User login
ONLINE EXCLUSIVE: Listen to Tosha Wetterneck and Keiki Hinami discuss burnout and career satisfaction
ONLINE EXCLUSIVE: Listen to David Meltzer and Scott Lundberg talk about HM efficiency
How proposed changes to personality disorders in DSM-5 will affect researchers
Crizotinib Approval Personalizes Lung Cancer Therapy
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
Venous thromboembolism: What to do after anticoagulation is started
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
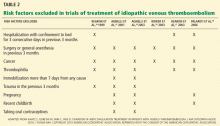
HOW LONG TO TREAT PROVOKED VTE
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
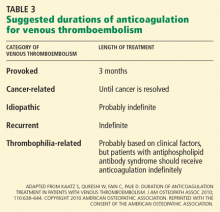
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
HOW LONG TO TREAT PROVOKED VTE
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
HOW LONG TO TREAT PROVOKED VTE
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
KEY POINTS
- A low-molecular-weight heparin for at least 6 months is the treatment of choice for cancer-related VTE.
- We recommend 3 months of anticoagulation for VTE caused by a reversible risk factor and indefinite treatment for idiopathic VTE in patients without risk factors for bleeding who can get anticoagulation monitoring.
- Clinical factors are more important in deciding the duration of anticoagulation therapy than evidence of an inherited thrombophilic state.
- Elastic compression stockings reduce the risk of postthrombotic syndrome substantially.
- Patients with idiopathic VTE should have a basic screening for malignancy.
Unmasking gastric cancer
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
Hepatic encephalopathy: Suspect it early in patients with cirrhosis
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
KEY POINTS
- Hepatic encephalopathy should be considered in any patient with cirrhosis who presents with neuropsychiatric manifestations in the absence of another brain disorder, such as stroke or brain tumor.
- “Minimal” hepatic encephalopathy may not be obvious on clinical examination but can be detected with neurophysiologic and neuropsychiatric testing.
- Every cirrhotic patient is at risk; potential precipitating factors should be addressed during regular clinic visits.
- Management requires prompt identification of precipitating factors and initiation of empiric medical therapy. Current treatments include drugs to prevent ammonia generation in the colon.
- Long-acting benzodiazepines should not be used to treat sleep disorders in patients with cirrhosis, as they may precipitate encephalopathy.
Oral plaques and dysphagia in a young man
A 23-year-old man presents with a sore throat, dysphagia, and general malaise that began 1 week ago. He also reports a 5-pound weight loss. He has not recently taken antibiotics or inhaled glucocorticoids, and he has no history of tobacco use or trauma to his mouth. He has no personal or family history of oral cancer. He uses cocaine on occasion. He reports feeling feverish and having a decreased appetite.
Q: Based on the history, the symptoms, and the physical examination, which of the following is the most likely diagnosis in this patient?
- Oral hairy leukoplakia
- Squamous cell carcinoma
- Oral candidiasis
- Herpetic gingivostomatitis
- Streptococcal pharyngitis
A: Oral candidiasis is correct.
Otherwise known as thrush, it is common in infants and in denture wearers, and it also can occur in diabetes mellitus, antibiotic therapy, chemotherapy, radiation therapy, and cellular immune deficiency states such as cancer or human immunodeficiency virus (HIV) infection.1 Patients using inhaled glucocorticoids are also at risk and should always be advised to rinse their mouth out with water after inhaled steroid use.
Although Candida albicans is the species most often responsible for candidal infections, other candidal species are increasingly responsible for infections in immunocompromised patients. Candida is part of the normal flora in many adults.
Oral hairy leukoplakia is caused by the Epstein-Barr virus and is often seen in HIV infection. It is a white, painless, corrugated lesion, typically found on the lateral aspect of the tongue, and it cannot be scraped from the adherent surfaces. It can also be found on the dorsum of the tongue, the buccal surfaces, and the floor of the mouth. In an asymptomatic patient with oral hairy leukoplakia, HIV infection with moderate immunosuppression is most likely present.2 Oral hairy leukoplakia is diagnosed by biopsy of suspected lesions. It is not a premalignant lesion, and how to best treat it is still being investigated.3
Squamous cell carcinoma of the oral cavity can present as nonhealing ulcers or masses, dental changes, or exophytic lesions with or without pain.1 They may be accompanied by cervical nodal disease. Malignancies of the oral cavity account for 14% of all head and neck cancers, with squamous cell carcinoma the predominant type.4 Alcohol and tobacco use increase the risk. Alcohol and tobacco together have a synergistic effect on the incidence of oral carcinoma.1,4 Predisposing lesions are leukoplakia, lichen planus of the erosive subtype, submucosal fibrosis, and erythroplakia. Oral infection with human papillomavirus has been shown to increase the risk of oral cancer by a factor of 14, and papillomavirus type 16 is detected in 72% of patients with oropharyngeal cancer.5
Herpetic gingivostomatitis is a manifestation of herpes simplex virus infection. The initial infection may be asymptomatic or may produce groups of vesicles that develop into shallow, painful, and superficial ulcerations on an erythematous base.1,3 If the gingiva is involved, it is erythematous, boggy, and tender.3 Infections are self-limited, lasting up to 2 weeks, but there is potential for recurrence because of the ability of herpes simplex virus to undergo latency. Recurrence is usually heralded by prodromal symptoms 24 hours before onset, with tingling, pain, or burning at the infected site. The diagnosis can be made clinically, but the Tzanck smear test, viral culture, direct fluorescent antibody test, or polymerase chain reaction test can be used to confirm the diagnosis. In patients who are immunocompromised, infections tend to be more severe and to last longer.
Streptococcal pharyngitis, most often caused by group A beta-hemolytic streptococci, is the most common type of bacterial pharyngitis in the clinical setting. The bacteria incubate for 2 to 5 days. The condition mainly affects younger children.6 Patients with “strep throat” often present with a sore throat and high-grade fever. Other symptoms include chills, myalgia, headache, and nausea. Findings on examination may include petechiae of the palate, pharyngeal and tonsillar erythema and exudates, and anterior cervical adenopathy.6 Children often present with coinciding abdominal complaints. A rapid antigen detection test for streptococcal infection can be performed in the office for quick diagnosis, but if clinical suspicion is high, a throat culture is necessary to confirm the diagnosis. Treatment is to prevent complications such as rheumatic fever.6
FEATURES AND DIAGNOSIS OF ORAL CANDIDIASIS
Lesions of oral candidiasis can vary in their appearance. The pseudomembranous form is the most characteristic, with white adherent “cottage-cheese-like” plaques that wipe away, causing minimal bleeding.1,7 The erythematous or atrophic form is associated with denture use and causes a “beefy” appearance on the dorsum of the tongue or on the mucosa that supports a denture.1,7 A third form affects the angles of the mouth, causing angular cheilitis (perlèche).7,8 Chronic infection appears as localized, firmly adherent plaques with an irregular surface similar to hyperkeratosis caused by chronic frictional irritation.7
Oral candidiasis can occur in different forms at the same time. Patients often describe minimal symptoms such as dysgeusia or dry mouth.1,7 Infections causing dysphagia or odynophagia warrant suspicion for involvement of the esophagus.
The diagnosis is made empirically if the lesions resolve with anticandidal therapy. A more definitive diagnosis can be made by microscopy with a potassium hydroxide preparation showing pseudohyphae. Formal culture can also determine the yeast’s susceptibility to medication in recurrent or resistant cases.2
Oral candidiasis may be the manifesting symptom of HIV infection, and more than 90% of patients with adult immunodeficiency syndrome have an episode of thrush.8 When candidiasis is diagnosed without obvious cause, HIV testing should be offered, regardless of a patient’s lack of obvious risk factors. Other oral lesions in HIV patients are oral hairy leukoplakia, Kaposi sarcoma, periodontal and gingival infections, aphthous ulcers, herpes simplex stomatitis, and xerostomia.2 With highly active antiretroviral therapy, the incidence of oral candidiasis has decreased by about 50%.2
Our patient was diagnosed with HIV when screened after this initial presentation. Lower CD4 counts and higher viral loads increase the patient’s risk for oral candidiasis and other lesions. This patient’s initial CD4 count was 524 cells/μL, and his viral load was 11,232 copies/mL.
TREATMENT
In HIV-negative patients or in HIV-positive patients with a CD4 count greater than 200 cells/μL, the treatment of oral candidiasis involves topical antifungal agents, including a nystatin suspension (Nystat-Rx) or clotrimazole (Mycelex) troches.3,7,9 Treatment should be continued for at least 7 days after resolution of the infection. If resolution does not occur, oral fluconazole (Diflucan) 200 mg daily should be given.
For HIV patients with CD4 counts below 200 cells/μL, oral fluconazole or itraconazole (Sporanox) is recommended, with posaconazole (Noxafil) as an alternative for refractory disease.3,9 Giving fluconazole prophylactically to prevent oral candidiasis is not recommended because of the risk of adverse effects, lack of survival benefit, associated cost, and potential to develop antifungal resistance.3,9
- Reichart PA. Clinical management of selected oral fungal and viral infections during HIV-disease. Int Dent J 1999; 49:251–259.
- Kim TB, Pletcher SD, Goldberg AN. Head and neck manifestations in the immunocompromised host. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:209–229(225–226).
- Sciubba JJ. Oral mucosal lesions. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1229–1231).
- Wein R. Malignant Neoplasms of the Oral Cavity. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1236).
- D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944–1956.
- Hayes CS, Williamson H. Management of group A betahemolytic streptococcal pharyngitis. Am Fam Physician 2001; 63:1557–1564.
- Coleman GC. Diseases of the mouth. In:Bope ET, Rakel RE, Kellerman R, editors. Conn’s Current Therapy. Philadelphia, PA: Saunders; 2010:861–867.
- Habif TP. Candidiasis (moniliasis). In: Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:523–536.
- Pappas PG, Rex JH, Sobel JD, et al; Infectious Diseases Society of America. Guidelines for treatment of candidiasis. Clin Infect Dis 2004; 38:161–189.
A 23-year-old man presents with a sore throat, dysphagia, and general malaise that began 1 week ago. He also reports a 5-pound weight loss. He has not recently taken antibiotics or inhaled glucocorticoids, and he has no history of tobacco use or trauma to his mouth. He has no personal or family history of oral cancer. He uses cocaine on occasion. He reports feeling feverish and having a decreased appetite.
Q: Based on the history, the symptoms, and the physical examination, which of the following is the most likely diagnosis in this patient?
- Oral hairy leukoplakia
- Squamous cell carcinoma
- Oral candidiasis
- Herpetic gingivostomatitis
- Streptococcal pharyngitis
A: Oral candidiasis is correct.
Otherwise known as thrush, it is common in infants and in denture wearers, and it also can occur in diabetes mellitus, antibiotic therapy, chemotherapy, radiation therapy, and cellular immune deficiency states such as cancer or human immunodeficiency virus (HIV) infection.1 Patients using inhaled glucocorticoids are also at risk and should always be advised to rinse their mouth out with water after inhaled steroid use.
Although Candida albicans is the species most often responsible for candidal infections, other candidal species are increasingly responsible for infections in immunocompromised patients. Candida is part of the normal flora in many adults.
Oral hairy leukoplakia is caused by the Epstein-Barr virus and is often seen in HIV infection. It is a white, painless, corrugated lesion, typically found on the lateral aspect of the tongue, and it cannot be scraped from the adherent surfaces. It can also be found on the dorsum of the tongue, the buccal surfaces, and the floor of the mouth. In an asymptomatic patient with oral hairy leukoplakia, HIV infection with moderate immunosuppression is most likely present.2 Oral hairy leukoplakia is diagnosed by biopsy of suspected lesions. It is not a premalignant lesion, and how to best treat it is still being investigated.3
Squamous cell carcinoma of the oral cavity can present as nonhealing ulcers or masses, dental changes, or exophytic lesions with or without pain.1 They may be accompanied by cervical nodal disease. Malignancies of the oral cavity account for 14% of all head and neck cancers, with squamous cell carcinoma the predominant type.4 Alcohol and tobacco use increase the risk. Alcohol and tobacco together have a synergistic effect on the incidence of oral carcinoma.1,4 Predisposing lesions are leukoplakia, lichen planus of the erosive subtype, submucosal fibrosis, and erythroplakia. Oral infection with human papillomavirus has been shown to increase the risk of oral cancer by a factor of 14, and papillomavirus type 16 is detected in 72% of patients with oropharyngeal cancer.5
Herpetic gingivostomatitis is a manifestation of herpes simplex virus infection. The initial infection may be asymptomatic or may produce groups of vesicles that develop into shallow, painful, and superficial ulcerations on an erythematous base.1,3 If the gingiva is involved, it is erythematous, boggy, and tender.3 Infections are self-limited, lasting up to 2 weeks, but there is potential for recurrence because of the ability of herpes simplex virus to undergo latency. Recurrence is usually heralded by prodromal symptoms 24 hours before onset, with tingling, pain, or burning at the infected site. The diagnosis can be made clinically, but the Tzanck smear test, viral culture, direct fluorescent antibody test, or polymerase chain reaction test can be used to confirm the diagnosis. In patients who are immunocompromised, infections tend to be more severe and to last longer.
Streptococcal pharyngitis, most often caused by group A beta-hemolytic streptococci, is the most common type of bacterial pharyngitis in the clinical setting. The bacteria incubate for 2 to 5 days. The condition mainly affects younger children.6 Patients with “strep throat” often present with a sore throat and high-grade fever. Other symptoms include chills, myalgia, headache, and nausea. Findings on examination may include petechiae of the palate, pharyngeal and tonsillar erythema and exudates, and anterior cervical adenopathy.6 Children often present with coinciding abdominal complaints. A rapid antigen detection test for streptococcal infection can be performed in the office for quick diagnosis, but if clinical suspicion is high, a throat culture is necessary to confirm the diagnosis. Treatment is to prevent complications such as rheumatic fever.6
FEATURES AND DIAGNOSIS OF ORAL CANDIDIASIS
Lesions of oral candidiasis can vary in their appearance. The pseudomembranous form is the most characteristic, with white adherent “cottage-cheese-like” plaques that wipe away, causing minimal bleeding.1,7 The erythematous or atrophic form is associated with denture use and causes a “beefy” appearance on the dorsum of the tongue or on the mucosa that supports a denture.1,7 A third form affects the angles of the mouth, causing angular cheilitis (perlèche).7,8 Chronic infection appears as localized, firmly adherent plaques with an irregular surface similar to hyperkeratosis caused by chronic frictional irritation.7
Oral candidiasis can occur in different forms at the same time. Patients often describe minimal symptoms such as dysgeusia or dry mouth.1,7 Infections causing dysphagia or odynophagia warrant suspicion for involvement of the esophagus.
The diagnosis is made empirically if the lesions resolve with anticandidal therapy. A more definitive diagnosis can be made by microscopy with a potassium hydroxide preparation showing pseudohyphae. Formal culture can also determine the yeast’s susceptibility to medication in recurrent or resistant cases.2
Oral candidiasis may be the manifesting symptom of HIV infection, and more than 90% of patients with adult immunodeficiency syndrome have an episode of thrush.8 When candidiasis is diagnosed without obvious cause, HIV testing should be offered, regardless of a patient’s lack of obvious risk factors. Other oral lesions in HIV patients are oral hairy leukoplakia, Kaposi sarcoma, periodontal and gingival infections, aphthous ulcers, herpes simplex stomatitis, and xerostomia.2 With highly active antiretroviral therapy, the incidence of oral candidiasis has decreased by about 50%.2
Our patient was diagnosed with HIV when screened after this initial presentation. Lower CD4 counts and higher viral loads increase the patient’s risk for oral candidiasis and other lesions. This patient’s initial CD4 count was 524 cells/μL, and his viral load was 11,232 copies/mL.
TREATMENT
In HIV-negative patients or in HIV-positive patients with a CD4 count greater than 200 cells/μL, the treatment of oral candidiasis involves topical antifungal agents, including a nystatin suspension (Nystat-Rx) or clotrimazole (Mycelex) troches.3,7,9 Treatment should be continued for at least 7 days after resolution of the infection. If resolution does not occur, oral fluconazole (Diflucan) 200 mg daily should be given.
For HIV patients with CD4 counts below 200 cells/μL, oral fluconazole or itraconazole (Sporanox) is recommended, with posaconazole (Noxafil) as an alternative for refractory disease.3,9 Giving fluconazole prophylactically to prevent oral candidiasis is not recommended because of the risk of adverse effects, lack of survival benefit, associated cost, and potential to develop antifungal resistance.3,9
A 23-year-old man presents with a sore throat, dysphagia, and general malaise that began 1 week ago. He also reports a 5-pound weight loss. He has not recently taken antibiotics or inhaled glucocorticoids, and he has no history of tobacco use or trauma to his mouth. He has no personal or family history of oral cancer. He uses cocaine on occasion. He reports feeling feverish and having a decreased appetite.
Q: Based on the history, the symptoms, and the physical examination, which of the following is the most likely diagnosis in this patient?
- Oral hairy leukoplakia
- Squamous cell carcinoma
- Oral candidiasis
- Herpetic gingivostomatitis
- Streptococcal pharyngitis
A: Oral candidiasis is correct.
Otherwise known as thrush, it is common in infants and in denture wearers, and it also can occur in diabetes mellitus, antibiotic therapy, chemotherapy, radiation therapy, and cellular immune deficiency states such as cancer or human immunodeficiency virus (HIV) infection.1 Patients using inhaled glucocorticoids are also at risk and should always be advised to rinse their mouth out with water after inhaled steroid use.
Although Candida albicans is the species most often responsible for candidal infections, other candidal species are increasingly responsible for infections in immunocompromised patients. Candida is part of the normal flora in many adults.
Oral hairy leukoplakia is caused by the Epstein-Barr virus and is often seen in HIV infection. It is a white, painless, corrugated lesion, typically found on the lateral aspect of the tongue, and it cannot be scraped from the adherent surfaces. It can also be found on the dorsum of the tongue, the buccal surfaces, and the floor of the mouth. In an asymptomatic patient with oral hairy leukoplakia, HIV infection with moderate immunosuppression is most likely present.2 Oral hairy leukoplakia is diagnosed by biopsy of suspected lesions. It is not a premalignant lesion, and how to best treat it is still being investigated.3
Squamous cell carcinoma of the oral cavity can present as nonhealing ulcers or masses, dental changes, or exophytic lesions with or without pain.1 They may be accompanied by cervical nodal disease. Malignancies of the oral cavity account for 14% of all head and neck cancers, with squamous cell carcinoma the predominant type.4 Alcohol and tobacco use increase the risk. Alcohol and tobacco together have a synergistic effect on the incidence of oral carcinoma.1,4 Predisposing lesions are leukoplakia, lichen planus of the erosive subtype, submucosal fibrosis, and erythroplakia. Oral infection with human papillomavirus has been shown to increase the risk of oral cancer by a factor of 14, and papillomavirus type 16 is detected in 72% of patients with oropharyngeal cancer.5
Herpetic gingivostomatitis is a manifestation of herpes simplex virus infection. The initial infection may be asymptomatic or may produce groups of vesicles that develop into shallow, painful, and superficial ulcerations on an erythematous base.1,3 If the gingiva is involved, it is erythematous, boggy, and tender.3 Infections are self-limited, lasting up to 2 weeks, but there is potential for recurrence because of the ability of herpes simplex virus to undergo latency. Recurrence is usually heralded by prodromal symptoms 24 hours before onset, with tingling, pain, or burning at the infected site. The diagnosis can be made clinically, but the Tzanck smear test, viral culture, direct fluorescent antibody test, or polymerase chain reaction test can be used to confirm the diagnosis. In patients who are immunocompromised, infections tend to be more severe and to last longer.
Streptococcal pharyngitis, most often caused by group A beta-hemolytic streptococci, is the most common type of bacterial pharyngitis in the clinical setting. The bacteria incubate for 2 to 5 days. The condition mainly affects younger children.6 Patients with “strep throat” often present with a sore throat and high-grade fever. Other symptoms include chills, myalgia, headache, and nausea. Findings on examination may include petechiae of the palate, pharyngeal and tonsillar erythema and exudates, and anterior cervical adenopathy.6 Children often present with coinciding abdominal complaints. A rapid antigen detection test for streptococcal infection can be performed in the office for quick diagnosis, but if clinical suspicion is high, a throat culture is necessary to confirm the diagnosis. Treatment is to prevent complications such as rheumatic fever.6
FEATURES AND DIAGNOSIS OF ORAL CANDIDIASIS
Lesions of oral candidiasis can vary in their appearance. The pseudomembranous form is the most characteristic, with white adherent “cottage-cheese-like” plaques that wipe away, causing minimal bleeding.1,7 The erythematous or atrophic form is associated with denture use and causes a “beefy” appearance on the dorsum of the tongue or on the mucosa that supports a denture.1,7 A third form affects the angles of the mouth, causing angular cheilitis (perlèche).7,8 Chronic infection appears as localized, firmly adherent plaques with an irregular surface similar to hyperkeratosis caused by chronic frictional irritation.7
Oral candidiasis can occur in different forms at the same time. Patients often describe minimal symptoms such as dysgeusia or dry mouth.1,7 Infections causing dysphagia or odynophagia warrant suspicion for involvement of the esophagus.
The diagnosis is made empirically if the lesions resolve with anticandidal therapy. A more definitive diagnosis can be made by microscopy with a potassium hydroxide preparation showing pseudohyphae. Formal culture can also determine the yeast’s susceptibility to medication in recurrent or resistant cases.2
Oral candidiasis may be the manifesting symptom of HIV infection, and more than 90% of patients with adult immunodeficiency syndrome have an episode of thrush.8 When candidiasis is diagnosed without obvious cause, HIV testing should be offered, regardless of a patient’s lack of obvious risk factors. Other oral lesions in HIV patients are oral hairy leukoplakia, Kaposi sarcoma, periodontal and gingival infections, aphthous ulcers, herpes simplex stomatitis, and xerostomia.2 With highly active antiretroviral therapy, the incidence of oral candidiasis has decreased by about 50%.2
Our patient was diagnosed with HIV when screened after this initial presentation. Lower CD4 counts and higher viral loads increase the patient’s risk for oral candidiasis and other lesions. This patient’s initial CD4 count was 524 cells/μL, and his viral load was 11,232 copies/mL.
TREATMENT
In HIV-negative patients or in HIV-positive patients with a CD4 count greater than 200 cells/μL, the treatment of oral candidiasis involves topical antifungal agents, including a nystatin suspension (Nystat-Rx) or clotrimazole (Mycelex) troches.3,7,9 Treatment should be continued for at least 7 days after resolution of the infection. If resolution does not occur, oral fluconazole (Diflucan) 200 mg daily should be given.
For HIV patients with CD4 counts below 200 cells/μL, oral fluconazole or itraconazole (Sporanox) is recommended, with posaconazole (Noxafil) as an alternative for refractory disease.3,9 Giving fluconazole prophylactically to prevent oral candidiasis is not recommended because of the risk of adverse effects, lack of survival benefit, associated cost, and potential to develop antifungal resistance.3,9
- Reichart PA. Clinical management of selected oral fungal and viral infections during HIV-disease. Int Dent J 1999; 49:251–259.
- Kim TB, Pletcher SD, Goldberg AN. Head and neck manifestations in the immunocompromised host. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:209–229(225–226).
- Sciubba JJ. Oral mucosal lesions. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1229–1231).
- Wein R. Malignant Neoplasms of the Oral Cavity. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1236).
- D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944–1956.
- Hayes CS, Williamson H. Management of group A betahemolytic streptococcal pharyngitis. Am Fam Physician 2001; 63:1557–1564.
- Coleman GC. Diseases of the mouth. In:Bope ET, Rakel RE, Kellerman R, editors. Conn’s Current Therapy. Philadelphia, PA: Saunders; 2010:861–867.
- Habif TP. Candidiasis (moniliasis). In: Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:523–536.
- Pappas PG, Rex JH, Sobel JD, et al; Infectious Diseases Society of America. Guidelines for treatment of candidiasis. Clin Infect Dis 2004; 38:161–189.
- Reichart PA. Clinical management of selected oral fungal and viral infections during HIV-disease. Int Dent J 1999; 49:251–259.
- Kim TB, Pletcher SD, Goldberg AN. Head and neck manifestations in the immunocompromised host. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:209–229(225–226).
- Sciubba JJ. Oral mucosal lesions. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1229–1231).
- Wein R. Malignant Neoplasms of the Oral Cavity. In:Flint PW, Haughey BH, Lund VJ, et al, editors. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:1222–1244(1236).
- D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944–1956.
- Hayes CS, Williamson H. Management of group A betahemolytic streptococcal pharyngitis. Am Fam Physician 2001; 63:1557–1564.
- Coleman GC. Diseases of the mouth. In:Bope ET, Rakel RE, Kellerman R, editors. Conn’s Current Therapy. Philadelphia, PA: Saunders; 2010:861–867.
- Habif TP. Candidiasis (moniliasis). In: Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:523–536.
- Pappas PG, Rex JH, Sobel JD, et al; Infectious Diseases Society of America. Guidelines for treatment of candidiasis. Clin Infect Dis 2004; 38:161–189.
Allergy blood testing: A practical guide for clinicians
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
KEY POINTS
- Specific IgE levels higher than 0.35 kU/L suggest sensitization, but that is not synonymous with clinical disease.
- Prospective studies have identified IgE levels that can predict clinical reactivity with greater than 95% certainty for certain foods, but similar studies have not been performed for most other foods, drugs, latex, or venom.
- The likelihood of an IgE-mediated clinical reaction often increases with the level of specific IgE, but these levels do not predict severity or guarantee a reaction will occur.
- The sensitivity of allergy blood tests ranges from 60% to 95%, and the specificity ranges from 30% to 95%.
- In the appropriate setting, these tests can help in identifying specific allergens and assessing allergic disease.
- Neither allergy blood testing nor skin testing should be used for screening: they may be most useful as confirmatory tests when the patient’s history is compatible with an IgE-mediated reaction.
Accountable care organizations, the patient-centered medical home, and health care reform: What does it all mean?
The US health care system cannot continue with “business as usual.” The current model is broken: it does not deliver the kind of care we want for our patients, ourselves, our families, and our communities. It is our role as professionals to help drive change and make medical care more cost-effective and of higher quality, with better satisfaction for patients as well as for providers.
Central to efforts to reform the system are two concepts. One is the “patient-centered medical home,” in which a single provider is responsible for coordinating care for individual patients. The other is “accountable care organizations,” a new way of organizing care along a continuum from doctor to hospital, mandated by the new health care reform law (technically known as the Patient Protection and Affordable Care Act).
CURRENT STATE OF HEALTH CARE: HIGH COST AND POOR QUALITY
Since health care reform was initially proposed in the 1990s, trends in the United States have grown steadily worse. Escalating health care costs have outstripped inflation, consuming an increasing percentage of the gross domestic product (GDP) at an unsustainable rate. Despite increased spending, quality outcomes are suboptimal. In addition, with the emergence of specialization and technology, care is increasingly fragmented and poorly coordinated, with multiple providers and poorly managed resources.
Over the last 15 years, the United States has far surpassed most countries in the developed world for total health care expenditures per capita.1,2 In 2009, we spent 17.4% of our GDP on health care, translating to $7,960 per capita, while Japan spent only 8.5% of its GDP, averaging $2,878 per capita.2 At the current rate, health care spending in the United States will increase from $2.5 trillion in 2009 to over $4.6 trillion in 2020.3
Paradoxically, costlier care is often of poorer quality. Many countries that spend far less per capita on health care achieve far better outcomes. Even within the United States, greater Medicare spending on a state and regional basis tends to correlate with poorer quality of care.4 Spending among Medicare beneficiaries is not standardized and varies widely throughout the country.5 The amount of care a patient receives also varies dramatically by region. The number of specialists involved in care during the last year of life is steadily increasing in many regions of the country, indicating poor care coordination.6
PATIENT-CENTERED MEDICAL HOMES: A POSITIVE TREND
The problems of high cost, poor quality, and poor coordination of care have led to the emergence of the concept of the patient-centered medical home. Originally proposed in 1967 by the American Academy of Pediatrics in response to the need for care coordination by a single physician, the idea did not really take root until the early 1990s. In 2002, the American Academy of Family Medicine embraced the concept and moved it forward.
According to the National Committee for Quality Assurance (NCQA), a nonprofit organization that provides voluntary certification for medical organizations, the patient-centered medical home is a model of care in which “patients have a direct relationship with a provider who coordinates a cooperative team of healthcare professionals, takes collective responsibility for the care provided to the patient, and arranges for appropriate care with other qualified providers as needed.”7
Patient-centered medical homes are supposed to improve quality outcomes and lower costs. In addition, they can compete for public or private incentives that reward this model of care and, as we will see later, are at the heart of ACO readiness.
Medical homes meet certification standards
NCQA first formally licensed patient-centered medical homes in 2008, based on nine standards and six key elements. A scoring system was used to rank the level of certification from level 1 (the lowest) to level 3. From 2008 to the end of 2010, the number of certified homes grew from 28 to 1,506. New York has the largest number of medical homes.
The new elements in the NCQA program align more closely with federal programs that are designed to drive quality, including the Centers for Medicare and Medicaid Services program to encourage the use of the electronic medical record, and with federal rule-making this last spring designed to implement accountable care organizations (ACOs).
Same-day access is now emphasized, as is managing patient populations—rather than just individual patients—with certain chronic diseases, such as diabetes and congestive heart failure. The requirements for tracking and coordinating care have profound implications about how resources are allocated. Ideally, coordinators of chronic disease management are embedded within practices to help manage high-risk patients, although the current reimbursement mechanism does not support this model. Population management may not be feasible for institutions that still rely on paper-based medical records.
Medical homes lower costs, improve quality
Integrated delivery system models such as patient-centered medical homes have demonstrated cost-savings while improving quality of care.8,9 Reducing hospital admissions and visits to the emergency department shows the greatest cost-savings in these models. Several projects have shown significant cost-savings10:
The Group Health Cooperative of Puget Sound reduced total costs by $10 per member per month (from $498 to $488, P = 0.76), with a 16% reduction in hospital admissions (P < .001) and a 29% reduction in emergency department visits (P < .001).
The Geisinger Health System Proven-Health Navigator in Pennsylvania reduced readmissions by 18% (P < .01). They also had a 7% reduction in total costs per member per month relative to a matched control group also in the Geisinger system but not in a medical home, although this difference did not reach statistical significance. Private payer demonstration projects of patient-centered medical homes have also shown cost-savings.
Blue Cross Blue Shield of South Carolina randomized patients to participate in either a patient-centered medical home or their standard system. The patient-centered medical home group had 36% fewer hospital days, 12.4% fewer emergency department visits, and a 6.5% reduction in total medical and pharmacy costs compared with controls.
Finally, the use of chronic care coordinators in a patient-centered medical home has been shown to be cost-effective and can lower the overall cost of care despite the investment to hire them. Johns Hopkins Guided Care program demonstrated a 24% reduction in hospital days, 15% fewer emergency department visits, and a 37% reduction in days in a skilled nursing facility. The annual net Medicare savings was $75,000 per coordinator nurse hired.
ACCOUNTABLE CARE ORGANIZATIONS: A NEW SYSTEM OF HEALTH CARE DELIVERY
While the patient-centered medical home is designed to improve the coordination of care among physicians, ACOs have the broader goal of coordinating care across the entire continuum of health care, from physicians to hospitals to other clinicians. The concept of ACOs was spawned in 2006 by Elliott S. Fisher, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice. The idea is that, by improving care coordination within an ACO and reducing fragmented care, costs can be controlled and outcomes improved. Of course, the devil is in the details.
As part of its health care reform initiative, the state of Massachusetts’ Special Commission on the Health Care Payment System defined ACOs as health care delivery systems composed of hospitals, physicians, and other clinician and nonclinician providers that manage care across the entire spectrum of care. An ACO could be a real (incorporated) or virtual (contractually networked) organization, for example, a large physician organization that would contract with one or more hospitals and ancillary providers.11
In a 2009 report to Congress, the Medicare Payment Advisory Committee (MedPac) similarly defined ACOs for the Medicare population. But MedPac also introduced the concept of financial risk: providers in the ACO would share in efficiency gains from improved care coordination and could be subjected to financial penalties for poor performance, depending on the structure of the ACO.12
But what has placed ACOs at center stage is the new health care reform law, which encourages the formation of ACOs. On March 31, 2011, the Centers for Medicare and Medicaid Services published proposed rules to implement ACOs for Medicare patients (they appeared in the Federal Register on April 7, 2011).13,14 Comments on the 129-page proposed rules were due by June 6, 2011. Final rules are supposed to be published later this year.
The proposed new rule has a three-part aim:
- Better care for individuals, as described by all six dimensions of quality in the Institute of Medicine report “Crossing the Quality Chasm”15: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity
- Better health for populations, with respect to educating beneficiaries about the major causes of ill health—poor nutrition, physical inactivity, substance abuse, and poverty—as well as about the importance of preventive services such as an annual physical examination and annual influenza vaccination
- Lower growth in expenditures by eliminating waste and inefficiencies while not withholding any needed care that helps beneficiaries.
DETAILS OF THE PROPOSED ACO RULE
Here are some of the highlights of the proposed ACO rule.
Two shared-savings options
Although the program could start as soon as January 1, 2012, the application process is formidable, so this timeline may not be realistic. Moreover, a final rule is pending.
The proposed rule requires at least a 3-year contract, and primary care physicians must be included. Shared savings will be available and will depend on an ACO’s ability to manage costs and to achieve quality target performances. Two shared-savings options will be available: one with no risk until the third year and the other with risk during all 3 years but greater potential benefit. In the one-sided model with no risk until year 3, an ACO would begin to accrue shared savings at a rate of 50% after an initial 2% of savings compared with a risk-adjusted per capita benchmark based on performance during the previous 3 years. In the second plan, an ACO would immediately realize shared savings at a rate of 60% as long as savings were achieved compared with prior benchmark performance. However, in this second model, the ACO would be at risk to repay a share of all losses that were more than 2% higher than the benchmark expenditures, with loss caps of 5%, 7.5%, and 10% above benchmark in years 1, 2, and 3, respectively.
Structure of an ACO
Under the proposed rule, the minimum population size of Medicare beneficiaries is 5,000 patients, with some exceptions in rural or other shortage areas, or areas with critical access hospitals. ACO founders can be primary care physicians, primary care independent practice associations, or employee groups. Participants may include hospitals, critical access hospitals, specialists, and other providers. The ACO must be a legal entity with its own tax identification number and its own governance and management structure.
Concerns have been expressed that, in some markets, certain groups may come together and achieve market dominance with more than half of the population. Proposed ACOs with less than 30% of the market share will be exempt from antitrust concerns, and those with greater than 50% of market share will undergo detailed review.
Patient assignment
Patients will be assigned to an ACO retrospectively, at the end of the 3 years. The Centers for Medicare and Medicaid Services argues that retrospective assignment will encourage the ACO to design a system to help all patients, not just those assigned to the ACO.
Patients may not opt out of being counted against ACO performance measures. Although Medicare will share beneficiaries’ data with the ACO retrospectively so that it can learn more about costs per patient, patients may opt out of this data-sharing. Patients also retain unrestricted choice to see other providers, with attribution of costs incurred to the ACO.
Quality and reporting
The proposed rule has 65 equally weighted quality measures, many of which are not presently reported by most health care organizations. The measures fall within five broad categories: patient and caregiver experience, care coordination, patient safety, preventive health, and managing at-risk populations, including the frail elderly. Bonus payments for cost-savings will be adjusted based on meeting the quality measures.
Governance and management
Under the proposed rule, an ACO must meet stringent governance requirements. It must be a distinct legal entity as governed by state law. There must be proportional representation of all participants (eg, hospitals, community organizations, providers), comprising at least 75% of its Board of Trustees. These members must have authority to execute statutory functions of the ACO. Medicare beneficiaries and community stakeholder organizations must also be represented on the Board.
ACO operations must be managed by an executive director, manager, or general partner, who may or may not be a physician. A board-certified physician who is licensed in the state in which the ACO is domiciled must serve on location as the full-time, senior-level medical director, overseeing and managing clinical operations. A leadership team must be able to influence clinical practice, and a physician-directed process-improvement and quality-assurance committee is required.
Infrastructure and policies
The proposed rule outlines a number of infrastructure and policy requirements that must be addressed in the application process. These include:
- Written performance standards for quality and efficiency
- Evidence-based practice guidelines
- Tools to collect, evaluate, and share data to influence decision-making at the point of care
- Processes to identify and correct poor performance
- Description of how shared savings will be used to further improve care.
CONCERNS ABOUT THE PROPOSED NEW ACO RULE
While there is broad consensus in the health care community that the current system of care delivery fails to achieve the desired outcomes and is financially unsustainable and in need of reform, many concerns have been expressed about the proposed new ACO rule.
The regulations are too detailed. The regulations are highly prescriptive with detailed application, reporting, and regulatory requirements that create significant administrative burdens. Small medical groups are unlikely to have the administrative infrastructure to become involved.
Potential savings are inadequate. The shared savings concept has modest upside gain when modeled with holdback.16 Moreover, a recent analysis from the University Health System Consortium suggested that 50% of ACOs with 5,000 or more attributed lives would sustain unwarranted penalties as a result of random fluctuation of expenditures in the population.17
Participation involves a big investment. Participation requires significant resource investment, such as hiring chronic-disease managers and, in some practices, creating a whole new concept of managing wellness and continuity of care.
Retrospective beneficiary assignment is unpopular. Groups would generally prefer to know beforehand for whom they are responsible financially. A prospective assignment model was considered for the proposed rule but was ultimately rejected.
The patient assignment system is too risky. The plurality rule requires only a single visit with the ACO in order to be responsible for a patient for the entire year. In addition, the fact that the patient has the freedom to choose care elsewhere with expense assigned to the ACO confers significant financial risk.
There are too many quality measures. The high number of quality metrics—65—required to be measured and reported is onerous for most organizations.
Advertising is micromanaged. All marketing materials that are sent to patients about the ACO and any subsequent revisions must first be approved by Medicare, a potentially burdensome and time-consuming requirement.
Specialists are excluded. Using only generalists could actually be less cost-effective for some patients, such as those with human immunodeficiency virus, end-stage renal disease, certain malignancies, or advanced congestive heart failure.
Provider replacement is prohibited. Providers cannot be replaced over the 3 years of the demonstration, but the departing physician’s patients are still the responsibility of the plan. This would be especially problematic for small practices.
PREDICTING ACO READINESS
I believe there are five core competencies that are required to be an ACO:
- Operational excellence in care delivery
- Ability to deliver care across the continuum
- Cultural alignment among participating organizations
- Technical and informatics support to manage individual and population data
- Physician alignment around the concept of the ACO.
Certain strategies will increase the chances of success of an ACO:
Reduce emergency department usage and hospitalization. Cost-savings in patient-centered medical homes have been greatest by reducing hospitalizations, rehospitalizations, and emergency department visits.
Develop a high-quality, efficient primary care network. Have enough of a share in the primary care physician network to deliver effective primary care. Make sure there is good access to care and effective communication between patients and the primary care network. Deliver comprehensive services and have good care coordination. Aggressively manage communication, care coordination, and “hand-offs” across the care continuum and with specialists.
Create an effective patient-centered medical home. The current reimbursement climate fails to incentivize all of the necessary elements, which ultimately need to include chronic-care coordinators for medically complex patients, pharmacy support for patient medication management, adequate support staff to optimize efficiency, and a culture of wellness and necessary resources to support wellness.
PHYSICIANS NEED TO DRIVE SOLUTIONS
Soaring health care costs in the United States, poor quality outcomes, and increasing fragmentation of care are the major drivers of health care reform. The Patient Centered Medical Home is a key component to the solution and has already been shown to improve outcomes and lower costs. Further refinement of this concept and implementation should be priorities for primary care physicians and health care organizations.
The ACO concept attempts to further improve quality and lower costs. The proposed ACO rule released by the Centers for Medicare and Medicaid Services on March 31, 2011, has generated significant controversy in the health care community. In its current form, few health care systems are likely to participate. A revised rule is awaited in the coming months. In the meantime, the Centers for Medicare and Medicaid Services has released a request for application for a Pioneer ACO model, which offers up to 30 organizations the opportunity to participate in an ACO pilot that allows for prospective patient assignment and greater shared savings.
Whether ACOs as proposed achieve widespread implementation remains to be seen. However, the current system of health care delivery in this country is broken. Physicians and health care systems need to drive solutions to the challenges we face about quality, cost, access, care coordination, and outcomes.
- The Concord Coalition. Escalating Health Care Costs and the Federal Budget. April 2, 2009. http://www.concordcoalition.org/files/uploaded_for_nodes/docs/Iowa_Handout_final.pdf. Accessed August 8, 2011.
- The Henry J. Kaiser Family Foundation. Snapshots: Health Care Costs. Health Care Spending in the United States and OECD Countries. April 2011. http://www.kff.org/insurance/snapshot/OECD042111.cfm. Accessed August 8, 2011.
- Centers for Medicare and Medicaid Services. National health expenditure projections 2010–2020. http://www.cms.gov/NationalHealthExpendData/downloads/proj2010.pdf. Accessed August 8, 2011.
- The Commonwealth Fund. Performance snapshots, 2006. http://www.cmwf.org/snapshots. Accessed August 8, 2011.
- Fisher E, Goodman D, Skinner J, Bronner K. Health care spending, quality, and outcomes. More isn’t always better. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2009. http://www.dartmouthatlas.org/downloads/reports/Spending_Brief_022709.pdf. Accessed August 8, 2011.
- Goodman DC, Esty AR, Fisher ES, Chang C-H. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2011. http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. Accessed August 8, 2011.
- National Committee for Quality Assurance (NCQA). Leveraging health IT to achieve ambulatory quality: the patient-centered medical home (PCMH). www.ncqa.org/Portals/0/Public%20Policy/HIMSS_NCQA_PCMH_Factsheet.pdf. Accessed August 8, 2011.
- Bodenheimer T. Lessons from the trenches—a high-functioning primary care clinic. N Eng J Med 2011; 365:5–8.
- Gabbay RA, Bailit MH, Mauger DT, Wagner EH, Siminerio L. Multipayer patient-centered medical home implementation guided by the chronic care model. Jt Comm J Qual Patient Saf 2011; 37:265–273.
- Grumbach K, Grundy P. Outcomes of implementing Patient Centered Medical Home interventions: a review of the evidence from prospective evaluation studies in the United States. Patient-Centered Primary Care Collaborative. November 16, 2010. http://www.pcpcc.net/files/evidence_outcomes_in_pcmh.pdf. Accessed August 8, 2011.
- Kirwan LA, Iselin S. Recommendations of the Special Commission on the Health Care Payment System. Commonwealth of Massachusetts, July 16, 2009. http://www.mass.gov/Eeohhs2/docs/dhcfp/pc/Final_Report/Final_Report.pdf. Accessed August 8, 2011.
- Medicare Payment Advisory Commission. Report to the Congress. Improving incentives in the Medicare Program. http://www.medpac.gov/documents/jun09_entirereport.pdf. Accessed August 8, 2011.
- National Archives and Records Administration. Federal Register Volume 76, Number 67, Thursday, April 7, 2011. http://edocket.access.gpo.gov/2011/pdf/2011-7880.pdf. Accessed August 8, 2011.
- Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med 2011; 364:e32.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academy Press; 2001.
- Fitch K, Mirkin D, Murphy-Barron C, Parke R, Pyenson B. A first look at ACOs’ risky business: quality is not enough. Seattle, WA: Millman, Inc; 2011. http://publications.milliman.com/publications/healthreform/pdfs/at-first-lookacos.pdf. Accessed August 10, 2011.
- University HealthSystem Consortium. Accountable care organizations: a measured view for academic medical centers. May 2011.
The US health care system cannot continue with “business as usual.” The current model is broken: it does not deliver the kind of care we want for our patients, ourselves, our families, and our communities. It is our role as professionals to help drive change and make medical care more cost-effective and of higher quality, with better satisfaction for patients as well as for providers.
Central to efforts to reform the system are two concepts. One is the “patient-centered medical home,” in which a single provider is responsible for coordinating care for individual patients. The other is “accountable care organizations,” a new way of organizing care along a continuum from doctor to hospital, mandated by the new health care reform law (technically known as the Patient Protection and Affordable Care Act).
CURRENT STATE OF HEALTH CARE: HIGH COST AND POOR QUALITY
Since health care reform was initially proposed in the 1990s, trends in the United States have grown steadily worse. Escalating health care costs have outstripped inflation, consuming an increasing percentage of the gross domestic product (GDP) at an unsustainable rate. Despite increased spending, quality outcomes are suboptimal. In addition, with the emergence of specialization and technology, care is increasingly fragmented and poorly coordinated, with multiple providers and poorly managed resources.
Over the last 15 years, the United States has far surpassed most countries in the developed world for total health care expenditures per capita.1,2 In 2009, we spent 17.4% of our GDP on health care, translating to $7,960 per capita, while Japan spent only 8.5% of its GDP, averaging $2,878 per capita.2 At the current rate, health care spending in the United States will increase from $2.5 trillion in 2009 to over $4.6 trillion in 2020.3
Paradoxically, costlier care is often of poorer quality. Many countries that spend far less per capita on health care achieve far better outcomes. Even within the United States, greater Medicare spending on a state and regional basis tends to correlate with poorer quality of care.4 Spending among Medicare beneficiaries is not standardized and varies widely throughout the country.5 The amount of care a patient receives also varies dramatically by region. The number of specialists involved in care during the last year of life is steadily increasing in many regions of the country, indicating poor care coordination.6
PATIENT-CENTERED MEDICAL HOMES: A POSITIVE TREND
The problems of high cost, poor quality, and poor coordination of care have led to the emergence of the concept of the patient-centered medical home. Originally proposed in 1967 by the American Academy of Pediatrics in response to the need for care coordination by a single physician, the idea did not really take root until the early 1990s. In 2002, the American Academy of Family Medicine embraced the concept and moved it forward.
According to the National Committee for Quality Assurance (NCQA), a nonprofit organization that provides voluntary certification for medical organizations, the patient-centered medical home is a model of care in which “patients have a direct relationship with a provider who coordinates a cooperative team of healthcare professionals, takes collective responsibility for the care provided to the patient, and arranges for appropriate care with other qualified providers as needed.”7
Patient-centered medical homes are supposed to improve quality outcomes and lower costs. In addition, they can compete for public or private incentives that reward this model of care and, as we will see later, are at the heart of ACO readiness.
Medical homes meet certification standards
NCQA first formally licensed patient-centered medical homes in 2008, based on nine standards and six key elements. A scoring system was used to rank the level of certification from level 1 (the lowest) to level 3. From 2008 to the end of 2010, the number of certified homes grew from 28 to 1,506. New York has the largest number of medical homes.
The new elements in the NCQA program align more closely with federal programs that are designed to drive quality, including the Centers for Medicare and Medicaid Services program to encourage the use of the electronic medical record, and with federal rule-making this last spring designed to implement accountable care organizations (ACOs).
Same-day access is now emphasized, as is managing patient populations—rather than just individual patients—with certain chronic diseases, such as diabetes and congestive heart failure. The requirements for tracking and coordinating care have profound implications about how resources are allocated. Ideally, coordinators of chronic disease management are embedded within practices to help manage high-risk patients, although the current reimbursement mechanism does not support this model. Population management may not be feasible for institutions that still rely on paper-based medical records.
Medical homes lower costs, improve quality
Integrated delivery system models such as patient-centered medical homes have demonstrated cost-savings while improving quality of care.8,9 Reducing hospital admissions and visits to the emergency department shows the greatest cost-savings in these models. Several projects have shown significant cost-savings10:
The Group Health Cooperative of Puget Sound reduced total costs by $10 per member per month (from $498 to $488, P = 0.76), with a 16% reduction in hospital admissions (P < .001) and a 29% reduction in emergency department visits (P < .001).
The Geisinger Health System Proven-Health Navigator in Pennsylvania reduced readmissions by 18% (P < .01). They also had a 7% reduction in total costs per member per month relative to a matched control group also in the Geisinger system but not in a medical home, although this difference did not reach statistical significance. Private payer demonstration projects of patient-centered medical homes have also shown cost-savings.
Blue Cross Blue Shield of South Carolina randomized patients to participate in either a patient-centered medical home or their standard system. The patient-centered medical home group had 36% fewer hospital days, 12.4% fewer emergency department visits, and a 6.5% reduction in total medical and pharmacy costs compared with controls.
Finally, the use of chronic care coordinators in a patient-centered medical home has been shown to be cost-effective and can lower the overall cost of care despite the investment to hire them. Johns Hopkins Guided Care program demonstrated a 24% reduction in hospital days, 15% fewer emergency department visits, and a 37% reduction in days in a skilled nursing facility. The annual net Medicare savings was $75,000 per coordinator nurse hired.
ACCOUNTABLE CARE ORGANIZATIONS: A NEW SYSTEM OF HEALTH CARE DELIVERY
While the patient-centered medical home is designed to improve the coordination of care among physicians, ACOs have the broader goal of coordinating care across the entire continuum of health care, from physicians to hospitals to other clinicians. The concept of ACOs was spawned in 2006 by Elliott S. Fisher, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice. The idea is that, by improving care coordination within an ACO and reducing fragmented care, costs can be controlled and outcomes improved. Of course, the devil is in the details.
As part of its health care reform initiative, the state of Massachusetts’ Special Commission on the Health Care Payment System defined ACOs as health care delivery systems composed of hospitals, physicians, and other clinician and nonclinician providers that manage care across the entire spectrum of care. An ACO could be a real (incorporated) or virtual (contractually networked) organization, for example, a large physician organization that would contract with one or more hospitals and ancillary providers.11
In a 2009 report to Congress, the Medicare Payment Advisory Committee (MedPac) similarly defined ACOs for the Medicare population. But MedPac also introduced the concept of financial risk: providers in the ACO would share in efficiency gains from improved care coordination and could be subjected to financial penalties for poor performance, depending on the structure of the ACO.12
But what has placed ACOs at center stage is the new health care reform law, which encourages the formation of ACOs. On March 31, 2011, the Centers for Medicare and Medicaid Services published proposed rules to implement ACOs for Medicare patients (they appeared in the Federal Register on April 7, 2011).13,14 Comments on the 129-page proposed rules were due by June 6, 2011. Final rules are supposed to be published later this year.
The proposed new rule has a three-part aim:
- Better care for individuals, as described by all six dimensions of quality in the Institute of Medicine report “Crossing the Quality Chasm”15: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity
- Better health for populations, with respect to educating beneficiaries about the major causes of ill health—poor nutrition, physical inactivity, substance abuse, and poverty—as well as about the importance of preventive services such as an annual physical examination and annual influenza vaccination
- Lower growth in expenditures by eliminating waste and inefficiencies while not withholding any needed care that helps beneficiaries.
DETAILS OF THE PROPOSED ACO RULE
Here are some of the highlights of the proposed ACO rule.
Two shared-savings options
Although the program could start as soon as January 1, 2012, the application process is formidable, so this timeline may not be realistic. Moreover, a final rule is pending.
The proposed rule requires at least a 3-year contract, and primary care physicians must be included. Shared savings will be available and will depend on an ACO’s ability to manage costs and to achieve quality target performances. Two shared-savings options will be available: one with no risk until the third year and the other with risk during all 3 years but greater potential benefit. In the one-sided model with no risk until year 3, an ACO would begin to accrue shared savings at a rate of 50% after an initial 2% of savings compared with a risk-adjusted per capita benchmark based on performance during the previous 3 years. In the second plan, an ACO would immediately realize shared savings at a rate of 60% as long as savings were achieved compared with prior benchmark performance. However, in this second model, the ACO would be at risk to repay a share of all losses that were more than 2% higher than the benchmark expenditures, with loss caps of 5%, 7.5%, and 10% above benchmark in years 1, 2, and 3, respectively.
Structure of an ACO
Under the proposed rule, the minimum population size of Medicare beneficiaries is 5,000 patients, with some exceptions in rural or other shortage areas, or areas with critical access hospitals. ACO founders can be primary care physicians, primary care independent practice associations, or employee groups. Participants may include hospitals, critical access hospitals, specialists, and other providers. The ACO must be a legal entity with its own tax identification number and its own governance and management structure.
Concerns have been expressed that, in some markets, certain groups may come together and achieve market dominance with more than half of the population. Proposed ACOs with less than 30% of the market share will be exempt from antitrust concerns, and those with greater than 50% of market share will undergo detailed review.
Patient assignment
Patients will be assigned to an ACO retrospectively, at the end of the 3 years. The Centers for Medicare and Medicaid Services argues that retrospective assignment will encourage the ACO to design a system to help all patients, not just those assigned to the ACO.
Patients may not opt out of being counted against ACO performance measures. Although Medicare will share beneficiaries’ data with the ACO retrospectively so that it can learn more about costs per patient, patients may opt out of this data-sharing. Patients also retain unrestricted choice to see other providers, with attribution of costs incurred to the ACO.
Quality and reporting
The proposed rule has 65 equally weighted quality measures, many of which are not presently reported by most health care organizations. The measures fall within five broad categories: patient and caregiver experience, care coordination, patient safety, preventive health, and managing at-risk populations, including the frail elderly. Bonus payments for cost-savings will be adjusted based on meeting the quality measures.
Governance and management
Under the proposed rule, an ACO must meet stringent governance requirements. It must be a distinct legal entity as governed by state law. There must be proportional representation of all participants (eg, hospitals, community organizations, providers), comprising at least 75% of its Board of Trustees. These members must have authority to execute statutory functions of the ACO. Medicare beneficiaries and community stakeholder organizations must also be represented on the Board.
ACO operations must be managed by an executive director, manager, or general partner, who may or may not be a physician. A board-certified physician who is licensed in the state in which the ACO is domiciled must serve on location as the full-time, senior-level medical director, overseeing and managing clinical operations. A leadership team must be able to influence clinical practice, and a physician-directed process-improvement and quality-assurance committee is required.
Infrastructure and policies
The proposed rule outlines a number of infrastructure and policy requirements that must be addressed in the application process. These include:
- Written performance standards for quality and efficiency
- Evidence-based practice guidelines
- Tools to collect, evaluate, and share data to influence decision-making at the point of care
- Processes to identify and correct poor performance
- Description of how shared savings will be used to further improve care.
CONCERNS ABOUT THE PROPOSED NEW ACO RULE
While there is broad consensus in the health care community that the current system of care delivery fails to achieve the desired outcomes and is financially unsustainable and in need of reform, many concerns have been expressed about the proposed new ACO rule.
The regulations are too detailed. The regulations are highly prescriptive with detailed application, reporting, and regulatory requirements that create significant administrative burdens. Small medical groups are unlikely to have the administrative infrastructure to become involved.
Potential savings are inadequate. The shared savings concept has modest upside gain when modeled with holdback.16 Moreover, a recent analysis from the University Health System Consortium suggested that 50% of ACOs with 5,000 or more attributed lives would sustain unwarranted penalties as a result of random fluctuation of expenditures in the population.17
Participation involves a big investment. Participation requires significant resource investment, such as hiring chronic-disease managers and, in some practices, creating a whole new concept of managing wellness and continuity of care.
Retrospective beneficiary assignment is unpopular. Groups would generally prefer to know beforehand for whom they are responsible financially. A prospective assignment model was considered for the proposed rule but was ultimately rejected.
The patient assignment system is too risky. The plurality rule requires only a single visit with the ACO in order to be responsible for a patient for the entire year. In addition, the fact that the patient has the freedom to choose care elsewhere with expense assigned to the ACO confers significant financial risk.
There are too many quality measures. The high number of quality metrics—65—required to be measured and reported is onerous for most organizations.
Advertising is micromanaged. All marketing materials that are sent to patients about the ACO and any subsequent revisions must first be approved by Medicare, a potentially burdensome and time-consuming requirement.
Specialists are excluded. Using only generalists could actually be less cost-effective for some patients, such as those with human immunodeficiency virus, end-stage renal disease, certain malignancies, or advanced congestive heart failure.
Provider replacement is prohibited. Providers cannot be replaced over the 3 years of the demonstration, but the departing physician’s patients are still the responsibility of the plan. This would be especially problematic for small practices.
PREDICTING ACO READINESS
I believe there are five core competencies that are required to be an ACO:
- Operational excellence in care delivery
- Ability to deliver care across the continuum
- Cultural alignment among participating organizations
- Technical and informatics support to manage individual and population data
- Physician alignment around the concept of the ACO.
Certain strategies will increase the chances of success of an ACO:
Reduce emergency department usage and hospitalization. Cost-savings in patient-centered medical homes have been greatest by reducing hospitalizations, rehospitalizations, and emergency department visits.
Develop a high-quality, efficient primary care network. Have enough of a share in the primary care physician network to deliver effective primary care. Make sure there is good access to care and effective communication between patients and the primary care network. Deliver comprehensive services and have good care coordination. Aggressively manage communication, care coordination, and “hand-offs” across the care continuum and with specialists.
Create an effective patient-centered medical home. The current reimbursement climate fails to incentivize all of the necessary elements, which ultimately need to include chronic-care coordinators for medically complex patients, pharmacy support for patient medication management, adequate support staff to optimize efficiency, and a culture of wellness and necessary resources to support wellness.
PHYSICIANS NEED TO DRIVE SOLUTIONS
Soaring health care costs in the United States, poor quality outcomes, and increasing fragmentation of care are the major drivers of health care reform. The Patient Centered Medical Home is a key component to the solution and has already been shown to improve outcomes and lower costs. Further refinement of this concept and implementation should be priorities for primary care physicians and health care organizations.
The ACO concept attempts to further improve quality and lower costs. The proposed ACO rule released by the Centers for Medicare and Medicaid Services on March 31, 2011, has generated significant controversy in the health care community. In its current form, few health care systems are likely to participate. A revised rule is awaited in the coming months. In the meantime, the Centers for Medicare and Medicaid Services has released a request for application for a Pioneer ACO model, which offers up to 30 organizations the opportunity to participate in an ACO pilot that allows for prospective patient assignment and greater shared savings.
Whether ACOs as proposed achieve widespread implementation remains to be seen. However, the current system of health care delivery in this country is broken. Physicians and health care systems need to drive solutions to the challenges we face about quality, cost, access, care coordination, and outcomes.
The US health care system cannot continue with “business as usual.” The current model is broken: it does not deliver the kind of care we want for our patients, ourselves, our families, and our communities. It is our role as professionals to help drive change and make medical care more cost-effective and of higher quality, with better satisfaction for patients as well as for providers.
Central to efforts to reform the system are two concepts. One is the “patient-centered medical home,” in which a single provider is responsible for coordinating care for individual patients. The other is “accountable care organizations,” a new way of organizing care along a continuum from doctor to hospital, mandated by the new health care reform law (technically known as the Patient Protection and Affordable Care Act).
CURRENT STATE OF HEALTH CARE: HIGH COST AND POOR QUALITY
Since health care reform was initially proposed in the 1990s, trends in the United States have grown steadily worse. Escalating health care costs have outstripped inflation, consuming an increasing percentage of the gross domestic product (GDP) at an unsustainable rate. Despite increased spending, quality outcomes are suboptimal. In addition, with the emergence of specialization and technology, care is increasingly fragmented and poorly coordinated, with multiple providers and poorly managed resources.
Over the last 15 years, the United States has far surpassed most countries in the developed world for total health care expenditures per capita.1,2 In 2009, we spent 17.4% of our GDP on health care, translating to $7,960 per capita, while Japan spent only 8.5% of its GDP, averaging $2,878 per capita.2 At the current rate, health care spending in the United States will increase from $2.5 trillion in 2009 to over $4.6 trillion in 2020.3
Paradoxically, costlier care is often of poorer quality. Many countries that spend far less per capita on health care achieve far better outcomes. Even within the United States, greater Medicare spending on a state and regional basis tends to correlate with poorer quality of care.4 Spending among Medicare beneficiaries is not standardized and varies widely throughout the country.5 The amount of care a patient receives also varies dramatically by region. The number of specialists involved in care during the last year of life is steadily increasing in many regions of the country, indicating poor care coordination.6
PATIENT-CENTERED MEDICAL HOMES: A POSITIVE TREND
The problems of high cost, poor quality, and poor coordination of care have led to the emergence of the concept of the patient-centered medical home. Originally proposed in 1967 by the American Academy of Pediatrics in response to the need for care coordination by a single physician, the idea did not really take root until the early 1990s. In 2002, the American Academy of Family Medicine embraced the concept and moved it forward.
According to the National Committee for Quality Assurance (NCQA), a nonprofit organization that provides voluntary certification for medical organizations, the patient-centered medical home is a model of care in which “patients have a direct relationship with a provider who coordinates a cooperative team of healthcare professionals, takes collective responsibility for the care provided to the patient, and arranges for appropriate care with other qualified providers as needed.”7
Patient-centered medical homes are supposed to improve quality outcomes and lower costs. In addition, they can compete for public or private incentives that reward this model of care and, as we will see later, are at the heart of ACO readiness.
Medical homes meet certification standards
NCQA first formally licensed patient-centered medical homes in 2008, based on nine standards and six key elements. A scoring system was used to rank the level of certification from level 1 (the lowest) to level 3. From 2008 to the end of 2010, the number of certified homes grew from 28 to 1,506. New York has the largest number of medical homes.
The new elements in the NCQA program align more closely with federal programs that are designed to drive quality, including the Centers for Medicare and Medicaid Services program to encourage the use of the electronic medical record, and with federal rule-making this last spring designed to implement accountable care organizations (ACOs).
Same-day access is now emphasized, as is managing patient populations—rather than just individual patients—with certain chronic diseases, such as diabetes and congestive heart failure. The requirements for tracking and coordinating care have profound implications about how resources are allocated. Ideally, coordinators of chronic disease management are embedded within practices to help manage high-risk patients, although the current reimbursement mechanism does not support this model. Population management may not be feasible for institutions that still rely on paper-based medical records.
Medical homes lower costs, improve quality
Integrated delivery system models such as patient-centered medical homes have demonstrated cost-savings while improving quality of care.8,9 Reducing hospital admissions and visits to the emergency department shows the greatest cost-savings in these models. Several projects have shown significant cost-savings10:
The Group Health Cooperative of Puget Sound reduced total costs by $10 per member per month (from $498 to $488, P = 0.76), with a 16% reduction in hospital admissions (P < .001) and a 29% reduction in emergency department visits (P < .001).
The Geisinger Health System Proven-Health Navigator in Pennsylvania reduced readmissions by 18% (P < .01). They also had a 7% reduction in total costs per member per month relative to a matched control group also in the Geisinger system but not in a medical home, although this difference did not reach statistical significance. Private payer demonstration projects of patient-centered medical homes have also shown cost-savings.
Blue Cross Blue Shield of South Carolina randomized patients to participate in either a patient-centered medical home or their standard system. The patient-centered medical home group had 36% fewer hospital days, 12.4% fewer emergency department visits, and a 6.5% reduction in total medical and pharmacy costs compared with controls.
Finally, the use of chronic care coordinators in a patient-centered medical home has been shown to be cost-effective and can lower the overall cost of care despite the investment to hire them. Johns Hopkins Guided Care program demonstrated a 24% reduction in hospital days, 15% fewer emergency department visits, and a 37% reduction in days in a skilled nursing facility. The annual net Medicare savings was $75,000 per coordinator nurse hired.
ACCOUNTABLE CARE ORGANIZATIONS: A NEW SYSTEM OF HEALTH CARE DELIVERY
While the patient-centered medical home is designed to improve the coordination of care among physicians, ACOs have the broader goal of coordinating care across the entire continuum of health care, from physicians to hospitals to other clinicians. The concept of ACOs was spawned in 2006 by Elliott S. Fisher, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice. The idea is that, by improving care coordination within an ACO and reducing fragmented care, costs can be controlled and outcomes improved. Of course, the devil is in the details.
As part of its health care reform initiative, the state of Massachusetts’ Special Commission on the Health Care Payment System defined ACOs as health care delivery systems composed of hospitals, physicians, and other clinician and nonclinician providers that manage care across the entire spectrum of care. An ACO could be a real (incorporated) or virtual (contractually networked) organization, for example, a large physician organization that would contract with one or more hospitals and ancillary providers.11
In a 2009 report to Congress, the Medicare Payment Advisory Committee (MedPac) similarly defined ACOs for the Medicare population. But MedPac also introduced the concept of financial risk: providers in the ACO would share in efficiency gains from improved care coordination and could be subjected to financial penalties for poor performance, depending on the structure of the ACO.12
But what has placed ACOs at center stage is the new health care reform law, which encourages the formation of ACOs. On March 31, 2011, the Centers for Medicare and Medicaid Services published proposed rules to implement ACOs for Medicare patients (they appeared in the Federal Register on April 7, 2011).13,14 Comments on the 129-page proposed rules were due by June 6, 2011. Final rules are supposed to be published later this year.
The proposed new rule has a three-part aim:
- Better care for individuals, as described by all six dimensions of quality in the Institute of Medicine report “Crossing the Quality Chasm”15: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity
- Better health for populations, with respect to educating beneficiaries about the major causes of ill health—poor nutrition, physical inactivity, substance abuse, and poverty—as well as about the importance of preventive services such as an annual physical examination and annual influenza vaccination
- Lower growth in expenditures by eliminating waste and inefficiencies while not withholding any needed care that helps beneficiaries.
DETAILS OF THE PROPOSED ACO RULE
Here are some of the highlights of the proposed ACO rule.
Two shared-savings options
Although the program could start as soon as January 1, 2012, the application process is formidable, so this timeline may not be realistic. Moreover, a final rule is pending.
The proposed rule requires at least a 3-year contract, and primary care physicians must be included. Shared savings will be available and will depend on an ACO’s ability to manage costs and to achieve quality target performances. Two shared-savings options will be available: one with no risk until the third year and the other with risk during all 3 years but greater potential benefit. In the one-sided model with no risk until year 3, an ACO would begin to accrue shared savings at a rate of 50% after an initial 2% of savings compared with a risk-adjusted per capita benchmark based on performance during the previous 3 years. In the second plan, an ACO would immediately realize shared savings at a rate of 60% as long as savings were achieved compared with prior benchmark performance. However, in this second model, the ACO would be at risk to repay a share of all losses that were more than 2% higher than the benchmark expenditures, with loss caps of 5%, 7.5%, and 10% above benchmark in years 1, 2, and 3, respectively.
Structure of an ACO
Under the proposed rule, the minimum population size of Medicare beneficiaries is 5,000 patients, with some exceptions in rural or other shortage areas, or areas with critical access hospitals. ACO founders can be primary care physicians, primary care independent practice associations, or employee groups. Participants may include hospitals, critical access hospitals, specialists, and other providers. The ACO must be a legal entity with its own tax identification number and its own governance and management structure.
Concerns have been expressed that, in some markets, certain groups may come together and achieve market dominance with more than half of the population. Proposed ACOs with less than 30% of the market share will be exempt from antitrust concerns, and those with greater than 50% of market share will undergo detailed review.
Patient assignment
Patients will be assigned to an ACO retrospectively, at the end of the 3 years. The Centers for Medicare and Medicaid Services argues that retrospective assignment will encourage the ACO to design a system to help all patients, not just those assigned to the ACO.
Patients may not opt out of being counted against ACO performance measures. Although Medicare will share beneficiaries’ data with the ACO retrospectively so that it can learn more about costs per patient, patients may opt out of this data-sharing. Patients also retain unrestricted choice to see other providers, with attribution of costs incurred to the ACO.
Quality and reporting
The proposed rule has 65 equally weighted quality measures, many of which are not presently reported by most health care organizations. The measures fall within five broad categories: patient and caregiver experience, care coordination, patient safety, preventive health, and managing at-risk populations, including the frail elderly. Bonus payments for cost-savings will be adjusted based on meeting the quality measures.
Governance and management
Under the proposed rule, an ACO must meet stringent governance requirements. It must be a distinct legal entity as governed by state law. There must be proportional representation of all participants (eg, hospitals, community organizations, providers), comprising at least 75% of its Board of Trustees. These members must have authority to execute statutory functions of the ACO. Medicare beneficiaries and community stakeholder organizations must also be represented on the Board.
ACO operations must be managed by an executive director, manager, or general partner, who may or may not be a physician. A board-certified physician who is licensed in the state in which the ACO is domiciled must serve on location as the full-time, senior-level medical director, overseeing and managing clinical operations. A leadership team must be able to influence clinical practice, and a physician-directed process-improvement and quality-assurance committee is required.
Infrastructure and policies
The proposed rule outlines a number of infrastructure and policy requirements that must be addressed in the application process. These include:
- Written performance standards for quality and efficiency
- Evidence-based practice guidelines
- Tools to collect, evaluate, and share data to influence decision-making at the point of care
- Processes to identify and correct poor performance
- Description of how shared savings will be used to further improve care.
CONCERNS ABOUT THE PROPOSED NEW ACO RULE
While there is broad consensus in the health care community that the current system of care delivery fails to achieve the desired outcomes and is financially unsustainable and in need of reform, many concerns have been expressed about the proposed new ACO rule.
The regulations are too detailed. The regulations are highly prescriptive with detailed application, reporting, and regulatory requirements that create significant administrative burdens. Small medical groups are unlikely to have the administrative infrastructure to become involved.
Potential savings are inadequate. The shared savings concept has modest upside gain when modeled with holdback.16 Moreover, a recent analysis from the University Health System Consortium suggested that 50% of ACOs with 5,000 or more attributed lives would sustain unwarranted penalties as a result of random fluctuation of expenditures in the population.17
Participation involves a big investment. Participation requires significant resource investment, such as hiring chronic-disease managers and, in some practices, creating a whole new concept of managing wellness and continuity of care.
Retrospective beneficiary assignment is unpopular. Groups would generally prefer to know beforehand for whom they are responsible financially. A prospective assignment model was considered for the proposed rule but was ultimately rejected.
The patient assignment system is too risky. The plurality rule requires only a single visit with the ACO in order to be responsible for a patient for the entire year. In addition, the fact that the patient has the freedom to choose care elsewhere with expense assigned to the ACO confers significant financial risk.
There are too many quality measures. The high number of quality metrics—65—required to be measured and reported is onerous for most organizations.
Advertising is micromanaged. All marketing materials that are sent to patients about the ACO and any subsequent revisions must first be approved by Medicare, a potentially burdensome and time-consuming requirement.
Specialists are excluded. Using only generalists could actually be less cost-effective for some patients, such as those with human immunodeficiency virus, end-stage renal disease, certain malignancies, or advanced congestive heart failure.
Provider replacement is prohibited. Providers cannot be replaced over the 3 years of the demonstration, but the departing physician’s patients are still the responsibility of the plan. This would be especially problematic for small practices.
PREDICTING ACO READINESS
I believe there are five core competencies that are required to be an ACO:
- Operational excellence in care delivery
- Ability to deliver care across the continuum
- Cultural alignment among participating organizations
- Technical and informatics support to manage individual and population data
- Physician alignment around the concept of the ACO.
Certain strategies will increase the chances of success of an ACO:
Reduce emergency department usage and hospitalization. Cost-savings in patient-centered medical homes have been greatest by reducing hospitalizations, rehospitalizations, and emergency department visits.
Develop a high-quality, efficient primary care network. Have enough of a share in the primary care physician network to deliver effective primary care. Make sure there is good access to care and effective communication between patients and the primary care network. Deliver comprehensive services and have good care coordination. Aggressively manage communication, care coordination, and “hand-offs” across the care continuum and with specialists.
Create an effective patient-centered medical home. The current reimbursement climate fails to incentivize all of the necessary elements, which ultimately need to include chronic-care coordinators for medically complex patients, pharmacy support for patient medication management, adequate support staff to optimize efficiency, and a culture of wellness and necessary resources to support wellness.
PHYSICIANS NEED TO DRIVE SOLUTIONS
Soaring health care costs in the United States, poor quality outcomes, and increasing fragmentation of care are the major drivers of health care reform. The Patient Centered Medical Home is a key component to the solution and has already been shown to improve outcomes and lower costs. Further refinement of this concept and implementation should be priorities for primary care physicians and health care organizations.
The ACO concept attempts to further improve quality and lower costs. The proposed ACO rule released by the Centers for Medicare and Medicaid Services on March 31, 2011, has generated significant controversy in the health care community. In its current form, few health care systems are likely to participate. A revised rule is awaited in the coming months. In the meantime, the Centers for Medicare and Medicaid Services has released a request for application for a Pioneer ACO model, which offers up to 30 organizations the opportunity to participate in an ACO pilot that allows for prospective patient assignment and greater shared savings.
Whether ACOs as proposed achieve widespread implementation remains to be seen. However, the current system of health care delivery in this country is broken. Physicians and health care systems need to drive solutions to the challenges we face about quality, cost, access, care coordination, and outcomes.
- The Concord Coalition. Escalating Health Care Costs and the Federal Budget. April 2, 2009. http://www.concordcoalition.org/files/uploaded_for_nodes/docs/Iowa_Handout_final.pdf. Accessed August 8, 2011.
- The Henry J. Kaiser Family Foundation. Snapshots: Health Care Costs. Health Care Spending in the United States and OECD Countries. April 2011. http://www.kff.org/insurance/snapshot/OECD042111.cfm. Accessed August 8, 2011.
- Centers for Medicare and Medicaid Services. National health expenditure projections 2010–2020. http://www.cms.gov/NationalHealthExpendData/downloads/proj2010.pdf. Accessed August 8, 2011.
- The Commonwealth Fund. Performance snapshots, 2006. http://www.cmwf.org/snapshots. Accessed August 8, 2011.
- Fisher E, Goodman D, Skinner J, Bronner K. Health care spending, quality, and outcomes. More isn’t always better. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2009. http://www.dartmouthatlas.org/downloads/reports/Spending_Brief_022709.pdf. Accessed August 8, 2011.
- Goodman DC, Esty AR, Fisher ES, Chang C-H. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2011. http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. Accessed August 8, 2011.
- National Committee for Quality Assurance (NCQA). Leveraging health IT to achieve ambulatory quality: the patient-centered medical home (PCMH). www.ncqa.org/Portals/0/Public%20Policy/HIMSS_NCQA_PCMH_Factsheet.pdf. Accessed August 8, 2011.
- Bodenheimer T. Lessons from the trenches—a high-functioning primary care clinic. N Eng J Med 2011; 365:5–8.
- Gabbay RA, Bailit MH, Mauger DT, Wagner EH, Siminerio L. Multipayer patient-centered medical home implementation guided by the chronic care model. Jt Comm J Qual Patient Saf 2011; 37:265–273.
- Grumbach K, Grundy P. Outcomes of implementing Patient Centered Medical Home interventions: a review of the evidence from prospective evaluation studies in the United States. Patient-Centered Primary Care Collaborative. November 16, 2010. http://www.pcpcc.net/files/evidence_outcomes_in_pcmh.pdf. Accessed August 8, 2011.
- Kirwan LA, Iselin S. Recommendations of the Special Commission on the Health Care Payment System. Commonwealth of Massachusetts, July 16, 2009. http://www.mass.gov/Eeohhs2/docs/dhcfp/pc/Final_Report/Final_Report.pdf. Accessed August 8, 2011.
- Medicare Payment Advisory Commission. Report to the Congress. Improving incentives in the Medicare Program. http://www.medpac.gov/documents/jun09_entirereport.pdf. Accessed August 8, 2011.
- National Archives and Records Administration. Federal Register Volume 76, Number 67, Thursday, April 7, 2011. http://edocket.access.gpo.gov/2011/pdf/2011-7880.pdf. Accessed August 8, 2011.
- Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med 2011; 364:e32.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academy Press; 2001.
- Fitch K, Mirkin D, Murphy-Barron C, Parke R, Pyenson B. A first look at ACOs’ risky business: quality is not enough. Seattle, WA: Millman, Inc; 2011. http://publications.milliman.com/publications/healthreform/pdfs/at-first-lookacos.pdf. Accessed August 10, 2011.
- University HealthSystem Consortium. Accountable care organizations: a measured view for academic medical centers. May 2011.
- The Concord Coalition. Escalating Health Care Costs and the Federal Budget. April 2, 2009. http://www.concordcoalition.org/files/uploaded_for_nodes/docs/Iowa_Handout_final.pdf. Accessed August 8, 2011.
- The Henry J. Kaiser Family Foundation. Snapshots: Health Care Costs. Health Care Spending in the United States and OECD Countries. April 2011. http://www.kff.org/insurance/snapshot/OECD042111.cfm. Accessed August 8, 2011.
- Centers for Medicare and Medicaid Services. National health expenditure projections 2010–2020. http://www.cms.gov/NationalHealthExpendData/downloads/proj2010.pdf. Accessed August 8, 2011.
- The Commonwealth Fund. Performance snapshots, 2006. http://www.cmwf.org/snapshots. Accessed August 8, 2011.
- Fisher E, Goodman D, Skinner J, Bronner K. Health care spending, quality, and outcomes. More isn’t always better. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2009. http://www.dartmouthatlas.org/downloads/reports/Spending_Brief_022709.pdf. Accessed August 8, 2011.
- Goodman DC, Esty AR, Fisher ES, Chang C-H. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness. The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2011. http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. Accessed August 8, 2011.
- National Committee for Quality Assurance (NCQA). Leveraging health IT to achieve ambulatory quality: the patient-centered medical home (PCMH). www.ncqa.org/Portals/0/Public%20Policy/HIMSS_NCQA_PCMH_Factsheet.pdf. Accessed August 8, 2011.
- Bodenheimer T. Lessons from the trenches—a high-functioning primary care clinic. N Eng J Med 2011; 365:5–8.
- Gabbay RA, Bailit MH, Mauger DT, Wagner EH, Siminerio L. Multipayer patient-centered medical home implementation guided by the chronic care model. Jt Comm J Qual Patient Saf 2011; 37:265–273.
- Grumbach K, Grundy P. Outcomes of implementing Patient Centered Medical Home interventions: a review of the evidence from prospective evaluation studies in the United States. Patient-Centered Primary Care Collaborative. November 16, 2010. http://www.pcpcc.net/files/evidence_outcomes_in_pcmh.pdf. Accessed August 8, 2011.
- Kirwan LA, Iselin S. Recommendations of the Special Commission on the Health Care Payment System. Commonwealth of Massachusetts, July 16, 2009. http://www.mass.gov/Eeohhs2/docs/dhcfp/pc/Final_Report/Final_Report.pdf. Accessed August 8, 2011.
- Medicare Payment Advisory Commission. Report to the Congress. Improving incentives in the Medicare Program. http://www.medpac.gov/documents/jun09_entirereport.pdf. Accessed August 8, 2011.
- National Archives and Records Administration. Federal Register Volume 76, Number 67, Thursday, April 7, 2011. http://edocket.access.gpo.gov/2011/pdf/2011-7880.pdf. Accessed August 8, 2011.
- Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med 2011; 364:e32.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academy Press; 2001.
- Fitch K, Mirkin D, Murphy-Barron C, Parke R, Pyenson B. A first look at ACOs’ risky business: quality is not enough. Seattle, WA: Millman, Inc; 2011. http://publications.milliman.com/publications/healthreform/pdfs/at-first-lookacos.pdf. Accessed August 10, 2011.
- University HealthSystem Consortium. Accountable care organizations: a measured view for academic medical centers. May 2011.
KEY POINTS
- Compared with other developed countries, health care in the United States is among the costliest and has poor quality measures.
- The patient-centered medical home is an increasingly popular model that emphasizes continuous coordinated patient care. It has been shown to lower costs while improving health care outcomes.
- Patient-centered medical homes are at the heart of ACOs, which establish a team approach to health care delivery systems that includes doctors and hospitals.
- Applications are now being accepted for participation in the Centers for Medicare and Medicaid Services’ ACO Proposed Rule. The 3-year minimum contract specifies numerous details regarding structure, governance, and management, and may or may not involve risk—as well as savings—according to the plan chosen.