User login
HM@15 - Is Hospital Medicine a Good Bet for Improving Patient Satisfaction?
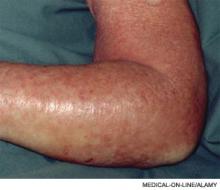
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
At first glance, the deck might seem hopelessly stacked against hospitalists with regard to patient satisfaction. HM practitioners lack the long-term relationship with patients that many primary-care physicians (PCPs) have established. Unlike surgeons and other specialists, they tend to care for those patients—more complicated, lacking a regular doctor, or admitted through the ED, for example—who are more inclined to rate their hospital stay unfavorably.1 They may not even be accurately remembered by patients who encounter multiple doctors during the course of their hospitalization.2 And hospital information systems can misidentify the treating physician, while the actual surveys used to gauge hospitalists have been imperfect at best.3
And yet, the hospitalist model has evolved substantially on the question of how it can impact patient perceptions of care.
Initially, hospitalist champions adopted a largely defensive posture: The model would not negatively impact patient satisfaction as it delivered on efficiency—and later on quality. The healthcare system, however, is beginning to recognize the hospitalist as part of a care “team” whose patient-centered approach might pay big dividends in the inpatient experience and, eventually, on satisfaction scores.
“I think the next phase, which is a focus on the hospitalist as a team member and team builder, is going to be key,” says William Southern, MD, MPH, SFHM, chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y.
Recent studies suggest that hospitalists are helping to design and test new tools that will not only improve satisfaction, but also more fairly assess the impact of individual doctors. As the maturation process continues, experts say, hospitalists have an opportunity to influence both provider-based interventions and more programmatic decision-making that can have far-reaching effects. Certainly, the hand dealt to hospitalists is looking more favorable even as the ante has been raised with Medicare programs like value-based purchasing, and its pot of money tied to patient perceptions of care.
So how have hospitalists played their cards so far?
A Look at the Evidence
In its early years, the HM model faced a persistent criticism: Replacing traditional caregivers with these new inpatient providers in the name of efficiency would increase handoffs and, therefore, discontinuities of care delivered by a succession of unfamiliar faces. If patients didn’t see their PCP in the hospital, the thinking went, they might be more disgruntled at being tended to by hospitalists, leading to lower satisfaction scores.4
A particularly heated exchange played out in 1999 in the New England Journal of Medicine. Farris A. Manian, MD, MPH, of Infectious Disease Consultants in St. Louis wrote in one letter, “I am particularly concerned about what impressionable house-staff members will learn from hospitalists who place an inordinate emphasis on cost rather than the quality of patient care or teaching.”5
A few subsequent studies, however, hinted that such concerns might be overstated. A 2000 analysis in the American Journal of Medicine that examined North Mississippi Health Services in Tupelo, for instance, found that care administered by hospitalists led to a shorter length of stay and lower costs than care delivered by internists. Importantly, the study found that patient satisfaction was similar for both models, while quality metrics were likewise equal or even tilted slightly toward hospitalists.6
In their influential 2002 review of a profession that was only a half-decade old, Robert Wachter, MD, MHM, and Lee Goldman, MD, MPH, FACP from the University of California at San Francisco reinforced the message that HM wouldn’t lead to unhappy patients. “Empirical research supports the premise that hospitalists improve inpatient efficiency without harmful effects on quality or patient satisfaction,” they asserted.7
Among pediatric patients, a 2005 review found that “none of the four studies that evaluated patient satisfaction found statistically significant differences in satisfaction with inpatient care. However, two of the three evaluations that did assess parents’ satisfaction with care provided to their children found that parents were more satisfied with some aspects of care provided by hospitalists.”8

—William Southern, MD, chief, division of hospital medicine, Montefiore Medical Center, Bronx, N.Y.
Similar findings were popping up around the country: Replacing an internal medicine residency program with a physician assistant/hospitalist model at Brooklyn, N.Y.’s Coney Island Hospital did not adversely impact patient satisfaction, while it significantly improved mortality.9 Brigham & Women’s Hospital in Boston likewise reported no change in patient satisfaction in a study comparing a physician assistant/hospitalist service with traditional house staff services.10
The shift toward a more proactive position on patient satisfaction is exemplified within a 2008 white paper, “Hospitalists Meeting the Challenge of Patient Satisfaction,” written by a group of 19 private-practice HM experts known as The Phoenix Group.3 The paper acknowledged the flaws and limitations of existing survey methodologies, including Medicare’s Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores. Even so, the authors urged practice groups to adopt a team-oriented approach to communicate to hospital administrations “the belief that hospitalists are in the best position to improve survey scores overall for the facility.”
Carle Foundation Hospital in Urbana, Ill., is now publicly advertising its HM service’s contribution to high patient satisfaction scores on its website, and underscoring the hospitalists’ consistency, accessibility, and communication skills. “The hospital is never without a hospitalist, and our nurses know that they can rely on them,” says Lynn Barnes, vice president of hospital operations. “They’re available, they’re within a few minutes away, and patients’ needs get met very efficiently and rapidly.”
As a result, she says, their presence can lead to higher scores in patients’ perceptions of communication.
Hospitalists also have been central to several safety initiatives at Carle. Napoleon Knight, MD, medical director of hospital medicine and associate vice president for quality, says the HM team has helped address undiagnosed sleep apnea and implement rapid responses, such as “Code Speed.” Caregivers or family members can use the code to immediately call for help if they detect a downturn in a patient’s condition.
The ongoing initiatives, Dr. Knight and Barnes say, are helping the hospital improve how patients and their loved ones perceive care as Carle adapts to a rapidly shifting healthcare landscape. “With all of the changes that seem to be coming from the external environment weekly, we want to work collaboratively to make sure we’re connected and aligned and communicating in an ongoing fashion so we can react to all of these changes,” Dr. Knight says.
Continued below...
A Hopeful Trend
So far, evidence that the HM model is more broadly raising patient satisfaction scores is largely anecdotal. But a few analyses suggest the trend is moving in the right direction. A recent study in the American Journal of Medical Quality, for instance, concludes that facilities with hospitalists might have an advantage in patient satisfaction with nursing and such personal issues as privacy, emotional needs, and response to complaints.11 The study also posits that teaching facilities employing hospitalists could see benefits in overall satisfaction, while large facilities with hospitalists might see gains in satisfaction with admissions, nursing, and tests and treatments.
Brad Fulton, PhD, a researcher at South Bend, Ind.-based healthcare consulting firm Press Ganey and the study’s lead author, says the 30,000-foot view of patient satisfaction at the facility level can get foggy in a hurry due to differences in the kind and size of hospitalist programs. “And despite all of that fog, we’re still able to see through that and find something,” he says.
One limitation is that the study findings could also reflect differences in the culture of facilities that choose to add hospitalists. That caveat means it might not be possible to completely untangle the effect of an HM group on inpatient care from the larger, hospitalwide values that have allowed the group to set up shop. The wrinkle brings its own fascinating questions, according to Fulton. For example, is that kind of culture necessary for hospitalists to function as well as they do?

—Lynn Barnes, vice president of hospital operations, Carle Foundation Hospital, Urbana, Ill.
Such considerations will become more important as the healthcare system places additional emphasis on patient satisfaction, as Medicare’s value-based purchasing program is doing through its HCAHPS scores. With all the changes, success or failure on the patient experience front is going to carry “not just a reputational import, but also a financial impact,” says Ethan Cumbler, MD, FACP, director of Acute Care for the Elderly (ACE) Service at the University of Colorado Denver.
So how can HM fairly and accurately assess its own practitioners? “I think one starts by trying to apply some of the rigor that we have learned from our experience as hospitalists in quality improvement to the more warm and fuzzy field of patient experience,” Dr. Cumbler says. Many hospitals employ surveys supplied by consultants like Press Ganey to track the global patient satisfaction for their institution, he says.
“But for an individual hospitalist or hospitalist group, that kind of tool often lacks both the specificity and the timeliness necessary to make good decisions about impact of interventions on patient satisfaction,” he says.
Mark Williams, MD, FACP, FHM, professor and chief of the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago, agrees that such imprecision could lead to unfair assessments. “You can imagine a scenario where a patient actually liked their hospitalist very much,” he says, “but when they got the survey, they said [their stay] was terrible and the reasons being because maybe the nurse call button was not answered and the food was terrible and medications were given to them incorrectly, or it was noisy at night so they couldn’t sleep.”
A recent study by Dr. Williams and his colleagues, in which they employed a new assessment method called the Communication Assessment Tool (CAT), confirmed the group’s suspicions: “that the results from the Press Ganey didn’t match up with the CAT, which was a direct assessment of the patient’s perception of the hospitalist’s communication skills,” he says.12
The validated tool, he adds, provides directed feedback to the physician based on the percentage of patients rating that provider as excellent, instead of on the average total score. Hospitalists have felt vindicated by the results. “They were very nervous because the hospital talked about basing an incentive off of the Press Ganey scores, and we said, ‘You can’t do that,’ because we didn’t feel they were accurate, and this study proved that,” Dr. Williams explains.
Fortunately, the message has reached researchers and consultants alike, and better tools are starting to reach hospitals around the country. At HM11 in May, Press Ganey unveiled a new survey designed to help patients assess the care delivered by two hospitalists, the average for inpatient stays. The item set is specific to HM functions, and includes the photo and name of each hospitalist, which Fulton says should improve the validity and accuracy of the data.
“The early response looks really good,” Fulton says, though it’s too early to say whether the tool, called Hospitalist Insight, will live up to its billing. If it proves its mettle, Fulton says, the survey could be used to reward top-performing hospitalists, and the growing dataset could allow hospitals to compare themselves with appropriate peer groups for fairer comparisons.
Meanwhile, researchers are testing out checklists to score hospitalist etiquette, and tracking and paging systems to help ensure continuity of care. They have found increased patient satisfaction when doctors engage in verbal communication during a discharge, in interdisciplinary team rounding, and in efforts to address religious and spiritual concerns.
Since 2000, when Montefiore’s hospitalist program began, Dr. Southern says the hospital has explained to patients the tradeoff accompanying the HM model. “I say something like this to every patient: ‘I know I’m not the doctor that you know, and you’re just meeting me. The downside is that you haven’t met me before and I’m a new face, but the upside is that if you need me during the day, I’m here all the time, I’m not someplace else. And so if you need something, I can be here quickly.’ ”
Being very explicit about that tradeoff, he says, has made patients very comfortable with the model of care, especially during a crisis moment in their lives. “I think it’s really important to say, ‘I know you don’t know me, but here’s the upside.’ And my experience is that patients easily understand that tradeoff and are very positive,” Dr. Southern says.
The Verdict
Available evidence suggests that practitioners of the HM model have pivoted from defending against early criticism that they may harm patient satisfaction to pitching themselves as team leaders who can boost facilitywide perceptions of care. So far, too little research has been conducted to suggest whether that optimism is fully warranted, but early signs look promising.
At facilities like Chicago’s Northwestern Memorial Hospital, medical floors staffed by hospitalists are beginning to beat out surgical floors for the traveling patient satisfaction award. And experts like Dr. Cumbler are pondering how ongoing initiatives to boost scores can follow in the footsteps of efficiency and quality-raising efforts by making the transition from focusing on individual doctors to adopting a more programmatic approach. “What’s happening to that patient during the 23 hours and 45 minutes of their hospital day that you are not sitting by the bedside? And what influence should a hospitalist have in affecting that other 23 hours and 45 minutes?” he says.
Handoffs, discharges, communication with PCPs, and other potential weak points in maintaining high levels of patient satisfaction, Dr. Cumbler says, all are amenable to systems-based improvement. “As hospitalists, we are in a unique position to influence not only our one-one-one interaction with the patient, but also to influence that system of care in a way that patients will notice in a real and tangible way,” he says. “I think we’ve recognized for some time that a healthy heart but a miserable patient is not a healthy person.”
Bryn Nelson is a freelance medical journalist based in Seattle.
References
- Williams M, Flanders SA, Whitcomb WF. Comprehensive hospital medicine: an evidence based approach. Elsevier;2007:971-976.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
- Singer AS, et al. Hospitalists meeting the challenge of patient satisfaction. The Phoenix Group. 2008;1-5.
- Manian FA. Whither continuity of care? N Engl J Med. 1999;340:1362-1363.
- Correspondence. Whither continuity of care? N Engl J Med. 1999;341:850-852.
- Davis KM, Koch KE, Harvey JK, et al. Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system. Amer J Med. 2000;108(8):621-626.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
- Coffman J, Rundall TG. The impact of hospitalists on the cost and quality of inpatient care in the United States (a research synthesis). Med Care Res Rev. 2005;62:379–406.
- Dhuper S, Choksi S. Replacing an academic internal medicine residency program with a physician assistant-hospitalist model: a comparative analysis study. Am J Med Qual. 2009;24(2):132-139.
- Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368.
- Fulton BR, Drevs KE, Ayala LJ, Malott DL Jr. Patient satisfaction with hospitalists: facility-level analyses. Am J Med Qual. 2011;26(2):95-102.
- Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527.
Mark Your Calendar
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists, SHM’s annual meeting is more than an educational conference; it’s an extended family reunion. And with HM12 located in sunny San Diego, the next meeting is a conference, vacation, and family reunion wrapped into one.
Like other family reunions, members of the HM family come to connect with others, catch up on recent experiences, and learn from each other.
“I'm really looking forward to the people,” says HM12 course director Jeff Glasheen, MD, SFHM, associate professor of medicine and director of the hospital medicine group at the University of Colorado Denver. “I attend a lot of CME meetings, and the one thing that sets HM12 apart is the people. It’s a chance for me to reconnect with old friends and make future old friends.”
For hospitalists who are new to SHM or considering going to their first annual meeting, Dr. Glasheen says the experience will be pivotal.
“There simply isn’t a better way to network, learn, and re-energize than coming to the annual meeting,” he says. “I can guarantee first-time attendees will find the annual meeting career-altering. I did, nine years ago, and I hear from new attendees every year that it happens for them as well.”
Registration is open at www.hospitalmedicine2012.org.

—Jeffrey Glasheen, MD, SFHM, HM12 course director
HM12: Off the Beaten Path
In recent years, SHM has presented educational content at the annual meeting in a series of tracks: clinical, academic, pediatric, evidence-based rapid fire, workshops, practice management, and quality. Those tracks help hospitalists identify the courses that will be most pertinent to their careers and daily life.
HM12 introduces a new innovation to the content: pathways. Not all courses fit squarely into the categories presented into the tracks, so pathways give hospitalists the chance to identify the most relevant talks from the different tracks.
To illustrate the pathways concept, Dr. Glasheen uses the example of a hospitalist who is interested in quality improvement (QI). Although there is a quality track, there are quality and safety presentations throughout the conference. The quality pathway will quickly allow the attendee to identify these out of the myriad talks contained in the four-day meeting.”
“Additionally, if you are a nurse practitioner or interested in palliative care, you'll be able to choose the NP or palliative-care pathway to immediately identify the sessions that might be most applicable to you,” he says. “You don't have to go to only those sessions, but the pathways will serve as an easy reference to identify the areas of most interest to you.”
And, in recognition of the broad spectrum of nonclinical topics that hospitalists cover, HM12 will present a “potpourri” track for the first time. This track will help round out the meeting by offering such nonclinical topics as “The History of Hospitals,” “Using Art to Improve Your Clinical Observation Skills,” and “Professionalism in the Digital Age”—topics that will help make the meeting, and hospitalists, more holistic.
Improvements aren’t limited to courses, either. HM12 organizers have split the popular Research, Innovation, and Clinical Vignettes (RIV) poster session into two sessions: one for research and innovations, the other for vignettes. Organizers say this will allow RIV participants more time to review the hundreds of posters presented at the annual meeting.
“We’ve heard the feedback that there just wasn’t enough time to get to the hundreds of posters that were presented at last year’s meeting,” Dr. Glasheen says. “By splitting this into two different sessions, we think this will make the poster sessions that much stronger.”
Networking
SHM’s annual meeting always serves as a forum for enterprising hospitalists to make connections and advance careers. For those hospitalists, HM12 will provide unprecedented time and opportunities to connect with peers and leaders in the specialty.
To many hospitalists, including Dr. Glasheen, the biggest benefit of attending SHM’s annual meeting isn’t the feeling in the conference center—it’s the feeling they take with them.
“Every year, I come away from the meeting reinvigorated and refreshed,” he says. “Much of that comes from the energy I get from spending four days with smart, motivated, and highly engaged hospitalists. It’s the one time every year where I feel firsthand how great it is to be a part of the society—small ‘s’—of hospital medicine.”
That sense of connection is what makes the specialty unique and full of energy, he adds. “These are my colleagues on a national level, this is our field, these people are our present and future, and it’s great to spend some time learning with—and from—all of them.”
Brendon Shank is SHM’s associate vice president of communications.
Survey Insights
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference
Those of you who are familiar with Medical Group Management Association’s reports know that MGMA uses medical group “ownership” categories that are similar to, but slightly different from, the employment model categories historically utilized by SHM. This year, we added the question: “Is your practice part of a multistate hospitalist group or management company?” to the SHM-MGMA Hospital Medicine Supplement. This question enables us to crosswalk from MGMA’s ownership categories to SHM’s traditional employment categories:
- Employed by a hospital or integrated delivery system;
- Employed by a multistate hospitalist group or management company;
- Employed by an independent multispecialty or primary-care medical group;
- Employed by an independent hospitalist-only group;
- Employed by an academic entity; and
- Employed by other.
The blue columns in the chart below show median annual direct compensation (light blue) and retirement benefits (dark blue) for all adult hospitalists by employment model, including the data for academic internal medicine hospitalists from the separate SHM-MGMA academic survey conducted in the fall of 2010.1 The median ratio of compensation to work RVUs for each employment type is represented by red squares.
Academic hospitalists report the lowest compensation but the highest compensation per unit of clinical work, even when production data is standardized to 100% billable clinical time.
“For most academic hospitalists, teaching and supervising residents is an integral part of our clinical work; this probably impedes our clinical efficiency relative to non-academicians,” explains Grace Huang, MD, a member of SHM’s Practice Analysis Committee (PAC). “On weekends, when only half the residents are present and I don’t spend as much time teaching, I can see two to three times more patients.”
Independent hospitalist-only groups saw both the highest direct compensation and the highest compensation per unit of work, while hospitalists employed by multistate groups and management companies had the second-lowest overall direct compensation and the lowest compensation per wRVU.
When including the value of employer retirement plan contributions, however, hospitalists employed by management companies received a combined total remuneration that was higher than for hospitalists employed by hospitals or “other” employers.
“If I’m a hospitalist working for a multistate group, I want to know I’m getting something good that I might not get working for a hospital,” says PAC member Troy Ahlstrom, MD, SFHM. “A better retirement contribution is an obvious example; a hospital can’t afford to give a high-powered retirement plan to all 5,000-plus employees, while a physician company with all ‘highly compensated’ employees can. It’s a perk of working for an independent company.”
Multispecialty/primary-care medical groups and independent hospitalist-only groups provided the highest direct compensation and total remuneration (including retirement contributions). “Keep in mind, though, that they have different responsibilities that come with the money,” Dr. Ahlstrom says. “Hospitalists in local groups have more management responsibilities and more ownership risk, so they should make more for the extra work of running a business. Hospitalists in multispecialty groups have the benefit of an investment in their salaries by their colleagues, but they also have to answer directly to their colleagues for the privilege.”
Leslie Flores, SHM senior advisor, practice management
Reference
Policy Corner
The 2010 Affordable Care Act (ACA) mandates a hospital value-based purchasing (VBP) program to begin this time next year. But hospitalists should start preparing now to be integral parts of the program in their hospitals.
Though the ACA provision states the VBP program for hospital payments will begin with discharges on Oct. 1, 2012, performance on clinical quality and patient experience measures began impacting hospitals’ bottom lines on July 1, 2011. The VBP’s “baseline period” actually lasted from July 1, 2009, through March 31, 2010. The performance period started July 1 and will last through March 31, 2012.
On Aug. 2, 2012, CMS will notify hospitals of estimated performance scores, delivering the actual performance scores on Nov. 1, 2012. The result: Payments for any discharge on or after Oct. 1, 2012 (the beginning of fiscal-year 2013), will be paid based on the performance period currently under way.
Hospitalists and program leaders might wonder how an ACA provision could start before the ACA was passed. The HVBP program actually is a transition of the well-established “Reporting Hospital Quality Data for Annual Payment Update,” or pay-for-reporting program, which in 2003 initially provided a 0.4% payment differential for public reporting through the Hospital Compare website. The 2005 Deficit Reduction Act increased the payment to 2%, and authorized CMS to develop a HVBP plan for FY2009—it just didn’t materialize.
The ACA created the HVBP program with the intention of transforming Medicare from a passive payor to an active purchaser of higher-quality, more efficient healthcare. In essence, Medicare wants to pay for performance rather than simply accurate reporting.
So hospitalists once again are faced with partnering with their hospitals to ensure payout. Reducing a hospital’s base operating Medicare Severity Diagnosis Related Groups (MS-DRG) by the applicable percentage, which will be phased in through 2017 (starting at 1% in 2013 and increasing 0.25% each year), will generate the HVBP’s source of ongoing incentive payments.
To help, SHM this month launched the “Hospital Value-Based Purchasing Toolkit.” It will help hospitalists and hospital executives gain a better understanding of what all the information above really means (including performance measures), and what to expect when your performance scores arrive.
The toolkit is different from any other product SHM has ever produced, as subscribers will be added to their own social collaboration network, similar to a tool like LinkedIn, putting them in touch with our panel of experts and other subscribers across the nation. We also will be putting on a series of roundtables: short presentations from a subject or quality-measure expert, followed by an opportunity to ask questions of our HVBP panel. All of the information will be based on best practices pulled from case studies we have spent the last 12 months scouring the country for. Most important, the best practices will be hospitalist-relevant. The free portal to the toolkit, which includes detailed background information on each piece of the program, can be accessed at www.hospitalmedicine.org/hvbp.
A subscription to the full toolkit can be purchased through the SHM store.
The 2010 Affordable Care Act (ACA) mandates a hospital value-based purchasing (VBP) program to begin this time next year. But hospitalists should start preparing now to be integral parts of the program in their hospitals.
Though the ACA provision states the VBP program for hospital payments will begin with discharges on Oct. 1, 2012, performance on clinical quality and patient experience measures began impacting hospitals’ bottom lines on July 1, 2011. The VBP’s “baseline period” actually lasted from July 1, 2009, through March 31, 2010. The performance period started July 1 and will last through March 31, 2012.
On Aug. 2, 2012, CMS will notify hospitals of estimated performance scores, delivering the actual performance scores on Nov. 1, 2012. The result: Payments for any discharge on or after Oct. 1, 2012 (the beginning of fiscal-year 2013), will be paid based on the performance period currently under way.
Hospitalists and program leaders might wonder how an ACA provision could start before the ACA was passed. The HVBP program actually is a transition of the well-established “Reporting Hospital Quality Data for Annual Payment Update,” or pay-for-reporting program, which in 2003 initially provided a 0.4% payment differential for public reporting through the Hospital Compare website. The 2005 Deficit Reduction Act increased the payment to 2%, and authorized CMS to develop a HVBP plan for FY2009—it just didn’t materialize.
The ACA created the HVBP program with the intention of transforming Medicare from a passive payor to an active purchaser of higher-quality, more efficient healthcare. In essence, Medicare wants to pay for performance rather than simply accurate reporting.
So hospitalists once again are faced with partnering with their hospitals to ensure payout. Reducing a hospital’s base operating Medicare Severity Diagnosis Related Groups (MS-DRG) by the applicable percentage, which will be phased in through 2017 (starting at 1% in 2013 and increasing 0.25% each year), will generate the HVBP’s source of ongoing incentive payments.
To help, SHM this month launched the “Hospital Value-Based Purchasing Toolkit.” It will help hospitalists and hospital executives gain a better understanding of what all the information above really means (including performance measures), and what to expect when your performance scores arrive.
The toolkit is different from any other product SHM has ever produced, as subscribers will be added to their own social collaboration network, similar to a tool like LinkedIn, putting them in touch with our panel of experts and other subscribers across the nation. We also will be putting on a series of roundtables: short presentations from a subject or quality-measure expert, followed by an opportunity to ask questions of our HVBP panel. All of the information will be based on best practices pulled from case studies we have spent the last 12 months scouring the country for. Most important, the best practices will be hospitalist-relevant. The free portal to the toolkit, which includes detailed background information on each piece of the program, can be accessed at www.hospitalmedicine.org/hvbp.
A subscription to the full toolkit can be purchased through the SHM store.
The 2010 Affordable Care Act (ACA) mandates a hospital value-based purchasing (VBP) program to begin this time next year. But hospitalists should start preparing now to be integral parts of the program in their hospitals.
Though the ACA provision states the VBP program for hospital payments will begin with discharges on Oct. 1, 2012, performance on clinical quality and patient experience measures began impacting hospitals’ bottom lines on July 1, 2011. The VBP’s “baseline period” actually lasted from July 1, 2009, through March 31, 2010. The performance period started July 1 and will last through March 31, 2012.
On Aug. 2, 2012, CMS will notify hospitals of estimated performance scores, delivering the actual performance scores on Nov. 1, 2012. The result: Payments for any discharge on or after Oct. 1, 2012 (the beginning of fiscal-year 2013), will be paid based on the performance period currently under way.
Hospitalists and program leaders might wonder how an ACA provision could start before the ACA was passed. The HVBP program actually is a transition of the well-established “Reporting Hospital Quality Data for Annual Payment Update,” or pay-for-reporting program, which in 2003 initially provided a 0.4% payment differential for public reporting through the Hospital Compare website. The 2005 Deficit Reduction Act increased the payment to 2%, and authorized CMS to develop a HVBP plan for FY2009—it just didn’t materialize.
The ACA created the HVBP program with the intention of transforming Medicare from a passive payor to an active purchaser of higher-quality, more efficient healthcare. In essence, Medicare wants to pay for performance rather than simply accurate reporting.
So hospitalists once again are faced with partnering with their hospitals to ensure payout. Reducing a hospital’s base operating Medicare Severity Diagnosis Related Groups (MS-DRG) by the applicable percentage, which will be phased in through 2017 (starting at 1% in 2013 and increasing 0.25% each year), will generate the HVBP’s source of ongoing incentive payments.
To help, SHM this month launched the “Hospital Value-Based Purchasing Toolkit.” It will help hospitalists and hospital executives gain a better understanding of what all the information above really means (including performance measures), and what to expect when your performance scores arrive.
The toolkit is different from any other product SHM has ever produced, as subscribers will be added to their own social collaboration network, similar to a tool like LinkedIn, putting them in touch with our panel of experts and other subscribers across the nation. We also will be putting on a series of roundtables: short presentations from a subject or quality-measure expert, followed by an opportunity to ask questions of our HVBP panel. All of the information will be based on best practices pulled from case studies we have spent the last 12 months scouring the country for. Most important, the best practices will be hospitalist-relevant. The free portal to the toolkit, which includes detailed background information on each piece of the program, can be accessed at www.hospitalmedicine.org/hvbp.
A subscription to the full toolkit can be purchased through the SHM store.
In the Literature: HM-Related Research You Need to Know
In This Edition
Literature At A Glance
A guide to this month’s studies
- PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
- CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
- Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
- CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
- Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
- MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
- New Left Bundle Branch Block Does Not Predict MI
- Acute Beta-Blocker Therapy for MI Increased Risk of Shock
PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
Clinical question: Is percutaneous coronary intervention (PCI) an acceptable alternative to coronary artery bypass grafting (CABG) in unprotected left main coronary artery disease (CAD)?
Background: The current standard of care for unprotected left main CAD is CABG. A sub-study from a large randomized trial suggests that PCI might be an alternative to CABG for patients with left main CAD. Outcomes after the two treatments have not been directly compared in an appropriately powered trial.
Study design: Prospective, open-label, randomized trial powered for noninferiority.
Setting: Thirteen sites in South Korea.
Synopsis: Six hundred patients with newly diagnosed left main disease with >50% stenosis were randomized to PCI with a sirolimus-eluting stent versus CABG. The primary endpoint of major adverse cardiac or cerebrovascular events occurred in 8.7% in the PCI group and 6.7% in the CABG group at one year (absolute risk difference 2 percentage points, 95% CI, -1.6 to 5.6; P=0.01), which was considered noninferior.
However, ischemia-driven target-vessel revascularization occurred in significantly more patients in the PCI group than in the CABG group. The wide noninferiority margin was due to an unexpectedly low rate of events, thus underpowering the study. Also, study duration was only two years.
Bottom line: PCI with a sirolimus-eluting stent was noninferior to CABG for unprotected left main CAD in this study, but the wide noninferiority margin and limited follow-up duration limit clinical application.
Reference: Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727.
CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
Clinical question: What role does coronary-artery bypass grafting (CABG) have in the treatment of patients with both coronary artery disease (CAD) and heart failure?
Background: Although CAD is the most common cause of heart failure, early trials that evaluated the use of CABG in relieving angina excluded patients who had left ventricular (LV) dysfunction with ejection fraction <35%. It is unknown whether CABG adds mortality benefit to intensive medical treatment in patients with CAD and LV dysfunction.
Study design: Multicenter, nonblinded, randomized trial.
Setting: One hundred twenty-seven sites in 26 countries.
Synopsis: From July 2002 to May 2007, 1,212 patients with known CAD amenable to CABG and LV ejection fraction <35% were randomized to medical therapy alone versus CABG plus medical therapy with an average follow-up of five years. The primary outcome of death from any cause occurred in 41% of the medical-therapy-alone group and 36% of the CABG-plus-medical-therapy group (hazard ratio with CABG 0.86; 95% CI 0.72 to 1.04; P=0.12).
Despite subgroup analysis suggesting decreased death rates from cardiovascular causes in the latter group, there was no significant difference in the primary endpoint of death from any cause.
Bottom line: The addition of CABG to medical therapy for patients with CAD and left ventricular dysfunction does not decrease mortality.
Reference: Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616.
Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
Clinical question: Is linezolid superior to glycopeptide antibiotics in the treatment of nosocomial pneumonia?
Background: Current ATS/IDSA guidelines suggest that linezolid might be preferred over glycopeptide antibiotics (i.e. vancomycin and teicoplanin) for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia, although this recommendation is based on a retrospective subgroup analysis of one randomized trial. No systematic reviews have looked at the comparative efficacy and safety of linezolid and glycopeptide antibiotics for nosocomial pneumonia.
Study design: Meta-analysis using a highly sensitive search method.
Setting: Eight multicenter, randomized controlled trials (RCTs).
Synopsis: The study authors retrieved 762 articles with a highly sensitive search strategy, from which eight RCTs were identified that met study criteria for a total of 1,641 patients. Primary outcome of clinical success at test-of-cure was not different between the two classes of antibiotics (pooled RR 1.04, 95% CI 0.97-1.11, P=0.28). Other endpoints, including mortality and microbiologic eradication, were similar between the two groups.
Clinical success in the subgroup of patients with culture-confirmed MRSA pneumonia was not different than those without culture-proven MRSA, although the study was not powered for subgroup analysis. Risk of thrombocytopenia and renal impairment were not statistically different in the limited subgroup of trials reporting this data.
The results should not be generalized to community-acquired MRSA or MRSA pneumonia with characteristics of PVL toxin-producing strain.
Bottom line: For the treatment of nosocomial pneumonia, there was no significant difference in clinical success or mortality between linezolid and glycopeptide antibiotics.
Citation: Walkey AJ, O’Donnell MR, Weiner RS. Linezolid vs. glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2011;139: 1148-1155.
CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
Clinical question: Are biomarkers such as CRP or procalcitonin useful in differentiating pneumonia from asthma or COPD exacerbation in hospitalized patients?
Background: Antibiotic overuse is associated with the emergence of drug resistance. One potential strategy to decrease antibiotic overuse is biomarker-guided therapy. Several randomized controlled trials (RCT) with procalcitonin-guided therapy have resulted in reduced antibiotic use for symptoms of acute respiratory tract infections (RTI). The use of CRP as a biomarker in acute RTI is not as well-described.
Study design: Prospective, observational, diagnostic accuracy study.
Setting: Winter months, 2006 to 2008, in two hospitals in England.
Synopsis: The study examined 319 patients: 62 with pneumonia, 96 with asthma exacerbation, and 161 with COPD exacerbation. Patients with pneumonia had significantly higher procalcitonin and CRP levels than those with COPD (P<0.0001) or asthma (P<0.0001). The area under receiver operator characteristic curve for distinguishing between pneumonia (requiring antibiotics) and asthma exacerbation (not requiring antibiotics) was 0.93 (0.88-0.98) for procalcitonin and 0.96 (0.93-1.00) for CRP. A CRP value >48 mg/L had a sensitivity of 91% (95% CI 80%-97%) and specificity of 93% (95% CI 86-98).
Using this CRP threshold, antibiotic use would have been reduced by 88% in asthma exacerbation, 76% in COPD exacerbation, and 9% in pneumonia cases.
This strategy was developed in a single-center study and requires further validation in a multicenter RCT.
Bottom line: Procalcitonin and CRP were elevated in patients with pneumonia compared to patients with asthma or COPD exacerbation and might be useful in guiding antibiotic usage.
Citation: Bafadhel, M, Clark TW, Reid, C, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410-1418.
Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
Clinical question: How does FOLFIRINOX compare to gemcitabine as first-line treatment of metastatic pancreatic cancer?
Background: Single-agent gemcitabine is the standard first-line treatment for metastatic pancreatic cancer. Preclinical studies followed by Phase 1 and Phase 2 studies have demonstrated response to the oxaliplatin, irinotecan, leucovorin, and fluorouracil regimen (FOLFIRINOX).
Study design: Multicenter, randomized, controlled Phase 2-3 trial.
Setting: Fifteen centers in France during Phase 2, which then expanded to 48 centers for Phase 3.
Synopsis: Three hundred forty-two patients with good performance status (ECOG 0 or 1) and age <76 were randomized to receive FOLFIRINOX or gemcitabine. Median survival in the FOLFIRINOX group was significantly increased, at 11.1 months, compared with 6.8 months in the gemcitabine group (HR 0.57, CI 95%, 0.45-0.73, P<000.1).
Median progression-free survival, objective response rate, and quality of life score at six months were significantly increased in the FOLFIRINOX group. Significantly more grade 3 or grade 4 toxicity was reported in the FOLFIRINOX group.
Patients with elevated bilirubin were excluded due to increased risk of irinotecan-induced toxicity, resulting in only 38% of study patients with carcinoma of the pancreatic head and low proportion of enrolled patients (14.3%) with biliary stents.
Bottom line: FOLFIRONOX was associated with a significant survival advantage compared with single-agent gemcitabine in carefully selected patients with advanced pancreatic cancer, although it was associated with increased toxicity.
Citation: Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
Clinical question: Can nationwide implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, influence healthcare-associated MRSA infection rates?
Background: MRSA is a common cause of nosocomial infection. A pilot project at a single Veterans Affairs (VA) hospital utilized a “MRSA bundle” developed from published guidelines, which resulted in decreased healthcare-associated MRSA infections. In October 2007, the MRSA bundle was implemented throughout VA hospitals nationwide.
Study design: Quality-improvement (QI) observational initiative.
Setting: One hundred fifty-eight acute-care VA hospitals in the U.S.
Synopsis: From October 2007 to June 2010, there were 1,934,598 admissions, transfers, or discharges, and 8,318,675 patient-days. Of this study group, 96% of patients were screened at admission and 93% were screened at transfer or discharge. MRSA colonization or infection at the time of admission was 13.6%. Rates of healthcare-associated MRSA infection declined 45% in the non-ICU setting (0.47 to 0.26 per 1,000 patient-days, P<0.001) and 62% in the ICU setting (1.64 to 0.62 per 1,000 patient days, P<0.001).
It is unclear how much each individual component of the MRSA bundle impacted the declining MRSA infection rate.
Bottom line: Implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, decreased the healthcare-associated MRSA infection rate in a large hospital system.
Citation: Jain R, Kralovi S, Evans M, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430.
New Left Bundle Branch Block Does Not Predict MI
Clinical question: How does the chronicity of left bundle branch block (LBBB) impact diagnosis and outcome in patients undergoing evaluation for acute myocardial infarction (MI)?
Background: ACA/AHA guidelines recommend that patients with new or presumed new LBBB undergo early reperfusion therapy. However, previous studies have shown that a minority of patients with new LBBB are diagnosed with MI.
Study design: Prospective cohort study.
Setting: University hospital in the U.S.
Synopsis: From 1994 to 2009, 401 consecutive patients undergoing evaluation for acute coronary syndrome with LBBB on initial ECG were included in the analysis. Of these patients, 64% had new (37%) or presumably new (27%) LBBB. Twenty-nine percent were diagnosed with MI, but there was no difference in frequency or size of MI between the new, presumably new, or chronic LBBB groups.
Concordant ST-T changes were an independent predictor of MI (OR 17, 95% CI 3.4-81, P<0.001) and mortality (OR 4.3, 95% CI 1.3-15, P<0.001), although this finding was present in only about 11% of the patient group.
Bottom line: Left bundle branch block is not a predictor of MI, although concordant ST-T changes were an independent predictor of MI and mortality.
Citation: Kontos MC, Aziz HA, Chau VQ, et al. Outcomes in patients with chronicity of left bundle-branch block with possible acute myocardial infarction. Am Heart J. 2011;161(4):698-704.
Acute Beta-Blocker Therapy for MI Increased Risk of Shock
Clinical question: How does acute beta-blocker therapy in myocardial infarction (MI) impact outcome?
Background: Long-term treatment with beta-blockers after myocardial infarction (MI) reduces mortality. However, data regarding outcome after acute use of beta-blockers in the first 24 hours of MI is conflicting. Updated ACA/AHA guidelines for STEMI and NSTEMI recommend caution when using beta-blockers in the first 24 hours, particularly in patients at risk for shock.
Study design: Observational registry study.
Setting: Two hundred ninety-one U.S hospitals.
Synopsis: More than 34,600 patients diagnosed with STEMI and NSTEMI from January 2007 to June 2008 were identified from a national QI MI registry. Patients were stratified by guideline-stated risk factors for shock; age >70, HR >110, and systolic BP <120 were associated with increased risk of composite outcome of shock or death.
At least one high-risk factor was present in 63% of the NSTEMI patients and 45% of STEMI patients; however, >90% of these patients received acute beta-blocker therapy. Nearly half (49%) of the NSTEMI patients received beta-blockers in the ED and 62% of the STEMI patients received beta-blockers before PCI.
In a multivariable model, NSTEMI patients receiving beta-blocker therapy in the ED were more likely to develop cardiogenic shock (OR 1.54, 95% CI 1.26-1.88, P<.001), as were STEMI patients receiving beta-blocker therapy prior to PCI (1.40, 95% CI 1.10-1.79, P=.006).
Bottom line: Caution should be exercised when using beta-blocker therapy during acute MI, particularly in the ED or prior to primary PCI.
Citation: Kontos MC, Diercks DB, Ho MP, Wang TY, Chen AY, Roe MT. Treatment and outcomes in patients with myocardial infarction treated with acute beta-blocker therapy: results from the American College of Cardiology’s NCDR. Am Heart J. 2011;161(5):864-870.
In This Edition
Literature At A Glance
A guide to this month’s studies
- PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
- CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
- Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
- CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
- Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
- MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
- New Left Bundle Branch Block Does Not Predict MI
- Acute Beta-Blocker Therapy for MI Increased Risk of Shock
PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
Clinical question: Is percutaneous coronary intervention (PCI) an acceptable alternative to coronary artery bypass grafting (CABG) in unprotected left main coronary artery disease (CAD)?
Background: The current standard of care for unprotected left main CAD is CABG. A sub-study from a large randomized trial suggests that PCI might be an alternative to CABG for patients with left main CAD. Outcomes after the two treatments have not been directly compared in an appropriately powered trial.
Study design: Prospective, open-label, randomized trial powered for noninferiority.
Setting: Thirteen sites in South Korea.
Synopsis: Six hundred patients with newly diagnosed left main disease with >50% stenosis were randomized to PCI with a sirolimus-eluting stent versus CABG. The primary endpoint of major adverse cardiac or cerebrovascular events occurred in 8.7% in the PCI group and 6.7% in the CABG group at one year (absolute risk difference 2 percentage points, 95% CI, -1.6 to 5.6; P=0.01), which was considered noninferior.
However, ischemia-driven target-vessel revascularization occurred in significantly more patients in the PCI group than in the CABG group. The wide noninferiority margin was due to an unexpectedly low rate of events, thus underpowering the study. Also, study duration was only two years.
Bottom line: PCI with a sirolimus-eluting stent was noninferior to CABG for unprotected left main CAD in this study, but the wide noninferiority margin and limited follow-up duration limit clinical application.
Reference: Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727.
CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
Clinical question: What role does coronary-artery bypass grafting (CABG) have in the treatment of patients with both coronary artery disease (CAD) and heart failure?
Background: Although CAD is the most common cause of heart failure, early trials that evaluated the use of CABG in relieving angina excluded patients who had left ventricular (LV) dysfunction with ejection fraction <35%. It is unknown whether CABG adds mortality benefit to intensive medical treatment in patients with CAD and LV dysfunction.
Study design: Multicenter, nonblinded, randomized trial.
Setting: One hundred twenty-seven sites in 26 countries.
Synopsis: From July 2002 to May 2007, 1,212 patients with known CAD amenable to CABG and LV ejection fraction <35% were randomized to medical therapy alone versus CABG plus medical therapy with an average follow-up of five years. The primary outcome of death from any cause occurred in 41% of the medical-therapy-alone group and 36% of the CABG-plus-medical-therapy group (hazard ratio with CABG 0.86; 95% CI 0.72 to 1.04; P=0.12).
Despite subgroup analysis suggesting decreased death rates from cardiovascular causes in the latter group, there was no significant difference in the primary endpoint of death from any cause.
Bottom line: The addition of CABG to medical therapy for patients with CAD and left ventricular dysfunction does not decrease mortality.
Reference: Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616.
Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
Clinical question: Is linezolid superior to glycopeptide antibiotics in the treatment of nosocomial pneumonia?
Background: Current ATS/IDSA guidelines suggest that linezolid might be preferred over glycopeptide antibiotics (i.e. vancomycin and teicoplanin) for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia, although this recommendation is based on a retrospective subgroup analysis of one randomized trial. No systematic reviews have looked at the comparative efficacy and safety of linezolid and glycopeptide antibiotics for nosocomial pneumonia.
Study design: Meta-analysis using a highly sensitive search method.
Setting: Eight multicenter, randomized controlled trials (RCTs).
Synopsis: The study authors retrieved 762 articles with a highly sensitive search strategy, from which eight RCTs were identified that met study criteria for a total of 1,641 patients. Primary outcome of clinical success at test-of-cure was not different between the two classes of antibiotics (pooled RR 1.04, 95% CI 0.97-1.11, P=0.28). Other endpoints, including mortality and microbiologic eradication, were similar between the two groups.
Clinical success in the subgroup of patients with culture-confirmed MRSA pneumonia was not different than those without culture-proven MRSA, although the study was not powered for subgroup analysis. Risk of thrombocytopenia and renal impairment were not statistically different in the limited subgroup of trials reporting this data.
The results should not be generalized to community-acquired MRSA or MRSA pneumonia with characteristics of PVL toxin-producing strain.
Bottom line: For the treatment of nosocomial pneumonia, there was no significant difference in clinical success or mortality between linezolid and glycopeptide antibiotics.
Citation: Walkey AJ, O’Donnell MR, Weiner RS. Linezolid vs. glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2011;139: 1148-1155.
CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
Clinical question: Are biomarkers such as CRP or procalcitonin useful in differentiating pneumonia from asthma or COPD exacerbation in hospitalized patients?
Background: Antibiotic overuse is associated with the emergence of drug resistance. One potential strategy to decrease antibiotic overuse is biomarker-guided therapy. Several randomized controlled trials (RCT) with procalcitonin-guided therapy have resulted in reduced antibiotic use for symptoms of acute respiratory tract infections (RTI). The use of CRP as a biomarker in acute RTI is not as well-described.
Study design: Prospective, observational, diagnostic accuracy study.
Setting: Winter months, 2006 to 2008, in two hospitals in England.
Synopsis: The study examined 319 patients: 62 with pneumonia, 96 with asthma exacerbation, and 161 with COPD exacerbation. Patients with pneumonia had significantly higher procalcitonin and CRP levels than those with COPD (P<0.0001) or asthma (P<0.0001). The area under receiver operator characteristic curve for distinguishing between pneumonia (requiring antibiotics) and asthma exacerbation (not requiring antibiotics) was 0.93 (0.88-0.98) for procalcitonin and 0.96 (0.93-1.00) for CRP. A CRP value >48 mg/L had a sensitivity of 91% (95% CI 80%-97%) and specificity of 93% (95% CI 86-98).
Using this CRP threshold, antibiotic use would have been reduced by 88% in asthma exacerbation, 76% in COPD exacerbation, and 9% in pneumonia cases.
This strategy was developed in a single-center study and requires further validation in a multicenter RCT.
Bottom line: Procalcitonin and CRP were elevated in patients with pneumonia compared to patients with asthma or COPD exacerbation and might be useful in guiding antibiotic usage.
Citation: Bafadhel, M, Clark TW, Reid, C, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410-1418.
Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
Clinical question: How does FOLFIRINOX compare to gemcitabine as first-line treatment of metastatic pancreatic cancer?
Background: Single-agent gemcitabine is the standard first-line treatment for metastatic pancreatic cancer. Preclinical studies followed by Phase 1 and Phase 2 studies have demonstrated response to the oxaliplatin, irinotecan, leucovorin, and fluorouracil regimen (FOLFIRINOX).
Study design: Multicenter, randomized, controlled Phase 2-3 trial.
Setting: Fifteen centers in France during Phase 2, which then expanded to 48 centers for Phase 3.
Synopsis: Three hundred forty-two patients with good performance status (ECOG 0 or 1) and age <76 were randomized to receive FOLFIRINOX or gemcitabine. Median survival in the FOLFIRINOX group was significantly increased, at 11.1 months, compared with 6.8 months in the gemcitabine group (HR 0.57, CI 95%, 0.45-0.73, P<000.1).
Median progression-free survival, objective response rate, and quality of life score at six months were significantly increased in the FOLFIRINOX group. Significantly more grade 3 or grade 4 toxicity was reported in the FOLFIRINOX group.
Patients with elevated bilirubin were excluded due to increased risk of irinotecan-induced toxicity, resulting in only 38% of study patients with carcinoma of the pancreatic head and low proportion of enrolled patients (14.3%) with biliary stents.
Bottom line: FOLFIRONOX was associated with a significant survival advantage compared with single-agent gemcitabine in carefully selected patients with advanced pancreatic cancer, although it was associated with increased toxicity.
Citation: Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
Clinical question: Can nationwide implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, influence healthcare-associated MRSA infection rates?
Background: MRSA is a common cause of nosocomial infection. A pilot project at a single Veterans Affairs (VA) hospital utilized a “MRSA bundle” developed from published guidelines, which resulted in decreased healthcare-associated MRSA infections. In October 2007, the MRSA bundle was implemented throughout VA hospitals nationwide.
Study design: Quality-improvement (QI) observational initiative.
Setting: One hundred fifty-eight acute-care VA hospitals in the U.S.
Synopsis: From October 2007 to June 2010, there were 1,934,598 admissions, transfers, or discharges, and 8,318,675 patient-days. Of this study group, 96% of patients were screened at admission and 93% were screened at transfer or discharge. MRSA colonization or infection at the time of admission was 13.6%. Rates of healthcare-associated MRSA infection declined 45% in the non-ICU setting (0.47 to 0.26 per 1,000 patient-days, P<0.001) and 62% in the ICU setting (1.64 to 0.62 per 1,000 patient days, P<0.001).
It is unclear how much each individual component of the MRSA bundle impacted the declining MRSA infection rate.
Bottom line: Implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, decreased the healthcare-associated MRSA infection rate in a large hospital system.
Citation: Jain R, Kralovi S, Evans M, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430.
New Left Bundle Branch Block Does Not Predict MI
Clinical question: How does the chronicity of left bundle branch block (LBBB) impact diagnosis and outcome in patients undergoing evaluation for acute myocardial infarction (MI)?
Background: ACA/AHA guidelines recommend that patients with new or presumed new LBBB undergo early reperfusion therapy. However, previous studies have shown that a minority of patients with new LBBB are diagnosed with MI.
Study design: Prospective cohort study.
Setting: University hospital in the U.S.
Synopsis: From 1994 to 2009, 401 consecutive patients undergoing evaluation for acute coronary syndrome with LBBB on initial ECG were included in the analysis. Of these patients, 64% had new (37%) or presumably new (27%) LBBB. Twenty-nine percent were diagnosed with MI, but there was no difference in frequency or size of MI between the new, presumably new, or chronic LBBB groups.
Concordant ST-T changes were an independent predictor of MI (OR 17, 95% CI 3.4-81, P<0.001) and mortality (OR 4.3, 95% CI 1.3-15, P<0.001), although this finding was present in only about 11% of the patient group.
Bottom line: Left bundle branch block is not a predictor of MI, although concordant ST-T changes were an independent predictor of MI and mortality.
Citation: Kontos MC, Aziz HA, Chau VQ, et al. Outcomes in patients with chronicity of left bundle-branch block with possible acute myocardial infarction. Am Heart J. 2011;161(4):698-704.
Acute Beta-Blocker Therapy for MI Increased Risk of Shock
Clinical question: How does acute beta-blocker therapy in myocardial infarction (MI) impact outcome?
Background: Long-term treatment with beta-blockers after myocardial infarction (MI) reduces mortality. However, data regarding outcome after acute use of beta-blockers in the first 24 hours of MI is conflicting. Updated ACA/AHA guidelines for STEMI and NSTEMI recommend caution when using beta-blockers in the first 24 hours, particularly in patients at risk for shock.
Study design: Observational registry study.
Setting: Two hundred ninety-one U.S hospitals.
Synopsis: More than 34,600 patients diagnosed with STEMI and NSTEMI from January 2007 to June 2008 were identified from a national QI MI registry. Patients were stratified by guideline-stated risk factors for shock; age >70, HR >110, and systolic BP <120 were associated with increased risk of composite outcome of shock or death.
At least one high-risk factor was present in 63% of the NSTEMI patients and 45% of STEMI patients; however, >90% of these patients received acute beta-blocker therapy. Nearly half (49%) of the NSTEMI patients received beta-blockers in the ED and 62% of the STEMI patients received beta-blockers before PCI.
In a multivariable model, NSTEMI patients receiving beta-blocker therapy in the ED were more likely to develop cardiogenic shock (OR 1.54, 95% CI 1.26-1.88, P<.001), as were STEMI patients receiving beta-blocker therapy prior to PCI (1.40, 95% CI 1.10-1.79, P=.006).
Bottom line: Caution should be exercised when using beta-blocker therapy during acute MI, particularly in the ED or prior to primary PCI.
Citation: Kontos MC, Diercks DB, Ho MP, Wang TY, Chen AY, Roe MT. Treatment and outcomes in patients with myocardial infarction treated with acute beta-blocker therapy: results from the American College of Cardiology’s NCDR. Am Heart J. 2011;161(5):864-870.
In This Edition
Literature At A Glance
A guide to this month’s studies
- PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
- CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
- Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
- CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
- Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
- MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
- New Left Bundle Branch Block Does Not Predict MI
- Acute Beta-Blocker Therapy for MI Increased Risk of Shock
PCI Not Inferior to CABG in Left Main Coronary Artery Stenosis at One Year, But Requires Further Study
Clinical question: Is percutaneous coronary intervention (PCI) an acceptable alternative to coronary artery bypass grafting (CABG) in unprotected left main coronary artery disease (CAD)?
Background: The current standard of care for unprotected left main CAD is CABG. A sub-study from a large randomized trial suggests that PCI might be an alternative to CABG for patients with left main CAD. Outcomes after the two treatments have not been directly compared in an appropriately powered trial.
Study design: Prospective, open-label, randomized trial powered for noninferiority.
Setting: Thirteen sites in South Korea.
Synopsis: Six hundred patients with newly diagnosed left main disease with >50% stenosis were randomized to PCI with a sirolimus-eluting stent versus CABG. The primary endpoint of major adverse cardiac or cerebrovascular events occurred in 8.7% in the PCI group and 6.7% in the CABG group at one year (absolute risk difference 2 percentage points, 95% CI, -1.6 to 5.6; P=0.01), which was considered noninferior.
However, ischemia-driven target-vessel revascularization occurred in significantly more patients in the PCI group than in the CABG group. The wide noninferiority margin was due to an unexpectedly low rate of events, thus underpowering the study. Also, study duration was only two years.
Bottom line: PCI with a sirolimus-eluting stent was noninferior to CABG for unprotected left main CAD in this study, but the wide noninferiority margin and limited follow-up duration limit clinical application.
Reference: Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727.
CABG Did Not Decrease Mortality in Patients with CAD and Left Ventricular Dysfunction
Clinical question: What role does coronary-artery bypass grafting (CABG) have in the treatment of patients with both coronary artery disease (CAD) and heart failure?
Background: Although CAD is the most common cause of heart failure, early trials that evaluated the use of CABG in relieving angina excluded patients who had left ventricular (LV) dysfunction with ejection fraction <35%. It is unknown whether CABG adds mortality benefit to intensive medical treatment in patients with CAD and LV dysfunction.
Study design: Multicenter, nonblinded, randomized trial.
Setting: One hundred twenty-seven sites in 26 countries.
Synopsis: From July 2002 to May 2007, 1,212 patients with known CAD amenable to CABG and LV ejection fraction <35% were randomized to medical therapy alone versus CABG plus medical therapy with an average follow-up of five years. The primary outcome of death from any cause occurred in 41% of the medical-therapy-alone group and 36% of the CABG-plus-medical-therapy group (hazard ratio with CABG 0.86; 95% CI 0.72 to 1.04; P=0.12).
Despite subgroup analysis suggesting decreased death rates from cardiovascular causes in the latter group, there was no significant difference in the primary endpoint of death from any cause.
Bottom line: The addition of CABG to medical therapy for patients with CAD and left ventricular dysfunction does not decrease mortality.
Reference: Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616.
Linezolid Not Superior to Glycopeptide Antibiotics in Treatment of Nosocomial Pneumonia
Clinical question: Is linezolid superior to glycopeptide antibiotics in the treatment of nosocomial pneumonia?
Background: Current ATS/IDSA guidelines suggest that linezolid might be preferred over glycopeptide antibiotics (i.e. vancomycin and teicoplanin) for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia, although this recommendation is based on a retrospective subgroup analysis of one randomized trial. No systematic reviews have looked at the comparative efficacy and safety of linezolid and glycopeptide antibiotics for nosocomial pneumonia.
Study design: Meta-analysis using a highly sensitive search method.
Setting: Eight multicenter, randomized controlled trials (RCTs).
Synopsis: The study authors retrieved 762 articles with a highly sensitive search strategy, from which eight RCTs were identified that met study criteria for a total of 1,641 patients. Primary outcome of clinical success at test-of-cure was not different between the two classes of antibiotics (pooled RR 1.04, 95% CI 0.97-1.11, P=0.28). Other endpoints, including mortality and microbiologic eradication, were similar between the two groups.
Clinical success in the subgroup of patients with culture-confirmed MRSA pneumonia was not different than those without culture-proven MRSA, although the study was not powered for subgroup analysis. Risk of thrombocytopenia and renal impairment were not statistically different in the limited subgroup of trials reporting this data.
The results should not be generalized to community-acquired MRSA or MRSA pneumonia with characteristics of PVL toxin-producing strain.
Bottom line: For the treatment of nosocomial pneumonia, there was no significant difference in clinical success or mortality between linezolid and glycopeptide antibiotics.
Citation: Walkey AJ, O’Donnell MR, Weiner RS. Linezolid vs. glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2011;139: 1148-1155.
CRP and Procalcitonin Independently Differentiated Pneumonia from Asthma or COPD Exacerbation
Clinical question: Are biomarkers such as CRP or procalcitonin useful in differentiating pneumonia from asthma or COPD exacerbation in hospitalized patients?
Background: Antibiotic overuse is associated with the emergence of drug resistance. One potential strategy to decrease antibiotic overuse is biomarker-guided therapy. Several randomized controlled trials (RCT) with procalcitonin-guided therapy have resulted in reduced antibiotic use for symptoms of acute respiratory tract infections (RTI). The use of CRP as a biomarker in acute RTI is not as well-described.
Study design: Prospective, observational, diagnostic accuracy study.
Setting: Winter months, 2006 to 2008, in two hospitals in England.
Synopsis: The study examined 319 patients: 62 with pneumonia, 96 with asthma exacerbation, and 161 with COPD exacerbation. Patients with pneumonia had significantly higher procalcitonin and CRP levels than those with COPD (P<0.0001) or asthma (P<0.0001). The area under receiver operator characteristic curve for distinguishing between pneumonia (requiring antibiotics) and asthma exacerbation (not requiring antibiotics) was 0.93 (0.88-0.98) for procalcitonin and 0.96 (0.93-1.00) for CRP. A CRP value >48 mg/L had a sensitivity of 91% (95% CI 80%-97%) and specificity of 93% (95% CI 86-98).
Using this CRP threshold, antibiotic use would have been reduced by 88% in asthma exacerbation, 76% in COPD exacerbation, and 9% in pneumonia cases.
This strategy was developed in a single-center study and requires further validation in a multicenter RCT.
Bottom line: Procalcitonin and CRP were elevated in patients with pneumonia compared to patients with asthma or COPD exacerbation and might be useful in guiding antibiotic usage.
Citation: Bafadhel, M, Clark TW, Reid, C, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410-1418.
Survival Benefit Demonstrated with FOLFIRINOX in Select Patients with Metastatic Pancreatic Cancer
Clinical question: How does FOLFIRINOX compare to gemcitabine as first-line treatment of metastatic pancreatic cancer?
Background: Single-agent gemcitabine is the standard first-line treatment for metastatic pancreatic cancer. Preclinical studies followed by Phase 1 and Phase 2 studies have demonstrated response to the oxaliplatin, irinotecan, leucovorin, and fluorouracil regimen (FOLFIRINOX).
Study design: Multicenter, randomized, controlled Phase 2-3 trial.
Setting: Fifteen centers in France during Phase 2, which then expanded to 48 centers for Phase 3.
Synopsis: Three hundred forty-two patients with good performance status (ECOG 0 or 1) and age <76 were randomized to receive FOLFIRINOX or gemcitabine. Median survival in the FOLFIRINOX group was significantly increased, at 11.1 months, compared with 6.8 months in the gemcitabine group (HR 0.57, CI 95%, 0.45-0.73, P<000.1).
Median progression-free survival, objective response rate, and quality of life score at six months were significantly increased in the FOLFIRINOX group. Significantly more grade 3 or grade 4 toxicity was reported in the FOLFIRINOX group.
Patients with elevated bilirubin were excluded due to increased risk of irinotecan-induced toxicity, resulting in only 38% of study patients with carcinoma of the pancreatic head and low proportion of enrolled patients (14.3%) with biliary stents.
Bottom line: FOLFIRONOX was associated with a significant survival advantage compared with single-agent gemcitabine in carefully selected patients with advanced pancreatic cancer, although it was associated with increased toxicity.
Citation: Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
MRSA Bundle Implementation at VA Hospitals Reduced Healthcare-Associated MRSA Infections
Clinical question: Can nationwide implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, influence healthcare-associated MRSA infection rates?
Background: MRSA is a common cause of nosocomial infection. A pilot project at a single Veterans Affairs (VA) hospital utilized a “MRSA bundle” developed from published guidelines, which resulted in decreased healthcare-associated MRSA infections. In October 2007, the MRSA bundle was implemented throughout VA hospitals nationwide.
Study design: Quality-improvement (QI) observational initiative.
Setting: One hundred fifty-eight acute-care VA hospitals in the U.S.
Synopsis: From October 2007 to June 2010, there were 1,934,598 admissions, transfers, or discharges, and 8,318,675 patient-days. Of this study group, 96% of patients were screened at admission and 93% were screened at transfer or discharge. MRSA colonization or infection at the time of admission was 13.6%. Rates of healthcare-associated MRSA infection declined 45% in the non-ICU setting (0.47 to 0.26 per 1,000 patient-days, P<0.001) and 62% in the ICU setting (1.64 to 0.62 per 1,000 patient days, P<0.001).
It is unclear how much each individual component of the MRSA bundle impacted the declining MRSA infection rate.
Bottom line: Implementation of a “MRSA bundle,” including universal surveillance, contact isolation, hand hygiene, and institutional culture change, decreased the healthcare-associated MRSA infection rate in a large hospital system.
Citation: Jain R, Kralovi S, Evans M, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430.
New Left Bundle Branch Block Does Not Predict MI
Clinical question: How does the chronicity of left bundle branch block (LBBB) impact diagnosis and outcome in patients undergoing evaluation for acute myocardial infarction (MI)?
Background: ACA/AHA guidelines recommend that patients with new or presumed new LBBB undergo early reperfusion therapy. However, previous studies have shown that a minority of patients with new LBBB are diagnosed with MI.
Study design: Prospective cohort study.
Setting: University hospital in the U.S.
Synopsis: From 1994 to 2009, 401 consecutive patients undergoing evaluation for acute coronary syndrome with LBBB on initial ECG were included in the analysis. Of these patients, 64% had new (37%) or presumably new (27%) LBBB. Twenty-nine percent were diagnosed with MI, but there was no difference in frequency or size of MI between the new, presumably new, or chronic LBBB groups.
Concordant ST-T changes were an independent predictor of MI (OR 17, 95% CI 3.4-81, P<0.001) and mortality (OR 4.3, 95% CI 1.3-15, P<0.001), although this finding was present in only about 11% of the patient group.
Bottom line: Left bundle branch block is not a predictor of MI, although concordant ST-T changes were an independent predictor of MI and mortality.
Citation: Kontos MC, Aziz HA, Chau VQ, et al. Outcomes in patients with chronicity of left bundle-branch block with possible acute myocardial infarction. Am Heart J. 2011;161(4):698-704.
Acute Beta-Blocker Therapy for MI Increased Risk of Shock
Clinical question: How does acute beta-blocker therapy in myocardial infarction (MI) impact outcome?
Background: Long-term treatment with beta-blockers after myocardial infarction (MI) reduces mortality. However, data regarding outcome after acute use of beta-blockers in the first 24 hours of MI is conflicting. Updated ACA/AHA guidelines for STEMI and NSTEMI recommend caution when using beta-blockers in the first 24 hours, particularly in patients at risk for shock.
Study design: Observational registry study.
Setting: Two hundred ninety-one U.S hospitals.
Synopsis: More than 34,600 patients diagnosed with STEMI and NSTEMI from January 2007 to June 2008 were identified from a national QI MI registry. Patients were stratified by guideline-stated risk factors for shock; age >70, HR >110, and systolic BP <120 were associated with increased risk of composite outcome of shock or death.
At least one high-risk factor was present in 63% of the NSTEMI patients and 45% of STEMI patients; however, >90% of these patients received acute beta-blocker therapy. Nearly half (49%) of the NSTEMI patients received beta-blockers in the ED and 62% of the STEMI patients received beta-blockers before PCI.
In a multivariable model, NSTEMI patients receiving beta-blocker therapy in the ED were more likely to develop cardiogenic shock (OR 1.54, 95% CI 1.26-1.88, P<.001), as were STEMI patients receiving beta-blocker therapy prior to PCI (1.40, 95% CI 1.10-1.79, P=.006).
Bottom line: Caution should be exercised when using beta-blocker therapy during acute MI, particularly in the ED or prior to primary PCI.
Citation: Kontos MC, Diercks DB, Ho MP, Wang TY, Chen AY, Roe MT. Treatment and outcomes in patients with myocardial infarction treated with acute beta-blocker therapy: results from the American College of Cardiology’s NCDR. Am Heart J. 2011;161(5):864-870.
What is the best approach to treat an upper-extremity DVT?
Case
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that such active interventions as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT.
However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC.
Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and pumonary embolism.
Dr. Hollberg is an assistant professor of medicine, Emory University School of Medicine, Atlanta, and medical director for information services, Emory Healthcare.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006; 4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
Case
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that such active interventions as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT.
However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC.
Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and pumonary embolism.
Dr. Hollberg is an assistant professor of medicine, Emory University School of Medicine, Atlanta, and medical director for information services, Emory Healthcare.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006; 4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
Case
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that such active interventions as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT.
However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC.
Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and pumonary embolism.
Dr. Hollberg is an assistant professor of medicine, Emory University School of Medicine, Atlanta, and medical director for information services, Emory Healthcare.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006; 4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
Small-Town Tonic
In mid-August, the White House released its “Jobs and Economic Security for Rural America” report (www.whitehouse.gov), which underlines what most hospitalists already know: Rural healthcare is ailing. As the report points out, rural residents are more likely to be uninsured or be covered through public sources, while mortality rates have dropped more slowly in rural areas than in urban ones.
One troubling statistic in particular highlights the disparity in access: In 2008, the report notes, rural counties had 62 primary-care physicians (PCPs) per 100,000 residents, while urban areas counted an average of 79.5 PCPs (28% more). Although a number of initiatives have specifically sought to narrow that gap, a lesser-known dynamic between primary care and HM might be exacerbating the shortage.
Over the past few years, several reports and media accounts have suggested that medical students increasingly want practices that are either hospital-based or office-based, but not both. The presence of hospitalists, then, helps rural facilities create an attractive office-hospital divide and place PCPs in practices frequently owned by the hospital. Hospitalists, in other words, might be necessary prerequisites to help lure and retain PCPs.

—Louis J. O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, P.C., Honesdale, Pa.
Meanwhile, many physicians already in private rural practices are burning out. According to the 2009 Rural Hospitalist Study by the Illinois Critical Access Health Network, “primary-care physicians in rural areas are throwing in the towel of managing their hospitalized patients. More and more, these PCPs unilaterally are announcing to their patients and to the local hospitals they will neither continue to take responsibility for hospitalized patients nor continue to ‘take call.’ ”
Ome Nwanze, MD, one of two hospitalists at the 42-bed Greenville Regional Hospital in Greenville, Ill., says the biggest benefit to being a rural hospitalist is the ability to make a difference in the lives of everyone in the community. Along with patients, Dr. Nwanze includes other doctors as beneficiaries: “The primary-care physicians and specialists are very happy with the program and the difference it makes in their lives.”
Competitive Business
If hospitalists are a natural solution, though, there’s a key problem: Rural communities are struggling to attract them as well. One sign of the difficulty is median salary. Similar to what surveys consistently show for other specialties, rural hospitalists outpace their urban counterparts in median annual salary, at roughly $206,000 versus $187,000, according to Becker’s Hospital Review (overall, hospitalists rank behind most other specialties in salary). The rural-urban divide can be attributed to that old real estate adage: location, location, location. Competition for hospitalist jobs in large cities is generally fierce, while rural communities often have to offer more incentives to attract and retain the doctors they need.
“The two biggest issues that I can see are recruitment and night coverage,” says Louis J. O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine (AIM), P.C., in Honesdale, Pa. He and AIM’s four other hospitalists work exclusively with the town’s 98-bed Wayne Memorial Hospital. “It is easier to recruit to a larger city, closer to more activities and residency programs,” Dr. O’Boyle says. “To get someone to come to our area almost always requires some form of local connection. That makes retention paramount.”
Night call can be a particular sticking point: Most rural hospitals aren’t busy enough to justify an FTE nocturnist, he says, putting the onus of night call on full-time hospitalists. Wayne Memorial Hospital is fortunate in that regard, as it averages only one or two admissions a day after 10 p.m., leaving the hospitalists “fresh enough to round the next day,” Dr. O’Boyle says. “However, this still makes rural programs less attractive compared to places that can boast a nocturnist team that eliminates night call.”
Government Assistance
So what has the government done to help address the growing need for more rural hospitalists and other healthcare providers? If the Affordable Care Act’s (ACA) measures proceed as expected, most experts predict a significant drop in the number of uninsured individuals—meaning a surge in both rural and urban demand for care.
According to the White House report, the Department of Health and Human Services has funded 444 rural community health centers since 2009. The ACA has expanded and extended the Medicare Rural Community Hospital Demonstration, providing “an estimated $52 million in enhanced reimbursement for inpatient services at 25 rural hospitals.” And the administration has expanded funding for the National Health Service Corps, which offers doctors scholarships and loan repayment in exchange for a commitment to practice medicine at underserved communities. The corps website boasts that more than 8,000 clinicians are in place, but it also notes that there are “more than 9,000 job vacancies for NHSC primary care medical, dental, and mental health clinicians.” (View the full report at http://nhsc.hrsa.gov/about.) Clearly, loan forgiveness isn’t enough.
Furthermore, the government might be facing a perception problem. Dr. Nwanze describes government support to rural programs as “poor,” while Dr. O’Boyle says he’s not aware of any specific efforts to support rural hospitalists. “There may be some areas, such as giving grants for telemedicine and other tertiary support, but I don’t think those of us in rural programs can sense any impact,” Dr. O’Boyle says. Wayne Memorial Hospital is in an underserved area, he says, and PCPs there do receive loan forgiveness. “However, I was disappointed to learn that those programs are not open to hospitalists.”
Meanwhile, many rural hospitalists face daunting responsibilities. Dr. Nwanze cites “the need to be a jack-of-all-trades and master of all,” and notes the pressure of providing a wide range of services and handling almost all situations with little or no specialist support.
But Dr. O’Boyle also sees opportunity in the autonomy, such as the ability to play a larger role in hospital management and more independence. “We don’t have a plethora of subspecialists looking for business,” he says. “That means much greater responsibility for our hospitalists, who will take care of much sicker patients without specialist backup being readily available.” As a result, advanced duties like ventilator management and the care of complex patients with such diagnoses as acute renal failure or new malignancies are all within the realm of the hospitalist.
“This is an attractive prospect for certain hospitalists who like the idea of taking care of patients without feeling like a captain who merely delegates to multiple specialists,” Dr. O’Boyle says. “Also, the group integrates into hospital committees at every level, and has an overall much larger say in the day-to-day operations, something largely out of the control of a hospitalist group at a large tertiary facility.”
Tech Solutions
Despite the challenges, many rural hospitals are gaining new tools to help them survive, and tech-savvy hospitalists might be big assets. Smaller facilities are increasingly gaining access to electronic health records, while many also are using video links to allow specialists hundreds of miles away to help with diagnoses without having to transfer the patients.
Recent research also suggests that hospital discharges could be better in rural communities.
Bryn Nelson is a freelance medical writer based in Seattle.
In mid-August, the White House released its “Jobs and Economic Security for Rural America” report (www.whitehouse.gov), which underlines what most hospitalists already know: Rural healthcare is ailing. As the report points out, rural residents are more likely to be uninsured or be covered through public sources, while mortality rates have dropped more slowly in rural areas than in urban ones.
One troubling statistic in particular highlights the disparity in access: In 2008, the report notes, rural counties had 62 primary-care physicians (PCPs) per 100,000 residents, while urban areas counted an average of 79.5 PCPs (28% more). Although a number of initiatives have specifically sought to narrow that gap, a lesser-known dynamic between primary care and HM might be exacerbating the shortage.
Over the past few years, several reports and media accounts have suggested that medical students increasingly want practices that are either hospital-based or office-based, but not both. The presence of hospitalists, then, helps rural facilities create an attractive office-hospital divide and place PCPs in practices frequently owned by the hospital. Hospitalists, in other words, might be necessary prerequisites to help lure and retain PCPs.

—Louis J. O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, P.C., Honesdale, Pa.
Meanwhile, many physicians already in private rural practices are burning out. According to the 2009 Rural Hospitalist Study by the Illinois Critical Access Health Network, “primary-care physicians in rural areas are throwing in the towel of managing their hospitalized patients. More and more, these PCPs unilaterally are announcing to their patients and to the local hospitals they will neither continue to take responsibility for hospitalized patients nor continue to ‘take call.’ ”
Ome Nwanze, MD, one of two hospitalists at the 42-bed Greenville Regional Hospital in Greenville, Ill., says the biggest benefit to being a rural hospitalist is the ability to make a difference in the lives of everyone in the community. Along with patients, Dr. Nwanze includes other doctors as beneficiaries: “The primary-care physicians and specialists are very happy with the program and the difference it makes in their lives.”
Competitive Business
If hospitalists are a natural solution, though, there’s a key problem: Rural communities are struggling to attract them as well. One sign of the difficulty is median salary. Similar to what surveys consistently show for other specialties, rural hospitalists outpace their urban counterparts in median annual salary, at roughly $206,000 versus $187,000, according to Becker’s Hospital Review (overall, hospitalists rank behind most other specialties in salary). The rural-urban divide can be attributed to that old real estate adage: location, location, location. Competition for hospitalist jobs in large cities is generally fierce, while rural communities often have to offer more incentives to attract and retain the doctors they need.
“The two biggest issues that I can see are recruitment and night coverage,” says Louis J. O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine (AIM), P.C., in Honesdale, Pa. He and AIM’s four other hospitalists work exclusively with the town’s 98-bed Wayne Memorial Hospital. “It is easier to recruit to a larger city, closer to more activities and residency programs,” Dr. O’Boyle says. “To get someone to come to our area almost always requires some form of local connection. That makes retention paramount.”
Night call can be a particular sticking point: Most rural hospitals aren’t busy enough to justify an FTE nocturnist, he says, putting the onus of night call on full-time hospitalists. Wayne Memorial Hospital is fortunate in that regard, as it averages only one or two admissions a day after 10 p.m., leaving the hospitalists “fresh enough to round the next day,” Dr. O’Boyle says. “However, this still makes rural programs less attractive compared to places that can boast a nocturnist team that eliminates night call.”
Government Assistance
So what has the government done to help address the growing need for more rural hospitalists and other healthcare providers? If the Affordable Care Act’s (ACA) measures proceed as expected, most experts predict a significant drop in the number of uninsured individuals—meaning a surge in both rural and urban demand for care.
According to the White House report, the Department of Health and Human Services has funded 444 rural community health centers since 2009. The ACA has expanded and extended the Medicare Rural Community Hospital Demonstration, providing “an estimated $52 million in enhanced reimbursement for inpatient services at 25 rural hospitals.” And the administration has expanded funding for the National Health Service Corps, which offers doctors scholarships and loan repayment in exchange for a commitment to practice medicine at underserved communities. The corps website boasts that more than 8,000 clinicians are in place, but it also notes that there are “more than 9,000 job vacancies for NHSC primary care medical, dental, and mental health clinicians.” (View the full report at http://nhsc.hrsa.gov/about.) Clearly, loan forgiveness isn’t enough.
Furthermore, the government might be facing a perception problem. Dr. Nwanze describes government support to rural programs as “poor,” while Dr. O’Boyle says he’s not aware of any specific efforts to support rural hospitalists. “There may be some areas, such as giving grants for telemedicine and other tertiary support, but I don’t think those of us in rural programs can sense any impact,” Dr. O’Boyle says. Wayne Memorial Hospital is in an underserved area, he says, and PCPs there do receive loan forgiveness. “However, I was disappointed to learn that those programs are not open to hospitalists.”
Meanwhile, many rural hospitalists face daunting responsibilities. Dr. Nwanze cites “the need to be a jack-of-all-trades and master of all,” and notes the pressure of providing a wide range of services and handling almost all situations with little or no specialist support.
But Dr. O’Boyle also sees opportunity in the autonomy, such as the ability to play a larger role in hospital management and more independence. “We don’t have a plethora of subspecialists looking for business,” he says. “That means much greater responsibility for our hospitalists, who will take care of much sicker patients without specialist backup being readily available.” As a result, advanced duties like ventilator management and the care of complex patients with such diagnoses as acute renal failure or new malignancies are all within the realm of the hospitalist.
“This is an attractive prospect for certain hospitalists who like the idea of taking care of patients without feeling like a captain who merely delegates to multiple specialists,” Dr. O’Boyle says. “Also, the group integrates into hospital committees at every level, and has an overall much larger say in the day-to-day operations, something largely out of the control of a hospitalist group at a large tertiary facility.”
Tech Solutions
Despite the challenges, many rural hospitals are gaining new tools to help them survive, and tech-savvy hospitalists might be big assets. Smaller facilities are increasingly gaining access to electronic health records, while many also are using video links to allow specialists hundreds of miles away to help with diagnoses without having to transfer the patients.
Recent research also suggests that hospital discharges could be better in rural communities.
Bryn Nelson is a freelance medical writer based in Seattle.
In mid-August, the White House released its “Jobs and Economic Security for Rural America” report (www.whitehouse.gov), which underlines what most hospitalists already know: Rural healthcare is ailing. As the report points out, rural residents are more likely to be uninsured or be covered through public sources, while mortality rates have dropped more slowly in rural areas than in urban ones.
One troubling statistic in particular highlights the disparity in access: In 2008, the report notes, rural counties had 62 primary-care physicians (PCPs) per 100,000 residents, while urban areas counted an average of 79.5 PCPs (28% more). Although a number of initiatives have specifically sought to narrow that gap, a lesser-known dynamic between primary care and HM might be exacerbating the shortage.
Over the past few years, several reports and media accounts have suggested that medical students increasingly want practices that are either hospital-based or office-based, but not both. The presence of hospitalists, then, helps rural facilities create an attractive office-hospital divide and place PCPs in practices frequently owned by the hospital. Hospitalists, in other words, might be necessary prerequisites to help lure and retain PCPs.

—Louis J. O’Boyle, DO, FACP, FHM, medical director, Advanced Inpatient Medicine, P.C., Honesdale, Pa.
Meanwhile, many physicians already in private rural practices are burning out. According to the 2009 Rural Hospitalist Study by the Illinois Critical Access Health Network, “primary-care physicians in rural areas are throwing in the towel of managing their hospitalized patients. More and more, these PCPs unilaterally are announcing to their patients and to the local hospitals they will neither continue to take responsibility for hospitalized patients nor continue to ‘take call.’ ”
Ome Nwanze, MD, one of two hospitalists at the 42-bed Greenville Regional Hospital in Greenville, Ill., says the biggest benefit to being a rural hospitalist is the ability to make a difference in the lives of everyone in the community. Along with patients, Dr. Nwanze includes other doctors as beneficiaries: “The primary-care physicians and specialists are very happy with the program and the difference it makes in their lives.”
Competitive Business
If hospitalists are a natural solution, though, there’s a key problem: Rural communities are struggling to attract them as well. One sign of the difficulty is median salary. Similar to what surveys consistently show for other specialties, rural hospitalists outpace their urban counterparts in median annual salary, at roughly $206,000 versus $187,000, according to Becker’s Hospital Review (overall, hospitalists rank behind most other specialties in salary). The rural-urban divide can be attributed to that old real estate adage: location, location, location. Competition for hospitalist jobs in large cities is generally fierce, while rural communities often have to offer more incentives to attract and retain the doctors they need.
“The two biggest issues that I can see are recruitment and night coverage,” says Louis J. O’Boyle, DO, FACP, FHM, medical director of Advanced Inpatient Medicine (AIM), P.C., in Honesdale, Pa. He and AIM’s four other hospitalists work exclusively with the town’s 98-bed Wayne Memorial Hospital. “It is easier to recruit to a larger city, closer to more activities and residency programs,” Dr. O’Boyle says. “To get someone to come to our area almost always requires some form of local connection. That makes retention paramount.”
Night call can be a particular sticking point: Most rural hospitals aren’t busy enough to justify an FTE nocturnist, he says, putting the onus of night call on full-time hospitalists. Wayne Memorial Hospital is fortunate in that regard, as it averages only one or two admissions a day after 10 p.m., leaving the hospitalists “fresh enough to round the next day,” Dr. O’Boyle says. “However, this still makes rural programs less attractive compared to places that can boast a nocturnist team that eliminates night call.”
Government Assistance
So what has the government done to help address the growing need for more rural hospitalists and other healthcare providers? If the Affordable Care Act’s (ACA) measures proceed as expected, most experts predict a significant drop in the number of uninsured individuals—meaning a surge in both rural and urban demand for care.
According to the White House report, the Department of Health and Human Services has funded 444 rural community health centers since 2009. The ACA has expanded and extended the Medicare Rural Community Hospital Demonstration, providing “an estimated $52 million in enhanced reimbursement for inpatient services at 25 rural hospitals.” And the administration has expanded funding for the National Health Service Corps, which offers doctors scholarships and loan repayment in exchange for a commitment to practice medicine at underserved communities. The corps website boasts that more than 8,000 clinicians are in place, but it also notes that there are “more than 9,000 job vacancies for NHSC primary care medical, dental, and mental health clinicians.” (View the full report at http://nhsc.hrsa.gov/about.) Clearly, loan forgiveness isn’t enough.
Furthermore, the government might be facing a perception problem. Dr. Nwanze describes government support to rural programs as “poor,” while Dr. O’Boyle says he’s not aware of any specific efforts to support rural hospitalists. “There may be some areas, such as giving grants for telemedicine and other tertiary support, but I don’t think those of us in rural programs can sense any impact,” Dr. O’Boyle says. Wayne Memorial Hospital is in an underserved area, he says, and PCPs there do receive loan forgiveness. “However, I was disappointed to learn that those programs are not open to hospitalists.”
Meanwhile, many rural hospitalists face daunting responsibilities. Dr. Nwanze cites “the need to be a jack-of-all-trades and master of all,” and notes the pressure of providing a wide range of services and handling almost all situations with little or no specialist support.
But Dr. O’Boyle also sees opportunity in the autonomy, such as the ability to play a larger role in hospital management and more independence. “We don’t have a plethora of subspecialists looking for business,” he says. “That means much greater responsibility for our hospitalists, who will take care of much sicker patients without specialist backup being readily available.” As a result, advanced duties like ventilator management and the care of complex patients with such diagnoses as acute renal failure or new malignancies are all within the realm of the hospitalist.
“This is an attractive prospect for certain hospitalists who like the idea of taking care of patients without feeling like a captain who merely delegates to multiple specialists,” Dr. O’Boyle says. “Also, the group integrates into hospital committees at every level, and has an overall much larger say in the day-to-day operations, something largely out of the control of a hospitalist group at a large tertiary facility.”
Tech Solutions
Despite the challenges, many rural hospitals are gaining new tools to help them survive, and tech-savvy hospitalists might be big assets. Smaller facilities are increasingly gaining access to electronic health records, while many also are using video links to allow specialists hundreds of miles away to help with diagnoses without having to transfer the patients.
Recent research also suggests that hospital discharges could be better in rural communities.
Bryn Nelson is a freelance medical writer based in Seattle.
A Brief History
Each visit category and level of service has corresponding documentation requirements.1 Selecting an evaluation and management (E/M) level is based upon 1) the content of the three “key” components: history, exam, and decision-making, or 2) time, but only when counseling or coordination of care dominates more than 50% of the physician’s total visit time. Failure to document any essential element in a given visit level (e.g. family history required but missing for 99222 and 99223) could result in downcoding or service denial. Be aware of what an auditor expects when reviewing the key component of “history.”
Documentation Options
Auditors recognize two sets of documentation guidelines: “1995” and “1997” guidelines.2,3,4 Each set of guidelines has received valid criticism. The 1995 guidelines undoubtedly are vague and subjective in some areas, whereas the 1997 guidelines are known for arduous specificity.
However, to benefit all physicians and specialties, both sets of guidelines apply to visit-level selection. In other words, physicians can utilize either set when documenting their services, and auditors must review provider records against both styles. The final audited outcome reflects the highest visit level supported upon comparison.
Elements of History2,3,4
Chief complaint. The chief complaint (CC) is the reason for the visit, as stated in the patient’s own words. Every encounter, regardless of visit type, must include a CC. The physician must personally document and/or validate the CC with reference to a specific condition or symptom (e.g. patient complains of abdominal pain).
History of present illness (HPI). The HPI is a description of the patient’s present illness as it developed. It characteristically is referenced as location, quality, severity, timing, context, modifying factors, and associated signs/symptoms, as related to the chief complaint. The 1997 guidelines allow physicians to receive HPI credit for providing the status of the patient’s chronic or inactive conditions, such as “extrinsic asthma without acute exacerbation in past six months.” An auditor will not assign HPI credit to a chronic or inactive condition that does not have a corresponding status (e.g. “asthma”). This will be considered “past medical history.”
The HPI is classified as brief (a comment on <3 HPI elements, or the status of <2 conditions) or extended (a comment on >4 HPI elements, or the status of >3 conditions). Consider these examples of an extended HPI:
- “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
- “Diabetes controlled by oral medication; hyperlipidemia stable on simvastatin with increased dietary efforts; hypertension stable with pressures ranging from 130-140/80-90.” (Status of three chronic conditions.)
Physicians receive credit for confirming and personally documenting the HPI, or linking to documentation recorded by residents (residents, fellows, interns) or nonphysician providers (NPPs) when performing services according to the Teaching Physician Rules or Split-Shared Billing Rules, respectively. An auditor will not assign physician credit for HPI elements documented by ancillary staff (registered nurses, medical assistants) or students.
Review of systems (ROS). The ROS is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient: constitutional; eyes, ears, nose, mouth, throat; cardiovascular; respiratory; gastrointestinal; genitourinary; musculoskeletal; integumentary (including skin and/or breast); neurological; psychiatric; endocrine; hematologic/ lymphatic; and allergic/immunologic. Auditors classify the ROS as brief (a comment on one system), extended (a comment on two to nine systems), or complete (a comment on >10 systems). Physicians can document a complete ROS by noting individual systems: “no fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular) or shortness of breath (respiratory); intermittent nausea (gastrointestinal); and occasional runny nose (ears, nose, mouth, throat),” or by eliciting a complete system review but documenting only the positive and pertinent negative findings related to the chief complaint, along with an additional comment that “all other systems are negative.”
Although the latter method is formally included in Medicare’s documentation guidelines and accepted by some Medicare contractors (e.g. Highmark, WPS), be aware that it is not universally accepted.5,6
Documentation involving the ROS can be provided by anyone, including the patient. The physician should reference ROS information that is completed by individuals other than residents or NPPs during services provided under the Teaching Physician Rules or Split-Shared Billing Rules. Physician duplication of ROS information is unnecessary unless an update or revision is required.
Past, family, and social history (PFSH). The PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH could be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). The physician merely needs a single comment associated with each history for the PFSH to be regarded as complete. Refrain from using “noncontributory” to describe any of the histories, as previous misuse of this term has resulted in its prohibition. An example of a complete PFSH documentation includes: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
Similar to the ROS, PFSH documentation can be provided by anyone, including the patient, and the physician should reference the documented PFSH in his own progress note. Redocumentation of the PFSH is not necessary unless a revision is required.
PFSH documentation is only required for initial care services (i.e. initial hospital care, initial observation care, consultations). It is not warranted in subsequent care services unless additional, pertinent information is obtained during the hospital stay that impacts care.
Considerations
When a physician cannot elicit historical information from the patient directly, and no other source is available, they should document “unable to obtain” the history. A comment regarding the circumstances surrounding this problem (e.g. patient confused, no caregiver present) should be provided, along with the available information from the limited resources (e.g. emergency medical technicians, previous hospitalizations at the same facility). Some contractors will not penalize the physician for the inability to ascertain complete historical information, as long as a proven attempt to obtain the information is evident.
Never document any item for the purpose of “getting paid.” Only document information that is clinically relevant, lends to the quality of care provided, or demonstrates the delivery of healthcare services. This prevents accusations of fraud and abuse, promotes billing compliance, and supports medical necessity for the services provided.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2010. Northbrook, IL: American College of Chest Physicians, 2009; 87-118.
- Centers for Medicare & Medicaid Services. 1995 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf. Accessed July 7, 2011.
- Centers for Medicare & Medicaid Services. 1997 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed July 7, 2011.
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2011.
- History of E/M (Q&As). WPS Health Insurance website. Available at: http://www.wpsmedicare.com/j5macpartb/resources/provider_types/2009_0526_emqahistory.shtml. Accessed July 11, 2011.
- Frequently Asked Questions: Evaluation and Management Services (Part B). Highmark Medicare Services website. Available at: www.highmarkmedicareservices.com/faq/partb/pet/lpet-evaluation_management_services.html. Accessed on July 11, 2011.
Each visit category and level of service has corresponding documentation requirements.1 Selecting an evaluation and management (E/M) level is based upon 1) the content of the three “key” components: history, exam, and decision-making, or 2) time, but only when counseling or coordination of care dominates more than 50% of the physician’s total visit time. Failure to document any essential element in a given visit level (e.g. family history required but missing for 99222 and 99223) could result in downcoding or service denial. Be aware of what an auditor expects when reviewing the key component of “history.”
Documentation Options
Auditors recognize two sets of documentation guidelines: “1995” and “1997” guidelines.2,3,4 Each set of guidelines has received valid criticism. The 1995 guidelines undoubtedly are vague and subjective in some areas, whereas the 1997 guidelines are known for arduous specificity.
However, to benefit all physicians and specialties, both sets of guidelines apply to visit-level selection. In other words, physicians can utilize either set when documenting their services, and auditors must review provider records against both styles. The final audited outcome reflects the highest visit level supported upon comparison.
Elements of History2,3,4
Chief complaint. The chief complaint (CC) is the reason for the visit, as stated in the patient’s own words. Every encounter, regardless of visit type, must include a CC. The physician must personally document and/or validate the CC with reference to a specific condition or symptom (e.g. patient complains of abdominal pain).
History of present illness (HPI). The HPI is a description of the patient’s present illness as it developed. It characteristically is referenced as location, quality, severity, timing, context, modifying factors, and associated signs/symptoms, as related to the chief complaint. The 1997 guidelines allow physicians to receive HPI credit for providing the status of the patient’s chronic or inactive conditions, such as “extrinsic asthma without acute exacerbation in past six months.” An auditor will not assign HPI credit to a chronic or inactive condition that does not have a corresponding status (e.g. “asthma”). This will be considered “past medical history.”
The HPI is classified as brief (a comment on <3 HPI elements, or the status of <2 conditions) or extended (a comment on >4 HPI elements, or the status of >3 conditions). Consider these examples of an extended HPI:
- “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
- “Diabetes controlled by oral medication; hyperlipidemia stable on simvastatin with increased dietary efforts; hypertension stable with pressures ranging from 130-140/80-90.” (Status of three chronic conditions.)
Physicians receive credit for confirming and personally documenting the HPI, or linking to documentation recorded by residents (residents, fellows, interns) or nonphysician providers (NPPs) when performing services according to the Teaching Physician Rules or Split-Shared Billing Rules, respectively. An auditor will not assign physician credit for HPI elements documented by ancillary staff (registered nurses, medical assistants) or students.
Review of systems (ROS). The ROS is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient: constitutional; eyes, ears, nose, mouth, throat; cardiovascular; respiratory; gastrointestinal; genitourinary; musculoskeletal; integumentary (including skin and/or breast); neurological; psychiatric; endocrine; hematologic/ lymphatic; and allergic/immunologic. Auditors classify the ROS as brief (a comment on one system), extended (a comment on two to nine systems), or complete (a comment on >10 systems). Physicians can document a complete ROS by noting individual systems: “no fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular) or shortness of breath (respiratory); intermittent nausea (gastrointestinal); and occasional runny nose (ears, nose, mouth, throat),” or by eliciting a complete system review but documenting only the positive and pertinent negative findings related to the chief complaint, along with an additional comment that “all other systems are negative.”
Although the latter method is formally included in Medicare’s documentation guidelines and accepted by some Medicare contractors (e.g. Highmark, WPS), be aware that it is not universally accepted.5,6
Documentation involving the ROS can be provided by anyone, including the patient. The physician should reference ROS information that is completed by individuals other than residents or NPPs during services provided under the Teaching Physician Rules or Split-Shared Billing Rules. Physician duplication of ROS information is unnecessary unless an update or revision is required.
Past, family, and social history (PFSH). The PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH could be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). The physician merely needs a single comment associated with each history for the PFSH to be regarded as complete. Refrain from using “noncontributory” to describe any of the histories, as previous misuse of this term has resulted in its prohibition. An example of a complete PFSH documentation includes: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
Similar to the ROS, PFSH documentation can be provided by anyone, including the patient, and the physician should reference the documented PFSH in his own progress note. Redocumentation of the PFSH is not necessary unless a revision is required.
PFSH documentation is only required for initial care services (i.e. initial hospital care, initial observation care, consultations). It is not warranted in subsequent care services unless additional, pertinent information is obtained during the hospital stay that impacts care.
Considerations
When a physician cannot elicit historical information from the patient directly, and no other source is available, they should document “unable to obtain” the history. A comment regarding the circumstances surrounding this problem (e.g. patient confused, no caregiver present) should be provided, along with the available information from the limited resources (e.g. emergency medical technicians, previous hospitalizations at the same facility). Some contractors will not penalize the physician for the inability to ascertain complete historical information, as long as a proven attempt to obtain the information is evident.
Never document any item for the purpose of “getting paid.” Only document information that is clinically relevant, lends to the quality of care provided, or demonstrates the delivery of healthcare services. This prevents accusations of fraud and abuse, promotes billing compliance, and supports medical necessity for the services provided.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2010. Northbrook, IL: American College of Chest Physicians, 2009; 87-118.
- Centers for Medicare & Medicaid Services. 1995 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf. Accessed July 7, 2011.
- Centers for Medicare & Medicaid Services. 1997 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed July 7, 2011.
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2011.
- History of E/M (Q&As). WPS Health Insurance website. Available at: http://www.wpsmedicare.com/j5macpartb/resources/provider_types/2009_0526_emqahistory.shtml. Accessed July 11, 2011.
- Frequently Asked Questions: Evaluation and Management Services (Part B). Highmark Medicare Services website. Available at: www.highmarkmedicareservices.com/faq/partb/pet/lpet-evaluation_management_services.html. Accessed on July 11, 2011.
Each visit category and level of service has corresponding documentation requirements.1 Selecting an evaluation and management (E/M) level is based upon 1) the content of the three “key” components: history, exam, and decision-making, or 2) time, but only when counseling or coordination of care dominates more than 50% of the physician’s total visit time. Failure to document any essential element in a given visit level (e.g. family history required but missing for 99222 and 99223) could result in downcoding or service denial. Be aware of what an auditor expects when reviewing the key component of “history.”
Documentation Options
Auditors recognize two sets of documentation guidelines: “1995” and “1997” guidelines.2,3,4 Each set of guidelines has received valid criticism. The 1995 guidelines undoubtedly are vague and subjective in some areas, whereas the 1997 guidelines are known for arduous specificity.
However, to benefit all physicians and specialties, both sets of guidelines apply to visit-level selection. In other words, physicians can utilize either set when documenting their services, and auditors must review provider records against both styles. The final audited outcome reflects the highest visit level supported upon comparison.
Elements of History2,3,4
Chief complaint. The chief complaint (CC) is the reason for the visit, as stated in the patient’s own words. Every encounter, regardless of visit type, must include a CC. The physician must personally document and/or validate the CC with reference to a specific condition or symptom (e.g. patient complains of abdominal pain).
History of present illness (HPI). The HPI is a description of the patient’s present illness as it developed. It characteristically is referenced as location, quality, severity, timing, context, modifying factors, and associated signs/symptoms, as related to the chief complaint. The 1997 guidelines allow physicians to receive HPI credit for providing the status of the patient’s chronic or inactive conditions, such as “extrinsic asthma without acute exacerbation in past six months.” An auditor will not assign HPI credit to a chronic or inactive condition that does not have a corresponding status (e.g. “asthma”). This will be considered “past medical history.”
The HPI is classified as brief (a comment on <3 HPI elements, or the status of <2 conditions) or extended (a comment on >4 HPI elements, or the status of >3 conditions). Consider these examples of an extended HPI:
- “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
- “Diabetes controlled by oral medication; hyperlipidemia stable on simvastatin with increased dietary efforts; hypertension stable with pressures ranging from 130-140/80-90.” (Status of three chronic conditions.)
Physicians receive credit for confirming and personally documenting the HPI, or linking to documentation recorded by residents (residents, fellows, interns) or nonphysician providers (NPPs) when performing services according to the Teaching Physician Rules or Split-Shared Billing Rules, respectively. An auditor will not assign physician credit for HPI elements documented by ancillary staff (registered nurses, medical assistants) or students.
Review of systems (ROS). The ROS is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient: constitutional; eyes, ears, nose, mouth, throat; cardiovascular; respiratory; gastrointestinal; genitourinary; musculoskeletal; integumentary (including skin and/or breast); neurological; psychiatric; endocrine; hematologic/ lymphatic; and allergic/immunologic. Auditors classify the ROS as brief (a comment on one system), extended (a comment on two to nine systems), or complete (a comment on >10 systems). Physicians can document a complete ROS by noting individual systems: “no fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular) or shortness of breath (respiratory); intermittent nausea (gastrointestinal); and occasional runny nose (ears, nose, mouth, throat),” or by eliciting a complete system review but documenting only the positive and pertinent negative findings related to the chief complaint, along with an additional comment that “all other systems are negative.”
Although the latter method is formally included in Medicare’s documentation guidelines and accepted by some Medicare contractors (e.g. Highmark, WPS), be aware that it is not universally accepted.5,6
Documentation involving the ROS can be provided by anyone, including the patient. The physician should reference ROS information that is completed by individuals other than residents or NPPs during services provided under the Teaching Physician Rules or Split-Shared Billing Rules. Physician duplication of ROS information is unnecessary unless an update or revision is required.
Past, family, and social history (PFSH). The PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH could be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). The physician merely needs a single comment associated with each history for the PFSH to be regarded as complete. Refrain from using “noncontributory” to describe any of the histories, as previous misuse of this term has resulted in its prohibition. An example of a complete PFSH documentation includes: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
Similar to the ROS, PFSH documentation can be provided by anyone, including the patient, and the physician should reference the documented PFSH in his own progress note. Redocumentation of the PFSH is not necessary unless a revision is required.
PFSH documentation is only required for initial care services (i.e. initial hospital care, initial observation care, consultations). It is not warranted in subsequent care services unless additional, pertinent information is obtained during the hospital stay that impacts care.
Considerations
When a physician cannot elicit historical information from the patient directly, and no other source is available, they should document “unable to obtain” the history. A comment regarding the circumstances surrounding this problem (e.g. patient confused, no caregiver present) should be provided, along with the available information from the limited resources (e.g. emergency medical technicians, previous hospitalizations at the same facility). Some contractors will not penalize the physician for the inability to ascertain complete historical information, as long as a proven attempt to obtain the information is evident.
Never document any item for the purpose of “getting paid.” Only document information that is clinically relevant, lends to the quality of care provided, or demonstrates the delivery of healthcare services. This prevents accusations of fraud and abuse, promotes billing compliance, and supports medical necessity for the services provided.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2010. Northbrook, IL: American College of Chest Physicians, 2009; 87-118.
- Centers for Medicare & Medicaid Services. 1995 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf. Accessed July 7, 2011.
- Centers for Medicare & Medicaid Services. 1997 Documentation Guidelines for Evaluation & Management Services. CMS website. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed July 7, 2011.
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2011.
- History of E/M (Q&As). WPS Health Insurance website. Available at: http://www.wpsmedicare.com/j5macpartb/resources/provider_types/2009_0526_emqahistory.shtml. Accessed July 11, 2011.
- Frequently Asked Questions: Evaluation and Management Services (Part B). Highmark Medicare Services website. Available at: www.highmarkmedicareservices.com/faq/partb/pet/lpet-evaluation_management_services.html. Accessed on July 11, 2011.
21st-Century Trainer
When Joshua Lenchus, DO, RPh, FACP, FHM, discussed his love for chemistry with his high school guidance counselors, they told him it could take him in one of two directions: teaching or a career in pharmacy.
Intrigued by the latter option, he decided to become a volunteer in the pharmacy department at a local hospital. Soon after, he became a full-time pharmacy technician and eventually enrolled in pharmacy school at the University of Florida.
“I always knew I wanted to be a physician, and everybody needs a bachelor’s degree in something,” Dr. Lenchus says. “I thought, ‘What better way to do it than to get a bachelor’s degree in pharmacy and then move into medicine?’ ”
After college, he worked as a retail pharmacist, then moved to the institutional setting, creating the position of clinical pharmacist at Wellington (Fla.) Regional Medical Center. Three years later, he entered medical school and ultimately pursued a career as an academic hospitalist.
Dr. Lenchus now serves as associate professor of clinical medicine at the University of Miami’s (UM) Miller School of Medicine, associate program director of Jackson Memorial Hospital’s (JMH) internal medicine residency training program, and associate director of the UM-JMH Center for Patient Safety, which trains about 1,000 medical students, residents, and interns each year.
“Pharmacy has provided an invaluable background for becoming a physician,” says Dr. Lenchus, who was appointed a member of Team Hospitalist in May. “Many physicians order a medication and have no idea what the other half of the equation entails. My experience gave me a solid footing from which I could springboard.”
Q: You spend considerable time mentoring the next generation of physicians. What’s the best advice you can give them?
A: Physicians have these altruistic notions about wanting to help people, but you really have to do what you love. There’s another hospital a mile and a half away from my house, whereas Jackson is 35 miles away and it takes me an hour in transit time each way. But I couldn’t do what I’m doing now at any other facility. I stay because I love what I’m doing.
Q: Why is the UM-JMH Center for Patient Safety so beneficial?
A: The greatest benefit is the ability to be exposed to and tackle real-life scenarios in a risk-free environment. We use life-size mannequins to re-create scenarios that medical personnel will see during their training. We try to re-create the chaos that will ensue.
Q: So it’s similar to a pilot using a flight simulator.
A: Exactly. When a plane crashes and the NTSB goes to see what happened, they perform what we in medicine call a root-cause analysis. They’re not blaming an individual; they want to see what they can change on a system level to prevent an error like that from happening again. We culminate our training with a debriefing that we approach the same way, so nobody walks away thinking they failed.
Q: How effective can simulation-based education be?
A: There will be some limitations because the technology simply cannot account for every aspect of a human. But there’s a wealth of data that supports this as a pretty good surrogate. The technology provides for an incredible amount of experience and exposure without any potential harm to a patient, and it provides [trainees] an opportunity to do things they otherwise would have to wait to do until a clinical scenario demanded it.
Q: Do you think this is the wave of the future?
A: Absolutely. And as the Accreditation Council for Graduate Medical Education promulgates new rules that limit the hours trainees can work, it’s going to be incumbent on training programs to be creative in providing equal or near-equal experience in a much shorter time. Simulation can help fill that bill.
Q: You created a crisis-management simulation course for IM residents. How did that come about?
A: When we have a crisis like a code blue, I witnessed the chaos that ensued. I thought some of the paltry resuscitation rate could be due to the fact there was no meaningful communication in that scenario.
Using full-scale mannequins, I put nurses and residents into those types of situations and videotaped what ensued. Frequently we saw the same chaos we see in reality, and many rather basic, commonsensical concepts went out the window.
Q: Can you offer an example?
A: A big one is situational awareness. If the head of the gurney is in a seated position, that’s not a conducive way to do chest compressions. If the side rails of the bed are up, you can lower them so you aren’t reaching over them. Was a team leader assigned or were roles delegated? These aren’t novel concepts, but when faced with a crisis, everybody tends to focus on their own thing. In a crisis, you need to break those silos down and operate as a team.
Q: How effective is the training?
A: After the first scenario, we show the video and debrief them for 10 or 15 minutes, keying in on some behaviors that can be employed in a crisis. Then we expose them to a different crisis scenario immediately thereafter. Often we see an immediate change in their behavior.
Q: You developed a curriculum through which residents are taught in a standardized manner how to perform invasive bedside procedures. How does it work?
A: They have 12 hours of hands-on instruction using fluid-filled, ultrasound-capable mannequins. A faculty attending teaches these procedures. We took it a step further and made a four-week rotation as a mandatory component of the residency program. They carry a beeper, and any service within the hospital can call the procedure team to do one of the procedures on which they were already trained.
Q: How successful is the effort?
A: This is the beginning of our fifth year, and we’ve been called more than 4,000 times to do procedures on hospitalized patients. We’ve published our curriculum. We’ve shown a significant improvement in knowledge, technical skills, and confidence level, and we have data we’re going to publish later this year that shows our complication rates are better than complication rates that are published elsewhere.
Q: What is your biggest professional reward?
A: The ability to impact the next generation. With the procedural training alone, we have just trained our 1,000th person. Each one of them is going to take care of thousands of patients in their professional careers. That’s an expansive influence.
Q: What is your biggest professional challenge?
A: The culture of medicine. It is infused with hundreds of years of tradition and, at times, it feels like trying to move a mountain. It may take a generation to do it, but there will come a time—at least within the field of patient safety—when more people are attuned to it and understand the concepts really are lifesaving. That doesn’t happen as fast as I would like it to, but if we keep plugging away one year at a time, we will be able to make an impact.
Mark Leiser is a freelance writer based in New Jersey.
When Joshua Lenchus, DO, RPh, FACP, FHM, discussed his love for chemistry with his high school guidance counselors, they told him it could take him in one of two directions: teaching or a career in pharmacy.
Intrigued by the latter option, he decided to become a volunteer in the pharmacy department at a local hospital. Soon after, he became a full-time pharmacy technician and eventually enrolled in pharmacy school at the University of Florida.
“I always knew I wanted to be a physician, and everybody needs a bachelor’s degree in something,” Dr. Lenchus says. “I thought, ‘What better way to do it than to get a bachelor’s degree in pharmacy and then move into medicine?’ ”
After college, he worked as a retail pharmacist, then moved to the institutional setting, creating the position of clinical pharmacist at Wellington (Fla.) Regional Medical Center. Three years later, he entered medical school and ultimately pursued a career as an academic hospitalist.
Dr. Lenchus now serves as associate professor of clinical medicine at the University of Miami’s (UM) Miller School of Medicine, associate program director of Jackson Memorial Hospital’s (JMH) internal medicine residency training program, and associate director of the UM-JMH Center for Patient Safety, which trains about 1,000 medical students, residents, and interns each year.
“Pharmacy has provided an invaluable background for becoming a physician,” says Dr. Lenchus, who was appointed a member of Team Hospitalist in May. “Many physicians order a medication and have no idea what the other half of the equation entails. My experience gave me a solid footing from which I could springboard.”
Q: You spend considerable time mentoring the next generation of physicians. What’s the best advice you can give them?
A: Physicians have these altruistic notions about wanting to help people, but you really have to do what you love. There’s another hospital a mile and a half away from my house, whereas Jackson is 35 miles away and it takes me an hour in transit time each way. But I couldn’t do what I’m doing now at any other facility. I stay because I love what I’m doing.
Q: Why is the UM-JMH Center for Patient Safety so beneficial?
A: The greatest benefit is the ability to be exposed to and tackle real-life scenarios in a risk-free environment. We use life-size mannequins to re-create scenarios that medical personnel will see during their training. We try to re-create the chaos that will ensue.
Q: So it’s similar to a pilot using a flight simulator.
A: Exactly. When a plane crashes and the NTSB goes to see what happened, they perform what we in medicine call a root-cause analysis. They’re not blaming an individual; they want to see what they can change on a system level to prevent an error like that from happening again. We culminate our training with a debriefing that we approach the same way, so nobody walks away thinking they failed.
Q: How effective can simulation-based education be?
A: There will be some limitations because the technology simply cannot account for every aspect of a human. But there’s a wealth of data that supports this as a pretty good surrogate. The technology provides for an incredible amount of experience and exposure without any potential harm to a patient, and it provides [trainees] an opportunity to do things they otherwise would have to wait to do until a clinical scenario demanded it.
Q: Do you think this is the wave of the future?
A: Absolutely. And as the Accreditation Council for Graduate Medical Education promulgates new rules that limit the hours trainees can work, it’s going to be incumbent on training programs to be creative in providing equal or near-equal experience in a much shorter time. Simulation can help fill that bill.
Q: You created a crisis-management simulation course for IM residents. How did that come about?
A: When we have a crisis like a code blue, I witnessed the chaos that ensued. I thought some of the paltry resuscitation rate could be due to the fact there was no meaningful communication in that scenario.
Using full-scale mannequins, I put nurses and residents into those types of situations and videotaped what ensued. Frequently we saw the same chaos we see in reality, and many rather basic, commonsensical concepts went out the window.
Q: Can you offer an example?
A: A big one is situational awareness. If the head of the gurney is in a seated position, that’s not a conducive way to do chest compressions. If the side rails of the bed are up, you can lower them so you aren’t reaching over them. Was a team leader assigned or were roles delegated? These aren’t novel concepts, but when faced with a crisis, everybody tends to focus on their own thing. In a crisis, you need to break those silos down and operate as a team.
Q: How effective is the training?
A: After the first scenario, we show the video and debrief them for 10 or 15 minutes, keying in on some behaviors that can be employed in a crisis. Then we expose them to a different crisis scenario immediately thereafter. Often we see an immediate change in their behavior.
Q: You developed a curriculum through which residents are taught in a standardized manner how to perform invasive bedside procedures. How does it work?
A: They have 12 hours of hands-on instruction using fluid-filled, ultrasound-capable mannequins. A faculty attending teaches these procedures. We took it a step further and made a four-week rotation as a mandatory component of the residency program. They carry a beeper, and any service within the hospital can call the procedure team to do one of the procedures on which they were already trained.
Q: How successful is the effort?
A: This is the beginning of our fifth year, and we’ve been called more than 4,000 times to do procedures on hospitalized patients. We’ve published our curriculum. We’ve shown a significant improvement in knowledge, technical skills, and confidence level, and we have data we’re going to publish later this year that shows our complication rates are better than complication rates that are published elsewhere.
Q: What is your biggest professional reward?
A: The ability to impact the next generation. With the procedural training alone, we have just trained our 1,000th person. Each one of them is going to take care of thousands of patients in their professional careers. That’s an expansive influence.
Q: What is your biggest professional challenge?
A: The culture of medicine. It is infused with hundreds of years of tradition and, at times, it feels like trying to move a mountain. It may take a generation to do it, but there will come a time—at least within the field of patient safety—when more people are attuned to it and understand the concepts really are lifesaving. That doesn’t happen as fast as I would like it to, but if we keep plugging away one year at a time, we will be able to make an impact.
Mark Leiser is a freelance writer based in New Jersey.
When Joshua Lenchus, DO, RPh, FACP, FHM, discussed his love for chemistry with his high school guidance counselors, they told him it could take him in one of two directions: teaching or a career in pharmacy.
Intrigued by the latter option, he decided to become a volunteer in the pharmacy department at a local hospital. Soon after, he became a full-time pharmacy technician and eventually enrolled in pharmacy school at the University of Florida.
“I always knew I wanted to be a physician, and everybody needs a bachelor’s degree in something,” Dr. Lenchus says. “I thought, ‘What better way to do it than to get a bachelor’s degree in pharmacy and then move into medicine?’ ”
After college, he worked as a retail pharmacist, then moved to the institutional setting, creating the position of clinical pharmacist at Wellington (Fla.) Regional Medical Center. Three years later, he entered medical school and ultimately pursued a career as an academic hospitalist.
Dr. Lenchus now serves as associate professor of clinical medicine at the University of Miami’s (UM) Miller School of Medicine, associate program director of Jackson Memorial Hospital’s (JMH) internal medicine residency training program, and associate director of the UM-JMH Center for Patient Safety, which trains about 1,000 medical students, residents, and interns each year.
“Pharmacy has provided an invaluable background for becoming a physician,” says Dr. Lenchus, who was appointed a member of Team Hospitalist in May. “Many physicians order a medication and have no idea what the other half of the equation entails. My experience gave me a solid footing from which I could springboard.”
Q: You spend considerable time mentoring the next generation of physicians. What’s the best advice you can give them?
A: Physicians have these altruistic notions about wanting to help people, but you really have to do what you love. There’s another hospital a mile and a half away from my house, whereas Jackson is 35 miles away and it takes me an hour in transit time each way. But I couldn’t do what I’m doing now at any other facility. I stay because I love what I’m doing.
Q: Why is the UM-JMH Center for Patient Safety so beneficial?
A: The greatest benefit is the ability to be exposed to and tackle real-life scenarios in a risk-free environment. We use life-size mannequins to re-create scenarios that medical personnel will see during their training. We try to re-create the chaos that will ensue.
Q: So it’s similar to a pilot using a flight simulator.
A: Exactly. When a plane crashes and the NTSB goes to see what happened, they perform what we in medicine call a root-cause analysis. They’re not blaming an individual; they want to see what they can change on a system level to prevent an error like that from happening again. We culminate our training with a debriefing that we approach the same way, so nobody walks away thinking they failed.
Q: How effective can simulation-based education be?
A: There will be some limitations because the technology simply cannot account for every aspect of a human. But there’s a wealth of data that supports this as a pretty good surrogate. The technology provides for an incredible amount of experience and exposure without any potential harm to a patient, and it provides [trainees] an opportunity to do things they otherwise would have to wait to do until a clinical scenario demanded it.
Q: Do you think this is the wave of the future?
A: Absolutely. And as the Accreditation Council for Graduate Medical Education promulgates new rules that limit the hours trainees can work, it’s going to be incumbent on training programs to be creative in providing equal or near-equal experience in a much shorter time. Simulation can help fill that bill.
Q: You created a crisis-management simulation course for IM residents. How did that come about?
A: When we have a crisis like a code blue, I witnessed the chaos that ensued. I thought some of the paltry resuscitation rate could be due to the fact there was no meaningful communication in that scenario.
Using full-scale mannequins, I put nurses and residents into those types of situations and videotaped what ensued. Frequently we saw the same chaos we see in reality, and many rather basic, commonsensical concepts went out the window.
Q: Can you offer an example?
A: A big one is situational awareness. If the head of the gurney is in a seated position, that’s not a conducive way to do chest compressions. If the side rails of the bed are up, you can lower them so you aren’t reaching over them. Was a team leader assigned or were roles delegated? These aren’t novel concepts, but when faced with a crisis, everybody tends to focus on their own thing. In a crisis, you need to break those silos down and operate as a team.
Q: How effective is the training?
A: After the first scenario, we show the video and debrief them for 10 or 15 minutes, keying in on some behaviors that can be employed in a crisis. Then we expose them to a different crisis scenario immediately thereafter. Often we see an immediate change in their behavior.
Q: You developed a curriculum through which residents are taught in a standardized manner how to perform invasive bedside procedures. How does it work?
A: They have 12 hours of hands-on instruction using fluid-filled, ultrasound-capable mannequins. A faculty attending teaches these procedures. We took it a step further and made a four-week rotation as a mandatory component of the residency program. They carry a beeper, and any service within the hospital can call the procedure team to do one of the procedures on which they were already trained.
Q: How successful is the effort?
A: This is the beginning of our fifth year, and we’ve been called more than 4,000 times to do procedures on hospitalized patients. We’ve published our curriculum. We’ve shown a significant improvement in knowledge, technical skills, and confidence level, and we have data we’re going to publish later this year that shows our complication rates are better than complication rates that are published elsewhere.
Q: What is your biggest professional reward?
A: The ability to impact the next generation. With the procedural training alone, we have just trained our 1,000th person. Each one of them is going to take care of thousands of patients in their professional careers. That’s an expansive influence.
Q: What is your biggest professional challenge?
A: The culture of medicine. It is infused with hundreds of years of tradition and, at times, it feels like trying to move a mountain. It may take a generation to do it, but there will come a time—at least within the field of patient safety—when more people are attuned to it and understand the concepts really are lifesaving. That doesn’t happen as fast as I would like it to, but if we keep plugging away one year at a time, we will be able to make an impact.
Mark Leiser is a freelance writer based in New Jersey.
Study: Rural Hospitals Behind IT Curve
Only a sliver of rural hospitals would meet the Center for Medicare & Medicaid Services’ (CMS) criteria to qualify for “meaningful use” of health information technology (HIT), according to a new study, but that could be a window for HM group leaders to take the reins of technology projects.
“[Hospitalists] could be very useful as a champion,” says Brock Slabach, MPH, FACHE, senior vice president for member services at the National Rural Health Association.
The new report showed that 5% of rural hospitals could demonstrate meaningful use of an electronic health record (EHR) system, as opposed to 9% of urban hospitals (J Rural Health. 2011;27(3):329-337). The number dips to 3% for critical-access hospitals. EHR usage often is used as a benchmark for HIT implementation.
CMS has allotted $20 billion for physicians and hospitals to adopt new technologies, but entities must prove they have met “meaningful use” requirements.
Slabach, who spent 20 years as an administrator at Field Memorial Community Hospital in Centreville, Miss., says the major hurdle for HIT implementation at rural hospitals is a lack of knowledge. But if hospitalists can show other physicians the value of HIT, others will follow, he adds.
“Somebody who may not have any informatics background, but is willing to grab a hold of the system, learn its applications, develop methods to spread the knowledge to the rest of the medical staff, is critical,” Slabach says. “It just takes that one or two [people] to get the momentum starting, in terms of a transition to what for a lot of middle-aged and older physicians is a completely new world.”
Only a sliver of rural hospitals would meet the Center for Medicare & Medicaid Services’ (CMS) criteria to qualify for “meaningful use” of health information technology (HIT), according to a new study, but that could be a window for HM group leaders to take the reins of technology projects.
“[Hospitalists] could be very useful as a champion,” says Brock Slabach, MPH, FACHE, senior vice president for member services at the National Rural Health Association.
The new report showed that 5% of rural hospitals could demonstrate meaningful use of an electronic health record (EHR) system, as opposed to 9% of urban hospitals (J Rural Health. 2011;27(3):329-337). The number dips to 3% for critical-access hospitals. EHR usage often is used as a benchmark for HIT implementation.
CMS has allotted $20 billion for physicians and hospitals to adopt new technologies, but entities must prove they have met “meaningful use” requirements.
Slabach, who spent 20 years as an administrator at Field Memorial Community Hospital in Centreville, Miss., says the major hurdle for HIT implementation at rural hospitals is a lack of knowledge. But if hospitalists can show other physicians the value of HIT, others will follow, he adds.
“Somebody who may not have any informatics background, but is willing to grab a hold of the system, learn its applications, develop methods to spread the knowledge to the rest of the medical staff, is critical,” Slabach says. “It just takes that one or two [people] to get the momentum starting, in terms of a transition to what for a lot of middle-aged and older physicians is a completely new world.”
Only a sliver of rural hospitals would meet the Center for Medicare & Medicaid Services’ (CMS) criteria to qualify for “meaningful use” of health information technology (HIT), according to a new study, but that could be a window for HM group leaders to take the reins of technology projects.
“[Hospitalists] could be very useful as a champion,” says Brock Slabach, MPH, FACHE, senior vice president for member services at the National Rural Health Association.
The new report showed that 5% of rural hospitals could demonstrate meaningful use of an electronic health record (EHR) system, as opposed to 9% of urban hospitals (J Rural Health. 2011;27(3):329-337). The number dips to 3% for critical-access hospitals. EHR usage often is used as a benchmark for HIT implementation.
CMS has allotted $20 billion for physicians and hospitals to adopt new technologies, but entities must prove they have met “meaningful use” requirements.
Slabach, who spent 20 years as an administrator at Field Memorial Community Hospital in Centreville, Miss., says the major hurdle for HIT implementation at rural hospitals is a lack of knowledge. But if hospitalists can show other physicians the value of HIT, others will follow, he adds.
“Somebody who may not have any informatics background, but is willing to grab a hold of the system, learn its applications, develop methods to spread the knowledge to the rest of the medical staff, is critical,” Slabach says. “It just takes that one or two [people] to get the momentum starting, in terms of a transition to what for a lot of middle-aged and older physicians is a completely new world.”