User login
Chronic suppurative otitis media
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
IVC Filters in Bariatric Surgery
The use of inferior vena cava (IVC) filters has increased substantially in recent years. These medical devices, which are used to prevent pulmonary embolism in patients considered to be at high risk of venous thromboembolism, were placed in 167,000 patients in 2007.1 In 2012, it is estimated that 259,000 patients will undergo placement of an IVC filter, an increase of 55%.[1] Increasing use of IVC filters is attributable to the development of retrievable versions of the devices, which have expanded indications for use such as in bariatric surgery.
Unfortunately, the increase in the use of IVC filters has been accompanied by an increase in reports of adverse events in patients receiving them. The United States Food and Drug Administration (FDA) has received more than 900 adverse event reports involving IVC filters, prompting the agency to issue a warning about their use.[2] A prior study by our group demonstrated a lack of benefit of IVC filter insertion for the prevention of pulmonary embolism among bariatric surgery patients but lacked statistical power to prove harms associated with this practice.[3]
In the current study, we analyzed data from the prospective, statewide, clinical registry of the Michigan Bariatric Surgery Collaborative. Our study population now includes 35,477 bariatric surgery patients from 32 hospitals whose procedures were performed between 2006 and 2012. Since the publication of our prior study, the use of IVC filters in bariatric surgery has decreased significantly in Michigan. For this reason, our study population now includes many more high‐risk patients who did not undergo IVC filter placement, allowing us to match IVC filter patients to similarly high‐risk patients who did not receive IVC filters. We used these data to compare outcomes within 30 days of surgery, including rates of venous thromboembolism, overall serious complications, and death among patients who did and not receive IVC filters.
METHODS
Study Setting
The Michigan Bariatric Surgery Collaborative (MBSC) is a regional voluntary consortium of hospitals and surgeons that perform bariatric surgery in Michigan. The goal of the project is to improve the quality of care for patients undergoing bariatric surgery. To do this, the participating hospitals submit data to the MBSC clinical outcomes registry, patient survey, and surgeon survey databases. Three times per year the group meets to examine these data and to design and implement changes in care to improve the outcomes of care for bariatric patients. The project is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network and coordinated by faculty and staff members from the Center for Healthcare Outcomes and Policy at the University of Michigan.
The MBSC held its first collaborative meeting in June 2005 and enrolled its first patient in June 2006. The MBSC now has the participation of all of the 32 bariatric programs in Michigan, enrolling approximately 6000 patients per year in its clinical registry. Participating hospitals submit data from a review of the medical records for all of their bariatric surgery patients. This review is conducted for each patient at 30 days after surgery. The information collected includes preoperative clinical characteristics and conditions as well as perioperative clinical care and outcomes. The medical record reviews are performed by centrally trained, nurse data abstractors using a standardized and validated instrument. Each participating hospital is site visited annually to verify the accuracy and completeness of their MBSC clinical registry data.
Study Population
This study includes data for 35,477 patients undergoing bariatric surgery, including: 9829 laparoscopic adjustable gastric band, 6068 sleeve gastrectomy, 19,141 gastric bypass, and 439 biliopancreatic diversion with duodenal switch procedures between June 2006 and September 2012. Patients undergoing revisional bariatric surgery were excluded from these analyses. Prior to surgery, 1077 (3.0%) of these patients had a prophylactic IVC filter placed for prevention of pulmonary embolism. Of the IVC filters placed, 39% were temporary IVC filters, 45% were permanent IVC filters, and the type of IVC filter was not known in 15%.
Baseline Clinical Characteristics
Data collected included patient demographic characteristics (age, gender, race, type of insurance), clinical characteristics (height, weight, history of cigarette smoking, mobility limitations), and obesity‐related and other comorbid conditions (lung disease, cardiovascular disease, hyperlipidemia, gastroesophageal reflux disease, peptic ulcer disease, cholelithiasis, urinary incontinence, renal disease, diabetes, liver disease, prior history of venous thromboembolism, sleep apnea, and psychological disorders).
Risk factors for VTE were empirically derived from our data base using multivariate statistical models. Risk factors for VTE included: age, body mass index, male sex, current or past smoking, mobility limitations, asthma, home oxygen use, peripheral vascular disease, prior history of VTE, bariatric procedure time, and procedure type. The baseline predicted risk for VTE was calculated for each patient based on these risk factors and was used to divide patients into low‐ (predicted risk <1%), medium‐ (predicted risk 1%2.5%), and high‐ (predicted risk 2.5%) risk groups. Among the 35,477 patients in the registry overall, 95% are in the low‐risk group, 4% are in the medium‐risk group, and 1% are in the high‐risk group. In the matched study cohorts, 69%, 22%, and 9% were in the high‐, medium‐, and low‐risk groups, respectively.
Medical Venous Thromboembolism Prophylaxis
Data were also collected regarding the type of medical venous thromboembolism prophylaxis (unfractionated vs low molecular weight heparin) used preoperatively, postoperatively, and whether the patient was discharged to home on low molecular weight heparin.
Outcomes
Our primary outcome measures included postoperative venous thromboembolism (deep vein thrombosis or pulmonary embolism requiring treatment). We also assessed overall rate of complications and complications according to severity as follows: non‐life threatening complications (surgical site infection including wound and port site infections treated with antibiotics and/or wound opening, anastomotic stricture requiring dilatation, bleeding requiring blood transfusion of <4 units, and pneumonia requiring treatment with antibiotics only); potentially life‐threatening complications (abdominal abscess requiring percutaneous drainage or reoperation, bowel obstruction requiring reoperation, leak requiring percutaneous drainage or reoperation, bleeding requiring transfusion >4 units, reoperation, or splenectomy, band‐related problems requiring reoperation, respiratory failure requiring 2 to 7 days intubation, renal failure requiring in‐hospital dialysis, wound infection/dehiscence requiring reoperation, and venous thromboembolism); and life‐threatening complications associated with residual and lasting disability or death (myocardial infarction or cardiac arrest, renal failure requiring long‐term dialysis, respiratory failure requiring >7 days intubation or tracheostomy, and death). Other complications that are not included in these categories (eg, IVC filter related) were assessed by an end points committee to determine their severity (non‐life threatening, potentially life threatening, or life threatening associated with residual and lasting disability or death).
Statistical Analyses
We used propensity score matching to assemble cohorts in which patients with and without IVC filters were balanced on baseline characteristics. The probability of IVC filter placement was estimated for each patient using a nonparsimonious multivariate logistic regression model, in which IVC filter was the dependent variable and all of the demographic, weight, medical history, weight‐related comorbidity, and procedure‐related variables (type, length, and year of procedure; and medical venous thromboembolism prophylaxis used) in our dataset were included as covariates. IVC filter patients were matched to control patients using a greedy, 1‐ to ‐1 matching without replacement protocol resulting in cohorts that were well balanced on all baseline characteristics.
Baseline characteristics and outcomes were then compared among the cohorts using [2] and t tests as appropriate. We used mixed effects logistic regression to compare outcomes between the 2 treatment groups while controlling for clustering at the hospital and surgeon level as random effects. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to compare outcomes among patients with and without IVC filters.
RESULTS
Matching resulted in cohorts of IVC filter and control patients who were well balanced on all baseline characteristics (Table 1). In contrast, there were large and significant differences between IVC filter patients and unmatched control patients. For example, mean body mass index was 58 and 57 in the matched cohorts and 47 in the unmatched control patients. Prior history of venous thromboembolism was present in 39%, 39%, and 2% of the IVC filter, matched control, and unmatched control patients, respectively. With regard to procedure mix, unmatched control patients were less likely to have open gastric bypass and more likely to have adjustable gastric band procedures than IVC filter or matched control patients.
| Variable | IVC Filter | Matched Controls | P Value | Unmatched Controls | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 1077 | 1077 | 33,323 | ||
| Age (mean, y) | 48 | 49 | 0.295 | 46 | <0.0001 |
| Body mass index (mean, kg/m2) | 58 | 57 | 0.061 | 47 | <0.0001 |
| Male gender (%) | 32 | 31 | 0.546 | 21 | <0.0001 |
| Black race (%) | 27 | 25 | 0.667 | 15 | <0.0001 |
| Private Insurance (%) | 62 | 64 | 0.305 | 74 | <0.0001 |
| Smoking in past year (%) | 2 | 2 | 0.883 | 2 | 0.440 |
| Mobility limitations (%) | 18 | 18 | 0.780 | 5 | <0.0001 |
| Lung dsease (%) | 43 | 43 | 1.000 | 25 | <0.0001 |
| Cardiovascular disease (%) | 21 | 21 | 0.874 | 10 | <0.0001 |
| Hypertension (%) | 72 | 72 | 0.737 | 53 | <0.0001 |
| Hyperlipidemia (%) | 59 | 59 | 0.930 | 50 | <0.0001 |
| GERD (%) | 50 | 52 | 0.490 | 49 | 0.417 |
| Peptic ulcer disease (%) | 5 | 4 | 0.228 | 3 | <0.0001 |
| Cholelithiasis (%) | 30 | 30 | 0.963 | 27 | 0.018 |
| Urinary incontinence (%) | 25 | 25 | 0.960 | 22 | 0.029 |
| Renal failure (%) | 0.4 | 0.6 | 0.526 | 0.2 | 0.298 |
| Diabetes (%) | 46 | 48 | 0.546 | 33 | <0.0001 |
| Liver disorder (%) | 4 | 4 | 0.584 | 5 | 0.184 |
| Prior history of VTE (%) | 39 | 39 | 0.965 | 2 | <0.0001 |
| Sleep apnea (%) | 70 | 68 | 0.209 | 43 | <0.0001 |
| Musculoskeletal disorder (%) | 78 | 80 | 0.221 | 77 | 0.189 |
| History of hernia repair (%) | 5 | 6 | 0.924 | 3 | <0.0001 |
| Psychological disorder (%) | 49 | 49 | 0.796 | 47 | 0.267 |
| Total comorbidities (mean, no.) | 6 | 6 | 0.922 | 4 | <0.0001 |
| Procedure | |||||
| Adjustable gastric banding (%) | 15 | 17 | 0.099 | 29 | <0.0001 |
| Sleeve gastrectomy (%) | 12 | 13 | 0.515 | 17 | <0.0001 |
| Gastric bypass (%) | 73 | 69 | 0.058 | 53 | <0.0001 |
| Duodenal switch (%) | 0.7 | 0.8 | 0.616 | 1.3 | <0.0001 |
| Procedure Length (mean, minutes) | 114 | 116 | 0.427 | 95 | <0.0001 |
| Medical VTE prophylaxis | |||||
| Preoperative heparin: | |||||
| Unfractionated (%) | 36 | 38 | 0.246 | 34 | 0.306 |
| Low molecular weight (%) | 60 | 54 | 0.017 | 53 | <0.0001 |
| Postoperative heparin: | |||||
| Unfractionated (%) | 7 | 10 | 0.023 | 19 | <0.0001 |
| Low molecular weight (%) | 70 | 68 | 0.326 | 64 | <0.0001 |
| Postdischarge heparin: | |||||
| Low molecular weight (%) | 72 | 66 | 0.003 | 16 | <0.0001 |
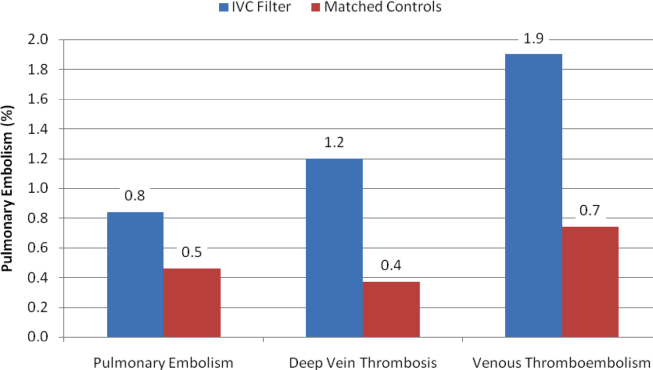
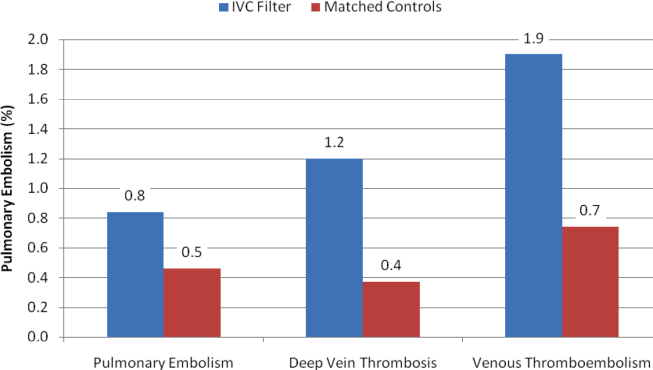
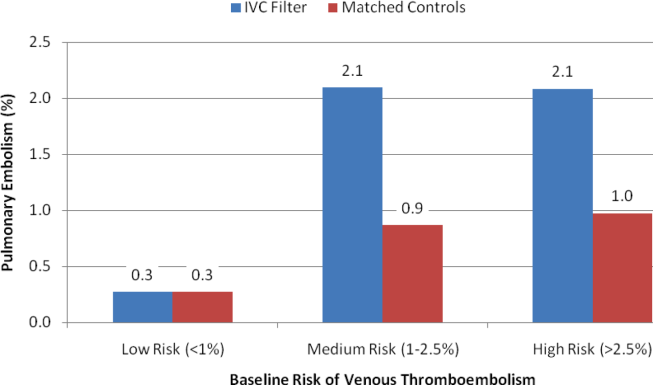
With regard to outcomes (Table 2, Figures 1 and 2), IVC filter patients had significantly higher rates of venous thromboembolism (1.9% vs 0.74%; OR, 2.7; 95% CI, 1.1‐6.3; P=0.027) and deep vein thrombosis (1.2% vs 0.37%, OR, 3.3; 95% CI, 1.1‐10.1; P=0.039) than matched control patients. Rates of pulmonary embolism were higher among IVC filter patients, but the difference was not statistically significant (0.84% vs 0.46%; OR, 2.0; 95% CI, 0.6‐6.5; P=0.232). Rates of pulmonary embolism were similar for patients with a low baseline risk of venous thromboembolism (0.27% vs 0.27%; OR, 1.0; 95% CI, 0.1‐7.7; P=0.965) but were higher for medium‐risk patients (2.1% vs 0.87%; OR, 2.5; 95% CI, 0.5‐12.7; P=0.288) and high‐risk patients (2.1% vs 0.97%; OR, 2.2; 95% CI, 0.2‐24.3; P=0.530).
| Outcomes | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Venous thromboembolism | 2.7 | 1.16.3 | 0.027 |
| Deep vein thrombosis | 3.3 | 1.110.1 | 0.039 |
| Pulmonary embolism | 2.0 | 0.66.5 | 0.232 |
| Low‐risk subgroup | 1.0 | 0.17.7 | 0.965 |
| Medium‐risk subgroup | 2.5 | 0.512.7 | 0.288 |
| High‐risk subgroup | 2.2 | 0.224.3 | 0.530 |
| Any complication | 1.3 | 1.01.7 | 0.048 |
| Serious complication | 1.6 | 1.02.4 | 0.031 |
| Permanently disabling complication | 4.3 | 1.215.6 | 0.028 |
| Death | 7.0 | 0.957.3 | 0.068 |
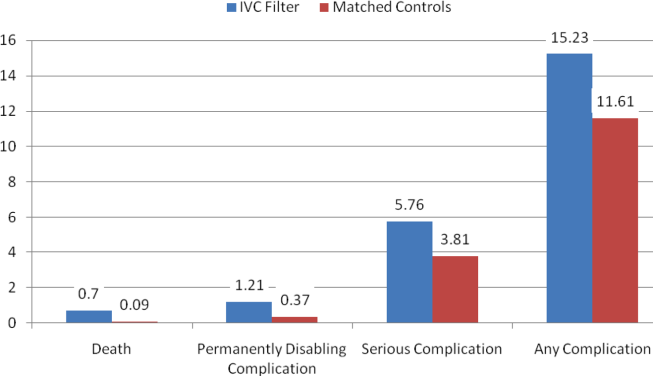
Rates of other complications were also higher among IVC filter than matched control patients (Table 2 and Figure 3). There were significantly higher rates of complications (15.2% vs 11.6%; OR, 1.3; 95% CI, 1.0‐1.7; P=0.048), serious complications (5.8% vs 3.8%; OR, 1.6; 95% CI, 1.0‐2.4; P=0.031), and permanently disabling complications (1.2% vs 0.4%; OR, 4.3; 95% CI, 1.2‐15.6; P=0.028) among IVC filter patients. Rates of death (0.7% vs 0.1%; OR, 7.0; 95% CI, 0.9‐57.3; P=0.068) were also higher among IVC filter patients than matched control patients, but this difference was not statistically significant.
Among the 7 IVC filter patients who died, 4 had fatal pulmonary embolism, and 2 had IVC filter thrombosis/occlusion. Other IVC filter‐specific complications included IVC filter migration requiring heart valve replacement surgery in 1 patient, contrast‐induced nephropathy in 1 patient, IVC filter incision site infection in 1 patient, and technical difficulties removing a temporary IVC filter requiring that the device stay in place in 1 patient.
DISCUSSION
In this propensity matched, observational cohort study, we assessed the safety and effectiveness of prophylactic IVC filters among bariatric surgery patients. We found that patients with IVC filters had significantly worse outcomes than comparably high‐risk patients without IVC filters. Rates of venous thromboembolism were higher in the IVC filter patients, and a large proportion of the other complications among IVC filter patients were device related.
Our current study of IVC filters was prompted by an FDA advisory report regarding complications in patients receiving IVC filters.[2] The FDA's report was in turn prompted by a study indicating a high prevalence of strut fracture and embolization among 80 patients who received a certain type of retrievable IVC filter.[4] The FDA conveyed receiving 921 adverse events reports involving IVC filters between 2005 and 2009. Thirty‐six percent of these reports involved migration of the device, 16% were related to breakage and embolization of parts of the device, and 8% involved perforation of the IVC.
Research on the safety and efficacy of IVC filters in bariatric surgery patients has largely been limited to small, single‐center, case series or cohort studies.[5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15] A systematic review of this literature concluded that the evidence was insufficient to recommend IVC filters for patients undergoing bariatric surgery.[16] A 2010 study by our group was the largest and only multicenter study of IVC filters in the bariatric surgery population. We found no benefit of IVC filters in a comparison of 542 gastric bypass patients with prophylactic IVC filters to 5,834 gastric bypass patients without prophylactic IVC filters.[3]
When interpreting the results of this study, a number of limitations should be considered. Our study was observational, so there is the potential for unmeasured confounding variables to have influenced our results. To minimize the risk of confounding, we used propensity scores to match IVC filter patients to comparably high‐risk control patients, resulting in study cohorts that were well balanced on all baseline variables. Although this method accounts for confounding on the variables for which there are data, there is still the possibility that an unknown confounder could affect our findings. For example, our clinical registry lacks data on hypercoagulable states, so it is possible that a higher proportion of IVC filter patients could have had this risk factor and therefore a higher baseline risk of venous thromboembolism. However, most patients with a hypercoagulable state would have had a prior history of venous thromboembolism, which is a variable included in our database that patients were matched on.
The effects of changes in clinical care occurring during the time frame of this study should be considered in interpreting our findings. For example, bariatric surgery has been getting safer in general over time. Rates of death have fallen both in Michigan and in the rest of the country as bariatric surgeons have gained experience with this procedure. In Michigan during this time period, our group has developed and implemented a risk‐stratified, standardized approach to venous thromboembolism prophylaxis for patients undergoing bariatric surgery. For these reasons, we included the year of the procedure and the type of medical venous thromboembolism prophylaxis (unfractionated or low molecular weight heparin) used perioperatively as a matching variable in our analysis.
Another limitation that should be considered in interpreting our findings is statistical power. Although our study is the largest in this study population to date, many of the outcomes of interest are relatively rare. Considering the entire bariatric surgery population, rates of venous thromboembolism and death within 30 days are each less than 1%. Even in the high‐risk patients included in this analysis, there were a total of just 28 (1.3%) venous thromboembolism events and 8 (0.37) deaths. Nonetheless, our study did find significantly greater risks of multiple types of complications among patients receiving IVC filters.
Finally, our study captures events occurring within 30 days of bariatric surgery. Complications, including venous thromboembolism and other complications directly related to IVC filters, frequently occur after 30 days of bariatric surgery. Therefore, our study may be a conservative estimate of the risks associated with the use of IVC filters in bariatric surgery patients. Furthermore, certain brands of filters have been shown to be associated with higher risks of complications. Our study lacks data on the brand of IVC filter used and so cannot assess the extent to which this would affect our results.
CONCLUSIONS
In conclusion, our study indicates that IVC filters do not reduce the risk of pulmonary embolism in high‐risk bariatric surgery patients. They are also associated with other complications attributable to malfunctions of the device itself. We believe that the use of IVC filters among bariatric surgery patients should be discouraged.
Disclosure
This study was supported by a grant from the Agency for Healthcare Research and Quality (HS018050) and was presented at the Annual Meeting of the American Society for Metabolic and Bariatric Surgery (ASMBS), San Diego, California, June 20, 2012.
- , , . Vena cava filters: a call to action. Chest Physician. 2011;16:18a.
- U.S. Food and Drug Administration. Removing retrievable inferior vena cava filters: initial communication. August 9, 2010. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221 676.htm. Accessed December 2, 2012.
- , , , et al. Preoperative placement of inferior vena cava filters and outcomes after gastric bypass surgery. Ann Surg. 2010;252:131–318.
- , , , et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170:1827–1831.
- , , , , , . Experience with inferior vena cava filter placement in patients undergoing open gastric bypass procedures. Ann Vasc Surg. 2006;44: 1301–1305.
- , . Preoperative placement of retrievable inferior vena cava filters in bariatric surgery. Surg Obes Relat Dis. 2007;3: 602–605.
- , , , et al. Safety and efficacy of intravascular ultrasound‐guided inferior vena cava filter in super obese bariatric patients. Surg Obes Relat Dis. 2008;4:50–54.
- , , , , , . Current indications for preoperative inferior vena cava filter insertion in patients undergoing surgery for morbid obesity. Obes Surg. 2005;15:1009–1012.
- , , , . Efficacy of prophylactic inferior vena cava filter placement in bariatric surgery. Surg Obes Relat Dis. 2007;3:606–610.
- , , , et al. Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients. J Vasc Surg. 2007;45:784–788.
- , , , . Retrievable inferior vena cava filters may be safely applied in gastric bypass surgery. Surg Endosc. 2007;21:2277–2279.
- , , , , . Inferior vena cava filter placement for pulmonary embolism risk reduction in super morbidly obese undergoing bariatric surgery. Surg Obes Relat Dis. 2007;3:461–464.
- , . A simple venous thromboembolism prophylaxis protocol for patients undergoing bariatric surgery. Obesity (Silver Spring). 2006;14:1961–1965.
- , , , et al. Risk‐group targeted inferior vena cava filter placemetn in gastric bypass patients. Obes Surg. 2009;19:451–455.
- , , , , , . Retreivable inferior vena cava filters in high‐risk patients undergoing bariatric surgery. Surg Endosc. 2009;23:2203–2207.
- , . Inferior vena caval filter insertion prior to bariatric surgery: A systematic review of the literature. J Thromb Haemost. 2010;8:1266–1270.
The use of inferior vena cava (IVC) filters has increased substantially in recent years. These medical devices, which are used to prevent pulmonary embolism in patients considered to be at high risk of venous thromboembolism, were placed in 167,000 patients in 2007.1 In 2012, it is estimated that 259,000 patients will undergo placement of an IVC filter, an increase of 55%.[1] Increasing use of IVC filters is attributable to the development of retrievable versions of the devices, which have expanded indications for use such as in bariatric surgery.
Unfortunately, the increase in the use of IVC filters has been accompanied by an increase in reports of adverse events in patients receiving them. The United States Food and Drug Administration (FDA) has received more than 900 adverse event reports involving IVC filters, prompting the agency to issue a warning about their use.[2] A prior study by our group demonstrated a lack of benefit of IVC filter insertion for the prevention of pulmonary embolism among bariatric surgery patients but lacked statistical power to prove harms associated with this practice.[3]
In the current study, we analyzed data from the prospective, statewide, clinical registry of the Michigan Bariatric Surgery Collaborative. Our study population now includes 35,477 bariatric surgery patients from 32 hospitals whose procedures were performed between 2006 and 2012. Since the publication of our prior study, the use of IVC filters in bariatric surgery has decreased significantly in Michigan. For this reason, our study population now includes many more high‐risk patients who did not undergo IVC filter placement, allowing us to match IVC filter patients to similarly high‐risk patients who did not receive IVC filters. We used these data to compare outcomes within 30 days of surgery, including rates of venous thromboembolism, overall serious complications, and death among patients who did and not receive IVC filters.
METHODS
Study Setting
The Michigan Bariatric Surgery Collaborative (MBSC) is a regional voluntary consortium of hospitals and surgeons that perform bariatric surgery in Michigan. The goal of the project is to improve the quality of care for patients undergoing bariatric surgery. To do this, the participating hospitals submit data to the MBSC clinical outcomes registry, patient survey, and surgeon survey databases. Three times per year the group meets to examine these data and to design and implement changes in care to improve the outcomes of care for bariatric patients. The project is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network and coordinated by faculty and staff members from the Center for Healthcare Outcomes and Policy at the University of Michigan.
The MBSC held its first collaborative meeting in June 2005 and enrolled its first patient in June 2006. The MBSC now has the participation of all of the 32 bariatric programs in Michigan, enrolling approximately 6000 patients per year in its clinical registry. Participating hospitals submit data from a review of the medical records for all of their bariatric surgery patients. This review is conducted for each patient at 30 days after surgery. The information collected includes preoperative clinical characteristics and conditions as well as perioperative clinical care and outcomes. The medical record reviews are performed by centrally trained, nurse data abstractors using a standardized and validated instrument. Each participating hospital is site visited annually to verify the accuracy and completeness of their MBSC clinical registry data.
Study Population
This study includes data for 35,477 patients undergoing bariatric surgery, including: 9829 laparoscopic adjustable gastric band, 6068 sleeve gastrectomy, 19,141 gastric bypass, and 439 biliopancreatic diversion with duodenal switch procedures between June 2006 and September 2012. Patients undergoing revisional bariatric surgery were excluded from these analyses. Prior to surgery, 1077 (3.0%) of these patients had a prophylactic IVC filter placed for prevention of pulmonary embolism. Of the IVC filters placed, 39% were temporary IVC filters, 45% were permanent IVC filters, and the type of IVC filter was not known in 15%.
Baseline Clinical Characteristics
Data collected included patient demographic characteristics (age, gender, race, type of insurance), clinical characteristics (height, weight, history of cigarette smoking, mobility limitations), and obesity‐related and other comorbid conditions (lung disease, cardiovascular disease, hyperlipidemia, gastroesophageal reflux disease, peptic ulcer disease, cholelithiasis, urinary incontinence, renal disease, diabetes, liver disease, prior history of venous thromboembolism, sleep apnea, and psychological disorders).
Risk factors for VTE were empirically derived from our data base using multivariate statistical models. Risk factors for VTE included: age, body mass index, male sex, current or past smoking, mobility limitations, asthma, home oxygen use, peripheral vascular disease, prior history of VTE, bariatric procedure time, and procedure type. The baseline predicted risk for VTE was calculated for each patient based on these risk factors and was used to divide patients into low‐ (predicted risk <1%), medium‐ (predicted risk 1%2.5%), and high‐ (predicted risk 2.5%) risk groups. Among the 35,477 patients in the registry overall, 95% are in the low‐risk group, 4% are in the medium‐risk group, and 1% are in the high‐risk group. In the matched study cohorts, 69%, 22%, and 9% were in the high‐, medium‐, and low‐risk groups, respectively.
Medical Venous Thromboembolism Prophylaxis
Data were also collected regarding the type of medical venous thromboembolism prophylaxis (unfractionated vs low molecular weight heparin) used preoperatively, postoperatively, and whether the patient was discharged to home on low molecular weight heparin.
Outcomes
Our primary outcome measures included postoperative venous thromboembolism (deep vein thrombosis or pulmonary embolism requiring treatment). We also assessed overall rate of complications and complications according to severity as follows: non‐life threatening complications (surgical site infection including wound and port site infections treated with antibiotics and/or wound opening, anastomotic stricture requiring dilatation, bleeding requiring blood transfusion of <4 units, and pneumonia requiring treatment with antibiotics only); potentially life‐threatening complications (abdominal abscess requiring percutaneous drainage or reoperation, bowel obstruction requiring reoperation, leak requiring percutaneous drainage or reoperation, bleeding requiring transfusion >4 units, reoperation, or splenectomy, band‐related problems requiring reoperation, respiratory failure requiring 2 to 7 days intubation, renal failure requiring in‐hospital dialysis, wound infection/dehiscence requiring reoperation, and venous thromboembolism); and life‐threatening complications associated with residual and lasting disability or death (myocardial infarction or cardiac arrest, renal failure requiring long‐term dialysis, respiratory failure requiring >7 days intubation or tracheostomy, and death). Other complications that are not included in these categories (eg, IVC filter related) were assessed by an end points committee to determine their severity (non‐life threatening, potentially life threatening, or life threatening associated with residual and lasting disability or death).
Statistical Analyses
We used propensity score matching to assemble cohorts in which patients with and without IVC filters were balanced on baseline characteristics. The probability of IVC filter placement was estimated for each patient using a nonparsimonious multivariate logistic regression model, in which IVC filter was the dependent variable and all of the demographic, weight, medical history, weight‐related comorbidity, and procedure‐related variables (type, length, and year of procedure; and medical venous thromboembolism prophylaxis used) in our dataset were included as covariates. IVC filter patients were matched to control patients using a greedy, 1‐ to ‐1 matching without replacement protocol resulting in cohorts that were well balanced on all baseline characteristics.
Baseline characteristics and outcomes were then compared among the cohorts using [2] and t tests as appropriate. We used mixed effects logistic regression to compare outcomes between the 2 treatment groups while controlling for clustering at the hospital and surgeon level as random effects. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to compare outcomes among patients with and without IVC filters.
RESULTS
Matching resulted in cohorts of IVC filter and control patients who were well balanced on all baseline characteristics (Table 1). In contrast, there were large and significant differences between IVC filter patients and unmatched control patients. For example, mean body mass index was 58 and 57 in the matched cohorts and 47 in the unmatched control patients. Prior history of venous thromboembolism was present in 39%, 39%, and 2% of the IVC filter, matched control, and unmatched control patients, respectively. With regard to procedure mix, unmatched control patients were less likely to have open gastric bypass and more likely to have adjustable gastric band procedures than IVC filter or matched control patients.
| Variable | IVC Filter | Matched Controls | P Value | Unmatched Controls | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 1077 | 1077 | 33,323 | ||
| Age (mean, y) | 48 | 49 | 0.295 | 46 | <0.0001 |
| Body mass index (mean, kg/m2) | 58 | 57 | 0.061 | 47 | <0.0001 |
| Male gender (%) | 32 | 31 | 0.546 | 21 | <0.0001 |
| Black race (%) | 27 | 25 | 0.667 | 15 | <0.0001 |
| Private Insurance (%) | 62 | 64 | 0.305 | 74 | <0.0001 |
| Smoking in past year (%) | 2 | 2 | 0.883 | 2 | 0.440 |
| Mobility limitations (%) | 18 | 18 | 0.780 | 5 | <0.0001 |
| Lung dsease (%) | 43 | 43 | 1.000 | 25 | <0.0001 |
| Cardiovascular disease (%) | 21 | 21 | 0.874 | 10 | <0.0001 |
| Hypertension (%) | 72 | 72 | 0.737 | 53 | <0.0001 |
| Hyperlipidemia (%) | 59 | 59 | 0.930 | 50 | <0.0001 |
| GERD (%) | 50 | 52 | 0.490 | 49 | 0.417 |
| Peptic ulcer disease (%) | 5 | 4 | 0.228 | 3 | <0.0001 |
| Cholelithiasis (%) | 30 | 30 | 0.963 | 27 | 0.018 |
| Urinary incontinence (%) | 25 | 25 | 0.960 | 22 | 0.029 |
| Renal failure (%) | 0.4 | 0.6 | 0.526 | 0.2 | 0.298 |
| Diabetes (%) | 46 | 48 | 0.546 | 33 | <0.0001 |
| Liver disorder (%) | 4 | 4 | 0.584 | 5 | 0.184 |
| Prior history of VTE (%) | 39 | 39 | 0.965 | 2 | <0.0001 |
| Sleep apnea (%) | 70 | 68 | 0.209 | 43 | <0.0001 |
| Musculoskeletal disorder (%) | 78 | 80 | 0.221 | 77 | 0.189 |
| History of hernia repair (%) | 5 | 6 | 0.924 | 3 | <0.0001 |
| Psychological disorder (%) | 49 | 49 | 0.796 | 47 | 0.267 |
| Total comorbidities (mean, no.) | 6 | 6 | 0.922 | 4 | <0.0001 |
| Procedure | |||||
| Adjustable gastric banding (%) | 15 | 17 | 0.099 | 29 | <0.0001 |
| Sleeve gastrectomy (%) | 12 | 13 | 0.515 | 17 | <0.0001 |
| Gastric bypass (%) | 73 | 69 | 0.058 | 53 | <0.0001 |
| Duodenal switch (%) | 0.7 | 0.8 | 0.616 | 1.3 | <0.0001 |
| Procedure Length (mean, minutes) | 114 | 116 | 0.427 | 95 | <0.0001 |
| Medical VTE prophylaxis | |||||
| Preoperative heparin: | |||||
| Unfractionated (%) | 36 | 38 | 0.246 | 34 | 0.306 |
| Low molecular weight (%) | 60 | 54 | 0.017 | 53 | <0.0001 |
| Postoperative heparin: | |||||
| Unfractionated (%) | 7 | 10 | 0.023 | 19 | <0.0001 |
| Low molecular weight (%) | 70 | 68 | 0.326 | 64 | <0.0001 |
| Postdischarge heparin: | |||||
| Low molecular weight (%) | 72 | 66 | 0.003 | 16 | <0.0001 |
With regard to outcomes (Table 2, Figures 1 and 2), IVC filter patients had significantly higher rates of venous thromboembolism (1.9% vs 0.74%; OR, 2.7; 95% CI, 1.1‐6.3; P=0.027) and deep vein thrombosis (1.2% vs 0.37%, OR, 3.3; 95% CI, 1.1‐10.1; P=0.039) than matched control patients. Rates of pulmonary embolism were higher among IVC filter patients, but the difference was not statistically significant (0.84% vs 0.46%; OR, 2.0; 95% CI, 0.6‐6.5; P=0.232). Rates of pulmonary embolism were similar for patients with a low baseline risk of venous thromboembolism (0.27% vs 0.27%; OR, 1.0; 95% CI, 0.1‐7.7; P=0.965) but were higher for medium‐risk patients (2.1% vs 0.87%; OR, 2.5; 95% CI, 0.5‐12.7; P=0.288) and high‐risk patients (2.1% vs 0.97%; OR, 2.2; 95% CI, 0.2‐24.3; P=0.530).


| Outcomes | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Venous thromboembolism | 2.7 | 1.16.3 | 0.027 |
| Deep vein thrombosis | 3.3 | 1.110.1 | 0.039 |
| Pulmonary embolism | 2.0 | 0.66.5 | 0.232 |
| Low‐risk subgroup | 1.0 | 0.17.7 | 0.965 |
| Medium‐risk subgroup | 2.5 | 0.512.7 | 0.288 |
| High‐risk subgroup | 2.2 | 0.224.3 | 0.530 |
| Any complication | 1.3 | 1.01.7 | 0.048 |
| Serious complication | 1.6 | 1.02.4 | 0.031 |
| Permanently disabling complication | 4.3 | 1.215.6 | 0.028 |
| Death | 7.0 | 0.957.3 | 0.068 |
Rates of other complications were also higher among IVC filter than matched control patients (Table 2 and Figure 3). There were significantly higher rates of complications (15.2% vs 11.6%; OR, 1.3; 95% CI, 1.0‐1.7; P=0.048), serious complications (5.8% vs 3.8%; OR, 1.6; 95% CI, 1.0‐2.4; P=0.031), and permanently disabling complications (1.2% vs 0.4%; OR, 4.3; 95% CI, 1.2‐15.6; P=0.028) among IVC filter patients. Rates of death (0.7% vs 0.1%; OR, 7.0; 95% CI, 0.9‐57.3; P=0.068) were also higher among IVC filter patients than matched control patients, but this difference was not statistically significant.

Among the 7 IVC filter patients who died, 4 had fatal pulmonary embolism, and 2 had IVC filter thrombosis/occlusion. Other IVC filter‐specific complications included IVC filter migration requiring heart valve replacement surgery in 1 patient, contrast‐induced nephropathy in 1 patient, IVC filter incision site infection in 1 patient, and technical difficulties removing a temporary IVC filter requiring that the device stay in place in 1 patient.
DISCUSSION
In this propensity matched, observational cohort study, we assessed the safety and effectiveness of prophylactic IVC filters among bariatric surgery patients. We found that patients with IVC filters had significantly worse outcomes than comparably high‐risk patients without IVC filters. Rates of venous thromboembolism were higher in the IVC filter patients, and a large proportion of the other complications among IVC filter patients were device related.
Our current study of IVC filters was prompted by an FDA advisory report regarding complications in patients receiving IVC filters.[2] The FDA's report was in turn prompted by a study indicating a high prevalence of strut fracture and embolization among 80 patients who received a certain type of retrievable IVC filter.[4] The FDA conveyed receiving 921 adverse events reports involving IVC filters between 2005 and 2009. Thirty‐six percent of these reports involved migration of the device, 16% were related to breakage and embolization of parts of the device, and 8% involved perforation of the IVC.
Research on the safety and efficacy of IVC filters in bariatric surgery patients has largely been limited to small, single‐center, case series or cohort studies.[5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15] A systematic review of this literature concluded that the evidence was insufficient to recommend IVC filters for patients undergoing bariatric surgery.[16] A 2010 study by our group was the largest and only multicenter study of IVC filters in the bariatric surgery population. We found no benefit of IVC filters in a comparison of 542 gastric bypass patients with prophylactic IVC filters to 5,834 gastric bypass patients without prophylactic IVC filters.[3]
When interpreting the results of this study, a number of limitations should be considered. Our study was observational, so there is the potential for unmeasured confounding variables to have influenced our results. To minimize the risk of confounding, we used propensity scores to match IVC filter patients to comparably high‐risk control patients, resulting in study cohorts that were well balanced on all baseline variables. Although this method accounts for confounding on the variables for which there are data, there is still the possibility that an unknown confounder could affect our findings. For example, our clinical registry lacks data on hypercoagulable states, so it is possible that a higher proportion of IVC filter patients could have had this risk factor and therefore a higher baseline risk of venous thromboembolism. However, most patients with a hypercoagulable state would have had a prior history of venous thromboembolism, which is a variable included in our database that patients were matched on.
The effects of changes in clinical care occurring during the time frame of this study should be considered in interpreting our findings. For example, bariatric surgery has been getting safer in general over time. Rates of death have fallen both in Michigan and in the rest of the country as bariatric surgeons have gained experience with this procedure. In Michigan during this time period, our group has developed and implemented a risk‐stratified, standardized approach to venous thromboembolism prophylaxis for patients undergoing bariatric surgery. For these reasons, we included the year of the procedure and the type of medical venous thromboembolism prophylaxis (unfractionated or low molecular weight heparin) used perioperatively as a matching variable in our analysis.
Another limitation that should be considered in interpreting our findings is statistical power. Although our study is the largest in this study population to date, many of the outcomes of interest are relatively rare. Considering the entire bariatric surgery population, rates of venous thromboembolism and death within 30 days are each less than 1%. Even in the high‐risk patients included in this analysis, there were a total of just 28 (1.3%) venous thromboembolism events and 8 (0.37) deaths. Nonetheless, our study did find significantly greater risks of multiple types of complications among patients receiving IVC filters.
Finally, our study captures events occurring within 30 days of bariatric surgery. Complications, including venous thromboembolism and other complications directly related to IVC filters, frequently occur after 30 days of bariatric surgery. Therefore, our study may be a conservative estimate of the risks associated with the use of IVC filters in bariatric surgery patients. Furthermore, certain brands of filters have been shown to be associated with higher risks of complications. Our study lacks data on the brand of IVC filter used and so cannot assess the extent to which this would affect our results.
CONCLUSIONS
In conclusion, our study indicates that IVC filters do not reduce the risk of pulmonary embolism in high‐risk bariatric surgery patients. They are also associated with other complications attributable to malfunctions of the device itself. We believe that the use of IVC filters among bariatric surgery patients should be discouraged.
Disclosure
This study was supported by a grant from the Agency for Healthcare Research and Quality (HS018050) and was presented at the Annual Meeting of the American Society for Metabolic and Bariatric Surgery (ASMBS), San Diego, California, June 20, 2012.
The use of inferior vena cava (IVC) filters has increased substantially in recent years. These medical devices, which are used to prevent pulmonary embolism in patients considered to be at high risk of venous thromboembolism, were placed in 167,000 patients in 2007.1 In 2012, it is estimated that 259,000 patients will undergo placement of an IVC filter, an increase of 55%.[1] Increasing use of IVC filters is attributable to the development of retrievable versions of the devices, which have expanded indications for use such as in bariatric surgery.
Unfortunately, the increase in the use of IVC filters has been accompanied by an increase in reports of adverse events in patients receiving them. The United States Food and Drug Administration (FDA) has received more than 900 adverse event reports involving IVC filters, prompting the agency to issue a warning about their use.[2] A prior study by our group demonstrated a lack of benefit of IVC filter insertion for the prevention of pulmonary embolism among bariatric surgery patients but lacked statistical power to prove harms associated with this practice.[3]
In the current study, we analyzed data from the prospective, statewide, clinical registry of the Michigan Bariatric Surgery Collaborative. Our study population now includes 35,477 bariatric surgery patients from 32 hospitals whose procedures were performed between 2006 and 2012. Since the publication of our prior study, the use of IVC filters in bariatric surgery has decreased significantly in Michigan. For this reason, our study population now includes many more high‐risk patients who did not undergo IVC filter placement, allowing us to match IVC filter patients to similarly high‐risk patients who did not receive IVC filters. We used these data to compare outcomes within 30 days of surgery, including rates of venous thromboembolism, overall serious complications, and death among patients who did and not receive IVC filters.
METHODS
Study Setting
The Michigan Bariatric Surgery Collaborative (MBSC) is a regional voluntary consortium of hospitals and surgeons that perform bariatric surgery in Michigan. The goal of the project is to improve the quality of care for patients undergoing bariatric surgery. To do this, the participating hospitals submit data to the MBSC clinical outcomes registry, patient survey, and surgeon survey databases. Three times per year the group meets to examine these data and to design and implement changes in care to improve the outcomes of care for bariatric patients. The project is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network and coordinated by faculty and staff members from the Center for Healthcare Outcomes and Policy at the University of Michigan.
The MBSC held its first collaborative meeting in June 2005 and enrolled its first patient in June 2006. The MBSC now has the participation of all of the 32 bariatric programs in Michigan, enrolling approximately 6000 patients per year in its clinical registry. Participating hospitals submit data from a review of the medical records for all of their bariatric surgery patients. This review is conducted for each patient at 30 days after surgery. The information collected includes preoperative clinical characteristics and conditions as well as perioperative clinical care and outcomes. The medical record reviews are performed by centrally trained, nurse data abstractors using a standardized and validated instrument. Each participating hospital is site visited annually to verify the accuracy and completeness of their MBSC clinical registry data.
Study Population
This study includes data for 35,477 patients undergoing bariatric surgery, including: 9829 laparoscopic adjustable gastric band, 6068 sleeve gastrectomy, 19,141 gastric bypass, and 439 biliopancreatic diversion with duodenal switch procedures between June 2006 and September 2012. Patients undergoing revisional bariatric surgery were excluded from these analyses. Prior to surgery, 1077 (3.0%) of these patients had a prophylactic IVC filter placed for prevention of pulmonary embolism. Of the IVC filters placed, 39% were temporary IVC filters, 45% were permanent IVC filters, and the type of IVC filter was not known in 15%.
Baseline Clinical Characteristics
Data collected included patient demographic characteristics (age, gender, race, type of insurance), clinical characteristics (height, weight, history of cigarette smoking, mobility limitations), and obesity‐related and other comorbid conditions (lung disease, cardiovascular disease, hyperlipidemia, gastroesophageal reflux disease, peptic ulcer disease, cholelithiasis, urinary incontinence, renal disease, diabetes, liver disease, prior history of venous thromboembolism, sleep apnea, and psychological disorders).
Risk factors for VTE were empirically derived from our data base using multivariate statistical models. Risk factors for VTE included: age, body mass index, male sex, current or past smoking, mobility limitations, asthma, home oxygen use, peripheral vascular disease, prior history of VTE, bariatric procedure time, and procedure type. The baseline predicted risk for VTE was calculated for each patient based on these risk factors and was used to divide patients into low‐ (predicted risk <1%), medium‐ (predicted risk 1%2.5%), and high‐ (predicted risk 2.5%) risk groups. Among the 35,477 patients in the registry overall, 95% are in the low‐risk group, 4% are in the medium‐risk group, and 1% are in the high‐risk group. In the matched study cohorts, 69%, 22%, and 9% were in the high‐, medium‐, and low‐risk groups, respectively.
Medical Venous Thromboembolism Prophylaxis
Data were also collected regarding the type of medical venous thromboembolism prophylaxis (unfractionated vs low molecular weight heparin) used preoperatively, postoperatively, and whether the patient was discharged to home on low molecular weight heparin.
Outcomes
Our primary outcome measures included postoperative venous thromboembolism (deep vein thrombosis or pulmonary embolism requiring treatment). We also assessed overall rate of complications and complications according to severity as follows: non‐life threatening complications (surgical site infection including wound and port site infections treated with antibiotics and/or wound opening, anastomotic stricture requiring dilatation, bleeding requiring blood transfusion of <4 units, and pneumonia requiring treatment with antibiotics only); potentially life‐threatening complications (abdominal abscess requiring percutaneous drainage or reoperation, bowel obstruction requiring reoperation, leak requiring percutaneous drainage or reoperation, bleeding requiring transfusion >4 units, reoperation, or splenectomy, band‐related problems requiring reoperation, respiratory failure requiring 2 to 7 days intubation, renal failure requiring in‐hospital dialysis, wound infection/dehiscence requiring reoperation, and venous thromboembolism); and life‐threatening complications associated with residual and lasting disability or death (myocardial infarction or cardiac arrest, renal failure requiring long‐term dialysis, respiratory failure requiring >7 days intubation or tracheostomy, and death). Other complications that are not included in these categories (eg, IVC filter related) were assessed by an end points committee to determine their severity (non‐life threatening, potentially life threatening, or life threatening associated with residual and lasting disability or death).
Statistical Analyses
We used propensity score matching to assemble cohorts in which patients with and without IVC filters were balanced on baseline characteristics. The probability of IVC filter placement was estimated for each patient using a nonparsimonious multivariate logistic regression model, in which IVC filter was the dependent variable and all of the demographic, weight, medical history, weight‐related comorbidity, and procedure‐related variables (type, length, and year of procedure; and medical venous thromboembolism prophylaxis used) in our dataset were included as covariates. IVC filter patients were matched to control patients using a greedy, 1‐ to ‐1 matching without replacement protocol resulting in cohorts that were well balanced on all baseline characteristics.
Baseline characteristics and outcomes were then compared among the cohorts using [2] and t tests as appropriate. We used mixed effects logistic regression to compare outcomes between the 2 treatment groups while controlling for clustering at the hospital and surgeon level as random effects. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to compare outcomes among patients with and without IVC filters.
RESULTS
Matching resulted in cohorts of IVC filter and control patients who were well balanced on all baseline characteristics (Table 1). In contrast, there were large and significant differences between IVC filter patients and unmatched control patients. For example, mean body mass index was 58 and 57 in the matched cohorts and 47 in the unmatched control patients. Prior history of venous thromboembolism was present in 39%, 39%, and 2% of the IVC filter, matched control, and unmatched control patients, respectively. With regard to procedure mix, unmatched control patients were less likely to have open gastric bypass and more likely to have adjustable gastric band procedures than IVC filter or matched control patients.
| Variable | IVC Filter | Matched Controls | P Value | Unmatched Controls | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 1077 | 1077 | 33,323 | ||
| Age (mean, y) | 48 | 49 | 0.295 | 46 | <0.0001 |
| Body mass index (mean, kg/m2) | 58 | 57 | 0.061 | 47 | <0.0001 |
| Male gender (%) | 32 | 31 | 0.546 | 21 | <0.0001 |
| Black race (%) | 27 | 25 | 0.667 | 15 | <0.0001 |
| Private Insurance (%) | 62 | 64 | 0.305 | 74 | <0.0001 |
| Smoking in past year (%) | 2 | 2 | 0.883 | 2 | 0.440 |
| Mobility limitations (%) | 18 | 18 | 0.780 | 5 | <0.0001 |
| Lung dsease (%) | 43 | 43 | 1.000 | 25 | <0.0001 |
| Cardiovascular disease (%) | 21 | 21 | 0.874 | 10 | <0.0001 |
| Hypertension (%) | 72 | 72 | 0.737 | 53 | <0.0001 |
| Hyperlipidemia (%) | 59 | 59 | 0.930 | 50 | <0.0001 |
| GERD (%) | 50 | 52 | 0.490 | 49 | 0.417 |
| Peptic ulcer disease (%) | 5 | 4 | 0.228 | 3 | <0.0001 |
| Cholelithiasis (%) | 30 | 30 | 0.963 | 27 | 0.018 |
| Urinary incontinence (%) | 25 | 25 | 0.960 | 22 | 0.029 |
| Renal failure (%) | 0.4 | 0.6 | 0.526 | 0.2 | 0.298 |
| Diabetes (%) | 46 | 48 | 0.546 | 33 | <0.0001 |
| Liver disorder (%) | 4 | 4 | 0.584 | 5 | 0.184 |
| Prior history of VTE (%) | 39 | 39 | 0.965 | 2 | <0.0001 |
| Sleep apnea (%) | 70 | 68 | 0.209 | 43 | <0.0001 |
| Musculoskeletal disorder (%) | 78 | 80 | 0.221 | 77 | 0.189 |
| History of hernia repair (%) | 5 | 6 | 0.924 | 3 | <0.0001 |
| Psychological disorder (%) | 49 | 49 | 0.796 | 47 | 0.267 |
| Total comorbidities (mean, no.) | 6 | 6 | 0.922 | 4 | <0.0001 |
| Procedure | |||||
| Adjustable gastric banding (%) | 15 | 17 | 0.099 | 29 | <0.0001 |
| Sleeve gastrectomy (%) | 12 | 13 | 0.515 | 17 | <0.0001 |
| Gastric bypass (%) | 73 | 69 | 0.058 | 53 | <0.0001 |
| Duodenal switch (%) | 0.7 | 0.8 | 0.616 | 1.3 | <0.0001 |
| Procedure Length (mean, minutes) | 114 | 116 | 0.427 | 95 | <0.0001 |
| Medical VTE prophylaxis | |||||
| Preoperative heparin: | |||||
| Unfractionated (%) | 36 | 38 | 0.246 | 34 | 0.306 |
| Low molecular weight (%) | 60 | 54 | 0.017 | 53 | <0.0001 |
| Postoperative heparin: | |||||
| Unfractionated (%) | 7 | 10 | 0.023 | 19 | <0.0001 |
| Low molecular weight (%) | 70 | 68 | 0.326 | 64 | <0.0001 |
| Postdischarge heparin: | |||||
| Low molecular weight (%) | 72 | 66 | 0.003 | 16 | <0.0001 |
With regard to outcomes (Table 2, Figures 1 and 2), IVC filter patients had significantly higher rates of venous thromboembolism (1.9% vs 0.74%; OR, 2.7; 95% CI, 1.1‐6.3; P=0.027) and deep vein thrombosis (1.2% vs 0.37%, OR, 3.3; 95% CI, 1.1‐10.1; P=0.039) than matched control patients. Rates of pulmonary embolism were higher among IVC filter patients, but the difference was not statistically significant (0.84% vs 0.46%; OR, 2.0; 95% CI, 0.6‐6.5; P=0.232). Rates of pulmonary embolism were similar for patients with a low baseline risk of venous thromboembolism (0.27% vs 0.27%; OR, 1.0; 95% CI, 0.1‐7.7; P=0.965) but were higher for medium‐risk patients (2.1% vs 0.87%; OR, 2.5; 95% CI, 0.5‐12.7; P=0.288) and high‐risk patients (2.1% vs 0.97%; OR, 2.2; 95% CI, 0.2‐24.3; P=0.530).


| Outcomes | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Venous thromboembolism | 2.7 | 1.16.3 | 0.027 |
| Deep vein thrombosis | 3.3 | 1.110.1 | 0.039 |
| Pulmonary embolism | 2.0 | 0.66.5 | 0.232 |
| Low‐risk subgroup | 1.0 | 0.17.7 | 0.965 |
| Medium‐risk subgroup | 2.5 | 0.512.7 | 0.288 |
| High‐risk subgroup | 2.2 | 0.224.3 | 0.530 |
| Any complication | 1.3 | 1.01.7 | 0.048 |
| Serious complication | 1.6 | 1.02.4 | 0.031 |
| Permanently disabling complication | 4.3 | 1.215.6 | 0.028 |
| Death | 7.0 | 0.957.3 | 0.068 |
Rates of other complications were also higher among IVC filter than matched control patients (Table 2 and Figure 3). There were significantly higher rates of complications (15.2% vs 11.6%; OR, 1.3; 95% CI, 1.0‐1.7; P=0.048), serious complications (5.8% vs 3.8%; OR, 1.6; 95% CI, 1.0‐2.4; P=0.031), and permanently disabling complications (1.2% vs 0.4%; OR, 4.3; 95% CI, 1.2‐15.6; P=0.028) among IVC filter patients. Rates of death (0.7% vs 0.1%; OR, 7.0; 95% CI, 0.9‐57.3; P=0.068) were also higher among IVC filter patients than matched control patients, but this difference was not statistically significant.

Among the 7 IVC filter patients who died, 4 had fatal pulmonary embolism, and 2 had IVC filter thrombosis/occlusion. Other IVC filter‐specific complications included IVC filter migration requiring heart valve replacement surgery in 1 patient, contrast‐induced nephropathy in 1 patient, IVC filter incision site infection in 1 patient, and technical difficulties removing a temporary IVC filter requiring that the device stay in place in 1 patient.
DISCUSSION
In this propensity matched, observational cohort study, we assessed the safety and effectiveness of prophylactic IVC filters among bariatric surgery patients. We found that patients with IVC filters had significantly worse outcomes than comparably high‐risk patients without IVC filters. Rates of venous thromboembolism were higher in the IVC filter patients, and a large proportion of the other complications among IVC filter patients were device related.
Our current study of IVC filters was prompted by an FDA advisory report regarding complications in patients receiving IVC filters.[2] The FDA's report was in turn prompted by a study indicating a high prevalence of strut fracture and embolization among 80 patients who received a certain type of retrievable IVC filter.[4] The FDA conveyed receiving 921 adverse events reports involving IVC filters between 2005 and 2009. Thirty‐six percent of these reports involved migration of the device, 16% were related to breakage and embolization of parts of the device, and 8% involved perforation of the IVC.
Research on the safety and efficacy of IVC filters in bariatric surgery patients has largely been limited to small, single‐center, case series or cohort studies.[5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15] A systematic review of this literature concluded that the evidence was insufficient to recommend IVC filters for patients undergoing bariatric surgery.[16] A 2010 study by our group was the largest and only multicenter study of IVC filters in the bariatric surgery population. We found no benefit of IVC filters in a comparison of 542 gastric bypass patients with prophylactic IVC filters to 5,834 gastric bypass patients without prophylactic IVC filters.[3]
When interpreting the results of this study, a number of limitations should be considered. Our study was observational, so there is the potential for unmeasured confounding variables to have influenced our results. To minimize the risk of confounding, we used propensity scores to match IVC filter patients to comparably high‐risk control patients, resulting in study cohorts that were well balanced on all baseline variables. Although this method accounts for confounding on the variables for which there are data, there is still the possibility that an unknown confounder could affect our findings. For example, our clinical registry lacks data on hypercoagulable states, so it is possible that a higher proportion of IVC filter patients could have had this risk factor and therefore a higher baseline risk of venous thromboembolism. However, most patients with a hypercoagulable state would have had a prior history of venous thromboembolism, which is a variable included in our database that patients were matched on.
The effects of changes in clinical care occurring during the time frame of this study should be considered in interpreting our findings. For example, bariatric surgery has been getting safer in general over time. Rates of death have fallen both in Michigan and in the rest of the country as bariatric surgeons have gained experience with this procedure. In Michigan during this time period, our group has developed and implemented a risk‐stratified, standardized approach to venous thromboembolism prophylaxis for patients undergoing bariatric surgery. For these reasons, we included the year of the procedure and the type of medical venous thromboembolism prophylaxis (unfractionated or low molecular weight heparin) used perioperatively as a matching variable in our analysis.
Another limitation that should be considered in interpreting our findings is statistical power. Although our study is the largest in this study population to date, many of the outcomes of interest are relatively rare. Considering the entire bariatric surgery population, rates of venous thromboembolism and death within 30 days are each less than 1%. Even in the high‐risk patients included in this analysis, there were a total of just 28 (1.3%) venous thromboembolism events and 8 (0.37) deaths. Nonetheless, our study did find significantly greater risks of multiple types of complications among patients receiving IVC filters.
Finally, our study captures events occurring within 30 days of bariatric surgery. Complications, including venous thromboembolism and other complications directly related to IVC filters, frequently occur after 30 days of bariatric surgery. Therefore, our study may be a conservative estimate of the risks associated with the use of IVC filters in bariatric surgery patients. Furthermore, certain brands of filters have been shown to be associated with higher risks of complications. Our study lacks data on the brand of IVC filter used and so cannot assess the extent to which this would affect our results.
CONCLUSIONS
In conclusion, our study indicates that IVC filters do not reduce the risk of pulmonary embolism in high‐risk bariatric surgery patients. They are also associated with other complications attributable to malfunctions of the device itself. We believe that the use of IVC filters among bariatric surgery patients should be discouraged.
Disclosure
This study was supported by a grant from the Agency for Healthcare Research and Quality (HS018050) and was presented at the Annual Meeting of the American Society for Metabolic and Bariatric Surgery (ASMBS), San Diego, California, June 20, 2012.
- , , . Vena cava filters: a call to action. Chest Physician. 2011;16:18a.
- U.S. Food and Drug Administration. Removing retrievable inferior vena cava filters: initial communication. August 9, 2010. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221 676.htm. Accessed December 2, 2012.
- , , , et al. Preoperative placement of inferior vena cava filters and outcomes after gastric bypass surgery. Ann Surg. 2010;252:131–318.
- , , , et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170:1827–1831.
- , , , , , . Experience with inferior vena cava filter placement in patients undergoing open gastric bypass procedures. Ann Vasc Surg. 2006;44: 1301–1305.
- , . Preoperative placement of retrievable inferior vena cava filters in bariatric surgery. Surg Obes Relat Dis. 2007;3: 602–605.
- , , , et al. Safety and efficacy of intravascular ultrasound‐guided inferior vena cava filter in super obese bariatric patients. Surg Obes Relat Dis. 2008;4:50–54.
- , , , , , . Current indications for preoperative inferior vena cava filter insertion in patients undergoing surgery for morbid obesity. Obes Surg. 2005;15:1009–1012.
- , , , . Efficacy of prophylactic inferior vena cava filter placement in bariatric surgery. Surg Obes Relat Dis. 2007;3:606–610.
- , , , et al. Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients. J Vasc Surg. 2007;45:784–788.
- , , , . Retrievable inferior vena cava filters may be safely applied in gastric bypass surgery. Surg Endosc. 2007;21:2277–2279.
- , , , , . Inferior vena cava filter placement for pulmonary embolism risk reduction in super morbidly obese undergoing bariatric surgery. Surg Obes Relat Dis. 2007;3:461–464.
- , . A simple venous thromboembolism prophylaxis protocol for patients undergoing bariatric surgery. Obesity (Silver Spring). 2006;14:1961–1965.
- , , , et al. Risk‐group targeted inferior vena cava filter placemetn in gastric bypass patients. Obes Surg. 2009;19:451–455.
- , , , , , . Retreivable inferior vena cava filters in high‐risk patients undergoing bariatric surgery. Surg Endosc. 2009;23:2203–2207.
- , . Inferior vena caval filter insertion prior to bariatric surgery: A systematic review of the literature. J Thromb Haemost. 2010;8:1266–1270.
- , , . Vena cava filters: a call to action. Chest Physician. 2011;16:18a.
- U.S. Food and Drug Administration. Removing retrievable inferior vena cava filters: initial communication. August 9, 2010. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221 676.htm. Accessed December 2, 2012.
- , , , et al. Preoperative placement of inferior vena cava filters and outcomes after gastric bypass surgery. Ann Surg. 2010;252:131–318.
- , , , et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170:1827–1831.
- , , , , , . Experience with inferior vena cava filter placement in patients undergoing open gastric bypass procedures. Ann Vasc Surg. 2006;44: 1301–1305.
- , . Preoperative placement of retrievable inferior vena cava filters in bariatric surgery. Surg Obes Relat Dis. 2007;3: 602–605.
- , , , et al. Safety and efficacy of intravascular ultrasound‐guided inferior vena cava filter in super obese bariatric patients. Surg Obes Relat Dis. 2008;4:50–54.
- , , , , , . Current indications for preoperative inferior vena cava filter insertion in patients undergoing surgery for morbid obesity. Obes Surg. 2005;15:1009–1012.
- , , , . Efficacy of prophylactic inferior vena cava filter placement in bariatric surgery. Surg Obes Relat Dis. 2007;3:606–610.
- , , , et al. Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients. J Vasc Surg. 2007;45:784–788.
- , , , . Retrievable inferior vena cava filters may be safely applied in gastric bypass surgery. Surg Endosc. 2007;21:2277–2279.
- , , , , . Inferior vena cava filter placement for pulmonary embolism risk reduction in super morbidly obese undergoing bariatric surgery. Surg Obes Relat Dis. 2007;3:461–464.
- , . A simple venous thromboembolism prophylaxis protocol for patients undergoing bariatric surgery. Obesity (Silver Spring). 2006;14:1961–1965.
- , , , et al. Risk‐group targeted inferior vena cava filter placemetn in gastric bypass patients. Obes Surg. 2009;19:451–455.
- , , , , , . Retreivable inferior vena cava filters in high‐risk patients undergoing bariatric surgery. Surg Endosc. 2009;23:2203–2207.
- , . Inferior vena caval filter insertion prior to bariatric surgery: A systematic review of the literature. J Thromb Haemost. 2010;8:1266–1270.
Copyright © 2013 Society of Hospital Medicine
Affordable Care Act Implementation
At the Centers for Medicare and Medicaid Services (CMS), we are charged with implementing many of the major provisions of the Affordable Care Act (ACA). Major policies and programs aimed at transforming the way care is delivered and paid for, testing and scaling innovative delivery system reforms, and expanding the number of Americans with health insurance will now move forward. The healthcare system is moving from paying for volume to paying for value. Hospitals and clinicians will need to be able to manage and be accountable for populations of patients and improving health outcomes. In this article, we highlight 4 broad provisions of the ACA that are either already implemented or under development for implementation in 2014, and are anticipated to have widespread impact on our health system. The potential impacts of each provision on hospitals and hospitalists are outlined in Table 1.
| Affordable Care Act Provision | Example of Potential Impacts on Hospitals and Hospitalists |
|---|---|
| |
| Expansion of insurance coverage | Care for fewer uninsured patients/fewer unreimbursed services |
| Patients have improved access to services after discharge | |
| Shorter lengths of stay due to better access to outpatient services and care | |
| Delivery system transformation | Financial incentives aligned between inpatient and outpatient providers to better coordinate care |
| Payment is at risk if performance rates do not meet benchmarks and if costs are not lowered | |
| Consolidation of hospitals and health systems within local markets | |
| Value‐based purchasing | Medicare FFS reimbursement increased or decreased based on quality and cost measure results |
| Opportunity to align incentives between hospitals and hospitalists | |
| Patient‐centered outcomes research | Emerging research on delivery system interventions relevant to hospitalists, such as care transitions |
| Funding for PCOR available for hospitalist researchers interested in delivery systems and outcomes research | |
EXPANSION OF INSURANCE COVERAGE
The central and perhaps most anticipated provision of the ACA is the expansion of insurance to the currently uninsured through the creation of state‐based health insurance exchanges. The exchanges are a competitive marketplace for purchasing private insurance products by individuals and small and large businesses. The individual mandate that accompanies the exchange provision requires that individuals purchase insurance. For those who cannot afford it, the government provides a subsidy. Any health plan that wishes to participate in an exchange marketplace must include at minimum a package of essential health benefits in each of their insurance products, which include benefits such as ambulatory care services, maternal and newborn services, and prescription drugs.[1] Importantly, health plans are required to implement quality improvement strategies and publicly report quality data. The ACA also requires the Secretary of Health and Human Services (HHS) to develop and administer a quality rating system and an enrollee satisfaction survey system, the results of which will be available to exchange consumers. All of these requirements will promote the delivery of high‐quality healthcare to millions of previously uninsured Americans.
Implementation of the exchanges in combination with the expansion of Medicaid is expected to provide insurance to approximately 30 million people who currently lack coverage. Prior to the Supreme Court ruling in June of 2012, states were required to expand Medicaid eligibility to a minimum of 133% of the federal poverty level. This expansion is subsidized 100% by the federal government through 2016, dropping to 90% by 2020. The Supreme Court ruled that the federal government could not require states to expand their Medicaid rolls, although it is expected that most states will do so given the generous federal subsidy and the significant cost to states, hospitals, and society to provide healthcare to the uninsured.
TRANSFORMATION OF HEALTHCARE DELIVERY
In addition to the expansion of insurance coverage, the ACA initiates a transformation in the way that healthcare will be delivered through the testing and implementation of innovative payment and care delivery models. The ACA authorized the creation of the Center for Medicare and Medicaid Innovation (CMMI, or The Innovation Center) within CMS. Payment and care delivery demonstrations or pilots that demonstrate a high quality of care at lower costs can be scaled up nationally at the discretion of the Secretary, rather than requiring authorization by Congress. The Innovation Center has already launched initiatives that test a variety of new models of care, all of which incentivize care coordination, provision of team‐based care, and use of data and quality metrics to drive systems‐based improvement. These programs include pilots that bundle payments to hospitals, physician group practices, and post‐acute care facilities for episodes of care across settings. This allows providers to innovate and redesign systems to deliver equivalent or higher quality of care at lower costs. Another CMMI model, called the comprehensive primary care initiative, involves CMS partnering with private insurers to provide payment to primary care practices for the delivery of chronic disease management and coordinated care to their entire population of patients, regardless of payer. Of great relevance to all hospitalists, CMMI and CMS, in partnership with other HHS agencies, launched the Partnership for Patients program in 2011. To date, approximately 4000 hospitals have signed on to the Partnership in a collective effort to significantly reduce hospital readmissions and hospital‐acquired conditions. Hospitalists are leading the charge related to Partnership for Patients in many hospitals. The Innovation Center is concurrently launching and rapidly evaluating current pilots, while considering what other new pilots might be needed to further test models aimed at the delivery of better healthcare and health outcomes at lower costs.
Perhaps the delivery system initiative that has received the most attention is the implementation of the Medicare Shared Savings Program (MSSP), or Accountable Care Organizations (ACO). Under the MSSP, ACOs are groups of providers (which may include hospitals) and suppliers of services who work together to coordinate care for the patients they serve. Participating ACOs must achieve performance benchmarks while lowering costs to share in the cost savings with CMS. Although this program is focused on Medicare fee‐for‐service (FFS) beneficiaries, it is expected that all patients will benefit from the infrastructure redesign and care coordination that is required under this program. The pioneer ACOs are large integrated health systems or other providers that have higher levels of shared risk in addition to shared savings. Hospitals that are a part of a participating ACO have greater financial incentives to work with their primary care and other outpatient providers to reduce readmissions and other adverse events and achieve quality benchmarks. With the degree of savings as well as financial risk that is on the table, it is possible that over time, hospitals and health systems may consolidate to capture a larger share of the market. Such a consequence could have a parallel effect on job opportunities and financial incentives and risk for hospitalists in local markets.
VALUE‐BASED PURCHASING
Improvement in the quality of care delivered to all patients is another central purpose of the Affordable Care Act. The law requires that the Secretary develop a National Quality Strategy that must be updated annually; the first version of this strategy was published in April of 2011.[2] The strategy identifies 3 aims for the nation: better healthcare for individuals, better health for populations and communities, and lower costs for all. One of the levers that CMS uses to achieve these 3 aims is value‐based purchasing (VBP). VBP is a way to link the National Quality Strategy with Medicare FFS payments on a national scale by adjusting payments based on performance. VBP rewards providers and health systems that deliver better outcomes in health and healthcare at lower cost to the beneficiaries and communities they serve, rather than rewarding them for the volume of services they provide. The ACA authorizes implementation of the Hospital Value‐Based Purchasing (HVBP) program as well as the Physician Value Modifier (PVM). The HVBP program began in 2011, and currently includes process, outcome, and patient experience quality metrics as well as a total cost metric, which includes 30 days postdischarge for beneficiaries admitted to the hospital. Hospitals are rewarded on either their improvement from baseline or achievement of a benchmark, whichever is higher.[3] The PVM program adjusts providers' Medicare FFS payments up or down beginning in 2015, based on quality metrics reported on care provided in 2013. In the first year of the program, groups of 100 or more physicians are eligible for the program, and are given a choice on metrics to report and whether to elect for quality tiering and the potential for payment adjustment[4]; by payment year 2017, all physicians must participate. To participate, physicians must report on quality metrics that they choose through the Physician Quality Reporting System (PQRS) or elect to have their quality assessed based on administrative claim measures. Measures currently in the PQRS program may not always be relevant for hospitalists; CMS is working to define and include metrics that would be most meaningful to hospitalists' scope of practice and is seeking comment on whether to allow hospital‐based physicians to align with and accept hospital quality measures to count as their performance metrics.
PATIENT‐CENTERED OUTCOMES RESEARCH
Building on the down payment on Comparative Effectiveness Research (CER) funded under the American Recovery and Reinvestment Act of 2009, the ACA authorized the creation of the Patient‐Centered Outcomes Research Institute (PCORI) and allocated funding for CER over 10 years. Rebranded as Patient‐Centered Outcomes Research (PCOR), CER has the potential to improve quality and reduce costs by identifying what works for different populations of patients (eg, children, elderly, patients with multiple chronic conditions, racial and ethnic minorities) in varied settings (eg, ambulatory, hospital, nursing home) under real‐world conditions. The PCORI governance board was created in 2010, and as required by law, developed a national agenda for patient‐centered outcomes research, which includes assessment of prevention, diagnosis, and treatment options; improving healthcare systems; communicating and disseminating research; addressing healthcare disparities; and accelerating PCOR and methodological research. The amount of funding available for research and PCOR infrastructure will ramp up over the next several years, eventually reaching approximately $500 million annually, with increasing funding opportunities for comparative research questions related to clinical and delivery system interventions using pragmatic, randomized, controlled trials; implementation science; and other novel research methodologies. Hospitalists have many roles within this realm, whether as researchers comparing delivery system or clinical interventions, as educators of students or healthcare professionals on the results of PCOR and their implications for practice, or as hospital leaders responsible for implementation of evidence‐based practices.[5]
CONCLUSION
The Affordable Care Act is a transformative piece of legislation, and our healthcare system is changing rapidly. Many of the ACA's provisions will change how care is delivered in the United States and will have a direct effect on practicing physicians, hospitals, and patients. Although CMS plays a major role in the implementation of the law, the government cannot be, and should not be, the primary force in transforming health care in this country. Through the provisions highlighted here as well as others, CMS can create a supportive environment, be a catalyst, and provide incentives for change; however, true transformation must occur on the front lines. For hospitalists, this means partnering with the hospital administration and other hospital personnel, local providers, and community organizations to drive systems‐based improvements that will ultimately achieve higher‐quality care at lower costs for all. It also calls for hospitalists to lead change in their local systems focused on better care, better health, and lower costs through improvement.
Disclosure
The views expressed in this manuscript represent the authors and not necessarily the policy or opinions of the Centers for Medicare and Medicaid Services.
- Department of Health and Human Services. Essential Health Benefits: HHS Informational Bulletin. Available at: http://www.healthcare.gov/news/factsheets/2011/12/essential‐health‐benefits12162011a.html. Accessed December 13, 2012.
- Department of Health and Human Services. Report to Congress: National Strategy for Quality Improvement in Healthcare. March 2011. Available at: http://www.healthcare.gov/law/resources/reports/quality03212011a.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. FY 2013 IPPS Final Rule Home Page. August 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/FY‐2013‐IPPS‐Final‐Rule‐Home‐Page.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. Physician Fee Schedule. November 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/PhysicianFeeSched/index.html. Accessed December 13, 2012.
- , . Comparative effectiveness research: implications for hospitalists. J Hosp Medicine. 2010;5(5):257–260.
At the Centers for Medicare and Medicaid Services (CMS), we are charged with implementing many of the major provisions of the Affordable Care Act (ACA). Major policies and programs aimed at transforming the way care is delivered and paid for, testing and scaling innovative delivery system reforms, and expanding the number of Americans with health insurance will now move forward. The healthcare system is moving from paying for volume to paying for value. Hospitals and clinicians will need to be able to manage and be accountable for populations of patients and improving health outcomes. In this article, we highlight 4 broad provisions of the ACA that are either already implemented or under development for implementation in 2014, and are anticipated to have widespread impact on our health system. The potential impacts of each provision on hospitals and hospitalists are outlined in Table 1.
| Affordable Care Act Provision | Example of Potential Impacts on Hospitals and Hospitalists |
|---|---|
| |
| Expansion of insurance coverage | Care for fewer uninsured patients/fewer unreimbursed services |
| Patients have improved access to services after discharge | |
| Shorter lengths of stay due to better access to outpatient services and care | |
| Delivery system transformation | Financial incentives aligned between inpatient and outpatient providers to better coordinate care |
| Payment is at risk if performance rates do not meet benchmarks and if costs are not lowered | |
| Consolidation of hospitals and health systems within local markets | |
| Value‐based purchasing | Medicare FFS reimbursement increased or decreased based on quality and cost measure results |
| Opportunity to align incentives between hospitals and hospitalists | |
| Patient‐centered outcomes research | Emerging research on delivery system interventions relevant to hospitalists, such as care transitions |
| Funding for PCOR available for hospitalist researchers interested in delivery systems and outcomes research | |
EXPANSION OF INSURANCE COVERAGE
The central and perhaps most anticipated provision of the ACA is the expansion of insurance to the currently uninsured through the creation of state‐based health insurance exchanges. The exchanges are a competitive marketplace for purchasing private insurance products by individuals and small and large businesses. The individual mandate that accompanies the exchange provision requires that individuals purchase insurance. For those who cannot afford it, the government provides a subsidy. Any health plan that wishes to participate in an exchange marketplace must include at minimum a package of essential health benefits in each of their insurance products, which include benefits such as ambulatory care services, maternal and newborn services, and prescription drugs.[1] Importantly, health plans are required to implement quality improvement strategies and publicly report quality data. The ACA also requires the Secretary of Health and Human Services (HHS) to develop and administer a quality rating system and an enrollee satisfaction survey system, the results of which will be available to exchange consumers. All of these requirements will promote the delivery of high‐quality healthcare to millions of previously uninsured Americans.
Implementation of the exchanges in combination with the expansion of Medicaid is expected to provide insurance to approximately 30 million people who currently lack coverage. Prior to the Supreme Court ruling in June of 2012, states were required to expand Medicaid eligibility to a minimum of 133% of the federal poverty level. This expansion is subsidized 100% by the federal government through 2016, dropping to 90% by 2020. The Supreme Court ruled that the federal government could not require states to expand their Medicaid rolls, although it is expected that most states will do so given the generous federal subsidy and the significant cost to states, hospitals, and society to provide healthcare to the uninsured.
TRANSFORMATION OF HEALTHCARE DELIVERY
In addition to the expansion of insurance coverage, the ACA initiates a transformation in the way that healthcare will be delivered through the testing and implementation of innovative payment and care delivery models. The ACA authorized the creation of the Center for Medicare and Medicaid Innovation (CMMI, or The Innovation Center) within CMS. Payment and care delivery demonstrations or pilots that demonstrate a high quality of care at lower costs can be scaled up nationally at the discretion of the Secretary, rather than requiring authorization by Congress. The Innovation Center has already launched initiatives that test a variety of new models of care, all of which incentivize care coordination, provision of team‐based care, and use of data and quality metrics to drive systems‐based improvement. These programs include pilots that bundle payments to hospitals, physician group practices, and post‐acute care facilities for episodes of care across settings. This allows providers to innovate and redesign systems to deliver equivalent or higher quality of care at lower costs. Another CMMI model, called the comprehensive primary care initiative, involves CMS partnering with private insurers to provide payment to primary care practices for the delivery of chronic disease management and coordinated care to their entire population of patients, regardless of payer. Of great relevance to all hospitalists, CMMI and CMS, in partnership with other HHS agencies, launched the Partnership for Patients program in 2011. To date, approximately 4000 hospitals have signed on to the Partnership in a collective effort to significantly reduce hospital readmissions and hospital‐acquired conditions. Hospitalists are leading the charge related to Partnership for Patients in many hospitals. The Innovation Center is concurrently launching and rapidly evaluating current pilots, while considering what other new pilots might be needed to further test models aimed at the delivery of better healthcare and health outcomes at lower costs.
Perhaps the delivery system initiative that has received the most attention is the implementation of the Medicare Shared Savings Program (MSSP), or Accountable Care Organizations (ACO). Under the MSSP, ACOs are groups of providers (which may include hospitals) and suppliers of services who work together to coordinate care for the patients they serve. Participating ACOs must achieve performance benchmarks while lowering costs to share in the cost savings with CMS. Although this program is focused on Medicare fee‐for‐service (FFS) beneficiaries, it is expected that all patients will benefit from the infrastructure redesign and care coordination that is required under this program. The pioneer ACOs are large integrated health systems or other providers that have higher levels of shared risk in addition to shared savings. Hospitals that are a part of a participating ACO have greater financial incentives to work with their primary care and other outpatient providers to reduce readmissions and other adverse events and achieve quality benchmarks. With the degree of savings as well as financial risk that is on the table, it is possible that over time, hospitals and health systems may consolidate to capture a larger share of the market. Such a consequence could have a parallel effect on job opportunities and financial incentives and risk for hospitalists in local markets.
VALUE‐BASED PURCHASING
Improvement in the quality of care delivered to all patients is another central purpose of the Affordable Care Act. The law requires that the Secretary develop a National Quality Strategy that must be updated annually; the first version of this strategy was published in April of 2011.[2] The strategy identifies 3 aims for the nation: better healthcare for individuals, better health for populations and communities, and lower costs for all. One of the levers that CMS uses to achieve these 3 aims is value‐based purchasing (VBP). VBP is a way to link the National Quality Strategy with Medicare FFS payments on a national scale by adjusting payments based on performance. VBP rewards providers and health systems that deliver better outcomes in health and healthcare at lower cost to the beneficiaries and communities they serve, rather than rewarding them for the volume of services they provide. The ACA authorizes implementation of the Hospital Value‐Based Purchasing (HVBP) program as well as the Physician Value Modifier (PVM). The HVBP program began in 2011, and currently includes process, outcome, and patient experience quality metrics as well as a total cost metric, which includes 30 days postdischarge for beneficiaries admitted to the hospital. Hospitals are rewarded on either their improvement from baseline or achievement of a benchmark, whichever is higher.[3] The PVM program adjusts providers' Medicare FFS payments up or down beginning in 2015, based on quality metrics reported on care provided in 2013. In the first year of the program, groups of 100 or more physicians are eligible for the program, and are given a choice on metrics to report and whether to elect for quality tiering and the potential for payment adjustment[4]; by payment year 2017, all physicians must participate. To participate, physicians must report on quality metrics that they choose through the Physician Quality Reporting System (PQRS) or elect to have their quality assessed based on administrative claim measures. Measures currently in the PQRS program may not always be relevant for hospitalists; CMS is working to define and include metrics that would be most meaningful to hospitalists' scope of practice and is seeking comment on whether to allow hospital‐based physicians to align with and accept hospital quality measures to count as their performance metrics.
PATIENT‐CENTERED OUTCOMES RESEARCH
Building on the down payment on Comparative Effectiveness Research (CER) funded under the American Recovery and Reinvestment Act of 2009, the ACA authorized the creation of the Patient‐Centered Outcomes Research Institute (PCORI) and allocated funding for CER over 10 years. Rebranded as Patient‐Centered Outcomes Research (PCOR), CER has the potential to improve quality and reduce costs by identifying what works for different populations of patients (eg, children, elderly, patients with multiple chronic conditions, racial and ethnic minorities) in varied settings (eg, ambulatory, hospital, nursing home) under real‐world conditions. The PCORI governance board was created in 2010, and as required by law, developed a national agenda for patient‐centered outcomes research, which includes assessment of prevention, diagnosis, and treatment options; improving healthcare systems; communicating and disseminating research; addressing healthcare disparities; and accelerating PCOR and methodological research. The amount of funding available for research and PCOR infrastructure will ramp up over the next several years, eventually reaching approximately $500 million annually, with increasing funding opportunities for comparative research questions related to clinical and delivery system interventions using pragmatic, randomized, controlled trials; implementation science; and other novel research methodologies. Hospitalists have many roles within this realm, whether as researchers comparing delivery system or clinical interventions, as educators of students or healthcare professionals on the results of PCOR and their implications for practice, or as hospital leaders responsible for implementation of evidence‐based practices.[5]
CONCLUSION
The Affordable Care Act is a transformative piece of legislation, and our healthcare system is changing rapidly. Many of the ACA's provisions will change how care is delivered in the United States and will have a direct effect on practicing physicians, hospitals, and patients. Although CMS plays a major role in the implementation of the law, the government cannot be, and should not be, the primary force in transforming health care in this country. Through the provisions highlighted here as well as others, CMS can create a supportive environment, be a catalyst, and provide incentives for change; however, true transformation must occur on the front lines. For hospitalists, this means partnering with the hospital administration and other hospital personnel, local providers, and community organizations to drive systems‐based improvements that will ultimately achieve higher‐quality care at lower costs for all. It also calls for hospitalists to lead change in their local systems focused on better care, better health, and lower costs through improvement.
Disclosure
The views expressed in this manuscript represent the authors and not necessarily the policy or opinions of the Centers for Medicare and Medicaid Services.
At the Centers for Medicare and Medicaid Services (CMS), we are charged with implementing many of the major provisions of the Affordable Care Act (ACA). Major policies and programs aimed at transforming the way care is delivered and paid for, testing and scaling innovative delivery system reforms, and expanding the number of Americans with health insurance will now move forward. The healthcare system is moving from paying for volume to paying for value. Hospitals and clinicians will need to be able to manage and be accountable for populations of patients and improving health outcomes. In this article, we highlight 4 broad provisions of the ACA that are either already implemented or under development for implementation in 2014, and are anticipated to have widespread impact on our health system. The potential impacts of each provision on hospitals and hospitalists are outlined in Table 1.
| Affordable Care Act Provision | Example of Potential Impacts on Hospitals and Hospitalists |
|---|---|
| |
| Expansion of insurance coverage | Care for fewer uninsured patients/fewer unreimbursed services |
| Patients have improved access to services after discharge | |
| Shorter lengths of stay due to better access to outpatient services and care | |
| Delivery system transformation | Financial incentives aligned between inpatient and outpatient providers to better coordinate care |
| Payment is at risk if performance rates do not meet benchmarks and if costs are not lowered | |
| Consolidation of hospitals and health systems within local markets | |
| Value‐based purchasing | Medicare FFS reimbursement increased or decreased based on quality and cost measure results |
| Opportunity to align incentives between hospitals and hospitalists | |
| Patient‐centered outcomes research | Emerging research on delivery system interventions relevant to hospitalists, such as care transitions |
| Funding for PCOR available for hospitalist researchers interested in delivery systems and outcomes research | |
EXPANSION OF INSURANCE COVERAGE
The central and perhaps most anticipated provision of the ACA is the expansion of insurance to the currently uninsured through the creation of state‐based health insurance exchanges. The exchanges are a competitive marketplace for purchasing private insurance products by individuals and small and large businesses. The individual mandate that accompanies the exchange provision requires that individuals purchase insurance. For those who cannot afford it, the government provides a subsidy. Any health plan that wishes to participate in an exchange marketplace must include at minimum a package of essential health benefits in each of their insurance products, which include benefits such as ambulatory care services, maternal and newborn services, and prescription drugs.[1] Importantly, health plans are required to implement quality improvement strategies and publicly report quality data. The ACA also requires the Secretary of Health and Human Services (HHS) to develop and administer a quality rating system and an enrollee satisfaction survey system, the results of which will be available to exchange consumers. All of these requirements will promote the delivery of high‐quality healthcare to millions of previously uninsured Americans.
Implementation of the exchanges in combination with the expansion of Medicaid is expected to provide insurance to approximately 30 million people who currently lack coverage. Prior to the Supreme Court ruling in June of 2012, states were required to expand Medicaid eligibility to a minimum of 133% of the federal poverty level. This expansion is subsidized 100% by the federal government through 2016, dropping to 90% by 2020. The Supreme Court ruled that the federal government could not require states to expand their Medicaid rolls, although it is expected that most states will do so given the generous federal subsidy and the significant cost to states, hospitals, and society to provide healthcare to the uninsured.
TRANSFORMATION OF HEALTHCARE DELIVERY
In addition to the expansion of insurance coverage, the ACA initiates a transformation in the way that healthcare will be delivered through the testing and implementation of innovative payment and care delivery models. The ACA authorized the creation of the Center for Medicare and Medicaid Innovation (CMMI, or The Innovation Center) within CMS. Payment and care delivery demonstrations or pilots that demonstrate a high quality of care at lower costs can be scaled up nationally at the discretion of the Secretary, rather than requiring authorization by Congress. The Innovation Center has already launched initiatives that test a variety of new models of care, all of which incentivize care coordination, provision of team‐based care, and use of data and quality metrics to drive systems‐based improvement. These programs include pilots that bundle payments to hospitals, physician group practices, and post‐acute care facilities for episodes of care across settings. This allows providers to innovate and redesign systems to deliver equivalent or higher quality of care at lower costs. Another CMMI model, called the comprehensive primary care initiative, involves CMS partnering with private insurers to provide payment to primary care practices for the delivery of chronic disease management and coordinated care to their entire population of patients, regardless of payer. Of great relevance to all hospitalists, CMMI and CMS, in partnership with other HHS agencies, launched the Partnership for Patients program in 2011. To date, approximately 4000 hospitals have signed on to the Partnership in a collective effort to significantly reduce hospital readmissions and hospital‐acquired conditions. Hospitalists are leading the charge related to Partnership for Patients in many hospitals. The Innovation Center is concurrently launching and rapidly evaluating current pilots, while considering what other new pilots might be needed to further test models aimed at the delivery of better healthcare and health outcomes at lower costs.
Perhaps the delivery system initiative that has received the most attention is the implementation of the Medicare Shared Savings Program (MSSP), or Accountable Care Organizations (ACO). Under the MSSP, ACOs are groups of providers (which may include hospitals) and suppliers of services who work together to coordinate care for the patients they serve. Participating ACOs must achieve performance benchmarks while lowering costs to share in the cost savings with CMS. Although this program is focused on Medicare fee‐for‐service (FFS) beneficiaries, it is expected that all patients will benefit from the infrastructure redesign and care coordination that is required under this program. The pioneer ACOs are large integrated health systems or other providers that have higher levels of shared risk in addition to shared savings. Hospitals that are a part of a participating ACO have greater financial incentives to work with their primary care and other outpatient providers to reduce readmissions and other adverse events and achieve quality benchmarks. With the degree of savings as well as financial risk that is on the table, it is possible that over time, hospitals and health systems may consolidate to capture a larger share of the market. Such a consequence could have a parallel effect on job opportunities and financial incentives and risk for hospitalists in local markets.
VALUE‐BASED PURCHASING
Improvement in the quality of care delivered to all patients is another central purpose of the Affordable Care Act. The law requires that the Secretary develop a National Quality Strategy that must be updated annually; the first version of this strategy was published in April of 2011.[2] The strategy identifies 3 aims for the nation: better healthcare for individuals, better health for populations and communities, and lower costs for all. One of the levers that CMS uses to achieve these 3 aims is value‐based purchasing (VBP). VBP is a way to link the National Quality Strategy with Medicare FFS payments on a national scale by adjusting payments based on performance. VBP rewards providers and health systems that deliver better outcomes in health and healthcare at lower cost to the beneficiaries and communities they serve, rather than rewarding them for the volume of services they provide. The ACA authorizes implementation of the Hospital Value‐Based Purchasing (HVBP) program as well as the Physician Value Modifier (PVM). The HVBP program began in 2011, and currently includes process, outcome, and patient experience quality metrics as well as a total cost metric, which includes 30 days postdischarge for beneficiaries admitted to the hospital. Hospitals are rewarded on either their improvement from baseline or achievement of a benchmark, whichever is higher.[3] The PVM program adjusts providers' Medicare FFS payments up or down beginning in 2015, based on quality metrics reported on care provided in 2013. In the first year of the program, groups of 100 or more physicians are eligible for the program, and are given a choice on metrics to report and whether to elect for quality tiering and the potential for payment adjustment[4]; by payment year 2017, all physicians must participate. To participate, physicians must report on quality metrics that they choose through the Physician Quality Reporting System (PQRS) or elect to have their quality assessed based on administrative claim measures. Measures currently in the PQRS program may not always be relevant for hospitalists; CMS is working to define and include metrics that would be most meaningful to hospitalists' scope of practice and is seeking comment on whether to allow hospital‐based physicians to align with and accept hospital quality measures to count as their performance metrics.
PATIENT‐CENTERED OUTCOMES RESEARCH
Building on the down payment on Comparative Effectiveness Research (CER) funded under the American Recovery and Reinvestment Act of 2009, the ACA authorized the creation of the Patient‐Centered Outcomes Research Institute (PCORI) and allocated funding for CER over 10 years. Rebranded as Patient‐Centered Outcomes Research (PCOR), CER has the potential to improve quality and reduce costs by identifying what works for different populations of patients (eg, children, elderly, patients with multiple chronic conditions, racial and ethnic minorities) in varied settings (eg, ambulatory, hospital, nursing home) under real‐world conditions. The PCORI governance board was created in 2010, and as required by law, developed a national agenda for patient‐centered outcomes research, which includes assessment of prevention, diagnosis, and treatment options; improving healthcare systems; communicating and disseminating research; addressing healthcare disparities; and accelerating PCOR and methodological research. The amount of funding available for research and PCOR infrastructure will ramp up over the next several years, eventually reaching approximately $500 million annually, with increasing funding opportunities for comparative research questions related to clinical and delivery system interventions using pragmatic, randomized, controlled trials; implementation science; and other novel research methodologies. Hospitalists have many roles within this realm, whether as researchers comparing delivery system or clinical interventions, as educators of students or healthcare professionals on the results of PCOR and their implications for practice, or as hospital leaders responsible for implementation of evidence‐based practices.[5]
CONCLUSION
The Affordable Care Act is a transformative piece of legislation, and our healthcare system is changing rapidly. Many of the ACA's provisions will change how care is delivered in the United States and will have a direct effect on practicing physicians, hospitals, and patients. Although CMS plays a major role in the implementation of the law, the government cannot be, and should not be, the primary force in transforming health care in this country. Through the provisions highlighted here as well as others, CMS can create a supportive environment, be a catalyst, and provide incentives for change; however, true transformation must occur on the front lines. For hospitalists, this means partnering with the hospital administration and other hospital personnel, local providers, and community organizations to drive systems‐based improvements that will ultimately achieve higher‐quality care at lower costs for all. It also calls for hospitalists to lead change in their local systems focused on better care, better health, and lower costs through improvement.
Disclosure
The views expressed in this manuscript represent the authors and not necessarily the policy or opinions of the Centers for Medicare and Medicaid Services.
- Department of Health and Human Services. Essential Health Benefits: HHS Informational Bulletin. Available at: http://www.healthcare.gov/news/factsheets/2011/12/essential‐health‐benefits12162011a.html. Accessed December 13, 2012.
- Department of Health and Human Services. Report to Congress: National Strategy for Quality Improvement in Healthcare. March 2011. Available at: http://www.healthcare.gov/law/resources/reports/quality03212011a.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. FY 2013 IPPS Final Rule Home Page. August 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/FY‐2013‐IPPS‐Final‐Rule‐Home‐Page.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. Physician Fee Schedule. November 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/PhysicianFeeSched/index.html. Accessed December 13, 2012.
- , . Comparative effectiveness research: implications for hospitalists. J Hosp Medicine. 2010;5(5):257–260.
- Department of Health and Human Services. Essential Health Benefits: HHS Informational Bulletin. Available at: http://www.healthcare.gov/news/factsheets/2011/12/essential‐health‐benefits12162011a.html. Accessed December 13, 2012.
- Department of Health and Human Services. Report to Congress: National Strategy for Quality Improvement in Healthcare. March 2011. Available at: http://www.healthcare.gov/law/resources/reports/quality03212011a.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. FY 2013 IPPS Final Rule Home Page. August 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/FY‐2013‐IPPS‐Final‐Rule‐Home‐Page.html. Accessed December 13, 2012.
- Centers for Medicare and Medicaid Services. Physician Fee Schedule. November 2012. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/PhysicianFeeSched/index.html. Accessed December 13, 2012.
- , . Comparative effectiveness research: implications for hospitalists. J Hosp Medicine. 2010;5(5):257–260.
Epidemiology of Organ System Dysfunction
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
Copyright © 2013 Society of Hospital Medicine
Nitrogen-binding agent approved for treating urea cycle disorders
Glycerol phenylbutyrate, a nitrogen-binding agent, has been approved for the chronic treatment of adults and children aged 2 years and older with urea cycle disorders, who cannot be managed with a protein-restricted diet or amino acid supplements alone, the Food and Drug Administration announced on Feb. 1.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
It will be marketed under the trade name Ravicti by Hyperion Therapeutics.
Approval was based on a study of 44 adults with urea cycle disorders, which found that glycerol phenylbutyrate was as effective as sodium phenylbutyrate (Buphenyl), in controlling ammonia levels, according to the FDA. In the study, patients were randomized to treatment with sodium phenylbutyrate, an approved treatment for urea cycle disorders, or glycerol phenylbutyrate for 2 weeks, then were switched to the other treatment for 2 weeks. Another three studies in children and adults "provided evidence supporting the long-term safety and effectiveness of Ravicti in patients 2 years and older," the FDA said.
Diarrhea, flatulence, and headache were the most common side effects associated with treatment, according to the FDA.
The manufacturer expects to launch the product by the end of April 2013 and will distribute it through two specialty pharmacies, according to a Hyperion statement.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
Glycerol phenylbutyrate, a nitrogen-binding agent, has been approved for the chronic treatment of adults and children aged 2 years and older with urea cycle disorders, who cannot be managed with a protein-restricted diet or amino acid supplements alone, the Food and Drug Administration announced on Feb. 1.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
It will be marketed under the trade name Ravicti by Hyperion Therapeutics.
Approval was based on a study of 44 adults with urea cycle disorders, which found that glycerol phenylbutyrate was as effective as sodium phenylbutyrate (Buphenyl), in controlling ammonia levels, according to the FDA. In the study, patients were randomized to treatment with sodium phenylbutyrate, an approved treatment for urea cycle disorders, or glycerol phenylbutyrate for 2 weeks, then were switched to the other treatment for 2 weeks. Another three studies in children and adults "provided evidence supporting the long-term safety and effectiveness of Ravicti in patients 2 years and older," the FDA said.
Diarrhea, flatulence, and headache were the most common side effects associated with treatment, according to the FDA.
The manufacturer expects to launch the product by the end of April 2013 and will distribute it through two specialty pharmacies, according to a Hyperion statement.
Glycerol phenylbutyrate, a nitrogen-binding agent, has been approved for the chronic treatment of adults and children aged 2 years and older with urea cycle disorders, who cannot be managed with a protein-restricted diet or amino acid supplements alone, the Food and Drug Administration announced on Feb. 1.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
It will be marketed under the trade name Ravicti by Hyperion Therapeutics.
Approval was based on a study of 44 adults with urea cycle disorders, which found that glycerol phenylbutyrate was as effective as sodium phenylbutyrate (Buphenyl), in controlling ammonia levels, according to the FDA. In the study, patients were randomized to treatment with sodium phenylbutyrate, an approved treatment for urea cycle disorders, or glycerol phenylbutyrate for 2 weeks, then were switched to the other treatment for 2 weeks. Another three studies in children and adults "provided evidence supporting the long-term safety and effectiveness of Ravicti in patients 2 years and older," the FDA said.
Diarrhea, flatulence, and headache were the most common side effects associated with treatment, according to the FDA.
The manufacturer expects to launch the product by the end of April 2013 and will distribute it through two specialty pharmacies, according to a Hyperion statement.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
The product, in a liquid formulation, helps eliminate excess ammonia that results from the accumulation of nitrogen in people with urea cycle disorders, which are inherited metabolic disorders. The drug is taken three times a day with a protein-restricted diet, according to the FDA statement announcing the approval. In some cases, it is used with dietary supplements, such as amino acids or arginine.
Doctors support bipartisan SGR repeal bill
A bill with bipartisan sponsors has been introduced in the U.S. House of Representatives to permanently repeal Medicare's Sustainable Growth Rate formula.
Rep. Joe Heck (R-Nev.) and Rep. Allyson Schwartz (D-Penn.) unveiled their proposal at a briefing with reporters on Feb. 6. They were surrounded by supporters, including representatives from the American College of Physicians, the American Academy of Family Physicians, the American College of Osteopathic Family Physicians, and the National Coalition on Health Care.
In addition to repealing the SGR, the bill also "stabilizes the current payment system for physicians, and it institutes measures to ensure access to primary care with increased updates for primary care physicians in the short term," said Rep. Schwartz, who added that it also "aggressively" tests new payment and delivery models and rewards high value, high quality health care.
Rep. Schwartz and Rep. Heck, an osteopathic physician trained in emergency medicine, also introduced the bill in the last Congress. But both said that they think that legislators are primed to act, in part because of the struggle to reduce health care spending and the deficit.
If the SGR is not replaced or repealed by the end of the year, physicians will see a 27% reduction in pay beginning in January 2014. Each year the cuts are delayed merely adds more on to the final tally for fixing the formula, noted Rep. Heck. The Congressional Budget Office estimated in its latest economic outlook released on Feb. 5 that it would cost about $138 billion to permanently repeal the SGR. That's less than the $245 billion in previous CBO estimates.
"The time right now is perfect to finally pass this legislation," said Rep. Heck.
"I think the imminent process of sequestration may add a little urgency to reform because across the board cuts are not going to get us where we need to go," said John Rother, president and CEO of the National Coalition on Health Care, an umbrella group representing medical societies, businesses, unions, health care providers, religious associations, insurers, and consumers. "And the alternative here is smarter and much more oriented toward value, and it provides a very practical and beneficial alternative to the kind of meat-axe approaches in sequestration," Mr. Rother said.
Physician groups said they are hopeful that the proposal has legs this year.
The constant uncertainty about whether SGR cuts will occur, "undermines the family doctor's ability to continue to keep doors open and to invest in their practices," said Dr. Jeffrey Cain, president of the AAFP. He praised the Heck-Schwartz bill, which had not been officially introduced in the House at press time, saying that it would put an end to "the annual question of whether physicians can continue to afford to practice in Medicare," and that it also "stabilizes the Medicare cost system and provides solutions that are based on successful and proven methods that can improve quality and incent value."
Dr. Chuck Cutler, chair-elect of the ACP Board of Regents, said that "the stability that this bill brings to the marketplace and to our practice is particularly encouraging." He also said that the ACP was happy that the bill would maintain 2013 payment levels through the end of 2014 and then provide "positive and predictable updates" through 2019.
That is especially important as physicians test out new delivery and payment models, said Dr. Cutler.
From 2015 to 2018, the bill calls for annual increases of 2.5% for primary care, preventive, and care-coordination services. All other physicians would get a 0.5% increase for the 4-year period.
By 2019, physicians who continue to use a volume-drive fee-for-service model would get a smaller increase than would those who have transitioned to new models.
In addition to the groups who participated in the briefing, the bill also is supported by the American College of Obstetricians and Gynecologists, the Society of Hospital Medicine, the American College of Rheumatology, the American College of Cardiology, the American Academy of Neurology, and the American Academy of Pediatrics.
On Twitter @aliciaault
A bill with bipartisan sponsors has been introduced in the U.S. House of Representatives to permanently repeal Medicare's Sustainable Growth Rate formula.
Rep. Joe Heck (R-Nev.) and Rep. Allyson Schwartz (D-Penn.) unveiled their proposal at a briefing with reporters on Feb. 6. They were surrounded by supporters, including representatives from the American College of Physicians, the American Academy of Family Physicians, the American College of Osteopathic Family Physicians, and the National Coalition on Health Care.
In addition to repealing the SGR, the bill also "stabilizes the current payment system for physicians, and it institutes measures to ensure access to primary care with increased updates for primary care physicians in the short term," said Rep. Schwartz, who added that it also "aggressively" tests new payment and delivery models and rewards high value, high quality health care.
Rep. Schwartz and Rep. Heck, an osteopathic physician trained in emergency medicine, also introduced the bill in the last Congress. But both said that they think that legislators are primed to act, in part because of the struggle to reduce health care spending and the deficit.
If the SGR is not replaced or repealed by the end of the year, physicians will see a 27% reduction in pay beginning in January 2014. Each year the cuts are delayed merely adds more on to the final tally for fixing the formula, noted Rep. Heck. The Congressional Budget Office estimated in its latest economic outlook released on Feb. 5 that it would cost about $138 billion to permanently repeal the SGR. That's less than the $245 billion in previous CBO estimates.
"The time right now is perfect to finally pass this legislation," said Rep. Heck.
"I think the imminent process of sequestration may add a little urgency to reform because across the board cuts are not going to get us where we need to go," said John Rother, president and CEO of the National Coalition on Health Care, an umbrella group representing medical societies, businesses, unions, health care providers, religious associations, insurers, and consumers. "And the alternative here is smarter and much more oriented toward value, and it provides a very practical and beneficial alternative to the kind of meat-axe approaches in sequestration," Mr. Rother said.
Physician groups said they are hopeful that the proposal has legs this year.
The constant uncertainty about whether SGR cuts will occur, "undermines the family doctor's ability to continue to keep doors open and to invest in their practices," said Dr. Jeffrey Cain, president of the AAFP. He praised the Heck-Schwartz bill, which had not been officially introduced in the House at press time, saying that it would put an end to "the annual question of whether physicians can continue to afford to practice in Medicare," and that it also "stabilizes the Medicare cost system and provides solutions that are based on successful and proven methods that can improve quality and incent value."
Dr. Chuck Cutler, chair-elect of the ACP Board of Regents, said that "the stability that this bill brings to the marketplace and to our practice is particularly encouraging." He also said that the ACP was happy that the bill would maintain 2013 payment levels through the end of 2014 and then provide "positive and predictable updates" through 2019.
That is especially important as physicians test out new delivery and payment models, said Dr. Cutler.
From 2015 to 2018, the bill calls for annual increases of 2.5% for primary care, preventive, and care-coordination services. All other physicians would get a 0.5% increase for the 4-year period.
By 2019, physicians who continue to use a volume-drive fee-for-service model would get a smaller increase than would those who have transitioned to new models.
In addition to the groups who participated in the briefing, the bill also is supported by the American College of Obstetricians and Gynecologists, the Society of Hospital Medicine, the American College of Rheumatology, the American College of Cardiology, the American Academy of Neurology, and the American Academy of Pediatrics.
On Twitter @aliciaault
A bill with bipartisan sponsors has been introduced in the U.S. House of Representatives to permanently repeal Medicare's Sustainable Growth Rate formula.
Rep. Joe Heck (R-Nev.) and Rep. Allyson Schwartz (D-Penn.) unveiled their proposal at a briefing with reporters on Feb. 6. They were surrounded by supporters, including representatives from the American College of Physicians, the American Academy of Family Physicians, the American College of Osteopathic Family Physicians, and the National Coalition on Health Care.
In addition to repealing the SGR, the bill also "stabilizes the current payment system for physicians, and it institutes measures to ensure access to primary care with increased updates for primary care physicians in the short term," said Rep. Schwartz, who added that it also "aggressively" tests new payment and delivery models and rewards high value, high quality health care.
Rep. Schwartz and Rep. Heck, an osteopathic physician trained in emergency medicine, also introduced the bill in the last Congress. But both said that they think that legislators are primed to act, in part because of the struggle to reduce health care spending and the deficit.
If the SGR is not replaced or repealed by the end of the year, physicians will see a 27% reduction in pay beginning in January 2014. Each year the cuts are delayed merely adds more on to the final tally for fixing the formula, noted Rep. Heck. The Congressional Budget Office estimated in its latest economic outlook released on Feb. 5 that it would cost about $138 billion to permanently repeal the SGR. That's less than the $245 billion in previous CBO estimates.
"The time right now is perfect to finally pass this legislation," said Rep. Heck.
"I think the imminent process of sequestration may add a little urgency to reform because across the board cuts are not going to get us where we need to go," said John Rother, president and CEO of the National Coalition on Health Care, an umbrella group representing medical societies, businesses, unions, health care providers, religious associations, insurers, and consumers. "And the alternative here is smarter and much more oriented toward value, and it provides a very practical and beneficial alternative to the kind of meat-axe approaches in sequestration," Mr. Rother said.
Physician groups said they are hopeful that the proposal has legs this year.
The constant uncertainty about whether SGR cuts will occur, "undermines the family doctor's ability to continue to keep doors open and to invest in their practices," said Dr. Jeffrey Cain, president of the AAFP. He praised the Heck-Schwartz bill, which had not been officially introduced in the House at press time, saying that it would put an end to "the annual question of whether physicians can continue to afford to practice in Medicare," and that it also "stabilizes the Medicare cost system and provides solutions that are based on successful and proven methods that can improve quality and incent value."
Dr. Chuck Cutler, chair-elect of the ACP Board of Regents, said that "the stability that this bill brings to the marketplace and to our practice is particularly encouraging." He also said that the ACP was happy that the bill would maintain 2013 payment levels through the end of 2014 and then provide "positive and predictable updates" through 2019.
That is especially important as physicians test out new delivery and payment models, said Dr. Cutler.
From 2015 to 2018, the bill calls for annual increases of 2.5% for primary care, preventive, and care-coordination services. All other physicians would get a 0.5% increase for the 4-year period.
By 2019, physicians who continue to use a volume-drive fee-for-service model would get a smaller increase than would those who have transitioned to new models.
In addition to the groups who participated in the briefing, the bill also is supported by the American College of Obstetricians and Gynecologists, the Society of Hospital Medicine, the American College of Rheumatology, the American College of Cardiology, the American Academy of Neurology, and the American Academy of Pediatrics.
On Twitter @aliciaault
Think outside the box─and outside your hospital─when planning your next hire
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Tighter rules for ad hoc PCI
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
Patient empowerment: A coming of age story
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
Obesity, diabetes fuel liver disease epidemic
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler