalist program director at Beth Israel Medical Center and in the middle of grand rounds when she first heard about the attacks on the twin towers. She has since moved to Battery Park, just a few blocks from the construction site for the new World Trade Center, and plans to participate in the 9/11 anniversary ceremony.
“I think that the memorial, the new building, and that whole area is just coming alive again. It is a real testament to the resilience of New Yorkers. Honoring the victims and their families is just so important. It’s such an incredible thing,” Dr. Rizk says. “I’m looking forward to the remembrance and celebrating the human spirit.”
Two physicians, two hospitalists, two human beings: They look back at 9/11 in diverse yet illuminating ways. These are their stories.
The Intern
A self-described New Yorker, Dr. Trosterman remembers Sept. 11, 2001, as a “beautiful, gorgeous morning” in which the sun was high and the temperature was pleasant. He, however, was in poor spirits, as everyone at NYU Bellevue “hated to be on trauma surgery” rotation. He was 29, single, and, as he puts it, “having a very good time” living in Manhattan.
He arrived at work at 6 a.m. and went about the basic duties of every first-year resident on trauma surgery rotation, rounding with two of his colleagues on 15 patients. At about 8:30 a.m., he ran into another surgery intern who informed him there was a “big trauma coming, something to do with a plane, you might want to check it out.”
Big traumas in New York City are a regular occurrence, and after nearly three months on, he says, he was “pretty well versed in how to run the trauma service. You grow up fast.”
“Back in 2001, you grew up really fast,” he adds. “There were no work-hour regulations; I was working 115 hours per week.”
Dr. Trosterman ran to his trauma slots in the ED—they were “acting weird,” he notes—and began setting up the four trauma beds for the unknown mass-casualty incident (MCI). “It took maybe five minutes,” he says. “Then I got a call from one of my colleagues who was a neurosurgery intern. He starts describing to me what happened, and tells me to come up to the ICU.”
—Adam Trosterman, MD, University of Colorado Denver
Bellevue’s ICU is on the 15th floor, with an unobstructed view of lower Manhattan. When he got there, Dr. Trosterman had a perfect view of the horror at the World Trade Center. “I was like, ‘Oh, my God,’ ” he recalls. “There was a humongous hole in the tower. At that point, I almost started laughing to myself. Not really, of course, but … we had to mobilize a whole different system, which, of course, I was a part of. But it was no longer my typical role for trauma.”
The first patient Dr. Trosterman saw that morning was pronounced dead on arrival. Ironically, he says, the patient looked a lot like his best friend’s stepfather, who worked in the World Trade Center. “They were like parents to me,” he says. “I couldn’t get through. It wasn’t until the second day that I could make a call. I don’t think I spoke to them until Sept. 13.” (Fortunately, everyone Dr. Trosterman knew who worked in the towers survived.)
The next patient Dr. Trosterman saw was a police officer who had a dislocated shoulder and a small fracture. He was screaming and it was difficult to tell if his outbursts were pain-related, Dr. Trosterman says. “He was ranting about what had happened—appropriately ranting,” he says. “He was saying, ‘My partner was at my side and I was trying to save him, but I knew I couldn’t get him out and save myself. I just had to run or I would’ve died. I left my partner to die. I left my partner to die.’ It was horrible. He probably still feels guilty about it right now.”
Contrary to some reports, Dr. Trosterman says, Bellevue and other New York City hospitals were overwhelmed with work, if not injured patients. Much of the work following the attacks was moving inpatients to free up space for casualties. The trauma service ballooned by 40 patients. “We saw more people than we ever see,” he says, “and, literally, the same number of doctors. I was, physically, unbelievably busy. I was emotionally worried about my friend and his family, and I hadn’t had contact with anyone for 48 hours. … I was frustrated that all I kept hearing on the radio was that there were no patients. I was like, ‘You need to come visit me and see what I’m doing!’ It was nonstop and nobody was alive.”

—Dahlia Rizk, DO, hospitalist program director, Beth Israel Medical Center, New York City
Dr. Trosterman cared for dozens of patients on 9/11, working into the wee hours of the night (see “The Most Interesting Patient,” below). He was told to go home at 3:30 a.m. but had to return to work at 6 a.m. He says walking out of the hospital that night was like walking through the morgue.
“Manhattan is one of the most happening places, and downtown Manhattan, it doesn’t matter what time of day it is, there’s always somebody in the street and there’s always something open,” he says. “Everything was closed, dead, silent, scary, barren. It was the most surreal thing I can ever remember in my life.”
In the midst of the chaos and confusion, loneliness and isolation replaced communication.
“Everyone was working, working, working, but no one was talking,” Dr. Trosterman says. “When I look back on that day, I feel angry, frustrated, scared, weird....While there weren’t 1,000 people [to save], those 10 or 15 lives that were saved, that were critically ill, were unbelievably important to the doctors who were taking care of them—no one knows about that.”
The Optimist
Ten years ago, Dr. Rizk was director of a three-hospitalist HM service at Beth Israel Medical Center; now the program has 26 FTE hospitalists and 15 physician assistants on staff. She was running late to grand rounds that day, coffee in hand as she passed a television and saw the first news reports of an airplane crashing into the first tower. Moments later, the hospital activated its disaster protocol, and Dr. Rizk rounded up her hospitalists.
“We very rapidly started discharging patients,” she recalls. “I actually went up to the 11th floor of our hospital and could see at that time that the second tower had been hit. It was almost like a dream, like a horrible nightmare. We could see the skyline changing when the first tower dropped. I could hear the sirens and see the smoke that was filling the air.
“We started to create triage stations outside our ED, and we had all the physicians at the hospital available. The ED was pretty chaotic in terms of the throughput. There wasn’t clear instruction; we didn’t know what was happening. ... There was a lot of debris and scratches and fractures that came through our ED.
continued below...
I remember very clearly standing outside of the ED as well, mostly greeting families who were looking for loved ones throughout the course of the day and collecting photographs that we posted on the wall for missing loved ones. And I remember these chilling feelings; there were so few people that were coming in that were in critical condition. I knew that this was not where they would find these patients.”
Beth Israel was not the Level 1 trauma center for lower Manhattan at the time; the now-shuttered St. Vincent’s Hospital was the go-to ED for mass casualty incidents. “They probably got the brunt of those patients, if there were any,” Dr. Rizk says. “I don’t know how many, but I can tell you from the hospitalist standpoint on the inpatient side, there was very little that was done.”
Most of the patients at Beth Israel were wheezing, needing eyewashes, or tending to scrapes and cuts. Dr. Rizk says many of the beds cleared for traumas sat empty. “We were ready, but so little happened in terms of activity on the inpatient side,” she says. “The saddest part really was the faces. I remember a college friend of mine actually coming and looking for his girlfriend’s family member at the time, and I just remember how horrified these family members were going from hospital to hospital throughout the city looking for loved ones.”
In the days and weeks that followed 9/11, Dr. Rizk says, a heavy feeling permeated the city. “Simple things like groceries and shops and restaurants—not that anyone felt like doing that—they just weren’t available,” she says. “Everybody was on foot trying to sort out what happened.”
Her brother-in-law, who worked in the building next to the towers, survived. Others she knew did not. An elementary school friend—a firefighter who rushed into the towers after the attacks—did not make it out. A close friend had an uncle, the head of the Brooklyn fire battalion, who lost his life, too. She attended his funeral.
The months that followed the attacks were “chilling” and “empty,” she says, as the soot covered the community and sorrow pierced those who lived and worked near ground zero.
Since then, Dr. Rizk has watched an “amazing” transformation in lower Manhattan. And it’s not just construction on the new 104-story Freedom Tower or the names of victims etched into the marble fountain walls, but the trees and momentum building for the 10-year anniversary.
“Just to see that renewed hope—it’s exciting,” she says. “I live down there now and am constantly reminded, every day, as I pass ground zero. I am amazed by how resilient the city is. The whole area is coming alive again.”
Dr. Rizk hopes to attend the 9/11 memorial service this month to honor the heroes and applaud New York’s future.
“[It’s] just a symbol of strength and hope for the future of people living together,” she says, “and to recognize that we all have the fundamental human commonality, and we really need to focus on how to move forward as a society—working together as a common goal.”
Jason Carris is editor of The Hospitalist.
HM@15 - Are You Living Up to High Expectations of Efficiency?
In 2002, a summary article in the Journal of the American Medical Association helped put the relatively small but rapidly growing HM profession on the map. Reviewing the available data, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, of the University of California at San Francisco (UCSF) concluded that implementing a hospitalist program yielded an average savings of 13.4% in hospital costs and a 16.6% reduction in the length of stay (LOS).1
A decade later, the idea of efficiency has become so intertwined with hospitalists that SHM has included the concept in its definition of a profession that now comprises more than 30,000 doctors, nurses, and other care providers. HM practitioners work to enhance hospital and healthcare performance, in part, through “efficient use of hospital and healthcare resources,” according to SHM.
The growth of any profession can create exceptions and outliers, and observers point out that HM programs have become as varied as the hospitals in which they reside, complicating any attempt at broad generalizations. As a core part of the job description, though, efficiency and its implied benefit on costs have been widely promoted as arguments for expanding HM’s reach.
So are hospitalists meeting the lofty expectations?
A Look at the Evidence
A large retrospective study that examined outcomes of care for nearly 77,000 patients in 45 hospitals found that those cared for by hospitalists had a “modestly shorter” stay (by 0.4 days) in the hospital than those cared for by either general internists or family physicians.2 Hospitalists saved about $270 per hospitalization compared with general internists but only about $125 per stay compared with family physicians, the latter of which was not deemed statistically significant.
A more recent review of 33 studies found general agreement that hospitalist care led to reduced costs and length of stay but revealed less uniformity in the impacts on quality and patient outcomes.3
A more dramatic—albeit smaller—affirmation of HM as an efficient force has come from a study of patients admitted to 200-bed Olive View-UCLA Medical Center in Sylmar, Calif. The study, led by assistant medical director Scott Lundberg, MD, concluded that the arrival of an academic hospitalist program led to a one-year increase of $2.3 million in reimbursements from Medi-Cal, California’s Medicaid program.4
“Most other places that have demonstrated the cost-effectiveness of hospitalists generally point to reducing length of stay, which therefore reduces the costs,” Dr. Lundberg says. Under Medicare’s diagnosis-based reimbursement (DRG) system, hospitals could get paid the same amount whether the patient stays one day or five.
Medi-Cal, however, uses a straight-up per diem reimbursement system. “So reducing someone’s length of stay is not necessarily desirable if Medi-Cal would have paid you for all of those days,” Dr. Lundberg says. The state’s Medicare program also can deny coverage for days deemed medically unnecessary after a review of patient charts.
Hospitalists, he says, helped boost revenue in two ways. First, the program helped the hospital avoid denied coverage days by ensuring that patients stayed only as long as necessary. Average LOS, in fact, dropped to 1.92 days from 2.48 days, decreasing the Medi-Cal denial rate to 31.8% (from 43.8%) and bumping up the average reimbursement per inpatient day to $955 from $787.
Hospitalists also helped alleviate the work-hour limits for residents imposed by the Accreditation Council for Graduate Medical Education (ACGME), which had effectively capped the number of inpatients the center could admit. Because Olive View-UCLA receives per diem payments from Medi-Cal, making room to accept more patients into the hospital has meant increased revenues. Among the other benefits, the program has improved patient satisfaction and relieved some of the pressure on teaching teams.
With $310,000 for salary outlay in the hospitalist program’s first year, the study found a net cost benefit of $2 million. “One of the real challenges in getting this hospitalist thing going was getting our administrators to shell out the money for the salaries,” Dr. Lundberg says. The study demonstrated that a hospitalist program not only pays for itself, but also can substantially ramp up revenue. “I’m guessing that others, especially at public hospitals, face the same challenges,” he says. “I’m hoping they can point to this analysis and say, ‘Look, here’s what L.A. County did. They were able to show a net increase in revenue from this hospitalist service.’ ”
On the opposite side of the country, hospitalists are pointing to a success story in pediatric care. At the 120-bed Children’s Hospital at Montefiore at Albert Einstein College of Medicine in the Bronx, N.Y., a recent study concluded that establishing a pediatric HM program led to a significant reduction in LOS for patients with asthma or bronchiolitis.5 Nora Esteban-Cruciani, MD, MS, assistant director of pediatric hospital medicine and lead author of the report, which was presented at HM11, says it’s the first study to demonstrate such an effect for asthma in an inner-city academic setting.
Compared to a traditional resident-attending team, care administered by a resident-physician’s assistant-hospitalist team reduced LOS for bronchiolitis by 15.5% and asthma by 11.8%. With the 378 hospital-bed days saved annually, Children’s Hospital at Montefiore achieved an estimated savings of about $944,000 before taking salaries into account. “We anticipate seeing similar benefits in other groups of patients, and the total savings will far exceed the hospitalist salaries,” Dr. Esteban-Cruciani says.
After the pediatric HM program launched, her study also documented a 17% to 25% decrease in rehospitalizations among asthmatic children at four, six, and 12 months after their initial hospital discharge. As a result of the demonstrated value, Dr. Esteban-Cruciani says, the children’s hospital is expanding its HM program and hiring another 4.5 full-time equivalents.
So how did hospitalists achieve the positive results?
“Knowing the most up-to-date and evidence-based treatment plans, understanding how to use the hospital systems in the most efficient manner, being on the ward for eight to 12 hours per day to respond to issues that arise, as well as 24-hour availability by phone for the residents,” she says. “The day-to-day continuity, as well as the ability to consistently improve systems of care, are distinctive advantages to hospital medicine.”
The case for HM as a model of efficiency comes with a major caveat, however. David Meltzer, MD, PhD, FHM, chief of the section of hospital medicine and an economist and public-policy expert at the University of Chicago, points out that healthcare costs don’t end with a patient’s hospital discharge. Could savings achieved during inpatient care be offset by greater costs afterward?
A new study in the Annals of Internal Medicine by researchers at the University of Texas Medical Branch in Galveston has sharpened that question with the suggestion that, at least in some cases, hospitalist-procured savings might not last.6 When compared to care delivered by primary-care physicians (PCPs), the researchers found that hospitalist care yielded an average inpatient savings of $282 per Medicare beneficiary. But that reduction was wiped out by an extra $332 average cost in the month after discharge, due to higher readmissions, more emergency department visits, and more patients sent to nursing facilities instead of to their own homes. An accompanying editorial raises the uncomfortable question: “Are hospitalists discharging their patients more quickly but less appropriately, such that some of their patients bounce back?”7

—David Meltzer, MD, PhD, FHM, chief, section of hospital medicine, economist, University of Chicago
The study itself has its own share of caveats: Data were collected only until 2006, before reducing 30-day readmissions became a widespread focal point. The editorial also highlights the possibility that hospitalists might care for patients whose weaker relationships with outpatient providers could be the true driver of increased readmissions. In a statement, SHM President Joe Li, MD, SFHM, adds that constructive talks about healthcare costs must include the notion of quality, something the organization has worked to improve with interventions like Project BOOST.
At the very least, the new research highlights the importance of context when considering HM impacts on cost and quality. Separate studies, meanwhile, suggest that the jury is still out on whether other hospitalist-led models can consistently improve outcomes and costs. At academic centers, for instance, work-hour limits for medical residents have provided a strong impetus for joint-care arrangements, such as comanagement systems. A 2004 study found that an orthopedics-hospitalist comanagement structure led to a modest reduction in complications after elective hip and knee surgery. But the report documented no difference in costs or actual length of stay.8
More recently, a study of nearly 7,600 patients at UCSF Medical Center found that an HM-neurosurgery comanagement model had no significant impact on the center’s patient mortality, readmissions, LOS, or patient satisfaction. The comanagement system, however, yielded an average savings of $1,439 per hospitalization and boosted physicians’ perceptions of quality and safety.9
Andrew Auerbach, MD, MPH, SFHM, associate professor of medicine at UCSF Medical Center, says the savings, while not dramatic, nevertheless can add up when applied to the thousands of patients seen by the service every year. “That’s compelling because I think one of the things that you’re arguing when you’re doing these services is what the return on investment is going to be,” he says. “Traditionally, these have been implemented without any specific financial return on investment being applied, but the large expectation that clinical improvement is going to happen.”
His study at UCSF found just the opposite: no clinical improvement but a net cost benefit. “We were a little disappointed in some ways, but in other ways not surprised because there are very few data out in the community that suggest comanagement improves any outcomes,” Dr. Auerbach says. Among complicated neurosurgery patients, the strongest determinants of outcome might be beyond the scope of hospitalist-aided medical care.
With hospitals nervously eyeing their bottom lines, however, any financial improvement that does not adversely affect quality can still be seen as a positive development, and Dr. Auerbach says his study was the first to demonstrate that benefit. At UCSF Medical Center, at least, comanagement has proven compelling enough to spur plans for extending the service to orthopedic surgery patients.
Regardless of the care model, other studies suggest that specific interventions at key moments can yield substantial savings. A small, randomized controlled study led by hospitalists at Johns Hopkins University in Baltimore, for example, supports the idea that “simply showing providers the cost of some diagnostic tests at the time of order entry can affect behavior.”10 Although the study didn’t focus exclusively on hospitalists, experts say they’re in the best position to take the lead in curbing unnecessary costs.
“Hospitalists, I think, have a better understanding of the impact of resource utilization on the total cost of care and can be more prudent in the use of technologies,” says Kenneth Epstein, MD, MBA, FHM, FACP, chief medical officer for Traverse City, Mich.-based Hospitalist Consultants Inc. One reason is that hospitalists aren’t beholden to any specific technology, whether endoscopies or cardiac catheterization.

—Scott Lundberg, MD, assistant medical director, Olive View-UCLA Medical Center, Sylmar, Calif.
Mark Graban, author of the book “Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction,” says hospitalists can play another critical role in controlling costs by mapping out and simplifying the discharge processes. He recalls how hospitalists helped coordinate the effort by one of his hospital clients to prevent discharge delays that would have unnecessarily kept patients in the hospital for an additional night or two.
“That length-of-stay reduction, especially in a fixed-reimbursement setting, can have a huge financial impact,” Graban says. “And, inarguably, it’s the right thing to do for the patient, because it’s patients that are medically ready to be discharged. It gets them home and it reduces their increased risk of picking up infections or being involved in hospital errors.”
Focusing on patient safety could translate into big cost savings under the new Medicare system that penalizes providers for certain hospital-acquired conditions, such as skin ulcers and urinary tract infections, Dr. Epstein says. “There’s an emphasis by hospitalists in understanding the system and being willing to put energy into things like documenting ‘present on admission,’ which then has a huge impact on the hospital,” he says. Close monitoring of patients and developing standardization of care can likewise minimize the risk of conditions, such as catheter-associated infections, from cropping up in the hospital.
Dr. Meltzer says his own research suggests that experienced hospitalists are most effective at controlling costs. “So a program that is structured in such a way as to hire or retain experienced hospitalists is likely to have a higher cost savings than one that doesn’t,” he says.
In a broader sense, the maturation of the HM model and more widespread adoption of effective methods by practitioners might be boosting the overall impact of hospitalist care. A study that examined nearly 2 million Medicare admissions over six years found that the effects of the hospitalist care model on LOS became progressively more pronounced over time, from an average reduction of only 0.02 inpatient days in 2001-2002 to a decrease of 0.35 days by 2005-2006.11
Interestingly, the study’s authors suggest that effects attributable to hospitalists were most pronounced among older, complicated, nonsurgical patients cared for at nonprofit community hospitals.
The Verdict
Despite the variable design and scope of individual programs, experts say, HM’s overall net positive on the efficiency of inpatient care is fairly well documented. Future considerations of hospitalists’ true effects on costs, however, will demand an accounting of healthcare across an entire system, where the HM impact is decidedly less certain. “The right comparison in some sense is, What are the total costs of care for a patient cared for in a system that uses hospitalists versus the totals costs of similar patients cared for in a system that doesn’t use hospitalists?” Dr. Meltzer says.
David Mitchell, MD, PhD, a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, is among those with an additional concern: Providers may not be taking full advantage of their position to control costs.
“The reason is primarily that the reimbursement structure is not set up to incentivize us to cut costs,” he says. Dr. Mitchell, who has worked in 12 hospitals in six states, argues that hospitalists still are too detached from the true price of ordered tests. “That’s what I fear in hospital medicine, that we just become robots: chest pain means CT scan without thinking,” he says. “This just doesn’t make sense.” Dr. Mitchell also contends that the focus of some HM programs on seeing as many patients as possible to maximize reimbursements is leading to less efficiency. At HM11 in May, he met another hospitalist who said he regularly saw 40 to 45 patients every day. “I know there’s absolutely no way you can see that many patients and do an efficient job,” Dr. Mitchell says.
If one of the clearest areas of success for hospitalists has been in reducing length of stay within a hospital, experts acknowledge that it may no longer be enough. “In the new payment model, success is going to be defined differently, and it will be in terms of reducing the total cost of care,” Dr. Meltzer says.
Over the next decade, hospitalists will need to respond to new set of incentives. “And I think one of the really interesting questions will be how hospitalists can best do that, and the extent to which it causes them to rethink the ways in which they organize their practice,” he says.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
- Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3): 248-254.
- Lundberg S, Balingit P, Wali S, Cope D. Cost-effectiveness of a hospitalist service in a public teaching hospital. Acad Med. 2010;85(8):1312-1315.
- Esteban-Cruciani N, Montejo J, Azzarone G, Douglas L, et al. Impact of a pediatric hospital medicine program on resource utilization for children with respiratory disorders. J Hosp Med. 2011;6(4)Supp 2:S27.
- Kuo Y-F, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3): 152-159.
- Chen LM, Saint S. Moments in time. Ann Intern Med. 2011;155(3):194-195.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38.
- Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22): 2004-2010.
- Feldman L, Thiemann D, Brotman D. Financial impact of presenting lab cost data to providers at the time of order entry: a randomized controlled clinical trial. J Hosp Med. 2011;6(4)Supp 2:S93.
- Kuo Y-F, Goodwin JS. Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58:1649-1657.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-8.
- Chandra S, Howell E, Wright S. CICLE: Creating incentives and continuity leading to efficiency. J Hosp Med. 2011;6(4)Supp 2:S17
In 2002, a summary article in the Journal of the American Medical Association helped put the relatively small but rapidly growing HM profession on the map. Reviewing the available data, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, of the University of California at San Francisco (UCSF) concluded that implementing a hospitalist program yielded an average savings of 13.4% in hospital costs and a 16.6% reduction in the length of stay (LOS).1
A decade later, the idea of efficiency has become so intertwined with hospitalists that SHM has included the concept in its definition of a profession that now comprises more than 30,000 doctors, nurses, and other care providers. HM practitioners work to enhance hospital and healthcare performance, in part, through “efficient use of hospital and healthcare resources,” according to SHM.
The growth of any profession can create exceptions and outliers, and observers point out that HM programs have become as varied as the hospitals in which they reside, complicating any attempt at broad generalizations. As a core part of the job description, though, efficiency and its implied benefit on costs have been widely promoted as arguments for expanding HM’s reach.
So are hospitalists meeting the lofty expectations?
A Look at the Evidence
A large retrospective study that examined outcomes of care for nearly 77,000 patients in 45 hospitals found that those cared for by hospitalists had a “modestly shorter” stay (by 0.4 days) in the hospital than those cared for by either general internists or family physicians.2 Hospitalists saved about $270 per hospitalization compared with general internists but only about $125 per stay compared with family physicians, the latter of which was not deemed statistically significant.
A more recent review of 33 studies found general agreement that hospitalist care led to reduced costs and length of stay but revealed less uniformity in the impacts on quality and patient outcomes.3
A more dramatic—albeit smaller—affirmation of HM as an efficient force has come from a study of patients admitted to 200-bed Olive View-UCLA Medical Center in Sylmar, Calif. The study, led by assistant medical director Scott Lundberg, MD, concluded that the arrival of an academic hospitalist program led to a one-year increase of $2.3 million in reimbursements from Medi-Cal, California’s Medicaid program.4
“Most other places that have demonstrated the cost-effectiveness of hospitalists generally point to reducing length of stay, which therefore reduces the costs,” Dr. Lundberg says. Under Medicare’s diagnosis-based reimbursement (DRG) system, hospitals could get paid the same amount whether the patient stays one day or five.
Medi-Cal, however, uses a straight-up per diem reimbursement system. “So reducing someone’s length of stay is not necessarily desirable if Medi-Cal would have paid you for all of those days,” Dr. Lundberg says. The state’s Medicare program also can deny coverage for days deemed medically unnecessary after a review of patient charts.
Hospitalists, he says, helped boost revenue in two ways. First, the program helped the hospital avoid denied coverage days by ensuring that patients stayed only as long as necessary. Average LOS, in fact, dropped to 1.92 days from 2.48 days, decreasing the Medi-Cal denial rate to 31.8% (from 43.8%) and bumping up the average reimbursement per inpatient day to $955 from $787.
Hospitalists also helped alleviate the work-hour limits for residents imposed by the Accreditation Council for Graduate Medical Education (ACGME), which had effectively capped the number of inpatients the center could admit. Because Olive View-UCLA receives per diem payments from Medi-Cal, making room to accept more patients into the hospital has meant increased revenues. Among the other benefits, the program has improved patient satisfaction and relieved some of the pressure on teaching teams.
With $310,000 for salary outlay in the hospitalist program’s first year, the study found a net cost benefit of $2 million. “One of the real challenges in getting this hospitalist thing going was getting our administrators to shell out the money for the salaries,” Dr. Lundberg says. The study demonstrated that a hospitalist program not only pays for itself, but also can substantially ramp up revenue. “I’m guessing that others, especially at public hospitals, face the same challenges,” he says. “I’m hoping they can point to this analysis and say, ‘Look, here’s what L.A. County did. They were able to show a net increase in revenue from this hospitalist service.’ ”
On the opposite side of the country, hospitalists are pointing to a success story in pediatric care. At the 120-bed Children’s Hospital at Montefiore at Albert Einstein College of Medicine in the Bronx, N.Y., a recent study concluded that establishing a pediatric HM program led to a significant reduction in LOS for patients with asthma or bronchiolitis.5 Nora Esteban-Cruciani, MD, MS, assistant director of pediatric hospital medicine and lead author of the report, which was presented at HM11, says it’s the first study to demonstrate such an effect for asthma in an inner-city academic setting.
Compared to a traditional resident-attending team, care administered by a resident-physician’s assistant-hospitalist team reduced LOS for bronchiolitis by 15.5% and asthma by 11.8%. With the 378 hospital-bed days saved annually, Children’s Hospital at Montefiore achieved an estimated savings of about $944,000 before taking salaries into account. “We anticipate seeing similar benefits in other groups of patients, and the total savings will far exceed the hospitalist salaries,” Dr. Esteban-Cruciani says.
After the pediatric HM program launched, her study also documented a 17% to 25% decrease in rehospitalizations among asthmatic children at four, six, and 12 months after their initial hospital discharge. As a result of the demonstrated value, Dr. Esteban-Cruciani says, the children’s hospital is expanding its HM program and hiring another 4.5 full-time equivalents.
So how did hospitalists achieve the positive results?
“Knowing the most up-to-date and evidence-based treatment plans, understanding how to use the hospital systems in the most efficient manner, being on the ward for eight to 12 hours per day to respond to issues that arise, as well as 24-hour availability by phone for the residents,” she says. “The day-to-day continuity, as well as the ability to consistently improve systems of care, are distinctive advantages to hospital medicine.”
The case for HM as a model of efficiency comes with a major caveat, however. David Meltzer, MD, PhD, FHM, chief of the section of hospital medicine and an economist and public-policy expert at the University of Chicago, points out that healthcare costs don’t end with a patient’s hospital discharge. Could savings achieved during inpatient care be offset by greater costs afterward?
A new study in the Annals of Internal Medicine by researchers at the University of Texas Medical Branch in Galveston has sharpened that question with the suggestion that, at least in some cases, hospitalist-procured savings might not last.6 When compared to care delivered by primary-care physicians (PCPs), the researchers found that hospitalist care yielded an average inpatient savings of $282 per Medicare beneficiary. But that reduction was wiped out by an extra $332 average cost in the month after discharge, due to higher readmissions, more emergency department visits, and more patients sent to nursing facilities instead of to their own homes. An accompanying editorial raises the uncomfortable question: “Are hospitalists discharging their patients more quickly but less appropriately, such that some of their patients bounce back?”7

—David Meltzer, MD, PhD, FHM, chief, section of hospital medicine, economist, University of Chicago
The study itself has its own share of caveats: Data were collected only until 2006, before reducing 30-day readmissions became a widespread focal point. The editorial also highlights the possibility that hospitalists might care for patients whose weaker relationships with outpatient providers could be the true driver of increased readmissions. In a statement, SHM President Joe Li, MD, SFHM, adds that constructive talks about healthcare costs must include the notion of quality, something the organization has worked to improve with interventions like Project BOOST.
At the very least, the new research highlights the importance of context when considering HM impacts on cost and quality. Separate studies, meanwhile, suggest that the jury is still out on whether other hospitalist-led models can consistently improve outcomes and costs. At academic centers, for instance, work-hour limits for medical residents have provided a strong impetus for joint-care arrangements, such as comanagement systems. A 2004 study found that an orthopedics-hospitalist comanagement structure led to a modest reduction in complications after elective hip and knee surgery. But the report documented no difference in costs or actual length of stay.8
More recently, a study of nearly 7,600 patients at UCSF Medical Center found that an HM-neurosurgery comanagement model had no significant impact on the center’s patient mortality, readmissions, LOS, or patient satisfaction. The comanagement system, however, yielded an average savings of $1,439 per hospitalization and boosted physicians’ perceptions of quality and safety.9
Andrew Auerbach, MD, MPH, SFHM, associate professor of medicine at UCSF Medical Center, says the savings, while not dramatic, nevertheless can add up when applied to the thousands of patients seen by the service every year. “That’s compelling because I think one of the things that you’re arguing when you’re doing these services is what the return on investment is going to be,” he says. “Traditionally, these have been implemented without any specific financial return on investment being applied, but the large expectation that clinical improvement is going to happen.”
His study at UCSF found just the opposite: no clinical improvement but a net cost benefit. “We were a little disappointed in some ways, but in other ways not surprised because there are very few data out in the community that suggest comanagement improves any outcomes,” Dr. Auerbach says. Among complicated neurosurgery patients, the strongest determinants of outcome might be beyond the scope of hospitalist-aided medical care.
With hospitals nervously eyeing their bottom lines, however, any financial improvement that does not adversely affect quality can still be seen as a positive development, and Dr. Auerbach says his study was the first to demonstrate that benefit. At UCSF Medical Center, at least, comanagement has proven compelling enough to spur plans for extending the service to orthopedic surgery patients.
Regardless of the care model, other studies suggest that specific interventions at key moments can yield substantial savings. A small, randomized controlled study led by hospitalists at Johns Hopkins University in Baltimore, for example, supports the idea that “simply showing providers the cost of some diagnostic tests at the time of order entry can affect behavior.”10 Although the study didn’t focus exclusively on hospitalists, experts say they’re in the best position to take the lead in curbing unnecessary costs.
“Hospitalists, I think, have a better understanding of the impact of resource utilization on the total cost of care and can be more prudent in the use of technologies,” says Kenneth Epstein, MD, MBA, FHM, FACP, chief medical officer for Traverse City, Mich.-based Hospitalist Consultants Inc. One reason is that hospitalists aren’t beholden to any specific technology, whether endoscopies or cardiac catheterization.

—Scott Lundberg, MD, assistant medical director, Olive View-UCLA Medical Center, Sylmar, Calif.
Mark Graban, author of the book “Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction,” says hospitalists can play another critical role in controlling costs by mapping out and simplifying the discharge processes. He recalls how hospitalists helped coordinate the effort by one of his hospital clients to prevent discharge delays that would have unnecessarily kept patients in the hospital for an additional night or two.
“That length-of-stay reduction, especially in a fixed-reimbursement setting, can have a huge financial impact,” Graban says. “And, inarguably, it’s the right thing to do for the patient, because it’s patients that are medically ready to be discharged. It gets them home and it reduces their increased risk of picking up infections or being involved in hospital errors.”
Focusing on patient safety could translate into big cost savings under the new Medicare system that penalizes providers for certain hospital-acquired conditions, such as skin ulcers and urinary tract infections, Dr. Epstein says. “There’s an emphasis by hospitalists in understanding the system and being willing to put energy into things like documenting ‘present on admission,’ which then has a huge impact on the hospital,” he says. Close monitoring of patients and developing standardization of care can likewise minimize the risk of conditions, such as catheter-associated infections, from cropping up in the hospital.
Dr. Meltzer says his own research suggests that experienced hospitalists are most effective at controlling costs. “So a program that is structured in such a way as to hire or retain experienced hospitalists is likely to have a higher cost savings than one that doesn’t,” he says.
In a broader sense, the maturation of the HM model and more widespread adoption of effective methods by practitioners might be boosting the overall impact of hospitalist care. A study that examined nearly 2 million Medicare admissions over six years found that the effects of the hospitalist care model on LOS became progressively more pronounced over time, from an average reduction of only 0.02 inpatient days in 2001-2002 to a decrease of 0.35 days by 2005-2006.11
Interestingly, the study’s authors suggest that effects attributable to hospitalists were most pronounced among older, complicated, nonsurgical patients cared for at nonprofit community hospitals.
The Verdict
Despite the variable design and scope of individual programs, experts say, HM’s overall net positive on the efficiency of inpatient care is fairly well documented. Future considerations of hospitalists’ true effects on costs, however, will demand an accounting of healthcare across an entire system, where the HM impact is decidedly less certain. “The right comparison in some sense is, What are the total costs of care for a patient cared for in a system that uses hospitalists versus the totals costs of similar patients cared for in a system that doesn’t use hospitalists?” Dr. Meltzer says.
David Mitchell, MD, PhD, a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, is among those with an additional concern: Providers may not be taking full advantage of their position to control costs.
“The reason is primarily that the reimbursement structure is not set up to incentivize us to cut costs,” he says. Dr. Mitchell, who has worked in 12 hospitals in six states, argues that hospitalists still are too detached from the true price of ordered tests. “That’s what I fear in hospital medicine, that we just become robots: chest pain means CT scan without thinking,” he says. “This just doesn’t make sense.” Dr. Mitchell also contends that the focus of some HM programs on seeing as many patients as possible to maximize reimbursements is leading to less efficiency. At HM11 in May, he met another hospitalist who said he regularly saw 40 to 45 patients every day. “I know there’s absolutely no way you can see that many patients and do an efficient job,” Dr. Mitchell says.
If one of the clearest areas of success for hospitalists has been in reducing length of stay within a hospital, experts acknowledge that it may no longer be enough. “In the new payment model, success is going to be defined differently, and it will be in terms of reducing the total cost of care,” Dr. Meltzer says.
Over the next decade, hospitalists will need to respond to new set of incentives. “And I think one of the really interesting questions will be how hospitalists can best do that, and the extent to which it causes them to rethink the ways in which they organize their practice,” he says.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
- Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3): 248-254.
- Lundberg S, Balingit P, Wali S, Cope D. Cost-effectiveness of a hospitalist service in a public teaching hospital. Acad Med. 2010;85(8):1312-1315.
- Esteban-Cruciani N, Montejo J, Azzarone G, Douglas L, et al. Impact of a pediatric hospital medicine program on resource utilization for children with respiratory disorders. J Hosp Med. 2011;6(4)Supp 2:S27.
- Kuo Y-F, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3): 152-159.
- Chen LM, Saint S. Moments in time. Ann Intern Med. 2011;155(3):194-195.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38.
- Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22): 2004-2010.
- Feldman L, Thiemann D, Brotman D. Financial impact of presenting lab cost data to providers at the time of order entry: a randomized controlled clinical trial. J Hosp Med. 2011;6(4)Supp 2:S93.
- Kuo Y-F, Goodwin JS. Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58:1649-1657.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-8.
- Chandra S, Howell E, Wright S. CICLE: Creating incentives and continuity leading to efficiency. J Hosp Med. 2011;6(4)Supp 2:S17
In 2002, a summary article in the Journal of the American Medical Association helped put the relatively small but rapidly growing HM profession on the map. Reviewing the available data, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, of the University of California at San Francisco (UCSF) concluded that implementing a hospitalist program yielded an average savings of 13.4% in hospital costs and a 16.6% reduction in the length of stay (LOS).1
A decade later, the idea of efficiency has become so intertwined with hospitalists that SHM has included the concept in its definition of a profession that now comprises more than 30,000 doctors, nurses, and other care providers. HM practitioners work to enhance hospital and healthcare performance, in part, through “efficient use of hospital and healthcare resources,” according to SHM.
The growth of any profession can create exceptions and outliers, and observers point out that HM programs have become as varied as the hospitals in which they reside, complicating any attempt at broad generalizations. As a core part of the job description, though, efficiency and its implied benefit on costs have been widely promoted as arguments for expanding HM’s reach.
So are hospitalists meeting the lofty expectations?
A Look at the Evidence
A large retrospective study that examined outcomes of care for nearly 77,000 patients in 45 hospitals found that those cared for by hospitalists had a “modestly shorter” stay (by 0.4 days) in the hospital than those cared for by either general internists or family physicians.2 Hospitalists saved about $270 per hospitalization compared with general internists but only about $125 per stay compared with family physicians, the latter of which was not deemed statistically significant.
A more recent review of 33 studies found general agreement that hospitalist care led to reduced costs and length of stay but revealed less uniformity in the impacts on quality and patient outcomes.3
A more dramatic—albeit smaller—affirmation of HM as an efficient force has come from a study of patients admitted to 200-bed Olive View-UCLA Medical Center in Sylmar, Calif. The study, led by assistant medical director Scott Lundberg, MD, concluded that the arrival of an academic hospitalist program led to a one-year increase of $2.3 million in reimbursements from Medi-Cal, California’s Medicaid program.4
“Most other places that have demonstrated the cost-effectiveness of hospitalists generally point to reducing length of stay, which therefore reduces the costs,” Dr. Lundberg says. Under Medicare’s diagnosis-based reimbursement (DRG) system, hospitals could get paid the same amount whether the patient stays one day or five.
Medi-Cal, however, uses a straight-up per diem reimbursement system. “So reducing someone’s length of stay is not necessarily desirable if Medi-Cal would have paid you for all of those days,” Dr. Lundberg says. The state’s Medicare program also can deny coverage for days deemed medically unnecessary after a review of patient charts.
Hospitalists, he says, helped boost revenue in two ways. First, the program helped the hospital avoid denied coverage days by ensuring that patients stayed only as long as necessary. Average LOS, in fact, dropped to 1.92 days from 2.48 days, decreasing the Medi-Cal denial rate to 31.8% (from 43.8%) and bumping up the average reimbursement per inpatient day to $955 from $787.
Hospitalists also helped alleviate the work-hour limits for residents imposed by the Accreditation Council for Graduate Medical Education (ACGME), which had effectively capped the number of inpatients the center could admit. Because Olive View-UCLA receives per diem payments from Medi-Cal, making room to accept more patients into the hospital has meant increased revenues. Among the other benefits, the program has improved patient satisfaction and relieved some of the pressure on teaching teams.
With $310,000 for salary outlay in the hospitalist program’s first year, the study found a net cost benefit of $2 million. “One of the real challenges in getting this hospitalist thing going was getting our administrators to shell out the money for the salaries,” Dr. Lundberg says. The study demonstrated that a hospitalist program not only pays for itself, but also can substantially ramp up revenue. “I’m guessing that others, especially at public hospitals, face the same challenges,” he says. “I’m hoping they can point to this analysis and say, ‘Look, here’s what L.A. County did. They were able to show a net increase in revenue from this hospitalist service.’ ”
On the opposite side of the country, hospitalists are pointing to a success story in pediatric care. At the 120-bed Children’s Hospital at Montefiore at Albert Einstein College of Medicine in the Bronx, N.Y., a recent study concluded that establishing a pediatric HM program led to a significant reduction in LOS for patients with asthma or bronchiolitis.5 Nora Esteban-Cruciani, MD, MS, assistant director of pediatric hospital medicine and lead author of the report, which was presented at HM11, says it’s the first study to demonstrate such an effect for asthma in an inner-city academic setting.
Compared to a traditional resident-attending team, care administered by a resident-physician’s assistant-hospitalist team reduced LOS for bronchiolitis by 15.5% and asthma by 11.8%. With the 378 hospital-bed days saved annually, Children’s Hospital at Montefiore achieved an estimated savings of about $944,000 before taking salaries into account. “We anticipate seeing similar benefits in other groups of patients, and the total savings will far exceed the hospitalist salaries,” Dr. Esteban-Cruciani says.
After the pediatric HM program launched, her study also documented a 17% to 25% decrease in rehospitalizations among asthmatic children at four, six, and 12 months after their initial hospital discharge. As a result of the demonstrated value, Dr. Esteban-Cruciani says, the children’s hospital is expanding its HM program and hiring another 4.5 full-time equivalents.
So how did hospitalists achieve the positive results?
“Knowing the most up-to-date and evidence-based treatment plans, understanding how to use the hospital systems in the most efficient manner, being on the ward for eight to 12 hours per day to respond to issues that arise, as well as 24-hour availability by phone for the residents,” she says. “The day-to-day continuity, as well as the ability to consistently improve systems of care, are distinctive advantages to hospital medicine.”
The case for HM as a model of efficiency comes with a major caveat, however. David Meltzer, MD, PhD, FHM, chief of the section of hospital medicine and an economist and public-policy expert at the University of Chicago, points out that healthcare costs don’t end with a patient’s hospital discharge. Could savings achieved during inpatient care be offset by greater costs afterward?
A new study in the Annals of Internal Medicine by researchers at the University of Texas Medical Branch in Galveston has sharpened that question with the suggestion that, at least in some cases, hospitalist-procured savings might not last.6 When compared to care delivered by primary-care physicians (PCPs), the researchers found that hospitalist care yielded an average inpatient savings of $282 per Medicare beneficiary. But that reduction was wiped out by an extra $332 average cost in the month after discharge, due to higher readmissions, more emergency department visits, and more patients sent to nursing facilities instead of to their own homes. An accompanying editorial raises the uncomfortable question: “Are hospitalists discharging their patients more quickly but less appropriately, such that some of their patients bounce back?”7

—David Meltzer, MD, PhD, FHM, chief, section of hospital medicine, economist, University of Chicago
The study itself has its own share of caveats: Data were collected only until 2006, before reducing 30-day readmissions became a widespread focal point. The editorial also highlights the possibility that hospitalists might care for patients whose weaker relationships with outpatient providers could be the true driver of increased readmissions. In a statement, SHM President Joe Li, MD, SFHM, adds that constructive talks about healthcare costs must include the notion of quality, something the organization has worked to improve with interventions like Project BOOST.
At the very least, the new research highlights the importance of context when considering HM impacts on cost and quality. Separate studies, meanwhile, suggest that the jury is still out on whether other hospitalist-led models can consistently improve outcomes and costs. At academic centers, for instance, work-hour limits for medical residents have provided a strong impetus for joint-care arrangements, such as comanagement systems. A 2004 study found that an orthopedics-hospitalist comanagement structure led to a modest reduction in complications after elective hip and knee surgery. But the report documented no difference in costs or actual length of stay.8
More recently, a study of nearly 7,600 patients at UCSF Medical Center found that an HM-neurosurgery comanagement model had no significant impact on the center’s patient mortality, readmissions, LOS, or patient satisfaction. The comanagement system, however, yielded an average savings of $1,439 per hospitalization and boosted physicians’ perceptions of quality and safety.9
Andrew Auerbach, MD, MPH, SFHM, associate professor of medicine at UCSF Medical Center, says the savings, while not dramatic, nevertheless can add up when applied to the thousands of patients seen by the service every year. “That’s compelling because I think one of the things that you’re arguing when you’re doing these services is what the return on investment is going to be,” he says. “Traditionally, these have been implemented without any specific financial return on investment being applied, but the large expectation that clinical improvement is going to happen.”
His study at UCSF found just the opposite: no clinical improvement but a net cost benefit. “We were a little disappointed in some ways, but in other ways not surprised because there are very few data out in the community that suggest comanagement improves any outcomes,” Dr. Auerbach says. Among complicated neurosurgery patients, the strongest determinants of outcome might be beyond the scope of hospitalist-aided medical care.
With hospitals nervously eyeing their bottom lines, however, any financial improvement that does not adversely affect quality can still be seen as a positive development, and Dr. Auerbach says his study was the first to demonstrate that benefit. At UCSF Medical Center, at least, comanagement has proven compelling enough to spur plans for extending the service to orthopedic surgery patients.
Regardless of the care model, other studies suggest that specific interventions at key moments can yield substantial savings. A small, randomized controlled study led by hospitalists at Johns Hopkins University in Baltimore, for example, supports the idea that “simply showing providers the cost of some diagnostic tests at the time of order entry can affect behavior.”10 Although the study didn’t focus exclusively on hospitalists, experts say they’re in the best position to take the lead in curbing unnecessary costs.
“Hospitalists, I think, have a better understanding of the impact of resource utilization on the total cost of care and can be more prudent in the use of technologies,” says Kenneth Epstein, MD, MBA, FHM, FACP, chief medical officer for Traverse City, Mich.-based Hospitalist Consultants Inc. One reason is that hospitalists aren’t beholden to any specific technology, whether endoscopies or cardiac catheterization.

—Scott Lundberg, MD, assistant medical director, Olive View-UCLA Medical Center, Sylmar, Calif.
Mark Graban, author of the book “Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction,” says hospitalists can play another critical role in controlling costs by mapping out and simplifying the discharge processes. He recalls how hospitalists helped coordinate the effort by one of his hospital clients to prevent discharge delays that would have unnecessarily kept patients in the hospital for an additional night or two.
“That length-of-stay reduction, especially in a fixed-reimbursement setting, can have a huge financial impact,” Graban says. “And, inarguably, it’s the right thing to do for the patient, because it’s patients that are medically ready to be discharged. It gets them home and it reduces their increased risk of picking up infections or being involved in hospital errors.”
Focusing on patient safety could translate into big cost savings under the new Medicare system that penalizes providers for certain hospital-acquired conditions, such as skin ulcers and urinary tract infections, Dr. Epstein says. “There’s an emphasis by hospitalists in understanding the system and being willing to put energy into things like documenting ‘present on admission,’ which then has a huge impact on the hospital,” he says. Close monitoring of patients and developing standardization of care can likewise minimize the risk of conditions, such as catheter-associated infections, from cropping up in the hospital.
Dr. Meltzer says his own research suggests that experienced hospitalists are most effective at controlling costs. “So a program that is structured in such a way as to hire or retain experienced hospitalists is likely to have a higher cost savings than one that doesn’t,” he says.
In a broader sense, the maturation of the HM model and more widespread adoption of effective methods by practitioners might be boosting the overall impact of hospitalist care. A study that examined nearly 2 million Medicare admissions over six years found that the effects of the hospitalist care model on LOS became progressively more pronounced over time, from an average reduction of only 0.02 inpatient days in 2001-2002 to a decrease of 0.35 days by 2005-2006.11
Interestingly, the study’s authors suggest that effects attributable to hospitalists were most pronounced among older, complicated, nonsurgical patients cared for at nonprofit community hospitals.
The Verdict
Despite the variable design and scope of individual programs, experts say, HM’s overall net positive on the efficiency of inpatient care is fairly well documented. Future considerations of hospitalists’ true effects on costs, however, will demand an accounting of healthcare across an entire system, where the HM impact is decidedly less certain. “The right comparison in some sense is, What are the total costs of care for a patient cared for in a system that uses hospitalists versus the totals costs of similar patients cared for in a system that doesn’t use hospitalists?” Dr. Meltzer says.
David Mitchell, MD, PhD, a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, is among those with an additional concern: Providers may not be taking full advantage of their position to control costs.
“The reason is primarily that the reimbursement structure is not set up to incentivize us to cut costs,” he says. Dr. Mitchell, who has worked in 12 hospitals in six states, argues that hospitalists still are too detached from the true price of ordered tests. “That’s what I fear in hospital medicine, that we just become robots: chest pain means CT scan without thinking,” he says. “This just doesn’t make sense.” Dr. Mitchell also contends that the focus of some HM programs on seeing as many patients as possible to maximize reimbursements is leading to less efficiency. At HM11 in May, he met another hospitalist who said he regularly saw 40 to 45 patients every day. “I know there’s absolutely no way you can see that many patients and do an efficient job,” Dr. Mitchell says.
If one of the clearest areas of success for hospitalists has been in reducing length of stay within a hospital, experts acknowledge that it may no longer be enough. “In the new payment model, success is going to be defined differently, and it will be in terms of reducing the total cost of care,” Dr. Meltzer says.
Over the next decade, hospitalists will need to respond to new set of incentives. “And I think one of the really interesting questions will be how hospitalists can best do that, and the extent to which it causes them to rethink the ways in which they organize their practice,” he says.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
- Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3): 248-254.
- Lundberg S, Balingit P, Wali S, Cope D. Cost-effectiveness of a hospitalist service in a public teaching hospital. Acad Med. 2010;85(8):1312-1315.
- Esteban-Cruciani N, Montejo J, Azzarone G, Douglas L, et al. Impact of a pediatric hospital medicine program on resource utilization for children with respiratory disorders. J Hosp Med. 2011;6(4)Supp 2:S27.
- Kuo Y-F, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3): 152-159.
- Chen LM, Saint S. Moments in time. Ann Intern Med. 2011;155(3):194-195.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38.
- Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22): 2004-2010.
- Feldman L, Thiemann D, Brotman D. Financial impact of presenting lab cost data to providers at the time of order entry: a randomized controlled clinical trial. J Hosp Med. 2011;6(4)Supp 2:S93.
- Kuo Y-F, Goodwin JS. Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58:1649-1657.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-8.
- Chandra S, Howell E, Wright S. CICLE: Creating incentives and continuity leading to efficiency. J Hosp Med. 2011;6(4)Supp 2:S17
Hospitalists On The Move -- September 2011
Eardly K. Wickramasinghe, MD, has been named the 2011 recipient of the Pennsylvania Medical Society’s Physician Award for Community Voluntary Service. Dr. Wickramasinghe, a general practitioner and hospitalist with Erie Physicians Network who works at Saint Vincent Health Center, was chosen for the award after the statewide organization’s board of trustees voted unanimously to award him the honor. Dr. Wickramasinghe has a long history of volunteerism in the Erie community. He organized the Start Your Day Right breakfast food collection drive. He also initiated the Sheltering the Community program, which created and administered a team of area physicians to conduct bimonthly medical clinics at homeless shelters.
Robert M. Wachter, MD, MHM, professor of medicine at the University of California at San Francisco (UCSF), associate chairman of UCSF’s Department of Medicine, chief of the medical service at UCSF Medical Center, and chief of UCSF’s division of hospital medicine, has been named chair-elect of the American Board of Internal Medicine’s (ABIM) board of directors. He also serves on ABIM’s executive committee.
Hospitalist Adeeb Jaber, MD, recently was chosen as Outstanding Physician of the Year at Anne Arundel Medical Center in Maryland—an honor bestowed by the hospital’s nurses. Dr. Jaber was the top vote-getter out of 1,000 cast by the center’s nurses, who were asked to nominate a doctor considered a role model and who “collaborates and communicates for care.” He has been on staff at the hospital since July 2008.
Tosha B. Wetterneck, MD, MS, FACP, has been named president-elect of the Wisconsin Medical Society. Dr. Wetterneck is associate professor of medicine at the University of Wisconsin School of Medicine and Public Health, and a general internist and hospitalist at UW Hospital and Clinics.
Julie Coffman Barnes, MD, has been named chief medical officer at Redmond Regional Medical Center in Georgia. Dr. Barnes will work alongside hospital personnel in patient safety and quality initiatives as well as evaluation of new clinical programs and technologies.
In Memoriam
Ryan L. Moore, MD, 36, a hospitalist at St. Francis Regional Medical Center in Topeka, Kan., died last month while kayaking along the Kansas River. Dr. Moore had recently accepted an appointment to become chief of staff at the hospital.
Dr. Moore was board-certified in internal medicine and pediatrics, and previously worked with Emergency Medical Services at Cushing Memorial Hospital in Leavenworth, Kan., and Emergency Medicine at Lawrence Memorial Hospital.
Eardly K. Wickramasinghe, MD, has been named the 2011 recipient of the Pennsylvania Medical Society’s Physician Award for Community Voluntary Service. Dr. Wickramasinghe, a general practitioner and hospitalist with Erie Physicians Network who works at Saint Vincent Health Center, was chosen for the award after the statewide organization’s board of trustees voted unanimously to award him the honor. Dr. Wickramasinghe has a long history of volunteerism in the Erie community. He organized the Start Your Day Right breakfast food collection drive. He also initiated the Sheltering the Community program, which created and administered a team of area physicians to conduct bimonthly medical clinics at homeless shelters.
Robert M. Wachter, MD, MHM, professor of medicine at the University of California at San Francisco (UCSF), associate chairman of UCSF’s Department of Medicine, chief of the medical service at UCSF Medical Center, and chief of UCSF’s division of hospital medicine, has been named chair-elect of the American Board of Internal Medicine’s (ABIM) board of directors. He also serves on ABIM’s executive committee.
Hospitalist Adeeb Jaber, MD, recently was chosen as Outstanding Physician of the Year at Anne Arundel Medical Center in Maryland—an honor bestowed by the hospital’s nurses. Dr. Jaber was the top vote-getter out of 1,000 cast by the center’s nurses, who were asked to nominate a doctor considered a role model and who “collaborates and communicates for care.” He has been on staff at the hospital since July 2008.
Tosha B. Wetterneck, MD, MS, FACP, has been named president-elect of the Wisconsin Medical Society. Dr. Wetterneck is associate professor of medicine at the University of Wisconsin School of Medicine and Public Health, and a general internist and hospitalist at UW Hospital and Clinics.
Julie Coffman Barnes, MD, has been named chief medical officer at Redmond Regional Medical Center in Georgia. Dr. Barnes will work alongside hospital personnel in patient safety and quality initiatives as well as evaluation of new clinical programs and technologies.
In Memoriam
Ryan L. Moore, MD, 36, a hospitalist at St. Francis Regional Medical Center in Topeka, Kan., died last month while kayaking along the Kansas River. Dr. Moore had recently accepted an appointment to become chief of staff at the hospital.
Dr. Moore was board-certified in internal medicine and pediatrics, and previously worked with Emergency Medical Services at Cushing Memorial Hospital in Leavenworth, Kan., and Emergency Medicine at Lawrence Memorial Hospital.
Eardly K. Wickramasinghe, MD, has been named the 2011 recipient of the Pennsylvania Medical Society’s Physician Award for Community Voluntary Service. Dr. Wickramasinghe, a general practitioner and hospitalist with Erie Physicians Network who works at Saint Vincent Health Center, was chosen for the award after the statewide organization’s board of trustees voted unanimously to award him the honor. Dr. Wickramasinghe has a long history of volunteerism in the Erie community. He organized the Start Your Day Right breakfast food collection drive. He also initiated the Sheltering the Community program, which created and administered a team of area physicians to conduct bimonthly medical clinics at homeless shelters.
Robert M. Wachter, MD, MHM, professor of medicine at the University of California at San Francisco (UCSF), associate chairman of UCSF’s Department of Medicine, chief of the medical service at UCSF Medical Center, and chief of UCSF’s division of hospital medicine, has been named chair-elect of the American Board of Internal Medicine’s (ABIM) board of directors. He also serves on ABIM’s executive committee.
Hospitalist Adeeb Jaber, MD, recently was chosen as Outstanding Physician of the Year at Anne Arundel Medical Center in Maryland—an honor bestowed by the hospital’s nurses. Dr. Jaber was the top vote-getter out of 1,000 cast by the center’s nurses, who were asked to nominate a doctor considered a role model and who “collaborates and communicates for care.” He has been on staff at the hospital since July 2008.
Tosha B. Wetterneck, MD, MS, FACP, has been named president-elect of the Wisconsin Medical Society. Dr. Wetterneck is associate professor of medicine at the University of Wisconsin School of Medicine and Public Health, and a general internist and hospitalist at UW Hospital and Clinics.
Julie Coffman Barnes, MD, has been named chief medical officer at Redmond Regional Medical Center in Georgia. Dr. Barnes will work alongside hospital personnel in patient safety and quality initiatives as well as evaluation of new clinical programs and technologies.
In Memoriam
Ryan L. Moore, MD, 36, a hospitalist at St. Francis Regional Medical Center in Topeka, Kan., died last month while kayaking along the Kansas River. Dr. Moore had recently accepted an appointment to become chief of staff at the hospital.
Dr. Moore was board-certified in internal medicine and pediatrics, and previously worked with Emergency Medical Services at Cushing Memorial Hospital in Leavenworth, Kan., and Emergency Medicine at Lawrence Memorial Hospital.